Fucoidan-Based Gold Nanoparticles: Antioxidant and Anticancer Potential from Turbinaria decurrens and Sargassum cinereum
Abstract
1. Introduction
2. Materials and Methods
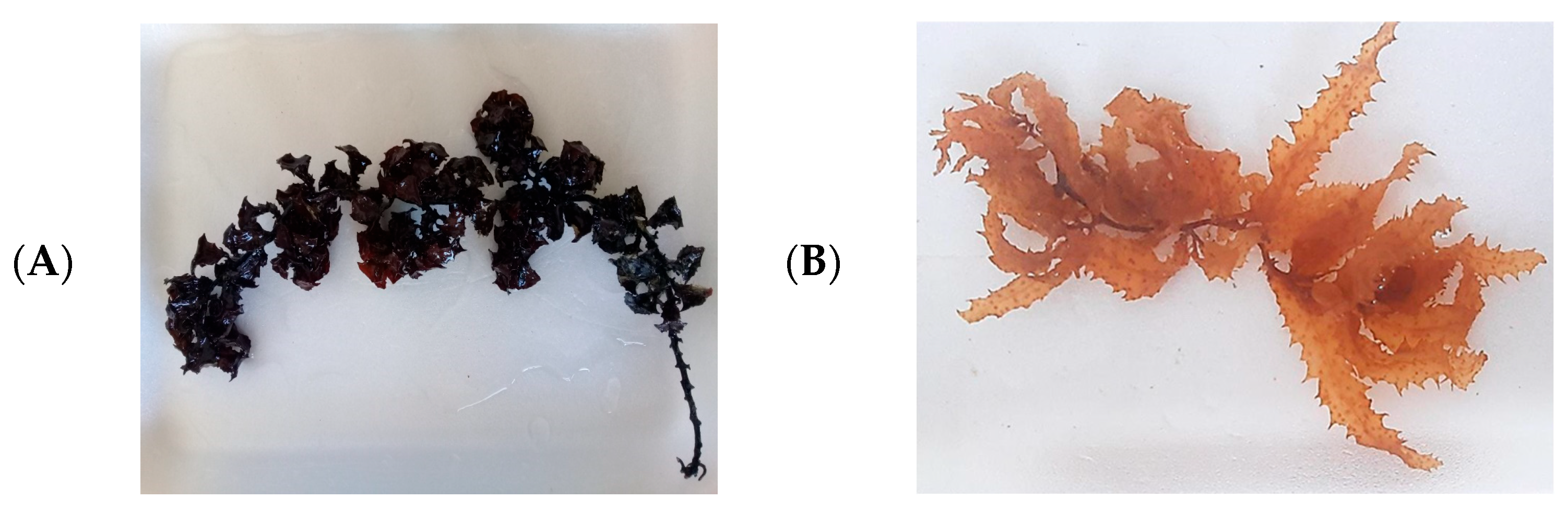
2.1. Collection and Identification of Macroalgae Samples
2.2. Extraction of Polysaccharide Rich in Fucoidan
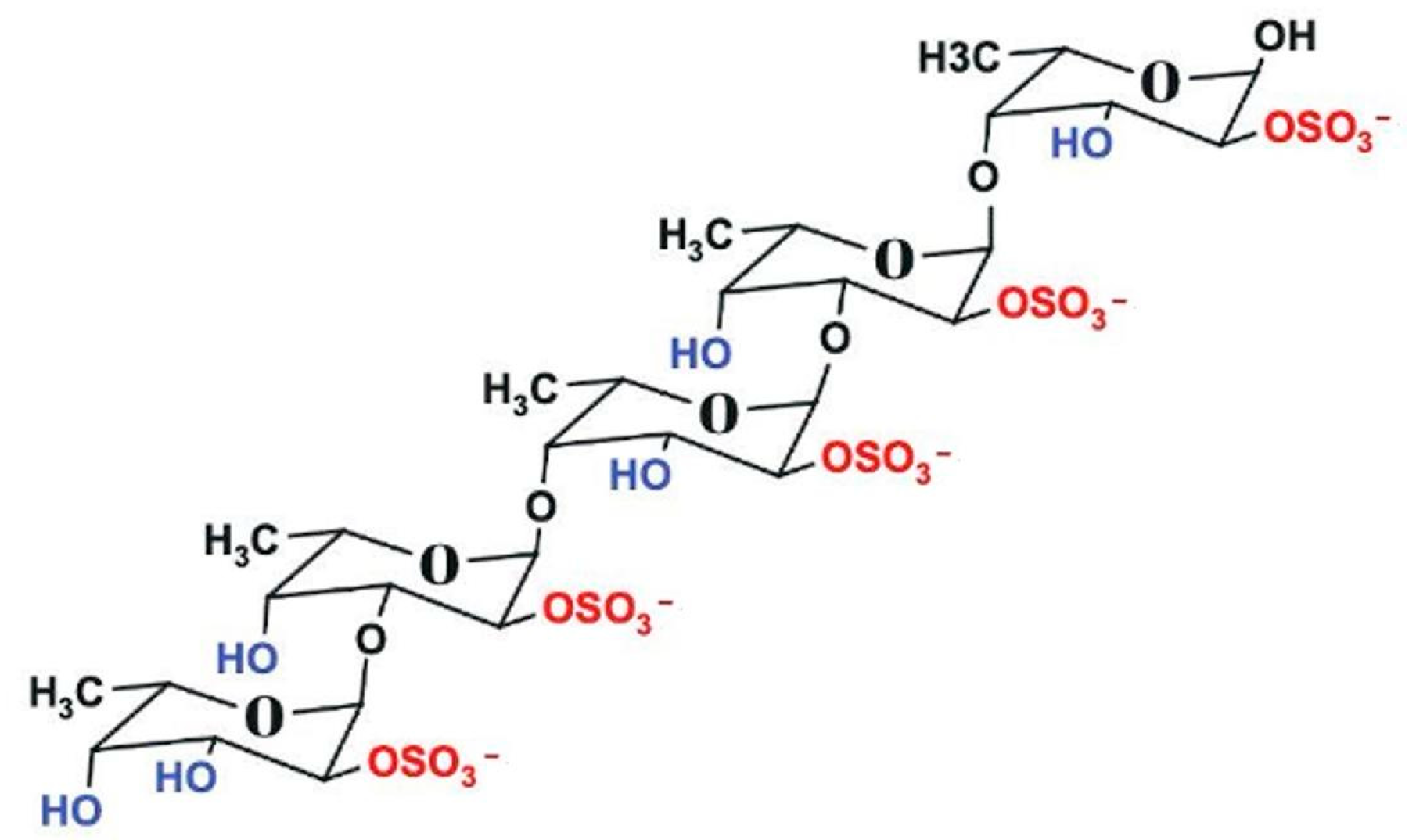
2.3. Characterization of Polysaccharide Rich in Fucoidan
2.3.1. Fourier-Transform Infrared Spectroscopy (FTIR) Spectrum
2.3.2. X-Ray Diffraction (EDX)
2.4. Preparation of Fucoidan Solution
2.5. Biosynthesis of Gold Nanoparticles (AuNPs)
2.6. Characterization of Au-NPs
2.7. In Vitro
2.7.1. Antioxidant Activity
DPPH (2,2-Diphenyl-1-Picryl-Hydrazyl-Hydrate) Free Radical Scavenging Assay
Ferric-Reducing Antioxidant Power (FRAP) Assay
2.7.2. Cytotoxicity and Anticancer Effect
Cell Culture and General Conditions
SRB Assay for Normal BNL Cells
MTT Assay for HepG2 Cells
WST-1 Assay for THP1 Cells
Cell Cycle Analysis by Flow Cytometry
2.8. In Silico Study
2.8.1. Target Prediction
2.8.2. Protein Preparation
2.8.3. Ligand Preparation
2.8.4. Binding Site Prediction
2.8.5. Molecular Docking
2.9. Statistical Analysis
3. Results
3.1. Identification of Macroalgae Samples
3.2. Extraction of Polysaccharides Rich in Fucoidan
3.3. Characterization of Polysaccharides Rich in Fucoidan
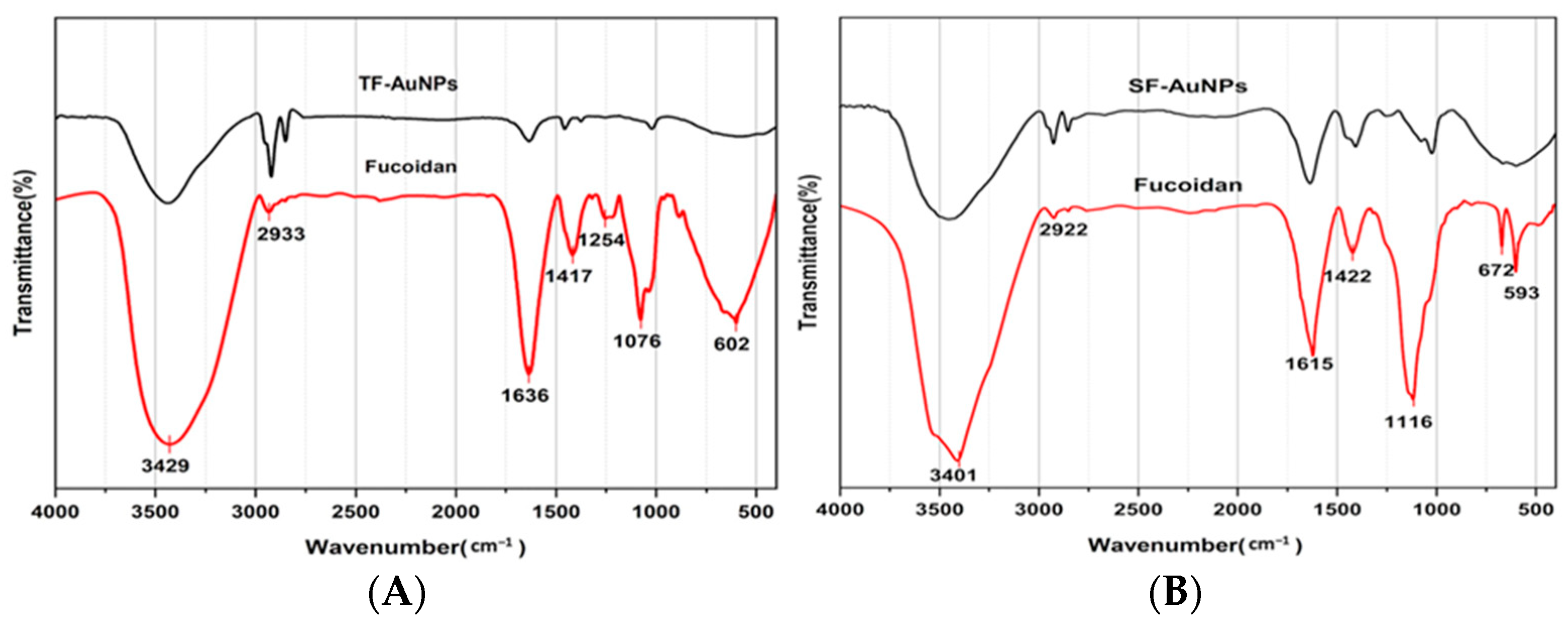
3.3.1. Fucoidan–Gold Nanoparticle FTIR Spectroscopy Analysis
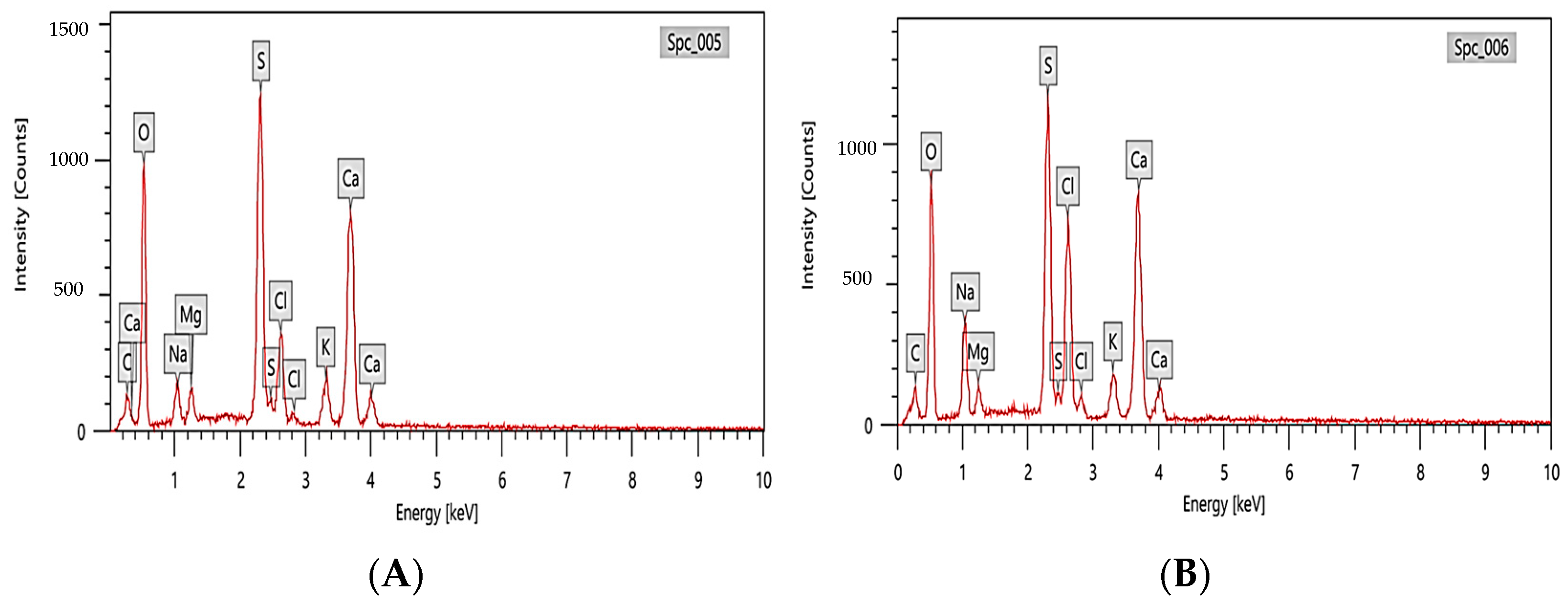
3.3.2. EDX Analysis of Fucoidan Polysaccharides Rich in Fucoidan
