CitH3, a Druggable Biomarker for Human Diseases Associated with Acute NETosis and Chronic Immune Dysfunction
Abstract
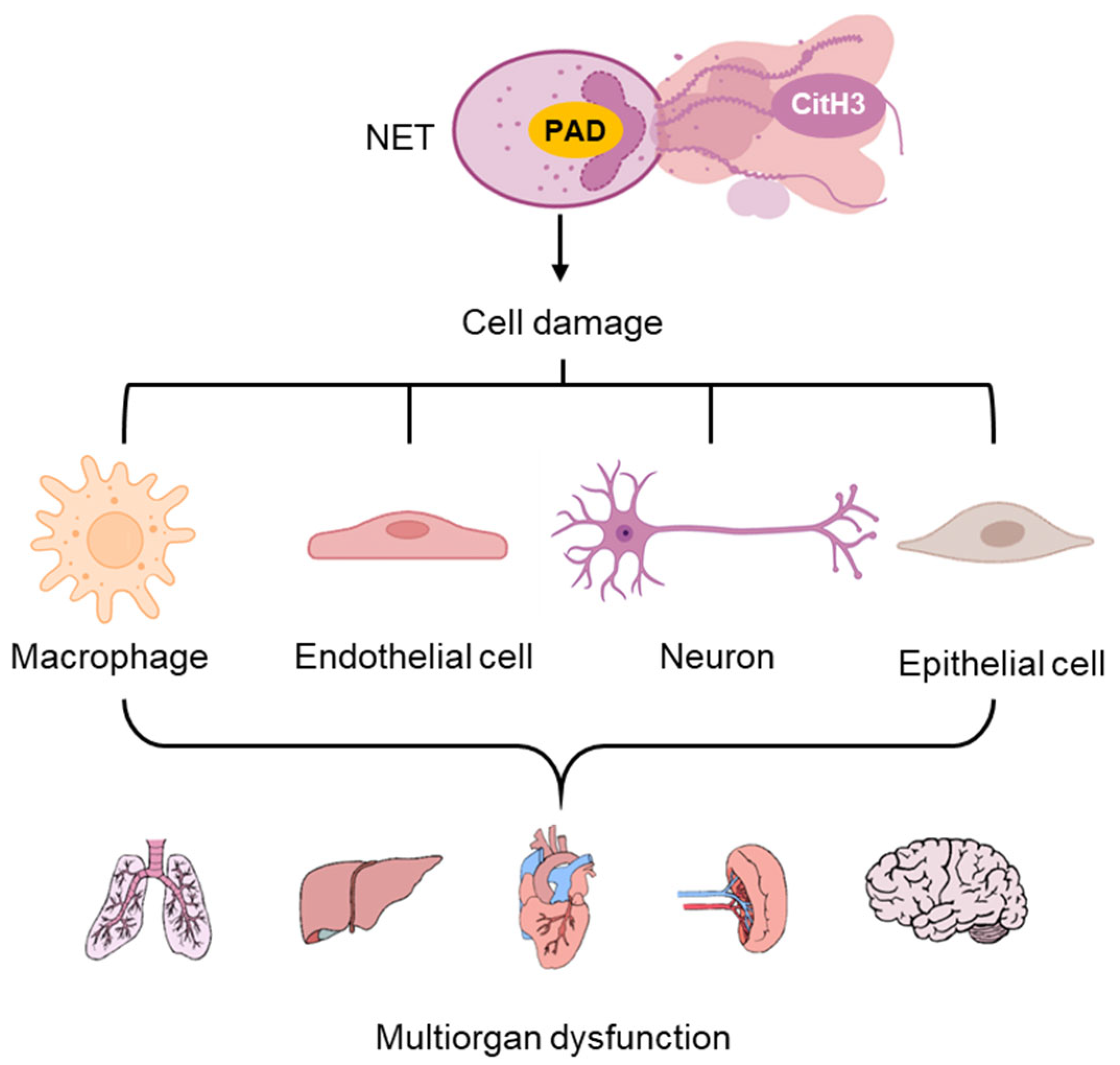
1. Introduction
2. Acute Inflammation
2.1. Non-Sterile Inflammation
2.1.1. Endotoxic Septic Shock (Sepsis)
2.1.2. Viral Infection
2.2. Sterile Inflammation
2.2.1. Ischemia–Reperfusion Injuries
2.2.2. Organ Transplantation
3. Chronic Immune Dysregulation
3.1. Diabetic Wounds
3.2. Autoimmune Disease
3.3. Cancer
4. Strategies for Targeting NETosis-Related Diseases
4.1. DNase
4.2. PAD Inhibitors
4.3. Other Approaches Targeting NETosis
4.4. Development of Antibody Against CitH3
5. Future Perspectives, Challenges, and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular Mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Kumar, S. Neutrophil and Remnant Clearance in Immunity and Inflammation. Immunology 2022, 165, 22–43. [Google Scholar] [CrossRef]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allerg. Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Scapini, P.; Cassatella, M.A. Social Networking of Human Neutrophils within the Immune System. Blood 2014, 124, 710–719. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid Killing of Human Neutrophils by the Potent Activator Phorbol 12-Myristate 13-Acetate (PMA) Accompanied by Changes Different from Typical Apoptosis or Necrosis. J. Leukoc. Biol. 1996, 59, 229–240. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil Extracellular Traps Enriched in Oxidized Mitochondrial DNA Are Interferogenic and Contribute to Lupus-like Disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida Albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef]
- Dwyer, M.; Shan, Q.; D’Ortona, S.; Maurer, R.; Mitchell, R.; Olesen, H.; Thiel, S.; Huebner, J.; Gadjeva, M. Cystic Fibrosis Sputum DNA Has NETosis Characteristics and Neutrophil Extracellular Trap Release Is Regulated by Macrophage Migration-Inhibitory Factor. J. Innate Immun. 2014, 6, 765–779. [Google Scholar] [CrossRef]
- Zhang, X.; Bolt, M.; Guertin, M.J.; Chen, W.; Zhang, S.; Cherrington, B.D.; Slade, D.J.; Dreyton, C.J.; Subramanian, V.; Bicker, K.L.; et al. Peptidylarginine Deiminase 2-Catalyzed Histone H3 Arginine 26 Citrullination Facilitates Estrogen Receptor α Target Gene Activation. Proc. Natl. Acad. Sci. USA 2012, 109, 13331–13336. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Alam, H.B.; Chong, W.; Mobley, J.; Liu, B.; Deng, Q.; Liang, Y.; Wang, Y.; Chen, E.; Wang, T.; et al. CitH3: A Reliable Blood Biomarker for Diagnosis and Treatment of Endotoxic Shock. Sci. Rep. 2017, 7, 8972. [Google Scholar] [CrossRef] [PubMed]
- Lewis, H.D.; Liddle, J.; Coote, J.E.; Atkinson, S.J.; Barker, M.D.; Bax, B.D.; Bicker, K.L.; Bingham, R.P.; Campbell, M.; Chen, Y.H.; et al. Inhibition of PAD4 Activity Is Sufficient to Disrupt Mouse and Human NET Formation. Nat. Chem. Biol. 2015, 11, 189–191. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Stadler, S.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone Hypercitrullination Mediates Chromatin Decondensation and Neutrophil Extracellular Trap Formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Martinod, K.; Demers, M.; Fuchs, T.A.; Wong, S.L.; Brill, A.; Gallant, M.; Hu, J.; Wang, Y.; Wagner, D.D. Neutrophil Histone Modification by Peptidylarginine Deiminase 4 Is Critical for Deep Vein Thrombosis in Mice. Proc. Natl. Acad. Sci. USA 2013, 110, 8674–8679. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, Q.; Pan, B.; Alam, H.B.; Tian, Y.; Bhatti, U.F.; Liu, B.; Mondal, S.; Thompson, P.R.; Li, Y. Inhibition of PAD2 Improves Survival in a Mouse Model of Lethal LPS-Induced Endotoxic Shock. Inflammation 2020, 43, 1436–1445. [Google Scholar] [CrossRef]
- Poli, V.; Zanoni, I. Neutrophil Intrinsic And Extrinsic Regulation of NETosis in Health And Disease. Trends Microbiol. 2023, 31, 280–293. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel Cell Death Program Leads to Neutrophil Extracellular Traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Jones, C.; La Flamme, A.; Larsen, P.; Hally, K. CPHEN-017: Comprehensive Phenotyping of Neutrophil Extracellular Traps (NETs) on Peripheral Human Neutrophils. Cytom. Part A 2024, 105, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Poto, R.; Shamji, M.; Marone, G.; Durham, S.R.; Scadding, G.W.; Varricchi, G. Neutrophil Extracellular Traps in Asthma: Friends or Foes? Cells 2022, 11, 3521. [Google Scholar] [CrossRef]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.H.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.V.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-Induced NETosis Is a Dynamic Process Involving Neutrophil Multitasking In Vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef]
- Mitsios, A.; Arampatzioglou, A.; Arelaki, S.; Mitroulis, I.; Ritis, K. NETopathies? Unraveling the Dark Side of Old Diseases through Neutrophils. Front. Immunol. 2016, 7, 678. [Google Scholar] [CrossRef]
