Nanoscale Porphyrin-Based Metal–Organic Frameworks for Enhanced Radiotherapy–Radiodynamic Therapy: A Comprehensive Review
Abstract
1. Introduction
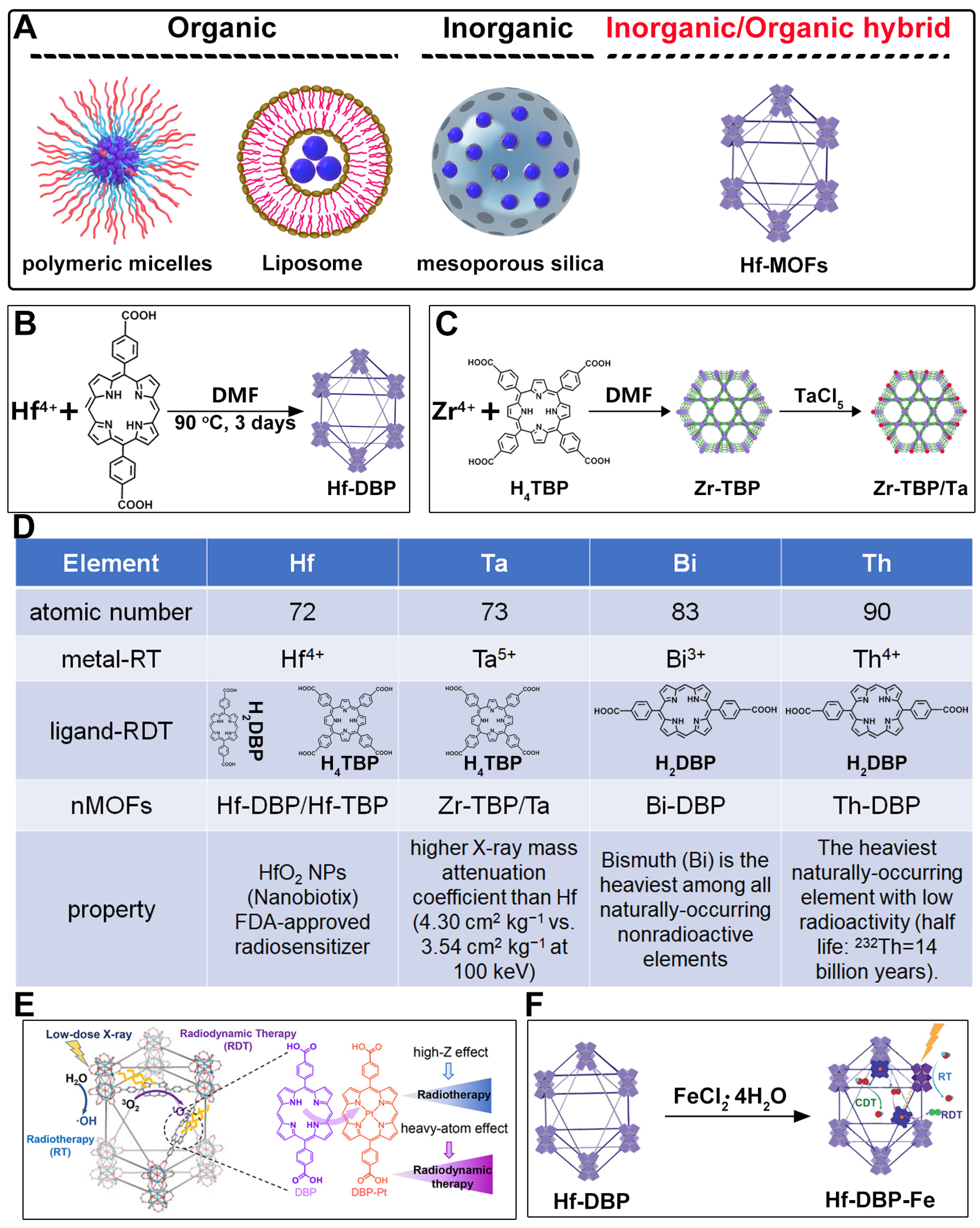
2. Design Rationale and Advantages of Porphyrin–MOFs for Combined RT-RDT
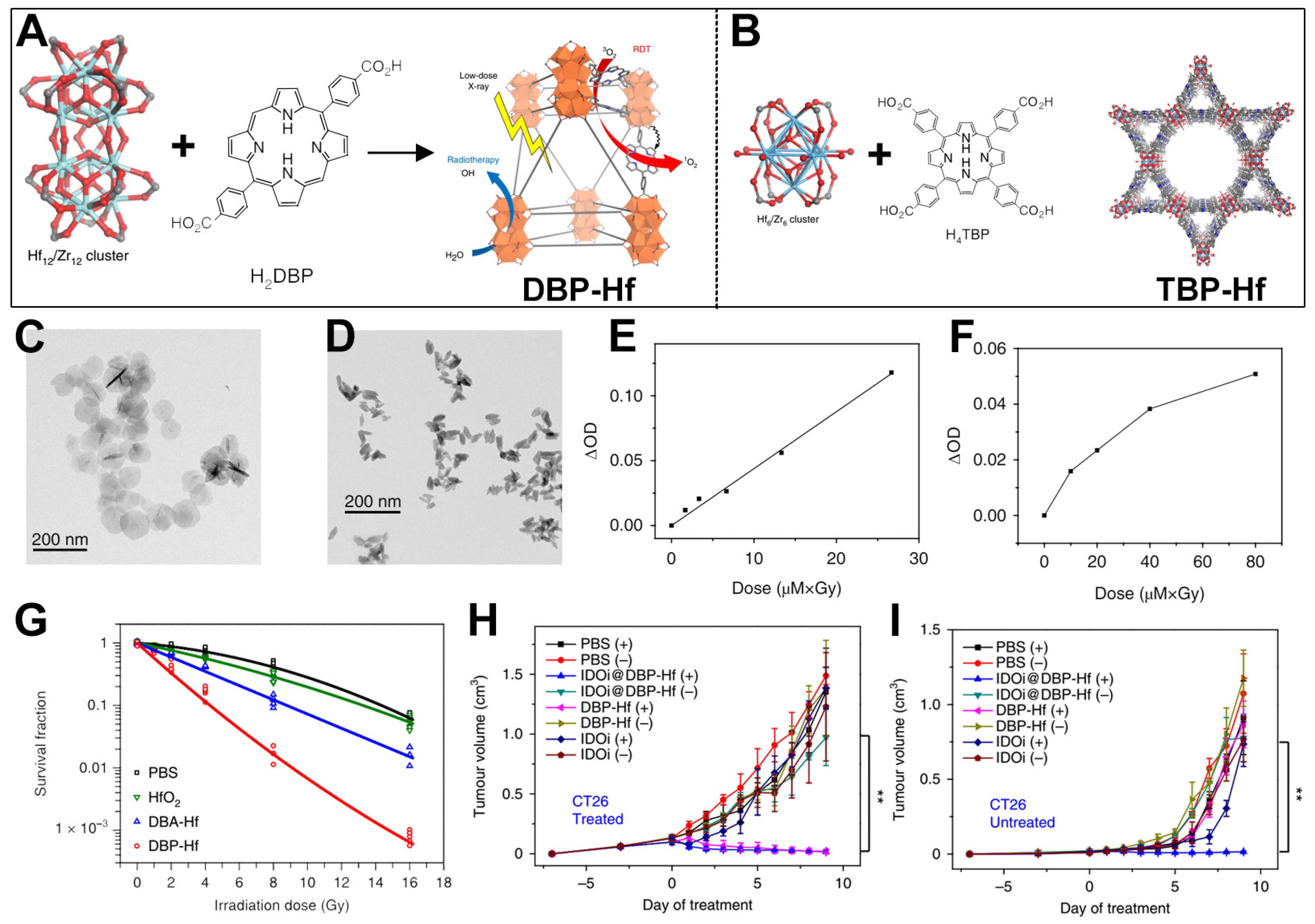
3. Hf-Based nMOFs for Enhanced RT-RDT and Synergistic Immunotherapy
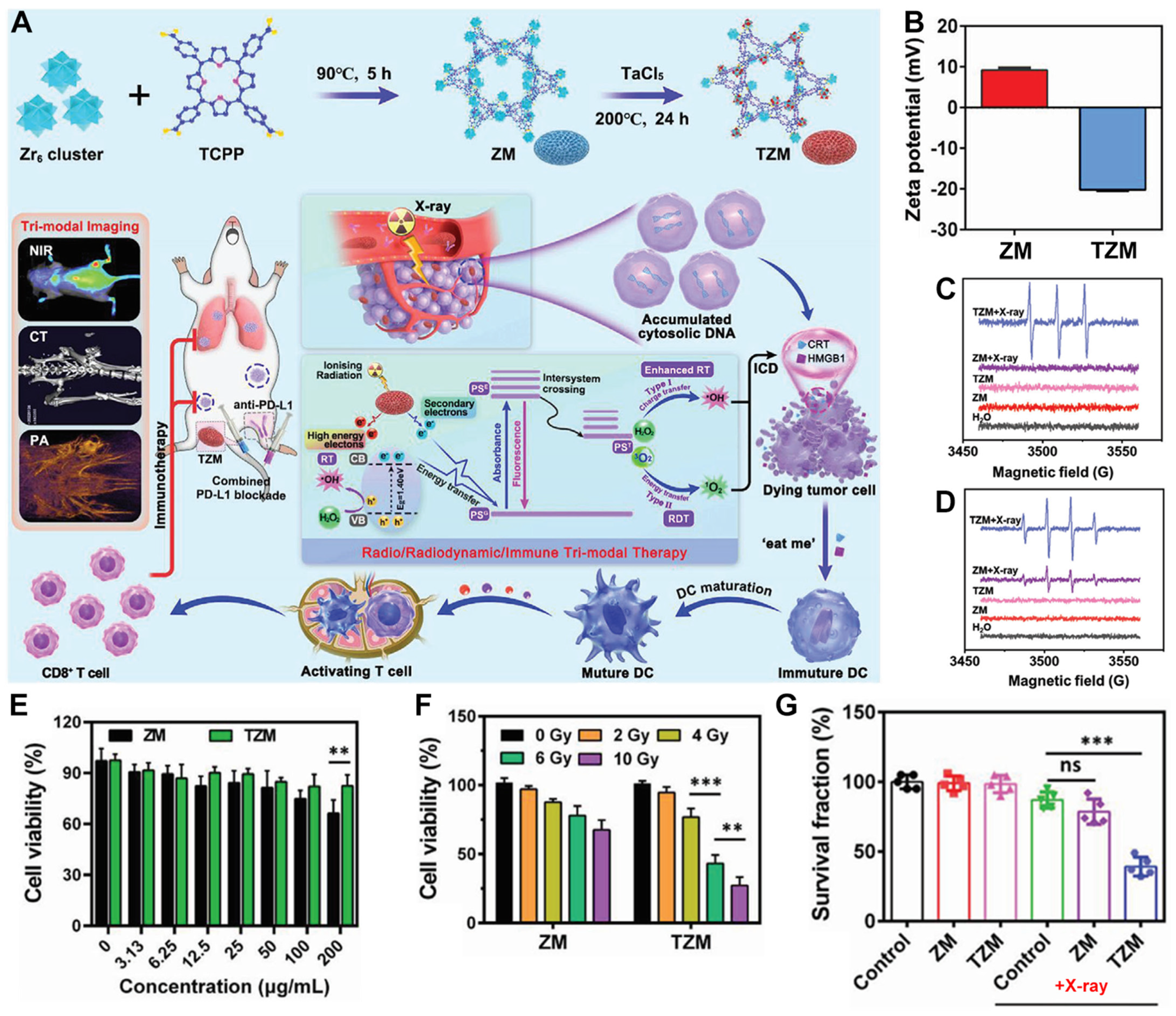
4. Ta-Zr-Co-Doped nMOFs for Combined RT and RDT
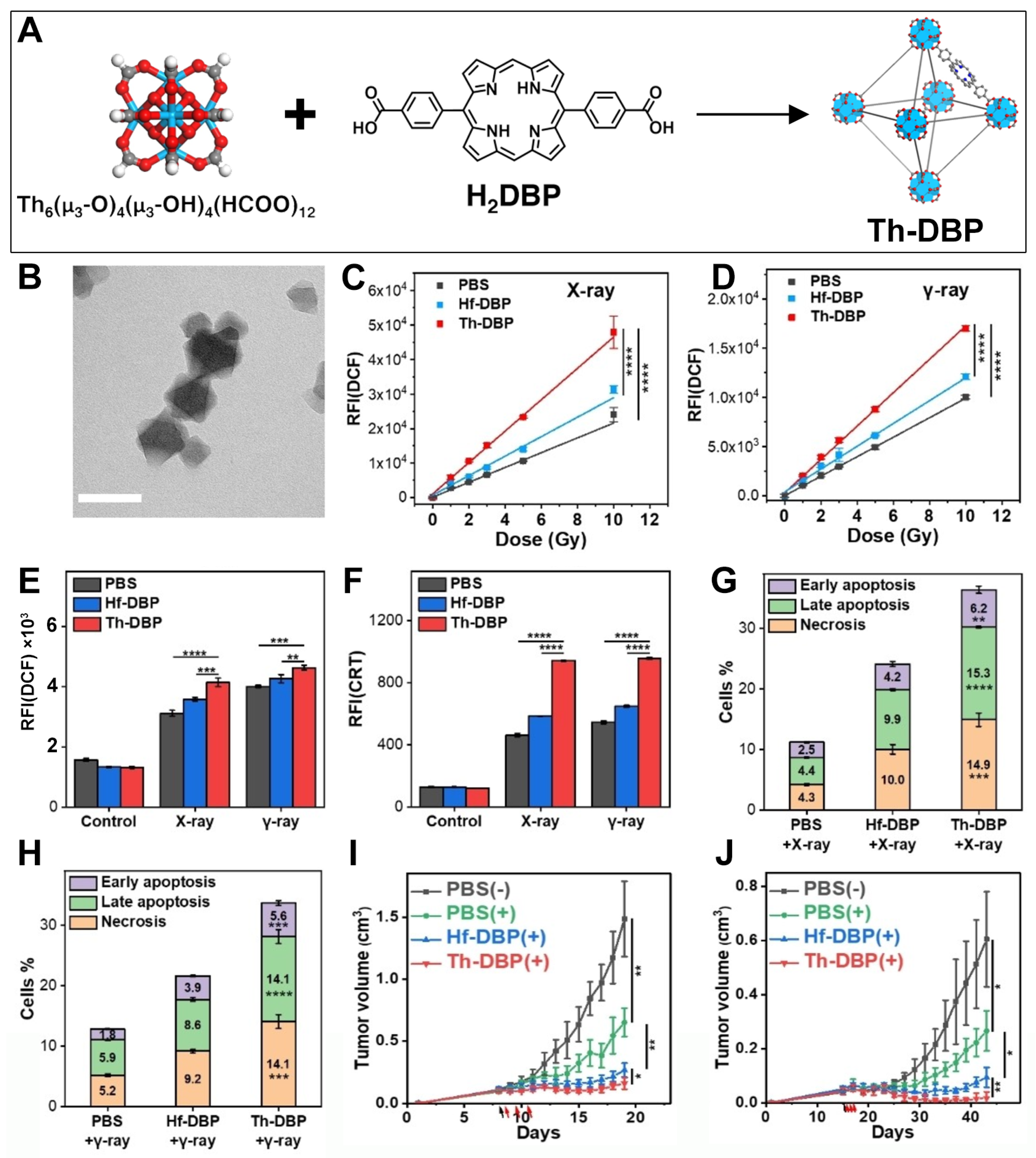
5. Th-Based nMOFs for Combined RT and RDT
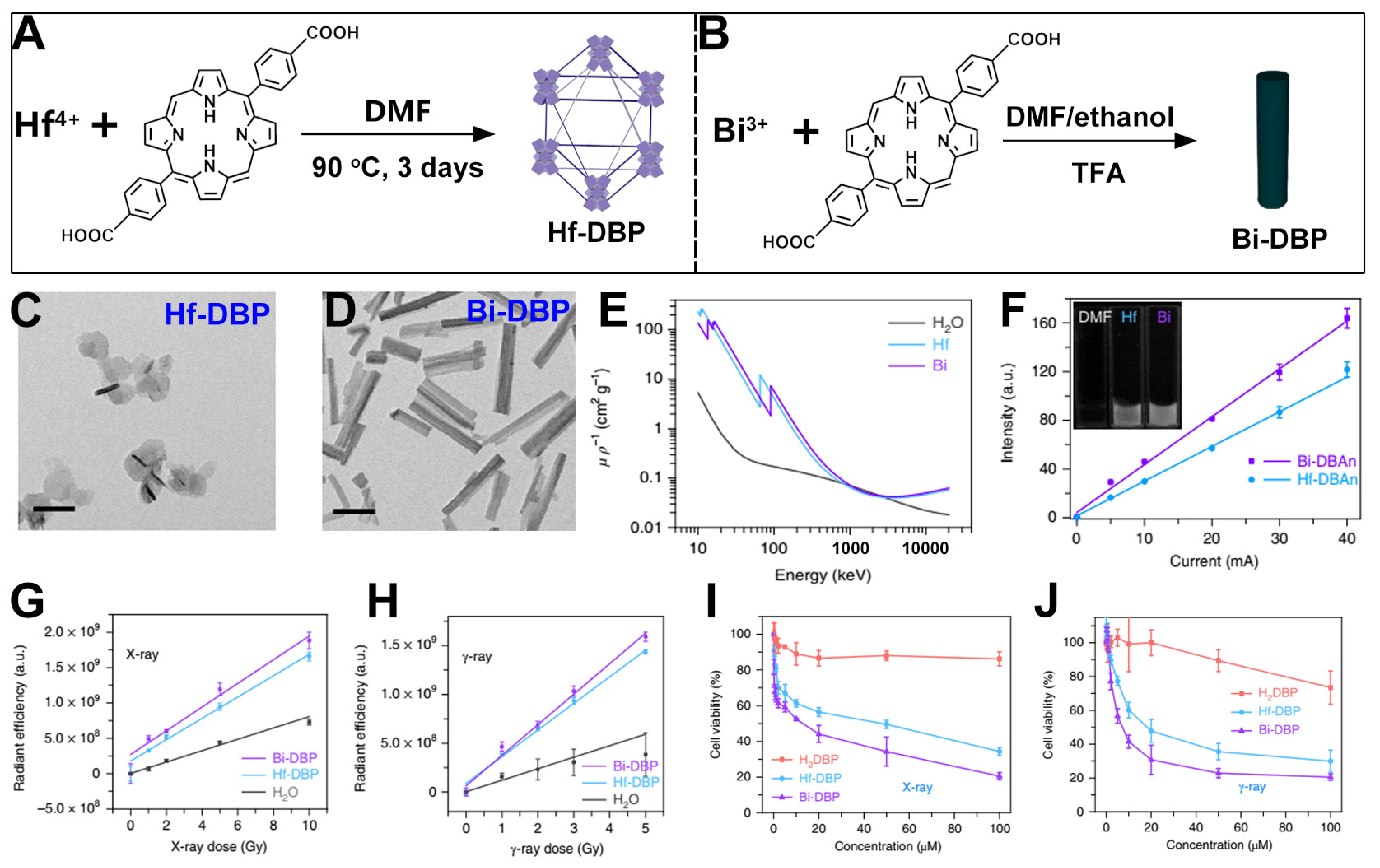
6. Bi-Based nMOFs for Combined RT and RDT
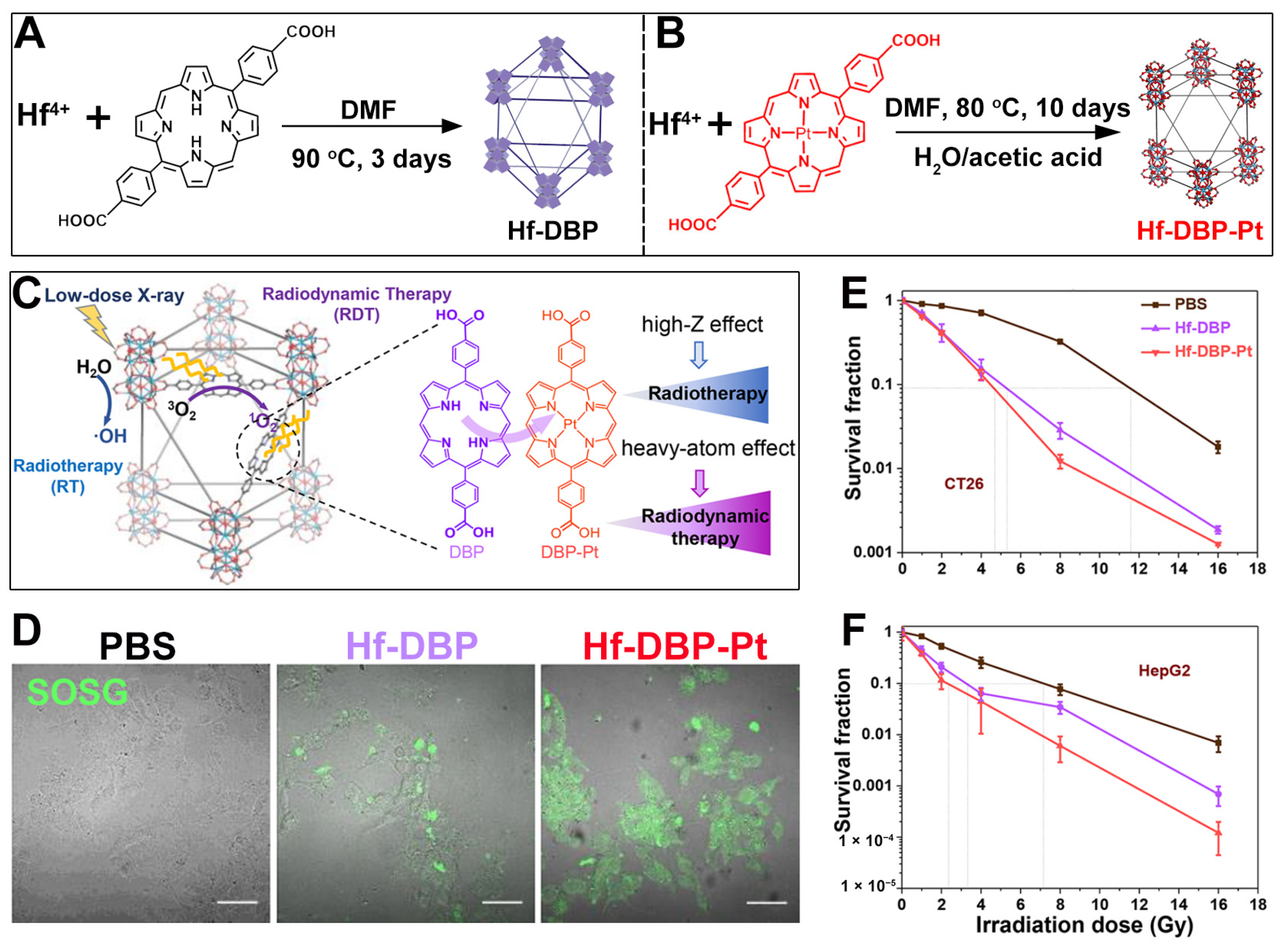
7. Hf-Based nMOFs: Boosting RT and RDT via Heavy Atom Effect
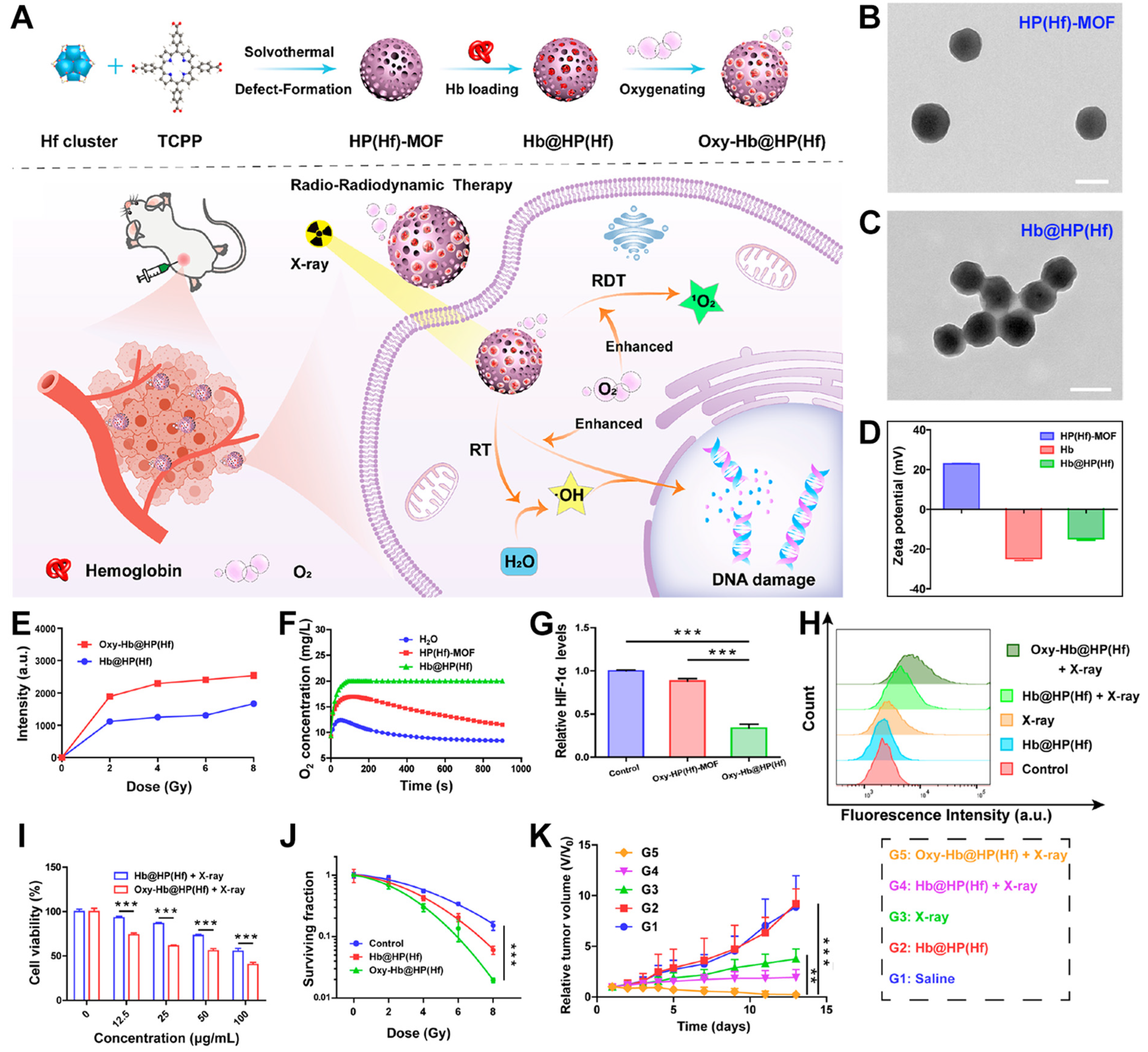
8. Hf-Based nMOFs: Boosting RT and RDT via Self-Oxygen-Carrying Function
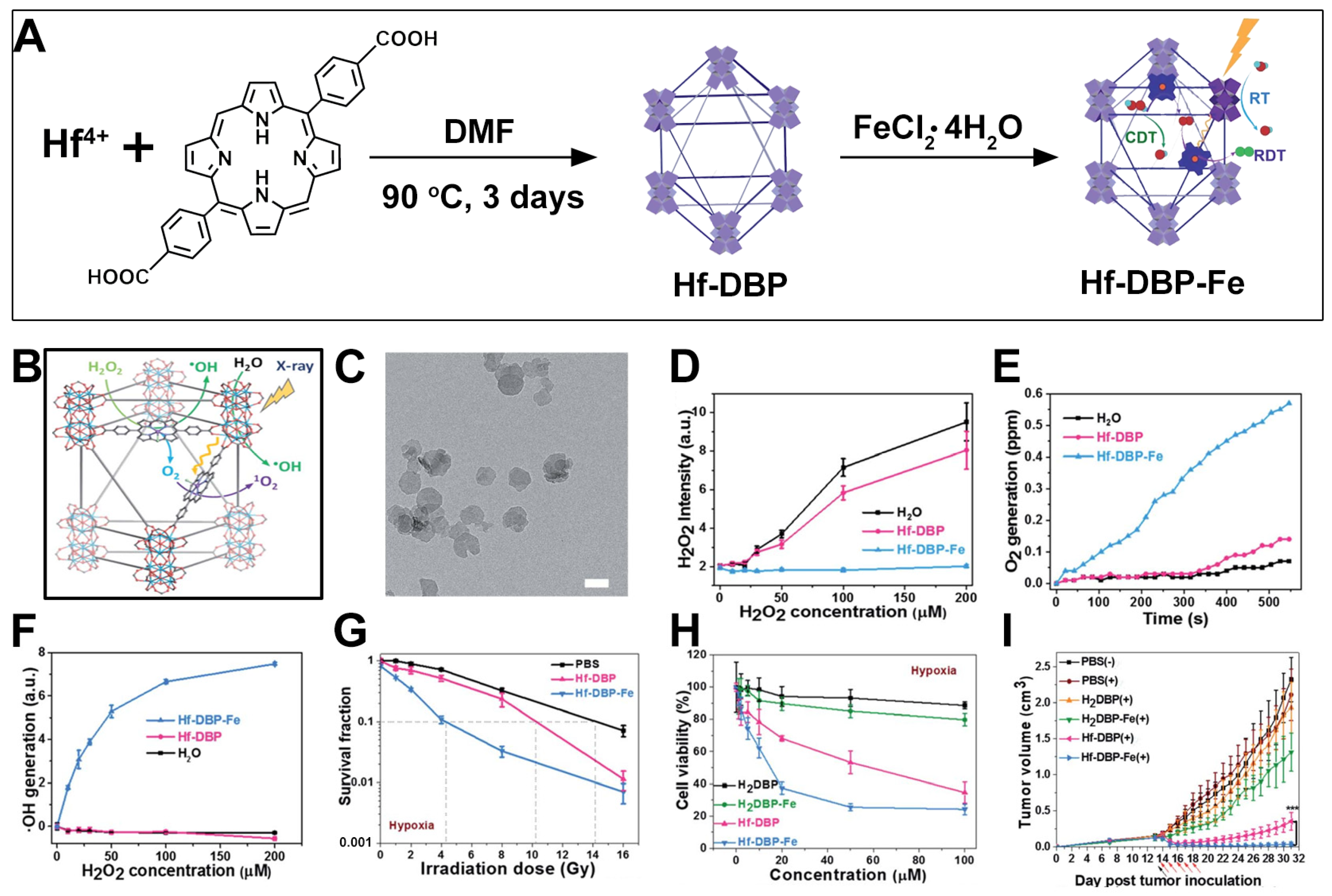
9. Hf-DBP-Fe nMOFs for RT-RDT Combined with CDT
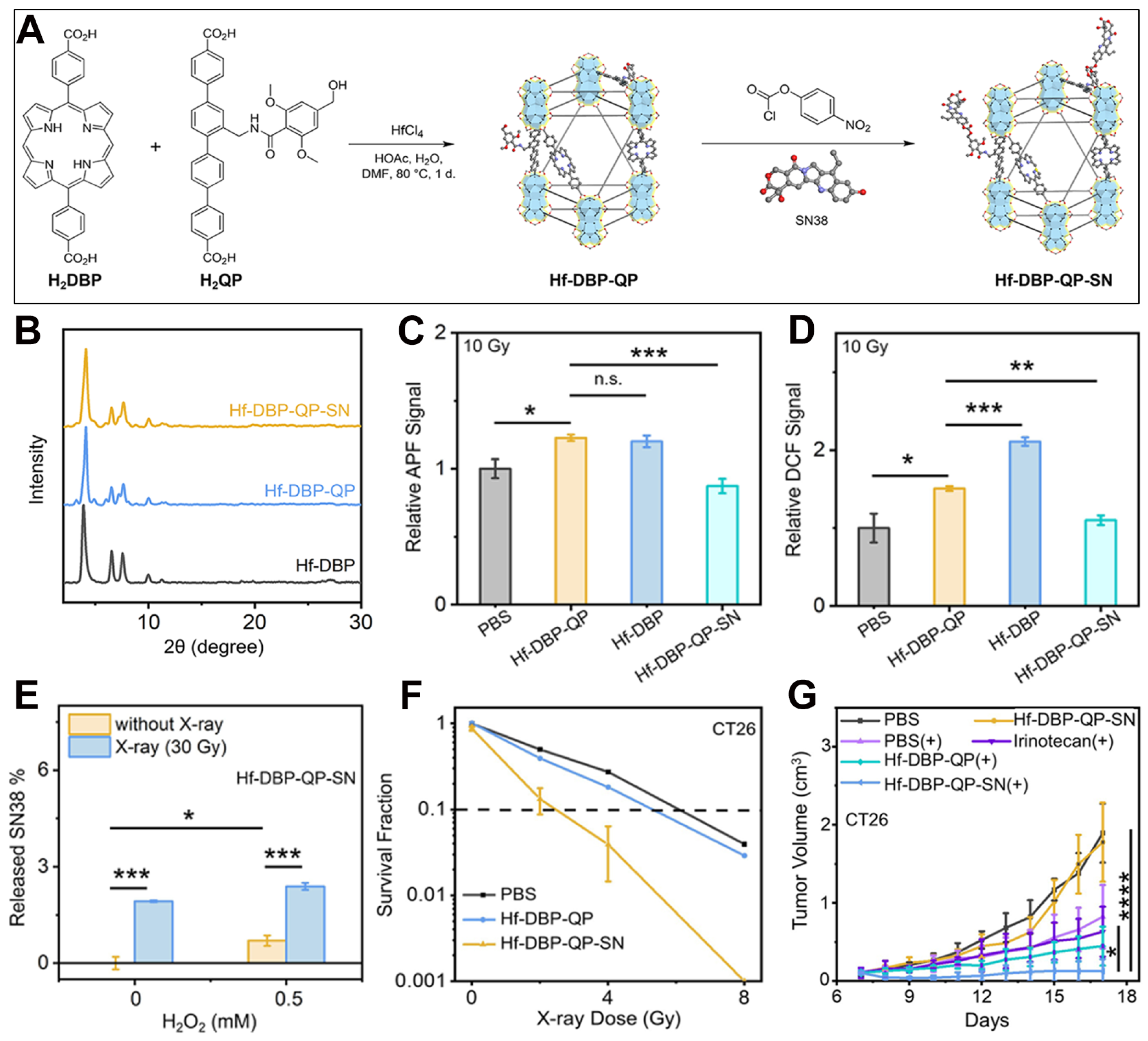
10. Mixed-Ligand nMOFs for RT-RDT Combined with Chemotherapy
11. Comparative Study of nMOFs Derived from Hf, Ta, Bi, and Th
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Chen, D.; Li, F.; Xiao, X.; Xu, Q. Metal-organic-framework-based materials as platforms for energy applications. Chem 2024, 10, 86–133. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Yang, S.; Garcia, H. Metal–organic framework heterojunctions for photocatalysis. Chem. Soc. Rev. 2024, 53, 3002–3035. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, X.; Wu, W.; Feng, X.; Kong, D.; Khan, U.; Ren, X.; Li, L. In Situ Encapsulation of Graphene Quantum Dots in Highly Stable Porphyrin Metal-Organic Frameworks for Efficient Photocatalytic CO2 Reduction. Molecules 2023, 28, 4703. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Kaskel, S. Porphyrin-Based Metal–Organic Frameworks for Biomedical Applications. Angew. Chem. Int. Ed. 2021, 60, 5010–5035. [Google Scholar] [CrossRef]
- Zhang, X.; Wasson, M.C.; Shayan, M.; Berdichevsky, E.K.; Ricardo-Noordberg, J.; Singh, Z.; Papazyan, E.K.; Castro, A.J.; Marino, P.; Ajoyan, Z.; et al. A historical perspective on porphyrin-based metal–organic frameworks and their applications. Coord. Chem. Rev. 2021, 429, 213615. [Google Scholar] [CrossRef]
- Xu, D.; Duan, Q.; Yu, H.; Dong, W. Photodynamic therapy based on porphyrin-based metal–organic frameworks. J. Mater. Chem. B 2023, 11, 5976–5989. [Google Scholar] [CrossRef]
- Qi, C.; Chen, J.; Qu, Y.; Luo, X.; Wang, W.; Zheng, X. Recent Advances in Porphyrin-Based Covalent Organic Frameworks for Synergistic Photodynamic and Photothermal Therapy. Pharmaceutics 2024, 16, 1625. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Chen, J.; Wu, J.; Zhou, M.; Xia, R.; Wang, W.; Zheng, X.; Xie, Z. Recent progress of porphyrin metal–organic frameworks for combined photodynamic therapy and hypoxia-activated chemotherapy. Chem. Commun. 2024, 60, 13641–13652. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Jurkovicova, D.; Neophytou, C.M.; Gašparović, A.Č.; Gonçalves, A.C. DNA Damage Response in Cancer Therapy and Resistance: Challenges and Opportunities. Int. J. Mol. Sci. 2022, 23, 14672. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy combined with immunotherapy: The dawn of cancer treatment. Signal Transduct. Target. Ther. 2022, 7, 258. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Lin, M.; Lu, C.; Kumar, A.; Pan, Y.; Liu, J.; Peng, Y. Current and promising applications of Hf(iv)-based MOFs in clinical cancer therapy. J. Mater. Chem. B 2023, 11, 5693–5714. [Google Scholar] [CrossRef]
- Mladenova, V.; Mladenov, E.; Stuschke, M.; Iliakis, G. DNA Damage Clustering after Ionizing Radiation and Consequences in the Processing of Chromatin Breaks. Molecules 2022, 27, 1540. [Google Scholar] [CrossRef]
- Haque, M.; Shakil, M.S.; Mahmud, K.M. The Promise of Nanoparticles-Based Radiotherapy in Cancer Treatment. Cancers 2023, 15, 1892. [Google Scholar] [CrossRef]
- He, L.; Yu, X.; Li, W. Recent Progress and Trends in X-ray-Induced Photodynamic Therapy with Low Radiation Doses. ACS Nano 2022, 16, 19691–19721. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, J.; Liu, R.; Xiang, H.; Zhang, W.; Chang, Q.; Wang, S.; Jiang, R.; Zhao, F.; Li, Q.; et al. Engineering Single-Atom Iron Nanozymes with Radiation-Enhanced Self-Cascade Catalysis and Self-Supplied H2O2 for Radio-enzymatic Therapy. ACS Nano 2022, 16, 18849–18862. [Google Scholar] [CrossRef]
- Pan, S.; Huang, G.; Sun, Z.; Chen, X.; Xiang, X.; Jiang, W.; Xu, Y.; Chen, T.; Zhu, X. X-Ray-Responsive Zeolitic Imidazolate Framework-Capped Nanotherapeutics for Cervical Cancer-Targeting Radiosensitization. Adv. Funct. Mater. 2023, 33, 2213364. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, Q.; Fu, H.; Cheng, J.; Xie, Y.; Liang, Q.; Xiao, X. A reactive oxygen species amplifier based on a Bi2WO6/BP heterojunction for high efficiency radiotherapy enhancement. J. Mater. Chem. B 2025, 13, 3128–3137. [Google Scholar] [CrossRef]
- Zhu, S.; Lin, S.; Han, R. Treating Deep-Seated Tumors with Radiodynamic Therapy: Progress and Perspectives. Pharmaceutics 2024, 16, 1135. [Google Scholar] [CrossRef]
