Liposomal Co-Delivery of Acteoside, CBD, and Naringenin: A Synergistic Strategy Against Gliomas
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Compounds and Reagents
2.2. Liposome Preparation
2.3. Liposome Size, PDI, and Zeta Potential Measurements
2.4. HPLC Assay for Encapsulation Efficiency
2.5. 1H Nuclear Magnetic Resonance
2.5.1. NMR Relaxation Measurements
2.5.2. NMR Off-Resonance
2.6. Biological Activity Assessment
2.6.1. Cell Culture and Viability Assay
2.6.2. Total Protein Lysate Preparation
2.6.3. Western Blot Assay
3. Results and Discussion
3.1. Liposome Size, PDI, EE, and Zeta Potential Measurements
3.2. 1H Nuclear Magnetic Resonance Results
3.2.1. Analysis of NMR Relaxation Measurements
3.2.2. NMR Off-Resonance Results
3.3. Biological Activity
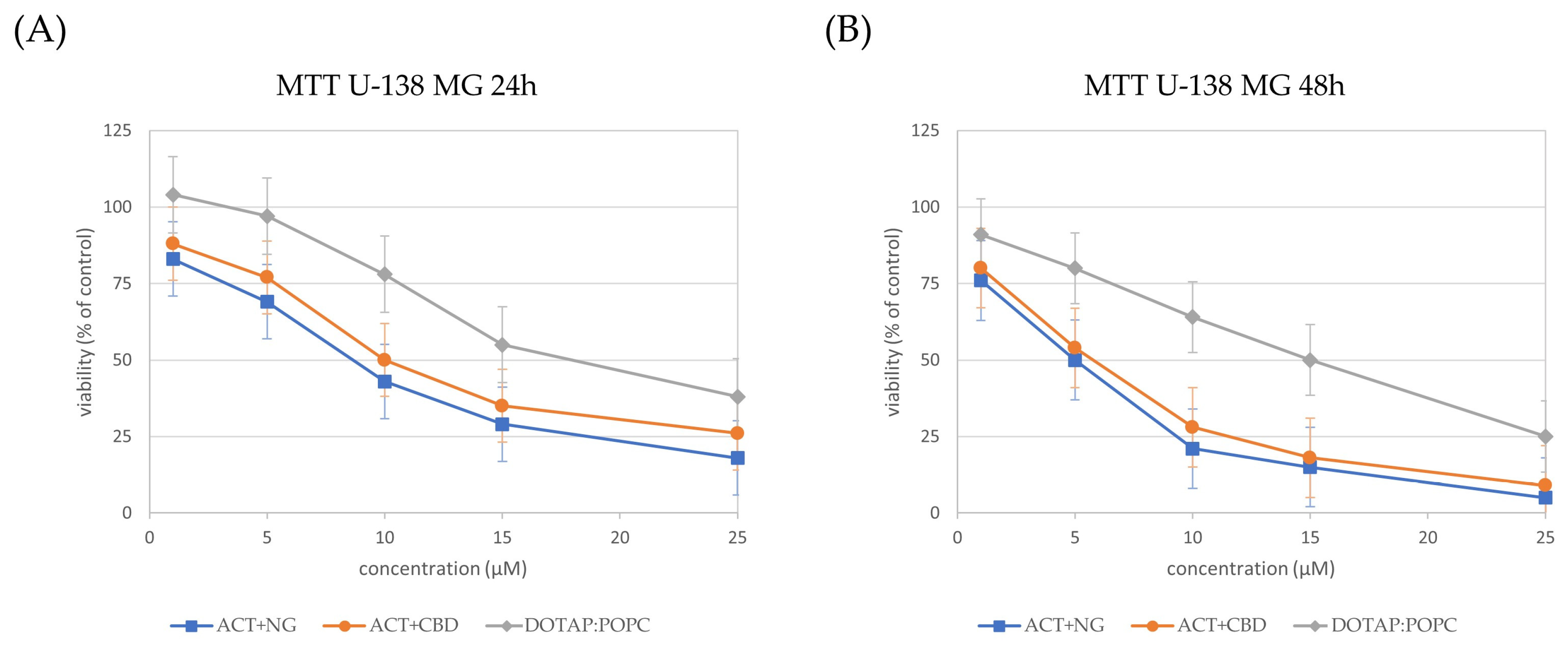
3.3.1. Impact of Nanoformulations on Cell Viability
3.3.2. Impact of Nanoformulations on Bax and Bcl-xL Protein Levels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACT | Acteoside |
| BBB | Blood-brain barrier |
| CBD | Cannabidiol |
| CUR | Curcumin |
| CNS | Central nervous system |
| DDS | Drug delivery system |
| DLS | Dynamic light scattering |
| DOTAP | 1,2-dioleoyl-3-trimethylammonium-propane |
| ELS | Electrophoretic light scattering |
| GBM | Glioblastoma |
| HRP | Horseradish peroxidase |
| LUVs | Large unilamellar vesicles |
| LDDS | Lipid based drug delivery systems |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MLVs | Multilamellar vesicles |
| NMR | Nuclear magnetic resonance |
| NG | Naringenin |
| ORI | Orientin |
| PBS | Phosphate-buffered saline |
| PDI | Polydispersity index |
| POPC | 1-Palmitoyl-2-oleoyl-glycero-3-phosphocholine |
| RQ | Relative quantification |
| SUVs | Small unilamellar vesicles |
| TMZ | Temozolomide |
References
- Yang, Y.-C.; Zhu, Y.; Sun, S.-J.; Zhao, C.-J.; Bai, Y.; Wang, J.; Ma, L.-T. ROS Regulation in Gliomas: Implications for Treatment Strategies. Front. Immunol. 2023, 14, 1259797. [Google Scholar] [CrossRef]
- Antonelli, M.; Poliani, P.L. Adult Type Diffuse Gliomas in the New 2021 WHO Classification. Pathologica 2022, 114, 397–409. [Google Scholar] [CrossRef]
- Paw, I.; Carpenter, R.C.; Watabe, K.; Debinski, W.; Lo, H.-W. Mechanisms Regulating Glioma Invasion. Cancer Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef]
- Rodriguez, B.; Brown, C.S.; Colan, J.A.; Zhang, J.Y.; Huq, S.; Rivera, D.; Young, T.; Williams, T.; Subramaniam, V.; Hadjipanayis, C. Fluorescence-Guided Surgery for Gliomas: Past, Present, and Future. Cancers 2025, 17, 1837. [Google Scholar] [CrossRef]
- Veviorskiy, A.; Mkrtchyan, G.V.; Osipov, A.N.; Izumchenko, E.; Ozerov, I.V.; Aliper, A.; Zhavoronkov, A.; Scheibye-Knudsen, M. Variability in Radiotherapy Outcomes across Cancer Types: A Comparative Study of Glioblastoma Multiforme and Low-Grade Gliomas. Aging 2025, 17, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Rominiyi, O.; Collis, S.J. DDRugging Glioblastoma: Understanding and Targeting the DNA Damage Response to Improve Future Therapies. Mol. Oncol. 2022, 16, 11–41. [Google Scholar] [CrossRef]
