Ivy Leaf Dry Extract EA 575® Is a Potent Immunomodulator Acting on Dendritic Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of EA 575® by HPLC Analysis
2.2. Cell Cultures
2.3. Cytotoxicity Assays
2.4. Autophagy and Reactive Oxygen Species Assays
2.5. Alloreactivity Assay
2.6. T Helper Polarization Assay
2.7. Induction of T Regulatory and Exhausted Cells
2.8. Cytokine Assays
2.9. Flow Cytometry
- Bio-Rad (Hercules, CA, USA): IgG1-PE (MCA928PE), IgG1-FITC (MCA928F).
- BioLegend (Basel, Switzerland): anti-CD1a-PerCP/Cy5.5 (HI149), HLA-DR-APC/Cy7 (L243), IL-4-PerCP/Cy5.5 and -PE (MP4-25D2), ILT-4-APC (42D1), CD25-PE (BC96), CD25-PerCP/Cy5.5 (M-A251), IL-10-APC and -PE (JES5-16E3), TGF-β-APC (TW4-6H10), IL-17A-Alexa Fluor 488 (BL168), IFN-γ-APC and -FITC (4S.B3), CD83-FITC (HB15e), PD-L1-PE (29E.2A3), IgG1-PerCP/Cy5.5 (HTK888).
- R&D Systems (Minneapolis, MN, USA): CD3-FITC and -PE, CD4-FITC and -APC (11830), IL-12p40/p70-PE (C11.5), TGF-β-PE (9016), IDO-1-APC (700838).
- Miltenyi Biotec (Gladbach, Germany): CD14-FITC (TUK4), IL-1β-PE (REA1172).
- Thermo Fisher Scientific (Dreieich, Germany): CD86-PE (IT2.2), streptavidin-PerCP and -APC, ILT3-PE (ZM4.1), IgG1-APC (MA5-18093), IL-17A-APC (eBio17B7).
- BD Biosciences (San Diego, CA, USA): CD40-APC (5C3), CD69-PE, FoxP3-PerCP/Cy5.5 and -Alexa Fluor 488 (236A/E7), granzyme B-APC, CD33-APC, IL-35-PerCP, PD-1-FITC.
- Elabscience (Houston, TX, USA): CD8-PerCP/Cy5.5 (HIT8a).
2.10. Statistical Analysis
3. Results
3.1. Cytotoxicity of EA 575®
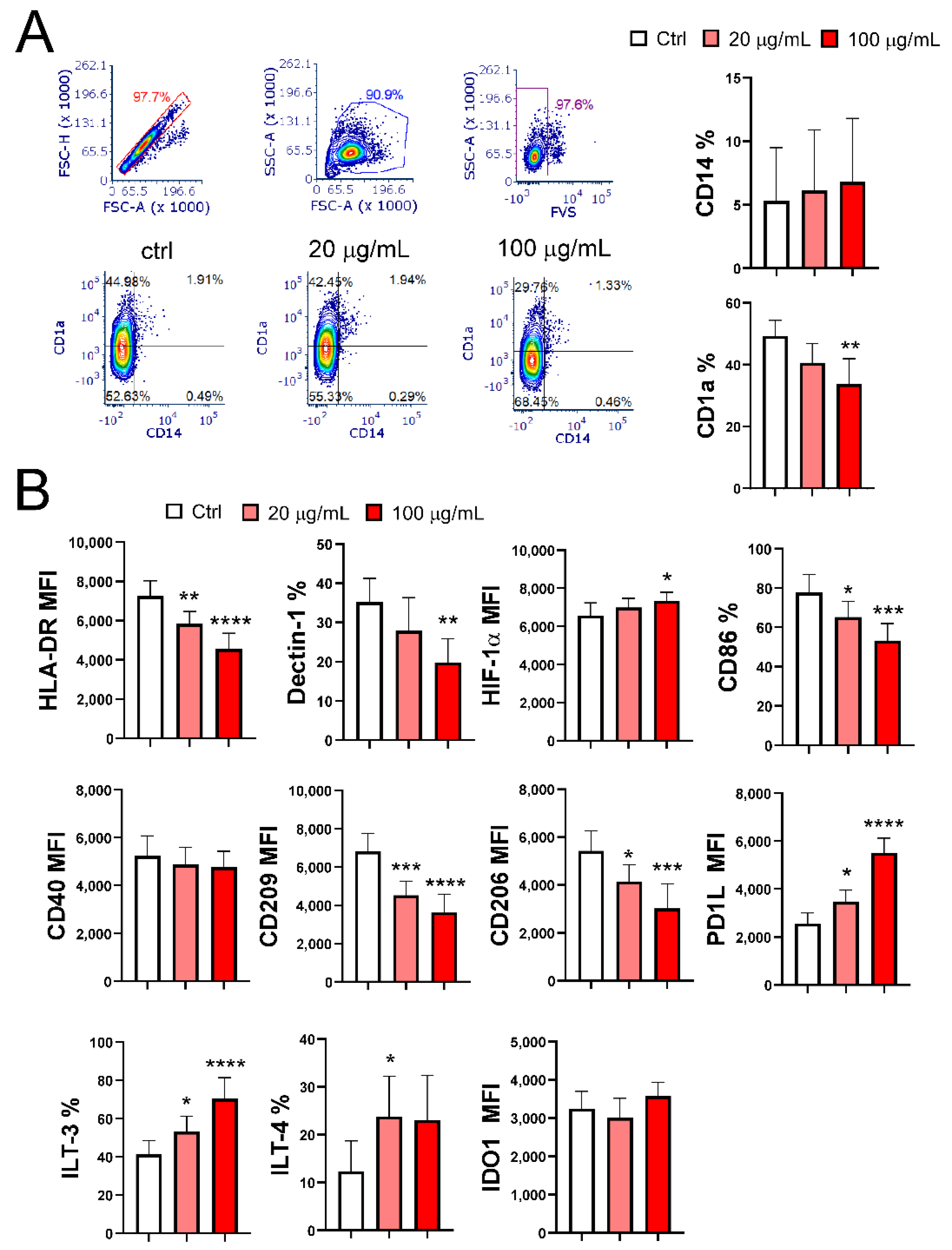
3.2. EA 575® Impairs the Differentiation of MoDCs
3.3. EA 575® Impairs the Phenotypic Maturation of MoDCs
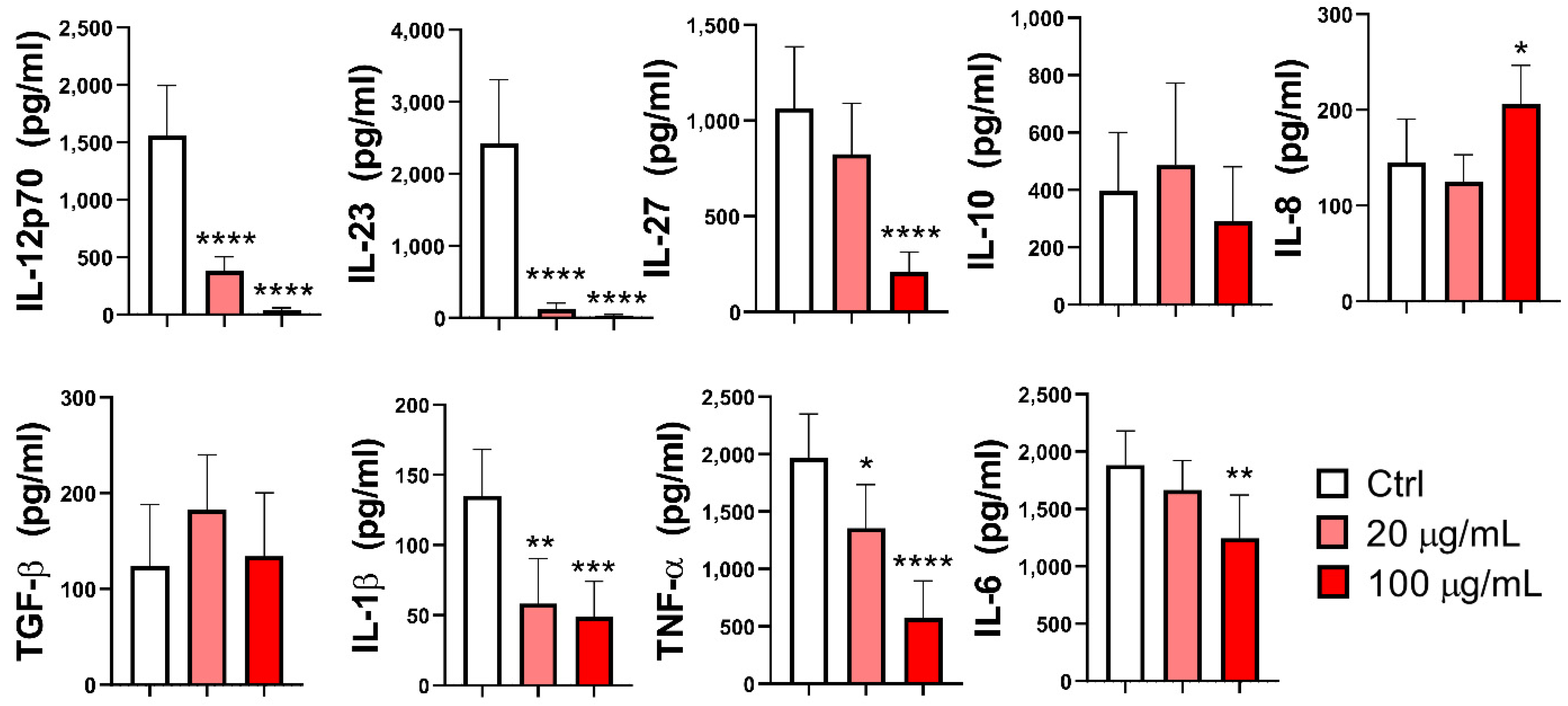
3.4. Effect of EA 575® on the Production of Cytokines by MoDCs
3.5. Effect of EA 575®-Treated MoDCs on the Proliferation of Alloreactive T Cells
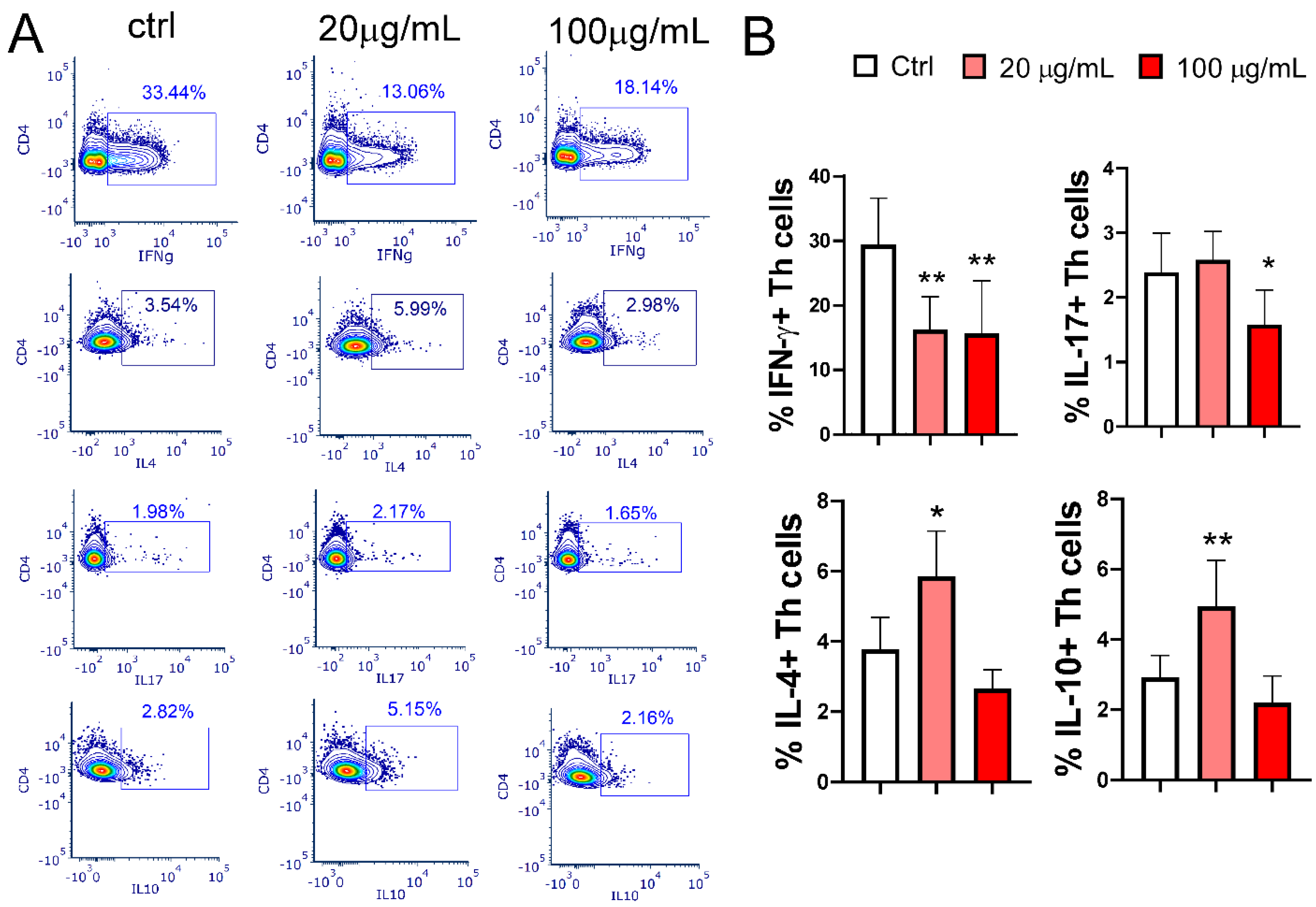
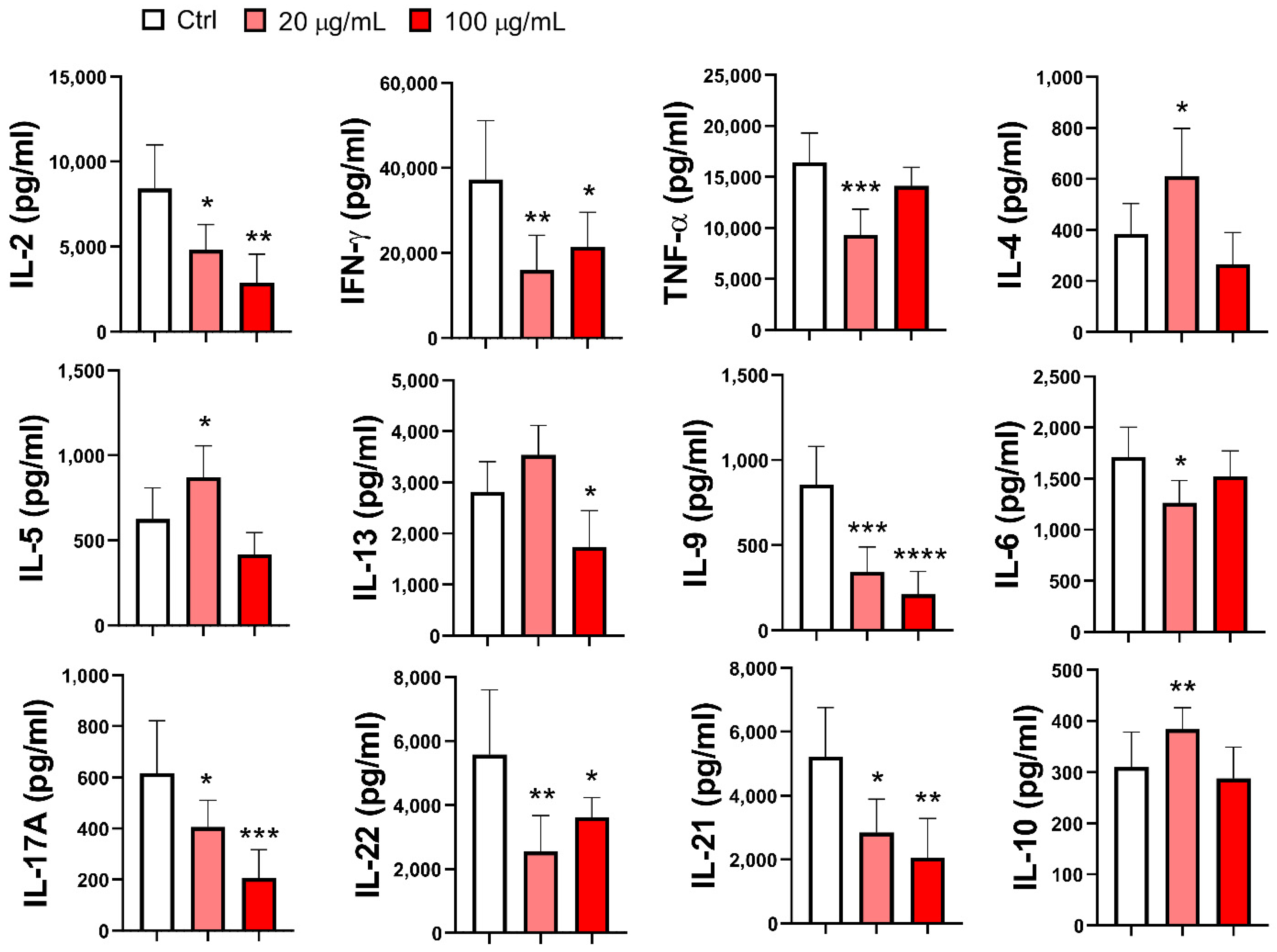
3.6. Effect of EA 575®-Treated MoDCs on Th Polarization
