Cationic Polymer Micelles as Carriers of Bioactive Sesquiterpene Lactones from Inula Helenium L. for Effective Treatment of Bacterial Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Inula helenium L. Root Extract
2.1.2. PMPP-PLA Cationic Block Copolymer
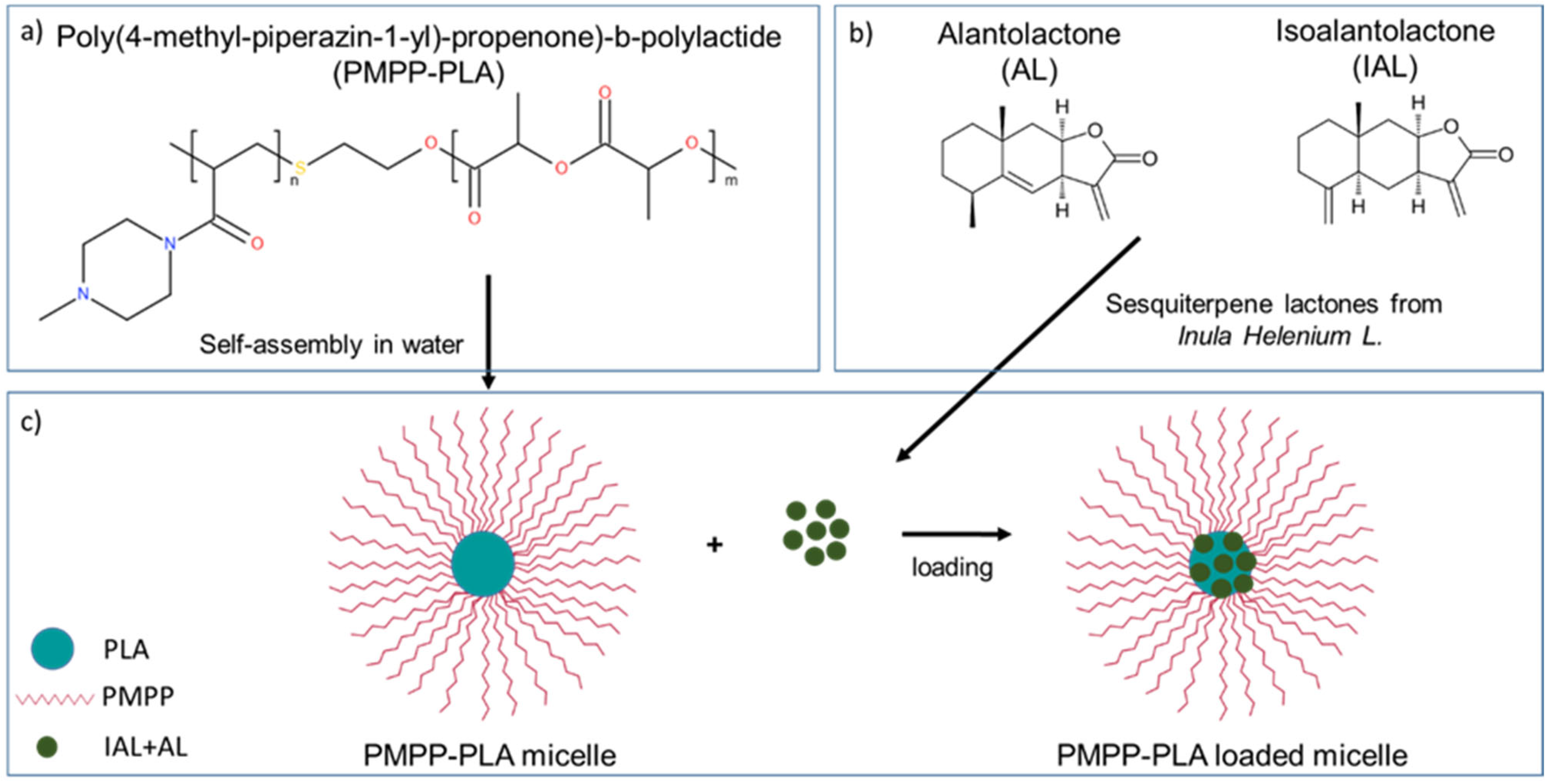
2.1.3. Preparation of PMPP-PLA Micelles
2.1.4. Loading of PMPP-PLA Micelles with Inula helenium Extract
2.1.5. Calculation of Solubility and Flory–Huggins Miscibility Parameters
2.1.6. In Vitro Release of Root Extract
2.2. Methods
2.2.1. High-Performance Liquid Chromatography (HPLC)
2.2.2. Dynamic and Electrophoretic Light Scattering (DLS and ELS)
2.2.3. Nanoparticle Tracking Analysis (NTA)
2.2.4. Transmission Electron Microscopy (TEM)
2.2.5. In Vitro Cytotoxicity
2.2.6. Biofilm Experiments
3. Results
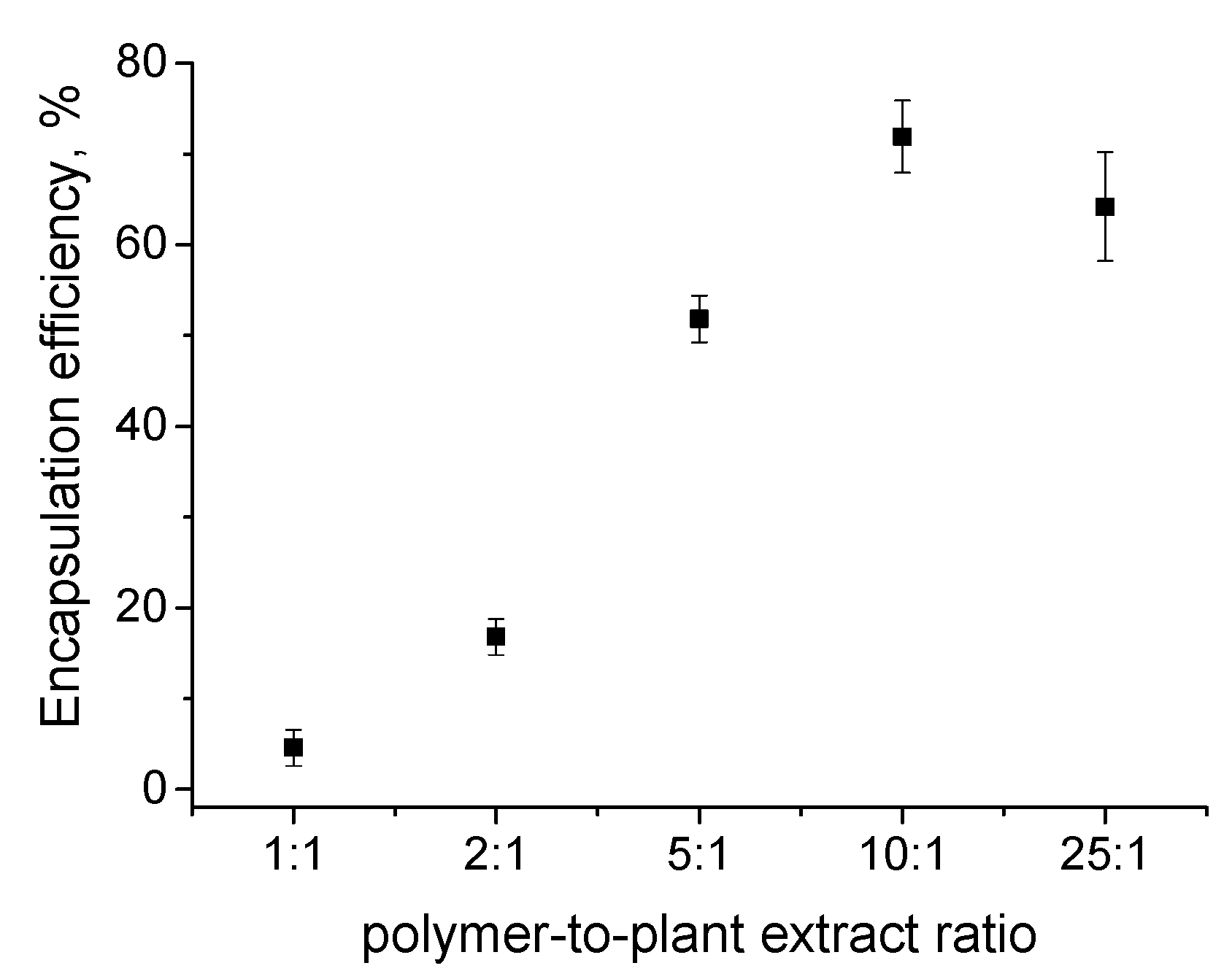
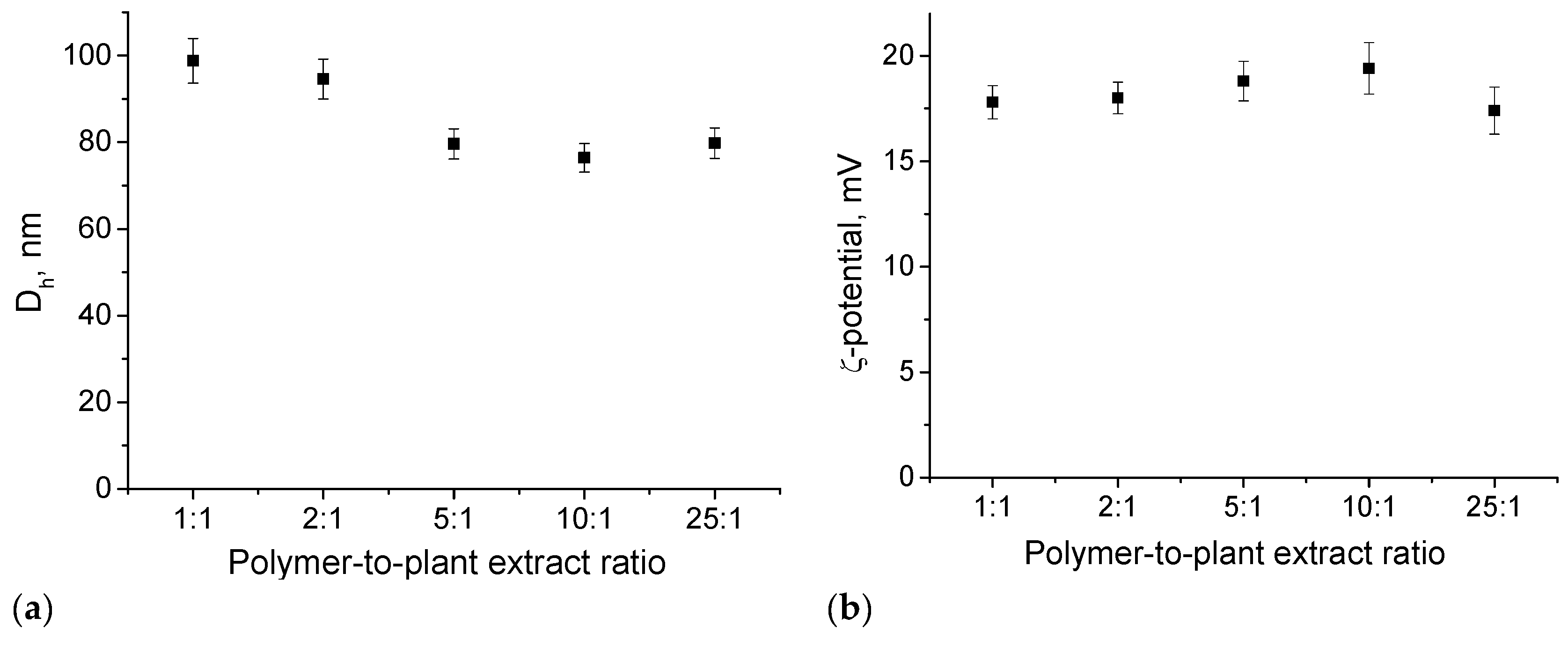
3.1. Preparation and Loading of PMPP-PLA PMs with Inula helenium L. Root Extract
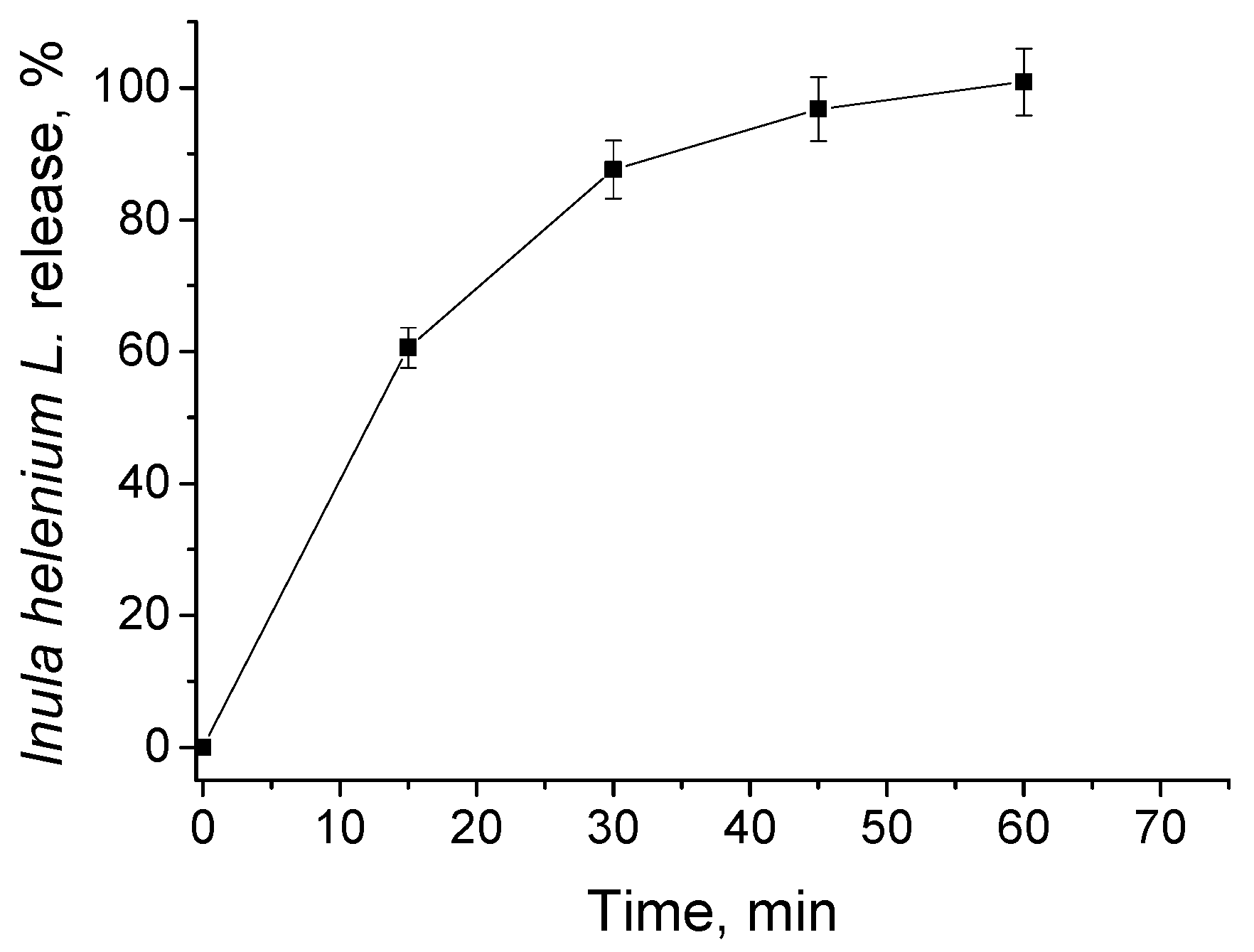
3.2. In Vitro Release of Inula helenium L. Root Extract
3.3. Cytotoxicity Evaluation
3.4. Bacterial Biofilm Destruction
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Barkhouse, A.; Hackenberger, D.; Wright, G.D. Antibiotic resistance: A key microbial survival mechanism that threatens public health. Cell Host Microbe 2024, 32, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Varela-Moreira, A.; Shi, Y.; Fens, M.H.; Lammers, T.; Hennink, W.E.; Schiffelers, R.M. Clinical application of polymeric micelles for the treatment of cancer. Mater. Chem. Front. 2017, 1, 1485–1501. [Google Scholar] [CrossRef]
- Toscanini, M.A.; Limeres, M.J.; Garrido, A.V.; Cagel, M.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A.; Cuestas, M.L. Polymeric micelles and nanomedicines: Shaping the future of next generation therapeutic strategies for infectious diseases. J. Drug Deliv. Sci. Technol. 2021, 66, 102927. [Google Scholar] [CrossRef]
