Micro and Nano Drug Delivery Systems for the Treatment of Oral Mucositis: A Review
Abstract
1. Introduction
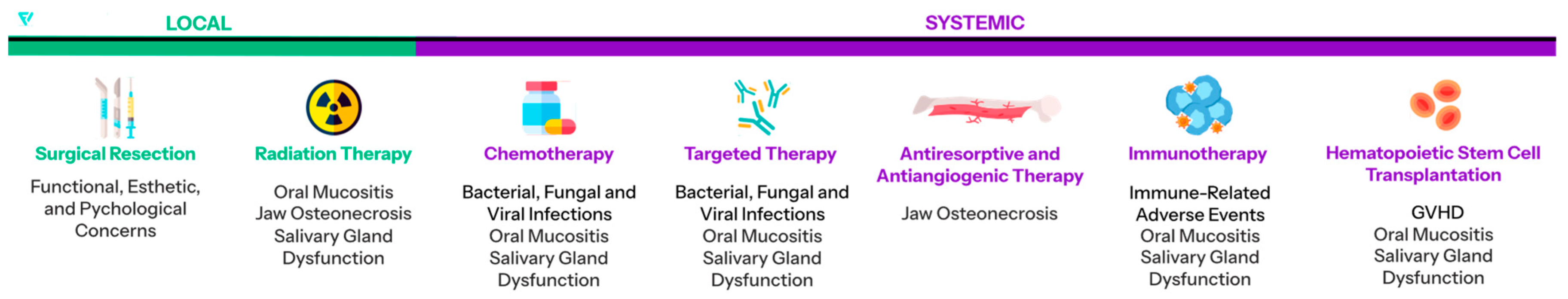
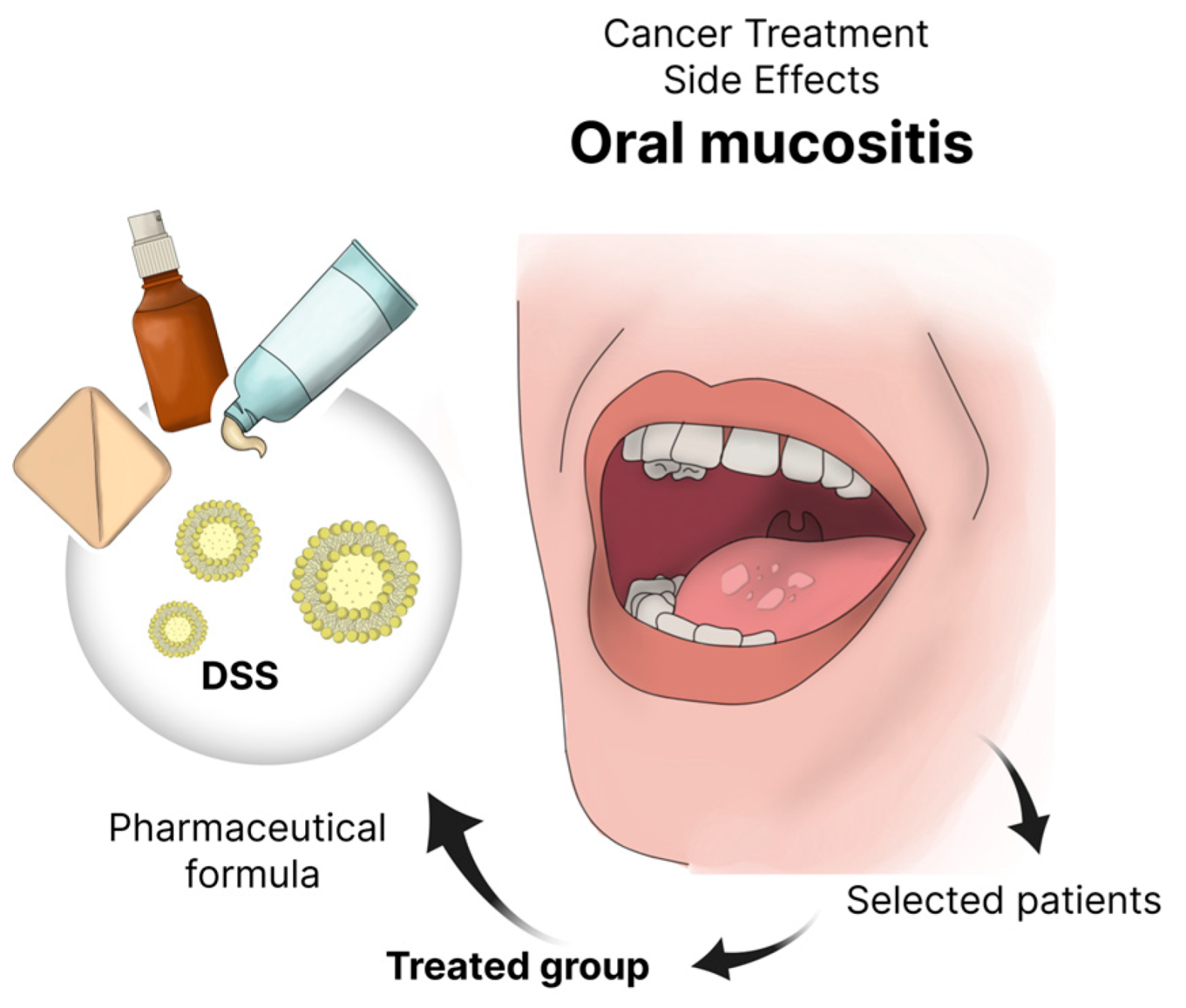
1.1. Oral Mucositis
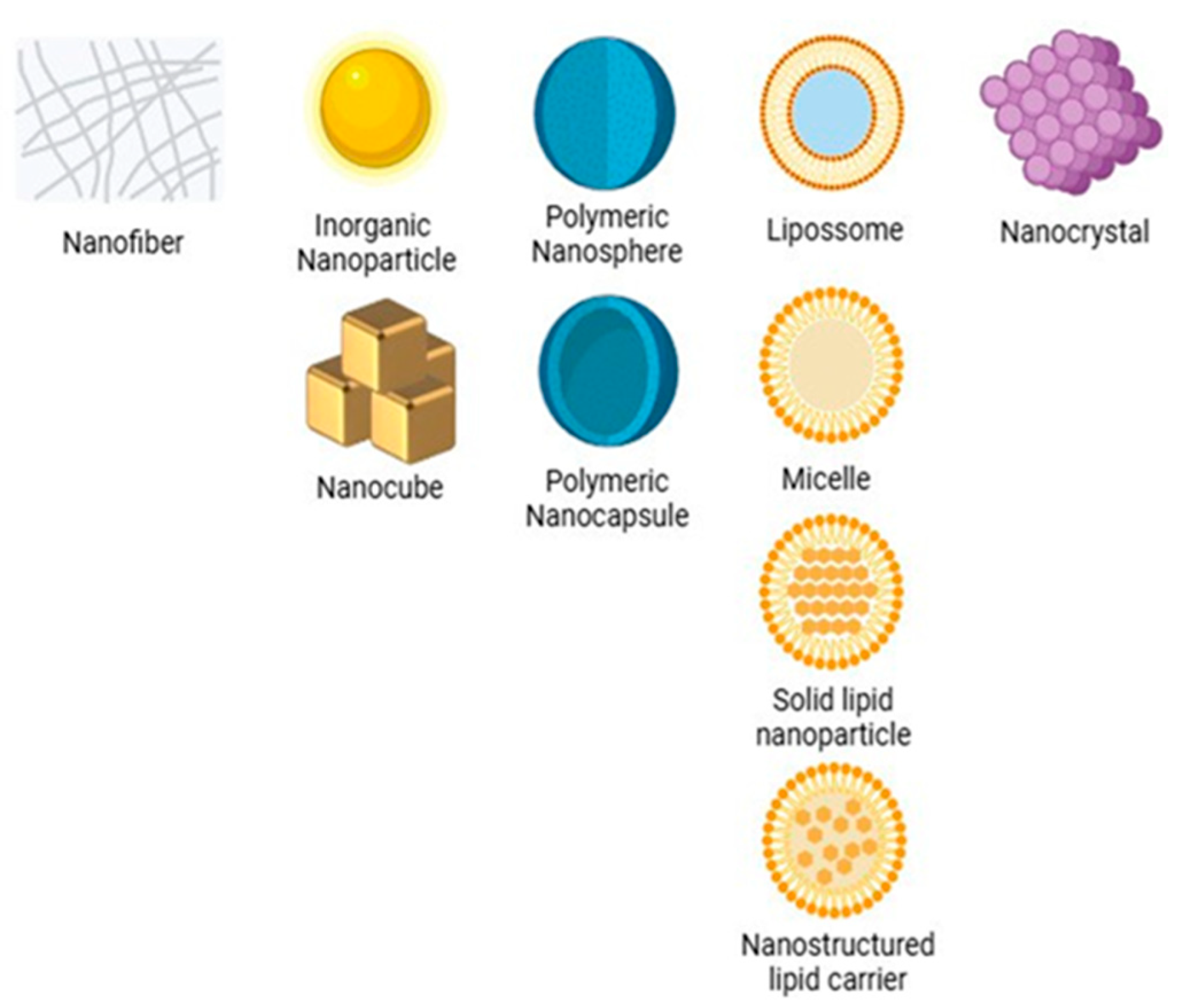
