Abstract
Background: Previous research has demonstrated that 20 kHz probe or 37 kHz bath sonication of poloxamers comprising polypropylene glycol (PPG) and polyethylene glycol (PEG) blocks can generate degradation byproducts that are toxic to mammalian cells and organisms. Herein, an investigation of a PEGylated phospholipid micelle was undertaken to identify low-molecular-weight sonolytic degradation byproducts that could be cytotoxic. The concern here lies with the fact that sonication is a frequently employed step in drug delivery manufacturing processes, during which PEGylated phospholipids can be subjected to shear forces and other extreme oxidative and thermal conditions. Methods: Control and 20 kHz-sonicated micelles of DSPE-mPEG2000 were analyzed using dynamic light scattering (DLS) and zeta potential analyses to study colloidal properties, matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) mass spectroscopy (MS) and proton nuclear magnetic resonance (1H-NMR) spectroscopy to study the structural integrity of DSPE-mPEG2000, and 1H-NMR spectroscopy and high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection to quantitate the formation of low-molecular-weight degradation byproducts. Results: MALDI-TOF-MS analyses of 20 kHz-sonicated DSPE-mPEG2000 revealed the loss of ethylene glycol moieties in accordance with depolymerization of the PEG chain; 1H-NMR spectroscopy showed the presence of formate, a known oxidative/thermal degradation product of PEG; and HPLC-UV showed that the generation of formate was dependent on 20 kHz probe sonication time between 5 and 60 min. Conclusions: It was found that 20 kHz sonication can degrade the PEG chain of DSPE-mPEG2000, altering the micelle’s PEG corona and generating formate, a known ocular toxicant.
1. Introduction
The administration of pharmaceuticals is often challenging due to their hydrophobic properties, which make them difficult to solubilize in aqueous bodily fluids, resulting in poor adsorption and reduced therapeutic effectiveness at the target site [1,2,3,4]. In light of this, numerous polymer- and phospholipid-based nanocarriers have been developed to augment the biophysiochemical characteristics of therapeutics [3,5,6,7,8]. A key component of most of these nanocarriers is an outer coating of polyethylene glycol (PEG) that enhances nanocarrier surface properties by minimizing their aggregation and making them stealth-like, thereby enabling them to avoid an immune response [8,9]. Figure 1 shows the structure of a common PEGylated lipid, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000], commonly known as DSPE-PEG2000-CH3 or DSPE-mPEG2000. The DSPE-PEG family of phospholipid–polymer conjugates is amphiphilic, comprising a hydrophobic core and a hydrophilic shell; these conjugates are biocompatible and biodegradable and can be functionalized with various biomolecules for specific functions in drug delivery and imaging [10,11]. DSPE-mPEG2000 micelles are advantageous because they are generally smaller in size relative to other polymer-based drug delivery nanoparticles and they possess a low (~1 μM) critical micelle concentration (CMC), which promotes micelle stability even after injection and subsequent dilution into the bloodstream [12]. DSPE-mPEG2000 is subsequently employed to improve the biopharmaceutical properties of micelles, liposomes, and nanoparticles by increasing their colloidal stability (e.g., by minimizing aggregation) and hindering interactions between colloidal nanocarriers and blood components (e.g., decreasing the adsorption of proteins), thereby reducing uptake mediated by macrophages of the reticuloendothelial system and leading to prolonged bloodstream circulation times [8,10,13,14]. In general, tailoring of these properties can be governed by the physiochemical properties of the PEG corona that coats the nanocarrier surface including the PEG surface density, as well as the chain length, branching, and terminal groups of the PEG moiety [3,8,13,15]. Given this, it is therefore imperative that the intended architecture of the PEG corona is maintained during nanocarrier manufacturing [16].
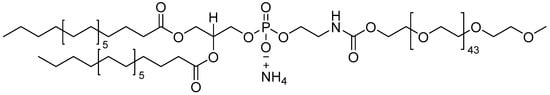
Figure 1.
Chemical structure of the ammonium salt of DSPE-PEG2000-CH3 (also known as DSPE-mPEG2000).
There are numerous PEGylated phospholipid drug delivery formulations, and many strategies have been developed that involve multiple processing steps depending on nanocarrier components and/or the envisioned biomedical application [11,17,18,19,20]. Of particular concern are manufacturing processes such as sonication, homogenization, or extrusion that subject components to shear forces as part of dispersing and/or sizing operations. In particular, PEGylated lipids such as DSPE-PEG2000 and DSPE-mPEG2000 are frequently sonicated in the process of preparing of micelles and liposomes [21,22,23,24,25,26,27,28,29,30,31]; for example, the “thin-film hydration and sonication” method to prepare liposomes comprising DSPE-mPEG2000 was performed by Khutoryanskiy and co-workers using a bath sonicator [32], and by Yao, Han, and co-workers using a probe sonicator [33].
Sonication is the application of sound energy at frequencies typically above 20 kHz. Briefly, as sound waves from an acoustic transducer radiate through a solution, they cause alternating high and low pressures in the solution. During the low-pressure stage, millions of microscopic bubbles form and grow in a process called cavitation, and during the high-pressure stage, the bubbles collapse, or implode, generating extreme local conditions including high heats, pressures, and shear forces at the bubble–water interface [34,35,36,37,38]. Sonolytic frequencies typically dictate application; for example, sonochemical syntheses and the purposeful degradation of pollutants is typically performed using frequencies in the range of 350 kHz–1 MHz [38,39,40,41]; the ultrasonic-mediated release of drugs from micelles/liposomes and uptake by biological cells is generally achieved using frequencies in the range of 1–5 MHz, while non-invasive medical imaging is typically performed with ultrasound frequencies in the range of 5–15 MHz [42,43].
Herein, this investigation focuses on the lower frequency ranges of benchtop probe (20–28 kHz) and bath (37–40 kHz) sonicators, which are commonly used to clean delicate instruments and materials, disrupt biological cell membranes, break down particles into smaller sizes, and disperse and mix particles in fluids [35]. Nonetheless, even at these low frequencies, certain probe and bath sonication conditions are well-known to degrade a variety of polymers; for example, 28 kHz probe sonication of PEG-PPG di-block co-polymers [44], 20 kHz probe and 37 kHz bath sonication of PEG-PPG-PEG tri-block co-polymers [45,46], 25–28 kHz probe sonication of PEG [47,48,49], 37 kHz bath sonication of PEG and branched-chain PEGs [45,50], 40 kHz bath sonication of DSPE-PEG2000-NH2 [16], and 37 kHz bath sonication of DSPE-PEG2000-NH2 and DSPE-PEG2000-COOH [50]. In these examples, the sonolytic transformation of polymeric materials in solution can stem from (i) attacks by reactive oxygen species and free radicals, which originate from the implosive collapse of bubbles, (ii) mechanochemical effects from shear forces generated around collapsing cavitation bubbles, and (iii) pyrolysis in the hot interfacial region between bubbles and the surrounding liquid [36,38,44,49,51,52].
