Cutting-Edge Approaches in the Co-Amorphization Process
Abstract
1. Introduction
2. Materials and Methods
3. Co-Former Types
3.1. Bile Acid
3.2. Organic Acid
3.3. Saccharides
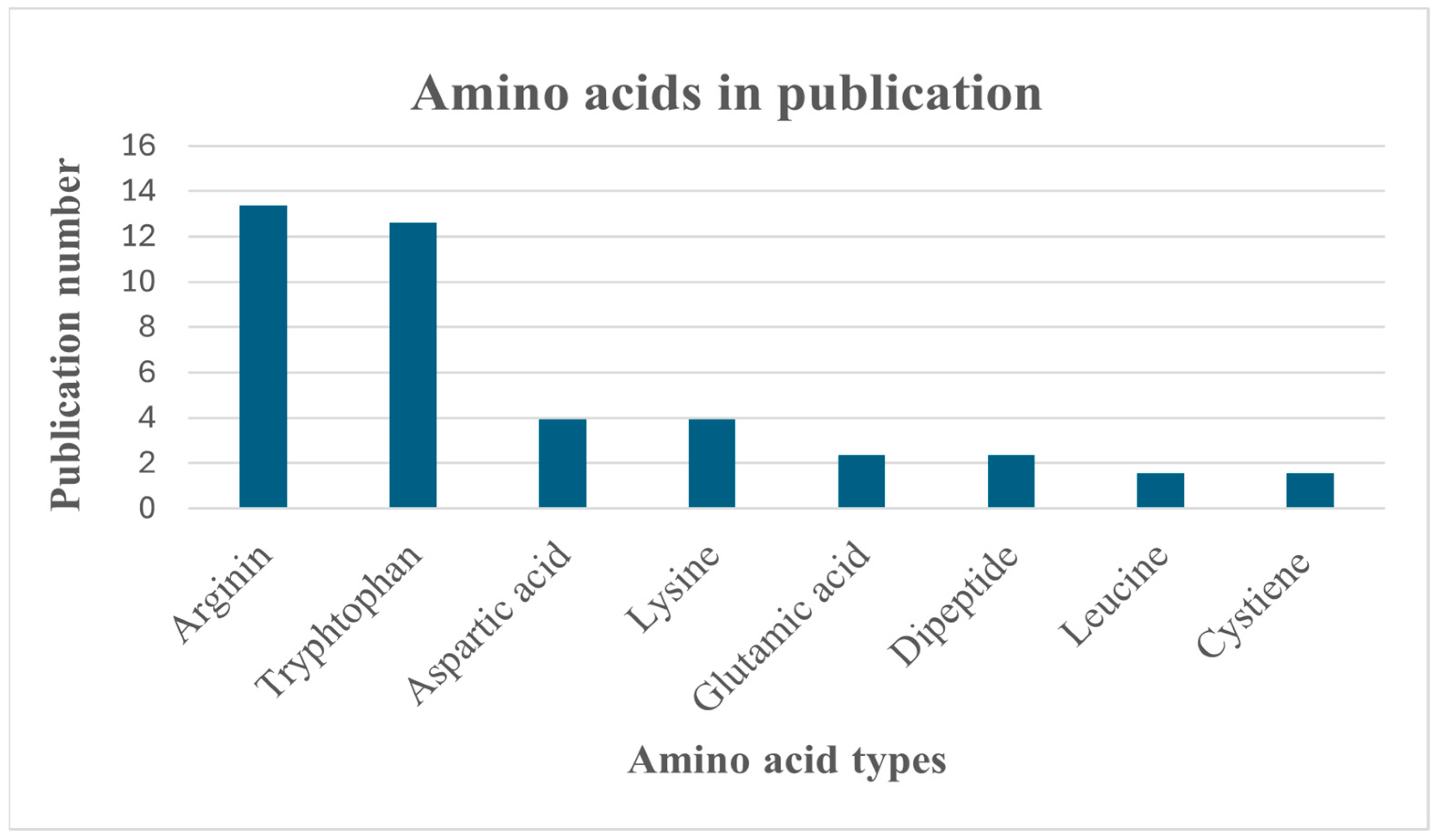
3.4. Amino Acids
3.4.1. Arginine
3.4.2. Lysine
3.4.3. Tryptophan
3.4.4. Leucine
3.4.5. Glutamic Acid
3.4.6. Aspartic Acid
3.4.7. Cysteine
3.4.8. Dipeptides
3.5. Drug–Drug Co-Former
3.6. Polyphenols and Alkaloids
3.7. Flavonoids
3.8. Nucleotides
3.9. Functionalized Calcium Carbonate (FCC)
4. Co-Amorphous Inhalation System
5. Ternary System
6. Co-Former Selection
6.1. Modern Computational Techniques
6.1.1. Molecular Descriptor and Partial Least Squares Regression (PLS)
6.1.2. Density Function Theory (DFT) and Quantum Mechanics
6.1.3. Molecular Docking
7. Process Evaluation
Principal Component Analysis (PCA)
8. Discussion
9. Conclusions
10. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adhikari, B.R.; Sinha, S.; Lyons, N.; Pletzer, D.; Lamont, I.; Gordon, K.C.; Das, S.C. Inhalable Ceftazidime-Roflumilast Powder Targeting Infection and Inflammation: Influence of Incorporating Roflumilast into Ceftazidime-Leucine Co-Amorphous Formulation. Eur. J. Pharm. Biopharm. 2022, 180, 260–268. [Google Scholar] [CrossRef]
- Han, J.; Zhang, C.; Zhang, Y.; Liu, X.; Wang, J. Mechanistic Insight into Gel Formation of Co-Amorphous Resveratrol and Piperine during Dissolution Process. Int. J. Pharm. 2023, 634, 122644. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.G.; Baldoni, H.A.; Dávila, Y.A.; Brusau, E.V.; Ellena, J.A.; Narda, G.E. Rational Design of a Famotidine-Ibuprofen Coamorphous System: An Experimental and Theoretical Study. J. Phys. Chem. B 2018, 122, 8772–8782. [Google Scholar] [CrossRef] [PubMed]
- Gniado, K.; Löbmann, K.; Rades, T.; Erxleben, A. The Influence of Co-Formers on the Dissolution Rates of Co-Amorphous Sulfamerazine/Excipient Systems. Int. J. Pharm. 2016, 504, 20–26. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Ueda, H.; Takata, Y.; Minamihata, K.; Wakabayashi, R.; Kamiya, N.; Goto, M. Co-Amorphous Formation of Piroxicam-Citric Acid to Generate Supersaturation and Improve Skin Permeation. Eur. J. Pharm. Sci. 2021, 158, 105667. [Google Scholar] [CrossRef]
- Di, R.; Liu, J.; Grohganz, H.; Rades, T. A Multivariate Approach for the Determination of the Optimal Mixing Ratio of the Non-Strong Interacting Co-Amorphous System Carvedilol-Tryptophan. Molecules 2021, 26, 801. [Google Scholar] [CrossRef] [PubMed]
- Moinuddin, S.M.; Ruan, S.; Huang, Y.; Gao, Q.; Shi, Q.; Cai, B.; Cai, T. Facile Formation of Co-Amorphous Atenolol and Hydrochlorothiazide Mixtures via Cryogenic-Milling: Enhanced Physical Stability, Dissolution and Pharmacokinetic Profile. Int. J. Pharm. 2017, 532, 393–400. [Google Scholar] [CrossRef]
- Lalge, R.; Kaur, N.; Duggirala, N.K.; Suryanarayanan, R. Dual Functionality of Bile Acid: Physical Stabilization of Drugs in the Amorphous Form and Solubility Enhancement in Solution. Mol. Pharm. 2022, 19, 2595–2606. [Google Scholar] [CrossRef]
- Tanida, S.; Yoshimoto, A.; Yoshida, M.; Uchiyama, H.; Kadota, K.; Tozuka, Y. Preparation of Amorphous Composite Particles of Drugs with Ursodeoxycholic Acid as Preclinical Formulations. Chem. Pharm. Bull. 2019, 67, 921–928. [Google Scholar] [CrossRef]
- Fael, H.; Demirel, A.L. Tannic Acid as a Co-Former in Co-Amorphous Systems: Enhancing Their Physical Stability, Solubility and Dissolution Behavior. Int. J. Pharm. 2020, 581, 119284. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, C.; Li, B.; Zhou, L.; Xu, R.; Guo, Y.; Cao, Y.; Cao, G.; Lu, S. A Novel Lurasidone Hydrochloride-Shikimic Acid Co-Amorphous System Formed by Hydrogen-Bonding Interaction with the Retained pH-Dependent Solubility Behavior. CrystEngComm 2020, 22, 5841–5853. [Google Scholar] [CrossRef]
- Wang, X.; Cao, J.; Li, Z.; Xu, R.; Guo, Y.; Pu, F.; Xiao, X.; Du, H.; He, J.; Lu, S. Co-Amorphous Mixture of Erlotinib Hydrochloride and Gallic Acid for Enhanced Antitumor Effects. J. Drug Deliv. Sci. Technol. 2024, 91, 105200. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.; Zhang, H.; Duan, Y.; Huang, Y. Sinomenine-Phenolic Acid Coamorphous Drug Systems: Solubilization, Sustained Release, and Improved Physical Stability. Int. J. Pharm. 2021, 598, 120389. [Google Scholar] [CrossRef]
- Han, J.; Wei, Y.; Li, L.; Song, Y.; Pang, Z.; Qian, S.; Zhang, J.; Gao, Y.; Heng, W. Gelation Elimination and Crystallization Inhibition by Co-Amorphous Strategy for Amorphous Curcumin. J. Pharm. Sci. 2023, 112, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ueda, H.; Löbmann, K.; Rades, T.; Grohganz, H. Organic Acids as Co-Formers for Co-Amorphous Systems—Influence of Variation in Molar Ratio on the Physicochemical Properties of the Co-Amorphous Systems. Eur. J. Pharm. Biopharm. 2018, 131, 25–32. [Google Scholar] [CrossRef]
- Shi, X.; Fan, B.; Gu, C.; Zhou, X.; Wang, C.; Ding, Z. Ibrutinib and Carboxylic Acid Coamorphous System with Increased Solubility and Dissolution: A Potential Interaction Mechanism. J. Drug Deliv. Sci. Technol. 2020, 59, 101875. [Google Scholar] [CrossRef]
