Maillard Reaction-Derived Carbon Nanodots: Food-Origin Nanomaterials with Emerging Functional and Biomedical Potential
Abstract
1. Introduction
2. Methodology of the Review
3. Understanding the Maillard Reaction (MR)
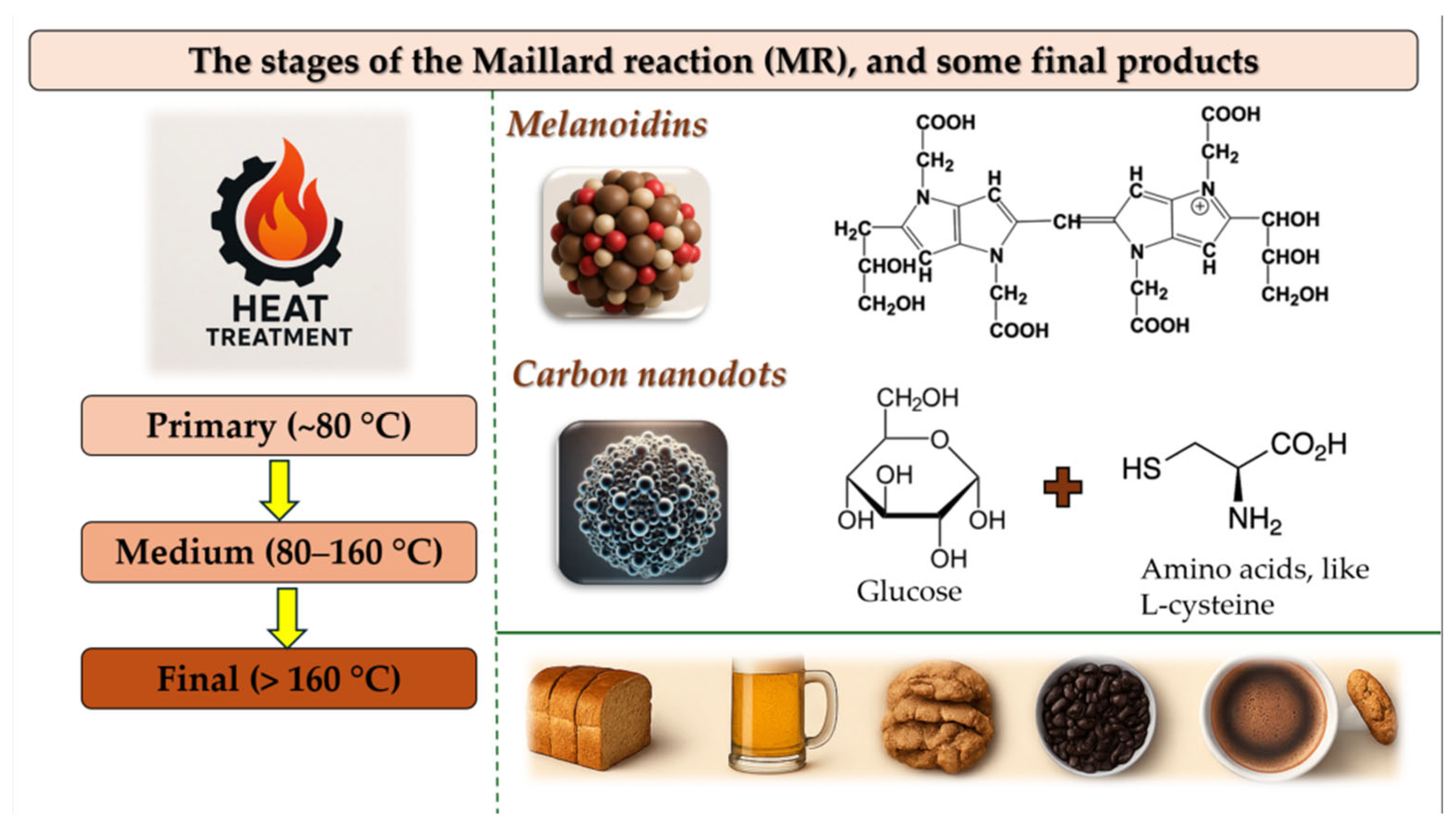
3.1. The Stages of MR
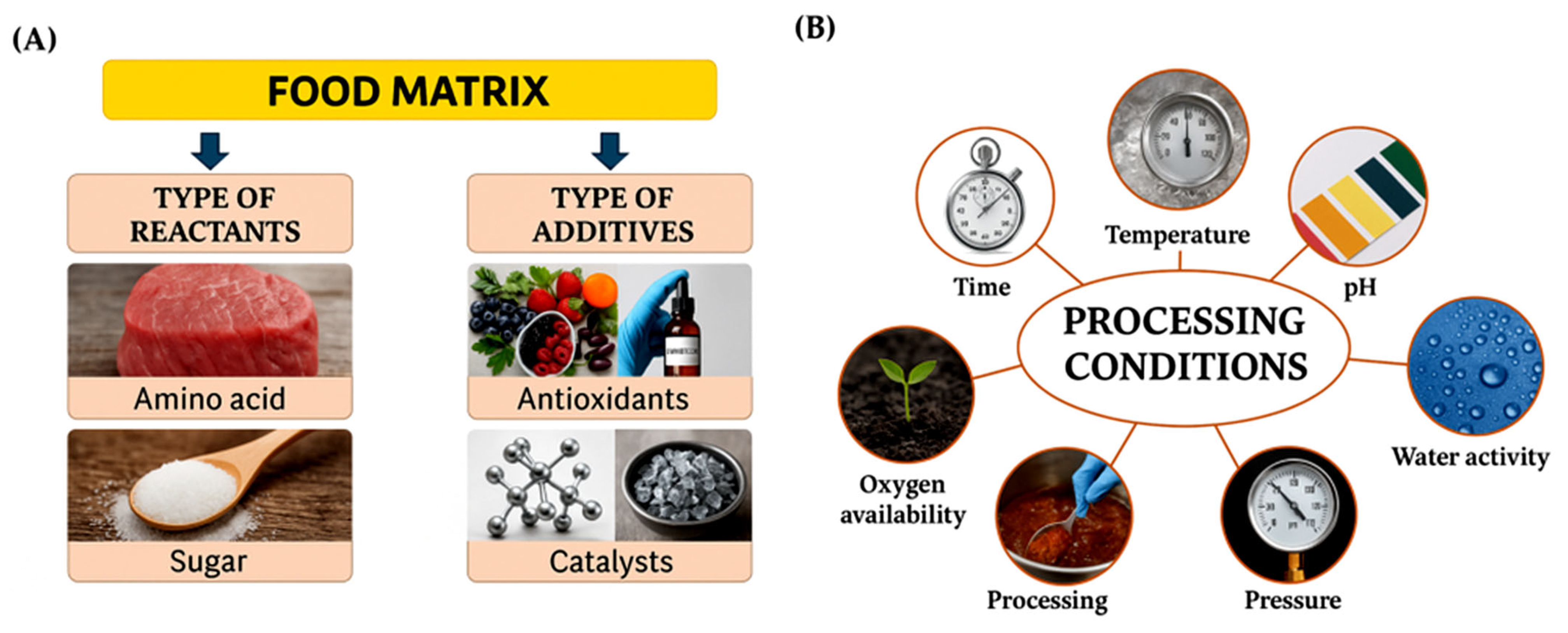
3.2. Factors Influencing the Maillard Reaction in Food Processing
4. Types of Maillard Reaction Products
4.1. Flavor Compounds
4.2. Color Compounds
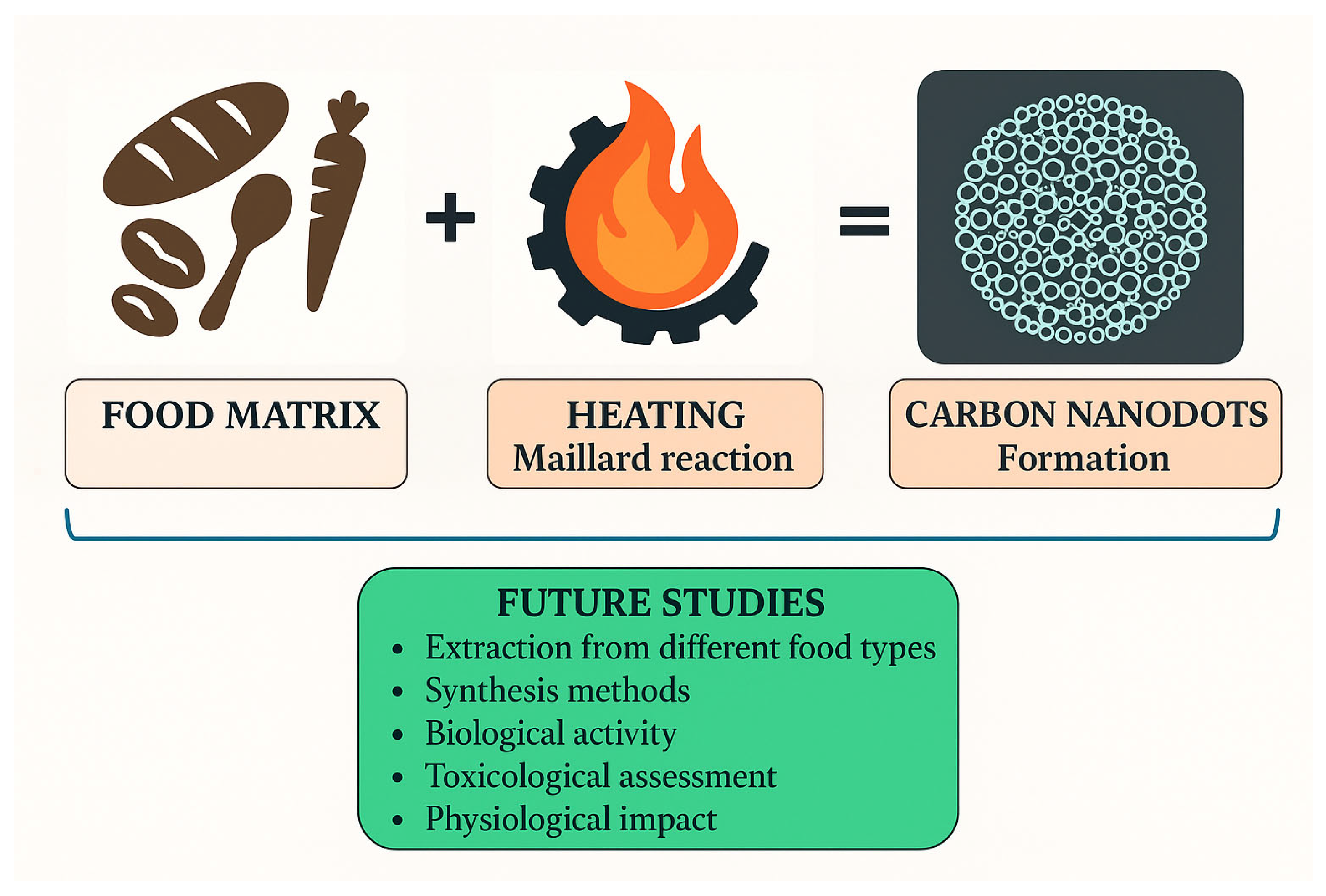
4.3. Recent Findings on the Formation of Carbon Nanodots (CNDs) Through the Maillard Reaction
- -
- In food science, CNDs could act as natural colorants, biosensors, or antioxidant carriers, providing functional benefits while enabling traceability and freshness indicators in packaging [67].
- -
- In biomedicine, their biocompatibility and ability to cross cell membranes support drug delivery, tumor imaging, and bio-sensing applications [68].
- -
- In environmental science, food-derived CNDs can have promise in pollutant adsorption, heavy metal chelation, and green catalysis [69].
5. Maillard Reaction in Different Food Types
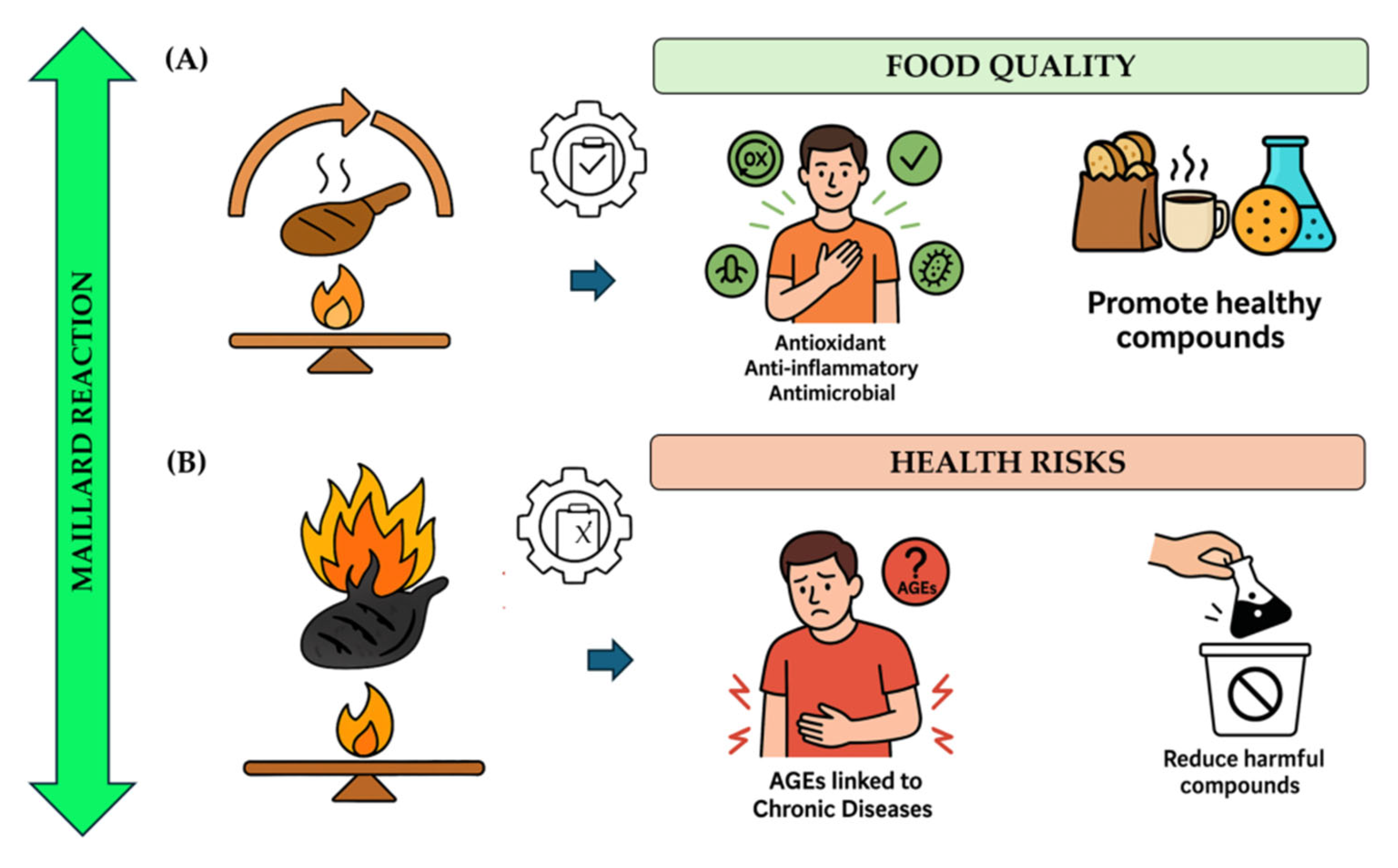
6. Health Implications and Consumer Perception
6.1. Positive Effects
6.2. Negative Effects on Health and Awareness
6.3. Potential Carcinogens
7. Fluorescent and Functional Characteristics of CND
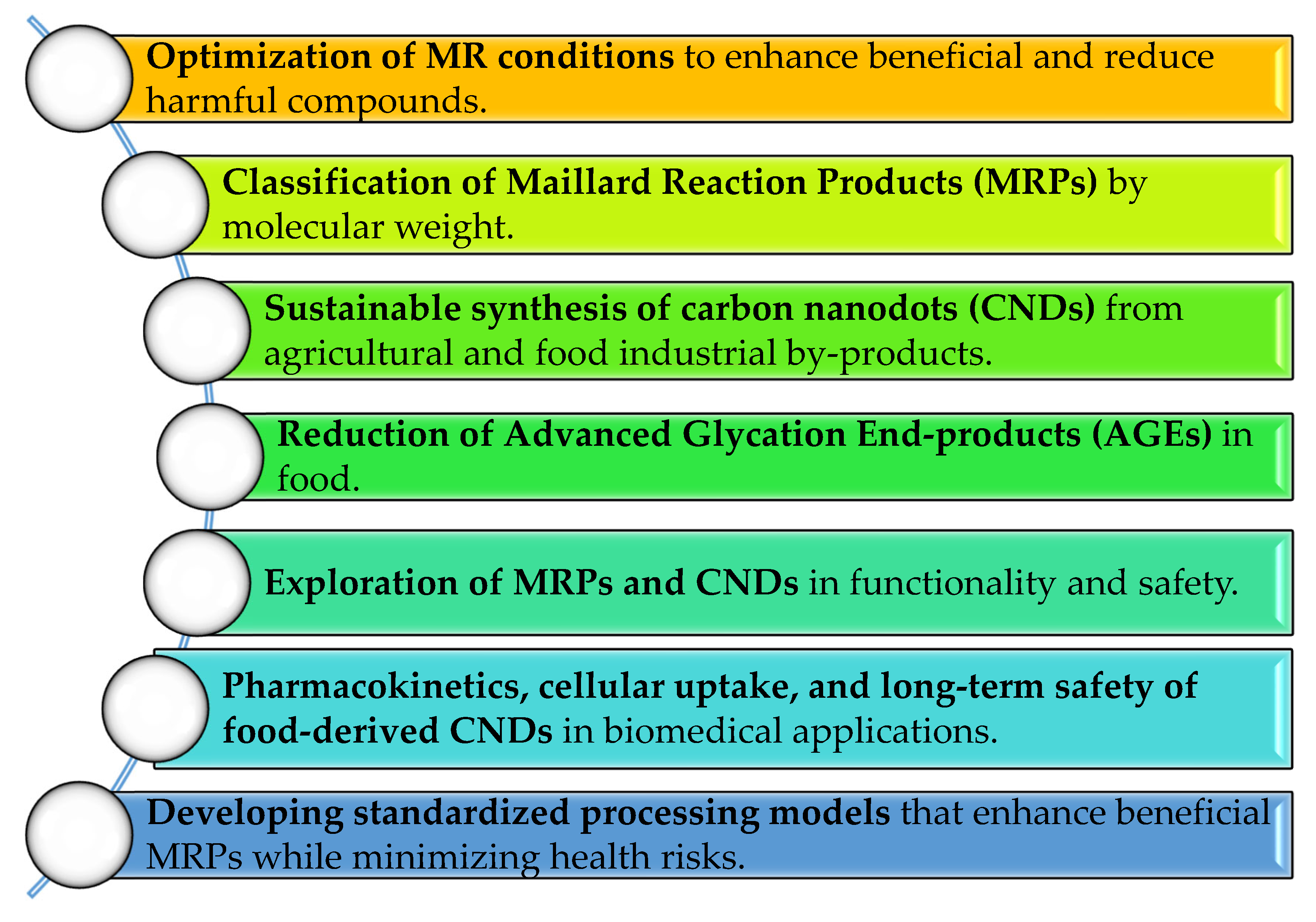
8. General Suggestions and Future Research Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murata, M. Browning and Pigmentation in Food Through the Maillard Reaction. Glycoconj. J. 2021, 38, 283–292. [Google Scholar] [CrossRef]
- Poojary, M.M.; Lund, M.N. Chemical Stability of Proteins in Foods: Oxidation and the Maillard Reaction. Annu. Rev. Food Sci. Technol. 2022, 13, 35–58. [Google Scholar] [CrossRef]
- Sun, A.; Wu, W.; Soladoye, O.P.; Aluko, R.E.; Bak, K.H.; Fu, Y.; Zhang, Y. Maillard Reaction of Food-Derived Peptides as a Potential Route to Generate Meat Flavor Compounds: A Review. Food Res. Int. 2022, 151, 110823. [Google Scholar] [CrossRef] [PubMed]
- Akib, H.; Al-Mamun, A.; Ashadujjaman Robin, M.; Habiba, U.; Sultana, R. Maillard Reaction: Food Processing Aspects. N. Am. Acad. Res. 2021, 4, 44–52. [Google Scholar] [CrossRef]
- Han, Z.; Zhu, M.; Wan, X.; Zhai, X.; Ho, C.-T.; Zhang, L. Food Polyphenols and Maillard Reaction: Regulation Effect and Chemical Mechanism. Crit. Rev. Food Sci. Nutr. 2024, 64, 4904–4920. [Google Scholar] [CrossRef] [PubMed]
- El Hosry, L.; Elias, V.; Chamoun, V.; Halawi, M.; Cayot, P.; Nehme, A.; Bou-Maroun, E. Maillard Reaction: Mechanism, Influencing Parameters, Advantages, Disadvantages, and Food Industrial Applications: A Review. Foods 2025, 14, 1881. [Google Scholar] [CrossRef]
- Al-Abbasy, O.Y.; Younus, S.A.; Rashan, A.I.; Ahmad, O.A.S. Maillard Reaction: Formation, Advantage, Disadvantage and Control. A Review. Food Sci. Appl. Biotechnol. 2024, 7, 145. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Ma, G.; Zhang, T.; Wang, L.; Pei, H.; Li, X.; Gao, L. Insights into Flavor and Key Influencing Factors of Maillard Reaction Products: A Recent Update. Front. Nutr. 2022, 9, 973677. [Google Scholar] [CrossRef]
- Lohinova, A.; Petrusha, O. Maillard Reaction in Food Technologies. Ukr. J. Food Sci. 2023, 11, 81–109. [Google Scholar] [CrossRef]
- Shakoor, A.; Zhang, C.; Xie, J.; Yang, X. Maillard Reaction Chemistry in Formation of Critical Intermediates and Flavour Compounds and Their Antioxidant Properties. Food Chem. 2022, 393, 133416. [Google Scholar] [CrossRef]
- Jia, W.; Guo, A.; Zhang, R.; Shi, L. Mechanism of Natural Antioxidants Regulating Advanced Glycosylation End Products of Maillard Reaction. Food Chem. 2023, 404, 134541. [Google Scholar] [CrossRef]
- Wang, W.; Sun, B.; Deng, J.; Ai, N. Addressing Flavor Challenges in Reduced-Fat Dairy Products: A Review from the Perspective of Flavor Compounds and Their Improvement Strategies. Food Res. Int. 2024, 188, 114478. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Akıllıoğlu, H.G.; Bevilacqua, M.; Abate, G.; Lund, M.N. Investigation of Maillard Reaction Products in Plant-Based Milk Alternatives. Food Res. Int. 2024, 198, 115418. [Google Scholar] [CrossRef] [PubMed]
- Shengbu, M.; Ai, L.; Shi, Q.; Zhao, Q.; Liu, X.; Lai, X. Research Progress of Maillard Reaction and Its Application in Processing of Traditional Chinese Medicine. Nat. Product. Commun. 2024, 19, 1934578X241290620. [Google Scholar] [CrossRef]
- Kaspchak, E.; Igarashi Mafra, L.; Mafra, M.R. Antinutrient and Sugar Type Effect on Structure and Maillard Reaction of Bovine Serum Albumin Submitted to Mild Heating. ACS Food Sci. Technol. 2022, 2, 1468–1476. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.-P.; Zhang, Y.-N.; Li, Y.; Gu, L.-T.; Sun, H.-H.; Liu, M.-D.; Zhou, H.-L.; Wang, Y.-S.; Xu, Z.-X. Short-Chain Fatty Acids in Diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Saltmarch, M.; Labuza, T.P. Nonenzymatic Browning via the Maillard Reaction in Foods. Diabetes 1982, 31 (Suppl. S3), 29–36. [Google Scholar] [CrossRef]
