Nasal Emulgel’s Role in Preventing Coronavirus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Nasal Emulgels
2.3. Characterization of the Nasal Emulgels
2.4. Physical Stability
2.5. Biological Assays
2.5.1. Cell Culture and Treatments
2.5.2. Cell Viability Assay
2.5.3. SARS-CoV-2 Infection
2.5.4. High-Content Confocal Imaging
2.6. Statistical Analysis
3. Results
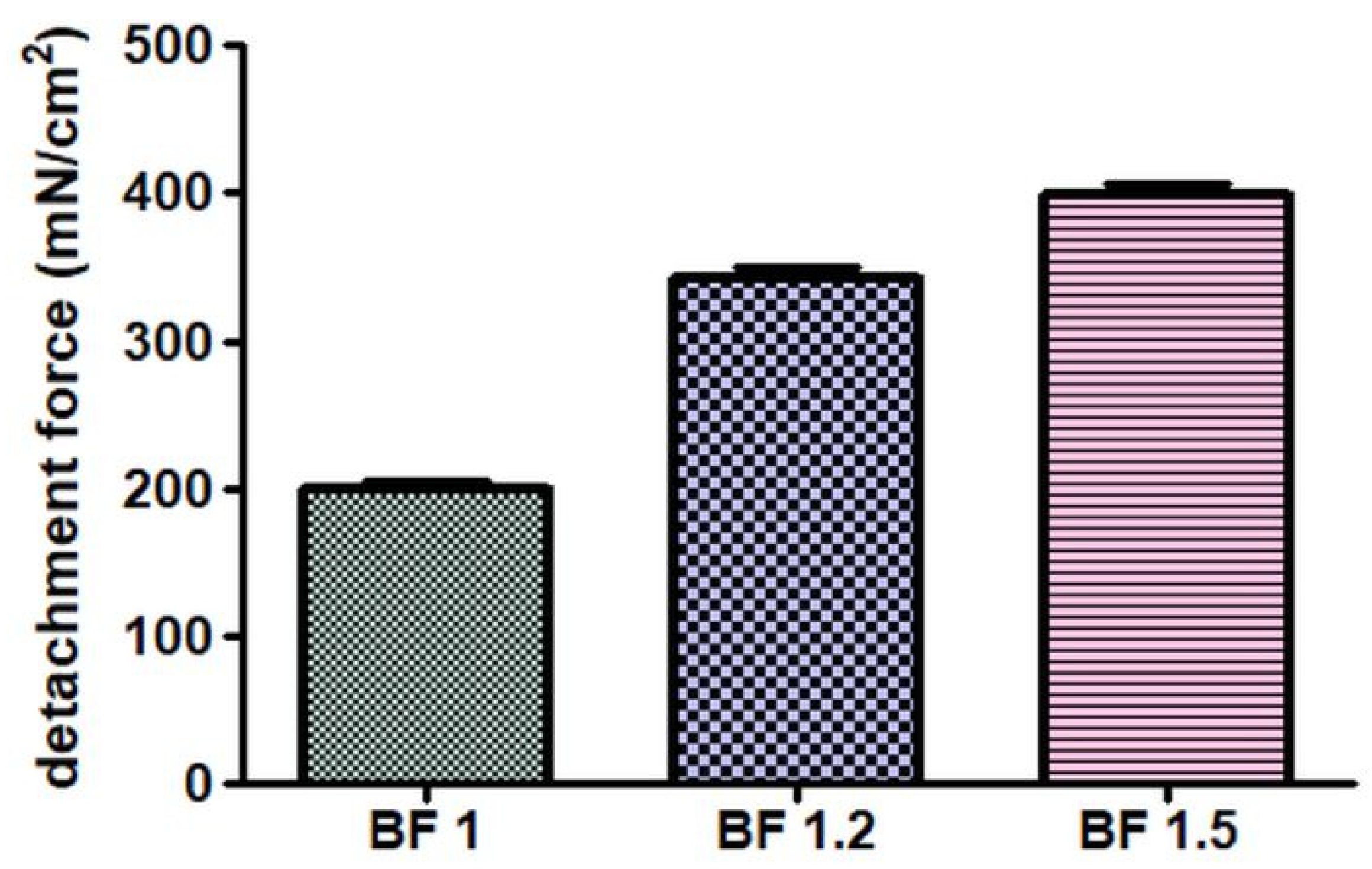
3.1. Characterization of the Nasal Emulgels
3.2. Physical Appearance and Stability
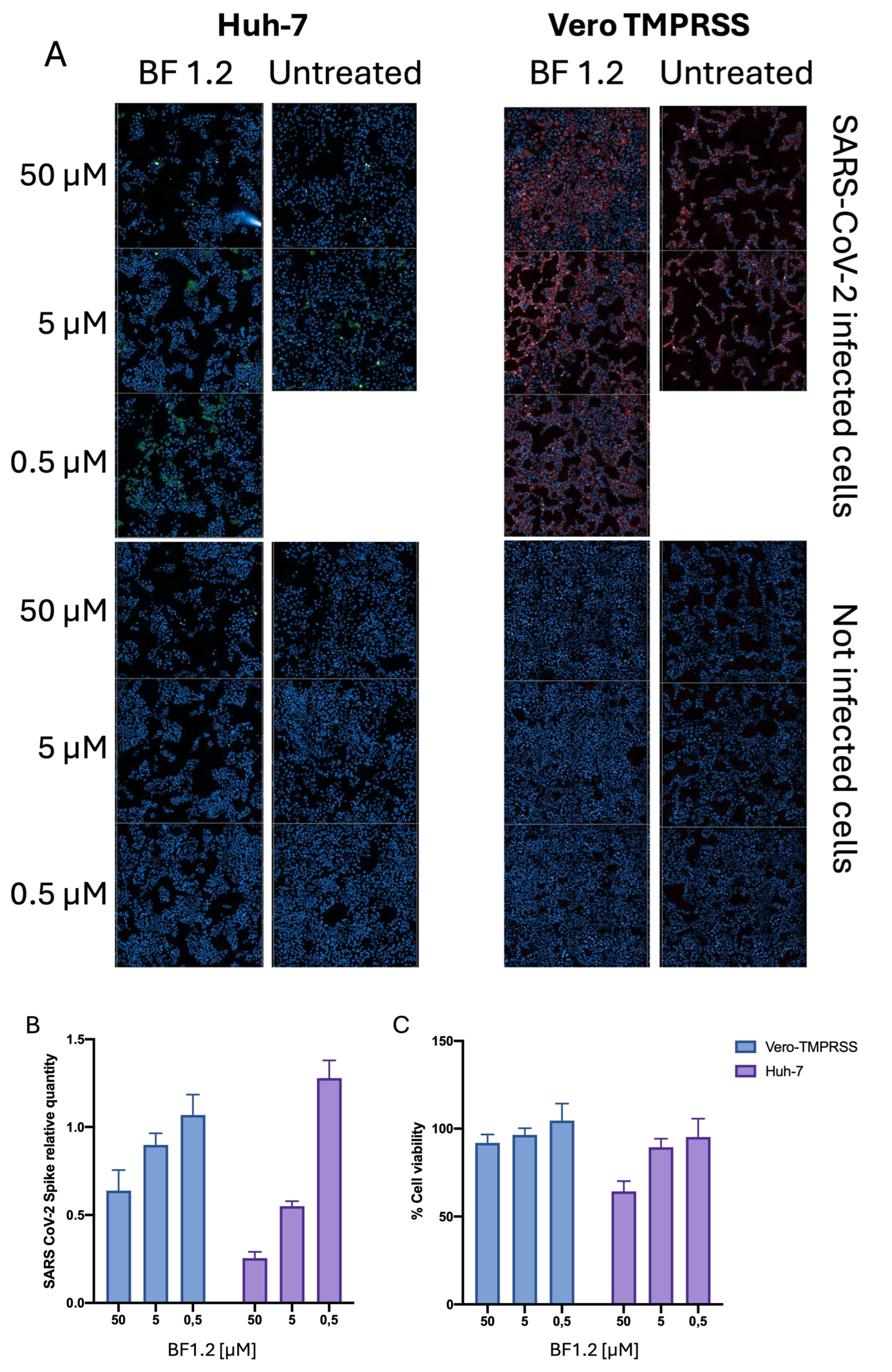
3.3. Biological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, X.; Xu, R.; Li, N. The Interplay between Airway Cilia and Coronavirus Infection, Implications for Prevention and Control of Airway Viral Infections. Cells 2024, 13, 1353. [Google Scholar] [CrossRef]
- Hoxha, I.; Agahi, R.; Bimbashi, A.; Aliu, M.; Raka, L.; Bajraktari, I.; Beqiri, P.; Adams, L.V. Higher COVID-19 Vaccination Rates Are Associated with Lower COVID-19 Mortality: A Global Analysis. Vaccines 2022, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, S. Influenza Virus Entry Inhibitors. In Virus Entry Inhibitors; Jiang, S., Lu, L., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2022; Volume 1366, pp. 123–135. ISBN 9789811687013. [Google Scholar]
- Liu, Y.; Qu, H.-Q.; Qu, J.; Tian, L.; Hakonarson, H. Expression Pattern of the SARS-CoV-2 Entry Genes ACE2 and TMPRSS2 in the Respiratory Tract. Viruses 2020, 12, 1174. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F. SARS-CoV-2 Entry Factors Are Highly Expressed in Nasal Epithelial Cells Together with Innate Immune Genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, J.; Hong, S.P.; Choi, S.Y.; Yang, M.J.; Ju, Y.S.; Kim, Y.T.; Kim, H.M.; Rahman, M.T.; Chung, M.K.; et al. Nasal Ciliated Cells Are Primary Targets for SARS-CoV-2 Replication in the Early Stage of COVID-19. J. Clin. Investig. 2021, 131, e148517. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, P.; Shi, X.; Wang, G.; Chen, J.; Zheng, A.; Wang, W.; Wang, Z.; Qu, X.; Luo, M.; et al. Highly Infectious SARS-CoV Pseudotyped Virus Reveals the Cell Tropism and Its Correlation with Receptor Expression. Biochem. Biophys. Res. Commun. 2004, 321, 994–1000. [Google Scholar] [CrossRef]
- Sherman, E.J.; Emmer, B.T. ACE2 Protein Expression within Isogenic Cell Lines Is Heterogeneous and Associated with Distinct Transcriptomes. Sci. Rep. 2021, 11, 15900. [Google Scholar] [CrossRef]
