Surface Functionalized Polyhydroxyalkanoate Nanoparticles via SpyTag–SpyCatcher System for Targeted Breast Cancer Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of SpyTag–Lipid Linker Conjugate
2.3. Preparation and Characterization of SpyTag-Decorated PHA NPs
2.4. Quantification of mEGFP-SpyTag on PHA NPs
2.5. Surface Functionalization of PHA NPs via the SpyTag–SpyCatcher System
2.6. Physicochemical Properties of PHA NPs
2.6.1. Size and Zeta Potential Measurement of PHA NPs
2.6.2. Scanning Electron Microscopic Analysis of the PHA NPs
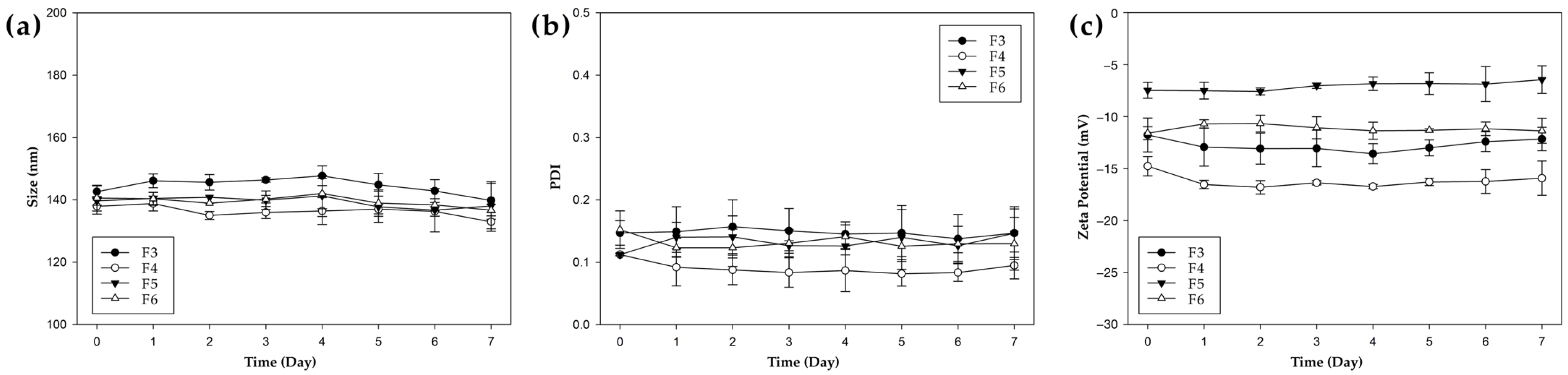
2.6.3. Storage Stability of PHA NPs
2.7. Determination of PTX Content in NPs
2.8. Serum Stability of PHA NPs
2.9. In Vitro Cell Culture
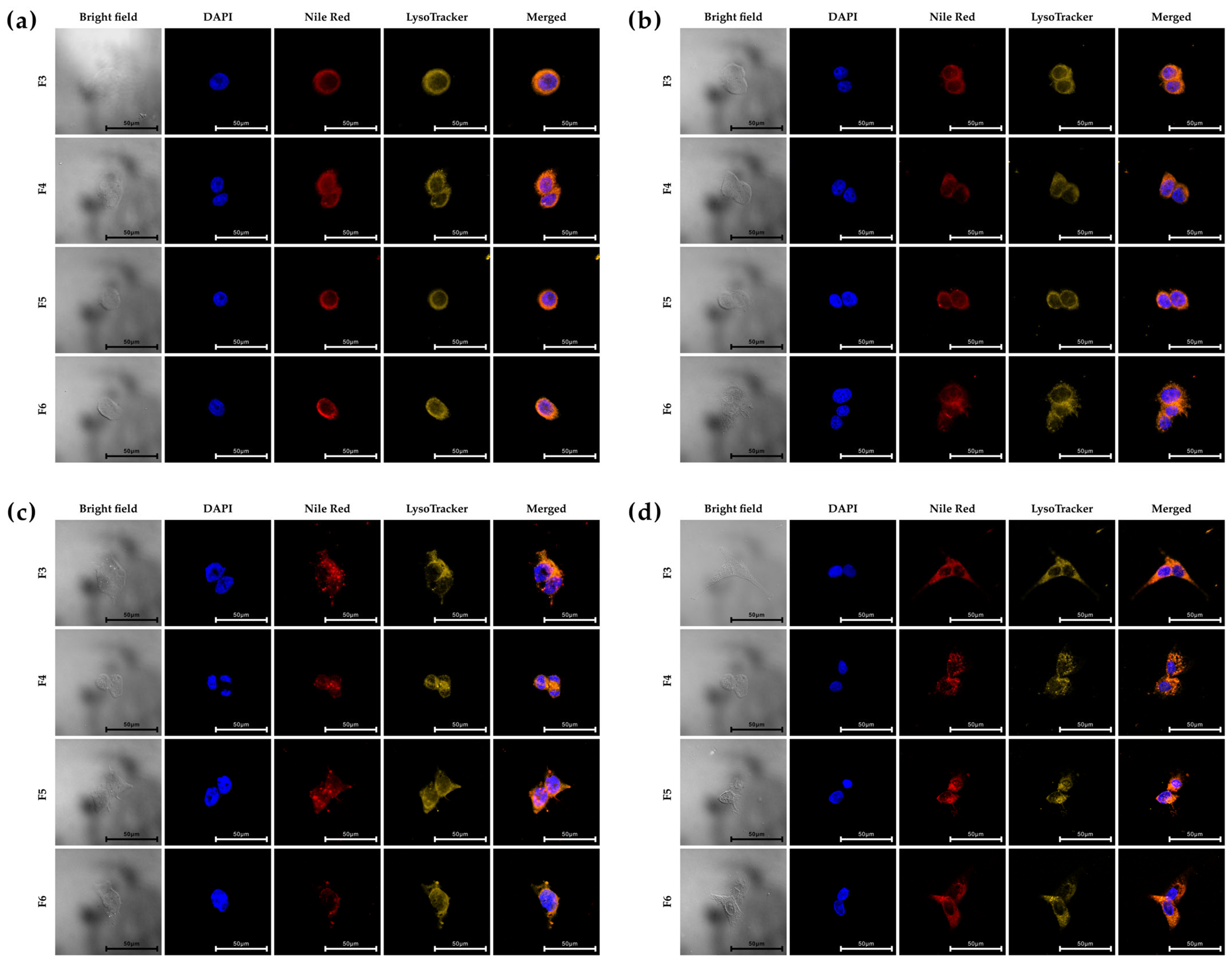
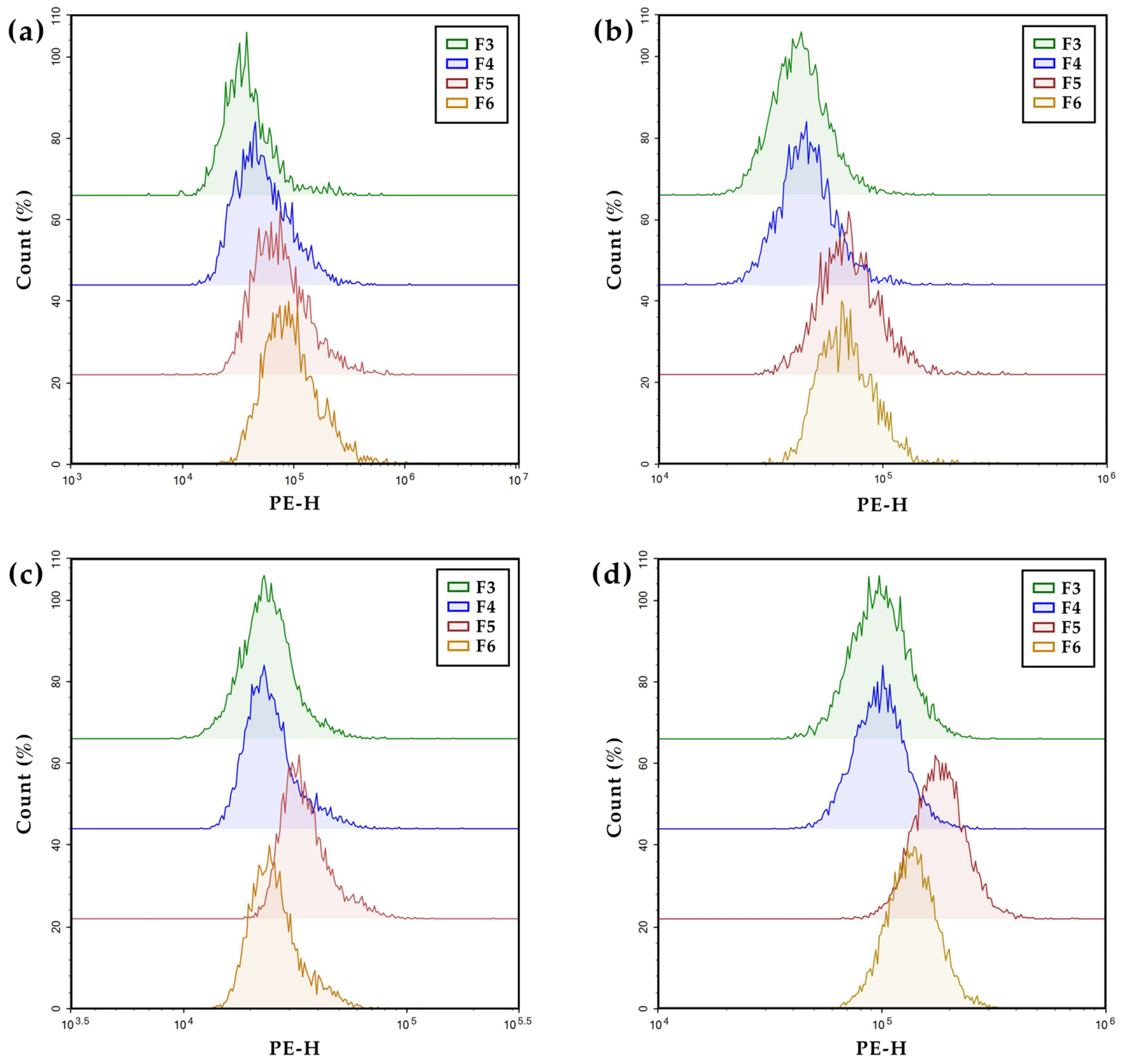
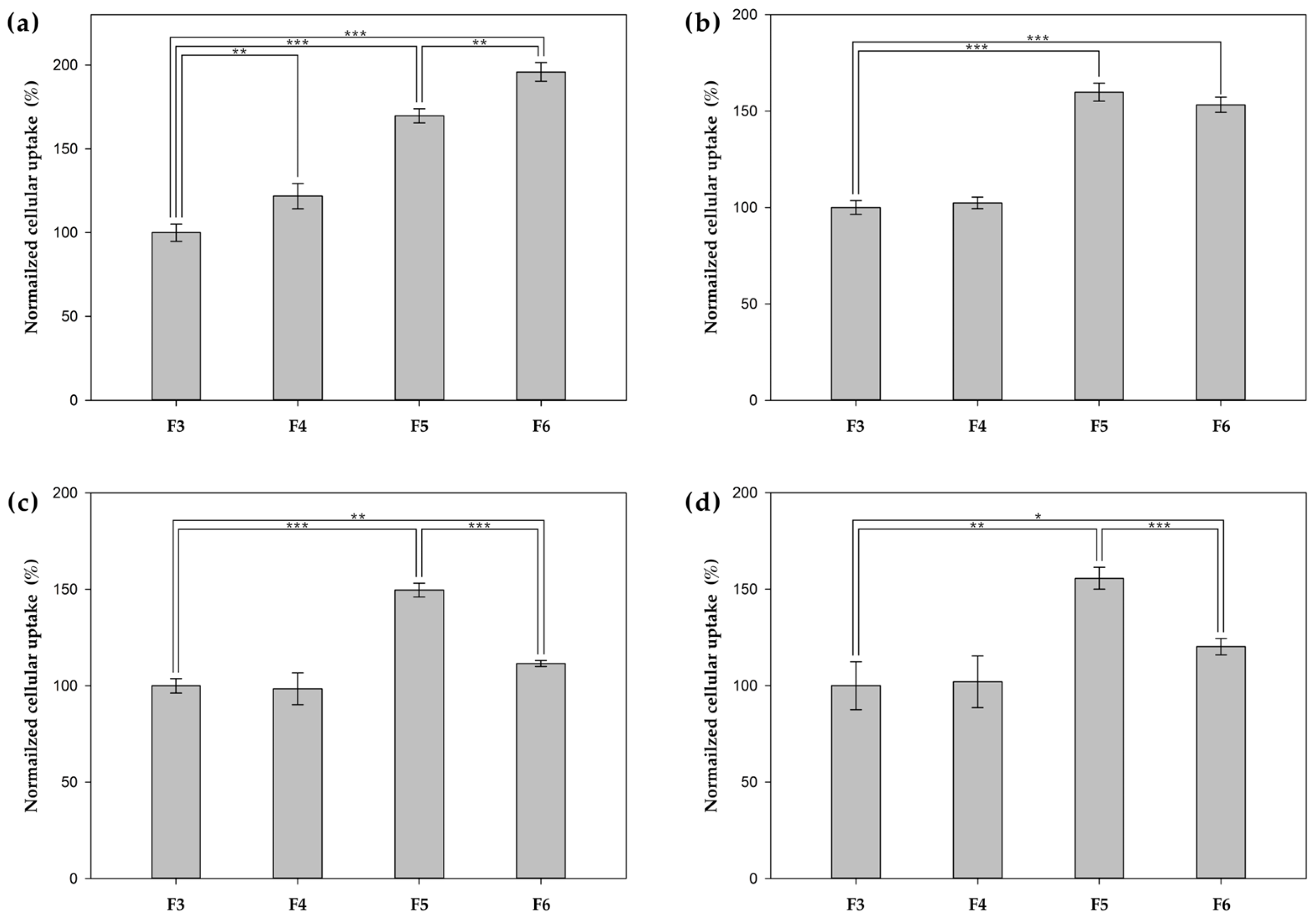
2.10. Cellular Uptake of Surface Functionalized PHA NPs
2.11. Cell Cytotoxicity Assay
2.12. Statistical Analysis
3. Results
3.1. Surface Immobilization Using Spy Tag–Lipid Linker Conjugation
3.2. Physicochemical Properties of NPs
3.3. Physicochemical Stability of NPs
3.4. Serum Stability of PHA NPs
3.5. In Vitro Cellular Uptake
3.6. In Vitro Cytotoxicity Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Čolnik, M.; Knez-Hrnčič, M.; Škerget, M.; Knez, Ž. Biodegradable Polymers, Current Trends of Research and Their Applications, a Review. Chem. Ind. Chem. Eng. Q. 2020, 26, 18. [Google Scholar] [CrossRef]
- Ha, C.-S.; Gardella, J.A. Surface Chemistry of Biodegradable Polymers for Drug Delivery Systems. Chem. Rev. 2005, 105, 4205–4232. [Google Scholar] [CrossRef] [PubMed]
