Biopharming of Lactoferrin: Current Strategies and Future Prospects
Abstract
1. Introduction
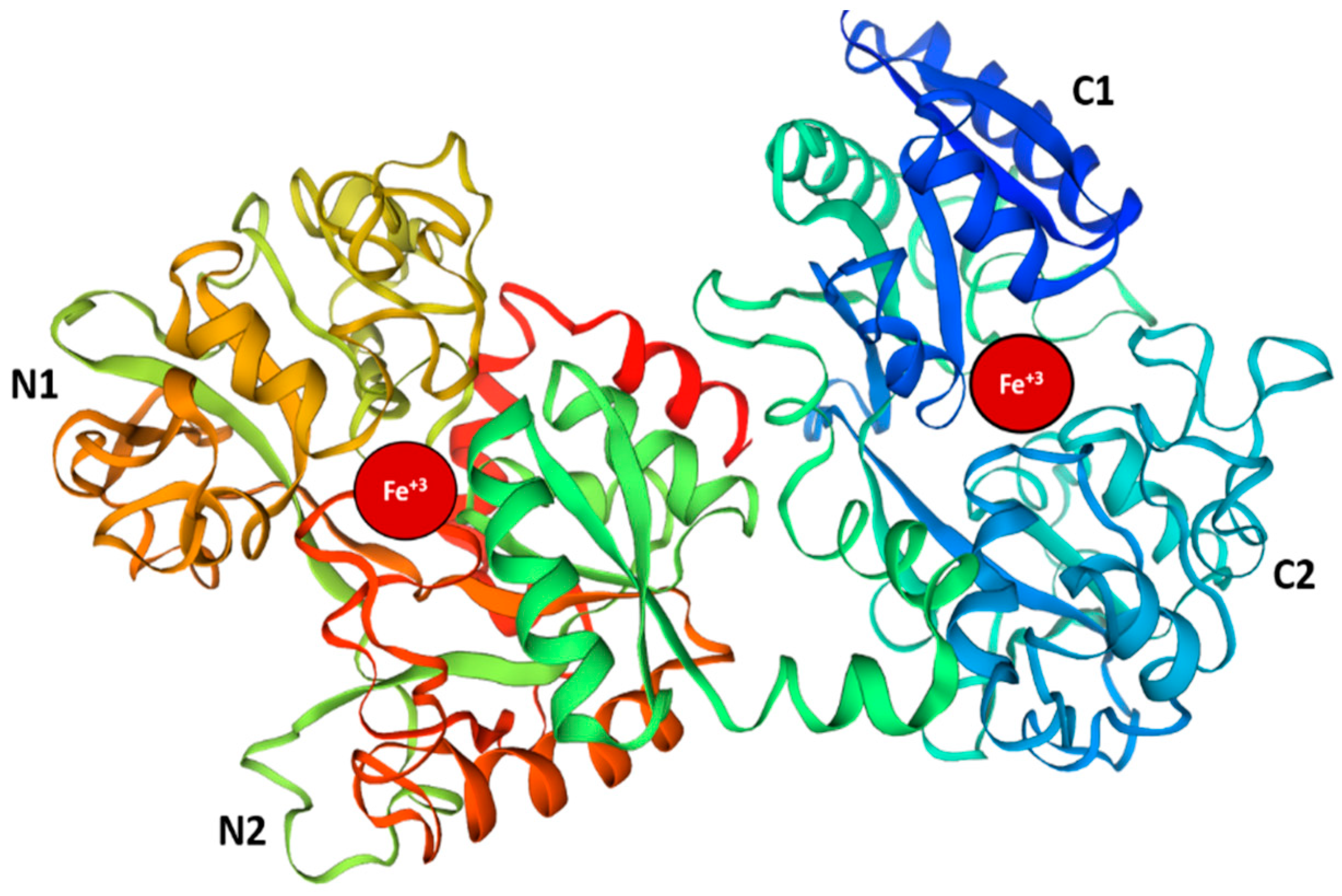
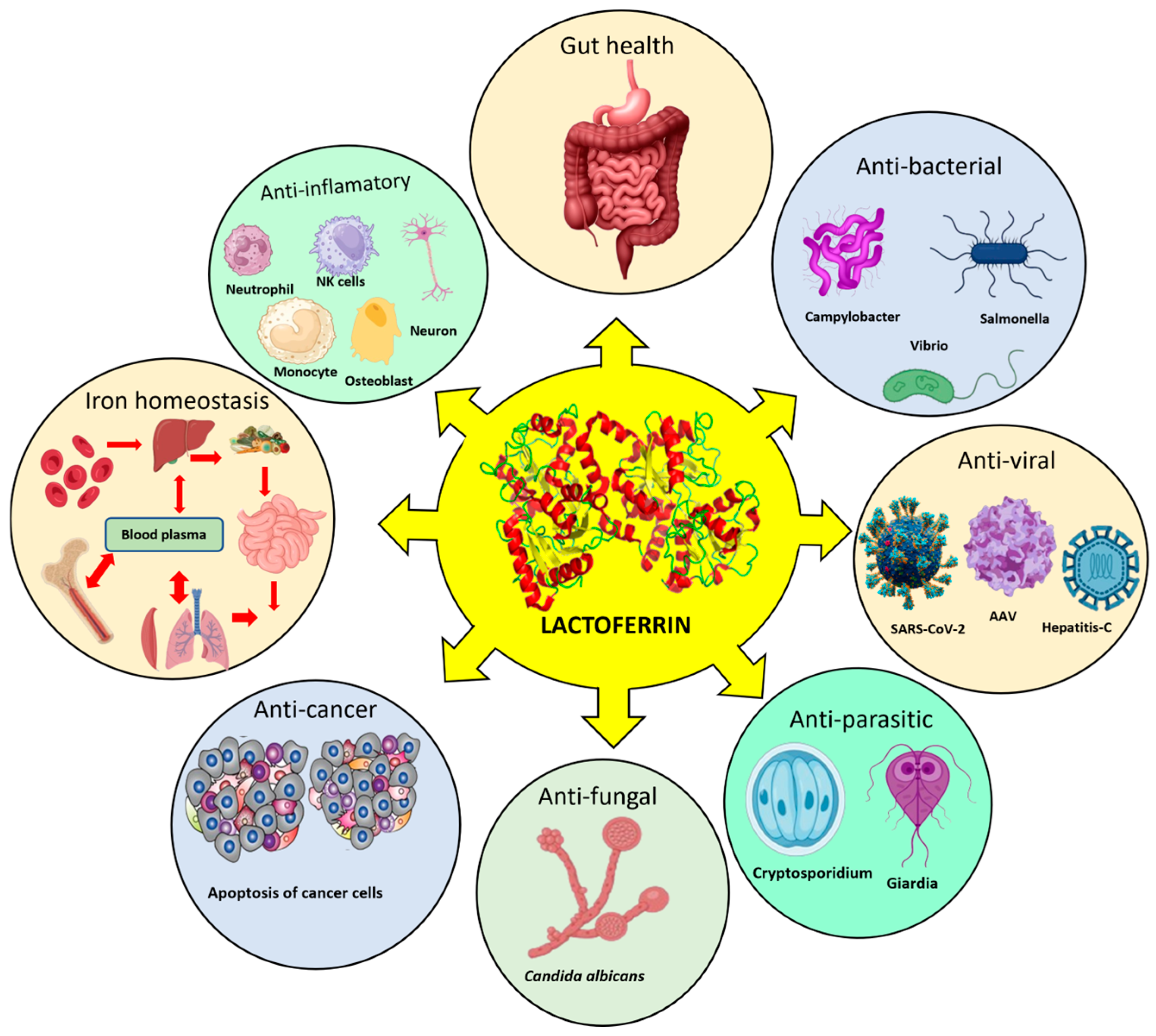
2. Therapeutic Properties of Lactoferrin
2.1. Antipathogenic Properties
2.2. Anti-Inflammatory Effects
2.3. Gut Health
2.4. Antiviral Activity
2.5. Anticancer Activities
3. Commercialization of Lactoferrin as a Food Supplement
4. Conventional Lactoferrin Purification Methods
4.1. Fractionation and Precipitation
4.2. Ion Exchange Chromatography
4.3. Size Exclusion and Affinity Chromatography
4.4. Ultrafiltration and Dialysis
4.5. High-Performance Liquid Chromatography (HPLC)
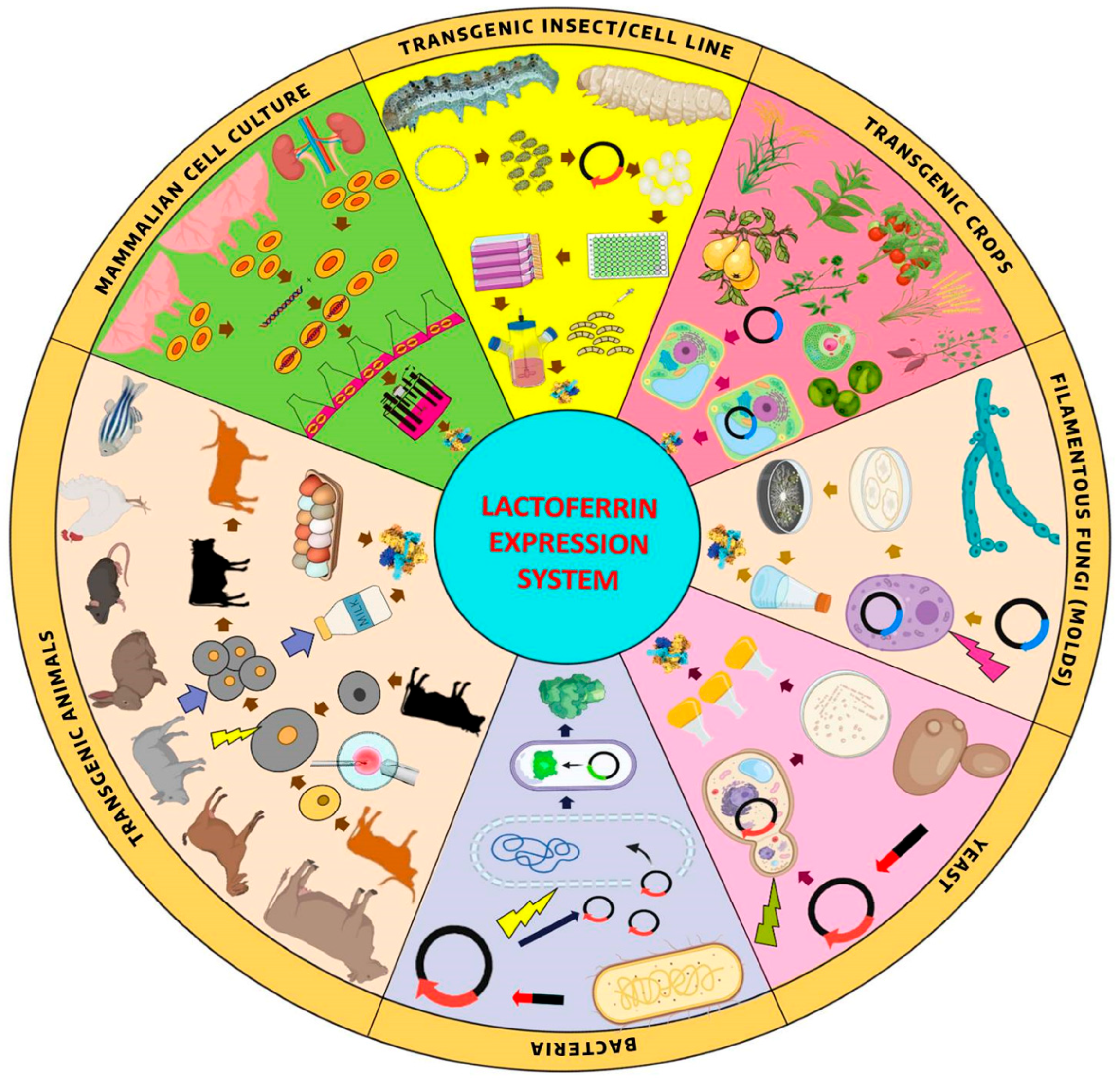
5. Recombinant Lactoferrin Biopharming Systems
5.1. Prokaryotic Expression Systems
5.2. Eukaryotic Expression Systems
5.2.1. Yeast
5.2.2. Filamentous Fungi (Molds)
5.2.3. Transgenic Insect and Insect Cell Line Expression Systems
5.2.4. Mammalian Cell Culture System
5.2.5. Animal Bioreactors
Transgenic Cattle
Transgenic Goats
Transgenic Swine
Transgenic Rabbits
Transgenic Mice
Transgenic Chickens
Transgenic Fish
5.2.6. Transgenic Crops
Transgenic Rice
Transgenic Tobacco
Transgenic Potatoes
Transgenic Tomato
Other Transgenic Crops
6. Future Prospects
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, B.; Baker, H.M.; Dodson, E.J.; Norris, G.E.; Rumball, S.V.; Waters, J.M.; Baker, E.N. Structure of Human Lactoferrin at 3.2-A Resolution. Proc. Natl. Acad. Sci. USA 1987, 84, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.F.; Baker, H.M.; Norris, G.E.; Rice, D.W.; Baker, E.N. Structure of Human Lactoferrin: Crystallographic Structure Analysis and Refinement at 2·8 Å Resolution. J. Mol. Biol. 1989, 209, 711–734. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.; Baker, H. A Structural Framework for Understanding the Multifunctional Character of Lactoferrin. Biochimie 2009, 91, 3–10. [Google Scholar] [CrossRef] [PubMed]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, Function and Applications. Int. J. Antimicrob. Agents 2008, 33, 301.e1–301.e8. [Google Scholar] [CrossRef]
- Cui, S.; Lv, X.; Sun, G.; Wu, W.; Xu, H.; Li, Y.; Liu, Y.; Li, J.; Du, G.; Wang, M.; et al. Recent Advances and Prospects in Purification and Heterologous Expression of Lactoferrin. Food Bioeng. 2022, 1, 58–67. [Google Scholar] [CrossRef]
- Baker, H.M.; Baker, E.N. Lactoferrin and Iron: Structural and Dynamic Aspects of Binding and Release. BioMetals 2004, 17, 209–216. [Google Scholar] [CrossRef]
- Reyes-López, M.; Ramírez-Rico, G.; Serrano-Luna, J.; de la Garza, M. Activity of Apo-Lactoferrin on Pathogenic Protozoa. Pharmaceutics 2022, 14, 1702. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Iyer, S. Lactoferrin: Molecular Structure and Biological Function. Annu. Rev. Nutr. 1995, 15, 93–110. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Georgieff, M.K.; Hernell, O. Developmental Physiology of Iron Absorption, Homeostasis, and Metabolism in the Healthy Term Infant. J. Pediatr. 2015, 167, S8–S14. [Google Scholar] [CrossRef]
- McCarthy, E.K.; O’Callaghan, T.F. Bovine Lactoferrin and Its Potential Use as a Functional Ingredient for Tackling the Global Challenge of Iron Deficiency. Curr. Opin. Food Sci. 2024, 59, 101211. [Google Scholar] [CrossRef]
- Hong, R.; Xie, A.; Jiang, C.; Guo, Y.; Zhang, Y.; Chen, J.; Shen, X.; Li, M.; Yue, X. A Review of the Biological Activities of Lactoferrin: Mechanisms and Potential Applications. Food Funct. 2024, 15, 8182–8199. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the Bactericidal Domain of Lactoferrin. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1992, 1121, 130–136. [Google Scholar] [CrossRef]
- Miura, K.; Nagai, Y.; Yokouchi, A.; Miwa, K. Expressing Recombinant Human Lactoferrin with Antibacterial Activity in Nicotiana benthamiana. Plant Biotechnol. 2023, 40, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Cutone, A.; Conte, M.P.; Campione, E.; Bianchi, L.; Valenti, P. An Overview on in vitro and in vivo Antiviral Activity of Lactoferrin: Its Efficacy against SARS-CoV-2 Infection. BioMetals 2023, 36, 417–436. [Google Scholar] [CrossRef]
- Graikini, D.; Conesa, C.; Abad, I.; Pérez, M.D.; Sánchez, L. Evaluation of in vitro Antirotaviral Activity of Lactoferrin from Different Species Using a Human Intestinal Model. Int. Dairy J. 2024, 149, 105818. [Google Scholar] [CrossRef]
- Stella, M.M.; Soetedjo, R.; Tandarto, K.; Arieselia, Z.; Regina, R. Bovine Lactoferrin and Current Antifungal Therapy against Candida Albicans: A Systematic Review and Meta-Analysis. Indian J. Dermatol. 2023, 68, 725. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Conneely, O.M. Anti-inflammatory Activities of Lactoferrin. J. Am. Coll. Nutr. 2013, 20 (Suppl. S5), 389S–395S. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Dong, Y.; Wang, C.; Cai, Z. Bovine Lactoferrin Inhibits Inflammatory Response and Apoptosis in Lipopolysaccharide-Induced Acute Lung Injury by Targeting the PPAR-γ Pathway. Mol. Biol. Rep. 2024, 51, 492. [Google Scholar] [CrossRef]
- Mastromarino, P.; Capobianco, D.; Campagna, G.; Laforgia, N.; Drimaco, P.; Dileone, A.; Baldassarre, M.E. Correlation between Lactoferrin and Beneficial Microbiota in Breast Milk and Infant’s Feces. BioMetals 2014, 27, 1077–1086. [Google Scholar] [CrossRef]
- Lu, J.; Haley, K.P.; Francis, J.D.; Guevara, M.A.; Doster, R.S.; Craft, K.M.; Moore, R.E.; Chambers, S.A.; Delgado, A.G.; Piazuelo, M.B.; et al. The Innate Immune Glycoprotein Lactoferrin Represses the Helicobacter pylori Cag Type IV Secretion System. ChemBioChem 2021, 22, 2783–2790. [Google Scholar] [CrossRef]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin against SARS-CoV-2: In vitro and in silico Evidences. Front. Pharmacol. 2021, 12, 666600. [Google Scholar] [CrossRef]
- Manzoni, P.; Messina, A.; Germano, C.; Picone, S.; Masturzo, B.; Sainaghi, P.P.; Sola, D.; Rizzi, M. Lactoferrin Supplementation in Preventing and Protecting from SARS-CoV-2 Infection: Is There Any Role in General and Special Populations? An Updated Review of Literature. Int. J. Mol. Sci. 2024, 25, 10248. [Google Scholar] [CrossRef]
- Gibbons, J.; Kanwar, R.K.; Kanwar, J.R. Lactoferrin and Cancer in Different Cancer Models. Front. Biosci. 2011, 3, 1080–1088. [Google Scholar] [CrossRef]
- Naidu, A.S. Activated Lactoferrin-A New Approach to Meat Safety. Food Technol. 2002, 56, 40–45. [Google Scholar]
- Future Market Insights. Bovine Lactoferrin Market by Product Type, Application, End User & Region|Forecast 2023 to 2033. Available online: https://www.futuremarketinsights.com/reports/bovine-lactoferrin-market (accessed on 20 March 2025).
- Luo, F.; Liu, C.G.; Cheng, L. Study on the Optimization of Technique of Crude Extracting Lactoferrin from Bovine Colostrum. Dairy Sci. Technol. 2010, 33, 11–14. [Google Scholar]
- Fan, F.; Tu, M.; Liu, M.; Shi, P.; Wang, Y.; Wu, D.; Du, M. Isolation and Characterization of Lactoferrin Peptides with Stimulatory Effect on Osteoblast Proliferation. J. Agric. Food Chem. 2017, 65, 7179–7185. [Google Scholar] [CrossRef]
- Baieli, M.F.; Urtasun, N.; Miranda, M.V.; Cascone, O.; Wolman, F.J. Isolation of Lactoferrin from Whey by Dye-Affinity Chromatography with Yellow HE-4R Attached to Chitosan Mini-Spheres. Int. Dairy J. 2014, 39, 53–59. [Google Scholar] [CrossRef]
- Li, N.; Yang, B.; Yang, P.H.; Wang, J.W. Method for Purifying Restructuring Lactoferrin from Transgenic Cow’s Milk. 2024. Available online: https://patents.google.com/patent/CN101117351B/en (accessed on 24 January 2025).