- Koosha, F.; Ahmadikamalabadi, M.; Mohammadi, M. Review of Recent Improvements in Carbon Ion Radiation Therapy in the Treatment of Glioblastoma. Adv. Radiat. Oncol. 2024, 9, 101465. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Marsden, P.A. Angiogenesis in Glioblastoma. N. Engl. J. Med. 2013, 369, 1561–1563. [Google Scholar] [CrossRef] [PubMed]
- Schmassmann, P.; Roux, J.; Dettling, S.; Hogan, S.; Shekarian, T.; Martins, T.A.; Ritz, M.-F.; Herter, S.; Bacac, M.; Hutter, G. Single-Cell Characterization of Human GBM Reveals Regional Differences in Tumor-Infiltrating Leukocyte Activation. eLife 2023, 12, RP92678. [Google Scholar] [CrossRef]
- Yasinjan, F.; Xing, Y.; Geng, H.; Guo, R.; Yang, L.; Liu, Z.; Wang, H. Immunotherapy: A Promising Approach for Glioma Treatment. Front. Immunol. 2023, 14, 1255611. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Rich, J.N.; Kay, S.A. Watching the Clock in Glioblastoma. Neuro-Oncology 2023, 25, 1932–1946. [Google Scholar] [CrossRef]
- Kanderi, T.; Munakomi, S.; Gupta, V. Glioblastoma Multiforme. In StatPearls; StatPearls Publishing: Florida, FL, USA, 2024. [Google Scholar]
- Dipasquale, A.; Franceschi, E.; Giordano, L.; Maccari, M.; Barigazzi, C.; Di Nunno, V.; Losurdo, A.; Persico, P.; Di Muzio, A.; Navarria, P.; et al. Dissecting the Prognostic Signature of Patients with Astrocytoma Isocitrate Dehydrogenase-Mutant Grade 4: A Large Multicenter, Retrospective Study. ESMO Open 2024, 9, 103485. [Google Scholar] [CrossRef]
- Majchrzak-Celińska, A.; Studzińska-Sroka, E. New Avenues and Major Achievements in Phytocompounds Research for Glioblastoma Therapy. Molecules 2024, 29, 1682. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, S.; Pan, J.; Ma, K. Verbascoside: A Neuroprotective Phenylethanoid Glycosides with Anti-Depressive Properties. Phytomedicine 2023, 120, 155027. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Peng, X.-M.; Huo, S.-X.; Liu, X.-M.; Yan, M. Memory Enhancement of Acteoside (Verbascoside) in a Senescent Mice Model Induced by a Combination of d-Gal and AlCl3. Phytother. Res. 2015, 29, 1131–1136. [Google Scholar] [CrossRef]
- Khan, R.A.; Hossain, R.; Roy, P.; Jain, D.; Mohammad Saikat, A.S.; Roy Shuvo, A.P.; Akram, M.; Elbossaty, W.F.; Khan, I.N.; Painuli, S.; et al. Anticancer Effects of Acteoside: Mechanistic Insights and Therapeutic Status. Eur. J. Pharmacol. 2022, 916, 174699. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ren, Q.; Wu, L. The Pharmacokinetic Property and Pharmacological Activity of Acteoside: A Review. Biomed. Pharmacother. 2022, 153, 113296. [Google Scholar] [CrossRef]
- Jia, W.-Q.; Wang, Z.-T.; Zou, M.-M.; Lin, J.-H.; Li, Y.-H.; Zhang, L.; Xu, R.-X. Verbascoside Inhibits Glioblastoma Cell Proliferation, Migration and Invasion While Promoting Apoptosis Through Upregulation of Protein Tyrosine Phosphatase SHP-1 and Inhibition of STAT3 Phosphorylation. Cell. Physiol. Biochem. 2018, 47, 1871–1882. [Google Scholar] [CrossRef]