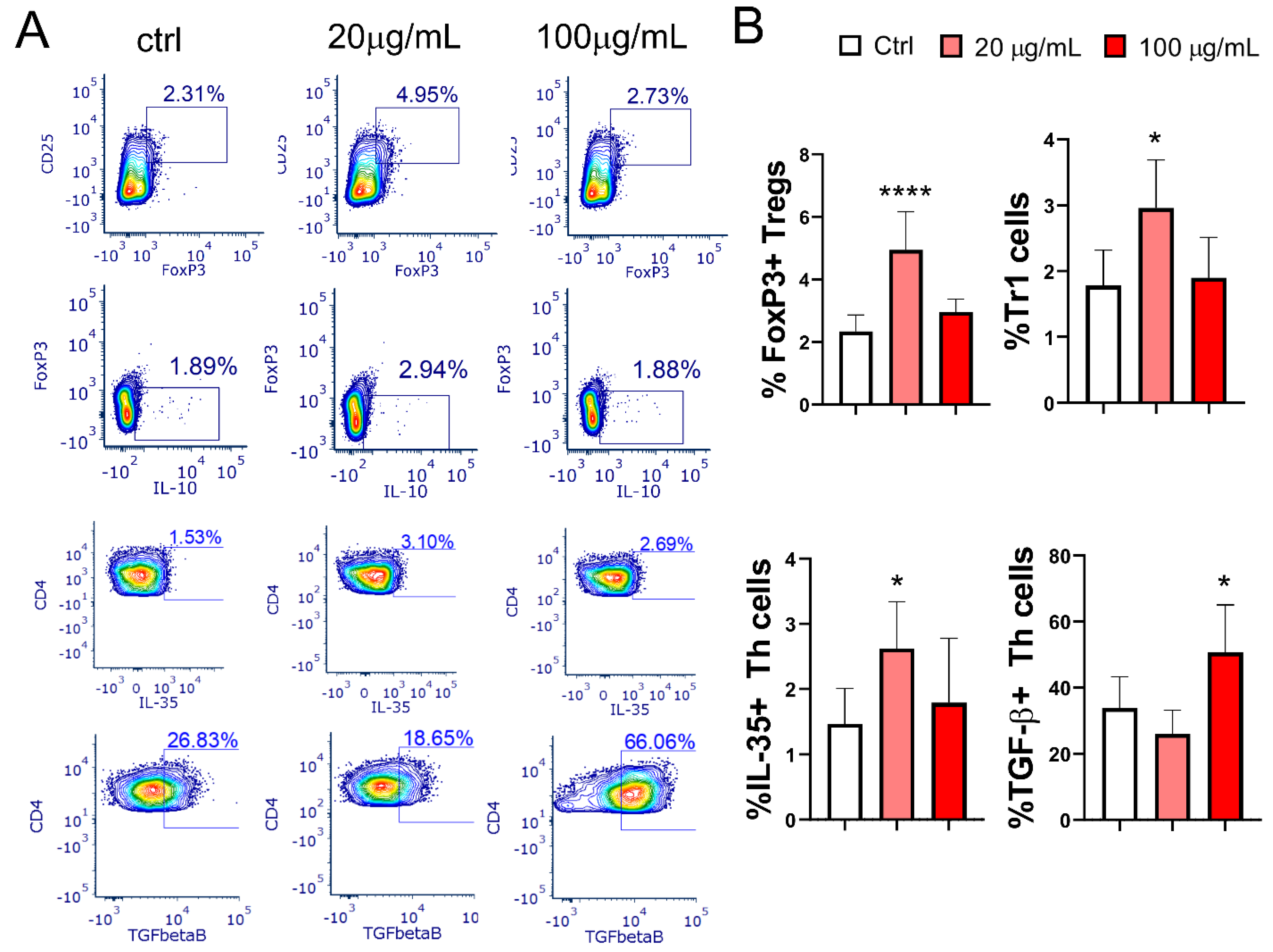
3.7. Effect of EA 575®-Treated mMoDCs on the Development of Treg Populations
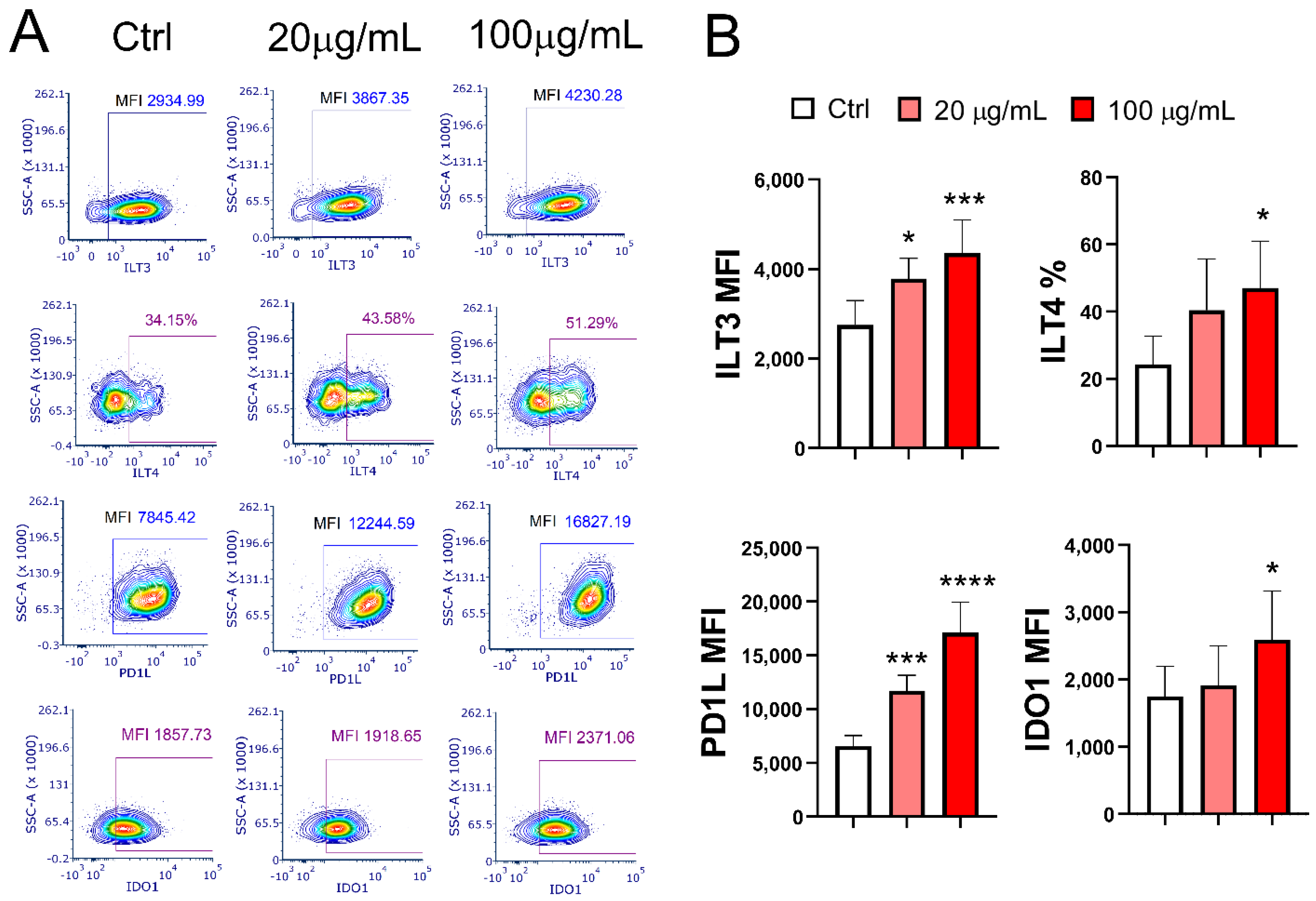
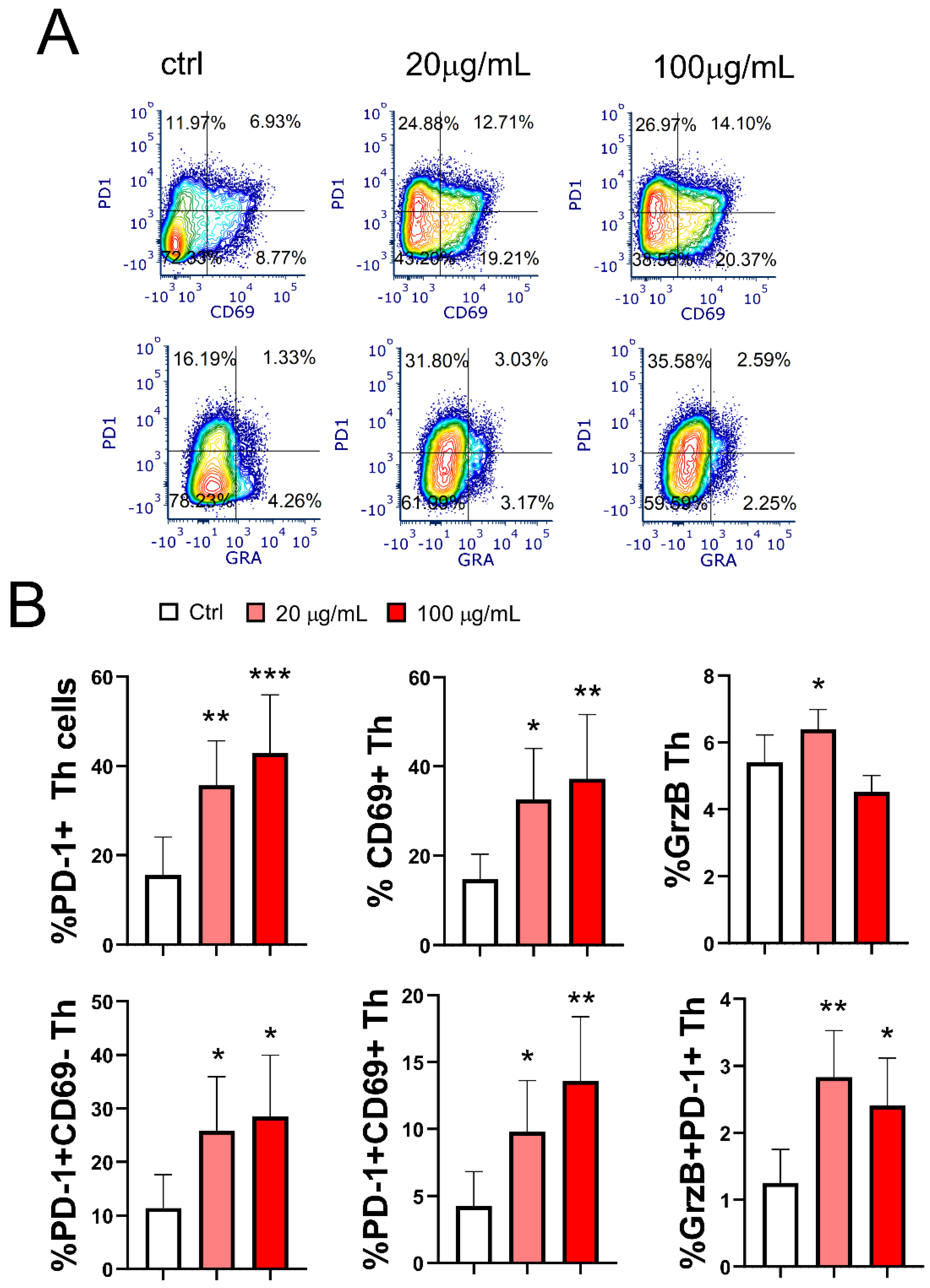
3.8. Effect of EA 575®-Treated mMoDCs on the Development of Exhausted PD1+CD4+ Cells and Cytotoxic CD4+ T Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fazio, S.; Pouso, J.; Dolinsky, D.; Fernandez, A.; Hernandez, M.; Clavier, G.; Hecker, M. Tolerance, safety and efficacy of Hedera helix extract in inflammatory bronchial diseases under clinical practice conditions: A prospective, open, multicentre postmarketing study in 9657 patients. Phytomedicine 2009, 16, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Cwientzek, U.; Ottillinger, B.; Arenberger, P. Acute bronchitis therapy with ivy leaves extracts in a two-arm study. A double-blind, randomised study vs. an other ivy leaves extract. Phytomedicine 2011, 18, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.; Kehr, M.S.; Giannetti, B.M.; Bulitta, M.; Staiger, C. A randomized, controlled, double-blind, multi-center trial to evaluate the efficacy and safety of a liquid containing ivy leaves dry extract (EA 575((R))) vs. placebo in the treatment of adults with acute cough. Pharmazie 2016, 71, 504–509. [Google Scholar] [PubMed]
- Schaefer, A.; Ludwig, F.; Giannetti, B.M.; Bulitta, M.; Wacker, A. Efficacy of two dosing schemes of a liquid containing ivy leaves dry extract EA 575 versus placebo in the treatment of acute bronchitis in adults. ERJ Open Res. 2019, 5, 00019–2019. [Google Scholar] [CrossRef]
- Lang, C.; Rottger-Luer, P.; Staiger, C. A Valuable Option for the Treatment of Respiratory Diseases: Review on the Clinical Evidence of the Ivy Leaves Dry Extract EA 575(R). Planta Med. 2015, 81, 968–974. [Google Scholar] [CrossRef]
- Seifert, G.; Upstone, L.; Watling, C.P.; Vogelberg, C. Ivy leaf dry extract EA 575 for the treatment of acute and chronic cough in pediatric patients: Review and expert survey. Curr. Med. Res. Opin. 2023, 39, 1407–1417. [Google Scholar] [CrossRef]
