Reprogramming Atherosclerosis: Precision Drug Delivery, Nanomedicine, and Immune-Targeted Therapies for Cardiovascular Risk Reduction
Abstract
1. Introduction
2. Mechanistic Pathways Driving Atherogenesis
2.1. Inflammatory Mechanisms in Atherogenesis
2.2. Oxidative Stress as a Central Driver of Atherogenesis
2.3. Dysregulated Lipid Metabolism in Atherogenesis
2.4. Mitochondrial Dysfunction in Atherogenesis
2.5. Endothelial Dysfunction in Atherogenesis
2.6. Role of Hemodynamic Forces in Atherosclerotic Plaque Development and Destabilization
2.7. Mechanisms of Atherosclerotic Plaque Progression and Opportunities for Targeted Therapeutic Intervention
3. Emerging Therapeutic Strategies and Targeted Drug Delivery in Atherosclerosis
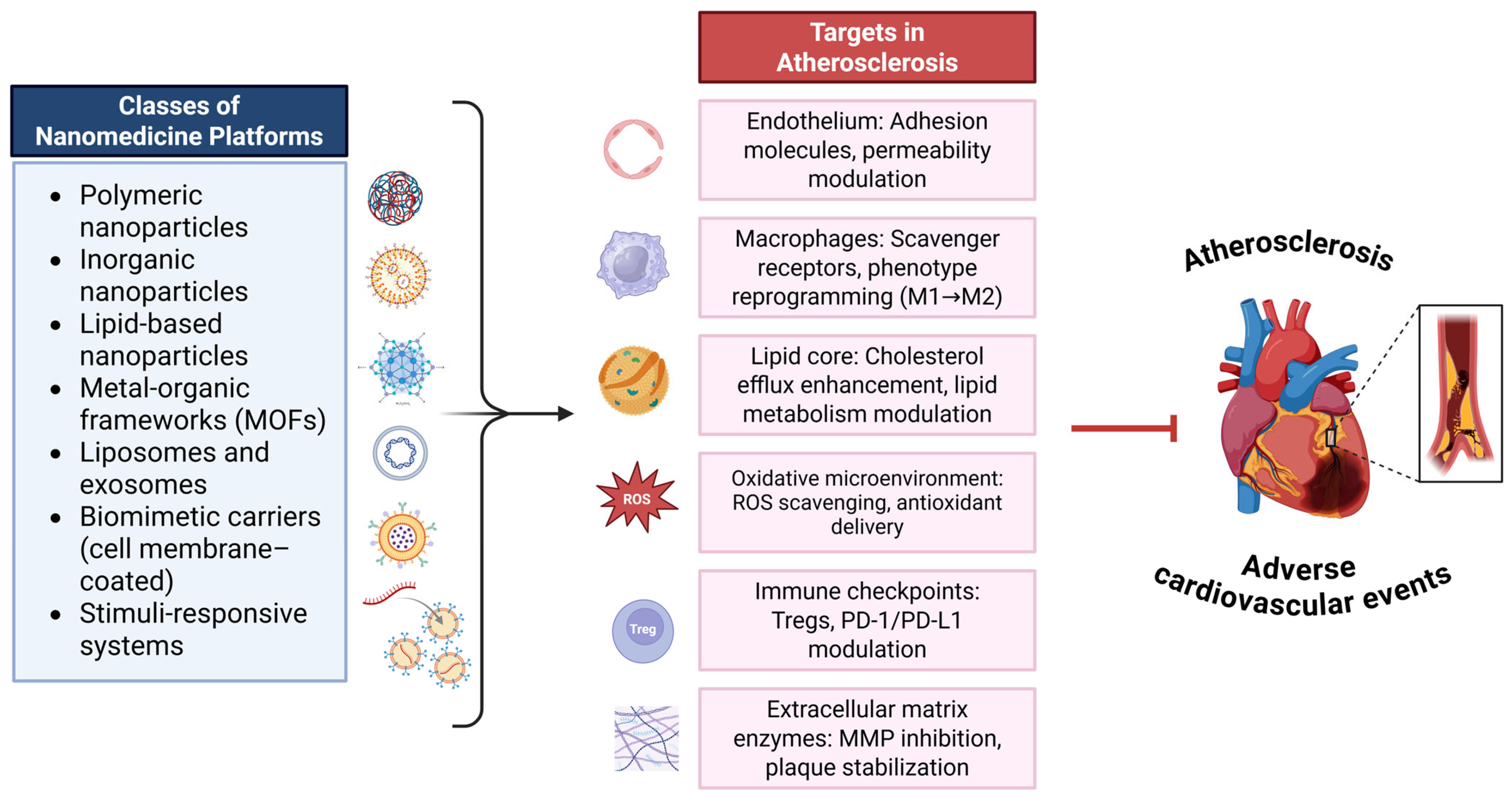
3.1. Nanomedicine in Atherosclerosis: Emerging Therapeutic and Theranostic Strategies
3.2. Immunomodulatory Therapies in Atherosclerosis
3.2.1. Active Immunization Approaches
3.2.2. Regulatory T Cell (Treg)–Enhancing Therapies
3.2.3. Monoclonal Antibodies and Cytokine Inhibition
3.2.4. T Cell–Targeting Therapies
3.2.5. B Cell Depletion Strategies
3.3. Anti-Inflammatory Therapies
3.4. mRNA-Based Therapeutics in Atherosclerosis
4. Targeted Therapeutics
4.1. Targeted Anti-Inflammatory Strategies
4.2. Targeted Antioxidant Strategies
4.3. Targeted Lipid Control
4.4. Targeting Macrophages
4.5. Targeting Enzymes and Modulating the Vascular Microenvironment in Atherosclerosis
4.6. Targeted Modulation of Signaling Pathways
4.6.1. Targeting Co-Stimulatory Pathways
4.6.2. Targeting the mTOR Signaling Pathway
4.6.3. Targeting the Nrf2/HO-1 Signaling Pathway in Atherosclerosis
4.7. Other Emerging Therapeutic Strategies
5. Future Directions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansson, G.K.; Libby, P. The Immune Response in Atherosclerosis: A Double-Edged Sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in Atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in Atherosclerosis: Pathophysiology and Mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef] [PubMed]
- Tasouli-Drakou, V.; Ogurek, I.; Shaikh, T.; Ringor, M.; DiCaro, M.V.; Lei, K. Atherosclerosis: A Comprehensive Review of Molecular Factors and Mechanisms. Int. J. Mol. Sci. 2025, 26, 1364. [Google Scholar] [CrossRef] [PubMed]
- Riksen, N.P.; Bekkering, S.; Mulder, W.J.M.; Netea, M.G. Trained Immunity in Atherosclerotic Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 799–811. [Google Scholar] [CrossRef]
- Amadori, L.; Calcagno, C.; Fernandez, D.M.; Koplev, S.; Fernandez, N.; Kaur, R.; Mury, P.; Khan, N.S.; Sajja, S.; Shamailova, R.; et al. Erratum: Publisher Correction: Systems Immunology-Based Drug Repurposing Framework to Target Inflammation in Atherosclerosis. Nat. Cardiovasc. Res. 2023, 2, 793. [Google Scholar] [CrossRef] [PubMed]
- Chu, R.; Wang, Y.; Kong, J.; Pan, T.; Yang, Y.; He, J. Lipid Nanoparticles as the Drug Carrier for Targeted Therapy of Hepatic Disorders. J. Mater. Chem. B 2024, 12, 4759–4784. [Google Scholar] [CrossRef]
- Gatto, M.S.; Johnson, M.P.; Najahi-Missaoui, W. Targeted Liposomal Drug Delivery: Overview of the Current Applications and Challenges. Life 2024, 14, 672. [Google Scholar] [CrossRef]
- Gu, X.; Majumder, J.; Taratula, O.; Kuzmov, A.; Garbuzenko, O.; Pogrebnyak, N.; Minko, T. Nanotechnology-Based Strategy for Enhancing Therapeutic Efficacy in Pancreatic Cancer: Receptor-Targeted Drug Delivery by Somatostatin Analog. Int. J. Mol. Sci. 2024, 25, 5545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sui, C.; Yang, W.; Luo, Q. Amino Acid Transporters: Emerging Roles in Drug Delivery for Tumor-Targeting Therapy. Asian J. Pharm. Sci. 2020, 15, 192–206. [Google Scholar] [CrossRef]
- Pala, R.; Anju, V.T.; Dyavaiah, M.; Busi, S.; Nauli, S.M. Nanoparticle-Mediated Drug Delivery for the Treatment of Cardiovascular Diseases. Int. J. Nanomed. 2020, 15, 3741–3769. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ren, J.; Yang, L. Nanoparticles in the New Era of Cardiovascular Therapeutics: Challenges and Opportunities. Int. J. Mol. Sci. 2023, 24, 5205. [Google Scholar] [CrossRef]
- Fu, E.; Pan, K.; Li, Z. Engineering Extracellular Vesicles for Targeted Therapeutics in Cardiovascular Disease. Front. Cardiovasc. Med. 2024, 11, 1503830. [Google Scholar] [CrossRef]
- Ding, H.; Liu, Y.; Xia, T.; Zhang, H.; Hao, Y.; Liu, B.; Jiang, Y. Biomimetic Membrane-Coated Nanoparticles for Targeted Synergistic Therapy of Homocysteine-Induced Atherosclerosis: Dual Modulation of Cholesterol Efflux and Reactive Oxygen Species Scavenging. Mater. Today Bio 2025, 33, 101938. [Google Scholar] [CrossRef]
- Bhat, A.; Malik, A.; Yadav, P.; Ware, W.J.; Kakalij, P.; Chand, S. Mesenchymal Stem Cell-derived Extracellular Vesicles: Recent Therapeutics and Targeted Drug Delivery Advances. J. Extracell. Biol. 2024, 3, e156. [Google Scholar] [CrossRef]
- Hossain, S.S.; Zhang, Y.; Liang, X.; Hussain, F.; Ferrari, M.; Hughes, T.J.R.; Decuzzi, P. In Silico Vascular Modeling for Personalized Nanoparticle Delivery. Nanomedicine 2013, 8, 343–357. [Google Scholar] [CrossRef]
- Shamloo, A.; Naseri, T.; Rahbary, A.; Bakhtiari, M.A.; Ebrahimi, S.; Mirafzal, I. In-Silico Study of Drug Delivery to Atherosclerosis in the Human Carotid Artery Using Metal-Organic Frameworks Based on Adhesion of Nanocarriers. Sci. Rep. 2023, 13, 21481. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Patoulias, D.; Vlachakis, P.K.; Antoniadis, A.P.; Fragakis, N. Diabetes-Driven Atherosclerosis: Updated Mechanistic Insights and Novel Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 2196. [Google Scholar] [CrossRef]
- Jia, Y.; Zou, L.; Xue, M.; Zhang, X.; Xiao, X. Evaluation of Peri-Plaque Pericoronary Adipose Tissue Attenuation in Coronary Atherosclerosis Using a Dual-Layer Spectral Detector CT. Front. Med. 2024, 11, 1357981. [Google Scholar] [CrossRef]
- Liu, B.; Su, L.; Loo, S.J.; Gao, Y.; Khin, E.; Kong, X.; Dalan, R.; Su, X.; Lee, K.-O.; Ma, J.; et al. Matrix Metallopeptidase 9 Contributes to the Beginning of Plaque and Is a Potential Biomarker for the Early Identification of Atherosclerosis in Asymptomatic Patients with Diabetes. Front. Endocrinol. 2024, 15, 1369369. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Papanikolaou, A.; Karakasis, P.; Dimitriadis, K.; Vlachakis, P.K.; Oikonomou, E.; Tsioufis, K.; Tousoulis, D. Coronary Atherosclerotic Plaque Modification: The Present and the Future. Expert Rev. Cardiovasc. Ther. 2025, 23, 65–71. [Google Scholar] [CrossRef]
- Abela, G.S.; Katkoori, V.R.; Pathak, D.R.; Bumpers, H.L.; Leja, M.; Abideen, Z.U.; Boumegouas, M.; Perry, D.; Al-Janadi, A.; Richard, J.E.; et al. Cholesterol Crystals Induce Mechanical Trauma, Inflammation, and Neo-Vascularization in Solid Cancers as in Atherosclerosis. Am. Heart J. Plus: Cardiol. Res. Pract. 2023, 35, 100317. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Z.-W.; Fang, L.-J.; Cheng, S.-Q.; Wang, X.; Liu, N.-F. Programmed Cell Death in Atherosclerosis and Vascular Calcification. Cell Death Dis. 2022, 13, 467. [Google Scholar] [CrossRef]
- Madaudo, C.; Coppola, G.; Parlati, A.L.M.; Corrado, E. Discovering Inflammation in Atherosclerosis: Insights from Pathogenic Pathways to Clinical Practice. Int. J. Mol. Sci. 2024, 25, 6016. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Yan, F.; Qin, X.; Zhang, K.; He, W.; Dong, M.; Wu, G. Mitochondrial Dysfunction in Vascular Endothelial Cells and Its Role in Atherosclerosis. Front. Physiol. 2022, 13, 1084604. [Google Scholar] [CrossRef]
- Xu, J.; Chen, C.; Yang, Y. Identification and Validation of Candidate Gene Module Along with Immune Cells Infiltration Patterns in Atherosclerosis Progression to Plaque Rupture via Transcriptome Analysis. Front. Cardiovasc. Med. 2022, 9, 894879. [Google Scholar] [CrossRef]
- Wang, S.; He, H.; Mao, Y.; Zhang, Y.; Gu, N. Advances in Atherosclerosis Theranostics Harnessing Iron Oxide-Based Nanoparticles. Adv. Sci. 2024, 11, e2308298. [Google Scholar] [CrossRef]
- Singh, D.; Rai, V.; Agrawal, D.K. Non-Coding RNAs in Regulating Plaque Progression and Remodeling of Extracellular Matrix in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 13731. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, X.; Wang, Y.; Zheng, Q. Caveolin-1-Mediated LDL Transcytosis across Endothelial Cells in Atherosclerosis. Atherosclerosis 2025, 402, 119113. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.K.; Fröbert, O.; Kindberg, J.; Stenvinkel, P.; Frostegård, J. Potential Natural Immunization against Atherosclerosis in Hibernating Bears. Sci. Rep. 2021, 11, 12120. [Google Scholar] [CrossRef] [PubMed]
- Hasheminasabgorji, E.; Jha, J.C. Dyslipidemia, Diabetes and Atherosclerosis: Role of Inflammation and ROS-Redox-Sensitive Factors. Biomedicines 2021, 9, 1602. [Google Scholar] [CrossRef]
- Zhang, H.; Ge, S.; Ni, B.; He, K.; Zhu, P.; Wu, X.; Shao, Y. Augmenting ATG14 Alleviates Atherosclerosis and Inhibits Inflammation via Promotion of Autophagosome-Lysosome Fusion in Macrophages. Autophagy 2021, 17, 4218–4230. [Google Scholar] [CrossRef]
- Gianopoulos, I.; Daskalopoulou, S.S. Macrophage Profiling in Atherosclerosis: Understanding the Unstable Plaque. Basic Res. Cardiol. 2024, 119, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Kobiyama, K.; Winkels, H.; Ghosheh, Y.; McArdle, S.; Mikulski, Z.; Kiosses, W.B.; Fan, Z.; Wen, L.; Jung, Y.; et al. Olfactory Receptor 2 in Vascular Macrophages Drives Atherosclerosis by NLRP3-Dependent IL-1 Production. Science 2022, 375, 214–221. [Google Scholar] [CrossRef]
- Dong, Z.; Hou, L.; Luo, W.; Pan, L.-H.; Li, X.; Tan, H.-P.; Wu, R.-D.; Lu, H.; Yao, K.; Mu, M.-D.; et al. Myocardial Infarction Drives Trained Immunity of Monocytes, Accelerating Atherosclerosis. Eur. Heart J. 2024, 45, 669–684. [Google Scholar] [CrossRef]
