Tumor Microenvironment Specifically Regulated Nano Chemoamplifier for Chemosensitization and Activation of Anti-Tumor Immune Response by Coordinating Intracellular Magnesium Overload
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of MMSN and MMSN@Dox
2.2. In Vitro Biodegradation and Drug Release
2.3. In Vitro Cytotoxicity and Anti-Tumor Assessment
2.4. Mitochondrial Membrane Potential and Intracellular ROS Evaluation
2.5. In Vitro Evaluation of ICD and DCs Maturation
2.6. In Vitro Evaluation of CSCs
2.7. In Vivo Anti-Tumor Evaluation
2.8. In Vivo Anti-Tumor Immune Response Evaluation
2.9. Statistical Analysis
3. Results
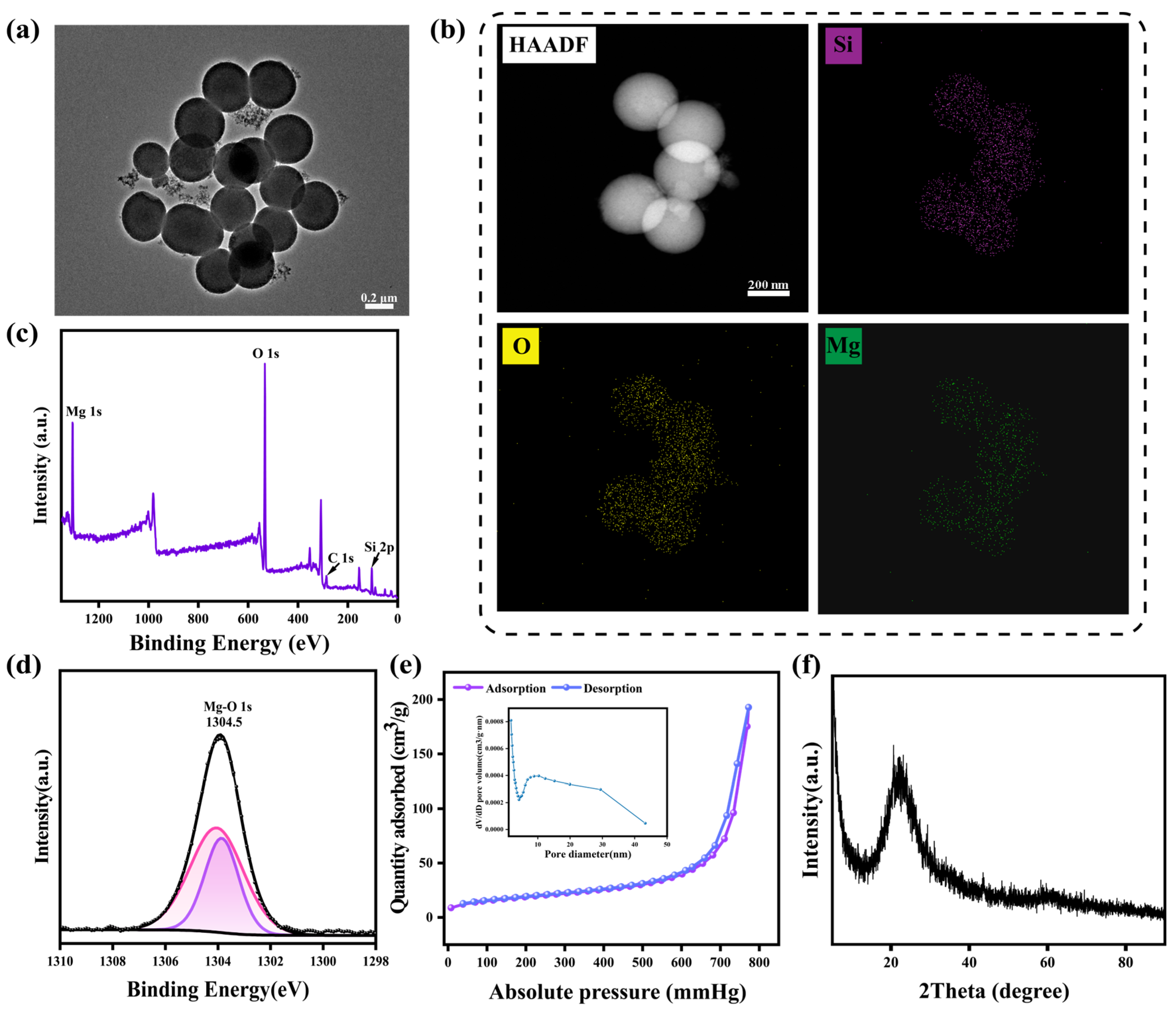
3.1. Preparation and Characterization of MMSN
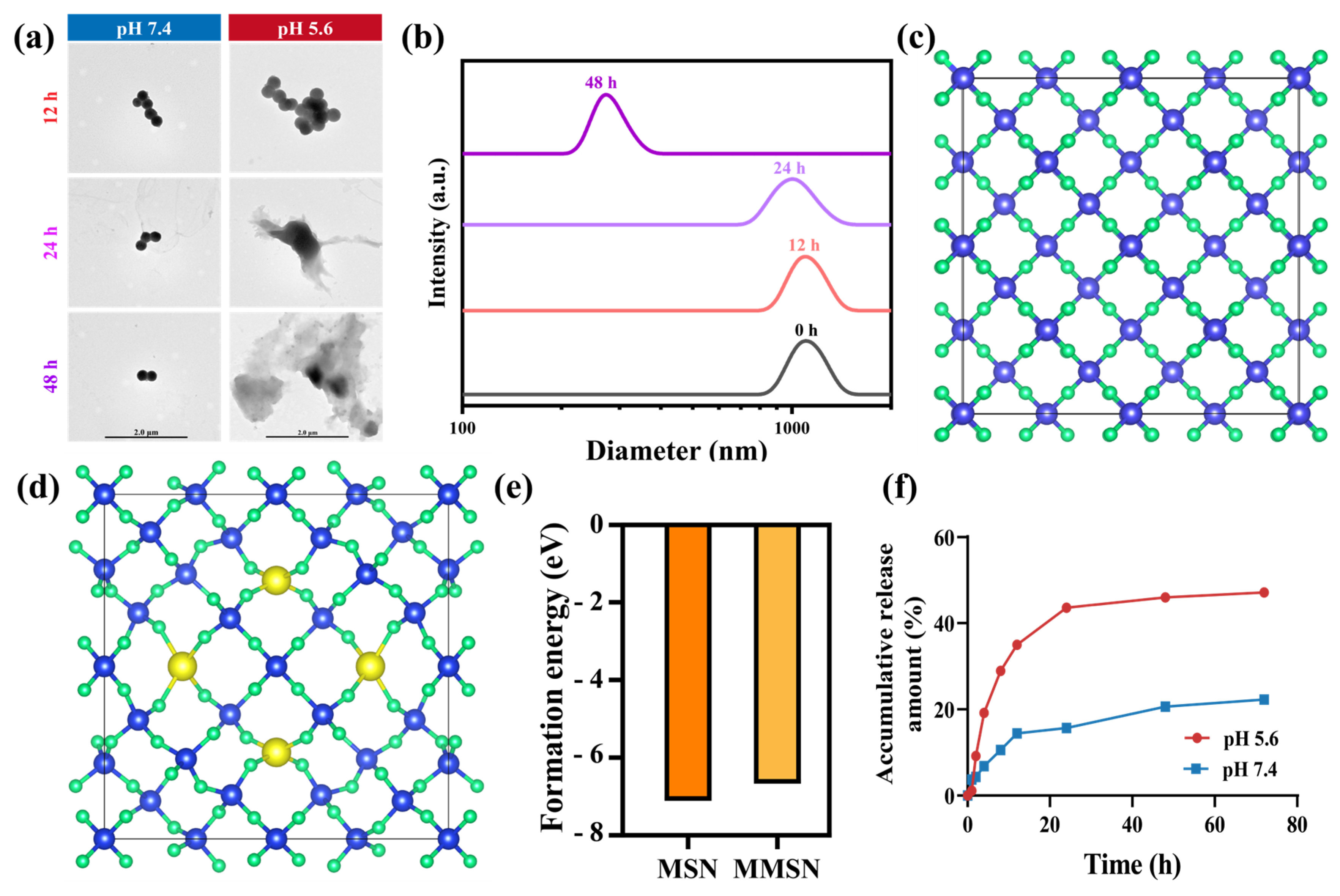
3.2. TME Stimuli Responsive Drug Release
3.3. In Vitro Anti-Tumor Evaluation
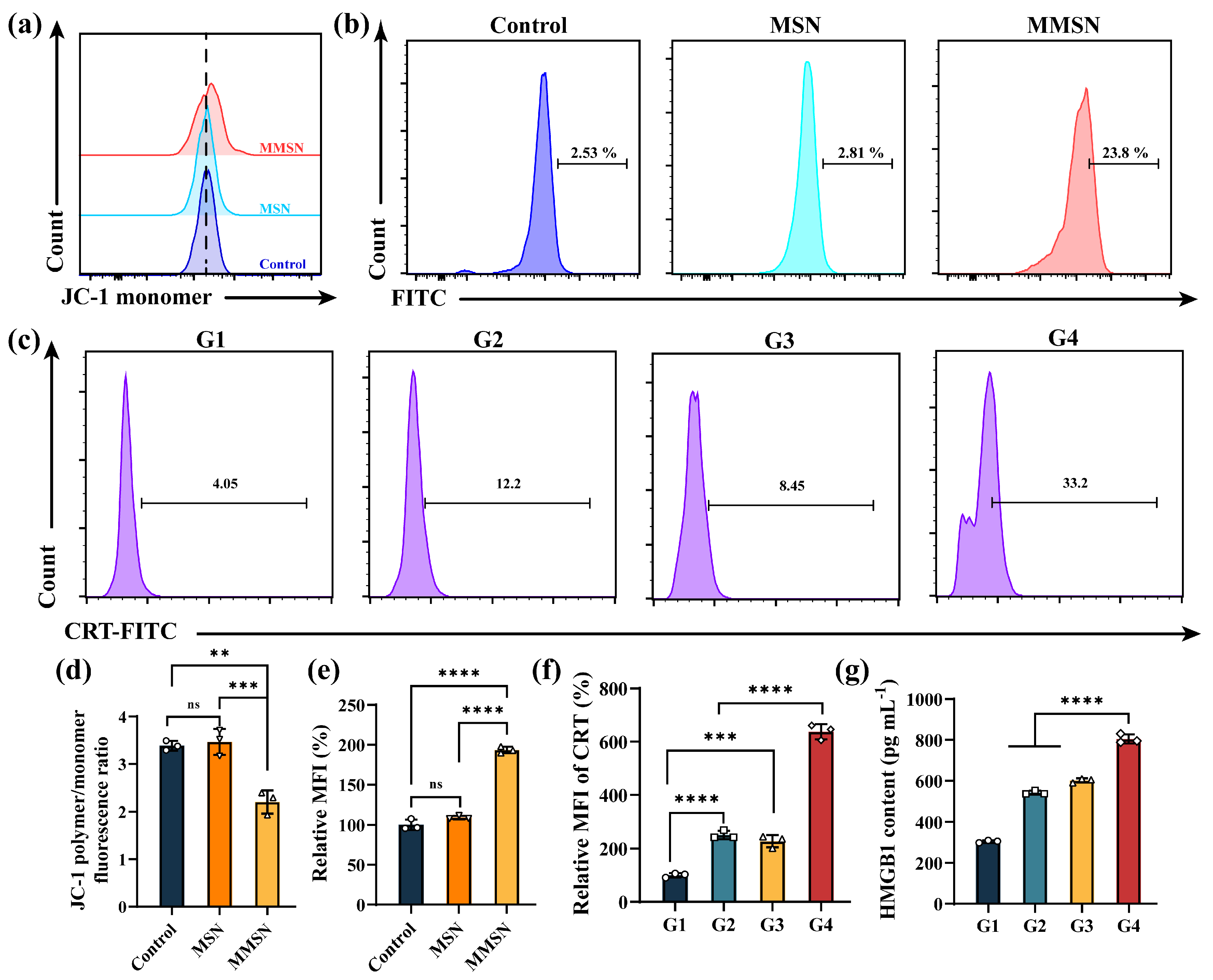
3.4. Magnesium Ions Disrupt Mitochondrial Function and Induce ROSs
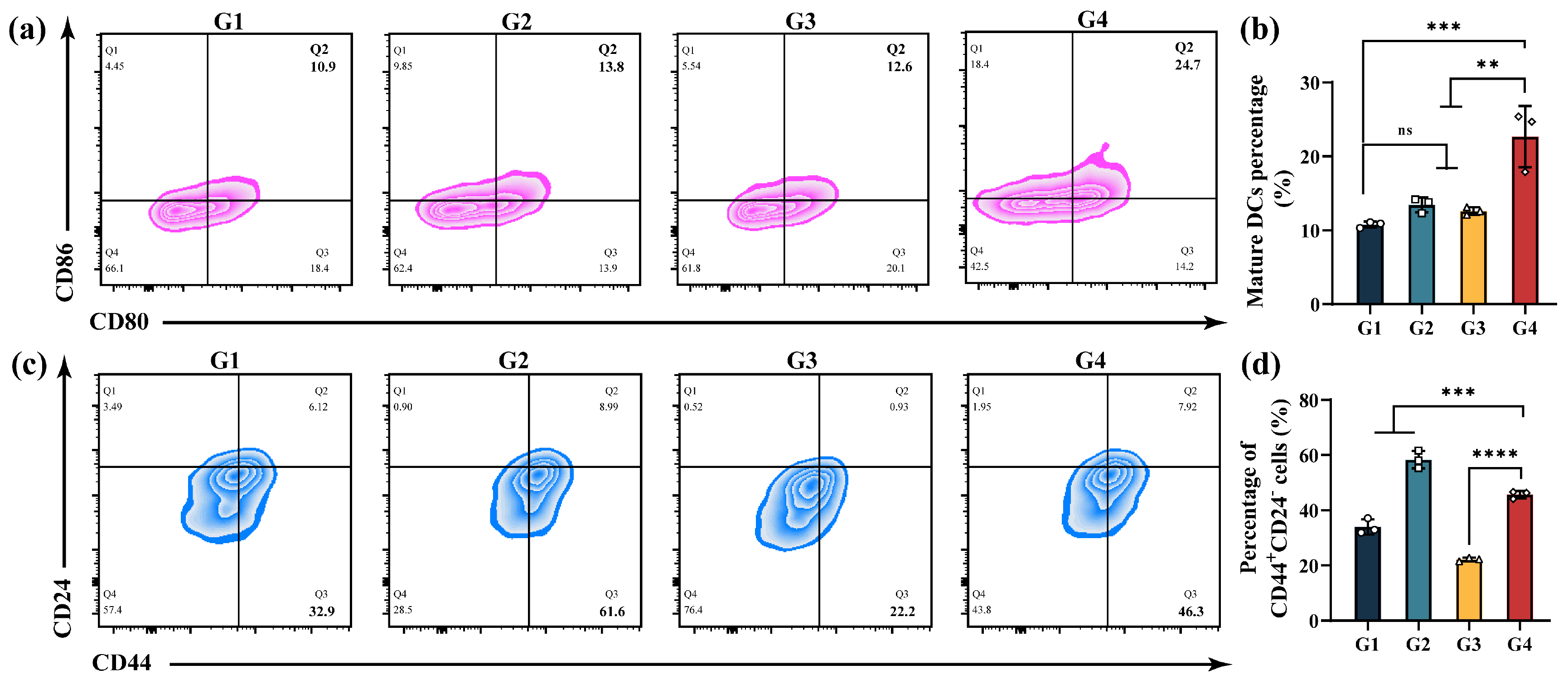
3.5. In Vitro ICD and Immune Activation
3.6. In Vitro CSCs Suppression
3.7. In Vivo Anti-Tumor Efficacy
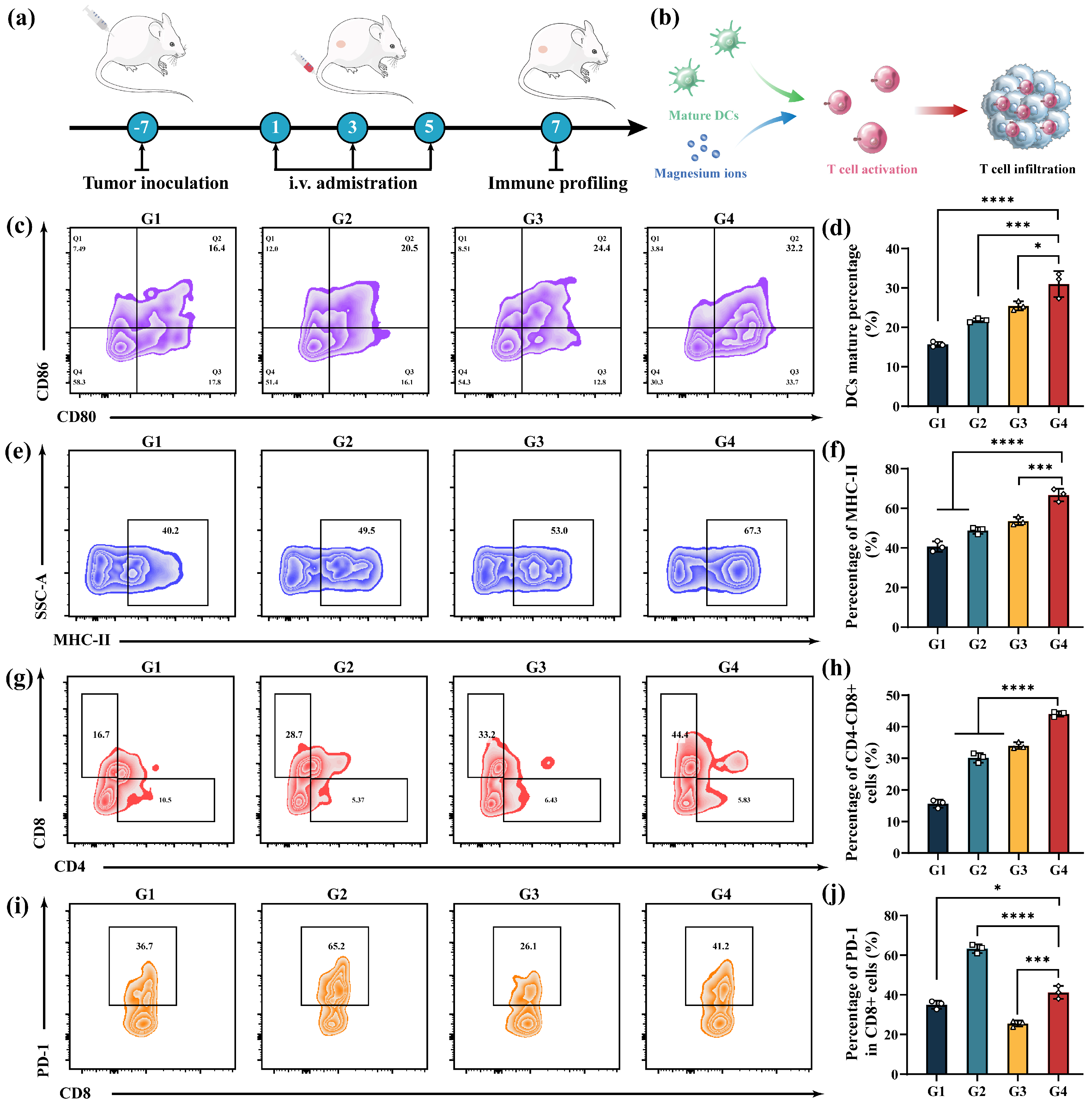
3.8. In Vivo Anti-Tumor Immune Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Luo, S.; Chu, Q.; Wu, K. Epidemiological trends of women’s cancers from 1990 to 2019 at the global, regional, and national levels: A population-based study. Biomark. Res. 2021, 9, 9–55. [Google Scholar] [CrossRef]
