ROS-Responsive Nanoplatforms for Targeted Tumor Immunomodulation: A Paradigm Shift in Precision Cancer Immunotherapy
Abstract
1. Introduction
2. ROS-Mediated Immunomodulation in the TME
2.1. DCs: ROS as Gatekeepers of Antigens’ Fate
2.2. Macrophage Polarization: Redox-Driven Functional Plasticity
2.3. T Cells: A Double-Edged Sword in Redox Signaling
2.4. NK Cells: Redox Regulation of Cytotoxicity and Survival
2.5. CAFs: Architects of ROS-Dependent Immunosuppression
3. ROS and Dysregulation of ICD
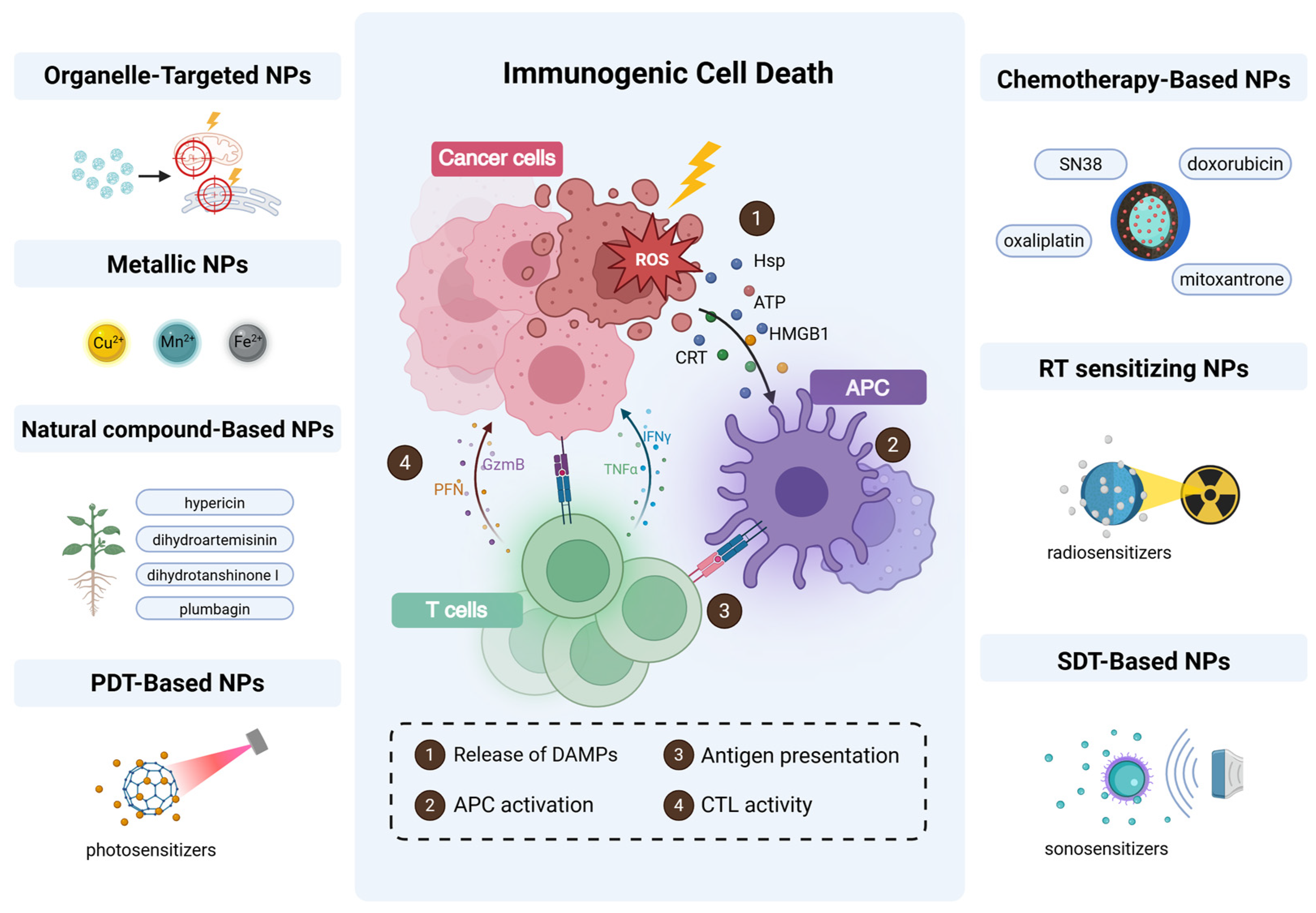
4. Nanoplatform Design Strategies for ROS Amplification to Enhance ICD
4.1. Organelle-Targeted ROS Delivery: Mitochondrial and ER-Centric Strategies
4.1.1. Mitochondria-Targeting ROS Nanogenerators
4.1.2. ER-Targeted ROS Amplification Systems
4.2. Metal-Based NPs
4.3. Natural Compound-Based NPs
4.4. PDT-Based NPs
4.4.1. Advanced Photosensitizer Design
4.4.2. ROS Amplification Mechanisms
4.4.3. Targeted Delivery and Immune Synergy
4.5. Chemotherapy-Based Nanomaterials
4.6. Radiotherapy-Sensitizing Nanomaterials
4.7. Sonodynamic Therapy (SDT)
5. ROS-Responsive Nanoplatforms for Targeted Immunomodulation
5.1. Restoring DC Antigen Cross-Presentation
5.2. Reinvigorating T Cell Activation
5.3. Reprogramming Macrophage Polarization
5.4. Disrupting CAF-Mediated Immunosuppression
6. Discussion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Almagro, J.; Fuchs, E. Beyond genetics: Driving cancer with the tumour microenvironment behind the wheel. Nat. Rev. Cancer 2024, 24, 274–286. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, M.; Xu, T.; Zhang, M.; Dai, H.; Wang, C.; Ding, D.; Zhong, Z. Reactive oxygen species-powered cancer immunotherapy: Current status and challenges. J. Control. Release 2023, 356, 623–648. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.H.; Devadas, S.; Kwon, J.; Pinto, L.A.; Williams, M.S. T cells express a phagocyte-type nadph oxidase that is activated after t cell receptor stimulation. Nat. Immunol. 2004, 5, 818–827. [Google Scholar] [CrossRef]
- Sena, L.A.; Li, S.; Jairaman, A.; Prakriya, M.; Ezponda, T.; Hildeman, D.A.; Wang, C.R.; Schumacker, P.T.; Licht, J.D.; Perlman, H.; et al. Mitochondria are required for antigen-specific t cell activation through reactive oxygen species signaling. Immunity 2013, 38, 225–236. [Google Scholar] [CrossRef]
- Savina, A.; Jancic, C.; Hugues, S.; Guermonprez, P.; Vargas, P.; Moura, I.C.; Lennon-Duménil, A.M.; Seabra, M.C.; Raposo, G.; Amigorena, S. Nox2 controls phagosomal ph to regulate antigen processing during crosspresentation by dendritic cells. Cell 2006, 126, 205–218. [Google Scholar] [CrossRef]
- Duwe, A.K.; Werkmeister, J.; Roder, J.C.; Lauzon, R.; Payne, U. Natural killer cell-mediated lysis involves an hydroxyl radical-dependent step. J. Immunol. 1985, 134, 2637–2644. [Google Scholar] [CrossRef]
- Cheng, A.N.; Cheng, L.C.; Kuo, C.L.; Lo, Y.K.; Chou, H.Y.; Chen, C.H.; Wang, Y.H.; Chuang, T.H.; Cheng, S.J.; Lee, A.Y. Mitochondrial lon-induced mtdna leakage contributes to pd-l1-mediated immunoescape via sting-ifn signaling and extracellular vesicles. J. Immunother. Cancer 2020, 8, e001372. [Google Scholar] [CrossRef]
- Liao, H.; Chang, X.; Gao, L.; Ye, C.; Qiao, Y.; Xie, L.; Lin, J.; Cai, S.; Dong, H. Il-17a promotes tumorigenesis and upregulates pd-l1 expression in non-small cell lung cancer. J. Transl. Med. 2023, 21, 828. [Google Scholar] [CrossRef]
- Bailly, C. Regulation of pd-l1 expression on cancer cells with ros-modulating drugs. Life Sci. 2020, 246, 117403. [Google Scholar] [CrossRef]
- Yu, X.; Lao, Y.; Teng, X.L.; Li, S.; Zhou, Y.; Wang, F.; Guo, X.; Deng, S.; Chang, Y.; Wu, X.; et al. Senp3 maintains the stability and function of regulatory t cells via bach2 desumoylation. Nat. Commun. 2018, 9, 3157. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Chou, H.Y.; Chiu, Y.C.; Cheng, A.N.; Fan, C.C.; Chang, Y.N.; Chen, C.H.; Jiang, S.S.; Chen, N.J.; Lee, A.Y. Mitochondrial oxidative stress by lon-pycr1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Lett. 2020, 474, 138–150. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ros in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and damps in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Alili, L.; Sack, M.; Karakoti, A.S.; Teuber, S.; Puschmann, K.; Hirst, S.M.; Reilly, C.M.; Zanger, K.; Stahl, W.; Das, S.; et al. Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor-stroma interactions. Biomaterials 2011, 32, 2918–2929. [Google Scholar] [CrossRef]
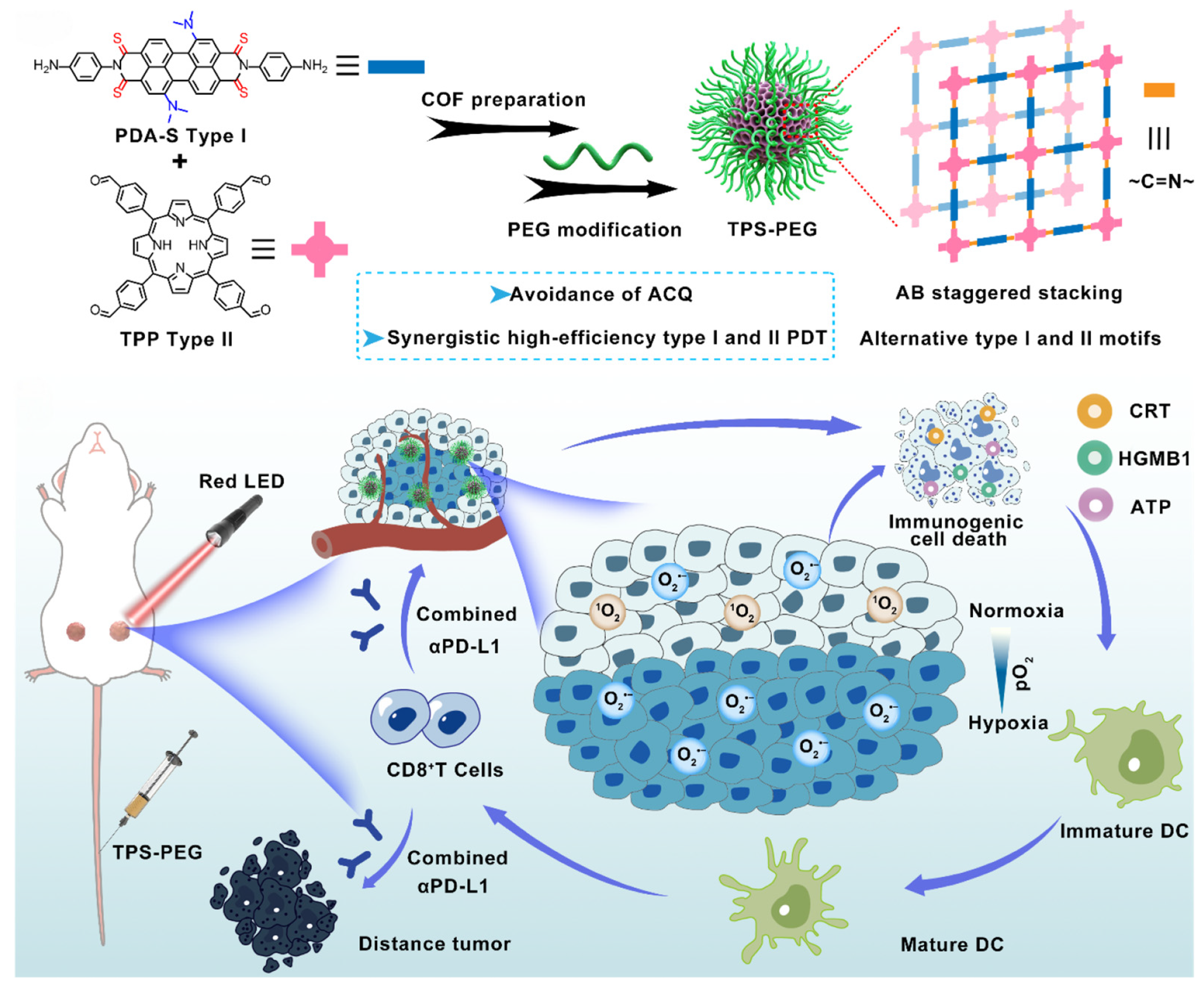
