Implications of Anaphylaxis Following mRNA-LNP Vaccines: It Is Urgent to Eliminate PEG and Find Alternatives
Abstract
1. Introduction
2. Immunogenicity of PEG
2.1. Immunogenicity of PEGylation
2.2. Factors Affecting PEGylation Immunogenicity
2.2.1. Structure Features
2.2.2. Grafting Density
2.2.3. Encapsulated Drug
2.2.4. Other Factors
2.3. Clinical Immune Responses Triggered by Anti-PEG Ab
2.4. Related Regulatory Measures
3. Anti-PEG Antibodies Detection
3.1. Western Blot
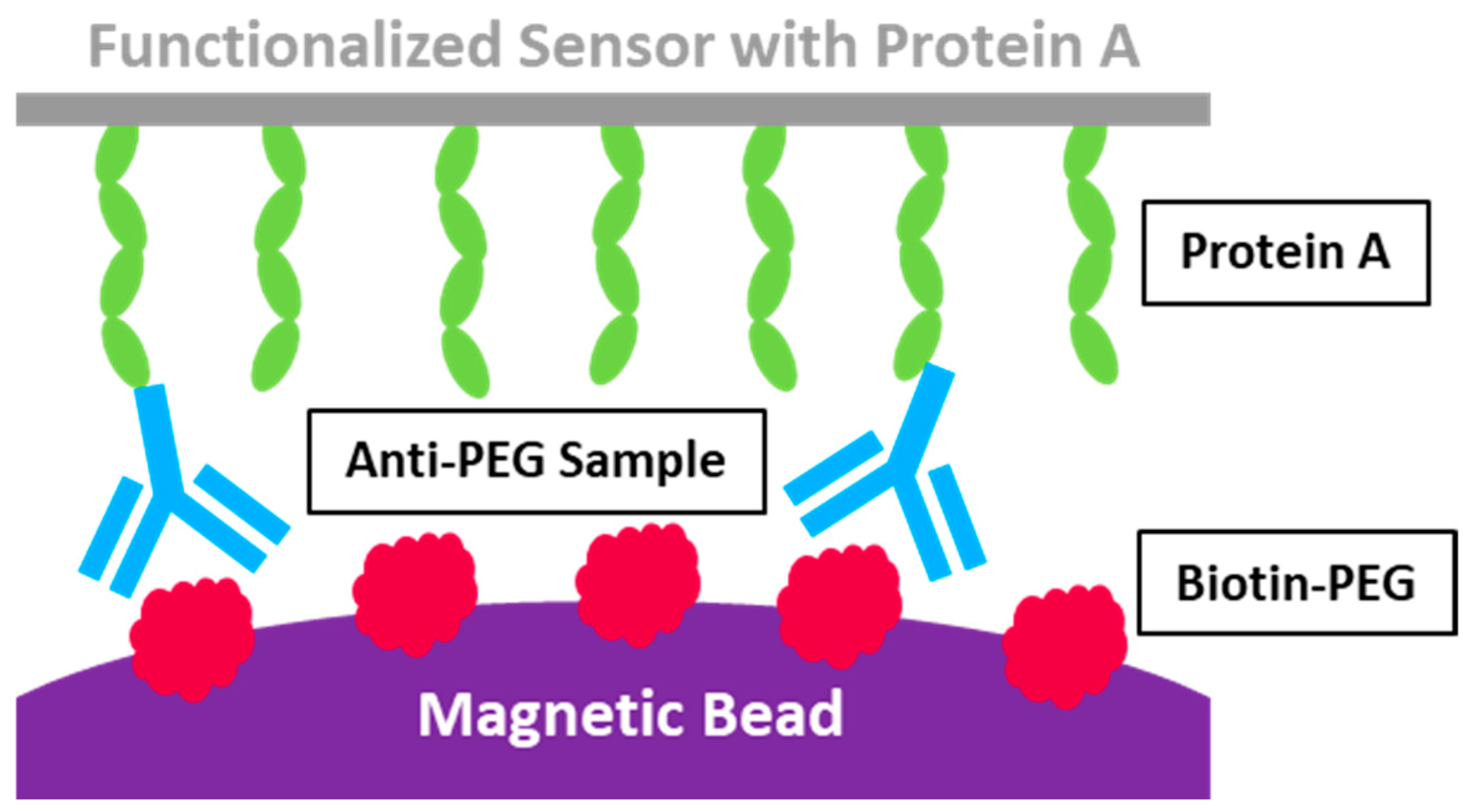
3.2. Acoustic Membrane Microparticle Technology
3.3. Enzyme-Linked Immunosorbent Assay
3.4. Flow Cytometry
3.5. Surface Plasmon Resonance Technology
| Method | Description | Pros | Cons | Detection Limit * | Ref |
|---|---|---|---|---|---|
| Western Blot | Main steps: Incubating the PEGylated antigen with samples to form antibody–antigen complex; separating the complex; separating the complex that is transferred to the membrane and blocked to prevent non-specific binding. It is incubated with anti-PEG Abs standards and detected for analysis. | High specificity; relatively lower detection limit | Accuracy compromised during the process; only one type of protein can be detected at once | 0.125 μg/mL for anti-PEG IgM 0.750 μg/mL for anti-PEG IgG | [90] |
| Acoustic Membrane Microparticle Technology | Main steps: Protein A isolation; recording the variations in acoustic frequency resulting from surface quality alterations before and after the acoustic sensor binds with the magnetic beads; calculating the results. | Superior detection sensitivity; broader detection range; quicker detection speed | It showed poor reproductivity for clinical samples. | 1000 ng/mL for anti-PEG IgG | [92] |
| Enzyme-linked Immunosorbent Assay | Main steps: Capturing anti-PEG Abs from test samples in 96 wells coated with PEGylated substances; attaching secondary antigens or Abs; visualizing through an enzyme reaction. The Results are quantified using a microplate reader. | High specificity; relatively lower detection limit; the most widely used for anti-PEG Abs quantitative detection | The results are relative values and vary related to standard curve changes. | 100 ng/mL for anti-PEG IgM 1 μg/mL for anti-PEG IgG | [102] |
| Flow Cytometry | Main steps: PEGylated polymer beads incubated with plasma samples; centrifugation to facilitate the binding of IgG or IgM with fluorescent dye-labeled anti-IgG or IgM; resuspension for flow cytometry analysis. | High sensitivity | Lack of fluorescence standard to compare results across studies. | 26 ng/mL for anti-PEG IgM 39 ng/mL for anti-PEG IgG | [91] |
| Surface Plasmon Resonance Technology | Main steps: PEGylated polymer is coated onto the sensor chips to capture anti-PEG Abs; quantitative analysis is achieved by recording the wavelength shift proportional to the anti-PEG Abs level on the sensor chip. | High sensitivity, quantification of absolute anti-PEG Abs concentrations | A special and expensive instrument is required; polymer coating on the sensor chips could influence the detection sensitivity | 10 ng/mL for anti-PEG IgM 50 ng/mL for anti-PEG IgG | [92] |
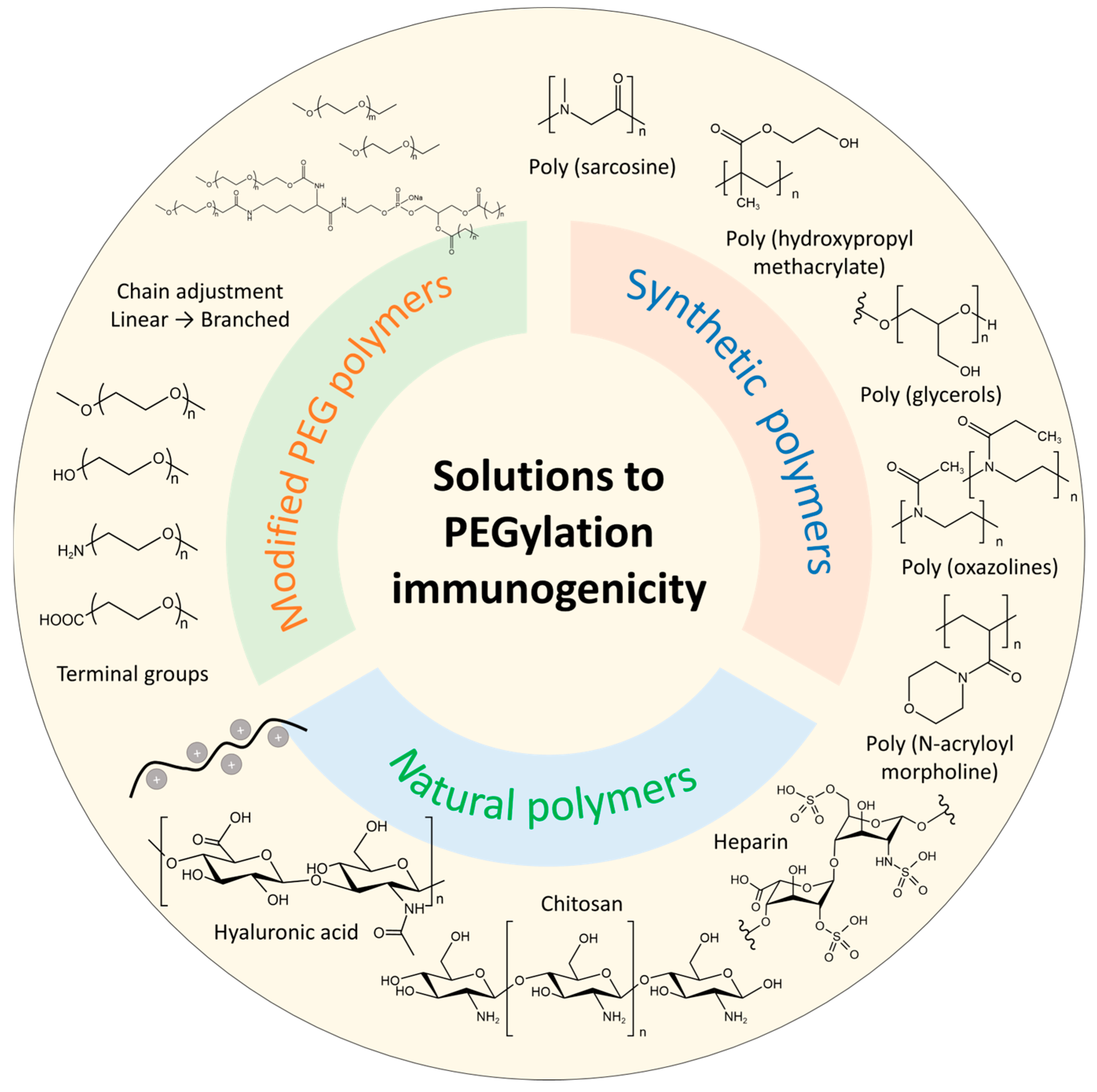
4. Solutions to PEGylation Immunogenicity
4.1. Modified PEG Polymers
4.2. Alternative Polymers
4.2.1. Synthetic Polymers
4.2.2. Natural Polymers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | Accelerating blood clearance |
| Abs | Antibodies |
| AMMP | Acoustic membrane microparticle technology |
| CHAPS | 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate |
| CDC | Centre of Disease Control |
| COVID-19 | Coronavirus disease 2019 |
| D | Distance |
| DSPE | 1,2-Distearoyl-sn-glycero-3-phospho-ethanolamine |
| DXR | Doxorubicin |
| ELISA | Enzyme-linked immunosorbent assay |
| GRAS | Generally Recognized As Safe |
| IC50 | half inhibition concentration |
| LNP | Lipid nanoparticles |
| mPEG | Methoxy-PEG |
| MW | Molecular weight |
| Oligo (ethylene glycol) | EG4 |
| PBS | Phosphate-buffered saline |
| PEG | Polyethylene glycol |
| PEGMA | Poly [poly (ethylene glycol) methyl ether methacrylate] |
| PGs | Poly (glycerols) |
| PHPMA | Poly (hydroxypropyl methacrylate) |
| POX | Poly (oxazolines) |
| PSar | Poly (sarcosine) |
