NLC-Based Rifampicin Delivery System: Development and Characterization for Improved Drug Performance Against Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NLCs
2.3. Incorporation Efficiency and Drug Loading
2.4. Physicochemical Characterization
2.5. Colloidal Stability Assessment
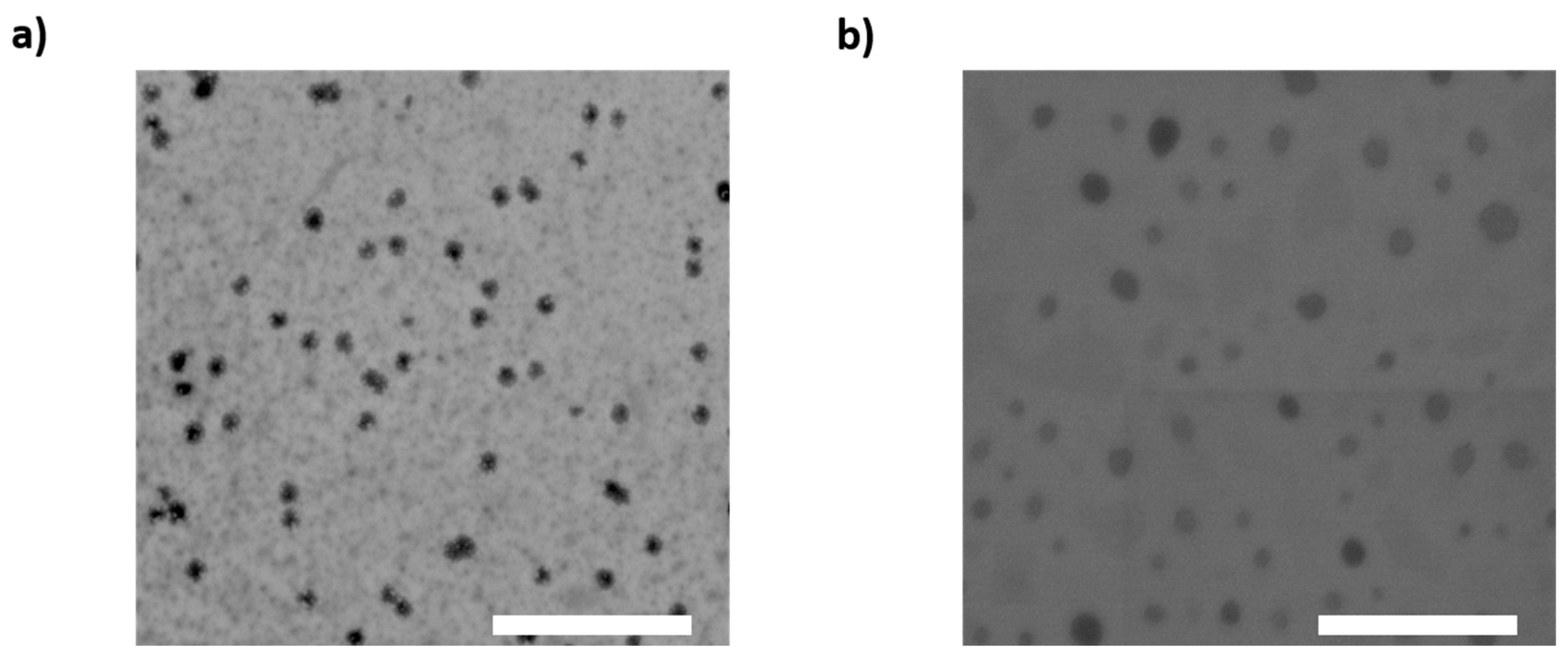
2.6. Morphology of Nanoparticles
2.7. Drug Release Studies
2.8. Bacterial Strain and Culture Conditions
2.9. Antibacterial Activity Assay
2.10. Metabolic Activity Assay
2.11. Data Analysis and Statistics
3. Results and Discussion
3.1. NLC Physicochemical Properties
3.2. Incorporation Efficiency and Drug Loading
3.3. Drug Release and Kinetic Fitting
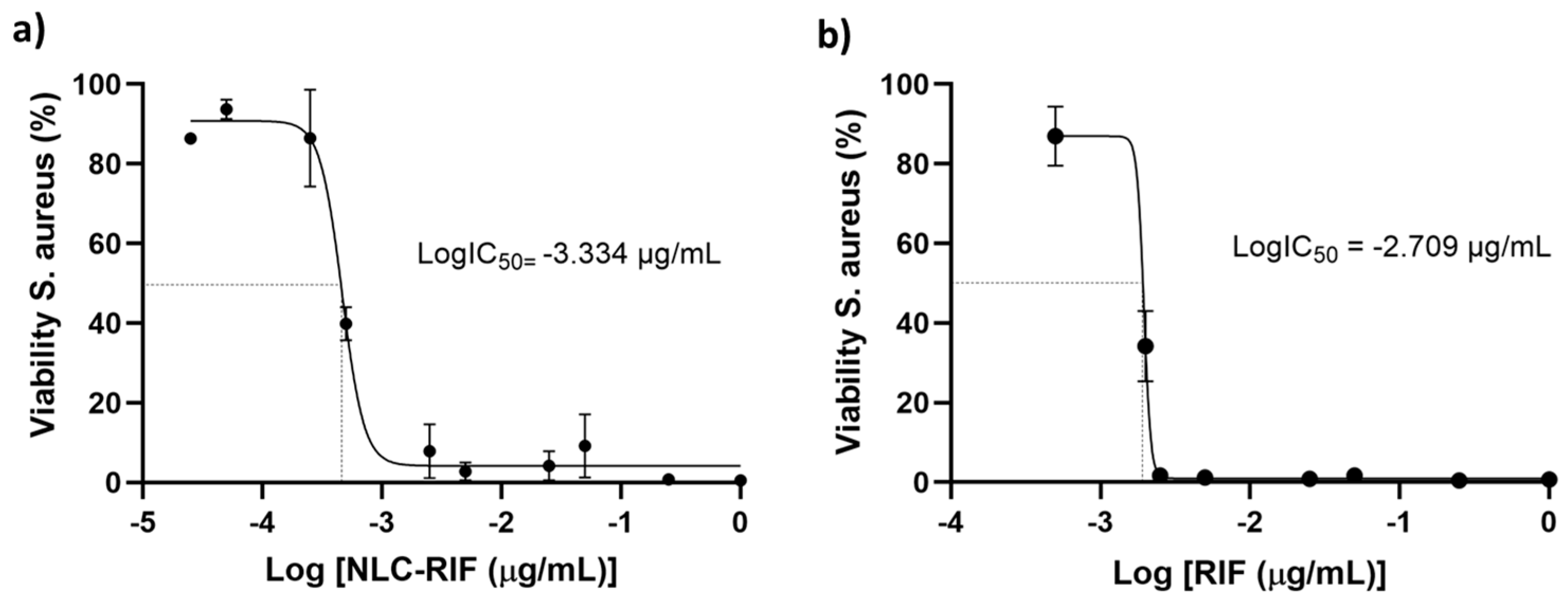
3.4. Antimicrobial Activity
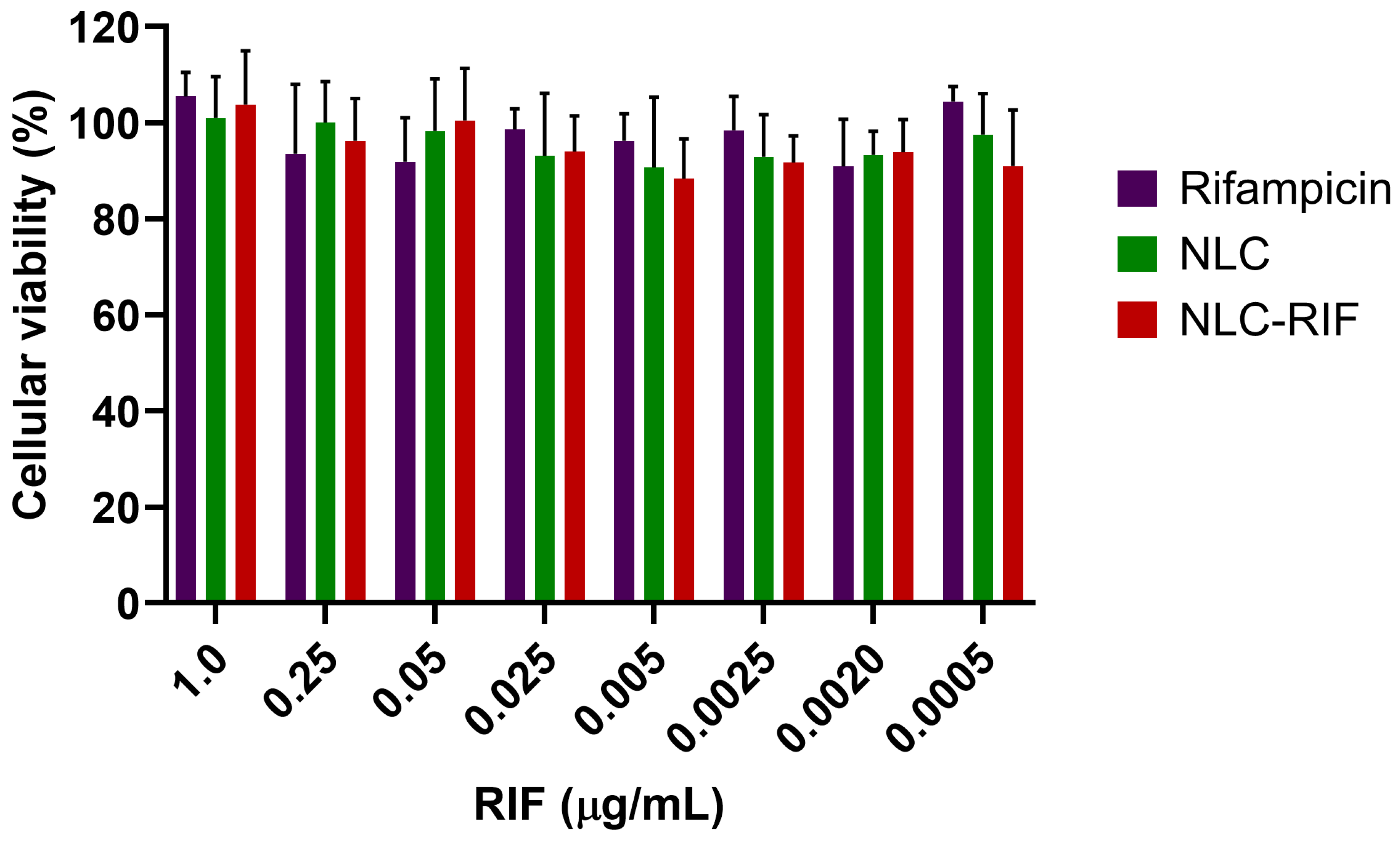
3.5. Cytotoxicity Outcomes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 28 March 2025).
- Hetta, H.F.; Ahmed, E.A.; Hemdan, A.G.; El-Deek, H.E.; Abd-Elregal, S.; Abd Ellah, N.H. Modulation of Rifampicin-Induced Hepatotoxicity Using Poly(Lactic-Co-Glycolic Acid) Nanoparticles: A Study on Rat and Cell Culture Models. Nanomedicine 2020, 15, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Zara, G.P.; Bargoni, A.; Cavalli, R.; Fundarò, A.; Vighetto, D.; Gasco, M.R. Pharmacokinetics and Tissue Distribution of Idarubicin-Loaded Solid Lipid Nanoparticles After Duodenal Administration to Rats. J. Pharm. Sci. 2002, 91, 1324–1333. [Google Scholar] [CrossRef]
- Mamun, M.M.; Sorinolu, A.J.; Munir, M.; Vejerano, E.P. Nanoantibiotics: Functions and Properties at the Nanoscale to Combat Antibiotic Resistance. Front. Chem. 2021, 9, 687660. [Google Scholar] [CrossRef]
- Wang, D.-Y.; van der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Lipid-Based Antimicrobial Delivery-Systems for the Treatment of Bacterial Infections. Front. Chem. 2020, 7, 872. [Google Scholar] [CrossRef]
- Pandey, R.; Sharma, S.; Khuller, G.K. Oral Solid Lipid Nanoparticle-Based Antitubercular Chemotherapy. Tuberculosis 2005, 85, 415–420. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, H.; Lan, T.; Chen, Y.; Chen, X.; Feng, Y.; Luo, Y.; Yao, Y.; Li, Y.; Pan, X.; et al. Co-Delivery of Antibiotic and Baicalein by Using Different Polymeric Nanoparticle Cargos with Enhanced Synergistic Antibacterial Activity. Int. J. Pharm. 2021, 599, 120419. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, A.; Thomas, N.; Barnes, T.J.; Subramaniam, S.; Loh, T.C.; Joyce, P.; Prestidge, C.A. Lipid Nanocarriers-Enabled Delivery of Antibiotics and Antimicrobial Adjuvants to Overcome Bacterial Biofilms. Pharmaceutics 2024, 16, 396. [Google Scholar] [CrossRef]