- Sritharan, S.; Sivalingam, N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021, 278, 119527–119536. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, H.; Xu, S.; Zhao, J.; Wang, Y.; Pan, X.; Zhao, B.; Sun, Z.; Yin, Y.; Xu, L.; et al. Injectable doxorubicin-loaded hyaluronic acid-based hydrogel for locoregional therapy and inhibiting metastasis of breast cancer. Colloids Surf B Biointerfaces 2025, 247, 114433. [Google Scholar] [CrossRef]
- Xiong, X.; Zheng, L.-W.; Ding, Y.; Chen, Y.-F.; Cai, Y.-W.; Wang, L.-P.; Huang, L.; Liu, C.-C.; Shao, Z.-M.; Yu, K.-D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.S.; Sharker, S.M.; Islam, M.T.; Khan, I.N.; Shaw, S.; Rahman, M.A.; Uddin, S.J.; Shill, M.C.; Rehman, S.; Das, N.; et al. Targeting cancer cells with nanotherapeutics and nanodiagnostics: Current status and future perspectives. Semin. Cancer Biol. 2021, 69, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Yeldag, G.; Rice, A.; Del Río Hernández, A. Chemoresistance and the Self-Maintaining Tumor Microenvironment. Cancers 2018, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zhang, K.; Zhang, X.; Zhu, Y.; Cai, B.; Wang, C.; Zhao, G.; Zhang, D.; Zhang, J. Arginine tagged liposomal carrier for the delivery of celastrol for ferroptosis-induced hepatocellular carcinoma therapy. Colloids Surf. B Biointerfaces 2025, 250, 114546. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Y.; Gao, Y.; He, J. Cuproptosis: Mechanisms and links with cancers. Mol. Cancer 2023, 22, 46. [Google Scholar] [CrossRef]
