Lipid-Polymer Hybrid Nanoparticles as a Smart Drug Delivery System for Peptide/Protein Delivery
Abstract
1. Introduction
2. Methodology
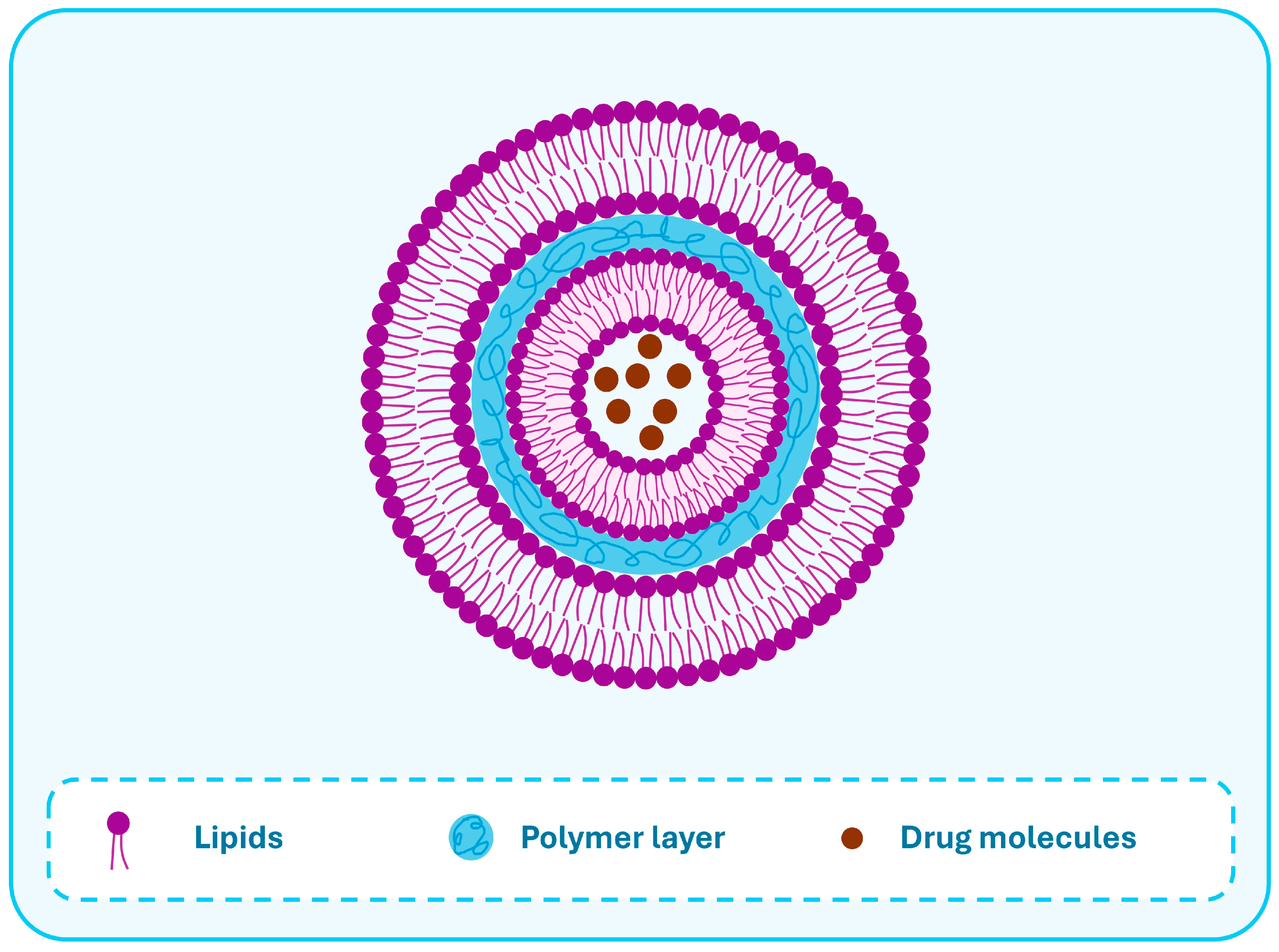
3. Lipid-Polymer Hybrid Nanoparticles
4. Gastrointestinal Behavior, Oral Absorption and Cellular Uptake Mechanisms of LPHNs
5. Preparation Methods
5.1. Two-Step Approach
5.2. Single-Step Approach
5.2.1. Self-Assembly Nanoprecipitation Method
5.2.2. Emulsification Solvent Evaporation/Extraction Method
- (1)
- The exposure of the protein to the hydrophobic organic solvent (e.g., DCM) in the primary emulsion as the maximum degree of deactivation occurs at the organic–aqueous interface.
- (2)
- Dehydration during the lyophilization process, which could be used to obtain a dry powder, might cause the denaturation of proteins as well.
- Organic phase composition.
- Volume of the external aqueous phase and its pH.
- Polymer type and concentration.
- Type of lipid or lipid combination and their ratios.
- Concentration of lipid/s in the organic phase.
- Polymer/lipid ratio.
- Type of the stabilizer and its concentration.
6. Examples of Peptides/Proteins Formulated in LPHNs
7. Conclusions and Future Opportunities
Author Contributions
Funding
Conflicts of Interest
References
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-Polymer Hybrid Nanoparticles: Synthesis Strategies and Biomedical Applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid–Polymer Hybrid Nanoparticles as a New Generation Therapeutic Delivery Platform: A Review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Kong, S.D.; Sartor, M.; Hu, C.-M.J.; Zhang, W.; Zhang, L.; Jin, S. Magnetic Field Activated Lipid–Polymer Hybrid Nanoparticles for Stimuli-Responsive Drug Release. Acta Biomater. 2013, 9, 5447–5452. [Google Scholar] [CrossRef]
- Sakuma, S.; Suzuki, N.; Kikuchi, H.; Hiwatari, K.; Arikawa, K.; Kishida, A.; Akashi, M. Oral Peptide Delivery Using Nanoparticles Composed of Novel Graft Copolymers Having Hydrophobic Backbone and Hydrophilic Branches. Int. J. Pharm. 1997, 149, 93–106. [Google Scholar] [CrossRef]
- Tang, B.; Qian, Y.; Fang, G. Development of Lipid–Polymer Hybrid Nanoparticles for Improving Oral Absorption of Enoxaparin. Pharmaceutics 2020, 12, 607. [Google Scholar] [CrossRef]
- Men, K.; Liu, W.; Li, L.; Duan, X.; Wang, P.; Gou, M.; Wei, X.; Gao, X.; Wang, B.; Du, Y.; et al. Delivering Instilled Hydrophobic Drug to the Bladder by a Cationic Nanoparticle and Thermo-Sensitive Hydrogel Composite System. Nanoscale 2012, 4, 6425. [Google Scholar] [CrossRef]
- Salvador-Morales, C.; Zhang, L.; Langer, R.; Farokhzad, O.C. Immunocompatibility Properties of Lipid–Polymer Hybrid Nanoparticles with Heterogeneous Surface Functional Groups. Biomaterials 2009, 30, 2231–2240. [Google Scholar] [CrossRef]
- Wakaskar, R.R. General Overview of Lipid–Polymer Hybrid Nanoparticles, Dendrimers, Micelles, Liposomes, Spongosomes and Cubosomes. J. Drug Target. 2018, 26, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Yasaswi, P.S.; Shetty, K.; Yadav, K.S. Temozolomide Nano Enabled Medicine: Promises Made by the Nanocarriers in Glioblastoma Therapy. J. Control. Release 2021, 336, 549–571. [Google Scholar] [CrossRef]
- Bou, S.; Wang, X.; Anton, N.; Bouchaala, R.; Klymchenko, A.S.; Collot, M. Lipid-Core/Polymer-Shell Hybrid Nanoparticles: Synthesis and Characterization by Fluorescence Labeling and Electrophoresis. Soft Matter 2020, 16, 4173–4181. [Google Scholar] [CrossRef]
- Hassan, D.; Omolo, C.A.; Fasiku, V.O.; Mocktar, C.; Govender, T. Novel Chitosan-Based pH-Responsive Lipid-Polymer Hybrid Nanovesicles (OLA-LPHVs) for Delivery of Vancomycin against Methicillin-Resistant Staphylococcus Aureus Infections. Int. J. Biol. Macromol. 2020, 147, 385–398. [Google Scholar] [CrossRef]
- Yalcin, T.E.; Ilbasmis-Tamer, S.; Takka, S. Development and Characterization of Gemcitabine Hydrochloride Loaded Lipid Polymer Hybrid Nanoparticles (LPHNs) Using Central Composite Design. Int. J. Pharm. 2018, 548, 255–262. [Google Scholar] [CrossRef]
- Sharma, B.; Chauhan, I. A Review: Bilosomes as Nanocarriers. CNANOM 2024, 14, 178–187. [Google Scholar] [CrossRef]
- Kumar, L.; Rana, R.; Kukreti, G.; Aggarwal, V.; Chaurasia, H.; Sharma, P.; Jyothiraditya, V. Overview of Spanlastics: A Groundbreaking Elastic Medication Delivery Device with Versatile Prospects for Administration via Various Routes. CPD 2024, 30, 2206–2221. [Google Scholar] [CrossRef]
- Fonte, P.; Nogueira, T.; Gehm, C.; Ferreira, D.; Sarmento, B. Chitosan-Coated Solid Lipid Nanoparticles Enhance the Oral Absorption of Insulin. Drug Deliv. Transl. Res. 2011, 1, 299–308. [Google Scholar] [CrossRef]
- Garciafuentes, M.; Torres, D.; Alonso, M. New Surface-Modified Lipid Nanoparticles as Delivery Vehicles for Salmon Calcitonin. Int. J. Pharm. 2005, 296, 122–132. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Murariu, A.; Oprea, O.; Bojin, D.; Meghea, A. Antioxidant Activity of Solid Lipid Nanoparticles Loaded with Umbelliferone. Soft Mater. 2013, 11, 75–84. [Google Scholar] [CrossRef]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.-W.; Langer, R.; Farokhzad, O.C. PLGA–Lecithin–PEG Core–Shell Nanoparticles for Controlled Drug Delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.; Madni, A.; Balasubramanian, V.; Rehman, M.; Correia, A.; Kashif, P.M.; Mäkilä, E.; Salonen, J.; Santos, H.A. Development and Optimization of Methotrexate-Loaded Lipid-Polymer Hybrid Nanoparticles for Controlled Drug Delivery Applications. Int. J. Pharm. 2017, 533, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, H.; Yu, M.; Liao, Z.; Wang, X.; Zhang, F.; Ji, W.; Wu, B.; Han, J.; Zhang, H.; et al. Paclitaxel Loaded Folic Acid Targeted Nanoparticles of Mixed Lipid-Shell and Polymer-Core: In Vitro and in Vivo Evaluation. Eur. J. Pharm. Biopharm. 2012, 81, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.J.; Runge, S.; Müller, R.H. Peptide-Loaded Solid Lipid Nanoparticles (SLN): Influence of Production Parameters. Int. J. Pharm. 1997, 149, 255–265. [Google Scholar] [CrossRef]