While it has been known for decades that the structural integrity of certain polymers can be sensitive to sonication, the demonstration that sonication of PEG-containing polymers can generate toxic byproducts is relatively recent. For example, Draper and co-workers first reported that the degradation byproducts of sonicated PEG-PPG-PEG tri-block co-polymers (also known as poloxamers, known by the trade name Pluronic®) can be toxic to mammalian cells and organisms [45,46]. Specifically, solutions of poloxamer 407 (i.e., Pluronic® F-127) or poloxamer 188 (i.e., Pluronic® F-68) at concentrations below their CMCs that were bath-sonicated for 15–240 min at 37 kHz (or probe-sonicated for 2–30 min at 20 kHz) were shown to be toxic to normal rat kidney (NRK) cells [45]; solutions of poloxamer 338 (i.e., Pluronic® F-108) at concentrations below its CMC that were bath-sonicated for 15–240 min at 37 kHz were shown to be toxic to mouse macrophage RAW 264.7 cells and zebrafish embryos [46]. An equally important aspect of these works was the development and validation of a simple dialysis purification method to detoxify these samples, whereby the resultant sonicated and dialyzed poloxamer solutions had no effect on cell proliferation or zebrafish embryo viability and development. Specifically, dialysis of sonicated poloxamer solutions with membranes possessing a molecular weight cut-off (MWCO) of 300 kDa was used to render sonicated poloxamer 407 and poloxamer 188 samples non-toxic to NRK cells [45], and membranes with an MWCO of 100 kDa were used to render sonicated poloxamer 338 samples non-toxic to RAW 264.7 cells and zebrafish embryos [46].
Herein, DSPE-mPEG2000 was chosen to expand upon previous reports of the 37–40 kHz sonolytic degradation of DSPE-PEG2000-COOH and DSPE-PEG2000-NH2 [16,50]. Specifically, the goals of this work were to investigate the structural integrity of DSPE-mPEG2000 micelles following 20 kHz sonication and to identify any low-molecular-weight degradation byproducts that could be potentially toxic to mammalian cells. MALDI-TOF-MS analyses of 20 kHz-sonicated DSPE-mPEG2000 revealed the loss of ethylene glycol moieties in accordance with depolymerization of the PEG chain, which was congruent with indicators of micelle disruption and reduced colloidal stability of sonicated DSPE-mPEG2000 micelles as measured by 1H-NMR and zeta potential analyses, respectively. 1H-NMR spectroscopy showed the presence of formate, a known ocular toxicant, in the spectrum of 20 kHz-sonicated DSPE-mPEG2000 micelles, and HPLC-UV showed that the generation of formate was dependent on 20kHz probe sonication time between 5 and 60 min. While formates are known oxidative/thermal degradation products of PEG [38,53,54,55,56,57,58], the actual detection of formates generated by sonication of PEG or PEG-containing molecules (using probe sonicators operated in the 20–28 kHz frequency regime or bath sonicators operated in the 37–40 kHz frequency regime) has not been demonstrated. In fact, to the best of our knowledge, the only demonstration of sonolytic production of formates from a surfactant containing a short ethylene oxide chain involved 358 kHz probe sonication of Triton-X-100 [40].
2. Materials and Methods
2.1. Chemicals
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-mPEG2000, product No. 880120) was purchased from Avanti Polar Lipids (Alabaster, AL, USA). Deuterium oxide (D2O, 100.0 atom% D) and α-cyano-4-hydroxycinnamic acid were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Trifluoroacetic acid (TFA, HPLC Grade), acetonitrile (ACN, HPLC Grade), sodium formate, and MALDI-TOF-MS standard (ProteoMASS™ adrenocorticotropic hormone (ACTH) fragment 18–39) were purchased from Sigma-Aldrich (Burlington, MA, USA). Polybead® carboxylate microspheres (0.10 µm diameter) were purchased from Polysciences (Warrington, PA, USA). Zeta-transfer standard (product No. ZTS1240) was purchased from Malvern Panalytical (Malvern, UK). Deionized water (18.3 MΩ cm) was obtained using a Nanopure® Infinity water purification system (Barnstead, Dubuque, IA, USA).
2.2. Preparation of DSPE-mPEG2000 Micelles
A 2.00 mM solution of DSPE-mPEG2000 was prepared by first weighing 0.0168 g (0.00600 mmol) of DSPE-mPEG2000 into a scintillation vial, followed by the slow addition of 3.00 mL of deionized water (or 3.00 mL of D2O for samples analyzed by NMR spectroscopy), and then stirring for 30 min at ~720 RPM.
2.3. Sonication of DSPE-mPEG2000 Micelles
Probe sonication was performed using a 3 mm diameter microtip sonicator probe attached to a Branson 250 Sonifier (Emerson Electric Co., St. Louis, MO, USA). An aqueous solution (1.5 mL) of DSPE-mPEG2000 was placed in a 15 mL falcon tube, which was submerged ~2.5 cm into an ice-water bath. The probe tip was centered and submerged ~0.5 cm below the surface of the DSPE-mPEG2000 solution. The sonicator was operated at 20 kHz in pulse mode (7.5-s on, 7.5-s off) at 50% amplitude, which corresponds to a power range of 30–42 W. Samples were sonicated for 5, 15, and 60 min; in the latter case, sonication was performed at 15 min intervals, and the bath’s ice-water was replaced with fresh ice-water after every interval. The mean effective calorimetric power delivered to the solution after 15 min was 14 W, as determined using the method of Hackley and co-workers [59].
Bath sonication was performed for 15 or 30 min using a Branson model 2510 bath sonicator operating at 130 W and 40 kHz. An aqueous solution (1.0 mL) of DSPE-mPEG2000 was placed in a 20 mL scintillation vial, which was positioned at the center of the bath, as detailed previously by Draper and co-workers [45]. The mean effective calorimetric power delivered to the solution after 30 min was 69 W, as determined using the method of Hackley and co-workers [59].
2.4. DLS and Zeta Potential
Particle size distributions and zeta potentials were determined at 25 °C using a Malvern Nano-ZS 3600 Zetasizer equipped with a 633 nm laser (Malvern Panalytical, Malvern, UK)and a fixed-angle detector position of 173°. Samples, control or sonicated DSPE-mPEG2000 solutions (both pH 6), or the calibration standard (1 mL each), were contained in a polystyrene DLS cuvette (Malvern product No. ZEN0040) or a folded capillary zeta potential cell (Malvern product No. DTS1070). For DLS analyses, three independent measurements of a sample or the Polybead® standard, each comprising ten runs at 30 s/run, were acquired and averaged. For zeta potential analyses, three consecutive measurements of a sample or the zeta-transfer standard were acquired and averaged using an automated number of runs. The hydrodynamic diameters and zeta potentials of deionized water and DSPE-mPEG2000 samples were calculated using a viscosity and refractive index of 0.8872 cP and 1.330, respectively, and an absorption and refractive index of 0.010 and 1.51, respectively.
2.5. MALDI-TOF-MS
MALDI-TOF-MS experiments were conducted using a Shimadzu AXIMA Confidence™ MALDI-TOF mass spectrometer equipped with a 337 nm nitrogen laser (Shimadzu, Columbia, MD, USA). The matrix solution was 10 mg α-cyano-4-hydroxycinnamic acid dissolved in 1.0 mL of a solution comprising 70% ACN and 30% (vol/vol) aqueous 0.1% TFA [16,60]. In all cases, the matrix solution was mixed (2:1 vol/vol) with the ACTH standard or with a sample (a control or sonicated DSPE-mPEG2000 solution). Subsequently, 2 µL of one of these mixtures was dispensed onto different regions of a stainless-steel target plate and left to dry for at least 30 min. Instrument calibration and sample analyses were performed in positive linear mode using the following automated parameters: a power setting of 120, a profile number of 500, and 2 shots. Spectral processing was performed with Shimadzu Launchpad Software version 2.9.4 using the following parameters: a peak width of 5, peak area set to the tip of the peak, smoothing method set to average, smoothing filter width of 10, baseline filter width of 1000, threshold-apex peak detection method, and a threshold offset of 0.010.