- Asri, N.A.; Sezali, N.A.A.; Ong, H.L.; Pisal, M.H.M.; Lim, Y.H.; Fang, J. Review on Biodegradable Aliphatic Polyesters: Development and Challenges. Macromol. Rapid Commun. 2024, 45, e2400475. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Wang, F.; Hu, X. Biodegradable Polymers: A Promising Solution for Green Energy Devices. Eur. Polym. J. 2024, 204, 112696. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Troy, E.; Tilbury, M.A.; Power, A.M.; Wall, J.G. Nature-Based Biomaterials and Their Application in Biomedicine. Polymers 2021, 13, 3321. [Google Scholar] [CrossRef]
- Redrado, M.; Xiao, Z.; Upitak, K.; Doan, B.; Thomas, C.M.; Gasser, G. Applications of Biodegradable Polymers in the Encapsulation of Anticancer Metal Complexes. Adv. Funct. Mater. 2024, 34, 2401950. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent Advances in Polymeric Drug Delivery Systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges. Polymers 2024, 16, 2536. [Google Scholar] [CrossRef]
- Zhao, K.; Li, D.; Shi, C.; Ma, X.; Rong, G.; Kang, H.; Wang, X.; Sun, B. Biodegradable Polymeric Nanoparticles as the Delivery Carrier for Drug. Curr. Drug Deliv. 2016, 13, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Saito, N. Biodegradable Polymers as Drug Delivery Systems for Bone Regeneration. Pharmaceutics 2020, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Thomas, T.J.; Tajmir-Riahi, H.-A.; Pillai, C.K.S. Biodegradable Polymers for Gene Delivery. Molecules 2019, 24, 3744. [Google Scholar] [CrossRef]
- Hetemi, D.; Pinson, J. Surface Functionalisation of Polymers. Chem. Soc. Rev. 2017, 46, 5701–5713. [Google Scholar] [CrossRef]
- Cano-Cortes, M.V.; Altea-Manzano, P.; Laz-Ruiz, J.A.; Unciti-Broceta, J.D.; Lopez-Delgado, F.J.; Espejo-Roman, J.M.; Diaz-Mochon, J.J.; Sanchez-Martin, R.M. An Effective Polymeric Nanocarrier That Allows for Active Targeting and Selective Drug Delivery in Cell Coculture Systems. Nanoscale 2021, 13, 3500–3511. [Google Scholar] [CrossRef]
