Application of Standardized Rosa damascena Stem Cell-Derived Exosomes in Dermatological Wound Healing and Scar Management: A Retrospective Case-Series Study with Long-Term Outcome Assessment
Abstract
1. Introduction
Traditional and Ethnopharmacological Applications of Rosa damascena
2. Methods
2.1. Study Design, Timeline, and Ethical Considerations
2.2. Materials
Product Characterization Overview
2.3. Mechanistic Rationale for RSCE Therapy
2.4. Documentation and Assessment
2.5. Standardized Efficacy Assessment Tools
2.5.1. Goodman and Baron Scarring Grading System
- Grade 1 (mild): Macular erythematous, pigmented, or depigmented flat marks visible to the patient or observer irrespective of distance.
- Grade 2 (moderate): Mild atrophy or hypertrophy that may not be obvious at social distances and may be covered with makeup or facial hair.
- Grade 3 (moderate to severe): Moderate atrophic or hypertrophic scarring that is obvious at social distances and not easily covered with makeup or facial hair.
- Grade 4 (severe): Severe atrophic or hypertrophic scarring that is evident at social distances and not concealable with makeup or facial hair.
2.5.2. Modified Vancouver Scar Scale (mVSS)
- Vascularity: 0 = normal, 1 = pink, 2 = red, and 3 = purple.
- Pigmentation: 0 = normal, 1 = hypopigmentation, and 2 = hyperpigmentation.
- Pliability: 0 = normal, 1 = supple, 2 = yielding, 3 = firm, 4 = ropes, and 5 = contracture.