- Zhou, Q.; Huang, G.; Si, J.; Wu, Y.; Jin, S.; Ji, Y.; Ge, Z. Potent covalent organic framework nanophotosensitizers with staggered type I/II motifs for photodynamic immunotherapy of hypoxic tumors. ACS Nano 2024, 18, 35671–35683. [Google Scholar] [CrossRef]
- Li, Z.; Chu, Z.; Yang, J.; Qian, H.; Xu, J.; Chen, B.; Tian, T.; Chen, H.; Xu, Y.; Wang, F. Immunogenic cell death augmented by manganese zinc sulfide nanoparticles for metastatic melanoma immunotherapy. ACS Nano 2022, 16, 15471–15483. [Google Scholar] [CrossRef] [PubMed]
- Dingjan, I.; Verboogen, D.R.; Paardekooper, L.M.; Revelo, N.H.; Sittig, S.P.; Visser, L.J.; Mollard, G.F.; Henriet, S.S.; Figdor, C.G.; Ter Beest, M.; et al. Lipid peroxidation causes endosomal antigen release for cross-presentation. Sci. Rep. 2016, 6, 22064. [Google Scholar] [CrossRef]
- Oberkampf, M.; Guillerey, C.; Mouriès, J.; Rosenbaum, P.; Fayolle, C.; Bobard, A.; Savina, A.; Ogier-Denis, E.; Enninga, J.; Amigorena, S.; et al. Mitochondrial reactive oxygen species regulate the induction of CD8+ T cells by plasmacytoid dendritic cells. Nat. Commun. 2018, 9, 2241. [Google Scholar] [CrossRef]
- Xie, D.; Han, C.; Chen, C.; Liao, Z.; Campos de Souza, S.; Niu, Y.; Mano, J.F.; Dong, L.; Wang, C. A scaffold vaccine to promote tumor antigen cross-presentation via sustained toll-like receptor-2 (TLR2) activation. Bioact. Mater. 2024, 37, 315–330. [Google Scholar] [CrossRef]
- Chougnet, C.A.; Thacker, R.I.; Shehata, H.M.; Hennies, C.M.; Lehn, M.A.; Lages, C.S.; Janssen, E.M. Loss of phagocytic and antigen cross-presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction. J. Immunol. 2015, 195, 2624–2632. [Google Scholar] [CrossRef]
- Hu, Z.; Teng, X.L.; Zhang, T.; Yu, X.; Ding, R.; Yi, J.; Deng, L.; Wang, Z.; Zou, Q. Senp3 senses oxidative stress to facilitate sting-dependent dendritic cell antitumor function. Mol. Cell 2021, 81, 940–952.e5. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The reactive oxygen species in macrophage polarization: Reflecting its dual role in progression and treatment of human diseases. Oxid. Med. Cell Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Wu, Q.; Chen, Y.; Deng, Y.; Yang, Z.; Zhang, L.; Liu, B. Tumoral nox4 recruits M2 tumor-associated macrophages via ros/pi3k signaling-dependent various cytokine production to promote nsclc growth. Redox Biol. 2019, 22, 101116. [Google Scholar] [CrossRef]
- Ghosh, S.; Mukherjee, S.; Choudhury, S.; Gupta, P.; Adhikary, A.; Baral, R.; Chattopadhyay, S. Reactive oxygen species in the tumor niche triggers altered activation of macrophages and immunosuppression: Role of fluoxetine. Cell Signal. 2015, 27, 1398–1412. [Google Scholar] [CrossRef] [PubMed]
- Griess, B.; Mir, S.; Datta, K.; Teoh-Fitzgerald, M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic. Biol. Med. 2020, 147, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Gülow, K.; Tümen, D.; Heumann, P.; Schmid, S.; Kandulski, A.; Müller, M.; Kunst, C. Unraveling the role of reactive oxygen species in T lymphocyte signaling. Int. J. Mol. Sci. 2024, 25, 6114. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, S.Q.; Li, C.; Lykken, E.; Jiang, S.; Wong, E.; Gong, Z.; Tao, Z.; Zhu, B.; Wan, Y.; et al. Microrna-23a curbs necrosis during early t cell activation by enforcing intracellular reactive oxygen species equilibrium. Immunity 2016, 44, 568–581. [Google Scholar] [CrossRef]
- Malmberg, K.J.; Arulampalam, V.; Ichihara, F.; Petersson, M.; Seki, K.; Andersson, T.; Lenkei, R.; Masucci, G.; Pettersson, S.; Kiessling, R. Inhibition of activated/memory (cd45ro(+)) t cells by oxidative stress associated with block of nf-kappab activation. J. Immunol. 2001, 167, 2595–2601. [Google Scholar] [CrossRef]
- Kotsafti, A.; Scarpa, M.; Castagliuolo, I.; Scarpa, M. Reactive oxygen species and antitumor immunity-from surveillance to evasion. Cancers 2020, 12, 1748. [Google Scholar] [CrossRef]
- Scharping, N.E.; Rivadeneira, D.B.; Menk, A.V.; Vignali, P.D.A.; Ford, B.R.; Rittenhouse, N.L.; Peralta, R.; Wang, Y.; Wang, Y.; DePeaux, K.; et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives t cell exhaustion. Nat. Immunol. 2021, 22, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Son, D.J.; Park, M.H.; Yoon, D.Y.; Han, S.B.; Hong, J.T. Glutathione peroxidase 1 deficiency attenuates concanavalin a-induced hepatic injury by modulation of t-cell activation. Cell Death Dis. 2016, 7, e2208. [Google Scholar] [CrossRef]
- Jin, F.; Wu, Z.; Hu, X.; Zhang, J.; Gao, Z.; Han, X.; Qin, J.; Li, C.; Wang, Y. The pi3k/akt/gsk-3β/ros/eif2b pathway promotes breast cancer growth and metastasis via suppression of nk cell cytotoxicity and tumor cell susceptibility. Cancer Biol. Med. 2019, 16, 38–54. [Google Scholar]