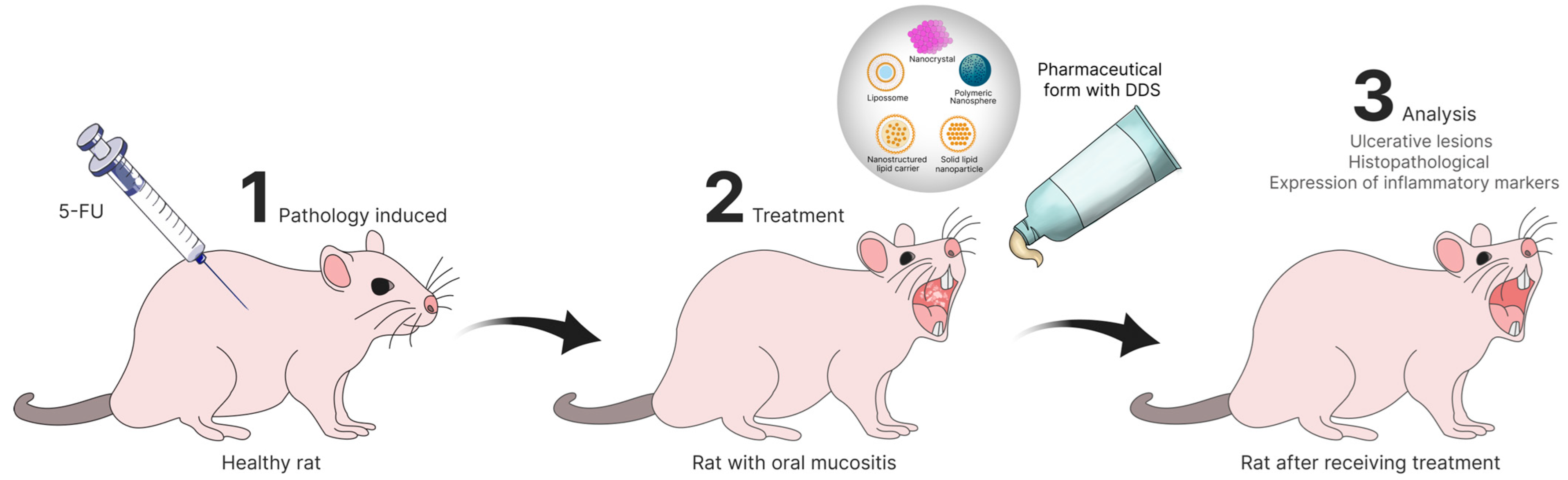
1.2. Micro- and Nanosystems
1.3. Existing Reviews
1.4. Motivation and Objectives
2. Materials and Methods
- Use of a micro- or nanosystem (nonmetallic) as a drug delivery vehicle.