- Arana, L.; Gallego, L.; Alkorta, I. Incorporation of Antibiotics into Solid Lipid Nanoparticles: A Promising Approach to Reduce Antibiotic Resistance Emergence. Nanomaterials 2021, 11, 1251. [Google Scholar] [CrossRef]
- Shegokar, R.; Nakach, M. Chapter 4—Large-Scale Manufacturing of Nanoparticles—An Industrial Outlook. In Drug Delivery Aspects; Shegokar, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 57–77. ISBN 978-0-12-821222-6. [Google Scholar]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: A Review Emphasizing on Particle Structure and Drug Release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Bazán Henostroza, M.A.; Diniz Tavares, G.; Nishitani Yukuyama, M.; De Souza, A.; José Barbosa, E.; Carlos Avino, V.; dos Santos Neto, E.; Rebello Lourenço, F.; Löbenberg, R.; Araci Bou-Chacra, N. Antibiotic-Loaded Lipid-Based Nanocarrier: A Promising Strategy to Overcome Bacterial Infection. Int. J. Pharm. 2022, 621, 121782. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, F.; Motavalizadehkakhky, A.; Mehrzad, J.; Zhiani, R.; Homayouni Tabrizi, M. Folic Acid Conjugated-Chitosan Modified Nanostructured Lipid Carriers as Promising Carriers for Delivery of Umbelliprenin to Cancer Cells: In Vivo and in Vitro. Eur. Polym. J. 2023, 186, 111849. [Google Scholar] [CrossRef]
- Muheem, A.; Wasim, M.; Aldosari, E.; Baboota, S.; Ali, J. Fabrication of TPGS Decorated Etravirine Loaded Lipidic Nanocarriers as a Neoteric Oral Bioavailability Enhancer for Lymphatic Targeting. Discov. Nano 2024, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Shirodkar, R.K.; Kumar, L.; Mutalik, S.; Lewis, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Emerging Lipid Based Drug Delivery Systems. Pharm. Chem. J. 2019, 53, 440–453. [Google Scholar] [CrossRef]
- Motiei, M.; Pleno de Gouveia, L.; Šopík, T.; Vícha, R.; Škoda, D.; Císař, J.; Khalili, R.; Domincová Bergerová, E.; Münster, L.; Fei, H.; et al. Nanoparticle-Based Rifampicin Delivery System Development. Molecules 2021, 26, 2067. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Glackin, C.A.; Horwitz, M.A.; Zink, J.I. Nanomachines and Other Caps on Mesoporous Silica Nanoparticles for Drug Delivery. Acc. Chem. Res. 2019, 52, 1531–1542. [Google Scholar] [CrossRef]
- Koch, A.; Mizrahi, V.; Warner, D.F. The Impact of Drug Resistance on Mycobacterium Tuberculosis Physiology: What Can We Learn from Rifampicin? Emerg. Microbes Infect. 2014, 3, 1–11. [Google Scholar] [CrossRef]