- Mithu, M.S.H.; Ross, S.A.; Hurt, A.P.; Douroumis, D. Effect of Mechanochemical Grinding Conditions on the Formation of Pharmaceutical Cocrystals and Co-Amorphous Solid Forms of Ketoconazole—Dicarboxylic Acid. J. Drug Deliv. Sci. Technol. 2021, 63, 102508. [Google Scholar] [CrossRef]
- Zhang, M.; Xiong, X.; Suo, Z.; Hou, Q.; Gan, N.; Tang, P.; Ding, X.; Li, H. Co-Amorphous Palbociclib-Organic Acid Systems with Increased Dissolution Rate, Enhanced Physical Stability and Equivalent Biosafety. RSC Adv. 2019, 9, 3946–3955. [Google Scholar] [CrossRef]
- Rédai, E.M.; Sipos, E.; Vlad, R.A.; Antonoaea, P.; Todoran, N.; Ciurba, A. Development of Co-Amorphous Loratadine–Citric Acid Orodispersible Drug Formulations. Processes 2022, 10, 2722. [Google Scholar] [CrossRef]
- Wu, H.; Ma, J.; Qian, S.; Jiang, W.; Liu, Y.; Li, J.; Ke, Z.; Feng, K. Co-Amorphization of Posaconazole Using Citric Acid as an Acidifier and a Co-Former for Solubility Improvement. J. Drug Deliv. Sci. Technol. 2023, 80, 104136. [Google Scholar] [CrossRef]
- Furuishi, T.; Sato-Hata, N.; Fukuzawa, K.; Yonemochi, E. Characterization of Co-Amorphous Carvedilol–Maleic Acid System Prepared by Solvent Evaporation. Pharm. Dev. Technol. 2023, 28, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Wicaksono, Y.; Rosidi, V.A.; Saragih, S.Y.; Fauziah, L.S.; Setyawan, D. Preparation of Spray Dried Coamorphous Solids to Improve the Solubility and Dissolution Rate of Atorvastatin Calcium. J. Teknol. 2021, 83, 77–83. [Google Scholar] [CrossRef]
- Pešić, N.; Dapčević, A.; Ivković, B.; Kachrimanis, K.; Mitrić, M.; Ibrić, S.; Medarević, D. Potential Application of Low Molecular Weight Excipients for Amorphization and Dissolution Enhancement of Carvedilol. Int. J. Pharm. 2021, 608, 121033. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.; Song, Y.; Luo, M.; Hu, E.; Wei, Y.; Gao, Y.; Pang, Z.; Zhang, J.; Qian, S. Mechanistic Insights into the Crystallization of Coamorphous Drug Systems. J. Control Release 2023, 354, 489–502. [Google Scholar] [CrossRef]
- da Costa, N.F.; Santos, I.A.; Fernandes, A.I.; Pinto, J.F. Sulfonic Acid Derivatives in the Production of Stable Co-Amorphous Systems for Solubility Enhancement. J. Pharm. Sci. 2022, 111, 3327–3339. [Google Scholar] [CrossRef]
- Samipillai, M.; Mirmehrabi, M.; Rohani, S. Co-Amorphous Solids of Dasatinib and Olanzapine by Saccharin with Promising Physicochemical Properties. J. Drug Deliv. Sci. Technol. 2021, 66, 102800. [Google Scholar] [CrossRef]
- da Costa, N.F.; Fernandes, A.I.; Pinto, J.F. Measurement of the Amorphous Fraction of Olanzapine Incorporated in a Co-Amorphous Formulation. Int. J. Pharm. 2020, 588, 119716. [Google Scholar] [CrossRef]
- Costa, N.F.; Daniels, R.; Fernandes, A.I.; Pinto, J.F. Downstream Processing of Amorphous and Co-Amorphous Olanzapine Powder Blends. Pharmaceutics 2022, 14, 1535. [Google Scholar] [CrossRef]
- Costa, N.F.; Daniels, R.; Fernandes, A.I.; Pinto, J.F. Amorphous and Co-Amorphous Olanzapine Stability in Formulations Intended for Wet Granulation and Pelletization. Int. J. Mol. Sci. 2022, 23, 10234. [Google Scholar] [CrossRef]
- Ueda, H.; Hirakawa, Y.; Miyano, T.; Imono, M.; Tse, J.Y.; Uchiyama, H.; Tozuka, Y.; Kadota, K. Design of a Stable Coamorphous System Using Lactose as an Antiplasticizing Agent for Diphenhydramine Hydrochloride with a Low Glass Transition Temperature. Mol. Pharm. 2022, 19, 1209–1218. [Google Scholar] [CrossRef]
- Minecka, A.; Kamińska, E.; Tarnacka, M.; Jurkiewicz, K.; Talik, A.; Wolnica, K.; Dulski, M.; Kasprzycka, A.; Spychalska, P.; Garbacz, G.; et al. Does the Molecular Mobility and Flexibility of the Saccharide Ring Affect the Glass-Forming Ability of Naproxen in Binary Mixtures? Eur. J. Pharm. Sci. 2020, 141, 105091. [Google Scholar] [CrossRef] [PubMed]
- Suleiman Alsalhi, M.; Royall, P.G.; Al-Obaidi, H.; Alsalhi, A.; Cilibrizzi, A.; Chan, K.L.A. Non-Salt Based Co-Amorphous Formulation Produced by Freeze-Drying. Int. J. Pharm. 2023, 645, 123404. [Google Scholar] [CrossRef]
- Pérez-Carreón, K.; Martínez, L.M.; Videa, M.; Cruz-Angeles, J.; Gómez, J.; Ramírez, E. Effect of Basic Amino Acids on Folic Acid Solubility. Pharmaceutics 2023, 15, 2544. [Google Scholar] [CrossRef] [PubMed]
- Garbiec, E.; Rosiak, N.; Zalewski, P.; Tajber, L.; Cielecka-Piontek, J. Genistein Co-Amorphous Systems with Amino Acids: An Investigation into Enhanced Solubility and Biological Activity. Pharmaceutics 2023, 15, 2653. [Google Scholar] [CrossRef]
- Schütz, D.; Timmerhaus, A.; Grohganz, H. Wet Granulation of Co-Amorphous Indomethacin Systems. Int. J. Pharm. 2023, 644, 123318. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Rades, T. Solventless Amorphization and Pelletization Using a High Shear Granulator. Part II.; Preparation of Co-Amorphous Mixture-Layered Pellets Using Indomethacin and Arginine. Eur. J. Pharm. Biopharm. 2022, 181, 183–194. [Google Scholar] [CrossRef]
