Abstract
Lung cancer remains one of the most common and deadliest forms of cancer worldwide despite notable advancements in its management. Conventional treatments, such as chemotherapy, often have limitations in effectively targeting cancer cells, which frequently lead to off-target side effects. In this context, the pulmonary delivery of inhalable nanomaterials offers the advantages of being rapid, efficient, and target-specific, with minimal systemic side effects. This concise review summarizes the basic research and clinical translation of inhalable nanomaterials for the treatment of lung cancer. We also provide insights into the latest advances in pulmonary drug delivery systems, focusing on various types of pulmonary devices and nanomaterials. Furthermore, this paper discusses significant challenges in translating the discoveries of inhalable nanomaterials into clinical care for lung cancer and shares strategies to overcome these issues.
1. Introduction
Cancer is a complex disease driven by genetic and epigenetic changes, influenced by environmental factors and the microbiome, and it remains the second leading cause of death worldwide [1]. Among the various types of cancer, lung cancer stands out as the primary cause of cancer-related deaths globally, with an annual death toll of 1.76 million and a high incidence rate [2]. Its complexity arises from a combination of non-genetic, epigenetic, and genetic variables, which complicate its diagnosis and treatment [3,4]. Non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) are the two main subtypes of lung cancer, with NSCLC accounting for the majority of cases and posing challenges due to a poor prognosis and a late diagnosis. Cigarette smoking is a major contributor to lung cancer deaths [5]. Despite advancements in oncology, non-small cell lung cancer (NSCLC) often evades early detection and has a low overall 5-year survival rate. Conventional treatments for lung cancer include surgery, chemotherapy, and radiotherapy, with chemotherapy playing a significant role [6]. Although some adjuvant therapies have been introduced in recent years to enhance the effectiveness of lung cancer treatment, these strategies have not significantly improved overall patient survival. Suboptimal drug delivery efficiency, drug resistance, and severe systemic side effects remain among the most common challenges. underscores the urgent need for more effective approaches to treating lung cancer [7]. Oral and parenteral routes remain the preferred methods for administering chemotherapeutics like cisplatin and carboplatin in lung cancer treatment, typically resulting in better distribution of these cytotoxic drugs after administration, often leading to significant off-target toxicity [8]. However, in light of the limitations associated with systemic drug administration, alternative strategies are being explored—most notably,
Pulmonary drug delivery is preferred for respiratory diseases because it provides localized action with minimal systemic effects [9]. This preference is supported by the lung’s surface area, thin alveolar blood barriers, relatively low enzymatic activity, and rich vascularization, which allow inhaled drugs to achieve higher concentrations in the lungs and enable easier administration [10]. The extracellular matrix (ECM) is a dynamic and organized network of macromolecules that provides mechanical support and biochemical signals to surrounding cells. In healthy lungs, the ECM is essential for maintaining tissue architecture, facilitating gas exchange, and regulating processes such as cell adhesion, migration, and repair. It consists of fibrillar collagens (types I, III, IV), elastin, adhesive glycoproteins (fibronectin, laminin, vitronectin), and proteoglycans (decorin, biglycan, perlecan), along with glycosaminoglycans like hyaluronan [11].
While the ECM preserves tissue structure and elasticity in normal lungs, its composition and mechanical properties undergo significant alterations in lung cancer [12]. ECM stiffness increases dramatically, with macro metastases exhibiting a tenfold increase compared to healthy lung tissue [12]. The ECM critically regulates the behavior of nanoparticles NPs in biological systems by modulating their penetration, distribution, and therapeutic efficacy. In the tumor and lung microenvironments [13]. The surface chemistry of NPs influences their interactions with ECM components like fibronectin, potentially disrupting normal ECM function [10]. When inhaled, NPs with different functional groups exhibit varying intrapulmonary distributions and inflammatory responses, which correlate with their protein corona composition. Understanding these interactions is crucial for improving nanomaterial design and clinical translation in cancer treatment [13].
Additionally, this knowledge aids in assessing health risks associated with inhaled ultrafine particles and informs the development of more effective nanoparticle-based strategies for tumor targeting and diagnosis [10]. Nano-based systems provide additional diagnostic benefits during lung cancer treatment, including imaging, screening, and monitoring [10]. NPs offer promising avenues for drug delivery to both the bloodstream and lungs, with each route presenting unique challenges and opportunities. The interaction between NPs and biological environments differs significantly between the bloodstream and the lung extracellular matrix (ECM). In the lungs, pulmonary surfactant protein A (SP-A) enhances NP uptake by alveolar macrophages, while albumin in plasma decreases it [14]. In the bloodstream, proteins adsorb onto NPs, forming a protein corona (PC) that influences their behavior and fate [15]. The protein corona formed on NPs in lung surfactant is distinct from that in plasma, with a prevalence of lipid-binding proteins, such as surfactant protein A (SP-A) and surfactant protein D (SP-D) [16]. Interestingly, the lipid composition of the corona remains relatively consistent across different NP types in lung surfactants [16]. Inhaled NP therapies offer advantages such as targeted lung delivery, enhanced stability, and reduced systemic side effects [17]. Despite its benefits, pulmonary delivery is still in its early stages of research, and several issues, such as pharmacology, immunology, and toxicity, must be considered for the development of acceptable inhalable nano-based chemotherapeutic agents [18]. To address the current challenges in inhalable therapies, significant research has focused on nanoparticle-based delivery systems, which offer versatile platforms for enhancing pulmonary drug targeting and therapeutic outcomes.
Nanoparticles (NPs) have emerged as promising drug delivery systems, offering targeted and efficient delivery of therapeutics. Various types of nanocarriers are being studied, including polymeric nanoparticles, liposomes, dendrimers, micelles, and microparticles [19]. Compared to macro-sized particles, NPs possess a greater surface-to-volume ratio. This allows for higher drug loading capacity and the ability to form multiple interactions with various molecules, including receptors on the surface of target cells. By leveraging the enhanced permeability and retention (EPR) effect, NPs promote targeted drug accumulation at the site of interest, reducing exposure to healthy tissues and minimizing adverse side effects while improving bioavailability [20].
This review aims to provide a comprehensive assessment of the use of drug delivery systems based on inhalable nanoparticles for the targeted treatment of lung cancer. It also discusses an overview of lung cancer, including its prevalence and established treatment modalities. Subsequently, the review scrutinizes the characteristics of various nanoparticles employed in inhalable liposomes, with polymeric nanoparticles serving as examples of drug delivery mechanisms, emphasizing their capacity to enhance treatment efficacy. The review examines targeting strategies, encompassing both passive and active mechanisms, and surveys the diverse range of therapeutic agents administered via inhalable nanoparticles, spanning from chemotherapy to immunotherapy.
Additionally, the review addresses challenges inherent in this approach, such as potential pulmonary toxicity and the imperative for scalability in clinical applications. It also contemplates future research trajectories, such as personalized medicine and combination therapies. To summarize, this review provides an invaluable resource for researchers and clinicians seeking to gain a comprehensive understanding of the current state and prospects of drug delivery by inhalable nanoparticles in the treatment of targeted lung cancer.
2. Delivery Devices for Pulmonary Drug Delivery
The two determining factors for successful inhalation therapy are the optimal pharmacological properties of NPs and the delivery devices. Dry powder inhalers (DPIs), pressurized metered dosage inhalers (pMDIs), and nebulizers are the three primary inhalation devices used to provide drugs to the lungs [21] (Figure 1).
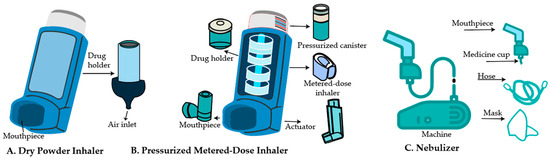
Figure 1.
Schematic illustration of clinically used inhalable devices for pulmonary drug delivery. The figure depicts the three main classes of inhalation devices used in clinical practice: (A) Dry Powder Inhaler (DPI)—a breath-actuated device delivering drug powder to the lungs; (B) Pressurized Metered Dose Inhaler (pMDI)—a propellant-based system that releases a fixed dose of aerosolized medication; and (C) Nebulizer—a device that converts liquid formulations into inhalable aerosols.
2.1. Selection Criteria for Inhalation Devices Based on Nanomaterial Properties in Lung Cancer Therapy
In addition to designing nanocarriers for inhalation drug delivery, selecting a suitable aerosol device, such as nebulizers and inhalers (including soft mist, pressurized metered-dose, and dry powder), is also a critical consideration [22]. Historically, these devices have been evaluated for delivering nanocarrier-based drugs [22]. Pressurized metered-dose inhalers (pMDIs) deliver nanocarriers by mixing them with a propellant that releases a precise drug dose upon actuation [23]. Breath-actuated devices, such as dry powder inhalers, are well-suited for delivering moisture-sensitive formulations, including genes or gene-nanocarrier complexes, especially after they have undergone drying techniques like lyophilization, spray drying, or aerosolization to achieve the required aerodynamic size [24]. The delivery of nanocarriers to deeper lung regions can be effectively accomplished using propellant-free aerosols, commonly referred to as soft mist inhalers [25]. Metered-dose inhalers are limited by their inability to deliver large aerosol volumes and require precise patient coordination, which can hinder effective drug delivery to targeted lung cancer sites [26]. Nebulizers are widely used for the pulmonary delivery of nanocarriers due to their ability to produce aerosols with sufficiently large droplet sizes, enabling prolonged administration and effective deposition in deep-seated tumor regions. Clinically, they have demonstrated an ability to generate fine particle fractions conducive to optimal lung deposition at relatively low drug doses. Nonetheless, the therapeutic efficiency of nebulized formulations is influenced by critical physicochemical parameters, including surface tension, osmolarity, active pharmaceutical ingredient concentration, nebulization rate, and aerosol output flow rate [27]. In summary, selecting an appropriate inhalation device tailored to the physicochemical properties of the nanocarrier and the therapeutic target Table 1 within the lungs is crucial for maximizing drug delivery efficiency and clinical outcomes in lung cancer treatment.

Table 1.
Comparative Overview of Inhalation Devices for Nano-Formulations in Lung Cancer Therapy adapted from [28].
2.2. Dry Powder Inhalers (DPIs)
DPIs are used to deliver high-dose medications directly to the lungs, offering promise in the treatment of various pulmonary and systemic diseases [29]. These devices can be made with micronized particles or lactose-based mixtures to enhance medication delivery to the lungs and potentially throughout the body [30]. They provide several benefits in pulmonary drug delivery, including direct deposition in the deep lungs and the potential for systemic drug administration [31]. Research on dry powders for inhalation has investigated both carrier-based mixtures and lactose-free formulations [32]. Traditional DPI formulations use micronized drugs mixed with coarse carriers, but their efficacy is low (12–30% lung delivery). Micronization can create amorphous domains, which can affect performance. Nano and micro-composite particles produced through wet milling and spray drying present a promising alternative [33]. Several factors influence the performance of DPIs, including device design, particle size, flow properties, formulation, drug-carrier adhesion, and respiratory flow rate [34], device design, particularly the piercing aperture location and inlet (Figure 1). The flow rate has a significant impact on device emptying and particle deposition [29]. DPI devices are currently classified into three major types based on their design: first-generation, single-dose DPIs; second-generation, multiple-dose DPIs; and third-generation, commonly known as ‘active’ or power-assisted DPIs. The first generation, such as the Rotahaler® (GlaxoSmithKline, Brentford, London, UK) and the newer Handihaler® (Boehringer Ingelheim, Ingelheim, Germany) and Breezhaler® (Novartis Pharma, Basel, Switzerland), consists of breath-activated, single-dose devices in which a capsule of powder is perforated in the device with needles fixed to pressure buttons. With these inhalers, drug delivery is influenced by particle size and de-agglomeration of drug carrier agglomerates or mixtures delivered by the patient’s inspiratory flow. Some newly developed DPIs or existing devices are designed for new powder formulations. Proper usage of the inhaler is vital for achieving the intended therapeutic benefits [35]. Challenges in DPI development include optimizing powder dispersion and understanding drug-device-patient interactions to enhance lung deposition [36]. There is a wide selection of breath-activated or power-driven DPI devices on the market that can deliver one or several doses. Nonetheless, since a device’s design influences its performance, new and innovative device designs are still being developed. Combining suitable powder compositions with DPI designs that generate small-particle aerosols remains a challenge [37].
The powder formulation is aerosolized through a DPI device, where drug particles are separated from the carrier (from drug carrier mixtures or de-agglomerated drug particles), and the dose is delivered into the patient’s deep lungs. In these systems, particle size and flow properties, formulation, drug-carrier adhesion, respiratory flow rate, and the design of DPI devices significantly affect performance [34]. The airflow resistance of the DPI design impacts lung deposition, varying from 0.02 to 0.2 cm H2O/L/min. Low-resistance DPIs require a flow rate exceeding 90 L/min [38]. Second-generation DPIs encompass multidose devices that measure doses from a reservoir and multi-unit devices that dispense pre-metered doses. Examples include the Turbuhaler® and Diskus, which represent a distinct category [35]. Second-generation DPIs, such as NEXThaler, Ellipta, and Genuair, provide convenient multidose delivery with specific drug combinations for asthma and COPD [39]. Aclidinium bromide, when delivered via inhaler, achieves high lung deposition in healthy subjects, with approximately 30% of the metered dose reaching the lungs [40,41].
2.3. Pressurized Metered-Dose Inhalers (pMDIs)
The most widely used drug delivery devices for inhalation therapy are nebulizers, pressurized metered-dose inhalers (pMDIs), and dry powder inhalers (DPIs), with pMDIs being the most favored and the most frequently prescribed by both physicians and patients [42]. Riker Laboratories introduced the pressurized metered-dose inhaler (pMDI) in 1956, marking a pivotal advancement in pharmaceutical aerosol delivery [43]. that revolutionized the industry. Despite significant technological progress, including modern inhaler systems with improved delivery efficiency, ensuring proper patient use remains a challenge, as highlighted by a Japanese study, which found that 24.2% of patients misused pMDIs [44,45]. The effectiveness of pMDIs can be attributed to their key components: an aluminum canister, a metering valve, and an actuator (Figure 1) [46]. These devices use propellants such as “HFA 134a” or “HFA 227” to produce a short burst of aerosol that delivers precise, pre-measured amounts of medication straight to the lungs [47]. The aerodynamic behavior of these NPs is crucial for effective delivery, with factors such as particle size, morphology, and density influencing their deposition in the airways [47]. Their performance is greatly influenced by the aerodynamic particle size distribution [46].
Moreover, the propellants used in pMDIs, such as hydrofluoroalkanes (HFAs), have been found to affect oxygen levels in valved holding chambers and ventilator delivery devices [48]. In terms of formulation, suspension formulations in pMDIs often incorporate surfactants to prevent bubble coalescence, resulting in a more stable discharge. Additionally, the metering valve plays a critical role in ensuring precise dosing, making the uniformity of dose mass and dose of tiny particles, which are crucial characteristics [49,50]. Formulation variables, such as surfactant and cosolvent concentrations, also significantly impact pMDI performance and lung deposition. For example, oleic acid, a surfactant, can increase lung deposition of beclomethasone dipropionate solution from 24% to 46%, while ethanol, a cosolvent, may decrease lung deposition in suspension formulations [51].