- Zhong, Y.; Meng, F.; Deng, C.; Zhong, Z. Ligand-Directed Active Tumor-Targeting Polymeric Nanoparticles for Cancer Chemotherapy. Biomacromolecules 2014, 15, 1955–1969. [Google Scholar] [CrossRef]
- Marcucci, F.; Lefoulon, F. Active Targeting with Particulate Drug Carriers in Tumor Therapy: Fundamentals and Recent Progress. Drug Discov. Today 2004, 9, 219–228. [Google Scholar] [CrossRef]
- Dilliard, S.A.; Siegwart, D.J. Passive, Active and Endogenous Organ-Targeted Lipid and Polymer Nanoparticles for Delivery of Genetic Drugs. Nat. Rev. Mater. 2023, 8, 282–300. [Google Scholar] [CrossRef]
- Kim, J.; Nam, H.Y.; Kim, T.; Kim, P.-H.; Ryu, J.; Yun, C.-O.; Kim, S.W. Active Targeting of RGD-Conjugated Bioreducible Polymer for Delivery of Oncolytic Adenovirus Expressing ShRNA against IL-8 MRNA. Biomaterials 2011, 32, 5158–5166. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing Nanoparticles with Cancer-Targeting Antibodies: A Comparison of Strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface Functionalization—The Way for Advanced Applications of Smart Materials. Coord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.T.; Sadat, S.M.A.; Walliser, M.; Haddadi, A. Targeted Therapeutic Nanoparticles: An Immense Promise to Fight against Cancer. J. Drug Deliv. 2017, 2017, 9090325. [Google Scholar] [CrossRef]
- Steinbach, J.M.; Seo, Y.-E.; Saltzman, W.M. Cell Penetrating Peptide-Modified Poly(lactic-co-glycolic acid) Nanoparticles with Enhanced Cell Internalization. Acta Biomater. 2016, 30, 49–61. [Google Scholar] [CrossRef]