- Garcıa-Fuentes, M.; Torres, D.; Alonso, M.J. Design of Lipid Nanoparticles for the Oral Delivery of Hydrophilic Macromolecules. Colloids Surf. B Biointerfaces 2003, 27, 159–168. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Pan, J.; Liu, B.; Feng, S.-S. Folic Acid Conjugated Nanoparticles of Mixed Lipid Monolayer Shell and Biodegradable Polymer Core for Targeted Delivery of Docetaxel. Biomaterials 2010, 31, 330–338. [Google Scholar] [CrossRef]
- Seedat, N.; Kalhapure, R.S.; Mocktar, C.; Vepuri, S.; Jadhav, M.; Soliman, M.; Govender, T. Co-Encapsulation of Multi-Lipids and Polymers Enhances the Performance of Vancomycin in Lipid–Polymer Hybrid Nanoparticles: In Vitro and in Silico Studies. Mater. Sci. Eng. C 2016, 61, 616–630. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, B.; Weecharangsan, W.; Piao, L.; Darby, M.; Mao, Y.; Koynova, R.; Yang, X.; Li, H.; Xu, S.; et al. Transferrin-Conjugated Lipid-Coated PLGA Nanoparticles for Targeted Delivery of Aromatase Inhibitor 7α-APTADD to Breast Cancer Cells. Int. J. Pharm. 2010, 390, 234–241. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Mieszawska, A.J.; Gianella, A.; Cormode, D.P.; Zhao, Y.; Meijerink, A.; Langer, R.; Farokhzad, O.C.; Fayad, Z.A.; Mulder, W.J.M. Engineering of Lipid-Coated PLGA Nanoparticles with a Tunable Payload of Diagnostically Active Nanocrystals for Medical Imaging. Chem. Commun. 2012, 48, 5835. [Google Scholar] [CrossRef]
- Le, L.; Bokare, A.; Erogbogbo, F. Hand Powered, Cost Effective, 3D Printed Nanoparticle Synthesizer: Effects of Polymer End Caps, Drugs, and Solvents on Lipid Polymer Hybrid Nanoparticles. Mater. Res. Express 2018, 6, 025403. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics 2022, 14, 2728. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Omri, A. Current Trends in PLGA Based Long-Acting Injectable Products: The Industry Perspective. Expert Opin. Drug Deliv. 2022, 19, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Khouri, N.G.; Bahú, J.O.; Blanco-Llamero, C.; Severino, P.; Concha, V.O.C.; Souto, E.B. Polylactic Acid (PLA): Properties, Synthesis, and Biomedical Applications—A Review of the Literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-Caprolactone (PCL), a Promising Polymer for Pharmaceutical and Biomedical Applications: Focus on Nanomedicine in Cancer. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Tan, S.; Sasada, T.; Bershteyn, A.; Yang, K.; Ioji, T.; Zhang, Z. Combinational Delivery of Lipid-Enveloped Polymeric Nanoparticles Carrying Different Peptides for Anti-Tumor Immunotherapy. Nanomedicine 2014, 9, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Madni, A.; Torchilin, V.; Filipczak, N.; Pan, J.; Tahir, N.; Shah, H. Lipid-Chitosan Hybrid Nanoparticles for Controlled Delivery of Cisplatin. Drug Deliv. 2019, 26, 765–772. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Mehta, S.; Yadav, S.; Singh, S.K.; Grobler, A.; Goyal, A.K.; Mehta, A. Pulmonary Delivery of Antitubercular Drugs Using Spray-Dried Lipid–Polymer Hybrid Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1544–1555. [Google Scholar] [CrossRef]
- Thevenot, J.; Troutier, A.-L.; David, L.; Delair, T.; Ladavière, C. Steric Stabilization of Lipid/Polymer Particle Assemblies by Poly(Ethylene Glycol)-Lipids. Biomacromolecules 2007, 8, 3651–3660. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid Polymer Hybrid Nanoparticles: A Custom-Tailored next-Generation Approach for Cancer Therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef]
- Boushra, M.; Tous, S.; Fetih, G.; Xue, H.-Y.; Tran, N.T.; Wong, H.L. Methocel-Lipid Hybrid Nanocarrier for Efficient Oral Insulin Delivery. J. Pharm. Sci. 2016, 105, 1733–1740. [Google Scholar] [CrossRef]
- Grigoras, A.G. Polymer-Lipid Hybrid Systems Used as Carriers for Insulin Delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2425–2437. [Google Scholar] [CrossRef]
- Cui, F.; Shi, K.; Zhang, L.; Tao, A.; Kawashima, Y. Biodegradable Nanoparticles Loaded with Insulin–Phospholipid Complex for Oral Delivery: Preparation, in Vitro Characterization and in Vivo Evaluation. J. Control. Release 2006, 114, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Rhodes, K.R.; Green, J.J.; Tzeng, S.Y. Poly(Beta-Amino Ester)s as Gene Delivery Vehicles: Challenges and Opportunities. Expert Opin. Drug Deliv. 2020, 17, 1395–1410. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, S.; Zhang, S.; Li, Y.; Li, J.; Dai, Y.; Wang, D. pH-Responsive Lipid Polymer Hybrid Nanoparticles (LPHNs) Based on Poly (β-Amino Ester) as a Promising Candidate to Resist Breast Cancers. J. Drug Deliv. Sci. Technol. 2021, 61, 102102. [Google Scholar] [CrossRef]