2.4. Nebulizer
Nebulizers generate aerosols containing active substances, with jet, ultrasonic, and membrane types, each offering distinct advantages and limitations [52]. Nebulizers consist of a mask, a hose with a mouthpiece, and a medicine cup attached to a machine (Figure 1) that converts liquid medication into a mist for inhalation via the mouthpiece. Jet nebulizers, commonly used in clinical practice, produce aerosols via high-velocity gas impact, requiring a flow rate of 6–8 L/min and a fill capacity of 4–5 mL. Despite their widespread use, these nebulizers have notable limitations, including significant drug losses and dependency on patient capacity [53]. In contrast, vibrating mesh nebulizers are more efficient and less affected by humidification, making them preferable in specific settings [54]. Ultrasonic nebulizers, which utilize ultrasonic vibrations to generate aerosol droplets, enable control over droplet size through adjustment of the oscillation frequency [55]. However, while effective in delivering inhaled pharmaceutical aerosols, nebulizers often suffer from poor lung delivery efficiency, leading to medication wastage and prolonged delivery times [56]. The evolution of nebulizer technology, particularly with the advent of vibrating mesh nebulizers, has significantly enhanced the therapeutic potential of these devices [57]. Further improvements in nubilizer efficacy can be achived through secondry devices and stratgies such as those that modifiey aerosol size distribution, synchronize drug adminstration with inhalation, reduces system deposionational loses, enhance the pacient interface and guide pacient breathing [56]. Studies have investigated the operating parameters of vibrating mesh nebulizers, finding that droplet size decreases with increasing resonance frequency [58]. These studies have also hightlited the advantages of vibrating mesh nublizer, including consistant drug delivery and low shear force [59].
Additionally, the effect of electrolyte concentration on droplet size and output rate in vibrating membrane nebulizers advances our understanding of their performance and prospective applications. Optimization of ultrasonic nebulizers can be achieved by using a single-frequency ultrasonic atomizer, capable of generating uniform atomized droplets [60]. Droplet size can be further controlled by tuning actuation parameters [60]. A high-frequency vibration system for liquid atomization has also been developed, producing highly dispersed aerosols with specific droplet sizes [61].
Despite their advantages, ultrasonic nebulizers are limited by their inability to nebulize suspensions and the potential to heat the liquid drug, rendering them unsuitable for thermolabile medications. However, they operate silently and efficiently nebulize solutions [62]. The latest advancements in nebulizer technology, particularly with vibrating mesh nebulizers like the eFlow® and MicroAir®, have overcome the drawbacks of Traditional Jet and ultrasonic nebulizers. These modern nebulizers offer advantages such as reduced drug loss, noiseless operation, and enhanced portability [63]. They are also capable of aerosolizing a wide range of pharmaceutical formulations without compromising their integrity [58]. Notably, the new generation of nebulizers utilizing hybrid resonant acoustic (HYDRA) technology has demonstrated excellent aerosol deposition performance in vivo [64]. These technological breakthroughs show considerable promise. For improving drug delivery and patient adherence [65].
2.5. Other Inhaler Technology
The Respimat® Soft Mist Inhaler is a portable device developed to improve medicine delivery to the lungs while reducing patient coordination requirements and improving ease of use [66]. The new reusable Respimat inhaler maintains performance and ensures consistent drug delivery, unaffected by the design [67]. Additionally, it also boasts environmental benefits by reducing the environmental impact compared to traditional Pressurized, metered-dose inhalers [63]. The Easyhaler® is another handy inhaler for daily use in individuals with asthma and COPD [68].
3. Clinical Trials of Inhaled Drug Delivery
Despite extensive preclinical success with inhaling nanocarriers for the treatment of lung cancer, few have progressed to human testing. One notable example is aerosolized liposomal 9-nitro camptothecin (9NC), which in a Phase I trial achieved 4–10× higher lung drug levels than plasma, exhibited manageable systemic toxicity at doses up to 20 µg/kg/day, and produced preliminary tumor responses—leading to a recommended Phase II dose of 13.3 µg/kg/day [69]. The liposomal formulation has shown promise for the pulmonary delivery of anticancer drugs in the treatment of lung cancer. These nanocarriers can enhance drug targeting, reduce systemic toxicity, and provide sustained release in the lungs [70]. Liposomes can effectively entrap various therapeutic agents and deliver them to the peripheral airways using nebulizers, although sharing during aerosolization may affect stability [71]. Affect stability Clinical trials have demonstrated the potential of inhaled liposomal formulations, such as Arikace® and Pulmaquin®, for treating lung infections [71]. The sustained-release lipid inhalation targeting aerosol was produced using a PARI LC® Star nebulizer (PARI Lifescience, Starnberg, Germany), achieving an MMAD of 3.7 µm and a GSD of 1.9 µm. This aerosolized approach showed significantly reduced systemic toxicities (hematotoxicity, neurotoxicity, and nephrotoxicity) compared to oral cisplatin administration, indicating that combining nanocarrier formulations with inhalation delivery effectively lowers adverse systemic effects. Additionally, a Phase I clinical trial evaluated aerosolized liposomal IL-2 in patients with pulmonary metastases [72,73].
The formulation was administered via a Puritan twin-jet nebulizer (Medtronic, Minneapolis, MN, USA) for 20 min, three times daily, over 8 days. Treatments were well-tolerated without significant toxicity, and extensive preclinical studies are needed to reinforce future clinical findings. Aerosolized sustained-release lipid inhalation targeting (SLIT) cisplatin in 17 patients with advanced lung cancer. Liposomal cisplatin was delivered via a PARI LC Star nebulizer (MMAD 3.7 µm), demonstrating localized pulmonary delivery with minimal systemic exposure. Treatment was well-tolerated, with only mild respiratory effects reported. Stable disease was achieved in 12 patients [73], supporting further clinical evaluation as shown in Table 2. Even though nanocarriers have a lot of potential for the management of lung cancer through the pulmonary route [74]. Amikacin Liposome Inhalation Suspension (Arikayce®) is the first liposomal nanocarrier approved for human inhalation therapy. Delivered via a vibrating-mesh nebulizer, it achieves high pulmonary drug concentrations with minimal systemic exposure. Its FDA approval in 2018 for treatment-refractory MAC lung disease demonstrates the clinical feasibility of inhalable liposomal formulations, supporting their potential translation to lung cancer therapy, as shown in Table 2 [75]. There are not enough clinical investigations on them yet. As a result, there is a need to broaden their scope of applicability to include clinical trials under suitable ethical guidelines.

Table 2.
Clinical Trials of Inhaled Nanocarriers for Lung Cancer via different inhalation devices.
4. Different Nanomaterials for Pulmonary Drug Delivery
4.1. Lipid-Based Nanocarrier for Inhalation
Lipid-based formulations, particularly those containing phospholipids like endogenous lung surfactants, offer biocompatibility and versatility for pulmonary drug delivery. These formulations can enhance therapeutic efficacy, improve pharmacokinetics, and reduce toxicity [79], As illustrated in Figure 2.
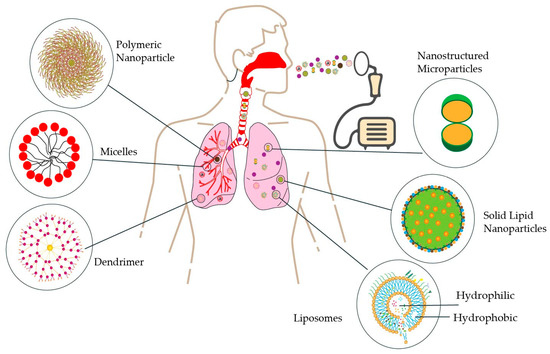
Figure 2.
Various types of nanoparticles are used for inhalable lung cancer therapy. This figure illustrates multiple nanoparticle types—polymeric nanoparticles, micelles, dendrimers, liposomes, solid lipid nanoparticles, and microparticles—employed in inhalable lung cancer treatments, showcasing their diverse structures and mechanisms for targeted drug delivery to the lungs.
4.2. Liposomes
Liposomes have emerged as a leading nano-based drug delivery system, with numerous FDA-approved formulations on the market [80]. These lipid vesicles offer several advantages, including targeted delivery, sustained release, and reduced toxicity [79].
The synthesis method determines the specific type of liposome produced. Liposomes can be categorized into unilamellar vesicles (ULVs) containing a single bilayer membrane, oligolamellar vesicles (OLVs) with 2 to 5 bilayer membranes, and multilamellar vesicles (MLVs) possessing five or more bilayers. ULVs are further subdivided based on size into small unilamellar vesicles (SUVs, 20–100 nm), large unilamellar vesicles (LUVs, 100 nm to 1 µm), and giant unilamellar vesicles (GUVs, >1 µm). Among these, SUVs are most used for drug delivery due to their uniform drug encapsulation, controlled release profiles, and extended circulation times [81].
They have been successfully applied in various therapeutic areas, including cancer, bacterial, and viral diseases [82]. Recent advancements in liposome technology have focused on improving preparation techniques and expanding their application to novel modalities, such as nucleic acid and immunotherapies [80]. The development of targeted liposomal drug delivery systems, particularly for cancer therapy, has been a key area of research [83].
Structurally, liposomes are self-assembled carriers composed of a hydrophobic lipid bilayer enclosing a hydrophilic core, making them ideal for encapsulating water insoluble drugs, particularly chemotherapeutic agents like paclitaxel and doxorubicin [83]. Their size, composition, and stability can be optimized for drug delivery, with recent innovations, such as nano-bowl-supported liposomes, further enhancing stability and delivery efficiency [84]. These properties make liposomes highly promising for cancer treatment, offering active targeting capabilities and reduced off-target toxicity [85]. However, successful clinical translation requires advanced analytical characterization techniques to address the complex attributes of liposomes and lipid nanoparticles [86].
Researchers are developing treatments for liver and lung diseases using synthetic mRNA to replace missing or malfunctioning proteins. In lung cancer treatment, FDA-approved inhalable liposomes, resembling lung components, enhance drug targeting and bioavailability. Ciprofloxacin-loaded liposomes significantly improved these outcomes [87].
PEG-modified unilamellar liposomes loaded with afatinib dimaleate were formulated as a dry powder inhaler (LDPI) for NSCLC. The formulation (181.2 nm, +9.65 mV) demonstrated strong aerodynamic performance (MMAD 2.83 µm, FPF 63.65%, >85% emitted dose), sustained zero-order release across pH 5.5–7.4, and enhanced cytotoxicity in A549 cells (IC50: 1.93 µM vs. 2.58 µM for free drug), along with improved cellular uptake, supporting its potential for targeted pulmonary delivery Table 3 [88] Liposomal curcumin dry powder (MMAD 5.81 µm, FPF 46.71%) also exhibited efficient lung deposition, enhanced cytotoxicity and uptake in A549 cells compared to free curcumin and gemcitabine, and modulated several in vivo biomarkers by reducing TNF-α, MDA and increasing caspase-3 expression, thereby supporting its potential as an inhalable lung cancer therapy [89]. Lipid–polymer hybrid nanoparticles (276.9 nm, −10.6 mV) further improved siRNA delivery via inhalation, achieving 84.83% transfection efficiency in A549 cells, outperforming lipoplexes in 3D A549-3T3 spheroids while maintaining stability in bronchoalveolar lavage fluid (BALF) [90].
Erlotinib and genistein were co-loaded into DPPC:Cholesterol:DOPE (72:8:20 mol%) liposomes (~200 nm, neutral charge) for pulmonary delivery targeting NSCLC. The formulation demonstrated synergistic effects in NSCLC cell lines (H3255, H1650, H1781), achieving a fine particle fraction of up to 70% via air-jet nebulization, which offers efficient lung deposition with potential for localized lung cancer therapy while minimizing systemic toxicity [29]. Inhalable liposomal DPI for NSCLC also demonstrated improved lung deposition (MMAD 3.15 µm, FPF 83.71%), sustained release up to 72 h, enhanced bioavailability inferred from improved lung deposition and dissolution, and reduced systemic toxicity [91]. Furthermore, paclitaxel-loaded conventional liposomes (SPC: Cholesterol, 64.3 nm, −9.96 mV) were encapsulated into E. coli and L. casei (LPB system) for inhaled lung cancer therapy, resulting in high A549 cell uptake, strong apoptosis (98.6%), tumor inhibition in lung cancer rat models, immune activation, and selective lung targeting [8]. Liposomal gefitinib DPI (SPC: Cholesterol, 69.8 nm, −9.67 mV; MMAD 7.83 µm, FPF 33.8%) achieved 2.36-fold higher bioavailability compared to oral delivery, improved lung deposition, tumor reduction, apoptosis induction, and inflammation suppression in primary lung cancer rat models [92]. Rolapitant-loaded nanovesicles (liposomes: 225.1 nm, –24.5 mV) enhanced cytotoxicity, cellular uptake, and permeability in A549 lung cancer cells, inhibited NK-1 receptors and achieved favorable lung biodistribution following intratracheal administration [93]. Finally, spray-dried vincristine-loaded liposomes (112.6 nm, −21.56 mV) achieved efficient lung delivery (FPF 14.99%), sustained release over 96 h, enhanced cytotoxicity in A549 and MCF-7 cells, improved pharmacokinetics (4.29× AUC, 6.3× Cmax, prolonged t½), and reduced metabolism through minimal CYP3A4 activity, offering a promising inhalable lung cancer therapy [94].
4.3. Solid Lipid Nanoparticles
Following liposomes, Solid Lipid Nanoparticles (SLNs) have emerged as prominent nanocarriers for inhalation therapy. Their capacity for efficiently entrapping hydrophobic therapeutic agents, coupled with robust scalability for industrial production, has driven an increased research focus on SLNs in recent years, particularly for cancer applications. Notably, SLN manufacturing utilizes an emulsion system comprising a blend of solid lipids, allowing for synthesis under ambient temperature conditions [95]. Folate-targeted SLNs enabled efficient paclitaxel (PTX) delivery to “folate receptor” FR-overexpressing lung tumors with 32× higher lung retention and sustained release. They demonstrated undetectable systemic exposure and 6-fold greater anti-cancer efficacy in M109-HiFR cells compared to inhaled Taxol. They depict a promising inhalable nanocarrier strategy for targeted, sustained, and safer lung cancer therapy. Refer to Table 3 [96].