- Ma, M.; Wang, J.; Jiang, H.; Chen, Q.; Xiao, Y.; Yang, H.; Lin, L. Transcranial deep-tissue phototherapy for Alzheimer’s disease using low-dose X-ray-activated long-afterglow scintillators. Acta Biomater. 2023, 155, 635–643. [Google Scholar] [CrossRef]
- Souris, J.S.; Leoni, L.; Zhang, H.J.; Pan, A.; Tanios, E.; Tsai, H.-M.; Balyasnikova, I.V.; Bissonnette, M.; Chen, C.-T. X-ray Activated Nanoplatforms for Deep Tissue Photodynamic Therapy. Nanomaterials 2023, 13, 673. [Google Scholar] [CrossRef]
- Chen, F.; Ruan, F.; Xie, X.; Lu, J.; Sun, W.; Shao, D.; Chen, M. Gold Nanocluster: A Photoelectric Converter for X-Ray-Activated Chemotherapy. Adv. Mater. 2024, 36, 2402966. [Google Scholar] [CrossRef]
- Li, H.; Zeng, J.; You, Q.; Zhang, M.; Shi, Y.; Yang, X.; Gu, W.; Liu, Y.; Hu, N.; Wang, Y.; et al. X-ray-activated nanoscintillators integrated with tumor-associated neutrophils polarization for improved radiotherapy in metastatic colorectal cancer. Biomaterials 2025, 316, 123031. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Zhang, Y.; Jiang, W. Immunogenicity-boosted cancer immunotherapy based on nanoscale metal-organic frameworks. J. Control. Release 2022, 347, 183–198. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Y.; Jin, Y.; Liu, X.; Shang, M.; Zheng, X.; Liu, T.; Xie, Z. Two-dimensional metal-organic frameworks: From synthesis to bioapplications. J. Nanobiotechnol. 2022, 20, 207. [Google Scholar] [CrossRef]
- Wei, Q.; Wu, Y.; Liu, F.; Cao, J.; Liu, J. Advances in antitumor nanomedicine based on functional metal–organic frameworks beyond drug carriers. J. Mater. Chem. B 2022, 10, 676–699. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Chen, X.; Ding, M.; Eskandari, A.; Fairen-Jimenez, D.; Giménez-Marqués, M.; Gref, R.; Lin, W.; Luo, T.; Forgan, R.S. Metal–organic frameworks for biological applications. Nat. Rev. Methods Primers 2024, 4, 42. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Guo, N.; Chan, C.; Ni, K.; Lan, G.; Tang, H.; Pelizzari, C.; Fu, Y.-X.; Spiotto, M.T.; et al. Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed. Eng. 2018, 2, 600–610. [Google Scholar] [CrossRef]
- Ni, K.; Xu, Z.; Culbert, A.; Luo, T.; Guo, N.; Yang, K.; Pearson, E.; Preusser, B.; Wu, T.; La Riviere, P.; et al. Synergistic checkpoint-blockade and radiotherapy–radiodynamic therapy via an immunomodulatory nanoscale metal–organic framework. Nat. Biomed. Eng. 2022, 6, 144–156. [Google Scholar] [CrossRef]
- Ding, S.; Chen, L.; Liao, J.; Huo, Q.; Wang, Q.; Tian, G.; Yin, W. Harnessing Hafnium-Based Nanomaterials for Cancer Diagnosis and Therapy. Small 2023, 19, 2300341. [Google Scholar] [CrossRef]
- Yang, S.; Gaohua, H.; Quan, C.; Lei, Y.; Peng, W.; Qi, Z.; Jiang, D.; Wei, Z.; Huang, J. Au-Pt Nanoparticle Formulation as a Radiosensitizer for Radiotherapy with Dual Effects. Int. J. Nanomed. 2021, 16, 239–248. [Google Scholar] [CrossRef]
- Ni, K.; Luo, T.; Nash, G.T.; Lin, W. Nanoscale Metal–Organic Frameworks for Cancer Immunotherapy. Acc. Chem. Res. 2020, 53, 1739–1748. [Google Scholar] [CrossRef]
- Xu, Z.; Luo, T.; Lin, W. Nanoscale Metal–Organic Layers for Biomedical Applications. Acc. Mater. Res. 2021, 2, 944–953. [Google Scholar] [CrossRef]
- Pan, Y.; Tang, W.; Fan, W.; Zhang, J.; Chen, X. Development of nanotechnology-mediated precision radiotherapy for anti-metastasis and radioprotection. Chem. Soc. Rev. 2022, 51, 9759–9830. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, J.; Zhang, Q.; Chen, J.; Luo, X.; Qu, Y.; Xia, R.; Wang, W.; Zheng, X. Recent advances in cell membrane-coated porphyrin-based nanoscale MOFs for enhanced photodynamic therapy. Front. Pharmacol. 2024, 15, 1505212. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, J.; Qu, Y.; Luo, X.; Wang, W.; Zheng, X. Evolution of nMOFs in photodynamic therapy: From porphyrins to chlorins and bacteriochlorins for better efficacy. Front. Pharmacol. 2025, 16, 1533040. [Google Scholar] [CrossRef]
- Mendes, R.F.; Figueira, F.; Leite, J.P.; Gales, L.; Almeida Paz, F.A. Metal–organic frameworks: A future toolbox for biomedicine? Chem. Soc. Rev. 2020, 49, 9121–9153. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Zhang, L.; Lei, J. Multifunctional metal–organic framework heterostructures for enhanced cancer therapy. Chem. Soc. Rev. 2021, 50, 1188–1218. [Google Scholar] [CrossRef]
- Elmehrath, S.; Nguyen, H.L.; Karam, S.M.; Amin, A.; Greish, Y.E. BioMOF-Based Anti-Cancer Drug Delivery Systems. Nanomaterials 2023, 13, 953. [Google Scholar] [CrossRef]
- Jiang, W.; Liang, C.-C.; Zhang, Y.-B. Multivariate Metal–Organic Frameworks for Programming Functions. Adv. Funct. Mater. 2024, 34, 2308946. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Chen, J.; Min, Y.; Park, G.B.; Shen, X.; Song, S.-S.; Sun, Y.-M.; Wang, H.; Long, W.; Xie, J.; et al. Metabolizable Bi2Se3 Nanoplates: Biodistribution, Toxicity, and Uses for Cancer Radiation Therapy and Imaging. Adv. Funct. Mater. 2014, 24, 1718–1729. [Google Scholar] [CrossRef]
- Cruje, C.; Yang, C.; Uertz, J.; van Prooijen, M.; Chithrani, B.D. Optimization of PEG coated nanoscale gold particles for enhanced radiation therapy. RSC Adv. 2015, 5, 101525–101532. [Google Scholar] [CrossRef]