- Articles published in English.
- The full texts are available.
- Review articles, editorials, or conference abstracts.
- Studies without clear methodological details or outcome data.
- Duplicate records identified across databases.
3. Results
3.1. Main Polymers Used
3.2. In Vitro Studies
| Micro and Nano | Polymer | Therapeutic Agent | Cell Model | Main Results | Ref. |
|---|---|---|---|---|---|
| NPs | - | P. indica leaf extract | HO-1-N-1 (human oral squamous cell carcinoma) | P. indica NPs promoted the migration of oral mucosal cells, indicating potential in the treatment of oral wounds. The spray formulation was found to be stable and efficient for long-term use. | [31] |
| Microparticles | Chitosan | BZH | - | Chitosan microparticles with different ratios of chitosan to BZH showed sustained release and strong mucoadhesive adhesion, which is promising for long-term treatment of oromucosal conditions. | [35] |
| Nanofibers | Sodium alginate and PEO | Glutamine | - | Nanofibers showed promise as a mucoadhesive for OM, remaining stable at 4–25 °C, but less stable at higher temperatures. Glutamine release was gradual, with more than 85% released after 4 h. | [36] |
| Nanogels | Starch and PEGDE 1 | Vancomycin (VNG) | - | VNG-loaded nanogels showed strong antibacterial activity against Staphylococcus aureus, Streptococcus pyogenes, and Streptococcus mutans, common pathogens in OM, showing promise for the treatment of oral infections. | [37] |
| Nanofibers | HA 2 | Glycyrrhizin | RAW 264.7 macrophages and human epithelial cells | The glycyrrhizin-loaded nanofiber reduced proinflammatory cytokines and maintained 83% viability in 5-FU-treated cells, showing potential with artificial saliva. | [38] |
| Solid Lipid NPs (SLN) | HA and Carbopol® | N-cetylglucosamine (NAG) | - | The NAG-enriched SLN gel demonstrated mucoadhesion efficacy and preserved epithelial integrity, suggesting potential for healing and protecting mucositis-affected oral mucosa. | [39] |
| NPs | HPC-L, SL and SSL 3 | Rebamipide | - | Rebamipide NPs were shown to be stable and adherent to the oral mucosa, providing localized, sustained release and potentially improving the quality of life of patients with stomatitis. | [40] |
| Microparticles | Eudragit | Chestnut shell extract rich in polyphenols | TR146 (squamous carcinoma of the oral mucosa) | Chestnut extract microparticles exhibited high antioxidant potential, biocompatibility, and effective polyphenol release, appearing promising for mucositis therapies. | [41] |
| Microparticles | Eudragit RS 30D | Actinidia arguta leaf extract | TR146 (buccal keratinocytes) and HSC-3 (tongue carcinoma) | Actinidia arguta microparticles had high phenolic content, strong antioxidant activity, and were well tolerated by oral epithelial cells, suggesting therapeutic potential for OM. | [42] |
| Hydrogels | HPMC, Carbopol® and Sodium hyaluronate | Budesonide and Lidocaine | - | The formulation demonstrated significant mucoadhesive capacity and prolonged drug release suitable for the management of mucositis. Tablets showed better adhesion, while films were more resistant and customizable for application. | [43] |
3.3. In Vivo Studies
| Micro and Nano | Polymer | Therapeutic Agent | Animal Model | Main Results | Ref. |
|---|---|---|---|---|---|
| NPs | PLGA and Chitosan | Rebamipide | Mouse mucositis model | Chitosan-coated NPs significantly reduced ulcer area and treatment time compared with the control and uncoated-NPs groups. The coating contributed to greater efficacy in the treatment of OM. | [44] |
| Nanomicelles | Fucoid (FD) | Cannabidiol (CBD) | Mouse mucositis model | CBD-FD micelles significantly reduced ulcer area and inflammation vs. free CBD, showing excellent retention and healing in chemotherapy-induced lesions. | [45] |
| Nanocrystals | Carbopol® and methylcellulose | Rebamipide | Hamster model | The rebamipide nanocrystal formulation improved healing and epithelial regeneration and reduced inflammation, likely through clathrin-mediated endocytosis. | [46] |
| Nanofibers | Eudragit and Chitosan | Human growth hormone (hGH) | Beagle dog model | hGH nanofibers with 0.5% chitosan fully regenerated ulcers, while the 1% chitosan version was less effective. The system allowed for controlled release of hGH, which enhanced healing. | [47] |