- Song, H.; Park, H.; Kim, Y.S.; Kim, K.D.; Lee, H.K.; Cho, D.H.; Yang, J.W.; Hur, D.Y. L-kynurenine-induced apoptosis in human nk cells is mediated by reactive oxygen species. Int. Immunopharmacol. 2011, 11, 932–938. [Google Scholar] [CrossRef]
- Izawa, S.; Kono, K.; Mimura, K.; Kawaguchi, Y.; Watanabe, M.; Maruyama, T.; Fujii, H. H2O2 production within tumor microenvironment inversely correlated with infiltration of cd56(dim) nk cells in gastric and esophageal cancer: Possible mechanisms of nk cell dysfunction. Cancer Immunol. Immunother. CII 2011, 60, 1801–1810. [Google Scholar] [CrossRef]
- Harmon, C.; Robinson, M.W.; Hand, F.; Almuaili, D.; Mentor, K.; Houlihan, D.D.; Hoti, E.; Lynch, L.; Geoghegan, J.; O’Farrelly, C. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident nk cells in colorectal liver metastasis. Cancer Immunol. Res. 2019, 7, 335–346. [Google Scholar] [CrossRef]
- Yang, Y.; Neo, S.Y.; Chen, Z.; Cui, W.; Chen, Y.; Guo, M.; Wang, Y.; Xu, H.; Kurzay, A.; Alici, E.; et al. Thioredoxin activity confers resistance against oxidative stress in tumor-infiltrating nk cells. J. Clin. Investig. 2020, 130, 5508–5522. [Google Scholar] [CrossRef]
- Mimura, K.; Kua, L.F.; Shimasaki, N.; Shiraishi, K.; Nakajima, S.; Siang, L.K.; Shabbir, A.; So, J.; Yong, W.P.; Kono, K. Upregulation of thioredoxin-1 in activated human nk cells confers increased tolerance to oxidative stress. Cancer Immunol. Immunother. CII 2017, 66, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Sampson, N.; Koziel, R.; Zenzmaier, C.; Bubendorf, L.; Plas, E.; Jansen-Dürr, P.; Berger, P. Ros signaling by nox4 drives fibroblast-to-myofibroblast differentiation in the diseased prostatic stroma. Mol. Endocrinol. 2011, 25, 503–515. [Google Scholar] [CrossRef]
- Sampson, N.; Brunner, E.; Weber, A.; Puhr, M.; Schäfer, G.; Szyndralewiez, C.; Klocker, H. Inhibition of nox4-dependent ros signaling attenuates prostate fibroblast activation and abrogates stromal-mediated protumorigenic interactions. Int. J. Cancer 2018, 143, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Ramil, C.P.; Hai, J.; Zhang, C.; Wang, H.; Watkins, A.A.; Afshar, R.; Georgiev, P.; Sze, M.A.; Song, X.S.; et al. Cancer-associated fibroblasts promote immunosuppression by inducing ros-generating monocytic mdscs in lung squamous cell carcinoma. Cancer Immunol. Res. 2020, 8, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Raber, P.; Ochoa, A.C.; Rodríguez, P.C. Metabolism of l-arginine by myeloid-derived suppressor cells in cancer: Mechanisms of t cell suppression and therapeutic perspectives. Immunol. Investig. 2012, 41, 614–634. [Google Scholar] [CrossRef]
- Pakravan, K.; Mossahebi-Mohammadi, M.; Ghazimoradi, M.H.; Cho, W.C.; Sadeghizadeh, M.; Babashah, S. Monocytes educated by cancer-associated fibroblasts secrete exosomal mir-181a to activate akt signaling in breast cancer cells. J. Transl. Med. 2022, 20, 559. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.; Hanley, C.J.; Mellone, M.; Szyndralewiez, C.; Heitz, F.; Wiesel, P.; Wood, O.; Machado, M.; Lopez, M.A.; Ganesan, A.P.; et al. Nox4 inhibition potentiates immunotherapy by overcoming cancer-associated fibroblast-mediated cd8 t-cell exclusion from tumors. Cancer Res. 2020, 80, 1846–1860. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, X.; Gao, M.; Zhang, Y.; Tang, B.Z. A self-reporting fluorescent salicylaldehyde-chlorambucil conjugate as a type-II icd inducer for cancer vaccines. Adv. Mater. 2022, 34, e2205701. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Yoon, B.; Dey, A.; Song, S.H.; Li, Y.; Joo, H.; Park, J.H. Self-immolative polymer-based immunogenic cell death inducer for regulation of redox homeostasis. Biomaterials 2023, 295, 122064. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, H.; Li, Y.; Xie, T.; Yan, S.; Wang, J.; Qu, J.; Ouyang, F.; Lv, S.; Guo, Z.; et al. Promotion of icd via nanotechnology. Macromol. Biosci. 2023, 23, e2300093. [Google Scholar] [CrossRef]
- Xu, W.; Zeng, Z.; Tang, Y.; Tian, J.; Hao, X.; Sun, P.; Peng, Y.; Tian, T.; Xiang, D.; Wang, R.; et al. Spatiotemporal-controllable ros-responsive camptothecin nano-bomb for chemo/photo/immunotherapy in triple-negative breast cancer. J. Nanobiotechnol. 2024, 22, 798. [Google Scholar] [CrossRef]