- Lei, H.; Li, Q.; Li, G.; Wang, T.; Lv, X.; Pei, Z.; Gao, X.; Yang, N.; Gong, F.; Yang, Y.; et al. Manganese molybdate nanodots with dual amplification of STING activation for “cycle” treatment of metalloimmunotherapy. Bioact. Mater. 2024, 31, 53–62. [Google Scholar] [CrossRef]
- Gong, F.; Xu, J.; Liu, B.; Yang, N.; Cheng, L.; Huang, P.; Wang, C.; Chen, Q.; Ni, C.; Liu, Z. Nanoscale CaH2 materials for synergistic hydrogen-immune cancer therapy. Chem 2022, 8, 268–286. [Google Scholar] [CrossRef]
- Li, W.; Tian, H.; Yan, Z.; Yu, X.; Li, B.; Dai, Y. Magnesium–Phenolic Nanoeditor Refining Gliomatous T Cells for Metalloimmunotherapy. ACS Nano 2024, 19, 1222–1237. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Guo, R.; Liu, M.; Hu, H.; Zhang, H. Multi-modal Ca2+ nanogenerator via reversing T cell exhaustion for enhanced chemo-immunotherapy. J. Control. Release 2024, 372, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.K.; Tungalag, T.; Lee, S.-J.; Kim, S.-J. Methyl Jasmonate-induced Increase in Intracellular Magnesium Promotes Apoptosis in Breast Cancer Cells. Anticancer Res. 2024, 44, 1087–1095. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Li, H.; Feng, X.; Li, H.; Ma, S.; Song, W.; Yang, B.; Jiang, T.; Yang, C. The Supplement of Magnesium Element to Inhibit Colorectal Tumor Cells. Biol. Trace Elem. Res. 2022, 201, 2895–2903. [Google Scholar] [CrossRef]