- Aref, Z.F.; Bazeed, S.E.E.S.; Hassan, M.H.; Hassan, A.S.; Rashad, A.; Hassan, R.G.; Abdelmaksoud, A.A. Clinical, Biochemical and Molecular Evaluations of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Reducing Upper Respiratory Symptoms of Mild COVID-19. Int. J. Nanomed. 2021, 16, 4063. [Google Scholar] [CrossRef]
- Shmuel, K.; Dalia, M.; Tair, L.; Yaakov, N. Low pH Hypromellose (Taffix) Nasal Powder Spray Could Reduce SARS-CoV-2 Infection Rate Post Mass-Gathering Event at a Highly Endemic Community: An Observational Prospective Open Label User Survey. Expert Rev. Anti Infect. Ther. 2021, 19, 1325–1330. [Google Scholar] [CrossRef]
- Bentley, K.; Stanton, R.J. Hydroxypropyl Methylcellulose-Based Nasal Sprays Effectively Inhibit In Vitro SARS-CoV-2 Infection and Spread. Viruses 2021, 13, 2345. [Google Scholar] [CrossRef] [PubMed]
- Fais, F.; Juskeviciene, R.; Francardo, V.; Mateos, S.; Guyard, M.; Viollet, C.; Constant, S.; Borelli, M.; Hohenfeld, I.P. Drug-Free Nasal Spray as a Barrier against SARS-CoV-2 and Its Delta Variant: In Vitro Study of Safety and Efficacy in Human Nasal Airway Epithelia. Int. J. Mol. Sci. 2022, 23, 4062. [Google Scholar] [CrossRef]
- Nocini, R.; Henry, B.M.; Mattiuzzi, C.; Lippi, G. Improving Nasal Protection for Preventing SARS-CoV-2 Infection. Biomedicines 2022, 10, 2966. [Google Scholar] [CrossRef]
- Figueroa, J.M.; Lombardo, M.E.; Dogliotti, A.; Flynn, L.P.; Giugliano, R.; Simonelli, G.; Valentini, R.; Ramos, A.; Romano, P.; Marcote, M.; et al. Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease. Int. J. Gen. Med. 2021, 14, 6277–6286. [Google Scholar] [CrossRef] [PubMed]
- Winchester, S.; John, S.; Jabbar, K.; John, I. Clinical Efficacy of Nitric Oxide Nasal Spray (NONS) for the Treatment of Mild COVID-19 Infection. J. Infect. 2021, 83, 237–279. [Google Scholar] [CrossRef]
- Du, J.; Shao, X.; Bouteiller, J.-M.C.; Lu, A.; Asante, I.; Louie, S.; Humayun, M.S.; Lazzi, G. Computational Optimization of Delivery Parameters to Guide the Development of Targeted Nasal Spray. Sci. Rep. 2023, 13, 4099. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G. Nasal Drug Delivery Devices: Characteristics and Performance in a Clinical Perspective—A Review. Drug Deliv. Transl. Res. 2013, 3, 42–62. [Google Scholar] [CrossRef]