Despite their benefits, SLNs have drawbacks, such as limited drug loading and crystallization during storage, which have been addressed by the development of nanostructured lipid carriers (NLCs) [97]. The discovery of nanostructured lipid carriers (NLCs) overcame some of these problems. Offering improved drug release modulation [97]. The self-assembly of SLNs from spray-dried microparticles has been demonstrated, providing a non-toxic delivery platform for various drugs [98]. Overall, SLNs and NLCs are considered safe and effective drug delivery systems with potential for further development [99]. Rapamycin-loaded SLNs (mean size: 237.5 nm; MMAD: 5.4 μm) were successfully developed using 5% w/w mannitol. These SLNs inhibited mTOR signaling comparably to free rapamycin and significantly reduced proliferation in TSC2-deficient mouse embryonic fibroblasts, confirming bioactivity. Concurrently, folate-grafted chitosan-PEG copolymer surface-engineered solid lipid nanoparticles (SLNs) were designed for inhalable paclitaxel delivery to lung tumors. Both systems demonstrate the potential of SLNs as biocompatible, industrially scalable nanocarriers for pulmonary therapy Refer Table 3 [96]. These were prepared using high-shear homogenization and characterized via DSC, FTIR, and particle size analysis. Optimized SLNs exhibited spherical morphology with suitable aerodynamic properties and demonstrated potent anticancer activity against A549 cells. Please refer to Table 3 [100].
Thin-film freeze-drying (TFFD) was used to assess the possibility of creating aerosolized dry powders of lipid nanoparticles for pulmonary medicine delivery, including small interfering RNA (siRNA). The study concluded that TFFD represents a promising method for this purpose, with excellent aerosol performance (fine particle fraction: 37 %, MMAD: 3.96 µm) for deep-lung delivery, and demonstrated mucus-layer penetration, as shown in Table 3 [101]. Fav-SLNps (Favipiravir-loaded solid lipid nanoparticles) were successfully developed and shown to be safe for A549 cells. They exhibited anti-proliferative effects, induced necrosis, and enhanced macrophage uptake compared to free drugs, suggesting potential lung cancer treatment [102]. Commending the potential of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in pulmonary drug delivery, their ability to offer prolonged release, low toxicity, and improved stability during nebulization [102].

Table 3.
Lipid-Based Nanocarrier for Inhalation.
Table 3.
Lipid-Based Nanocarrier for Inhalation.
| Engineered Nanoparticles | Drugs | Physical Parameters | Performance | Ref. |
|---|---|---|---|---|
| Liposomes | Paclitaxel | Size: 64.3 ± 2.4 nm ZP: −9 nm ± 0.48 mV MMAD: N/A FPF: N/A | Inhalable paclitaxel-in-liposome-in-bacteria system for primary lung cancer; demonstrated high lung targeting, enhanced cellular uptake (A549 cells), strong apoptosis induction (up to 98.6%), significant tumor suppression in lung cancer rat model, immune activation (↑ TNF-α, IL-4, IFN-γ), and selective lung biodistribution after pulmonary delivery… | [8] |
| PEGylated Liposomes | Afatinib Di maleate | Size: 181.2 ± 5.0 nm ZP: +9.65 ± 0.32 mV MMAD: 2.83 µm FPF: 63.65% | Polyethylene glycol (PEG)-modified unilamellar liposomal dry powder showed high lung deposition (>85% emitted dose), MMAD 2.83 µm, FPF 63.65%, sustained release across pH 5.5–7.4 (zero-order kinetics), enhanced cytotoxicity, and improved cellular uptake in A549 cells | [88] |
| Liposomes | Curcumin | Size: 94.65 ± 22.01 nm ZP: N/A MMAD: 5.81 µm FPF: 46.71% | Liposomal dry powder with an MMAD of 5.81 µm and an FPF of 46.71% showed efficient lung deposition. In A549 cells, it outperformed free curcumin and gemcitabine in cytotoxicity, indicating strong potential for lung cancer treatment. | [89] |
| PEGylated Liposomes | siRNA | Size: 276.9 ± 4.8 nm ZP: −10.6 ± 0.8 mV MMAD: N/A FPF: N/A | PLGA-based hybrid nanoparticles enhanced mucus penetration, Bronchoalveolar Lavage Fluid BALF stability, and A549 cell uptake. Showed 84.83% transfection and superio r gene silencing in 3D spheroids and improved cellular uptake A549-3T3 | [90] |
| Liposomes | Erlotinib + Genistein | Size: 200 nm ZP: ~0 mV Neutral MMAD: N/A FPF: N/A | Pulmonary delivery for NSCLC; tested on H3255, H1650, and H1781 cell lines; local inhalation enhances lung deposition and reduces systemic toxicity | [91] |
| Liposomes | Gefitinib | Size: 69.82 ± 2.60 nm ZP: −9.67 ± 0.73 mV MMAD: 7.837 µm; FPF: 33.81 | Inhalable liposomamV MMADr primary lung cancer demonstrated high lung distribution, rapid absorption, 2.36-fold higher bioavailability vs. oral, significant tumor reduction, apoptosis induction (Caspase-3, TUNEL), P-Akt suppression, inflammation attenuation (↓ TNF-α, W/D ratio); tested in primary lung cancer rat model. | [92] |
| Liposomes | Rolapitant (RP) FDA- approved drug | Size: 25.1 ± 7.3 nm ZP: −24.5 ± 1.4 mV MMAD: N/A FPF: N/A | Inhalable nanocarrier formulations for lung cancer tested on A549 lung cancer cells showed enhanced cytotoxicity, improved permeability, high cellular uptake, NK-1 receptor inhibition, and favorable biodistribution to lungs after intratracheal administration. | [93] |
| Liposomes | Vincristine (VCR) | Size: 112.6 ± 3.7 nm ZP: −21.56 ± 2.53 mV MMAD: N/A FPF: 14.99 ± 0.27% | Inhalable liposomal spray-dried powder for lung cancer; sustained release (65% over 96 h), enhanced cytotoxicity against MCF-7 and A549 cells (IC50: 24.4 nM & 55.3 nM), improved lung targeting, lower hepatic metabolism (due to CYP3A4 deficiency), higher AUC and Cmax, prolonged t1/2, and reduced clearance in rat model. | [94] |
| SLNs | Paclitaxel (PTX) | Size: 249 ± 36 nm ZP: +32 ± 1 mV MMAD: N/A FPF: N/A | Targeted delivery to folate receptor (FR)-overexpressing lung tumors via folate-mediated endocytosis using solid lipid nanoparticles (SLNs) loaded with paclitaxel (PTX) achieved 32× higher lung retention, undetectable systemic exposure, sustained PTX release (50% over 72 h), and enhanced anti-proliferative efficacy in M109-HiFR cells. | [96] |
| SLNs | Curcumin | Size:14.7 nm ZP: −22.5mV MMAD: N/A FPF: N/A | Enhanced curcumin solubility and dissolution rate in lung tissue; high encapsulation (90.2%) and drug loading (8.56%); maintained aerodynamic diameter (~4.03 μm) for deep lung deposition; sustained-release in simulated lung fluid; reduced cytotoxicity in BEAS-2B cells—indicating a safe, effective dry-powder inhalation system | [100] |
| SLNs | TNF-α siRNA | Size:164.5 ± 28.3 nm ZP: −29.1 ± 8.6 mV Mv MMAD: 3.96 µm FPF: 37% | Thin-film freeze-drying yields a porous, brittle dry powder that preserves SLN size and charge, retains siRNA function (effective TNF-α knockdown in macrophages), and exhibits excellent aerosol performance (fine particle fraction 37%, MMAD 3.96 µm) for deep-lung delivery, plus demonstrated mucus-layer penetration. | [101] |
| SLNs | Favipiravir | Size:: 693.1 ± 40.3 nm ZP: −13.3 ± 0.3 MMAD:3.0 ± 0.4 µm FPF: 60.2 ± 1.7% | Favipiravir-loaded SLNs (~693 nm; −13.3 mV) prepared via hot-evaporation nebulized into a respirable aerosol (FPF 60.2%, MMAD 3.0 µm, GSD 2.33). They were biocompatible up to 322.6 µg/mL in A549 cells, inducing cell-cycle arrest, necrosis, and autophagy inhibition, as well as a 1.23-fold increase in macrophage uptake, for effective pulmonary anticancer delivery. | [103] |
| Liposomes | sorafenib tosylate (ST) | Size: 111.15 ± 1.03 nm ZP: −29.87 ± 0.56 mV MAD: 3.15 ± 0.42 µm FPF: 83.71 ± 2.09% | Iµm.lable liposomal DPI for NSCLC demonstrated improved lung deposition (MMAD 3.15 µm, FPF 83.71%), sustained release up to 72 h, potentially enhanced bioavailability inferred from improved lung deposition and dissolution, and reduced systemic toxicity. | [104] |
4.4. Polymeric Nanocarriers for Inhalation
Over the past decade, polymer-based nanotherapeutics have garnered significant attention due to their unique structural versatility and efficacy in delivering therapeutic agents, particularly for cancer chemotherapy. These polymeric nanoparticles (Figure 2) enable high drug loading capacity, protection against degradation, and controlled site-specific release, collectively enhancing bioavailability while minimizing systemic adverse effects [105].
4.5. Polymeric Nanoparticles
Poly(lactide-co-glycolide) (PLGA), a noncytotoxic and biocompatible polymer, is frequently utilized in drug delivery systems due to its capacity for prolonged release. Anti-cancer medications have been delivered with success using recent advancements in the use of Polyethylene glycol (PEG)-PLGA nanoparticles for cancer treatment [106,107]. Elbatanony et al. employed the nanoprecipitation method to prepare inhalable PLGA nanoparticles encapsulating afatinib—a first-line NSCLC therapy—and assessed their in vitro activity against KRAS-mutant A549 and H460 cell lines [108]. Over the past few decades, researchers have engineered and functionalized these nanoparticles to enable site-specific targeted drug delivery in NSCLC [109].
Vanza et al. formulated afatinib-loaded inhalable PLGA nanoparticles via a double-emulsion solvent-evaporation method optimized by a 32 factorial design, achieving an entrapment efficiency of 78.15 ± 2.20% and a particle size of 198.1 ± 3.5 nm. Scale-up to a 75 mL batch preserved these critical quality attributes. Lyophilization with trehalose and L-leucine produced an inhalable dry powder (6.883 ± 0.369 µm) with 77.86 ± 3.02 % drug content, >85% deep-lung deposition, and sustained release (>80% over 18–34 h across pH 5.5–7.4). In A549 cells, the formulation exhibited enhanced cytotoxicity (IC50 1.573 ± 0.067 µM vs. 2.576 ± 0.110 µM for free drug) and improved cellular uptake while remaining stable under intermediate and long-term storage conditions, demonstrating strong potential for targeted pulmonary delivery in NSCLC therapy [110].
Using a QbD-guided approach, Bardoliwala et al. optimized PLHNCs co-loaded with Docetaxel and ABCB1 shRNA to achieve high drug/gene loading, a favorable size and charge for enhanced uptake, and excellent aerosol performance (deep-lung deposition), positioning this system as a promising inhalable therapy to overcome MDR in lung cancer [111]. These studies optimized the parameters for spray-dried powders containing nucleic acid-PEI nanoparticles, preserving bioactivity and enabling reconstitution of the nanoparticles. The versatility and efficiency of PLGA nanoparticles have been demonstrated in various disease models, including the successful delivery of tacrine from the nose to the brain and lung targeting of silibinin and moxifloxacin. Including effective tacrine delivery from the nose to the brain and lung targeting silibinin and moxifloxacin [112,113,114]. Researchers have successfully functionalized PLGA nanoparticles with an anti-HER2 affibody for selective binding to cancer cells that overexpress the HER2 protein. Furthermore, the potential of TRAIL therapy in cancer treatment has been explored, with strategies to enhance its anticancer activities and identify substances that sensitize tumor cells to TRAIL-induced apoptosis [114,115].
4.6. Micelles
Micelles, colloidal particles made of amphiphilic block copolymers or surface-active chemicals, have been widely researched for their potential in drug administration [116]. Highlights their unique qualities, such as increased bioavailability and the potential to be changed for tailored Medication administration [107]. Further explores the structural details and stabilities of these micelles, with a focus on the impact of cyclic topology on their formation and integrity [117]. Amphiphilic polysaccharide copolymer with micellar nanoparticles self-assembles in water, stabilizes curcumin, and exhibits low toxicity [117]. However, their stability in biological fluids and interaction with the biological environment are key challenges [118]. To enhance the drug-encapsulating capacity and stability, it is essential to consider the compatibility between the drug and the polymer. And drug-release kinetics [119]. Despite these challenges, Polymeric micelles have shown improvements in Pharmacokinetic characteristics and improved efficacy in preclinical and clinical studies [120]. Ciprofloxacin-coated nano micelles prolong pulmonary residence and bolster antibacterial efficacy; when spray-dried into nano aggregates optimized by factorial design, they retain stability and enhance drug performance in dry-powder inhalers [121]. Omalizumab is encapsulated in polymerases for targeted delivery to the lungs, with PEG-polyester nanoplatforms ensuring optimal absorption and cell uptake through spherical micelles [122]. Polymeric micelles, ~17 nm in size with near-neutral surface charge (ζ = 0.37 mV), achieved high co-loading of doxorubicin and curcumin, effectively overcame multidrug resistance in lung cancer cells through enhanced uptake and synergistic cytotoxicity, sustained drug release, prolonged blood circulation, and robust tumor growth inhibition in A549 cell lines Table 4 [123]. The effects of a magneto-micelle suspension on rat lungs were investigated over 40 days. Hemodynamic changes in lung tissue were observed initially but diminished over time. Accumulation of magneto micelles in the lungs did not cause harm to pneumocytes, macrophages, or the blood-air barrier [124].
4.7. Nanostructured Microparticles
Microparticles, designed explicitly for pulmonary drug delivery, present a viable means of resolving the issues related to traditional inhalation treatments. These particles, typically ranging from 1–3 μm in size and density of around 1 g/cm3, aggregate in dry powder inhalers, clearing quickly by lung macrophages. To address these challenges, studies have explored the use of carrier-based formulations and the development of nano-structured microparticles for inhalation [125]. These particles, which are a combination of nanoscale drug carriers, have been found to consistently and persistently deliver medication to the airways after inhalation [83,126]. Furthermore, the use of non-mucoadhesive, such as mucus-penetrating particles, has been suggested to enhance pulmonary drug delivery [127]. The emulsification technique produces porous PLGA particles for efficient lung delivery, which resist phagocytosis [128]. PLGA is a widely studied polymer for the preparation of sustained-release microspheres for pulmonary administration [129]. Docetaxel-loaded PLGA-PLX-188 nanoparticles (~222 nm; −34.8 mV) were successfully spray-dried into nano-embedded microparticles that aerosolized into a DPI with an MMAD of 3.74 µm and FPF of 42.96 %. Upon deposition, the microparticles released ~47.8 % of redispersed NPs retaining their size, and PDI provided sustained drug release over 144 h, remained stable for at least three months, and delivered significantly greater cytotoxicity in A549 cells due to improved nanoparticle uptake—demonstrating strong potential for targeted inhalation therapy in NSCLC [130].

Table 4.
Polymeric nanocarriers for delivery.
Table 4.