- Yılmaz, E.Ş.; Aslantaş, Ö. Antimicrobial Resistance and Underlying Mechanisms in Staphylococcus aureus Isolates. Asian Pac. J. Trop. Med. 2017, 10, 1059–1064. [Google Scholar] [CrossRef]
- Ellis, T.; Chiappi, M.; García-Trenco, A.; Al-Ejji, M.; Sarkar, S.; Georgiou, T.K.; Shaffer, M.S.P.; Tetley, T.D.; Schwander, S.; Ryan, M.P.; et al. Multimetallic Microparticles Increase the Potency of Rifampicin against Intracellular Mycobacterium tuberculosis. ACS Nano 2018, 12, 5228–5240. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, Y.; Yan, J.; Li, Y.; Wang, J.; Yang, Y.Y.; Yuan, P.; Ding, X. Bacterial Outer Membrane-Coated Mesoporous Silica Nanoparticles for Targeted Delivery of Antibiotic Rifampicin against Gram-Negative Bacterial Infection In Vivo. Adv. Funct. Mater. 2021, 31, 2103442. [Google Scholar] [CrossRef]
- Chaudhary, N.; Aggarwal, B.; Saini, V.; Yavvari, P.S.; Sharma, P.; Srivastava, A.; Bajaj, A. Polyaspartate-Derived Synthetic Antimicrobial Polymer Enhances the Activity of Rifampicin against Multidrug-Resistant Pseudomonas Aeruginosa Infections. Biomater. Sci. 2022, 10, 5158–5171. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Ahmad, T.; Khan, A.; Sarwar, R.; Shafiq, J.; Raza, Y.; Ahmed, A.; Ullah, S.; Ur Rehman, N.; Al-Harrasi, A. Rifampicin Conjugated Silver Nanoparticles: A New Arena for Development of Antibiofilm Potential against Methicillin Resistant Staphylococcus aureus and Klebsiella Pneumoniae. Int. J. Nanomed. 2019, 14, 3983–3993. [Google Scholar] [CrossRef]
- Scolari, I.R.; Páez, P.L.; Musri, M.M.; Petiti, J.P.; Torres, A.; Granero, G.E. Rifampicin Loaded in Alginate/Chitosan Nanoparticles as a Promising Pulmonary Carrier against Staphylococcus aureus. Drug Deliv. Transl. Res. 2020, 10, 1403–1417. [Google Scholar] [CrossRef]
- Banerjee, S.; Roy, S.; Bhaumik, K.N.; Pillai, J. Mechanisms of the Effectiveness of Lipid Nanoparticle Formulations Loaded with Anti-Tubercular Drugs Combinations toward Overcoming Drug Bioavailability in Tuberculosis. J. Drug Target. 2020, 28, 55–69. [Google Scholar] [CrossRef]
- Pinheiro, M.; Ribeiro, R.; Vieira, A.; Andrade, F.; Reis, S. Design of a Nanostructured Lipid Carrier Intended to Improve the Treatment of Tuberculosis. Drug Des. Dev. Ther. 2016, 10, 2467–2475. [Google Scholar] [CrossRef]