- Trenkel, M.; Scherließ, R. Nasal Powder Formulations: In-Vitro Characterisation of the Impact of Powders on Nasal Residence Time and Sensory Effects. Pharmaceutics 2021, 13, 385. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Abou-Taleb, H.A.; Naguib, D.M. Nanosized Nasal Emulgel of Resveratrol: Preparation, Optimization, in Vitro Evaluation and in Vivo Pharmacokinetic Study. Drug Dev. Ind. Pharm. 2019, 45, 1624–1634. [Google Scholar] [CrossRef]
- Javed, H.; Shah, S.N.H.; Iqbal, F.M. Formulation Development and Evaluation of Diphenhydramine Nasal Nano-Emulgel. AAPS PharmSciTech 2018, 19, 1730–1743. [Google Scholar] [CrossRef] [PubMed]
- Umekar, M.; Wadher, K.; Bute, S.; Chandewar, A.; Kochar, N.; Amgaonkar, Y. Overview of Emulgel as Emergent Topical Delivery: Recent Applications and Advancement. J. Pharm. Res. Int. 2021, 33, 258–268. [Google Scholar]
- Talat, M.; Zaman, M.; Khan, R.; Jamshaid, M.; Akhtar, M.; Mirza, A.Z. Emulgel: An Effective Drug Delivery System. Drug Dev. Ind. Pharm. 2021, 47, 1193–1199. [Google Scholar] [CrossRef]
- Mohamed, M.I. Optimization of Chlorphenesin Emulgel Formulation. AAPS J. 2004, 6, 81–87. [Google Scholar] [CrossRef]
- Ajazuddin; Alexander, A.; Khichariya, A.; Gupta, S.; Patel, R.J.; Giri, T.K.; Tripathi, D.K. Recent Expansions in an Emergent Novel Drug Delivery Technology: Emulgel. J. Control. Release 2013, 171, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Raeisi Estabragh, M.A.; Sajadi Bami, M.; Dehghannoudeh, G.; Noudeh, Y.D.; Moghimipour, E. Cellulose Derivatives and Natural Gums as Gelling Agents for Preparation of Emulgel-Based Dosage Forms: A Brief Review. Int. J. Biol. Macromol. 2023, 241, 124538. [Google Scholar] [CrossRef]
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; Al Zarzour, R. COVID-19 and Therapy with Essential Oils Having Antiviral, Anti-Inflammatory, and Immunomodulatory Properties. Inflammopharmacology 2020, 28, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Shuai, X.; Dai, T.; Chen, M.; Liu, C.; Ruan, R.; Liu, Y.; Chen, J. Characterization of Lipid Compositions, Minor Components and Antioxidant Capacities in Macadamia (Macadamia integrifolia) Oil from Four Major Areas in China. Food Biosci. 2022, 50, 102009. [Google Scholar] [CrossRef]
- Ulandari Natasia, Y.; Wahyuni Nasution, S.; Suci, T. Test the Potential of Macadamia Nut Oil (Macadamia F. Muell) As Sunscreen in Cream Preparations In Vitro. Int. J. Health Pharm. IJHP 2022, 3, 281–293. [Google Scholar] [CrossRef]