- Jia, W.-Q.; Zhu, J.-W.; Yang, C.-Y.; Ma, J.; Pu, T.-Y.; Han, G.-Q.; Zou, M.-M.; Xu, R.-X. Verbascoside Inhibits Progression of Glioblastoma Cells by Promoting Let-7g-5p and down-Regulating HMGA2 via Wnt/Beta-Catenin Signalling Blockade. J. Cell. Mol. Med. 2020, 24, 2901–2916. [Google Scholar] [CrossRef]
- Heider, C.G.; Itenberg, S.A.; Rao, J.; Ma, H.; Wu, X. Mechanisms of Cannabidiol (CBD) in Cancer Treatment: A Review. Biology 2022, 11, 817. [Google Scholar] [CrossRef]
- Mokoena, D.; George, B.P.; Abrahamse, H. Cannabidiol Combination Enhances Photodynamic Therapy Effects on MCF-7 Breast Cancer Cells. Cells 2024, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, A.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V. Targeting Nrf2 Signaling Pathway in Cancer Prevention and Treatment: The Role of Cannabis Compounds. Antioxidants 2023, 12, 2052. [Google Scholar] [CrossRef]
- Solinas, M.; Massi, P.; Cinquina, V.; Valenti, M.; Bolognini, D.; Gariboldi, M.; Monti, E.; Rubino, T.; Parolaro, D. Cannabidiol, a Non-Psychoactive Cannabinoid Compound, Inhibits Proliferation and Invasion in U87-MG and T98G Glioma Cells through a Multitarget Effect. PLoS ONE 2013, 8, e76918. [Google Scholar] [CrossRef] [PubMed]
- de Santana, M.R.; Argolo, D.S.; Lima, I.S.; dos Santos, C.C.; Victor, M.M.; Ramos, G.d.S.; do Nascimento, R.P.; Ulrich, H.; Costa, S.L. Naringenin Exhibits Antiglioma Activity Related to Aryl Hydrocarbon Receptor Activity and IL-6, CCL2, and TNF-α Expression. Brain Sci. 2025, 15, 325. [Google Scholar] [CrossRef]
- Atoki, A.V.; Aja, P.M.; Shinkafi, T.S.; Ondari, E.N.; Awuchi, C.G. Naringenin: Its Chemistry and Roles in Neuroprotection. Nutr. Neurosci. 2024, 27, 637–666. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guo, X.; Li, S.; Wu, Y.; Zhu, F.; Qin, C.; Zhang, Q.; Yang, Y. Naringenin Ameliorates Amyloid-β Pathology and Neuroinflammation in Alzheimer’s Disease. Commun. Biol. 2024, 7, 912. [Google Scholar] [CrossRef]
- Uçar, K.; Göktaş, Z. Biological Activities of Naringenin: A Narrative Review Based on in Vitro and in Vivo Studies. Nutr. Res. 2023, 119, 43–55. [Google Scholar] [CrossRef]
- Dhiman, A.; Shah, Y.; Rana, D.; Garkhal, K. Comprehensive Review on Glioblastoma: Nanotechnology, Immunotherapy and Combined Therapeutic Approaches. RSC Pharm. 2025, 2, 207–234. [Google Scholar] [CrossRef]
- Hong, J.P.; Choi, R.J.; Shim, J.-K.; Kim, K.; Kim, R.N.; Cho, H.; Kim, S.J.; Kim, S.; Kim, N.H.; Park, H.H.; et al. Synergistic Combination of Perphenazine and Temozolomide Suppresses Patient-Derived Glioblastoma Tumorspheres. Neuro-Oncology 2025, 27, 654–667. [Google Scholar] [CrossRef]