- Nielsen, R.V.; Fuster, V.; Bundgaard, H.; Fuster, J.J.; Johri, A.M.; Kofoed, K.F.; Douglas, P.S.; Diederichsen, A.; Shapiro, M.D.; Nicholls, S.J.; et al. Personalized Intervention Based on Early Detection of Atherosclerosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2024, 83, 2112–2127. [Google Scholar] [CrossRef]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, N.; Xi, D.; Feng, Q.; Liu, Y.; Wang, L.; Tang, Y.; Zhong, H.; He, F. Human Cytomegalovirus Promoting Endothelial Cell Proliferation by Targeting Regulator of G-Protein Signaling 5 Hypermethylation and Downregulation. Sci. Rep. 2020, 10, 2252. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, A.; Maryukhnich, E.; Grivel, J.-C.; Vasilieva, E.; Margolis, L.; Shpektor, A. Productive Cytomegalovirus Infection Is Associated with Impaired Endothelial Function in ST-Elevation Myocardial Infarction. Am. J. Med. 2020, 133, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Didion, S.P. Chlamydophila Pneumoniae and Endothelial Activation: The Smoke That Precedes the Fire of Atherosclerosis? Circ. Res. 2008, 102, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Liuba, P.; Karnani, P.; Pesonen, E.; Paakkari, I.; Forslid, A.; Johansson, L.; Persson, K.; Wadström, T.; Laurini, R. Endothelial Dysfunction after Repeated Chlamydia Pneumoniae Infection in Apolipoprotein E-Knockout Mice. Circulation 2000, 102, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, L.; Chi, J.; Li, H.; Liu, X.; Hu, T.; Li, R.; Guo, Y.; Zhang, X.; Wang, H.; et al. Helicobacter Pylori Infection Impairs Endothelial Function Through an Exosome-Mediated Mechanism. J. Am. Heart Assoc. 2020, 9, e014120. [Google Scholar] [CrossRef]
- Higashi, Y.; Goto, C.; Jitsuiki, D.; Umemura, T.; Nishioka, K.; Hidaka, T.; Takemoto, H.; Nakamura, S.; Soga, J.; Chayama, K.; et al. Periodontal Infection Is Associated with Endothelial Dysfunction in Healthy Subjects and Hypertensive Patients. Hypertension 2008, 51, 446–453. [Google Scholar] [CrossRef]
- Chee, Y.J.; Dalan, R.; Cheung, C. The Interplay Between Immunity, Inflammation and Endothelial Dysfunction. Int. J. Mol. Sci. 2025, 26, 1708. [Google Scholar] [CrossRef]
- Pickett, J.R.; Wu, Y.; Zacchi, L.F.; Ta, H.T. Targeting Endothelial Vascular Cell Adhesion Molecule-1 in Atherosclerosis: Drug Discovery and Development of Vascular Cell Adhesion Molecule-1-Directed Novel Therapeutics. Cardiovasc. Res. 2023, 119, 2278–2293. [Google Scholar] [CrossRef]
- Gáll, T.; Nagy, P.; Garai, D.; Potor, L.; Balla, G.J.; Balla, G.; Balla, J. Overview on Hydrogen Sulfide-Mediated Suppression of Vascular Calcification and Hemoglobin/Heme-Mediated Vascular Damage in Atherosclerosis. Redox Biol. 2022, 57, 102504. [Google Scholar] [CrossRef]
- He, Z.; Chen, W.; Hu, K.; Luo, Y.; Zeng, W.; He, X.; Li, T.; Ouyang, J.; Li, Y.; Xie, L.; et al. Resolvin D1 Delivery to Lesional Macrophages Using Antioxidative Black Phosphorus Nanosheets for Atherosclerosis Treatment. Nat. Nanotechnol. 2024, 19, 1386–1398. [Google Scholar] [CrossRef]
- Hettwer, J.; Hinterdobler, J.; Miritsch, B.; Deutsch, M.-A.; Li, X.; Mauersberger, C.; Moggio, A.; Braster, Q.; Gram, H.; Robertson, A.A.B.; et al. Interleukin-1β Suppression Dampens Inflammatory Leucocyte Production and Uptake in Atherosclerosis. Cardiovasc. Res. 2022, 118, 2778–2791. [Google Scholar] [CrossRef]
- Soltani, S.; Boozari, M.; Cicero, A.F.G.; Jamialahmadi, T.; Sahebkar, A. Effects of Phytochemicals on Macrophage Cholesterol Efflux Capacity: Impact on Atherosclerosis. Phytother. Res. 2021, 35, 2854–2878. [Google Scholar] [CrossRef]
- Wei, X.; Lin, H.; Zhang, B.; Li, M.; Chen, Y.; Huang, Y.; Zhang, J.; Yang, Y.; Guo, Z.; Li, W.; et al. Phoenixin-20 Prevents Ox-LDL-Induced Attachment of Monocytes to Human Aortic Endothelial Cells (HAECs): A Protective Implication in Atherosclerosis. ACS Chem. Neurosci. 2021, 12, 990–997. [Google Scholar] [CrossRef]
- Amponsah-Offeh, M.; Ciliberti, G.; Polycarpou-Schwarz, M.; Stamatelopoulos, K.; Sperandio, M.; Turchinovich, A.; Tual-Chalot, S.; Stellos, K. Role of ADAR2-Mediated Innate Immune Responses in Vascular Inflammation and Atherosclerosis. Cardiovasc. Res. 2024, 120, cvae088-169. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Patoulias, D.; Vlachakis, P.K.; Pamporis, K.; Sagris, M.; Ktenopoulos, N.; Kassimis, G.; Antoniadis, A.P.; Fragakis, N. Sodium–Glucose Cotransporter 2 Inhibitors in Aortic Stenosis: Toward a Comprehensive Cardiometabolic Approach. Int. J. Mol. Sci. 2025, 26, 4494. [Google Scholar] [CrossRef] [PubMed]
- Kłósek, M.; Kurek-Górecka, A.; Balwierz, R.; Krawczyk-Łebek, A.; Kostrzewa-Susłow, E.; Bronikowska, J.; Jaworska, D.; Czuba, Z.P. The Effect of Methyl-Derivatives of Flavanone on MCP-1, MIP-1β, RANTES, and Eotaxin Release by Activated RAW264.7 Macrophages. Molecules 2024, 29, 2239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Pang, C.; Xu, M.; Zhao, Q.; Hu, Z.; Jiang, X.; Guo, M. The Role of Immune System in Atherosclerosis: Molecular Mechanisms, Controversies, and Future Possibilities. Hum. Immunol. 2024, 85, 110765. [Google Scholar] [CrossRef]
- Natarajan, N.; Florentin, J.; Johny, E.; Xiao, H.; O’Neil, S.P.; Lei, L.; Shen, J.; Ohayon, L.; Johnson, A.R.; Rao, K.; et al. Aberrant Mitochondrial DNA Synthesis in Macrophages Exacerbates Inflammation and Atherosclerosis. Nat. Commun. 2024, 15, 7337. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wu, H.; Liu, Y.; Huang, H.; Liu, L.; Yang, Y.; Jiang, T.; Zhou, M.; Dai, M. Inhibiting Vascular Smooth Muscle Cell Proliferation Mediated by Osteopontin via Regulating Gut Microbial Lipopolysaccharide: A Novel Mechanism for Paeonol in Atherosclerosis Treatment. Front. Pharmacol. 2022, 13, 936677. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Liu, J.; Hu, W.; Chen, Z.; Lan, J.; Zhang, T.; Zhang, Y.; Wu, X.; Zhong, Z.; Zhang, D.; et al. Targeting Pro-Inflammatory T Cells as a Novel Therapeutic Approach to Potentially Resolve Atherosclerosis in Humans. Cell Res. 2024, 34, 407–427. [Google Scholar] [CrossRef]
- Langer, H.F. Chronic Inflammation in Atherosclerosis-The CD40L/CD40 Axis Belongs to Dendritic Cells and T Cells, Not Platelets. J. Thromb. Haemost. 2022, 20, 3–5. [Google Scholar] [CrossRef]
- Yang, B.; Hang, S.; Xu, S.; Gao, Y.; Yu, W.; Zang, G.; Zhang, L.; Wang, Z. Macrophage Polarisation and Inflammatory Mechanisms in Atherosclerosis: Implications for Prevention and Treatment. Heliyon 2024, 10, e32073. [Google Scholar] [CrossRef] [PubMed]
- van der Vorst, E.P.C.; Maas, S.L.; Theodorou, K.; Peters, L.J.F.; Jin, H.; Rademakers, T.; Gijbels, M.J.; Rousch, M.; Jansen, Y.; Weber, C.; et al. Endothelial ADAM10 Controls Cellular Response to OxLDL and Its Deficiency Exacerbates Atherosclerosis with Intraplaque Hemorrhage and Neovascularization in Mice. Front. Cardiovasc. Med. 2023, 10, 974918. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Li, L.; Liu, Y.; Liang, Y.; Qi, X. Silencing Ferritin Alleviates Atherosclerosis in Mice via Regulating the Expression Levels of Matrix Metalloproteinases and Interleukins. Acta Biochim. Pol. 2021, 68, 705–710. [Google Scholar] [CrossRef]
- Xiang, P.; Blanchard, V.; Francis, G.A. Smooth Muscle Cell-Macrophage Interactions Leading to Foam Cell Formation in Atherosclerosis: Location, Location, Location. Front. Physiol. 2022, 13, 921597. [Google Scholar] [CrossRef]
- Li, S.; He, R.-C.; Wu, S.-G.; Song, Y.; Zhang, K.-L.; Tang, M.-L.; Bei, Y.-R.; Zhang, T.; Lu, J.-B.; Ma, X.; et al. LncRNA PSMB8-AS1 Instigates Vascular Inflammation to Aggravate Atherosclerosis. Circ. Res. 2024, 134, 60–80. [Google Scholar] [CrossRef]
- Fang, F.; Xiao, C.; Li, C.; Liu, X.; Li, S. Tuning Macrophages for Atherosclerosis Treatment. Regen. Biomater. 2023, 10, rbac103. [Google Scholar] [CrossRef]
- Omelchenko, A.; Borodko, D.; Soplenkova, A.; Sukhorukov, V. Association of Atherosclerosis-Related Mitochondrial Mutations with the Mitochondrial Dysfunction. Atherosclerosis 2023, 379, S13. [Google Scholar] [CrossRef]
- Guo, J.; Ma, J.; Cai, K.; Chen, H.; Xie, K.; Xu, B.; Quan, D.; Du, J. Isoflavones from Semen Sojae Preparatum Improve Atherosclerosis and Oxidative Stress by Modulating Nrf2 Signaling Pathway through Estrogen-Like Effects. Evid. Based. Complement. Alternat. Med. 2022, 2022, 4242099. [Google Scholar] [CrossRef]
- Violi, F.; Pignatelli, P.; Valeriani, E. Oxidative Stress and Atherosclerosis: Basic and Clinical Open Issues. Kardiol. Pol. 2024, 82, 689–691. [Google Scholar] [CrossRef]
- Shiina, K.; Tomiyama, H.; Tanaka, A.; Yoshida, H.; Eguchi, K.; Kario, K.; Kato, T.; Teragawa, H.; Toyoda, S.; Ohishi, M.; et al. Differential Effect of a Xanthine Oxidase Inhibitor on Arterial Stiffness and Carotid Atherosclerosis: A Subanalysis of the PRIZE Study. Hypertens. Res. 2022, 45, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cao, H.; Chen, B.; Xu, X.; Zhang, Q.; Chen, H.; Zhang, X.-B.; Song, G. Simultaneous In Vivo Imaging of Neutrophil Elastase and Oxidative Stress in Atherosclerotic Plaques Using a Unimolecular Photoacoustic Probe. Angew. Chemie Int. Ed. 2024, 63, e202411840. [Google Scholar] [CrossRef]
- Becker, P.-H.; Le Guillou, E.; Duque, M.; Blondel, A.; Gons, C.; Ben Souna, H.; Imbard, A.; Fournier, N.; Gaignard, P.; Thérond, P. Cholesterol Accumulation Induced by Acetylated LDL Exposure Modifies the Enzymatic Activities of the TCA Cycle without Impairing the Respiratory Chain Functionality in Macrophages. Biochimie 2022, 200, 87–98. [Google Scholar] [CrossRef]
- Wang, X.Q.; Chen, J.W.; Lu, L.; Yang, C.D. Increased 12/15-Lipoxygenase by Disturbed Flow Promotes Endothelial Dysfunction and the Development of Atherosclerosis. Eur. Heart J. 2023, 44, ehad655-3266. [Google Scholar] [CrossRef]
- Mathew, A.V.; Zeng, L.; Atkins, K.B.; Sadri, K.N.; Byun, J.; Fujiwara, H.; Reddy, P.; Pennathur, S. Deletion of Bone Marrow Myeloperoxidase Attenuates Chronic Kidney Disease Accelerated Atherosclerosis. J. Biol. Chem. 2021, 296, 100120. [Google Scholar] [CrossRef]
- Altahrawi, A.Y.; James, A.W.; Shah, Z.A. The Role of Oxidative Stress and Inflammation in the Pathogenesis and Treatment of Vascular Dementia. Cells 2025, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative Stress and Inflammation in the Pathogenesis of Neurological Disorders: Mechanisms and Implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Liao, Y.; Gou, L.; Chen, L.; Zhong, X.; Zhang, D.; Zhu, H.; Lu, X.; Zeng, T.; Deng, X.; Li, Y. NADPH Oxidase 4 and Endothelial Nitric Oxide Synthase Contribute to Endothelial Dysfunction Mediated by Histone Methylations in Metabolic Memory. Free Radic. Biol. Med. 2018, 115, 383–394. [Google Scholar] [CrossRef]