2.6. 1H-NMR Spectroscopy
1H-NMR spectroscopy experiments involving control and sonicated DSPE-mPEG2000 samples were performed at 25 °C using a Bruker Avance III™ 500-MHz spectrometer (Bruker, Billerica, MA, USA). Briefly, 1 mL aliquots of a sample or a D2O blank was placed into a Wilmad® NMR tube, and 400 scans were acquired. Data were processed through MestreNOVA Software version 16.0.0
2.7. HPLC-UV
Quantitative analyses were based on established HPLC-UV methodologies for the detection of formates [38,61] and were conducted using an Agilent Technologies 1260 Infinity II HPLC system equipped with a variable-wavelength detector set at 220 nm (Agilent Technologies, Inc., Santa Clara, CA, USA). A reverse-phase C18 column (Kinetex XB-C18 core–shell column, Phenomenex, Princeton, NJ, USA) was used with a mobile phase comprising 90% of a 25 mM monobasic potassium phosphate solution and 10% (vol/vol) aqueous ACN. All experiments were run for 30 min in isocratic mode at an instrument flow rate of 1.0 mL/min. Samples, control or sonicated DSPE-mPEG2000 solutions, or sonicated DSPE-mPEG2000 solutions spiked with 7.35 mM sodium formate, were prepared using an aqueous 10% (vol/vol) ACN solution. Sodium formate standards (1.0 mg/mL, 0.1 mg/mL, and 0.01 mg/mL) were prepared using the mobile phase. All sample and standard injection volumes were 20.0 µL, and the injector was rinsed with 1.0 mL of deionized water between injections.
3. Results
3.1. DLS and Zeta Potential Analyses
The thermodynamics of DSPE-based phospholipid micelles with varying lengths of PEG appendages have been the subject of many experimental and computational investigations [12,24,50,62]. In the case of aqueous DSPE-based micelles modified with PEG2000, it has been shown that spherical micelles are formed, with an aggregation number of 76 at 25 °C, and an ~11 nm micelle comprises 20 DSPE-mPEG2000 phospholipids [12,62]. Herein, DLS and zeta potential analyses were used to study the colloidal properties of DSPE-mPEG2000 micelles subjected to sonication. Figure 2 shows a representative DLS particle size distribution of an aqueous solution of DSPE-mPEG2000 before and after 1 h of 20 kHz probe sonication. DSPE-mPEG2000 is known to spontaneously assemble in water into uniform micelles [12]. Indeed, the non-sonicated control is characterized by a single peak representing a micelle structure with an average hydrodynamic diameter of 8.2 ± 1.2 nm (n = 3 independent samples),which is in agreement with ~10 nm diameter sizes reported for various DSPE-PEG2000 micelles [12,24,50,62]. Following 1 h of sonication, the micelle peak was no longer observed, and only larger structures with hydrodynamic diameters > 90 nm were detected. These findings are similar to those reported by Draper and co-workers, who observed significant declines in the intensity of the DLS micelle peaks of DSPE-PEG2000-NH2 and DSPE-PEG2000-COOH as a function of 37 kHz bath sonication time, concomitant with the appearance of larger particles with hydrodynamic diameters > 200 nm, believed to be aggregates of hydrophobic degradation fragments [50]. The zeta potential of control DSPE-mPEG2000 micelle solutions was −30.1 ± 6.3 mV (n = 3), corresponding to a moderately stable colloidal system, which is to be expected since the steric stabilization provided by PEG is known to reduce the tendency of particles to aggregate [13]. Conversely, the zeta potential of 1 h probe-sonicated solutions of DSPE-mPEG2000 was less negative (−23.1 ± 5.5 mV (n = 3)), symptomatic of colloidal nanoparticle instability, which could be a result of the formation of aggregates of DSPE-mPEG2000 degradation fragments.
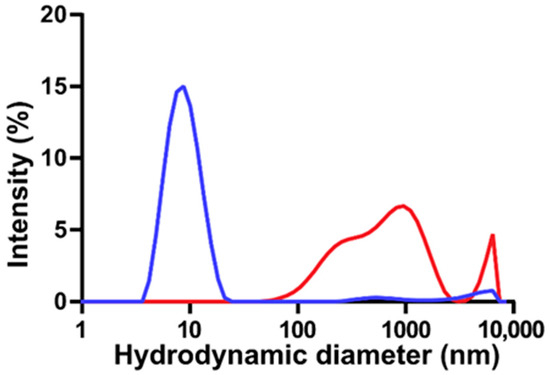
Figure 2.
Representative DLS particle size distributions of aqueous 2.00 mM solutions of DSPE-mPEG2000 before (blue) and after (red) 1 h of 20 kHz probe sonication. Before sonication, DSPE-mPEG2000 is characterized primarily as a single major peak representing a micelle with a hydrated diameter of ~8 nm; following sonication, the micelle peak is no longer observed and a series of new structures with sizes > 90 nm appear.
3.2. MALDI-TOF-MS Analyses
MALDI-TOF-MS has previously been demonstrated to be a useful tool in assessing the structural integrity of PEG and PEGylated molecules subjected to sonication [44,49,60]; most pertinently, Zeineldin and co-workers used MALDI-TOF-MS to demonstrate the degradation of DSPE-PEG2000-NH2 into smaller fragments following 40 kHz bath sonication [16]. Figure 3 shows representative MALDI-TOF-MS spectra of aqueous DSPE-mPEG2000 solutions before and after 1 h of 20 kHz probe sonication. Both spectra display the expected symmetry characteristic of a PEGylated phospholipid with a polydisperse PEG appendage, and both display the expected spacing between adjacent peaks (Δm) that correspond to the mass unit difference between repeating units (m/z = 44.05) of ethylene glycol monomers (CH2–CH2–O)n. However, examination of the peak areas for the non-sonicated (17,896 ± 375 (n = 3)) and sonicated (13,755 ± 646 (n = 3)) DSPE-mPEG2000 samples in the m/z range of 2428 to 3678 reveals a 14% decrease in mass. Since the peaks in the sonicated spectrum continue to represent a homologous series of ethylene glycol monomers, the most probable sonolytic degradation mechanism involves the successive depolymerization of ethylene glycol moieties [38], in accordance with the loss of repeating units of ethylene glycol moieties observed previously in the MALDI-TOF-MS analyses of 28 kHz probe-sonicated PEG [49] and 40 kHz bath-sonicated DSPE-PEG2000-NH2 [16].
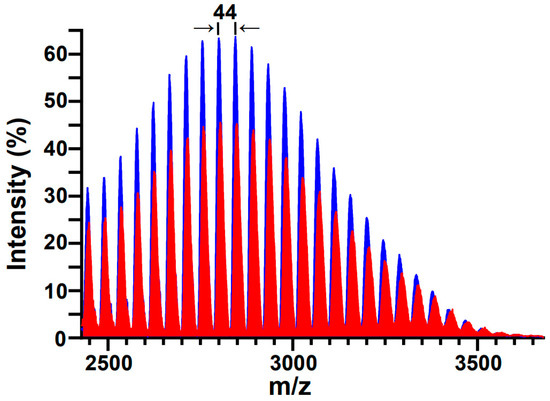
Figure 3.