- Bezerra, D.L.C.; Mendes, P.M.V.; Melo, S.R.S.; Dos Santos, L.R.; Santos, R.O.; Vieira, S.C.; Henriques, G.S.; Freitas, B.J.E.S.A.; Marreiro, D.D.N. Hypomagnesemia and Its Relationship with Oxidative Stress Markers in Women with Breast Cancer. Biol. Trace Elem. Res. 2021, 199, 4466–4474. [Google Scholar] [CrossRef]
- Winter, J.M.; Yadav, T.; Rutter, J. Stressed to death: Mitochondrial stress responses connect respiration and apoptosis in cancer. Mol. Cell 2022, 82, 3321–3332. [Google Scholar] [CrossRef]
- Isha, H.; Jain, L.Z.; Goli, R.; Alexa, K.; Schatzman-Bone, S.; Dhillon, H.; Goldberger, O.; Peng, J.; Shalem, O.; Sanjana, N.E.; et al. Hypoxia as a therapy for mitochondrial disease. Science 2016, 352, 54–61. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Liu, J.; Kang, R.; Klionsky, D.J.; Tang, D. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy 2020, 17, 948–960. [Google Scholar] [CrossRef]
- Lee, J.; Yesilkanal, A.E.; Wynne, J.P.; Frankenberger, C.; Liu, J.; Yan, J.; Elbaz, M.; Rabe, D.C.; Rustandy, F.D.; Tiwari, P.; et al. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature 2019, 568, 254–258. [Google Scholar] [CrossRef]
- Daw, C.C.; Ramachandran, K.; Enslow, B.T.; Maity, S.; Bursic, B.; Novello, M.J.; Rubannelsonkumar, C.S.; Mashal, A.H.; Ravichandran, J.; Bakewell, T.M.; et al. Lactate Elicits ER-Mitochondrial Mg2+ Dynamics to Integrate Cellular Metabolism. Cell 2020, 183, 474–489.e17. [Google Scholar] [CrossRef]