- Zaidi, A.K.; Dehgani-Mobaraki, P. The Mechanisms of Action of Ivermectin against SARS-CoV-2—An Extensive Review. J. Antibiot. 2022, 75, 60–71. [Google Scholar] [CrossRef]
- Jang, Y.; Shin, H.; Lee, M.K.; Kwon, O.S.; Shin, J.S.; Kim, Y.; Kim, C.W.; Lee, H.-R.; Kim, M. Antiviral Activity of Lambda-Carrageenan against Influenza Viruses and Severe Acute Respiratory Syndrome Coronavirus 2. Sci. Rep. 2021, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Kim, G.; Desai, K.-G.H.; Patel, H.; Olsen, K.F.; Curtis-Fisk, J.; Tocce, E.; Jordan, S.; Schwendeman, S.P. Feasibility Investigation of Cellulose Polymers for Mucoadhesive Nasal Drug Delivery Applications. Mol. Pharm. 2015, 12, 2732–2741. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients; Libros Digitales-Pharmaceutical Press: London, UK, 2009; ISBN 1-58212-135-4. [Google Scholar]
- Popov, T.A.; Åberg, N.; Emberlin, J.; Josling, P.; Ilyina, N.I.; Nikitin, N.P.; Church, M. Methyl-Cellulose Powder for Prevention and Management of Nasal Symptoms. Expert Rev. Respir. Med. 2017, 11, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Chaudhary, S.; Shah, H.; Jacob, S.; Mewada, V.; Shinu, P.; Aldhubiab, B.; Sreeharsha, N.; Venugopala, K.N.; Attimarad, M.; et al. Intranasal Delivery of Darunavir-Loaded Mucoadhesive In Situ Gel: Experimental Design, In Vitro Evaluation, and Pharmacokinetic Studies. Gels 2022, 8, 342. [Google Scholar] [CrossRef]
- Khan, B.A.; Ahmad, S.; Khan, M.K.; Hosny, K.M.; Bukhary, D.M.; Iqbal, H.; Murshid, S.S.; Halwani, A.A.; Alissa, M.; Menaa, F. Fabrication and Characterizations of Pharmaceutical Emulgel Co-Loaded with Naproxen-Eugenol for Improved Analgesic and Anti-Inflammatory Effects. Gels 2022, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Corredor-Chaparro, M.Y.; Vargas-Riveros, D.; Mora-Huertas, C.E. Hypromellose—Collagen Hydrogels/Sesame Oil Organogel Based Bigels as Controlled Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2022, 75, 103637. [Google Scholar] [CrossRef]
- Sah, S.K.; Badola, A.; Mukhopadhyay, S. Development and Evaluation of Tioconazole Loaded Emulgel. Int. J. Appl. Pharm. 2017, 9, 83. [Google Scholar] [CrossRef]
- Jelvehgari, M.; Rashidi, M.R. Adhesive and Spreading Properties of Pharmaceutical Gel Composed of Cellulose Polymer. Jundishapur J. Nat. Pharm. Prod. 2007, 2, 45–58. [Google Scholar]