- Hu, L.; Cao, Z.; Ma, L.; Liu, Z.; Liao, G.; Wang, J.; Shen, S.; Li, D.; Yang, X. The potentiated checkpoint blockade immunotherapy by ros-responsive nanocarrier-mediated cascade chemo-photodynamic therapy. Biomaterials 2019, 223, 119469. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, G.; Wang, S.; Yu, G.; Yang, Z.; Lin, L.; Zhou, Z.; Liu, Y.; Dai, Y.; Zhang, F.; et al. In situ dendritic cell vaccine for effective cancer immunotherapy. ACS Nano 2019, 13, 3083–3094. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, M.; Tan, Y.; Shen, J.; Jin, Q.; Deng, W.; Sun, J.; Wang, C.; Liu, Z.; Chen, Q. Injectable reactive oxygen species-responsive sn38 prodrug scaffold with checkpoint inhibitors for combined chemoimmunotherapy. ACS Appl. Mater. Interfaces 2020, 12, 50248–50259. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.L.; Lee, H.M.; Li, C.P.; Lin, M.W.; Chou, M.Y.; Yen, Y.T.; Wu, T.H.; Lian, Y.C.; Shih, Y.C.; Chiang, C.S.; et al. Robust and sustained sting pathway activation via hydrogel-based in situ vaccination for cancer immunotherapy. ACS Nano 2024, 18, 29439–29456. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Z.; Li, G.; Zhan, M.; Xiao, T.; Wang, J.; van Hest, J.C.M.; Shi, X.; Shen, M. A polymer nanogel-based therapeutic nanovaccine for prophylaxis and direct treatment of tumors via a full-cycle immunomodulation. Bioact. Mater. 2025, 43, 129–144. [Google Scholar] [CrossRef]
- Yu, H.; Tiemuer, A.; Yao, X.; Zuo, M.; Wang, H.Y.; Liu, Y.; Chen, X. Mitochondria-specific near-infrared photoactivation of peroxynitrite upconversion luminescent nanogenerator for precision cancer gas therapy. Acta Pharm. Sinica. B 2024, 14, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, M.; Quan, C.; Ren, S.; Chen, W.; Wang, J. Ros-responsive and triple-synergistic mitochondria-targeted polymer micelles for efficient induction of icd in tumor therapeutics. Bioact. Mater. 2024, 36, 490–507. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat. Commun. 2019, 10, 3349. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Li, X.; Huang, J.; Guo, X.; Zhang, J.; Luo, Z.; Shi, Y.; Jiang, M.; Qin, B.; et al. Er-targeting PDT converts tumors into in situ therapeutic tumor vaccines. ACS Nano 2022, 16, 9240–9253. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhou, Z.; Yang, W.; Lin, L.S.; Wang, S.; Niu, G.; Song, J.; Chen, X. Endoplasmic reticulum targeting to amplify immunogenic cell death for cancer immunotherapy. Nano Lett. 2020, 20, 1928–1933. [Google Scholar] [CrossRef]
- Pashootan, P.; Saadati, F.; Fahimi, H.; Rahmati, M.; Strippoli, R.; Zarrabi, A.; Cordani, M.; Moosavi, M.A. Metal-based nanoparticles in cancer therapy: Exploring photodynamic therapy and its interplay with regulated cell death pathways. Int. J. Pharm. 2024, 649, 123622. [Google Scholar] [CrossRef]
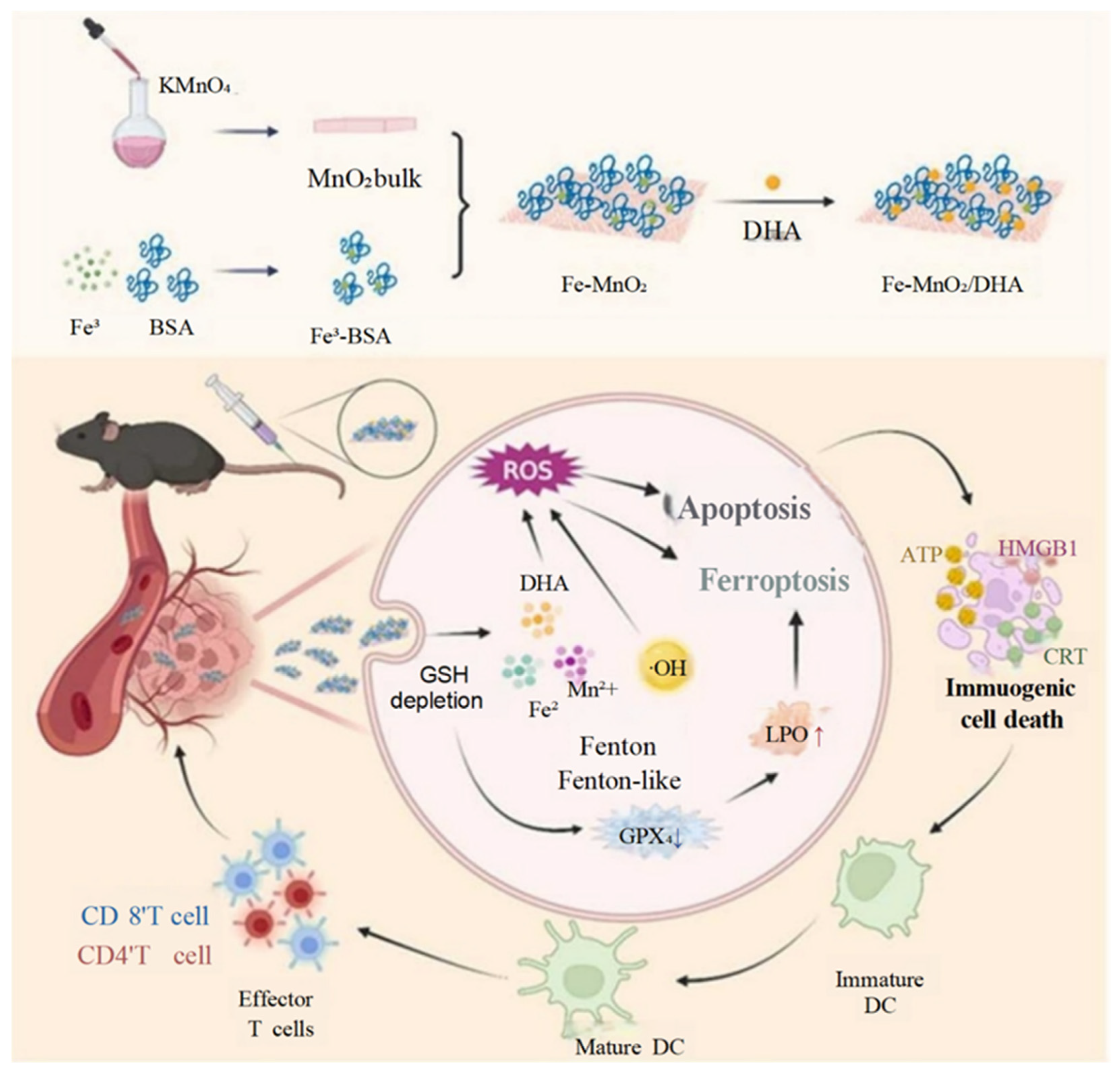
- Huang, D.; Xu, D.; Chen, W.; Wu, R.; Wen, Y.; Liu, A.; Lin, L.; Lin, X.; Wang, X. Fe-MnO2 nanosheets loading dihydroartemisinin for ferroptosis and immunotherapy. Biomed. Pharmacother. 2023, 161, 114431. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, X.; Ru, Y.; Zhou, X.; Liu, D.; Huang, Q.; Linghu, M.; Wu, Y.; Lv, Z.; Chen, M.; et al. Copper(II)-based nano-regulator correlates cuproptosis burst and sequential immunogenic cell death for synergistic cancer immunotherapy. Biomater. Res. 2024, 28, 0039. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, Y.; Yu, Y. Glutathione-scavenging celastrol-cu nanoparticles induce self-amplified cuproptosis for augmented cancer immunotherapy. Adv. Mater. 2024, 36, e2404971. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Xie, Z.; Wang, X.; Sun, J.; Ran, F.; Jiang, W.; Liu, Y.; Wang, Z.; Ran, H.; et al. Nir-responsive copper nanoliposome composites for cascaded ferrotherapy via ferroptosis actived icd and ifn-γ released. Biomaterials 2024, 308, 122570. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liang, S.; Liu, D.; Ma, K.; Yun, K.; Yao, J.; Peng, Y.; Hai, L.; Zhang, Q.; Wang, Z. Manganese-enriched zinc peroxide functional nanoparticles for potentiating cancer immunotherapy. Nano Lett. 2023, 23, 10350–10359. [Google Scholar] [CrossRef]
- Xu, J.; Pei, Z.; Wang, Y.; Jiang, N.; Gong, Y.; Gong, F.; Ni, C.; Cheng, L. Bioactive microspheres to enhance sonodynamic-embolization-metalloimmune therapy for orthotopic liver cancer. Biomaterials 2025, 317, 123063. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, T.; Li, Y.; Zhang, Y.; Chen, J.; Zhao, X.; Yang, H. Carrageenan-ferrocene-eicosapentaenoic acid composite hydrogel induce ferroptosis and apoptosis for anti-tumor recurrence and metastasis. Int. J. Biol. Macromol. 2024, 276, 133942. [Google Scholar] [CrossRef]