- Height: 0 = flat, 1 ≤ mm, 2 = 2–5 mm, and 3 ≥ 5 mm.
2.5.3. Wound Healing Assessment Scale (WHAS)
- Wound size: 0 = complete closure, 1 ≤ 1 cm diameter, 2 = 1–2 cm diameter, 3 = 2–4 cm diameter, and 4 ≥ 4 cm diameter.
- Exudate: 0 = none, 1 = minimal, 2 = moderate, 3 = copious, and 4 = very copious.
- Erythema: 0 = none, 1 = minimal, 2 = mild, 3 = severe, and 4 = very severe.
- Edema: 0 = none, 1 = minimal, 2 = moderate, 3 = significant, and 4 = very significant.
- Epithelialization: 0 = complete, 1 ≥ 75%, 2 = 50–75%, 3 = 25–49%, and 4 ≤ 25% or none.
- Granulation: 0 = optimal, 1 = minimal, 2 = moderate, 3 = poor, and 4 = none.
2.5.4. Visual Analog Scale (VAS) for Pain
- 0 = no pain;
- 1–3 = mild discomfort;
- 4–6 = moderate pain;
- 7–9 = severe pain;
- 10 = worst possible pain/unbearable pain.
2.5.5. Patient and Observer Scar Assessment Scale (POSAS)
- Observer component—evaluating vascularization, pigmentation, thickness, relief, pliability, and surface area.
- Patient component—assessing pain, itching, color, stiffness, thickness, and irregularity.
2.5.6. Patient Satisfaction Assessment
3. Results
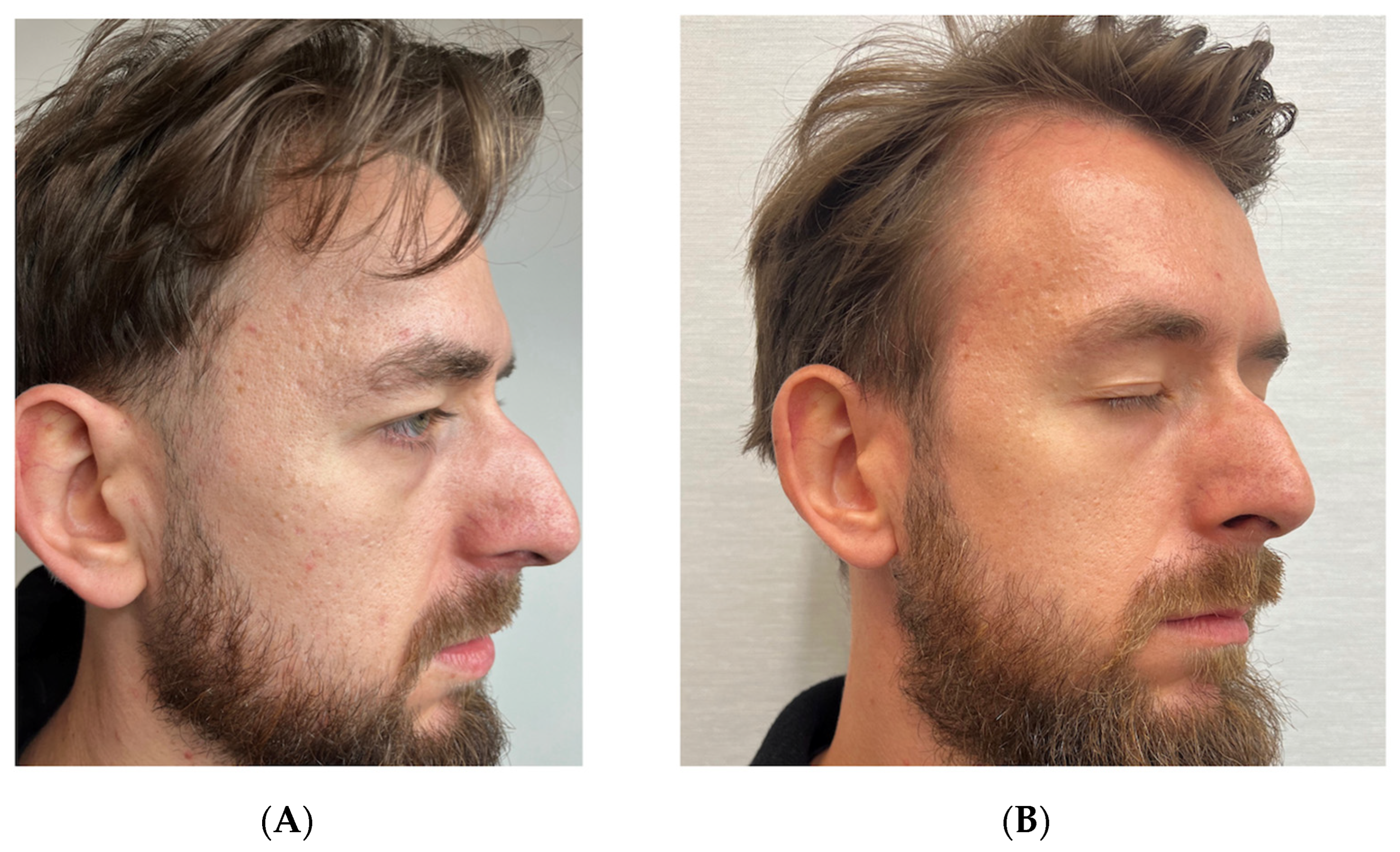
3.1. Case 1: Traumatic Facial Injury in a 24-Year-Old Male
Standardized Assessment Outcomes
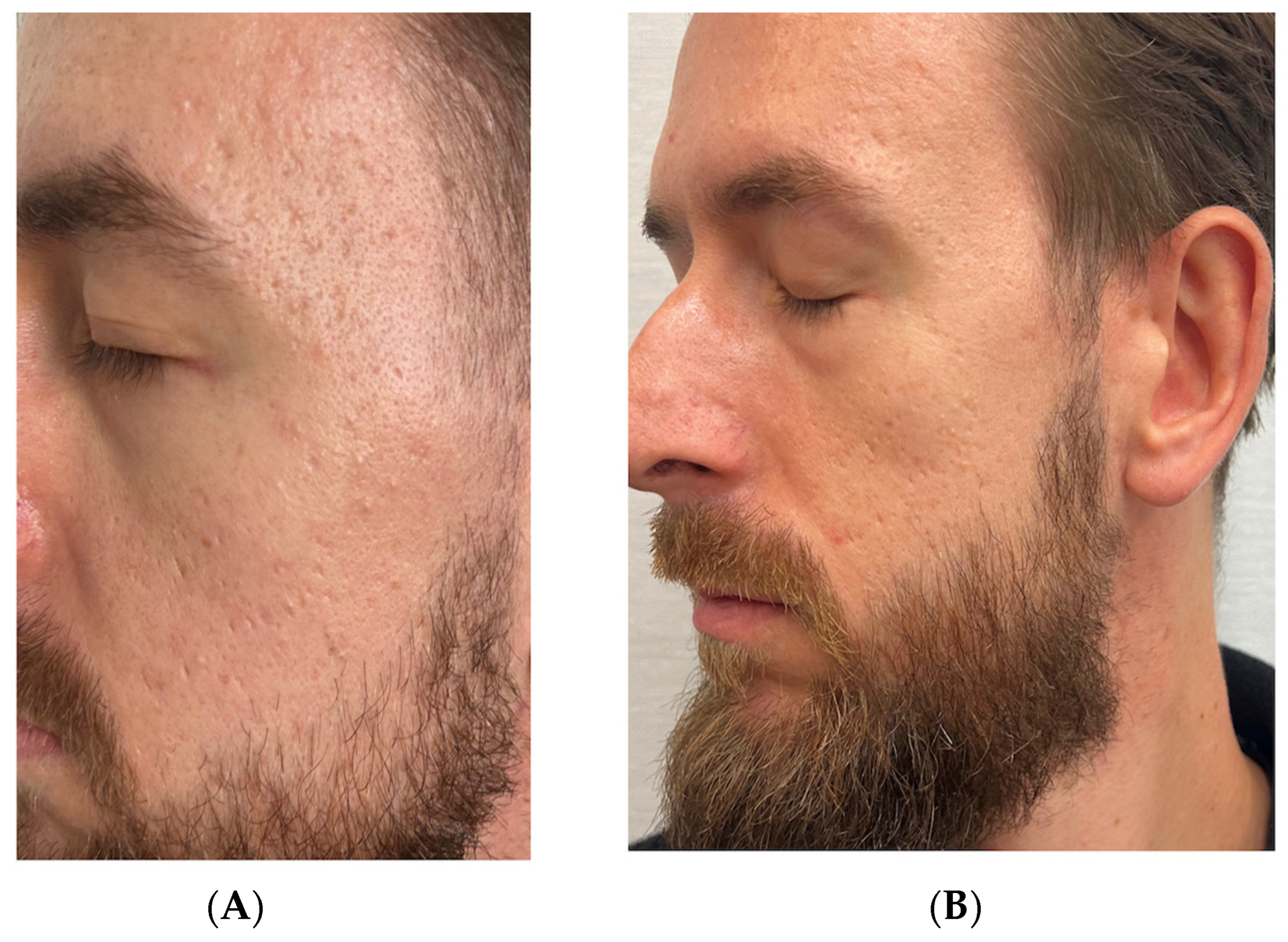
3.2. Case 2: Atrophic Acne Scars in a 32-Year-Old Male
Standardized Assessment Outcomes
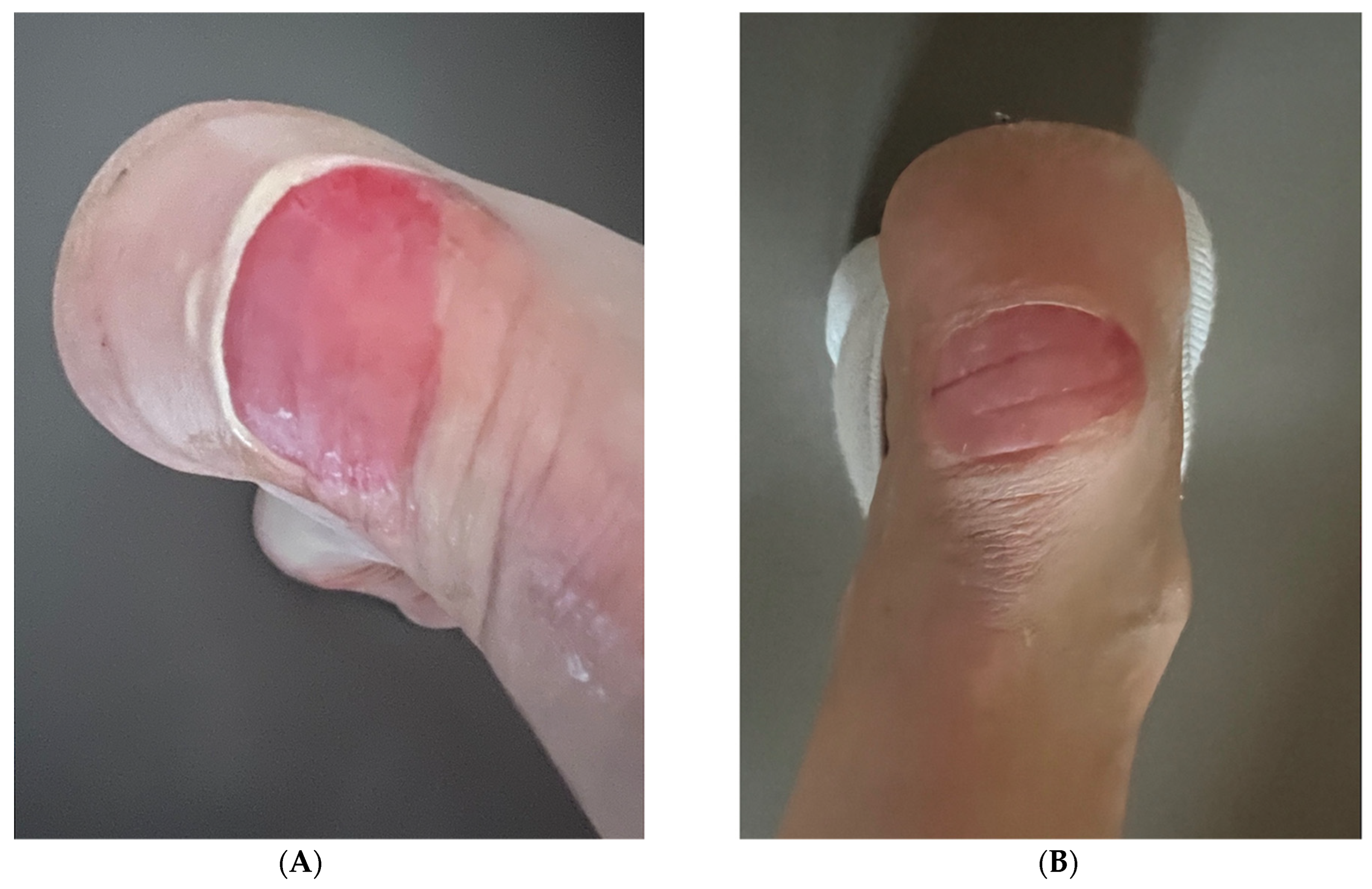