- Tsakali, E.; Chatzilazarou, A.; Houhoula, D.; Koulouris, S.; Tsaknis, J.; Van Impe, J. A Rapid HPLC Method for the Determination of Lactoferrin in Milk of Various Species. J. Dairy Res. 2019, 86, 238–241. [Google Scholar] [CrossRef]
- Pang, J.; Xiao, Q.; Yan, H.; Cao, Y.; Miao, J.; Wang, S.; Li, X.; Li, H.; Cheng, Z. Bovine Lactoferrin Quantification in Dairy Products by a Simple Immunoaffinity Magnetic Purification Method Coupled with High-Performance Liquid Chromatography with Fluorescence Detection. J. Agric. Food Chem. 2019, 68, 892–898. [Google Scholar] [CrossRef]
- Liu, K.; Tong, Z.; Zhang, X.; Dahmani, M.; Zhao, M.; Hu, M.; Li, X.; Xue, Z. A Review: Development of a Synthetic Lactoferrin Biological System. Biodes. Res. 2024, 6, 0040. [Google Scholar] [CrossRef]
- Karav, S.; German, J.B.; Rouquié, C.; Le Parc, A.; Barile, D. Studying Lactoferrin N-Glycosylation. Int. J. Mol. Sci. 2017, 18, 870. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, A.; Goyal, S.; Dubey, P.K.; Mishra, S.K.; Ahlawat, S.; Kataria, R.S. Evaluation of Therapeutic Potential of Recombinant Buffalo Lactoferrin N-Lobe Expressed in E coli. Anim. Biotechnol. 2019, 31, 181–187. [Google Scholar] [CrossRef]
- Novoselova, M.V.; Prosekov, A.Y. Technological options for the production of lactoferrin. Foods Raw Mater. 2016, 4, 90–101. [Google Scholar] [CrossRef]
- Feng, X.-J.; Xing, L.-W.; Liu, D.; Song, X.-Y.; Liu, C.-L.; Li, J.; Xu, W.-S.; Li, Z.-Q. Design and High-Level Expression of a Hybrid Antimicrobial Peptide LF15-CA8 in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2014, 41, 527–534. [Google Scholar] [CrossRef]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a Multiple Bioactive Protein: An Overview. Biochim. Biophys. Acta 2012, 1820, 226–236. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Z.; Teng, D.; Yang, Y.; Hu, J.; Wang, J. Cloning, Expression and Characterization of Kunming Mice Lactoferrin and Its N-Lobe. BioMetals 2010, 23, 523–530. [Google Scholar] [CrossRef]
- Tian, Z.; Teng, D.; Yang, Y.; Luo, J.; Feng, X.; Fan, Y.; Zhang, F.; Wang, J. Multimerization and Fusion Expression of Bovine Lactoferricin Derivative LfcinB15-W4,10 in Escherichia coli. Appl. Microbiol. Biotechnol. 2007, 75, 117–124. [Google Scholar] [CrossRef]
- Kim, H.-K.; Chun, D.-S.; Kim, J.-S.; Yun, C.-H.; Lee, J.-H.; Hong, S.-K.; Kang, D.-K. Expression of the Cationic Antimicrobial Peptide Lactoferricin Fused with the Anionic Peptide in Escherichia coli. Appl. Microbiol. Biotechnol. 2006, 72, 330–338. [Google Scholar] [CrossRef]
- Feng, X.; Wang, J.; Shan, A.; Teng, D.; Yang, Y.; Yao, Y.; Yang, G.; Shao, Y.; Liu, S.; Zhang, F. Fusion Expression of Bovine Lactoferricin in Escherichia coli. Protein Expr. Purif. 2006, 47, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Hsieh, C.-Y.; Yang, C.-Y.; Chang, Y.-K.; Shih, W.-L.; Yeh, C.-M.; Hu, N.-J.; Chen, M.-S.; Nielsen, B.L.; Liu, H.-J. Sortase a Fusion Expression and MIFc2 Co-Expression of Bovine Lactoferricin and Analysis of Its Antibacterial Activity. Processes 2022, 10, 2470. [Google Scholar] [CrossRef]
- Kim, W.-S.; Shimazaki, K.; Tamura, T. Expression of Bovine Lactoferrin C-Lobe in Rhodococcus erythropolis and Its Purification and Characterization. Biosci. Biotechnol. Biochem. 2006, 70, 2641–2645. [Google Scholar] [CrossRef][Green Version]
- Lee, B.; Tsai, J.; Hung, C.; Lin, C.; Sheu, J.; Tsai, H. High Antimicrobial Activity of Lactoferricin-Expressing Bacillus subtilis Strains. Microb. Biotechnol. 2022, 15, 1895–1909. [Google Scholar] [CrossRef]
- Jin, L.; Li, L.; Zhang, W.; Zhang, R.; Xu, Y. Heterologous Expression of Bovine Lactoferrin C-Lobe in Bacillus subtilis and Comparison of Its Antibacterial Activity with N-Lobe. Syst. Microbiol. Biomanufacturing 2022, 2, 345–354. [Google Scholar] [CrossRef]
- Jin, L.; Li, L.; Zhou, L.; Zhang, R.; Xu, Y.; Li, J. Improving Expression of Bovine Lactoferrin N-Lobe by Promoter Optimization and Codon Engineering in Bacillus subtilis and Its Antibacterial Activity. J. Agric. Food Chem. 2019, 67, 9749–9756. [Google Scholar] [CrossRef]
- Tanhaeian, A.; Mirzaii, M.; Pirkhezranian, Z.; Sekhavati, M.H. Generation of an Engineered Food-Grade Lactococcus Lactis Strain for Production of an Antimicrobial Peptide: In vitro and in silico Evaluation. BMC Biotechnol. 2020, 20, 19. [Google Scholar] [CrossRef]
- Xu, Y.-G.; Yu, H.; Zhang, L.; Liu, M.; Qiao, X.-Y.; Cui, W.; Jiang, Y.-P.; Wang, L.; Li, Y.-J.; Tang, L.-J. Probiotic Properties of Genetically Engineered Lactobacillus plantarum Producing Porcine Lactoferrin Used as Feed Additive for Piglets. Process Biochem. 2016, 51, 719–724. [Google Scholar] [CrossRef]
- Yu, H.; Yu, J.; Zhu, Y.; Li, Y.; Tang, L. Comparison of Improved Effect of Antibacterial and Antiviral Activity of Four Probiotic Lactobacillus Expressing Porcine Lactoferrin in Mice. Pak. Vet. J. 2015, 35, 274–278. [Google Scholar]
- Chen, H.-L.; Lai, Y.-W.; Chen, C.-S.; Chu, T.-W.; Lin, W.; Yen, C.-C.; Lin, M.-F.; Tu, M.-Y.; Chen, C.-M. Probiotic Lactobacillus casei Expressing Human Lactoferrin Elevates Antibacterial Activity in the Gastrointestinal Tract. BioMetals 2010, 23, 543–554. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant Protein Expression in Escherichia coli: Advances and Challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef]
- Yen, C.-C.; Wu, P.-Y.; Ou-Yang, H.; Chen, H.-L.; Chong, K.-Y.; Chang, R.-L.; Chen, C.-M. Production of Bioactive Porcine Lactoferrin through a Novel Glucose-Inducible Expression System in Pichia pastoris: Unveiling Antimicrobial and Anticancer Functionalities. Int. J. Mol. Sci. 2024, 25, 1818. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, Y.; Wang, L.; Cui, S.; Liu, Y.; Li, J.; Du, G.; Liu, L. Expression and Antimicrobial Activity of the Recombinant Bovine Lactoferricin in Pichia pastoris. Synth. Syst. Biotechnol. 2024, 9, 26–32. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Cui, S.; Xu, X.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Engineering a Transport Pathway to Boost Extracellular Production of Human Lactoferrin in Komagataella phaffii. Food Biosci. 2024, 59, 104057. [Google Scholar] [CrossRef]
- Zhang, X.; Xi, Z.; Zhao, H.; Zhang, W.; Xu, Y.; Zhang, R. Efficient Heterologous Expression of Bovine Lactoferrin in Pichia pastoris and Characterization of Antibacterial Activity. Syst. Microbiol. Biomanufacturing 2024, 5, 237–248. [Google Scholar] [CrossRef]
- Lu, X.; Cummings, C.; Osuala, U.A.; Yennawar, N.H.; Namitz, K.E.W.; Hellner, B.; Besada-Lombana, P.B.; Peterson, R.D.; Clark, A.J. Characterization of Recombinant Human Lactoferrin Expressed in Komagataella phaffii. Analyst 2024, 149, 3636–3650. [Google Scholar] [CrossRef]
- Won, S.J.; Jo, J.H.; Kim, S.H.; Kwon, H.J.; Lee, H.H. High Expression of Bovine Lactoferrin N-Lobe in Pichia pastoris and Its Antibacterial Activity. Acta Microbiol. Sin. 2022, 62, 1425–1437. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.-L.; Lv, Z.-L.; Zhang, E.; Guo, A. Design of Bovine Lactoferricin-Derived Peptide and Its Expression and Activity in Pichia Pastoris. Biochem. Biophys. Res. Commun. 2021, 534, 822–829. [Google Scholar] [CrossRef]
- Iglesias-Figueroa, B.; Valdiviezo-Godina, N.; Siqueiros-Cendón, T.; Sinagawa-García, S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. High-Level Expression of Recombinant Bovine Lactoferrin in Pichia Pastoris with Antimicrobial Activity. Int. J. Mol. Sci. 2016, 17, 902. [Google Scholar] [CrossRef]
- Chahardooli, M.; Niazi, A.; Aram, F.; Sohrabi, S.M. Expression of Recombinant Arabian Camel Lactoferricin-Related Peptide in Pichia pastoris and Its Antimicrobial Identification. J. Sci. Food Agric. 2016, 96, 569–575. [Google Scholar] [CrossRef]