- Frey, R.S.; Ushio-Fukai, M.; Malik, A.B. NADPH Oxidase-Dependent Signaling in Endothelial Cells: Role in Physiology and Pathophysiology. Antioxid. Redox Signal. 2009, 11, 791–810. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Cross-Talk between NADPH Oxidase and Mitochondria: Role in ROS Signaling and Angiogenesis. Cells 2020, 9, 1849. [Google Scholar] [CrossRef] [PubMed]
- Burtenshaw, D.; Kitching, M.; Redmond, E.M.; Megson, I.L.; Cahill, P.A. Reactive Oxygen Species (ROS), Intimal Thickening, and Subclinical Atherosclerotic Disease. Front. Cardiovasc. Med. 2019, 6, 89. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, X.; Xu, W.; Li, J.; Sun, Y.; Cui, S.; Xu, R.; Li, W.; Jiao, L.; Wang, T. ROS-Induced Endothelial Dysfunction in the Pathogenesis of Atherosclerosis. Aging Dis. 2024, 16, 250–268. [Google Scholar] [CrossRef]
- Wang, Y.; Tabas, I. Emerging Roles of Mitochondria ROS in Atherosclerotic Lesions: Causation or Association? J. Atheroscler. Thromb. 2014, 21, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Nowak, W.N.; Deng, J.; Ruan, X.Z.; Xu, Q. Reactive Oxygen Species Generation and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e41–e52. [Google Scholar] [CrossRef]
- Korashy, H.M.; El-Kadi, A.O.S. The Role of Redox-Sensitive Transcription Factors NF-KappaB and AP-1 in the Modulation of the Cyp1a1 Gene by Mercury, Lead, and Copper. Free Radic. Biol. Med. 2008, 44, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Ortega, Á.L.; Guryanova, S.V.; Maksimova, T.V.; Azova, M.M.; Shemyakin, M.M.; Ovchinnikov, Y.A. Transcription Factors and Methods for the Pharmacological Correction of Their Activity. Int. J. Mol. Sci. 2025, 26, 6394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, J.; Hu, W. The Complex Interplay between Ferroptosis and Atherosclerosis. Biomed. Pharmacother. 2024, 178, 117183. [Google Scholar] [CrossRef]
- He, C.; Kim, H.I.; Park, J.; Guo, J.; Huang, W. The Role of Immune Cells in Different Stages of Atherosclerosis. Int. J. Med. Sci. 2024, 21, 1129–1143. [Google Scholar] [CrossRef]
- Karakasis, P.; Sagris, M.; Patoulias, D.; Koufakis, T.; Theofilis, P.; Klisic, A.; Fragakis, N.; El Tanani, M.; Rizzo, M. Mitigating Increased Cardiovascular Risk in Patients with Obstructive Sleep Apnea Using GLP-1 Receptor Agonists and SGLT2 Inhibitors: Hype or Hope? Biomedicines 2024, 12, 2503. [Google Scholar] [CrossRef]
- Queiroz, M.I.C.; Lazaro, C.M.; Dos Santos, L.M.B.; Rentz, T.; Virgilio-da-Silva, J.V.; Moraes-Vieira, P.M.M.; Cunha, F.A.S.; Santos, J.C.C.; Vercesi, A.E.; Leite, A.C.R.; et al. In Vivo Chronic Exposure to Inorganic Mercury Worsens Hypercholesterolemia, Oxidative Stress and Atherosclerosis in the LDL Receptor Knockout Mice. Ecotoxicol. Environ. Saf. 2024, 275, 116254. [Google Scholar] [CrossRef] [PubMed]
- Sajja, A.; Li, H.-F.; Spinelli, K.J.; Blumenthal, R.S.; Virani, S.S.; Martin, S.S.; Gluckman, T.J. Discordance Between Standard Equations for Determination of LDL Cholesterol in Patients with Atherosclerosis. J. Am. Coll. Cardiol. 2022, 79, 530–541. [Google Scholar] [CrossRef]
- Cao, Y.; Song, N.; Wang, Y.; Leng, X.; Wang, Q.; Ma, Y.; Chen, S.; Ju, X.; Jia, L. The Potential Association of TFR1/SLC11A2/GPX4 with Ferroptosis in Mediating Lipid Metabolism Disorders in Atherosclerosis. Comb. Chem. High Throughput Screen. 2025, 28, 467–477. [Google Scholar] [CrossRef]
- Ansari, A.; Yadav, P.K.; Zhou, L.; Prakash, B.; Gangula, B.; Ijaz, L.; Christiano, A.; Ahmad, S.; Rimbert, A.; Hussain, M.M. MicroRNA-541-3p Alters Lipoproteins to Reduce Atherosclerosis by Degrading Znf101 and Casz1 Transcription Factors. bioRxiv 2023. bioRxiv:2023.11.01.565110. [Google Scholar] [CrossRef]
- Marchini, T.; Hansen, S.; Wolf, D. ApoB-Specific CD4(+) T Cells in Mouse and Human Atherosclerosis. Cells 2021, 10, 446. [Google Scholar] [CrossRef]
- Hartley, A.; Greene, M.; Caga-Anan, M.; Owen, S.; Mullin, M.; Pericleous, C.; Scott, C.; Mason, J.; Haskard, D.O.; Khamis, R. Molecular Imaging of Experimental Atherosclerosis Using Anti-Malondialdehyde-Modified Low-Density Lipoprotein Humanised Antibody Fragment Targeted Nanoparticles. Eur. Heart J. 2022, 43, ehac544-3040. [Google Scholar] [CrossRef]
- Chen, H.; Wu, B.; Guan, K.; Chen, L.; Chai, K.; Ying, M.; Li, D.; Zhao, W. Identification of Lipid Metabolism Related Immune Markers in Atherosclerosis through Machine Learning and Experimental Analysis. Front. Immunol. 2025, 16, 1549150. [Google Scholar] [CrossRef]
- Qiao, Y.-N.; Zou, Y.-L.; Guo, S.-D. Low-Density Lipoprotein Particles in Atherosclerosis. Front. Physiol. 2022, 13, 931931. [Google Scholar] [CrossRef] [PubMed]
- Demina, E.P.; Smutova, V.; Pan, X.; Fougerat, A.; Guo, T.; Zou, C.; Chakraberty, R.; Snarr, B.D.; Shiao, T.C.; Roy, R.; et al. Neuraminidases 1 and 3 Trigger Atherosclerosis by Desialylating Low-Density Lipoproteins and Increasing Their Uptake by Macrophages. J. Am. Heart Assoc. 2021, 10, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2020, 11, 613780. [Google Scholar] [CrossRef]
- Mushenkova, N.V.; Bezsonov, E.E.; Orekhova, V.A.; Popkova, T.V.; Starodubova, A.V.; Orekhov, A.N. Recognition of Oxidized Lipids by Macrophages and Its Role in Atherosclerosis Development. Biomedicines 2021, 9, 915. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Zhao, Y.; Li, Y.; Zhang, X.; Bao, L.; Yan, R.; Yang, Y.; Zhou, H.; Zhang, J.; et al. Research Progress and Clinical Translation Potential of Coronary Atherosclerosis Diagnostic Markers from a Genomic Perspective. Genes 2025, 16, 98. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, B.; Guo, Y.; Zhang, A.; Yang, K.; He, Y.; Wang, J.; Cheng, Y.; Cui, D. SR-A-Targeted Nanoplatform for Sequential Photothermal/Photodynamic Ablation of Activated Macrophages to Alleviate Atherosclerosis. ACS Appl. Mater. Interfaces 2021, 13, 29349–29362. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Wang, Y.-R.; Li, R.; Xiao, L.; Xie, Y.; Xie, N.-C.; Liu, H.-B. Single Nucleotide Polymorphisms Rs102313, Rs118231 and Rs201832 of CTEP TaqIB Gene Correlated with Lipid Metabolism Abnormalities and Cerebral Infarction in Patients with Atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7402–7408. [Google Scholar] [CrossRef] [PubMed]
- Chehaitly, A.; Guihot, A.-L.; Proux, C.; Grimaud, L.; Aurrière, J.; Legouriellec, B.; Rivron, J.; Vessieres, E.; Tétaud, C.; Zorzano, A.; et al. Altered Mitochondrial Opa1-Related Fusion in Mouse Promotes Endothelial Cell Dysfunction and Atherosclerosis. Antioxidants 2022, 11, 1078. [Google Scholar] [CrossRef]
- Wu, X.; Pan, J.; Yu, J.J.; Kang, J.; Hou, S.; Cheng, M.; Xu, L.; Gong, L.; Li, Y. DiDang Decoction Improves Mitochondrial Function and Lipid Metabolism via the HIF-1 Signaling Pathway to Treat Atherosclerosis and Hyperlipidemia. J. Ethnopharmacol. 2023, 308, 116289. [Google Scholar] [CrossRef]
- Nevoit, G.; Jarusevicius, G.; Potyazhenko, M.; Mintser, O.; Bumblyte, I.A.; Vainoras, A. Mitochondrial Dysfunction and Atherosclerosis: The Problem and the Search for Its Solution. Biomedicines 2025, 13, 963. [Google Scholar] [CrossRef]
- Xu, M.; Wang, W.; Cheng, J.; Qu, H.; Xu, M.; Wang, L. Effects of Mitochondrial Dysfunction on Cellular Function: Role in Atherosclerosis. Biomed. Pharmacother. 2024, 174, 116587. [Google Scholar] [CrossRef]
- Ciccarelli, G.; Conte, S.; Cimmino, G.; Maiorano, P.; Morrione, A.; Giordano, A. Mitochondrial Dysfunction: The Hidden Player in the Pathogenesis of Atherosclerosis? Int. J. Mol. Sci. 2023, 24, 1086. [Google Scholar] [CrossRef] [PubMed]
- Gielecińska, A.; Kciuk, M.; Kontek, R. The Impact of Calcium Overload on Cellular Processes: Exploring Calcicoptosis and Its Therapeutic Potential in Cancer. Int. J. Mol. Sci. 2024, 25, 13727. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Gao, M.; Liu, B.; Qin, Y.; Chen, L.; Liu, H.; Wu, H.; Gong, G. Mitochondrial Autophagy: Molecular Mechanisms and Implications for Cardiovascular Disease. Cell Death Dis. 2022, 13, 444. [Google Scholar] [CrossRef]
- Wang, X.; Luo, D.; Wu, S. Molecular Dysfunctions of Mitochondria-Associated Endoplasmic Reticulum Contacts in Atherosclerosis. Oxid. Med. Cell. Longev. 2021, 2021, 2424509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Weng, J.; Huan, L.; Sheng, S.; Xu, F. Mitophagy in Atherosclerosis: From Mechanism to Therapy. Front. Immunol. 2023, 14, 1165507. [Google Scholar] [CrossRef] [PubMed]
- Fedotova, E.I.; Berezhnov, A.V.; Popov, D.Y.; Shitikova, E.Y.; Vinokurov, A.Y. The Role of MtDNA Mutations in Atherosclerosis: The Influence of Mitochondrial Dysfunction on Macrophage Polarization. Int. J. Mol. Sci. 2025, 26, 1019. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Wu, W.-K.; Kirichenko, T.V.; Orekhov, A.N. Autophagy and Mitophagy as Essential Components of Atherosclerosis. Cells 2021, 10, 443. [Google Scholar] [CrossRef]
- An, C.; Sun, F.; Liu, C.; Huang, S.; Xu, T.; Zhang, C.; Ge, S. IQGAP1 Promotes Mitochondrial Damage and Activation of the MtDNA Sensor CGAS-STING Pathway to Induce Endothelial Cell Pyroptosis Leading to Atherosclerosis. Int. Immunopharmacol. 2023, 123, 110795. [Google Scholar] [CrossRef]
- Campolo, J.; Canale, P.; Gazzaniga, G.; Parolini, M.; Piccaluga, E.; Bossi, I.; Oreglia, J.; Borghini, A.; Marinaro, I.; Andreassi, M.G. The Mitochondrial Dysfunction, alongside the Modifiable Burden of Traditional Risk Factors, Drives the Development of Early-Onset Coronary Artery Disease. Front. Cardiovasc. Med. 2025, 12, 1538202. [Google Scholar] [CrossRef]
- Ren, H.; Hu, W.; Jiang, T.; Yao, Q.; Qi, Y.; Huang, K. Mechanical Stress Induced Mitochondrial Dysfunction in Cardiovascular Diseases: Novel Mechanisms and Therapeutic Targets. Biomed. Pharmacother. 2024, 174, 116545. [Google Scholar] [CrossRef]
- Vendrov, A.E.; Lozhkin, A.; Hayami, T.; Levin, J.; Silveira Fernandes Chamon, J.; Abdel-Latif, A.; Runge, M.S.; Madamanchi, N.R. Mitochondrial Dysfunction and Metabolic Reprogramming Induce Macrophage Pro-Inflammatory Phenotype Switch and Atherosclerosis Progression in Aging. Front. Immunol. 2024, 15, 1410832. [Google Scholar] [CrossRef]