- Dhungel, L.; Rowsey, M.E.; Harris, C.; Raucher, D. Synergistic Effects of Temozolomide and Doxorubicin in the Treatment of Glioblastoma Multiforme: Enhancing Efficacy through Combination Therapy. Molecules 2024, 29, 840. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.W.; Kim, D.H.; Kim, D.B.; Jang, T.W.; Kim, G.-H.; Moon, M.; Yoon, K.A.; Choi, D.E.; Park, J.H.; Kim, J.-J. Synergistic Anticancer Effect of Acteoside and Temozolomide-Based Glioblastoma Chemotherapy. Int. J. Mol. Med. 2019, 43, 1478–1486. [Google Scholar] [CrossRef]
- Soroceanu, L.; Singer, E.; Dighe, P.; Sidorov, M.; Limbad, C.; Rodriquez-Brotons, A.; Rix, P.; Woo, R.W.L.; Dickinson, L.; Desprez, P.-Y.; et al. Cannabidiol Inhibits RAD51 and Sensitizes Glioblastoma to Temozolomide in Multiple Orthotopic Tumor Models. Neurooncol Adv. 2022, 4, vdac019. [Google Scholar] [CrossRef]
- Gautam, M.; Gabrani, R. Combinatorial Effect of Temozolomide and Naringenin in Human Glioblastoma Multiforme Cell Lines. Nutr. Cancer 2022, 74, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Grzegorzewski, J.; Michalak, M.; Wołoszczuk, M.; Bulicz, M.; Majchrzak-Celińska, A. Nanotherapy of Glioblastoma—Where Hope Grows. Int. J. Mol. Sci. 2025, 26, 1814. [Google Scholar] [CrossRef]
- Yaroslavov, A.A.; Efimova, A.A.; Krasnikov, E.A.; Trosheva, K.S.; Popov, A.S.; Melik-Nubarov, N.S.; Krivtsov, G.G. Chitosan-Based Multi-Liposomal Complexes: Synthesis, Biodegradability and Cytotoxicity. Int. J. Biol. Macromol. 2021, 177, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, B.; Wu, H.; Li, B.; Xu, J.; Duan, L.; Jiang, C.; Zhao, X.; Yuan, Y.; Zhang, G.; et al. Anti-Tumor Activity of Verbascoside Loaded Gold Nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhao, X.; Jiang, C.; Yu, J.; Wu, J.; Zeng, X. Gold Nanoshells with Verbascoside Induce the Apoptosis of Drug-Resistant Leukemia Cells Through Caspases Pathway and Inhibit Tumor Growth. J. Nanosci. Nanotechnol. 2016, 16, 7118–7124. [Google Scholar] [CrossRef]
- Zhao, X.-H.; Yue, H.-L.; Li, P.; Zeng, X.; Zhang, G. Evaluation of the Antitumor Activity by CdTe QDs with Verbascoside. Nano 2013, 8, 1350031. [Google Scholar] [CrossRef]
- Alcantara, K.P.; Malabanan, J.W.T.; Nalinratana, N.; Thitikornpong, W.; Rojsitthisak, P.; Rojsitthisak, P. Cannabidiol-Loaded Solid Lipid Nanoparticles Ameliorate the Inhibition of Proinflammatory Cytokines and Free Radicals in an In Vitro Inflammation-Induced Cell Model. Int. J. Mol. Sci. 2024, 25, 4744. [Google Scholar] [CrossRef]
- Qayum, S.; Schmitt, R.R.; Machhar, J.S.; Garg, S.; Bass, C.; Muthaiah, V.P.K.; Ignatowski, T.A.; Mahajan, S.D. Biodegradable Cannabidiol: A Potential Nanotherapeutic for Neuropathic Pain. NeuroImmune Pharmacol. Ther. 2024, 3, 221–236. [Google Scholar] [CrossRef]
- Mahanta, A.K.; Chaulagain, B.; Trivedi, R.; Singh, J. Mannose-Functionalized Chitosan-Coated PLGA Nanoparticles for Brain-Targeted Codelivery of CBD and BDNF for the Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2024, 15, 4021–4032. [Google Scholar] [CrossRef] [PubMed]
- Taha, I.E.; ElSohly, M.A.; Radwan, M.M.; Elkanayati, R.M.; Wanas, A.; Joshi, P.H.; Ashour, E.A. Enhancement of Cannabidiol Oral Bioavailability through the Development of Nanostructured Lipid Carriers: In Vitro and in Vivo Evaluation Studies. Drug Deliv. Transl. Res. 2025, 15, 2722–2732. [Google Scholar] [CrossRef]