3.4. Characterization of Au-NPs
3.4.1. Morphological Visual Observations
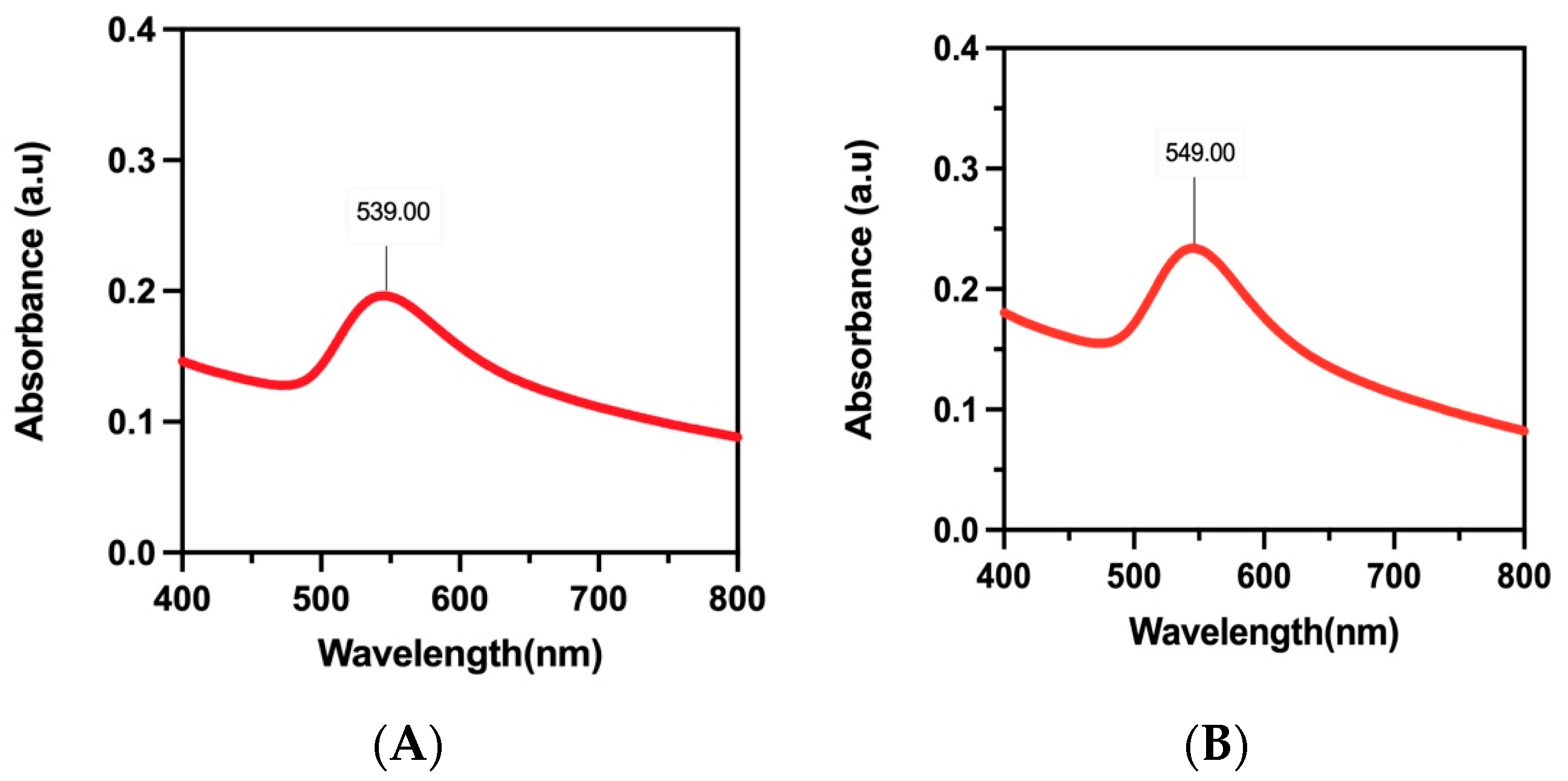
3.4.2. UV–Vis Spectral Studies
3.4.3. Fucoidan–Gold Nanoparticle FTIR Spectroscopy Analysis
3.4.4. X-Ray Diffraction Analysis of F-AuNPs
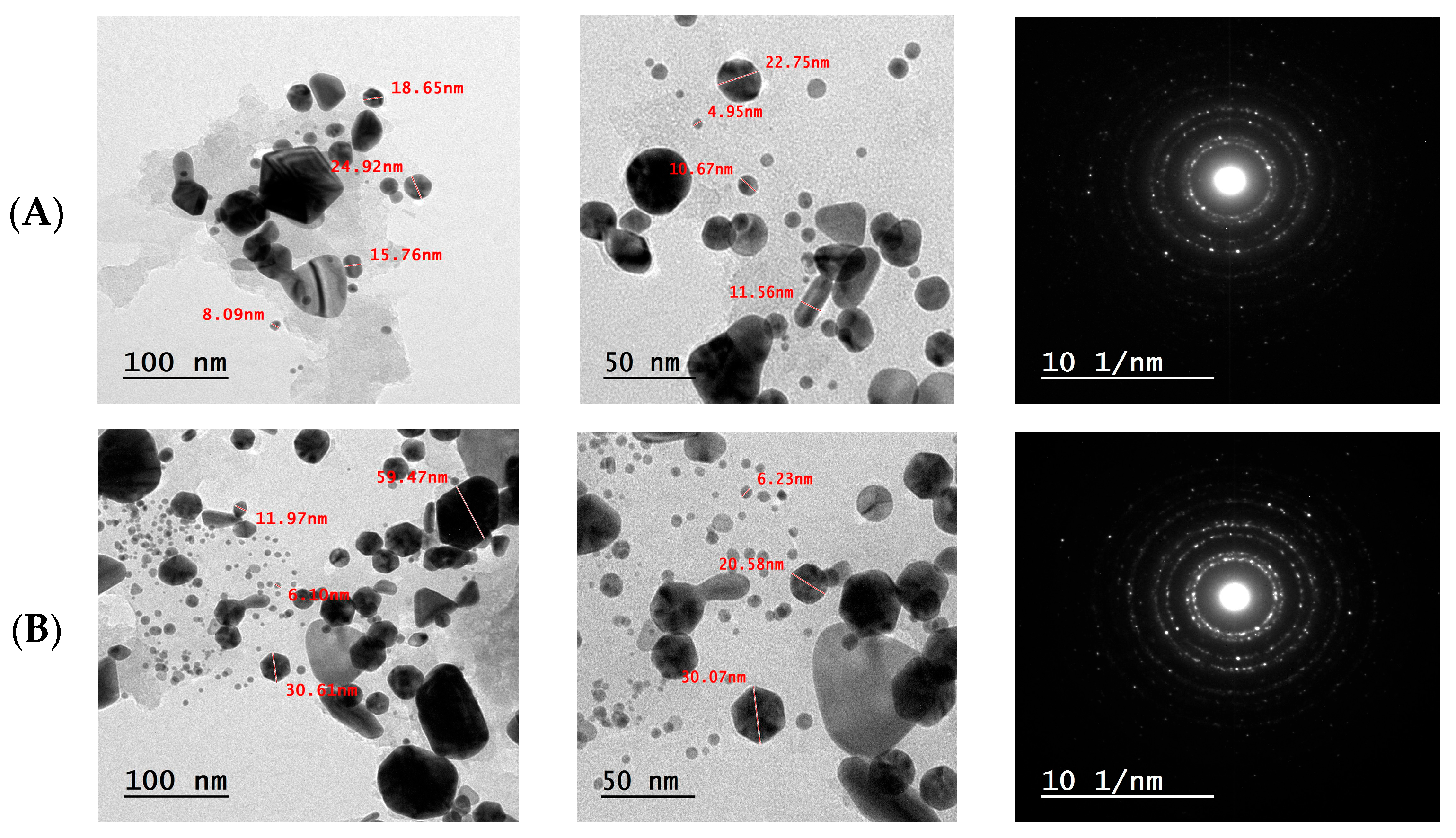
3.4.5. Transmission Electron Microscopy (TEM) Analysis of F-AuNPs
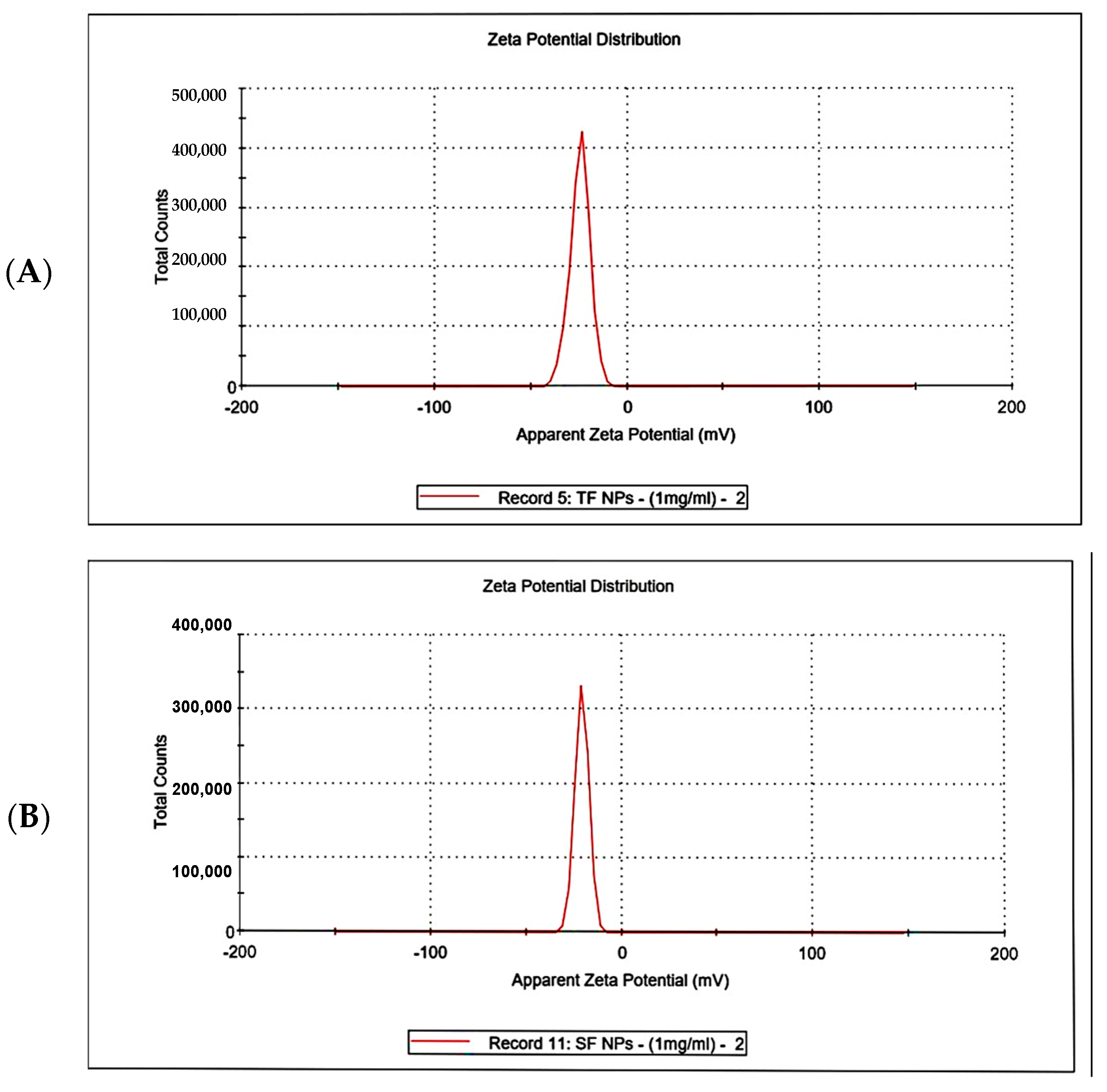
3.4.6. Zeta Potential Analysis
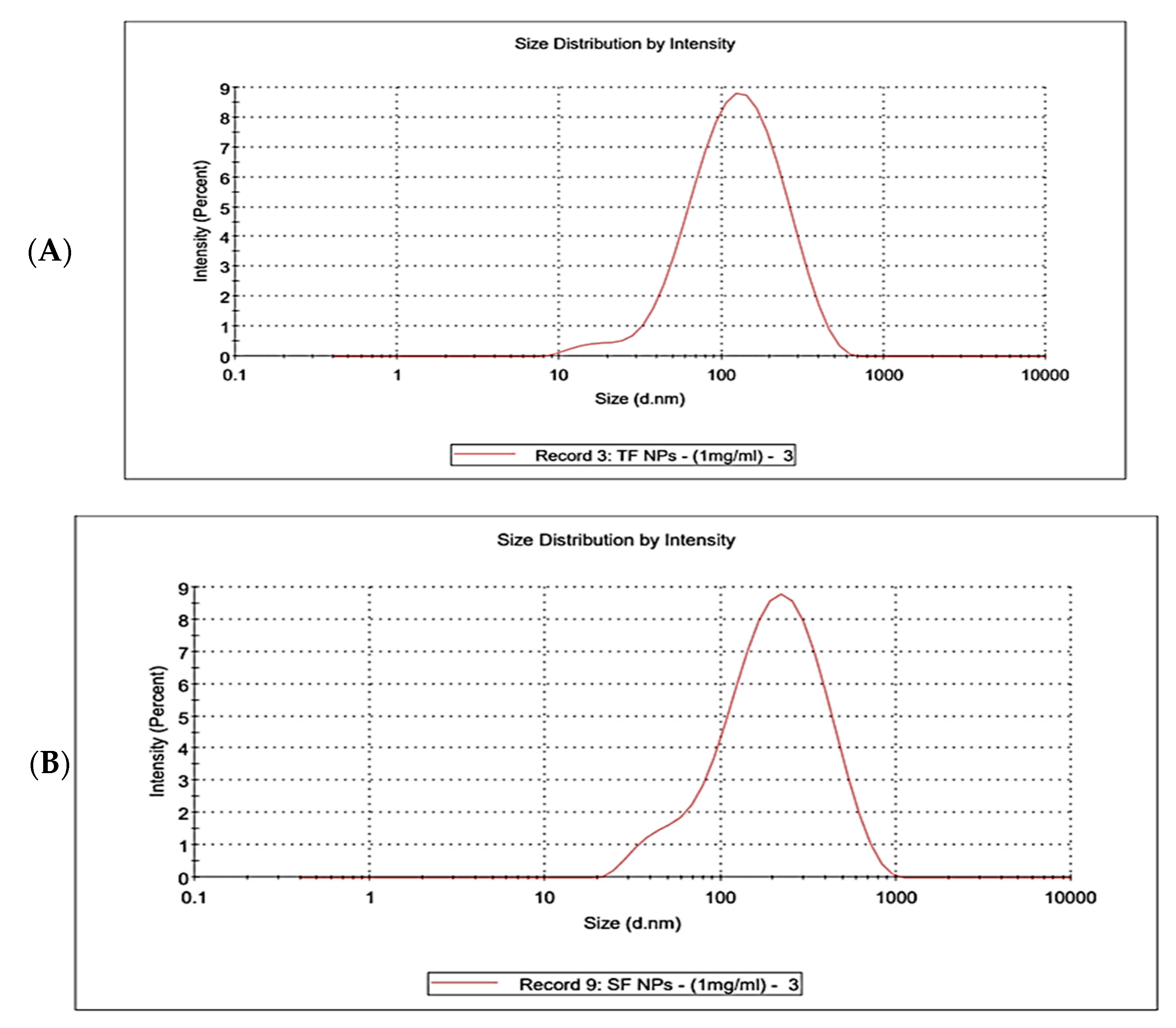
3.4.7. Dynamic Light Scattering (DLS)
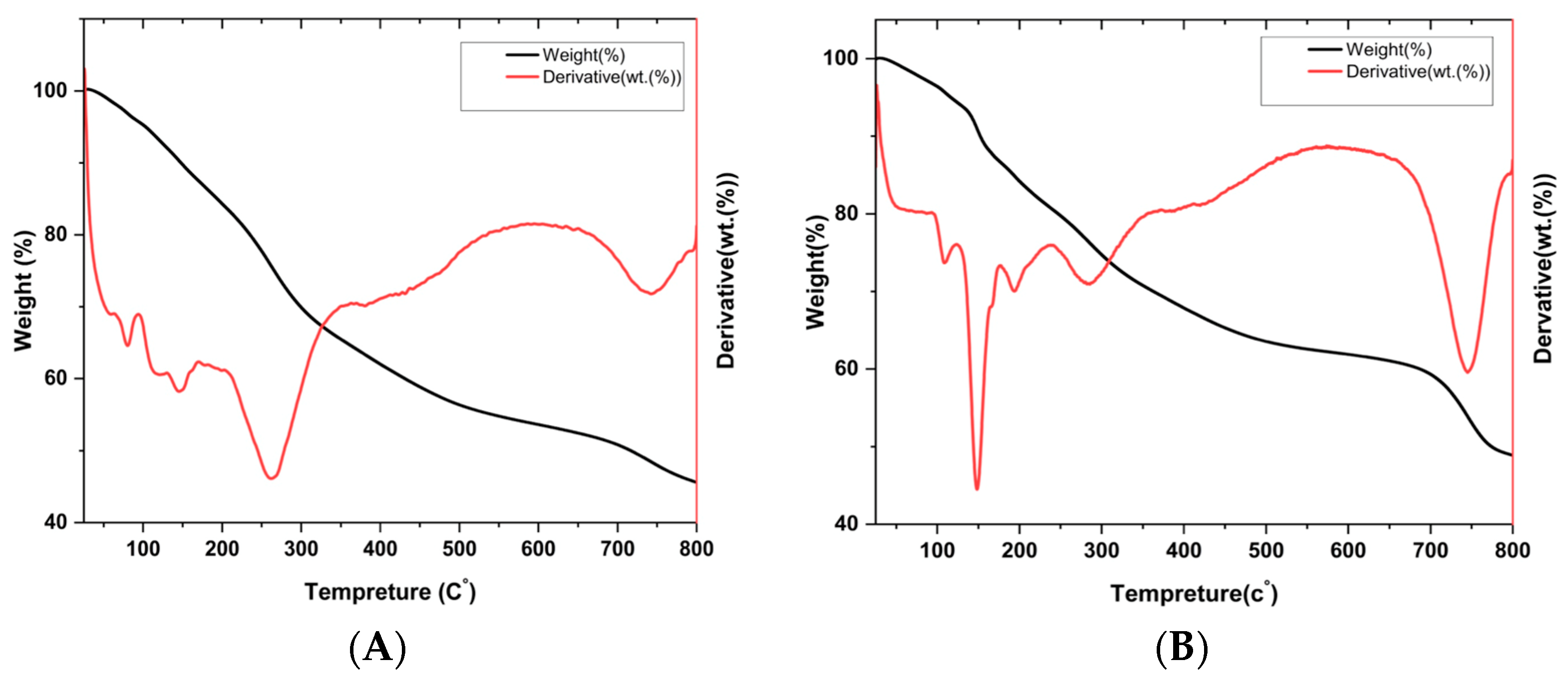
3.4.8. Thermogravimetric Analysis (TGA)
3.5. Antioxidant Activity
3.5.1. DPPH Free Radical Scavenging Assay
3.5.2. Ferric-Reducing Antioxidant Power (FRAP) Assay
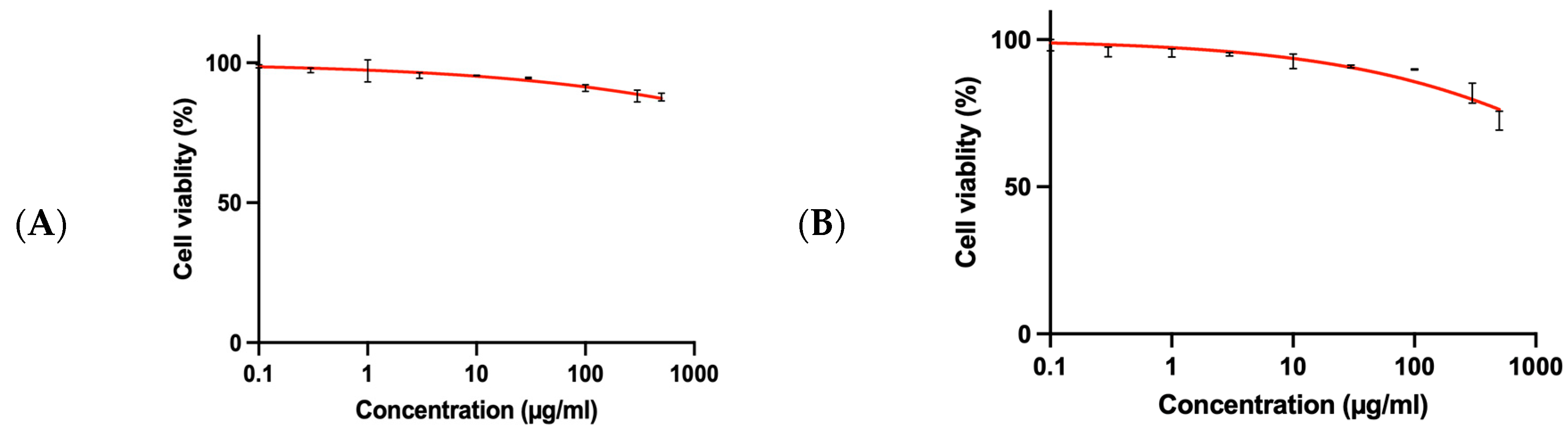
3.6. SRB Assay for Normal BNL Cells
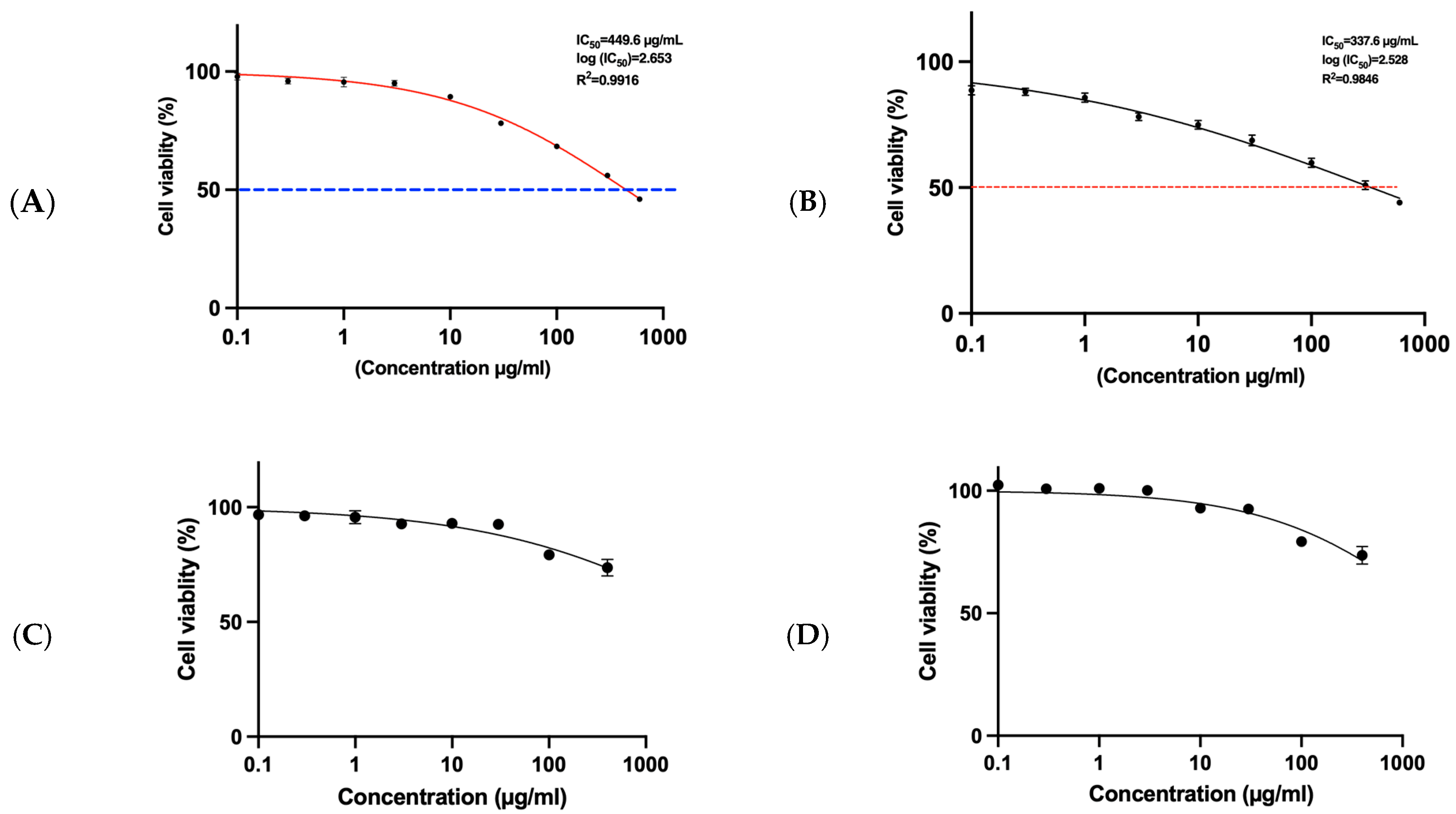
3.7. MTT Assay for HepG2 Cells and SRB Assay for THP1
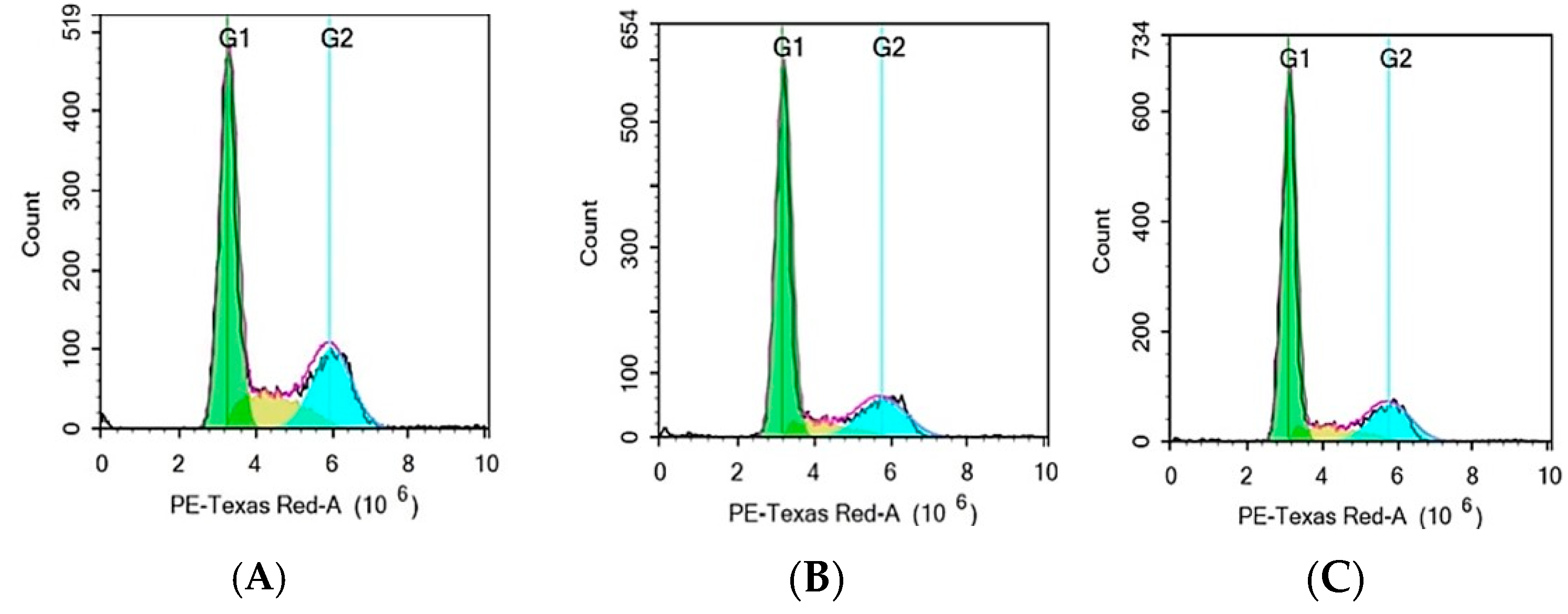
3.8. Cell Cycle Analysis by Flow Cytometry
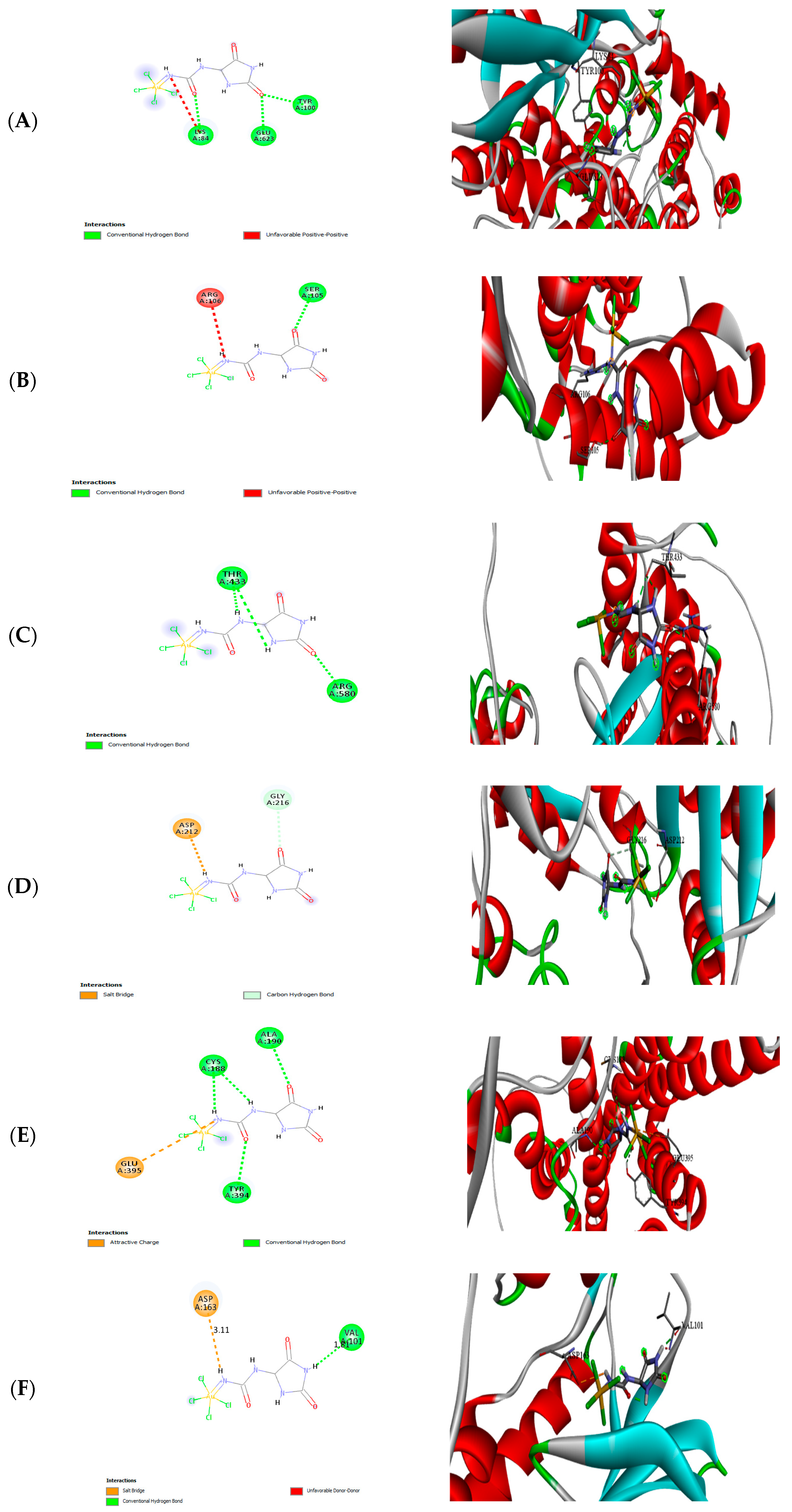
3.9. Molecular Docking
4. Discussion
5. Study Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koul, B. Herbs for Cancer Treatment; Springer Nature: Berlin/Heidelberg, Germany, 2020; ISBN 978-981-329-147-8. [Google Scholar]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring Treatment Options in Cancer: Tumor Treatment Strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K. The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery. In Comprehensive Pharmacology; ResearchGate: Berlin, Germany, 2022. [Google Scholar]