- Figueiras, A.; Domingues, C.; Jarak, I.; Santos, A.I.; Parra, A.; Pais, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Kabanov, A.; Cabral, H.; et al. New Advances in Biomedical Application of Polymeric Micelles. Pharmaceutics 2022, 14, 1700. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Nishiyama, N.; Bae, Y.; Miyata, K.; Fukushima, S.; Kataoka, K. Smart polymeric micelles for gene and drug delivery. Drug Discov. Today Technol. 2005, 2, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Thotakura, N.; Parashar, P.; Raza, K. Assessing the pharmacokinetics and toxicology of polymeric micelle conjugated therapeutics. Expert Opin. Drug. Metab. Toxicol. 2021, 17, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mundra, V.; Peng, Y.; Wang, Y.; Tan, C.; Mahato, R.I. Pharmacokinetics and biodistribution of polymeric micelles containing miRNA and small-molecule drug in orthotopic pancreatic tumor-bearing mice. Theranostics 2018, 8, 4033. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, F.; Liu, Q.; Du, J. Antibacterial polymeric nanostructures for biomedical applications. Chem. Commun. 2014, 50, 14482–14493. [Google Scholar] [CrossRef]
- Lam, S.J.; Wong, E.H.; Boyer, C.; Qiao, G.G. Antimicrobial polymeric nanoparticles. Prog. Polym. Sci. 2018, 76, 40–64. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Chen, M.; Wei, J.; Xie, S.; Tao, X.; Zhang, Z.; Ran, P.; Li, X. Bacterial biofilm destruction by size/surface charge-adaptive micelles. Nanoscale 2019, 11, 1410–1422. [Google Scholar] [CrossRef]
- Stancheva, R.; Paunova-Krasteva, T.; Topouzova-Hristova, T.; Stoitsova, S.; Petrov, P.; Haladjova, E. Ciprofloxacin-Loaded Mixed Polymeric Micelles as Antibiofilm Agents. Pharmaceutics 2023, 15, 1147. [Google Scholar] [CrossRef]
- Damyanova, T.; Dimitrova, P.D.; Borisova, D.; Topouzova-Hristova, T.; Haladjova, E.; Paunova-Krasteva, T. An Overview of Biofilm-Associated Infections and the Role of Phytochemicals and Nanomaterials in Their Control and Prevention. Pharmaceutics 2024, 16, 162. [Google Scholar] [CrossRef]