- Urango, A.C.M.; Meireles, M.A.A.; Silva, E.K. Maillard Conjugates Produced from Proteins and Prebiotic Dietary Fibers: Technological Properties, Health Benefits and Challenges. Trends Food Sci. Technol. 2024, 147, 104438. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Y.; Hou, T.; Ning, Q.; Han, W.; Zhao, X.; Cui, F.; Li, H. Formation of Advanced Glycation End Products in Glucose–Amino Acid Models of Maillard Reaction Under Dry- and Wet-heating Conditions. J. Sci. Food Agric. 2025, 105, 2342–2351. [Google Scholar] [CrossRef]
- Agarwal, N.; Mason, A.; Pradhan, R.; Kemper, J.; Bosley, A.; Serfiotis-Mitsa, D.; Wang, J.; Lindo, V.; Ahuja, S.; Hatton, D.; et al. Kinetic Modeling as a Tool to Understand the Influence of Cell Culture Process Parameters on the Glycation of Monoclonal Antibody Biotherapeutics. Biotechnol. Progress. 2019, 35, e2865. [Google Scholar] [CrossRef]
- Alonso-Riaño, P.; Illera, A.E.; Benito-Román, O.; Melgosa, R.; Bermejo-López, A.; Beltrán, S.; Sanz, M.T. Degradation Kinetics of Sugars (Glucose and Xylose), Amino Acids (Proline and Aspartic Acid) and Their Binary Mixtures in Subcritical Water: Effect of Maillard Reaction. Food Chem. 2024, 442, 138421. [Google Scholar] [CrossRef]
- Feng, J.; Berton-Carabin, C.C.; Ataç Mogol, B.; Schroën, K.; Fogliano, V. Glycation of Soy Proteins Leads to a Range of Fractions with Various Supramolecular Assemblies and Surface Activities. Food Chem. 2021, 343, 128556. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Chen, S.; Shi, Y.; Wang, P.; Wu, Y.; Li, G. Dietary Advanced Glycation End Products (dAGEs): An Insight between Modern Diet and Health. Food Chem. 2023, 415, 135735. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Liu, F.; Wang, B.; Chen, L.; Liu, W.; Tan, S. A Literature Review on Maillard Reaction Based on Milk Proteins and Carbohydrates in Food and Pharmaceutical Products: Advantages, Disadvantages, and Avoidance Strategies. Foods 2021, 10, 1998. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Woo, M.W.; Hu, J.; Xiong, H.; Zhao, Q. The Role of Heating Time on the Characteristics, Functional Properties and Antioxidant Activity of Enzyme-Hydrolyzed Rice Proteins-Glucose Maillard Reaction Products. Food Biosci. 2021, 43, 101225. [Google Scholar] [CrossRef]
- Kathuria, D.; Hamid; Gautam, S.; Thakur, A. Maillard Reaction in Different Food Products: Effect on Product Quality, Human Health and Mitigation Strategies. Food Control 2023, 153, 109911. [Google Scholar] [CrossRef]
- Kitts, D.D.; Chen, X.-M.; Jing, H. Demonstration of Antioxidant and Anti-Inflammatory Bioactivities from Sugar–Amino Acid Maillard Reaction Products. J. Agric. Food Chem. 2012, 60, 6718–6727. [Google Scholar] [CrossRef]
- Fay, L.B.; Brevard, H. Contribution of Mass Spectrometry to the Study of the Maillard Reaction in Food. Mass Spectrom. Rev. 2005, 24, 487–507. [Google Scholar] [CrossRef]
- Meng, L.; Nie, Y.; Zhou, Q.; Zheng, T.; Song, J.; Zhang, C.; Chen, H.; Lin, D.; Cao, S.; Xu, S. Effect of Hot-Air Drying Processing on the Volatile Organic Compounds and Maillard Precursors of Dictyophora Rubrovalvata Based on GC-IMS, HPLC and LC-MS. Food Chem. 2025, 463, 141074. [Google Scholar] [CrossRef]
- Bureau, J.-A.; Oliva, M.E.; Dong, Y.; Ignea, C. Engineering Yeast for the Production of Plant Terpenoids Using Synthetic Biology Approaches. Nat. Prod. Rep. 2023, 40, 1822–1848. [Google Scholar] [CrossRef]
- Lovato, K.; Fier, P.S.; Maloney, K.M. The Application of Modern Reactions in Large-Scale Synthesis. Nat. Rev. Chem. 2021, 5, 546–563. [Google Scholar] [CrossRef]
- Rui, X.; Fu, K.; Wang, H.; Pan, T.; Wang, W. Formation Mechanisms of Protein Coronas on Food-Related Nanoparticles: Their Impact on Digestive System and Bioactive Compound Delivery. Foods 2025, 14, 512. [Google Scholar] [CrossRef]
- Han, L.; Wang, L.-H.; Wen, Y.-J.; Zhang, X.-J.; Liu, P.-P.; Zhao, X.-D.; Zheng, Q.-X.; Chai, G.-B.; Zhang, Q.-D.; Yu, Y.-J.; et al. A Study on the Dynamic Changes and Relationships of Volatile and Semi-Volatile Compounds in Flaxseed during the Roasting Procedure by Using Untargeted GC–MS Combined with Advanced Chemometrics. Microchem. J. 2025, 211, 113130. [Google Scholar] [CrossRef]
- Pielech-Przybylska, K.; Balcerek, M.; Dziekońska-Kubczak, U.; Patelski, P.; Różański, M. Effect of Starch Liberation Method and Initial pH of Sweet Mashes on Higher Alcohols Content in Distillates Obtained from Different Starchy Raw Materials. Process Biochem. 2018, 73, 29–37. [Google Scholar] [CrossRef]