| Nanocubes | Starch and Dextrin | Apremilast | Kunming mice model | The formulation demonstrated potent anti-inflammatory and antioxidant capabilities, promoted healing of OM induced, demonstrated excellent adhesion and comfort, accelerated healing and promoted epithelial proliferation. | [48] |
| NPs | PLGA and PVA | Dexamethasone | Syrian golden hamsters | NPs reduced the severity of mucositis, with less inflammation and tissue damage, especially at a dose of 0.1 mg/kg. The use of PLGA allowed therapeutic efficacy with a reduced dose of dexamethasone, minimizing potential side effects. | [49] |
| NPs | PLGA and HPMC | Benzydamine (BZN) | Rabbit mucositis model | BZN-PLGA-NPs-HG reduced ulcer area in a mucositis model compared to the BZN-only and control groups. The PLGA-NPs-HG system had shorter healing time than other formulations. | [50] |
| NPs | PDA and PLGA | Dexamethasone | Rat model | The mussel-inspired mucoadhesive system enhanced film retention in a moist environment, tripling bioavailability and accelerating wound closure, showing potential for OM treatment. | [51] |
| NPs | HA and BSP | Triamcinolone acetonide (TA) | Rat mucositis model | Application of TA@MPDA 1-HA/BSP significantly reduced inflammation and accelerated healing of oral ulcers in rats, proving a promising alternative for treating oral inflammatory diseases | [52] |
| NPs | Carbopol® and HPMC | Troxipide | Hamster mucositis model | The NP gel healed significantly faster than the microparticle gel, enhancing local drug absorption and retention. The therapeutic effect was mediated by the CME 2 endocytosis pathway, which proved effective in the mucositis model. | [53] |
| Nanoemulsion | - | Quercetin | Mouse mucositis model | Quercetin nanoemulsion reduced inflammation and tissue damage compared to the control group, with a remarkable protective effect against OM, showing potential for mucositis prevention. | [54] |
| Mucoadhesive microparticles | PVA 3 | Indomethacin (IM) | Mouse oral mucosa retention model | The IM-NK(50) 4 gel formulation, using a specific type of PVA, showed the highest concentration of IM in the oral mucosa without a significant increase in plasma concentrations, indicating its potential to relieve pain without systemic effects. | [55] |
| Freeze-dried wafer | Chitosan, CMC 5, HPMC and PLX | Benzydamine hydrochloride (BZH) | Rat mucositis model | BZH-loaded wafer significantly reduced the severity of mucositis compared to controls, promoted accelerated healing, and decreased inflammatory cell infiltration. | [56] |
| Submicronized crystals of rebamipide | HPMC and HPC | Rebamipide | Rat model | Submicronized rebamipide crystals significantly reduced ulcers versus control. Intraoral administration led to higher mucosal drug concentration than intragastric delivery, enhancing therapeutic efficacy. | [57] |
| Nanofibers | Eudragit L100 and S100 | Ketoprofen | Rabbit model | The formulation with ketoprofen (EL-NF) 6 relieved mucositis and promoted re-epithelialization in rabbits, remained adherent for up to 2 h, and provided sustained release and rapid healing. | [58] |
| Nanoemulsion | HPMC | Diclofenac and Lidocaine | Albino Rat and Mice Model | The formulation showed a strong anti-inflammatory effect (up to 87.99% reduction in edema) and effective analgesia in vivo, presenting a high acceptance rate and rapid pain relief. | [59] |
3.4. Clinical Studies
| Micro and Nano | Therapeutic Agent | Concentration | Dose and Frequency | Main Results | Ref. |
|---|---|---|---|---|---|
| Micelles | Silymarin | 70 mg/5 mL | 5 mL, 3× daily for 6 weeks | The nanosilymarin formulation slightly reduced mucositis progression in four weeks but was not statistically significant. It was well tolerated, although some reported unpleasant taste and mild gastrointestinal reactions. | [19] |
| NPs | Curcumin | NPs with 0.1% curcumin | 10 mL, 3× daily (without dilution), for 7 days | The use of curcumin mouthwash reduced the risk of mucositis onset by 50% and delayed its onset by an average of two weeks compared to the control group (benzydamine), with less severity observed. | [30] |