- Marine Natural Products: A Source of Novel Anticancer Drugs. Available online: https://www.mdpi.com/1660-3397/17/9/491 (accessed on 11 June 2025).
- Osman, M.E.H.; Elshobary, M.E.; Abu-Shady, A.M. The Seasonal Fluctuation of the Antimicrobial Activity of Some Macroalgae Collected from Alexandria Coast, Egypt. In Salmonella—Distribution, Adaptation, Control Measures and Molecular Technologies; Annous, B., Ed.; InTech: Tokyo, Japan, 2012; ISBN 978-953-51-0661-6. [Google Scholar]
- El-Sapagh, S.; El-Shenody, R.; Pereira, L.; Elshobary, M. Unveiling the Potential of Algal Extracts as Promising Antibacterial and Antibiofilm Agents against Multidrug-Resistant Pseudomonas Aeruginosa: In Vitro and In Silico Studies Including Molecular Docking. Plants 2023, 12, 3324. [Google Scholar] [CrossRef]
- Ashour, M.; Al-Souti, A.S.; Hassan, S.M.; Ammar, G.A.G.; Goda, A.M.A.-S.; El-Shenody, R.; Abomohra, A.E.-F.; El-Haroun, E.; Elshobary, M.E. Commercial Seaweed Liquid Extract as Strawberry Biostimulants and Bioethanol Production. Life 2022, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Golberg, A.; Vitkin, E.; Yakhini, Z. Seaweed biorefineries: Exergy efficiency, fermentation and sustainability implications; example of potential production of bioethanol from Kappaphycus alvarezzi in Philippines. In Proceedings of the ECOS 2015—28th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, Pau, France, 29 June–3 July 2015; pp. 1–12. [Google Scholar]