- Petersen, A.L.; Henriksen, J.R.; Binderup, T.; Elema, D.R.; Rasmussen, P.H.; Hag, A.M.; Kjær, A.; Andresen, T.L. In vivo evaluation of PEGylated 64Cu-liposomes with theranostic and radiotherapeutic potential using micro PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 941–952. [Google Scholar] [CrossRef]
- Mohammadian, M.; Emamgholizadeh Minaei, S.; Shiralizadeh Dezfuli, A. Improve the cytotoxic effects of megavoltage radiation treatment by Fe3O4@Cus–PEG nanoparticles as a novel radiosensitizer in colorectal cancer cells. Cancer Nanotechnol. 2022, 13, 25. [Google Scholar] [CrossRef]
- Liu, H.; Cai, Y.; Zhang, Y.; Xie, Y.; Qiu, H.; Hua, L.; Liu, X.; Li, Y.; Lu, J.; Zhang, L.; et al. Development of a Hypoxic Radiosensitizer-Prodrug Liposome Delivery DNA Repair Inhibitor Dbait Combination with Radiotherapy for Glioma Therapy. Adv. Healthc. Mater. 2017, 6, 1601377. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Z.; Xie, Y.; Liu, Y.; Yang, K.; Li, T.; Shen, H.; Zhao, M.; Jin, J.; Xiao, H.; et al. Radiation-Triggered Selenium-Engineered Mesoporous Silica Nanocapsules for RNAi Therapy in Radiotherapy-Resistant Glioblastoma. ACS Nano 2023, 17, 4062–4076. [Google Scholar] [CrossRef]
- Wu, H.; Yuan, Y.; Kang, S.; Zhou, G.; Gu, Y.; Yuan, X.; Li, J.; Gu, N. Autophagy inhibitor-loaded mesoporous AgNPs@SiO2 nanoplatform for synergistically enhanced glioma radiotherapy. Sci. China Mater. 2023, 66, 2902–2912. [Google Scholar] [CrossRef]
- Stewart, C.; Konstantinov, K.; McKinnon, S.; Guatelli, S.; Lerch, M.; Rosenfeld, A.; Tehei, M.; Corde, S. First proof of bismuth oxide nanoparticles as efficient radiosensitisers on highly radioresistant cancer cells. Phys. Medica 2016, 32, 1444–1452. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef]
- Li, T.; Gao, M.; Wu, Z.; Yang, J.; Mo, B.; Yu, S.; Gong, X.; Liu, J.; Wang, W.; Luo, S.; et al. Tantalum–Zirconium Co-Doped Metal–Organic Frameworks Sequentially Sensitize Radio–Radiodynamic–Immunotherapy for Metastatic Osteosarcoma. Adv. Sci. 2023, 10, 2206779. [Google Scholar] [CrossRef]
- Xu, Z.; Luo, T.; Mao, J.; McCleary, C.; Yuan, E.; Lin, W. Monte Carlo Simulation-Guided Design of a Thorium-Based Metal–Organic Framework for Efficient Radiotherapy-Radiodynamic Therapy. Angew. Chem. Int. Ed. 2022, 61, e202208685. [Google Scholar] [CrossRef]
- Lu, M.; Wu, H.; Liu, D.; Wang, F.; Wang, Y.; Wang, M.; Cui, Q.; Zhang, H.; Zang, F.; Ma, M.; et al. Camouflaged Nanoreactors Mediated Radiotherapy-Adjuvant Chemodynamic Synergistic Therapy. ACS Nano 2023, 17, 24170–24186. [Google Scholar] [CrossRef]
- Hu, H.; Zheng, S.; He, C.; Zheng, Y.; Wei, Q.; Chen, S.; Wu, Z.; Xu, Y.; Zhao, B.; Yan, C. Radiotherapy-sensitized cancer immunotherapy via cGAS-STING immune pathway by activatable nanocascade reaction. J. Nanobiotechnol. 2024, 22, 234. [Google Scholar] [CrossRef]
- Liu, D.; Cao, F.; Xu, Z.; Zhao, C.; Liu, Z.; Pang, J.; Liu, Z.-X.; Moghiseh, M.; Butler, A.; Liang, S.; et al. Selective Organ-Targeting Hafnium Oxide Nanoparticles with Multienzyme-Mimetic Activities Attenuate Radiation-Induced Tissue Damage. Adv. Mater. 2024, 36, 2308098. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Liao, Y.; Guo, X.; Song, Q.; Liu, W.; Gu, C.; Du, S.; Sun, B.; Gu, Z. Hafnium oxide-based sensitizer with radiation-triggered cuproptosis for radiotherapy. Nano Today 2025, 61, 102626. [Google Scholar] [CrossRef]
- Ji, C.; Zhao, M.; Wang, C.; Liu, R.; Zhu, S.; Dong, X.; Su, C.; Gu, Z. Biocompatible Tantalum Nanoparticles as Radiosensitizers for Enhancing Therapy Efficacy in Primary Tumor and Metastatic Sentinel Lymph Nodes. ACS Nano 2022, 16, 9428–9441. [Google Scholar] [CrossRef]
- Li, R.; Zhao, W.; Wu, T.; Wang, A.; Li, Q.; Liu, Y.; Xiong, H. Tantalum-carbon-integrated nanozymes as a nano-radiosensitizer for radiotherapy enhancement. Front. Bioeng. Biotechnol. 2022, 10, 1042646. [Google Scholar] [CrossRef]
- Zhao, M.; Ji, C.; Dai, H.; Wang, C.; Liu, R.; Xie, J.; Wang, Y.; Gu, Z. Mussel-Inspired Tantalum Nanocomposite Hydrogels for In Situ Oral Cancer Treatment. ACS Appl. Mater. Interfaces 2023, 15, 4984–4995. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Christopher, A.T.; Lekan, O.K.; Aworinde, O.R.; Faderin, E.; Obembe, O.; Abdulsalam_Akanji, T.F.; Igboanugo, J.C.; Udogu, U.; Ogidi, G.O.; et al. Advancements in tantalum based nanoparticles for integrated imaging and photothermal therapy in cancer management. RSC Adv. 2024, 14, 33681–33740. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Liu, L.; Li, M.; He, J.; Meng, Q.; Kang, J.; Zhou, D.; Gao, L.; Bai, J.; et al. Enhancing Chordoma Radiotherapy: Ta@PVP Nanoparticles as Potent Radiosensitizers. ACS Appl. Mater. Interfaces 2025, 17, 750–762. [Google Scholar] [CrossRef]
- Huang, H.; He, L.; Zhou, W.; Qu, G.; Wang, J.; Yang, N.; Gao, J.; Chen, T.; Chu, P.K.; Yu, X.-F. Stable black phosphorus/Bi2O3 heterostructures for synergistic cancer radiotherapy. Biomaterials 2018, 171, 12–22. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, S.; Zhang, N.; Kuang, Y.; Li, W.; Gai, S.; He, F.; Gulzar, A.; Yang, P. X-ray-triggered NO-released Bi–SNO nanoparticles: All-in-one nano-radiosensitizer with photothermal/gas therapy for enhanced radiotherapy. Nanoscale 2020, 12, 19293–19307. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Liu, H.; Zhang, K.; Zeng, Q.; Yang, S.; Jiang, Z.; Zhang, X.; Chen, T.; Li, D.; et al. Bi/Se-Based Nanotherapeutics Sensitize CT Image-Guided Stereotactic Body Radiotherapy through Reprogramming the Microenvironment of Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2021, 13, 42473–42485. [Google Scholar] [CrossRef]