- Leitner, V.M.; Marschütz, M.K.; Bernkop-Schnürch, A. Mucoadhesive and Cohesive Properties of Poly(Acrylic Acid)-Cysteine Conjugates with Regard to Their Molecular Mass. Eur. J. Pharm. Sci. 2003, 18, 89–96. [Google Scholar] [CrossRef]
- Juliano, C.; Cossu, M.; Pigozzi, P.; Rassu, G.; Giunchedi, P. Preparation, In Vitro Characterization and Preliminary In Vivo Evaluation of Buccal Polymeric Films Containing Chlorhexidine. AAPS PharmSciTech 2008, 9, 1153–1158. [Google Scholar] [CrossRef]
- Gavini, E.; Rassu, G.; Sanna, V.; Cossu, M.; Giunchedi, P. Mucoadhesive Microspheres for Nasal Administration of an Antiemetic Drug, Metoclopramide: In-Vitro/Ex-Vivo Studies. J. Pharm. Pharmacol. 2010, 57, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Storti, B.; Quaranta, P.; Di Primio, C.; Clementi, N.; Mancini, N.; Criscuolo, E.; Spezia, P.G.; Carnicelli, V.; Lottini, G.; Paolini, E.; et al. A Spatial Multi-Scale Fluorescence Microscopy Toolbox Discloses Entry Checkpoints of SARS-CoV-2 Variants in Vero E6 Cells. Comput. Struct. Biotechnol. J. 2021, 19, 6140–6156. [Google Scholar] [CrossRef]
- Lai, M.; Iacono, E.; Spezia, P.G.; Lottini, G.; La Rocca, V.; Quaranta, P.; Pistello, M.; Freer, G. A Low-Cost Simple Test for Weekly Detection of Mycoplasma Hyorhinis and Arginini Contaminations in Cell Cultures and Viral Preparations. J. Virol. Methods 2022, 299, 114327. [Google Scholar] [CrossRef] [PubMed]
- Moscato, G.; Mazzetti, P.; Lucenteforte, E.; Rosellini, A.; Cara, A.; Quaranta, P.; Mainardi, V.; Villa, P.; Focosi, D.; Lanza, M.; et al. Assessment of Automated High-Throughput Serological Assays for Prediction of High-Titer SARS-CoV-2 Neutralizing Antibody. J. Clin. Virol. Plus 2021, 1, 100016. [Google Scholar] [CrossRef]
- Hull, D.; Rennie, P.; Noronha, A.; Poore, C.; Harrington, N.; Fearnley, V.; Passàli, D. Effects of Creating a Non-Specific, Virus-Hostile Environment in the Nasopharynx on Symptoms and Duration of Common Cold. Acta Otorhinolaryngol. Ital. 2007, 27, 73. [Google Scholar]
- Mann, B.J.; Moreau, G.B.; Lapidot, T.; Megiddo, D. TaffiX Nasal Powder Forms an Effective Barrier against SARS-CoV-2. Biomed. J. Sci. Tech. Res. 2021, 33, 25483–25485. [Google Scholar] [CrossRef]
- Megiddo, D.; Pharma, N. Taffix- Mechanism of Action. Available online: https://www.mygenomics.eu/img/cms/documenti%20blog/TaffiX%20materiale%20scientificopdf%20(2)%20(6).pdf (accessed on 18 April 2025).