3.3. Case 3: Heel Wound in a 51-Year-Old Female
Standardized Assessment Outcomes
3.4. Case 4: Lacerated Leg Wound in a 42-Year-Old Female
Standardized Assessment Outcomes
3.5. RSCE Characterization Results
3.6. Mechanistic Observations
4. Discussion
4.1. Microneedling as a Complementary Approach in Scar Management and Skin Regeneration
4.2. Standardized Assessment of Treatment Outcomes
4.3. Limitations
- The small sample size and case-series design limit the generalizability of our observations and preclude statistical analysis of efficacy or safety.
- The multimodal treatment approaches used in most cases make it difficult to isolate the specific effects of RSCE therapy alone versus their combination with other interventions.
- While we employed standardized assessment scales to improve objectivity, the absence of blinded assessors may have introduced bias in scoring, particularly for observer-dependent measurements.
- The variability in follow-up periods between cases affects the comparability of long-term outcomes, and the specific mechanisms of action of RSCEs in human tissue have not been fully elucidated.
- The absence of control groups in three of four cases limits our ability to determine the specific contribution of RSCE therapy versus natural healing processes or other concurrent interventions. Only Case 2 employed a split-face design that allowed for direct comparison, though this comparison was between RSCE-enhanced treatment versus standard multimodal therapy rather than RSCE alone versus placebo.
- Future studies should incorporate proper control groups, including microneedling without RSCE therapy, and RSCE therapy without microneedling, to better isolate the therapeutic effects of each intervention component.
- We did not perform independent characterization or verification of the RSCE product composition, bioactivity, or physical properties, relying instead on manufacturer specifications and the published literature. Independent verification of product characteristics would strengthen the scientific rigor of clinical outcome assessments.
5. Conclusions
- Potential impact on fibrotic tissue: In the traumatic scar case, we observed changes in scar appearance that might indicate reduced fibrotic adhesions, potentially contributing to improved skin flexibility and reduced contractures.
- Possible influence on dermal remodeling: RSCE-treated areas appeared to show changes in skin texture and elasticity that might be associated with collagen remodeling, though specific mechanisms require further study.
- Observed improvements in tissue quality: Treated areas showed apparent improvements in hydration and reduced inflammation, though the extent to which these effects can be attributed specifically to RSCE therapy versus other treatment components remains to be determined.