- Denning, N.-L.; Aziz, M.; Gurien, S.D.; Wang, P. DAMPs and NETs in Sepsis. Front. Immunol. 2019, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, J.; Zhang, Y.; Huang, H.; Zhang, L.; Femel, J.; Dimberg, A.; Olsson, A.-K. Neutrophil Extracellular Traps Accumulate in Peripheral Blood Vessels and Compromise Organ Function in Tumor-Bearing Animals. Cancer Res. 2015, 75, 2653–2662. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular Histones Are Major Mediators of Death in Sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Fukudome, E.Y.; Lu, J.; Chong, W.; Jin, G.; Liu, Z.; Velmahos, G.C.; Demoya, M.; King, D.R.; et al. Identification of Citrullinated Histone H3 as a Potential Serum Protein Biomarker in a Lethal Model of Lipopolysaccharide-Induced Shock. Surgery 2011, 150, 442–451. [Google Scholar] [CrossRef]
- Tian, Y.; Russo, R.M.; Li, Y.; Karmakar, M.; Liu, B.; Puskarich, M.A.; Jones, A.E.; Stringer, K.A.; Standiford, T.J.; Alam, H.B. Serum Citrullinated Histone H3 Concentrations Differentiate Patients with Septic Verses Non-Septic Shock and Correlate with Disease Severity. Infection 2021, 49, 83–93. [Google Scholar] [CrossRef]
- Pan, B.; Li, Y.; Liu, Y.; Wang, W.; Huang, G.; Ouyang, Y. Circulating CitH3 Is a Reliable Diagnostic and Prognostic Biomarker of Septic Patients in Acute Pancreatitis. Front. Immunol. 2021, 12, 766391. [Google Scholar] [CrossRef]
- Silk, E.; Zhao, H.; Weng, H.; Ma, D. The Role of Extracellular Histone in Organ Injury. Cell Death Dis. 2017, 8, e2812. [Google Scholar] [CrossRef]
- Liang, Y.; Pan, B.; Alam, H.B.; Deng, Q.; Wang, Y.; Chen, E.; Liu, B.; Tian, Y.; Williams, A.M.; Duan, X.; et al. Inhibition of Peptidylarginine Deiminase Alleviates LPS-Induced Pulmonary Dysfunction and Improves Survival in a Mouse Model of Lethal Endotoxemia. Eur. J. Pharmacol. 2018, 833, 432–440. [Google Scholar] [CrossRef]
- Deng, Q.; Pan, B.; Alam, H.B.; Liang, Y.; Wu, Z.; Liu, B.; Mor-Vaknin, N.; Duan, X.; Williams, A.M.; Tian, Y.; et al. Citrullinated Histone H3 as a Therapeutic Target for Endotoxic Shock in Mice. Front. Immunol. 2020, 10, 2957. [Google Scholar] [CrossRef] [PubMed]
- Tsourouktsoglou, T.-D.; Warnatsch, A.; Ioannou, M.; Hoving, D.; Wang, Q.; Papayannopoulos, V. Histones, DNA, and Citrullination Promote Neutrophil Extracellular Trap Inflammation by Regulating the Localization and Activation of TLR4. Cell Rep. 2020, 31, 107602. [Google Scholar] [CrossRef]
- Park, Y.; Ryu, B.; Deng, Q.; Pan, B.; Song, Y.; Tian, Y.; Alam, H.B.; Li, Y.; Liang, X.; Kurabayashi, K. An Integrated Plasmo-Photoelectronic Nanostructure Biosensor Detects an Infection Biomarker Accompanying Cell Death in Neutrophils. Small 2020, 16, e1905611. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Liu, B.; Zhao, T.; Chong, W.; Wang, Y.; Alam, H.B. Citrullinated Histone H3: A Novel Target for the Treatment of Sepsis. Surgery 2014, 156, 229–234. [Google Scholar] [CrossRef]
- Siddiqui, A.Z.; Bhatti, U.F.; Deng, Q.; Biesterveld, B.E.; Tian, Y.; Wu, Z.; Dahl, J.; Liu, B.; Xu, J.; Koike, Y.; et al. Cl-Amidine Improves Survival and Attenuates Kidney Injury in a Rabbit Model of Endotoxic Shock. Surg. Infect. 2021, 22, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of Hypotension before Initiation of Effective Antimicrobial Therapy Is the Critical Determinant of Survival in Human Septic Shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Chang, P.; Li, Y. Editorial: Targeting Protein Post-Translational Modifications (PTMs) for Diagnosis and Treatment of Sepsis. Front. Immunol. 2022, 13, 856146. [Google Scholar] [CrossRef]
- Foster, D.M.; Kellum, J.A. Endotoxic Septic Shock: Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 16185. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhou, P.; Chang, Z.; Liu, B.; Liu, X.; Wang, Y.; Li, Y.; Alam, H.B. Inhibition of Peptidylarginine Deiminase Attenuates Inflammation and Improves Survival in a Rat Model of Hemorrhagic Shock. J. Surg. Res. 2016, 200, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Bergman, Z.R.; Kiberenge, R.K.; Bianco, R.W.; Beilman, G.J.; Brophy, C.M.; Hocking, K.M.; Alvis, B.D.; Wise, E.S. Norepinephrine Infusion and the Central Venous Waveform in a Porcine Model of Endotoxemic Hypotension with Resuscitation: A Large Animal Study. J. Investig. Surg. 2025, 38, 2445603. [Google Scholar] [CrossRef]
- Martinod, K.; Fuchs, T.A.; Zitomersky, N.L.; Wong, S.L.; Demers, M.; Gallant, M.; Wang, Y.; Wagner, D.D. PAD4-Deficiency Does Not Affect Bacteremia in Polymicrobial Sepsis and Ameliorates Endotoxemic Shock. Blood 2015, 125, 1948–1956. [Google Scholar] [CrossRef]
- Zhou, J.; Biesterveld, B.E.; Li, Y.; Wu, Z.; Tian, Y.; Williams, A.M.; Tian, S.; Gao, W.; Bhatti, U.F.; Duan, X.; et al. Peptidylarginine Deiminase 2 Knockout Improves Survival in Hemorrhagic Shock. Shock 2020, 54, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Pan, B.; Alam, H.B.; Liu, B.; Bronson, R.T.; Deng, Q.; Wu, E.; Li, Y. Protective Effect of Cl-Amidine against CLP-Induced Lethal Septic Shock in Mice. Sci. Rep. 2016, 6, 36696. [Google Scholar] [CrossRef]
- Ho, J.W.; Quan, C.; Gauger, M.A.; Alam, H.B.; Li, Y. ROLE OF PEPTIDYLARGININE DEIMINASE AND NEUTROPHIL EXTRACELLULAR TRAPS IN INJURIES: FUTURE NOVEL DIAGNOSTICS AND THERAPEUTIC TARGETS. Shock 2023, 59, 247–255. [Google Scholar] [CrossRef]