- Nie, Y.; Kong, Y.; Peng, J.; Sun, J.; Fan, B. Enhanced Oral Bioavailability of Cannabidiol by Flexible Zein Nanoparticles: In Vitro and Pharmacokinetic Studies. Front. Nutr. 2024, 11, 1431620. [Google Scholar] [CrossRef]
- Maity, S.; Chakraborti, A.S. Formulation, Physico-Chemical Characterization and Antidiabetic Potential of Naringenin-Loaded Poly D, L Lactide-Co-Glycolide (N-PLGA) Nanoparticles. Eur. Polym. J. 2020, 134, 109818. [Google Scholar] [CrossRef]
- Gera, S.; Talluri, S.; Rangaraj, N.; Sampathi, S. Formulation and Evaluation of Naringenin Nanosuspensions for Bioavailability Enhancement. AAPS PharmSciTech 2017, 18, 3151–3162. [Google Scholar] [CrossRef]
- Nouri, Z.; Sajadimajd, S.; Hoseinzadeh, L.; Bahrami, G.; Arkan, E.; Moradi, S.; Abdi, F.; Farzaei, M.H. Neuroprotective Effect of Naringenin-Loaded Solid Lipid Nanoparticles against Streptozocin-Induced Neurotoxicity through Autophagy Blockage. J. Food Biochem. 2022, 46, e14408. [Google Scholar] [CrossRef]
- Pour, P.M.; Nouri, Z.; Ghasemi, D.; Sajadimajd, S.; Farzaei, M.H. Cytotoxic Impact of Naringenin-Loaded Solid Lipid Nanoparticles on RIN5F Pancreatic β Cells via Autophagy Blockage. Recent Adv. Drug Deliv. Formul. 2024, 18, 304–314. [Google Scholar] [CrossRef]
- Mobaleghol Eslam, H.; Hataminia, F.; Esmaeili, F.; Salami, S.A.; Ghanbari, H.; Amani, A. Preparation of a Nanoemulsion Containing Active Ingredients of Cannabis Extract and Its Application for Glioblastoma: In Vitro and in Vivo Studies. BMC Pharmacol. Toxicol. 2024, 25, 73. [Google Scholar] [CrossRef] [PubMed]
- Juhairiyah, F.; de Lange, E.C.M. Understanding Drug Delivery to the Brain Using Liposome-Based Strategies: Studies That Provide Mechanistic Insights Are Essential. AAPS J. 2021, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Singh-Moon, R.P.; Ellis, J.A.; Chaudhuri, D.B.; Wang, M.; Reif, R.; Bruce, J.N.; Bigio, I.J.; Straubinger, R.M. Cerebral Hypoperfusion-Assisted Intra-Arterial Deposition of Liposomes in Normal and Glioma-Bearing Rats. Neurosurgery 2015, 76, 92. [Google Scholar] [CrossRef]
- Piwowarczyk, L.; Mlynarczyk, D.T.; Krajka-Kuźniak, V.; Majchrzak-Celińska, A.; Budzianowska, A.; Tomczak, S.; Budzianowski, J.; Woźniak-Braszak, A.; Pietrzyk, R.; Baranowski, M.; et al. Natural Compounds in Liposomal Nanoformulations of Potential Clinical Application in Glioblastoma. Cancers 2022, 14, 6222. [Google Scholar] [CrossRef]
- Piwowarczyk, L.; Kucinska, M.; Tomczak, S.; Mlynarczyk, D.T.; Piskorz, J.; Goslinski, T.; Murias, M.; Jelinska, A. Liposomal Nanoformulation as a Carrier for Curcumin and pEGCG—Study on Stability and Anticancer Potential. Nanomaterials 2022, 12, 1274. [Google Scholar] [CrossRef]
- ICH Q2(R2) Guideline on Validation of Analytical Procedures–Step 5. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 26 June 2025).