| PSMA | Prostate-specific membrane antigen |
| RF | Flory radius |
| SPR | Surface plasmon resonance technology |
| TD | Thymus-dependent |
| TFH | Follicular helper T |
| TI | Thymus-independent |
| WB | Western blot |
References
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.; Wang, C.; Bernardini, S.; Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.; et al. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Abrams, E.M.; Greenhawt, M.; Shaker, M.; Pinto, A.D.; Sinha, I.; Singer, A. The COVID-19 pandemic adverse effects on the social determinants of health in children and families. Ann. Allergy Asthma Immunol. 2022, 128, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Forni, G.; Mantovani, A. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- Thanh Le, T.; Andreadakis, Z.; Kumar, A.; Gómez Román, R.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Mo, K.; Newbert, H.; Song, X. mRNA vaccines do not stop with COVID-19. Lancet 2023, 402, 526. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Bavli, Y.; Chen, B.-M.; Gross, G.; Hershko, A.; Turjeman, K.; Roffler, S.; Barenholz, Y. Anti-PEG antibodies before and after a first dose of comirnaty® (mRNA-LNP-Based SARS-CoV-2 Vaccine). J. Control. Release 2023, 354, 316–322. [Google Scholar] [CrossRef]
- Cabanillas, B.; Novak, N.; Akdis, C.A. The form of PEG matters: PEG conjugated with lipids and not PEG alone could be the specific form involved in allergic reactions to COVID-19 vaccines. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y. mRNA Vaccine: A potential therapeutic strategy. Mol. Cancer. 2021, 20, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Schlake, T.; Thran, M.; Fiedler, K.; Heidenreich, R.; Petsch, B. mRNA: A novel avenue to antibody therapy? Mol. Ther. 2019, 27, 773–784. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid nanoparticles-from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Mendonça, M.C.P.; Kont, A.; Kowalski, P.S.; O’Driscoll, C.M. Design of lipid-based nanoparticles for delivery of therapeutic nucleic acids. Drug Discov. Today 2023, 28, 103505. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ramadan, E.; Elsadek, N.E.; Emam, S.E.; Shimizu, T.; Ishida, T. Polyethylene glycol (PEG): The nature, immunogenicity, and role in the hypersensitivity of pegylated products. J. Control. Release 2022, 351, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, S.A.; Shimizu, T.; Ando, H.; Takata, H.; Emam, S.E.; Ramadan, E.; Naguib, Y.W.; Mady, F.M.; Khaled, K.A.; Ishida, T. Treatment-induced and pre-existing anti-PEG antibodies: Prevalence, clinical implications, and future perspectives. J. Pharm. Sci. 2024, 113, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Pitoc, G.A.; Ganson, N.J.; Layzer, J.M.; Hershfield, M.S.; Tarantal, A.F.; Sullenger, B.A.; Moreno, A.; Pitoc, G.A.; Ganson, N.J.; et al. Anti-PEG antibodies inhibit the anticoagulant activity of PEGylated aptamers. Cell Chem. Biol. 2019, 26, 634–644.e3. [Google Scholar] [CrossRef]
- Lenders, M.; Feidicker, L.M.; Brand, S.; Brand, E. Characterization of pre-existing anti-PEG and anti-AGAL antibodies towards PRX-102 in patients with Fabry disease. Front. Immunol. 2023, 14, 1266082. [Google Scholar] [CrossRef]
- Estapé Senti, M.; de Jongh, C.A.; Dijkxhoorn, K.; Verhoef, J.J.F.; Szebeni, J.; Storm, G.; Hack, C.E.; Schiffelers, R.M.; Fens, M.H.; Boross, P. Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J. Control. Release 2022, 341, 475–486. [Google Scholar] [CrossRef]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef]
- Sa, S.; Lee, C.W.; Shim, S.R.; Yoo, H.; Choi, J.; Kim, J.H.; Lee, K.; Hong, M.; Han, H.W. The safety of mRNA-1273, BNT162b2 and JNJ-78436735 COVID-19 vaccines: Safety monitoring for adverse events using real-world data. Vaccines 2022, 10, 320. [Google Scholar] [CrossRef]
- Yuan, Z.; McMullen, P.; Luozhong, S.; Sarker, P.; Tang, C.; Wei, T.; Jiang, S. Hidden hydrophobicity impacts polymer immunogenicity. Chem. Sci. 2023, 14, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Ma, L.L.; Pan, J.; Venkatraman, S. ABA and BAB type triblock copolymers of PEG and PLA: A comparative study of drug release properties and “stealth” particle characteristics. Int. J. Pharm. 2007, 334, 48–55. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Cui, J.; Caruso, F.; Ju, Y. Engineering poly (ethylene glycol) particles for targeted drug delivery. Chem. Commun. 2024, 60, 2591–2604. [Google Scholar] [CrossRef]