- El-Sayed, H.S.; Elshobary, M.E.; Barakat, K.M.; Khairy, H.M.; El-Sheikh, M.A.; Czaja, R.; Allam, B.; Senousy, H.H. Ocean Acidification Induced Changes in Ulva Fasciata Biochemistry May Improve Dicentrarchus Labrax Aquaculture via Enhanced Antimicrobial Activity. Aquaculture 2022, 560, 738474. [Google Scholar] [CrossRef]
- Pereira, L.; Valado, A. Harnessing the Power of Seaweed: Unveiling the Potential of Marine Algae in Drug Discovery. Explor. Drug Sci. 2023, 1, 475–496. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O. Antiproliferative and Apoptosis-Inducing Effects of Aqueous Extracts from Ecklonia Maxima and Ulva Rigida on HepG2 Cells. J. Food Biochem. 2022, 46, e14498. [Google Scholar] [CrossRef]
- Cao, J.; Liu, J.; Zhao, S.; Tong, Y.; Li, S.; Xia, Z.; Hu, M.; Sun, Y.; Zhang, J.; He, P. Advances in the Research on Micropropagules and Their Role in Green Tide Outbreaks in the Southern Yellow Sea|Request PDF. Mar. Pollut. Bull. 2023, 188, 114710. [Google Scholar] [CrossRef]
- Mensah, E.; Kanwugu, O.; Panda, P.; Adadi, P. Marine Fucoidans: Structural, Extraction, Biological Activities and Their Applications in the Food Industry. Food Hydrocoll. 2023, 142, 108784. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and Cancer: A Multifunctional Molecule with Anti-Tumor Potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jeon, Y.-J. Fucoidans as Scientifically and Commercially Important Algal Polysaccharides. Mar. Drugs 2021, 19, 284. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Wang, H.; Chen, J.; Zan, X.; Zhang, C.; Qian, J.; Zhu, F.; Ma, H.; Elshobary, M. A Preliminary Study on Polysaccharide Extraction, Purification, and Antioxidant Properties of Sugar-Rich Filamentous Microalgae Tribonema Minus. J. Appl. Phycol. 2022, 34, 2755–2767. [Google Scholar] [CrossRef]