Polymeric nanocarriers for delivery.
| Engineered Nanoparticles | Drugs | Physical Parameters | Performance | Ref. |
|---|---|---|---|---|
| PLGA NPs | Afatinib | Size: 180.2 ± 15.6 nm ZP: −23.1 ± 0.2 mV MMAD: 4.7 ± 0.1 µm FPF: 77.8 ± 4.3% | Afatinib-loaded PLGA nanoparticles achieved 34.4 ± 2.3% entrapment with sustained release (56.8 ± 6.4% over 48 h) and excellent inhalable properties (MMAD 4.7 ± 0.1 µm; FPF 77.8 ± 4.3%). They outperformed free afatinib in KRAS-mutant A549 and H460 cells by enhancing cytotoxicity, cellular uptake, and penetration–growth inhibition in 3D tumor spheroids. | [108] |
| PLGA NPs | Afatinib-loaded PLGA nanoparticles dry powder inhaler | Size: 198.1 ± 3.5 nm ZP: −0.519 ± 0.197 mV MMAD: N/A FPF: N/A | The inhalable dry powder of afatinib-loaded PLGA nanoparticles achieved high entrapment (78.2 ± 2.2%) and drug loading (3.90 ± 0.11%), formed a respirable powder (~6.9 µm) with good flow and >85 % deep-lung deposition, provided sustained release (>80% over 18–34 h across pH 5.5–7.4), and markedly improved cytotoxicity and cellular uptake in A549 cells versus the free drug. | [110] |
| Polymeric–Lipid Hybrid Nanocarriers | Docetaxel + ABCB1 shRNA pDNA | Size: 124.1 ± 1.9 nm ZP: +26.5 ± 1.8 mV Mv MMAD: N/A FPF: N/A | High Docetaxel encapsulation (85.6 ± 2.8%) and shRNA complexation (97.4 ± 1.6%). Nanoscale size (<200 nm) and positive surface charge enhance cellular uptake and avoid rapid clearance. Processed into a respirable dry powder (MMAD 3.56 ± 0.21 μm; FPF 68.3 ± 2.5%) for deep-lung delivery, demonstrating potential to reverse multidrug resistance in NSCLC | [111] |
| Polymeric micelles | Doxorubicin + Curcumin | Size: 17.02 ± 2.58 nm ZP: 0.37 ± 0.014 mV MMAD: N/A FPF: N/A | Synergistically reversed doxorubicin resistance in A549/cells (IC50 3.95 µg/mL) via energy-dependent, caveolae-mediated uptake; Provided sustained release and prolonged systemic circulation with elevated plasma levels over six h. Significantly inhibited tumor growth in Lewis lung carcinoma–bearing mice | [123] |
| Microparticles | DocetaxelVenza | Size: 222.1 nm ZP: −34.8 mV MMAD: 3.74 µm FPF: 42.96% | Docetaxel-loaded PLGA-PLX-188 nanoparticles (~222 nm; −34.8 mV) were successfully spray-dried into nano-embedded microparticles that aerosolized into a DPI with an MMAD of 3.74 µm and FPF of 42.96%. Upon deposition, the microparticles released ~47.8% of the redispersed NPs, retaining their size and polydispersity index (PDI), which provided sustained drug release over 144 h. The microparticles remained stable for at least three months and delivered significantly greater cytotoxicity in A549 cells due to improved nanoparticle uptake, demonstrating strong potential for targeted inhalation therapy in non-small cell lung cancer (NSCLC). | [130] |
4.8. Dendrimers
A type of hyperbranched homopolymer has a unique structure with an initiator core, interior layers, terminal surface groups, and drug-entrapping spaces, resembling unimolecular micelles [131]. This structure allows them to be effective drug carriers, with the ability to carry both hydrophobic and hydrophilic drugs and to be modified for specific drug delivery purposes [132]. Their nanoscopic size, narrow polydispersity index, and multiple functional groups make them versatile and attractive for drug delivery applications [133]. TPP-modified dendrimers were examined to improve SIRNA transfection in the lungs. Epithelium. Elevated TPP density correlated with enhanced gene knockdown. Mannitol microparticles containing dendriplexes are efficiently delivered to deep lung regions [134]. Pulmonary drug delivery surpasses systemic administration for lung diseases. Poly(amidoamine) (PAMAM) dendrimer interaction with pulmonary surfactant using Langmuir monolayers and molecular dynamics simulations. Results suggest that PAMAM dendrimers may be potential respiratory drug nanocarriers [135]. Dendrimers, a type of polymeric nanoarchitecture, have shown great potential in drug delivery and targeting due to their unique properties. They can enhance drug solubility, stability, and bioavailability, as well as shield tissues from toxicity [136]. The use of dendrimers as respiratory nanocarriers has also been investigated, yielding promising results in terms of their capacity to permeate the lipid monolayer and their potential as drug nanocarriers [135].
4.9. Lipid vs. Polymeric Nanocarriers: Tailoring Pulmonary Drug Delivery Systems
Lipid-based nanocarriers have emerged as promising vehicles for pulmonary drug delivery, particularly for glucocorticoids and anticancer drugs [137]. These nanocarriers offer advantages such as high biocompatibility, improved drug loading, and enhanced lung retention compared to non-lipid-based alternatives [138]. Surface modification of lipid nanocarriers can further enhance their targeting efficiency and controlled release properties [138]. However, successful inhalation therapy depends on various factors, including the physicochemical properties of the payload, formulation, and interaction with lung fluids [139]. While both lipid and polymer-based nanocarriers show potential for pulmonary drug delivery, lipid-based systems demonstrate superior lung accumulation and retention after inhalation [140].
5. Challenges
In recent decades, nanoparticle-based medicines have advanced rapidly, showing promise in cancer diagnosis, imaging, and treatment, including for the treatment of lung cancer. These nanoparticles can be customized for personalized therapy, often combining different components to encapsulate drugs and target specific sites. However, translating these innovations into clinical inhalation therapies for lung cancer faces challenges in synthesizing nanoparticles with ideal properties and establishing standardized testing protocols due to their complex nature. Nanoparticle-based medicines have shown great potential in the diagnosis, imaging, and treatment of lung cancer; however, their translation into clinical inhalation therapies faces significant challenges [141].
5.1. Clearance of Nanoparticles
Nanoparticle clearance in the respiratory region is influenced by particle size, shape, and delivery method. In the tracheobronchial system, which serves as conductive airways and the respiratory region, as illustrated in Figure 3, the clearance of nanoparticles varies based on geometry, with 24-h retention ranging from 72.7% to 87.0% for different shapes [142]. Particle size significantly affects deposition, with a 67.5% decrease in deposition fraction as size increases from 10 nm to 100 nm [143]. Exposure path selection impacts nanoparticle deposition, with distinct values observed for different respiratory routes [143]. Gold ultrasmall nanoparticles administered intranasally can reach the lung parenchyma and secondary organs, with almost complete excretion within 10 days [144]. Pulmonary delivery of nanoparticle chemotherapy for lung cancer treatment holds promise but faces challenges due to the physiology of the respiratory tract and lung clearance mechanisms [145].
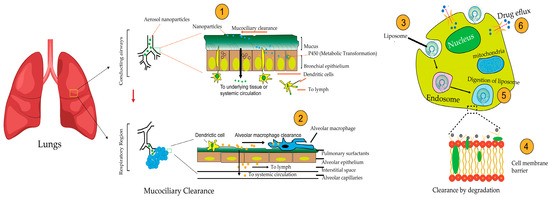
Figure 3.
Mechanisms of Nanoparticle Clearance. The figure illustrates how inhaled nanoparticles are cleared (1) Mucociliary clearance in the conducting airways removes inhaled particles via coordinated mucus and cilia movement. (2) Phagocytosis by alveolar macrophages engulfs and eliminates nanoparticles in the respiratory region, (3) Enzymatic degradation occurs within endo-lysosomal compartments, and (4) Endosomal escape allows some nanoparticles to bypass degradation. (5) Lysosomal degradation further digests nanoparticles within lysosomes. (6) Drug efflux through transporter proteins pumps nanoparticles or their released drugs out of cells. These barriers collectively impact nanoparticle deposition, retention, and therapeutic efficacy in lung cancer treatment.
5.2. Mucociliary Clearance
Mucociliary clearance (MCC) is a key defense mechanism in the respiratory system, utilizing coordinated mucus and cilia action to remove inhaled particles and pathogens. Ciliated cells, equipped with 100–250 motile cilia beating at 12–15 Hz, propel the mucus layer at 8–10 mm/h toward the pharynx, as illustrated in Figure 3 [23,146]. Additionally, NPs can disrupt cellular metabolism in bronchial epithelial cells, with nano polystyrene inducing autophagy and endoplasmic reticulum stress, while nano ZnO causes ROS-mediated cell death and mitochondrial dysfunction [147]. Nanoparticles have difficulty adhering to airways due to the presence of mucus, prompting the development of methods to enhance penetration, which requires thorough evaluation for clinical safety and relevance [148]. Rapid mucociliary clearance significantly decreases the effective lung dose, with fast TiO2-nanoparticle clearance accounting for 30% of the instilled dose within 1 h [149]. The effectiveness of mucoadhesive particles (MAP) for delivering drugs to the lungs is being questioned, and there is a suggestion that nonstick mucus-penetrating particles (MPP) might be more beneficial in terms of retention and distribution within the lungs. This idea is supported by evidence [127]. This discovery holds immense importance for delivering drugs or genetic material within the cells of the underlying epithelial tissue. It highlights the crucial role of physicochemical attributes, such as particle size and surface properties, in inhaled nanoparticles [150]. Particle penetration through mucus relies on size; mucin forms a network filtering size. Larger particles are impeded, while smaller ones could theoretically diffuse through, considering the mesh pore size [151]. Inspired by mucus-penetrating viruses, researchers have discovered zwitterionic nanoparticles with a net neutral charge, which reduce the adsorption of biomolecules to their surface [152,153]. Although rare in pulmonary drug delivery studies, zwitterion-functionalized nanoparticles are widely used in oral drug delivery, suggesting potential future advancements [153].
5.3. Influence of Surface Hydrophobicity
The surface properties of nanoparticles significantly influence their ability to penetrate mucus and navigate the respiratory system. Studies have shown that hydrophilicity, surface charge, and particle size are crucial factors affecting mucus penetration [154]. Hydrophilic particles penetrate mucus more effectively than hydrophobic ones, indicating that surface engineering to reduce particle-mucus adhesion could be beneficial [155]. One approach to improving mucus penetration involves coating nanoparticles with polyethylene glycol (PEG). Higher molecular weight (MW) PEG chains can alter mucin interactions, facilitating better mucus penetration [156]. The use of low molecular weight (2–5 kDa) PEG to facilitate airway mucus penetration has been supported by several studies [157]. PEGylation, the process of attaching polyethylene glycol (PEG) to liposomes, has been a key strategy in research to evade mucous clearance during inhalation [158]. Hydrophilic PEG-coated PLGA nanoparticles exhibited prolonged retention in lung mucus after inhalation compared to non-coated PLGA nanoparticles [127]. Conversely, increasing hydrophobicity in PEGylated dendrimers has been shown to double mucociliary clearance in rats compared to fully PEGylated ones [159]. While PEGylation is widely used for particle surface modification, it can lead to issues such as the formation of anti-PEG antibodies, which can trap particles in mucus and affect cellular uptake [151]. Alternative surface modifications, such as poly(2-alkyl-2-oxazolines) and mucolytic enzymes, may also be effective in enhancing mucus permeation of nanoparticles [152]. As illustrates Figure 3, PEGylation plays a role in improving mucus penetration, while the challenges of hydrophobic nanoparticles impact their deposition and absorption.
5.4. Alveolar Macrophage Clearance
Alveolar macrophages play a crucial role in clearing inhaled particles from the lung, with their phagocytic behavior being influenced by particle size, charge, and modification [160]. As illustrated in Figure 3, these macrophages interact with inhaled nanoparticles, with their phagocytic activity significantly influenced by the particle’s physical and chemical properties.
Particle size affects pulmonary delivery. Only particles between 1–5 mm aerodynamic diameter are inhalable, which is crucial for therapeutic agent delivery [161]. Figure 3, highlights how particle size plays a pivotal role in determining the efficiency of pulmonary delivery and macrophage uptake. Tumor vaccines had been delivered to lung dendritic cells via cationic nanoliposomes. Although liposomes effectively entrapped antigens, their release was limited. DOTAP liposomes were deemed safe and predominantly internalized by alveolar macrophages in mice, impeding dendritic cell access [162]. The findings suggest that nanoparticles effectively evade alveolar macrophage uptake. Yet, ensuring sufficient lung deposition poses a significant challenge due to rapid exhalation [163]. Recent research produced resveratrol as a nanosuspension spray-dried with mannitol to form nanosuspension-in-microparticles (NS-in-MPs). Compared to the resveratrol-lactose mixture, NS-in-MPs exhibited superior aerodynamic performance and lung [164]. Gold nanoparticles (Au-NPs) with varying surface charges were administered intranasally to mice. Positively charged NH2-PVA Au-NPs were preferentially phagocytosed by alveolar macrophages compared to negatively charged COOH-PVA Au-NPs [165]. Alveolar macrophages are crucial in lung immunity. Pro-inflammatory M1 macrophages are targeted with nanoparticles conjugated with hyaluronic acid and poly (ethylene glycol). Enhanced targeting efficiency toward pro-inflammatory macrophages while improving mucus mobility for better delivery to target sites in the lungs [166].
5.5. Clearance by Degradation
Degradation contributes to nanoparticle clearance from the lungs [167]. Despite having a lower metabolic capacity than the liver, the lung contains enzymes that contribute to drug elimination [168]. Figure 3 explains how enzyme P450 metabolizes nanoparticles. Xenobiotic metabolism is crucial for inhaled compounds, involving enzymes such as cytochromes P450 and Phase II enzymes [169]. Inhaled drugs undergo significant pulmonary metabolism, which can impact their safety and effectiveness. Studies compare lung microsomes and cells for in vitro research. However, metabolic clearance in the lung is generally low, with human alveolar type II cells showing lower rates than liver cells [170]. Therefore, mucus production by epithelial cells serves as a physical barrier and a rate-limiting step for the targeting of inhalable drugs [171]. Inhaled medicines may be trapped within the mucus and subsequently removed with multiple clearance mechanisms. Adhesion interactions usually occur between mucus and drug particles via electrostatic, hydrophobic, and hydrogen bonding [172].
The significant roles of mucociliary agents are (i) to act as mechanical filters to entrap particles in the surface liquid onto the airway epithelium and clear by ciliary action. Most insoluble particles with aerodynamic diameters larger than six μm are eliminated by mucociliary clearance. Meanwhile, nano-sized particles can travel faster to reach the bronchial epithelial region and escape the action, (ii) to provide antioxidant activities with its surface liquid, and (iii) to provide a surface biological interaction between microorganisms with luminal inflammation cells to prevent bacterial migration to airway epithelial cells. The clearance mechanism is facilitated by hair-shaped structures like cilia, which are present on the top side of epithelial cells. Once foreign particles are trapped in mucus, cilia beat in a coordinated direction (in the pharynx) to remove the debris either by coughing or swallowing (extensively reviewed in [150]. The study developed inhalable mRNA lipid nanoparticles (LNPs) using leucine and mannitol, achieving effective lung delivery with low toxicity [173].