- Vieira, A.C.; Magalhaes, J.; Rocha, S.; Cardoso, M.S.; Santos, S.G.; Borges, M.; Pinheiro, M.; Reis, S. Targeted Macrophages Delivery of Rifampicin-Loaded Lipid Nanoparticles to Improve Tuberculosis Treatment. Nanomedicine 2017, 12, 2721–2736. [Google Scholar] [CrossRef]
- Ortiz, A.C.; Yañez, O.; Salas-Huenuleo, E.; Morales, J.O. Development of a Nanostructured Lipid Carrier (NLC) by a Low-Energy Method, Comparison of Release Kinetics and Molecular Dynamics Simulation. Pharmaceutics 2021, 13, 531. [Google Scholar] [CrossRef]
- Goutal, S.; Auvity, S.; Legrand, T.; Hauquier, F.; Cisternino, S.; Chapy, H.; Saba, W.; Tournier, N. Validation of a Simple HPLC-UV Method for Rifampicin Determination in Plasma: Application to the Study of Rifampicin Arteriovenous Concentration Gradient. J. Pharm. Biomed. Anal. 2016, 123, 173–178. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A New Paradigm for Treating Infectious Diseases Using Nanomaterials in the Antibiotics Resistant Era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Fazly Bazzaz, B.S.; Khameneh, B.; Zarei, H.; Golmohammadzadeh, S. Antibacterial Efficacy of Rifampin Loaded Solid Lipid Nanoparticles against Staphylococcus epidermidis Biofilm. Microb. Pathog. 2016, 93, 137–144. [Google Scholar] [CrossRef]
- Carneiro, S.P.; Carvalho, K.V.; de Oliveira Aguiar Soares, R.D.; Carneiro, C.M.; de Andrade, M.H.G.; Duarte, R.S.; dos Santos, O.D.H. Functionalized Rifampicin-Loaded Nanostructured Lipid Carriers Enhance Macrophages Uptake and Antimycobacterial Activity. Colloids Surf. B Biointerfaces 2019, 175, 306–313. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Domínguez-Robles, J.; Utomo, E.; Font, M.; Martínez-Ohárriz, M.C.; Permana, A.D.; Cárcamo-Martínez, Á.; Larrañeta, E.; Donnelly, R.F. Inclusion Complexes of Rifampicin with Native and Derivatized Cyclodextrins: In Silico Modeling, Formulation, and Characterization. Pharmaceuticals 2022, 15, 20. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, X.G.; Zhang, J.; Liu, C.S.; Xue, Y.P.; Sun, G.Z.; Zhang, W.F. In Vitro Release of Rifampicin and Biocompatibility of Oleoylchitosan Nanoparticles. J. Appl. Polym. Sci. 2009, 111, 2269–2274. [Google Scholar] [CrossRef]
- Mistry, N.; Bandyopadhyaya, R.; Mehra, S. Enhancement of Antimycobacterial Activity of Rifampicin Using Mannose-Anchored Lipid Nanoparticles against Intramacrophage Mycobacteria. ACS Appl. Bio Mater. 2022, 5, 5779–5789. [Google Scholar] [CrossRef]
- Bunjes, H. Structural Properties of Solid Lipid Based Colloidal Drug Delivery Systems. Curr. Opin. Colloid Interface Sci. 2011, 16, 405–411. [Google Scholar] [CrossRef]
