Insights on Natural Membrane Characterization for the Rational Design of Biomimetic Drug Delivery Systems
Abstract
1. Introduction
2. Cell Membrane Characteristics
2.1. Lipid Composition
2.2. Protein Composition
2.3. Lipid–Protein Interaction
2.4. Physical Characteristics
2.4.1. Atomic Force Microscopy (AFM)
2.4.2. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
2.4.3. Small Angle and Wide Angle X-Ray Scattering
3. Extracellular Vesicles: Membrane Characteristics
4. Development of Exosome-Mimetic Drug Delivery Systems
5. Conclusions
Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| α-syn | α-synuclein |
| AFM | atomic force microscopy |
| BSE | back-scatter electron |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| chol | cholesterol |
| CHS | cholesteryl succinate |
| DC-chol | 3-(N-(N′,N’-dimethylaminoethane)carbamoyl)cholesterol |
| DGK | diacylglycerol kinase |
| DHP | dihexadecyl phosphate |
| DLPC | 1,2-dilauroyl-sn-glycero-3-phosphatidylcholine |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine |
| DOPE | 1,2-dioleoyl-sn-glycero-3-phosphatidylethanolamine |
| DOPG | 1,2-dioleoyl-sn-glycero-3-phosphoglycerol |
| DOPS | 1,2-dioleoyl-sn-glycero-3-phosphatidylserine |
| DOTAP | 1,2-dioleoyl-3-trimethylammonium-propane |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphatidylcholine |
| DSPS | 1,2-distearoyl-sn-glycero-3-phosphatidylserine |
| EE% | encapsulation efficiency |
| EMN | exosome-mimetic nanosystem |
| EV | extracellular vesicle |
| Fb | breakthrough force |
| GPMV | giant plasma membrane vesicle |
| HEV | Hepatitis E virus |
| His-pDNA | Pigment Epithelium-Derived Factor genes linked with a histone |
| LUV | large unilamellar vesicle |
| NP | nanoparticle |
| NSAID | nonsteroidal anti-inflammatory drug |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PG | phosphatidylglycerol |
| PI | phosphatidylinositol |
| PS | phosphatidylserine |
| PSPD | position-sensitive photo diode |
| SAXS | small angle X-ray scattering |
| SE | secondary electron |
| SEM | scanning electron microscopy |
| siVEGF | vascular endothelial growth factor-targeting siRNA |
| SM | sphingomyelin |
| TEM | transmission electron microscopy |
| VEGF | vascular endothelial growth factor |
| WAXS | wide angle X-ray scattering |
References
- Mishra, S.; Khurana, M. A Snapshot Review: In Vitro Characterization of Lipid Membranes and Their Applications. MRS Adv. 2022, 7, 551–561. [Google Scholar] [CrossRef]
- Bechtel, T.J.; Reyes-Robles, T.; Fadeyi, O.O.; Oslund, R.C. Strategies for Monitoring Cell–Cell Interactions. Nat. Chem. Biol. 2021, 17, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Bolla, J.R.; Agasid, M.T.; Mehmood, S.; Robinson, C.V. Membrane Protein–Lipid Interactions Probed Using Mass Spectrometry. Annu. Rev. Biochem. 2019, 88, 85–111. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef]
- Tan, C.; Wang, J.; Sun, B. Biopolymer-Liposome Hybrid Systems for Controlled Delivery of Bioactive Compounds: Recent Advances. Biotechnol. Adv. 2021, 48, 107727. [Google Scholar] [CrossRef]
- Rodriguez, P.L.; Harada, T.; Christian, D.A.; Pantano, D.A.; Tsai, R.K.; Discher, D.E. Minimal “Self” Peptides That Inhibit Phagocytic Clearance and Enhance Delivery of Nanoparticles. Science 2013, 339, 971–975. [Google Scholar] [CrossRef]
- Reading, E.; Hall, Z.; Martens, C.; Haghighi, T.; Findlay, H.; Ahdash, Z.; Politis, A.; Booth, P.J. Interrogating Membrane Protein Conformational Dynamics within Native Lipid Compositions. Angew. Chem. Int. Ed. 2017, 56, 15654–15657. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the Diversity of Membrane Lipid Composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Shimizu, T. Lipid Mediators in Health and Disease: Enzymes and Receptors as Therapeutic Targets for the Regulation of Immunity and Inflammation. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [Google Scholar] [CrossRef]
- Saliba, A.-E.; Vonkova, I.; Gavin, A.-C. The Systematic Analysis of Protein–Lipid Interactions Comes of Age. Nat. Rev. Mol. Cell Biol. 2015, 16, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Resh, M.D. Fatty Acylation of Proteins: The Long and the Short of It. Prog. Lipid Res. 2016, 63, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Hoejholt, K.L.; Mužić, T.; Jensen, S.D.; Dalgaard, L.T.; Bilgin, M.; Nylandsted, J.; Heimburg, T.; Frandsen, S.K.; Gehl, J. Calcium Electroporation and Electrochemotherapy for Cancer Treatment: Importance of Cell Membrane Composition Investigated by Lipidomics, Calorimetry and in Vitro Efficacy. Sci. Rep. 2019, 9, 4758. [Google Scholar] [CrossRef] [PubMed]
- Berlin, E.; Lork, A.A.; Bornecrantz, M.; Ernst, C.; Phan, N.T.N. Lipid Organization and Turnover in the Plasma Membrane of Human Differentiating Neural Progenitor Cells Revealed by Time-of-Flight Secondary Ion Mass Spectrometry Imaging. Talanta 2024, 272, 125762. [Google Scholar] [CrossRef]
- Lamari, F.; Mochel, F.; Sedel, F.; Saudubray, J.M. Disorders of Phospholipids, Sphingolipids and Fatty Acids Biosynthesis: Toward a New Category of Inherited Metabolic Diseases. J. Inherit. Metab. Dis. 2013, 36, 411–425. [Google Scholar] [CrossRef]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid Classification, Structures and Tools. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2011, 1811, 637–647. [Google Scholar] [CrossRef]