- Völp, A.; Schmitz, J.; Bulitta, M.; Raskopf, E.; Acikel, C.; Mosges, R. Ivy leaves extract EA 575 in the treatment of cough during acute respiratory tract infections: Meta-analysis of double-blind, randomized, placebo-controlled trials. Sci. Rep. 2022, 12, 20041. [Google Scholar] [CrossRef]
- Stauss-Grabo, M.; Atiye, S.; Warnke, A.; Wedemeyer, R.S.; Donath, F.; Blume, H.H. Observational study on the tolerability and safety of film-coated tablets containing ivy extract (Prospan(R) Cough Tablets) in the treatment of colds accompanied by coughing. Phytomedicine 2011, 18, 433–436. [Google Scholar] [CrossRef]
- Hocaoglu, A.B.; Karaman, O.; Erge, D.O.; Erbil, G.; Yilmaz, O.; Kivcak, B.; Bagriyanik, H.A.; Uzuner, N. Effect of Hedera helix on lung histopathology in chronic asthma. Iran. J. Allergy Asthma Immunol. 2012, 11, 316–323. [Google Scholar]
- Suleyman, H.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Acute and chronic antiinflammatory profile of the ivy plant, Hedera helix, in rats. Phytomedicine 2003, 10, 370–374. [Google Scholar] [CrossRef]
- Shokry, A.A.; El-Shiekh, R.A.; Kamel, G.; Bakr, A.F.; Ramadan, A. Bioactive phenolics fraction of Hedera helix L. (Common Ivy Leaf) standardized extract ameliorates LPS-induced acute lung injury in the mouse model through the inhibition of proinflammatory cytokines and oxidative stress. Heliyon 2022, 8, e09477. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Michels, J.; Runkel, F.; Gokorsch, S.; Häberlein, H. Ivy leaves dry extract EA 575(R) decreases LPS-induced IL-6 release from murine macrophages. Pharmazie 2016, 71, 158–161. [Google Scholar]
- Schulte-Michels, J.; Keksel, C.; Häberlein, H.; Franken, S. Anti-inflammatory effects of ivy leaves dry extract: Influence on transcriptional activity of NF-kappaB. Inflammopharmacology 2019, 27, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Meurer, F.; Häberlein, H.; Franken, S. Ivy Leaf Dry Extract EA 575((R)) Has an Inhibitory Effect on the Signalling Cascade of Adenosine Receptor A(2B). Int. J. Mol. Sci. 2023, 24, 12373. [Google Scholar] [CrossRef]