- Barakat, K.M.; Ismail, M.M.; Abou El Hassayeb, H.E.; El Sersy, N.A.; Elshobary, M.E. Chemical Characterization and Biological Activities of Ulvan Extracted from Ulva Fasciata (Chlorophyta). Rendiconti Lincei Sci. Fis. E Nat. 2022, 33, 829–841. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown Seaweed Fucoidan: Biological Activity and Apoptosis, Growth Signaling Mechanism in Cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Bui, N.Q.; Jang, B.; Oh, Y.-O.; Lim, I.G.; Oh, J. Doxorubicin-Loaded Fucoidan Capped Gold Nanoparticles for Drug Delivery and Photoacoustic Imaging. Int. J. Biol. Macromol. 2016, 91, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Jiang, Z. Platinum–Iron Nanoparticles for Oxygen-Enhanced Sonodynamic Tumor Cell Suppression. Inorganics 2024, 12, 331. [Google Scholar] [CrossRef]
- Nie, Y.; Li, D.; Peng, Y.; Wang, S.; Hu, S.; Liu, M.; Ding, J.; Zhou, W. Metal Organic Framework Coated MnO2 Nanosheets Delivering Doxorubicin and Self-Activated DNAzyme for Chemo-Gene Combinatorial Treatment of Cancer. Int. J. Pharm. 2020, 585, 119513. [Google Scholar] [CrossRef]
- Green Synthesis of Gold Nanoparticles Using Plant Extracts as Reducing|IJN. Available online: https://www.dovepress.com/green-synthesis-of-gold-nanoparticles-using-plant-extracts-as-reducing-peer-reviewed-fulltext-article-IJN (accessed on 12 June 2025).