- Putta, P.; Rankenberg, J.; Korver, R.A.; Van Wijk, R.; Munnik, T.; Testerink, C.; Kooijman, E.E. Phosphatidic Acid Binding Proteins Display Differential Binding as a Function of Membrane Curvature Stress and Chemical Properties. Biochim. Biophys. Acta BBA Biomembr. 2016, 1858, 2709–2716. [Google Scholar] [CrossRef]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid Composition of the Cancer Cell Membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Phosphatidylinositol-3,4,5-Triphosphate and Cellular Signaling: Implications for Obesity and Diabetes. Cell. Physiol. Biochem. 2015, 35, 1253–1275. [Google Scholar] [CrossRef]
- Yeagle, P.L. Chapter 3—Biogenesis of Membrane Lipids. In The Membranes of Cells, 3rd ed.; Yeagle, P.L., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 57–71. ISBN 978-0-12-800047-2. [Google Scholar]
- Birge, R.B.; Boeltz, S.; Kumar, S.; Carlson, J.; Wanderley, J.; Calianese, D.; Barcinski, M.; Brekken, R.A.; Huang, X.; Hutchins, J.T.; et al. Phosphatidylserine Is a Global Immunosuppressive Signal in Efferocytosis, Infectious Disease, and Cancer. Cell Death Differ. 2016, 23, 962–978. [Google Scholar] [CrossRef]
- Segawa, K.; Nagata, S. An Apoptotic ‘Eat Me’ Signal: Phosphatidylserine Exposure. Trends Cell Biol. 2015, 25, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Pulica, R.; Aquib, A.; Varsanyi, C.; Gadiyar, V.; Wang, Z.; Frederick, T.; Calianese, D.C.; Patel, B.; De Dios, K.V.; Poalasin, V.; et al. Dys-Regulated Phosphatidylserine Externalization as a Cell Intrinsic Immune Escape Mechanism in Cancer. Cell Commun. Signal. 2025, 23, 131. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Kanwar, S.S. Phosphatidylserine: A Cancer Cell Targeting Biomarker. Semin. Cancer Biol. 2018, 52, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Stasi, I.; Cappuzzo, F. Profile of Bavituximab and Its Potential in the Treatment of Non-Small-Cell Lung Cancer. Lung Cancer 2014, 5, 43–50. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Wang, H.; Xu, C.; Shi, G.; Zhao, J.; Zhang, L.; Zeng, M.; Jiang, L. Biomimetic Exosome Harnessing Exosomal Lipidomics and Functional Proteins for PEDF-pDNA Delivery in High Altitude Pulmonary Edema Intervention. J. Control. Release 2025, 379, 652–677. [Google Scholar] [CrossRef]
- Zech, T.; Ejsing, C.S.; Gaus, K.; de Wet, B.; Shevchenko, A.; Simons, K.; Harder, T. Accumulation of Raft Lipids in T-cell Plasma Membrane Domains Engaged in TCR Signalling. EMBO J. 2009, 28, 466–476. [Google Scholar] [CrossRef]
- Calzada, E.; Onguka, O.; Claypool, S.M. Phosphatidylethanolamine Metabolism in Health and Disease. Int. Rev. Cell Mol. Biol. 2016, 321, 29–88. [Google Scholar] [CrossRef]
- Van Der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The Critical Role of Phosphatidylcholine and Phosphatidylethanolamine Metabolism in Health and Disease. Biochim. Biophys. Acta BBA Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Moradi, S.; Nowroozi, A.; Shahlaei, M. Shedding Light on the Structural Properties of Lipid Bilayers Using Molecular Dynamics Simulation: A Review Study. RSC Adv. 2019, 9, 4644–4658. [Google Scholar] [CrossRef]
- Hoekstra, D.; Maier, O.; Van Der Wouden, J.M.; Slimane, T.A.; Van IJzendoorn, S.C.D. Membrane Dynamics and Cell Polarity: The Role of Sphingolipids. J. Lipid Res. 2003, 44, 869–877. [Google Scholar] [CrossRef]
- Aguilera-Romero, A.; Lucena, R.; Sabido-Bozo, S.; Muñiz, M. Impact of Sphingolipids on Protein Membrane Trafficking. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2023, 1868, 159334. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Hayashi, Y.; Nemoto-Sasaki, Y.; Ito, M.; Oka, S.; Tanikawa, T.; Waku, K.; Sugiura, T. Acyltransferases and Transacylases That Determine the Fatty Acid Composition of Glycerolipids and the Metabolism of Bioactive Lipid Mediators in Mammalian Cells and Model Organisms. Prog. Lipid Res. 2014, 53, 18–81. [Google Scholar] [CrossRef]
- Grösch, S.; Schiffmann, S.; Geisslinger, G. Chain Length-Specific Properties of Ceramides. Prog. Lipid Res. 2012, 51, 50–62. [Google Scholar] [CrossRef] [PubMed]
- De Craene, J.-O.; Bertazzi, D.L.; Bär, S.; Friant, S. Phosphoinositides, Major Actors in Membrane Trafficking and Lipid Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 634. [Google Scholar] [CrossRef]
- Ernst, R.; Ejsing, C.S.; Antonny, B. Homeoviscous Adaptation and the Regulation of Membrane Lipids. J. Mol. Biol. 2016, 428, 4776–4791. [Google Scholar] [CrossRef]
- Fajardo, V.A.; McMeekin, L.; LeBlanc, P.J. Influence of Phospholipid Species on Membrane Fluidity: A Meta-Analysis for a Novel Phospholipid Fluidity Index. J. Membr. Biol. 2011, 244, 97–103. [Google Scholar] [CrossRef]
- Pekker, M.; Shneider, M.N. Interaction between Electrolyte Ions and the Surface of a Cell Lipid Membrane. J. Phys. Chem. Biophys. 2015, 5, 2. [Google Scholar] [CrossRef]
- Pekker, M.; Shneider, M.N.; Keidar, M. Charged Cell Membrane in Electrolyte. arXiv 2017, arXiv:1709.09293. [Google Scholar]
- Bacalum, M.; Radu, M. Electric Charges of the Lipid Headgroup Modulate Melittin Adsorption to Lipid Vesicle Membranes. Rom. Rep. Phys. 2024, 76, 603. [Google Scholar] [CrossRef]
- Shukla, S.; Baumgart, T. Enzymatic Trans-Bilayer Lipid Transport: Mechanisms, Efficiencies, Slippage, and Membrane Curvature. Biochim. Biophys. Acta BBA Biomembr. 2021, 1863, 183534. [Google Scholar] [CrossRef]