| Micelles | Curcumin | 80 mg of curcumin in micelles per capsule | 1 capsule a day during radiotherapy | Curcumin micelles reduced mucositis severity during radiotherapy, halving grade 4 incidence vs. control, proving effective for prevention and management. | [32] |
| Micelles | Curcumin | 0.1% w/v for mouthwash; 40 mg curcuminoids for curcumin capsule | 10 mL, 3× daily for 21 days for mouthwash; 1 capsule a day for 21 days | Curcumin, orally or topically, significantly reduced pain and inflammation in radiation-induced mucositis. Over 33% using mouthwash and 15% using capsules remained ulcer-free. No significant differences occurred between forms. | [33] |
| Micelles | Curcumin | 80 mg of curcumin in micelles per capsule | Two capsules a day, after meals, for 7 weeks | Patients receiving curcumin micelles had less mucositis progression and pain over seven weeks. Its anti-inflammatory and antioxidant effects helped control mucositis, especially in chemotherapy-only patients. | [34] |
| Nanogel | Doxepin | 0.2% w/v chitosan with 5 mg/mL doxepin | Nanogel application on the lesions with a cotton swab, 4× a day | Chitosan nanogel with doxepin reduced markers of inflammation and pain more effectively than conventional treatments, demonstrating positive clinical results for the treatment of mucositis. | [60] |
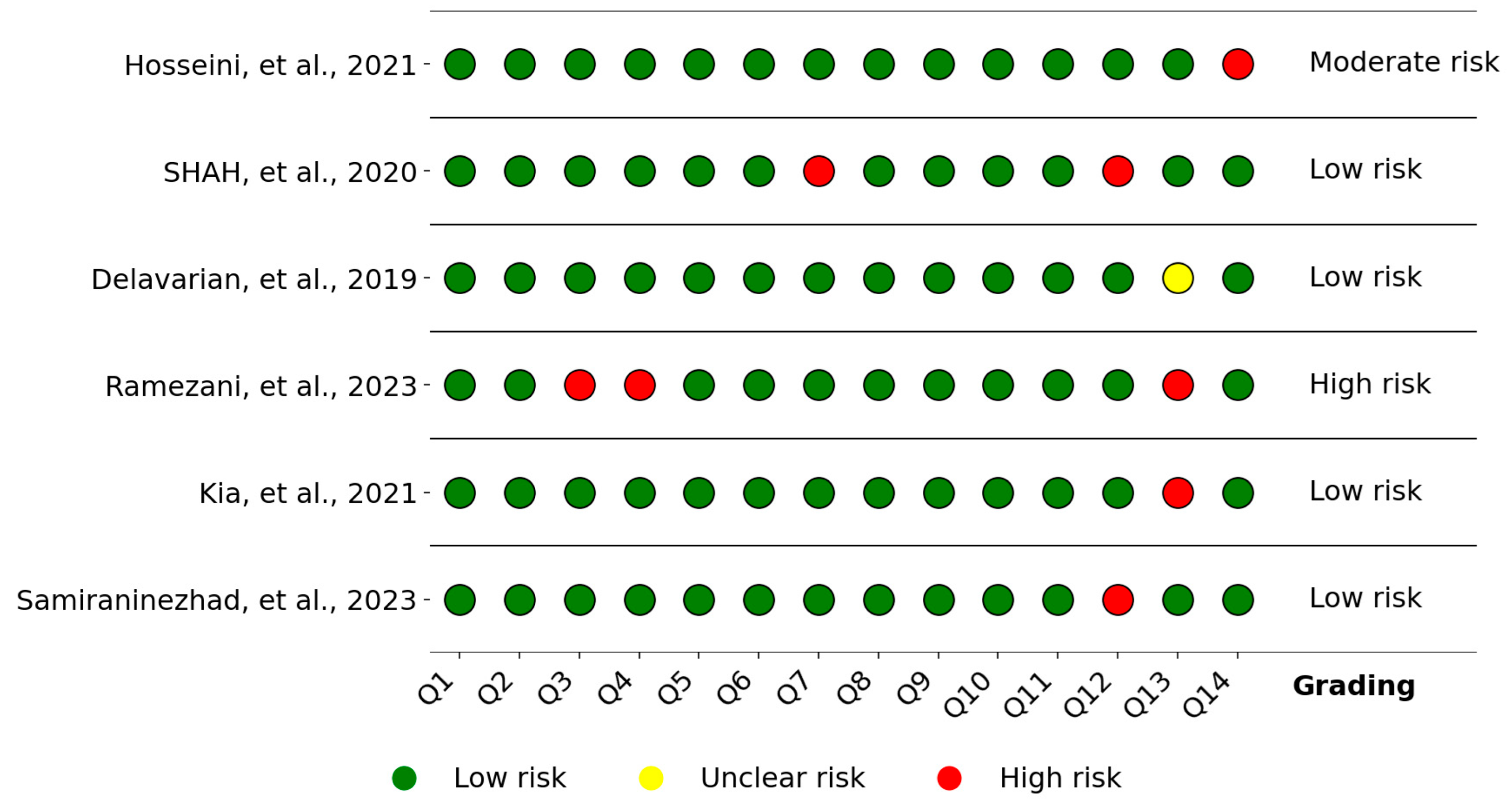
3.5. Quality Assessment and Risk of Bias
4. Discussion
4.1. Drug Delivery Systems
4.2. In Vitro Studies
4.3. In Vivo Studies
4.4. Clinical Trial Studies
4.5. Limitations of the Review
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, J.A.; Ottaviani, G.; Treister, N.S.; Hanna, G.J. An overview of clinical oncology and impact on oral health. Front. Oral Health 2022, 3, 874332. [Google Scholar] [CrossRef]
- Curra, M.; Soares Junior, L.A.V.; Martins, M.D.; Santos, P.S.D.S. Chemotherapy protocols and incidence of oral mucositis: An integrative review. Einstein 2018, 16, eRW4007. [Google Scholar] [CrossRef]
- Nathan, C.-A.O.; Asarkar, A.A.; Entezami, P.; Corry, J.; Strojan, P.; Poorten, V.V.; Makitie, A.; Eisbruch, A.; Robbins, K.T.; Smee, R.; et al. Current management of xerostomia in head and neck cancer patients. Am. J. Otolaryngol. 2023, 44, 103867. [Google Scholar] [CrossRef]
- Sonis, S.T.; Costa, J.W.; Evitts, S.M., Jr.; Lindquist, L.E.; Nicolson, M. Effect of epidermal growth factor on ulcerative mucositis in hamsters that receive cancer chemotherapy. Oral Surg. Oral Med. Oral Pathol. 1992, 74, 749–755. [Google Scholar] [CrossRef]
- Sonis, S.T. Oral mucositis. Anti-Cancer Drugs 2011, 22, 607–612. [Google Scholar] [CrossRef]
- Raber-Durlacher, J.E.; Elad, S.; Barasch, A. Oral mucositis. Oral Oncol. 2010, 46, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Cauwels, R.G.E.C.; Martens, L.C. Low level laser therapy in oral mucositis: A pilot study. Eur. Arch. Pediatr. Dent. 2011, 12, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Heydari, A.; Sharifi, H.; Salek, R. Effect of oral cryotherapy on combination chemotherapy-induced oral mucositis: A randomized clinical trial. Middle East J. Cancer 2012, 3, 55–56. [Google Scholar]
- Noé, J.E. L-glutamine use in the treatment and prevention of mucositis and cachexia: A naturopathic perspective. Integr. Cancer Ther. 2009, 8, 409–415. [Google Scholar] [CrossRef]
- Manish, G.; Vimukta, S. Targeted drug delivery system: A review. Res. J. Chem. Sci. 2011, 1, 135–138. [Google Scholar]
- Hosseinpour-Moghadam, R.; Mehryab, F.; Torshabi, M.; Haeri, A. Applications of novel and nanostructured drug delivery systems for the treatment of oral cavity diseases. Clin. Ther. 2021, 43, e377–e402. [Google Scholar] [CrossRef]
- Choudhury, M.; Brunton, P.; Schwass, D.; Pletzer, D.; Ratnayake, J.; Dias, G.; Tompkins, G. Effectiveness of gold nanoparticles in prevention and treatment of oral mucositis in animal models: A systematic review. Syst. Rev. 2024, 13, 39. [Google Scholar] [CrossRef]
- Hasirci, N. Micro and nano systems in biomedicine and drug delivery. In Nanomaterials and Nanosystems for Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–26. [Google Scholar]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Zarei, M.; Hashemi, S. Polydopamine for biomedical application and drug delivery system. Med. Chem. 2018, 8, 218–229. [Google Scholar] [CrossRef]