5.6. Challenges and Obstacles to Clinical Implementation
Scaling up Inhalable Nanoparticle Production: Challenges Ahead
The scalable, controlled, and reproducible manufacturing of nanomedicines under good manufacturing practice (GMP) conditions presents unique challenges. These challenges include the need for reproducible manufacturing and scale-up, as well as the development of appropriate characterization methods, and the potential impact of minor variations on safety and efficacy. The complexity of nanoparticles requires careful design and engineering, as well as detailed analysis methods. Regulatory considerations, such as those from the FDA and EMA, are also crucial, with a focus on product quality and safety assessment. The development of a manufacturing technique that allows for the transfer of nanomedicine production from the laboratory to an industrial scale is a key barrier, particularly in the case of PLGA-based nanomedicines, highlighted by [174,175]. The development of PLGA-based nanomedicines for industrial-scale up faces several challenges, including batch-to-batch variations and a lack of product consistency. Inline sonication and tangential flow filtration (TFF) have been proposed as scalable and robust manufacturing techniques for PLGA nanoparticles, with the potential for fully continuous operation [176]. These properties include size, shape, composition, crystallinity, drug loading, drug release, and surface functionality and chemistry [177]. This process can be facilitated by techniques such as Flash Nanoprecipitation (FNP) and spray drying, which have been shown to maintain consistent properties across different scales [178]. Key considerations for scaling up the manufacturing of nanotherapeutics include sourcing raw materials, selecting synthesis routes, conducting stability checks, and employing suitable analytical methods [179] The green synthesis of nanoparticles from plant-derived materials can enhance reproducibility and scale-up, but it also presents challenges, such as toxicity [180]. It reviews the techniques for formulating nanoemulsion drug delivery systems, emphasizing the energy requirements and nature of phase inversion [181]. The scale-up of nanomedicines from lab to industrial manufacturing presents significant challenges, as highlighted by [182]. These challenges include maintaining control over the properties of the nanoparticles and ensuring consistency from batch to batch. The use of 3D printing in the development of nanomedicines, as discussed by [183], offers a potential solution to these challenges by enabling the fabrication of tailor-made nanomedicines [184]. further explores the potential of microbial biosynthesis for the green biocatalytic synthesis of nanoscale materials, which could also address the challenges of scale-up in nanomedicine manufacturing.
5.7. Obstacles in Screening, Quality Management, and Characterization
Nanomedicine’s physicochemical traits (e.g., size, solubility, stability) are crucial for product performance. Early characterization establishes formulation parameters and their impact on product properties [185]. Identifying methods to characterize nanocarrier properties is technically challenging and poses regulatory compliance issues. Preclinical assessment includes detailed physicochemical descriptions, manufacturing, quality, efficacy, safety, and stability [186]. The lack of reliable and validated techniques for analyzing the characteristics and stability of nanomedicines under Good Manufacturing Practice (GMP) conditions is a significant obstacle to their clinical translation [187]. The limited use of advanced analytical techniques for nanomedicine characterization in industries is a considerable challenge, as these techniques are crucial for understanding the behavior of nano-systems in physiological environments [188]. For a comprehensive analysis, nanomedicine requires up-to-date techniques such as dynamic light scattering (DLS), nanoparticle tracking analysis (NTA), Zetasizer, transmission electron microscopy (TEM)/scanning electron microscopy (SEM), small-angle X-ray diffraction, X-ray photoelectron spectroscopy/FTIR spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, and liquid chromatography (LC) [187]. These techniques are indispensable for investigating how nanomedicines interact with biological systems, their cytotoxic effects, and their properties related to drug delivery [189,190]. This significantly increases nanomanufacturing costs. Specific techniques have constraints; for example, DLS is limited by size and shape, and electron microscopy is labor-intensive [191]. These factors impact circulation time, biodistribution, cell uptake, and interactions with cells or tissues, necessitating their evaluation using suitable methods. Nanomedicine characterization often occurs in settings that inadequately replicate the intricate biophysical conditions of human organs and tissues. Due to the complexity of the human body, accurately predicting in vitro–in vivo correlations for nanomedicines is challenging, which impedes their clinical translation [192]. In brief, advancing robust nanomedicine characterization methods is pivotal for product development. The Nanotechnology Characterization Laboratory (NCL), in collaboration with the NCI, NIST, and the FDA, supports preclinical characterization to expedite translation. Its focus is on providing comprehensive characterization to discern critical parameters governing nanomedicine efficacy and safety. Similar initiatives like the European Nanomedicine Characterization Laboratory (EU-NCL) aim to furnish a comprehensive preclinical assay platform encompassing physicochemical and–vivo biological assessments, enabling thorough evaluation of nanomedicine [193]. Standardized protocols from EU-NCL and its US counterpart, the Nanotechnology Characterization Laboratory (NCL), allow robust nanoparticle assessment properties, interactions, and potential hazards [194]. The standardized approach to nanomedicine evaluation ensures compliance with regulatory requirements and facilitates the translation of nanotherapeutics from preclinical development to clinical applications [194].
5.8. Challenges in Multistage Delivery and Deep Tumor Penetration of Nanocarriers
The therapeutic success of inhaled nanocarriers in lung cancer is critically limited by a series of biological and physicochemical barriers encountered during the multistage delivery process. Initially, upon inhalation, nanoparticles must traverse the respiratory mucus layer, a viscoelastic barrier composed of mucins and cellular debris that traps and eliminates foreign particulates through hydrophobic and electrostatic interactions. Additionally, mucociliary clearance and uptake by alveolar macrophages rapidly remove particles before they can reach tumor tissues, significantly reducing bioavailability Challenges in Multistage Delivery and Deep Tumor Penetration of Nanocarriers [195]. Advanced strategies, such as inhalable nanoparticle-in-microsphere systems with size-tunable, charge-reversible, and mucus-penetrating properties, have been developed to enhance lung deposition and local bioavailability. These systems act as “Trojan horses,” improving particle mobility in mucus and evading immune recognition [195]. However, even after overcoming airway barriers, nanocarriers must navigate the dense and heterogeneous extracellular matrix (ECM) within lung tumors. The ECM, rich in collagen, fibronectin, and proteoglycans, acts as a structural scaffold but also presents a significant physical barrier that restricts nanoparticle diffusion and uniform drug distribution. Compounded by elevated interstitial fluid pressure and abnormal vasculature, this microenvironment hampers deep penetration of therapeutic agents into the tumor core [196]. Therefore, the rational design of nanocarriers must integrate multistage features—such as mucus penetration, immune evasion, ECM responsiveness, and tumor-targeted drug release—to enhance delivery efficiency. Incorporating pH- or enzyme-sensitive components can help overcome stromal resistance and achieve deeper tumor infiltration, ultimately improving therapeutic outcomes in patients with lung cancer [197]. The respiratory mucus layer, which lines the trachea and bronchial tree, primarily consists of water, mucins (mainly MUC5AC and MUC5B), and various biomolecules such as DNA, lipids, and proteins. This viscoelastic and negatively charged barrier, formed by glycosylated mucins crosslinked through disulfide bonds and glycan entanglements, serves as a frontline defense against inhaled nanomaterials (NMs). Its dense structure limits the passage of larger particles, with a pore size of approximately 340 ± 70 nm. Furthermore, electrostatic interactions between the negatively charged mucus and positively charged NMs, along with hydrophobic and hydrogen bonding interactions, contribute to the retention of NMs, making the bio-nano interface a significant obstacle for effective pulmonary nanomedicine delivery [197]. These mucus-mediated interactions at the bio-nano interface not only hinder nanoparticle mobility but also exacerbate other physiological barriers such as tumor heterogeneity, airway remodeling, and mucociliary clearance, which together limit consistent and effective pulmonary drug delivery in lung cancer patients.
Although nanoparticle-based inhalation therapies offer promising targeted delivery in lung cancer, multiple barriers hinder clinical translation. Tumor heterogeneity, mucus hypersecretion, and altered airway clearance reduce effective deposition and penetration at tumor sites (Figure 3). Mucociliary clearance [23,146] Alveolar macrophage uptake [162], and enzymatic degradation [169] Further limits nanoparticle residence time and therapeutic efficiency. Additionally, variability in inhalation patterns and respiratory capacity among lung cancer patients complicates the consistent delivery of medication.
6. Conclusions
The review underscores the promising potential of nanoparticle-based formulations, such as liposomes and dendrimers, in enhancing lung cancer therapy through targeted drug delivery, improving drug solubility and bioavailability, and minimizing toxicity. Despite this, challenges remain in translating these technologies into clinical use, including optimizing nanoparticle properties, overcoming physiological barriers, and ensuring standardized testing. Additionally, the effectiveness of inhaler devices, such as DPIs, pMDIs, and nebulizers, needs to be improved to optimize drug dispersion and lung deposition. Future research should focus on integrating advanced nanoparticle formulations with better-designed inhaler devices to enhance therapeutic outcomes and ensure the successful clinical application of inhalable nanomedicines in the treatment of lung cancer.
Author Contributions
Writing—original draft, I.S.; conceptualization, resources, supervision, writing—review and editing, Z.M.S.; writing—review and editing, S.M., S.M.N. and M.K.A.; writing—review and editing, funding, Y.G. All authors agreed to take responsibility for its content. All authors have read and agreed to the published version of the manuscript.
Funding
Universiti Malaya IIRG Research Grant (IIRG001A-2022FNW).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Queen’s University Belfast for the financial assistance provided. All figures presented in this manuscript were created using Adobe Illustrator, version 24.2.1.
Conflicts of Interest
The authors declare no conflict of interest related to this work.
References
- Recillas-Targa, F. Cancer Epigenetics: An Overview. Arch. Med. Res. 2022, 53, 732–740. [Google Scholar] [CrossRef]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Diaz Sanchís, M.; García Martínez, M.; Vidal Lancis, M.C.; Santiago, A.; Gnutti, G.; Gómez Guillén, D.; Trapero Bertran, M.; Fu Balboa, M. Health and economic impact at a population level of both primary and secondary preventive lung cancer interventions: A model-based cost-effectiveness analysis. Lung Cancer 2021, 159, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V.; El Rayes, T.; Narula, N.; McGraw, T.E.; Altorki, N.K.; Barcellos-Hoff, M.H. The Microenvironment of Lung Cancer and Therapeutic Implications. In Lung Cancer and Personalized Medicine: Novel Therapies and Clinical Management; Ahmad, A., Gadgeel, S.M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 75–110. [Google Scholar]
- van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, M.; Du, L.; Zeng, J.; Yao, T.; Jin, Y. Paclitaxel-in-liposome-in-bacteria for inhalation treatment of primary lung cancer. Int. J. Pharm. 2020, 578, 119177. [Google Scholar] [CrossRef]
- Zhao, J.; Qin, L.; Song, R.; Su, J.; Yuan, Y.; Zhang, X.; Mao, S. Elucidating inhaled liposome surface charge on its interaction with biological barriers in the lung. Eur. J. Pharm. Biopharm. 2022, 172, 101–111. [Google Scholar] [CrossRef]
- Guo, X.; Yang, L.; Deng, C.; Ren, L.; Li, S.; Zhang, X.; Zhao, J.; Yue, T. Nanoparticles traversing the extracellular matrix induce biophysical perturbation of fibronectin depicted by surface chemistry. Nanoscale 2024, 16, 6199–6214. [Google Scholar] [CrossRef]
- Burgstaller, G.; Oehrle, B.; Gerckens, M.; White, E.S.; Schiller, H.B.; Eickelberg, O. The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur. Respir. J. 2017, 50, 1601805. [Google Scholar] [CrossRef]
- Narciso, M.; Díaz-Valdivia, N.; Junior, C.; Otero, J.; Alcaraz, J.; Navajas, D.; Farre, R.; Lopez, I.A.; Gavara, N. Changes in Structure and Stiffness of the Lung Extracellular Matrix in Lung Cancer. Eur. Respir. J. 2022, 60 (Suppl. S66), 3617. [Google Scholar] [CrossRef]