- Elnaz, A.; Ali, N.; Adel, Y.; Ali, M.; Farzaneh, A. Expression of a Recombinant Therapeutic Protein, Lactoferrin, in PichiaPinkTM: A Powerful Antimicrobial Protein. Biol. Forum 2016, 8, 471–478. [Google Scholar]
- Won, S.-J.; Jo, J.-H.; Kim, S.-H.; Kwon, H.-J.; Lee, H.-H. Expression of Human Lactoferrin N-Lobe in Pichia pastoris and Its Antibacterial Activity. Korean J. Microbiol. 2015, 51, 271–279. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Zhu, W.; Luo, M.; Ren, H.; Tang, L.; Liao, H.; Wang, Y. Molecular Cloning, Expression and Purification of Lactoferrin from Tibetan Sheep Mammary Gland Using a Yeast Expression System. Protein Expr. Purif. 2015, 109, 35–39. [Google Scholar] [CrossRef]
- Xi, D.; Teng, D.; Wang, X.; Mao, R.; Yang, Y.; Xiang, W.; Wang, J. Design, Expression and Characterization of the Hybrid Antimicrobial Peptide LHP7, Connected by a Flexible Linker, against Staphylococcus and Streptococcus. Process Biochem. 2013, 48, 453–461. [Google Scholar] [CrossRef]
- Tang, X.-S.; Tang, Z.-R.; Wang, S.-P.; Feng, Z.-M.; Zhou, D.; Li, T.-J.; Yin, Y.-L. Expression, Purification, and Antibacterial Activity of Bovine Lactoferrampin–Lactoferricin in Pichia pastoris. Appl. Biochem. Biotechnol. 2012, 166, 640–651. [Google Scholar] [CrossRef]
- Jo, J.-H.; Im, E.-M.; Kim, S.-H.; Lee, H.-H. Surface Display of Human Lactoferrin Using a Glycosylphosphatidylinositol-Anchored Protein of Saccharomyces Cerevisiae in Pichia pastoris. Biotechnol. Lett. 2011, 33, 1113–1120. [Google Scholar] [CrossRef]
- Bai, X.; Teng, D.; Tian, Z.; Zhu, Y.; Yang, Y.; Wang, J. Contribution of Bovine Lactoferrin Inter-Lobe Region to Iron Binding Stability and Antimicrobial Activity against Staphylococcus aureus. BioMetals 2010, 23, 431–439. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, L.; Jia, S.; Chen, L.; Ma, Y. High-Level Expression and Production of Human Lactoferrin in Pichia pastoris. Dairy Sci. Technol. 2008, 88, 173–181. [Google Scholar] [CrossRef]
- Choi, B.-K.; Actor, J.K.; Rios, S.; d’Anjou, M.; Stadheim, T.A.; Warburton, S.; Giaccone, E.; Cukan, M.; Li, H.; Kull, A.; et al. Recombinant Human Lactoferrin Expressed in Glycoengineered Pichia pastoris: Effect of Terminal N-Acetylneuraminic Acid on in vitro Secondary Humoral Immune Response. Glycoconj. J. 2008, 25, 581–593. [Google Scholar] [CrossRef]
- Chen, G.-H.; Yin, L.-J.; Chiang, I.-H.; Jiang, S.-T. Expression and Purification of Goat Lactoferrin from Pichia pastoris Expression System. J. Food Sci. 2007, 72, M67–M71. [Google Scholar] [CrossRef]
- Dong, Z.-Y.; Zhang, Y.-Z. Molecular Cloning and Expression of Yak (Bos grunniens) Lactoferrin CDNA in Pichia pastoris. Biotechnol. Lett. 2006, 28, 1285–1292. [Google Scholar] [CrossRef]
- Pecorini, C.; Savazzini, F.; Martino, P.A.; Fusi, E.; Fogher, C.; Baldi, A. Heterologous Expression of Biologically Active Porcine Lactoferrin in Pichia pastoris Yeast. Vet. Res. Commun. 2005, 29 (Suppl. S2), 379–382. [Google Scholar] [CrossRef]
- Chen, H.-L.; Lai, Y.-W.; Yen, C.-C.; Lin, Y.-Y.; Lu, C.-Y.; Yang, S.-H.; Tsai, T.-C.; Lin, Y.-J.; Lin, C.-W.; Chen, C.-M. Production of Recombinant Porcine Lactoferrin Exhibiting Antibacterial Activity in Methylotrophic Yeast, Pichia pastoris. Microb. Physiol. 2004, 8, 141–149. [Google Scholar] [CrossRef]
- Ying, G.; Wu, S.; Wang, J.; Zhao, X.; Chen, J.; Zhang, X.; Hou, Y. Producing Human Lactoferrin by High-Density Fermentation Recombinant Pichia pastoris. Chin. J. Exp. Clin. Virol. 2004, 18, 181–185. [Google Scholar]
- Wang, S.-H.; Yang, T.-S.; Lin, S.-M.; Tsai, M.-S.; Wu, S.-C.; Mao, S.J.T. Expression, Characterization, and Purification of Recombinant Porcine Lactoferrin in Pichia pastoris. Protein Expr. Purif. 2002, 25, 41–49. [Google Scholar] [CrossRef]
- Paramasivam, M.; Saravanan, K.; Uma, K.; Sharma, S.; Singh, T.P.; Srinivasan, A. Expression, Purification, and Characterization of Equine Lactoferrin in Pichia pastoris. Protein Expr. Purif. 2002, 26, 28–34. [Google Scholar] [CrossRef]
- Shan, T.; Wang, Y.; Liu, G.; Huang, H. Expression of Recombinant Porcine Lactoferrin N-Lobe in Pichia methanolica and Its Antibacterial Activity. J. Anim. Feed Sci. 2007, 16, 283–292. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Lu, F. Heterologous Expression of Bovine Lactoferricin in Pichia methanolica. Biochemistry 2007, 72, 640–643. [Google Scholar] [CrossRef]
- Ho, C.L.; Hwang, I.Y.; Loh, K.; Chang, M.W. Matrix-Immobilized Yeast for Large-Scale Production of Recombinant Human Lactoferrin. MedChemComm 2015, 6, 486–491. [Google Scholar] [CrossRef]
- Liang, Q.; Richardson, T. Expression and Characterization of Human Lactoferrin in Yeast Saccharomyces cerevisiae. J. Agric. Food Chem. 1993, 41, 1800–1807. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Lv, B.; Li, C. Regulating Strategies for Producing Carbohydrate Active Enzymes by Filamentous Fungal Cell Factories. Front. Bioeng. Biotechnol. 2020, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.P.; Lo, J.-Y.; Duke, M.; May, G.S.; Headon, D.R.; Conneely, O.M. Production of Biologically Active Recombinant Human Lactoferrin in Aspergillus oryzae. Nat. Biotechnol. 1992, 10, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Baker, H.M.; Shewry, S.C.; Jameson, G.B.; Baker, E.N. Structure of Recombinant Human Lactoferrin Expressed in Aspergillus awamori. Acta Crystallogr. Sect. D Struct. Biol. 1999, 55, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.P.; Piddington, C.S.; Cunningham, G.A.; Zhou, X.; Wyatt, R.D.; Conneely, O.M. A System for Production of Commercial Quantities of Human Lactoferrin: A Broad Spectrum Natural Antibiotic. Nat. Biotechnol. 1995, 13, 498–503. [Google Scholar] [CrossRef]
- Ward, P.P.; Chu, H.; Zhou, X.; Conneely, O.M. Expression and Characterization of Recombinant Murine Lactoferrin. Gene 1997, 204, 171–176. [Google Scholar] [CrossRef]
- Ward, P.P.; May, G.S.; Headon, D.R.; Conneely, O.M. An Inducible Expression System for the Production of Human Lactoferrin in Aspergillus nidulans. Gene 1992, 122, 219–223. [Google Scholar] [CrossRef]
- Xu, S.; Wang, F.; Wang, Y.; Wang, R.; Hou, K.; Tian, C.; Ji, Y.; Yang, Q.; Zhao, P.; Xia, Q. A Silkworm Based Silk Gland Bioreactor for High-Efficiency Production of Recombinant Human Lactoferrin with Antibacterial and Anti-Inflammatory Activities. J. Biol. Eng. 2019, 13, 61. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Y.-Z.; Wu, X.-F. Recombinant Functional Human Lactoferrin Expressed in Baculovirus System. Acta Biochim. Biophys. Sin. 2006, 38, 201–206. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Liu, G.; Cao, C.; Huang, H.; Xu, Z.; Liu, J. Expression of Porcine Lactoferrin by Using Recombinant Baculovirus in Silkworm, Bombyx mori L., and Its Purification and Characterization. Appl. Microbiol. Biotechnol. 2005, 69, 385–389. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Y.-Z.; Wu, X.-F. High Level Expression of Functionally Active Human Lactoferrin in Silkworm Larvae. J. Biotechnol. 2005, 118, 246–256. [Google Scholar] [CrossRef]
- Nakamura, I.; Watanabe, A.; Tsunemitsu, H.; Lee, N.-Y.; Kumura, H.; Shimazaki, K.; Yagi, Y. Production of Recombinant Bovine Lactoferrin N-Lobe in Insect Cells and Its Antimicrobial Activity. Protein Expr. Purif. 2001, 21, 424–431. [Google Scholar] [CrossRef]
- Zhang, D.-B.; Jiang, Y.-L.; Wu, X.-F.; Hong, M.-M. Expression of Human Lactoferrin CDNA in Insect Cells. Acta Biochim. Biophys. Sin. 1998, 30, 575–578. [Google Scholar]
- Salmon, V.; Legrand, D.; Georges, B.; Slomianny, M.-C.; Coddeville, B.; Spik, G. Characterization of Human Lactoferrin Produced in the Baculovirus Expression System. Protein Expr. Purif. 1997, 9, 203–210. [Google Scholar] [CrossRef]