- Xie, F.; Jiang, L.; Xiao, X.; Lu, Y.; Liu, R.; Jiang, W.; Cai, J. Quaternized Polysaccharide-Based Cationic Micelles as a Macromolecular Approach to Eradicate Multidrug-Resistant Bacterial Infections while Mitigating Antimicrobial Resistance. Small 2022, 18, 2104885. [Google Scholar] [CrossRef]
- Si, Z.; Zheng, W.; Prananty, D.; Li, J.; Koh, C.H.; Kang, E.T.; Pethe, K.; Chan-Park, M.B. Polymers as advanced antibacterial and antibiofilm agents for direct and combination therapies. Chem. Sci. 2022, 13, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers 2020, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Borisova, D.; Haladjova, E.; Kyulavska, M.; Petrov, P.; Pispas, S.; Stoitsova, S.; Paunova-Krasteva, T. Application of cationic polymer micelles for the dispersal of bacterial biofilms. Eng. Life Sci. 2018, 18, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Caputo, G.A. Antimicrobial polymers as synthetic mimics of host-defense peptides. Wiley Interdiscip. Rev. Nanomed. Nanobiotech. 2013, 5, 49–66. [Google Scholar] [CrossRef]
- Milović, N.M.; Wang, J.; Lewis, K.; Klibanov, A.M. Immobilized N-alkylated polyethylenimine avidly kills bacteria by rupturing cell membranes with no resistance developed. Biotechnol. Bioeng. 2005, 90, 715–722. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Weiss, A.M.; Lopez, M.A.; Rawe, B.W.; Manna, S.; Chen, Q.; Mulder, E.J.; Rowan, S.J.; Esser-Kahn, A.P. Understanding how cationic polymers’ properties inform toxic or immunogenic responses via parametric analysis. Macromolecules 2023, 56, 7286–7299. [Google Scholar] [CrossRef]