- Wilde, B.R.; Christofk, H.R. O-Mg! Lactate Drives Mg2+ Mobilization. Mol. Cell 2020, 80, 762–763. [Google Scholar] [CrossRef]
- Shao, X.; Qu, C.; Song, G.; Wang, B.; Tao, Q.; Jia, R.; Li, J.; Wang, J.; An, H. NIR-II Photothermal Activation of TRPV1 Channels for Intracellular Magnesium Regulation by Porous Pd@Pt Core–Shell Nanostructure to Reverse Tumor Multidrug Resistance. Adv. Funct. Mater. 2023, 33, 2306585. [Google Scholar] [CrossRef]
- Lötscher, J.; Martí i Líndez, A.-A.; Kirchhammer, N.; Cribioli, E.; Giordano Attianese, G.M.P.; Trefny, M.P.; Lenz, M.; Rothschild, S.I.; Strati, P.; Künzli, M.; et al. Magnesium sensing via LFA-1 regulates CD8+ T cell effector function. Cell 2022, 185, 585–602.e29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Song, Y.; Yao, J.; Qin, H.; Ye, Y.; Gao, J.; Zhang, C.; Han, D.; Gao, M.; Chen, H.; et al. RNA-Seq Reveals the Mechanism of Synergistic Hydrogen-Chemotherapy Based on Active Magnesium Micromotors for Inhibiting Glioblastoma Recurrence by Modulating Tumor Microenvironment. Small 2025, 21, e2408809. [Google Scholar] [CrossRef]
- Chen, M.; Lu, Q.; Gong, F.; Yang, Y.; Pei, Z.; Huang, X.; Shu, G.; Shen, L.; Yan, P.; Guo, X.; et al. A Gas Nanobomb to Promote Drug Penetration and Amplify TACE Therapy for Orthotopic Liver Tumor. Adv Mater 2025, e2505770. [Google Scholar] [CrossRef]