- Du, W.; Wang, Y.; Yan, Q.; Bai, S.; Huang, Y.; Li, L.; Mu, Y.; Shakoor, A.; Fan, B.; Wang, F. The Number and Position of Unsaturated Bonds in Aliphatic Aldehydes Affect the Cysteine-Glucose Maillard Reaction: Formation Mechanism and Comparison of Volatile Compounds. Food Res. Int. 2023, 173, 113337. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhang, S.; Zhou, P.; Zhang, W.; Luo, X.; Cao, J.; Sun, D. Analysis of Volatile Flavor Substances in the Enzymatic Hydrolysate of Lanmaoa Asiatica Mushroom and Its Maillard Reaction Products Based on E-Nose and GC-IMS. Foods 2022, 11, 4056. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Xia, X.; Cui, H.; Hayat, K.; Zhang, X.; Ho, C.-T. Competitive Formation of 2,3-Butanedione and Pyrazines through Intervention of Added Cysteine during Thermal Processing of Alanine-Xylose Amadori Compounds. J. Agric. Food Chem. 2022, 70, 15202–15212. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Cao, Y. Effect of Maillard Reaction Products Derived from Cysteine on the Formation of Dimethyl Disulfide and Dimethyl Trisulfide during Storage. J. Agric. Food Chem. 2023, 71, 13043–13053. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. How Maillard Reaction Influences Sensorial Properties (Color, Flavor and Texture) of Food Products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, H.; Tian, L.; Shi, C.; Zheng, Y.; Wang, J.; Tan, Y.; Luo, Y.; Hong, H. Gut Microbiota and Metabolic Profile as Affected by Maillard Reaction Products Derived from Bighead Carp Meat Hydrolysates with Galactose and Galacto-Oligosaccharides during in Vitro Pig Fecal Fermentation. Food Chem. 2023, 398, 133905. [Google Scholar] [CrossRef]
- Yanagimoto, K.; Lee, K.-G.; Ochi, H.; Shibamoto, T. Antioxidative Activity of Heterocyclic Compounds Formed in Maillard Reaction Products. Int. Congr. Ser. 2002, 1245, 335–340. [Google Scholar] [CrossRef]
- Nath, P.; Pandey, N.; Samota, M.; Sharma, K.; Kale, S.; Kannaujia, P.; Sethi, S.; Chauhan, O.P. Browning Reactions in Foods. In Advances in Food Chemistry; Chauhan, O.P., Ed.; Springer Nature: Singapore, 2022; pp. 117–159. ISBN 978-981-19-4795-7. [Google Scholar]
- Echavarría, A.P.; Pagán, J.; Ibarz, A. Melanoidins Formed by Maillard Reaction in Food and Their Biological Activity. Food Eng. Rev. 2012, 4, 203–223. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, V.; Kumar, S.; Majid, I.; Aggarwal, P.; Suri, S. 5-Hydroxymethylfurfural (HMF) Formation, Occurrence and Potential Health Concerns: Recent Developments. Toxin Rev. 2021, 40, 545–561. [Google Scholar] [CrossRef]
- Bork, L.V.; Stobernack, T.; Rohn, S.; Kanzler, C. Browning Reactions of Hydroxycinnamic Acids and Heterocyclic Maillard Reaction Intermediates–Formation of Phenol-Containing Colorants. Food Chem. 2024, 449, 139189. [Google Scholar] [CrossRef]
- Bayat, A.; Dondapati, J.S.; Ahmed, S.R.; Srinivasan, S.; Rajabzadeh, A.R. Electrochemical Detection of 4(5)-Methylimidazole in Aqueous Solutions. Food Chem. 2024, 450, 139320. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, S.; Zhang, Q.; Wang, N.; Yang, Q.; Hao, J. Development and Standardization of Spectrophotometric Assay for Quantification of Thermal Hydrolysis-Origin Melanoidins and Its Implication in Antioxidant Activity Evaluation. J. Hazard. Mater. 2024, 476, 135021. [Google Scholar] [CrossRef]
- Ren, A.; Zhang, Y.; Bian, Y.; Liu, Y.; Zhang, Y.; Ren, C.; Zhou, Y.; Zhang, T.; Feng, X. Pyrazines in Food Samples: Recent Update on Occurrence, Formation, Sampling, Pretreatment and Analysis Methods. Food Chem. 2024, 430, 137086. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, J.; Ye, B.; Shen, Y.; Liu, L. Inhibition Mechanism against Hemoglobin Oxidation of Volatile Pyrroles from Maillard Reaction. Food Chem. 2025, 480, 143870. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Zhang, Q.; Chai, G.; Yang, C.; Meng, Y.; Xu, H.; Chen, S. Color Characteristics and Pyrolysis Volatile Properties of Main Colored Fractions from the Maillard Reaction Models of Glucose with Three Amino Acids. LWT 2024, 192, 115739. [Google Scholar] [CrossRef]
- Törős, G.; Béni, Á.; Balláné, A.K.; Semsey, D.; Ferroudj, A.; Prokisch, J. Production of Myco-Nanomaterial Products from Pleurotus Ostreatus (Agaricomycetes) Mushroom via Pyrolysis. Pharmaceutics 2025, 17, 591. [Google Scholar] [CrossRef]
- Nguyen, D.H.H.; Muthu, A.; El-Ramady, H.; Béni, Á.; Prokisch, J. Detection and Formation of Fluorescent Carbon Nanodots in Coffee Brews and Its Relationship with Other Compositions. J. Food Compos. Anal. 2024, 132, 106347. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. Engl. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Semsey, D.; Nguyen, D.H.H.; Törős, G.; Muthu, A.; Labidi, S.; El-Ramady, H.; Béni, Á.; Rai, M.; József, P. Analysis of Fluorescent Carbon Nanodot Formation during Pretzel Production. Nanomaterials 2024, 14, 1142. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Na, X.; Wang, H.; Wang, C.; Yuan, Z.; Zhu, B.-W.; Tan, M. The Effects of Carbon Dots Produced by the Maillard Reaction on the HepG2 Cell Substance and Energy Metabolism. Food Funct. 2020, 11, 6487–6495. [Google Scholar] [CrossRef]