- Hänsel, R.; Keller, K.; Rimpler, H.; Schneider, G. Drogen EO; Springer: Berlin, Germany, 1993. [Google Scholar]
- Lutsenko, Y.; Bylka, W.; Matlawska, I.; Darmohray, R. Hedera helix as a medicinal plant. Herba Pol. 2010, 56, 83–96. [Google Scholar]
- Passos, F.R.S.; Araujo-Filho, H.G.; Monteiro, B.S.; Shanmugam, S.; Araujo, A.A.S.; Almeida, J.; Thangaraj, P.; Junior, L.J.Q.; Quintans, J.S.S. Anti-inflammatory and modulatory effects of steroidal saponins and sapogenins on cytokines: A review of pre-clinical research. Phytomedicine 2022, 96, 153842. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Mamun, M.A.A.; Rakib, A.; Mandal, M.; Kumar, S.; Singla, B.; Singh, U.P. Polyphenols: Role in Modulating Immune Function and Obesity. Biomolecules 2024, 14, 221. [Google Scholar] [CrossRef]
- Focaccetti, C.; Izzi, V.; Benvenuto, M.; Fazi, S.; Ciuffa, S.; Giganti, M.G.; Potenza, V.; Manzari, V.; Modesti, A.; Bei, R. Polyphenols as Immunomodulatory Compounds in the Tumor Microenvironment: Friends or Foes? Int. J. Mol. Sci. 2019, 20, 1714. [Google Scholar] [CrossRef]
- Lasso, P.; Gomez-Cadena, A.; Uruena, C.; Donda, A.; Martinez-Usatorre, A.; Barreto, A.; Romero, P.; Fiorentino, S. Prophylactic vs. Therapeutic Treatment with P2Et Polyphenol-Rich Extract Has Opposite Effects on Tumor Growth. Front. Oncol. 2018, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Rajput, Z.I.; Hu, S.H.; Xiao, C.W.; Arijo, A.G. Adjuvant effects of saponins on animal immune responses. J. Zhejiang Univ. Sci. B 2007, 8, 153–161. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.T.G.; Da Silva Baldivia, D.; de Castro, D.T.H.; Dos Santos, H.F.; Dos Santos, C.M.; Oliveira, A.S.; Alfredo, T.M.; Vilharva, K.N.; de Picoli Souza, K.; Dos Santos, E.L. The immunoregulatory function of polyphenols: Implications in cancer immunity. J. Nutr. Biochem. 2020, 85, 108428. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef]