- Duration of observed changes: The patients in this series exhibited maintenance of improvements through the follow-up period, with observed benefits at six to ten months post-treatment, suggesting potential durability of effect that deserves further investigation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- desJardins-Park, H.E.; Gurtner, G.C.; Wan, D.C.; Longaker, M.T. From Chronic Wounds to Scarring: The Growing Health Care Burden of Under- and Over-Healing Wounds. Adv. Wound Care 2022, 11, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Meretsky, C.R.; Polychronis, A.; Schiuma, A.T. A Comparative Analysis of the Advances in Scar Reduction: Techniques, Technologies, and Efficacy in Plastic Surgery. Cureus 2024, 16, e66806. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Qin, X.; Lin, W.; Wang, C.; Yin, X.; Wu, J.; Chen, Y.; Chen, X.; Chen, T. Microneedle-Based Approaches for Skin Disease Treatment. Nanomicro Lett. 2025, 17, 132. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Waheed, S.; Wang, C.; Shekh, M.; Li, Z.; Wu, J. Exosomes and Their Bioengineering Strategies in the Cutaneous Wound Healing and Related Complications: Current Knowledge and Future Perspectives. Int. J. Biol. Sci. 2023, 19, 1430–1454. [Google Scholar] [CrossRef]
- Hao, M.; Duan, M.; Yang, Z.; Zhou, H.; Li, S.; Xiang, J.; Wu, H.; Liu, H.; Chang, L.; Wang, D.; et al. Engineered Stem Cell Exosomes for Oral and Maxillofacial Wound Healing. Front. Bioeng. Biotechnol. 2022, 10, 1038261. [Google Scholar] [CrossRef]
- Lotvall, J. ISEV2020 Abstract Book. J. Extracell. Vesicles 2020, 9 (Suppl. 1), 1784511. [Google Scholar] [CrossRef]
- Zhu, H.; Chang, M.; Wang, Q.; Chen, J.; Liu, D.; He, W. Identifying the Potential of miRNAs in Houttuynia Cordata-Derived Exosome-Like Nanoparticles Against Respiratory RNA Viruses. Int. J. Nanomed. 2023, 18, 5983–6000. [Google Scholar] [CrossRef]
- Yang, S.; Li, W.; Bai, X.; Di Nunzio, G.; Fan, L.; Zhao, Y.; Ren, L.; Zhao, R.; Bian, S.; Liu, M.; et al. Ginseng-Derived Nanoparticles Alleviate Inflammatory Bowel Disease via the TLR4/MAPK and p62/Nrf2/Keap1 Pathways. J. Nanobiotechnol. 2024, 22, 48. [Google Scholar] [CrossRef]
- Ocansey, D.K.; Zhang, L.; Wang, Y.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Exosome-Mediated Effects and Applications in Inflammatory Bowel Disease. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1287–1307. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Li, C.; Yu, Y.; Yi, Y.; Wang, J.; Chen, D. Exosome-Induced Regulation in Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 1464. [Google Scholar] [CrossRef]
- Gao, C.; Zhou, Y.; Chen, Z.; Li, H.; Xiao, Y.; Hao, W.; Zhu, Y.; Vong, C.T.; Farag, M.A.; Wang, Y.; et al. Turmeric-Derived Nanovesicles as Novel Nanobiologics for Targeted Therapy of Ulcerative Colitis. Theranostics 2022, 12, 5596–5614. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, B. Insight into Endothelial Cell-Derived Extracellular Vesicles in Cardiovascular Disease: Molecular Mechanisms and Clinical Implications. Pharmacol. Res. 2024, 207, 107309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, J.; Ding, H.; Qiu, M.; Xue, L.; Ge, D.; Wen, G.; Ren, H.; Li, P.; Wang, J. Applications of Plant-Derived Extracellular Vesicles in Medicine. MedComm 2024, 5, e741. [Google Scholar] [CrossRef]
- Lotvall, J. ISEV2024 Abstract Book. J. Extracell. Vesicles 2024, 13 (Suppl. 1), e12444. [Google Scholar] [CrossRef]
- Widrlechner, M.P. History and Utilization of Rosa damascena. Econ. Bot. 1981, 35, 42–58. [Google Scholar] [CrossRef]
- Mahboubi, M. Rosa damascena as Holy Ancient Herb with Novel Applications. J. Tradit. Complement. Med. 2016, 6, 10–16. [Google Scholar] [CrossRef]
- Nayebi, N.; Khalili, N.; Kamalinejad, M.; Emtiazy, M. A Systematic Review of the Efficacy and Safety of Rosa damascena Mill. with an Overview on Its Phytopharmacological Properties. Complement. Ther. Med. 2017, 34, 129–140. [Google Scholar] [CrossRef]
- Boskabady, M.H.; Shafei, M.N.; Saberi, Z.; Amini, S. Pharmacological Effects of Rosa damascena. Iran. J. Basic Med. Sci. 2011, 14, 295–307. [Google Scholar]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A Review on Exosomes Application in Clinical Trials: Perspective, Questions, and Challenges. Cell Commun. Signal. 2022, 20, 145. [Google Scholar] [CrossRef]
- Mu, N.; Li, J.; Zeng, L.; You, J.; Li, R.; Qin, A.; Zhou, Z. Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar] [CrossRef]
- ExoCoBio Inc. Technical Materials: ExoSCRT™ Technology Overview. Available online: http://www.exocobio.com/default/eng/02/01.php?top=2&sub=1 (accessed on 3 May 2025).