- Khodaei, M.; Rostamizadeh, K.; Taromchi, A.H.; Monirinasab, H.; Fathi, M. DDAB Cationic Lipid-mPEG, PCL Copolymer Hybrid Nano-Carrier Synthesis and Application for Delivery of siRNA Targeting IGF-1R into Breast Cancer Cells. Clin. Transl. Oncol. 2021, 23, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, X.; Su, H.; Zhou, D.; Song, H.; Wang, L.; Jiang, X. Poly(Ethylene Glycol)-Block-Poly(ε-Caprolactone—and Phospholipid-Based Stealth Nanoparticles with Enhanced Therapeutic Efficacy on Murine Breast Cancer by Improved Intracellular Drug Delivery. IJN 2015, 10, 1791–1804. [Google Scholar] [CrossRef]
- Wang, C.; Sui, X.; Simbar, N.; Sjollema, K.A.; van Hest, J.C.; Zuhorn, I.S. Hybrid Nanoparticles Composed of pH-Sensitive PDPA-b-PCL-b-PEG Copolymer and DOTAP Cationic Lipid Show a Two-Step Mechanism of Endosomal Escape. In Biomimetic Drug Nanocarriers to Overcome Biological Barriers: Inspiration from Pathogen Invasion; University of Groningen: Groningen, The Netherlands, 2016. [Google Scholar]
- Liu, J.-L.; Li, J.; Zhang, L.-Y.; Zhang, P.-L.; Zhou, J.-L.; Liu, B. Preparation of N, N, N-Trimethyl Chitosan-Functionalized Retinoic Acid-Loaded Lipid Nanoparticles for Enhanced Drug Delivery to Glioblastoma. Trop. J. Pharm. Res. 2017, 16, 1765. [Google Scholar] [CrossRef]
- Santos, J.C.C.; Moreno, P.M.D.; Mansur, A.A.P.; Leiro, V.; Mansur, H.S.; Pêgo, A.P. Functionalized Chitosan Derivatives as Nonviral Vectors: Physicochemical Properties of Acylated N,N,N-Trimethyl Chitosan/Oligonucleotide Nanopolyplexes. Soft Matter 2015, 11, 8113–8125. [Google Scholar] [CrossRef] [PubMed]
- Massadeh, S.; Omer, M.E.; Alterawi, A.; Ali, R.; Alanazi, F.H.; Almutairi, F.; Almotairi, W.; Alobaidi, F.F.; Alhelal, K.; Almutairi, M.S.; et al. Optimized Polyethylene Glycolylated Polymer–Lipid Hybrid Nanoparticles as a Potential Breast Cancer Treatment. Pharmaceutics 2020, 12, 666. [Google Scholar] [CrossRef] [PubMed]
- Mutlu-Agardan, N.B.; Han, S. In Vitro and in Vivo Evaluations on Nanoparticle and Phospholipid Hybrid Nanoparticles with Absorption Enhancers for Oral Insulin Delivery. Pharm. Dev. Technol. 2021, 26, 157–166. [Google Scholar] [CrossRef]
- Ling, G.; Zhang, P.; Zhang, W.; Sun, J.; Meng, X.; Qin, Y.; Deng, Y.; He, Z. Development of Novel Self-Assembled DS-PLGA Hybrid Nanoparticles for Improving Oral Bioavailability of Vincristine Sulfate by P-Gp Inhibition. J. Control. Release 2010, 148, 241–248. [Google Scholar] [CrossRef]
- Qin, L.; Wu, H.; Xu, E.; Zhang, X.; Guan, J.; Zhao, R.; Mao, S. Exploring the Potential of Functional Polymer-Lipid Hybrid Nanoparticles for Enhanced Oral Delivery of Paclitaxel. Asian J. Pharm. Sci. 2021, 16, 387–395. [Google Scholar] [CrossRef]
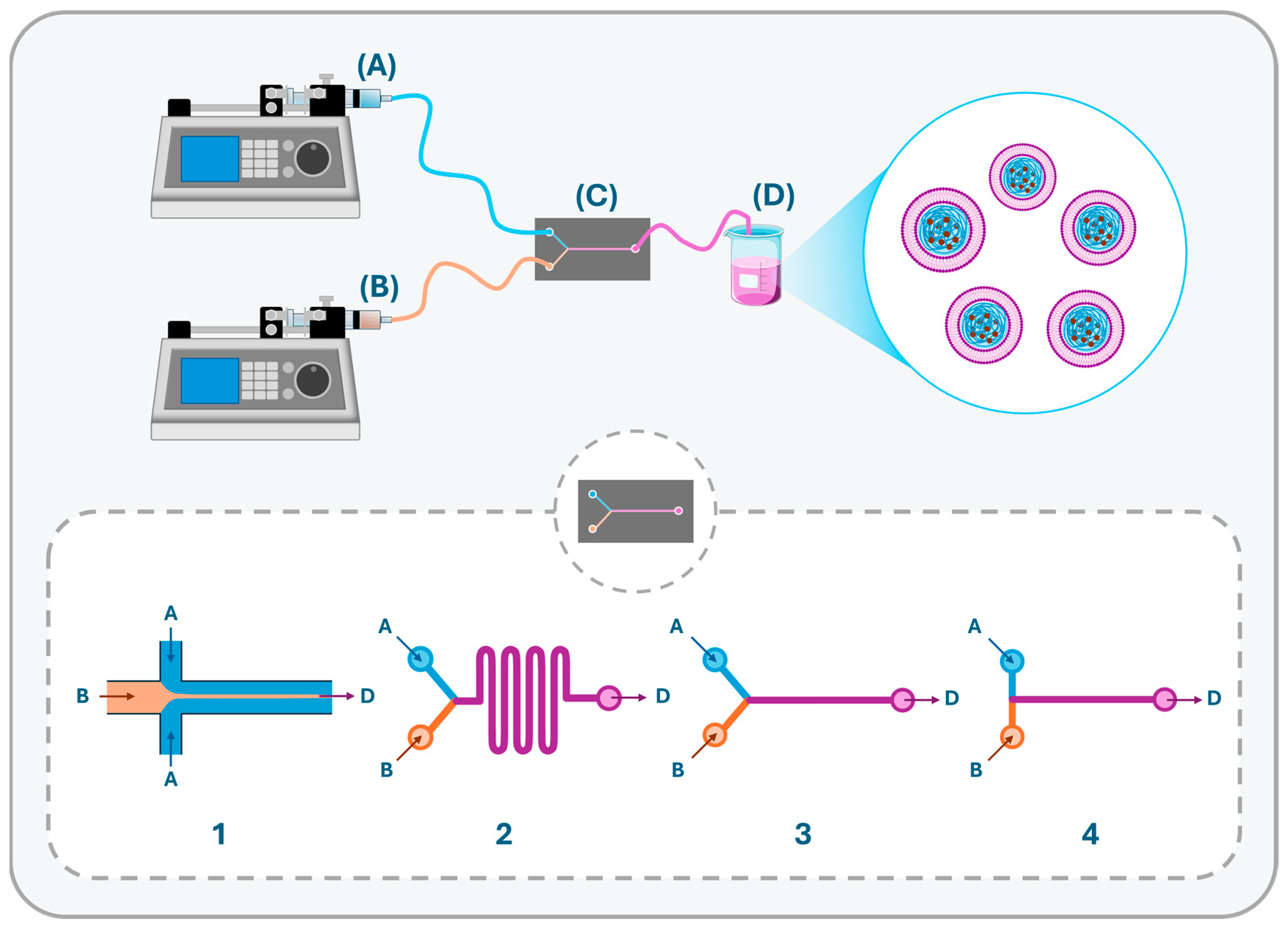
- Feng, Q.; Zhang, L.; Liu, C.; Li, X.; Hu, G.; Sun, J.; Jiang, X. Microfluidic Based High Throughput Synthesis of Lipid-Polymer Hybrid Nanoparticles with Tunable Diameters. Biomicrofluidics 2015, 9, 052604. [Google Scholar] [CrossRef]
- González-García, D.; Tapia, O.; Évora, C.; García-García, P.; Delgado, A. Conventional and Microfluidic Methods: Design and Optimization of Lipid-Polymeric Hybrid Nanoparticles for Gene Therapy. Drug Deliv. Transl. Res. 2025, 15, 908–924. [Google Scholar] [CrossRef]
- Boushra, M.; Tous, S.; Fetih, G.; Xue, H.-Y.; Wong, H.-L. Development of Bi-Polymer Lipid Hybrid Nanocarrier (BLN) to Improve the Entrapment and Stability of Insulin for Efficient Oral Delivery. J. Drug Deliv. Sci. Technol. 2019, 49, 632–641. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Kaushal, S.; Cao, H.S.T.; Aryal, S.; Sartor, M.; Esener, S.; Bouvet, M.; Zhang, L. Half-Antibody Functionalized Lipid−Polymer Hybrid Nanoparticles for Targeted Drug Delivery to Carcinoembryonic Antigen Presenting Pancreatic Cancer Cells. Mol. Pharm. 2010, 7, 914–920. [Google Scholar] [CrossRef]
- Valencia, P.M.; Basto, P.A.; Zhang, L.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Single-Step Assembly of Homogenous Lipid−Polymeric and Lipid−Quantum Dot Nanoparticles Enabled by Microfluidic Rapid Mixing. ACS Nano 2010, 4, 1671–1679. [Google Scholar] [CrossRef]
- Parveen, S.; Gupta, P.; Kumar, S.; Banerjee, M. Lipid Polymer Hybrid Nanoparticles as Potent Vehicles for Drug Delivery in Cancer Therapeutics. Med. Drug Discov. 2023, 20, 100165. [Google Scholar] [CrossRef]
- Kassaee, S.N.; Richard, D.; Ayoko, G.A.; Islam, N. Lipid Polymer Hybrid Nanoparticles against Lung Cancer and Their Application as Inhalable Formulation. Nanomedicine 2024, 19, 2113–2133. [Google Scholar] [CrossRef]
- Pang, J.; Xing, H.; Sun, Y.; Feng, S.; Wang, S. Non-Small Cell Lung Cancer Combination Therapy: Hyaluronic Acid Modified, Epidermal Growth Factor Receptor Targeted, pH Sensitive Lipid-Polymer Hybrid Nanoparticles for the Delivery of Erlotinib plus Bevacizumab. Biomed. Pharmacother. 2020, 125, 109861. [Google Scholar] [CrossRef]
- Guo, P.; Xue, H.Y.; Buttaro, B.A.; Tran, N.T.; Wong, H.L. Enhanced Eradication of Intracellular and Biofilm-Residing Methicillin-Resistant Staphylococcus Aureus (MRSA) Reservoirs with Hybrid Nanoparticles Delivering Rifampicin. Int. J. Pharm. 2020, 589, 119784. [Google Scholar] [CrossRef]
- Khan, M.M.; Madni, A.; Tahir, N.; Parveen, F.; Khan, S.; Jan, N.; Ali, A.; Abdurrahim, M.; Farooq, U.; Khan, M.I. Co-Delivery of Curcumin and Cisplatin to Enhance Cytotoxicity of Cisplatin Using Lipid-Chitosan Hybrid Nanoparticles. IJN 2020, 15, 2207–2217. [Google Scholar] [CrossRef]