- Hudiyanti, D.; Al Khafiz, M.F.; Anam, K.; Siahaan, P.; Christa, S.M. In Vitro Evaluation of Curcumin Encapsulation in Gum Arabic Dispersions under Different Environments. Molecules 2022, 27, 3855. [Google Scholar] [CrossRef]
- Ma, J.; Guo, C.; Tang, Y.; Xiang, J.; Chen, S.; Wang, J.; Liu, H. Micellization in Aqueous Solution of an Ethylene Oxide–Propylene Oxide Triblock Copolymer, Investigated with 1H NMR Spectroscopy, Pulsed-Field Gradient NMR, and NMR Relaxation. J. Colloid Interface Sci. 2007, 312, 390–396. [Google Scholar] [CrossRef]
- Inoue, T. Micelle Formation of Polyoxyethylene-Type Nonionic Surfactants in bmimBF4 Studied by 1H NMR and Dynamic Light-Scattering. J. Colloid Interface Sci. 2009, 337, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.; Rögnvaldsson, S.; Fossheim, S.; Nilssen, E.A.; Topgaard, D. Dynamic and Structural Aspects of PEGylated Liposomes Monitored by NMR. J. Colloid Interface Sci. 2008, 325, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Niklasson, M.; Otten, R.; Ahlner, A.; Andresen, C.; Schlagnitweit, J.; Petzold, K.; Lundström, P. Comprehensive Analysis of NMR Data Using Advanced Line Shape Fitting. J. Biomol. NMR 2017, 69, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Slichter, C.P. Principles of Magnetic Resonance; Springer: Berlin, Germany; New York, NY, USA, 1978; ISBN 978-0-387-08476-3. [Google Scholar]
- Faid, K.; Fox, R.F. Stochastic Theory of Line Shape and Relaxation. Phys. Rev. A 1986, 34, 4286–4302. [Google Scholar] [CrossRef]
- Weiger, M.; Pruessmann, K.P. Short-T2 MRI: Principles and Recent Advances. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 114–115, 237–270. [Google Scholar] [CrossRef]
- Lasic, D.D. Magnetic Resonance Methods in The Studies of Liposomes. Bull. Magn. Reason. 1991, 13, 3–13. [Google Scholar]
- Baranowski, M.; Woźniak-Braszak, A.; Jurga, K. High Homogeneity B1 30.2 MHz Nuclear Magnetic Resonance Probe for off-Resonance Relaxation Times Measurements. J. Magn. Reson. 2011, 208, 163–166. [Google Scholar] [CrossRef]
- Desvaux, H.; Goldman, M. A New NMR Method for Measuring the Rotational Correlation Time of Molecules in the Liquid State. Mol. Phys. 1994, 81, 955–974. [Google Scholar] [CrossRef]
- Gilani, I.A.; Sepponen, R. Quantitative Rotating Frame Relaxometry Methods in MRI. NMR Biomed. 2016, 29, 841–861. [Google Scholar] [CrossRef]
- Kimmich, R.; Fatkullin, N. Self-Diffusion Studies by Intra- and Inter-Molecular Spin-Lattice Relaxometry Using Field-Cycling: Liquids, Plastic Crystals, Porous Media, and Polymer Segments. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 101, 18–50. [Google Scholar] [CrossRef]
- Jurga, K.; Fojud, Z.; Woźniak-Braszak, A. NMR Strong Off-Resonance Irradiation without Sample Overheating. Solid State Nucl. Magn. Reson. 2004, 25, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Makrocka-Rydzyk, M.; Woźniak-Braszak, A.; Jurga, K.; Jurga, S. Local Motions in Poly(Ethylene-Co-Norbornene) Studied by 1H NMR Relaxometry. Solid State Nucl. Magn. Reson. 2015, 71, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Lin, L.-C.; Sung, J.-S.; Tsai, T.-H. Determination of Acteoside in Cistanche Deserticola and Boschniakia Rossica and Its Pharmacokinetics in Freely-Moving Rats Using LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 844, 89–95. [Google Scholar] [CrossRef]