- Randolph, G.J.; Inaba, K.; Robbiani, D.F.; Steinman, R.M.; Muller, W.A. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 1999, 11, 753–761. [Google Scholar] [CrossRef]
- Leon, B.; Lopez-Bravo, M.; Ardavin, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 2007, 26, 519–531. [Google Scholar] [CrossRef]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Greunke, C.; Hage-Hülsmann, A.; Sorkalla, T.; Keksel, N.; Häberlein, F.; Häberlein, H. A systematic study on the influence of the main ingredients of an ivy leaves dry extract on the β2-adrenergic responsiveness of human airway smooth muscle cells. Pulm. Pharmacol. Ther. 2015, 31, 92–98. [Google Scholar] [CrossRef][Green Version]
- Sierocinski, E.; Holzinger, F.; Chenot, J.F. Ivy leaf (Hedera helix) for acute upper respiratory tract infections: An updated systematic review. Eur. J. Clin. Pharmacol. 2021, 77, 1113–1122. [Google Scholar] [CrossRef]
- Yenigün, V.B.; Kocyigit, A.; Kanımdan, E.; Durmus, E.; Koktasoglu, F. Hedera helix (Wall Ivy) leaf ethanol extract shows cytotoxic and apoptotic effects in glioblastoma cells by generating reactive oxygen species. Acta Med. 2023, 54, 295–303. [Google Scholar] [CrossRef]
- Liu, B.X.; Zhou, J.Y.; Li, Y.; Zou, X.; Wu, J.; Gu, J.F.; Yuan, J.R.; Zhao, B.J.; Feng, L.; Jia, X.B.; et al. Hederagenin from the leaves of ivy (Hedera helix L.) induces apoptosis in human LoVo colon cells through the mitochondrial pathway. BMC Complement. Altern. Med. 2014, 14, 412. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Baek, S.; Shin, D.; Lee, J.; Roh, J.L. Hederagenin Induces Apoptosis in Cisplatin-Resistant Head and Neck Cancer Cells by Inhibiting the Nrf2-ARE Antioxidant Pathway. Oxid. Med. Cell Longev. 2017, 2017, 5498908. [Google Scholar] [CrossRef]