- Fadeel, B.; Xue, D. The Ins and Outs of Phospholipid Asymmetry in the Plasma Membrane: Roles in Health and Disease. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Kihara, A.; Denpoh, A.; Igarashi, Y. The Rim101 Pathway Is Involved in Rsb1 Expression Induced by Altered Lipid Asymmetry. Mol. Biol. Cell 2008, 19, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.; Matuschewski, K.; Maier, A.G. The Enemy within: Lipid Asymmetry in Intracellular Parasite–Host Interactions. Emerg. Top. Life Sci. 2023, 7, 67–79. [Google Scholar] [CrossRef]
- Pabst, G.; Keller, S. Exploring Membrane Asymmetry and Its Effects on Membrane Proteins. Trends Biochem. Sci. 2024, 49, 333–345. [Google Scholar] [CrossRef]
- London, E. Membrane Structure–Function Insights from Asymmetric Lipid Vesicles. Acc. Chem. Res. 2019, 52, 2382–2391. [Google Scholar] [CrossRef]
- Bartoš, L.; Vácha, R. Peptide Translocation across Asymmetric Phospholipid Membranes. Biophys. J. 2024, 123, 693–702. [Google Scholar] [CrossRef]
- Gardea-Gutiérrez, D.; Núñez-García, E.; Oseguera-Guerra, B.E.; Román-Aguirre, M.; Montes-Fonseca, S.L. Asymmetric Lipid Vesicles: Techniques, Applications, and Future Perspectives as an Innovative Drug Delivery System. Pharmaceuticals 2023, 16, 777. [Google Scholar] [CrossRef]
- Kristanc, L.; Božič, B.; Jokhadar, Š.Z.; Dolenc, M.S.; Gomišček, G. The Pore-Forming Action of Polyenes: From Model Membranes to Living Organisms. Biochim. Biophys. Acta BBA Biomembr. 2019, 1861, 418–430. [Google Scholar] [CrossRef]
- Singh, P.; Saxena, R.; Srinivas, G.; Pande, G.; Chattopadhyay, A. Cholesterol Biosynthesis and Homeostasis in Regulation of the Cell Cycle. PLoS ONE 2013, 8, e58833. [Google Scholar] [CrossRef]
- Needham, D.; Nunn, R.S. Elastic Deformation and Failure of Lipid Bilayer Membranes Containing Cholesterol. Biophys. J. 1990, 58, 997–1009. [Google Scholar] [CrossRef]
- Gumí-Audenis, B.; Costa, L.; Carlá, F.; Comin, F.; Sanz, F.; Giannotti, M. Structure and Nanomechanics of Model Membranes by Atomic Force Microscopy and Spectroscopy: Insights into the Role of Cholesterol and Sphingolipids. Membranes 2016, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Dunina-Barkovskaya, A.Y. Cell Membrane Cholesterol and Regulation of Cellular Processes: New and the Same Old Thing. Biochem. Mosc. Suppl. Ser. Membr. Cell Biol. 2024, 18, 224–240. [Google Scholar] [CrossRef]
- Steck, T.L.; Lange, Y. Transverse Distribution of Plasma Membrane Bilayer Cholesterol: Picking Sides. Traffic 2018, 19, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J. Lipid Rafts: Bringing Order to Chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid Rafts As a Membrane-Organizing Principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef]
- Fuentes, N.R.; Kim, E.; Fan, Y.-Y.; Chapkin, R.S. Omega-3 Fatty Acids, Membrane Remodeling and Cancer Prevention. Mol. Aspects Med. 2018, 64, 79–91. [Google Scholar] [CrossRef]
- Hryniewicz-Jankowska, A.; Augoff, K.; Sikorski, A.F. The Role of Cholesterol and Cholesterol-Driven Membrane Raft Domains in Prostate Cancer. Exp. Biol. Med. 2019, 244, 1053–1061. [Google Scholar] [CrossRef]
- Zhuang, L.; Kim, J.; Adam, R.M.; Solomon, K.R.; Freeman, M.R. Cholesterol Targeting Alters Lipid Raft Composition and Cell Survival in Prostate Cancer Cells and Xenografts. J. Clin. Investig. 2005, 115, 959–968. [Google Scholar] [CrossRef]
- Steinkühler, J.; Sezgin, E.; Urbančič, I.; Eggeling, C.; Dimova, R. Mechanical Properties of Plasma Membrane Vesicles Correlate with Lipid Order, Viscosity and Cell Density. Commun. Biol. 2019, 2, 337. [Google Scholar] [CrossRef]
- Saher, G.; Quintes, S.; Nave, K.-A. Cholesterol: A Novel Regulatory Role in Myelin Formation. Neuroscientist 2011, 17, 79–93. [Google Scholar] [CrossRef]
- Aureli, M.; Grassi, S.; Prioni, S.; Sonnino, S.; Prinetti, A. Lipid Membrane Domains in the Brain. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2015, 1851, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Ingólfsson, H.I.; Carpenter, T.S.; Bhatia, H.; Bremer, P.-T.; Marrink, S.J.; Lightstone, F.C. Computational Lipidomics of the Neuronal Plasma Membrane. Biophys. J. 2017, 113, 2271–2280. [Google Scholar] [CrossRef]
- Nakahara, K.; Ohkuni, A.; Kitamura, T.; Abe, K.; Naganuma, T.; Ohno, Y.; Zoeller, R.A.; Kihara, A. The Sjögren-Larsson Syndrome Gene Encodes a Hexadecenal Dehydrogenase of the Sphingosine 1-Phosphate Degradation Pathway. Mol. Cell 2012, 46, 461–471. [Google Scholar] [CrossRef]
- Wigger, L.; Cruciani-Guglielmacci, C.; Nicolas, A.; Denom, J.; Fernandez, N.; Fumeron, F.; Marques-Vidal, P.; Ktorza, A.; Kramer, W.; Schulte, A.; et al. Plasma Dihydroceramides Are Diabetes Susceptibility Biomarker Candidates in Mice and Humans. Cell Rep. 2017, 18, 2269–2279. [Google Scholar] [CrossRef]
- Escribá, P.V.; Busquets, X.; Inokuchi, J.; Balogh, G.; Török, Z.; Horváth, I.; Harwood, J.L.; Vígh, L. Membrane Lipid Therapy: Modulation of the Cell Membrane Composition and Structure as a Molecular Base for Drug Discovery and New Disease Treatment. Prog. Lipid Res. 2015, 59, 38–53. [Google Scholar] [CrossRef]
- Engel, A.; Gaub, H.E. Structure and Mechanics of Membrane Proteins. Annu. Rev. Biochem. 2008, 77, 127–148. [Google Scholar] [CrossRef]
- Luchini, A.; Vitiello, G. Mimicking the Mammalian Plasma Membrane: An Overview of Lipid Membrane Models for Biophysical Studies. Biomimetics 2020, 6, 3. [Google Scholar] [CrossRef]
- Antonny, B. Mechanisms of Membrane Curvature Sensing. Annu. Rev. Biochem. 2011, 80, 101–123. [Google Scholar] [CrossRef]