- Diederich, M. Natural compound inducers of immunogenic cell death. Arch. Pharm. Res. 2019, 42, 629–645. [Google Scholar] [CrossRef]
- Zhou, Z.; Lei, J.; Fang, J.; Chen, P.; Zhou, J.; Wang, H.; Sun, Z.; Chen, Y.; Yin, L. Dihydroartemisinin remodels tumor micro-environment and improves cancer immunotherapy through inhibiting cyclin-dependent kinases. Int. Immunopharmacol. 2024, 139, 112637. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Bi, S.; Guo, T.; Sun, D.; Zou, Y.; Wang, L.; Song, L.; Chu, D.; Liao, A.; Song, X.; et al. Nano co-delivery of plumbagin and dihydrotanshinone i reverses immunosuppressive tme of liver cancer. J. Control. Release Off. J. Control. Release Soc. 2022, 348, 250–263. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.H.; Yang, X.; Hu, J.Q.; Zhu, Y.Z.; Yan, P.Y.; Xie, Y. Novel nano-drug delivery system for natural products and their application. Pharmacol. Res. 2024, 201, 107100. [Google Scholar] [CrossRef]
- Yan, X.; Meng, L.; Zhang, X.; Deng, Z.; Gao, B.; Zhang, Y.; Yang, M.; Ma, Y.; Zhang, Y.; Tu, K.; et al. Reactive oxygen species-responsive nanocarrier ameliorates murine colitis by intervening colonic innate and adaptive immune responses. Mol. Ther. J. Am. Soc. Gene Ther. 2023, 31, 1383–1401. [Google Scholar] [CrossRef]
- Kianamiri, S.; Dinari, A.; Sadeghizadeh, M.; Rezaei, M.; Daraei, B.; Bahsoun, N.E.; Nomani, A. Mitochondria-targeted polyamidoamine dendrimer-curcumin construct for hepatocellular cancer treatment. Mol. Pharm. 2020, 17, 4483–4498. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, Z.; Shen, H.; Jian, Z.; Li, J.; Chen, Y.; Shen, Y.; Dai, X. Topically applicated curcumin/gelatin-blended nanofibrous mat inhibits pancreatic adenocarcinoma by increasing ros production and endoplasmic reticulum stress mediated apoptosis. J. Nanobiotechnol. 2020, 18, 126. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ming, H.; Li, B.; Liu, S.; Chen, L.; Zhang, T.; Gao, Y.; He, T.; Huang, C.; Du, Z. A ph and glutathione-responsive carbon monoxide-driven nano-herb delivery system for enhanced immunotherapy in colorectal cancer. J. Control. Release Off. J. Control. Release Soc. 2024, 376, 659–677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fan, T.; Yan, Y.; Chen, Y.; Ma, X.; Yang, T.; Xiang, G.; Lu, Y. A manganese-oxide nano-rambutan as the intrinsic modifier for hypericin delivery and triple-negative breast cancer treatment. Int. J. Pharm. 2024, 666, 124824. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, Z.; Xiao, X.; Mo, L.; Yao, W.; Yang, H.; Wang, J.; Wei, X.; Yuan, Y.; Yang, R.; et al. Ros-responsive self-activatable photosensitizing agent for photodynamic-immunotherapy of cancer. Acta Biomater. 2023, 164, 511–521. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Tang, K.; Pan, W.; Xu, H.; Li, Y.; Gao, Y.; Li, N.; Tang, B. Cu2+ embedded three-dimensional covalent organic framework for multiple ros-based cancer immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 30618–30625. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y.; Yao, Z.; Su, J.; Wang, Z.; Xia, H.; Liu, S. 2D copper(II) metalated metal-organic framework nanocomplexes for dual-enhanced photodynamic therapy and amplified antitumor immunity. ACS Appl. Mater. Interfaces 2022, 14, 44199–44210. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Guan, S.; Wang, Z.; Cao, W.; Luo, G.; Ling, X. Precisely amplifying intracellular oxidative storm by metal-organic coordination polymers to augment anticancer immunity. ACS Nano 2023, 17, 15165–15179. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.L.; Wan, S.C.; Yang, Q.C.; Xiao, Y.; Deng, H.; Sun, Z.J. Three-dimensional covalent organic frameworks with cross-linked pores for efficient cancer immunotherapy. Nano Lett. 2021, 21, 7979–7988. [Google Scholar] [CrossRef]
- Mao, C.; Yeh, S.; Fu, J.; Porosnicu, M.; Thomas, A.; Kucera, G.L.; Votanopoulos, K.I.; Tian, S.; Ming, X. Delivery of an ectonucleotidase inhibitor with ros-responsive nanoparticles overcomes adenosine-mediated cancer immunosuppression. Sci. Transl. Med. 2022, 14, eabh1261. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yang, S.B.; Park, S.J.; Kweon, S.; Ma, G.; Seo, M.; Kim, H.R.; Kang, T.B.; Lim, J.H.; Park, J. Cell-penetrating peptide like anti-programmed cell death-ligand 1 peptide conjugate-based self-assembled nanoparticles for immunogenic photodynamic therapy. ACS Nano 2025, 19, 2870–2889. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, R.; Kuang, F.; Hui, T.; Fu, C.; Zhang, L.; Zhou, C.; Qiu, M.; Yue, B. Schottky heterojunction CeO2@mxene nanosheets with synergistic type I and type II PDT for anti-osteosarcoma. J. Mater. Chem. B 2024, 12, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, F.; Zheng, B.; Yang, Y.; Yang, J.; Xiong, H. Electron-withdrawing substituents enhance the type I PDT and NIR-II fluorescence of bodipy J aggregates for bioimaging and cancer therapy. Nano Lett. 2024, 24, 8287–8295. [Google Scholar] [CrossRef]