- Canato, E.; Grigoletto, A.; Zanotto, I.; Tedeschini, T.; Campara, B.; Quaglio, G.; Toffoli, G.; Mandracchia, D.; Dinarello, A.; Tiso, N.; et al. Anti-HER2 super stealth immunoliposomes for targeted-chemotherapy. Adv. Healthc. Mater. 2023, 12, e2301650. [Google Scholar] [CrossRef]
- Cui, J.; Björnmalm, M.; Ju, Y.; Caruso, F. Nanoengineering of poly (ethylene glycol) particles for stealth and targeting. Langmuir 2018, 34, 10817–10827. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lai, S.K. Anti-PEG Immunity: Emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 655–677. [Google Scholar] [CrossRef]
- Miao, G.; He, Y.; Lai, K.; Zhao, Y.; He, P.; Tan, G.; Wang, X. Accelerated blood clearance of PEGylated nanoparticles induced by PEG-based pharmaceutical excipients. J. Control. Release 2023, 363, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Chen, B.; Wu, J.; Cheng, T.; Roffler, S. Impact of anti-PEG antibody affinity on accelerated blood clearance of PEGylated epoetin beta in mice. Biomed. Pharmacother. 2022, 146, 112502. [Google Scholar] [CrossRef]
- Iheanacho, C.O.; Eze, U.I.H. Immunogenicity and clinical features relating to BNT162b2 messenger RNA COVID-19 vaccine, Ad26.COV2.S and ChAdOx1 adenoviral vector COVID-19 vaccines: A systematic review of non-interventional studies. Futur. J. Pharm. Sci. 2022, 8, 20. [Google Scholar] [CrossRef]
- Beatty, A.L.; Peyser, N.D.; Butcher, X.E.; Cocohoba, J.M.; Lin, F.; Olgin, J.E. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw. Open 2021, 4, e2140364. [Google Scholar] [CrossRef]
- Shimizu, T.; Lila, A.S.A.; Fujita, R.; Awata, M.; Kawanishi, M.; Hashimoto, Y.; Okuhira, K.; Ishima, Y.; Ishida, T. A hydroxyl PEG version of PEGylated liposomes and its impact on anti-PEG IgM induction and on the accelerated clearance of PEGylated liposomes. Eur. J. Pharm. Biopharm. 2018, 127, 142–149. [Google Scholar] [CrossRef]
- Kong, Y.W.; Dreaden, E.C. PEG: Will it come back to you? Polyethelyne glycol immunogenicity, COVID vaccines, and the case for new PEG derivatives and alternatives. Front. Bioeng. Biotechnol. 2022, 10, 879988. [Google Scholar] [CrossRef] [PubMed]
- Judge, A.; McClintock, K.; Phelps, J.R.; MacLachlan, I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol. Ther. 2006, 13, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, A.; Miller, I.; Starnes, R.; Talkington, A.; Stone, C.A.; Phillips, E.J.; Choudhary, S.K.; Commins, S.P.; Lai, S.K. Development of anti-PEG IgG / IgM / IgE ELISA assays for profiling anti-PEG immunoglobulin response in PEG-sensitized individuals and patients with alpha-gal allergy. J. Control. Release 2024, 366, 342–348. [Google Scholar] [CrossRef]
- Shimizu, T.; Mima, Y.; Hashimoto, Y.; Ukawa, M.; Ando, H.; Kiwada, H.; Ishida, T. Anti-PEG IgM and complement system are required for the association of second doses of PEGylated liposomes with splenic marginal zone B cells. Immunobiology 2015, 220, 1151–1160. [Google Scholar] [CrossRef]
- Gabizon, A.; Szebeni, J. Complement activation: A potential threat on the safety of poly (ethylene glycol)-coated nanomedicines. ACS Nano 2020, 14, 7682–7688. [Google Scholar] [CrossRef]
- Chen, B.M.; Cheng, T.L.; Roffler, S.R. Polyethylene glycol immunogenicity: Theoretical, clinical, and practical aspects of anti-polyethylene glycol antibodies. ACS Nano 2021, 15, 14022–14048. [Google Scholar] [CrossRef]
- Stone, C.A., Jr.; Liu, Y.; Relling, M.V.; Krantz, M.S.; Pratt, A.L.; Abreo, A.; Hemler, J.A.; Phillips, E.J. Immediate hypersensitivity to polyethylene glycols and polysorbates: More common than we have recognized. J. Allergy Clin. Immunol. Pract. 2019, 7, 1533–1540.e8. [Google Scholar] [CrossRef]