- Ojarinta, R.; Saarinen, J.; Strachan, C.J.; Korhonen, O.; Laitinen, R. Preparation and Characterization of Multi-Component Tablets Containing Co-Amorphous Salts: Combining Multimodal Non-Linear Optical Imaging with Established Analytical Methods. Eur. J. Pharm. Biopharm. 2018, 132, 112–126. [Google Scholar] [CrossRef]
- Wu, W.; Löbmann, K.; Rades, T.; Grohganz, H. On the Role of Salt Formation and Structural Similarity of Co-Formers in Co-Amorphous Drug Delivery Systems. Int. J. Pharm. 2018, 535, 86–94. [Google Scholar] [CrossRef]
- Khanfar, M.; Al-Remawi, M.; Al-Akayleh, F.; Hmouze, S. Preparation and Evaluation of Co-Amorphous Formulations of Telmisartan-Amino Acids as a Potential Method for Solubility and Dissolution Enhancement. AAPS PharmSciTech 2021, 22, 112. [Google Scholar] [CrossRef]
- Elkholy, N.E.; Sultan, A.A.; Elosaily, G.H.; El Maghraby, G.M. Acetone-Assisted Co-Processing of Meloxicam with Amino Acids for Enhanced Dissolution Rate. Pharm. Dev. Technol. 2020, 25, 882–891. [Google Scholar] [CrossRef]
- Wostry, M.; Plappert, H.; Grohganz, H. Preparation of Co-Amorphous Systems by Freeze-Drying. Pharmaceutics 2020, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Ojarinta, R.; Lerminiaux, L.; Laitinen, R. Spray Drying of Poorly Soluble Drugs from Aqueous Arginine Solution. Int. J. Pharm. 2017, 532, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Kassem, F.A.; Abdelaziz, A.E.; El Maghraby, G.M. Ethanol-Assisted Kneading of Apigenin with Arginine for Enhanced Dissolution Rate of Apigenin: Development of Rapidly Disintegrating Tablets. Pharm. Dev. Technol. 2021, 26, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Minode, M.; Kadota, K.; Kawabata, D.; Yoshida, M.; Shirakawa, Y. Enhancement in Dissolution Behavior and Antioxidant Capacity of Quercetin with Amino Acids Following Radical Formation via Mechanochemical Technique. Adv. Powder Technol. 2022, 33, 103582. [Google Scholar] [CrossRef]
- Ruponen, M.; Visti, M.; Ojarinta, R.; Laitinen, R. Permeability of Glibenclamide through a PAMPA Membrane: The Effect of Co-Amorphization. Eur. J. Pharm. Biopharm. 2018, 129, 247–256. [Google Scholar] [CrossRef]
- Ruponen, M.; Rusanen, H.; Laitinen, R. Dissolution and Permeability Properties of Co-Amorphous Formulations of Hydrochlorothiazide. J. Pharm. Sci. 2020, 109, 2252–2261. [Google Scholar] [CrossRef]
- Kasten, G.; Lobo, L.; Dengale, S.; Grohganz, H.; Rades, T.; Löbmann, K. In Vitro and in Vivo Comparison between Crystalline and Co-Amorphous Salts of Naproxen-Arginine. Eur. J. Pharm. Biopharm. 2018, 132, 192–199. [Google Scholar] [CrossRef]
- Kasten, G.; Duarte, Í.; Paisana, M.; Löbmann, K.; Rades, T.; Grohganz, H. Process Optimization and Upscaling of Spray-Dried Drug-Amino Acid Co-Amorphous Formulations. Pharmaceutics 2019, 11, 24. [Google Scholar] [CrossRef]
- Kasten, G.; Nouri, K.; Grohganz, H.; Rades, T.; Löbmann, K. Performance Comparison between Crystalline and Co-Amorphous Salts of Indomethacin-Lysine. Int. J. Pharm. 2017, 533, 138–144. [Google Scholar] [CrossRef]
- Gunnam, A.; Nangia, A.K. Solubility Improvement of Curcumin with Amino Acids. CrystEngComm 2021, 23, 3398–3410. [Google Scholar] [CrossRef]
- Silva, J.F.C.; Pereira Silva, P.S.; Ramos Silva, M.; Fantechi, E.; Chelazzi, L.; Ciattini, S.; Eusébio, M.E.S.; Rosado, M.T.S. Amorphous Solid Forms of Ranolazine and Tryptophan and Their Relaxation to Metastable Polymorphs. Cryst. Growth Des. 2023, 23, 6679–6691. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, K.; Rades, T.; Leopold, C.S. Co-Amorphous Systems Consisting of Indomethacin and the Chiral Co-Former Tryptophan: Solid-State Properties and Molecular Mobilities. Int. J. Pharm. 2023, 636, 122840. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, C.-M.; Rantanen, J.; Grohganz, H. Compaction Behavior of Co-Amorphous Systems. Pharmaceutics 2023, 15, 858. [Google Scholar] [CrossRef]
- Vasilev, N.A.; Voronin, A.P.; Surov, A.O.; Perlovich, G.L. Influence of Co-Amorphization on the Physical Stability and Dissolution Performance of an Anthelmintic Drug Flubendazole. Mol. Pharm. 2023, 20, 1657–1669. [Google Scholar] [CrossRef]
- Li, M.; Jin, Z.; Han, D.; Wu, S.; Gong, J. Co-Former Selection for Coamorphous Amino Acid/Spironolactone Formulations and Exploration of the Amorphization Kinetics of Systems. Cryst. Growth Des. 2023, 23, 1511–1521. [Google Scholar] [CrossRef]
- França, M.T.; Marcos, T.M.; Pereira, R.N.; Stulzer, H.K. Could the Small Molecules Such as Amino Acids Improve Aqueous Solubility and Stabilize Amorphous Systems Containing Griseofulvin? Eur. J. Pharm. Sci. 2020, 143, 105178. [Google Scholar] [CrossRef]
- Guinet, Y.; Paccou, L.; Hédoux, A. Mechanism for Stabilizing an Amorphous Drug Using Amino Acids within Co-Amorphous Blends. Pharmaceutics 2023, 15, 337. [Google Scholar] [CrossRef]
- Partheniadis, I.; Nikolakakis, I. Development and Characterization of Co-Amorphous Griseofulvin/L-Leucin by Modified Solvent Processing Hot-Melt Extrusion. Int. J. Pharm. 2024, 652, 123824. [Google Scholar] [CrossRef] [PubMed]