- Li, R.T.; Chen, M.; Yang, Z.C.; Chen, Y.J.; Huang, N.H.; Chen, W.H.; Chen, J.; Chen, J.X. Aie-based gold nanostar-berberine dimer nanocomposites for PDT and PTT combination therapy toward breast cancer. Nanoscale 2022, 14, 9818–9831. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Y.; Zang, Y.; Han, J.; Xiong, Q.; Xiong, J. Photodynamic therapy and multi-modality imaging of up-conversion nanomaterial doped with AuNPs. Int. J. Mol. Sci. 2022, 23, 1227. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, Y.; Sun, X.; Leng, J.; Lin, S.; He, X.; Zhang, C.; Yuan, C. A multifunctional CaCO3 bioreactor coated with coordination polymers enhances cancer immunotherapy. J. Control. Release Off. J. Control. Release Soc. 2024, 368, 780–796. [Google Scholar] [CrossRef]
- Qian, Y.; Mao, J.; Leng, X.; Zhu, L.; Rui, X.; Jin, Z.; Jiang, H.; Liu, H.; Zhang, F.; Bi, X.; et al. Co-delivery of proanthocyanidin and mitoxantrone induces synergistic immunogenic cell death to potentiate cancer immunotherapy. Biomater. Sci. 2022, 10, 4549–4560. [Google Scholar] [CrossRef]
- Gu, Z.; Hao, Y.; Schomann, T.; Ossendorp, F.; Ten Dijke, P.; Cruz, L.J. Enhancing anti-tumor immunity through liposomal oxaliplatin and localized immunotherapy via sting activation. J. Control. Release Off. J. Control. Release Soc. 2023, 357, 531–544. [Google Scholar] [CrossRef]
- Liu, X.; Liang, S.; Sang, X.; Chang, L.; Fu, S.; Yang, H.; Yang, H.; Liu, Y.; Zhang, N. On-demand integrated nano-engager converting cold tumors to hot via increased DNA damage and dual immune checkpoint inhibition. Acta Pharm. Sinica. B 2023, 13, 1740–1754. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Ren, J.; He, X.; Shi, H.; Zhang, F.; Li, H.; Tong, R. Ros-triggered nanoinducer based on dermatan sulfate enhances immunogenic cell death in melanoma. J. Control. Release Off. J. Control. Release Soc. 2022, 348, 22–33. [Google Scholar] [CrossRef]
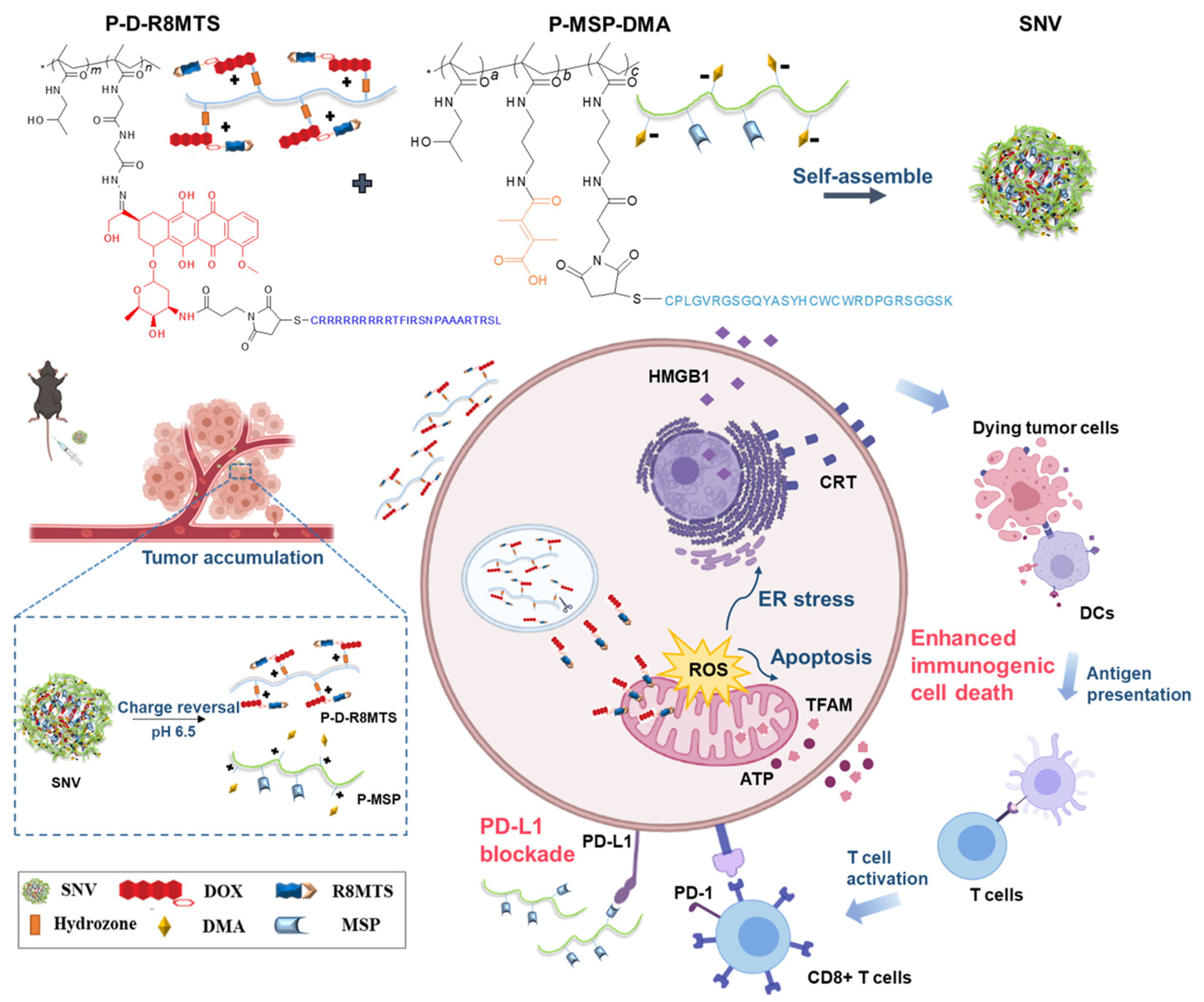
- Li, Q.; Chen, C.; Kong, J.; Li, L.; Li, J.; Huang, Y. Stimuli-responsive nano vehicle enhances cancer immunotherapy by coordinating mitochondria-targeted immunogenic cell death and pd-l1 blockade. Acta Pharm. Sinica. B 2022, 12, 2533–2549. [Google Scholar] [CrossRef]
- Guo, J.; Yu, Z.; Sun, D.; Zou, Y.; Liu, Y.; Huang, L. Two nanoformulations induce reactive oxygen species and immunogenetic cell death for synergistic chemo-immunotherapy eradicating colorectal cancer and hepatocellular carcinoma. Mol. Cancer 2021, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Cao, J.; Cheng, W.; Ming, H.; He, B.; Duan, X.; Li, L.; Tian, Y.; Nice, E.C.; Zhang, Z.; et al. Manganese dioxide-based in situ vaccine boosts antitumor immunity via simultaneous activation of immunogenic cell death and the sting pathway. Acta Biomater. 2025, 194, 467–482. [Google Scholar] [CrossRef]
- Zheng, Z.; Su, J.; Bao, X.; Wang, H.; Bian, C.; Zhao, Q.; Jiang, X. Mechanisms and applications of radiation-induced oxidative stress in regulating cancer immunotherapy. Front. Immunol. 2023, 14, 1247268. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, M.; Zhang, J.; Yin, Y.; Fan, X.; Zhang, Y.; Qin, S.; Zhang, H.; Yu, F. Immunogenic cell death induction by ionizing radiation. Front. Immunol. 2021, 12, 705361. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yang, C.; Xu, H.; Zhang, R.; Zhang, D.; Tu, J.; Guo, Y.; Niu, B.; Kong, L.; Zhang, Z. Cell-derived biogenetic gold nanoparticles for sensitizing radiotherapy and boosting immune response against cancer. Small 2021, 17, e2103984. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yao, D.; Ye, Q.; Jiang, H.; Gu, R.; Ji, C.; Wu, J.; Hu, Y.; Yuan, A. Zoledronic acid-gadolinium coordination polymer nanorods for improved tumor radioimmunotherapy by synergetically inducing immunogenic cell death and reprogramming the immunosuppressive microenvironment. ACS Nano 2021, 15, 8450–8465. [Google Scholar] [CrossRef]
- Fu, S.; Li, Y.; Shen, L.; Chen, Y.; Lu, J.; Ran, Y.; Zhao, Y.; Tang, H.; Tan, L.; Lin, Q.; et al. Cu2WS4-peg nanozyme as multifunctional sensitizers for enhancing immuno-radiotherapy by inducing ferroptosis. Small 2024, 20, e2309537. [Google Scholar] [CrossRef]