- Liu, V.X.; Fielding-Singh, V.; Greene, J.D.; Baker, J.M.; Iwashyna, T.J.; Bhattacharya, J.; Escobar, G.J. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 856–863. [Google Scholar] [CrossRef]
- Seymour, C.W.; Gesten, F.; Prescott, H.C.; Friedrich, M.E.; Iwashyna, T.J.; Phillips, G.S.; Lemeshow, S.; Osborn, T.; Terry, K.M.; Levy, M.M. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N. Engl. J. Med. 2017, 376, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Ashar, H.; Mueller, N.; Rudd, J.; Snider, T.; Achanta, M.; Prasanthi, M.; Sivasami, P.; Thomas, P.; Ramachandran, A.; Malayer, J.; et al. The Role of Extracellular Histones in Influenza Virus Pathogenesis. Am. J. Pathol. 2017, 188, 135–148. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Park, H.H.; Park, W.; Kim, H.; Jang, J.G.; Hong, K.S.; Lee, J.-Y.; Seo, H.S.; Na, D.H.; Kim, T.-H.; et al. Long-Acting Nanoparticulate DNase-1 for Effective Suppression of SARS-CoV-2-Mediated Neutrophil Activities and Cytokine Storm. Biomaterials 2021, 267, 120389. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil Extracellular Traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil Extracellular Traps Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liang, S.; Wang, L.; Guan, X.; Wang, J.; Gong, P. Predictive Value of Neutrophil Extracellular Traps Components for 28-Day All-Cause Mortality in Patients with Cardiac Arrest: A Pilot Observational Study. Shock 2023, 60, 664–670. [Google Scholar] [CrossRef]
- Huang, H.; Tohme, S.; Al-Khafaji, A.B.; Tai, S.; Loughran, P.; Chen, L.; Wang, S.; Kim, J.; Billiar, T.; Wang, Y.; et al. Damage-Associated Molecular Pattern–Activated Neutrophil Extracellular Trap Exacerbates Sterile Inflammatory Liver Injury. Hepatology 2015, 62, 600–614. [Google Scholar] [CrossRef]
- Kim, S.-W.; Lee, H.; Lee, H.-K.; Kim, I.-D.; Lee, J.-K. Neutrophil Extracellular Trap Induced by HMGB1 Exacerbates Damages in the Ischemic Brain. Acta Neuropathol. Commun. 2019, 7, 94. [Google Scholar] [CrossRef]
- Yokoyama, A.P.H.; Kutner, J.M.; Moraes Mazetto Fonseca, B.; Mesquita, G.L.T.V.; Sakashita, A.M.; Santos, A.P.R.; Nakazawa, C.Y.; Almeida, M.D.; Orsi, F.L. de A. Neutrophil Extracellular Traps (NETs), Transfusion Requirements and Cl Inical Outcomes in Orthotopic Liver Transplantation. J. Thromb. Thrombolysis 2023, 56, 253–263. [Google Scholar] [CrossRef]
- Sayah, D.M.; Mallavia, B.; Liu, F.; Ortiz-Muñoz, G.; Caudrillier, A.; DerHovanessian, A.; Ross, D.J.; Iii, J.P.L.; Saggar, R.; Ardehali, A.; et al. Neutrophil Extracellular Traps Are Pathogenic in Primary Graft Dysfunction after Lung Transplantation. Am. J. Respir. Crit. Care Med. 2015, 191, 455–463. [Google Scholar] [CrossRef]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes Primes Neutrophils to Undergo NETosis, Which Impairs Wound Healing. Nat. Med. 2015, 21, 815–819. [Google Scholar] [CrossRef]
- Sabbatini, M.; Magnelli, V.; Renò, F. NETosis in Wound Healing: When Enough Is Enough. Cells 2021, 10, 494. [Google Scholar] [CrossRef]
- Liu, D.; Yang, P.; Gao, M.; Yu, T.; Shi, Y.; Zhang, M.; Yao, M.; Liu, Y.; Zhang, X. NLRP3 Activation Induced by Neutrophil Extracellular Traps Sustains Inflammatory Response in the Diabetic Wound. Clin. Sci. 2019, 133, 565–582. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, Y.; Ren, Y.; Xu, L.; Wang, H.; Ling, X.; Jin, L.; Hu, Y.; Zhang, H.; Miao, C.; et al. The Emerging Roles of Neutrophil Extracellular Traps in Wound Healing. Cell Death Dis. 2021, 12, 984. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil Migration in Infection and Wound Repair: Going Forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef]
- van der Linden, M.; Kumari, S.; Montizaan, D.; van Dalen, S.; Kip, A.; Foster, M.; Reinieren-Beeren, I.; Neubert, E.; Erpenbeck, L.; Waaijenberg, K.; et al. Anti-Citrullinated Histone Monoclonal Antibody CIT-013, a Dual Action Therapeutic for Neutrophil Extracellular Trap-Associated Autoimmune Diseases. MAbs 2023, 15, 2281763. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Arfman, T.; Wichapong, K.; Reutelingsperger, C.P.M.; Voorberg, J.; Nicolaes, G.A.F. PAD4 Takes Charge during Neutrophil Activation: Impact of PAD4 Mediated NET Formation on Immune-Mediated Disease. J. Thromb. Haemost. 2021, 19, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, Y.; Xiao, J.; Hong, Y.; Zhu, Y. The Emerging Role of Neutrophil Extracellular Traps in the Progression of Rheumatoid Arthritis. Front. Immunol. 2024, 15, 1438272. [Google Scholar] [CrossRef]
- Wu, Z.; Li, P.; Tian, Y.; Ouyang, W.; Ho, J.W.-Y.; Alam, H.B.; Li, Y. Peptidylarginine Deiminase 2 in Host Immunity: Current Insights and Perspectives. Front. Immunol. 2021, 12, 761946. [Google Scholar] [CrossRef]
- Henning, S.; Reimers, T.; Abdulahad, W.; Fierro, J.J.; Doornbos-van der Meer, B.; Bootsma, H.; Horvath, B.; Leeuw, K.; Westra, J. Low-Density Granulocytes and Neutrophil Extracellular Trap Formation a Re Increased in Incomplete Systemic Lupus Erythematosus. Rheumatology 2024, 64, 1234–1242. [Google Scholar] [CrossRef]