- Wang, D.; Yan, Z.; Ren, L.; Jiang, Y.; Zhou, K.; Li, X.; Cui, F.; Li, T.; Li, J. Carbon Dots as New Antioxidants: Synthesis, Activity, Mechanism and Application in the Food Industry. Food Chem. 2025, 475, 143377. [Google Scholar] [CrossRef]
- Uzcan, F.; Soylak, M. Milk-Derived Carbon Nanodots for Dispersive Micro-Solid Phase Extraction of Copper at Trace Levels from Vegetable Samples. Anal. Lett. 2025, 1–15. [Google Scholar] [CrossRef]
- Semsey, D.; Nguyen, D.H.H.; Törős, G.; Papp, V.; Pénzes, J.; Vida, T.; Béni, Á.; Rai, M.; Prokisch, J. Analysis of Fluorescent Carbon Nanodots Synthesized from Spices Through Thermal Processes Treatment. Nanomaterials 2025, 15, 625. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Understanding the Interaction between Nanomaterials Originated from High-Temperature Processed Starch/Myristic Acid and Human Monocyte Cells. Foods 2024, 13, 554. [Google Scholar] [CrossRef]
- Ahmed, A.; Shahadat, M.; Islam, S.U.; Adnan, R.; Mohamad Ibrahim, M.N.; Ullah, Q. Synthesis, Characterization, and Properties of Green Carbon Nanodots. In ACS Symposium Series; Islam, S.U., Hussain, C.M., Eds.; American Chemical Society: Washington, DC, USA, 2023; Volume 1441, pp. 25–39. ISBN 978-0-8412-9714-2. [Google Scholar]
- Chauhan, D.S.; Quraishi, M.A.; Verma, C. Carbon Nanodots: Recent Advances in Synthesis and Applications. Carbon Lett. 2022, 32, 1603–1629. [Google Scholar] [CrossRef]
- Lu, B.; Chen, X.; Ouyang, X.; Li, Z.; Yang, X.; Khan, Z.; Duan, S.; Shen, H. The Roles of Novel Chitooligosaccharide-Peanut Oligopeptide Carbon Dots in Improving the Flavor Quality of Chinese Cabbage. Food Chem. X 2023, 20, 100963. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, T.; Pu, H.; Sun, D.-W. Determination of Acrylamide in Food Products Based on the Fluorescence Enhancement Induced by Distance Increase between Functionalized Carbon Quantum Dots. Talanta 2020, 218, 121152. [Google Scholar] [CrossRef]
- Wei, W.; Xu, C.; Wu, L.; Wang, J.; Ren, J.; Qu, X. Non-Enzymatic-Browning-Reaction: A Versatile Route for Production of Nitrogen-Doped Carbon Dots with Tunable Multicolor Luminescent Display. Sci. Rep. 2014, 4, 3564. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, H. Novel Properties and Applications of Carbon Nanodots. Nanoscale Horiz. 2018, 3, 565–597. [Google Scholar] [CrossRef]
- Smart Micro- and Nanomaterials for Pharmaceutical Applications. In Emerging Materials and Technologies, 1st ed.; Behera, A., Nayak, A.K., Mohapatra, R.K., Rabaan, A.A., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2025; ISBN 978-1-003-46843-1. [Google Scholar]
- Bhatlawande, A.R.; Ghatge, P.U.; Shinde, G.U.; Anushree, R.K.; Patil, S.D. Unlocking the Future of Smart Food Packaging: Biosensors, IoT, and Nano Materials. Food Sci. Biotechnol. 2024, 33, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Dhamodharan, D.; Byun, H.-S.; Varsha Shree, M.; Veeman, D.; Natrayan, L.; Stalin, B. Carbon Nanodots: Synthesis, Mechanisms for Bio-Electrical Applications. J. Ind. Eng. Chem. 2022, 110, 68–83. [Google Scholar] [CrossRef]
- Bhattacharjee, T.; Konwar, A.; Boruah, J.S.; Chowdhury, D.; Majumdar, G. A Sustainable Approach for Heavy Metal Remediation from Water Using Carbon Dot Based Composites: A Review. J. Hazard. Mater. Adv. 2023, 10, 100295. [Google Scholar] [CrossRef]
- Perez-Locas, C.; Yaylayan, V.A. The Maillard Reaction and Food Quality Deterioration. In Chemical Deterioration and Physical Instability of Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2010; pp. 70–94. ISBN 978-1-84569-495-1. [Google Scholar]
- Tamanna, N.; Mahmood, N. Food Processing and Maillard Reaction Products: Effect on Human Health and Nutrition. Int. J. Food Sci. 2015, 2015, 26762. [Google Scholar] [CrossRef]
- Yang, F.; Huang, X.; Wang, P.; Xue, W.; Cheng, J.; Yu, D.; Shi, Y. Preparation of Meaty Flavor Additive from Soybean Meal Through the Maillard Reaction. Food Chem. X 2023, 19, 100780. [Google Scholar] [CrossRef]
- Hu, H.; Wang, Y.; Shen, M.; Huang, Y.; Li, C.; Nie, S.; Xie, M. Effects of Baking Factors and Recipes on the Quality of Butter Cookies and the Formation of Advanced Glycation End Products (AGEs) and 5-Hydroxymethylfurfural (HMF). Curr. Res. Food Sci. 2022, 5, 940–948. [Google Scholar] [CrossRef]
- Cadwallader, K.R.; Drake, M.; McGorrin, R.J. (Eds.) Flavor of Dairy Products; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2007; Volume 971, ISBN 978-0-8412-3968-5. [Google Scholar]
- Fardet, A.; Richonnet, C. Nutrient Density and Bioaccessibility, and the Antioxidant, Satiety, Glycemic, and Alkalinizing Potentials of Fruit-Based Foods According to the Degree of Processing: A Narrative Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3233–3258. [Google Scholar] [CrossRef]