- Gong, Z.; Fu, Y.; Gao, Y.; Jiao, F.; Su, Q.; Sang, X.; Chen, B.; Deng, X.; Liu, X. “Abraxane-like” radiosensitizer for in situ oral cancer therapy. Adv. Sci. 2024, 11, e2309569. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, J.; Liang, N.; Zhang, X.; Shabiti, S.; Wang, Z.; Yu, S.; Pan, Z.Y.; Li, W.; Cai, L. Bioorthogonal/ultrasound activated oncolytic pyroptosis amplifies in situ tumor vaccination for boosting antitumor immunity. ACS Nano 2024, 18, 9413–9430. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, D.; Huang, S.; Yu, A. Dual-targeting aie polymer micelles mediate immunogenic sonodynamic therapy for tumor cell growth inhibition and macrophage reprogramming. Acta Biomater. 2025, 195, 321–337. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, F.; Han, Z.; Lei, H.; Zhou, Y.; Cheng, S.; Yang, X.; Wang, T.; Wang, L.; Yang, N.; et al. Oxygen-deficient molybdenum oxide nanosensitizers for ultrasound-enhanced cancer metalloimmunotherapy. Angew. Chem. (Int. Ed. Engl.) 2023, 62, e202215467. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xiao, H.; Hu, L.; Huang, Y.; Cao, Z.; Shuai, X.; Su, Z. 19F mri/ceus dual imaging-guided sonodynamic therapy enhances immune checkpoint blockade in triple-negative breast cancer. Adv. Sci. 2024, 11, e2401182. [Google Scholar] [CrossRef]
- Jiao, X.; Sun, L.; Zhang, W.; Ren, J.; Zhang, L.; Cao, Y.; Xu, Z.; Kang, Y.; Xue, P. Engineering oxygen-deficient ZrO2−x nanoplatform as therapy-activated “immunogenic cell death (ICD)” inducer to synergize photothermal-augmented sonodynamic tumor elimination in NIR-II biological window. Biomaterials 2021, 272, 120787. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Li, Y.; Liao, Z.; Wang, Z.; Dai, C.; Wang, W.; Yang, E.; Guo, F.; Wright, I.R.; Martin, L.L.; et al. Live bio-nano-sonosensitizer targets malignant tumors in synergistic therapy. Acta Biomater. 2023, 155, 491–506. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Ding, M.; Yao, W.; Wang, K.; Ullah, I.; Bulatov, E.; Yuan, Y. Ultrasound-activated protac prodrugs overcome immunosuppression to actuate efficient deep-tissue sono-immunotherapy in orthotopic pancreatic tumor mouse models. Nano Lett. 2024, 24, 8741–8751. [Google Scholar] [CrossRef]
- Gao, J.; Zhai, Y.; Lu, W.; Jiang, X.; Zhou, J.; Wu, L.; Du, L.; Ou, C.; Zhang, X.; He, H.; et al. Ros-sensitive PD-L1 sirna cationic selenide nanogels for self-inhibition of autophagy and prevention of immune escape. Bioact. Mater. 2024, 41, 597–610. [Google Scholar] [CrossRef]
- Jin, F.; Qi, J.; Liu, D.; You, Y.; Shu, G.; Du, Y.; Wang, J.; Xu, X.; Ying, X.; Ji, J.; et al. Cancer-cell-biomimetic upconversion nanoparticles combining chemo-photodynamic therapy and CD73 blockade for metastatic triple-negative breast cancer. J. Control. Release Off. J. Control. Release Soc. 2021, 337, 90–104. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Wang, Q.; Hang, L.; Wang, Y.; Wang, Y. Ros-sensitive biomimetic nanocarriers modulate tumor hypoxia for synergistic photodynamic chemotherapy. Biomater. Sci. 2019, 7, 3706–3716. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Liu, L.; Pan, H.; Li, H.; Cai, L.; Ma, Y. Self-adjuvanted nanovaccine for cancer immunotherapy: Role of lysosomal rupture-induced ros in mhc class I antigen presentation. Biomaterials 2016, 79, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dong, Y.; Zhang, J.; Chen, C.; Gao, L.; Shi, C.; Fu, Z.; Han, M.; Tang, C.; Sun, P.; et al. Reversing immune evasion using a DNA nano-orchestrator for pancreatic cancer immunotherapy. Acta Biomater. 2023, 166, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Ni, J.S.; Li, Y.; Zha, M.; Tu, Y.; Li, K. Acceptor engineering for optimized ros generation facilitates reprogramming macrophages to m1 phenotype in photodynamic immunotherapy. Angew. Chem. (Int. Ed. Engl.) 2021, 60, 5386–5393. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Liu, S.; Zhao, X.; Wei, F.; Li, Y.; Sheng, X.; Cao, W.; Ding, M.; Zhang, W.; Chen, X.; et al. Ros scavenging nanozyme modulates immunosuppression for sensitized cancer immunotherapy. Adv. Healthc. Mater. 2023, 12, e2300191. [Google Scholar] [CrossRef]
- Schmidt, K.T.; Karzai, F.; Bilusic, M.; Cordes, L.M.; Chau, C.H.; Peer, C.J.; Wroblewski, S.; Huitema, A.D.R.; Schellens, J.H.M.; Gulley, J.L.; et al. A single-arm phase II study combining NLG207, a nanoparticle camptothecin, with enzalutamide in advanced metastatic castration-resistant prostate cancer post-enzalutamide. Oncologist 2022, 27, 718-e694. [Google Scholar] [CrossRef]