- Huang, X.; Zha, F.; Zou, J.; Li, Y.; Wang, F.; Chen, X. Photoacoustic Imaging-Guided Synergistic Photothermal/Radiotherapy Using Plasmonic Bi/Bi2O3−x Nanoparticles. Adv. Funct. Mater. 2022, 32, 2113353. [Google Scholar] [CrossRef]
- Nosrati, H.; Ghaffarlou, M.; Salehiabar, M.; Mousazadeh, N.; Abhari, F.; Barsbay, M.; Ertas, Y.N.; Rashidzadeh, H.; Mohammadi, A.; Nasehi, L.; et al. Magnetite and bismuth sulfide Janus heterostructures as radiosensitizers for in vivo enhanced radiotherapy in breast cancer. Biomater. Adv. 2022, 140, 213090. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, G.; Wang, Q.; Du, J.; Wu, S.; Lu, J.; Liu, B.; Miao, Y.; Li, Y. High-Z elements dominated bismuth-based heterojunction nano-semiconductor for radiotherapy-enhanced sonodynamic breast cancer therapy. J. Colloid Interface Sci. 2024, 662, 914–927. [Google Scholar] [CrossRef]
- Anderson, P.M.; Subbiah, V.; Trucco, M.M. Current and future targeted alpha particle therapies for osteosarcoma: Radium-223, actinium-225, and thorium-227. Front. Med. 2022, 9, 1030094. [Google Scholar] [CrossRef]
- Karlsson, J.; Schatz, C.A.; Wengner, A.M.; Hammer, S.; Scholz, A.; Cuthbertson, A.; Wagner, V.; Hennekes, H.; Jardine, V.; Hagemann, U.B. Targeted thorium-227 conjugates as treatment options in oncology. Front. Med. 2023, 9, 1071086. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Wang, Q.; Zhang, Z.; Liu, J.; Zhang, C.; Shi, J. Recent Progress on High- Metal-Based Nanomaterials for Cancer Radiosensitization. Chin. J. Chem. 2023, 41, 2545–2556. [Google Scholar] [CrossRef]
- Lunj, S.; Smith, T.A.D.; Reeves, K.J.; Currell, F.; Honeychurch, J.; Hoskin, P.; Choudhury, A. Immune effects of α and β radionuclides in metastatic prostate cancer. Nat. Rev. Urol. 2024, 21, 651–661. [Google Scholar] [CrossRef]
- Zhen, W.; Fan, Y.; Germanas, T.; Tillman, L.; Li, J.; Blenko, A.L.; Weichselbaum, R.R.; Lin, W. Digitonin-Loaded Nanoscale Metal–Organic Framework for Mitochondria-Targeted Radiotherapy-Radiodynamic Therapy and Disulfidptosis. Adv. Mater. 2024. ahead of print. [Google Scholar] [CrossRef]
- Woods, J.J.; Rigby, A.; Wacker, J.N.; Arino, T.; Alvarenga Vasquez, J.V.; Cosby, A.; Martin, K.E.; Abergel, R.J. Synthesis and Evaluation of a Bifunctional Chelator for Thorium-227 Targeted Radiotherapy. J. Med. Chem. 2025, 68, 1682–1692. [Google Scholar] [CrossRef]
- Bodedla, G.B.; Dong, Y.; Tang, G.; Zhao, J.; Zhang, F.; Zhu, X.; Wong, W.-Y. Long-lived excited states of platinum(ii)-porphyrins for highly efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2022, 10, 13402–13409. [Google Scholar] [CrossRef]
- Feng, H.; Li, H.; Liu, X.; Huang, Y.; Pan, Q.; Peng, R.; Du, R.; Zheng, X.; Yin, Z.; Li, S.; et al. Porphyrin-based Ti-MOFs conferred with single-atom Pt for enhanced photocatalytic hydrogen evolution and NO removal. Chem. Eng. J. 2022, 428, 132045. [Google Scholar] [CrossRef]
- Kou, M.; Qin, F.; Wang, Y.; Zhang, X.; Zhao, H.; Tian, Y.; Zhang, Z. Intrinsic Characterization Method on the Heavy Atom Effect of Metalloporphyrins. Inorg. Chem. 2022, 61, 15175–15181. [Google Scholar] [CrossRef]
- Scoditti, S.; Chiodo, F.; Mazzone, G.; Richeter, S.; Sicilia, E. Porphyrins and Metalloporphyrins Combined with N-Heterocyclic Carbene (NHC) Gold(I) Complexes for Photodynamic Therapy Application: What Is the Weight of the Heavy Atom Effect? Molecules 2022, 27, 4046. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; He, Y.; Feng, H.; Zhang, Y.; Liu, C.; Wu, Z. 2D porphyrin-based MOFs with highly dispersed Pt nanoparticles via in-situ partial reduction strategy from porphyrin embedded with single-atom Pt for enhancing photocatalytic hydrogen production. Fuel 2023, 338, 127369. [Google Scholar] [CrossRef]
- Tan, C.; Li, X.; Li, Z.; Lu, S.; Wang, F.; Liu, Y.; Wen, F.; Li, R.; Tu, D.; Chen, Z.; et al. Near-infrared-responsive nanoplatforms integrating dye-sensitized upconversion and heavy-atom effect for enhanced photodynamic therapy efficacy. Nano Today 2024, 54, 102089. [Google Scholar] [CrossRef]
- Guo, N.; Ni, K.; Luo, T.; Lan, G.; Arina, A.; Xu, Z.; Mao, J.; Weichselbaum, R.R.; Spiotto, M.; Lin, W. Reprogramming of Neutrophils as Non-canonical Antigen Presenting Cells by Radiotherapy–Radiodynamic Therapy to Facilitate Immune-Mediated Tumor Regression. ACS Nano 2021, 15, 17515–17527. [Google Scholar] [CrossRef]
- Ni, K.; Lan, G.; Song, Y.; Hao, Z.; Lin, W. Biomimetic nanoscale metal–organic framework harnesses hypoxia for effective cancer radiotherapy and immunotherapy. Chem. Sci. 2020, 11, 7641–7653. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, C.; Mei, Z.; Yang, H.; Wang, B.; Xie, C.; Xu, Y.; Tian, J. Oxygen-Enriched MOF-Hemoglobin X-ray Nanosensitizer for Enhanced Cancer Radio–Radiodynamic Therapy. ACS Mater. Lett. 2023, 5, 3237–3247. [Google Scholar] [CrossRef]