- Park, J.; Mattessich, T.; Jay, S.M.; Agawu, A.; Saltzman, W.M.; Fahmy, T.M. Enhancement of Surface Ligand Display on PLGA Nanoparticles with Amphiphilic Ligand Conjugates. J. Control. Release 2011, 156, 109–115. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, S.Y.; Ku, S.H.; Park, E.J.; Jang, D.-J.; Kim, S.T.; Kim, S.-B. Polyhydroxyalkanoate Decelerates the Release of Paclitaxel from Poly(lactic-co-glycolic acid) Nanoparticles. Pharmaceutics 2022, 14, 1618. [Google Scholar] [CrossRef]
- Quevedo, P.D.; Behnke, T.; Resch-Genger, U. Streptavidin Conjugation and Quantification—A Method Evaluation for Nanoparticles. Anal. Bioanal. Chem. 2016, 408, 4133–4149. [Google Scholar] [CrossRef]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide Tag Forming a Rapid Covalent Bond to a Protein, through Engineering a Bacterial Adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef]
- Mu, L.; Feng, S.S. A Novel Controlled Release Formulation for the Anticancer Drug Paclitaxel (Taxol®): PLGA Nanoparticles Containing Vitamin E TPGS. J. Control. Release 2003, 86, 33–48. [Google Scholar] [CrossRef]
- Ruozi, B.; Belletti, D.; Sharma, H.S.; Sharma, A.; Muresanu, D.F.; Mössler, H.; Forni, F.; Vandelli, M.A.; Tosi, G. PLGA Nanoparticles Loaded Cerebrolysin: Studies on Their Preparation and Investigation of the Effect of Storage and Serum Stability with Reference to Traumatic Brain Injury. Mol. Neurobiol. 2015, 52, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Albarki, M.A.; Donovan, M.D. Uptake of Cationic PAMAM-PLGA Nanoparticles by the Nasal Mucosa. Sci. Pharm. 2022, 90, 72. [Google Scholar] [CrossRef]
- Kim, S.Y.; Guk, D.; Jeong, Y.; Kim, E.; Kim, H.; Kim, S.T. Engineered Hybrid Vesicles and Cellular Internalization in Mammary Cancer Cells. Pharmaceutics 2024, 16, 440. [Google Scholar] [CrossRef]