- Laganowsky, A.; Reading, E.; Allison, T.M.; Ulmschneider, M.B.; Degiacomi, M.T.; Baldwin, A.J.; Robinson, C.V. Membrane Proteins Bind Lipids Selectively to Modulate Their Structure and Function. Nature 2014, 510, 172–175. [Google Scholar] [CrossRef]
- Daumke, O.; Roux, A.; Haucke, V. BAR Domain Scaffolds in Dynamin-Mediated Membrane Fission. Cell 2014, 156, 882–892. [Google Scholar] [CrossRef]
- Hedger, G.; Sansom, M.S.P. Lipid Interaction Sites on Channels, Transporters and Receptors: Recent Insights from Molecular Dynamics Simulations. Biochim. Biophys. Acta BBA Biomembr. 2016, 1858, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Barelli, H.; Antonny, B. Lipid Unsaturation and Organelle Dynamics. Curr. Opin. Cell Biol. 2016, 41, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Eliezer, D. Membrane Interactions of Intrinsically Disordered Proteins: The Example of Alpha-Synuclein. Biochim. Biophys. Acta BBA Proteins Proteom. 2019, 1867, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Martel, A.; Antony, L.; Gerelli, Y.; Porcar, L.; Fluitt, A.; Hoffmann, K.; Kiesel, I.; Vivaudou, M.; Fragneto, G.; de Pablo, J.J. Membrane Permeation versus Amyloidogenicity: A Multitechnique Study of Islet Amyloid Polypeptide Interaction with Model Membranes. J. Am. Chem. Soc. 2017, 139, 137–148. [Google Scholar] [CrossRef]
- Ren, K.; Feng, J.; Bi, H.; Sun, Q.; Li, X.; Han, D. AFM-Based Poroelastic@Membrane Analysis of Cells and Its Opportunities for Translational Medicine. Small 2023, 19, 2303610. [Google Scholar] [CrossRef]
- Zills, G.; Datta, T.; Malmi-Kakkada, A.N. Enhanced Mechanical Heterogeneity of Cell Collectives Due to Temporal Fluctuations in Cell Elasticity. Phys. Rev. E 2023, 107, 014401. [Google Scholar] [CrossRef]
- Wang, B.; Wang, W.; Wang, Y.; Liu, B.; Liu, L. Dynamical Modeling and Analysis of Viscoelastic Properties of Single Cells. Micromachines 2017, 8, 171. [Google Scholar] [CrossRef]
- Moraille, P.; Abdali, Z.; Ramkaran, M.; Polcari, D.; Patience, G.S.; Dorval Courchesne, N.-M.; Badia, A. Experimental Methods in Chemical Engineering: Atomic Force Microscopy—AFM. Can. J. Chem. Eng. 2022, 100, 2778–2806. [Google Scholar] [CrossRef]
- LeClaire, M.; Gimzewski, J.; Sharma, S. A Review of the Biomechanical Properties of Single Extracellular Vesicles. Nano Sel. 2021, 2, 1–15. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, J.; Xu, G.-K.; Wang, G.-F. Are Elastic Moduli of Biological Cells Depth Dependent or Not? Another Explanation Using a Contact Mechanics Model with Surface Tension. Soft Matter 2018, 14, 7534–7541. [Google Scholar] [CrossRef]
- Ren, K.; Gao, J.; Han, D. AFM Force Relaxation Curve Reveals That the Decrease of Membrane Tension Is the Essential Reason for the Softening of Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 663021. [Google Scholar] [CrossRef] [PubMed]
- Masters, T.A.; Pontes, B.; Viasnoff, V.; Li, Y.; Gauthier, N.C. Plasma Membrane Tension Orchestrates Membrane Trafficking, Cytoskeletal Remodeling, and Biochemical Signaling during Phagocytosis. Proc. Natl. Acad. Sci. USA 2013, 110, 11875–11880. [Google Scholar] [CrossRef] [PubMed]
- Prévost, C.; Zhao, H.; Manzi, J.; Lemichez, E.; Lappalainen, P.; Callan-Jones, A.; Bassereau, P. IRSp53 Senses Negative Membrane Curvature and Phase Separates along Membrane Tubules. Nat. Commun. 2015, 6, 8529. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.; Di Giacinto, F.; Nardini, M.; Mazzini, A.; Rossi, C.; Porceddu, E.; Papi, M.; Grieco, A.; De Spirito, M.; Ciasca, G. Erythrocyte Viscoelastic Recovery after Liver Transplantation in a Cirrhotic Patient Affected by Spur Cell Anaemia. J. Microsc. 2020, 280, 287–296. [Google Scholar] [CrossRef]
- Lekka, M.; Gil, D.; Pogoda, K.; Dulińska-Litewka, J.; Jach, R.; Gostek, J.; Klymenko, O.; Prauzner-Bechcicki, S.; Stachura, Z.; Wiltowska-Zuber, J.; et al. Cancer Cell Detection in Tissue Sections Using AFM. Arch. Biochem. Biophys. 2012, 518, 151–156. [Google Scholar] [CrossRef]
- Herrero, Y.R.; Camas, K.L.; Ullah, A. Characterization of Biobased Materials. In Advanced Applications of Biobased Materials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 111–143. ISBN 978-0-323-91677-6. [Google Scholar]
- Begemann, I.; Keller, U.; Nüsse, H.; Klingauf, J.; Galic, M. Parallel Acquisition of Plasma Membrane Ultrastructure and Cytosolic Protein Localisation in Cultured Cells via Correlated Immunogold SEM. Cells 2020, 9, 1329. [Google Scholar] [CrossRef]
- Alqaheem, Y.; Alomair, A.A. Microscopy and Spectroscopy Techniques for Characterization of Polymeric Membranes. Membranes 2020, 10, 33. [Google Scholar] [CrossRef]
- Graham, L.; Orenstein, J.M. Processing Tissue and Cells for Transmission Electron Microscopy in Diagnostic Pathology and Research. Nat. Protoc. 2007, 2, 2439–2450. [Google Scholar] [CrossRef]
- Boldon, L.; Laliberte, F.; Liu, L. Review of the Fundamental Theories behind Small Angle X-Ray Scattering, Molecular Dynamics Simulations, and Relevant Integrated Application. Nano Rev. 2015, 6, 25661. [Google Scholar] [CrossRef]
- Baroni, D.; Zegarra-Moran, O.; Moran, O. Functional and Pharmacological Induced Structural Changes of the Cystic Fibrosis Transmembrane Conductance Regulator in the Membrane Solved Using SAXS. Cell. Mol. Life Sci. CMLS 2014, 72, 1363–1375. [Google Scholar] [CrossRef]