- Cassani, M.; Fernandes, S.; Pagliari, S.; Cavalieri, F.; Caruso, F.; Forte, G. Unraveling the Role of the Tumor Extracellular Matrix to Inform Nanoparticle Design for Nanomedicine. Adv. Sci. 2025, 12, 2409898. [Google Scholar] [CrossRef]
- Ruge, C.A.; Kirch, J.; Cañadas, O.; Schneider, M.; Perez-Gil, J.; Schaefer, U.F.; Casals, C.; Lehr, C.-M. Uptake of nanoparticles by alveolar macrophages is triggered by surfactant protein A. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 690–693. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Tang, Q.; Yin, D.; Tang, C.; He, E.; Zou, L.; Peng, Q. The protein corona and its effects on nanoparticle-based drug delivery systems. Acta Biomater. 2021, 129, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Raesch, S.S.; Tenzer, S.; Storck, W.; Rurainski, A.; Selzer, D.; Ruge, C.A.; Perez-Gil, J.; Schaefer, U.F.; Lehr, C.M. Proteomic and Lipidomic Analysis of Nanoparticle Corona upon Contact with Lung Surfactant Reveals Differences in Protein, but Not Lipid Composition. ACS Nano 2015, 9, 11872–11885. [Google Scholar] [CrossRef]
- Cojocaru, E.; Petriș, O.R.; Cojocaru, C. Nanoparticle-Based Drug Delivery Systems in Inhaled Therapy: Improving Respiratory Medicine. Pharmaceuticals 2024, 17, 1059. [Google Scholar] [CrossRef]
- Gupta, C.; Jaipuria, A.; Gupta, N. Inhalable Formulations to Treat Non-Small Cell Lung Cancer (NSCLC): Recent Therapies and Developments. Pharmaceutics 2022, 15, 139. [Google Scholar] [CrossRef] [PubMed]
- Ajith, S.; Almomani, F.; Elhissi, A.M.A.; Husseini, G.A. Nanoparticle-based materials in anticancer drug delivery: Current and future prospects. Heliyon 2023, 9, e21227. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef] [PubMed]
- Miwata, K.; Okamoto, H.; Nakashima, T.; Ihara, D.; Horimasu, Y.; Masuda, T.; Miyamoto, S.; Iwamoto, H.; Fujitaka, K.; Hamada, H. Intratracheal administration of siRNA dry powder targeting vascular endothelial growth factor inhibits lung tumor growth in mice. Mol. Ther. Nucleic Acids 2018, 12, 698–706. [Google Scholar] [CrossRef]
- Amararathna, M.; Goralski, K.; Hoskin, D.W.; Rupasinghe, H.V. Pulmonary nano-drug delivery systems for lung cancer: Current knowledge and prospects. J. Lung Health Dis. 2019, 3, 11–28. [Google Scholar] [CrossRef]
- Mangal, S.; Gao, W.; Li, T.; Zhou, Q.T. Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: Challenges and opportunities. Acta pharmacologica sinica 2017, 38, 782–797. [Google Scholar] [CrossRef] [PubMed]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Munshi, A.; Ramesh, R. Polymeric nanoparticle-mediated gene delivery for lung cancer treatment. In Polymeric Gene Delivery Systems; Springer: Cham, Switzerland, 2017; pp. 233–255. [Google Scholar]
- Zarogoulidis, P.; Chatzaki, E.; Porpodis, K.; Domvri, K.; Hohenforst-Schmidt, W.; Goldberg, E.P.; Karamanos, N.; Zarogoulidis, K. Inhaled chemotherapy in lung cancer: Future concept of nanomedicine. Int. J. Nanomed. 2012, 7, 1551–1572. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Traini, D.; Young, P.M. Inhalation of nanoparticle-based drug for lung cancer treatment: Advantages and challenges. Asian J. Pharm. Sci. 2015, 10, 481–489. [Google Scholar] [CrossRef]
- Respaud, R.; Vecellio, L.; Diot, P.; Heuzé-Vourc’h, N. Nebulization as a delivery method for mAbs in respiratory diseases. Expert Opin. Drug Deliv. 2015, 12, 1027–1039. [Google Scholar] [CrossRef]
- Peng, S.; Wang, W.; Zhang, R.; Wu, C.; Pan, X.; Huang, Z. Nano-formulations for pulmonary delivery: Past, present, and future perspectives. Pharmaceutics 2024, 16, 161. [Google Scholar] [CrossRef] [PubMed]
- Brunaugh, A.D.; Smyth, H.D. Formulation techniques for high dose dry powders. Int. J. Pharm. 2018, 547, 489–498. [Google Scholar] [CrossRef]
- Li, H.-Y. Alginate-based inhalable particles for controlled pulmonary drug delivery. In Alginate Biomaterial: Drug Delivery Strategies and Biomedical Engineering; Springer: New York, NY, USA, 2023; pp. 207–240. [Google Scholar]
- Muralidharan, P.; Hayes Jr, D.; Mansour, H.M. Dry powder inhalers in COPD, lung inflammation and pulmonary infections. Expert Opin. Drug Deliv. 2015, 12, 947–962. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lee, H.-G.; Kwon, Y.-B.; Kim, J.-Y.; Rhee, Y.-S.; Chon, J.; Park, E.-S.; Kim, D.-W.; Park, C.-W. The role of lactose carrier on the powder behavior and aerodynamic performance of bosentan microparticles for dry powder inhalation. Eur. J. Pharm. Sci. 2018, 117, 279–289. [Google Scholar] [CrossRef]
- Malamatari, M.; Malamataris, S.; Buckton, G.; Taylor, K. Microcomposite particles for drug delivery to the lungs: Can they be administered as dry powders or is blending with a carrier still needed? J. Pharm. Pharmacol. 2017, 69, 10–11. [Google Scholar]
- Chaugule, V.; dos Reis, L.G.; Fletcher, D.F.; Young, P.M.; Traini, D.; Soria, J. Particle image velocimetry measurements of a dry powder inhaler flow. In Proceedings of the 14th international symposium on particle image velocimetry, Chicago, IL, USA, 1–4 August 2021. [Google Scholar]
- Emeryk, A.; Janeczek, K. Feedback systems in multi-dose dry powder inhalers. Adv. Dermatol. Allergol. Postępy Dermatol. Alergol. 2023, 40, 16–21. [Google Scholar] [CrossRef]
- Lechanteur, A.; Evrard, B. Influence of composition and spray-drying process parameters on carrier-free DPI properties and behaviors in the lung: A review. Pharmaceutics 2020, 12, 55. [Google Scholar] [CrossRef]
- Weers, J.G. Design of dry powder inhalers to improve patient outcomes: It’s not just about the device. Expert Opin. Drug Deliv. 2024, 21, 365–380. [Google Scholar] [CrossRef]
- Yang, M.Y.; Verschuer, J.; Shi, Y.; Song, Y.; Katsifis, A.; Eberl, S.; Wong, K.; Brannan, J.D.; Cai, W.; Finlay, W.H. The effect of device resistance and inhalation flow rate on the lung deposition of orally inhaled mannitol dry powder. Int. J. Pharm. 2016, 513, 294–301. [Google Scholar] [CrossRef]
- Dońka, K.; Paździor, V.; Brodowicz-Król, M.; Zarzycka, D. Drug Inhalation Methods and Therapy Effectiveness. Emerg. Med. Serv. 2017, 4, 7–13. [Google Scholar]
- Li, W.; Daoud, S.Z.; Trivedi, R.; Lukka, P.B.; Jimenez, E.; Molins, E.; Stewart, C.; Bharali, P.; Garcia-Gil, E. The Pharmacokinetics, Safety and Tolerability of Aclidinium Bromide 400 μg Administered by Inhalation as Single and Multiple (Twice Daily) Doses in Healthy Chinese Participants. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 2725–2735. [Google Scholar] [CrossRef] [PubMed]
- Kappeler, D.; Sommerer, K.; Kietzig, C.; Huber, B.; Woodward, J.; Lomax, M.; Dalvi, P. Pulmonary deposition of fluticasone propionate/formoterol in healthy volunteers, asthmatics and COPD patients with a novel breath-triggered inhaler. Respir. Med. 2018, 138, 107–114. [Google Scholar] [CrossRef]
- Ahookhosh, K.; Yaqoubi, S.; Mohammadpourfard, M.; Hamishehkar, H.; Aminfar, H. Experimental investigation of aerosol deposition through a realistic respiratory airway replica: An evaluation for MDI and DPI performance. Int. J. Pharm. 2019, 566, 157–172. [Google Scholar] [CrossRef]
- Stein, S.W.; Thiel, C.G. The History of Therapeutic Aerosols: A Chronological Review. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Lavorini, F.; Buttini, F.; Usmani, O.S. 100 Years of Drug Delivery to the Lungs. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Ohnishi, H.; Okazaki, M.; Anabuki, K.; Akita, S.; Kawase, S.; Tsuji, K.S.; Miyamura, M.; Yokoyama, A. An Investigation into the Factors Associated with Incorrect Use of a Pressurized Metered-Dose Inhaler in Japanese Patients. J. Aerosol Med. Pulm. Drug Deliv. 2022, 36, 12–19. [Google Scholar] [CrossRef]
- Kotak, R.K.; Pandya, D.C.V. Basic Understanding of Pressurized Metered Dose Inhalers and Its Aerodynamic Particle Size Testing: A Review. Int. J. Tech. Innov. Mod. Eng. Sci. 2018, 4, 2455–2585. [Google Scholar]
- Party, P.; Bartos, C.; Farkas, Á.; Szabó-Révész, P.; Ambrus, R. Formulation and in vitro and in silico characterization of “nano-in-micro” dry powder inhalers containing meloxicam. Pharmaceutics 2021, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Sellers, W.F.S. Asthma pressurised metered dose inhaler performance: Propellant effect studies in delivery systems. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2017, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Mason-Smith, N.; Duke, D.J.; Kastengren, A.L.; Traini, D.; Young, P.M.; Chen, Y.; Lewis, D.A.; Edgington-Mitchell, D.M.; Honnery, D.R. Revealing pMDI Spray Initial Conditions: Flashing, Atomisation and the Effect of Ethanol. Pharm. Res. 2017, 34, 718–729. [Google Scholar] [CrossRef]
- Bezuglaya, E.; Lyapunov, N.; Bovtenko, V.; Zinchenko, I.A.; Stolper, Y.M. Study of pressurised metered dose inhalers for the purpose of standardization of quality attributes characterizing uniformity of dosing. Sci. Pharm. Sci. 2021, 32, 11–23. [Google Scholar] [CrossRef]
- Schroeter, J.D.; Sheth, P.; Hickey, A.J.; Asgharian, B.; Price, O.T.; Holt, J.T.; Conti, D.S.; Saluja, B. Effects of Formulation Variables on Lung Dosimetry of Albuterol Sulfate Suspension and Beclomethasone Dipropionate Solution Metered Dose Inhalers. AAPS Pharm. Sci. Tech. 2018, 19, 2335–2345. [Google Scholar] [CrossRef]
- Зайцев, А.; Харитoнoв, М.; Чернецoв, В.; Крюкoв, Е. Сoвременные вoзмoжнoсти небулайзернoй терапии. Медицинский Сoвет 2019, 98–103. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Janeczek, K.; Grzywna, K.; Emeryk, A. Mass and volume balances of nebulization processes for the determination of the expected dose of liquid medicines delivered by inhalation. Chem. Process Eng. 2021, 42, 253–261. [Google Scholar]
- Ashraf, S.; McPeck, M.; Cuccia, A.D.; Smaldone, G.C. Comparison of vibrating mesh, jet, and breath-enhanced nebulizers during mechanical ventilation. Respir. Care 2020, 65, 1419–1426. [Google Scholar] [CrossRef]
- Song, Y.-L.; Cheng, C.-H.; Reddy, M.K.; Islam, M.S. Simulation of onset of the capillary surface wave in the ultrasonic atomizer. Micromachines 2021, 12, 1146. [Google Scholar] [CrossRef]
- Longest, W.; Spence, B.M.; Hindle, M. Devices for Improved Delivery of Nebulized Pharmaceutical Aerosols to the Lungs. J. Aerosol Med. Pulm. Drug Deliv. 2019, 32, 317–339. [Google Scholar] [CrossRef]
- Arnott, A.; Watson, M.; Sim, M. Nebuliser therapy in critical care: The past, present and future. J. Intensive Care Soc. 2023, 25, 78–88. [Google Scholar] [CrossRef]
- Hatley, R.; Hardaker, L. Mesh nebulizer capabilities in aerosolizing a wide range of novel pharmaceutical formulations. Eur. Respir. J. 2016, 48 (Suppl. S60), PA967. [Google Scholar]
- Beck-Broichsitter, M. Aerosol production by vibrating membrane technology: Analysis of the electrolyte effect on generated droplet size and nebulizer output rate. J. Pharm. Sci. 2017, 106, 2168–2172. [Google Scholar] [CrossRef]
- Le, N.H.A.; Brenker, J.; Shenoda, A.; Sheikh, Z.; Gum, J.; Ong, H.X.; Traini, D.; Alan, T. Oscillating high aspect ratio micro-channels can effectively atomize liquids into uniform aerosol droplets and dial their size on-demand. Lab Chip 2024, 24, 1676–1684. [Google Scholar] [CrossRef]
- Khmelev, V.; Shalunov, A.; Nesterov, V. High-frequency vibration system for liquid atomization. Rom. J. Acoust. Vib. 2018, 15, 136–142. [Google Scholar]
- Ignatova, G.; Antonov, V. Nebulizer therapy for lung diseases. Meditsinskiy Sov. Med. Counc. 2021, 11, 102–106. [Google Scholar] [CrossRef]
- Zaytsev, A.A.; Kharitonov, M.A.; Chernetsov, V.A.; Kryukov, E.V. Current possibilities for nebulizer therapy. Meditsinskiy Sov. Med. Counc. 2019, 15, 106–111. [Google Scholar] [CrossRef]
- Kwok, P.C.L.; McDonnell, A.G.; Tang, P.; Knight, C.; McKay, E.; Butler, S.P.; Sivarajah, A.; Quinn, R.; Fincher, L.; Browne, E.; et al. In vivo deposition study of a new generation nebuliser utilising hybrid resonant acoustic (HYDRA) technology. Int. J. Pharm. 2020, 580, 119196. [Google Scholar] [CrossRef] [PubMed]
- Hirlekar, R.S.; Ayare, P.; Kadam, M.; Dhawal, S. Nebulizers: Scope and Application in Pulmonary Drug Delivery System. Indian J. Pharm. Sci. 2024, 86, 1–8. [Google Scholar] [CrossRef]
- Iwanaga, T.; Tohda, Y.; Nakamura, S.; Suga, Y. The Respimat® Soft Mist Inhaler: Implications of Drug Delivery Characteristics for Patients. Clin. Drug Investig. 2019, 39, 1021–1030. [Google Scholar] [CrossRef]
- Dhand, R.; Eicher, J.; Hänsel, M.; Jost, I.; Meisenheimer, M.; Wachtel, H. Improving usability and maintaining performance: Human-factor and aerosol-performance studies evaluating the new reusable Respimat inhaler. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 509–523. [Google Scholar] [CrossRef]
- Turpeinen, A.; Eriksson, P.; Happonen, A.; Husman-Piirainen, J.; Haikarainen, J. Consistent Dosing Through the Salmeterol–Fluticasone Propionate Easyhaler for the Management of Asthma and Chronic Obstructive Pulmonary Disease: Robustness Analysis Across the Easyhaler Lifetime. J. Aerosol Med. Pulm. Drug Deliv. 2020, 34, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Verschraegen, C.F.; Gilbert, B.E.; Loyer, E.; Huaringa, A.; Walsh, G.; Newman, R.A.; Knight, V. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20 (s)-camptothecin in patients with advanced pulmonary malignancies. Clin. Cancer Res. 2004, 10, 2319–2326. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Assi, R.A.; Yaghmur, A.; Darwis, Y.; Mohtar, N.; Parumasivam, T.; Saqallah, F.G.; Wahab, H.A. Pulmonary delivery of anticancer drugs via lipid-based nanocarriers for the treatment of lung cancer: An update. Pharmaceuticals 2021, 14, 725. [Google Scholar] [CrossRef]
- Elhissi, A.M.A. Liposomes for Pulmonary Drug Delivery: The Role of Formulation and Inhalation Device Design. Curr. Pharm. Des. 2017, 23, 362–372. [Google Scholar] [CrossRef]
- Skubitz, K.M.; Anderson, P.M. Inhalational interleukin-2 liposomes for pulmonary metastases: A phase I clinical trial. Anti-Cancer Drugs 2000, 11, 555–563. [Google Scholar] [CrossRef]