- Hu, G.; Song, M.; Wang, Y.; Hao, K.; Wang, J.; Zhang, Y. Using a Modified PiggyBac Transposon-Combined Cre/LoxP System to Produce Selectable Reporter-Free Transgenic Bovine Mammary Epithelial Cells for Somatic Cell Nuclear Transfer. Genesis 2023, 61, e23510. [Google Scholar] [CrossRef]
- Kenigsberg, Z.; Welch, R.C.; Bejoy, J.; Williams, F.M.; Veach, R.A.; Jarrett, I.; Thompson, T.K.; Wilson, M.H.; Woodard, L.E. Genome Engineering of Human Urine-Derived Stem Cells to Express Lactoferrin and Deoxyribonuclease. Tissue Eng. Part A 2023, 29, 372–383. [Google Scholar] [CrossRef]
- Sharma, N.; Huynh, D.L.; Kim, S.W.; Ghosh, M.; Sodhi, S.S.; Singh, A.K.; Kim, N.E.; Lee, S.J.; Hussain, K.; Oh, S.J.; et al. A PiggyBac Mediated Approach for Lactoferricin Gene Transfer in Bovine Mammary Epithelial Stem Cells for Management of Bovine Mastitis. Oncotarget 2017, 8, 104272–104285. [Google Scholar] [CrossRef]
- Yuan, Y.; Song, S.; Zhu, M.-M.; He, Z.; Lü, R.; Zhang, T.; Mi, F.; Wang, J.; Cheng, Y. Human Lactoferrin Efficiently Targeted into Caprine Beta-Lactoglobulin Locus with Transcription Activator-like Effector Nucleases. Asian-Australas. J. Anim. Sci. 2016, 30, 1175–1182. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Actor, J.K.; Zimecki, M.; Wise, J.; Płoszaj, P.; Mirza, S.; Kruzel, M.; Hwang, S.-A.; Ba, X.; Boldogh, I. Novel Recombinant Human Lactoferrin: Differential Activation of Oxidative Stress Related Gene Expression. J. Biotechnol. 2013, 168, 666–675. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Xu, X.; Wang, Z.; Yan, Y.; Pang, X.; Zhong, B.; Huang, R.; Song, Y.; Wang, J.; et al. Construction and Identification of Mammary Expressional Vector for cDNA of Human Lactoferrin. Chin. J. Biotechnol. 2011, 27, 253–261. [Google Scholar]
- Shu, J.; Zhang, Y.; Pan, Z.; Peng, S.; Cao, J.; Li, X. Construction of Expression Vector of Human Lactoferrin and Its Expression in Bovine Mammary Epithelial Cells. Belg. J. Zool. 2007, 137, 231–237. [Google Scholar]
- Tutykhina, I.L.; Bezborodova, O.A.; Verkhovskaya, L.V.; Shmarov, M.M.; Logunov, D.Y.; Nemtsova, E.R.; Naroditskii, B.S.; Yakubovskaya, R.I.; Ginzburg, A.L. Recombinant Pseudoadenovirus Nanostructure with the Human Lactoferrin Gene: Production and Study of Lactoferrin Expression and Properties in vivo. Mol. Genet. Microbiol. Virol. 2009, 24, 32–36. [Google Scholar] [CrossRef]
- Kumura, H.; Hiramatsu, Y.; Ukai, Y.; Mikawa, K.; Shimazaki, K. Expression of Human Lactoferrin in Transfected Rat Mammary Epithelial Cells. In Advances in Lactoferrin Research; Springer Nature: Boston, MA, USA, 1998; Volume 443, pp. 85–89. [Google Scholar] [CrossRef]
- Van Berkel, P.H.C.; Geerts, M.E.J.; Van Veen, H.A.; Kooiman, P.M.; Pieper, F.R.; De Boer, H.A.; Nuijens, J.H. Glycosylated and Unglycosylated Human Lactoferrins Both Bind Iron and Show Identical Affinities towards Human Lysozyme and Bacterial Lipopolysaccharide, but Differ in Their Susceptibilities towards Tryptic Proteolysis. Biochem. J. 1995, 312, 107–114. [Google Scholar] [CrossRef]
- Kim, S.-J.; Yu, D.-Y.; Lee, K.-W.; Cho, Y.-Y.; Lee, C.-S.; Han, Y.-M.; Lee, K.-K. Heterologous Introns Enhanced Expression of Human Lactoferrin CDNA in Mouse Mammary Epithelial Cells. Korean Soc. Biochem. Mol. Biol. 1995, 28, 57–61. [Google Scholar]
- Stowell, K.M.; Rado, T.A.; Funk, W.D.; Tweedie, J.W. Expression of Cloned Human Lactoferrin in Baby-Hamster Kidney Cells. Biochem. J. 1991, 276, 349–355. [Google Scholar] [CrossRef]
- Wang, M.; Sun, Z.; Yu, T.; Ding, F.; Li, L.; Wang, X.; Fu, M.; Wang, H.; Huang, J.; Li, N.; et al. Large-Scale Production of Recombinant Human Lactoferrin from High-Expression, Marker-Free Transgenic Cloned Cows. Sci. Rep. 2017, 7, 10733. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, G.G.; Mucci, N.C.; González, V.; Sánchez, L.; Parrón, J.A.; Pérez, M.D.; Calvo, M.; Aller, J.F.; Hozbor, F.A.; Mutto, A.A. Detection of Recombinant Human Lactoferrin and Lysozyme Produced in a Bitransgenic Cow. J. Dairy Sci. 2017, 100, 1605–1617. [Google Scholar] [CrossRef]
- Yang, P.; Wang, J.; Gong, G.; Sun, X.; Zhang, R.; Du, Z.; Liu, Y.; Li, R.; Ding, F.; Tang, B.; et al. Cattle Mammary Bioreactor Generated by a Novel Procedure of Transgenic Cloning for Large-Scale Production of Functional Human Lactoferrin. PLoS ONE 2008, 3, e3453. [Google Scholar] [CrossRef]
- Van Berkel, P.H.C.; Welling, M.M.; Geerts, M.; van Veen, H.A.; Ravensbergen, B.; Salaheddine, M.; Pauwels, E.K.J.; Pieper, F.; Nuijens, J.H.; Nibbering, P.H. Large Scale Production of Recombinant Human Lactoferrin in the Milk of Transgenic Cows. Nat. Biotechnol. 2002, 20, 484–487. [Google Scholar] [CrossRef]
- Krimpenfort, P.; Rademakers, A.; Eyestone, W.; van der Schans, A.; van den Broek, S.; Kooiman, P.; Kootwijk, E.; Platenburg, G.; Pieper, F.; Strijker, R. Generation of Transgenic Dairy Cattle Using “in vitro” Embryo Production. Nat. Biotechnol. 1991, 9, 844–847. [Google Scholar] [CrossRef]
- Bogdanovich, D.M.; Radchikov, V.F.; Kuznetsova, V.N.; Petrushko, E.V.; Spivak, M.E.; Sivko, A.N. Goats Producing Biosimilar Human Lactoferrin. IOP Conf. Ser. Earth Environ. Sci. 2021, 848, 012080. [Google Scholar] [CrossRef]
- Semak, I.; Budzevich, A.; Maliushkova, E.; Kuzniatsova, V.; Popkov, N.; Zalutsky, I.; Ivashkevich, O. Development of Dairy Herd of Transgenic Goats as Biofactory for Large-Scale Production of Biologically Active Recombinant Human Lactoferrin. Transgenic Res. 2019, 28, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yuan, Y.; Lu, R.; Xu, S.; Zhou, M.; Yuan, T.; Lu, Y.; Yan, K.; Cheng, Y. The Goat β-Casein/CMV Chimeric Promoter Drives the Expression of HLF in Transgenic Goats Produced by Cell Transgene Microinjection. Int. J. Mol. Med. 2019, 44, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hu, L.; Liu, J.; Chen, H.; Cui, C.; Song, Y.; Jin, Y.; Zhang, Y. Generation of β-Lactoglobulin-Modified Transgenic Goats by Homologous Recombination. FEBS J. 2016, 283, 4600–4613. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, G.M.; Wan, Y.J.; Jia, R.X.; Li, P.Z.; Han, L.; Wang, F.; Huang, M.R. Identification of Transgenic Cloned Dairy Goats Harboring Human Lactoferrin and Methylation Status of the Imprinted Gene IGF2R in Their Lungs. Genet. Mol. Res. 2015, 14, 11099–11108. [Google Scholar] [CrossRef]
- Cui, C.; Song, Y.; Liu, J.; Ge, H.; Li, Q.; Kang, Z.; Hu, L.; Zhu, H.; Jin, Y.-P.; Zhang, Y. Gene Targeting by TALEN-Induced Homologous Recombination in Goats Directs Production of β-Lactoglobulin-Free, High-Human Lactoferrin Milk. Sci. Rep. 2015, 5, 10482. [Google Scholar] [CrossRef]
- Meng, L.; Wan, Y.; Sun, Y.; Zhang, Y.; Wang, Z.; Song, Y.; Wang, F. Generation of Five Human Lactoferrin Transgenic Cloned Goats Using Fibroblast Cells and Their Methylation Status of Putative Differential Methylation Regions of IGF2R and H19 Imprinted Genes. PLoS ONE 2013, 8, e77798. [Google Scholar] [CrossRef]
- An, L.-Y.; Yuan, Y.-G.; Yu, B.-L.; Yang, T.-J.; Cheng, Y. Generation of Human Lactoferrin Transgenic Cloned Goats Using Donor Cells with Dual Markers and a Modified Selection Procedure. Theriogenology 2012, 78, 1303–1311. [Google Scholar] [CrossRef]
- Yu, H.; Chen, J.; Sun, W.; Liu, S.; Zhang, A.; Xu, X.; Wang, X.; He, Z.; Liu, G.; Cheng, G. The Dominant Expression of Functional Human Lactoferrin in Transgenic Cloned Goats Using a Hybrid Lactoferrin Expression Construct. J. Biotechnol. 2012, 161, 198–205. [Google Scholar] [CrossRef]