- Hameed, I.H.; Altameme, H.J.; Mohammed, G.J. Evaluation of Antifungal and Antibacterial Activity and Analysis of Bioactive Phytochemical Compounds of Cinnamomum Zeylanicum (Cinnamon Bark) Using Gas Chromatography-Mass Spectrometry. Orient. J. Chem. 2016, 32, 1769–1788. [Google Scholar] [CrossRef]
- Corso, G.; Deng, A.; Fry, B.; Polizzi, N.; Barzilay, R.; Jaakkola, T. Deep Confident Steps to New Pockets: Strategies for Docking Generalization. arXiv 2024, arXiv:2402.18396v1. [Google Scholar]
- F.J.R. “Max” Taylor. Available online: http://www.dinophyta.org/literature/bibliographies/max-taylor/ (accessed on 12 June 2025).
- Taylor, F. The taxonomy and relationships of red tide flagellates. In Toxic Dinoflagelletas; Elsevier: Amsterdam, The Netherlands, 1985; pp. 11–26. [Google Scholar]
- Oza, R.M.; Zaidi, S. A revised checklist of Indian marine algae. CSMCRI Bhavnagar 2001, 296. [Google Scholar]
- Aleem, A.A. 1993: AlgaeBase. Available online: https://www.algaebase.org/search/bibliography/detail/?biblio_id=q7e6cea4cf2f83c44 (accessed on 12 June 2025).
- About AlgaeBase: AlgaeBase. Available online: https://www.algaebase.org/about/ (accessed on 12 June 2025).
- Tako, M. Rheological Characteristics of Fucoidan Isolated from Commercially Cultured Cladosiphon Okamuranus. Bot. Mar. 2003, 46, 461–465. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C.; Gnanajobitha, G.; Paulkumar, K.; Vanaja, M.; Kannan, C.; Annadurai, G. Seaweed-Mediated Synthesis of Gold Nanoparticles Using Turbinaria Conoides and Its Characterization. J. Nanostructure Chem. 2013, 3, 44. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.; van Dam, J.E. Characterisation of Structure-Dependent Functional Properties of Lignin with Infrared Spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Boly, R.; Boly, A.G.L.; Belem-Kabré, W.L.M.E.; Traoré, K.T.; Kaboré, B.; Youl, O.; Sawadogo, L.; Nitièma, M.; Koala, M.; Ouédraogo, N. Anti-inflammatory and antioxidant properties of the aqueous extracts of the leaves of Opilia amentacea (Opiliaceae). Pharmacol. Pharm. 2023, 14, 329–347. [Google Scholar] [CrossRef]
- Elkholy, N.S.; Hariri, M.L.M.; Mohammed, H.S.; Shafaa, M.W. Lutein and β-Carotene Characterization in Free and Nanodispersion Forms in Terms of Antioxidant Activity and Cytotoxicity. J. Pharm. Innov. 2023, 18, 1727–1744. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ahmed, A.H.H.; Mohamed, M.F.A.; Allam, R.M.; Nafady, A.; Mohamed, S.K.; Gouda, A.E.; Beshr, E.A.M. Design, Synthesis, and Molecular Docking of Novel Pyrazole-Chalcone Analogs of Lonazolac as 5-LOX, iNOS and Tubulin Polymerization Inhibitors with Potential Anticancer and Anti-Inflammatory Activities. Bioorganic Chem. 2022, 129, 106171. [Google Scholar] [CrossRef]
- Allam, R.M.; Al-Abd, A.M.; Khedr, A.; Sharaf, O.A.; Nofal, S.M.; Khalifa, A.E.; Mosli, H.A.; Abdel-Naim, A.B. Fingolimod Interrupts the Cross Talk between Estrogen Metabolism and Sphingolipid Metabolism within Prostate Cancer Cells. Toxicol. Lett. 2018, 291, 77–85. [Google Scholar] [CrossRef]
- Cytotoxicity of Thymoquinone Alone or in Combination with Cisplatin (CDDP) Against Oral Squamous Cell Carcinoma In Vitro|Scientific Reports. Available online: https://www.nature.com/articles/s41598-017-13357-5 (accessed on 12 June 2025).
- Sharma, A.; Marceau, C.; Hamaguchi, R.; Burridge, P.W.; Rajarajan, K.; Churko, J.M.; Wu, H.; Sallam, K.I.; Matsa, E.; Sturzu, A.C.; et al. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes as an in Vitro Model for Coxsackievirus B3-Induced Myocarditis and Antiviral Drug Screening Platform. Circ. Res. 2014, 115, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Fekry, M.I.; Ezzat, S.M.; Salama, M.M.; Alshehri, O.Y.; Al-Abd, A.M. Bioactive Glycoalkaloides Isolated from Solanum Melongena Fruit Peels with Potential Anticancer Properties against Hepatocellular Carcinoma Cells. Sci. Rep. 2019, 9, 1746. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Liu, X.; Jin, X.; Ou, H.; Qian, C.; Wu, H.; Zuo, C.; Ren, Y.; Fu, M.; Zhang, T.; Zhang, L.; et al. The Direct STAT3 Inhibitor 6-Ethoxydihydrosanguinarine Exhibits Anticancer Activity in Gastric Cancer. Acta Mater. Medica 2022, 1, 365–380. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Vranken, S.; Robuchon, M.; Dekeyzer, S.; Bárbara, I.; Bartsch, I.; Blanfuné, A.; Boudouresque, C.-F.; Decock, W.; Destombe, C.; De Reviers, B.; et al. AlgaeTraits: A Trait Database for (European) Seaweeds. Earth Syst. Sci. Data 2023, 15, 2711–2754. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from Brown Seaweeds: An Update on Structures, Extraction Techniques and Use of Enzymes as Tools for Structural Elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Alwaleed, E.A.; Kassem, W.M.A.; Saber, H. Optimizing the Fucoidan Extraction Using Box-Behnken Design and Its Potential Bioactivity. Int. J. Biol. Macromol. 2024, 277, 134490. [Google Scholar] [CrossRef]
- Lutfia, F.N.L.; Isnansetyo, A.; Susidarti, R.A.; Nursid, M. Chemical Composition Diversity of Fucoidans Isolated from Three Tropical Brown Seaweeds (Phaeophyceae) Species. Biodivers. J. Biol. Divers. 2020, 21. [Google Scholar] [CrossRef]
- Souza, A.O.; Oliveira, J.W.d.F.; Moreno, C.J.G.; de Medeiros, M.J.C.; Fernandes-Negreiros, M.M.; Souza, F.R.M.; Pontes, D.L.; Silva, M.S.; Rocha, H.A.O. Silver Nanoparticles Containing Fucoidan Synthesized by Green Method Have Anti-Trypanosoma Cruzi Activity. Nanomaterials 2022, 12, 2059. [Google Scholar] [CrossRef] [PubMed]
- El-Sapagh, S.H.; El-Zawawy, N.A.; Elshobary, M.E.; Alquraishi, M.; Zabed, H.M.; Nouh, H.S. Harnessing the Power of Neobacillus Niacini AUMC-B524 for Silver Oxide Nanoparticle Synthesis: Optimization, Characterization, and Bioactivity Exploration. Microb. Cell Factories 2024, 23, 220. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of Fucoidan from Sargassum Polycystum Brown Algae: Structural Characterization, in Vitro Antioxidant and Anticancer Activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanasenthil, S.; Vinothkumar, T.; Geetharamani, D.; Marudhupandi, T.; Suja, G.; Sindhu, N.S. Fucoidan—A Novel α-Amylase Inhibitor from Turbinaria Ornata with Relevance to NIDDM Therapy. Biocatal. Agric. Biotechnol. 2014, 3, 66–70. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Fernando, I.P.S.; Lee, W.W.; Sanjeewa, K.K.A.; Kim, H.-S.; Lee, D.-S.; Jeon, Y.-J. Isolation and Purification of Fucoidan Fraction in Turbinaria Ornata from the Maldives; Inflammation Inhibitory Potential under LPS Stimulated Conditions in in-Vitro and in-Vivo Models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef]
- Singthong, J.; Cui, S.W.; Ningsanond, S.; Douglas Goff, H. Structural Characterization, Degree of Esterification and Some Gelling Properties of Krueo Ma Noy (Cissampelos pareira) Pectin. Carbohydr. Polym. 2004, 58, 391–400. [Google Scholar] [CrossRef]
- Khan, F.; Manivasagan, P.; Lee, J.-W.; Pham, D.T.N.; Oh, J.; Kim, Y.-M. Fucoidan-Stabilized Gold Nanoparticle-Mediated Biofilm Inhibition, Attenuation of Virulence and Motility Properties in Pseudomonas Aeruginosa PAO1. Mar. Drugs 2019, 17, 208. [Google Scholar] [CrossRef]
- Puhari, S.S.M.; Yuvaraj, S.; Vasudevan, V.; Ramprasath, T.; Rajkumar, P.; Arunkumar, K.; Amutha, C.; Selvam, G.S. Isolation and Characterization of Fucoidan from Four Brown Algae and Study of the Cardioprotective Effect of Fucoidan from Sargassum Wightii against High Glucose-Induced Oxidative Stress in H9c2 Cardiomyoblast Cells. J. Food Biochem. 2022, 46, e14412. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kumar, R.; Alhan, S.; Sharma, H.; Singh, N.; Yogi, R.; Chhokar, V.; Beniwal, V.; Kumar Ghosh, M.; Kumar Chandraker, S.; et al. Lycium shawii Mediated Green Synthesis of Silver Nanoparticles, Characterization and Assessments of Their Phytochemical, Antioxidant, Antimicrobial Properties. Inorg. Chem. Commun. 2024, 159, 111735. [Google Scholar] [CrossRef]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; et al. Biosynthesis of Silver and Gold Nanoparticles by Novel Sundried Cinnamomum Camphora Leaf. Nanotechnology 2007, 18, 105104. [Google Scholar] [CrossRef]
- Surface Plasmon Spectroscopy of Nanosized Metal|PDF|Plasmon|Adsorption. Available online: https://www.scribd.com/document/93435203/Surface-Plasmon-Spectroscopy-of-Nanosized-Metal (accessed on 13 June 2025).