- Qiao, L.; Hu, S.; Huang, K.; Su, T.; Li, Z.; Vandergriff, A.; Cores, J.; Dinh, P.-U.; Allen, T.; Shen, D.; et al. Tumor Cell-Derived Exosomes Home to Their Cells of Origin and Can Be Used as Trojan Horses to Deliver Cancer Drugs. Theranostics 2020, 10, 3474–3487. [Google Scholar] [CrossRef]
- Lee, S.S.; Lee, Y.B.; Oh, I.J. Cellular Uptake of Poly(DL-lactide-co-glycolide) Nanoparticles: Effects of Drugs and Surface Characteristics of Nanoparticles. J. Pharm. Investig. 2015, 45, 659–667. [Google Scholar] [CrossRef]
- Xu, P.; Gullotti, E.; Tong, L.; Highley, C.B.; Errabelli, D.R.; Hasan, T.; Cheng, J.-X.; Kohane, D.S.; Yeo, Y. Intracellular Drug Delivery by Poly(lactic-co-glycolic acid) Nanoparticles, Revisited. Mol. Pharm. 2009, 6, 190–201. [Google Scholar] [CrossRef]
- Kim, H.; Choi, H.; Bae, Y.; Kang, S. Development of Target-tunable P22 VLP-based Delivery Nanoplatforms Using Bacterial Superglue. Biotechnol. Bioeng. 2019, 116, 2843–2851. [Google Scholar] [CrossRef]
- Danışman-Kalındemirtaş, F.; Karïper, İ.A.; Hepokur, C.; Erdem-Kuruca, S. Selective Cytotoxicity of Paclitaxel Bonded Silver Nanoparticle on Different Cancer Cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102265. [Google Scholar] [CrossRef]
- Cabeza, L.; El-Hammadi, M.M.; Ortiz, R.; Cayero-Otero, M.D.; Jiménez-López, J.; Perazzoli, G.; Martin-Banderas, L.; Baeyens, J.M.; Melguizo, C.; Prados, J. Evaluation of Poly (lactic-co-glycolic acid) Nanoparticles to Improve the Therapeutic Efficacy of Paclitaxel in Breast Cancer. BioImpacts 2022, 12, 515–531. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and Chemical Stability of Drug Nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef]