- Nunes, C.; Brezesinski, G.; Lima, J.L.F.C.; Reis, S.; Lúcio, M. Synchrotron SAXS and WAXS Study of the Interactions of NSAIDs with Lipid Membranes. J. Phys. Chem. B 2011, 115, 8024–8032. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, L.M.; Wang, Z.-M.; Romero, J.J.; Ulloa, C.; Perez, J.C.; Giraud, M.-N.; Barreto, J.C. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Associate with Zwitterionic Phospholipids: Insight into the Mechanism and Reversal of NSAID-Induced Gastrointestinal Injury. Nat. Med. 1995, 1, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Chang, W.-H.; Cerione, R.A.; Antonyak, M.A. Extracellular Vesicles and Their Roles in Cancer Progression. In Cancer Cell Signaling; Robles-Flores, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2174, pp. 143–170. ISBN 978-1-0716-0758-9. [Google Scholar]
- Paskeh, M.D.A.; Entezari, M.; Mirzaei, S.; Zabolian, A.; Saleki, H.; Naghdi, M.J.; Sabet, S.; Khoshbakht, M.A.; Hashemi, M.; Hushmandi, K.; et al. Emerging Role of Exosomes in Cancer Progression and Tumor Microenvironment Remodeling. J. Hematol. Oncol. 2022, 15, 83. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal Lipid Composition and the Role of Ether Lipids and Phosphoinositides in Exosome Biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Laulagnier, K.; Motta, C.; Hamdi, S.; Roy, S.; Fauvelle, F.; Pageaux, J.-F.; Kobayashi, T.; Salles, J.-P.; Perret, B.; Bonnerot, C.; et al. Mast Cell- and Dendritic Cell-Derived Exosomes Display a Specific Lipid Composition and an Unusual Membrane Organization. Biochem. J. 2004, 380, 161–171. [Google Scholar] [CrossRef]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J.O. Extracellular Vesicles: Lipids as Key Components of Their Biogenesis and Functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef]
- Sakai-Kato, K.; Yoshida, K.; Takechi-Haraya, Y.; Izutsu, K. Physicochemical Characterization of Liposomes That Mimic the Lipid Composition of Exosomes for Effective Intracellular Trafficking. Langmuir 2020, 36, 12735–12744. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in Exosomes: Current Knowledge and the Way Forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Liaw, W.-S.; Chen, C.-A.; Zhou, Q.A. Exosomes—Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. ACS Nano 2022, 16, 17802–17846. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Watanabe, H.; Hirosawa, K.M.; Suzuki, K.G.N.; Suga, K.; Hanashima, S. Fluorescence Spectroscopic Analysis of Lateral and Transbilayer Fluidity of Exosome Membranes. Langmuir 2022, 38, 14695–14703. [Google Scholar] [CrossRef] [PubMed]
- Cebecauer, M.; Amaro, M.; Jurkiewicz, P.; Sarmento, M.J.; Šachl, R.; Cwiklik, L.; Hof, M. Membrane Lipid Nanodomains. Chem. Rev. 2018, 118, 11259–11297. [Google Scholar] [CrossRef]
- Subra, C.; Laulagnier, K.; Perret, B.; Record, M. Exosome Lipidomics Unravels Lipid Sorting at the Level of Multivesicular Bodies. Biochimie 2007, 89, 205–212. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current Knowledge of Their Composition, Biological Functions, and Diagnostic and Therapeutic Potentials. Biochim. Biophys. Acta BBA Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Wubbolts, R.; Leckie, R.S.; Veenhuizen, P.T.M.; Schwarzmann, G.; Möbius, W.; Hoernschemeyer, J.; Slot, J.-W.; Geuze, H.J.; Stoorvogel, W. Proteomic and Biochemical Analyses of Human B Cell-Derived Exosomes. J. Biol. Chem. 2003, 278, 10963–10972. [Google Scholar] [CrossRef]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH Is a Key Factor for Exosome Traffic in Tumor Cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef]
- Donoso-Quezada, J.; Ayala-Mar, S.; González-Valdez, J. The Role of Lipids in Exosome Biology and Intercellular Communication: Function, Analytics and Applications. Traffic 2021, 22, 204–220. [Google Scholar] [CrossRef]
- Chapuy-Regaud, S.; Dubois, M.; Plisson-Chastang, C.; Bonnefois, T.; Lhomme, S.; Bertrand-Michel, J.; You, B.; Simoneau, S.; Gleizes, P.-E.; Flan, B.; et al. Characterization of the Lipid Envelope of Exosome Encapsulated HEV Particles Protected from the Immune Response. Biochimie 2017, 141, 70–79. [Google Scholar] [CrossRef]
- Durcin, M.; Fleury, A.; Taillebois, E.; Hilairet, G.; Krupova, Z.; Henry, C.; Truchet, S.; Trötzmüller, M.; Köfeler, H.; Mabilleau, G.; et al. Characterisation of Adipocyte-Derived Extracellular Vesicle Subtypes Identifies Distinct Protein and Lipid Signatures for Large and Small Extracellular Vesicles. J. Extracell. Vesicles 2017, 6, 1305677. [Google Scholar] [CrossRef]
- Suga, K.; Matsui, D.; Watanabe, N.; Okamoto, Y.; Umakoshi, H. Insight into the Exosomal Membrane: From Viewpoints of Membrane Fluidity and Polarity. Langmuir 2021, 37, 11195–11202. [Google Scholar] [CrossRef]
- Hazan-Halevy, I.; Rosenblum, D.; Weinstein, S.; Bairey, O.; Raanani, P.; Peer, D. Cell-Specific Uptake of Mantle Cell Lymphoma-Derived Exosomes by Malignant and Non-Malignant B-Lymphocytes. Cancer Lett. 2015, 364, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, N.; Yan, T.; Shi, Y.-N.; Chen, J.; Zhang, C.-J.; Xie, X.-J.; Liao, D.-F.; Qin, L. The Crosstalk: Exosomes and Lipid Metabolism. Cell Commun. Signal. 2020, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.J.; Redzic, J.S.; Graner, M.W.; Anchordoquy, T.J. Examination of the Specificity of Tumor Cell Derived Exosomes with Tumor Cells in Vitro. Biochim. Biophys. Acta BBA Biomembr. 2014, 1838, 2954–2965. [Google Scholar] [CrossRef]
- Van Der Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthc. Mater. 2021, 11, 2100639. [Google Scholar] [CrossRef]