- Goldman, I.L.; Georgieva, S.G.; Gurskiy, Y.G.; Krasnov, A.N.; Deykin, A.V.; Popov, A.N.; Ermolkevich, T.G.; Budzevich, A.I.; Chernousov, A.D.; Sadchikova, E.R. Production of Human Lactoferrin in Animal milk. Biochem. Cell Biol. 2012, 90, 513–519. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Zhang, Y.-L.; Zhou, Z.-R.; Jia, R.-X.; Li, M.; Song, H.; Wang, Z.-Y.; Wang, L.-Z.; Zhang, G.-M.; You, J.-H.; et al. Efficiency of Donor Cell Preparation and Recipient Oocyte Source for Production of Transgenic Cloned Dairy Goats Harboring Human Lactoferrin. Theriogenology 2012, 78, 583–592. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Cai, Y.; Xu, X.; Chen, J.; Wu, Y.; Yu, H.; Yu, G.; Liu, S.; Zhang, A.; et al. Expression of Active Recombinant Human Lactoferrin in the Milk of Transgenic Goats. Protein Expr. Purif. 2008, 57, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shen, W.; Min, L.; Pan, Q.; Sun, Y.; Deng, J.; Pan, Q. Nuclear Transfer of Goat Somatic Cells Transgenic for Human Lactoferrin Gene. Front. Biol. China 2008, 3, 269–274. [Google Scholar] [CrossRef]
- Han, Z.-S.; Li, Q.-W.; Zhang, Z.-Y.; Xiao, B.; Gao, D.-W.; Wu, S.-Y.; Li, J.; Zhao, H.-W.; Jiang, Z.-L.; Hu, J.-H. High-Level Expression of Human Lactoferrin in the Milk of Goats by Using Replication-Defective Adenoviral Vectors. Protein Expr. Purif. 2007, 53, 225–231. [Google Scholar] [CrossRef]
- Han, X.; Gao, Y.; Li, G.; Xiong, Y.; Zhao, C.; Ruan, J.; Ma, Y.; Li, X.; Li, C.; Zhao, S.; et al. Enhancing the Antibacterial Activities of Sow Milk via Site-Specific Knock-in of a Lactoferrin Gene in Pigs Using CRISPR/Cas9 Technology. Cell Biosci. 2020, 10, 133. [Google Scholar] [CrossRef]
- Cui, D.; Li, J.; Zhang, L.; Liu, S.; Wen, X.; Li, Q.; Zhao, Y.; Hu, X.; Zhang, R.; Li, N. Generation of Bi-Transgenic Pigs Overexpressing Human Lactoferrin and Lysozyme in Milk. Transgenic Res. 2014, 24, 365–373. [Google Scholar] [CrossRef]
- Han, Z.-S.; Li, Q.-W.; Zhang, Z.-Y.; Yu, Y.-S.; Xiao, B.; Wu, S.-Y.; Jiang, Z.-L.; Zhao, H.-W.; Zhao, R.; Li, J. Adenoviral Vector Mediates High Expression Levels of Human Lactoferrin in the Milk of Rabbits. J. Microbiol. Biotechnol. 2008, 18, 153–159. [Google Scholar]
- Li, L.; Shen, W.; Min, L.; Dong, H.; Sun, Y.; Pan, Q. Human Lactoferrin Transgenic Rabbits Produced Efficiently Using Dimethylsulfoxide—Sperm-Mediated Gene Transfer. Reprod. Fertil. Dev. 2006, 18, 689–695. [Google Scholar] [CrossRef]
- Schmidhauser, C.; Bissell, M.J.; Myers, C.A.; Casperson, G.F. Extracellular Matrix and Hormones Transcriptionally Regulate Bovine Beta-Casein 5’ Sequences in Stably Transfected Mouse Mammary Cells. Proc. Natl. Acad. Sci. USA 1990, 87, 9118–9122. [Google Scholar] [CrossRef]
- Bühler, T.A.; Bruyère, T.; Went, D.F.; Stranzinger, G.; Bürki, K. Rabbit β-Casein Promoter Directs Secretion of Human Interleukin-2 into the Milk of Transgenic Rabbits. Nat. Biotechnol. 1990, 8, 140–143. [Google Scholar] [CrossRef]
- Cheng, Y.; An, L.; Yuan, Y.; Wang, Y.; Du, F.; Yu, B.; Zhang, Z.; Huang, Y.; Yang, T. Hybrid Expression Cassettes Consisting of a Milk Protein Promoter and a Cytomegalovirus Enhancer Significantly Increase Mammary-Specific Expression of Human Lactoferrin in Transgenic Mice. Mol. Reprod. Dev. 2012, 79, 573–585. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Lu, D.; Shang, S.; Wang, M.; Zheng, M.; Zhang, R.; Tang, B.; Li, Q.; Dai, Y.; et al. High-Level Expression of Bioactive Recombinant Human Lysozyme in the Milk of Transgenic Mice Using a Modified Human Lactoferrin BAC. Transgenic Res. 2012, 21, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.; Lin, C.; Chong, K.; Tsai, T.; Shen, C.; Lin, M.; Su, C.; Chen, H.; Chen, C. Lactoferrin as a Natural Regimen for Selective Decontamination of the Digestive Tract: Recombinant Porcine Lactoferrin Expressed in the Milk of Transgenic Mice Protects Neonates from Pathogenic Challenge in the Gastrointestinal Tract. J. Infect. Dis. 2009, 199, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Chen, H.; Wu, X.; Zhou, Y.; Liu, Z.; Zheng, T.; Huang, P. A MWAP–HLF Hybrid Gene Locus Gave Extremely High-Level Expression of Human Lactoferrin in the Milk of Transgenic Mice. Transgenic Res. 2009, 18, 573–582. [Google Scholar] [CrossRef]
- Deykin, A.V.; Ermolkevich, T.G.; Gursky, Y.G.; Krasnov, A.N.; Georgieva, S.G.; Kuznetsov, S.L.; Derevyanko, V.G.; Novikova, N.I.; Murashev, A.N.; Goldman, I.L.; et al. The State of Health and the Reproductive Potential of Transgenic Mice Secreting Recombinant Human Lactoferrin in Milk. Dokl. Biochem. Biophys. 2009, 427, 195–198. [Google Scholar] [CrossRef]
- Wu, S.-C.; Chen, H.-L.; Yen, C.-C.; Kuo, M.-F.; Yang, T.-S.; Wang, S.-R.; Weng, C.-N.; Chen, C.-M.; Cheng, W.T. Recombinant Porcine Lactoferrin Expressed in the Milk of Transgenic Mice Enhances Offspring Growth Performance. J. Agric. Food Chem. 2007, 55, 4670–4677. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Pulina, M.O.; Kristiyan, A.V.; Zakharova, E.T.; Runova, O.L.; Vasil’ev, V.B.; Gurskii, Y.G.; Minashkin, M.M.; Krasnov, A.N.; Kadulin, S.G.; et al. A Study of Recombinant Human Lactoferrin Secreted in Milk of Transgenic Mice. Dokl. Biochem. Biophys. 2006, 411, 336–338. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, C.; Fan, B.; Dai, Y.; Zhao, Z.; Wang, L.; Zheng, M.; Feng, J.; Chen, Y.; Duan, Y.; et al. Variable Expression of Human Lactoferrin Gene in Mice Milk Driven by Its 90 KB Upstream Flanking Sequences. Anim. Biotechnol. 2004, 15, 21–31. [Google Scholar] [CrossRef]
- Kim, S.J.; Sohn, B.H.; Jeong, S.; Pak, K.-W.; Park, J.-S.; Park, I.-Y.; Lee, T.-H.; Choi, Y.-H.; Lee, C.-S.; Han, Y.-M.; et al. High-Level Expression of Human Lactoferrin in Milk of Transgenic Mice Using Genomic Lactoferrin Sequence. J. Biochem. 1999, 126, 320–325. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, K.-W.; Yu, D.-Y.; Han, Y.-M.; Lee, C.-S.; Nam, M.-S.; Moon, H.-B.; Lee, K.-K. Expression Analysis of a Bovine β-Casein/Human Lactoferrin Hybrid Gene in Transgenic Mice. J. Reprod. Dev. 1997, 43, 143–149. [Google Scholar] [CrossRef][Green Version]
- Nuijens, J.H.; van Berkel, P.H.; Geerts, M.E.; Hartevelt, P.P.; de Boer, H.A.; van Veen, H.A.; Pieper, F.R. Characterization of Recombinant Human Lactoferrin Secreted in Milk of Transgenic Mice. J. Biol. Chem. 1997, 272, 8802–8807. [Google Scholar] [CrossRef]
- Platenburg, G.J.; Kootwijk, E.P.A.; Kooiman, P.M.; Woloshuk, S.L.; Nuijens, J.H.; Krimpenfort, P.J.A.; Pieper, F.R.; de Boer, H.A.; Strijker, R. Expression of Human Lactoferrin in Milk of Transgenic Mice. Transgenic Res. 1994, 3, 99–108. [Google Scholar] [CrossRef]
- Kim, S.J.; Cho, Y.-Y.; Lee, K.-W.; Yu, D.-Y.; Lee, C.-S.; Han, Y.-M.; Lee, K.-K. Expression of Human Lactoferrin in Milk of Transgenic Mice Using Bovine β-Casein/Human Lactoferrin cDNA Fusion Gene. Mol. Cells 1994, 4, 355–360. [Google Scholar] [CrossRef]
- Tutykhina, I.L.; Oa, B.; Shmarov, M.M.; Logunov, D.Y.; Neugodova, G.L.; Nemtsova, E.R.; Naroditsky, B.S.; Yakubovskaya, R.I.; Gintsburg, A.L. Production of Recombinant Human Lactoferrin in the Allantoic Fluid of Embryonated Chicken Eggs and Its Characteristics. Protein Expr. Purif. 2009, 65, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Yang, P.H.; Kao, C.L.; Huang, H.I.; Tsai, H.J. Transgenic Zebrafish Eggs Containing Bactericidal Peptide Is a Novel Food Supplement Enhancing Resistance to Pathogenic Infection of Fish. Fish Shellfish Immunol. 2010, 28, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Shi, S.; Chou, Y.; He, G.; Oh, J.; Oh, Y. A Transgenic Zebrafish for Mass Production of Human Lactoferrin and a Process of Producing Human Lactoferrin Using the Same. Available online: https://patents.google.com/patent/WO2010005273A2/en?oq=WO2010005273A2/ (accessed on 26 December 2024).