Representative MALDI-TOF-MS spectra of DSPE-mPEG2000 before (blue) and after (red) 1 h of 20 kHz probe sonication. Both spectra display the expected symmetry distinctive of a polydisperse polymer; the differences between adjacent peaks (Δm) correspond to the mass unit difference (m/z = 44.05) between repeating units of (CH2-CH2-O)n. Post sonication, the loss of mass observed in the m/z range of 2428 to 3678 was 14%.
3.3. 1H-NMR Spectroscopic Analyses
One of the first complete chemical shift assignments of DSPE-mPEG2000 protons was reported by Shively and co-workers [12]; these proton assignments are identified by lowercase letters adjacent to the DSPE-mPEG2000 structure shown Figure 4, which also shows the 1H-NMR spectra of aqueous DSPE-mPEG2000 solutions before and after 1 h of 20 kHz probe sonication. Several noteworthy differences are observed in the post-sonicated spectrum relative to the control spectrum. The first is a ~2-fold narrowing of the full width at half maximum (FWHM) of peaks “a” and “b” (i.e., the methyl- and methylene-protons of the hydrocarbon chain, respectively), and the other is a ~3-fold narrowing of the FWHM of peak “g” (i.e., the methylene protons alpha to the phosphate group). These differences are significant because appreciable narrowing of 1H-NMR peaks is indicative of micelle disruption [63,64,65], which is congruent with the zeta potentials recorded for sonicated DSPE-mPEG2000 samples vs. controls.
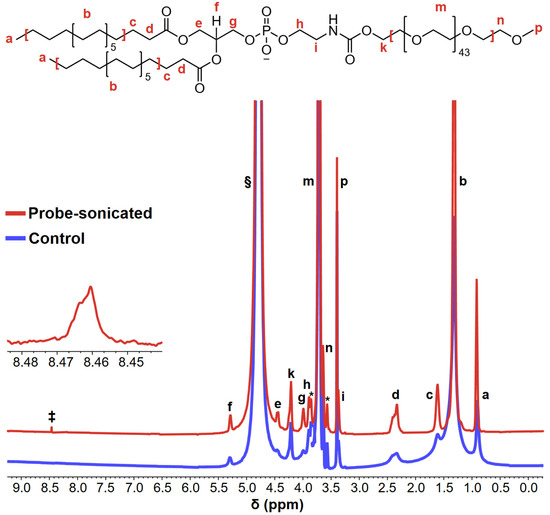
Figure 4.
Representative 1H-NMR spectra of aqueous 2.00 mM solutions of DSPE-mPEG2000 before (blue) and after (red) 1 h of 20 kHz probe sonication. The lowercase letters above/adjacent to peaks correspond to the protons denoted in the DSPE-mPEG2000 structure. The peaks indicated with asterisks (*) represent J-coupling splitting between 1H and the natural 13C abundance of strong ethylene glycol units [12]; the § symbol represents the D2O peak; the peak denoted with the ‡ symbol at δ ~8.4 ppm represents the generation of formate in the sonicated sample. The inset represents a ~65× magnified view of the formate peak.
The most noteworthy result of the 1H-NMR experiments, as shown in Figure 4 in the spectrum of the probe-sonicated sample, is the appearance of a new peak at δ~8.4 ppm, which can be ascribed to formate HCO2− [58,66,67]. This is a key finding because formates are known oxidative/thermal degradation products of PEG [38,53,54,55,56,57,58]; therefore, formate generation supports the sonolytic degradation of the PEG chain of DSPE-mPEG2000, congruent with the MALDI-TOF-MS results.
3.4. HPLC-UV Analyses
HPLC-UV analyses were employed to quantitate sonolytic formate production. Figure 5A,B show representative HPLC-UV chromatograms of DSPE-mPEG2000 before and after 1 h of 20 kHz probe sonication, respectively. The first noteworthy peak in both chromatograms appears at a retention time of ~8.0 min and corresponds to DSPE-mPEG2000. The second peak, at a retention time of 1.4 min, represents formate and appears only in the sonicated chromatogram, which also shows a corresponding decrease in the area of the DSPE-mPEG2000 peak. To verify that the 1.4 min peak was indeed formate, a sample of DSPE-mPEG2000 that was sonicated for 1 h was spiked with 7.35 mM aqueous sodium formate. As shown in Figure 5C, the only observable change in this chromatogram (up to a retention time of 30 min) was an increase in the signal intensity of the peak at 1.4 min. Next, a series of sodium formate standards between 14.7 mM and 0.147 mM were analyzed, and a calibration curve was generated where the areas of the peak at 1.4 min were found to be linear (R2 = 0.9999) with respect to the concentration of formate. Using this calibration curve, the concentration of formate detected in the chromatogram of the DSPE- mPEG2000 sample that was probe-sonicated for 1 h (Figure 5B) was determined to be 2.26 mM. This value is also shown in Table 1, along with those for DSPE-mPEG2000 samples that were probe-sonicated for 5 and 15 min, and the combined data indicate that formate generation is dependent on 20 kHz probe sonication time between 5 and 60 min. Table 1 also shows the results for two DSPE-mPEG2000 samples that were sonicated using a bath sonicator—the more commonly used apparatus in pharmaceutical research and development work. These results are for comparative purposes and indicate that 15 min of 40 kHz bath sonication did not produce detectable levels of formate, and that the concentration of formate detected after 30 min of bath sonication (0.25 mM) was similar to the concentration of formate detected after merely 5 min of 20 kHz probe sonication (0.29 mM).
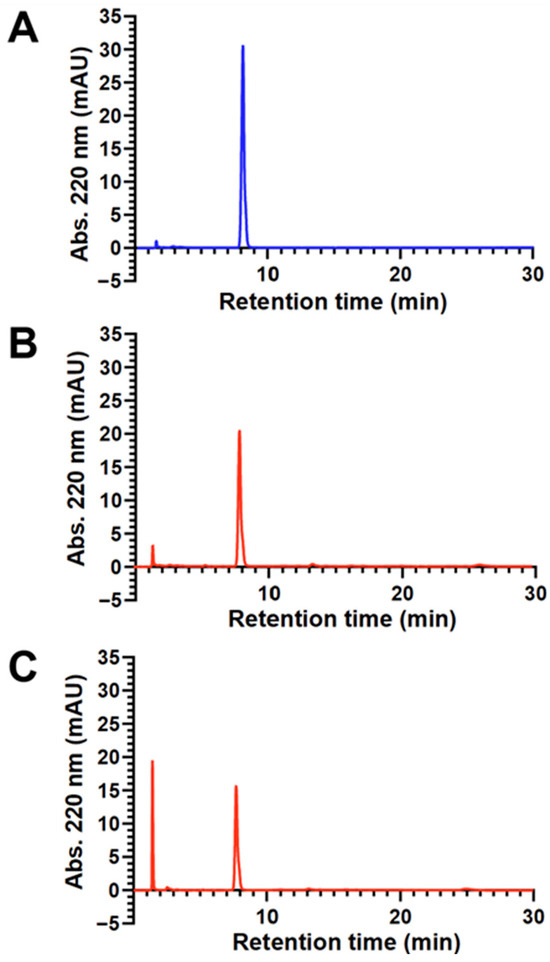
Figure 5.
Representative HPLC-UV chromatograms (normalized to the same y-axis scale) of aqueous 2.00 mM solutions of DSPE-mPEG2000 before sonication (A), after 1 h of 20 kHz probe sonication (B), and a 1 h probe-sonicated sample spiked with 7.35 mM sodium formate (C). In all chromatograms, the peak with a retention time of ~8 min represents DSPE-mPEG2000, and the peak with a retention time of 1.4 min represents formate. The peak with a retention time of 1.6 min in chromatogram A represents the solvent front.