- ExoCoBio Inc. Press Release, 2023: ExoCoBio Obtained a US Patent for Rose Stem Cell-Derived Exosome (RSCE™). Available online: https://parsers.vc/startup/exocobio.com/ (accessed on 3 May 2025).
- ASCEplus Product Documentation, ExoCoBio Inc. Available online: http://www.asceplus.com (accessed on 15 May 2025).
- Asadpour, A.; Yahaya, B.H.; Bicknell, K.; Cottrell, G.S.; Widera, D. Uncovering the gray zone: Mapping the global landscape of direct-to-consumer businesses offering interventions based on secretomes, extracellular vesicles, and exosomes. Stem Cell Res. Ther. 2023, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- ExoCoBio Patent Documentation: Method for Manufacturing Rose Stem CELL-Derived Exosomes. Available online: http://www.exocobio.com/default/eng/05/01.php?top=5&sub=1 (accessed on 15 May 2025).
- Won, Y.J.; Lee, E.; Min, S.Y.; Cho, B.S. Biological Function of Exosome-Like Particles Isolated from Rose (Rosa Damascena) Stem Cell Culture Supernatant. bioRxiv. 2023. Available online: https://www.biorxiv.org/content/10.1101/2023.04.21.537865v1 (accessed on 3 May 2025).
- Rodriguez, C.; Porcello, A.; Chemali, M.; Raffoul, W.; Marques, C.; Scaletta, C.; Lourenço, K.; Abdel-Sayed, P.; Applegate, L.A.; Pelissier Vatter, F.; et al. Medicalized Aesthetic Uses of Exosomes and Cell Culture-Conditioned Media: Opening an Advanced Care Era for Biologically Inspired Cutaneous Prejuvenation and Rejuvenation. Cosmetics 2024, 11, 154. [Google Scholar] [CrossRef]
- Goodman, G.J.; Baron, J.A. Postacne Scarring—A Quantitative Global Scarring Grading System. J. Cosmet. Dermatol. 2006, 5, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Goodman, G.J.; Baron, J.A. The Management of Postacne Scarring. Dermatol. Surg. 2007, 33, 1175–1188. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wei, S.; Xu, Q.; Sun, Y.; Ning, X.; Wang, Z. Application of ADSCs and Their Exosomes in Scar Prevention. Stem Cell Rev. Rep. 2022, 18, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.I.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes Derived from Human Adipose Mesenchymal Stem Cells Accelerate Cutaneous Wound Healing via Optimizing the Characteristics of Fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Z.; Li, S.; Xie, X.; Qin, L.; Zhang, Q.; Yang, Y.; Wang, T.; Zhang, Y. Exosomes: Compositions, Biogenesis, and Mechanisms in Diabetic Wound Healing. J. Nanobiotechnol. 2024, 22, 398. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, Y.; Zhang, Y.; Zhou, J.; Cao, K. Adipose-Derived Mesenchymal Stem Cell Exosomes: A Novel Pathway for Tissue Repair. Cell Tissue Bank. 2019, 20, 153–161. [Google Scholar] [CrossRef]
- Dong, Z.; Fu, Y.; Cai, Z.; Dai, H.; He, Y. Recent Advances in Adipose-Derived Mesenchymal Stem Cell-Derived Exosomes for Regulating Macrophage Polarization. Front. Immunol. 2025, 16, 1525466. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Guo, S.; Ren, X.; Wu, Z.; Liu, S.; Yao, X. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells 2023, 12, 1416. [Google Scholar] [CrossRef]
- Fan, I.H.; Pi, J.K.; Zou, C.Y.; Jiang, Y.L.; Li, Q.J.; Zhang, X.Z.; Xing, F.; Nie, R.; Han, C.; Xie, H.Q. Hydrogel-Exosome System in Tissue Engineering: A Promising Therapeutic Strategy. Bioact. Mater. 2024, 38, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Tsai, T.H.; Lee, C.H. Regulation of Exosomes as Biologic Medicines: Regulatory Challenges Faced in Exosome Development and Manufacturing Processes. Clin. Transl. Sci. 2024, 17, e13904. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products. Official Journal of the European Union. 2009. Available online: https://health.ec.europa.eu/system/files/2016-11/cosmetic_1223_2009_regulation_en_0.pdf (accessed on 20 February 2025).