- Tahir, N.; Madni, A.; Li, W.; Correia, A.; Khan, M.M.; Rahim, M.A.; Santos, H.A. Microfluidic Fabrication and Characterization of Sorafenib-Loaded Lipid-Polymer Hybrid Nanoparticles for Controlled Drug Delivery. Int. J. Pharm. 2020, 581, 119275. [Google Scholar] [CrossRef]
- Bokare, A.; Takami, A.; Kim, J.H.; Dong, A.; Chen, A.; Valerio, R.; Gunn, S.; Erogbogbo, F. Herringbone-Patterned 3D-Printed Devices as Alternatives to Microfluidics for Reproducible Production of Lipid Polymer Hybrid Nanoparticles. ACS Omega 2019, 4, 4650–4657. [Google Scholar] [CrossRef]
- Sawant, R.M.; Hurley, J.P.; Salmaso, S.; Kale, A.; Tolcheva, E.; Levchenko, T.S.; Torchilin, V.P. “SMART” Drug Delivery Systems: Double-Targeted pH-Responsive Pharmaceutical Nanocarriers. Bioconj. Chem. 2006, 17, 943–949. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Care, A.; Deng, W. Targeted Polymer Lipid Hybrid Nanoparticles for In-Vitro siRNA Therapy in Triple-Negative Breast Cancer. J. Drug Deliv. Sci. Technol. 2024, 98, 105911. [Google Scholar] [CrossRef]
- Hu, Y.; Hoerle, R.; Ehrich, M.; Zhang, C. Engineering the Lipid Layer of Lipid–PLGA Hybrid Nanoparticles for Enhanced in Vitro Cellular Uptake and Improved Stability. Acta Biomater. 2015, 28, 149–159. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid–Polymer Hybrid Nanoparticles as a next-Generation Drug Delivery Platform: State of the Art, Emerging Technologies, and Perspectives. IJN 2019, 14, 1937–1952. [Google Scholar] [CrossRef]
- Ferreira Soares, D.C.; Domingues, S.C.; Viana, D.B.; Tebaldi, M.L. Polymer-Hybrid Nanoparticles: Current Advances in Biomedical Applications. Biomed. Pharmacother. 2020, 131, 110695. [Google Scholar] [CrossRef]
- Ramasamy, T.; Tran, T.H.; Choi, J.Y.; Cho, H.J.; Kim, J.H.; Yong, C.S.; Choi, H.-G.; Kim, J.O. Layer-by-Layer Coated Lipid–Polymer Hybrid Nanoparticles Designed for Use in Anticancer Drug Delivery. Carbohydr. Polym. 2014, 102, 653–661. [Google Scholar] [CrossRef]
- Elder, D. ICH Q6A Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances. ICH Qual. Guidel. Implement. Guide 2017, 433–466. [Google Scholar]
- European Medicines Agency. ICH Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities) Q11; European Medicines Agency: London, UK, 2011. [Google Scholar]
- Hallan, S.S.; Kaur, P.; Kaur, V.; Mishra, N.; Vaidya, B. Lipid Polymer Hybrid as Emerging Tool in Nanocarriers for Oral Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 334–349. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.; Chen, H.; Hou, X.; He, Y.; Shen, J.; Shi, J.; Feng, N. Advances in Next-Generation Lipid-Polymer Hybrid Nanocarriers with Emphasis on Polymer-Modified Functional Liposomes and Cell-Based-Biomimetic Nanocarriers for Active Ingredients and Fractions from Chinese Medicine Delivery. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102237. [Google Scholar] [CrossRef]
- Bangera, P.D.; Kara, D.D.; Tanvi, K.; Tippavajhala, V.K.; Rathnanand, M. Highlights on Cell-Penetrating Peptides and Polymer-Lipid Hybrid Nanoparticle: Overview and Therapeutic Applications for Targeted Anticancer Therapy. AAPS PharmSciTech 2023, 24, 124. [Google Scholar] [CrossRef]
- Rahman, M.; Alharbi, K.S.; Alruwaili, N.K.; Anfinan, N.; Almalki, W.H.; Padhy, I.; Sambamoorthy, U.; Swain, S.; Beg, S. Nucleic Acid-Loaded Lipid-Polymer Nanohybrids as Novel Nanotherapeutics in Anticancer Therapy. Expert Opin. Drug Deliv. 2020, 17, 805–816. [Google Scholar] [CrossRef]
- Ruttala, H.B.; Ramasamy, T.; Ruttala, R.R.T.; Tran, T.H.; Jeong, J.-H.; Choi, H.-G.; Ku, S.K.; Yong, C.S.; Kim, J.O. Mitochondria-Targeting Multi-Metallic ZnCuO Nanoparticles and IR780 for Efficient Photodynamic and Photothermal Cancer Treatments. J. Mater. Sci. Technol. 2021, 86, 139–150. [Google Scholar] [CrossRef]
- Cheow, W.S.; Hadinoto, K. Factors Affecting Drug Encapsulation and Stability of Lipid–Polymer Hybrid Nanoparticles. Colloids Surf. B Biointerfaces 2011, 85, 214–220. [Google Scholar] [CrossRef]
- Mandal, B.; Mittal, N.K.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Development and in Vitro Evaluation of Core–Shell Type Lipid–Polymer Hybrid Nanoparticles for the Delivery of Erlotinib in Non-Small Cell Lung Cancer. Eur. J. Pharm. Sci. 2016, 81, 162–171. [Google Scholar] [CrossRef]
- Gameiro, M.; Mano, J.F.; Gaspar, V.M. Emerging Lipid–Polymer Hybrid Nanoparticles for Genome Editing. Polym. Chem. 2024, 15, 3436–3468. [Google Scholar] [CrossRef]
- Almawash, S. Oral Bioavailability Enhancement of Anti-Cancer Drugs Through Lipid Polymer Hybrid Nanoparticles. Pharmaceutics 2025, 17, 381. [Google Scholar] [CrossRef]
- Subramanian, D.A.; Langer, R.; Traverso, G. Mucus Interaction to Improve Gastrointestinal Retention and Pharmacokinetics of Orally Administered Nano-Drug Delivery Systems. J. Nanobiotechnol. 2022, 20, 362. [Google Scholar] [CrossRef]
- Imam, S.S.; Gilani, S.J.; Bin Jumah, M.N.; Rizwanullah, M.; Zafar, A.; Ahmed, M.M.; Alshehri, S. Harnessing Lipid Polymer Hybrid Nanoparticles for Enhanced Oral Bioavailability of Thymoquinone: In Vitro and In Vivo Assessments. Polymers 2022, 14, 3705. [Google Scholar] [CrossRef]
- Cheng, H.; Cui, Z.; Guo, S.; Zhang, X.; Huo, Y.; Mao, S. Mucoadhesive versus Mucopenetrating Nanoparticles for Oral Delivery of Insulin. Acta Biomater. 2021, 135, 506–519. [Google Scholar] [CrossRef]
- Lemmer, H.J.R.; Hamman, J.H. Paracellular Drug Absorption Enhancement through Tight Junction Modulation. Expert Opin. Drug Deliv. 2013, 10, 103–114. [Google Scholar] [CrossRef]