- Wang, M.; Lv, X.; Wang, Y.; Li, Y.; Li, H.; Shen, Z.; Zhao, L. Biomarkers of Peripheral Blood Neutrophil Extracellular Traps in the Diagnosis and Progression of Malignant Tumors. Cancer Med. 2024, 13, e6935. [Google Scholar] [CrossRef]
- Thålin, C.; Lundström, S.; Seignez, C.; Daleskog, M.; Lundström, A.; Henriksson, P.; Helleday, T.; Phillipson, M.; Wallén, H.; Demers, M. Citrullinated Histone H3 as a Novel Prognostic Blood Marker in Patient s with Advanced Cancer. PLoS ONE 2018, 13, e0191231. [Google Scholar] [CrossRef]
- Grilz, E.; Mauracher, L.-M.; Posch, F.; Königsbrügge, O.; Zöchbauer-Müller, S.; Marosi, C.; Lang, I.; Pabinger, I.; Ay, C. Citrullinated Histone H3, a Biomarker for Neutrophil Extracellular Trap Formation, Predicts the Risk of Mortality in Patients with Cancer. Br. J. Haematol. 2019, 186, 311–320. [Google Scholar] [CrossRef]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Source of Chronic Inflammation in Aging. Front. Cardiovasc. Med. 2018, 5, 12. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Q.; Wang, W.; Lu, S. Increased Neutrophil Extracellular Traps Caused by Diet-induced Obesity Delay Fracture Healing. FASEB J. 2024, 38, e70126. [Google Scholar] [CrossRef]
- Meegan, J.E.; Yang, X.; Beard, R.S.; Jannaway, M.; Chatterjee, V.; Taylor-Clark, T.E.; Yuan, S.Y. Citrullinated Histone 3 Causes Endothelial Barrier Dysfunction. Biochem. Biophys. Res. Commun. 2018, 503, 1498–1502. [Google Scholar] [CrossRef]
- Flannery, D.D.; Dysart, K.; Cook, A.; Greenspan, J.; Aghai, Z.H.; Jensen, E.A. Association between Early Antibiotic Exposure and Bronchopulmonary Dysplasia or Death. J. Perinatol. 2018, 38, 1227–1234. [Google Scholar] [CrossRef]
- Reed, B.D.; Schibler, K.R.; Deshmukh, H.; Ambalavanan, N.; Morrow, A.L. The Impact of Maternal Antibiotics on Neonatal Disease. J. Pediatr. 2018, 197, 97–103.e3. [Google Scholar] [CrossRef]
- Ambalavanan, N.; Jauk, V.; Szychowski, J.M.; Boggess, K.A.; Saade, G.; Longo, S.; Esplin, S.; Cleary, K.; Wapner, R.; Letson, K.; et al. Epidemiology of Readmissions in Early Infancy Following Nonelective Cesarean Delivery. J. Perinatol. 2021, 41, 24–31. [Google Scholar] [CrossRef]
- Song, Y.; Sandford, E.; Tian, Y.; Yin, Q.; Kozminski, A.G.; Su, S.-H.; Cai, T.; Ye, Y.; Chung, M.T.; Lindstrom, R.; et al. Rapid Single-Molecule Digital Detection of Protein Biomarkers for Continuous Monitoring of Systemic Immune Disorders. Blood 2021, 137, 1591–1602. [Google Scholar] [CrossRef]
- Tian, Y.; Qu, S.; Alam, H.B.; Williams, A.M.; Wu, Z.; Deng, Q.; Pan, B.; Zhou, J.; Liu, B.; Duan, X.; et al. Peptidylarginine Deiminase 2 Has Potential as Both a Biomarker and Therapeutic Target of Sepsis. JCI Insight 2020, 5, e138873. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, Y.; Wang, Z.-H.; Zhang, H.; Meng, X.-T.; Wu, Y.-Q.; Qian, Z.-Z.; Ding, Y.-H.; Li, J.; Ma, T.-T.; et al. Neutrophil Extracellular Traps Promote Acetaminophen-Induced Acute Liver Injury in Mice via AIM2. Acta Pharmacol. Sin. 2024, 45, 1660–1672. [Google Scholar] [CrossRef]
- Wang, H.; Gao, T.; Zhang, R.; Hu, J.; Gao, S.; Wang, Y.; Qi, X.; Zhou, Y.; Zheng, G.; Dong, H. Neutrophil Extracellular Traps Aggravate Contrast-Induced Acute Kidney Injury by Damaging Glomeruli and Peritubular Capillaries. J. Inflamm. Res. 2023, 16, 5629–5646. [Google Scholar] [CrossRef]
- Ma, Y. Role of Neutrophils in Cardiac Injury and Repair Following Myocardial Infarction. Cells 2021, 10, 1676. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Regulation of the Inflammatory Response in Cardiac Repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef]
- Ferré-Vallverdú, M.; Latorre, A.M.; Fuset, M.P.; Sánchez, E.; Madrid, I.; Ten, F.; Vallés, J.; Santos, M.T.; Bonanad, S.; Moscardó, A. Neutrophil Extracellular Traps (NETs) in Patients with STEMI. Association with Percutaneous Coronary Intervention and Antithrombotic Treatments. Thromb. Res. 2022, 213, 78–83. [Google Scholar] [CrossRef]
- Saisorn, W.; Santiworakul, C.; Phuengmaung, P.; Siripen, N.; Rianthavorn, P.; Leelahavanichkul, A. Extracellular Traps in Peripheral Blood Mononuclear Cell Fraction in Childhood-Onset Systemic Lupus Erythematosus. Sci. Rep. 2024, 14, 23177. [Google Scholar] [CrossRef]
- Vander Cruyssen, B.; Peene, I.; Cantaert, T.; Hoffman, I.E.A.; De Rycke, L.; Veys, E.M.; De Keyser, F. Anti-Citrullinated Protein/Peptide Antibodies (ACPA) in Rheumatoid Arthritis: Specificity and Relation with Rheumatoid Factor. Autoimmun. Rev. 2005, 4, 468–474. [Google Scholar] [CrossRef]
- Trier, N.H.; Houen, G. Anti-Citrullinated Protein Antibodies as Biomarkers in Rheumatoid Arthritis. Expert Rev. Mol. Diagn. 2023, 23, 895–911. [Google Scholar] [CrossRef]
- Bryk, A.H.; Prior, S.M.; Plens, K.; Konieczynska, M.; Hohendorff, J.; Malecki, M.T.; Butenas, S.; Undas, A. Predictors of Neutrophil Extracellular Traps Markers in Type 2 Diabetes Mellitus: Associations with a Prothrombotic State and Hypofibrinolysis. Cardiovasc. Diabetol. 2019, 18, 49. [Google Scholar] [CrossRef]