- Camacho-Encina, M.; Booth, L.K.; Redgrave, R.E.; Folaranmi, O.; Spyridopoulos, I.; Richardson, G.D. Cellular Senescence, Mitochondrial Dysfunction, and Their Link to Cardiovascular Disease. Cells 2024, 13, 353. [Google Scholar] [CrossRef] [PubMed]
- Sazonova, M.A.; Sinyov, V.V.; Ryzhkova, A.I.; Sazonova, M.D.; Kirichenko, T.V.; Khotina, V.A.; Khasanova, Z.B.; Doroschuk, N.A.; Karagodin, V.P.; Orekhov, A.N.; et al. Some Molecular and Cellular Stress Mechanisms Associated with Neurodegenerative Diseases and Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 699. [Google Scholar] [CrossRef] [PubMed]
- Khotina, V.A.; Vinokurov, A.Y.; Sinyov, V.V.; Zhuravlev, A.D.; Popov, D.Y.; Sukhorukov, V.N.; Sobenin, I.A.; Orekhov, A.N. Mitochondrial Dysfunction Associated with MtDNA Mutation: Mitochondrial Genome Editing in Atherosclerosis Research. Curr. Med. Chem. 2024. ahead of print. [Google Scholar] [CrossRef]
- Peng, X.; Sun, B.; Tang, C.; Shi, C.; Xie, X.; Wang, X.; Jiang, D.; Li, S.; Jia, Y.; Wang, Y.; et al. HMOX1-LDHB Interaction Promotes Ferroptosis by Inducing Mitochondrial Dysfunction in Foamy Macrophages during Advanced Atherosclerosis. Dev. Cell 2025, 60, 1070–1086.e8. [Google Scholar] [CrossRef]
- Fock, E.M.; Parnova, R.G. Protective Effect of Mitochondria-Targeted Antioxidants against Inflammatory Response to Lipopolysaccharide Challenge: A Review. Pharmaceutics 2021, 13, 144. [Google Scholar] [CrossRef]
- Roşian, Ş.H.; Boarescu, I.; Boarescu, P.-M. Antioxidant and Anti-Inflammatory Effects of Bioactive Compounds in Atherosclerosis. Int. J. Mol. Sci. 2025, 26, 1379. [Google Scholar] [CrossRef] [PubMed]
- Noone, S.; Schubert, R.; Fichtlscherer, S.; Hilberg, T.; Alesci, S.; Miesbach, W.; Klophaus, N.; Wehmeier, U.F. Endothelial Dysfunction and Atherosclerosis Related MiRNA-Expression in Patients with Haemophilia. Haemophilia 2023, 29, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.; Ni, L.; Lin, Y. Treatment of Endothelial Cell Dysfunction in Atherosclerosis: A New Perspective Integrating Traditional and Modern Approaches. Front. Physiol. 2025, 16, 1555118. [Google Scholar] [CrossRef]
- Wang, X.; He, B. Endothelial Dysfunction: Molecular Mechanisms and Clinical Implications. MedComm 2024, 5, e651. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Kouskouras, K.; Karamitsos, T.; Patoulias, D.; Rizzo, M. Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Acute Coronary Syndrome: A Modern Cinderella? Clin. Ther. 2024, 46, 841–850. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Kassimis, G.; Koufakis, T.; Klisic, A.; Doumas, M.; Fragakis, N.; Rizzo, M. Therapeutic Potential of Sodium-Glucose Co-Transporter-2 Inhibitors and Glucagon-like Peptide-1 Receptor Agonists for Patients with Acute Coronary Syndrome: A Review of Clinical Evidence. Curr. Pharm. Des. 2024, 30, 2109–2119. [Google Scholar] [CrossRef]
- Donadini, M.P.; Calcaterra, F.; Romualdi, E.; Ciceri, R.; Cancellara, A.; Lodigiani, C.; Bacci, M.; Della Bella, S.; Ageno, W.; Mavilio, D. The Link Between Venous and Arterial Thrombosis: Is There a Role for Endothelial Dysfunction? Cells 2025, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Lefkou, E.; Pamporis, K.; Nevras, V.; Bougioukas, K.I.; Haidich, A.-B.; Fragakis, N. Risk of Subclinical Atherosclerosis in Patients with Antiphospholipid Syndrome and Subjects with Antiphospholipid Antibody Positivity: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101672. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Stachteas, P.; Lefkou, E.; Dimitroulas, T.; Fragakis, N. Accelerated Atherosclerosis and Management of Cardiovascular Risk in Autoimmune Rheumatic Diseases: An Updated Review. Curr. Probl. Cardiol. 2023, 48, 101999. [Google Scholar] [CrossRef]
- Jimenez-Trinidad, F.R.; Calvo-Gomez, S.; Sabaté, M.; Brugaletta, S.; Campuzano, V.; Egea, G.; Dantas, A.P. Extracellular Vesicles as Mediators of Endothelial Dysfunction in Cardiovascular Diseases. Int. J. Mol. Sci. 2025, 26, 1008. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Zhang, L.; Xia, H. Experimental Study on Alleviating Atherosclerosis through Intervention of Mitochondrial Calcium Transport and Calcium-Induced Membrane Permeability Transition. J. Investig. Med.Off. Publ. Am. Fed. Clin. Res. 2021, 69, 1156–1160. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, Y.; Chen, R.; Liu, X.; Hu, Y.; Ma, Z.; Gao, L.; Jian, W.; Wang, L. Wall Shear Stress and Its Role in Atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1083547. [Google Scholar] [CrossRef]
- Wentzel, J.J.; Chatzizisis, Y.S.; Gijsen, F.J.H.; Giannoglou, G.D.; Feldman, C.L.; Stone, P.H. Endothelial Shear Stress in the Evolution of Coronary Atherosclerotic Plaque and Vascular Remodelling: Current Understanding and Remaining Questions. Cardiovasc. Res. 2012, 96, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Korteland, S.-A.; Tardajos-Ayllon, B.; Wu, J.; Chambers, E.; Weninck, J.; Simons, M.; Dunning, M.; Schenkel, T.; Diagbouga, M.; et al. Shear Stress Is Uncoupled from Atheroprotective KLK10 in Atherosclerotic Plaques. Atherosclerosis 2024, 398, 118622. [Google Scholar] [CrossRef]
- Saib, Z.A.; Abed, F.; Ghayesh, M.H.; Amabili, M. A Review of Fluid-Structure Interaction: Blood Flow in Arteries. Biomed. Eng. Adv. 2025, 9, 100171. [Google Scholar] [CrossRef]
- Caro, C.G. Discovery of the Role of Wall Shear in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, Y.; Mu, X. The Role of Fluid Mechanics in Coronary Atherosclerotic Plaques: An Up-to-Date Review. Rev. Cardiovasc. Med. 2024, 25, 49. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Deng, Y.; Ma, Y.; Man, S.; Yang, X.; Yu, C.; Lv, J.; Wang, B.; Li, L. National and Provincial-Level Prevalence and Risk Factors of Carotid Atherosclerosis in Chinese Adults. JAMA Netw. Open 2024, 7, e2351225. [Google Scholar] [CrossRef]
- Pepin, M.E.; Gupta, R.M. The Role of Endothelial Cells in Atherosclerosis: Insights from Genetic Association Studies. Am. J. Pathol. 2024, 194, 499–509. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.-Y.; Shi, S.-R.; Ma, C.-N.; Lin, Y.-P.; Song, W.-G.; Guo, S.-D. Natural Products in Atherosclerosis Therapy by Targeting PPARs: A Review Focusing on Lipid Metabolism and Inflammation. Front. Cardiovasc. Med. 2024, 11, 1372055. [Google Scholar] [CrossRef] [PubMed]
- Martos-Rodríguez, C.J.; Albarrán-Juárez, J.; Morales-Cano, D.; Caballero, A.; MacGrogan, D.; de la Pompa, J.L.; Carramolino, L.; Bentzon, J.F. Fibrous Caps in Atherosclerosis Form by Notch-Dependent Mechanisms Common to Arterial Media Development. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e427–e439. [Google Scholar] [CrossRef]
- Zhao, N.; Yu, X.; Zhu, X.; Song, Y.; Gao, F.; Yu, B.; Qu, A. Diabetes Mellitus to Accelerated Atherosclerosis: Shared Cellular and Molecular Mechanisms in Glucose and Lipid Metabolism. J. Cardiovasc. Transl. Res. 2024, 17, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Gao, W.; Zhang, J.; Dong, X.; Zhu, D.; Ma, G. Engineering Nanoparticles-Enabled Tumor-Associated Macrophages Repolarization and Phagocytosis Restoration for Enhanced Cancer Immunotherapy. J. Nanobiotechnol. 2024, 22, 341. [Google Scholar] [CrossRef]
- Desai, O.; Kumar, S.; Köster, M.; Ullah, S.; Sarker, S.; Hagemann, V.; Habib, M.; Klaassen, N.; Notter, S.; Feldmann, C.; et al. Macrophages Co-Loaded with Drug-Associated and Superparamagnetic Nanoparticles for Triggered Drug Release by Alternating Magnetic Fields. Drug Deliv. Transl. Res. 2025, 15, 2779–2793. [Google Scholar] [CrossRef]
- Guo, Q.; Qian, Z.-M. Macrophage Based Drug Delivery: Key Challenges and Strategies. Bioact. Mater. 2024, 38, 55–72. [Google Scholar] [CrossRef]
- Hartmann, F.; Gorski, D.J.; Newman, A.A.C.; Homann, S.; Petz, A.; Owsiany, K.M.; Serbulea, V.; Zhou, Y.-Q.; Deaton, R.A.; Bendeck, M.; et al. SMC-Derived Hyaluronan Modulates Vascular SMC Phenotype in Murine Atherosclerosis. Circ. Res. 2021, 129, 992–1005. [Google Scholar] [CrossRef]
- Młynarska, E.; Czarnik, W.; Fularski, P.; Hajdys, J.; Majchrowicz, G.; Stabrawa, M.; Rysz, J.; Franczyk, B. From Atherosclerotic Plaque to Myocardial Infarction-The Leading Cause of Coronary Artery Occlusion. Int. J. Mol. Sci. 2024, 25, 7295. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent Developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Chen, L.; Li, Q.; Zhao, Y.; Yin, D.; Sun, S. Relationship between Serum FGF21 and VWF Expression and Carotid Atherosclerosis in Elderly Patients with Hypertension. J. Healthc. Eng. 2022, 2022, 6777771. [Google Scholar] [CrossRef]
- Kong, W.; Ma, J.; Lin, Y.; Chen, W. Positive Association of Plasma Trimethylamine-N-Oxide and Atherosclerosis in Patient with Acute Coronary Syndrome. Cardiovasc. Ther. 2022, 2022, 2484018. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Xue, C.; Auerbach, B.J.; Fan, J.; Bashore, A.C.; Cui, J.; Yang, D.Y.; Trignano, S.B.; Liu, W.; Shi, J.; et al. Single-Cell Genomics Reveals a Novel Cell State During Smooth Muscle Cell Phenotypic Switching and Potential Therapeutic Targets for Atherosclerosis in Mouse and Human. Circulation 2020, 142, 2060–2075. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Liu, Y.; Yuan, L. Exosomes in Atherosclerosis: Performers, Bystanders, Biomarkers, and Therapeutic Targets. Theranostics 2021, 11, 3996–4010. [Google Scholar] [CrossRef]
- Ouyang, S.; You, J.; Zhi, C.; Li, P.; Lin, X.; Tan, X.; Ma, W.; Li, L.; Xie, W. Ferroptosis: The Potential Value Target in Atherosclerosis. Cell Death Dis. 2021, 12, 782. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, R.; Lin, H.B.; Shen, S. Nanomedicine-Based Drug Delivery Strategies for the Treatment of Atherosclerosis. Med. Drug Discov. 2024, 22, 100189. [Google Scholar] [CrossRef]
- Omidian, H.; Babanejad, N.; Cubeddu, L.X. Nanosystems in Cardiovascular Medicine: Advancements, Applications, and Future Perspectives. Pharmaceutics 2023, 15, 1935. [Google Scholar] [CrossRef]