- Zhen, W.; Xu, Z.; Mao, Y.; McCleary, C.; Jiang, X.; Weichselbaum, R.R.; Lin, W. Nanoscale Mixed-Ligand Metal–Organic Framework for X-ray Stimulated Cancer Therapy. J. Am. Chem. Soc. 2024, 146, 33149–33158. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Guo, Z.; Tang, C.; Sa, B.; Miao, N.; Zhou, J.; Sun, Z. Breaking the linear scaling relations in MXene catalysts for efficient CO2 reduction. Chem. Eng. J. 2022, 429, 132171. [Google Scholar] [CrossRef]
- Zhang, P.-X.; Ye, L.; Chen, F.-H.; Han, W.-J.; Wu, Y.-H.; Zhao, T. Stability, mechanical, and thermodynamic behaviors of (TiZrHfTaM)C (M = Nb, Mo, W, V, Cr) high-entropy carbide ceramics. J. Alloys Compd. 2022, 903, 163868. [Google Scholar] [CrossRef]
- Min, R.; Gao, Y.; Jiang, X.; Yang, X.; Li, L.; Kang, H.; Guo, E.; Chen, Z.; Wang, T. Enhancement in the thermoelectric performance of ZrNiSn-based alloys through extra Zr-rich nanoprecipitates with superstructures. Chem. Eng. J. 2023, 464, 142531. [Google Scholar] [CrossRef]
- Neuer, A.L.; Jessernig, A.; Gerken, L.R.H.; Gogos, A.; Starsich, F.H.L.; Anthis, A.H.C.; Herrmann, I.K. Cellular fate and performance of group IV metal organic framework radioenhancers. Biomater. Sci. 2022, 10, 6558–6569. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Z.; Wang, N.; Chen, X.; Sun, X.; Cheng, Y. Hafnium-Based Metal–Organic Framework Nanoparticles as a Radiosensitizer to Improve Radiotherapy Efficacy in Esophageal Cancer. ACS Omega 2022, 7, 12021–12029. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, Z.; Wang, J.; Deng, L.; Wang, H.; Tang, W.; Ni, D. Interfering biosynthesis by nanoscale metal-organic frameworks for enhanced radiation therapy. Biomaterials 2023, 295, 122035. [Google Scholar] [CrossRef]
- Jerozal, R.T.; Pitt, T.A.; MacMillan, S.N.; Milner, P.J. High-Concentration Self-Assembly of Zirconium- and Hafnium-Based Metal–Organic Materials. J. Am. Chem. Soc. 2023, 145, 13273–13283. [Google Scholar] [CrossRef]
- Kang, L.-L.; Xing, C.; Jin, Y.-X.; Xie, L.-X.; Li, Z.-F.; Li, G. Two Dual-Function Zr/Hf-MOFs as High-Performance Proton Conductors and Amines Impedance Sensors. Inorg. Chem. 2023, 62, 3036–3046. [Google Scholar] [CrossRef]
- Hong, Y.-L.; Xu, Z.; Du, J.; Shi, Z.-Q.; Zuo, Y.-H.; Hu, H.-L.; Li, G. Prominent Intrinsic Proton Conduction in Two Robust Zr/Hf Metal–Organic Frameworks Assembled by Bithiophene Dicarboxylate. Inorg. Chem. 2024, 63, 10786–10797. [Google Scholar] [CrossRef]
- Hu, M.; Zhou, W.; Wang, Y.; Yao, D.; Ye, T.; Yao, Y.; Chen, B.; Liu, G.; Yang, X.; Wang, W.; et al. Discovery of the first potent proteolysis targeting chimera (PROTAC) degrader of indoleamine 2,3-dioxygenase 1. Acta Pharm. Sin. B 2020, 10, 1943–1953. [Google Scholar] [CrossRef]
- Guo, R.; Li, J.; Hu, J.; Fu, Q.; Yan, Y.; Xu, S.; Wang, X.; Jiao, F. Combination of epidrugs with immune checkpoint inhibitors in cancer immunotherapy: From theory to therapy. Int. Immunopharmacol. 2023, 120, 110417. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, F.; Wang, W.; Du, G.; Yu, P.; Ye, L.; Wang, H.; Yang, Y.; Tian, J. Design, synthesis, and biological evaluation of novel molecules as potent inhibitors of indoleamine 2,3-dioxygenase 1. Mol. Divers. 2024. ahead of print. [Google Scholar] [CrossRef]
- Zhang, M.-R.; Lin-Lin, F.; Yang, G.; Qin, W.; You-Jie, L.; Hong-Fang, S.; Shu-Yang, X.; Liang, Y. Advancements in Stimulus-Responsive Co-Delivery Nanocarriers for Enhanced Cancer Immunotherapy. Int. J. Nanomed. 2024, 19, 3387–3404. [Google Scholar] [CrossRef]
- Xiong, S.; Song, D.; Xiang, Y.; Li, Y.; Zhong, Y.; Li, H.; Zhang, P.; Zhou, W.; Zeng, X.; Zhang, X. Reactive oxygen species, not Ca2+, mediates methotrexate-induced autophagy and apoptosis in spermatocyte cell line. Basic Clin. Pharmacol. Toxicol. 2020, 126, 144–152. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, J.; Guo, H.; Lu, J.-l.; Zhou, J.; Guo, X.-y.; Shi, Y.; Zhang, Y.; Yu, S.; Zhang, Q.; et al. Pyrroloquinoline quinone promotes mitochondrial biogenesis in rotenone-induced Parkinson’s disease model via AMPK activation. Acta Pharmacol. Sin. 2021, 42, 665–678. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Wei, H.; Zhou, S.; Chen, W.; Yan, X.; Cai, J.; Chen, X.; Chen, B.; Liao, M.; et al. Neurite Extension and Orientation of Spiral Ganglion Neurons Can Be Directed by Superparamagnetic Iron Oxide Nanoparticles in a Magnetic Field. Int. J. Nanomed. 2021, 16, 4515–4526. [Google Scholar] [CrossRef]
- Wei, H.; Yangnan, H.; Junguo, W.; Xia, G.; Xiaoyun, Q.; Tang, M. Superparamagnetic Iron Oxide Nanoparticles: Cytotoxicity, Metabolism, and Cellular Behavior in Biomedicine Applications. Int. J. Nanomed. 2021, 16, 6097–6113. [Google Scholar] [CrossRef]
- de Krafft, K.E.; Boyle, W.S.; Burk, L.M.; Zhou, O.Z.; Lin, W. Zr- and Hf-based nanoscale metal–organic frameworks as contrast agents for computed tomography. J. Mater. Chem. 2012, 22, 18139–18144. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Zhao, D. The chemistry and applications of hafnium and cerium(iv) metal–organic frameworks. Chem. Soc. Rev. 2021, 50, 4629–4683. [Google Scholar] [CrossRef]
- Wang, J.; Pan, J.; Tang, Y.; Chen, J.; Fei, X.; Xue, W.; Liu, X. Advances of hafnium based nanomaterials for cancer theranostics. Front. Chem. 2023, 11, 1283924. [Google Scholar] [CrossRef]