- Structural Characterization and Anti-Inflammatory Activity of Fucoidan Isolated from Ecklonia Maxima Stipe. Available online: https://www.e-algae.org/journal/view.php?number=2979 (accessed on 12 June 2025).
- Firdhouse, M.J.; Lalitha, P. Biogenic Silver Nanoparticles—Synthesis Characterization and Its Potential Against Cancer Inducing Bacteria. J. Mol. Liq. 2016, 222, 1041–1050. [Google Scholar] [CrossRef]
- Rokkarukala, S.; Cherian, T.; Ragavendran, C.; Mohanraju, R.; Kamaraj, C.; Almoshari, Y.; Albariqi, A.; Sultan, M.H.; Alsalhi, A.; Mohan, S. One-pot green synthesis of gold nanoparticles using Sarcophyton crassocaule, a marine soft coral: Assessing biological potentialities of antibacterial, antioxidant, anti-diabetic and catalytic degradation of toxic organic pollutants. Heliyon 2023, 9, e14668. [Google Scholar] [CrossRef]
- Manivasagan, P.; Oh, J. Production of a Novel Fucoidanase for the Green Synthesis of Gold Nanoparticles by Streptomyces Sp. and Its Cytotoxic Effect on HeLa Cells. Mar. Drugs 2015, 13, 6818–6837. [Google Scholar] [CrossRef]
- Venkatesan, J.; Murugan, S.S.; Seong, G.H. Fucoidan-Based Nanoparticles: Preparations and Applications. Int. J. Biol. Macromol. 2022, 217, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Ali Dheyab, M.; Abdul Aziz, A.; Jameel, M.S.; Moradi Khaniabadi, P.; Oglat, A.A. Rapid Sonochemically-Assisted Synthesis of Highly Stable Gold Nanoparticles as Computed Tomography Contrast Agents. Appl. Sci. 2020, 10, 7020. [Google Scholar] [CrossRef]
- Paul, K.G.; Frigo, T.B.; Groman, J.Y.; Groman, E.V. Synthesis of Ultrasmall Superparamagnetic Iron Oxides Using Reduced Polysaccharides. Bioconjug. Chem. 2004, 15, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dixit, C.K. 3—Methods for Characterization of Nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chandra, R., Gupta, N., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 43–58. ISBN 978-0-08-100557-6. [Google Scholar]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Khaniabadi, P.M.; Mehrdel, B. Simple Rapid Stabilization Method through Citric Acid Modification for Magnetite Nanoparticles. Sci. Rep. 2020, 10, 10793. [Google Scholar] [CrossRef]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Bagheri, S.; Aghaei, H.; Ghaedi, M.; Asfaram, A.; Monajemi, M.; Bazrafshan, A.A. Synthesis of Nanocomposites of Iron Oxide/Gold (Fe3O4/Au) Loaded on Activated Carbon and Their Application in Water Treatment by Using Sonochemistry: Optimization Study. Ultrason. Sonochem. 2018, 41, 279–287. [Google Scholar] [CrossRef]
- Jang, H.; Kang, K.; El-Sayed, M.A. Facile size-controlled synthesis of fucoidan-coated gold nanoparticles and cooperative anticancer effect with doxorubicin. J. Mater. Chem. B 2017, 5, 6147–6153. [Google Scholar] [CrossRef]
- Kumar, M.; Jha, A.; Bharti, K.; Manjit; Mishra, B. Fucoidan-Based Bosutinib Nanocrystals for Pulmonary Drug Delivery: Solid State Characterization and In-Vitro Assessment. Chem. Phys. Impact 2024, 8, 100644. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important Determinants for Fucoidan Bioactivity: A Critical Review of Structure-Function Relations and Extraction Methods for Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Bourakhouadar, M. Kinetics and Mechanism of the Thermal Degradation of Biopolymers Chitin and Chitosan Using Thermogravimetric Analysis. Polym. Degrad. Stab. 2016, 130, 1–9. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from Fucoidan; Multifunctional Marine Polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, Z.; He, X.; Song, M.; Westerhoff, P.; Doudrick, K.; Hanigan, D. Critical Review of Thermal Decomposition of Per- and Polyfluoroalkyl Substances: Mechanisms and Implications for Thermal Treatment Processes. Environ. Sci. Technol. 2022, 56, 5355–5370. [Google Scholar] [CrossRef]
- Griffiths, M.B.E.; Dubrawski, Z.S.; Bačić, G.; Japahuge, A.; Masuda, J.D.; Zeng, T.; Barry, S.T. Controlling the Thermal Stability and Volatility of Organogold(I) Compounds for Vapor Deposition with Complementary Ligand Design. Eur. J. Inorg. Chem. 2019, 2019, 4927–4938. [Google Scholar] [CrossRef]
- Hasan, E.A.; El-Hashash, M.A.; Zahran, M.K.; El-Rafie, H.M. Comparative Study of Chemical Composition, Antioxidant and Anticancer Activities of Both Turbinaria Decurrens Bory Methanol Extract and Its Biosynthesized Gold Nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 67, 103005. [Google Scholar] [CrossRef]
- Husni, A.; Izmi, N.; Ayunani, F.; Kartini, A.; Husnayain, N.; Isnansetyo, A. Characteristics and Antioxidant Activity of Fucoidan from Sargassum Hystrix: Effect of Extraction Method. Int. J. Food Sci. 2022, 2022, 3689724. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Simchi, A.; Milani, A.S.; Stroeve, P. Cell Toxicity of Superparamagnetic Iron Oxide Nanoparticles. J. Colloid Interface Sci. 2009, 336, 510–518. [Google Scholar] [CrossRef]
- Demirgan, R.; Karagöz, A.; Pekmez, M.; Önay-Uçar, E.; Artun, F.T.; Gürer, Ç.; Mat, A. In Vitro Anticancer Activity and Cytotoxicity of Some Papaver Alkaloids on Cancer and Normal Cell Lines. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 22–26. [Google Scholar] [CrossRef]
- Zbakh, H.; Zubía, E.; de los Reyes, C.; Calderón-Montaño, J.M.; López-Lázaro, M.; Motilva, V. Meroterpenoids from the Brown Alga Cystoseira Usneoides as Potential Anti-Inflammatory and Lung Anticancer Agents. Mar. Drugs 2020, 18, 207. [Google Scholar] [CrossRef]
- El-Deeb, N.M.; Khattab, S.M.; Abu-Youssef, M.A.; Badr, A.M.A. Green Synthesis of Novel Stable Biogenic Gold Nanoparticles for Breast Cancer Therapeutics via the Induction of Extrinsic and Intrinsic Pathways. Sci. Rep. 2022, 12, 11518. [Google Scholar] [CrossRef] [PubMed]
- Agasti, S.S.; Chompoosor, A.; You, C.-C.; Ghosh, P.; Kim, C.K.; Rotello, V.M. Photoregulated Release of Caged Anticancer Drugs from Gold Nanoparticles. J. Am. Chem. Soc. 2009, 131, 5728–5729. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jia, J.; Jiang, C.; Zhai, S. Gold Nanoparticle-Induced Cell Death and Potential Applications in Nanomedicine. Int. J. Mol. Sci. 2018, 19, 754. [Google Scholar] [CrossRef] [PubMed]
- Vairavel, M.; Devaraj, E.; Shanmugam, R. An Eco-Friendly Synthesis of Enterococcus Sp.-Mediated Gold Nanoparticle Induces Cytotoxicity in Human Colorectal Cancer Cells. Environ. Sci. Pollut. Res. Int. 2020, 27, 8166–8175. [Google Scholar] [CrossRef]
- Kamala Priya, M.R.; Iyer, P.R. Anticancer Studies of the Synthesized Gold Nanoparticles against MCF 7 Breast Cancer Cell Lines. Appl. Nanosci. 2015, 5, 443–448. [Google Scholar] [CrossRef]
- Rattanata, N.; Daduang, S.; Wongwattanakul, M.; Leelayuwat, C.; Limpaiboon, T.; Lekphrom, R.; Sandee, A.; Boonsiri, P.; Chio-Srichan, S.; Daduang, J. Gold Nanoparticles Enhance the Anticancer Activity of Gallic Acid against Cholangiocarcinoma Cell Lines. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 7143–7147. [Google Scholar] [CrossRef]