Table 1.
Concentrations of formate detected in 20 kHz probe-sonicated and 40 kHz bath-sonicated DSPE-mPEG2000 solutions.
4. Discussion
Herein, MALDI-TOF-MS analyses of 20 kHz-sonicated DSPE-mPEG2000 micelles revealed the loss of ethylene glycol moieties, consistent with depolymerization of the PEG chain; this finding aligned with indicators of micelle disruption and reduced colloidal stability of sonicated DSPE-mPEG2000 micelles as measured by 1H-NMR and zeta potential analyses, respectively. 1H-NMR spectroscopy of 20 kHz-sonicated DSPE-mPEG2000 micelles showed the presence of formate, a known oxidative/thermal degradation product of PEG. Formate is generated following the random scission of C-O bonds that produce end products such as ethylene glycol, which can further be oxidized to various C2 carboxylic acids and ultimately formate [38,53,54,55,56,57,58]. The presence of formate is consistent with the oxidative, thermal, and mechanochemical processes involved in the sonolytic degradation of polymers [36,38,44,49,51,52]. Detection of formates was also demonstrated using an HPLC-UV method, which additionally showed that the generation of formates was dependent on 20 kHz probe sonication time between 5 and 60 min.
There are two main implications regarding the intended biopharmaceutical performance of a DSPE-mPEG2000 micelle if the PEG chain is transformed by 20 kHz sonication. The first is the potential alteration of the micelle’s PEG corona, whose physiochemical properties are directly involved in influencing the micelle’s colloidal stability, circulation time, etc. [8,10,13,14]. The second concerns PEG degradation byproducts, such as formate, and the need to compare sonolytically generated formate levels to those reported in the literature that could elicit toxicity and/or a deleterious biological response. In the case of formate, this is a particular concern for ocular cells, such as those in the retina, optic nerve, and basal ganglia, which are quite sensitive due to their limited ability to metabolize it [68,69,70,71,72,73,74,75]. For example, an investigation by Treichel, Burke, and co-workers using cultured mouse retinal photoreceptor (661 W) cells revealed cytotoxicity after 6 h of exposure to 30 mM sodium formate [74], a concentration ~13× higher than that in the 1 h probe-sonicated DSPE-mPEG2000 samples and roughly two orders of magnitude higher than that in 30 min bath-sonicated DSPE-mPEG2000 samples (Table 1).
The ability to investigate the structural integrity of PEGylated phospholipid nanocarriers following sonication and to identify potentially toxic degradation byproducts is increasingly relevant for the ocular drug delivery field. This is because, in the case of eye drops, a drug formulation (and a potential unintended toxicant) could be administered repeatedly on a daily basis, while in the case of an intravitreal injection, the drug formulation is delivered into the vitreous humor, a space that represents a ~1000-fold smaller volume (and a ~1000-fold less dilution of potential toxicant amounts) than a formulation injected into the bloodstream. Indeed, when one considers polymer- and lipid-based colloidal nanocarrier systems that are currently being developed for ocular drug delivery applications [76,77], several approaches involve micelles or liposomes comprising DSPE-mPEG2000 that were subjected to sonication. For example, Khutoryanskiy and co-workers prepared liposomes where 30 min of sonication was employed using a bath sonicator operating at a frequency of 35–45 kHz and a power of 120 W [32], and Yao, Han and co-workers prepared liposomes where 2 min of sonication was employed using a probe sonicator operating at a frequency of 20–25 kHz and a power of 50 W [33].
It should be additionally appreciated that while these two examples represent works where the experimental details of sonication were well documented, this is not always the case. For example, a non-exhaustive survey of sixteen works since 2007 that described the preparation of DSPE-PEG2000/DSPE-mPEG2000 micelles and liposomes (and a few hybrid lipid–polymer nanoparticles or phospholipid nanoparticles containing DSPE-PEG2000 or DSPE-mPEG2000), which employed either bath sonication frequencies, powers, and times spanning 35–45 kHz, 100–120 W, and 3–30 min, respectively, or probe sonication frequencies, powers, and times spanning 20–25 kHz, 50–400 W, and 3–15 min, respectively, revealed five works that did not define the sonication power, four that did not define the sonication frequency, and seven that did not report the sonication temperature [21,22,23,24,25,26,27,28,29,30,31,32,33,78,79,80]. Furthermore, seven of the sixteen works with undefined sonication powers and frequencies, and with reported sonication times ranging from 2 to 240 min, did not even distinguish whether sonication was performed using a bath sonicator or a probe sonicator. This is important because the omission of even one critical parameter, such as sonication frequency, which is a major determinate of cavitation bubble size, makes it difficult to predict whether any sonolytic degradation of PEG could have potentially occurred, and also makes it difficult for researchers to reproduce these experiments. Coincidently, almost all of the sixteen works performed some degree of sample purification following sonication, including four works that employed dialysis that would have undoubtedly removed any low-molecular-weight sonolytic degradation byproducts (if present). Disappointingly, three of the seven works that did not distinguish whether sonication was performed using either a bath sonicator or a probe sonicator additionally did not perform any purification procedures. Finally, it should also be noted that reproducing sonication operations across different laboratories is hardly straightforward due to the spectrum of sonication devices currently available [59]. For example, with bath sonication, the age of acoustic transducers and the position of the sample in the bath are important factors in determining the effective acoustic energy delivered to a sample, while with probe sonication, the condition of the probe tip and the volume of liquid are two of the important factors [59,81].
In conclusion, despite the documentation of sonolytic degradation of PEG and PEG-containing molecules in the peer-reviewed literature, there remain works where nanocarriers containing PEGylated lipids are sonicated without evaluating the integrity of PEG or applying protocols designed to remove any sonolytic degradation products that were potentially generated. It would therefore be prudent in studies involving sonicated PEGylated phospholipid micelles and liposomes to test for the generation of low-molecular-weight sonolytic degradation products that may elicit unwanted biological responses and may indicate alteration of the intended architecture of the PEG corona.