- Li, Q.-R.; Niu, M.-T.; Liu, L.-M.; Zeng, J.-Y.; Ji, P.; Zhou, H.; He, J.-L.; Chen, W.-H.; Zhang, X.-Z. Bioinspired Hf-based metal-organic framework radiosensitizer for nitric oxide-assisted radio-immunotherapy. Nano Today 2024, 58, 102447. [Google Scholar] [CrossRef]
- Gong, L.; Yujie, Z.; Chengcheng, L.; Mingzhen, Z.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Jia, S.; Shengfang, G.; Xianqun, F.; Leong, K.W.; Ruan, J. Promoting Reactive Oxygen Species Generation: A Key Strategy in Nanosensitizer-Mediated Radiotherapy. Nanomedicine 2021, 16, 759–778. [Google Scholar] [CrossRef]
- Dubey, P.; Sertorio, M.; Takiar, V. Therapeutic Advancements in Metal and Metal Oxide Nanoparticle-Based Radiosensitization for Head and Neck Cancer Therapy. Cancers 2022, 14, 514. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Liu, Y.; Younis, M.H.; Cai, W.; Bu, W. Catalytic radiosensitization: Insights from materials physicochemistry. Mater. Today 2022, 57, 262–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, X.; Liu, Y.; Wang, S.; Han, X.; Cheng, C. Research progress on nano-sensitizers for enhancing the effects of radiotherapy. Mater. Adv. 2022, 3, 3709–3725. [Google Scholar] [CrossRef]
- Taheri, A.; Khandaker, M.U.; Rabus, H.; Moradi, F.; Bradley, D.A. The influence of atomic number on the radiosensitization efficiency of metallic nanorods: A Monte Carlo simulation study. Radiat. Phys. Chem. 2025, 230, 112589. [Google Scholar] [CrossRef]
- Wen, S.; Ovais, M.; Li, X.; Ren, J.; Liu, T.; Wang, Z.; Cai, R.; Chen, C. Tailoring bismuth-based nanoparticles for enhanced radiosensitivity in cancer therapy. Nanoscale 2022, 14, 8245–8254. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Chen, D.; Miao, S.; Wen, J.; Liu, C.; Xue, S.; Liu, Y.; Zhang, Q.; Shen, Y. “Cluster Bomb” Based Bismuth Nano-in-Micro Spheres Formed Dry Powder Inhalation for Thermo-Radio Sensitization Effects of Lung Metastatic Breast Cancer. Adv. Funct. Mater. 2023, 12, 2202622. [Google Scholar] [CrossRef]
- Wang, Q.-X.; Li, G. Bi(iii) MOFs: Syntheses, structures and applications. Inorg. Chem. Front. 2021, 8, 572–589. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Peng, L.; Wang, Q.; Wang, Y.; Xu, X. Radiation-Responsive Metal–Organic Frameworks: Fundamentals and Applications. Adv. Funct. Mater. 2024, 34, 2310270. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Faghfouri, L.; Ferreira, M.P.A.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H.A. The versatile biomedical applications of bismuth-based nanoparticles and composites: Therapeutic, diagnostic, biosensing, and regenerative properties. Chem. Soc. Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef]
- Badrigilan, S.; Fatemeh, H.; Jalal, C.; Mehdi, J.; Hadi, S.; Mojtaba, H.-G.; Webster, T.J.; Tayebi, L. A Review on the Biodistribution, Pharmacokinetics and Toxicity of Bismuth-Based Nanomaterials. Int. J. Nanomed. 2020, 15, 7079–7096. [Google Scholar] [CrossRef]
- Griffith, D.M.; Li, H.; Werrett, M.V.; Andrews, P.C.; Sun, H. Medicinal chemistry and biomedical applications of bismuth-based compounds and nanoparticles. Chem. Soc. Rev. 2021, 50, 12037–12069. [Google Scholar] [CrossRef]
- Gomez, C.; Hallot, G.; Laurent, S.; Port, M. Medical Applications of Metallic Bismuth Nanoparticles. Pharmaceutics 2021, 13, 1793. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Chen, Y.; He, W.; Guo, Z. Recent advances in noble metal complex based photodynamic therapy. Chem. Sci. 2022, 13, 5085–5106. [Google Scholar] [CrossRef]
- Lee, S.; Wu, H.; Yeo, S.; Lee, W.K.; Yoon, I. Transition metal-dependent heavy-atom effect of metallochlorin photosensitizers for enhanced photodynamic therapy. Inorg. Chem. Commun. 2023, 152, 110693. [Google Scholar] [CrossRef]
- Wu, L.-L.; Meng, X.; Zhang, Q.; Han, X.; Yang, F.; Wang, Q.; Hu, H.-Y.; Xing, N. Heavy-atom engineered hypoxia-responsive probes for precisive photoacoustic imaging and cancer therapy. Chin. Chem. Lett. 2024, 35, 108663. [Google Scholar] [CrossRef]
- Li, Q.; Pang, Y.; Mei, L.; Liang, S.; Wang, H.; Jiao, Y.; Qiu, S.; Chen, H.; Xing, X.; Sun, Y. Heavy atom engineering of Ru(ii) complex based sonosensitizers for enhancing antifungal therapy. Inorg. Chem. Front. 2025, 12, 2668–2677. [Google Scholar] [CrossRef]
- Sheng, Y.; Nesbitt, H.; Callan, B.; Taylor, M.A.; Love, M.; McHale, A.P.; Callan, J.F. Oxygen generating nanoparticles for improved photodynamic therapy of hypoxic tumours. J. Control. Release 2017, 264, 333–340. [Google Scholar] [CrossRef]
- Li, X.; Kwon, N.; Guo, T.; Liu, Z.; Yoon, J. Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 11522–11531. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Wu, J.; Yu, L.; Singh, N.; Yang, K.; Lan, M.; Wang, P.; Kim, J.S. Photodynamic therapy for hypoxic tumors: Advances and perspectives. Coord. Chem. Rev. 2021, 438, 213888. [Google Scholar] [CrossRef]
- Shen, Z.; Ma, Q.; Zhou, X.; Zhang, G.; Hao, G.; Sun, Y.; Cao, J. Strategies to improve photodynamic therapy efficacy by relieving the tumor hypoxia environment. NPG Asia Mater. 2021, 13, 39. [Google Scholar] [CrossRef]
- Wan, Y.; Fu, L.-H.; Li, C.; Lin, J.; Huang, P. Conquering the Hypoxia Limitation for Photodynamic Therapy. Adv. Mater. 2021, 33, 2103978. [Google Scholar] [CrossRef]