- Ziegler, A.; Nervi, P.; Dürrenberger, M.; Seelig, J. The Cationic Cell-Penetrating Peptide CPPTAT Derived from the HIV-1 Protein TAT Is Rapidly Transported into Living Fibroblasts: Optical, Biophysical, and Metabolic Evidence. Biochemistry 2005, 44, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, I.M.; Wadia, J.S.; Dowdy, S.F. Cationic TAT Peptide Transduction Domain Enters Cells by Macropinocytosis. J. Control. Release 2005, 102, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.; Jun, H.; Kim, E.; Min, D.; Kim, H.; Kang, S. Developing Porous Protein Cage Nanoparticles as Cargo-Loadable and Ligand-Displayable Modular Delivery Nanoplatforms. ACS Appl. Mater. Interfaces 2024, 16, 58464–58476. [Google Scholar] [CrossRef]
- Gall, V.A.; Philips, A.V.; Qiao, N.; Clise-Dwyer, K.; Perakis, A.A.; Zhang, M.; Clifton, G.T.; Sukhumalchandra, P.; Ma, Q.; Reddy, S.M.; et al. Trastuzumab Increases HER2 Uptake and Cross-Presentation by Dendritic Cells. Cancer Res. 2017, 77, 5374–5383. [Google Scholar] [CrossRef]
- Weinberg, F.; Peckys, D.B.; Jonge, N. de EGFR Expression in HER2-Driven Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9008. [Google Scholar] [CrossRef]
- Xu, T.; Ding, H.; Vorobyeva, A.; Oroujeni, M.; Orlova, A.; Tolmachev, V.; Gräslund, T. Drug Conjugates Based on a Monovalent Affibody Targeting Vector Can Efficiently Eradicate HER2 Positive Human Tumors in an Experimental Mouse Model. Cancers 2020, 13, 85. [Google Scholar] [CrossRef]
- Westerberg, C.; Borras, A.M.; Ståhl, S.; Löfblom, J. Affibody-Based HER2 Prodrug Shows Conditional Cytotoxic Effect on HER2-Positive Cancer Cells. Biochem. Biophys. Res. Commun. 2025, 758, 151660. [Google Scholar] [CrossRef]