- Jansen, M.A.A.; Klausen, L.H.; Thanki, K.; Lyngsø, J.; Skov Pedersen, J.; Franzyk, H.; Nielsen, H.M.; van Eden, W.; Dong, M.; Broere, F.; et al. Lipidoid-Polymer Hybrid Nanoparticles Loaded with TNF siRNA Suppress Inflammation after Intra-Articular Administration in a Murine Experimental Arthritis Model. Eur. J. Pharm. Biopharm. 2019, 142, 38–48. [Google Scholar] [CrossRef]
- Wang, J. Combination Treatment of Cervical Cancer Using Folate-Decorated, pH-Sensitive, Carboplatin and Paclitaxel Co-Loaded Lipid-Polymer Hybrid Nanoparticles. DDDT 2020, 14, 823–832. [Google Scholar] [CrossRef]
- Prajapati, J.B.; Katariya, H.; Patel, R. Peyer’e Patch Targeting of Isradipine Loaded Solid Lipid Nanoparticles: It’s Cellular Uptake Study. J. Drug Deliv. Sci. Technol. 2018, 43, 318–326. [Google Scholar] [CrossRef]
- Patel, M.; Desai, A.; Kansara, V.; Vyas, B. Core Shell Lipid-Polymer Hybrid Nanoparticles for Oral Bioavailability Enhancement of Ibrutinib via Lymphatic Uptake. AAPS PharmSciTech 2023, 24, 142. [Google Scholar] [CrossRef]
- He, Y.; Cheng, M.; Yang, R.; Li, H.; Lu, Z.; Jin, Y.; Feng, J.; Tu, L. Research Progress on the Mechanism of Nanoparticles Crossing the Intestinal Epithelial Cell Membrane. Pharmaceutics 2023, 15, 1816. [Google Scholar] [CrossRef]
- Bose, R.J.; Arai, Y.; Ahn, J.C.; Park, H.; Lee, S.-H. Influence of Cationic Lipid Concentration on Properties of Lipid–Polymer Hybrid Nanospheres for Gene Delivery. IJN 2015, 10, 5367–5382. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Wang, X.; Li, X.; Xiao, B. Effect of Particle Size on the Cellular Uptake and Anti-Inflammatory Activity of Oral Nanotherapeutics. Colloids Surf. B Biointerfaces 2020, 187, 110880. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, M.; Kumar, P.; Verma, J.; Rosenholm, J.M.; Bansal, K.K.; Vaidya, A. Lipid–Polymer Hybrid Nanosystems: A Rational Fusion for Advanced Therapeutic Delivery. J. Funct. Biomater. 2023, 14, 437. [Google Scholar] [CrossRef]
- Raman, S.; Mahmood, S.; Rahman, A. A Review on Lipid-Polymer Hybrid Nanoparticles and Preparation with Recent Update. MSF 2020, 981, 322–327. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L. Lipid–Polymer Hybrid Nanoparticles: Synthesis, Characterization and Applications. Nano LIFE 2010, 1, 163–173. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Y.; Jin, S.; Hui, Y.; Wang, X.; Xu, L.; Chen, D.; Weitz, D.; Zhao, C.-X. Phase Separation-Induced Nanoprecipitation for Making Polymer Nanoparticles with High Drug Loading. Aggregate 2023, 4, e314. [Google Scholar] [CrossRef]
- Khalili, L.; Dehghan, G.; Sheibani, N.; Khataee, A. Smart Active-Targeting of Lipid-Polymer Hybrid Nanoparticles for Therapeutic Applications: Recent Advances and Challenges. Int. J. Biol. Macromol. 2022, 213, 166–194. [Google Scholar] [CrossRef]
- Craparo, E.F.; Cabibbo, M.; Scialabba, C.; Giammona, G.; Cavallaro, G. Inhalable Formulation Based on Lipid–Polymer Hybrid Nanoparticles for the Macrophage Targeted Delivery of Roflumilast. Biomacromolecules 2022, 23, 3439–3451. [Google Scholar] [CrossRef]
- Meyer, R.A.; Hussmann, G.P.; Peterson, N.C.; Santos, J.L.; Tuesca, A.D. A Scalable and Robust Cationic Lipid/Polymer Hybrid Nanoparticle Platform for mRNA Delivery. Int. J. Pharm. 2022, 611, 121314. [Google Scholar] [CrossRef]
- Shiraishi, K.; Kawano, K.; Maitani, Y.; Aoshi, T.; Ishii, K.J.; Sanada, Y.; Mochizuki, S.; Sakurai, K.; Yokoyama, M. Exploring the Relationship between Anti-PEG IgM Behaviors and PEGylated Nanoparticles and Its Significance for Accelerated Blood Clearance. J. Control. Release 2016, 234, 59–67. [Google Scholar] [CrossRef]
- Dehaini, D.; Fang, R.H.; Luk, B.T.; Pang, Z.; Hu, C.-M.J.; Kroll, A.V.; Yu, C.L.; Gao, W.; Zhang, L. Ultra-Small Lipid–Polymer Hybrid Nanoparticles for Tumor-Penetrating Drug Delivery. Nanoscale 2016, 8, 14411–14419. [Google Scholar] [CrossRef]
- Korucu Aktas, P.; Baysal, I.; Yabanoglu-Ciftci, S.; Arica, B. Development and In Vitro Evaluation of Crizotinib-Loaded Lipid–Polymer Hybrid Nanoparticles Using Box–Behnken Design in Non-Small Cell Lung Cancer. AAPS PharmSciTech 2023, 24, 178. [Google Scholar] [CrossRef]
- Gusmão, L.A.; Tedesco, A.C. Polymer–Lipid Hybrid Nanostructures for Drug Delivery. In Hybrid Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 101–127. [Google Scholar]
- Verma, J.; Singh, N.K.; Bansal, K.K. Recent Patents in Polymer–Lipid Hybrid Nanoparticles Technology. Ther. Deliv. 2024, 15, 489–493. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.-W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-Assembled Lipid−Polymer Hybrid Nanoparticles: A Robust Drug Delivery Platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef]
- Hazari, S.A.; Sheikh, A.; Abourehab, M.A.S.; Tulbah, A.S.; Kesharwani, P. Self-Assembled Gallic Acid Loaded Lecithin-Chitosan Hybrid Nanostructured Gel as a Potential Tool against Imiquimod-Induced Psoriasis. Environ. Res. 2023, 234, 116562. [Google Scholar] [CrossRef]
- Sun, Y.; Lee, R.J.; Meng, F.; Wang, G.; Zheng, X.; Dong, S.; Teng, L. Microfluidic Self-Assembly of High Cabazitaxel Loading Albumin Nanoparticles. Nanoscale 2020, 12, 16928–16933. [Google Scholar] [CrossRef]
- Fabozzi, A.; Della Sala, F.; di Gennaro, M.; Barretta, M.; Longobardo, G.; Solimando, N.; Pagliuca, M.; Borzacchiello, A. Design of Functional Nanoparticles by Microfluidic Platforms as Advanced Drug Delivery Systems for Cancer Therapy. Lab. A Chip 2023, 23, 1389–1409. [Google Scholar] [CrossRef]