- Gulcin, I.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Antioxidant activity of saponins isolated from ivy: Alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F. Planta Med. 2004, 70, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Mba Gachou, C.; Laget, M.; Guiraud-Dauriac, H.; De Meo, M.; Elias, R.; Dumenil, G. The protective activity of alpha-hederine against H2O2 genotoxicity in HepG2 cells by alkaline comet assay. Mutat. Res. 1999, 445, 9–20. [Google Scholar] [CrossRef]
- Cabeza-Cabrerizo, M.; Cardoso, A.; Minutti, C.M.; Pereira da Costa, M.; Reis e Sousa, C. Dendritic Cells Revisited. Annu. Rev. Immunol. 2021, 39, 131–166. [Google Scholar] [CrossRef]
- Gieseler, R.; Heise, D.; Soruri, A.; Schwartz, P.; Peters, J.H. In-vitro differentiation of mature dendritic cells from human blood monocytes. Dev. Immunol. 1998, 6, 25–39. [Google Scholar] [CrossRef]
- Takenaka, M.C.; Quintana, F.J. Tolerogenic dendritic cells. Semin. Immunopathol. 2017, 39, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Han, T.H.; Jin, P.; Ren, J.; Slezak, S.; Marincola, F.M.; Stroncek, D.F. Evaluation of 3 clinical dendritic cell maturation protocols containing lipopolysaccharide and interferon-gamma. J. Immunother. 2009, 32, 399–407. [Google Scholar] [CrossRef]
- Gee, K.; Guzzo, C.; Che Mat, N.F.; Ma, W.; Kumar, A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm. Allergy Drug Targets 2009, 8, 40–52. [Google Scholar] [CrossRef]
- Tait Wojno, E.D.; Hunter, C.A.; Stumhofer, J.S. The Immunobiology of the Interleukin-12 Family: Room for Discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, J. Properties of immature and mature dendritic cells: Phenotype, morphology, phagocytosis, and migration. RSC Adv. 2019, 9, 11230–11238. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Li, Z.; Zhang, X.; Deng, R.; Ma, Y. Inhibition of Dectin-1 on Dendritic Cells Prevents Maturation and Prolongs Murine Islet Allograft Survival. J. Inflamm. Res. 2021, 14, 63–73. [Google Scholar] [CrossRef]