- Barba-Ostria, C.; Carrera-Pacheco, S.E.; Gonzalez-Pastor, R.; Heredia-Moya, J.; Mayorga-Ramos, A.; Rodríguez-Pólit, C.; Zúñiga-Miranda, J.; Arias-Almeida, B.; Guamán, L.P. Evaluation of Biological Activity of Natural Compounds: Current Trends and Methods. Molecules 2022, 27, 4490. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; Moraes, A.A.B.d.; Costa, K.S.d.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Faria, L.J.G.d. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Kausar, N.; Muratza, S.; Raza, M.A.; Rafique, H.; Arshad, M.N.; Altaf, A.A.; Asiri, A.M.; Shafqat, S.S.; Shafqat, S.R. Sulfonamide hybrid schiff bases of anthranilic acid: Synthesis, characterization and their biological potential. J. Mol. Struct. 2019, 1185, 8–20. [Google Scholar] [CrossRef]
- Thakur, L.; Ghodasra, U.; Patel, N.; Dabhi, M. Novel approaches for stability improvement in natural medicines. Pharmacogn. Rev. 2011, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Unde, J.S.; Shukla, R. Polymeric micelles in the delivery of therapeutic phytoconstituents. In Polymeric Micelles: Principles, Perspectives and Practices; Singh, S.K., Gulati, M., Mutalik, S., Dhanasekaran, M., Dua, K., Eds.; Springer: Singapore, 2023; pp. 175–201. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef] [PubMed]
- Haladjova, E.; Dimitrov, I.; Davydova, N.; Todorova, J.; Ugrinova, I.; Forys, A.; Trzebicka, B.; Rangelov, S. Cationic (Co)polymers Based on N-Substituted Polyacrylamides as Carriers of Bio-macromolecules: Polyplexes, Micelleplexes, and Spherical Nucleic Acidlike Structures. Biomacromolecules 2021, 22, 971. [Google Scholar] [CrossRef] [PubMed]
- Stancheva, R.; Haladjova, E.; Petrova, M.; Ugrinova, I.; Dimitrov, I.; Rangelov, S. Polypiperazine-Based Micelles of Mixed Composition for Gene Delivery. Polymers 2024, 16, 3100. [Google Scholar] [CrossRef]
- Chun, J.; Park, S.-M.; Lee, M.; Ha, I.J.; Jeong, M.-K. The Sesquiterpene Lactone-Rich Fraction of Inula helenium L. Enhances the Antitumor Effect of Anti-PD-1 Antibody in Colorectal Cancer: Integrative Phytochemical, Transcriptomic, and Experimental Analyses. Cancers 2023, 15, 653. [Google Scholar] [CrossRef]