- Bose, R.J.C.; Ravikumar, R.; Karuppagounder, V.; Bennet, D.; Rangasamy, S.; Thandavarayan, R.A. Lipid–Polymer Hybrid Nanoparticle-Mediated Therapeutics Delivery: Advances and Challenges. Drug Discov. Today 2017, 22, 1258–1265. [Google Scholar] [CrossRef]
- Liu, Z.; Fontana, F.; Python, A.; Hirvonen, J.T.; Santos, H.A. Microfluidics for Production of Particles: Mechanism, Methodology, and Applications. Small 2020, 16, 1904673. [Google Scholar] [CrossRef]
- Kim, Y.; Lee Chung, B.; Ma, M.; Mulder, W.J.M.; Fayad, Z.A.; Farokhzad, O.C.; Langer, R. Mass Production and Size Control of Lipid–Polymer Hybrid Nanoparticles through Controlled Microvortices. Nano Lett. 2012, 12, 3587–3591. [Google Scholar] [CrossRef]
- Li, S.; Yang, B.; Ye, L.; Hu, S.; Li, B.; Yang, Y.; Jia, X.; Feng, L.; Xiong, Z. Timescale Dependent Homogeneous Assembly of Lipid-Polymer Hybrid Nanoparticles in Laminar Mixing for Enhanced Lung Cancer Treatment. Chem. Eng. J. 2024, 498, 155625. [Google Scholar] [CrossRef]
- Mandal, B.; Bhattacharjee, H.; Mittal, N.; Sah, H.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Core–Shell-Type Lipid–Polymer Hybrid Nanoparticles as a Drug Delivery Platform. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 474–491. [Google Scholar] [CrossRef]
- Devrim, B.; Kara, A.; Vural, İ.; Bozkır, A. Lysozyme-Loaded Lipid-Polymer Hybrid Nanoparticles: Preparation, Characterization and Colloidal Stability Evaluation. Drug Dev. Ind. Pharm. 2016, 42, 1865–1876. [Google Scholar] [CrossRef]
- Garcia-Fuentes, M.; Alonso, M.J.; Torres, D. Design and Characterization of a New Drug Nanocarrier Made from Solid–Liquid Lipid Mixtures. J. Colloid Interface Sci. 2005, 285, 590–598. [Google Scholar] [CrossRef]
- Shafique, M.; Ur Rehman, M.; Kamal, Z.; Alzhrani, R.M.; Alshehri, S.; Alamri, A.H.; Bakkari, M.A.; Sabei, F.Y.; Safhi, A.Y.; Mohammed, A.M.; et al. Formulation Development of Lipid Polymer Hybrid Nanoparticles of Doxorubicin and Its In-Vitro, in-Vivo and Computational Evaluation. Front. Pharmacol. 2023, 14, 1025013. [Google Scholar] [CrossRef]
- Yalcin, T.E.; Ilbasmis-Tamer, S.; Takka, S. Antitumor Activity of Gemcitabine Hydrochloride Loaded Lipid Polymer Hybrid Nanoparticles (LPHNs): In Vitro and in Vivo. Int. J. Pharm. 2020, 580, 119246. [Google Scholar] [CrossRef]
- Shi, J.; Xiao, Z.; Votruba, A.R.; Vilos, C.; Farokhzad, O.C. Differentially Charged Hollow Core/Shell Lipid-Polymer-Lipid Hybrid Nanoparticles for Small Interfering RNA Delivery. Angew. Chem. Int. Ed. 2011, 50, 7027–7031. [Google Scholar] [CrossRef]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan Coated Nanostructured Lipid Carriers for Brain Delivery of Proteins by Intranasal Administration. Colloids Surf. B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef]
- García-Díaz, M.; Foged, C.; Nielsen, H.M. Improved Insulin Loading in Poly(Lactic-Co-Glycolic) Acid (PLGA) Nanoparticles upon Self-Assembly with Lipids. Int. J. Pharm. 2015, 482, 84–91. [Google Scholar] [CrossRef]
- Asuman Bozkır, B.D. Preparation and Characterization of Protein-Loaded Lipid-Polymer Hybrid Nanoparticles with Polycaprolactone as Polymeric Core Material. J. Biomol. Res. Ther. 2014, 3, 2. [Google Scholar] [CrossRef]
- Srinivasan, C.; Katare, Y.K.; Muthukumaran, T.; Panda, A.K. Effect of Additives on Encapsulation Efficiency, Stability and Bioactivity of Entrapped Lysozyme from Biodegradable Polymer Particles. J. Microencapsul. 2005, 22, 127–138. [Google Scholar] [CrossRef]
- Zafar, A.; Yasir, M.; Panda, D.S.; Khalid, M.; Singh, L.; Quazi, A.M. Development of Lipid Polymer Hybrid Nanoparticles of Abietic Acid: Optimization, In-Vitro and Preclinical Evaluation. AAPS PharmSciTech 2024, 25, 145. [Google Scholar] [CrossRef]
- Kasif, M.; Gupta, R.; Singh, P.P.; Bhardwaj, P.; Goyal, R.; Bansal, K.K.; Mahor, A.K. Development of Biocompatible Lipid-Polymer Hybrid Nanoparticles for Enhanced Oral Absorption of Posaconazole: A Mechanistic in Vitro and in Silico Assessment. J. Drug Deliv. Sci. Technol. 2024, 101, 106109. [Google Scholar] [CrossRef]
- Asuman Bozkır, B.D. Design and Evaluation of Hydrophobic Ion-Pairing Complexation of Lysozyme with Sodium Dodecyl Sulfate for Improved Encapsulation of Hydrophilic Peptides/Proteins by Lipid-Polymer Hybrid Nanoparticles. J. Nanomed. Nanotechnol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Sarmento, B.; Mazzaglia, D.; Bonferoni, M.C.; Neto, A.P.; do Céu Monteiro, M.; Seabra, V. Effect of Chitosan Coating in Overcoming the Phagocytosis of Insulin Loaded Solid Lipid Nanoparticles by Mononuclear Phagocyte System. Carbohydr. Polym. 2011, 84, 919–925. [Google Scholar] [CrossRef]
- Boushra, M.; Tous, S.; Fetih, G.; Korzekwa, K.; Lebo, D.B.; Xue, H.Y.; Wong, H.L. Development and Evaluation of Viscosity-Enhanced Nanocarrier (VEN) for Oral Insulin Delivery. Int. J. Pharm. 2016, 511, 462–472. [Google Scholar] [CrossRef]
- Ebrahimian, M.; Mahvelati, F.; Malaekeh-Nikouei, B.; Hashemi, E.; Oroojalian, F.; Hashemi, M. Bromelain Loaded Lipid-Polymer Hybrid Nanoparticles for Oral Delivery: Formulation and Characterization. Appl. Biochem. Biotechnol. 2022, 194, 3733–3748. [Google Scholar] [CrossRef]
- Hanson, L.R.; Frey, W.H. Intranasal Delivery Bypasses the Blood-Brain Barrier to Target Therapeutic Agents to the Central Nervous System and Treat Neurodegenerative Disease. BMC Neurosci. 2008, 9, S5. [Google Scholar] [CrossRef]