- Haneef, J.; Ali, S. Multicomponent Amorphous Solid Forms of Telmisartan: Insights into Mechanochemical Activation and Physicochemical Attributes. AAPS PharmSciTech 2024, 25, 84. [Google Scholar] [CrossRef]
- Mishra, J.; Löbmann, K.; Grohganz, H.; Rades, T. Influence of Preparation Technique on Co-Amorphization of Carvedilol with Acidic Amino Acids. Int. J. Pharm. 2018, 552, 407–413. [Google Scholar] [CrossRef]
- Liu, J.; Rades, T.; Grohganz, H. Determination of the Optimal Molar Ratio in Amino Acid-Based Coamorphous Systems. Mol. Pharm. 2020, 17, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Chishti, N.A.H.; Pathan, I.B.; Dehghan, M.H.G.; Bairagi, S.M. Design and Development of Immediate Release Pellets Formulation Containing Co Amorphous Mixture of Aceclofenac: In-Vitro and In-Vivo Study. J. Pharm. Innov. 2024, 19, 13. [Google Scholar] [CrossRef]
- Kitayama, A.; Kadota, K.; Fujioka, S.; Konishi, Y.; Uchiyama, H.; Tozuka, Y.; Shimosaka, A.; Yoshida, M.; Shirakawa, Y. Assessment of Amorphization Behavior of a Drug during Co-Grinding with an Amino Acid by Discrete Element Method Simulation. J. Ind. Eng. Chem. 2018, 62, 436–445. [Google Scholar] [CrossRef]
- Wu, W.; Grohganz, H.; Rades, T.; Löbmann, K. Comparison of Co-Former Performance in Co-Amorphous Formulations: Single Amino Acids, Amino Acid Physical Mixtures, Amino Acid Salts and Dipeptides as Co-Formers. Eur. J. Pharm. Sci. 2021, 156, 105582. [Google Scholar] [CrossRef]
- Wu, W.; Löbmann, K.; Schnitzkewitz, J.; Knuhtsen, A.; Pedersen, D.S.; Rades, T.; Grohganz, H. Dipeptides as Co-Formers in Co-Amorphous Systems. Eur. J. Pharm. Biopharm. 2019, 134, 68–76. [Google Scholar] [CrossRef]
- Wu, W.; Löbmann, K.; Schnitzkewitz, J.; Knuhtsen, A.; Pedersen, D.S.; Grohganz, H.; Rades, T. Aspartame as a Co-Former in Co-Amorphous Systems. Int. J. Pharm. 2018, 549, 380–387. [Google Scholar] [CrossRef]
- Shu, Y.; Jia, J.; Zhao, P.; Bao, R.; Li, W.; Yang, L.; Tang, X.; He, Z.; Fu, Q. Stoichiometric-Dependent Physical Stability of Atorvastatin-Lisinopril Co-Amorphous in Stress Testing. J. Ind. Eng. Chem. 2024, 139, 175–184. [Google Scholar] [CrossRef]
- Li, W.; Song, J.; Li, J.; Li, M.; Tian, B.; He, Z.; Liu, X.; Fu, Q. Co-Amorphization of Atorvastatin by Lisinopril as a Co-Former for Solubility Improvement. Int. J. Pharm. 2021, 607, 120971. [Google Scholar] [CrossRef]
- Oyama, S.; Ogawa, N.; Kawai, K.; Iwai, K.; Yasunaga, T.; Yamamoto, H. Improved Dissolution Properties of Co-Amorphous Probucol with Atorvastatin Calcium Trihydrate Prepared by Spray-Drying. Chem. Pharm. Bull. 2024, 72, 190–199. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, X.; Shen, S.; Chen, Q.; Song, S.; Gu, C.; Wang, C. Improved in Vitro and in Vivo Properties of Telmisartan in the Co-Amorphous System with Hydrochlorothiazide: A Potential Drug-Drug Interaction Mechanism Prediction. Eur. J. Pharm. Sci. 2021, 161, 105773. [Google Scholar] [CrossRef]
- Hatanaka, Y.; Uchiyama, H.; Kaneko, S.; Ueda, K.; Higashi, K.; Moribe, K.; Furukawa, S.; Takase, M.; Yamanaka, S.; Kadota, K.; et al. Designing a Novel Coamorphous Salt Formulation of Telmisartan with Amlodipine to Enhance Permeability and Oral Absorption. Mol. Pharm. 2023, 20, 4071–4085. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, M.; McArdle, P.; Erxleben, A. Dual-Drug Amorphous Formulation of Gliclazide. Drug Dev. Ind. Pharm. 2021, 47, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, M.; MacFhionnghaile, P.; McArdle, P.; Erxleben, A. Investigation of the Formation of Drug-Drug Cocrystals and Coamorphous Systems of the Antidiabetic Drug Gliclazide. Int. J. Pharm. 2019, 561, 35–42. [Google Scholar] [CrossRef]
- Fael, H.; Demirel, A.L. Indomethacin Co-Amorphous Drug-Drug Systems with Improved Solubility, Supersaturation, Dissolution Rate and Physical Stability. Int. J. Pharm. 2021, 600, 120448. [Google Scholar] [CrossRef] [PubMed]
- Di, R.; Rades, T.; Grohganz, H. Destabilization of Indomethacin-Paracetamol Co-Amorphous Systems by Mechanical Stress. Pharmaceutics 2024, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, R.; Waraya, H.; Hirakura, Y. Application of Co-Amorphous Technology for Improving the Physicochemical Properties of Amorphous Formulations. Mol. Pharm. 2019, 16, 2142–2152. [Google Scholar] [CrossRef]
- Pardhi, E.; Tomar, D.S.; Khemchandani, R.; Samanthula, G.; Singh, P.K.; Mehra, N.K. Design, Development and Characterization of the Apremilast and Indomethacin Coamorphous System. J. Mol. Struct. 2024, 1299, 137045. [Google Scholar] [CrossRef]
- Queiroz, L.H.S.; Barros, R.S.; de Sousa, F.F.; Lage, M.R.; Sarraguça, M.C.; Ribeiro, P.R.S. Preparation and Characterization of a Rifampicin Coamorphous Material with Tromethamine Coformer: An Experimental-Theoretical Study. Mol. Pharm. 2024, 21, 1272–1284. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, S.; Jiang, Y.; Grohganz, H.; Rades, T. Salt Hydrates as a Source to Form Co-Amorphous Systems When Prepared in the Absence of Water: Hydrogen Bond Analysis. Chem. Eng. Sci. 2024, 296, 120232. [Google Scholar] [CrossRef]