- Özcan, F.Ş.; Özcan, N.; Dikmen Meral, H.; Çetin, Ö.; Çelik, M.; Trendafilova, A. Extraction of Sesquiterpene Lactones from Inula helenium Roots by High-Pressure Homogenization and Effects on Antimicrobial, Antioxidant, and Antiglycation Activities. Food Bioprocess Technol. 2024, 17, 4071–4082. [Google Scholar] [CrossRef]
- Zeng, W.; Du, Y.; Xue, Y.; Frisch, H.L. Solubility Parameters. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007; pp. 289–303. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Huo, Y.; Shi, H.; Li, W.; Wang, M.; Li, X. HPLC determination and NMR structural elucidation of sesquiterpene lactones in Inula helenium. J. Pharm. Biomed. Anal. 2010, 51, 942–946. [Google Scholar] [CrossRef]
- De Soyza, A.; Hall, A.J.; Mahenthiralingam, E.; Drevinek, P.; Kaca, W.; Drulis-Kawa, Z.; Stoitsova, S.R.; Toth, V.; Coenye, T.; Zlosnik, J.E.A.; et al. Developing an international Pseudomonas aeruginosa reference panel. Microbiologyopen 2013, 2, 1010–1023. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Rijcken, C.J.F.; Soga, O.; Hennink, W.E.; Van Nostrum, C.F. Triggered destabilisation of polymeric micelles and vesicles by changing polymers polarity: An attractive tool for drug delivery. J. Control. Release 2007, 120, 131–148. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; YT, K. Progress in Polymeric Micelles for Drug Delivery Applications. Pharmaceutics 2022, 14, 1636. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Ren, Y.; Wang, X. New advances in the biodegradation of Poly(lactic) acid. Intern. Biodeterior. Biodegrad. 2017, 117, 215–223. [Google Scholar] [CrossRef]
- Dinarvand, R.; Sepehri, N.; Manoochehri, S.; Rouhani, H.; Atyabi, F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Hausig-Punke, F.; Dekevic, G.; Sobotta, F.H.; Solomun, J.I.; Richter, F.; Salzig, D.; Traeger, A.; Brendel, J.C. Efficient Transfection via an Unexpected Mechanism by Near Neutral Polypiperazines with Tailored Response to Endosomal pH. Macromol. Biosci. 2023, 23, 2200517. [Google Scholar] [CrossRef]