- Isacchi, B.; Bergonzi, M.C.; Iacopi, R.; Ghelardini, C.; Galeotti, N.; Bilia, A.R. Liposomal Formulation to Increase Stability and Prolong Antineuropathic Activity of Verbascoside. Planta Medica 2016, 83, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Sitovs, A.; Logviss, K.; Lauberte, L.; Mohylyuk, V. Oral Delivery of Cannabidiol: Revealing the Formulation and Absorption Challenges. J. Drug Deliv. Sci. Technol. 2024, 92, 105316. [Google Scholar] [CrossRef]
- Zgair, A.; Wong, J.C.; Lee, J.B.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; Barrett, D.A.; Constantinescu, C.S.; Fischer, P.M.; et al. Dietary Fats and Pharmaceutical Lipid Excipients Increase Systemic Exposure to Orally Administered Cannabis and Cannabis-Based Medicines. Am. J. Transl. Res. 2016, 8, 3448–3459. [Google Scholar]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Huang, Y.-B.; Fang, J.-W.; Fu, Y.-S.; Wu, P.-C. Preparation and Characterization of Naringenin-Loaded Elastic Liposomes for Topical Application. PLoS ONE 2015, 10, e0131026. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Firempong, C.K.; Zhang, H.; Wang, M.; Zhang, Y.; Zhu, Y.; Yu, J.; Xu, X. Enhanced Solubility and Bioavailability of Naringenin via Liposomal Nanoformulation: Preparation and In Vitro and In Vivo Evaluations. AAPS PharmSciTech 2017, 18, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, L.; Tripathy, N.S.; Dilnawaz, F. Naringenin Nanoformulations for Neurodegenerative Diseases. Curr. Pharm. Biotechnol. 2024, 25, 2108–2124. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Kumar, E.M.; Chavali, M.S. Updates on Responsive Drug Delivery Based on Liposome Vehicles for Cancer Treatment. Pharmaceutics 2022, 14, 2195. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta Potential: A Case Study of Cationic, Anionic, and Neutral Liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical Characterization of Liposomes and Other Lipid Nanoparticles for Drug Delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef]
- Woźniak-Braszak, A.; Jurga, K.; Baranowski, M. The Lipari-Szabo Model-Free Analysis as a Method for Study of Molecular Motion in Solid State Heteronuclear Systems Using NMR Off-Resonance. Appl. Magn. Reson. 2016, 47, 567–574. [Google Scholar] [CrossRef][Green Version]
- Singh, K.; Bhushan, B.; Chanchal, D.K.; Sharma, S.K.; Rani, K.; Yadav, M.K.; Porwal, P.; Kumar, S.; Sharma, A.; Virmani, T.; et al. Emerging Therapeutic Potential of Cannabidiol (CBD) in Neurological Disorders: A Comprehensive Review. Behav. Neurol. 2023, 2023, 8825358. [Google Scholar] [CrossRef]
- Dakkak, B.E.; Taneera, J.; El-Huneidi, W.; Abu-Gharbieh, E.; Hamoudi, R.; Semreen, M.H.; Soares, N.C.; Abu-Rish, E.Y.; Alkawareek, M.Y.; Alkilany, A.M.; et al. Unlocking the Therapeutic Potential of BCL-2 Associated Protein Family: Exploring BCL-2 Inhibitors in Cancer Therapy. Biomol. Ther. 2024, 32, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-K.; Chang, K.-C.; Li, C.-Y.; Lieu, A.-S.; Lin, C.-L. AKR1B1 Represses Glioma Cell Proliferation through P38 MAPK-Mediated Bcl-2/BAX/Caspase-3 Apoptotic Signaling Pathways. Curr. Issues Mol. Biol. 2023, 45, 3391–3405. [Google Scholar] [CrossRef] [PubMed]
- Daisy, P.S.; Shreyas, S.K.; Sathish, R.; Anitha, T.S. Synergistic Apoptotic Effect of Naringenin on Enhancing the Anti-Glioma Efficacy of Temozolomide in an in Vitro Experimental Model. Res. J. Biotechnol. 2021, 16, 43–49. [Google Scholar] [CrossRef]