- Mamot, C.; Wicki, A.; Hasler-Strub, U.; Riniker, S.; Li, Q.; Holer, L.; Bärtschi, D.; Zaman, K.; von Moos, R.; Dedes, K.J.; et al. A multicenter phase II trial of anti-egfr-immunoliposomes loaded with doxorubicin in patients with advanced triple negative breast cancer. Sci. Rep. 2023, 13, 3705. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Takácsi-Nagy, Z.; Nuyts, S.; Thureau, S.; Liu, F.; Hoffmann, C.; Hackman, T.G.; Lesnik, M.; Debard, A.; Finzi, L.; et al. Nanoray-312: Phase III study of NBTXR3 + radiotherapy ± cetuximab in elderly, platinum-ineligible locally advanced hnscc. Future Oncol. 2025, 21, 1489–1499. [Google Scholar] [CrossRef]
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.P.; Agoston, P.; Hong, A.M.; Mervoyer, A.; Rastrelli, M.; et al. Final safety and health-related quality of life results of the phase 2/3 act.In.Sarc study with preoperative NBTXR3 plus radiation therapy versus radiation therapy in locally advanced soft-tissue sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 422–432. [Google Scholar] [CrossRef]
- Kasenda, B.; König, D.; Manni, M.; Ritschard, R.; Duthaler, U.; Bartoszek, E.; Bärenwaldt, A.; Deuster, S.; Hutter, G.; Cordier, D.; et al. Targeting immunoliposomes to egfr-positive glioblastoma. ESMO Open 2022, 7, 100365. [Google Scholar] [CrossRef]




| Drugs/NPs | Cancer Model | Antitumor Mechanisms | Immunity Activation In Vivo | Ref. |
|---|---|---|---|---|
| Fe-MnO2/DHA | HCC | ICD, ferroptosis, apoptosis | ~3.36-fold increase in CD8+ T cell infiltration; promotes macrophage polarization | [60] |
| ES-Cu-MOF | Fibrosarcoma | ICD, cuproptosis | ~1-fold increase in CD8+ T cell infiltration; systemic antitumor immunity | [61] |
| Cel-Cu | Breast cancer | ICD, cuproptosis | ~0.5-fold increase in CD8+ T cell infiltration; promotes the polarization of TAMs | [62] |
| MONPs | Breast cancer | ICD, activate STING pathway | ~1.83-fold increase in CD8+ T cell infiltration in spleen; reduces Tregs; polarizes M2 macrophages to M1 type | [64] |
| Mn-GMSs | Liver cancer | ICD; activate cGAS-STING pathway | ~1.21-fold increase in CD8+ T cell infiltration | [65] |
| CE-Fc-Gel | Breast cancer | ICD, ferroptosis, apoptosis | Promotes the maturation of DCs; increases the infiltration of CD8+ T cells | [66] |
| Drugs/NPs | Cancer Model | Antitumor Mechanisms | Immunity Activation In Vivo | Ref. |
|---|---|---|---|---|
| Cu-TCPP(Al)-Pt-FA | Lung cancer | ICD | ~5-fold increase in CD8+ T cell infiltration; polarizes M2 macrophages to the M1 type | [78] |
| MOCPs | Breast cancer | ICD, ferroptosis | Increase in cytotoxic T cell infiltration | [79] |
| COF-609 | Breast cancer | ICD | Increase in the ratio of T cells | [80] |
| 3D Cu@COF-TATB | Breast cancer | ICD | Increase in cytotoxic T cell infiltration | [77] |
| TPS-PEG | Breast cancer | ICD, apoptosis | ~0.83-fold increase in CD8+ T cell infiltration | [16] |
| NP700-ARL | Oral cancer, colon cancer, breast cancer | ICD | ~2.59/2.7-fold increase in IFN-γ–CD8+ T cell infiltration | [81] |
| CPPD1 | Colon cancer | ICD | Increase in tumor-responsive T cells; blocks PD-1/PD-L1 interactions | [82] |
| Drugs/Nps | Cancer Type | Combination Therapy | NCT | Phase | Ref. |
|---|---|---|---|---|---|
| NBTXR3 | HNSCC | RT/cetuximab | NCT04892173 | Phase III | [117] |
| NBTXR3 | Soft-tissue sarcoma | RT | NCT02379845 | Phase II/III | [118] |
| Anti-EGFR-ILs-dox | TNBC | - | NCT02833766 | Phase II | [116] |
| Anti-EGFR-ILs-dox | Glioblastoma | - | NCT03603379 | Phase I | [119] |
| NLG207 | Prostate cancer | Enzalutamide | NCT03531827 | Phase II | [115] |
| NLG207 | NSCLC | - | NCT01380769 | Phase II | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.-Y.; Wu, H.; Xu, C. ROS-Responsive Nanoplatforms for Targeted Tumor Immunomodulation: A Paradigm Shift in Precision Cancer Immunotherapy. Pharmaceutics 2025, 17, 886. https://doi.org/10.3390/pharmaceutics17070886
Fan Y-Y, Wu H, Xu C. ROS-Responsive Nanoplatforms for Targeted Tumor Immunomodulation: A Paradigm Shift in Precision Cancer Immunotherapy. Pharmaceutics. 2025; 17(7):886. https://doi.org/10.3390/pharmaceutics17070886
Chicago/Turabian StyleFan, Yuan-Yuan, Hong Wu, and Chuan Xu. 2025. "ROS-Responsive Nanoplatforms for Targeted Tumor Immunomodulation: A Paradigm Shift in Precision Cancer Immunotherapy" Pharmaceutics 17, no. 7: 886. https://doi.org/10.3390/pharmaceutics17070886
APA StyleFan, Y.-Y., Wu, H., & Xu, C. (2025). ROS-Responsive Nanoplatforms for Targeted Tumor Immunomodulation: A Paradigm Shift in Precision Cancer Immunotherapy. Pharmaceutics, 17(7), 886. https://doi.org/10.3390/pharmaceutics17070886