- Mao, Y.; Ren, J.; Yang, L. Advances of Nanomedicine in Treatment of Atherosclerosis and Thrombosis. Environ. Res. 2023, 238, 116637. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, Y.; Liu, Z.; Guo, L.; Zhang, L.; Rong, H.; Duan, Z.; Liang, H.; Zhang, A.; Wang, L.; et al. Selenopeptide Nanomedicine Ameliorates Atherosclerosis by Reducing Monocyte Adhesions and Inflammations. Nano Res. 2024, 17, 6332–6341. [Google Scholar] [CrossRef]
- Li, D.; Chen, J.; Lu, Y.; Yan, X.; Yang, X.; Zhang, F.; Tang, Y.; Cao, M.; Wang, J.; Pan, M.; et al. Codelivery of Dual Gases with Metal-Organic Supramolecular Cage-Based Microenvironment-Responsive Nanomedicine for Atherosclerosis Therapy. Small 2024, 20, e2402673. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.; Guo, L.; Cui, Y.; Zou, C.; Hu, J.; Zhang, H.; Yang, G.; Zhou, W. Multifunctional Nanomedicine for Targeted Atherosclerosis Therapy: Activating Plaque Clearance Cascade and Suppressing Inflammation. ACS Nano 2025, 19, 3339–3361. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, W.; Tang, C.; Rong, X.; Liu, Y.; Luo, Y.; Xu, L.; Xu, Z.; Wang, J.; Wang, Y.; et al. Macrophage Membrane-Functionalized Manganese Dioxide Nanomedicine for Synergistic Treatment of Atherosclerosis by Mitigating Inflammatory Storms and Promoting Cholesterol Efflux. J. Nanobiotechnol. 2024, 22, 664. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.; Kastantin, M.; Kotamraju, V.R.; Karmali, P.P.; Gujraty, K.; Tirrell, M.; Ruoslahti, E. Targeting Atherosclerosis by Using Modular, Multifunctional Micelles. Proc. Natl. Acad. Sci. USA 2009, 106, 9815–9819. [Google Scholar] [CrossRef]
- Zhou, Z.; Yeh, C.-F.; Mellas, M.; Oh, M.-J.; Zhu, J.; Li, J.; Huang, R.-T.; Harrison, D.L.; Shentu, T.-P.; Wu, D.; et al. Targeted Polyelectrolyte Complex Micelles Treat Vascular Complications in Vivo. Proc. Natl. Acad. Sci. USA 2021, 118, e2114842118. [Google Scholar] [CrossRef]
- Peng, Y.; Feng, W.; Huang, H.; Chen, Y.; Yang, S. Macrophage-Targeting Antisenescence Nanomedicine Enables in-Situ NO Induction for Gaseous and Antioxidative Atherosclerosis Intervention. Bioact. Mater. 2025, 48, 294–312. [Google Scholar] [CrossRef]
- Gu, X.; Du, L.; Lin, R.; Ding, Z.; Guo, Z.; Wei, J.; Li, Y. How Advanced Is Nanomedicine for Atherosclerosis? Int. J. Nanomedicine 2025, 20, 3445–3470. [Google Scholar] [CrossRef]
- Hu, B.; Boakye-Yiadom, K.O.; Yu, W.; Yuan, Z.-W.; Ho, W.; Xu, X.; Zhang, X.-Q. Nanomedicine Approaches for Advanced Diagnosis and Treatment of Atherosclerosis and Related Ischemic Diseases. Adv. Healthc. Mater. 2020, 9, e2000336. [Google Scholar] [CrossRef]
- Cui, H.; Soga, K.; Tamehiro, N.; Adachi, R.; Hachisuka, A.; Hirose, A.; Kondo, K.; Nishimaki-Mogami, T. Statins Repress Needle-like Carbon Nanotube- or Cholesterol Crystal-Stimulated IL-1β Production by Inhibiting the Uptake of Crystals by Macrophages. Biochem. Pharmacol. 2021, 188, 114580. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, H.; Chen, Y.; Wu, R.; Cheng, J.; Wu, R.; Huang, H.; Chen, Y.; Lab, M. Nanomedicine for Diagnosis and Treatment of Atherosclerosis. Adv. Sci. 2023, 10, 2304294. [Google Scholar] [CrossRef]
- Luo, X.; Fu, H.; Xu, C.; Dong, Y.; Wu, Z.; Li, D.; Sun, Y.; Shen, M.; Wang, L.; Li, Z.; et al. Efficient Treatment of Atherosclerosis by Dexamethasone Acetate and Rapamycin Co-Loaded MPEG-DSPE Calcium Phosphate Nanoparticles. J. Biomed. Nanotechnol. 2020, 16, 810–826. [Google Scholar] [CrossRef]
- Kim, H.; Kumar, S.; Kang, D.-W.; Jo, H.; Park, J.-H. Affinity-Driven Design of Cargo-Switching Nanoparticles to Leverage a Cholesterol-Rich Microenvironment for Atherosclerosis Therapy. ACS Nano 2020, 14, 6519–6531. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Yurdagul, A.J.; Kong, N.; Li, W.; Wang, X.; Doran, A.C.; Feng, C.; Wang, J.; Islam, M.A.; Farokhzad, O.C.; et al. SiRNA Nanoparticles Targeting CaMKIIγ in Lesional Macrophages Improve Atherosclerotic Plaque Stability in Mice. Sci. Transl. Med. 2020, 12, eaay1063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, R.; Liu, C.; Pan, L.; Qi, Y.; Cheng, J.; Guo, J.; Jia, Y.; Ding, J.; Zhang, J.; et al. A PH/ROS Dual-Responsive and Targeting Nanotherapy for Vascular Inflammatory Diseases. Biomaterials 2020, 230, 119605. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Ma, X.; Zhang, B.; Huang, Y.; Zhao, J.; Wang, S.; Li, Y.; Zhu, Y.; Xiong, J.; et al. Synthesis and Characterization of Fucoidan-Chitosan Nanoparticles Targeting P-Selectin for Effective Atherosclerosis Therapy. Oxid. Med. Cell. Longev. 2022, 2022, 8006642. [Google Scholar] [CrossRef]
- Deng, H.; Konopka, C.J.; Prabhu, S.; Sarkar, S.; Medina, N.G.; Fayyaz, M.; Arogundade, O.H.; Vidana Gamage, H.E.; Shahoei, S.H.; Nall, D.; et al. Dextran-Mimetic Quantum Dots for Multimodal Macrophage Imaging In Vivo, Ex Vivo, and In Situ. ACS Nano 2022, 16, 1999–2012. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, W.; Gao, P.; Chen, W.; Zhou, Q. Construction of Dual Nanomedicines for the Imaging and Alleviation of Atherosclerosis. Artif. Cells Nanomed. Biotechnol. 2020, 48, 169–179. [Google Scholar] [CrossRef]
- Kingwell, B.A.; Nicholls, S.J.; Velkoska, E.; Didichenko, S.A.; Duffy, D.; Korjian, S.; Michael Gibson, C. Antiatherosclerotic Effects of CSL112 Mediated by Enhanced Cholesterol Efflux Capacity. J. Am. Heart Assoc. 2022, 11, e024754. [Google Scholar] [CrossRef]
- Scisciola, L.; Cataldo, V.; Taktaz, F.; Fontanella, R.A.; Pesapane, A.; Ghosh, P.; Franzese, M.; Puocci, A.; De Angelis, A.; Sportiello, L.; et al. Anti-Inflammatory Role of SGLT2 Inhibitors as Part of Their Anti-Atherosclerotic Activity: Data from Basic Science and Clinical Trials. Front. Cardiovasc. Med. 2022, 9, 1008922. [Google Scholar] [CrossRef]
- Chen, J.; Xiang, X.; Nie, L.; Guo, X.; Zhang, F.; Wen, C.; Xia, Y.; Mao, L. The Emerging Role of Th1 Cells in Atherosclerosis and Its Implications for Therapy. Front. Immunol. 2022, 13, 1079668. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Lavillegrand, J.-R.; Tedgui, A. Regulatory T Cell-Enhancing Therapies to Treat Atherosclerosis. Cells 2021, 10, 723. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Kanagasooriyan, R.; Subramanian, M. Tackling Inflammation in Atherosclerosis: Are We There yet and What Lies Beyond? Curr. Opin. Pharmacol. 2022, 66, 102283. [Google Scholar] [CrossRef]
- Li, T.; Safitri, M.; Zhang, K.; Wang, Y.; Huang, L.; Zhu, Y.; Daniel, R.; Wu, L.J.; Qiu, J.; Wang, G. Downregulation of G3BP2 Reduces Atherosclerotic Lesions in ApoE-/- Mice. Atherosclerosis 2020, 310, 64–74. [Google Scholar] [CrossRef]
- Mulholland, M.; Jakobsson, G.; Lei, Y.; Sundius, L.; Ljungcrantz, I.; Rattik, S.; Tietge, U.J.F.; Engelbertsen, D. IL-2Rβγ Signalling in Lymphocytes Promotes Systemic Inflammation and Reduces Plasma Cholesterol in Atherosclerotic Mice. Atherosclerosis 2021, 326, 1–10. [Google Scholar] [CrossRef]
- He, X.; Fan, X.; Bai, B.; Lu, N.; Zhang, S.; Zhang, L. Pyroptosis Is a Critical Immune-Inflammatory Response Involved in Atherosclerosis. Pharmacol. Res. 2021, 165, 105447. [Google Scholar] [CrossRef] [PubMed]
- Schlöder, J.; Shahneh, F.; Schneider, F.-J.; Wieschendorf, B. Boosting Regulatory T Cell Function for the Treatment of Autoimmune Diseases—That’s Only Half the Battle! Front. Immunol. 2022, 13, 973813. [Google Scholar] [CrossRef] [PubMed]
- Munjal, A.; Khandia, R. Atherosclerosis: Orchestrating Cells and Biomolecules Involved in Its Activation and Inhibition. Adv. Protein Chem. Struct. Biol. 2020, 120, 85–122. [Google Scholar] [CrossRef]
- Lotfy, H.; Moaaz, M.; Moaaz, M. The Novel Role of IL-37 to Enhance the Anti-Inflammatory Response of Regulatory T Cells in Patients with Peripheral Atherosclerosis. Vascular 2020, 28, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Milward, K.F.; Wood, K.J.; Hester, J. Enhancing Human Regulatory T Cells in Vitro for Cell Therapy Applications. Immunol. Lett. 2017, 190, 139–147. [Google Scholar] [CrossRef]
- Vos, W.G.; van Os, B.W.; den Toom, M.; Beckers, L.; van Roomen, C.P.A.A.; van Tiel, C.M.; Mohapatra, B.C.; Band, H.; Nitz, K.; Weber, C.; et al. T Cell Specific Deletion of Casitas B Lineage Lymphoma-b Reduces Atherosclerosis, but Increases Plaque T Cell Infiltration and Systemic T Cell Activation. Front. Immunol. 2024, 15, 1297893. [Google Scholar] [CrossRef]
- Jones, P.W.; Mallat, Z.; Nus, M. T-Cell/B-Cell Interactions in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1502–1511. [Google Scholar] [CrossRef]
- Obare, L.M.; Bonami, R.H.; Doran, A.C.; Wanjalla, C.N. B Cells and Atherosclerosis: A HIV Perspective. J. Cell. Physiol. 2024, 239, e31270. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Jia, Y.; Tan, F.; Yuan, X.; Du, J. The Roles of B Cells in Cardiovascular Diseases. Mol. Immunol. 2024, 171, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Blincoe, A.; Labrosse, R.; Abraham, R.S. Acquired B-Cell Deficiency Secondary to B-Cell-Depleting Therapies. J. Immunol. Methods 2022, 511, 113385. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.S.; Kraakman, M.J.; Dragoljevic, D.; Hanssen, N.M.J.; Flynn, M.C.; Al-Sharea, A.; Sreejit, G.; Bertuzzo-Veiga, C.; Cooney, O.D.; Baig, F.; et al. Apoptotic Ablation of Platelets Reduces Atherosclerosis in Mice with Diabetes. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1167–1178. [Google Scholar] [CrossRef]
- Nevado, R.M.; Hamczyk, M.R.; Gonzalo, P.; Andrés-Manzano, M.J.; Andrés, V. Premature Vascular Aging with Features of Plaque Vulnerability in an Atheroprone Mouse Model of Hutchinson-Gilford Progeria Syndrome with Ldlr Deficiency. Cells 2020, 9, 2252. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.; Newland, S.A.; Jiang, W.; Giakomidi, D.; Zhao, X.; Clement, M.; Masters, L.; Corovic, A.; Zhang, X.; Drago, F.; et al. Marginal Zone B Cells Produce “natural” Atheroprotective IgM Antibodies in a T Cell-Dependent Manner. Cardiovasc. Res. 2024, 120, 318–328. [Google Scholar] [CrossRef]
- O’Brien, J.W.; Case, A.; Kemper, C.; Zhao, T.X.; Mallat, Z. Therapeutic Avenues to Modulate B-Cell Function in Patients With Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1512–1522. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, C.; Chen, H.; Chen, X.; Liu, T.; Gu, D. C-Reactive Protein: An Important Inflammatory Marker of Coronary Atherosclerotic Disease. Angiology 2024, 00033197241273360. [Google Scholar] [CrossRef]