- Kajani, A.A.; Bordbar, A.-K.; Esfahani, S.H.Z.; Razmjou, A. Gold Nanoparticles as Potent Anticancer Agent: Green Synthesis, Characterization, and in Vitro Study. RSC Adv. 2016, 6, 63973–63983. [Google Scholar] [CrossRef]
- Nandhini, J.T.; Ezhilarasan, D.; Rajeshkumar, S. An Ecofriendly Synthesized Gold Nanoparticles Induces Cytotoxicity via Apoptosis in HepG2 Cells. Environ. Toxicol. 2021, 36, 24–32. [Google Scholar] [CrossRef]
- Islam, M.S.; Kusumoto, Y.; Al-Mamun, M.A. Cytotoxicity and Cancer (HeLa) Cell Killing Efficacy of Aqueous Garlic (Allium Sativum) Extract. J. Sci. Res. 2011, 3, 375–382. [Google Scholar] [CrossRef]
- Raghunandan, D.; Ravishankar, B.; Sharanbasava, G.; Mahesh, D.B.; Harsoor, V.; Yalagatti, M.S.; Bhagawanraju, M.; Venkataraman, A. Anti-Cancer Studies of Noble Metal Nanoparticles Synthesized Using Different Plant Extracts. Cancer Nanotechnol. 2011, 2, 57–65. [Google Scholar] [CrossRef]
- Hamed, M.M.; Abdelftah, L.S. Biosynthesis of Gold Nanoparticles Using Marine Streptomyces Griseus Isolate (M8) and Evaluating Its Antimicrobial and Anticancer Activity. Egypt. J. Aquat. Biol. Fish. 2019, 23, 173–184. [Google Scholar] [CrossRef]
- Zahariev, N.; Katsarov, P.; Lukova, P.; Pilicheva, B. Novel Fucoidan Pharmaceutical Formulations and Their Potential Application in Oncology—A Review. Polymers 2023, 15, 3242. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.-Y.; Zhou, H.-H.; Mao, X.-Y. Emerging Roles of 5-lipoxygenase Phosphorylation in Inflammation and Cell Death. Oxid. Med. Cell. Longev. 2019, 2019, 2749173. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.Y.; Hurst, E.A.; Argyle, D.J. Cyclooxygenase-2: A Role in Cancer Stem Cell Survival and Repopulation of Cancer Cells during Therapy. Stem Cells Int. 2016, 2016, 2048731. [Google Scholar] [CrossRef]
- Yuan, X.; Larsson, C.; Xu, D. Mechanisms Underlying the Activation of TERT Transcription and Telomerase Activity in Human Cancer: Old Actors and New Players. Oncogene 2019, 38, 6172–6183. [Google Scholar] [CrossRef]
- Liu, N.-B.; Peng, T.; Pan, C.; Yao, Y.-Y.; Shen, B.; Leng, J. Overexpression of Cyclooxygenase-2 in Human HepG2, Bel-7402 and SMMC-7721 Hepatoma Cell Lines and Mechanism of Cyclooxygenase-2 Selective Inhibitor Celecoxib-Induced Cell Growth Inhibition and Apoptosis. World J. Gastroenterol. WJG 2005, 11, 6281–6287. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, D.; Fan, L.; Li, Y.; Liu, Y.; Yu, H.; Wang, H.; Liu, J.; Sun, G. COX-2 Induces Apoptosis-Resistance in Hepatocellular Carcinoma Cells via the HIF-1α/PKM2 Pathway. Int. J. Mol. Med. 2019, 43, 475–488. [Google Scholar] [CrossRef]
- in der Stroth, L.; Tharehalli, U.; Günes, C.; Lechel, A. Telomeres and Telomerase in the Development of Liver Cancer. Cancers 2020, 12, 2048. [Google Scholar] [CrossRef]
- Cevik, D.; Yildiz, G.; Ozturk, M. Common Telomerase Reverse Transcriptase Promoter Mutations in Hepatocellular Carcinomas from Different Geographical Locations. World J. Gastroenterol. WJG 2015, 21, 311–317. [Google Scholar] [CrossRef]
- Chen, J.; Hu, X.-Y. Inhibition of Histamine Receptor H3R Suppresses Prostate Cancer Growth, Invasion and Increases Apoptosis via the AR Pathway. Oncol. Lett. 2018, 16, 4921–4928. [Google Scholar] [CrossRef] [PubMed]
- Bhambri, S.; Jha, P.C. Targeting Cyclin-Dependent Kinase 11: A Computational Approach for Natural Anti-Cancer Compound Discovery. Mol. Divers. 2025; online ahead of print. [Google Scholar]
- Haggag, Y.A.; Abd Elrahman, A.A.; Ulber, R.; Zayed, A. Fucoidan in Pharmaceutical Formulations: A Comprehensive Review for Smart Drug Delivery Systems. Mar. Drugs 2023, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H.; Stringer, D.N.; Park, A.Y.; Karpiniec, S.S. Therapies from Fucoidan: New Developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef] [PubMed]
- Abdollah, M.R.A.; Carter, T.J.; Jones, C.; Kalber, T.L.; Rajkumar, V.; Tolner, B.; Gruettner, C.; Zaw-Thin, M.; Baguña Torres, J.; Ellis, M.; et al. Fucoidan Prolongs the Circulation Time of Dextran-Coated Iron Oxide Nanoparticles. ACS Nano 2018, 12, 1156–1169. [Google Scholar] [CrossRef]




| Inhibition (%) | |||||
|---|---|---|---|---|---|
| Concentrations (µg mL−1) | Trolox | S. cinereum Fucoidan | S. cinereum F-AuNPs | T. decurrens Fucoidan | T. decurrens F-AuNPs |
| 3.90 | 11.73 ± 0.21 | 7.92 ± 0.10 | 10.82 ± 0.11 | 9.15 ± 0.25 | 12.05 ± 0.41 |
| 7.81 | 25.92 ± 0.39 | 17.51 ± 0.05 | 21.61 ± 0.08 | 13.17 ± 0.14 | 24.07 ± 0.36 |
| 15.60 | 47.15 ± 0.42 | 37.13 ± 0.08 | 43.23 ± 0.13 | 35.16 ± 0.12 | 39.06 ± 0.12 |
| 25.00 | 68.64 ± 0.15 | 59.17 ± 0.14 | 66.19 ± 0.09 | 59.35 ± 0.26 | 62.49 ± 0.31 |
| 31.25 | 83.80 ± 0.18 | 71.36 ± 0.21 | 80.49 ± 0.19 | 73.09 ± 0.23 | 78.11 ± 0.31 |
| Inhibition (%) | |||||
|---|---|---|---|---|---|
| Concentrations (µg mL−1) | Trolox | S. cinereum Fucoidan | S. cinereum F-AuNPs | T. decurrens Fucoidan | T. decurrens F-AuNPs |
| 25 | 0.09 ± 0.01 | 0.02 ± 0.04 | 0.07 ± 0.02 | 0.04 ± 0.15 | 0.07 ± 0.00 |
| 50 | 0.15 ± 0.02 | 0.11 ± 0.12 | 0.14 ± 0.03 | 0.09 ± 0.04 | 0.14 ± 0.01 |
| 100 | 0.34 ± 0.06 | 0.21 ± 0.13 | 0.28 ± 0.04 | 0.16 ± 0.09 | 0.28 ± 0.07 |
| 200 | 0.65 ± 0.00 | 0.27 ± 0.06 | 0.56 ± 0.03 | 0.32 ± 0.21 | 0.57 ± 0.02 |
| 400 | 1.40 ± 0.10 | 1.09 ± 0.12 | 1.12 ± 0.18 | 1.11 ± 0.01 | 1.14 ± 0.19 |
| No. | Receptors | Nanoparticles | Binding Affinity (Kcal mol−1) |
|---|---|---|---|
| 1 | Arachidonate 5-lipoxygenase | F-Au NAPs | −4.07 |
| 2 | Cyclooxygenase-2 | F-Au NAPs | −7.1 |
| 3 | Telomerase reverse transcriptase | F-Au NAPs | −5.4 |
| 4 | Thymidylate synthase | F-Au NAPs | −4.06 |
| 5 | Histamine H3 receptor | F-Au NAPs | −5.07 |
| 6 | Cyclin-Dependent Kinases | F-Au NAPs | −1.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newehy, A.S.E.; Gheda, S.F.; Ismail, M.M.; Aldisi, D.; Abulmeaty, M.M.A.; Elshobary, M.E. Fucoidan-Based Gold Nanoparticles: Antioxidant and Anticancer Potential from Turbinaria decurrens and Sargassum cinereum. Pharmaceutics 2025, 17, 826. https://doi.org/10.3390/pharmaceutics17070826
Newehy ASE, Gheda SF, Ismail MM, Aldisi D, Abulmeaty MMA, Elshobary ME. Fucoidan-Based Gold Nanoparticles: Antioxidant and Anticancer Potential from Turbinaria decurrens and Sargassum cinereum. Pharmaceutics. 2025; 17(7):826. https://doi.org/10.3390/pharmaceutics17070826
Chicago/Turabian StyleNewehy, Ahmed S. El, Saly F. Gheda, Mona M. Ismail, Dara Aldisi, Mahmoud M. A. Abulmeaty, and Mostafa E. Elshobary. 2025. "Fucoidan-Based Gold Nanoparticles: Antioxidant and Anticancer Potential from Turbinaria decurrens and Sargassum cinereum" Pharmaceutics 17, no. 7: 826. https://doi.org/10.3390/pharmaceutics17070826
APA StyleNewehy, A. S. E., Gheda, S. F., Ismail, M. M., Aldisi, D., Abulmeaty, M. M. A., & Elshobary, M. E. (2025). Fucoidan-Based Gold Nanoparticles: Antioxidant and Anticancer Potential from Turbinaria decurrens and Sargassum cinereum. Pharmaceutics, 17(7), 826. https://doi.org/10.3390/pharmaceutics17070826