Author Contributions
Conceptualization, P.P. (Perouza Parsamian) and P.P. (Paul Pantano); methodology, P.P. (Perouza Parsamian) and P.P. (Paul Pantano); formal analysis, P.P. (Perouza Parsamian) and P.P. (Paul Pantano); investigation, P.P. (Perouza Parsamian) and P.P. (Paul Pantano); data curation, P.P. (Perouza Parsamian) and P.P. (Paul Pantano); validation, P.P. (Perouza Parsamian) and P.P. (Paul Pantano); writing—original draft preparation, P.P. (Perouza Parsamian); writing—review and editing, P.P. (Perouza Parsamian) and P.P. (Paul Pantano); visualization, P.P. (Paul Pantano); supervision, P.P. (Paul Pantano); project administration, P.P. (Paul Pantano); funding acquisition, P.P. (Perouza Parsamian) and P.P. (Paul Pantano). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the UT Dallas Department of Chemistry and Biochemistry, an Eric Moore UT Dallas Graduate Student Scholarship, an Armenian General Benevolent Union Student Scholarship, and an Instrumentation Grant from the National Science Foundation (Award No. 1126177 to Mihaela C. Stefan, UT Dallas Department of Chemistry and Biochemistry). The APC was funded by UT Dallas and the UT Dallas Department of Chemistry and Biochemistry.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed in this work are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Mihaela C. Stefan, Michael C. Biewer, Ronald A. Smaldone, Sheena D’Arcy, Hien Nguyen, Jing Ge, and Stacy Chanakira for their contributions to this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PPG | polypropylene glycol |
| PEG | polyethylene glycol |
| DLS | dynamic light scattering |
| MALDI | matrix-assisted laser desorption/ionization |
| TOF | time of flight |
| MS | mass spectroscopy |
| 1H-NMR | proton nuclear magnetic resonance |
| DSPE- | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine |
| mPEG | methoxy PEG |
| HPLC | high-performance liquid chromatography |
| UV | ultraviolet |
| CMC | critical micelle concentration |
| NRK | normal rat kidney |
| MWCO | molecular weight cut-off |
| D2O | deuterium oxide |
| TFA | trifluoroacetic acid |
| ACN | acetonitrile |
| ACTH | adrenocorticotropic hormone |
| FWHM | full width at half maximum |
References
- Kumar, A.; Sahoo, S.K.; Padhee, K.; Kochar, P.P.S.; Sathapathy, A.; Pathak, N. Review On Solubility Enhancement Techniques For Hydrophobic Drugs. Pharm. Glob. 2011, 3, 1–7. [Google Scholar]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. Int. Sch. Res. Netw. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Gill, K.K.; Kaddoumi, A.; Nazzal, S. PEG-lipid micelles as drug carriers: Physiochemical attributes, formulation principles and biological implication. J. Drug Target. 2015, 23, 222–231. [Google Scholar] [CrossRef]
- Vimalson, D.C.; Parimalakrishnan, S.; Jeganathan, N.S.; Anbazhagan, S. Techniques to Enhance Solubility of Hydrophobic Drugs: An Overview. Asian J. Pharm. (AJP) 2016, 10, 195727. [Google Scholar]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles horizontal line From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Zhou, Q.A. PEGylated Lipid Nanoparticle Formulations: Immunological Safety and Efficiency Perspective. Bioconjugate Chem. 2023, 34, 941–960. [Google Scholar] [CrossRef]
- Davis, F.F. The origin of pegnology. Adv. Drug Deliv. Rev. 2002, 54, 457–458. [Google Scholar] [CrossRef]
- Che, J.; IOkeke, C.; Hu, Z.-B.; Xu, J. DSPE-PEG: A Distinctive Component in Drug Delivery System. Curr. Pharm. Des. 2015, 21, 1598–1605. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Mao, A.; Wong, P.; Larsen, A.; Yazaki, P.J.; Wong, J.Y.C.; Shively, J.E. Characterization of 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[Methoxy(polyethylene glycerol)-2000] and Its Complex with Doxorubicin Using Nuclear Magnetic Resonance Spectroscopy and Molecular Dynamics. Bioconjugate Chem. 2017, 28, 1777–1790. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as well as Potential Alternatives. Angew. Chem. Int. Ed. Engl. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Paolino, D.; Accolla, M.L.; Cilurzo, F.; Cristiano, M.C.; Cosco, D.; Castelli, F.; Sarpietro, M.G.; Fresta, M.; Celia, C. Interaction between PEG lipid and DSPE/DSPC phospholipids: An insight of PEGylation degree and kinetics of de-PEGylation. Colloids Surf. B Biointerfaces 2017, 155, 266–275. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef]
- Zeineldin, R.; Al-Haik, M.; Hudson, L.G. Role of Polyethylene Glycol Integrity in Specific Receptor Targeting of Carbon Nanotubes to Cancer Cells. Nano Lett. 2009, 9, 751–757. [Google Scholar] [CrossRef]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J.Y. Emerging Research and Clinical Development Trends of Liposome and Lipid Nanoparticle Drug Delivery Systems. J. Pharm. Sci. 2013, 103, 29–52. [Google Scholar] [CrossRef]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Jain, S.; Cherukupalli, S.K.; Mahmood, A.; Gorantla, S.; Rapalli, V.K.; Dubey, S.K.; Singhvi, G. Emerging nanoparticulate systems: Preparation techniques and stimuli responsive release characteristics. J. Appl. Pharm. Sci. 2019, 9, 130–143. [Google Scholar] [CrossRef]
- Ojha, S.; Saikia, D.; Bora, U. Nanopharmaceuticals: Synthesis, Characterization, and Challenges. Nanopharmaceuticals Princ. Appl. 2020, 3, 81–138. [Google Scholar]
- Terada, T.; Mizobata, M.; Kawakami, S.; Yamashita, F.; Hashida, M. Optimization of tumor-selective targeting by basic fibroblast growth factor-binding peptide grafted PEGylated liposomes. J. Control. Release 2007, 119, 262–270. [Google Scholar] [CrossRef]
- Rathore, S.S.; Ghosh, P.C. Effect of surface charge and density of distearyl-phosphatidylethanolamine-mPEG-2000 (DSPE-mPEG-2000) on the cytotoxicity of liposome-entrapped ricin: Effect of lysosomotropic agents. Int. J. Pharm. 2008, 350, 79–94. [Google Scholar] [CrossRef]
- Yousefi, A.; Esmaeili, F.; Rahimian, S.; Atyabi, F.; Dinarvand, R. Preparation and In Vitro Evaluation of a PEGylated Nano-Liposomal Formulation Containing Docetaxel. Sci. Pharm. 2009, 77, 453–464. [Google Scholar] [CrossRef]
- Vukovic, L.; Khatib, F.A.; Drake, S.P.; Madriaga, A.; Brandenburg, K.S.; Kral, P.; Onyuksel, H. Structure and Dynamics of Hghly PEG-ylated Sterically Stabilized Micelles in Aqueous Media. J. Am. Chem. Soc. 2011, 133, 13481–13488. [Google Scholar] [CrossRef]
- Wang, D.; Qian, J.; He, S.; Park, J.S.; Lee, K.-S.; Han, S.; Mu, Y. Aggregation-enhanced fluorescence in PEGylated phospholipid nanomicelles for in vivo imaging. Biomaterials 2011, 32, 5880–5888. [Google Scholar] [CrossRef]