- Zhong, J.; Wang, Y.; Zhu, Z. Introduction of the Human Lactoferrin Gene into Grass Carp (Ctenopharyngodon idellus) to Increase Resistance against GCH Virus. Aquaculture 2002, 214, 93–101. [Google Scholar] [CrossRef]
- Lee, K.-C.; Hsieh, K.-T.; Chen, R.-B.; Lin, W.-C.; Wang, C.-S.; Lee, T.-T.; Chen, L.-J. Expression and Characterization of Rice-Produced Recombinant Porcine Lactoferrin and Its Antioxidant Activities. Open Biotechnol. J. 2020, 14, 94–106. [Google Scholar] [CrossRef]
- Funakoshi, T.; Hosaka, T.; Inoue, E.; Anzai, H. Gene Expression of Lactoferrin-Derived Antimicrobial Peptides in Rice. Plant Physiol. Biochem. 2017, 123, 112–113. [Google Scholar]
- Lee, T.-T.; Chang, C.-C.; Juang, R.-S.; Chen, R.-B.; Yang, H.-Y.; Chu, L.-W.; Wang, S.-R.; Tseng, T.-H.; Wang, C.-S.; Chen, L.-J.; et al. Porcine Lactoferrin Expression in Transgenic Rice and Its Effects as a Feed Additive on Early Weaned Piglets. J. Agric. Food Chem. 2010, 58, 5166–5173. [Google Scholar] [CrossRef]
- Lin, C.; Nie, P.; Lu, W.; Zhang, Q.; Li, J.; Shen, Z. A Selectively Terminable Transgenic Rice Line Expressing Human Lactoferrin. Protein Expr. Purif. 2010, 74, 60–64. [Google Scholar] [CrossRef]
- Rachmawati, D.; Mori, T.; Hosaka, T.; Takaiwa, F.; Inoue, E.; Anzai, H. Expression and Localization of Recombinant Human Lactoferrin in Transgenic Javanica Rice Cv. Rojolele. Milk Sci. 2004, 53, 247–249. [Google Scholar]
- Takase, K.; Hagiwara, K.; Onodera, H.; Nishizawa, Y.; Ugaki, M.; Omura, T.; Numata, S.; Akutsu, K.; Kumura, H.; Shimazaki, K. Constitutive Expression of Human Lactoferrin and Its N-Lobe in Rice Plants to Confer Disease Resistance. Biochem. Cell Biol. 2005, 83, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Kelleher, S.L.; Yalda, D.; Wu, L.; Huang, J.; Huang, N.; Lönnerdal, B. Expression, Characterization, and Biologic Activity of Recombinant Human Lactoferrin in Rice. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Suzuki, Y.A.; Huang, J.; Yalda, D.; Pham, P.; Wu, L.; Bartley, G.; Huang, N.; Lönnerdal, B. Expression of Human Lactoferrin in Transgenic Rice Grains for the Application in Infant Formula. Plant Sci. 2002, 163, 713–722. [Google Scholar] [CrossRef]
- Sohrabi, S.M.; Niazi, A.; Chahardooli, M.; Aram, F. Isolation and Expression of Antimicrobial Camel Lactoferrin (“CLf”) Gene in Tobacco. Plant Omics 2014, 7, 298–307. [Google Scholar]
- Nguyen, T.C.; Lakshman, D.K.; Han, J.; Galvez, L.C.; Mitra, A. Transgenic Plants Expressing Antimicrobial Lactoferrin Protein Are Resistant to a Fungal Pathogen. J. Plant Mol. Biol. Biotechnol. 2011, 2, 1–8. [Google Scholar]
- Li, Y.; Geng, Y.; Song, H.; Zheng, G.; Huan, L.; Qiu, B. Expression of a Human Lactoferrin N-Lobe in Nicotiana Benthamiana with Potato Virus X-Based Agroinfection. Biotechnol. Lett. 2004, 26, 953–957. [Google Scholar] [CrossRef]
- Choi, S.-M.; Lee, O.-S.; Kwon, S.-Y.; Kwak, S.-S.; Yu, D.-Y.; Lee, H.-S. High Expression of a Human Lactoferrin in Transgenic Tobacco Cell Cultures. Biotechnol. Lett. 2003, 25, 213–218. [Google Scholar] [CrossRef]
- Spik, G.; Theisen, M. Characterization of the Post-Translational Biochemical Processing of Human Lactoferrin Expressed in Transgenic Tobacco. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2000, 43, 104–109. [Google Scholar] [CrossRef]
- Zhang, Z.; Coyne, D.P.; Vidaver, A.K.; Mitra, A. Expression of Human Lactoferrin CDNA Confers Resistance to Ralstonia solanacearum in Transgenic Tobacco Plants. Phytopathology 1998, 88, 730–734. [Google Scholar] [CrossRef]
- Salmon, V.; Legrand, D.; Slomianny, M.-C.; Yazidi, I.E.; Spik, G.; Gruber, V.; Bournat, P.; Olagnier, B.; Mison, D.; Theisen, M.; et al. Production of Human Lactoferrin in Transgenic Tobacco Plants. Protein Expr. Purif. 1998, 13, 127–135. [Google Scholar] [CrossRef]
- Mitra, A.; Zhang, Z. Expression of a Human Lactoferrin CDNA in Tobacco Cells Produces Antibacterial Protein(S). Plant Physiol. 1994, 106, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Buziashvili, A.; Cherednichenko, L.; Kropyvko, S.; Blume, Y.B.; Yemets, A. Obtaining Transgenic Potato Plants Expressing the Human Lactoferrin Gene and Analysis of Their Resistance to Phytopathogens. Cytol. Genet. 2020, 54, 179–188. [Google Scholar] [CrossRef]
- Chong, D.K.X.; Langridge, W.H.R. Expression of Full-Length Bioactive Antimicrobial Human Lactoferrin in Potato Plants. Transgenic Res. 2000, 9, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Buziashvili, A.; Cherednichenko, L.; Kropyvko, S.; Yemets, A. Transgenic Tomato Lines Expressing Human Lactoferrin Show Increased Resistance to Bacterial and Fungal Pathogens. Biocatal. Agric. Biotechnol. 2020, 25, 101602. [Google Scholar] [CrossRef]
- Lee, T.-J.; Coyne, D.P.; Clemente, T.E.; Mitra, A. Partial Resistance to Bacterial Wilt in Transgenic Tomato Plants Expressing Antibacterial Lactoferrin Gene. J. Am. Soc. Hortic. Sci. 2002, 127, 158–164. [Google Scholar] [CrossRef]
- Malnoy, M.; Venisse, J.-S.; Brisset, M.-N.; Chevreau, E. Expression of Bovine Lactoferrin CDNA Confers Resistance to Erwinia Amylovora in Transgenic Pear. Mol. Breed. 2003, 12, 231–244. [Google Scholar] [CrossRef]
- Jo, S.-H.; Kwon, S.-Y.; Park, D.-S.; Yang, K.-S.; Kim, J.-W.; Lee, K.-T.; Kwak, S.-S.; Lee, H.-S. High-Yield Production of Functional Human Lactoferrin in Transgenic Cell Cultures of Siberian Ginseng (Acanthopanax senticosus). Biotechnol. Bioprocess Eng. 2006, 11, 442–448. [Google Scholar] [CrossRef]
- Kwon, S.-Y.; Jo, S.-H.; Lee, O.-S.; Choi, S.-M.; Kwak, S.-S.; Lee, H.-S. Transgenic Ginseng Cell Lines That Produce High Levels of a Human Lactoferrin. Planta Medica 2003, 69, 1005–1008. [Google Scholar] [CrossRef]
- Han, J.; Lakshman, D.K.; Galvez, L.C.; Mitra, S.; Baenziger, P.S.; Mitra, A. Transgenic Expression of Lactoferrin Imparts Enhanced Resistance to Head Blight of Wheat Caused by Fusarium Graminearum. BMC Plant Biol. 2012, 12, 33. [Google Scholar] [CrossRef]
- Min, S.R.; Woo, J.W.; Jeong, W.J.; Han, S.K.; Lee, Y.B.; Liu, J.R. Production of Human Lactoferrin in Transgenic Cell Suspension Cultures of Sweet Potato. Biol. Plant. 2005, 50, 131–134. [Google Scholar] [CrossRef]
- Tanasienko, I.V.; Yemets, A.I.; Pirko, Y.V.; Korhkovyy, V.I.; Abumhadi, N.; Blume, Y.B. Generation of Transgenic Barley Lines Producing Human Lactoferrin Using Mutant Alpha-Tubulin Gene as the Selective Marker. Cytol. Genet. 2011, 45, 1–6. [Google Scholar] [CrossRef]
- Kamenarova, K.; Gecheff, K.; Stoyanova, M.; Muhovski, Y.; Anzai, H.; Atanassov, A. Production of Recombinant Human Lactoferin in Transgenic Barley. Biotechnol. Biotechnol. Equip. 2007, 21, 18–27. [Google Scholar] [CrossRef]
- Stefanova, G.; Slavov, S.; Gecheff, K.; Vlahova, M.; Atanassov, A. Expression of Recombinant Human Lactoferrin in Transgenic Alfalfa Plants. Biol. Plant. 2013, 57, 457–464. [Google Scholar] [CrossRef]
- Pang, X.; Tong, Y.; Xue, W.; Yang, Y.; Chen, X.; Liu, J.; Chen, D. Expression and Characterization of Recombinant Human Lactoferrin in Edible Alga Chlamydomonas reinhardtii. Biosci. Biotechnol. Biochem. 2019, 83, 851–859. [Google Scholar] [CrossRef]
- Koo, J.; Park, D.; Kim, H. Expression of Bovine Lactoferrin N-Lobe by the Green Alga, Chlorella Vulgaris. ALGAE 2013, 28, 379–387. [Google Scholar] [CrossRef]
- Zhao, X.; Kruzel, M.; Ting, S.-M.; Sun, G.; Savitz, S.I.; Aronowski, J. Optimized Lactoferrin as a Highly Promising Treatment for Intracerebral Hemorrhage: Pre-Clinical Experience. J. Cereb. Blood Flow Metab. 2020, 41, 53–66. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, G.; Ahmad, T.; Kaur, B.; Hakeem, K.R. Tailoring Cellular Metabolism in Lactic Acid Bacteria through Metabolic Engineering. J. Microbiol. Methods 2020, 170, 105862. [Google Scholar] [CrossRef]
- Alias, N.A.R.; Song, A.A.L.; Alitheen, N.B.; Rahim, R.A.; Othman, S.S.; In, L.L.A. Optimization of Signal Peptide via Site-Directed mutagenesis for enhanced secretion of Heterologous Proteins in Lactococcus lactis. Int. J. Mol. Sci. 2022, 17, 10044. [Google Scholar] [CrossRef]
- Estabragh, A.M.; Sadeghi, H.M.M.; Akbari, V. Co-expression of chaperones for improvement of soluble expression and purification of ananti-her2 scfv in Escherichia coli. Adv. Biomed. Res. 2022, 11, 117. [Google Scholar] [CrossRef]
- Shakiba, N.; Jones, R.D.; Weiss, R.; Del Vecchio, D. Context-aware synthetic biology by controller design: Engineering the mammalian cell. Cell Syst. 2021, 12, 561–592. [Google Scholar] [CrossRef]
- Utsugi, Y.; Miyamae, Y. Strategies for post-translational control of protein expression and their applications. Appl. Sci. 2021, 11, 8300. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Huang, Z.; Zhai, Y.; Li, H.; Wu, J. Lactoferrin Alleviates Lipopolysaccharide-Induced Infantile Intestinal Immune Barrier Damage by Regulating an ELAVL1-Related Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 13719. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Yamauchi, K.; Takase, M. Lactoferrin Research, Technology and Applications. Int. Dairy J. 2006, 16, 1241–1251. [Google Scholar] [CrossRef]
- Bak, S.; Olsen, C.E.; Halkier, B.A.; Møller, B.L. Transgenic tobacco and Arabidopsis plants expressing the two multifunctional sorghum cytochrome P450 enzymes, CYP79A1 and CYP71E1, are cyanogenic and accumulate metabolites derived from intermediates in dhurrin biosynthesis. Plant Physiol. 2000, 123, 1437–1448. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, Z.; Wang, X.; An, Q.; Huang, K.; Dai, Y.; Meng, Q.; Zhang, Y. Lactoferrin Deficiency during Lactation Increases the Risk of Depressive-like Behavior in Adult Mice. BMC Biol. 2023, 21, 242. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, C.; Chen, Q.; Zhou, Z.; Zhou, J. Rational modification of the iron ion-binding site to improve the expression of human lactoferrin in Pichia pastoris and validation of the mutant protein function. Food Biosci. 2025, 64, 105984. [Google Scholar] [CrossRef]
- Glinšek, K.; Bozovičar, K.; Bratkovič, T. CRISPR technologies in chinese hamster ovary cell line engineering. Int. J. Mol. Sci. 2023, 24, 8144. [Google Scholar] [CrossRef]
- Byrne, G.; O’Rourke, S.M.; Alexander, D.L.; Yu, B.; Doran, R.C.; Wright, M.; Chen, Q.; Azadi, P.; Berman, P.W. CRISPR/Cas9 gene editing for the creation of an MGAT1-deficient CHO cell line to control HIV-1 vaccine glycosylation. PLoS Biol. 2018, 16, e2005817. [Google Scholar] [CrossRef]