- Conceição, L.D.S.; Almeida, B.S.D.; Souza, S.F.D.; Martinez, V.O.; Matos, M.F.R.D.; Andrade, L.L.; Ruggiero, W.F.; Pinto Matos, L.C. Critical Conditions for the Formation of Maillard Reaction Products (MRP) in Bread: An Integrative Review. J. Cereal Sci. 2024, 118, 103985. [Google Scholar] [CrossRef]
- Ateş, E.; Unal, K. The Effects of Deep-Frying Deep Frying, Microwave, Oven and Sous Vide Cooking on the Acrylamide Formation of Gluten-Free Chicken Nuggets. Int. J. Gastron. Food Sci. 2023, 31, 100666. [Google Scholar] [CrossRef]
- Li, H.; Murugesan, A.; Shoaib, M.; Chen, Q. Emerging Trends and Future Prospects of Peptide-Based Hydrogels: Revolutionizing Food Technology Applications. Compr. Rev. Food Sci. Food Safe 2025, 24, e70187. [Google Scholar] [CrossRef]
- Xiong, K.; Li, M.; Chen, Y.; Hu, Y.; Jin, W. Formation and Reduction of Toxic Compounds Derived from the Maillard Reaction During the Thermal Processing of Different Food Matrices. J. Food Prot. 2024, 87, 100338. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-M.; Kitts, D.D. Antioxidant and Anti-Inflammatory Activities of Maillard Reaction Products Isolated from Sugar–Amino Acid Model Systems. J. Agric. Food Chem. 2011, 59, 11294–11303. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D. Antioxidant and Functional Activities of MRPs Derived from Different Sugar–Amino Acid Combinations and Reaction Conditions. Antioxidants 2021, 10, 1840. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, W.; Wang, K.; Hu, Z.; Xiao, H.; Du, B.; Zhao, L. Structural and Inflammatory Characteristics of Maillard Reaction Products from Litchi Thaumatin-like Protein and Fructose. Food Chem. 2022, 374, 131821. [Google Scholar] [CrossRef]
- Wang, W.; Yang, W.; Dai, Y.; Liu, J.; Chen, Z.-Y. Production of Food-Derived Bioactive Peptides with Potential Application in the Management of Diabetes and Obesity: A Review. J. Agric. Food Chem. 2023, 71, 5917–5943. [Google Scholar] [CrossRef]
- Abdisa, K.B.; Szerdahelyi, E.; Molnár, M.A.; Friedrich, L.; Lakner, Z.; Koris, A.; Toth, A.; Nath, A. Metabolic Syndrome and Biotherapeutic Activity of Dairy (Cow and Buffalo) Milk Proteins and Peptides: Fast Food-Induced Obesity Perspective—A Narrative Review. Biomolecules 2024, 14, 478. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, M.; Gong, Z.; Jiao, W.; Li, L.; Dong, M.; Xiang, T.; Feng, N.; Wu, Q. Inhibition of Polyphenols on Maillard Reaction Products and Their Induction of Related Diseases: A Comprehensive Review. Phytomedicine 2024, 128, 155589. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.-J. Advanced Glycation End Products (AGEs) and Other Adducts in Aging-Related Diseases and Alcohol-Mediated Tissue Injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef]
- Xing, Z.; He, Z.; Wang, S.; Yan, Y.; Zhu, H.; Gao, Y.; Zhao, Y.; Zhang, L. Ameliorative Effects and Possible Molecular Mechanisms of Action of Fibrauretine from Fibraurea Recisa Pierre on D -Galactose/AlCl3 -Mediated Alzheimer’s Disease. RSC Adv. 2018, 8, 31646–31657. [Google Scholar] [CrossRef]
- Nie, C.; Li, Y.; Qian, H.; Ying, H.; Wang, L. Advanced Glycation End Products in Food and Their Effects on Intestinal Tract. Crit. Rev. Food Sci. Nutr. 2022, 62, 3103–3115. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Šebeková, K.; Simon Klenovics, K.; Brouder Šebeková, K. Advanced Glycation End Products in Infant Formulas. In Handbook of Dietary and Nutritional Aspects of Bottle Feeding; Preedy, V.R., Watson, R.R., Zibadi, S., Eds.; Brill|Wageningen Academic: Wageningen, The Netherlands, 2014; pp. 421–440. ISBN 978-90-8686-223-8. [Google Scholar]
- Uribarri, J.; del Castillo, M.D.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Markowicz Bastos, D.H.; Medrano, A.; Menini, T.; et al. Dietary Advanced Glycation End Products and Their Role in Health and Disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Amico, A.; Wootan, M.G.; Jacobson, M.F.; Leung, C.; Willett, A.W. The Demise of Artificial Trans Fat: A History of a Public Health Achievement. Milbank Q. 2021, 99, 746–770. [Google Scholar] [CrossRef]
- Teixeira, R.F.; Balbinot Filho, C.A.; Oliveira, D.D.; Zielinski, A.A.F. Prospects on Emerging Eco-Friendly and Innovative Technologies to Add Value to Dry Bean Proteins. Crit. Rev. Food Sci. Nutr. 2024, 64, 10256–10280. [Google Scholar] [CrossRef]
- Eckel, R.H.; Borra, S.; Lichtenstein, A.H.; Yin-Piazza, S.Y. Understanding the Complexity of Trans. Fatty Acid Reduction in the American Diet: American Heart Association Trans. Fat Conference 2006: Report of the Trans. Fat Conference Planning Group. Circulation 2007, 115, 2231–2246. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, X.; Yang, Y.; Zheng, L.; Xiao, D.; Ai, B.; Sheng, Z. Emerging Technologies in Reducing Dietary Advanced Glycation End Products in Ultra-processed Foods: Formation, Health Risks, and Innovative Mitigation Strategies. Compr. Rev. Food Sci. Food Safe 2025, 24, e70130. [Google Scholar] [CrossRef]