- Kim, C.-E.; Lim, S.-K.; Kim, J.-S. In vivo antitumor effect of cromolyn in PEGylated liposomes for pancreatic cancer. J. Control. Release 2012, 157, 190–195. [Google Scholar] [CrossRef]
- Cho, H.-Y.; Lee, C.K.; Lee, Y.-B. Preparation and Evaluation of PEGylated and Folate-PEGylated Liposomes Containing Paclitaxel for Lymphatic Delivery. J. Nanomater. 2015, 2015, 471283. [Google Scholar] [CrossRef]
- Korani, M.; Ghaffari, S.; Attar, H.; Mashreghi, M.; Jaafari, M.R. Preparation and characterization of nanoliposomal bortezomib formulations and evaluation of their anti-cancer efficacy in mice bearing C26 colon carcinoma and B16F0 melanoma. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102013. [Google Scholar] [CrossRef]
- Sai, N.; Dong, X.; Huang, P.; You, L.; Yang, C.; Liu, Y.; Wang, W.; Wu, H.; Yu, Y.; Du, Y.; et al. A Novel Gel-Forming Solution Based on PEG-DSPE/Solutol HS 15 Mixed Micelles and Gellan Gum for Ophthalmic Delivery of Curcumin. Molecules 2019, 25, 81. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, W.; Zhang, L.; Zhang, C.; Zhou, C.; Wei, W.; Li, Y.; Zhou, Q.; Chen, W.; Tang, Y. Targeting Peptide, Fluorescent Reagent Modified Magnetic Liposomes Coated with Rapamycin Target Early Atherosclerotic Plaque and Therapy. Pharmaceutics 2022, 14, 1083. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.; Zhao, D.; Yan, N.; Zhang, H.; Liu, M.; Tang, X.; Hu, Y.; Ding, J.; Zhang, N.; et al. Branched PEG-modification: A new strategy for nanocarriers to evade of the accelerated blood clearance phenomenon and enhance anti-tumor efficacy. Biomaterials 2022, 283, 121415. [Google Scholar] [CrossRef]
- Moiseev, R.V.; Kaldybekov, D.B.; Filippov, S.K.; Radulescu, A.; Khutoryanskiy, V.V. Maleimide-Decorated PEGylated Mucoadhesive Liposomes for Ocular Drug Delivery. Langmuir 2022, 38, 13870–13879. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, L.; Zhong, Y.; Zhang, Y.; Yin, Q.; Li, S.; Zhang, X.; Han, H.; Yao, K. Ferrostatin-1-loaded liposome for treatment of corneal alkali burn via targeting ferroptosis. Bioeng. Transl. Med. 2022, 7, e10276. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Suslick, K.S.; Price, G.J. Applications of Ultrasound to Materials Chemistry. Annu. Rev. Mater. Res. 1999, 29, 295–326. [Google Scholar] [CrossRef]
- Rokita, B.; Ulański, P. Studies on the spatial distribution of polymeric reagents in sonochemical reactions-application of competitive kinetics. Polimery 2005, 50, 29–36. [Google Scholar] [CrossRef][Green Version]
- Suslick, K.S.; Flannigan, D.J. Inside a Collapsing Bubble: Sonoluminescence and the Conditions During Cavitation. Annu. Rev. Phys. Chem. 2008, 59, 659–683. [Google Scholar] [CrossRef]
- Singla, R.; Grieser, F.; Ashokkumar, M. Kinetics and Mechanism for the Sonochemical Degradation of a Nonionic Surfactant. J. Phys. Chem. A 2009, 113, 2865–2872. [Google Scholar] [CrossRef]
- Koda, S.; Mori, H.; Matsumoto, K.; Nomura, H. Ultrasonic degradation of water soluble polymers. Polymer 1994, 34, 30–36. [Google Scholar] [CrossRef]
- Destaillats, H.; Hung, H.-M.; Hoffmann, M.R. Degradation of Alkylphenol Ethoxylate Surfactants in Water with Ultrasonic Iirradiation. Environ. Sci. Technol. 2000, 34, 311–317. [Google Scholar] [CrossRef]
- Awoyemi, O.S.; Luo, Y.; Niu, J.; Naidu, R.; Fang, C. Ultrasonic degradation of per-and polyfluoroalkyl substances (PFAS), aqueous film-forming foam (AFFF) and foam fractionate (FF). Chemosphere 2024, 360, 142420. [Google Scholar] [CrossRef]
- Marin, A.; Sun, H.; Husseini, G.A.; Pitt, W.G.; Christensen, D.A.; Rapoport, N.Y. Drug delivery in pluronic micelles: Effect of high-frequency ultrasound on drug release from micelles and intracellular uptake. J. Control. Release 2002, 84, 39–47. [Google Scholar] [CrossRef]
- Husseini, G.A.; Pitt, W.G.; Martins, A.M. Ultrasonically triggered drug delivery: Breaking the barrier. Colloids Surf. B Biointerfaces 2014, 123, 364–386. [Google Scholar] [CrossRef]
- Watanabe, T.; Okabayashi, M.; Kurokawa, D.; Nishimoto, Y.; Ozawa, T.; Kawasaki, H.; Arakawa, R. Determination of primary bond scissions by mass spectrometric analysis of ultrasonic degradation products of poly (ethylene oxide-block-propylene oxide) copolymers. J. Mass Spectrom. 2010, 45, 799–805. [Google Scholar] [CrossRef]
- Wang, R.; Hughes, T.; Beck, S.; Vakil, S.; Li, S.; Pantano, P.; Draper, R.K. Generation of toxic degradation products by sonication of Pluronic® dispersants: Implications for nanotoxicity testing. Nanotoxicology 2013, 7, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; N Meredith, A.; Lee, M., Jr.; Deutsch, D.; Miadzvedskaya, L.; Braun, E.; Pantano, P.; Harper, S.; Draper, R. Toxicity assessment and bioaccumulation in zebrafish embryos exposed to carbon nanotubes suspended in Pluronic® F-108. Nanotoxicology 2016, 10, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, S.P.; Madras, G. Effect of temperature on the ultrasonic degradation of polyacrylamide and poly (ethylene oxide). Polym. Degrad. Stab. 2004, 84, 341–344. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.P.; Madras, G. Effect of initial molecular weight and solvents on the ultrasonic degradation of poly (ethylene oxide). Polym. Degrad. Stab. 2005, 90, 116–122. [Google Scholar] [CrossRef]
- Kawasaki, H.; Takeda, Y.; Arakawa, R. Mass Spectrometric Analysis for High Molecular Weight Synthetic Polymers Using Ultrasonic Degradation and the Mechanism of Degradation. Anal. Chem. 2007, 79, 4182–4187. [Google Scholar] [CrossRef]
- Murali, V.S.; Wang, R.; Mikoryak, C.A.; Pantano, P.; Draper, R. Rapid detection of polyethylene glycol sonolysis upon functionalization of carbon nanomaterials. Exp. Biol. Med. 2015, 240, 1147–1151. [Google Scholar] [CrossRef]
- Weissler, A. Depolymerization by Ultrasonic Irradiation: The Role of Cavitation. J. Appl. Phys. 1950, 21, 171–173. [Google Scholar] [CrossRef]
- Berkowski, K.L.; Potisek, S.L.; Hickenboth, C.R.; Moore, J.S. Ultrasound-Induced Site-Specific Cleavage of Azo-Functionalized Poly(ethylene glycol). Macromolecules 2005, 38, 8975–8978. [Google Scholar] [CrossRef]
- Donbrow, M.; Hamburger, R.; Azaz, E.; Pillersdorf, A. Development of Acidity in Non-ionic Surfactants: Formic and Acetic Acid. Analyst 1978, 103, 400–402. [Google Scholar] [CrossRef]
- Yang, L.; Heatley, F.; Blease, T.G.; Thompson, R.I.G. A Study of the Mechanism of the Oxidative Thermal Degradation of Poly(Ethylene Oxide) and Poly(Propylene Oxide) using 1H- and 13C-NMR. Eur. Polym. J. 1996, 32, 535–547. [Google Scholar] [CrossRef]