- Wayman, J.A.; Glasscock, C.; Mansell, T.J.; DeLisa, M.P.; Varner, J.D. Improving designer glycan production in Escherichia coli through model-guided metabolic engineering. Metab. Eng. Commun. 2019, 9, e00088. [Google Scholar] [CrossRef]
- Mansour, A.; Kipper, K.; Pulk, A. Optimizing Human Cell-Free System for Efficient Protein Production. J. Microbiol. Biotechnol. 2025, 35, e2410026. [Google Scholar] [CrossRef]
| Trade Name | Lactoferrin Type (LF) | Manufacturing Firm, Country |
|---|---|---|
| SureStart™ Lactoferrin 7200 | Bovine lactoferrin (bLF) | New Zealand Milk Products (NZMP), Auckland, New Zealand |
| Effera™ | Human lactoferrin (hLF) | Helaina Inc., New York, NY, USA |
| Vitalarmor® Lactoferrin | bLF | Armor Protéines, Saint-Herblain, Loire-Atlantique, France |
| Bioferrin® | bLF | Glanbia Nutritionals, Kilkenny, Ireland |
| Vivinal® Lactoferrin | bLF | Royal Friesland Campina, Amersfoort, Utrecht, The Netherlands |
| Synlait lactoferrin | bLF | Synlait Milk Ltd., Dunsandel, Canterbury, New Zealand |
| Inferrin® | bLF | Bega Cheese Limited, Bega, NSW, Australia |
| PUREnFERRIN™ | bLF | Freedom food group, Stanbridge, NSW, Australia |
| Proferrin® | bLF | Ingredia SA, Arras, Hauts-de-France, France |
| LF+ | bLF | Turtle tree, Singapore |
| Valpalf® | bLF | Pharmaguida, Milan, Lombardy, Italy |
| Species (Strain) | Vector/Promoter | Type of Lactoferrin | Yield/Culture | References |
|---|---|---|---|---|
| Escherichia coli BL21(DE3) | pQE30 | Buffalo LF N-lobe | 01 mg/mL | [36] |
| pET28a+ | hLF | 2.9 mg/mL | [37] | |
| pGEX-4T-2 | Hybrid peptide LF15-CA8 Bovine lactoferricin (LfcinB)-Cecropin A(CA8) | 5.1–10 mg/L | [38] | |
| pET32a | bLF | 15.3 mg/L | [39] | |
| pET28a | Mouse lactoferrin (mLF) (full length) | 17 mg/L | [40] | |
| pET32a | bLF oligomeric peptide-(LfcinB15-W4,10) | 74 mg/L | [41] | |
| pET21d | LfcinB fusion peptide | 60 mg/L | [42] | |
| pGEX-4T-2 | LfcinB | 02 mg/L | [43] | |
| E. coli C43(DE3) | pET32a | Fusion peptide SrtA-LfcinB Staphylococcus aureus sortase A (SrtA)-N-terminus of LfcinB | LfcinB-1.32 ± 0.07 mg/500 mL | [44] |
| pET21b | pET21b-(fLfcinB-bmIFc2)2 co-expressionbmIFc2 | 4.1 ± 01 mg/500 mL | ||
| Rhodococcus erythropolis | pTipLCH1.2 | bLF C-lobe | 3.6 mg/mL | [45] |
| Bacillus subtilis P245 and P263 | ptrnQ promoter | Six tandem repeats of Lfcin | Antimicrobial activity | [46] |
| Bacillus subtilis | pMA0911/ Pveg promoter | bLF C-lobe | 7.5 mg/L | [47] |
| pMA0911/ Pveg promoter | bLF N-lobe | 29.6 mg/L | [48] | |
| Lactococcus lactis (P170) | pAMJ1653 expression vector | Camel lactoferrin (cLF) chimeric peptide (lactoferrampin–lactoferricin) | 0.13 mg/mL | [49] |
| Lactobacillus plantarum | pPG-pLF | Porcine lactoferrin (pLF) | 8.8 mg/L (36 h) | [50] |
| L. plantarum | pPG612.1 expression vector | pLF | 27.2 µg/mL | [51] |
| Lactobacillus casei | 20.5 µg/mL | |||
| Lactobacillus paracasei | 21.6 µg/mL | |||
| Lactobacillus pentosus | 21.6 µg/mL | |||
| L. casei | pSD | hLF | 10.6 mg/mL | [52] |
| Species (Strain) | Vector/Promoter | Type of Lactoferrin | Yield/Culture | References |
|---|---|---|---|---|
| 1. Pichia pastoris (Komagataella phaffii) | ||||
| P. pastoris (GS115) | pPICZαC/G1 (PG1) | pLF | 2.8 g/L | [54] |
| P. pastoris (X-33) | X33-pPICZɑA-PAOX1- Lfcin | LfcinB | 19.3 mg/L | [55] |
| X33-pPICZɑA-PAOX1-0030-α * Lfcin | 28.8 mg/L | |||
| X33-pPICZɑA-PAOX1-0030-α-PEP1/PEP2 # Lfcin | 150–193 mg/L | |||
| Komagataella phaffii (X33) | - | hLF | 137.6–304.6 mg/L | [56] |
| P. pastoris (GS115) | pPIC9K/AOX1 | bLF | 824.93 mg/L | [57] |
| Komagataella phaffii (GS115) | - | hLF (Effera™) | >98% purity | [58] |
| P. pastoris (GS115) | pPIC9K/AOX1 | bLF N-lobe | 50.5 mg/L | [59] |
| pPIC9K/AOX1 | bLF peptide (LfcinB) | Antimicrobial property | [60] | |
| P. pastoris (KM71-H) | pJ902/AOX1 | bLF | 3.5 g/L | [61] |
| P. pastoris (KM71) | PPICZαA/AOX1 | Camel lactoferricin (LfcinC) | Antimicrobial property | [62] |
| P. pastoris (PichiaPinkTM) | pPINKα-HC/AOX1 | Arabian LfcinC | Antimicrobial property | [63] |
| P. Pastoris (SMD1168) | pPIC9K/AOX1 | hLF- N lobe | 458 μg/mL | [64] |
| P. pastoris (GS115) | pPICZaA/AOX1 | Ovine LF | >60 mg/L | [65] |
| P. pastoris (X-33) | pPICZA/AOX1 | Lfcin tri-hybrid peptide (LHP7) | 0.906 g/L | [66] |
| P. pastoris (KM71) | pPICZαA/AOX1 | (Fusion peptide) LFA-LFC | 0.27 ± 0.12 mg/L | [67] |
| P. pastoris (SMD1168) | pPIC9K/AOX1 | hLF | Antimicrobial property | [68] |
| P. pastoris (X-33) | pMD18-T/AOX1 | bLF (full length) | 88 mg/L | [69] |
| bLFA: N-lobe + inter lobe region | 485 mg/L | |||
| P. pastoris (KM71) | pPIC9K/AOX1 | hLF | 1200 mg/L | [70] |
| P. pastoris (yAS309) | pPICZA/GAPDH | hLF | 99.8 mg/L | [71] |
| P. pastoris (X-33) | pGAPZαC/GAP | Goat lactoferrin (gLF) | 2.0 mg/L | [72] |
| P. pastoris (JM109) | pPICZαC/AOX1 | Chinese Yak lactoferrin | 40 mg/L | [73] |
| P. pastoris (GS115) | pPIC9/AOX1 | pLF | 0.1 mg/mL | [74] |
| P. pastoris (GS115) | pPICZαC/AOX1 | pLF (cytoplasm) | 760 mg/L | [75] |
| P. pastoris (KM71) | pPIC 3.5 K/AOX1 | hLF | 115 mg/L | [76] |
| P. pastoris (SMD 1168) | pGAPZa B/GAPDH | pLF | 12 mg/L | [77] |
| P. pastoris (GS115) | pPIC9K/AOX1 | Equine LF | 40 mg/L | [78] |
| 2.Pichia methanolica | ||||
| P. methanolica (PMAD11) | pGEM-3Z/AUG1 | pLF N-lobe | Antimicrobial property | [79] |
| P. methanolica (pMAD16) | pMETα A/AUG1 | LfcinB | 90 mg/L | [80] |
| 3.Saccharomyces cerevisiae (Baker’s yeast) | ||||
| S. cerevisiae (BY4741) | pGAL-MF/GAL (Galactose-inducible) | hLF | 18.6 mg/L | [81] |
| S. cerevisiae (AB116) | pRL1 vector | hLF | 1.5–2 mg/L | [82] |
| Species (Strain) | Vector/Promoter | Type of Lactoferrin | Yield/Culture | References |
|---|---|---|---|---|
| Aspergillus oryzae | pAhLFG/α-amylase promoter | hLF | 25 mg/L | [18] |
| Aspergillus nidulans | pGEM4/alcohol dehydrogenase (alcA) | |||
| Aspergillus awamori | pPLF-19/gluco amylase (GAP) promoter | hLF | 2 g/L | [85] |
| A. awamori | p26mLF/SP6 promoter | Murine LF | 12 mg/L | [87] |
| A. awamori | pPLF-19/gluco amylase (GAP) promoter | hLF | 2 g/L | [86] |
| A. oryzae | pAhLFG/α-amylase promoter | hLF | 25 mg/L | [84] |
| A. nidulans | pGEM4/alcohol dehydrogenase (alcA) | hLF | 5 μg/mL | [88] |
| Species (Strain) | Vector/Promoter | Type of Lactoferrin | Yield/Culture | References |
|---|---|---|---|---|
| Silkworm strain 34 (Bombyx mori) | piggyBac-based transgenic vector (phSrhLFSer1)/Sericin 1 promoter | hLF expressed in silk glands | 12.07 mg/g hLF cocoon shell weight | [89] |
| Silkworm ovary cell line (B. mori) | Recombinant virus generated by co-transfecting pBacPAK-hLf, BacPAK6 vectors to cells. | hLF | 13.5 μg/1–2 × 105 cells | [90] |
| B. mori | pBlueBacHisc and HyNPVbaculovirus DNA was co-transfected into Sf-9 cells | pLF | 205 μg of rPLF/pupae | [91] |
| B. mori | B. mori NPB expression system | hLF | 65 μg hLF/mL hemolymph | [92] |
| Spodoptera frugiperda (Sf9) cells | VL1392 vector and NPB * expression system | bLF (N lobe) | 10 mg bLF N lobe/mL culture | [93] |
| p8hLFc vector and NPB * expression system | hLF | 9.5 mg/L | [94] | |
| VL1392 vector and NPB * expression system | hLF | 10–15 mg/L | [95] |
| Cell Culture Type | Vector/Promoter | Type of Lactoferrin | Yield/Culture | References |
|---|---|---|---|---|
| Bovine Mammary Epithelial Cells (BMEC) | PiggyBac transposon + Cre/loxP system/bovine β-casein promoter | hLF | Expressed in culture supernatant | [96] |
| Human Urine-Derived Stem Cells (HUDSC) | piggyBac transposon | hLF | Higher levels of lactoferrin found | [97] |
| Bovine Mammary Epithelial Stem Cells (BMESC) | PiggyBac vector | bLF | 06 µg/mL | [98] |
| Goat Fetal Fibroblast Cells (GFFC) | pBLC-TK vector-TALEN-mediated knock in | hLF | (Targeted mutagenesis) | [99] |
| Chinese Hamster Ovary Cells (CHO) | pTT5 vector | hLF | >200 mg/L | [100] |
| Goat Mammary Gland Epithelial Cells (GMGEC) | pBC1-hLF-Neo/goat beta-casein gene promoter | hLF | LF expressed in cell culture medium | [101] |
| HEK293 * | pShuttle-CMV Vector pMD | hLF | 1135.8 ± 534.3 µg/mL | [102] |
| BMEC | 18-T, pEGFP-C1/CMV promoter | [103] | ||
| Rat Mammary Epithelial Cells (RMEC) | pBL1vector | hLF | hLF detected in culture supernatant | [104] |
| HEK293 | pαS1/bovine αS1 casein promoter | hLF | 0.6 µg/mL | [105] |
| Mouse Mammary Epithelium Cells (HC11) | pBL1vector | hLF | 150–200 µg/mL | [106] |
| BHK Cell culture | pNUT/Metallothionein promoter | hLF | 20 mg/L | [107] |
| Vector/Promoter | Lactoferrin | Transgenesis | Expression Level/Site | References |
|---|---|---|---|---|
| 1. Cattle | ||||
| BAC * | hLF | SCNT | 4.5–13.6 g/L (milk) | [108] |
| pIRES2-EGFP/goat β-casein | hLF | SCNT | 0.0098–0.011 mg/mL (milk) | [109] |
| BAC vector/b-casein | hLF | Microinjection | 2.5–3.4 g/L (milk) | [110] |
| Bovine αS1 casein | hLF | Microinjection | 1.5–2.0 g/L (milk) | [111] |
| Bovine αS1 casein | hLF | Microinjection | Successfully expressed | [112] |
| 2. Goat | ||||
| pBC1/goat β-casein promoter | hLF | Microinjection | 2.60 g/L (milk) | [113] |
| 16 g/L (milk) | [114] | |||
| pCL25/goat β-casein-CMV chimeric promoter | hLF | Microinjection | Avg concentration of 3.89 ± 0.82 mg/mL (milk) | [115] |
| pBLC-TK vector/TALEN-mediated biallelic knock-in | hLF | Electroporation | (Targeted mutagenesis) | [99] |
| pIRES2-EGFP/goat β-casein promoter | hLF | Microinjection | 1.6 g/L in milk | [116] |
| pBC1/goat β-casein promoter | hLF | SCNT | Transgenic kids generated | [117] |
| TALEN mediated knock-in of construct (phosphoglycerol kinase (PGK) promoter-hLF-Neo) | hLF | SCNT | 2.3–2.4 mg/mL (milk) | [118] |
| pBC1/goat β-casein | hLF | SCNT | Transgenic kids generated | [119] |
| pBLC14/bovine alpha1-casein | hLF | SCNT | 2.1 g/L (milk) | [120] |
| pBC1/goat β-casein promoter | hLF | Microinjection | 30 g/L (milk) | [121] |
| 10 g/L (milk) | [122] | |||
| SCNT and liposome transfection | Transgenic kids generated | [123] | ||
| Microinjection | 0.765 mg/mL (milk) | [124] | ||
| Lipofection | LF expressed in cell culture | [101] | ||
| pGBC2LF/goat β-casein gene promoter | hLF | SCNT | Cloned embryos developed to blastocyst stage | [125] |
| Adenovirus expression vector (pAd)/CMV promoter | hLF | Adenovirus mediated transduction | 2 g/L (milk) | [126] |
| pBHC (a bacterial artificial chromosome) /Bovine β-casein promoter | hLF | TALEN-induced homologous recombination | 1.3 g/L (milk) | [118] |