- Shete, S.; Reddy, S.C.; Lakshman, Y.D.; Vullendula, S.K.A.; Mehta, C.H.; Nayak, U.Y.; Dengale, S. Implications of Phase Solubility/Miscibility and Drug-Rich Phase Formation on the Performance of Co-Amorphous Materials: The Case of Darunavir Co-Amorphous Materials with Ritonavir and Indomethacin as Co-Formers. Int. J. Pharm. 2021, 608, 121119. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.; Zhang, H.; Duan, Y.; Huang, Y. Co-Amorphous Systems of Sinomenine with Platensimycin or Sulfasalazine: Physical Stability and Excipient-Adjusted Release Behavior. Mol. Pharm. 2022, 19, 4370–4381. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.; Zhang, H.; Duan, Y.; Huang, Y. Co-Amorphous Systems of Sinomenine with Nonsteroidal Anti-Inflammatory Drugs: A Strategy for Solubility Improvement, Sustained Release, and Drug Combination Therapy against Rheumatoid Arthritis. Int. J. Pharm. 2021, 606, 120894. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, D.; Duan, Y.; Huang, Y. Characterization of Co-Amorphous Sinomenine-Tranilast Systems with Strong Intermolecular Interactions and Sustained Release Profiles. J. Drug Deliv. Sci. Technol. 2022, 71, 103296. [Google Scholar] [CrossRef]
- Ueda, H.; Kadota, K.; Imono, M.; Ito, T.; Kunita, A.; Tozuka, Y. Co-Amorphous Formation Induced by Combination of Tranilast and Diphenhydramine Hydrochloride. J. Pharm. Sci. 2017, 106, 123–128. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Feng, Y.; Yuan, D.; Xu, R.; Jiang, C.; Xiao, X.; Lu, S. Design and Molecular Insights of Drug-Active Metabolite Based Co-Amorphous Formulation: A Case Study of Toltrazuril-Ponazuril Co-Amorphous. Int. J. Pharm. 2022, 615, 121475. [Google Scholar] [CrossRef]
- Ueda, H.; Peter Bøtker, J.; Edinger, M.; Löbmann, K.; Grohganz, H.; Müllertz, A.; Rades, T.; Østergaard, J. Formulation of Co-Amorphous Systems from Naproxen and Naproxen Sodium and in Situ Monitoring of Physicochemical State Changes during Dissolution Testing by Raman Spectroscopy. Int. J. Pharm. 2020, 587, 119662. [Google Scholar] [CrossRef]
- Mannava, M.K.C.; Suresh, K.; Bommaka, M.K.; Konga, D.B.; Nangia, A. Curcumin-Artemisinin Coamorphous Solid: Xenograft Model Preclinical Study. Pharmaceutics 2018, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Kissi, E.O.; Khorami, K.; Rades, T. Determination of Stable Co-Amorphous Drug–Drug Ratios from the Eutectic Behavior of Crystalline Physical Mixtures. Pharmaceutics 2019, 11, 628. [Google Scholar] [CrossRef]
- Wang, R.; Han, J.; Jiang, A.; Huang, R.; Fu, T.; Wang, L.; Zheng, Q.; Li, W.; Li, J. Involvement of Metabolism-Permeability in Enhancing the Oral Bioavailability of Curcumin in Excipient-Free Solid Dispersions Co-Formed with Piperine. Int. J. Pharm. 2019, 561, 9–18. [Google Scholar] [CrossRef]
- Han, J.; Li, L.; Yu, Q.; Zheng, D.; Song, Y.; Zhang, J.; Gao, Y.; Heng, W.; Qian, S.; Pang, Z. Self-Gelation Involved in the Transformation of Resveratrol and Piperine from a Co-Amorphous System into a Co-Crystal System. Cryst. Eng. Comm. 2022, 24, 5733–5747. [Google Scholar] [CrossRef]
- Hu, D.; Chen, X.; Li, D.; Zhang, H.; Duan, Y.; Huang, Y. Tranilast-Matrine Co-Amorphous System: Strong Intermolecular Interactions, Improved Solubility, and Physiochemical Stability. Int. J. Pharm. 2023, 635, 122707. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Chen, X.; Li, D.; Zhang, H.; Duan, Y.; Huang, Y. Sustained Release of Co-Amorphous Matrine-Type Alkaloids and Resveratrol with Anti-COVID-19 Potential. Pharmaceutics 2022, 14, 603. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hu, Y.; Guo, Y.; Xu, R.; Fang, X.; Xiao, X.; Jiang, C.; Lu, S. Coamorphous System of Florfenicol-Oxymatrine for Improving the Solubility and Dissolution Rate of Florfenicol: Preparation, Characterization and Molecular Dynamics Simulation. J. Pharm. Sci. 2021, 110, 2544–2554. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Li, X.; Yuan, D.; Cheng, H.; Ke, Y.; Cheng, J.; Wang, Z.; Chen, J.; Li, J. Co-Amorphous Systems Using Epigallocatechin-3-Gallate as a Co-Former: Stability, in Vitro Dissolution, in Vivo Bioavailability and Underlying Molecular Mechanisms. Eur. J. Pharm. Biopharm. 2022, 178, 82–93. [Google Scholar] [CrossRef]
- Huang, P.; Yan, H.; Wu, H.; Liu, L.; Niu, W.; Zhai, B.; Hu, Z.; Li, J.; Du, Q.; Zhou, Y. Application of Curcumin as a Co-Former and an Efflux Inhibitor in Paclitaxel Co-Amorphous Mixture. J. Drug Deliv. Sci. Technol. 2023, 84, 104513. [Google Scholar] [CrossRef]
- Prajapati, B.; Maji, I.; Kumar, R.; Tomar, D.; Khatri, D.K.; Madan, J.; Singh, P.K. Strategy to Counteract the Pyrazinamide Induced Hepatotoxicity by Developing Naringin Based Co-Amorphous System with Supplementary Benefits. J. Drug Deliv. Sci. Technol. 2022, 69, 103181. [Google Scholar] [CrossRef]
- Pardhi, E.; Tomar, D.S.; Khemchandani, R.; Bazaz, M.R.; Dandekar, M.P.; Samanthula, G.; Singh, S.B.; Mehra, N.K. Monophasic Coamorphous Sulpiride: A Leap in Physicochemical Attributes and Dual Inhibition of GlyT1 and P-Glycoprotein, Supported by Experimental and Computational Insights. J. Biomol. Struct. Dyn. 2025, 43, 4297–4326. [Google Scholar] [CrossRef]