- Hadipour, E.; Kafash, M.R.; Emami, S.A.; Asili, J.; Boghrati, Z.; Tayarani-Najaran, Z. Evaluation of Anti-Oxidant and Antimelanogenic Effects of the Essential Oil and Extracts of Rosa ×damascena in B16F10 Murine Melanoma Cell Line. Iran. J. Basic Med. Sci. 2023, 26, 1076–1082. [Google Scholar] [CrossRef]
- Gavra, D.I.; Kósa, D.; Pető, Á.; Józsa, L.; Ujhelyi, Z.; Fehér, P.; Pallag, A.; Ghitea, T.C.; Frățilă, S.; Jurca, T.; et al. Exploring the Correlation between the PASI and DLQI Scores in Psoriasis Treatment with Topical Ointments Containing Rosa ×damascena Mill. Extract. Pharm. 2024, 17, 1092. [Google Scholar] [CrossRef]
- Proietti, I.; Battilotti, C.; Svara, F.; Innocenzi, C.; Spagnoli, A.; Potenza, C. Efficacy and Tolerability of a Microneedling Device Plus Exosomes for Treating Melasma. Appl. Sci. 2024, 14, 7252. [Google Scholar] [CrossRef]
- Lueangarun, S.; Cho, B.S.; Tempark, T. Topical Moisturizer with Rose Stem Cell-Derived Exosomes (RSCEs) for Recalcitrant Seborrheic Dermatitis: A Case Report with 6 Months of Follow-Up. J. Cosmet. Dermatol. 2024, 23, 3128–3132. [Google Scholar] [CrossRef]
- Majewska, L.; Dorosz, K.; Kijowski, J. Efficacy of Rose Stem Cell-Derived Exosomes (RSCEs) in Skin Treatment: From Healing to Hyperpigmentation Management: Case Series and Review. J. Cosmet. Dermatol. 2025, 24, e16776. [Google Scholar] [CrossRef]
- Albalat, W.; Ghonemy, S.; Saleh, A.; Elradi, M. Microneedling Combined with Botulinum Toxin-A Versus Microneedling Combined with Platelet-Rich Plasma in Treatment of Atrophic Acne Scars: A Comparative Split-Face Study. Arch. Dermatol. Res. 2023, 315, 839–846. [Google Scholar] [CrossRef]
- Pakla-Misiur, A.; Grochowiec, M.; Lesiak, A.; Bednarski, I.A. Double-Blind, Randomized Controlled Trial Comparing the Use of Microneedling Alone Versus Chemical Peeling Alone Versus a Combination of Microneedling and Chemical Peeling in the Treatment of Atrophic Post-Acne Scars: An Assessment of Clinical Effectiveness and Patients’ Quality-of-Life. Postępy Dermatol. I Alergol. 2021, 38, 629–635. [Google Scholar] [CrossRef]
- Kim, C.N.T.; Thi, L.P.; Van, T.N.; Minh, P.P.T.; Nguyet, M.V.; Le Thi, M.; Huu, N.D.; Hau, K.T.; Gandolfi, M.; Satolli, F.; et al. Successful Treatment of Facial Atrophic Acne Scars by Fractional Radiofrequency Microneedle in Vietnamese Patients. Open Access Maced. J. Med. Sci. 2019, 7, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Dukharan, V.; Broughton, L.; Stegura, C.; Schur, N.; Samman, L.; Garcia-Meraz, C.; Macaraeg-Jimenez, J.L.D.; Belo, V.G.; Majewska, L.; et al. Exosomes for Aesthetic Dermatology: A Comprehensive Literature Review and Update. J. Cosmet. Dermatol. 2025, 24, e16766. [Google Scholar] [CrossRef] [PubMed]
- Burshtein, J.; Wei, J.; Majewska, L.; Schlesinger, T. Plant-Derived Extracellular Vesicles in Dermatology: A Review of Emerging Therapeutic Applications. JCAD 2025, in press.
- Majewska, L.; Dorosz, K.; Kijowski, J. Exosome-Based Therapy for Skin Complications in Oncology Patients Treated with EGFR Inhibitors: A Case Report Highlighting the Need for Coordinated Dermato-Oncologic Care. Pharmaceuticals 2025, in press.