| pBC1 vector/β-casein promoter | hLF | SCNT | 30 mg/mL (milk) | [121] |
| Vector/Promoter | Lactoferrin | Transgenesis | Expression Level | References |
|---|---|---|---|---|
| 1. Swine | ||||
| CRISPR/Cas9-based site-specific knock-in of LF gene in CSN1S1 locus | pLF | SCNT | Sustainable LF production | [127] |
| pBAC/bovine β-casein promoter (Bi-transgenic swine) | hLF | SCNT | 6.5 g/L (milk) | [128] |
| 2. Rabbit | ||||
| pShuttle-Cytomegalovirus (CMV) vector/CMV promoter (adenovirus-mediated gene transfer into mammary gland) | hLF | Virus-mediated transduction | 2.3 mg/mL (milk) | [129] |
| pEGFP-N1/eCMV promoter | hLF | SMGT * | 103 ± 20 µg/L | [130] |
| pCMV/CMV promoter | hLF | Microinjection | 2.3 mg/mL (milk) | [131] |
| pRB1/rabbit β-casein promoter | hLF | Microinjection | 0.2 mg/mL (milk) | [132] |
| 3. Mice | ||||
| pBC1/goat β-casein promoter | hLF | Microinjection | 15.3–160 g/L (milk) | [122] |
| Hybrid multiplex promoter/ CMV enhancer | hLF | Microinjection | 1.17–8.10 mg/mL (milk) | [133] |
| hLF | Microinjection | 7–40 ng/mL (milk) | ||
| BAC vector | hLF | SCNT | 0.21–1.76 g/L | [134] |
| pBC1/β -casein gene promoter | hLF | Lipofection | Cell culture medium | [101] |
| T-protruding pCR3 vector/ bovine α-lactoalbumin promoter | pLF | Microinjection | 120 × 13.6 mg/L | [135] |
| pGEM-3Zf (+)/Bovine β-casein promoter (three-step “gap-repair” strategy) | hLF | Microinjection | 16.7 to 29.8 g/L | [136] |
| pBC1 vector/β-casein promoter | hLF | Microinjection | 0.22–40 g/L (milk) | [137] |
| T-protruding pCR3 vector/bovine R-lactalbumin (RLA) promoter | pLF | Microinjection | 10 to 106 µg/mL (milk) | [138] |
| pBC1 vector | hLF | Microinjection | 30 mg/mL (milk) | [139] |
| BAC vector | hLF | Microinjection | 1–8.02 mg/mL (milk) | [140] |
| pWE cosmid/Bovine β-casein promoter | hLF | Microinjection | 1–200 μg/mL | [141] |
| pBL1vector/chimeric promoter * | hLF | Microinjection | >1 to 200 μg/mL | [142] |
| pBLC/bovine alpha S1-casein | hLF | Microinjection | 0.1 to 36 µg/L (milk) | [143,144] |
| pBL1vector/chimeric promoter * | hLF | Microinjection | 150–200 µg/mL | [145] |
| 4. Chicken | ||||
| pBluescript II KS (+) vector/ human cytomegalovirus (CMV) promoter | hLF | Chicken Embryo Lethal Orphan(CELO) adenovirus | 0.1 to 0.3 mg/mL (culture medium) | [146] |
| 5. Fish | ||||
| Danio rerio (Zebra Fish) | ||||
| pZBGFP/beta-actin promoter | LfcinB | Microinjection | Expressed protein | [147] |
| pCS2 (+) vector/sCMV IE94 promoter | hLF | Microinjection | 64 ng/mL (fry stage) | [148] |
| Ctenopharyngodon idellus (Chinese grass carp) | ||||
| pCAgcGH/common carp β-actin promoter | hLF | SMGT | Transgenic fish resistance to GCHV # | [149] |
| Genus/Species | Vector/Promoter | Type of Lactoferrin | Yield | References |
|---|---|---|---|---|
| 1. Oryza sativa | ||||
| Oryza sativa | pCAMBIA-1300/rice actin | pLF | 0.12–100% purity | [150] |
| O. sativa L. cv. Nipponbare | Binary vector (pIG260 and pIG261)/maize ubiquitin-1 promoter | LFcinH | Antimicrobial activity against B. subtilis and E. coli | [151] |
| O. sativa | pCAMBIA1300/cauliflower mosaic virus 35S promoter (CaMV 35S) | pLF | 0.1% of rice bran weight | [152] |
| O. sativa | pCAMBIA1300/CaMV 35S | pLF | 0.1% of rice bran weight | [152] |
| O. sativa | pCAMBIA1300/CaMV 35S | hLF | 0.45% of total dry weight | [153] |
| O. sativa | Binary vector (pIG200 and pIG211)/maize ubiquitin-1 Promoter | hLF | 2.0 mg/g of dehusked seeds | [154] |
| O. sativa | pCAMBIA 1301/CMV35S | hLF-N lobe | 2.1 mg/g of dehusked seeds | [155] |
| O. sativa cell culture | pAPI137/rice actin promoter | hLF | 2–4% of the total soluble protein | [156] |
| O. sativa | pAPI135/rice actin promoter | hLF | 0.5–5.0 g/kg of dehusked rice | [157] |
| 2. Nicotiana tabacum/Nicotiana benthamiana | ||||
| Nicotiana benthamiana | pTKB3/CMV35S | hLF | 40 µg/g fresh mass (gFM) | [13] |
| Nicotiana tabacum | pART27/CaMV 35S | Camel LF | 1.5% of the total soluble protein (TSP) | [158] |
| N. tabacum var Xanthi | pCAMBIA 1301/CaMV 35S | bLF | 0.5% of TSP | [159] |
| N. benthamiana | Potexvirus potato virus X (PVX) vector/CaMV 35S | hLF (N-lobe) | 0.6% of TSP | [160] |
| N. tabacum cell culture | pCAMBIA 1301/CaMV 35S | hLF | 0.7–2.7% of TSP | [161] |
| Nicotiana tabacum xanthi | pBIOC21/CaMV 35S | hLF | 0.1 to 0.3% of TSP | [162] |
| N. tabacum | pAM1400/CaMV 35S | hLF | 0.1 to 0.8% of TSP | [163] |
| N. tabacum | pBI121/CaMV 35S | hLF | 0.1–0.3% of TSP | [164] |
| N. tabacum | pBI121/CaMV 35S | hLF | 1.8% of total cellular protein | [165] |
| 3. Solanum tuberosum | ||||
| Solanum tuberosum | pBIN35LF vector/CaMV 35S | hLF | 0.05% of TSP | [166] |
| S. tuberosum | Auxin-inducible manopine synthase (mas) P2 promoter/CaMV 35S | hLF | 0.01–0.1% of TSP | [167] |
| 4. Lycopersicon esculentum | ||||
| Lycopersicon esculentum | pBI121/CaMV 35S | hLF | 0.5% of TSP | [168] |
| L. esculentum | pBI121/CaMV 35S | hLF | 0.1% of TSP | [169] |
| 5. Pear (Pyrus sp.) | ||||
| Pear (Pyrus sp.) | pBI121/CaMV 35S | bLF | 0.3% of TSP | [170] |
| 6. Panax ginseng | ||||
| Siberian ginseng plant (Acanthopanax senticosis) | PCAMBIA2300/oxidative stress-inducible peroxidase (SWPA2) promoter | hLF | 3.6% of TSP | [171] |
| Korean ginseng cell line (Panax ginseng) | pCAMBIA/oxidative stress-inducible peroxidase (SWPA2) promoter | hLF | 3% of TSP | [172] |
| 7. Triticum aestivum (Wheat) | ||||
| Triticum aestivum | pAM4424/CaMV 35S | bLF | 21 to 67 ng/mg tissue | [173] |
| 8. Sweet potato | ||||
| Ipomoea batatas (cell culture) | Binary vector pLSM1/ CaMV 35S | hLF | 3.2 µg/mg (total protein) | [174] |
| 9. Hordeum vulgare (Barley) | ||||
| Three commercial cultivars of barley (Oksamytoviy, Vodogray, Hetman) | pHLFTuBA vector/ Rice glutelin B-1 (GluB-1) promoter | bLF | 0.5–1.2% of TSP | [175] |
| Hordeum vulgare | pAHC25/Ubiquitin (Ubi) promoter. | hLF | 3 ng/mg of TSP | [176] |
| 10. Alfalfa | ||||
| Alfalfa (Medicago sativa) | pBI/CaMV 35S | hLF | 0.0047% of TSP | [177] |
| 11. Edible algae | ||||
| Chlamydomonas reinhardtii | pCAMBIA1301C/ CaMV 35S | hLF | 1.82% of TSP | [178] |
| Chlorella vulgaris (green algae) | pCAMBIA1304/ CaMV 35S | bLF (N-lobe) | 0.5% of TSP | [179] |
| Expression System | Range of Expression (mg/mL) | Advantages | Disadvantages |
|---|---|---|---|
| 1. Prokaryotic expression | |||
| Bacteria | 0.002–10.6 | Highly optimized, short production timeline | Lack of glycosylation, Inclusion body formation |
| 2. Eukaryotic expression | |||
| Yeast | 0.00027–3.5 | High density fermentation, glycosylated proteins | High rate of translocation, N-glycosylation |
| Filamentous fungi | 0.005–2 | High yield, proper protein folding, and mammal like glycosylation patterns. | Improper glycosylation, proteolytic degradation of expressed protein. |
| Insect and insect cell line | 0.0095–10 | High expression levels, better post-translation modifications than bacteria | Non-human glycosylation, high production costs |
| Mammalian cell culture | 0.0006–1.6701 | Accurate glycosylation and proper protein folding | High production cost and lower yields compared to other systems |
| 3. Transgenic animals | |||
| Cattle | 0.0098–13.6 (milk) | Cost effective and enhanced nutritional value | Animal welfare and ethical issues |
| Goat | 0.765–30 (milk) | Large scale, cost-effective production system | Animal welfare and ethical issues |
| Swine | 6.5 (milk) | Dual animal model such as bioreactor and human disease models | Low milk yield, animal welfare issues |
| Rabbits | 0.2–2.3 (milk) | High reproductive rate and fast maturation, efficient bioreactor | Lower milk yield compared to larger animals |
| Mice | 0.0000001–30 (milk) | High reproducibility, cost-effective genetic studies | Low expression levels in milk, high variability in protein expression |
| Chicken | 0.1–0.3 (culture) | Fast growth, high reproductive rate, easy to handle | Ethical concerns with genetic modifications |
| 4. Transgenic plants/crops | |||
| Rice | 0.05–100% | High scalability, easy to harvest in large quantities | Environmental impact, regulatory issues |
| Tobacco | 0.1–2.7% TSP | Easy genetic manipulation | Potential for allergens |
| Potato | 0.01–0.1% TSP | Cost-effective, easy to grow | Low protein yield compared to other crops |
| Tomato | 0.1–0.5% TSP | Easy genetic manipulation | Low protein yield compared to other crops |
| Pear | 0.3% TSP | High yield, easy to harvest | Environmental concerns |
| Panax ginseng | 3–3.6% TSP | High potential for pharmaceutical applications | Low yield in controlled environments |
| Wheat | 21 to 67 ng/mg tissue | Highly abundant, high transformation efficiency | Lower protein concentration |
| Sweet potato | 3.2 µg/mg (total protein) | High yield, easy to grow | Lower protein concentration compared to other crops |
| Barley | 0.0003–1.2% TSP | High yield, adaptable to a variety of environmental conditions | Low protein concentration, difficult to scale up. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konadaka Sri, R.; Balasamudram Chandrasekhar, P.; Sirisilla, A.; Mohammed, Q.K.Q.; Jakkoju, T.; Bheemreddy, R.R.; Bhattacharya, T.K.; Ullengala, R.; Chatterjee, R.N. Biopharming of Lactoferrin: Current Strategies and Future Prospects. Pharmaceutics 2025, 17, 1023. https://doi.org/10.3390/pharmaceutics17081023
Konadaka Sri R, Balasamudram Chandrasekhar P, Sirisilla A, Mohammed QKQ, Jakkoju T, Bheemreddy RR, Bhattacharya TK, Ullengala R, Chatterjee RN. Biopharming of Lactoferrin: Current Strategies and Future Prospects. Pharmaceutics. 2025; 17(8):1023. https://doi.org/10.3390/pharmaceutics17081023
Chicago/Turabian StyleKonadaka Sri, Rajaravindra, Parthasarathi Balasamudram Chandrasekhar, Architha Sirisilla, Qudrathulla Khan Quadri Mohammed, Thejasri Jakkoju, Rajith Reddy Bheemreddy, Tarun Kumar Bhattacharya, Rajkumar Ullengala, and Rudra Nath Chatterjee. 2025. "Biopharming of Lactoferrin: Current Strategies and Future Prospects" Pharmaceutics 17, no. 8: 1023. https://doi.org/10.3390/pharmaceutics17081023
APA StyleKonadaka Sri, R., Balasamudram Chandrasekhar, P., Sirisilla, A., Mohammed, Q. K. Q., Jakkoju, T., Bheemreddy, R. R., Bhattacharya, T. K., Ullengala, R., & Chatterjee, R. N. (2025). Biopharming of Lactoferrin: Current Strategies and Future Prospects. Pharmaceutics, 17(8), 1023. https://doi.org/10.3390/pharmaceutics17081023