- Yuan, P.; Deng, F.-A.; Liu, Y.-B.; Zheng, R.-R.; Rao, X.-N.; Qiu, X.-Z.; Zhang, D.-W.; Yu, X.-Y.; Cheng, H.; Li, S.-Y. Mitochondria Targeted O2 Economizer to Alleviate Tumor Hypoxia for Enhanced Photodynamic Therapy. Adv. Healthc. Mater. 2021, 10, 2100198. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Zhao, L.-P.; Zhou, X.; Liu, L.-S.; Zheng, R.-R.; Deng, F.-A.; Liu, Y.-B.; Yu, X.-Y.; Li, S.-Y.; Cheng, H. Carrier Free O2-Economizer for Photodynamic Therapy Against Hypoxic Tumor by Inhibiting Cell Respiration. Small 2022, 18, 2107467. [Google Scholar] [CrossRef]
- Cen, Y.; Chen, X.; Liu, Y.; Yu, B.; Yan, M.; Yang, N.; Kong, R.; Li, S.; Ti, H.; Cheng, H. Drug induced mitochondria dysfunction to enhance photodynamic therapy of hypoxic tumors. J. Control. Release 2023, 358, 654–666. [Google Scholar] [CrossRef]
- Yu, J.; Li, Q.; Wei, Z.; Fan, G.; Wan, F.; Tian, L. Ultra-stable MOF@MOF nanoplatform for photodynamic therapy sensitized by relieved hypoxia due to mitochondrial respiration inhibition. Acta Biomater. 2023, 170, 330–343. [Google Scholar] [CrossRef]
- Wei, J.; Li, J.; Sun, D.; Li, Q.; Ma, J.; Chen, X.; Zhu, X.; Zheng, N. A Novel Theranostic Nanoplatform Based on Pd@Pt-PEG-Ce6 for Enhanced Photodynamic Therapy by Modulating Tumor Hypoxia Microenvironment. Adv. Funct. Mater. 2018, 28, 1706310. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, X.; Zhang, C.; Huang, W.; Zhou, Y.; Yan, D. Oxygen and Pt(II) self-generating conjugate for synergistic photo-chemo therapy of hypoxic tumor. Nat. Commun. 2018, 9, 2053. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Y.; Li, N.; Luo, C.; Hou, X. Anthracene-based fluorescent MOFs decorated by platinum nanozymes as a multifunctional nanoplatform for enhanced photodynamic therapy and self-monitoring of real-time singlet oxygen. Chem. Eng. J. 2022, 446, 137333. [Google Scholar] [CrossRef]
- Gu, X.; Wu, B.; Feng, G.; Chen, Z.; Ren, F.; Chen, X.; Hong, W.; Li, W. PD-L1 Blockade Peptide-Modified Polymeric Nanoparticles for Oxygen-Independent-Based Hypoxic Tumor Photo/Thermodynamic Immunotherapy. Mol. Pharm. 2023, 20, 4007–4020. [Google Scholar] [CrossRef]
- Liang, Y.; Ping-Yu, W.; Ze-Yun, L.; Hong-Fang, S.; Qin, W.; Guang-Bin, S.; Xia, Z.; You-Jie, L.; Xie, S.-Y. Dual Stimuli-Responsive Micelles for Imaging-Guided Mitochondrion-Targeted Photothermal/Photodynamic/Chemo Combination Therapy-Induced Immunogenic Cell Death. Int. J. Nanomed. 2023, 18, 4381–4402. [Google Scholar] [CrossRef]
- Fu, L.; Huang, Y.; Shan, X.; Sun, X.; Wang, X.; Wang, X.; Chen, L.; Yu, S. NIR-activatable nitric oxide generator based on nanoparticles loaded small-molecule photosensitizers for synergetic photodynamic/gas therapy. J. Nanobiotechnol. 2024, 22, 595. [Google Scholar] [CrossRef]
- Gong, B.; Zhang, Q.; Chen, J.; Qu, Y.; Luo, X.; Wang, W.; Zheng, X. Recent Advances in Glutathione Depletion-Enhanced Porphyrin-Based nMOFs for Photodynamic Therapy. Pharmaceutics 2025, 17, 244. [Google Scholar] [CrossRef]
- Arabzadeh, A.; Mortezazadeh, T.; Aryafar, T.; Gharepapagh, E.; Majdaeen, M.; Farhood, B. Therapeutic potentials of resveratrol in combination with radiotherapy and chemotherapy during glioblastoma treatment: A mechanistic review. Cancer Cell Int. 2021, 21, 391. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Ismaila, N.; Chua, M.L.K.; Colevas, A.D.; Haddad, R.; Huang, S.H.; Wee, J.T.S.; Whitley, A.C.; Yi, J.-L.; Yom, S.S.; et al. Chemotherapy in Combination With Radiotherapy for Definitive-Intent Treatment of Stage II-IVA Nasopharyngeal Carcinoma: CSCO and ASCO Guideline. J. Clin. Oncol. 2021, 39, 840–859. [Google Scholar] [CrossRef]
- Mortezaee, K.; Narmani, A.; Salehi, M.; Bagheri, H.; Farhood, B.; Haghi-Aminjan, H.; Najafi, M. Synergic effects of nanoparticles-mediated hyperthermia in radiotherapy/chemotherapy of cancer. Life Sci. 2021, 269, 119020. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Fang, F.; Xu, T.; Lan, M.; Zhang, J. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials 2021, 268, 120557. [Google Scholar] [CrossRef]
- Li, G.; Sun, B.; Li, Y.; Luo, C.; He, Z.; Sun, J. Small-Molecule Prodrug Nanoassemblies: An Emerging Nanoplatform for Anticancer Drug Delivery. Small 2021, 17, 2101460. [Google Scholar] [CrossRef]
- Liu, S.; Khan, A.R.; Yang, X.; Dong, B.; Ji, J.; Zhai, G. The reversal of chemotherapy-induced multidrug resistance by nanomedicine for cancer therapy. J. Control. Release 2021, 335, 1–20. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Desai, A.; Lustberg, M.B.; Lyman, G.H. Mitigating acute chemotherapy-associated adverse events in patients with cancer. Nat. Rev. Clin. Oncol. 2022, 19, 681–697. [Google Scholar] [CrossRef]
- Wei, D.; Sun, Y.; Zhu, H.; Fu, Q. Stimuli-Responsive Polymer-Based Nanosystems for Cancer Theranostics. ACS Nano 2023, 17, 23223–23261. [Google Scholar] [CrossRef]
- Zhuang, F.; Xiang, H.; Huang, B.; Chen, Y. Ultrasound-Triggered Cascade Amplification of Nanotherapy. Adv. Mater. 2023, 35, 2303158. [Google Scholar] [CrossRef]
- Liu, Z.-S.; Wen, J.; Huang, C.-Y.; Zhang, P.-W.; Miao, Y.-L.; Cheng, H.; Li, S.-Y. Nanomedicines Based on Responsive Nanocarriers for Cancer Therapy. Adv. Ther. 2024, 7, 2300223. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B. Polymersomes as Innovative, Stimuli-Responsive Platforms for Cancer Therapy. Pharmaceutics 2024, 16, 463. [Google Scholar] [CrossRef]