- Stitham, J.; Rodriguez-Velez, A.; Zhang, X.; Jeong, S.-J.; Razani, B. Inflammasomes: A Preclinical Assessment of Targeting in Atherosclerosis. Expert Opin. Ther. Targets 2020, 24, 825–844. [Google Scholar] [CrossRef]
- Tall, A.R.; Bornfeldt, K.E. Inflammasomes and Atherosclerosis: A Mixed Picture. Circ. Res. 2023, 132, 1505–1520. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Melnichenko, A.A.; Wetzker, R.; Gerasimova, E.V.; Orekhov, A.N. NLPR3 Inflammasomes and Their Significance for Atherosclerosis. Biomedicines 2020, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, X.; Zhang, R.; Ma, B.; Niu, S.; Di, X.; Ni, L.; Liu, C. Melatonin Attenuates Smoking-Induced Atherosclerosis by Activating the Nrf2 Pathway via NLRP3 Inflammasomes in Endothelial Cells. Aging 2021, 13, 11363–11380. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, R.; Chen, X.; Sun, T.; Yan, J. Paeonol Suppresses the Effect of Ox-LDL on Mice Vascular Endothelial Cells by Regulating MiR-338-3p/TET2 Axis in Atherosclerosis. Mol. Cell. Biochem. 2020, 475, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Ran, X.; Wang, D.; Zheng, X.; Zhang, M.; Yu, B.; Sun, Y.; Wu, J. Mettl14 Mediates the Inflammatory Response of Macrophages in Atherosclerosis through the NF-ΚB/IL-6 Signaling Pathway. Cell. Mol. Life Sci. 2022, 79, 311. [Google Scholar] [CrossRef]
- Kovanen, P.T. Inhibition of Chymase-Dependent Production of IL-1β by Smooth Muscle Cells in the Fibrous Caps of Human Atherosclerotic Plaques: A Reasonable Approach to Prevent Cap Rupture? Atherosclerosis 2024, 390, 117412. [Google Scholar] [CrossRef]
- Luo, P.; Shi, W.; Wang, Y.; Ma, H.; Liu, T.; Yan, D.; Huo, S.; Guo, J.; Wang, M.; Li, C.; et al. Raloxifene Inhibits IL-6/STAT3 Signaling Pathway and Protects against High-Fat-Induced Atherosclerosis in ApoE-/- Mice. Life Sci. 2020, 261, 118304. [Google Scholar] [CrossRef]
- Edsfeldt, A.; Gonçalves, I.; Vigren, I.; Jovanović, A.; Engström, G.; Shore, A.C.; Natali, A.; Khan, F.; Nilsson, J. Circulating Soluble IL-6 Receptor Associates with Plaque Inflammation but Not with Atherosclerosis Severity and Cardiovascular Risk. Vascul. Pharmacol. 2023, 152, 107214. [Google Scholar] [CrossRef]
- Rai, M.K.; Jain, N.; Mohindra, N.; Kumar, S.; Agarwal, V.; Misra, D.P. Clinical and Serological Associations of Subclinical Atherosclerosis in Spondyloarthropathy. Indian J. Rheumatol. 2024, 19, 25–32. [Google Scholar] [CrossRef]
- Cyr, Y.; Bozal, F.K.; Barcia Durán, J.G.; Newman, A.A.C.; Amadori, L.; Smyrnis, P.; Gourvest, M.; Das, D.; Gildea, M.; Kaur, R.; et al. The IRG1-Itaconate Axis Protects from Cholesterol-Induced Inflammation and Atherosclerosis. Proc. Natl. Acad. Sci. USA 2024, 121, e2400675121. [Google Scholar] [CrossRef]
- Monaco, C.; Dib, L. Atheroimmunology: Keeping the Immune System in Atherosclerosis in Check. Nat. Rev. Cardiol. 2024, 21, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Chen, Y.; Zong, Q.; Liu, C.; Xie, J.; Wang, Y.; Fisher, D.; Hien, N.T.T.; Pronyuk, K.; Musabaev, E.; et al. Corilagin Alleviates Atherosclerosis by Inhibiting NLRP3 Inflammasome Activation via the Olfr2 Signaling Pathway in Vitro and in Vivo. Front. Immunol. 2024, 15, 1364161. [Google Scholar] [CrossRef]
- Jia, D.; Zhao, M.; Zhang, X.; Cheng, X.; Wei, Q.; Lou, L.; Zhao, Y.; Jin, Q.; Chen, M.; Zhang, D. Transcriptomic Analysis Reveals the Critical Role of Chemokine Signaling in the Anti-Atherosclerosis Effect of Xuefu Zhuyu Decoction. J. Ethnopharmacol. 2024, 332, 118245. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Tang, M.; Ho, W.; Teng, Y.; Chen, Q.; Bu, L.; Xu, X.; Zhang, X.-Q. Modulating Plaque Inflammation via Targeted MRNA Nanoparticles for the Treatment of Atherosclerosis. ACS Nano 2023, 17, 17721–17739. [Google Scholar] [CrossRef]
- Kishore, R.; Magadum, A. Cell-Specific MRNA Therapeutics for Cardiovascular Diseases and Regeneration. J. Cardiovasc. Dev. Dis. 2024, 11, 38. [Google Scholar] [CrossRef]
- Bu, T.; Li, Z.; Hou, Y.; Sun, W.; Zhang, R.; Zhao, L.; Wei, M.; Yang, G.; Yuan, L. Exosome-Mediated Delivery of Inflammation-Responsive Il-10 MRNA for Controlled Atherosclerosis Treatment. Theranostics 2021, 11, 9988–10000. [Google Scholar] [CrossRef]
- Kettunen, S.; Ruotsalainen, A.-K.; Ylä-Herttuala, S. RNA Interference-Based Therapies for the Control of Atherosclerosis Risk Factors. Curr. Opin. Cardiol. 2022, 37, 364–371. [Google Scholar] [CrossRef]
- Kim, T.K.; Jeon, S.; Park, S.; Sonn, S.-K.; Seo, S.; Suh, J.; Jin, J.; Kweon, H.Y.; Kim, S.; Moon, S.H.; et al. 2’-5’ Oligoadenylate Synthetase-like 1 (OASL1) Protects against Atherosclerosis by Maintaining Endothelial Nitric Oxide Synthase MRNA Stability. Nat. Commun. 2022, 13, 6647. [Google Scholar] [CrossRef] [PubMed]
- Bejar, N.; Tat, T.T.; Kiss, D.L. RNA Therapeutics: The Next Generation of Drugs for Cardiovascular Diseases. Curr. Atheroscler. Rep. 2022, 24, 307–321. [Google Scholar] [CrossRef]
- Khair, M.; Khair, M.; Vangaveti, V.N.; Malabu, U.H. The Role of the NLRP3 Inflammasome in Atherosclerotic Disease: Systematic Review and Meta-Analysis. J. Cardiol. 2024, 84, 14–21. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, G.; Tang, Q.; Song, Y.; Liu, D.; Wang, H.; Ma, J. Methyltransferase-like 14 Silencing Relieves the Development of Atherosclerosis via m(6)A Modification of P65 MRNA. Bioengineered 2022, 13, 11832–11843. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, Z.; Yang, H.; Yang, Y.; Geng, C.; Liu, B.; Zhang, T.; Liu, S.; Xue, Y.; Zhang, H.; et al. YB1 Dephosphorylation Attenuates Atherosclerosis by Promoting CCL2 MRNA Decay. Front. Cardiovasc. Med. 2022, 9, 945557. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Wu, D.; Sun, Y.; Suo, Y.; Yu, Q.; Zeng, M.; Gao, Q.; Yu, B.; Jiang, X.; Wang, Y. The Selective NLRP3 Inhibitor MCC950 Hinders Atherosclerosis Development by Attenuating Inflammation and Pyroptosis in Macrophages. Sci. Rep. 2021, 11, 19305. [Google Scholar] [CrossRef]
- Ismailani, U.S.; Buchler, A.; MacMullin, N.; Abdirahman, F.; Adi, M.; Rotstein, B.H. Synthesis and Evaluation of [(11)C]MCC950 for Imaging NLRP3-Mediated Inflammation in Atherosclerosis. Mol. Pharm. 2023, 20, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Choi, J.S.Y.; Stefanovic, N.; Al-Sharea, A.; Simpson, D.S.; Mukhamedova, N.; Jandeleit-Dahm, K.; Murphy, A.J.; Sviridov, D.; Vince, J.E.; et al. Specific NLRP3 Inhibition Protects Against Diabetes-Associated Atherosclerosis. Diabetes 2021, 70, 772–787. [Google Scholar] [CrossRef]
- Lunding, L.P.; Skouras, D.B.; Vock, C.; Dinarello, C.A.; Wegmann, M. The NLRP3 Inflammasome Inhibitor, OLT1177®, Ameliorates Experimental Allergic Asthma in Mice. Allergy 2022, 77, 1035–1038. [Google Scholar] [CrossRef]
- Fidler, T.P.; Xue, C.; Yalcinkaya, M.; Hardaway, B.; Abramowicz, S.; Xiao, T.; Liu, W.; Thomas, D.G.; Hajebrahimi, M.A.; Pircher, J.; et al. The AIM2 Inflammasome Exacerbates Atherosclerosis in Clonal Haematopoiesis. Nature 2021, 592, 296–301. [Google Scholar] [CrossRef]
- Myszko, M.; Bychowski, J.; Skrzydlewska, E.; Łuczaj, W. The Dual Role of Oxidative Stress in Atherosclerosis and Coronary Artery Disease: Pathological Mechanisms and Diagnostic Potential. Antioxidants 2025, 14, 275. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.; Dastmalchi, L.N.; Gulati, M.; Michos, E.D. A Heart-Healthy Diet for Cardiovascular Disease Prevention: Where Are We Now? Vasc. Health Risk Manag. 2023, 19, 237–253. [Google Scholar] [CrossRef]
- Ostrominski, J.W.; Plutzky, J.; Sattar, N.; Vaduganathan, M.; Aroda, V.R. Obesity Management: A Foundational Cardiovascular Health Priority. Eur. J. Prev. Cardiol. 2025, zwaf043. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Calabriso, N.; Carluccio, M.A.; Hugenholtz, P.; De Caterina, R. Nutrients and Gene Expression in Cardiovascular Disease. Princ. Nutr. Nutr. Fundam. Individ. Nutr. 2020, 2020, 469–481. [Google Scholar] [CrossRef]
- Li, H.; Bai, L.; Qin, Q.; Feng, B.-L.; Zhang, L.; Wei, F.-Y.; Yang, X.-F. Research progress on anti-atherosclerosis effect and mechanism of flavonoids compounds mediated by macrophages. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 2020, 45, 2827–2834. [Google Scholar] [CrossRef]
- Bolea, G.; Philouze, C.; Dubois, M.; Risdon, S.; Humberclaude, A.; Ginies, C.; Charles, A.-L.; Geny, B.; Reboul, C.; Arnaud, C.; et al. Digestive N-6 Lipid Oxidation, a Key Trigger of Vascular Dysfunction and Atherosclerosis in the Western Diet: Protective Effects of Apple Polyphenols. Mol. Nutr. Food Res. 2021, 65, e2000487. [Google Scholar] [CrossRef]
- Alonso-Piñeiro, J.A.; Gonzalez-Rovira, A.; Sánchez-Gomar, I.; Moreno, J.A.; Durán-Ruiz, M.C. Nrf2 and Heme Oxygenase-1 Involvement in Atherosclerosis Related Oxidative Stress. Antioxidants 2021, 10, 1463. [Google Scholar] [CrossRef]
- El Hadri, K.; Smith, R.; Duplus, E.; El Amri, C. Inflammation, Oxidative Stress, Senescence in Atherosclerosis: Thioredoxine-1 as an Emerging Therapeutic Target. Int. J. Mol. Sci. 2021, 23, 77. [Google Scholar] [CrossRef]
- Leong, X.-F. Lipid Oxidation Products on Inflammation-Mediated Hypertension and Atherosclerosis: A Mini Review. Front. Nutr. 2021, 8, 717740. [Google Scholar] [CrossRef] [PubMed]
- Perez-Araluce, M.; Jüngst, T.; Sanmartin, C.; Prosper, F.; Plano, D.; Mazo, M.M. Biomaterials-Based Antioxidant Strategies for the Treatment of Oxidative Stress Diseases. Biomimetics 2024, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Nocella, C.; Loffredo, L.; Carnevale, R.; Pignatelli, P. Interventional Study with Vitamin E in Cardiovascular Disease and Meta-Analysis. Free Radic. Biol. Med. 2022, 178, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Nakamura, J.; Hiller, S.; Simington, S.; Holley, D.W.; Mota, R.; Willis, M.S.; Bultman, S.J.; Luft, J.C.; DeSimone, J.M.; et al. New Insights into Immunomodulation via Overexpressing Lipoic Acid Synthase as a Therapeutic Potential to Reduce Atherosclerosis. Vascul. Pharmacol. 2020, 133–134, 106777. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Q.; Zhou, J. Mechanisms of Abnormal Lipid Metabolism in the Pathogenesis of Disease. Int. J. Mol. Sci. 2024, 25, 8465. [Google Scholar] [CrossRef]