- Uppala, S.; Vullendula, S.K.A.; Yarlagadda, D.L.; Dengale, S.J. Exploring the Utility of Co-Amorphous Materials to Concurrently Improve the Solubility and Permeability of Fexofenadine. J. Drug Deliv. Sci. Technol. 2022, 72, 103431. [Google Scholar] [CrossRef]
- KS, N.S.; Dengale, S.J.; Mutalik, S.; Bhat, K. Raloxifene HCl–Quercetin Co-Amorphous System: Preparation, Characterization, and Investigation of Its Behavior in Phosphate Buffer. Drug Dev. Ind. Pharm. 2022, 48, 227–238. [Google Scholar]
- Liu, X.; Shen, L.; Zhou, L.; Wu, W.; Liang, G.; Zhao, Y.; Wu, W. Nucleotides as New Co-Formers in Co-Amorphous Systems: Enhanced Dissolution Rate, Water Solubility and Physical Stability. Eur. J. Pharm. Biopharm. 2024, 200, 114333. [Google Scholar] [CrossRef]
- Liu, J.; Rades, T.; Tho, I.; Kissi, E.O. Functionalised Calcium Carbonate as a Coformer to Stabilize Amorphous Drugs by Mechanochemical Activation. Eur. J. Pharm. Biopharm. 2020, 155, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.R.; Sinha, S.; Gordon, K.C.; Das, S.C. Amino Acids Improve Aerosolization and Chemical Stability of Potential Inhalable Amorphous Spray-Dried Ceftazidime for Pseudomonas Aeruginosa Lung Infection. Int. J. Pharm. 2022, 621, 121799. [Google Scholar] [CrossRef]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Development and Characterization of a Spray-Dried Inhalable Ciprofloxacin-Quercetin Co-Amorphous System. Int. J. Pharm. 2022, 618, 121657. [Google Scholar] [CrossRef]
- Gabelmann, A.; Lehr, C.-M.; Grohganz, H. Preparation of Co-Amorphous Levofloxacin Systems for Pulmonary Application. Pharmaceutics 2023, 15, 1574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Grohganz, H.; Rades, T. Effects of Polymer Addition on the Non-Strongly Interacting Binary Co-Amorphous System Carvedilol-Tryptophan. Int. J. Pharm. 2022, 617, 121625. [Google Scholar] [CrossRef]
- Liu, J.; Grohganz, H.; Rades, T. Influence of Polymer Addition on the Amorphization, Dissolution and Physical Stability of Co-Amorphous Systems. Int. J. Pharm. 2020, 588, 119768. [Google Scholar] [CrossRef] [PubMed]
- Iemtsev, A.; Zemánková, A.; Hassouna, F.; Mathers, A.; Klajmon, M.; Slámová, M.; Malinová, L.; Fulem, M. Ball Milling and Hot-Melt Extrusion of Indomethacin–L-Arginine–Vinylpyrrolidone-Vinyl Acetate Copolymer: Solid-State Properties and Dissolution Performance. Int. J. Pharm. 2022, 613, 121424. [Google Scholar] [CrossRef]
- El-Maradny, H.; Saab, M. Spray-Dried Co-Amorphous Tadalafil Ternary Mixtures: A Promising Platform towards the Enhancement of Solubility and Bioavailability. Braz. J. Pharm. Sci. 2022, 58, 20622. [Google Scholar]
- Fang, X.; Hu, Y.; Yang, G.; Shi, W.; Lu, S.; Cao, Y. Improving Physicochemical Properties and Pharmacological Activities of Ternary Co-Amorphous Systems. Eur. J. Pharm. Biopharm. 2022, 181, 22–35. [Google Scholar] [CrossRef]
- Kasten, G.; Grohganz, H.; Rades, T.; Löbmann, K. Development of a Screening Method for Co-Amorphous Formulations of Drugs and Amino Acids. Eur. J. Pharm. Sci. 2016, 95, 28–35. [Google Scholar] [CrossRef]
- Kasten, G.; Löbmann, K.; Grohganz, H.; Rades, T. Co-Former Selection for Co-Amorphous Drug-Amino Acid Formulations. Int. J. Pharm. 2019, 557, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Ruponen, M.; Kettunen, K.; Pires, M.S.; Laitinen, R. Co-Amorphous Formulations of Furosemide with Arginine and p-Glycoprotein Inhibitor Drugs. Pharmaceutics 2021, 13, 171. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Liu, Y.; Ouyang, R.; Liu, M.; Han, D.; Gong, J. Co-Amorphization Story of Furosemide-Amino Acid Systems: Protonation and Aromatic Stacking Insights for Promoting Compatibility and Stability. Cryst. Growth Des. 2021, 21, 3280–3289. [Google Scholar] [CrossRef]
- Deng, Y.; Deng, W.; Huang, W.; Zheng, Z.; Zhang, R.; Liu, S.; Jiang, Y. Norfloxacin Co-Amorphous Salt Systems: Effects of Molecular Descriptors on the Formation and Physical Stability of Co-Amorphous Systems. Chem. Eng. Sci. 2022, 253, 117549. [Google Scholar] [CrossRef]
- Meng-Lund, H.; Kasten, G.; Jensen, K.T.; Poso, A.; Pantsar, T.; Rades, T.; Rantanen, J.; Grohganz, H. The Use of Molecular Descriptors in the Development of Co-Amorphous Formulations. Eur. J. Pharm. Sci. 2018, 119, 31–38. [Google Scholar] [CrossRef]
- Chambers, L.I.; Grohganz, H.; Palmelund, H.; Löbmann, K.; Rades, T.; Musa, O.M.; Steed, J.W. Predictive Identification of Co-Formers in Co-Amorphous Systems. Eur. J. Pharm. Sci. 2021, 157, 105636. [Google Scholar] [CrossRef]
- Chambers, L.I.; Musa, O.M.; Steed, J.W. Prediction and Preparation of Coamorphous Phases of a Bislactam. Mol. Pharm. 2022, 19, 2651–2661. [Google Scholar] [CrossRef] [PubMed]
- Fink, E.; Brunsteiner, M.; Mitsche, S.; Schröttner, H.; Paudel, A.; Zellnitz-Neugebauer, S. Data-Driven Prediction of the Formation of Co-Amorphous Systems. Pharmaceutics 2023, 15, 347. [Google Scholar] [CrossRef]