- Zhou, Z.-H.; Stone, C.A., Jr.; Jakubovic, B.; Phillips, E.J.; Sussman, G.; Park, J.; Hoang, U.; Kirshner, S.L.; Levin, R.; Kozlowski, S. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J. Allergy Clin. Immunol. Pract. 2021, 9, 1731–1733.e3. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, B.M.; Tran, T.T.M.; Chang, T.C.; Al-Qaisi, T.S.; Roffler, S.R. Accelerated clearance by antibodies against methoxy PEG depends on PEGylation architecture. J. Control. Release 2023, 354, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Carla, V.; Mosqueira, F.; Legrand, P.; Morgat, J.; Vert, M.; Mysiakine, E.; Gref, R.; Devissaguet, J.; Barratt, G. Biodistribution of long-circulating PEG-grafted nanocapsules in mice: Effects of PEG chain length and density. Pharm. Res. 2001, 18, 1411–1419. [Google Scholar]
- Povsic, T.J.; Vavalle, J.P.; Aberle, L.H.; Kasprzak, J.D.; Cohen, M.G.; Mehran, R.; Bode, C.; Buller, C.E.; Montalescot, G.; Cornel, J.H.; et al. A Phase 2, randomized, partially blinded, active-controlled study assessing the efficacy and safety of variable anticoagulation reversal using the reg1 system in patients with acute coronary syndromes: Results of the RADAR trial. Eur. Heart J. 2013, 34, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Steffensmeier, A.C.G.; Azar, A.E.; Fuller, J.J.; Muller, B.A.; Russell, S.R. Vitreous injections of pegaptanib sodium triggering allergic reactions. Am. J. Ophthalmol. 2007, 143, 512–513. [Google Scholar] [CrossRef]
- Weinhandl, E.D.; Gilbertson, D.T.; Collins, A.J.; Foley, R.N. Relative safety of peginesatide and epoetin alfa. Pharmacoepidemiol. Drug Saf. 2014, 23, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly (ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chemie Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Wenande, E.; Garvey, L.H. Immediate-type hypersensitivity to polyethylene glycols: A review. Clin. Exp. Allergy 2016, 46, 907–922. [Google Scholar] [CrossRef]
- Sellaturay, P.; Nasser, S.; Ewan, P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis). J. Allergy Clin. Immunol. Pract. 2021, 9, 670–675. [Google Scholar] [CrossRef]
- Jiao, J.; Luo, X.; Wang, C.; Jiao, X.; Liu, M.; Liu, X.; Wei, L.; Deng, Y.; Song, Y. Effects of uncleavable and cleavable PEG-lipids with different molecular weights on accelerated blood clearance of PEGylated emulsions in beagle dogs. AAPS PharmSciTech 2020, 21, 106. [Google Scholar] [CrossRef]
- Pannuzzo, M.; Esposito, S.; Wu, L.; Key, J.; Aryal, S.; Celia, C.; Marzio, L.; Moghimi, S.M.; Decuzzi, P. Overcoming nanoparticle-mediated complement activation by surface PEG-pairing. Nano Lett. 2020, 20, 4312–4321. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, D.; Yan, N.; Li, J.; Zhang, H.; Liu, M.; Tang, X.; Liu, X.; Deng, Y.; Song, Y.; et al. Evasion of the accelerated blood clearance phenomenon by branched PEG lipid derivative coating of nanoemulsions. Int. J. Pharm. 2022, 612, 121365. [Google Scholar] [CrossRef]
- Mastrotto, F.; Brazzale, C.; Bellato, F.; De Martin, S.; Grange, G.; Mahmoudzadeh, M.; Magarkar, A.; Bunker, A.; Salmaso, S.; Caliceti, P. In vitro and in vivo behavior of liposomes decorated with PEGs with different chemical features. Mol. Pharm. 2020, 17, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Hamano, M.; Ma, H.; Kawano, K.; Maitani, Y.; Aoshi, T.; Ishii, K.J.; Yokoyama, M. Hydrophobic blocks of PEG-conjugates play a significant role in the accelerated blood clearance (ABC) phenomenon. J. Control. Release 2013, 165, 183–190. [Google Scholar] [CrossRef]
- Xu, H.; Deng, Y.; Chen, D.; Hong, W.; Lu, Y.; Dong, X. Esterase-catalyzed dePEGylation of pH-sensitive vesicles modified with cleavable PEG-lipid derivatives. J. Control. Release 2008, 130, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, W.; Shen, Y.; Mu, H.; Zhang, Y.; Liang, R.; Wang, A.; Sun, K.; Fu, F. Effects of a novel pH-sensitive liposome with cleavable esterase-catalyzed and pH-responsive double smart mPEG lipid derivative on ABC phenomenon. Int. J. Nanomed. 2011, 6, 2053–2061. [Google Scholar] [CrossRef]
- Kohli, A.G.; Kierstead, P.H.; Venditto, V.J.; Walsh, C.L.; Szoka, F.C. Designer lipids for drug delivery: From heads to tails. J. Control. Release 2014, 190, 274–287. [Google Scholar] [CrossRef]
- Maeda, T.; Fujimoto, K. A reduction-triggered delivery by a liposomal carrier possessing membrane-permeable ligands and a detachable coating. Colloids Surf. B Biointerfaces 2006, 49, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, S.W.; Park, T.G. Novel intracellular delivery system of antisense oligonucleotide by self-assembled hybrid micelles composed of DNA/PEG conjugate and cationic fusogenic peptide. Bioconjug. Chem. 2003, 14, 473–479. [Google Scholar] [CrossRef]