- Furuhashi, M. Fatty Acid-Binding Protein 4 in Cardiovascular and Metabolic Diseases. J. Atheroscler. Thromb. 2019, 26, 216–232. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Wu, W.-K.; Melnichenko, A.A.; Wetzker, R.; Sukhorukov, V.; Markin, A.M.; Khotina, V.A.; Orekhov, A.N. Signaling Pathways and Key Genes Involved in Regulation of Foam Cell Formation in Atherosclerosis. Cells 2020, 9, 584. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Y.; Wang, W.; Han, X.; Han, J.; Chen, M.; Zhang, J.; Wang, C.; Li, S.; Luo, J.; et al. Nuclear Factor Erythroid 2 Related Factor 2 Activator JC-5411 Inhibits Atherosclerosis Through Suppression of Inflammation and Regulation of Lipid Metabolism. Front. Pharmacol. 2020, 11, 532568. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Staurenghi, E.; Giannelli, S.; Sottero, B.; Gargiulo, S.; Poli, G.; Gamba, P.; Leonarduzzi, G. Up-Regulation of PCSK6 by Lipid Oxidation Products: A Possible Role in Atherosclerosis. Biochimie 2021, 181, 191–203. [Google Scholar] [CrossRef]
- Khan, A.A.; Gupta, V.; Mahapatra, N.R. Key Regulatory MiRNAs in Lipid Homeostasis: Implications for Cardiometabolic Diseases and Development of Novel Therapeutics. Drug Discov. Today 2022, 27, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xi, M.; Li, Y.; Zheng, R.; Ding, X.; Li, X.; Zhang, X.; Wang, L.; Xing, J.; Hong, B. Tilianin Improves Lipid Profile and Alleviates Atherosclerosis in ApoE-/- Mice through up-Regulation of SREBP2-Mediated LDLR Expression. Phytomedicine 2023, 109, 154577. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Ren, P.; Yang, Y.; Li, S.; Qin, X.; Zhang, M.; Zhou, M.; Liu, W. Qing-Xue-Xiao-Zhi Formula Attenuates Atherosclerosis by Inhibiting Macrophage Lipid Accumulation and Inflammatory Response via TLR4/MyD88/NF-ΚB Pathway Regulation. Phytomedicine 2021, 93, 153812. [Google Scholar] [CrossRef]
- Wang, W.; Liang, M.; Wang, L.; Bei, W.; Rong, X.; Xu, J.; Guo, J. Role of Prostaglandin E2 in Macrophage Polarization: Insights into Atherosclerosis. Biochem. Pharmacol. 2023, 207, 115357. [Google Scholar] [CrossRef]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-Targeted Nanomedicine for the Diagnosis and Treatment of Atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 228–249. [Google Scholar] [CrossRef]
- Flores, A.M.; Hosseini-Nassab, N.; Jarr, K.-U.; Ye, J.; Zhu, X.; Wirka, R.; Koh, A.L.; Tsantilas, P.; Wang, Y.; Nanda, V.; et al. Pro-Efferocytic Nanoparticles Are Specifically Taken up by Lesional Macrophages and Prevent Atherosclerosis. Nat. Nanotechnol. 2020, 15, 154–161. [Google Scholar] [CrossRef]
- Rahman, K.; Vengrenyuk, Y.; Ramsey, S.A.; Vila, N.R.; Girgis, N.M.; Liu, J.; Gusarova, V.; Gromada, J.; Weinstock, A.; Moore, K.J.; et al. Inflammatory Ly6Chi Monocytes and Their Conversion to M2 Macrophages Drive Atherosclerosis Regression. J. Clin. Investig. 2017, 127, 2904–2915. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, K.; Li, T.; Maruf, A.; Qin, X.; Luo, L.; Zhong, Y.; Qiu, J.; McGinty, S.; Pontrelli, G.; et al. Macrophage Membrane Functionalized Biomimetic Nanoparticles for Targeted Anti-Atherosclerosis Applications. Theranostics 2021, 11, 164–180. [Google Scholar] [CrossRef]
- Kuznetsova, T.; Prange, K.H.M.; Glass, C.K.; de Winther, M.P.J. Transcriptional and Epigenetic Regulation of Macrophages in Atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhang, J.; Zhao, Q.; Zhuang, W.; Ding, J.; Zhang, C.; Gao, H.; Pang, D.W.; Pu, K.; Xie, H.Y. Molecularly Engineered Macrophage-Derived Exosomes with Inflammation Tropism and Intrinsic Heme Biosynthesis for Atherosclerosis Treatment. Angew. Chemie Int. Ed. 2020, 59, 4068–4074. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Schlegel, M.P.; Afonso, M.S.; Brown, E.J.; Rahman, K.; Weinstock, A.; Sansbury, B.E.; Corr, E.M.; van Solingen, C.; Koelwyn, G.J.; et al. Regulatory T Cells License Macrophage Pro-Resolving Functions During Atherosclerosis Regression. Circ. Res. 2020, 127, 335–353. [Google Scholar] [CrossRef]
- Gharavi, A.T.; Hanjani, N.A.; Movahed, E.; Doroudian, M. The Role of Macrophage Subtypes and Exosomes in Immunomodulation. Cell. Mol. Biol. Lett. 2022, 27, 83. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Tang, Z.; Song, Z.; Meng, S.; Yang, X.; Guo, H.; Zhu, Y.; Wang, X. Hyaluronic Acid-Functionalized Mesoporous Silica Nanoparticles Loading Simvastatin for Targeted Therapy of Atherosclerosis. Pharmaceutics 2022, 14, 1265. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Paone, S.; Chan, E.; Poon, I.K.H.; Baxter, A.A.; Thomas, S.R.; Hulett, M.D. Heparanase: A Novel Therapeutic Target for the Treatment of Atherosclerosis. Cells 2022, 11, 3198. [Google Scholar] [CrossRef] [PubMed]
- Mamoudou, H.; Başaran, B.; Mune, M.A.M.; Abubakar, A.M.; Nandwa, J.O.; Raimi, M.K.Z.; Hashmi, M.Z. Bioactive Peptides Derived from the Enzymatic Hydrolysis of Cowhide Collagen for the Potential Treatment of Atherosclerosis: A Computational Approach. Intell. Pharm. 2024, 2, 456–466. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Grechko, A.V.; Orekhova, V.A.; Chegodaev, Y.S.; Wu, W.-K.; Orekhov, A.N. Oxidative Stress and Antioxidants in Atherosclerosis Development and Treatment. Biology 2020, 9, 60. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G. Synergistic Anti-Atherosclerotic Role of Combined Treatment of Omega-3 and Co-Enzyme Q10 in Hypercholesterolemia-Induced Obese Rats. Heliyon 2020, 6, e03659. [Google Scholar] [CrossRef]
- Bantwal, A.; Singh, A.; Menon, A.R.; Kumar, N. Pathogenesis of Atherosclerosis and Its Influence on Local Hemodynamics: A Comparative FSI Study in Healthy and Mildly Stenosed Carotid Arteries. Int. J. Eng. Sci. 2021, 167, 103525. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Morino, K. Perivascular Mechanical Environment: A Narrative Review of the Role of Externally Applied Mechanical Force in the Pathogenesis of Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 944356. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; He, Y.; Hong, T.; Bi, C.; Li, J.; Xia, M. Piezo1 in Vascular Remodeling of Atherosclerosis and Pulmonary Arterial Hypertension: A Potential Therapeutic Target. Front. Cardiovasc. Med. 2022, 9, 1021540. [Google Scholar] [CrossRef]
- Sundqvist, K.-G. T Cell Co-Stimulation: Inhibition of Immunosuppression? Front. Immunol. 2018, 9, 974. [Google Scholar] [CrossRef]
- Shah, K.; Al-Haidari, A.; Sun, J.; Kazi, J.U. T Cell Receptor (TCR) Signaling in Health and Disease. Signal Transduct. Target. Ther. 2021, 6, 412. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T Cells in Health and Disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef]
- Bosmans, L.A.; Shami, A.; Atzler, D.; Weber, C.; Gonçalves, I.; Lutgens, E. Glucocorticoid Induced TNF Receptor Family-Related Protein (GITR)—A Novel Driver of Atherosclerosis. Vascul. Pharmacol. 2021, 139, 106884. [Google Scholar] [CrossRef]
- Winkels, H.; Meiler, S.; Lievens, D.; Engel, D.; Spitz, C.; Bürger, C.; Beckers, L.; Dandl, A.; Reim, S.; Ahmadsei, M.; et al. CD27 Co-Stimulation Increases the Abundance of Regulatory T Cells and Reduces Atherosclerosis in Hyperlipidaemic Mice. Eur. Heart J. 2017, 38, 3590–3599. [Google Scholar] [CrossRef]
- Cao, Q.; Du, H.; Fu, X.; Duan, N.; Liu, C.; Li, X. Artemisinin Attenuated Atherosclerosis in High-Fat Diet-Fed ApoE-/- Mice by Promoting Macrophage Autophagy Through the AMPK/MTOR/ULK1 Pathway. J. Cardiovasc. Pharmacol. 2020, 75, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Y.; Cheng, S.; Zhou, X.; Zhou, F.; He, P.; Hu, F.; Zhang, L.; Chen, Y.; Jia, Y. Geniposide Combined with Notoginsenoside R1 Attenuates Inflammation and Apoptosis in Atherosclerosis via the AMPK/MTOR/Nrf2 Signaling Pathway. Front. Pharmacol. 2021, 12, 687394. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Sukhorukov, V.N.; Zhuravlev, A.; Orekhov, N.A.; Kalmykov, V.; Orekhov, A.N. Modulating MTOR Signaling as a Promising Therapeutic Strategy for Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 1153. [Google Scholar] [CrossRef] [PubMed]
- Toner, Y.C.; Munitz, J.; Prevot, G.; Morla-Folch, J.; Wang, W.; van Elsas, Y.; Priem, B.; Deckers, J.; Anbergen, T.; Beldman, T.J.; et al. Targeting MTOR in Myeloid Cells Prevents Infection-Associated Inflammation. iScience 2025, 28, 112163. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Duan, Q.; Niu, S.; Li, P.; Feng, Y.; Zhang, Y.; Xu, X.; Gong, S.-P.; Cao, H. Autophagy and Its Association with Macrophages in Clonal Hematopoiesis Leading to Atherosclerosis. Int. J. Mol. Sci. 2025, 26, 3252. [Google Scholar] [CrossRef]
- Martinet, W.; De Loof, H.; De Meyer, G.R.Y. MTOR Inhibition: A Promising Strategy for Stabilization of Atherosclerotic Plaques. Atherosclerosis 2014, 233, 601–607. [Google Scholar] [CrossRef]
- Wang, L.; Wu, T.; Si, C.; Wang, H.; Yue, K.; Shang, S.; Li, X.; Chen, Y.; Guan, H. Danlou Tablet Activates Autophagy of Vascular Adventitial Fibroblasts Through PI3K/Akt/MTOR to Protect Cells from Damage Caused by Atherosclerosis. Front. Pharmacol. 2021, 12, 730525. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Du, M.; Li, S.; Zhang, Y.; Ding, J.; Wang, J.; Wang, Y.; Liu, P. Hydroxysafflor Yellow A Regulates Lymphangiogenesis and Inflammation via the Inhibition of PI3K on Regulating AKT/MTOR and NF-ΚB Pathway in Macrophages to Reduce Atherosclerosis in ApoE-/- Mice. Phytomedicine 2023, 112, 154684. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 Signaling: An Important Molecular Mechanism of Herbal Medicine in the Treatment of Atherosclerosis via the Protection of Vascular Endothelial Cells from Oxidative Stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Jiang, X.-S.; Liu, T.; Xia, Y.-F.; Gan, H.; Ren, W.; Du, X.-G. Activation of the Nrf2/ARE Signaling Pathway Ameliorates Hyperlipidemia-Induced Renal Tubular Epithelial Cell Injury by Inhibiting MtROS-Mediated NLRP3 Inflammasome Activation. Front. Immunol. 2024, 15, 1342350. [Google Scholar] [CrossRef]