- Patil, A.A.; Rhee, W.J. Exosomes: Biogenesis, Composition, Functions, and Their Role in Pre-Metastatic Niche Formation. Biotechnol. Bioprocess Eng. 2019, 24, 689–701. [Google Scholar] [CrossRef]
- Rana, S.; Yue, S.; Stadel, D.; Zöller, M. Toward Tailored Exosomes: The Exosomal Tetraspanin Web Contributes to Target Cell Selection. Int. J. Biochem. Cell Biol. 2012, 44, 1574–1584. [Google Scholar] [CrossRef]
- Jankovičová, J.; Sečová, P.; Michalková, K.; Antalíková, J. Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction. Int. J. Mol. Sci. 2020, 21, 7568. [Google Scholar] [CrossRef]
- Schorey, J.S.; Bhatnagar, S. Exosome Function: From Tumor Immunology to Pathogen Biology. Traffic 2008, 9, 871–881. [Google Scholar] [CrossRef]
- Otto, G.P.; Nichols, B.J. The Roles of Flotillin Microdomains—Endocytosis and Beyond. J. Cell Sci. 2011, 124, 3933–3940. [Google Scholar] [CrossRef]
- Chernomordik, L.V.; Kozlov, M.M. Mechanics of Membrane Fusion. Nat. Struct. Mol. Biol. 2008, 15, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ma, X.; Yu, J. Exosomes and Organ-Specific Metastasis. Mol. Ther. Methods Clin. Dev. 2021, 22, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, Biogenesis and Function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Baldassari, S.; Balboni, A.; Drava, G.; Donghia, D.; Canepa, P.; Ailuno, G.; Caviglioli, G. Phytochemicals and Cancer Treatment: Cell-Derived and Biomimetic Vesicles as Promising Carriers. Pharmaceutics 2023, 15, 1445. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, J.; Zhu, Y.; Wang, X.; Han, Y.; Zhang, D. Recent Advances in Extracellular Vesicles for Therapeutic Cargo Delivery. Exp. Mol. Med. 2024, 56, 836–849. [Google Scholar] [CrossRef]
- Lu, M.; Huang, Y. Bioinspired Exosome-like Therapeutics and Delivery Nanoplatforms. Biomaterials 2020, 242, 119925. [Google Scholar] [CrossRef]
- Mougenot, M.F.; Pereira, V.S.; Costa, A.L.R.; Lancellotti, M.; Porcionatto, M.A.; da Silveira, J.C.; de la Torre, L.G. Biomimetic Nanovesicles—Sources, Design, Production Methods, and Applications. Pharmaceutics 2022, 14, 2008. [Google Scholar] [CrossRef]
- Liu, H.; Su, Y.-Y.; Jiang, X.-C.; Gao, J.-Q. Cell Membrane-Coated Nanoparticles: A Novel Multifunctional Biomimetic Drug Delivery System. Drug Deliv. Transl. Res. 2023, 13, 716–737. [Google Scholar] [CrossRef]
- Balboni, A.; Ailuno, G.; Baldassari, S.; Drava, G.; Petretto, A.; Grinovero, N.; Cavalleri, O.; Angeli, E.; Lagomarsino, A.; Canepa, P.; et al. Human Glioblastoma-Derived Cell Membrane Nanovesicles: A Novel, Cell-Specific Strategy for Boron Neutron Capture Therapy of Brain Tumors. Sci. Rep. 2024, 14, 19225. [Google Scholar] [CrossRef]
- Liu, A.; Yang, G.; Liu, Y.; Liu, T. Research Progress in Membrane Fusion-Based Hybrid Exosomes for Drug Delivery Systems. Front. Bioeng. Biotechnol. 2022, 10, 939441. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.; Forbes, N.; Roces, C.B.; Anderluzzi, G.; Lou, G.; Abraham, S.; Ingalls, L.; Marshall, K.; Leaver, T.J.; Watts, J.A.; et al. Using Microfluidics for Scalable Manufacturing of Nanomedicines from Bench to GMP: A Case Study Using Protein-Loaded Liposomes. Int. J. Pharm. 2020, 582, 119266. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xu, L.; Zhang, W. Biomimetic Synthesis and Optimization of Extracellular Vesicles for Bone Regeneration. J. Control. Release 2023, 355, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Wiest, E.F.; Zubair, A.C. Generation of Current Good Manufacturing Practices-Grade Mesenchymal Stromal Cell-Derived Extracellular Vesicles Using Automated Bioreactors. Biology 2025, 14, 313. [Google Scholar] [CrossRef]
- Rosso, G.; Cauda, V. Biomimicking Extracellular Vesicles with Fully Artificial Ones: A Rational Design of EV-BIOMIMETICS toward Effective Theranostic Tools in Nanomedicine. ACS Biomater. Sci. Eng. 2023, 9, 5924–5932. [Google Scholar] [CrossRef]
- Tsering, T.; Nadeau, A.; Wu, T.; Dickinson, K.; Burnier, J.V. Extracellular Vesicle-Associated DNA: Ten Years since Its Discovery in Human Blood. Cell Death Dis. 2024, 15, 668. [Google Scholar] [CrossRef]
- Chen, Y.; Douanne, N.; Wu, T.; Kaur, I.; Tsering, T.; Erzingatzian, A.; Nadeau, A.; Juncker, D.; Nerguizian, V.; Burnier, J.V. Leveraging Nature’s Nanocarriers: Translating Insights from Extracellular Vesicles to Biomimetic Synthetic Vesicles for Biomedical Applications. Sci. Adv. 2025, 11, eads5249. [Google Scholar] [CrossRef]
- Yi, X.; Shi, X.; Gao, H. Cellular Uptake of Elastic Nanoparticles. Phys. Rev. Lett. 2011, 107, 098101. [Google Scholar] [CrossRef]
- Yue, T.; Zhang, X. Molecular Modeling of the Pathways of Vesicle–Membrane Interaction. Soft Matter 2013, 9, 559–569. [Google Scholar] [CrossRef]
- Banquy, X.; Suarez, F.; Argaw, A.; Rabanel, J.-M.; Grutter, P.; Bouchard, J.-F.; Hildgen, P.; Giasson, S. Effect of Mechanical Properties of Hydrogel Nanoparticles on Macrophage Cell Uptake. Soft Matter 2009, 5, 3984. [Google Scholar] [CrossRef]
- Beningo, K.A.; Wang, Y. Fc-Receptor-Mediated Phagocytosis Is Regulated by Mechanical Properties of the Target. J. Cell Sci. 2002, 115, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of Nanoparticles Influences Their Blood Circulation, Phagocytosis, Endocytosis, and Targeting. ACS Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, X.; Mao, Z.; Yu, D.; Wang, B.; Gao, C. Uptake of Hydrogel Particles with Different Stiffness and Its Influence on HepG2 Cell Functions. Soft Matter 2012, 8, 9235. [Google Scholar] [CrossRef]