- Ronchetti, L.; Boubaker, N.S.; Barba, M.; Vici, P.; Gurtner, A.; Piaggio, G. Neutrophil Extracellular Traps in Cancer: Not Only Catching Microbes. J. Exp. Clin. Cancer Res. 2021, 40, 231. [Google Scholar] [CrossRef]
- Ploeger, D.T.; Hosper, N.A.; Schipper, M.; Koerts, J.A.; De Rond, S.; Bank, R.A. Cell Plasticity in Wound Healing: Paracrine Factors of M1/ M2 Polarized Macrophages Influence the Phenotypical State of Dermal Fibroblasts. Cell Commun. Signal 2013, 11, 29. [Google Scholar] [CrossRef]
- Phillipson, M.; Kubes, P. The Healing Power of Neutrophils. Trends Immunol. 2019, 40, 635–647. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and Impaired Wound Healing: Pathophysiology and Current Methods for Drug Delivery, Part 1: Normal and Chronic Wounds: Biology, Causes, and Approaches to Care. Adv. Ski. Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, C.-X.; Xu, B.; Yu, Z. Diabetic Foot Ulcers: Classification, Risk Factors and Management. World J. Diabetes 2022, 13, 1049–1065. [Google Scholar] [CrossRef]
- Chen, L.; Sun, S.; Gao, Y.; Ran, X. Global Mortality of Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis of Observational Studies. Diabetes Obes. Metab. 2023, 25, 36–45. [Google Scholar] [CrossRef]
- Hirota, T.; Levy, J.H.; Iba, T. The Influence of Hyperglycemia on Neutrophil Extracellular Trap Formation and Endothelial Glycocalyx Damage in a Mouse Model of Type 2 Diabetes. Microcirculation 2020, 27, e12617. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Zhong, L.; Ye, D.; Zhang, J.; Tu, Y.; Bornstein, S.R.; Zhou, Z.; Lam, K.S.L.; Xu, A. Increased Neutrophil Elastase and Proteinase 3 and Augmented NETosis Are Closely Associated with β-Cell Autoimmunity in Patients with Type 1 Diabetes. Diabetes 2014, 63, 4239–4248. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Peng, W.; Wu, S.; Wang, W. Correlation of Serum Citrullinated Histone H3 Levels with Disease Activity in Patients with Rheumatoid Arthritis. Clin. Exp. Rheumatol. 2023, 41, 1792–1800. [Google Scholar] [CrossRef]
- Venetsanopoulou, A.I.; Ntinopoulou, M.; Papagianni, E.; Koletsos, N.; Voulgari, P.V.; Chrysanthopoulou, A. Neutrophil Extracellular Traps as Immunofibrotic Mediators in RA-ILD. Pilot Evaluation of the Nintedanib Therapy. Front. Immunol. 2024, 15, 1480594. [Google Scholar] [CrossRef]
- Dinallo, V.; Marafini, I.; Di Fusco, D.; Laudisi, F.; Franzè, E.; Di Grazia, A.; Figliuzzi, M.M.; Caprioli, F.; Stolfi, C.; Monteleone, I.; et al. Neutrophil Extracellular Traps Sustain Inflammatory Signals in Ulcerative Colitis. J. Crohn’s Colitis 2019, 13, 772–784. [Google Scholar] [CrossRef]
- Schroder, A.L.; Chami, B.; Liu, Y.; Doyle, C.M.; El Kazzi, M.; Ahlenstiel, G.; Ahmad, G.; Pathma-Nathan, N.; Collins, G.; Toh, J.; et al. Neutrophil Extracellular Trap Density Increases with Increasing Histopathological Severity of Crohn’s Disease. Inflamm. Bowel Dis. 2022, 28, 586–598. [Google Scholar] [CrossRef]
- Li, T.; Wang, C.; Liu, Y.; Li, B.; Zhang, W.; Wang, L.; Yu, M.; Zhao, X.; Du, J.; Zhang, J.; et al. Neutrophil Extracellular Traps Induce Intestinal Damage and Thrombotic Tendency in Inflammatory Bowel Disease. J. Crohn’s Colitis 2020, 14, 240–253. [Google Scholar] [CrossRef]
- Lin, E.Y.-H.; Lai, H.-J.; Cheng, Y.-K.; Leong, K.-Q.; Cheng, L.-C.; Chou, Y.-C.; Peng, Y.-C.; Hsu, Y.-H.; Chiang, H.-S. Neutrophil Extracellular Traps Impair Intestinal Barrier Function during Experimental Colitis. Biomedicines 2020, 8, 275. [Google Scholar] [CrossRef]
- Manjili, M.H. Tumor Dormancy and Relapse: From a Natural Byproduct of Evolution to a Disease State. Cancer Res. 2017, 77, 2564–2569. [Google Scholar] [CrossRef]
- Subhan, M.A.; Torchilin, V.P. Neutrophils as an Emerging Therapeutic Target and Tool for Cancer Therapy. Life Sci. 2021, 285, 119952. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, Y.; Wang, S. Histone Citrullination: A New Target for Tumors. Mol. Cancer 2021, 20, 90. [Google Scholar] [CrossRef]
- Berger-Achituv, S.; Brinkmann, V.; Abed, U.A.; Kühn, L.I.; Ben-Ezra, J.; Elhasid, R.; Zychlinsky, A. A Proposed Role for Neutrophil Extracellular Traps in Cancer Immunoediting. Front. Immunol. 2013, 4, 48. [Google Scholar] [CrossRef]
- Tohme, S.; Yazdani, H.O.; Al-Khafaji, A.B.; Chidi, A.P.; Loughran, P.; Mowen, K.; Wang, Y.; Simmons, R.L.; Huang, H.; Tsung, A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016, 76, 1367–1380. [Google Scholar] [CrossRef]
- Adrover, J.M.; McDowell, S.A.C.; He, X.-Y.; Quail, D.F.; Egeblad, M. NETworking with Cancer: The Bidirectional Interplay between Cancer and Neutrophil Extracellular Traps. Cancer Cell 2023, 41, 505–526. [Google Scholar] [CrossRef] [PubMed]
- He, X.-Y.; Gao, Y.; Ng, D.; Michalopoulou, E.; George, S.; Adrover, J.M.; Sun, L.; Albrengues, J.; Daßler-Plenker, J.; Han, X.; et al. Chronic Stress Increases Metastasis via Neutrophil-Mediated Changes to the Microenvironment. Cancer Cell 2024, 42, 474–486.e12. [Google Scholar] [CrossRef]