- Sherafudeen, S.P.; Vasantha, P.V. Development and Evaluation of in Situ Nasal Gel Formulations of Loratadine. Res. Pharm. Sci. 2015, 10, 466. [Google Scholar]
- Quay, S.C.; Aprile, P.C.; Go, Z.O.; Sileno, A.P. Cyanocobalamin Low Viscosity Aqueous Formulations for Intranasal Delivery. US20150004198A1, 1 January 2015. [Google Scholar]
- Bom, S.; Gouveia, L.F.; Pinto, P.; Martins, A.M.; Ribeiro, H.M.; Marto, J. A Mathematical Modeling Strategy to Predict the Spreading Behavior on Skin of Sustainable Alternatives to Personal Care Emollients. Colloids Surf. B Biointerfaces 2021, 205, 111865. [Google Scholar] [CrossRef] [PubMed]
- Kharenko, E.A.; Larionova, N.I.; Demina, N.B. Mucoadhesive Drug Delivery Systems (Review). Pharm. Chem. J. 2009, 43, 200–208. [Google Scholar] [CrossRef]
- Varese, A.; Paletta, A.; Ceballos, A.; Palacios, C.A.; Figueroa, J.M.; Dugour, A.V. Iota-Carrageenan Prevents the Replication of SARS-CoV-2 in a Human Respiratory Epithelium Cell Line in Vitro. Front. Virol. 2021, 1, 746824. [Google Scholar] [CrossRef]
- Grassauer, A.; Weinmuellner, R.; Meier, C.; Pretsch, A.; Prieschl-Grassauer, E.; Unger, H. Iota-Carrageenan Is a Potent Inhibitor of Rhinovirus Infection. Virol. J. 2008, 5, 107. [Google Scholar] [CrossRef] [PubMed]
| HPMC (%) | pH | Viscosity (cp) | Spreadability (cm) | |
|---|---|---|---|---|
| BF 1 | 1 | 3.9 ± 0.1 | 212 ± 11 | 8.37 ± 0.12 |
| BF 1.2 | 1.2 | 3.8 ± 0.2 | 1080 ± 83 | 7.27 ± 0.06 |
| BF 1.5 | 1.5 | 3.7 ± 0.1 | 2884 ± 11 | 6.43 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accioni, F.; Rassu, G.; Brunetti, A.; Plicanti, E.; Freer, G.; Carta, A.; Giunchedi, P.; Gavini, E. Nasal Emulgel’s Role in Preventing Coronavirus Infection. Pharmaceutics 2025, 17, 795. https://doi.org/10.3390/pharmaceutics17060795
Accioni F, Rassu G, Brunetti A, Plicanti E, Freer G, Carta A, Giunchedi P, Gavini E. Nasal Emulgel’s Role in Preventing Coronavirus Infection. Pharmaceutics. 2025; 17(6):795. https://doi.org/10.3390/pharmaceutics17060795
Chicago/Turabian StyleAccioni, Francesca, Giovanna Rassu, Antonio Brunetti, Erika Plicanti, Giulia Freer, Antonio Carta, Paolo Giunchedi, and Elisabetta Gavini. 2025. "Nasal Emulgel’s Role in Preventing Coronavirus Infection" Pharmaceutics 17, no. 6: 795. https://doi.org/10.3390/pharmaceutics17060795
APA StyleAccioni, F., Rassu, G., Brunetti, A., Plicanti, E., Freer, G., Carta, A., Giunchedi, P., & Gavini, E. (2025). Nasal Emulgel’s Role in Preventing Coronavirus Infection. Pharmaceutics, 17(6), 795. https://doi.org/10.3390/pharmaceutics17060795