- Rayer, A.V.; Sumon, K.Z.; Jaffari, L.; Henni, A. Dissociation constants (pK a) of tertiary and cyclic amines: Structural and temperature dependences. J. Chem. Eng. Data 2014, 59, 3805–3813. [Google Scholar] [CrossRef]
- Seca, A.M.; Grigore, A.; Pinto, D.C.; Silva, A.M. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J. Ethnopharmacol. 2014, 154, 286–310. [Google Scholar] [CrossRef]
- Flemming, H.C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef]
- Cendra, M.D.M.; Torrents, E. Pseudomonas aeruginosa biofilms and their partners in crime. Biotechnol Adv. 2021, 49, 107734. [Google Scholar] [CrossRef]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Damyanova, T.; Paunova-Krasteva, T. What We Still Don’t Know About Biofilms—Current Overview and Key Research Information. Microbiol. Res. 2025, 16, 46. [Google Scholar] [CrossRef]
- Ivanova, V.; Nedialkov, P.; Dimitrova, P.; Paunova-Krasteva, T.; Trendafilova, A. Inula salicina L.: Insights into its polyphenolic constituents and biological activity. Pharmaceuticals 2024, 17, 844. [Google Scholar] [CrossRef] [PubMed]
- Peltola, H.; Pääkkönen, M. Acute osteomyelitis in children. N. Engl. J. Med. 2014, 370, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Song, K.H.; Park, K.H.; Kim, M.; Choe, P.G.; Lee, S.H.; Oh, M.D.; Lee, S.H.; Jang, H.C.; Kang, S.J.; et al. Impact of antimicrobial treatment duration on outcome of Staphylococcus aureus bacteraemia: A cohort study. Clin. Microbiol. Infect. 2019, 25, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.E.; Wagner, N.J.; Li, L.; Beam, J.E.; Wilkinson, A.D.; Radlinski, L.C.; Zhang, Q.; Miao, E.A.; Conlon, B.P. Reactive oxygen species induce antibiotic tolerance during systemic Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 282–290. [Google Scholar] [CrossRef]