- He, X.-Y.; Ng, D.; Egeblad, M. Caught in a Web: Emerging Roles of Neutrophil Extracellular Traps in Cancer. Annu. Rev. Cancer Biol. 2022, 6, 223–243. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The Prognostic Landscape of Genes and Infiltrating Immune Cells across Human Cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jin, J. Neutrophil Extracellular Traps: New Players in Cancer Research. Front. Immunol. 2022, 13, 937565. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.; Mantovani, A.; Selmi, C. The Other Side of the Innate Immune System: Humoral Arms Favoring Cancer. Cell. Mol. Immunol. 2020, 17, 1024–1025. [Google Scholar] [CrossRef]
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps That Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef]
- Wang, L.; Song, G.; Zhang, X.; Feng, T.; Pan, J.; Chen, W.; Yang, M.; Bai, X.; Pang, Y.; Yu, J.; et al. PADI2-Mediated Citrullination Promotes Prostate Cancer Progression. Cancer Res. 2017, 77, 5755–5768. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, X.; Xu, Y.; He, G.; Liu, Q.; Zhu, J.; Zhang, C. Elevated Histone H3 Citrullination Is Associated with Increased Beclin1 Expression in HBV-Related Hepatocellular Carcinoma. J. Med. Virol. 2020, 92, 1221–1230. [Google Scholar] [CrossRef]
- Modestino, L.; Cristinziano, L.; Poto, R.; Ventrici, A.; Trocchia, M.; Ferrari, S.M.; Fallahi, P.; Paparo, S.R.; Marone, G.; Antonelli, A.; et al. Neutrophil Extracellular Traps and Neutrophil-Related Mediators in Human Thyroid Cancer. Front. Immunol. 2023, 14, 1167404. [Google Scholar] [CrossRef]
- Fang, Q.; Stehr, A.M.; Naschberger, E.; Knopf, J.; Herrmann, M.; Stürzl, M. No NETs No TIME: Crosstalk between Neutrophil Extracellular Traps and the Tumor Immune Microenvironment. Front. Immunol. 2022, 13, 1075260. [Google Scholar] [CrossRef]
- Wang, H.; Kim, S.J.; Lei, Y.; Wang, S.; Wang, H.; Huang, H.; Zhang, H.; Tsung, A. Neutrophil Extracellular Traps in Homeostasis and Disease. Sig. Transduct. Target. Ther. 2024, 9, 235. [Google Scholar] [CrossRef]
- Dong, T.; Barasa, L.; Yu, X.; Ouyang, W.; Shao, L.; Quan, C.; Wang, S.H.; Zhang, J.; Salmon, M.; Tsung, A.; et al. AFM41a: A Novel PAD2 Inhibitor for Sepsis Treatment—Efficacy and Mechanism. Int. J. Biol. Sci. 2024, 20, 5043–5055. [Google Scholar] [CrossRef]
- Yadav, R.; Momin, A.; Godugu, C. DNase Based Therapeutic Approaches for the Treatment of NETosis Related Inflammatory Diseases. Int. Immunopharmacol. 2023, 124, 110846. [Google Scholar] [CrossRef]
- Rossaint, J.; Herter, J.M.; Van Aken, H.; Napirei, M.; Döring, Y.; Weber, C.; Soehnlein, O.; Zarbock, A. Synchronized Integrin Engagement and Chemokine Activation Is Crucial in Neutrophil Extracellular Trap-Mediated Sterile Inflammation. Blood 2014, 123, 2573–2584. [Google Scholar] [CrossRef]
- Lefrançais, E.; Mallavia, B.; Zhuo, H.; Calfee, C.S.; Looney, M.R. Maladaptive Role of Neutrophil Extracellular Traps in Pathogen-Induced Lung Injury. JCI Insight 2018, 3, e98178. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An Emerging Role for Neutrophil Extracellular Traps in Noninfectious Disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Jenne, C.N.; Surewaard, B.G.J.; Thanabalasuriar, A.; Lee, W.-Y.; Sanz, M.-J.; Mowen, K.; Opdenakker, G.; Kubes, P. Molecular Mechanisms of NET Formation and Degradation Revealed by Intravital Imaging in the Liver Vasculature. Nat. Commun. 2015, 6, 6673. [Google Scholar] [CrossRef]
- Gan, T.; Yang, Y.; Hu, F.; Chen, X.; Zhou, J.; Li, Y.; Xu, Y.; Wang, H.; Chen, Y.; Zhang, M. TLR3 Regulated Poly I:C-Induced Neutrophil Extracellular Traps and Acute Lung Injury Partly Through P38 MAP Kinase. Front. Microbiol. 2018, 9, 3174. [Google Scholar] [CrossRef]
- Englert, H.; Göbel, J.; Khong, D.; Omidi, M.; Wolska, N.; Konrath, S.; Frye, M.; Mailer, R.K.; Beerens, M.; Gerwers, J.C.; et al. Targeting NETs Using Dual-Active DNase1 Variants. Front. Immunol. 2023, 14, 1181761. [Google Scholar] [CrossRef]
- Thammavongsa, V.; Missiakas, D.M.; Schneewind, O. Staphylococcus aureus Degrades Neutrophil Extracellular Traps to Promote Immune Cell Death. Science 2013, 342, 863. [Google Scholar] [CrossRef]
- Khan, M.A.; Ali, Z.S.; Sweezey, N.; Grasemann, H.; Palaniyar, N. Progression of Cystic Fibrosis Lung Disease from Childhood to Adulthood: Neutrophils, Neutrophil Extracellular Trap (NET) Formation, and NET Degradation. Genes 2019, 10, 183. [Google Scholar] [CrossRef]
- Chamardani, T.M.; Amiritavassoli, S. Inhibition of NETosis for Treatment Purposes: Friend or Foe? Mol. Cell. Biochem. 2022, 477, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 Is Essential for Antibacterial Innate Immunity Mediated by Neutrophil Extracellular Traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef]
- Bicker, K.L.; Thompson, P.R. The Protein Arginine Deiminases (PADs): Structure, Function, Inhibition, and Disease. Biopolymers 2013, 99, 155. [Google Scholar] [CrossRef]
- Green, R.M.; Thompson, P.R. Current Insights into the Role of Citrullination in Thrombosis. Curr. Opin. Chem. Biol. 2023, 75, 102313. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, C.; Ueda, K.; Suzuki, A.; Iida, A.; Nakamura, R.; Atsuta, N.; Tohnai, G.; Sobue, G.; Saichi, N.; Momozawa, Y.; et al. Citrullination of RGG Motifs in FET Proteins by PAD4 Regulates Protein Aggregation and ALS Susceptibility. Cell Rep. 2018, 22, 1473–1483. [Google Scholar] [CrossRef]