- Walker, G.F.; Fella, C.; Pelisek, J.; Fahrmeir, J.; Boeckle, S.; Ogris, M.; Wagner, E. Toward synthetic viruses: Endosomal pH-triggered deshielding of targeted polyplexes greatly enhances gene transfer in vitro and in vivo. Mol. Ther. 2005, 11, 418–425. [Google Scholar] [CrossRef]
- Shin, J.; Shum, P.; Thompson, D.H. Acid-triggered release via dePEGylation of DOPE Liposomes Containing acid-labile vinyl ether PEG-lipids. J. Control. Release 2003, 91, 187–200. [Google Scholar] [CrossRef]
- Charoensit, P.; Pompimon, W.; Khorana, N.; Sungthongjeen, S. Effect of amide linkage of PEG-lipid conjugates on the stability and cytotoxic activity of goniodiol loaded in PEGylated liposomes. J. Drug Deliv. Sci. Technol. 2019, 50, 1–8. [Google Scholar] [CrossRef]
- Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Tan, W.S. Stealth coating of nanoparticles in drug-delivery systems. Nanomaterials 2020, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jones, S.W.; Parker, C.L.; Zamboni, W.C.; Bear, J.E.; Lai, S.K. Evading immune cell uptake and clearance requires PEG grafting at densities substantially exceeding the minimum for brush conformation. Mol. Pharm. 2014, 11, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Xiao, Y.; Lian, X.; Sun, Y.; Sung, Y.-C.; Vaidya, A.; Chen, Z.; Gupta, A.; Chatterjee, S.; Zheng, L.; Guerrero, E.; et al. High-density brush-shaped polymer lipids reduce anti-PEG antibody binding for repeated administration of mRNA therapeutics. Nat. Mater. 2025. [Google Scholar] [CrossRef]
- Tagami, T.; Nakamura, K.; Shimizu, T.; Ishida, T.; Kiwada, H. Effect of siRNA in PEG-coated siRNA lipoplex on anti-PEG IgM production. J. Control. Release 2009, 137, 234–240. [Google Scholar] [CrossRef]
- La-Beck, N.M.; Zamboni, B.A.; Gabizon, A.; Schmeeda, H.; Amantea, M.; Gehrig, P.A.; Zamboni, W.C. Factors affecting the pharmacokinetics of pegylated liposomal doxorubicin in patients. Cancer Chemother. Pharmacol. 2012, 69, 43–50. [Google Scholar] [CrossRef]
- Ishida, T.; Atobe, K.; Wang, X.; Kiwada, H. Accelerated blood clearance of PEGylated liposomes upon repeated injections: Effect of doxorubicin-encapsulation and high-dose first injection. J. Control. Release 2006, 115, 251–258. [Google Scholar] [CrossRef]
- Ju, Y.; Lee, W.S.; Pilkington, E.H.; Kelly, H.G.; Li, S.; Selva, K.J.; Wragg, K.M.; Subbarao, K.; Nguyen, T.H.O.; Rowntree, L.C.; et al. Anti-PEG antibodies boosted in humans by SARS-CoV-2 lipid nanoparticle mRNA Vaccine. ACS Nano 2022, 16, 11769–11780. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.C.; Harasym, T.O.; Clow, K.A.; Ansell, S.M.; Klimuk, S.K.; Hope, M.J. Immunogenicity and rapid blood clearance of liposomes containing polyethylene glycol-lipid conjugates and nucleic acid. J. Pharmacol. Exp. Ther. 2005, 312, 1020–1026. [Google Scholar] [CrossRef]
- Saadati, R.; Dadashzadeh, S.; Abbasian, Z.; Soleimanjahi, H. Accelerated blood clearance of PEGylated PLGA nanoparticles following repeated injections: Effects of polymer dose, PEG coating, and encapsulated anticancer drug. Pharm. Res. 2013, 30, 985–995. [Google Scholar] [CrossRef]
- Ganson, N.J.; Povsic, T.J.; Sullenger, B.A.; Alexander, J.H.; Zelenkofske, S.L.; Sailstad, J.M.; Rusconi, C.P.; Hershfield, M.S. Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J. Allergy Clin. Immunol. 2016, 137, 1610–1613.e7. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-M.; Su, Y.-C.; Chang, C.-J.; Burnouf, P.-A.; Chuang, K.-H.; Chen, C.-H.; Cheng, T.-L.; Chen, Y.-T.; Wu, J.-Y.; Roffler, S.R. Measurement of pre-existing IgG and IgM antibodies against polyethylene glycol in healthy individuals. Anal. Chem. 2016, 88, 10661–10666. [Google Scholar] [CrossRef]