- Cholakova, D.; Denkov, N. Polymorphic Phase Transitions in Triglycerides and Their Mixtures Studied by SAXS/WAXS Techniques: In Bulk and in Emulsions. Adv. Colloid Interface Sci. 2024, 323, 103071. [Google Scholar] [CrossRef]
- Aboutaleb, E.; Noori, M.; Gandomi, N.; Atyabi, F.; Fazeli, M.R.; Jamalifar, H.; Dinarvand, R. Improved Antimycobacterial Activity of Rifampin Using Solid Lipid Nanoparticles. Int. Nano Lett. 2012, 2, 33. [Google Scholar] [CrossRef]
- Aliyazdi, S.; Frisch, S.; Neu, T.; Veldung, B.; Karande, P.; Schaefer, U.F.; Loretz, B.; Vogt, T.; Lehr, C.-M. A Novel 3D Printed Model of Infected Human Hair Follicles to Demonstrate Targeted Delivery of Nanoantibiotics. ACS Biomater. Sci. Eng. 2024, 10, 4947–4957. [Google Scholar] [CrossRef] [PubMed]
- Woźniak-Budych, M.J.; Przysiecka, Ł.; Langer, K.; Peplińska, B.; Jarek, M.; Wiesner, M.; Nowaczyk, G.; Jurga, S. Green Synthesis of Rifampicin-Loaded Copper Nanoparticles with Enhanced Antimicrobial Activity. J. Mater. Sci. Mater. Med. 2017, 28, 42. [Google Scholar] [CrossRef] [PubMed]
- Aubry-Damon, H.; Soussy, C.-J.; Courvalin, P. Characterization of Mutations in therpoB Gene That Confer Rifampin Resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1998, 42, 2590–2594. [Google Scholar] [CrossRef]
- Mlynarczyk-Bonikowska, B.; Kowalewski, C.; Krolak-Ulinska, A.; Marusza, W. Molecular Mechanisms of Drug Resistance in Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 8088. [Google Scholar] [CrossRef]
- Abedi, E.; Akhavan, H.-R.; Mohammadi, H.; Banasaz, S. Structure-Based Modifications of Nano Lipid Carriers: Comparative Review on Release Properties and Anti-Microbial Activities of Bioactive Compounds. Food Control 2024, 159, 110237. [Google Scholar] [CrossRef]
- Dyett, B.P.; Yu, H.; Sarkar, S.; Strachan, J.B.; Drummond, C.J.; Conn, C.E. Uptake Dynamics of Cubosome Nanocarriers at Bacterial Surfaces and the Routes for Cargo Internalization. ACS Appl. Mater. Interfaces 2021, 13, 53530–53540. [Google Scholar] [CrossRef]
- Tran, N.; Hocquet, M.; Eon, B.; Sangwan, P.; Ratcliffe, J.; Hinton, T.M.; White, J.; Ozcelik, B.; Reynolds, N.P.; Muir, B.W. Non-Lamellar Lyotropic Liquid Crystalline Nanoparticles Enhance the Antibacterial Effects of Rifampicin against Staphylococcus aureus. J. Colloid Interface Sci. 2018, 519, 107–118. [Google Scholar] [CrossRef]