- Iyer, A.M.; Dadlani, V.; Pawar, H.A. Review on Acrylamide: A Hidden Hazard in Fried Carbohydrate-rich Food. Curr. Nutr. Food Sci. 2022, 18, 274–286. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Ismail, T.; Sestili, P.; Lorenzo, J.M.; Ranjha, M.M.A.N.; Jooste, L.; Hano, C.; Aadil, R.M. Heterocyclic Aromatic Amines in Meat: Formation, Isolation, Risk Assessment, and Inhibitory Effect of Plant Extracts. Foods 2021, 10, 1466. [Google Scholar] [CrossRef] [PubMed]
- Crews, C.; Castle, L. A Review of the Occurrence, Formation and Analysis of Furan in Heat-Processed Foods. Trends Food Sci. Technol. 2007, 18, 365–372. [Google Scholar] [CrossRef]
- Wang, K.; Gao, Z.; Gao, G.; Wo, Y.; Wang, Y.; Shen, G.; Cui, D. Systematic Safety Evaluation on Photoluminescent Carbon Dots. Nanoscale Res. Lett. 2013, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, N.; Marinovic, A.; Yoshizawa, N.; Goode, A.E.; Fay, M.; Khlobystov, A.; Titirici, M.-M.; Sapelkin, A. Structure and Solvents Effects on the Optical Properties of Sugar-Derived Carbon Nanodots. Sci. Rep. 2018, 8, 6559. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.J. Carbon Quantum Dots and Their Biomedical and Therapeutic Applications: A Review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Yan, T.; Zhong, W.; Yu, R.; Yi, G.; Liu, Z.; Liu, L.; Wang, X.; Jiang, J. Nitrogen-Doped Fluorescent Carbon Dots Used for the Imaging and Tracing of Different Cancer Cells. RSC Adv. 2019, 9, 24852–24857. [Google Scholar] [CrossRef]
- Jiwanti, P.K.; Wardhana, B.Y.; Sutanto, L.G.; Dewi, D.M.M.; Putri, I.Z.D.; Savitri, I.N.I. Recent Development of Nano-Carbon Material in Pharmaceutical Application: A Review. Molecules 2022, 27, 7578. [Google Scholar] [CrossRef]
- Bolchini, S.; Nardin, T.; Morozova, K.; Scampicchio, M.; Larcher, R. Antioxidant Maillard Reaction Products from Milk Whey: A Food By-Product Valorisation. Foods 2025, 14, 450. [Google Scholar] [CrossRef]
- Truskewycz, A.; Beker, S.; Ball, A.S.; Cole, I. Photoluminescence Measurements of Carbon Quantum Dots within Three-Dimensional Hydrogel Matrices Using a High Throughput 96 Well Plate Method. MethodsX 2019, 6, 437–441. [Google Scholar] [CrossRef]
- Bolchini, S.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Assessing Antioxidant Properties of Maillard Reaction Products: Methods and Potential Applications as Food Preservatives. Eur. Food Res. Technol. 2025. [Google Scholar] [CrossRef]
- Yu, A.-N.; Tan, Z.-W.; Wang, F.-S. Mechanism of Formation of Sulphur Aroma Compounds from L-Ascorbic Acid and l-Cysteine during the Maillard Reaction. Food Chem. 2012, 132, 1316–1323. [Google Scholar] [CrossRef]
- Zhu, K.-X.; Li, J.; Li, M.; Guo, X.-N.; Peng, W.; Zhou, H.-M. Functional Properties of Chitosan–Xylose Maillard Reaction Products and Their Application to Semi-Dried Noodle. Carbohydr. Polym. 2013, 92, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Tavernaro, I.; Dekkers, S.; Soeteman-Hernández, L.G.; Herbeck-Engel, P.; Noorlander, C.; Kraegeloh, A. Safe-by-Design Part II: A Strategy for Balancing Safety and Functionality in the Different Stages of the Innovation Process. NanoImpact 2021, 24, 100354. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Yadav, N. Effect of Plant Extracts on the Reduction of Acrylamide and Hydroxymethylfurfural Formation in French Fries. Food Chem. Adv. 2024, 4, 100708. [Google Scholar] [CrossRef]
- Lund, M.N.; Ray, C.A. Control of Maillard Reactions in Foods: Strategies and Chemical Mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef]
- Račkauskienė, I.; Pukalskas, A.; Fiore, A.; Troise, A.D.; Venskutonis, P.R. Phytochemical-Rich Antioxidant Extracts of Vaccinium Vitis-idaea L. Leaves Inhibit the Formation of Toxic Maillard Reaction Products in Food Models. J. Food Sci. 2019, 84, 3494–3503. [Google Scholar] [CrossRef]
- Törős, G.; Peles, F.; Elramady, H.; Prokisch, J. To What Extent Can Maillard Reaction Products Influence the Probiotic and Harmful Bacteria? Egypt. J. Soil Sci. 2023, 63, 177–185. [Google Scholar] [CrossRef]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.-J. Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.-Q.; Li, L.; Guo, M.; He, Y.; Dong, Y.; Meng, H.; Yi, F. Advanced Glycation End Products in the Skin: Molecular Mechanisms, Methods of Measurement, and Inhibitory Pathways. Front. Med. 2022, 9, 837222. [Google Scholar] [CrossRef]
- Younessi, P.; Yoonessi, A. Advanced Glycation End-Products and Their Receptor-Mediated Roles: Inflammation and Oxidative Stress. Iran. J. Med. Sci. 2011, 36, 154–166. [Google Scholar]
- Wen, K.; Zhang, Q.; Xie, J.; Xue, B.; Li, X.; Bian, X.; Sun, T. Effect of Mono- and Polysaccharide on the Structure and Property of Soy Protein Isolate during Maillard Reaction. Foods 2024, 13, 2832. [Google Scholar] [CrossRef]