- Kol, N.; Shi, Y.; Tsvitov, M.; Barlam, D.; Shneck, R.Z.; Kay, M.S.; Rousso, I. A Stiffness Switch in Human Immunodeficiency Virus. Biophys. J. 2007, 92, 1777–1783. [Google Scholar] [CrossRef]
- Zumbuehl, A.; Dobner, B.; Brezesinski, G. Phase Behavior of Selected Artificial Lipids. Curr. Opin. Colloid Interface Sci. 2014, 19, 17–24. [Google Scholar] [CrossRef]
- López, R.R.; Ben El Khyat, C.Z.; Chen, Y.; Tsering, T.; Dickinson, K.; Bustamante, P.; Erzingatzian, A.; Bartolomucci, A.; Ferrier, S.T.; Douanne, N.; et al. A Synthetic Model of Bioinspired Liposomes to Study Cancer-Cell Derived Extracellular Vesicles and Their Uptake by Recipient Cells. Sci. Rep. 2025, 15, 8430. [Google Scholar] [CrossRef]
- Golubovic, A.; Tsai, S.; Li, B. Bioinspired Lipid Nanocarriers for RNA Delivery. ACS Bio Med. Chem. Au 2023, 3, 114–136. [Google Scholar] [CrossRef]
- Martinez-Lostao, L.; García-Alvarez, F.; Basáñez, G.; Alegre-Aguarón, E.; Desportes, P.; Larrad, L.; Naval, J.; Martínez-Lorenzo, M.J.; Anel, A. Liposome-bound APO2L/TRAIL Is an Effective Treatment in a Rabbit Model of Rheumatoid Arthritis. Arthritis Rheum. 2010, 62, 2272–2282. [Google Scholar] [CrossRef]
- Li, K.; Chang, S.; Wang, Z.; Zhao, X.; Chen, D. A Novel Micro-Emulsion and Micelle Assembling Method to Prepare DEC205 Monoclonal Antibody Coupled Cationic Nanoliposomes for Simulating Exosomes to Target Dendritic Cells. Int. J. Pharm. 2015, 491, 105–112. [Google Scholar] [CrossRef]
- Lu, M.; Zhao, X.; Xing, H.; Xun, Z.; Zhu, S.; Lang, L.; Yang, T.; Cai, C.; Wang, D.; Ding, P. Comparison of Exosome-Mimicking Liposomes with Conventional Liposomes for Intracellular Delivery of siRNA. Int. J. Pharm. 2018, 550, 100–113. [Google Scholar] [CrossRef]
- Vázquez-Ríos, A.J.; Molina-Crespo, Á.; Bouzo, B.L.; López-López, R.; Moreno-Bueno, G.; De La Fuente, M. Exosome-Mimetic Nanoplatforms for Targeted Cancer Drug Delivery. J. Nanobiotechnol. 2019, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Yahata, S.; Hirose, M.; Ueno, T.; Nagumo, H.; Sakai-Kato, K. Effect of Sample Concentration on Nanoparticle Tracking Analysis of Small Extracellular Vesicles and Liposomes Mimicking the Physicochemical Properties of Exosomes. Chem. Pharm. Bull. 2021, 69, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Okami, K.; Fumoto, S.; Yamashita, M.; Nakashima, M.; Miyamoto, H.; Kawakami, S.; Nishida, K. One-Step Formation Method of Plasmid DNA-Loaded, Extracellular Vesicles-Mimicking Lipid Nanoparticles Based on Nucleic Acids Dilution-Induced Assembly. Cells 2024, 13, 1183. [Google Scholar] [CrossRef]
- Arduino, I.; Di Fonte, R.; Tiboni, M.; Porcelli, L.; Serratì, S.; Fondaj, D.; Rafaschieri, T.; Cutrignelli, A.; Guida, G.; Casettari, L.; et al. Microfluidic Development and Biological Evaluation of Targeted Therapy-Loaded Biomimetic Nano System to Improve the Metastatic Melanoma Treatment. Int. J. Pharm. 2024, 650, 123697. [Google Scholar] [CrossRef]
- Kong, D.; Qian, Y.; Yu, B.; Hu, Z.; Cheng, C.; Wang, Y.; Fang, Z.; Yu, J.; Xiang, S.; Cao, L.; et al. Interaction of Human Dendritic Cell Receptor DEC205/CD205 with Keratins. J. Biol. Chem. 2024, 300, 105699. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of Cationic Lipids and Cationic Polymers in Gene Delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- De Gassart, A.; Géminard, C.; Février, B.; Raposo, G.; Vidal, M. Lipid Raft-Associated Protein Sorting in Exosomes. Blood 2003, 102, 4336–4344. [Google Scholar] [CrossRef]
- Rahman, M.S.; Ghorai, S.; Panda, K.; Santiago, M.J.; Aggarwal, S.; Wang, T.; Rahman, I.; Chinnapaiyan, S.; Unwalla, H.J. Dr. Jekyll or Mr. Hyde: The Multifaceted Roles of miR-145-5p in Human Health and Disease. Non-Coding RNA Res. 2025, 11, 22–37. [Google Scholar] [CrossRef]
- Miyanishi, M.; Tada, K.; Koike, M.; Uchiyama, Y.; Kitamura, T.; Nagata, S. Identification of Tim4 as a Phosphatidylserine Receptor. Nature 2007, 450, 435–439. [Google Scholar] [CrossRef]
- Briuglia, M.-L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of Cholesterol on Liposome Stability and on in Vitro Drug Release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Rayamajhi, S.; Nguyen, T.D.T.; Marasini, R.; Aryal, S. Macrophage-Derived Exosome-Mimetic Hybrid Vesicles for Tumor Targeted Drug Delivery. Acta Biomater. 2019, 94, 482–494. [Google Scholar] [CrossRef] [PubMed]
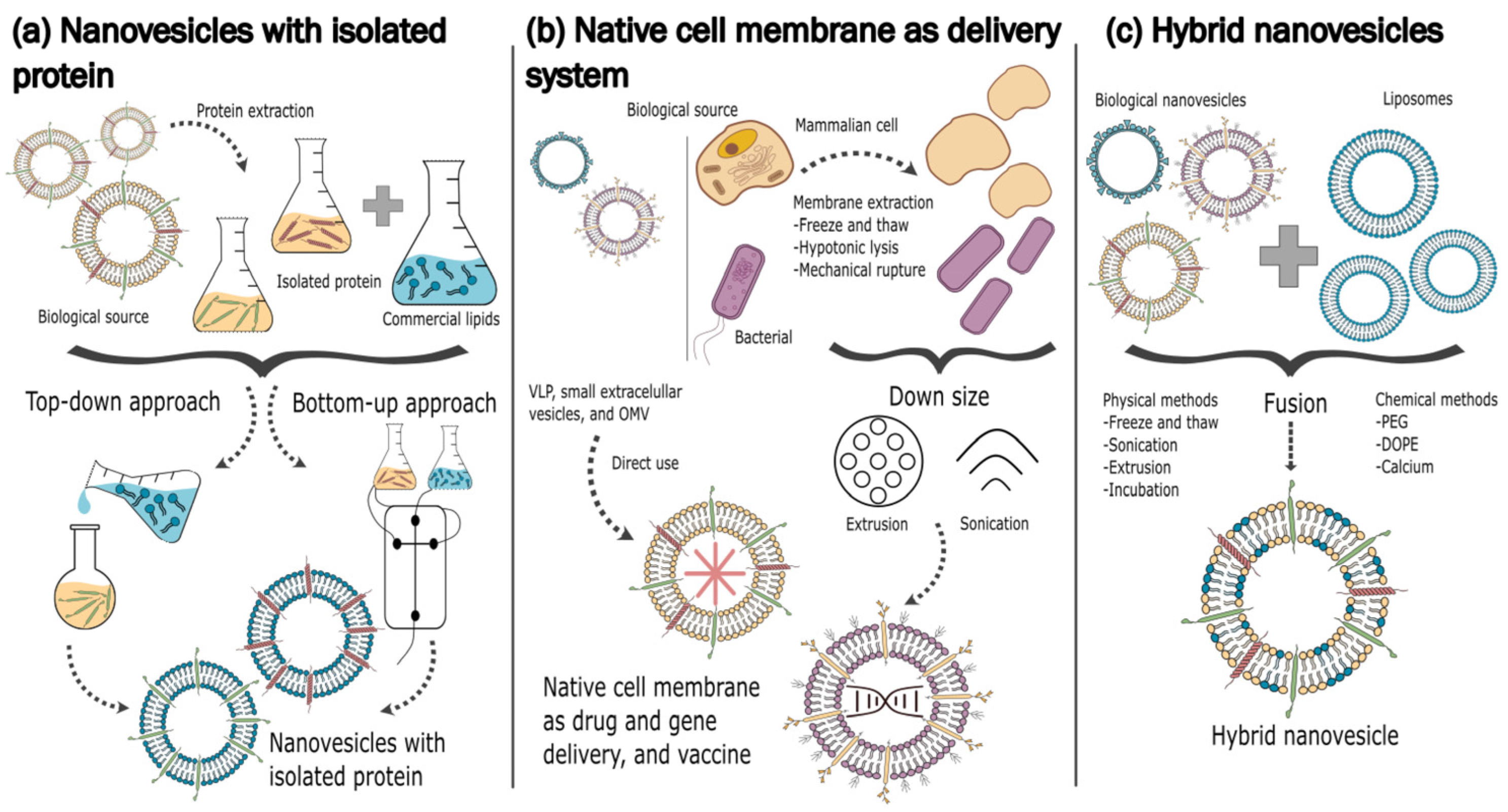
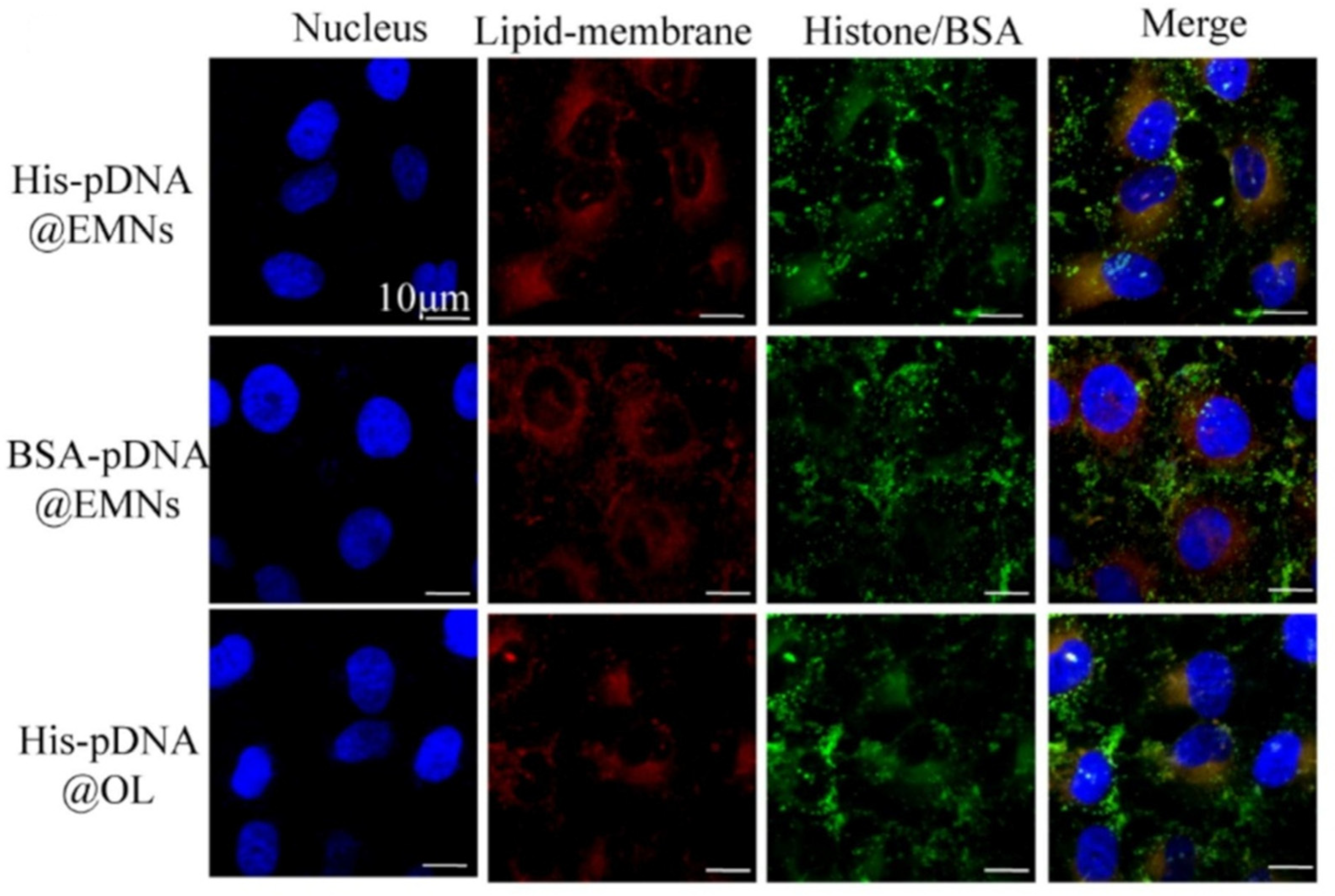
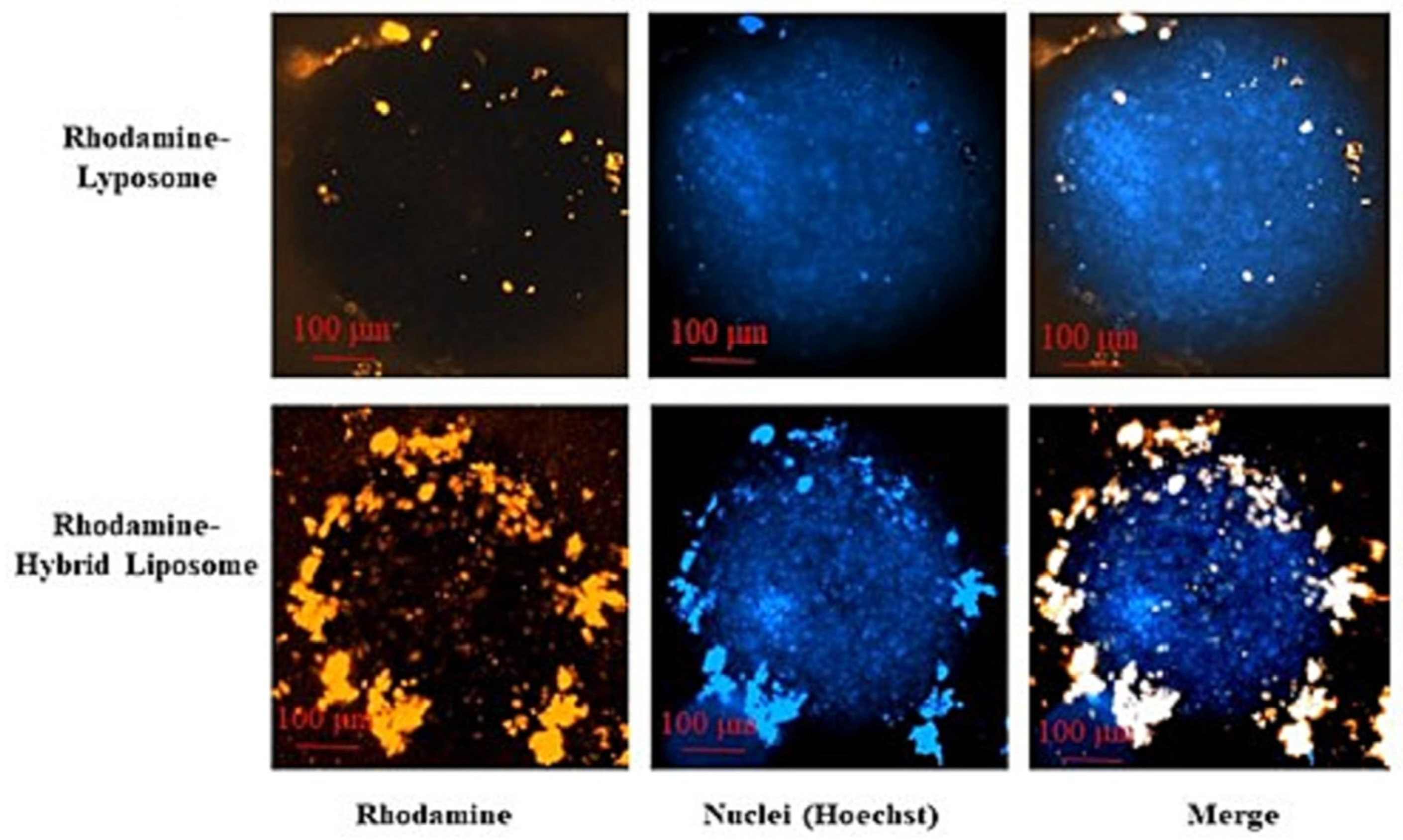
| Lipid | Ratio (mol%) | Membrane Leaflet | Mechanism Maintaining the Asymmetric Distribution | Properties |
|---|---|---|---|---|
| Phosphatidic acid | 1 | Inner leaflet | - | Levels regulated by diacylglycerol kinase and phospholipase D, elevated levels activate kinases involved in cancer cell metabolism and proliferation. |
| Phosphatidylinositol | 5–12 | Inner leaflet | - | Substrate for phospholipase C or phosphoinositide-3 kinase, regulating role in membrane trafficking and proliferation. |
| Phosphatidylglycerol | 1 | Inner leaflet | - | High level observed in increased viral replication and cancer development. |
| Phosphatidylserine | 6 | Inner-outer leaflet | Calcium ion-dependent flippase moves PS to the outer leaflet; exposed on the outer leaflet during cellular oxidative stress | Role in apoptosis and protecting cells from immune detection; in some cases, it can be considered a tumor marker. |