- Povsic, T.J.; Lawrence, M.G.; Lincoff, A.M.; Mehran, R.; Rusconi, C.P.; Zelenkofske, S.L.; Huang, Z.; Sailstad, J.; Armstrong, P.W.; Steg, P.G.; et al. Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J. Allergy Clin. Immunol. 2016, 138, 1712–1715. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A.; Abu Lila, A.S.; Ishida, T.; Kiwada, H. Abrogation of the accelerated blood clearance phenomenon by SOXL regimen: Promise for clinical application. Int. J. Pharm. 2013, 441, 395–401. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Kiwada, H.; Ishida, T. The accelerated blood clearance (ABC) phenomenon: Clinical challenge and approaches to manage. J. Control. Release 2013, 172, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Maeda, R.; Ichihara, M.; Irimura, K.; Kiwada, H. Accelerated clearance of PEGylated liposomes in rats after repeated injections. J. Control. Release 2003, 88, 35–42. [Google Scholar] [CrossRef]
- Ishida, T.; Harada, M.; Wang, X.Y.; Ichihara, M.; Irimura, K.; Kiwada, H. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: Effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. J. Control. Release 2005, 105, 305–317. [Google Scholar] [CrossRef]
- Guerrini, G.; Gioria, S.; Sauer, A.V.; Lucchesi, S.; Montagnani, F.; Pastore, G.; Ciabattini, A.; Medaglini, D.; Calzolai, L. Monitoring anti-PEG antibodies level upon repeated lipid nanoparticle-based COVID-19 Vaccine administration. Int. J. Mol. Sci. 2022, 23, 8838. [Google Scholar] [CrossRef]
- Castells, M.C.; Phillips, E.J. Maintaining safety with SARS-CoV-2 vaccines. N. Engl. J. Med. 2021, 384, 643–649. [Google Scholar] [CrossRef]
- Holme, P.A.; Poulsen, L.H.; Tueckmantel, C.; Maas Enriquez, M.; Alvarez Román, M.T.; De Cristofaro, R. Safety and efficacy of damoctocog alfa pegol prophylaxis in patients with severe haemophilia A: Results of an interventional, post-marketing study. Haemophilia 2024, 30, 388–394. [Google Scholar] [CrossRef]
- Patrawala, M.; Kuruvilla, M.; Li, H. Successful desensitization of Pegvaliase (Palynziq®) in a patient with phenylketonuria. Mol. Genet. Metab. Rep. 2020, 23, 100575. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, D.L.; Putz, V.; Heger, L.; Reinöhl, J.; Hortmann, M.; Zelenkofske, S.L.; Becker, R.C.; Rusconi, C.P.; Bode, C.; Ahrens, I. Direct factor IXa inhibition with the RNA-aptamer pegnivacogin reduces platelet reactivity in vitro and residual platelet aggregation in patients with acute coronary syndromes. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.E.; Nowak, J.; Larsen, J.M.; Moore, E.; Bell, D.; Liu, K.C.; Sorensen, N.S.; Kappers, W.A.; Krogh-Meibom, T.; Offenberg, H. Long-term safety of PEGylated coagulation factor VIII in the immune-deficient rowett nude rat. J. Toxicol. 2017, 2017, 8496246. [Google Scholar] [CrossRef]
- Male, C.; Königs, C.; Dey, S.; Matsushita, T.; Millner, A.H.; Zak, M.; Young, G.; Kenet, G. The safety and efficacy of N8-GP (turoctocog alfa pegol) in previously untreated pediatric patients with hemophilia A. Blood Adv. 2023, 7, 620–629. [Google Scholar] [CrossRef]
- He, H.; Liu, L.; Morin, E.E.; Liu, M.; Schwendeman, A. Survey of clinical translation of cancer nanomedicines-lessons learned from successes and failures. Acc. Chem. Res. 2019, 52, 2445–2461. [Google Scholar] [CrossRef]
- El Sayed, M.M.; Takata, H.; Shimizu, T.; Kawaguchi, Y.; Abu Lila, A.S.; Elsadek, N.E.; Alaaeldin, E.; Ishima, Y.; Ando, H.; Kamal, A.; et al. Hepatosplenic phagocytic cells indirectly contribute to anti-PEG IgM production in the accelerated blood clearance (ABC) phenomenon against PEGylated liposomes: Appearance of an unexplained mechanism in the ABC phenomenon. J. Control. Release 2020, 323, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Lindner, J.R.; Belcik, T.; Main, M.L.; Montanaro, A.; Mulvagh, S.L.; Olson, J.; Olyaei, A.; Porter, T.R.; Senior, R. Expert consensus statement from the American Society of Echocardiography on hypersensitivity reactions to ultrasound enhancing agents in patients with allergy to polyethylene glycol. J. Am. Soc. Echocardiogr. 2021, 34, 707–708. [Google Scholar] [CrossRef]
- Soni, M.; McGovern, M.; Jacob, R.; Weiss, R.; Adusumalli, S.; Litwin, S.; Jagasia, D.; Scherrer-Crosbie, M. Ultrasound-enhancing agents and associated adverse reactions: A potential connection to the COVID-19 vaccines? J. Am. Soc. Echocardiogr. 2022, 35, 241–242. [Google Scholar] [CrossRef]
- Bell, G. Quantifying western blots: None more black. BMC Biol. 2016, 14, 116. [Google Scholar] [CrossRef]