- Long, F.; Wang, Y.-X.; Liu, L.; Zhou, J.; Cui, R.-Y.; Jiang, C.-L. Rapid Nongenomic Inhibitory Effects of Glucocorticoids on Phagocytosis and Superoxide Anion Production by Macrophages. Steroids 2005, 70, 55–61. [Google Scholar] [CrossRef]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil Function in Inflammation and Inflammatory Diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Glynn, R.J.; Karlson, E.W.; Lu, F.; Corrigan, C.; Colls, J.; Xu, C.; MacFadyen, J.; Barbhaiya, M.; Berliner, N.; et al. Adverse Effects of Low-Dose Methotrexate: A Randomized Trial. Ann. Intern. Med. 2020, 172, 369–380. [Google Scholar] [CrossRef]
- Lee, J. Use of Antioxidants to Prevent Cyclosporine a Toxicity. Toxicol. Res. 2010, 26, 163–170. [Google Scholar] [CrossRef]
- Saha, P.; Yeoh, B.S.; Xiao, X.; Golonka, R.M.; Singh, V.; Wang, Y.; Vijay-Kumar, M. PAD4-Dependent NETs Generation Are Indispensable for Intestinal Clearance of Citrobacter Rodentium. Mucosal Immunol. 2019, 12, 761–771. [Google Scholar] [CrossRef]
- Knight, J.S.; Luo, W.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Subramanian, V.; Guo, C.; Grenn, R.C.; Thompson, P.R.; Eitzman, D.T.; et al. Peptidylarginine Deiminase Inhibition Reduces Vascular Damage and Modulates Innate Immune Responses in Murine Models of Atherosclerosis. Circ. Res. 2014, 114, 947–956. [Google Scholar] [CrossRef]
- Hartmann, G. Chapter Four—Nucleic Acid Immunity. In Advances in Immunology; Alt, F.W., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 133, pp. 121–169. [Google Scholar]
- Angeletti, A.; Volpi, S.; Bruschi, M.; Lugani, F.; Vaglio, A.; Prunotto, M.; Gattorno, M.; Schena, F.; Verrina, E.; Ravelli, A.; et al. Neutrophil Extracellular Traps-DNase Balance and Autoimmunity. Cells 2021, 10, 2667. [Google Scholar] [CrossRef]
- Bonilha, C.S.; Veras, F.P.; de Queiroz Cunha, F. NET-Targeted Therapy: Effects, Limitations, and Potential Strategies to Enhance Treatment Efficacy. Trends Pharmacol. Sci. 2023, 44, 622–634. [Google Scholar] [CrossRef]
- Holliday, Z.M.; Earhart, A.P.; Alnijoumi, M.M.; Krvavac, A.; Allen, L.-A.H.; Schrum, A.G. Non-Randomized Trial of Dornase Alfa for Acute Respiratory Distress Syndrome Secondary to COVID-19. Front. Immunol. 2021, 12, 714833. [Google Scholar] [CrossRef]
- Ali, R.A.; Gandhi, A.A.; Meng, H.; Yalavarthi, S.; Vreede, A.P.; Estes, S.K.; Palmer, O.R.; Bockenstedt, P.L.; Pinsky, D.J.; Greve, J.M.; et al. Adenosine Receptor Agonism Protects against NETosis and Thrombosis in Antiphospholipid Syndrome. Nat. Commun. 2019, 10, 1916. [Google Scholar] [CrossRef] [PubMed]
- Friebe, D.; Yang, T.; Schmidt, T.; Borg, N.; Steckel, B.; Ding, Z.; Schrader, J. Purinergic Signaling on Leukocytes Infiltrating the LPS-Injured Lung. PLoS ONE 2014, 9, e95382. [Google Scholar] [CrossRef]
- Schälter, F.; Dürholz, K.; Bucci, L.; Burmester, G.; Caporali, R.; Figuereido, C.; Cobra, J.F.; Manger, B.; Zaiss, M.M.; Schett, G. Does Methotrexate Influence COVID-19 Infection? Case Series and Mechanistic Data. Arthritis Res. Ther. 2021, 23, 166. [Google Scholar] [CrossRef]
- Weinmann, P.; Moura, R.A.; Caetano-Lopes, J.R.; Pereira, P.A.; Canhão, H.; Queiroz, M.V.; Fonseca, J.E. Delayed Neutrophil Apoptosis in Very Early Rheumatoid Arthritis Patients Is Abrogated by Methotrexate Therapy. Clin. Exp. Rheumatol. 2007, 25, 885–887. [Google Scholar] [PubMed]
- Sperling, R.I.; Benincaso, A.I.; Anderson, R.J.; Coblyn, J.S.; Austen, K.F.; Weinblatt, M.E. Acute and Chronic Suppression of Leukotriene B4 Synthesis Ex Vivo in Neutrophils from Patients with Rheumatoid Arthritis Beginning Treatment with Methotrexate. Arthritis Rheum. 1992, 35, 376–384. [Google Scholar] [CrossRef]
- Laurindo, I.M.; Mello, S.B.; Cossermelli, W. Influence of Low Doses of Methotrexate on Superoxide Anion Production by Polymorphonuclear Leukocytes from Patients with Rheumatoid Arthritis. J. Rheumatol. 1995, 22, 633–638. [Google Scholar]
- Ruiz-Limón, P.; Ortega, R.; Arias de la Rosa, I.; Abalos-Aguilera, M.D.C.; Perez-Sanchez, C.; Jimenez-Gomez, Y.; Peralbo-Santaella, E.; Font, P.; Ruiz-Vilches, D.; Ferrin, G.; et al. Tocilizumab Improves the Proatherothrombotic Profile of Rheumatoid Arthritis Patients Modulating Endothelial Dysfunction, NETosis, and Inflammation. Transl. Res. 2017, 183, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.N.; Hernández-Sánchez, J.; Nagel, S.; Feng, Y.; Cai, F.; Rabin, J.; Morse, C.G.; Nadig, N.R.; Ashraf, O.; Gotur, D.B.; et al. Safety and Efficacy of Tocilizumab 4 or 8 Mg/Kg in Hospitalized Patients with Moderate to Severe Coronavirus Disease 2019 Pneumonia: A Randomized Clinical Trial. Open Forum Infect. Dis. 2022, 9, ofab608. [Google Scholar] [CrossRef] [PubMed]
- Kraaij, T.; Kamerling, S.W.A.; de Rooij, E.N.M.; van Daele, P.L.A.; Bredewold, O.W.; Bakker, J.A.; Bajema, I.M.; Scherer, H.U.; Toes, R.E.M.; Huizinga, T.J.W.; et al. The NET-Effect of Combining Rituximab with Belimumab in Severe Systemic Lupus Erythematosus. J. Autoimmun. 2018, 91, 45–54. [Google Scholar] [CrossRef]