- Sun, H.; Liu, K.; Liu, W.; Wang, W.; Guo, C.; Tang, B.; Gu, J.; Zhang, J.; Li, H.; Mao, X.; et al. Development and characterization of a novel nanoemulsion drug-delivery system for potential application in oral delivery of protein drugs. Int. J. Nanomed. 2012, 7, 5529–5543. [Google Scholar] [CrossRef]
- Amirmahani, N.; Mahmoodi, N.O.; Galangash, M.M.; Ghavidast, A. Advances in nanomicelles for sustained drug delivery. J. Ind. Eng. Chem. 2017, 55, 21–34. [Google Scholar] [CrossRef]
- Hosseini, S.; Rezaei, S.; Moghaddam, M.R.N.; Elyasi, S.; Karimi, G. Evaluation of oral nanosilymarin formulation efficacy on prevention of radiotherapy induced mucositis: A randomized, double-blinded, placebo-controlled clinical trial. PharmaNutrition 2021, 15, 100253. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Monfared, M.; Taghizadeh, S.; Zare-Hoseinabadi, A.; Mousavi, S.M.; Hashemi, S.A.; Ranjbar, S.; Amani, A.M. Emerging frontiers in drug release control by core–shell nanofibers: A review. Drug Metab. Rev. 2019, 51, 589–611. [Google Scholar] [CrossRef]
- Lengyel, M.; Ka’llai-Szabo’, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; El-Baz, N.M.; Yacoub, M.H. Inhaled nano-and microparticles for drug delivery. Glob. Cardiol. Sci. Pract. 2015, 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Colella, G.; Boschetti, C.E.; Vitagliano, R.; Colella, C.; Jiao, L.; King-Smith, N.; Li, C.; Nuoh Lau, Y.; Lai, Z.; Mohammed, A.I.; et al. Interventions for the prevention of oral mucositis in patients receiving cancer treatment: Evidence from randomised controlled trials. Curr. Oncol. 2023, 30, 967–980. [Google Scholar] [CrossRef]
- Gholizadeh, N.; Sheykhbahaei, N.; Sadrzadeh-Afshar, M.-S. New treatment approaches of oral mucositis: A review of literature. Adv. Hum. Biol. 2016, 6, 66–72. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Macedo, C.; Silva, A.M.; Delerue-Matos, C.; Costa, P.; Rodrigues, F. Natural products for the prevention and treatment of oral mucositis—A review. Int. J. Mol. Sci. 2022, 23, 4385. [Google Scholar] [CrossRef]
- Choudhury, M.; Brunton, P.; Dias, G.; Schwass, D.; Meledandri, C.; Ratnayake, J.; Pletzer, D.; Tompkins, G. Gold nanoparticles as innovative therapeutics for oral mucositis: A review of current evidence. Drug Deliv. Transl. Res. 2024, 15, 2323–2353. [Google Scholar] [CrossRef]
- Sreedevi, D.; Gopal, M.; Rajeshkumar, S.; Karpagavalli, S. Current perspectives of nanotherapies in the prevention and treatment of radiotherapy/chemotherapy-induced oral mucositis in head and neck cancer—A narrative review. J. Int. Oral Health 2023, 15, 491–499. [Google Scholar]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Shah, S.; Rath, H.; Sharma, G.; Senapati, S.N.; Mishra, E. Effectiveness of curcumin mouthwash on radiation-induced oral mucositis among head and neck cancer patients: A triple-blind, pilot randomized controlled trial. Indian J. Dent. Res. 2020, 31, 718–727. [Google Scholar]
- Buranasukhon, W.; Athikomkulchai, S.; Tadtong, S.; Chittasupho, C. Wound healing activity of pluchea indica leaf extract in oral mucosal cell line and oral spray formulation containing nanoparticles of the extract. Pharm. Biol. 2017, 55, 1767–1774. [Google Scholar] [CrossRef]
- Delavarian, Z.; Pakfetrat, A.; Ghazi, A.; Jaafari, M.R.; Homaei Shandiz, F.; Dalirsani, Z.; Mohammadpour, A.H.; Rahimi, H.R. Oral administration of nanomicelle curcumin in the prevention of radiotherapy-induced mucositis in head and neck cancers. Spec. Care Dent. 2019, 39, 166–172. [Google Scholar] [CrossRef]
- Ramezani, V.; Ghadirian, S.; Shabani, M.; Boroumand, M.A.; Daneshvar, R.; Saghafi, F. Efficacy of curcumin for amelioration of radiotherapy-induced oral mucositis: A preliminary randomized controlled clinical trial. BMC Cancer 2023, 23, 354. [Google Scholar] [CrossRef]
- Kia, S.J.; Basirat, M.; Saedi, H.S.; Arab, S.A. Effects of nanomicelle curcumin capsules on prevention and treatment of oral mucositis in patients under chemotherapy with or without head and neck radiotherapy: A randomized clinical trial. BMC Complement. Med. Ther. 2021, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-Z.; Cai, Y.; Li, H.-Y. Chitosan-based spray-dried mucoadhesive micro- spheres for sustained oromucosal drug delivery. Powder Technol. 2017, 312, 124–132. [Google Scholar] [CrossRef]