- Damiani, I.; Castiglioni, S.; Sochaj-Gregorczyk, A.; Bonacina, F.; Colombo, I.; Rusconi, V.; Otlewski, J.; Corsini, A.; Bellosta, S. Purification and In Vitro Evaluation of an Anti-HER2 Affibody-Monomethyl Auristatin E Conjugate in HER2-Positive Cancer Cells. Biology 2021, 10, 758. [Google Scholar] [CrossRef]
- Moradian, C.; Rahbarizadeh, F. PE38-Based Gene Therapy of HER2-Positive Breast Cancer Stem Cells via VHH-Redirected Polyamidoamine Dendrimers. Sci. Rep. 2021, 11, 15517. [Google Scholar] [CrossRef]
- Cruz, E.; Kayser, V. Synthesis and Enhanced Cellular Uptake In Vitro of Anti-HER2 Multifunctional Gold Nanoparticles. Cancers 2019, 11, 870. [Google Scholar] [CrossRef]
- Brooks, H.; Lebleu, B.; Vivès, E. Tat Peptide-Mediated Cellular Delivery: Back to Basics. Adv. Drug Deliv. Rev. 2005, 57, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Derakhshankhah, H.; Jafari, S. Cell Penetrating Peptides: A Concise Review with Emphasis on Biomedical Applications. Biomed. Pharmacother. 2018, 108, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Wang, H.; Liu, Y.; Hui, Y.; Sun, Q.; Seth, A.; Wibowo, D.; Chen, D.; Zhao, C.-X. Microfluidic Self-Assembly of a Combinatorial Library of Single- and Dual-Ligand Liposomes for in Vitro and in Vivo Tumor Targeting. Eur. J. Pharm. Biopharm. 2018, 130, 1–10. [Google Scholar] [CrossRef]
- Zhang, Q.; Reinhard, B.M. Ligand Density and Nanoparticle Clustering Cooperate in the Multivalent Amplification of Epidermal Growth Factor Receptor Activation. ACS Nano 2018, 12, 10473–10485. [Google Scholar] [CrossRef]

| PHA NPs | Size (nm) | Polydiversity Index (PDI) | Zeta Potential Value (ζ, mV) | |
|---|---|---|---|---|
| F1 | Blank NP | 120.07 ± 0.25 | 0.06 ± 0.01 | −16.37 ± 0.59 |