- Han, X.; Sun, S.; Yang, N.; Han, Z.; Pei, Z.; Yu, Q.; Nie, J.; Wang, L.; Liu, A.; Meng, X.; et al. Nano-Engineered Magnesium Implants for Magnetothermal Enhanced Pyroptosis to Boost Immunotherapy. Adv. Funct. Mater. 2024, 34, 2405836. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Song, S.; Yu, X.; Xu, C.; Wan, L.; Yao, F.; Yang, K.; Witte, F.; Yang, S. Mechanism and application prospect of magnesium-based materials in cancer treatment. J. Magnes. Alloys 2025, 13, 982–1011. [Google Scholar] [CrossRef]
- Lammers, T. Nanomedicine Tumor Targeting. Adv. Mater. 2024, 36, 2312169–2312188. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Li, H.; Hu, T.Y. Recent advances in the bench-to-bedside translation of cancer nanomedicines. Acta Pharm. Sin. B 2025, 15, 97–122. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Yan, J.; Siwakoti, P.; Shaw, S.; Bose, S.; Kokil, G.; Kumeria, T. Porous silicon and silica carriers for delivery of peptide therapeutics. Drug Deliv. Transl. Res. 2024, 14, 3549–3567. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhao, X.; Duan, Y.; Shi, J. Hollow mesoporous silica nanoparticles for drug formulation and delivery: Opportunities for cancer therapy. Colloids Surf. B Biointerfaces 2025, 249, 114534. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Schüth, F.; Lozano, D.; Colilla, M.; Manzano, M. Engineering mesoporous silica nanoparticles for drug delivery: Where are we after two decades? Chem. Soc. Rev. 2022, 51, 5365–5451. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, C.; Chang, J. Ca-Doped mesoporous SiO2/dental resin composites with enhanced mechanical properties, bioactivity and antibacterial properties. J. Mater. Chem. B 2018, 6, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2019, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Highlights the recent important findings in cancer heterogeneity. Holist. Integr. Oncol. 2023, 2, 2–15. [Google Scholar] [CrossRef]
- Nassar, D.; Blanpain, C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 47–76. [Google Scholar] [CrossRef]
- Launonen, I.-M.; Niemiec, I.; Hincapié-Otero, M.; Erkan, E.P.; Junquera, A.; Afenteva, D.; Falco, M.M.; Liang, Z.; Salko, M.; Chamchougia, F.; et al. Chemotherapy induces myeloid-driven spatially confined T cell exhaustion in ovarian cancer. Cancer Cell 2024, 42, 2045–2063.e10. [Google Scholar] [CrossRef]
- Karachi, A.; Dastmalchi, F.; Nazarian, S.; Huang, J.; Sayour, E.J.; Jin, L.; Yang, C.; Mitchell, D.A.; Rahman, M. Optimizing T Cell-Based Therapy for Glioblastoma. Front. Immunol. 2021, 12, 705580. [Google Scholar] [CrossRef]
- Liu, N.; Lv, J.; Qi, W.; Sun, L.; Guo, J.; Zhao, S.; Qiu, W.; Liang, J. Programmed death 1 induces cell chemoresistance to 5-fluorouracil in gastric cancer cell lines. Transl. Cancer Res. 2016, 5, 781–788. [Google Scholar] [CrossRef]