| Phosphatidylethanolamine | 15–25 | Inner leaflet | Change from lamellar to hexagonal II phase to enhance the fusion of lipid bilayers with lysosomal membranes, reverse distribution in cancer cells | Lipid chaperone that assists membrane protein folding, involved in cell signaling pathways; promotes autophagy and regulates protein interactions. |
| Phosphatidylcholine | 40–50 | Outer leaflet | - | Choline kinase alpha and phospholipases C and D regulate its metabolism; its level can affect cell proliferation and energy metabolism in cancer cells. |
| Sphingomyelin | 10–20 | Outer leaflet | - | Involved in molecular sorting, cell-cell interaction, intracellular transport and signaling. |
| Cholesterol | 20–50 | Depends on the affinity with other components | Stronger interaction with sphingolipids: outer leaflet/major affinity with PE and PS: inner leaflet (60%) | Regulates membrane permeability, affects stiffness, thickness and thermosensitivity. |
| Ref. | Type of System | Composition | Inspiring Vesicles | Application | In Vitro/In Vivo Tests |
|---|---|---|---|---|---|
| [114] | Liposomes | (1) Chol/milk SM/POPC 40:15:45 mol%, (2) Chol/milk SM/POPC/cardiolipin 40:15:30:15 mol% | Bovine milk-derived exosomes | Drug delivery of nucleic acid | |
| [151] | Large unilamellar vesicles | PC/SM/ovine wool chol in 55:30:10 weight ratio + different amounts of 1,2-dioleoyl-sn-glycero-3-{[N-(5-amino-1-carboxypentyl)-iminodiacetic acid]succinyl}(nickel salt) | Exosomes of unspecified origin | Treatment of autoimmune diseases | In vivo tests on a rabbit model of antigen-induced arthritis |
| [152] | Liposomes | PC/Cremophor EL in different weight ratios from 1:9 to 9:1 + bovine serum albumin + DOPE/DC-chol/cholesteryl-succinate in 8:2:1 weight ratio | Exosomes of unspecified origin | Dendritic cell targeting | In vitro: cytotoxicity (by MTT) and internalization (flow cytometry) on dendritic cells |
| [153] | Liposomes | (1) DOPC/SM/chol/DOPS/DOPE in 21:17.5:30:14:17.5 mol%, (2) DOTAP/DOPC/chol in 40:40:20 mol%, (3) DOPC/chol in 70:30 mol% | Exosomes of unspecified origin | Delivery of a VEGF-targeting siRNA | In vitro: cytotoxicity (by MTT), internalization on A549 (lung adenocarcinoma) and HUVEC cells (confocal laser scanning microscopy), uptake efficiency (flow cytometry and fluorescence microscopy) and gene silencing efficacy studies |
| [154] | Exosome-mimetic nanosystem | Chol/PC/SM/C16 ceramide in 0.9:1:0.4:0.03 weight ratio + protein functionalization | Tumor-derived exosomes | Delivery of miR145, as an anticancer active | In vitro: internalization on SW480 (colorectal cancer), PC-3 (prostatic adenocarcinoma) and A549 (lung adenocarcinoma) cells by confocal microscopy |
| [102,155] | Liposomes | (1) DSPC/chol/DOPS in 40:40:20 mol%, (2) SM/DSPC/chol/DOPS in 10:30:40:20 mol%, (3) DSPC/chol/DSPS in 40:40:20 mol%, (4) SM/DSPC/chol/DSPS in 10:30:40:20 mol%, (5) SM/DSPC/chol/DOPG in 10:30:40:20 mol%, (6) DSPC/chol in 60:40 mol% | Exosomes deriving from human hepatocellular HepG2 carcinoma cells | Targeted drug delivery and intracellular trafficking studies | In vitro: internalization on HeLa cells by confocal microscopy |
| [156] | EV-mimicking lipid nanoparticles | DOPC/DOPE/DOPS/SM/chol in 18:7:13:17:45 mol% + N-octanoyl-sphingosine-1-{succinyl[methoxy(polyethyleneglycol)2000]}, 1 mol% of total lipids | Lipidomic study on extracellular vesicles | Delivery of plasmid DNA for transfection | In vitro: internalization on Hep-G2 cells by measuring the transfection efficiency |
| [26] | Liposomes | Chol/SM/PC/PS/PE in 10:10:15:10:35 molar ratio and formulations lacking SM, PC, PS or PE | Lipidomics analysis of HUVEC-derived exosomes | Delivery of Pigment Epithelium-Derived Factor genes linked with a histone (aimed at gene transfection) | In vitro: Internalization on HUVEC by flow cytometry; in vivo: tests on a mouse model of High Altitude Pulmonary Edema |
| [157] | Hybrid liposomes | Cell membranes isolated from BRAF wild type metastatic melanoma cell line, L-α-PC and cholesterol in 70:30 mol% | / | Delivery of cobimetinib or lenvatinib for melanoma treatment | In vitro: internalization on the parent melanoma cells by flow cytometry and confocal microscopy (on 2D cell cultures and 3D spheroids), hemolysis test on whole human blood, cytotoxicity on melanoma cells (by MTT) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donghia, D.; Baldassari, S.; Drava, G.; Ailuno, G.; Caviglioli, G. Insights on Natural Membrane Characterization for the Rational Design of Biomimetic Drug Delivery Systems. Pharmaceutics 2025, 17, 841. https://doi.org/10.3390/pharmaceutics17070841
Donghia D, Baldassari S, Drava G, Ailuno G, Caviglioli G. Insights on Natural Membrane Characterization for the Rational Design of Biomimetic Drug Delivery Systems. Pharmaceutics. 2025; 17(7):841. https://doi.org/10.3390/pharmaceutics17070841
Chicago/Turabian StyleDonghia, Daniela, Sara Baldassari, Giuliana Drava, Giorgia Ailuno, and Gabriele Caviglioli. 2025. "Insights on Natural Membrane Characterization for the Rational Design of Biomimetic Drug Delivery Systems" Pharmaceutics 17, no. 7: 841. https://doi.org/10.3390/pharmaceutics17070841
APA StyleDonghia, D., Baldassari, S., Drava, G., Ailuno, G., & Caviglioli, G. (2025). Insights on Natural Membrane Characterization for the Rational Design of Biomimetic Drug Delivery Systems. Pharmaceutics, 17(7), 841. https://doi.org/10.3390/pharmaceutics17070841