- Kumar, M.; Misra, A.; Mishra, A.K.; Mishra, P.; Pathak, K. Mucoadhesive Nanoemulsion-Based Intranasal Drug Delivery System of Olanzapine for Brain Targeting. J. Drug Target. 2008, 16, 806–814. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as Biomaterial in Drug Delivery and Tissue Engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Olah, I.; Lasher, J.; Regdon, G.; Pintye-Hodi, K.; Baki, G.; Sovany, T. Evaluating Superdisintegrants for Their Performance in Orally Disintegrating Tablets Containing Lysozyme Enzyme. J. Drug Deliv. Sci. Technol. 2019, 49, 396–404. [Google Scholar] [CrossRef]
- Oderinde, B.; Agbede, O.; Iheukwumere, I.; Ghamba, P.; Medugu, J.; Oku, E. Antiviral Activity of Hen Egg-White Lysozyme on Polio Virus. Sokoto J. Med. Lab. Sci. 2017, 2, 109–120. [Google Scholar]
- Oliver, W.T.; Wells, J.E. Lysozyme as an Alternative to Growth Promoting Antibiotics in Swine Production. J. Anim. Sci. Biotechnol. 2015, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Borges, O.; Borchard, G.; Verhoef, J.C.; de Sousa, A.; Junginger, H.E. Preparation of Coated Nanoparticles for a New Mucosal Vaccine Delivery System. Int. J. Pharm. 2005, 299, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, C.H.; Azria, M.; Silverman, S.; Engelhardt, M.; Olson, M.; Mindeholm, L. Salmon Calcitonin: A Review of Current and Future Therapeutic Indications. Osteoporos. Int. 2008, 19, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Plosker, G.L.; McTavish, D. Intranasal Salcatonin (Salmon Calcitonin). Drugs Aging 1996, 8, 378–400. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Xiong, X.Y.; Tian, Y.; Li, Z.L.; Gong, Y.C.; Li, Y.P. A Review of Biodegradable Polymeric Systems for Oral Insulin Delivery. Drug Deliv. 2016, 23, 1882–1891. [Google Scholar] [CrossRef]
- Németh, Z.; Pallagi, E.; Dobó, D.G.; Csóka, I. A Proposed Methodology for a Risk Assessment-Based Liposome Development Process. Pharmaceutics 2020, 12, 1164. [Google Scholar] [CrossRef]
- Giovino, C.; Ayensu, I.; Tetteh, J.; Boateng, J.S. An Integrated Buccal Delivery System Combining Chitosan Films Impregnated with Peptide Loaded PEG-b-PLA Nanoparticles. Colloids Surf. B Biointerfaces 2013, 112, 9–15. [Google Scholar] [CrossRef]
- Hu, S.; Pei, X.; Duan, L.; Zhu, Z.; Liu, Y.; Chen, J.; Chen, T.; Ji, P.; Wan, Q.; Wang, J. A Mussel-Inspired Film for Adhesion to Wet Buccal Tissue and Efficient Buccal Drug Delivery. Nat. Commun. 2021, 12, 1689. [Google Scholar] [CrossRef]
- Morales, J.O.; Huang, S.; Williams, R.O.; McConville, J.T. Films Loaded with Insulin-Coated Nanoparticles (ICNP) as Potential Platforms for Peptide Buccal Delivery. Colloids Surf. B Biointerfaces 2014, 122, 38–45. [Google Scholar] [CrossRef]
- Morales, J.O.; Ross, A.C.; McConville, J.T. Protein-Coated Nanoparticles Embedded in Films as Delivery Platforms. J. Pharm. Pharmacol. 2013, 65, 827–838. [Google Scholar] [CrossRef]
- Mortazavian, E.; Dorkoosh, F.A.; Rafiee-Tehrani, M. Design, Characterization and Ex Vivo Evaluation of Chitosan Film Integrating of Insulin Nanoparticles Composed of Thiolated Chitosan Derivative for Buccal Delivery of Insulin. Drug Dev. Ind. Pharm. 2014, 40, 691–698. [Google Scholar] [CrossRef] [PubMed]



| Feature | Lipid-Based NCs | Polymeric NCs | LPHNs |
|---|---|---|---|
| Stability | Moderate (lipid oxidation, drug leakage) | High (rigid matrix) | High (rigid polymer core) |
| Biocompatibility | Excellent | Moderate (depending on the polymer nature) | Excellent (lipid shields polymer) |
| Encapsulation | Moderate for proteins | Low for hydrophilic macromolecules | Higher for hydrophilic/hydrophobic peptides/proteins |
| Cellular Uptake | High (membrane fusion, endocytosis) | Variable | High (lipid-mimic + polymer protection) |
| Bioavailability | Limited for peptide/protein due to stability issues | Moderate due to lower transcellular absorption | Improved due to combined stability and biocompatibility |
| Drug Release | Fast, burst due to fragile lipid structure | Controlled, tunable | Controlled due to polymer core matrix |
| Preparation | Simple self-assembly | More complex | Most complex due to hybrid structure, often require multiple steps |
| Scalability | Established with moderate challenges | Established with moderate challenges | Still emerging with more difficult challenges |
| Single-Step Approach | Two-Step Approach | |
|---|---|---|
| Definition | Simultaneous formation of lipid and polymer components in one process | Separate formation of polymer core and lipid shell, followed by their combination |
| Complexity | Relatively simple | More complex and time-consuming |
| Control over structure | Limited control over core–shell architecture | Greater control over nanoparticle architecture |
| Encapsulation efficiency | May be lower or variable | Generally higher due to optimized core loading before lipid coating |
| Scalability | More scalable and suitable for industrial production | Less scalable due to complexity |
| Reproducibility | Higher, due to fewer steps and reduced variability | Lower, due to multiple processing stages |
| Stability of the API | May be compromised during the preparation due to harsh conditions and/or organic solvent exposure | Improved stability due to drug encapsulation during the polymer core formation in gentler conditions |