| Parameter | Baseline | 30 Days | 110 Days |
|---|---|---|---|
| Vascularity (0–3) | 2 (red) | 2 (red) | 1 (pink) |
| Pigmentation (0–2) | 0 (normal) | 0 (normal) | 0 (normal) |
| Pliability (0–5) | 4 (rope–like) | 3 (firm) | 1 (supple) |
| Height (0–3) | 2 (2–5 mm) | 1 (<2 mm) | 0 (flat) |
| Total mVSS (0–13) | 8/13 | 6/13 | 2/13 |
| Improvement (%) | - | 25% | 75% |
| POSAS Component | Baseline | 30 Days | 110 Days | Improvement (%) |
|---|---|---|---|---|
| Observer Score (6–60) | 45/60 | 34/60 | 16/60 | 64% |
| Patient Score (6–60) | 49/60 | 34/60 | 14/60 | 71% |
| Assessment Parameter | Right Side (RSCE-Enhanced) | Right Side (RSCE-Enhanced) | Left Side (Standard) | Left Side (Standard) |
|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | Pre-Treatment | Post-Treatment | |
| Goodman and Baron Grade | 4 (severe) | 2 (mild–moderate) | 3 (moderate–severe) | 2 (mild–moderate) |
| Grade Improvement | - | 2 grades | - | 1 grade |
| Scar Depth Reduction | - | 68% | - | 42% |
| Texture Improvement | - | 73% | - | 51% |
| Overall Smoothness | - | 76% | - | 54% |
| Patient Satisfaction (1–5) | - | 5/5 | - | 4/5 |
| Parameter | 2 Weeks Post-Injury | 18 Days Post-Injury (4 Days Post-RSCE) | 6 Months | 8 Months |
|---|---|---|---|---|
| Vascularity (0–3) | 2 (red) | 1 (pink) | 1 (pink) | 0 (normal) |
| Pigmentation (0–2) | 1 (hypopigmentation) | 1 (hypopigmentation) | 0 (normal) | 0 (normal) |
| Pliability (0–5) | 3 (firm) | 2 (yielding) | 1 (supple) | 0 (normal) |
| Height (0–3) | 1 (<2 mm) | 1 (<2 mm) | 0 (flat) | 0 (flat) |
| Total mVSS (0–13) | 7/13 | 5/13 | 2/13 | 0/13 |
| Improvement (%) | - | 29% | 71% | 100% |
| POSAS Component | Baseline | 30 Days | 110 Days | Improvement (%) |
|---|---|---|---|---|
| Observer Score (6–60) | 45/60 | 34/60 | 16/60 | 64% |
| Patient Score (6–60) | 49/60 | 34/60 | 14/60 | 71% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewska, L.; Kondraciuk, A.; Dorosz, K.; Budzyńska, A. Application of Standardized Rosa damascena Stem Cell-Derived Exosomes in Dermatological Wound Healing and Scar Management: A Retrospective Case-Series Study with Long-Term Outcome Assessment. Pharmaceutics 2025, 17, 910. https://doi.org/10.3390/pharmaceutics17070910
Majewska L, Kondraciuk A, Dorosz K, Budzyńska A. Application of Standardized Rosa damascena Stem Cell-Derived Exosomes in Dermatological Wound Healing and Scar Management: A Retrospective Case-Series Study with Long-Term Outcome Assessment. Pharmaceutics. 2025; 17(7):910. https://doi.org/10.3390/pharmaceutics17070910
Chicago/Turabian StyleMajewska, Lidia, Agnieszka Kondraciuk, Karolina Dorosz, and Agnieszka Budzyńska. 2025. "Application of Standardized Rosa damascena Stem Cell-Derived Exosomes in Dermatological Wound Healing and Scar Management: A Retrospective Case-Series Study with Long-Term Outcome Assessment" Pharmaceutics 17, no. 7: 910. https://doi.org/10.3390/pharmaceutics17070910
APA StyleMajewska, L., Kondraciuk, A., Dorosz, K., & Budzyńska, A. (2025). Application of Standardized Rosa damascena Stem Cell-Derived Exosomes in Dermatological Wound Healing and Scar Management: A Retrospective Case-Series Study with Long-Term Outcome Assessment. Pharmaceutics, 17(7), 910. https://doi.org/10.3390/pharmaceutics17070910