- Ehlinger, C.; Spear, N.; Doddareddy, R.; Shankar, G.; Schantz, A. A generic method for the detection of polyethylene glycol specific IgG and IgM antibodies in human serum. J. Immunol. Methods 2019, 474, 112669. [Google Scholar] [CrossRef]
- Fang, J.-L.; Beland, F.A.; Tang, Y.; Roffler, S.R. Flow cytometry analysis of anti-polyethylene glycol antibodies in human plasma. Toxicol. Rep. 2021, 8, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Mora, J.R.; Brockus, C.; Chilewski, S.D.; Dodge, R.; Merrifield, C.; Dickerson, W.M.; DeSilva, B. Development of a generic anti-PEG antibody assay using BioScale’s Acoustic Membrane MicroParticle technology. AAPS J. 2015, 17, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Chilewski, S.D.; Shields, J.; Mora, J.R.; Myler, H. Generic anti-PEG antibody assay on ProterixBio’s (formerly BioScale) ViBE platform shows poor reproducibility. AAPS J. 2018, 20, 3–7. [Google Scholar] [CrossRef]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef]
- Williams, W.T.; Kathryn, L.; Hong, L.; Lynn, K.; Michelle, M.; Amanda, H.; Sailstad, J. Development of a validated novel bead extraction method for the detection of anti-PEG antibodies in human serum. Bioanalysis 2025, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kozma, G.T.; Mészáros, T.; Vashegyi, I.; Fülöp, T.; Örfi, E.; Dézsi, L.; Rosivall, L.; Bavli, Y.; Urbanics, R.; Mollnes, T.E.; et al. Pseudo-anaphylaxis to polyethylene glycol (PEG)-coated liposomes: Roles of anti-PEG IgM and complement activation in a porcine model of human infusion reactions. ACS Nano 2019, 13, 9315–9324. [Google Scholar] [CrossRef]
- Omata, D.; Kawahara, E.; Munakata, L.; Tanaka, H.; Akita, H.; Yoshioka, Y.; Suzuki, R. Effect of Anti-PEG Antibody on Immune Response of MRNA-Loaded Lipid Nanoparticles. Mol. Pharm. 2024, 21, 5672–5680. [Google Scholar] [CrossRef]
- Neun, B.W.; Barenholz, Y.; Szebeni, J.; Dobrovolskaia, M.A. Understanding the Role of Anti-PEG Antibodies in the Complement Activation by Doxil in Vitro. Molecules 2018, 23, 1700. [Google Scholar] [CrossRef]
- Kloos, R.; van der Sluis, I.M.; Mastrobattista, E.; Hennink, W.; Pieters, R.; Verhoef, J.-J. Acute Lymphoblastic Leukaemia patients treated with PEGasparaginase develop antibodies to PEG and the succinate linker. Br. J. Haematol. 2020, 189, 442–451. [Google Scholar] [CrossRef]
- Armstrong, J.K.; Hempel, G.; Koling, S.; Chan, L.S.; Fisher, T.; Meiselman, H.J.; Garratty, G. Antibody against poly (ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer 2007, 110, 103–111. [Google Scholar] [CrossRef]
- Armstrong, J.K. The occurrence, induction, specificity and potential effect of antibodies against poly(ethylene glycol). In PEGylated Protein Drugs: Basic Science and Clinical Applications, 1st ed.; Veronese, F.M., Ed.; Birkhäuser: Basel, Switzerland, 2009; pp. 147–168. [Google Scholar]
- Zhang, P.; Sun, F.; Hung, H.-C.; Jain, P.; Leger, K.J.; Jiang, S. Sensitive and quantitative detection of anti-poly (ethylene glycol) (PEG) antibodies by methoxy-PEG-coated surface plasmon resonance sensors. Anal. Chem. 2017, 89, 8217–8222. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.R.; Williams, L.D.; Sobczyk, M.A.; Michaels, S.J.; Saifer, M.G.P. Role of the methoxy group in immune responses to mPEG-protein conjugates. Bioconjug. Chem. 2012, 23, 485–499. [Google Scholar] [CrossRef]
- Saifer, M.G.P.; Williams, L.D.; Sobczyk, M.A.; Michaels, S.J.; Sherman, M.R. Selectivity of binding of PEGs and PEG-like oligomers to anti-PEG antibodies induced by methoxyPEG-proteins. Mol. Immunol. 2014, 57, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Swainson, S.M.E.; Styliari, I.D.; Taresco, V.; Garnett, M.C. Poly (glycerol adipate) (PGA), an enzymatically synthesized functionalizable polyester and versatile drug delivery carrier: A literature update. Polymers 2019, 11, 1561. [Google Scholar] [CrossRef]
- Zahoranová, A.; Luxenhofer, R. Poly(2-oxazoline)- and poly(2-oxazine)-based self-assemblies, polyplexes, and drug nanoformulations-an update. Adv. Healthc. Mater. 2021, 10, e2001382. [Google Scholar] [CrossRef] [PubMed]
- Hayes, G.; Dias-Barbieri, B.; Yilmaz, G.; Shattock, R.J.; Becer, C.R. Poly(2-oxazoline)/saRNA polyplexes for targeted and nonviral gene delivery. Biomacromolecules 2023, 24, 5142–5151. [Google Scholar] [CrossRef]