| Suitable applications | When stability of the API is not a concern | More suitable for peptide/protein encapsulation due to the less harsh conditions and/or organic solvent exposure compared to the one-step approach |
| Type of LPHNs | Peptide/Protein | Lipid | Polymer | Preparation Method | Disease/Route | Results | Ref. |
|---|---|---|---|---|---|---|---|
| LC-PS | Insulin | Dynasan® 116 + Lecithin | Cholic acid or Synperonic® F68 or PEG-2000 stearate or PEG-4500 stearate | Single-step DESE | Diabetes mellitus/Oral | Ps: 198 ± 4.7 nm PDI: 0.24–0.28 Zeta P.: −19.9 ± 0.9 mV EE%: ----- | [23] |
| M-LPHNs | Insulin | SPC | PLGA (Av.Mw 9500) or PLGA (Av.Mw 18,000) or PLA | A- Ins-SPC complex by anhydrous co-solvent lyophilization. B- Reverse-micelle + ESE | Diabetes mellitus/Oral | Ps: 201 ± 25 nm PDI:----- Zeta P.: −17.8 ± 8.6 mV EE%: 88.5 ± 9.7% RB:7.7% | [41] |
| LC-PC | Insulin | Witepsol 85E/or Compritol 888 ATO/or Precirol ATO 5 | Low Mw Chitosan | Two-step DESE for SLN + coating by incubation under stirring | Diabetes mellitus/Oral | Ps: 191 ± 24 nm PDI:------ Zeta P.: +28.2 ± 1 mV EE%: 57.3 ± 0.2% | [126] |
| LC-PC | Insulin | Witepsol 85E | Low Mw Chitosan | Two-step DESE for SLN + coating by incubation under stirring | Diabetes mellitus/Oral | Ps: 470 ± 32 nm PDI: 0.63 ± 0.02 Zeta P.: +34.2 ± 3.4 mV EE%: 52.2 ± 5.3% RB.: 17.7% | [16] |
| M-LPHNs | Insulin | SPC or NaC 10 | PLGA | A- Ins-lipid complex by self-assembly and lyophilisation. B- DESE | Diabetes mellitus/Oral | (SPC) Ps: 202 ± 10 nm PDI: 0.10 ± 0.03 Zeta P.: −30 ± 5 mV EE%: 90 ± 7% | [120] |
| (NaC10)Ps: 222 ± 8 nm PDI: 0.12 ± 0.04 Zeta P.: −30 ± 2 mV EE%: 91 ± 7% | |||||||
| M-LPHNs | Insulin | SPC + trimyristin | Methocel A | Single-step DESE | Diabetes mellitus/Oral | Ps: 367.43 ± 9.85 nm PDI: 0.28 ± 0.01 Zeta P.: −39.4 ± 1.44 mV EE%: 40% | [39] |
| M-LPHNs | Insulin | SPC + trimyristin | PEG400 or PEG600 or propylene glycol | Single-step DESE + Filtration under vacuum/freeze drying | Diabetes mellitus/Oral | Ps: 203.6 ± 21.3 nm PDI: 0.175 ± 0.029 Zeta P.: −43.3 ± 0.6 mV EE%: 54.5% RB.: 5.1% | [127] |
| PC-LS | Lysozyme | Tripalmitin + PC | PCL | Single-step DESE | - | Ps: 110.43 ± 1.68 nm PDI: 0.29 ± 0.024 Zeta P.: −21.77 ± 0.93 mV EE%:--------- | [121] |
| PC-LS | Lysozyme | Tripalmitin + PC | PCL | Two-step ESE (Solid-in-oil-in-water emulsification) | - | Ps: 112.53 ± 0.133 nm PDI: 0.164 ± 0.012 Zeta P.: −2.9 ± 0.04 mV EE%: 42.85 ± 0.08% | [125] |
| PC-LS | Lysozyme | Tripalmitin + PC | PCL | Single-step DESE | - | Ps: 58.04 ± 1.95 nm PDI: 0.15 ± 0.015 Zeta P.: −32.91 ± 2.94 mV EE%: 55.36 ± 0.21% | [114] |
| LC-PS | Salmon calcitonin | Tripalmitin + Lecithin ± Miglyol 812 (In case of PEG) | PEG-2000 stearate or Chitosan | Single-step DESE (PEG) | Oral | (PEG without Miglyol) Ps: 226.4 ± 7.5 nm PDI: 0.25 ± 0.07 Zeta P.: −34.8 ± 2.8 mV EE%: ≈90% | [17] |
| (PEG with Miglyol) Ps: 207.4 ± 19.1 nm PDI: 0.18 ± 0.08 Zeta P.: −36.6 ± 2.5 mV EE%: ≈90% | |||||||
| Two-steps DESE for SLN + coating by incubation (chitosan) | (Chitosan) Ps: 537.7 ± 16.6 nm PDI: 0.43 ± 0.14 Zeta P.: +29.2 ± 6.7 mV EE%: 30.7 ± 2.3% | ||||||
| LC-PS | - | Tripalmitin + Lecithin + Miglyol 812 | PEG40-stearate | Single-step DESE | - | Ps: 187 ± 5 nm PDI: 0.21–0.23 Zeta P:------- | [115] |
| PC-LS | TRP2/hgp100/p15E (peptides) | DOPC + DSPE-PEG-2000 | PLGA | Single-step DESE | Cancer vaccination/intradermal | Ps: 102 ± 12 nm PDI: -------- Zeta P:------- EE%: 30.1 ± 5.1% (TRP2) EE%: 12.1 ± 2.4% (p15E) EE%: 2.3 ± 0.7% (hgp100) | [34] |
| PC-LS | BSA | DOTAP + cholesterol + DSPE-PEG(2000) amine | PLGA | Two-steps A- DESE + freeze drying (BSA-containing PLGA NPs) B- Lipid film hydration (Liposomes). C- Homogenization + sonication + freeze drying (LPHNs) | - | Ps: 114 nm PDI: 0.067–0.109 Zeta P.: +4.78–+5.58 mV EE%: 88.5–98.3% | [67] |
| LC-PS | Neurotrophic factor hIGF-I | Precirol ATO5 or Dynasan114 and Miglyol® | Chitosan | Two-steps A-Emulsification to form NLC B-coating by incubation + lyophilisation | Brain disorder/Brain drug delivery after intranasal administration | Ps: 114.48 ± 20.20 nm PDI: 0.287 ± 0.05 Zeta P.: +28.40 ± 2.83 mV EE%: 90.28 ± 0.4% RB:-------- | [119] |
| PC-LS | Bromelain: combination of proteolytic enzymes | Phosphatidylcholine | PLGA | Single-step DESE + centrifugation, washing, and resuspension | Oral | Ps: 209.4 ± 7.30 nm PDI: 0.134 Zeta P.: −42.1 ± 0.55 mV EE: 83% | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, A.A.A.; Ramadan, E.; Kristó, K.; Regdon, G., Jr.; Sovány, T. Lipid-Polymer Hybrid Nanoparticles as a Smart Drug Delivery System for Peptide/Protein Delivery. Pharmaceutics 2025, 17, 797. https://doi.org/10.3390/pharmaceutics17060797
Hassan AAA, Ramadan E, Kristó K, Regdon G Jr., Sovány T. Lipid-Polymer Hybrid Nanoparticles as a Smart Drug Delivery System for Peptide/Protein Delivery. Pharmaceutics. 2025; 17(6):797. https://doi.org/10.3390/pharmaceutics17060797
Chicago/Turabian StyleHassan, Alharith A. A., Eslam Ramadan, Katalin Kristó, Géza Regdon, Jr., and Tamás Sovány. 2025. "Lipid-Polymer Hybrid Nanoparticles as a Smart Drug Delivery System for Peptide/Protein Delivery" Pharmaceutics 17, no. 6: 797. https://doi.org/10.3390/pharmaceutics17060797
APA StyleHassan, A. A. A., Ramadan, E., Kristó, K., Regdon, G., Jr., & Sovány, T. (2025). Lipid-Polymer Hybrid Nanoparticles as a Smart Drug Delivery System for Peptide/Protein Delivery. Pharmaceutics, 17(6), 797. https://doi.org/10.3390/pharmaceutics17060797