- Pang, Q.; Xu, Z.; Sun, T.; Yue, S.; Yu, Y.; Lu, H.; He, M.; Chen, X.; Lu, Y.; Li, J. Strategic chemical synthesis and application of nanocarriers responsive to the tumor microenvironment. Nano Today 2024, 58, 102421. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.; Liu, H.; Wang, A.; Ren, T.; Gou, J.; Zhang, Y.; Yin, T.; He, H.; Tang, X. Toxicity Reduction and Efficacy Promotion of Doxorubicin in the Treatment of Breast Tumors Assisted by Enhanced Oral Absorption of Curcumin-Loaded Lipid–Polyester Mixed Nanoparticles. Mol. Pharm. 2020, 17, 4533–4547. [Google Scholar] [CrossRef]
- Yong, L.; Ji, L.; Jianqiang, Q.; Chi, M.; Jing, G.; Weijie, G.; Biao, X.; Changchun, L.; Yanan, Z. Recent Advances in Multi-target Drugs Targeting Protein Kinases and Histone Deacetylases in Cancer Therapy. Curr. Med. Chem. 2020, 27, 7264–7288. [Google Scholar] [CrossRef]
- Fan, Z.; Wu, C.; Chen, M.; Jiang, Y.; Wu, Y.; Mao, R.; Fan, Y. The generation of PD-L1 and PD-L2 in cancer cells: From nuclear chromatin reorganization to extracellular presentation. Acta Pharm. Sin. B 2022, 12, 1041–1053. [Google Scholar] [CrossRef]
- Wu, Z.-X.; Teng, Q.-X.; Yang, Y.; Acharekar, N.; Wang, J.-Q.; He, M.; Yoganathan, S.; Lin, J.; Wang, J.; Chen, Z.-S. MET inhibitor tepotinib antagonizes multidrug resistance mediated by ABCG2 transporter: In vitro and in vivo study. Acta Pharm. Sin. B 2022, 12, 2609–2618. [Google Scholar] [CrossRef]
- Guo, J.; Niu, Z.; Lv, R.; Yuan, J.; Zhang, Z.; Guan, X.; Li, D.; Zhang, H.; Zhao, A.; Feng, J.; et al. A novel GARP humanized mouse model for efficacy assessment of GARP-targeting therapies. Int. Immunopharmacol. 2024, 130, 111782. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, J.; Liu, Y.; Zhao, Y.; Li, Z.; Xu, B.; Chen, D.; Wang, B.; Wang, Q.; Shen, Y. Hybrid nanopotentiators with dual cascade amplification for glioma combined interventional therapy. J. Control. Release 2024, 372, 95–112. [Google Scholar] [CrossRef]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Sang, M.; Dong, A.; Wu, S.; Li, F.; Wang, J.; Griffin, C.; Wu, R. A graph model of combination therapies. Drug Discov. Today 2022, 27, 1210–1217. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, X.; Xu, H.; Jiao, P.; Zhao, L.-X.; Su, G. Nanomaterial-Based Drug Delivery Systems for Pain Treatment and Relief: From the Delivery of a Single Drug to Co-Delivery of Multiple Therapeutics. Pharmaceutics 2023, 15, 2309. [Google Scholar] [CrossRef]

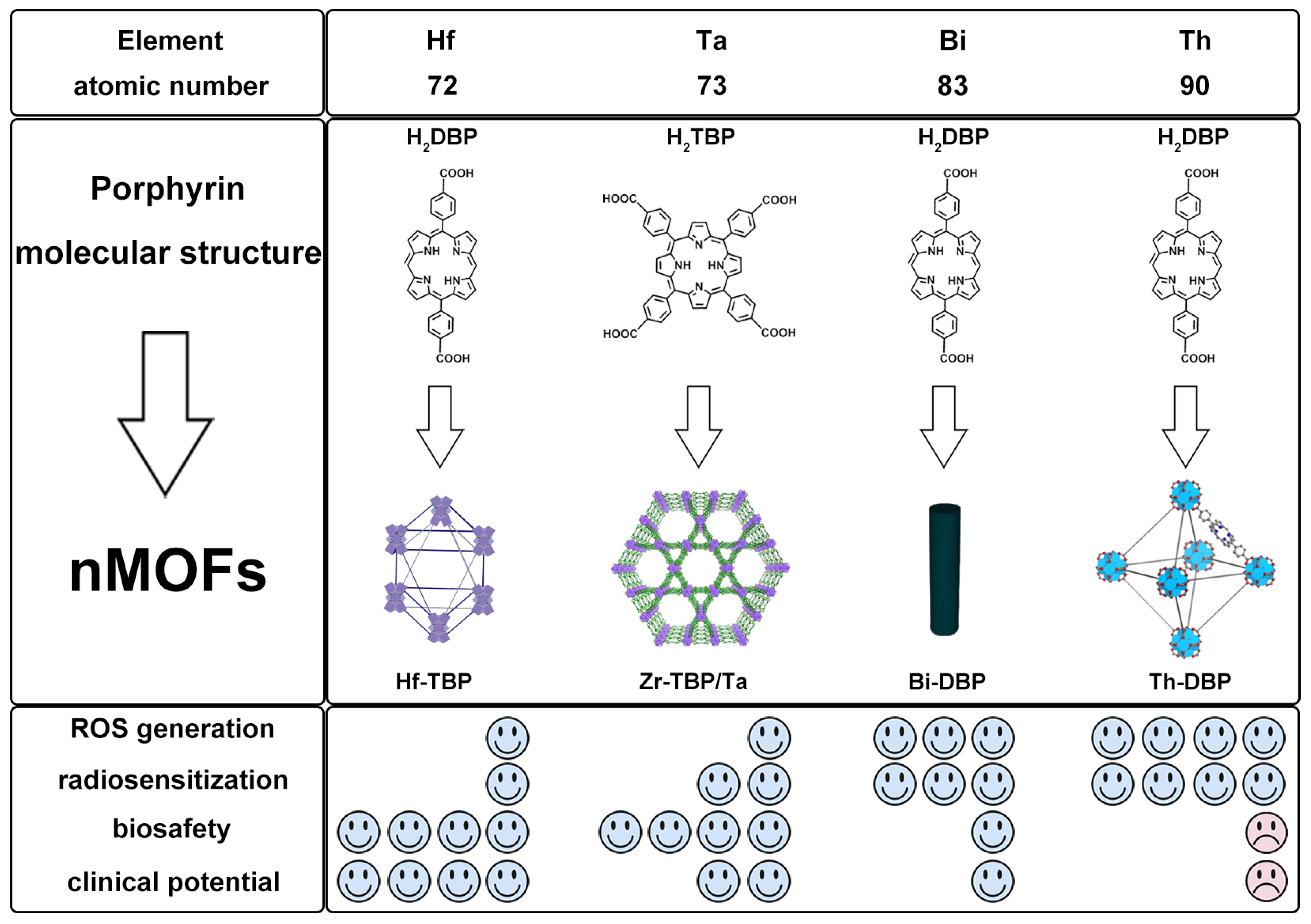
| Material | Structure | Properties and Therapeutic Advantages | Ref. | |
|---|---|---|---|---|
| Porphyrin | nMOF | |||
| Hf-DBP, Hf-TBP | 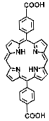 |  | Hf-DBP, nanoplate size = 72.3 nm, 630 nm, RT (1O2)-RDT (·OH); Hf-TBP, fusiform, size = 72.7 nm, 650 nm, RT (1O2)-RDT (·OH), RT/RDT for CT26 | [28] |
| Zr-TBP/Ta, Zr/Ta co-doped MOFs (TZM) | 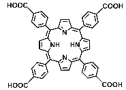 | 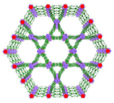 | 120 nm in width and 300 nm in length, RT (1O2)-RDT (·OH), RT/RDT for metastatic osteosarcoma | [50] |
| Th-DBP, Th-DBP@PEG | 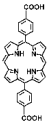 | 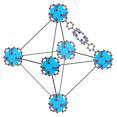 | nano-octahedron, 80 nm, RT (1O2)-RDT (·OH), RT/RDT for CT26 and Panc02 cells | [51] |
| Bi-DBP |  |  | nanorod, diameter (~20 nm) and length (~180 nm), RT (1O2)-RDT (·OH), RT/RDT for TRAMP-C2 cells | [29] |
| Pt—heavy atom effect, Hf-DBP-Pt | 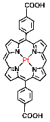 |  | nanoplate, diameter (~100 nm) and thickness (~8 nm), RT (1O2)-RDT (·OH), RT/RDT for CT26 and HepG2 cells | [79] |
| Hf-TBP, HP(Hf)-MOF, Hb@HP(Hf) | 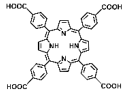 |  | spherical structure, 200 nm, O2-carrying, RT (1O2)-RDT (·OH), RT/RDT for 4T1 and CT26 cells | [81] |
| Fe(III)-mediated CDT, Hf-DBP, Hf-DBP-Fe |  |  | nanoplate, ~100 nm, CDT (O2 generation, ·OH), RT (1O2)-RDT (·OH), RT/RDT for MC38 cells | [80] |
| Hf-DBP-QP, Hf-DBP-QP-SN | 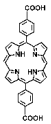 |  | nanoplate, diameter (~120 nm) and thickness (~20 nm), chemotherapy, RT (1O2)-RDT (·OH), RT/RDT for CT26 cells | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, B.; Zhang, Q.; Qu, Y.; Zheng, X.; Wang, W. Nanoscale Porphyrin-Based Metal–Organic Frameworks for Enhanced Radiotherapy–Radiodynamic Therapy: A Comprehensive Review. Pharmaceutics 2025, 17, 883. https://doi.org/10.3390/pharmaceutics17070883
Gong B, Zhang Q, Qu Y, Zheng X, Wang W. Nanoscale Porphyrin-Based Metal–Organic Frameworks for Enhanced Radiotherapy–Radiodynamic Therapy: A Comprehensive Review. Pharmaceutics. 2025; 17(7):883. https://doi.org/10.3390/pharmaceutics17070883
Chicago/Turabian StyleGong, Bin, Qiuyun Zhang, Yijie Qu, Xiaohua Zheng, and Weiqi Wang. 2025. "Nanoscale Porphyrin-Based Metal–Organic Frameworks for Enhanced Radiotherapy–Radiodynamic Therapy: A Comprehensive Review" Pharmaceutics 17, no. 7: 883. https://doi.org/10.3390/pharmaceutics17070883
APA StyleGong, B., Zhang, Q., Qu, Y., Zheng, X., & Wang, W. (2025). Nanoscale Porphyrin-Based Metal–Organic Frameworks for Enhanced Radiotherapy–Radiodynamic Therapy: A Comprehensive Review. Pharmaceutics, 17(7), 883. https://doi.org/10.3390/pharmaceutics17070883