| F2 | NP with PTX | 134.60 ± 1.67 | 0.07 ± 0.03 | −17.13 ± 1.63 |
| F3 | GFP-SpyTag decorated NP with PTX | 142.60 ± 1.87 | 0.15 ± 0.02 | −11.77 ± 0.42 |
| F4 | HER2 Affibody functionalized * NP with PTX | 137.90 ± 2.51 | 0.11 ± 0.01 | −14.77 ± 0.93 |
| F5 | TAT functionalized * NP with PTX | 139.67 ± 1.39 | 0.11 ± 0.01 | −7.47 ± 0.77 |
| F6 | HER2 Affibody & TAT ** functionalized * NP with PTX | 140.53 ± 4.13 | 0.15 ± 0.03 | −11.60 ± 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, J.Y.; Sung, M.K.; Jang, S.; Kim, H.; Jeong, Y.; Jang, D.-J.; Lee, S.-J.; Kim, S.-B.; Kim, S.T. Surface Functionalized Polyhydroxyalkanoate Nanoparticles via SpyTag–SpyCatcher System for Targeted Breast Cancer Treatment. Pharmaceutics 2025, 17, 721. https://doi.org/10.3390/pharmaceutics17060721
Heo JY, Sung MK, Jang S, Kim H, Jeong Y, Jang D-J, Lee S-J, Kim S-B, Kim ST. Surface Functionalized Polyhydroxyalkanoate Nanoparticles via SpyTag–SpyCatcher System for Targeted Breast Cancer Treatment. Pharmaceutics. 2025; 17(6):721. https://doi.org/10.3390/pharmaceutics17060721
Chicago/Turabian StyleHeo, Jin Young, Min Kyung Sung, Seonhye Jang, Hansol Kim, Youngdo Jeong, Dong-Jin Jang, Sang-Jae Lee, Seong-Bo Kim, and Sung Tae Kim. 2025. "Surface Functionalized Polyhydroxyalkanoate Nanoparticles via SpyTag–SpyCatcher System for Targeted Breast Cancer Treatment" Pharmaceutics 17, no. 6: 721. https://doi.org/10.3390/pharmaceutics17060721
APA StyleHeo, J. Y., Sung, M. K., Jang, S., Kim, H., Jeong, Y., Jang, D.-J., Lee, S.-J., Kim, S.-B., & Kim, S. T. (2025). Surface Functionalized Polyhydroxyalkanoate Nanoparticles via SpyTag–SpyCatcher System for Targeted Breast Cancer Treatment. Pharmaceutics, 17(6), 721. https://doi.org/10.3390/pharmaceutics17060721