- Ghaderkhani, J.; Yousefimashouf, R.; Arabestani, M.; Roshanaei, G.; Asl, S.S.; Abbasalipourkabir, R. Improved Antibacterial Function of Rifampicin-Loaded Solid Lipid Nanoparticles on Brucella Abortus. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1181–1193. [Google Scholar] [CrossRef] [PubMed]
- Khatak, S.; Mehta, M.; Awasthi, R.; Paudel, K.R.; Singh, S.K.; Gulati, M.; Hansbro, N.G.; Hansbro, P.M.; Dua, K.; Dureja, H. Solid Lipid Nanoparticles Containing Anti-Tubercular Drugs Attenuate the Mycobacterium marinum Infection. Tuberculosis 2020, 125, 102008. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Baek, K.-H. Phyto-Mediated Biosynthesis of Silver Nanoparticles Using the Rind Extract of Watermelon (Citrullus lanatus) under Photo-Catalyzed Condition and Investigation of Its Antibacterial, Anticandidal and Antioxidant Efficacy. J. Photochem. Photobiol. B Biol. 2016, 161, 200–210. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Ghitulica, C.D.; Voicu, G.; Huang, K.-S.; Yang, C.-H.; Ficai, A.; Vasile, B.S.; Grumezescu, V.; Bleotu, C.; Chifiriuc, M.C. New Silica Nanostructure for the Improved Delivery of Topical Antibiotics Used in the Treatment of Staphylococcal Cutaneous Infections. Int. J. Pharm. 2014, 463, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Suciati, T.; Nafisa, S.; Nareswari, T.L.; Juniatik, M.; Julianti, E.; Wibowo, M.S.; Yudhistira, T.; Ihsanawati, I.; Triyani, Y.; Khairurrijal, K. ArtinM Grafted Phospholipid Nanoparticles for Enhancing Antibiotic Cellular Uptake Against Intracellular Infection. Int. J. Nanomed. 2020, 15, 8829–8843. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tang, X.; Yi, S.; Guo, T.; Liao, Y.; Wang, Y.; Zhang, X. Maltodextrin-Derived Nanoparticles Resensitize Intracellular Dormant Staphylococcus aureus to Rifampicin. Carbohydr. Polym. 2025, 348, 122843. [Google Scholar] [CrossRef]
- Aguilar-Colomer, A.; Colilla, M.; Izquierdo-Barba, I.; Jiménez-Jiménez, C.; Mahillo, I.; Esteban, J.; Vallet-Regí, M. Impact of the Antibiotic-Cargo from MSNs on Gram-Positive and Gram-Negative Bacterial Biofilms. Microporous Mesoporous Mater. 2021, 311, 110681. [Google Scholar] [CrossRef]
- Amarnath Praphakar, R.; Sumathra, M.; Sam Ebenezer, R.; Vignesh, S.; Shakila, H.; Rajan, M. Fabrication of Bioactive Rifampicin Loaded κ-Car-MA-INH/Nano Hydroxyapatite Composite for Tuberculosis Osteomyelitis Infected Tissue Regeneration. Int. J. Pharm. 2019, 565, 543–556. [Google Scholar] [CrossRef]
- Jia, D.; Zou, Y.; Cheng, J.; Zhang, Y.; Zhang, H.; Lu, K.; Chen, H.; Zhang, Y.; Yu, Q. A Multifunctional Nanoplatform with “Disruption and Killing” Function to Improve the Efficiency of Conventional Antibiotics for Biofilm Eradication. J. Mater. Sci. Technol. 2025, 205, 98–108. [Google Scholar] [CrossRef]