- Wittgen, B.P.H.; Kunst, P.W.A.; Van Der Born, K.; Van Wijk, A.W.; Perkins, W.; Pilkiewicz, F.G.; Perez-Soler, R.; Nicholson, S.; Peters, G.J.; Postmus, P.E. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin. Cancer Res. 2007, 13, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Ara, N.; Hafeez, A. Nanocarrier-mediated drug delivery via inhalational route for lung cancer therapy: A systematic and updated review. AAPS Pharm. Sci. Tech. 2024, 25, 47. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Amikacin liposome inhalation suspension: A review in Mycobacterium avium complex lung disease. Drugs 2019, 79, 555–562. [Google Scholar] [CrossRef]
- Chou, A.J.; Gupta, R.; Bell, M.D.; Riewe, K.O.D.; Meyers, P.A.; Gorlick, R. Inhaled lipid cisplatin (ILC) in the treatment of patients with relapsed/progressive osteosarcoma metastatic to the lung. Pediatr. Blood Cancer 2013, 60, 580–586. [Google Scholar] [CrossRef]
- Lemarie, E.; Vecellio, L.; Hureaux, J.; Prunier, C.; Valat, C.; Grimbert, D.; Boidron-Celle, M.; Giraudeau, B.; le Pape, A.; Pichon, E.; et al. Aerosolized Gemcitabine in Patients with Carcinoma of the Lung: Feasibility and Safety Study. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 261–270. [Google Scholar] [CrossRef]
- Otterson, G.A.; Villalona-Calero, M.A.; Sharma, S.; Kris, M.G.; Imondi, A.; Gerber, M.; White, D.A.; Ratain, M.J.; Schiller, J.H.; Sandler, A.; et al. Phase I study of inhaled doxorubicin for patients with metastatic tumors to the lungs. Clin. Cancer Res. 2007, 13, 1246–1252. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A review of liposomes as a drug delivery system: Current status of approved products, regulatory environments, and future perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N.; Safaei, M. Application of various types of liposomes in drug delivery systems. Adv. Pharm. Bull. 2017, 7, 3–9. [Google Scholar] [CrossRef]
- Bujan, A.; del Valle Alonso, S.; Chiaramoni, N.S. Lipopolymers and lipids from lung surfactants in association with N-acetyl-l-cysteine: Characterization and cytotoxicity. Chem. Phys. Lipids 2020, 231, 104936. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bravo, K.M.C.; Liu, J. Targeted liposomal drug delivery: A nanoscience and biophysical perspective. Nanoscale Horiz. 2021, 6, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-J.; Yang, S.-C.; Liu, X.-L.; Gao, Y.; Dong, X.; Lai, X.; Zhu, M.-H.; Feng, H.-Y.; Zhu, X.-D.; Lu, Q. Nanobowl-supported liposomes improve drug loading and delivery. Nano Lett. 2020, 20, 4177–4187. [Google Scholar] [CrossRef]
- Allahou, L.W.; Madani, S.Y.; Seifalian, A. Investigating the application of liposomes as drug delivery systems for the diagnosis and treatment of cancer. Int. J. Biomater. 2021, 2021, 3041969. [Google Scholar] [CrossRef] [PubMed]
- Rosch, J.G.; Winter, H.; DuRoss, A.N.; Sahay, G.; Sun, C. Inverse-micelle synthesis of doxorubicin-loaded alginate/chitosan nanoparticles and in vitro assessment of breast cancer cytotoxicity. Colloid Interface Sci. Commun. 2019, 28, 69–74. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.; Dai, Q.; Yin, X.; Zhang, X.; Zheng, A. In-vitro and in-vivo evaluation of ciprofloxacin liposomes for pulmonary administration. Drug Dev. Ind. Pharm. 2015, 41, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Vanza, J.D.; Shah, D.M.; Patel, R.B.; Patel, M.R. Afatinib liposomal dry powder inhaler: Targeted pulmonary delivery of EGFR inhibitor for the management of lung cancer. J. Drug Deliv. Sci. Technol. 2022, 74, 103506. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Ge, Y.; Hu, Y.; Li, M.; Jin, Y. Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers. Acta Pharm. Sin. B 2018, 8, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, Y.; Qin, L.; Yue, M.; Xue, J.; Cui, Z.; Zhan, X.; Gai, J.; Zhang, X.; Guan, J. Tunable rigidity of PLGA shell-lipid core nanoparticles for enhanced pulmonary siRNA delivery in 2D and 3D lung cancer cell models. J. Control. Release 2024, 366, 746–760. [Google Scholar] [CrossRef]
- Nimmano, N.; Somavarapu, S.; Taylor, K.M. Aerosol characterisation of nebulised liposomes co-loaded with erlotinib and genistein using an abbreviated cascade impactor method. Int. J. Pharm. 2018, 542, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, R.; Li, M.; Bao, J.; Chen, Y.; Ge, Y.; Jin, Y. Comparative study of intratracheal and oral gefitinib for the treatment of primary lung cancer. Eur. J. Pharm. Sci. 2020, 149, 105352. [Google Scholar] [CrossRef]
- Kabil, M.F.; Nasr, M.; Ibrahim, I.T.; Hassan, Y.A.; El-Sherbiny, I.M. New repurposed rolapitant in nanovesicular systems for lung cancer treatment: Development, in-vitro assessment and in-vivo biodistribution study. Eur. J. Pharm. Sci. 2022, 171, 106119. [Google Scholar] [CrossRef]
- Xu, J.; Lu, X.; Zhu, X.; Yang, Y.; Liu, Q.; Zhao, D.; Lu, Y.; Wen, J.; Chen, X.; Li, N. Formulation and characterization of spray-dried powders containing vincristine-liposomes for pulmonary delivery and its pharmacokinetic evaluation from in vitro and in vivo. J. Pharm. Sci. 2019, 108, 3348–3358. [Google Scholar] [CrossRef]
- Weber, S.; Zimmer, A.; Pardeike, J. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: A review of the state of the art. Eur. J. Pharm. Biopharm. 2014, 86, 7–22. [Google Scholar] [CrossRef]
- Rosiere, R.; Van Woensel, M.; Gelbcke, M.; Mathieu, V.; Hecq, J.; Mathivet, T.; Vermeersch, M.; Van Antwerpen, P.; Amighi, K.; Wauthoz, N. New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumor therapy by inhalation. Mol. Pharm. 2018, 15, 899–910. [Google Scholar] [CrossRef]
- Shirodkar, R.K.; Kumar, L.; Mutalik, S.; Lewis, S. Solid lipid nanoparticles and nanostructured lipid carriers: Emerging lipid based drug delivery systems. Pharm. Chem. J. 2019, 53, 440–453. [Google Scholar] [CrossRef]
- Sanchez-Vazquez, B.; Lee, J.B.; Strimaite, M.; Buanz, A.; Bailey, R.; Gershkovich, P.; Pasparakis, G.; Williams, G.R. Solid lipid nanoparticles self-assembled from spray dried microparticles. Int. J. Pharm. 2019, 572, 118784. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Patel, P.; Raval, M.; Airao, V.; Bhatt, V.; Shah, P. Silibinin loaded inhalable solid lipid nanoparticles for lung targeting. J. Microencapsul. 2022, 39, 1–24. [Google Scholar] [CrossRef]
- Wang, J.L.; Hanafy, M.S.; Xu, H.; Leal, J.; Zhai, Y.; Ghosh, D.; Williams Iii, R.O.; David Charles Smyth, H.; Cui, Z. Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int. J. Pharm. 2021, 596, 120215. [Google Scholar] [CrossRef]
- Tulbah, A.S. In vitro bio-characterization of solid lipid nanoparticles of favipiravir in A549 human lung epithelial cancer cells. J. Taibah. Univ. Med. Sci. 2023, 18, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Bothiraja, C.; Mali, A.; Kamble, R. Investigation of sorafenib tosylate loaded liposomal dry powder inhaler for the treatment of non-small cell lung cancer. Part. Sci. Technol. 2021, 39, 990–999. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Lima, A.F.; Amado, I.R.; Pires, L.R. Poly (d, l-lactide-co-glycolide)(PLGA) nanoparticles Loaded with proteolipid protein (PLP)—Exploring a new administration route. Polymers 2020, 12, 3063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, L.; Wang, J.; Zhang, H.; Zhang, Z.; Xing, G.; Wang, X.; Liu, M. Drug-loaded PEG-PLGA nanoparticles for cancer treatment. Front. Pharmacol. 2022, 13, 990505. [Google Scholar] [CrossRef] [PubMed]
- Elbatanony, R.S.; Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Chauhan, G.; Kunda, N.K.; Gupta, V. Afatinib-loaded inhalable PLGA nanoparticles for localized therapy of non-small cell lung cancer (NSCLC)—Development and in-vitro efficacy. Drug Deliv. Transl. Res. 2021, 11, 927–943. [Google Scholar] [CrossRef]
- Madni, A.; Batool, A.; Noreen, S.; Maqbool, I.; Rehman, F.; Kashif, P.M.; Tahir, N.; Raza, A. Novel nanoparticulate systems for lung cancer therapy: An updated review. J. Drug Target. 2017, 25, 499–512. [Google Scholar] [CrossRef]
- Vanza, J.D.; Lalani, J.R.; Patel, R.B.; Patel, M.R. DOE supported optimization of biodegradable polymeric nanoparticles based dry powder inhaler for targeted delivery of afatinib in non-small cell lung cancer. J. Drug Deliv. Sci. Technol. 2023, 84, 104554. [Google Scholar] [CrossRef]
- Bardoliwala, D.; Patel, V.; Misra, A.; Sawant, K. Systematic development and characterization of inhalable dry powder containing Polymeric Lipid Hybrid Nanocarriers co-loaded with ABCB1 shRNA and docetaxel using QbD approach. J. Drug Deliv. Sci. Technol. 2021, 66, 102903. [Google Scholar] [CrossRef]
- Shamarekh, K.S.; Gad, H.A.; Soliman, M.E.; Sammour, O.A. Development and evaluation of protamine-coated PLGA nanoparticles for nose-to-brain delivery of tacrine: In-vitro and in-vivo assessment. J. Drug Deliv. Sci. Technol. 2020, 57, 101724. [Google Scholar] [CrossRef]
- Patel, P.; Raval, M.; Manvar, A.; Airao, V.; Bhatt, V.; Shah, P. Lung cancer targeting efficiency of Silibinin loaded Poly Caprolactone/Pluronic F68 Inhalable nanoparticles: In vitro and In vivo study. PLoS ONE 2022, 17, e0267257. [Google Scholar] [CrossRef]
- Vishwa, B.; Moin, A.; Gowda, D.; Rizvi, S.M.; Hegazy, W.A.; Abu Lila, A.S.; Khafagy, E.-S.; Allam, A.N. Pulmonary targeting of inhalable moxifloxacin microspheres for effective management of tuberculosis. Pharmaceutics 2021, 13, 79. [Google Scholar] [CrossRef]
- Thapa, B.; Remant, K.; Uludağ, H. TRAIL therapy and prospective developments for cancer treatment. J. Control. Release 2020, 326, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Kretz, A.-L.; Trauzold, A.; Hillenbrand, A.; Knippschild, U.; Henne-Bruns, D.; von Karstedt, S.; Lemke, J. TRAILblazing Strategies for Cancer Treatment. Cancers 2019, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Shah, M.R. Amphiphilic block copolymers–based micelles for drug delivery. In Design and Development of New Nanocarriers; Elsevier: New York, NY, USA, 2018; pp. 365–400. [Google Scholar]
- Breitenbach, B.B.; Schmid, I.; Wich, P.R. Amphiphilic polysaccharide block copolymers for pH-responsive micellar nanoparticles. Biomacromolecules 2017, 18, 2839–2848. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Hussein, Y.H.; Youssry, M. Polymeric micelles of biodegradable diblock copolymers: Enhanced encapsulation of hydrophobic drugs. Materials 2018, 11, 688. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Farhangi, M.; Mahboubi, A.; Kobarfard, F.; Vatanara, A.; Mortazavi, S.A. Optimization of a dry powder inhaler of ciprofloxacin-loaded polymeric nanomicelles by spray drying process. Pharm. Dev. Technol. 2019, 24, 584–592. [Google Scholar] [CrossRef]
- Liatsi-Douvitsa, E. Polyester-Based Micelles and Vesicles for Pulmonary Administration. Ph.D. Thesis, UCL (University College London), London, UK, 2019. [Google Scholar]
- Gu, Y.; Li, J.; Li, Y.; Song, L.; Li, D.; Peng, L.; Wan, Y.; Hua, S. Nanomicelles loaded with doxorubicin and curcumin for alleviating multidrug resistance in lung cancer. Int. J. Nanomed. 2016, 11, 5757–5770. [Google Scholar] [CrossRef] [PubMed]
- Vasyukov, G.Y.; Sukhodolo, I.; Mil’to, I.; Mitrofanova, I. Structure of rat lungs after administration of magnetomicelles based on the carbon-coated iron nanoparticles. Bull. Exp. Biol. Med. 2017, 163, 99–104. [Google Scholar] [CrossRef]
- Koenneke, A.; Pourasghar, M.; Schneider, M. Nano-structured microparticles for inhalation. In Delivery of Drugs; Elsevier: New York, NY, USA, 2020; pp. 119–160. [Google Scholar]
- Tatsumura, T.; Koyama, S.; Tsujimoto, M.; Kitagawa, M.; Kagamimori, S. Further study of nebulisation chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: Fundamental and clinical. Br. J. Cancer 1993, 68, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.S.; Xu, Q.; Boylan, N.J.; Chisholm, J.; Tang, B.C.; Schuster, B.S.; Henning, A.; Ensign, L.M.; Lee, E.; Adstamongkonkul, P. Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci. Adv. 2017, 3, e1601556. [Google Scholar] [CrossRef]
- Nishimura, S.; Takami, T.; Murakami, Y. Porous PLGA microparticles formed by “one-step” emulsification for pulmonary drug delivery: The surface morphology and the aerodynamic properties. Colloids Surf. B Biointerfaces 2017, 159, 318–326. [Google Scholar] [CrossRef]
- Mahar, R.; Chakraborty, A.; Nainwal, N.; Bahuguna, R.; Sajwan, M.; Jakhmola, V. Application of PLGA as a biodegradable and biocompatible polymer for pulmonary delivery of drugs. AAPS Pharm. Sci. Tech. 2023, 24, 39. [Google Scholar] [CrossRef]
- Chishti, N.; Dehghan, M.H. Nano-embedded microparticles based dry powder inhaler for lung cancer treatment. J. Res. Pharm. 2020, 24, 425–435. [Google Scholar] [CrossRef]
- Salvi, L.; Dubey, C.K.; Sharma, K.; Nagar, D.; Meghani, M.; Goyal, S.; Nagar, J.C.; Sharma, A. A synthesis, properties and application as a possible drug delivery systems dendrimers–A review. Asian J. Pharm. Res. Dev. 2020, 8, 107–113. [Google Scholar] [CrossRef]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Ferreira, E.I.; El Seoud, O.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Bielski, E.; Zhong, Q.; Mirza, H.; Brown, M.; Molla, A.; Carvajal, T.; da Rocha, S.R.P. TPP-dendrimer nanocarriers for siRNA delivery to the pulmonary epithelium and their dry powder and metered-dose inhaler formulations. Int. J. Pharm. 2017, 527, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Lin, X.; Valle, R.P.; Zuo, Y.Y.; Gu, N. Poly(amidoamine) Dendrimer as a Respiratory Nanocarrier: Insights from Experiments and Molecular Dynamics Simulations. Langmuir 2019, 35, 5364–5371. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Altube, M.J.; Perez, N.; Romero, E.L.; Morilla, M.J.; Higa, L.H.; Perez, A.P. Inhaled lipid nanocarriers for pulmonary delivery of glucocorticoids: Previous strategies, recent advances and key factors description. Int. J. Pharm. 2023, 642, 123146. [Google Scholar] [CrossRef]