- Watowich, M.B.; Gilbert, M.R.; Larion, M. T cell exhaustion in malignant gliomas. Trends Cancer 2023, 9, 270–292. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEPcode. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154122. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Huang, G.; Zhu, L.; Li, S.; Yang, K.; Alifu, N.; Duan, Y. Tumor Microenvironment Specifically Regulated Nano Chemoamplifier for Chemosensitization and Activation of Anti-Tumor Immune Response by Coordinating Intracellular Magnesium Overload. Pharmaceutics 2025, 17, 1034. https://doi.org/10.3390/pharmaceutics17081034
Liu C, Huang G, Zhu L, Li S, Yang K, Alifu N, Duan Y. Tumor Microenvironment Specifically Regulated Nano Chemoamplifier for Chemosensitization and Activation of Anti-Tumor Immune Response by Coordinating Intracellular Magnesium Overload. Pharmaceutics. 2025; 17(8):1034. https://doi.org/10.3390/pharmaceutics17081034
Chicago/Turabian StyleLiu, Chao, Gaofei Huang, Lu Zhu, Shasha Li, Kun Yang, Nuernisha Alifu, and Yingni Duan. 2025. "Tumor Microenvironment Specifically Regulated Nano Chemoamplifier for Chemosensitization and Activation of Anti-Tumor Immune Response by Coordinating Intracellular Magnesium Overload" Pharmaceutics 17, no. 8: 1034. https://doi.org/10.3390/pharmaceutics17081034
APA StyleLiu, C., Huang, G., Zhu, L., Li, S., Yang, K., Alifu, N., & Duan, Y. (2025). Tumor Microenvironment Specifically Regulated Nano Chemoamplifier for Chemosensitization and Activation of Anti-Tumor Immune Response by Coordinating Intracellular Magnesium Overload. Pharmaceutics, 17(8), 1034. https://doi.org/10.3390/pharmaceutics17081034