- He, X.; Dai, L.; Ye, L.; Sun, X.; Enoch, O.; Hu, R.; Zan, X.; Lin, F.; Shen, J. A vehicle-free antimicrobial polymer hybrid gold nanoparticle as synergistically therapeutic platforms for Staphylococcus aureus infected wound healing. Adv. Sci. 2022, 9, 2105223. [Google Scholar] [CrossRef]
- Zhang, S.; Qu, X.; Tang, H.; Wang, Y.; Yang, H.; Yuan, W.; Yue, B. Diclofenac Resensitizes Methicillin-Resistant Staphylococcus aureus to β-Lactams and Prevents Implant Infections. Adv. Sci. 2021, 8, 2100681. [Google Scholar] [CrossRef]
- van Dijk, B.; Hooning van Duyvenbode, J.F.F.; de Vor, L.; Nurmohamed, F.R.H.; Lam, M.G.; Poot, A.J.; Ramakers, R.M.; Koustoulidou, S.; Beekman, F.J.; van Strijp, J.; et al. Evaluating the targeting of a Staphylococcus-aureus-infected implant with a radiolabeled antibody In Vivo. Int. J. Mol. Sci. 2023, 24, 4374. [Google Scholar] [CrossRef]
- Samia, N.I.; Robicsek, A.; Heesterbeek, H.; Peterson, L.R. Methicillin-resistant staphylococcus aureus nosocomial infection has a distinct epidemiological position and acts as a marker for overall hospital-acquired infection trends. Sci. Rep. 2022, 12, 17007. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev. Anti. Infect. Ther. 2015, 13, 1499–1516. [Google Scholar] [CrossRef]
- Teper, P.; Sotirova, A.; Kowalczuk, A.; Mendrek, B.; Paunova-Krasteva, T. Effects of cationic polymers on the viability of microbial biofilms. Folia Med. 2023, 65, 124–130. [Google Scholar] [CrossRef]
- Paunova-Krasteva, T.; Hemdan, B.A.; Dimitrova, P.D.; Damyanova, T.; El-Feky, A.M.; Elbatanony, M.M.; Stoitsova, S.; El-Liethy, M.A.; El-Taweel, G.E.; El Nahrawy, A.M. Hybrid chitosan/CaO-based nanocomposites doped with plant extracts from Azadirachta indica and Melia azedarach: Evaluation of antibacterial and antibiofilm activities. Bionanoscience 2023, 13, 88–102. [Google Scholar] [CrossRef]