- Tort, S.; Acartürk, F. Preparation and characterization of electrospun nanofibers containing glutamine. Carbohydr. Polym. 2016, 152, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Saracoglu, P.; Dokuz, S.; Ozbek, T.; Topuzogullari, M.; Ozmen, M.M. Starch nanogels as promising drug nanocarriers in the management of oral bacterial infections. J. Drug Deliv. Sci. Technol. 2023, 88, 104973. [Google Scholar] [CrossRef]
- Halder, J.; Rajwar, T.K.; Pradhan, D.; Rai, V.K.; Dubey, D.; Kar, B.; Ghosh, G.; Rath, G. Glycyrrhizin loaded hyaluronic acid nanofiber-based artificial saliva for the management of oral mucositis: Preparation, optimization and in vitro evaluation. J. Drug Deliv. Sci. Technol. 2023, 87, 104777. [Google Scholar] [CrossRef]
- Capra, P.; Bleve, M.; Musitelli, G.; Pavanetto, F.; Perugini1, P. Multimethodological approach to evaluate performances of mucoadhesive oral gel based on nanoparticles. J. Pharm. Pharmacol. 2016, 4, 307–317. [Google Scholar] [CrossRef]
- Kawano, Y.; Ishii, N.; Shimizu, Y.; Hanawa, T. Development and characterization of a suspension containing nanoparticulated rebamipide for a mouth wash for stomatitis. J. Pharma-Ceutical Sci. Technol. Jpn. 2017, 77, 104–115. [Google Scholar][Green Version]
- Ferreira, A.S.; Silva, A.M.; Laveriano-Santos, E.P.; Lozano-Castellón, J.; Lamuela-Raventós, R.M.; Švarc-Gajíc, J.; Delerue-Matos, C.; Estevinho, B.N.; Costa, P.C.; Rodrigues, F. Development and characterization of spray-drying microparticles loaded with chestnut shells extract: New insights for oral mucositis therapy. Powder Technol. 2024, 446, 120182. [Google Scholar] [CrossRef]
- Teixeira, F.; Silva, A.M.; Sut, S.; Dall’Acqua, S.; Delerue-Matos, C.; Estevinho, B.; Costa, P.C.; Rodrigues, F. Development and characterization of microparticles with actinidia arguta leaves extract by spray-drying: A new mind-set regarding healthy compounds for oral mucositis. Antioxidants 2023, 12, 1496. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.C.; Cunha, D.; Ferreira, D.C.; Reis, S.; Costa, P.J. Oromucosal precursors of in loco hydrogels for wound-dressing and drug delivery in oral mucositis: Retain, resist, and release. Mater. Sci. Eng. C 2021, 118, 111413. [Google Scholar] [CrossRef]
- Takeuchi, I.; Kamiki, Y.; Makino, K. Therapeutic efficacy of rebamipide-loaded plga nanoparticles coated with chitosan in a mouse model for oral mucositis induced by cancer chemotherapy. Colloids Surf. B Biointerfaces 2018, 167, 468–473. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, X.; Wang, Y.; Li, M.; Yuan, Q.; Zhao, Z. Inflammation-targeted cannabidiol-loaded nanomicelles for enhanced oral mucositis treatment. Drug Deliv. 2022, 29, 1272–1281. [Google Scholar] [CrossRef]
- Nagai, N.; Seiriki, R.; Deguchi, S.; Otake, H.; Hiramatsu, N.; Sasaki, H.; Yamamoto, N. Hydrogel formulations incorporating drug nanocrystals enhance the therapeutic effect of rebamipide in a hamster model for oral mucositis. Pharmaceutics 2020, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Han, S.-H.; Hyun, C.; Yoo, H.S. Buccal adhesive nanofibers containing human growth hormone for oral mucositis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1396–1406. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Dong, X.; Zhu, C.; Peng, Q.; Liu, C.; Zhang, Y.; Chen, F.; Zhang, K. Salivary amylase-responsive buccal tablets wipe out chemotherapy- rooted refractory oral mucositis. Adv. Sci. 2024, 11, e2308439. [Google Scholar] [CrossRef]
- Ribeiro, S.B.; de Araújo, A.A.; Oliveira, M.M.B.; Santos Silva, A.M.D.; da Silva-Júnior, A.A.; Guerra, G.C.B.; Brito, G.A.C.; Leitão, R.F.C.; Araújo Júnior, R.F.; Garcia, V.B.; et al. Effect of dexamethasone-loaded plga nanoparticles on oral mucositis induced by 5-fluorouracil. Pharmaceutics 2021, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- El-Feky, G.S.; Zayed, G.M. Plga nanoparticles loaded mucoadhesive and thermosensitive hydrogel as a potential platform for the treatment of oral mucositis. Int. J. Appl. Pharm. 2019, 11, 106. [Google Scholar] [CrossRef]