- Zioga, E.; Tøstesen, M.; Kjærulf Madsen, S.; Shetty, R.; Bang-Berthelsen, C.H. Bringing Plant-Based Cli-Meat Closer to Original Meat Experience: Insights in Flavor. Future Foods 2022, 5, 100138. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Soladoye, O.P.; Aluko, R.E. Maillard Reaction Products Derived from Food Protein-Derived Peptides: Insights into Flavor and Bioactivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 3429–3442. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yang, C.; Zhang, J.; Zhang, L.; Tsao, R. Amadori Compounds: Analysis, Composition in Food and Potential Health Beneficial Functions. Crit. Rev. Food Sci. Nutr. 2025, 65, 406–428. [Google Scholar] [CrossRef] [PubMed]
- Törős, G.; Gulyás, G.; El-Ramady, H.; Alibrahem, W.; Muthu, A.; Gangakhedkar, P.; Atieh, R.; Prokisch, J. Sustainable Nanotechnology Strategies for Modulating the Human Gut Microbiota. Int. J. Mol. Sci. 2025, 26, 5433. [Google Scholar] [CrossRef]
- Afonso, A.J.G.; Aquino, F.T.; Dalmônico, G.M.L.; Nascimento, M.V.; Wrasse, E.; De Aguiar, K.M.F.R. Green Synthesis of Carbon Nanodots from Agro-Industrial Residues. Carbon Lett. 2022, 32, 131–141. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Danquah, M.K. Agricultural Waste-Derived Carbon Nanomaterials for Biomedical Applications. In Waste-Derived Carbon Nanostructures; Talreja, N., Chauhan, D., Ashfaq, M., Eds.; Nanostructure Science and Technology; Springer Nature: Cham, Switzerland, 2025; pp. 213–232. ISBN 978-3-031-75246-9. [Google Scholar]
- Chan, M.-H.; Chen, B.-G.; Ngo, L.T.; Huang, W.-T.; Li, C.-H.; Liu, R.-S.; Hsiao, M. Natural Carbon Nanodots: Toxicity Assessment and Theranostic Biological Application. Pharmaceutics 2021, 13, 1874. [Google Scholar] [CrossRef]
- Cho, S.; Kim, H.; Song, D.; Jung, J.; Park, S.; Jo, H.; Seo, S.; Han, C.; Park, S.; Kwon, W.; et al. Insights into Glucose-Derived Carbon Dot Synthesis via Maillard Reaction: From Reaction Mechanism to Biomedical Applications. Sci. Rep. 2024, 14, 31325. [Google Scholar] [CrossRef]
- Habelreeh, H.H.; Athinarayanan, J.; Periasamy, V.S.; Alshatwi, A.A. Maillard Reaction-Derived S-Doped Carbon Dots Promotes Downregulation of PPARγ, C/EBPα, and SREBP-1 Genes In-Vitro. Molecules 2024, 29, 2008. [Google Scholar] [CrossRef]
- Aksu, M.; Güzdemir, Ö. Food Waste-Derived Carbon Quantum Dots and Their Applications in Food Technology: A Critical Review. Food Bioprocess Technol. 2025, 18, 6753–6778. [Google Scholar] [CrossRef]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A Review of Carbon Dots Produced from Biomass Wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Ndraha, N.; Wu, R.-S.; Liu, H.-Y.; Lin, S.-W.; Yang, K.-M.; Lin, H.-Y. An Overview of the Potential of Food-Based Carbon Dots for Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 16579. [Google Scholar] [CrossRef]
- Abekoon, T.; Buthpitiya, B.L.S.K.; Sajindra, H.; Samarakoon, E.R.J.; Jayakody, J.A.D.C.A.; Kantamaneni, K.; Rathnayake, U. A Comprehensive Review to Evaluate the Synergy of Intelligent Food Packaging with Modern Food Technology and Artificial Intelligence Field. Discov. Sustain. 2024, 5, 160. [Google Scholar] [CrossRef]
| Compound Type | Examples | Ref. |
|---|---|---|
| Acids | Butyric acid, isovaleric acid | [40] |
| Alcohols | 1-hexanol, 2-phenylethanol | [34] |
| Aldehydes | Hexanal, nonanal, furfural | [35] |
| Carbonyl Compounds | Acetoin, diacetyl (2,3-butanedione) | [37] |
| Heterocyclic Compounds | Pyrazines, pyrroles, furans | [41] |
| Ketones | 2-heptanone, 3-octanone, 2-pentanone | [36] |
| Sulfur Compounds | Dimethyl disulfide, methional | [38] |
| Compound Type | Examples | Ref. |
|---|---|---|
| Furan Derivatives | Furfural, 5-hydroxymethylfurfural (HMF) | [45] |
| Imidazoles | 4(5)-methylimidazole | [46] |
| Nitrogenous Polymers | Melanoidins (high molecular weight, dark-brown pigments) | [47] |
| Phenolic Compounds | Phenol, hydroxyphenylacetaldehyde | [5] |
| Pyrazines | 2-ethyl-3,5-dimethylpyrazine, methylpyrazine | [48] |
| Pyrroles | Pyrrole, substituted pyrroles | [49] |
| Reaction Products | Glucose–lysine browning products, caramel-like pigments | [50] |
| Food Matrix | Formation/Methods | Key Findings | Ref. |
|---|---|---|---|
| Bakery products | Baking (NaOH immersion step involved) | CNDs < 10 nm formed during baking; NaOH pretreatment facilitated CND formation and improved yield. | [54] |
| Coffee beans | Roasting process | Fluorescent CNDs identified; their presence correlated with caffeine content and roast intensity. | [52] |
| Milk | Synthesized via the hydrothermal method | The resulting CNDs demonstrated good sensitivity for detecting copper ions, with potential applications in food safety monitoring. | [57] |
| Mushroom powder | Pyrolysis of Pleurotus ostreatus | A strong positive correlation between the carbon/nitrogen ratio and CND yield; mushroom biomass is a viable precursor. | [51] |
| Spices | Pyrolysis of black pepper, turmeric, cysteine, clove, ginger, and chili spices | CNDs showed enhanced bioavailability, potent antioxidant activity, and improved biological functionality. | [58] |