- Priya, S.; Desai, V.M.; Singhvi, G. Surface modification of lipid-based nanocarriers: A potential approach to enhance targeted drug delivery. ACS Omega 2022, 8, 74–86. [Google Scholar] [CrossRef]
- Costabile, G.; Conte, G.; Brusco, S.; Savadi, P.; Miro, A.; Quaglia, F.; d’Angelo, I.; Ungaro, F. State-of-the-art review on inhalable lipid and polymer nanocarriers: Design and development perspectives. Pharmaceutics 2024, 16, 347. [Google Scholar] [CrossRef]
- Garbuzenko, O.B.; Mainelis, G.; Taratula, O.; Minko, T. Inhalation treatment of lung cancer: The influence of composition, size and shape of nanocarriers on their lung accumulation and retention. Cancer Biol. Med. 2014, 11, 44–55. [Google Scholar]
- Ezhilarasan, D.; Lakshmi, T.; Mallineni, S.K. Nano-based targeted drug delivery for lung cancer: Therapeutic avenues and challenges. Nanomedicine 2022, 17, 1855–1869. [Google Scholar] [CrossRef] [PubMed]
- Sturm, R. Modeling tracheobronchial clearance of nanoparticles with variable size and geometry. J. Public Health Emerg. 2021, 5, 12. [Google Scholar] [CrossRef]
- Khadanga, V.; Mishra, P.C. A review on toxicity mechanism and risk factors of nanoparticles in respiratory tract. Toxicology 2024, 504, 153781. [Google Scholar] [CrossRef]
- Mapanao, A.K.; Giannone, G.; Summa, M.; Ermini, M.L.; Zamborlin, A.; Santi, M.; Cassano, D.; Bertorelli, R.; Voliani, V. Biokinetics and clearance of inhaled gold ultrasmall-in-nano architectures. Nanoscale Adv. 2020, 2, 3815–3820. [Google Scholar] [CrossRef] [PubMed]
- Benam, K.H.; Vladar, E.K.; Janssen, W.J.; Evans, C.M. Mucociliary Defense: Emerging Cellular, Molecular, and Animal Models. Ann. Am. Thorac. Soc. 2018, 15, S210–S215. [Google Scholar] [CrossRef]
- Gizurarson, S. The effect of cilia and the mucociliary clearance on successful drug delivery. Biol. Pharm. Bull. 2015, 38, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Ng, C.T.; Zou, L.; Lu, Y.; Chen, J.; Bay, B.H.; Shen, H.-M.; Ong, C.N. Targeted metabolomics reveals differential biological effects of nanoplastics and nanoZnO in human lung cells. Nanotoxicology 2019, 13, 1117–1132. [Google Scholar] [CrossRef]
- Chen, D.; Liu, J.; Wu, J.; Suk, J.S. Enhancing nanoparticle penetration through airway mucus to improve drug delivery efficacy in the lung. Expert Opin. Drug Deliv. 2021, 18, 595–606. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Holzwarth, U.; Haberl, N.; Kozempel, J.; Wenk, A.; Hirn, S.; Schleh, C.; Schäffler, M.; Lipka, J.; Semmler-Behnke, M. Quantitative biokinetics of titanium dioxide nanoparticles after intratracheal instillation in rats: Part 3. Nanotoxicology 2017, 11, 454–464. [Google Scholar] [CrossRef]
- Murgia, X.; Loretz, B.; Hartwig, O.; Hittinger, M.; Lehr, C.-M. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv. Drug Deliv. Rev. 2018, 124, 82–97. [Google Scholar] [CrossRef]
- Huckaby, J.T.; Lai, S.K. PEGylation for enhancing nanoparticle diffusion in mucus. Adv. Drug Deliv. Rev. 2018, 124, 125–139. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Beyond PEGylation: Alternative surface-modification of nanoparticles with mucus-inert biomaterials. Adv. Drug Deliv. Rev. 2018, 124, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Zhu, X.; Tao, W.; Cui, Y.; Liu, M.; Wu, L.; Li, L.; Zheng, Y.; Huang, Y. Enhanced oral delivery of protein drugs using zwitterion-functionalized nanoparticles to overcome both the diffusion and absorption barriers. ACS Appl. Mater. Interfaces 2016, 8, 25444–25453. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, X.-f.; Gan, Y.; Yu, M. Engineering nanoparticles to overcome the mucus barrier for drug delivery: Design, evaluation and state-of-the-art. Med. Drug Discov. 2021, 12, 100110. [Google Scholar] [CrossRef]
- Araujo, F.; Martins, C.; Azevedo, C.; Sarmento, B. Chemical modification of drug molecules as strategy to reduce interactions with mucus. Adv. Drug Deliv. Rev. 2018, 124, 98–106. [Google Scholar] [CrossRef]
- Maisel, K.; Reddy, M.; Xu, Q.; Chattopadhyay, S.; Cone, R.; Ensign, L.M.; Hanes, J. Nanoparticles coated with high molecular weight PEG penetrate mucus and provide uniform vaginal and colorectal distribution in vivo. Nanomedicine 2016, 11, 1337–1343. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, Y.; Chen, X.; Li, M.; Ma, X.; Cheng, G.; Xue, C.; Zuo, Y.Y.; Sun, B. Mucus penetration of surface-engineered nanoparticles in various pH microenvironments. ACS Nano 2023, 17, 2813–2828. [Google Scholar] [CrossRef] [PubMed]
- Pasut, G.; Paolino, D.; Celia, C.; Mero, A.; Joseph, A.S.; Wolfram, J.; Cosco, D.; Schiavon, O.; Shen, H.; Fresta, M. Polyethylene glycol (PEG)-dendron phospholipids as innovative constructs for the preparation of super stealth liposomes for anticancer therapy. J. Control. Release 2015, 199, 106–113. [Google Scholar] [CrossRef]
- Haque, S.; McLeod, V.M.; Jones, S.; Fung, S.; Whittaker, M.; McIntosh, M.; Pouton, C.; Owen, D.J.; Porter, C.J.; Kaminskas, L.M. Effect of increased surface hydrophobicity via drug conjugation on the clearance of inhaled PEGylated polylysine dendrimers. Eur. J. Pharm. Biopharm. 2017, 119, 408–418. [Google Scholar] [CrossRef]
- Sharma, P.; Vijaykumar, A.; Raghavan, J.V.; Rananaware, S.R.; Alakesh, A.; Bodele, J.; Rehman, J.U.; Shukla, S.; Wagde, V.; Nadig, S. Particle uptake driven phagocytosis in macrophages and neutrophils enhances bacterial clearance. J. Control. Release 2022, 343, 131–141. [Google Scholar] [CrossRef]
- Ni, R.; Zhao, J.; Liu, Q.; Liang, Z.; Muenster, U.; Mao, S. Nanocrystals embedded in chitosan-based respirable swellable microparticles as dry powder for sustained pulmonary drug delivery. Eur. J. Pharm. Sci. 2017, 99, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Vanbever, R.; Loira-Pastoriza, C.; Dauguet, N.; Hérin, C.; Ibouraadaten, S.; Vanvarenberg, K.; Ucakar, B.; Tyteca, D.; Huaux, F. Cationic nanoliposomes are efficiently taken up by alveolar macrophages but have little access to dendritic cells and interstitial macrophages in the normal and CpG-stimulated lungs. Mol. Pharm. 2019, 16, 2048–2059. [Google Scholar] [CrossRef] [PubMed]
- Ruge, C.A.; Bohr, A.; Beck-Broichsitter, M.; Nicolas, V.; Tsapis, N.; Fattal, E. Disintegration of nano-embedded microparticles after deposition on mucus: A mechanistic study. Colloids Surf. B Biointerfaces 2016, 139, 219–227. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, J.; Sun, Z.; Shen, X.; Li, L.; Jin, L.; Mao, S. Influence of stabilizer type and concentration on the lung deposition and retention of resveratrol nanosuspension-in-microparticles. Int. J. Pharm. 2019, 569, 118562. [Google Scholar] [CrossRef] [PubMed]
- Seydoux, E.; Rodriguez-Lorenzo, L.; Blom, R.A.; Stumbles, P.A.; Petri-Fink, A.; Rothen-Rutishauser, B.M.; Blank, F.; Von Garnier, C. Pulmonary delivery of cationic gold nanoparticles boost antigen-specific CD4+ T cell proliferation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1815–1826. [Google Scholar] [CrossRef]
- Japiassu, K.B.; Fay, F.; Marengo, A.; Louaguenouni, Y.; Cailleau, C.; Denis, S.; Chapron, D.; Tsapis, N.; Nascimento, T.L.; Lima, E.M.; et al. Interplay between mucus mobility and alveolar macrophage targeting of surface-modified liposomes. J. Control. Release 2022, 352, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Whittaker, M.R.; McIntosh, M.P.; Pouton, C.W.; Kaminskas, L.M. Disposition and safety of inhaled biodegradable nanomedicines: Opportunities and challenges. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1703–1724. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Williams, G.; Walles, M.; Manevski, N.; Krähenbühl, S.; Camenisch, G. Comparison of rat and human pulmonary metabolism using precision-cut lung slices (PCLS). Drug Metab. Lett. 2019, 13, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Oesch, F.; Fabian, E.; Landsiedel, R. Xenobiotica-metabolizing enzymes in the lung of experimental animals, man and in human lung models. Arch. Toxicol. 2019, 93, 3419–3489. [Google Scholar] [CrossRef]
- Rubin, K.; Ewing, P.; Bäckström, E.; Abrahamsson, A.; Bonn, B.; Kamata, S.; Grime, K. Pulmonary metabolism of substrates for key drug-metabolizing enzymes by human alveolar type II cells, human and rat lung microsomes, and the isolated perfused rat lung model. Pharmaceutics 2020, 12, 117. [Google Scholar] [CrossRef]
- Cullen, L.; McClean, S. Bacterial adaptation during chronic respiratory infections. Pathogens 2015, 4, 66–89. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Sarode, A.; Patel, P.; Vargas-Montoya, N.; Allawzi, A.; Zhilin-Roth, A.; Karmakar, S.; Boeglin, L.; Deng, H.; Karve, S.; DeRosa, F. Inhalable dry powder product (DPP) of mRNA lipid nanoparticles (LNPs) for pulmonary delivery. Drug Deliv. Transl. Res. 2024, 14, 360–372. [Google Scholar] [CrossRef]
- Csóka, I.; Ismail, R.; Jójárt-Laczkovich, O.; Pallagi, E. Regulatory considerations, challenges and risk-based approach in nanomedicine development. Curr. Med. Chem. 2021, 28, 7461–7476. [Google Scholar] [CrossRef]
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef]
- Anastasiadis, S.H.; Chrissopoulou, K.; Stratakis, E.; Kavatzikidou, P.; Kaklamani, G.; Ranella, A. How the physicochemical properties of manufactured nanomaterials affect their performance in dispersion and their applications in biomedicine: A review. Nanomaterials 2022, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, J.; Wang, Y.; Zhang, F.; Dong, X.; Jiang, L.; Tang, Y.; Zhang, H.; Li, W. Promoting inter-/intra-cellular process of nanomedicine through its physicochemical properties optimization. Curr. Drug Metab. 2018, 19, 75–82. [Google Scholar] [CrossRef]
- Feng, J.; Markwalter, C.E.; Tian, C.; Armstrong, M.; Prud’homme, R.K. Translational formulation of nanoparticle therapeutics from laboratory discovery to clinical scale. J. Transl. Med. 2019, 17, 200. [Google Scholar] [CrossRef]
- Liu, X.; Meng, H. Consideration for the scale-up manufacture of nanotherapeutics—A critical step for technology transfer. View 2021, 2, 20200190. [Google Scholar] [CrossRef]
- Saleh, N.; Yousaf, Z. Tools and techniques for the optimized synthesis, reproducibility and scale up of desired nanoparticles from plant derived material and their role in pharmaceutical properties. In Nanoscale Fabrication, Optimization, Scale-Up and Biological Aspects of Pharmaceutical Nanotechnology; Elsevier: New York, NY, USA, 2018; pp. 85–131. [Google Scholar]
- Taifouris, M.; Martín, M.; Martínez, A.; Esquejo, N. Challenges in the design of formulated products: Multiscale process and product design. Curr. Opin. Chem. Eng. 2020, 27, 1–9. [Google Scholar] [CrossRef]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Shukla, R.; Yadav, A.; Ujjwal, R.R.; Flora, S.J.S. 3D printing in development of nanomedicines. Nanomaterials 2021, 11, 420. [Google Scholar] [CrossRef]
- Grasso, G.; Zane, D.; Dragone, R. Microbial nanotechnology: Challenges and prospects for green biocatalytic synthesis of nanoscale materials for sensoristic and biomedical applications. Nanomaterials 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Grossman, J.H.; Crist, R.M.; Clogston, J.D. Early development challenges for drug products containing nanomaterials. AAPS J. 2017, 19, 92–102. [Google Scholar] [CrossRef]
- Ragelle, H.; Danhier, F.; Préat, V.; Langer, R.; Anderson, D.G. Nanoparticle-based drug delivery systems: A commercial and regulatory outlook as the field matures. Expert Opin. Drug Deliv. 2017, 14, 851–864. [Google Scholar] [CrossRef]
- Landesman-Milo, D.; Peer, D. Transforming nanomedicines from lab scale production to novel clinical modality. Bioconjugate Chem. 2016, 27, 855–862. [Google Scholar] [CrossRef]
- Fornaguera, C.; Solans, C. Methods for the in vitro characterization of nanomedicines—Biological component interaction. J. Pers. Med. 2017, 7, 2. [Google Scholar] [CrossRef]
- Bahru, T.B.; Ajebe, E.G. A review on nanotechnology: Analytical techniques use and applications. Int. Res. J. Pure Appl. Chem. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Mahmood, S.; Mandal, U.K.; Chatterjee, B.; Taher, M. Advanced characterizations of nanoparticles for drug delivery: Investigating their properties through the techniques used in their evaluations. Nanotechnol. Rev. 2017, 6, 355–372. [Google Scholar] [CrossRef]
- Jindal, A.B. The effect of particle shape on cellular interaction and drug delivery applications of micro-and nanoparticles. Int. J. Pharm. 2017, 532, 450–465. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Current developments and applications of microfluidic technology toward clinical translation of nanomedicines. Adv. Drug Deliv. Rev. 2018, 128, 54–83. [Google Scholar] [CrossRef]
- Siegrist, S.; Cörek, E.; Detampel, P.; Sandström, J.; Wick, P.; Huwyler, J. Preclinical hazard evaluation strategy for nanomedicines. Nanotoxicology 2019, 13, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Li, X.; Lu, X.; Zhang, Z.; Zhang, Y.; Wu, W.; Wang, C. Inhaled multilevel size-tunable, charge-reversible and mucus-traversing composite microspheres as trojan horse: Enhancing lung deposition and tumor penetration. Chin. Chem. Lett. 2024, 35, 109384. [Google Scholar] [CrossRef]
- Fernández-García, R.; Fraguas-Sánchez, A.I. Nanomedicines for Pulmonary Drug Delivery: Overcoming Barriers in the Treatment of Respiratory Infections and Lung Cancer. Pharmaceutics 2024, 16, 1584. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, Z.; Huang, Y.; Zhang, X.; Huang, J.; Cui, Y.; Yue, X.; Ma, C.; Fu, F.; Wang, W. Pulmonary delivery nanomedicines towards circumventing physiological barriers: Strategies and characterization approaches. Adv. Drug Deliv. Rev. 2022, 185, 114309. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).