- Gallet, G.; Carroccio, S.; Rizzarelli, P.; Karlsson, S. Thermal degradation of poly (ethylene oxide–propylene oxide–ethylene oxide) triblock copolymer: Comparative study by SEC/NMR, SEC/MALDI-TOF-MS and SPME/GC-MS. Polymer 2002, 43, 1081–1094. [Google Scholar] [CrossRef]
- Gallet, G.; Erlandsson, B.; Albertsson, A.-C.; Karlsson, S. Thermal oxidation of poly (ethylene oxide–propylene oxide–ethylene oxide) triblock copolymer: Focus on low molecular weight degradation products. Polym. Degrad. Stab. 2002, 77, 55–66. [Google Scholar] [CrossRef]
- de Sainte Claire, P. Degradation of PEO in the Solid State: A Theoretical Kinetic Model. Macromolecules 2009, 42, 3469–3482. [Google Scholar] [CrossRef]
- Payne, M.E.; Kareem, O.O.; Williams-Pavlantos, K.; Wesdemiotis, C.; Grayson, S.M. Mass spectrometry investigation into the oxidative degradation of poly (ethylene glycol). Polym. Degrad. Stab. 2021, 183, 109388. [Google Scholar] [CrossRef]
- Taurozzi, J.S.; Hackley, V.A.; Wiesner, M.R. Ultrasonic dispersion of nanoparticles for environmental, health and safety assessment--issues and recommendations. Nanotoxicology 2011, 5, 711–729. [Google Scholar] [CrossRef]
- Watanabe, T.; Kawasaki, H.; Kimoto, T.; Arakawa, R. Characterization of polyether mixtures using thin-layer chromatography and matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 787–791. [Google Scholar] [CrossRef]
- Hemenway, J.N.; Carvalho, T.C.; Rao, V.M.; Wu, Y.; Levons, J.K.; Narang, A.S.; Paruchuri, S.R.; Stamato, H.J.; Varia, S.A. Formation of reactive impurities in aqueous and neat polyethylene glycol 400 and effects of antioxidants and oxidation inducers. J. Pharm. Sci. 2012, 101, 3305–3318. [Google Scholar] [CrossRef]
- Johnsson, M.; Hansson, P.; Edwards, K. Spherical Micelles and Other Self-Assembled Structures in Dilute Aqueous Mixtures of Poly(Ethylene Glycol) Lipids. J. Phys. Chem. B 2001, 105, 8420–8430. [Google Scholar] [CrossRef]
- Staples, E.J.; Tiddy, G.J.T. Nuclear Magnetic Resonance Technique to Distinguish between Micelle Size Changes and Secondary Aggregation in Anionic and Nonionic Surfactant Solutions. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1978, 74, 2530–2541. [Google Scholar] [CrossRef]
- Leal, C.; Rögnvaldsson, S.; Fossheim, S.; Nilssen, E.A.; Topgaard, D. Dynamic and structural aspects of PEGylated liposomes monitored by NMR. J. Colloid Interface Sci. 2008, 325, 485–493. [Google Scholar] [CrossRef]
- Khashami, F. Molecular Motion, Correlation, and Relaxation Time. In Fundamentals of NMR and MRI: From Quantum Principles to Medical Applications; Khashami, F., Ed.; Springer Nature: Cham, Switzerland, 2023; Chapter 5; pp. 91–106. [Google Scholar]
- Berregi, I.; Del Campo, G.; Caracena, R.; Miranda, J.I. Quantitative determination of formic acid in apple juices by 1H NMR spectrometry. Talanta 2007, 72, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Intrator, J.A.; Velazquez, D.A.; Fan, S.; Mastrobattista, E.; Yu, C.; Marinescu, S.C. Electrocatalytic CO2 reduction to formate by a cobalt phosphino–thiolate complex. Chem. Sci. 2024, 15, 6385–6396. [Google Scholar] [CrossRef]
- Hayreh, M.S.; Hayreh, S.S.; Baumbach, G.L.; Cancilla, P.; Martin-Amat, G.; Tephly, T.R.; McMartin, K.E.; Makar, A.B. Methyl alcohol poisoning: III. Ocular toxicity. Arch. Ophthalmol. 1977, 95, 1851–1858. [Google Scholar] [CrossRef]
- Sharpe, J.A.; Hostovsky, M.; Bilbao, J.M.; Rewcastle, N.B. Methanol optic neuropathy: A histopathological study. Neurology 1982, 32, 1093. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.G.; Burton, T.C.; Rajani, C.; Lewandowski, M.F.; Burke, J.M.; Eells, J.T. Methanol Poisoning: A Rodent Model with Structural and Functional Evidence for Retinal Involvement. Arch. Ophthalmol. 1991, 109, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Eells, J.T.; Salzman, M.M.; Lewandowski, M.F.; Murray, T.G. Formate-Induced Alterations in Retinal Function in Methanol-Intoxicated Rats. Toxicol. Appl. Pharmacol. 1996, 140, 58–69. [Google Scholar] [CrossRef]
- Wallace, K.B.; Eells, J.T.; Madeira, V.M.C.; Cortopassi, G.; Jones, D.P. Symposium overview: Mitochondria-mediated cell injury. Fundam. Appl. Toxicol. 1997, 38, 23–37. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Hayasaka, S.; Nagaki, Y. Ocular Changes after Intravitreal Injection of Methanol, Formaldehyde, or Formate in Rabbits. Pharmacol. Toxicol. 2001, 89, 74–78. [Google Scholar] [CrossRef]
- Treichel, J.L.; Henry, M.M.; Skumatz, C.M.B.; Eells, J.T.; Burke, J.M. Formate, the Toxic Metabolite of Methanol, in Cultured Ocular Cells. Neurotoxicology 2003, 24, 825–834. [Google Scholar] [CrossRef]
- Johnson, W., Jr.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Formic Acid and Sodium Formate as Used in Cosmetics. Int. J. Toxicol. 2016, 35 (Suppl. S2), 41S–54S. [Google Scholar] [CrossRef]
- Almeida, H.; Lobão, P.; Frigerio, C.; Fonseca, J.; Silva, R.; Sousa Lobo, J.M.; Amaral, M.H. Preparation, characterization and biocompatibility studies of thermoresponsive eyedrops based on the combination of nanostructured lipid carriers (NLC) and the polymer Pluronic F-127 for controlled delivery of ibuprofen. Pharm. Dev. Technol. 2017, 22, 336–349. [Google Scholar] [CrossRef]
- Han, H.; Li, S.; Xu, M.; Zhong, Y.; Fan, W.; Xu, J.; Zhou, T.; Ji, J.; Ye, J.; Yao, K. Polymer- and lipid-based nanocarriers for ocular drug delivery: Current status and future perspectives. Adv. Drug Deliv. Rev. 2023, 196, 114770. [Google Scholar] [CrossRef]
- Fang, R.H.; Aryal, S.; Hu, C.-M.J.; Zhang, L. Quick Synthesis of Lipid−Polymer Hybrid Nanoparticles with Low Polydispersity Using a Single-Step Sonication Method. Langmuir 2010, 26, 16958–16962. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Min, C.-N.; Wu, H.-C.; Lin, C.-T.; Hsieh, W.-Y. In vitro evaluation of the L-peptide modified magnetic lipid nanoparticles as targeted magnetic resonance imaging contrast agent for the nasopharyngeal cancer. J. Biomater. Appl. 2012, 28, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Zhou, X.; Sun, J.; Fan, X.; Guan, Z.; Zhang, L.; Yang, Z. Construction of a Targeting Nanoparticle of 3′,3″-Bis-Peptide-siRNA Conjugate/Mixed Lipid with Postinserted DSPE-PEG2000-cRGD. Mol. Pharm. 2019, 16, 4920–4928. [Google Scholar] [CrossRef]
- Nascentes, C.C.; Korn, M.; Sousa, C.S.; Arruda, M.A.Z. Use of Ultrasonic Baths for Analytical Applications: A New Approach for Optimisation Conditions. J. Braz. Chem. Soc. 2001, 12, 57–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