- Holick, C.T.; Klein, T.; Mehnert, C.; Adermann, F.; Anufriev, I.; Streiber, M.; Harder, L.; Traeger, A.; Hoeppener, S.; Franke, C.; et al. Poly(2-ethyl-2-oxazoline) (POx) as poly (ethylene glycol) (PEG)-lipid substitute for lipid nanoparticle formulations. Small 2025, 21, 2411354. [Google Scholar] [CrossRef]
- Cho, S.; Hori, M.; Ueki, R.; Saito, Y.; Nagai, Y.; Iki, H.; Tsuchiya, A.; Konno, T.; Owari, K.; Piao, H.; et al. Zwitterionic polymer with minimal reactivity against PEG antibodies to enhance the therapeutic effects of cytokine-targeting DNA aptamer. Biomater. Sci. 2025, 13, 1347–1353. [Google Scholar] [CrossRef]
- Tian, Y.; Lv, H.; Ju, Y.; Hao, J.; Cui, J. Zwitterionic poly (ethylene glycol) nanoparticles minimize protein adsorption and immunogenicity for improved biological fate. ACS Appl. Mater. Interfaces 2025, 17, 6125–6133. [Google Scholar] [CrossRef]
- Kang, D.D.; Hou, X.; Wang, L.; Xue, Y.; Li, H.; Zhong, Y.; Wang, S.; Deng, B.; McComb, D.W.; Dong, Y. Engineering LNPs with polysarcosine lipids for mRNA delivery. Bioact. Mater. 2024, 37, 86–93. [Google Scholar] [CrossRef]
- Sentoukas, T.; Pispas, S. Poly(2-[dimethylamino]ethyl methacrylate)-b-poly (hydroxypropyl methacrylate)/DNA polyplexes in aqueous solutions. J. Polym. Sci. 2020, 58, 2335–2346. [Google Scholar] [CrossRef]
- Birke, A.; Ling, J.; Barz, M. Polysarcosine-containing copolymers: Synthesis, characterization, self-assembly, and applications. Prog. Polym. Sci. 2018, 81, 163–208. [Google Scholar] [CrossRef]
- Hu, Y.; Hou, Y.; Wang, H.; Lu, H. Polysarcosine as an alternative to PEG for therapeutic protein conjugation. Bioconjug. Chem. 2018, 29, 2232–2238. [Google Scholar] [CrossRef]
- Wilhelmy, C.; Keil, I.S.; Uebbing, L.; Schroer, M.A.; Franke, D.; Nawroth, T.; Barz, M.; Sahin, U.; Haas, H.; Diken, M.; et al. Polysarcosine-functionalized mRNA lipid nanoparticles tailored for immunotherapy. Pharmaceutics 2023, 15, 2068. [Google Scholar] [CrossRef]
- Bi, D.; Unthan, D.M.; Hu, L.; Bussmann, J.; Remaut, K.; Barz, M.; Zhang, H. Polysarcosine-based lipid formulations for intracranial delivery of mRNA. J. Control. Release 2023, 356, 1–13. [Google Scholar] [CrossRef]
- Nogueira, S.; Schlegel, A.; Maxeiner, K.; Weber, B.; Barz, M.; Schroer, M.A.; Blanchet, E.; Svergun, D.; Ramishetti, S.; Peer, D.; et al. Polysarcosine-functionalized lipid nanoparticles for therapeutic mRNA delivery. ACS Appl. Nano Mater. 2020, 3, 10634–10645. [Google Scholar] [CrossRef]
- Caprifico, A.E.; Foot, P.J.S.; Polycarpou, E.; Calabrese, G. Advances in chitosan-based CRISPR/Cas9 delivery systems. Pharmaceutics 2022, 14, 1840. [Google Scholar] [CrossRef]
- Yu, Q.; Qiu, Y.; Chen, X.; Wang, X.; Mei, L.; Wu, H.; Liu, K.; Liu, Y.; Li, M.; Zhang, Z.; et al. Chemotherapy priming of the pancreatic tumor microenvironment promotes delivery and anti-metastasis efficacy of intravenous low-molecular-weight heparin-coated lipid-siRNA complex. Theranostics 2019, 9, 355–368. [Google Scholar] [CrossRef]
- Chuan, D.; Jin, T.; Fan, R.; Zhou, L.; Guo, G. Chitosan for gene delivery: Methods for improvement and applications. Adv. Colloid. Interface Sci. 2019, 268, 25–38. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Su, D.; Wu, H.; Guo, J. Implications of Anaphylaxis Following mRNA-LNP Vaccines: It Is Urgent to Eliminate PEG and Find Alternatives. Pharmaceutics 2025, 17, 798. https://doi.org/10.3390/pharmaceutics17060798
Song J, Su D, Wu H, Guo J. Implications of Anaphylaxis Following mRNA-LNP Vaccines: It Is Urgent to Eliminate PEG and Find Alternatives. Pharmaceutics. 2025; 17(6):798. https://doi.org/10.3390/pharmaceutics17060798
Chicago/Turabian StyleSong, Jinxing, Dihan Su, Hongbing Wu, and Jeremy Guo. 2025. "Implications of Anaphylaxis Following mRNA-LNP Vaccines: It Is Urgent to Eliminate PEG and Find Alternatives" Pharmaceutics 17, no. 6: 798. https://doi.org/10.3390/pharmaceutics17060798
APA StyleSong, J., Su, D., Wu, H., & Guo, J. (2025). Implications of Anaphylaxis Following mRNA-LNP Vaccines: It Is Urgent to Eliminate PEG and Find Alternatives. Pharmaceutics, 17(6), 798. https://doi.org/10.3390/pharmaceutics17060798