| Starch-rich cooked foods | High-Temperature Processed Starch/Myristic Acid | Produced CNDs exhibited strong fluorescence; demonstrated potential for immunomodulation via cytokine regulation. | [59] |
| Food Type | Key Findings | Impact | Ref. |
|---|---|---|---|
| Meat Products | A meaty flavor additive was developed using soybean meal hydrolysate and xylose via the Maillard reaction at 120 °C for 120 min with 10% cysteine. The product contained 4.941 μmol/mL of free amino acids and 50 volatile compounds, including mercaptans, sulfur-substituted furans, pyrazines, aldehydes, and esters. | High antioxidant activity; rich in volatile flavor compounds; potential as a food additive | [72] |
| Baked Goods | MR during baking leads to the formation of color and flavor compounds and potentially toxic substances like AGEs and HMF. Ingredients like butter, sugar, and eggs influence MR extent and sensory quality. | Flavor and color formation; risk of toxic MRPs | [73] |
| Dairy Products | Non-enzymatic browning and MR contribute to caramel and roasted flavors in milk powders but can also result in off-flavors and sedimentation. Browning issues in skim milk powders can lead to consumer complaints. | Both desirable and undesirable effects: flavor, off-odors, browning | [74] |
| Vegetables | MR in processed vegetables can enhance flavor but also produce toxic compounds. Reactions involve proteins, polysaccharides, and polyamines, especially during storage and thermal processing. | Flavor enhancement: potential health risks | [71] |
| Fruits | While MR can improve the sensory quality of fruit-based products, it can also lead to the formation of toxic Heterocyclic Aromatic Amines (HAAs). Advances suggest MRs can occur without heat, through green processing methods, challenging traditional assumptions. | Sensory improvement and potential toxicity also occur in non-thermal processes. | [75] |
| Shynthesis Method | Optical Properties | Toxicity | Drug Delivery Potential | Key Findings | Ref. |
|---|---|---|---|---|---|
| Dry and solution-based techniques | Size- and wavelength-dependent luminescence; resistant to photobleaching; non-blinking | Generally non-toxic, but certain forms may pose risks | Highlights potential use but notes need for further testing | Introduced foundational knowledge of C-dot fluorescence and synthesis; raised awareness of potential health concerns tied to specific structures | [99] |
| Sugar-derived C-dots in various solvents | Emission is strongly influenced by the solvent environment; tunable fluorescence | Low toxicity; highlights the need for safety assessments | Supports application in bioimaging and drug delivery | Demonstrated how structural and solvent variables influence C-dot behavior; encouraged deeper study of formation and emission mechanisms for food and drug safety | [100] |
| Various methods, with focus on functionalization | Fluorescent emission is useful for imaging and therapeutic tracking | Emphasizes the low cytotoxicity of CQDs | Strong drug loading and release capabilities via covalent bonding | Highlighted CQDs’ promise in multifunctional roles, including simultaneous imaging and drug release; discussed controllable delivery methods | [101] |
| One-pot hydrothermal synthesis | Strong fluorescence with cell-type specificity; stable in aqueous media | Low cytotoxicity confirmed in cancer cells. | Effective for imaging and drug tracing. | Nitrogen-doped CNDs have been shown to differentiate cancer cells with low toxicity and high water stability | [102] |
| Review of multiple synthesis approaches | Describes diverse fluorescence behaviors for targeting and imaging | Calls for detailed toxicological evaluation | Responsive to pH/temperature triggers as nanocarriers | Summarized nano-carbon drug carriers; emphasized stimulus-responsiveness and rigorous safety evaluation required | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Törős, G.; Prokisch, J. Maillard Reaction-Derived Carbon Nanodots: Food-Origin Nanomaterials with Emerging Functional and Biomedical Potential. Pharmaceutics 2025, 17, 1050. https://doi.org/10.3390/pharmaceutics17081050
Törős G, Prokisch J. Maillard Reaction-Derived Carbon Nanodots: Food-Origin Nanomaterials with Emerging Functional and Biomedical Potential. Pharmaceutics. 2025; 17(8):1050. https://doi.org/10.3390/pharmaceutics17081050
Chicago/Turabian StyleTörős, Gréta, and József Prokisch. 2025. "Maillard Reaction-Derived Carbon Nanodots: Food-Origin Nanomaterials with Emerging Functional and Biomedical Potential" Pharmaceutics 17, no. 8: 1050. https://doi.org/10.3390/pharmaceutics17081050
APA StyleTörős, G., & Prokisch, J. (2025). Maillard Reaction-Derived Carbon Nanodots: Food-Origin Nanomaterials with Emerging Functional and Biomedical Potential. Pharmaceutics, 17(8), 1050. https://doi.org/10.3390/pharmaceutics17081050