- Topsakal, A.; Ekren, N.; Kilic, O.; Oktar, F.N.; Mahirogullari, M.; Ozkan, O.; Sasmazel, H.T.; Turk, M.; Bogdan, I.M.; Stan, G.E.; et al. Synthesis and Characterization of Antibacterial Drug Loaded β-Tricalcium Phosphate Powders for Bone Engineering Applications. J. Mater. Sci. Mater. Med. 2020, 31, 16. [Google Scholar] [CrossRef] [PubMed]
- Kranthi Kiran, A.S.; Kizhakeyil, A.; Ramalingam, R.; Verma, N.K.; Lakshminarayanan, R.; Kumar, T.S.S.; Doble, M.; Ramakrishna, S. Drug Loaded Electrospun Polymer/Ceramic Composite Nanofibrous Coatings on Titanium for Implant Related Infections. Ceram. Int. 2019, 45, 18710–18720. [Google Scholar] [CrossRef]
- Chambin, O.; and Jannin, V. Interest of Multifunctional Lipid Excipients: Case of Gelucire® 44/14. Drug Dev. Ind. Pharm. 2005, 31, 527–534. [Google Scholar] [CrossRef]
- Traul, K.A.; Driedger, A.; Ingle, D.L.; Nakhasi, D. Review of the Toxicologic Properties of Medium-Chain Triglycerides. Food Chem. Toxicol. 2000, 38, 79–98. [Google Scholar] [CrossRef]
- Buss, N.; Ryan, P.; Baughman, T.; Roy, D.; Patterson, C.; Gordon, C.; Dixit, R. Nonclinical Safety and Pharmacokinetics of Miglyol 812: A Medium Chain Triglyceride in Exenatide Once Weekly Suspension. J. Appl. Toxicol. 2018, 38, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.J.; Onyuksel, H. Mechanistic Studies on Surfactant-Induced Membrane Permeability Enhancement. Pharm. Res. 2000, 17, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Rege, B.D.; Kao, J.P.Y.; Polli, J.E. Effects of Nonionic Surfactants on Membrane Transporters in Caco-2 Cell Monolayers. Eur. J. Pharm. Sci. 2002, 16, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yang, K.; Tang, X.; Bi, Y.; Ding, Y.; Deng, M.; Xia, D.; Zhao, Y.; Chen, T. Norcantharidin Nanostructured Lipid Carrier (NCTD-NLC) Suppresses the Viability of Human Hepatocellular Carcinoma HepG2 Cells and Accelerates the Apoptosis. J. Immunol. Res. 2022, 2022, 3851604. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, E.; Demir, E.-S.; Ekinci, M.; Ozgenc, E.; Ilem-Ozdemir, D.; Senyigit, Z.; Gonzalez-Alvarez, I.; Bermejo, M. An Innovative Formulation Based on Nanostructured Lipid Carriers for Imatinib Delivery: Pre-Formulation, Cellular Uptake and Cytotoxicity Studies. Nanomaterials 2022, 12, 250. [Google Scholar] [CrossRef]