- Deng, Y.; Luo, W.; Zheng, Z.; Wei, G.; Liu, S.; Jiang, Y.; Yang, H. Prediction of Co-Amorphous Formation Using Non-Bonded Interaction Energy: Molecular Dynamic Simulation and Experimental Validation. Chem. Eng. Sci. 2023, 272, 118618. [Google Scholar] [CrossRef]
- Silva, J.F.C.; Rosado, M.T.S.; Eusébio, M.E.S. Structure and Energetics of Intermolecular Association in Two Lurasidone Co-Amorphous Drug Systems. J. Mol. Struc. 2021, 1242, 130709. [Google Scholar] [CrossRef]
- Han, J.; Yang, Y.; Hou, Y.; Tang, M.; Zhang, Y.; Zhu, Y.; Liu, X.; Wang, J.; Gao, Y. Insight into Formation, Synchronized Release and Stability of Co-Amorphous Curcumin-Piperine by Integrating Experimental-Modeling Techniques. J. Pharm. Sci. 2024, 113, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, D.L.; Anand, V.S.K.; Nair, A.R.; Dengale, S.J.; Pandiyan, S.; Mehta, C.H.; Manandhar, S.; Nayak, U.Y.; Bhat, K. A Computational-Based Approach to Fabricate Ceritinib Co-Amorphous System Using a Novel Co-Former Rutin for Bioavailability Enhancement. Eur. J. Pharm. Biopharm. 2023, 190, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Garbiec, E.; Rosiak, N.; Tykarska, E.; Zalewski, P.; Cielecka-Piontek, J. Sinapic Acid Co-Amorphous Systems with Amino Acids for Improved Solubility and Antioxidant Activity. Int. J. Mol. Sci. 2023, 24, 5533. [Google Scholar] [CrossRef]
- Ekawa, B.; Diogo, H.P.; Castro, R.A.E.; Caires, F.J.; Eusébio, M.E.S. Coamorphous Systems of Valsartan: Thermal Analysis Contribution to Evaluate Intermolecular Interactions Effects on the Structural Relaxation. Molecules 2023, 28, 6240. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, H.; Babu, S.; Garad, S. Co-Amorphous Formation of High-Dose Zwitterionic Compounds with Amino Acids To Improve Solubility and Enable Parenteral Delivery. Mol. Pharm. 2018, 15, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Huang, Q.; Li, X.; Liu, X.; Yin, L.; Zhao, Y.; Liang, G.; Wu, W. Dissolution Changes in Drug-Amino Acid/Biotin Co-Amorphous Systems: Decreased/Increased Dissolution during Storage without Recrystallization. Eur. J. Pharm. Sci. 2023, 188, 106526. [Google Scholar] [CrossRef]
- Adhikari, B.R.; Bērziņš, K.; Fraser-Miller, S.J.; Gordon, K.C.; Das, S.C. Co-Amorphization of Kanamycin with Amino Acids Improves Aerosolization. Pharmaceutics 2020, 12, 715. [Google Scholar] [CrossRef]

| Co-Former | Type |
|---|---|
| cholic acid, ursodeoxy cholic acid, deoxycholic acid | bile salt |
| tannic acid, shikimic acid, gallic acid, salicylic acid, 2,3-dihydroxy benzoic acid, 2,4-dihydroxy benzoic acid, citric acid, malic acid, tartaric acid, succinic acid, fumaric acid | organic acids |
| lactose, acetylated glucose | saccharides |
| arginine, lysine, tryptophan, leucine, glutamic acid, aspartic acid, cysteine | amino acids |
| piperine, sophoridine, matrine, oxymatrine | alkaloids |
| epigallocatechin-3-gallate, curcumin | polyphenols |
| naringin, quercetin | flavonoids |
| adenosine monophosphate and diphosphate | nucleotides |
| functionalized calcium carbonate (FCC) | miscellaneous |
| Co-Amorphous System | Preparation Method and Molar Ratio | Dissolution Characteristics | Physical Stability |
|---|---|---|---|
| Atorvastatin-lisinopril [67] | Cryo-milling 1:4,1:2, 1:1, 2:1 | 1:2 and 1:1 higher than 2:1 and 4:1. | - |
| Atorvastatin-lisinopril [68] | Cryo-milling 1:4, 1:2, 1:1, 2:1 | - 1:2 and 1:1 had a higher by 1.6 times and 2.7 times than amorphous and crystalline atorvastatin. - 2:1 and 4:1 had slow dissolution rate. | 1:2 and 1:1 at (40 °C) and had lower moisture absorption than amorphous drugs and dissolution rate did not decrease within 10 days of storage. |
| Atorvastatin-probucol [69] | Spray drying 1:1 | - | 1:1 for 35 days at 40 °C/75% RH. |
| Atorvastatin-isonicotinamide [22] | Spray drying 1:1 | Solubility and dissolution of atorvastatin were increased by 2- and 1.94-fold, respectively. | - |
| Telmisartan-hydrochlorothiazide [70] | Solvent evaporation 1:1, 1:2, 1:3, 2:3 | 1:3 had 10 times higher than crystalline telmisartan. | 1:3 for 180 days at 25 °C, 90 days at 40 °C, and 30 days at 60 °C at 40 °C and 30 days at 60 °C. |
| Telmisartan-amlodipine [71] | Ball milling 3:1, 2:1, 1:1, 1:2, 1:3 | 1:1 had a faster dissolution rate. | - |
| Atenolol-hydrochlorothiazide [7] | Cryogenic milling 2:1, 1:1, 1:2 | - Intrinsic dissolution rate of 1:1 is higher than physical mixture and crystalline hydrochlorothiazide by 2.2- and 12.5-fold, respectively. - Molar ratio 1:1 improved Cmax physical mixture and crystalline hydrochlorothiazide by 1.7 and 7.3, and AUC(0–24h) by 1.4 and 2.6, respectively. | 1:1 for 30 days at 4 and 25 °C. |
| - Gliclazide-valsartan - Gliclazide-valsartan—α-lactose monohydrate - Gliclazide-valsartan-microcrystalline cellulose [72] | - Binary system:ball milling (1:1) - Ternary systems: cryomilling (1:2) | Gliclazide-valsartan-α-lactose monohydrate has a dissolution release percentage higher than 80%. | - Binary systems (1:1) and (1:2) stay amorphous for 4 months at 20 °C/65% RH. - Gliclazide-valsartan-α-lactose monohydrate was recrystallized within 2 months. - Gliclazide-valsartan-microcrystalline cellulose stayed amorphous for 2 months. |