- Patel, N.; Kommineni, N.; Surapaneni, S.K.; Kalvala, A.; Yaun, X.; Gebeyehu, A.; Arthur, P.; Duke, L.C.; York, S.B.; Bagde, A.; et al. Cannabidiol Loaded Extracellular Vesicles Sensitize Triple-Negative Breast Cancer to Doxorubicin in Both In-Vitro and In Vivo Models. Int. J. Pharm. 2021, 607, 120943. [Google Scholar] [CrossRef]













| Time | Liposomes (DOTAP:POPC) | Particle Size (±SD) [nm] | PDI (±SD) | Zeta Potential (±SD) [mV] |
|---|---|---|---|---|
| T0 | Control DOTAP:POPC | 128.9 ± 5.0 | 0.21 ± 0.01 | 34.3 ± 1.7 |
| ACT | 126.0 ± 7.7 | 0.17 ± 0.07 | 33.3 ± 3.6 | |
| ACT + CBD | 1273 ± 6.0 | 0.19 ± 0.05 | 37.3 ± 0.6 | |
| ACT + NG | 129.3 ± 25.1 | 0.17 ± 0.07 | 33.9 ± 3.3 | |
| T7 | Control DOTAP:POPC | 141.8 ± 40.3 | 0.21 ± 0.04 | 39.7 ± 0.4 |
| ACT | 133.4 ± 16.3 | 0.21 ± 0.03 | 37.3 ± 2.8 | |
| ACT + CBD | 144.6 ± 9.6 | 0.24 ± 0.02 | 36.0 ± 2.5 | |
| ACT + NG | 142.2 ± 28.7 | 0.21 ± 0.07 | 34.6 ± 3.9 | |
| T14 | Control DOTAP:POPC | 154.3 ± 40.5 | 0.25 ± 0.03 | 38.6 ± 2.1 |
| ACT | 190.6 ± 54.5 | 0.23 ± 0.03 | 38.7 ± 1.3 | |
| ACT + CBD | 153.4 ± 29.3 | 0.25 ± 0.04 | 37.3 ± 0.5 | |
| ACT + NG | 182.1 ± 59.3 | 0.23 ± 0.04 | 37.2 ± 3.8 | |
| T21 | Control DOTAP:POPC | 146.7 ± 39.1 | 0.23 ± 0.06 | 39.5 ± 4.1 |
| ACT | 136.9 ± 6.4 | 0.21 ± 0.04 | 39.9 ± 2.1 | |
| ACT + CBD | 142.9 ± 18.5 | 0.22 ± 0.03 | 39.5 ± 3.2 | |
| ACT + NG | 147.8 ± 6.6 | 0.22 ± 0.08 | 34.1 ± 2.6 |
| Material | T1 (s) | T2G (ms) | T2L (ms) | ||
|---|---|---|---|---|---|
| ACT | 2.5 | 58 | 184 | 42 | 300.0 |
| ACT + CBD | 2.6 | 77 | 232 | 23 | 7.6 |
| ACT + NG | 2.7 | 86 | 188 | 14 | 118.0 |
| DOTAP:POPC | 2.6 | 62 | 186 | 38 | 352.0 |
| Liposomal Systems | K | (ns) |
|---|---|---|
| ACT | 1.22 ± 0.17 | 1.8 |
| ACT + CBD | 1.46 ± 0.27 | 2.8 |
| ACT + NG | 1.72 ± 0.21 | 3.6 |
| DOTAP:POPC | 1.61 ± 0.22 | 3.2 |
| Nanoformulations | U-87 MG 24 h | U-87 MG 48 h | U-138 MG 24 h | U-138 MG 48 h | MRC-524 h | MRC-548 h |
|---|---|---|---|---|---|---|
| DOTAP:POPC | 18 | 16 | 17 | 15 | 20 | 19 |
| ACT + CBD | 11 | 7 | 10 | 6 | 12 | 9 |
| ACT + NG | 9 | 6 | 8 | 5 | 10 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkudlarek, J.; Piwowarczyk, L.; Krajka-Kuźniak, V.; Majchrzak-Celińska, A.; Tomczak, S.; Baranowski, M.; Pietrzyk, R.; Woźniak-Braszak, A.; Jelińska, A. Liposomal Co-Delivery of Acteoside, CBD, and Naringenin: A Synergistic Strategy Against Gliomas. Pharmaceutics 2025, 17, 1026. https://doi.org/10.3390/pharmaceutics17081026
Szkudlarek J, Piwowarczyk L, Krajka-Kuźniak V, Majchrzak-Celińska A, Tomczak S, Baranowski M, Pietrzyk R, Woźniak-Braszak A, Jelińska A. Liposomal Co-Delivery of Acteoside, CBD, and Naringenin: A Synergistic Strategy Against Gliomas. Pharmaceutics. 2025; 17(8):1026. https://doi.org/10.3390/pharmaceutics17081026
Chicago/Turabian StyleSzkudlarek, Jagoda, Ludwika Piwowarczyk, Violetta Krajka-Kuźniak, Aleksandra Majchrzak-Celińska, Szymon Tomczak, Mikołaj Baranowski, Rafał Pietrzyk, Aneta Woźniak-Braszak, and Anna Jelińska. 2025. "Liposomal Co-Delivery of Acteoside, CBD, and Naringenin: A Synergistic Strategy Against Gliomas" Pharmaceutics 17, no. 8: 1026. https://doi.org/10.3390/pharmaceutics17081026
APA StyleSzkudlarek, J., Piwowarczyk, L., Krajka-Kuźniak, V., Majchrzak-Celińska, A., Tomczak, S., Baranowski, M., Pietrzyk, R., Woźniak-Braszak, A., & Jelińska, A. (2025). Liposomal Co-Delivery of Acteoside, CBD, and Naringenin: A Synergistic Strategy Against Gliomas. Pharmaceutics, 17(8), 1026. https://doi.org/10.3390/pharmaceutics17081026