- Chandan, C.; Phani Kumar, G.; Jawahar, N.; Sushma, B.V.; Amachawadi, R.G.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Prasad, S.K.; Shivamallu, C.; et al. Design, Development and Characterization of Papain-Loaded Nanostructured Lipid Carriers for Enhanced Stability and Bio-Accessibility in Acidic Environments. Results Chem. 2024, 8, 101571. [Google Scholar] [CrossRef]


| Nanomaterial | Hydrodynamic Size ± SD (nm) | Polydispersity Index ± SD | Zeta Potential ± SD (mV) | Particle Concentration ± SD (Particle/mL) |
|---|---|---|---|---|
| NLC | 98.6 ± 2.2 | 0.06 ± 0.03 | −4.9 ± 0.1 | 2.1 × 1017 ± 1.8 × 1016 |
| NLC-RIF | 121.5 ± 2.0 | 0.18 ± 0.03 | −3.4 ± 1.6 | 8.7 × 1016 ± 1.2 × 1015 |
| Nanosystem | Nanoparticle Tracking Analysis (nm ± SD) | ||
|---|---|---|---|
| D10 | D50 | D90 | |
| NLC | 84.3 ± 1.1 | 80.0 ± 0.2 | 100.4 ± 2.6 |
| NLC-RIF | 75.3 ± 2.2 | 101.7 ± 4.6 | 159.1 ± 8.9 |
| Zero-Order | First Order | Korsmeyer–Peppas | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k0 (%h−1) | R2 | AIC | k1 (h−1) | R2 | AIC | kKP | n | R2 | AIC | |
| NLC-RIF | 1.64 | 0.879 | 130.2 | 0.04 | 0.995 | 75.3 | 4.07 | 0.99 | 0.986 | 56.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco-Rojas, J.; Sandoval, F.I.; Schuh, C.M.A.P.; Lagos, C.F.; Morales, J.O.; Arriagada, F.; Ortiz, A.C. NLC-Based Rifampicin Delivery System: Development and Characterization for Improved Drug Performance Against Staphylococcus aureus. Pharmaceutics 2025, 17, 799. https://doi.org/10.3390/pharmaceutics17060799
Carrasco-Rojas J, Sandoval FI, Schuh CMAP, Lagos CF, Morales JO, Arriagada F, Ortiz AC. NLC-Based Rifampicin Delivery System: Development and Characterization for Improved Drug Performance Against Staphylococcus aureus. Pharmaceutics. 2025; 17(6):799. https://doi.org/10.3390/pharmaceutics17060799
Chicago/Turabian StyleCarrasco-Rojas, Javiera, Felipe I. Sandoval, Christina M. A. P. Schuh, Carlos F. Lagos, Javier O. Morales, Francisco Arriagada, and Andrea C. Ortiz. 2025. "NLC-Based Rifampicin Delivery System: Development and Characterization for Improved Drug Performance Against Staphylococcus aureus" Pharmaceutics 17, no. 6: 799. https://doi.org/10.3390/pharmaceutics17060799
APA StyleCarrasco-Rojas, J., Sandoval, F. I., Schuh, C. M. A. P., Lagos, C. F., Morales, J. O., Arriagada, F., & Ortiz, A. C. (2025). NLC-Based Rifampicin Delivery System: Development and Characterization for Improved Drug Performance Against Staphylococcus aureus. Pharmaceutics, 17(6), 799. https://doi.org/10.3390/pharmaceutics17060799