- Dimitrova, P.D.; Ivanova, V.; Trendafilova, A.; Paunova-Krasteva, T. Anti-Biofilm and Anti-Quorum-Sensing Activity of Inula Extracts: A Strategy for Modulating Chromobacterium violaceum Virulence Factors. Pharmaceuticals 2024, 17, 573. [Google Scholar] [CrossRef] [PubMed]



| Polymer/Extract | δ [(MPa)1/2] * | χAL | χIAL |
|---|---|---|---|
| PLA | 21.33 | 1.22 | 1.28 |
| AL | 17.6 | - | - |
| IAL | 17.5 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stancheva, R.; Damyanova, T.; Paunova-Krasteva, T.; Veleva, R.; Topouzova-Hristova, T.; Ivanova, V.; Trendafilova, A.; Dimitrov, I.; Rangelov, S.; Haladjova, E. Cationic Polymer Micelles as Carriers of Bioactive Sesquiterpene Lactones from Inula Helenium L. for Effective Treatment of Bacterial Biofilms. Pharmaceutics 2025, 17, 800. https://doi.org/10.3390/pharmaceutics17060800
Stancheva R, Damyanova T, Paunova-Krasteva T, Veleva R, Topouzova-Hristova T, Ivanova V, Trendafilova A, Dimitrov I, Rangelov S, Haladjova E. Cationic Polymer Micelles as Carriers of Bioactive Sesquiterpene Lactones from Inula Helenium L. for Effective Treatment of Bacterial Biofilms. Pharmaceutics. 2025; 17(6):800. https://doi.org/10.3390/pharmaceutics17060800
Chicago/Turabian StyleStancheva, Rumena, Tsvetozara Damyanova, Tsvetelina Paunova-Krasteva, Ralitsa Veleva, Tanya Topouzova-Hristova, Viktoria Ivanova, Antoaneta Trendafilova, Ivaylo Dimitrov, Stanislav Rangelov, and Emi Haladjova. 2025. "Cationic Polymer Micelles as Carriers of Bioactive Sesquiterpene Lactones from Inula Helenium L. for Effective Treatment of Bacterial Biofilms" Pharmaceutics 17, no. 6: 800. https://doi.org/10.3390/pharmaceutics17060800
APA StyleStancheva, R., Damyanova, T., Paunova-Krasteva, T., Veleva, R., Topouzova-Hristova, T., Ivanova, V., Trendafilova, A., Dimitrov, I., Rangelov, S., & Haladjova, E. (2025). Cationic Polymer Micelles as Carriers of Bioactive Sesquiterpene Lactones from Inula Helenium L. for Effective Treatment of Bacterial Biofilms. Pharmaceutics, 17(6), 800. https://doi.org/10.3390/pharmaceutics17060800