| - Gliclazide-hydrochlorothiazide - Gliclazide- triamterene [73] | Ball milling 5:1, 1:1, 1:5 | - 1:1 no improvement in dissolution rate - Gliclazide-triamterene with taurocholic acid sodium improved the dissolution of triamterene. | 1:1 for 6 months at 25 °C/56% RH. |
| Indomethacin-paracetamol [74] | Quench cooling 2:1, 1:1, 1:2 | The intrinsic dissolution rate of 2:1 was increased. | 2:1 for 7 and 3 months at 4 and 40 °C. |
| Indomethacin-paracetamol [75] | Quench cooling 10–90% and 90–10% | - | 50:50 for 9 weeks. |
| Indomethacin-felodipine [76] | Melting method 5:1, 3:1, 1:3, 1:1, 1:5 | - | 1:1 had the highest stability while 5:1 and 1:5 had the lowest stability at 40 °C. |
| Indomethacin-apremilast [77] | Quench cooling 1:1 | - Solubility increased by 1.64- and 1.85-fold. - Dissolution increased by 14.3- and 49.47-fold. - Drug flux increased by 8.15- and 5.82-fold. | 5 months at 40 °C/75% RH. |
| Rifimpacin-tromethacin [78] | Solvent evaporation 3:1, 2:1, 1:1, 1:2, 1:3 | 3.5 and 2 folds higher than crystalline form at 30 and 60 min. | 2:1 for 180 days at 40 °C/75% RH. |
| - Gefitinib-bumethanide - Gefitinib-uresemide [79] | - Solvent evaporation - Neat ball milling - Liquid-assisted grinding - Quench cooling (1:1) | 2.3 and 3.8 folds higher than crystalline gefitinib. | For 15 months at dry conditions (0 °C and 40 °C) and humid conditions (25 °C/60% RH). |
| Famotidine-ibuprofen [3] | Cryo-milling 3:7, 1:1, 7:3 | - | 1:1 for 60 days at 4, 25 and 40 °C. |
| - Darunavir-ritonavir - Darunavir-indomethacin [80] | Quench cooling 2:1, 1:1, 1:2 | higher than crystalline darunavir and lower than amorphous darunavir. | - |
| - Sinomenine-suphasalazine - Sinomenine-platensimycin [81] | Reduced pressure evaporation | Lower by 20%. | Both were stable for 6 months at 25 °C/75% RH. |
| - Sinomenine-sulindac - Sinomenine-indomethacin - Sinomenine-naproxen [82] | Solvent evaporation 1:1 | The dissolution release percent of sinomenin in all co-amorphous preparations was lower than in crystalline drugs. | All co-amorphous systems had good stability for 4 months at 25 °C/75% RH. |
| Sinomenine-tranilast [83] | Solvent evaporation 2:1, 1:1, 1:2 | - Sinomenin in all co-amorphous preparations exhibited sustained release behaviour. - Tranilast in 1:1 and 1:2 co-amorphous preparations was lower than crystalline tranilast and 2:1. | All saturated with sodium chloride for 6 months at 25 °C/75%RH. |
| Tranilast-diphenyldramine [84] | Grinding (mortar and pestle) 2:1, 1:1, 1:2 | - | For 30 days at 40 °C. |
| Tolmisartan-ponazural [85] | Solvent evaporation 1:1 | Higher than crystalline telmisartan. | The co-amorphous system remained stable for 30 days at 40 °C/75% RH. |
| Naproxone-naproxone sodium [86] | Ball milling 2:1, 1:1, 1:2 | Higher by 2.875-fold over pure naproxone. | 1:1 for 2 months at 40 °C. |
| Curcumin-artemisinin [87] | Solvent evaporation 1:1 | Higher solubility and dissolution release than curcumin. The co-amorphous system had two-fold higher bioavailability than the co-crystal system of curcumin-pyrogallol. | - |
| - Indomethacin-nifedipine - Nifedipine-paracetamol - Paracetamol-celecoxib [88] | Quench cooling different molar ratios | - | - Indomethacin-nifedipine (40% and 60%) for 31–38 days. - Nifedipine-paracetamol (30–50%) for 13–20 days. - Paracetamol-celecoxib (50–50%) for 86–114 days |
| Selection Criteria | Properties | Identification Tool |
|---|---|---|
| Drug and co-former molecular interaction | Ability to form ionic interaction or hydrogen or π–π bond | Density function theory and molecular docking |
| Physiochemical and thermal properties | Hansen solubility parameter, molecular weight, LogP, pKa, mixing enthalpy difference (ΔHmix), Van der Waals molecular volume, Van der Waals surface area of the molecules, number of donors and acceptors of hydrogen bond | Molecular descriptor combined with partial least squares regression (PLS) |
| Pharmacological properties | Compatibility, synergistic effect, and toxicity | Literature review and in silico modeling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, A.A.K.; Regdon, G., Jr.; Kristó, K. Cutting-Edge Approaches in the Co-Amorphization Process. Pharmaceutics 2025, 17, 850. https://doi.org/10.3390/pharmaceutics17070850
Mahmoud AAK, Regdon G Jr., Kristó K. Cutting-Edge Approaches in the Co-Amorphization Process. Pharmaceutics. 2025; 17(7):850. https://doi.org/10.3390/pharmaceutics17070850
Chicago/Turabian StyleMahmoud, Azza A. K., Géza Regdon, Jr., and Katalin Kristó. 2025. "Cutting-Edge Approaches in the Co-Amorphization Process" Pharmaceutics 17, no. 7: 850. https://doi.org/10.3390/pharmaceutics17070850
APA StyleMahmoud, A. A. K., Regdon, G., Jr., & Kristó, K. (2025). Cutting-Edge Approaches in the Co-Amorphization Process. Pharmaceutics, 17(7), 850. https://doi.org/10.3390/pharmaceutics17070850