- Hu, S.; Pei, X.; Duan, L.; Zhu, Z.; Liu, Y.; Chen, J.; Chen, T.; Ji, P.; Wan, Q.; Wang, J. A mussel-inspired film for adhesion to wet buccal tissue and efficient buccal drug delivery. Nat. Commun. 2021, 12, 1689. [Google Scholar] [CrossRef]
- Qu, X.; Guo, X.; Zhu, T.; Zhang, Z.; Wang, W.; Hao, Y. Microneedle patches containing mesoporous polydopamine nanoparticles loaded with triamcinolone acetonide for the treatment of oral mucositis. Front. Bioeng. Biotechnol. 2023, 11, 1203709. [Google Scholar] [CrossRef]
- Kadowaki, R.; Ogata, F.; Nishida, M.; Komatsu, M.; Otake, H.; Nakazawa, Y.; Yamamoto, N.; Kawasaki, N.; Nagai, N. Therapeutic effects of hydrogel formulations incorporating troxipide nanoparticles on oral mucositis in hamsters. Drug Des. Dev. Ther. 2023, 15, 3349–3361. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, M.; Kazemi, S.; Ebrahimpour, A.; Shirafkan, F.; Pirzadeh, M.; Hosseini, M.A.A. Moghadamnia. Protective effect of quercetin nanoemulsion on 5-fluorouracil-induced oral mucositis in mice. J. Oncol. 2021, 2021, 5598230. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Ikeuchi-Takahashi, Y.; Kobayashi, A.; Yoshimura, N.; Ishihara, C.; Aomori, T.; Onishi, H. Formulation development of mucoadhesive microparticle-laden gels for oral mucositis: An in vitro and in vivo study. Pharmaceutics 2020, 12, 603. [Google Scholar] [CrossRef] [PubMed]
- Mehravaran, M.; Haeri, A.; Rabbani, S.; Mortazavi, S.A.; Torshabi, M. Preparation and characterization of benzydamine hydrochloride-loaded lyophilized mucoadhesive wafers for the treatment of oral mucositis. J. Drug Deliv. Sci. Technol. 2022, 78, 103944. [Google Scholar] [CrossRef]
- Nakashima, T.; Sako, N.; Matsuda, T.; Uematsu, N.; Sakurai, K.; Ishida, T. Novel submicronized rebamipide liquid with moderate viscosity: Significant effects on oral mucositis in animal models. Biol. Pharm. Bull. 2014, 37, 671–678. [Google Scholar] [CrossRef]
- Reda, R.I.; Wen, M.M.; El-Kamel, A.H. Ketoprofen-loaded eudragit electrospun nanofibers for the treatment of oral mucositis. Int. J. Nanomed. 2017, 12, 2335–2351. [Google Scholar] [CrossRef]
- Enin, H.A.A.; El Nabarawy, N.A.; Elmonem, R.A.A. Treatment of radiation- induced oral mucositis using a novel accepted taste of prolonged release mucoadhesive bimedicated double-layer buccal films. AAPS PharmSciTech 2017, 18, 563–575. [Google Scholar] [CrossRef]
- Samiraninezhad, N.; Rezaee, M.; Gholami, A.; Amanati, A.; Mardani, M. A novel chitosan-based doxepin nanoformulation for chemotherapy-induced oral mucositis: A randomized, double-blinded, placebo- controlled clinical trial. Inflammopharmacology 2023, 31, 2411–2420. [Google Scholar] [CrossRef]
- Carlini, A.; Scarpi, E.; Bettini, C.; Ardizzoni, A.; Donati, C.M.; Fabbri, L.; Ghetti, F.; Martini, F.; Ricci, M.; Sansoni, E.; et al. Transdermal Fentanyl in Patients with Cachexia—A Scoping Review. Cancers 2024, 16, 3094. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Boddu, S.H.S.; Gorain, B.; Sreeharsha, N.; Shah, J. An updated overview of the emerging role of patch and film-based buccal delivery systems. Pharmaceutics 2021, 13, 1206. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties; characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maia, L.Â.S.; Siqueira, T.T.A.; Bezerra, C.A.A.S.; Farias, J.H.P.d.; Oliveira, E.E. Micro and Nano Drug Delivery Systems for the Treatment of Oral Mucositis: A Review. Pharmaceutics 2025, 17, 1025. https://doi.org/10.3390/pharmaceutics17081025
Maia LÂS, Siqueira TTA, Bezerra CAAS, Farias JHPd, Oliveira EE. Micro and Nano Drug Delivery Systems for the Treatment of Oral Mucositis: A Review. Pharmaceutics. 2025; 17(8):1025. https://doi.org/10.3390/pharmaceutics17081025
Chicago/Turabian StyleMaia, Luciana Ângela Soares, Tâmara Thaiane Almeida Siqueira, Carlos Alberto Arcelly Santos Bezerra, Jéssica Horana Pereira de Farias, and Elquio Eleamen Oliveira. 2025. "Micro and Nano Drug Delivery Systems for the Treatment of Oral Mucositis: A Review" Pharmaceutics 17, no. 8: 1025. https://doi.org/10.3390/pharmaceutics17081025
APA StyleMaia, L. Â. S., Siqueira, T. T. A., Bezerra, C. A. A. S., Farias, J. H. P. d., & Oliveira, E. E. (2025). Micro and Nano Drug Delivery Systems for the Treatment of Oral Mucositis: A Review. Pharmaceutics, 17(8), 1025. https://doi.org/10.3390/pharmaceutics17081025