- Sarad, K.; Stefańska, M.; Kraszewska, I.; Burda, G.; Szade, K.; Błyszczuk, P.; Dulak, J.; Jaźwa-Kusior, A. Endothelial Nrf2 Deficiency Promotes Atherosclerotic Lesion Formation by Shaping a Proinflammatory Niche. Life Sci. 2025, 375, 123725. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Khan, S.U.; Suleman, M.; Khan, M.U.; Khan, M.S.; Arbi, F.M.; Hussain, T.; Mohammed Alsuhaibani, A.; Refat, M.S. Natural Allies for Heart Health: Nrf2 Activation and Cardiovascular Disease Management. Curr. Probl. Cardiol. 2024, 49, 102084. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xu, H.; Zhuang, W.; Wang, Y.; Li, G.; Wang, Y. ROS Responsive Nanoplatform with Two-Photon AIE Imaging for Atherosclerosis Diagnosis and “Two-Pronged” Therapy. Small 2020, 16, e2003253. [Google Scholar] [CrossRef]
- Zheng, S.; Huang, H.; Li, Y.; Wang, Y.; Zheng, Y.; Liang, J.; Zhang, S.; Liu, M.; Fang, Z. Yin-Xing-Tong-Mai Decoction Attenuates Atherosclerosis via Activating PPARγ-LXRα-ABCA1/ABCG1 Pathway. Pharmacol. Res. 2021, 169, 105639. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Tan, W.R.; Low, Z.S.; Marvalim, C.; Lee, J.Y.H.; Tan, N.S. Exploration and Development of PPAR Modulators in Health and Disease: An Update of Clinical Evidence. Int. J. Mol. Sci. 2019, 20, 5055. [Google Scholar] [CrossRef]
- Hossaini Nasr, S.; Huang, X. Nanotechnology for Targeted Therapy of Atherosclerosis. Front. Pharmacol. 2021, 12, 755569. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Q.; Wang, Y.; Jin, M.; Tao, Z.; Wan, J. Identification of HMOX1 as a Critical Ferroptosis-Related Gene in Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 833642. [Google Scholar] [CrossRef]
- Suero-Abreu, G.A.; Zanni, M.V.; Neilan, T.G. Atherosclerosis with Immune Checkpoint Inhibitor Therapy: Evidence, Diagnosis, and Management: JACC: CardioOncology State-of-the-Art Review. JACC. CardioOncology 2022, 4, 598–615. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Ji, H.-H.; Li, Y.-J.; Guo, S.-D. Macrophage Plasticity and Atherosclerosis Therapy. Front. Mol. Biosci. 2021, 8, 679797. [Google Scholar] [CrossRef]
- Fernandez, D.M.; Giannarelli, C. Immune Cell Profiling in Atherosclerosis: Role in Research and Precision Medicine. Nat. Rev. Cardiol. 2022, 19, 43–58. [Google Scholar] [CrossRef]
- Talev, J.; Kanwar, J.R. Iron Oxide Nanoparticles as Imaging and Therapeutic Agents for Atherosclerosis. Semin. Thromb. Hemost. 2020, 46, 553–562. [Google Scholar] [CrossRef]
- Dash, U.C.; Nayak, V.; Navani, H.S.; Samal, R.R.; Agrawal, P.; Singh, A.K.; Majhi, S.; Mogare, D.G.; Duttaroy, A.K.; Jena, A.B. Understanding the Molecular Bridges between the Drugs and Immune Cell. Pharmacol. Ther. 2025, 267, 108805. [Google Scholar] [CrossRef] [PubMed]
- Mhaimeed, O.; Burney, Z.A.; Schott, S.L.; Kohli, P.; Marvel, F.A.; Martin, S.S. The Importance of LDL-C Lowering in Atherosclerotic Cardiovascular Disease Prevention: Lower for Longer Is Better. Am. J. Prev. Cardiol. 2024, 18, 100649. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Lepor, N.E.; Michos, E.D. Evolving Management of Low-Density Lipoprotein Cholesterol: A Personalized Approach to Preventing Atherosclerotic Cardiovascular Disease Across the Risk Continuum. J. Am. Heart Assoc. 2023, 12, e028892. [Google Scholar] [CrossRef] [PubMed]
- Fujino, M.; Di Giovanni, G.; Nicholls, S.J. New Approaches to Lipoproteins for the Prevention of Cardiovascular Events. J. Atheroscler. Thromb. 2025, 32, 265–280. [Google Scholar] [CrossRef]
| Nanomaterial | Drug/Agent Carried | Targeting/Physiological Activity | Mechanism of Action | Reference |
|---|---|---|---|---|
| Multi-walled carbon nanotubes (MWCNTs) | Statins (pitavastatin, atorvastatin, fluvastatin, lovastatin) | Inhibition of IL-1β production in macrophages; targeting NLRP3 inflammasome pathway | Inhibits internalization of MWCNTs and cholesterol crystals into macrophages, suppressing NLRP3-mediated IL-1β release | Cui et al., 2021 [170] |
| Lipid-based nanoparticles (rHDL NPs) | Cholesteryl esters; hydrophobic and hydrophilic drugs | Targeting macrophages and foam cells; promoting cholesterol efflux; anti-inflammatory effects | Reverse cholesterol transport via ABCA1, ABCG1, SR-B1; reduces lipid burden and inflammation in plaques | Cheng et al., 2023 [171] |
| Polymeric nanoparticles (PLGA, PEG, PAMAM, etc.) | Various drugs (e.g., rapamycin, dexamethasone, siRNA) | Targeting macrophages, endothelial cells, and foam cells; controlled drug release | Sustained drug release; reduces phagocytosis by RES; enables longer circulation time, and enhances plaque targeting | Cheng et al., 2023 [171] |
| Biomimetic nanomaterials (Macrophage membrane-coated NPs) | Rapamycin, siRNA, or other drugs | Targeting inflamed plaques and foam cells via macrophage membrane antigens | Inhibits lipid uptake by foam cells; sequesters pro-inflammatory cytokines; enhances circulation time; reduces inflammation | Cheng et al., 2023 [171] |
| Inorganic nanoparticles (Iron oxide NPs, AuNPs, MSNs) | Imaging agents, antioxidants, drugs | Targeting macrophages, inflamed endothelium, and thrombus | Enables multimodal imaging (MRI, CT, PA), ROS scavenging, phototherapy, targeted drug delivery | Cheng et al., 2023 [171] |
| mPEG-DSPE Calcium Phosphate (CaP) nanoparticles | Dexamethasone acetate (DEX) and Rapamycin (RAPA) | Targeting atherosclerotic plaques; protection of endothelial cells; foam cell apoptosis; plaque regression | DEX protects endothelial cells from oxidative stress; RAPA induces foam cell apoptosis via autophagy; DR-NPs accumulate at plaques, reduce lipid core and necrotic core size, and downregulate adhesion molecules (MMP-2, MMP-9, ICAM-1) | Luo et al., 2020 [172] |
| Cargo-switching nanoparticles (CSNP) with cyclodextrin–statin core and phospholipid shell | Simvastatin; cyclodextrin as cholesterol scavenger | Targeting cholesterol-rich atherosclerotic plaques; reducing plaque cholesterol and macrophages; promoting plaque regression | Cyclodextrin binds cholesterol in plaque, displacing statin; statin is released locally → anti-inflammatory and antiproliferative effects; cholesterol is scavenged from plaques; synergistic effect | Kim et al., [173] |
| Lipid-polymer hybrid nanoparticles (PLGA core + lipid-PEG shell + S2P peptide) | siRNA targeting Camk2g (CaMKIIγ) | Targeting macrophages in atherosclerotic plaques; reduction of plaque necrosis; promotion of fibrous cap stability; increased efferocytosis | siRNA silencing of Camk2g in plaque macrophages → restores MerTK-mediated efferocytosis → reduces necrotic core size; increases fibrous cap thickness; enhances plaque stability | Tao et al., 2020 [174] |
| pH/ROS dual-responsive cyclodextrin-based nanoparticles (AOCD NP and TAOCD NP) | Rapamycin (RAP) | Targeting vascular inflammatory sites and injured arteries; Type IV collagen-targeted version enhances arterial accumulation | pH and ROS dual-responsive release of rapamycin → inhibits VSMC proliferation and migration; reduces oxidative stress and inflammation; prevents neointimal hyperplasia | Zhang et al., 2020 [175] |
| Chitosan–fucoidan nanoparticles (CFNs) | No exogenous drug—intrinsic antioxidant and anti-inflammatory activity of fucoidan + chitosan | Targeting P-selectin in atherosclerotic plaques; inhibition of ROS, inflammation, foam cell formation; plaque stabilization | Fucoidan binds P-selectin; CFNs scavenge ROS, reduce IL-6, IL-1β, TNF-α, inhibit foam cell formation, promote plaque stabilization | Liu et al., 2022 [176] |
| Dextran-mimetic Quantum Dots (Q-Dex) | No exogenous drug—intrinsic macrophage-targeting and imaging capability | Targeting macrophages in inflamed tissues (e.g., visceral adipose tissue, atherosclerotic plaques); multimodal imaging (PET, fluorescence) | Dextran coating enables macrophage-specific uptake via lectin receptors; Q-Dex allows long-circulating, photostable, high-resolution multimodal macrophage imaging | Deng et al., 2022 [177] |
| Profilin-1 antibody-conjugated, cyclodextrin-modified magnetic iron oxide nanoparticles (RAP@PFN1-CD-MNPs) | Rapamycin | Targeting vascular smooth muscle cells (VSMCs) in atherosclerotic plaques; dual imaging (MRI, NIRF) and therapeutic activity | pH-responsive release of rapamycin in acidic plaque microenvironment → inhibition of VSMC proliferation and migration → increased fibrous cap collagen content → plaque stabilization | Zhang et al., 2020 [178] |
| CSL112 (human plasma-derived apoA-I + phosphatidylcholine discs) | ApoA-I (native human plasma-derived) | Targets plaque macrophages via ABCA1-mediated cholesterol efflux; promotes plaque stabilization; reduces inflammation | Enhances ABCA1-dependent cholesterol efflux → reduces macrophage lipid content → ↓ inflammation, ↓ foam cell apoptosis, ↑ efferocytosis, ↑ collagen in fibrous cap → stabilizes plaques | Kingwell et al., 2022 [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Grigoriou, K.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Reprogramming Atherosclerosis: Precision Drug Delivery, Nanomedicine, and Immune-Targeted Therapies for Cardiovascular Risk Reduction. Pharmaceutics 2025, 17, 1028. https://doi.org/10.3390/pharmaceutics17081028
Karakasis P, Theofilis P, Vlachakis PK, Grigoriou K, Patoulias D, Antoniadis AP, Fragakis N. Reprogramming Atherosclerosis: Precision Drug Delivery, Nanomedicine, and Immune-Targeted Therapies for Cardiovascular Risk Reduction. Pharmaceutics. 2025; 17(8):1028. https://doi.org/10.3390/pharmaceutics17081028
Chicago/Turabian StyleKarakasis, Paschalis, Panagiotis Theofilis, Panayotis K. Vlachakis, Konstantinos Grigoriou, Dimitrios Patoulias, Antonios P. Antoniadis, and Nikolaos Fragakis. 2025. "Reprogramming Atherosclerosis: Precision Drug Delivery, Nanomedicine, and Immune-Targeted Therapies for Cardiovascular Risk Reduction" Pharmaceutics 17, no. 8: 1028. https://doi.org/10.3390/pharmaceutics17081028
APA StyleKarakasis, P., Theofilis, P., Vlachakis, P. K., Grigoriou, K., Patoulias, D., Antoniadis, A. P., & Fragakis, N. (2025). Reprogramming Atherosclerosis: Precision Drug Delivery, Nanomedicine, and Immune-Targeted Therapies for Cardiovascular Risk Reduction. Pharmaceutics, 17(8), 1028. https://doi.org/10.3390/pharmaceutics17081028