- Li, J.; Yin, L.; Chen, S.; Li, Z.; Ding, J.; Wu, J.; Yang, K.; Xu, J. The Perspectives of NETosis on the Progression of Obesity and Obesity-Related Diseases: Mechanisms and Applications. Front. Cell Dev. Biol. 2023, 11, 1221361. [Google Scholar] [CrossRef]
- Bukong, T.N.; Cho, Y.; Iracheta-Vellve, A.; Saha, B.; Lowe, P.; Adejumo, A.; Furi, I.; Ambade, A.; Gyongyosi, B.; Catalano, D.; et al. Abnormal Neutrophil Traps and Impaired Efferocytosis Contribute to Liver Injury and Sepsis Severity after Binge Alcohol Use. J. Hepatol. 2018, 69, 1145–1154. [Google Scholar] [CrossRef]
- Fadini, G.P.; Menegazzo, L.; Rigato, M.; Scattolini, V.; Poncina, N.; Bruttocao, A.; Ciciliot, S.; Mammano, F.; Ciubotaru, C.D.; Brocco, E.; et al. NETosis Delays Diabetic Wound Healing in Mice and Humans. Diabetes 2016, 65, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Neeli, I.; Radic, M. Current Challenges and Limitations in Antibody-Based Detection of Citrullinated Histones. Front. Immunol. 2016, 7, 528. [Google Scholar] [CrossRef]
- Wang, Y.; Wysocka, J.; Sayegh, J.; Lee, Y.-H.; Perlin, J.R.; Leonelli, L.; Sonbuchner, L.S.; McDonald, C.H.; Cook, R.G.; Dou, Y.; et al. Human PAD4 Regulates Histone Arginine Methylation Levels via Demethylimination. Science 2004, 306, 279–283. [Google Scholar] [CrossRef]
- Mohan, C.; Zhang, T.; Putterman, C. Pathogenic Cellular and Molecular Mediators in Lupus Nephritis. Nat. Rev. Nephrol. 2023, 19, 491–508. [Google Scholar] [CrossRef]
- Kostin, S.; Richter, M.; Krizanic, F.; Sasko, B.; Kelesidis, T.; Pagonas, N. NETosis Is an Important Component of Chronic Myocardial Inflammation in Patients with Heart Failure. Circ. Heart Fail. 2025, 18, e012231. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Shi, Q.; Wu, P.; Zhang, X.; Kambara, H.; Su, J.; Yu, H.; Park, S.-Y.; Guo, R.; Ren, Q.; et al. Single-Cell Transcriptome Profiling Reveals Neutrophil Heterogeneity in Homeostasis and Infection. Nat. Immunol. 2020, 21, 1119–1133. [Google Scholar] [CrossRef]
- Yu, X.; Song, Y.; Dong, T.; Ouyang, W.; Shao, L.; Quan, C.; Lee, K.E.; Tan, T.; Tsung, A.; Kurabayashi, K.; et al. Loss of PADI2 and PADI4 Ameliorates Sepsis-Induced Acute Lung Injury by Suppressing NLRP3+ Macrophages. JCI Insight 2024, 9, e181686. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, J.; Cai, T.; Stephens, A.; Su, S.-H.; Sandford, E.; Flora, C.; Singer, B.H.; Ghosh, M.; Choi, S.W.; et al. Machine Learning-Based Cytokine Microarray Digital Immunoassay Analysis. Biosens. Bioelectron. 2021, 180, 113088. [Google Scholar] [CrossRef]

| Approach | Objective | Mechanism | Type of Study | References |
|---|---|---|---|---|
| Anti-CitH3 antibodies | Target the harmful CitH3 released during dysregulated NETosis | Capture circulating CitH3 released during NETosis, preventing excess inflammation and cytokine storm | Preclinical | [34,64] |
| Inhibitors against PAD2, PAD4, MPO, NE | Inhibit the function of proteins involved in NETs formation | Inhibit PAD2, PAD4, MPO, NE to suppress chromatin decondensation and nuclear material release | Preclinical | [65,132,141,142] |
| DNases I | Removal of NETs to alleviate harmful effects of NETosis | DNases degrade NETs | Phase 3; a more extensive clinical trial is warranted (NCT04402970) | [143,144,145,146] |
| Methotrexate, Prednisolone, Cyclosporine | Inhibit the function of activated neutrophils | Inhibit ROS and IL-8 production, TLR-9 expression. Indirectly inhibit NET production | Preclinical/Phase 3 with no results posted (NCT04227366) | [137,147,148,149,150,151,152] |
| Tocilizumab | Target inflammatory cytokines | Bind to IL-6 receptor to inhibit the activation of IL-6 downstream signaling pathways | Phase 2 (NCT04363736) | [153,154] |
| Rituximab and Belimumab | Target B cells | B cell depletion via inhibiting the survival and activation of B cells | Phase 2 (NCT02284984) | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Tetz, Z.A.; Zeng, X.; Go, S.J.; Ouyang, W.; Lee, K.E.; Dong, T.; Li, Y.; Ma, J. CitH3, a Druggable Biomarker for Human Diseases Associated with Acute NETosis and Chronic Immune Dysfunction. Pharmaceutics 2025, 17, 809. https://doi.org/10.3390/pharmaceutics17070809
Chen Y, Tetz ZA, Zeng X, Go SJ, Ouyang W, Lee KE, Dong T, Li Y, Ma J. CitH3, a Druggable Biomarker for Human Diseases Associated with Acute NETosis and Chronic Immune Dysfunction. Pharmaceutics. 2025; 17(7):809. https://doi.org/10.3390/pharmaceutics17070809
Chicago/Turabian StyleChen, Yuchen, Zoe Ann Tetz, Xindi Zeng, Sophia Jihye Go, Wenlu Ouyang, Kyung Eun Lee, Tao Dong, Yongqing Li, and Jianjie Ma. 2025. "CitH3, a Druggable Biomarker for Human Diseases Associated with Acute NETosis and Chronic Immune Dysfunction" Pharmaceutics 17, no. 7: 809. https://doi.org/10.3390/pharmaceutics17070809
APA StyleChen, Y., Tetz, Z. A., Zeng, X., Go, S. J., Ouyang, W., Lee, K. E., Dong, T., Li, Y., & Ma, J. (2025). CitH3, a Druggable Biomarker for Human Diseases Associated with Acute NETosis and Chronic Immune Dysfunction. Pharmaceutics, 17(7), 809. https://doi.org/10.3390/pharmaceutics17070809