- van der Zande, H.J.P.; Nitsche, D.; Schlautmann, L.; Guigas, B.; Burgdorf, S. The Mannose Receptor: From Endocytic Receptor and Biomarker to Regulator of (Meta)Inflammation. Front. Immunol. 2021, 12, 765034. [Google Scholar] [CrossRef] [PubMed]
- Relloso, M.; Puig-Kroger, A.; Pello, O.M.; Rodriguez-Fernandez, J.L.; de la Rosa, G.; Longo, N.; Navarro, J.; Munoz-Fernandez, M.A.; Sanchez-Mateos, P.; Corbi, A.L. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J. Immunol. 2002, 168, 2634–2643. [Google Scholar] [CrossRef]
- Labiod, N.; Luczkowiak, J.; Tapia, M.M.; Lasala, F.; Delgado, R. The role of DC-SIGN as a trans-receptor in infection by MERS-CoV. Front. Cell Infect. Microbiol. 2023, 13, 1177270. [Google Scholar] [CrossRef]
- Adamik, J.; Munson, P.V.; Hartmann, F.J.; Combes, A.J.; Pierre, P.; Krummel, M.F.; Bendall, S.C.; Arguello, R.J.; Butterfield, L.H. Distinct metabolic states guide maturation of inflammatory and tolerogenic dendritic cells. Nat. Commun. 2022, 13, 5184. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, C.M.; Holowka, T.; Sun, J.; Blagih, J.; Amiel, E.; DeBerardinis, R.J.; Cross, J.R.; Jung, E.; Thompson, C.B.; Jones, R.G.; et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 2010, 115, 4742–4749. [Google Scholar] [CrossRef]
- Hosseinzade, A.; Sadeghi, O.; Naghdipour Biregani, A.; Soukhtehzari, S.; Brandt, G.S.; Esmaillzadeh, A. Immunomodulatory Effects of Flavonoids: Possible Induction of T CD4+ Regulatory Cells Through Suppression of mTOR Pathway Signaling Activity. Front. Immunol. 2019, 10, 51. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Qari, H.A.; Oves, M. Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharmaceuticals 2021, 14, 1069. [Google Scholar] [CrossRef]
- Thiyagarajan, V.; Lee, K.W.; Leong, M.K.; Weng, C.F. Potential natural mTOR inhibitors screened by in silico approach and suppress hepatic stellate cells activation. J. Biomol. Struct. Dyn. 2018, 36, 4220–4234. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, Y.; Bai, H.; Wang, Y.; Yang, B.; Wang, Q.; Kuang, H. Effects of saponins from Chinese herbal medicines on signal transduction pathways in cancer: A review. Front. Pharmacol. 2023, 14, 1159985. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.C.; Shen, J.; Si, Y.; Liu, X.W.; Zhang, L.; Wen, J.; Zhang, T.; Yu, Q.Q.; Lu, J.F.; Xiang, K.; et al. Paris saponin VII, a direct activator of AMPK, induces autophagy and exhibits therapeutic potential in non-small-cell lung cancer. Chin. J. Nat. Med. 2021, 19, 195–204. [Google Scholar] [CrossRef]
- Sun, J.; Feng, Y.; Wang, Y.; Ji, Q.; Cai, G.; Shi, L.; Wang, Y.; Huang, Y.; Zhang, J.; Li, Q. Alpha-hederin induces autophagic cell death in colorectal cancer cells through reactive oxygen species dependent AMPK/mTOR signaling pathway activation. Int. J. Oncol. 2019, 54, 1601–1612. [Google Scholar]
- Akhtar, M.; Shaukat, A.; Zahoor, A.; Chen, Y.; Wang, Y.; Yang, M.; Umar, T.; Guo, M.; Deng, G. Anti-inflammatory effects of Hederacoside-C on Staphylococcus aureus induced inflammation via TLRs and their downstream signal pathway in vivo and in vitro. Microb. Pathog. 2019, 137, 103767. [Google Scholar] [CrossRef] [PubMed]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, C.E. T helper cell subsets: Diversification of the field. Eur. J. Immunol. 2023, 53, e2250218. [Google Scholar] [CrossRef]
- Walker, J.A.; McKenzie, A.N.J. T(H)2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef]
- Brogdon, J.L.; Xu, Y.; Szabo, S.J.; An, S.; Buxton, F.; Cohen, D.; Huang, Q. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood 2007, 109, 1123–1130. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Yang, T.; Ye, Y.; Shan, J.; Yin, Z.; Luo, L. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury 2010, 41, 746–752. [Google Scholar] [CrossRef]
- Jang, G.; Lee, S.; Hong, J.; Park, B.; Kim, D.; Kim, C. Anti-Inflammatory Effect of 4,5-Dicaffeoylquinic Acid on RAW264.7 Cells and a Rat Model of Inflammation. Nutrients 2021, 13, 3537. [Google Scholar] [CrossRef]
- Han, E.H.; Kim, J.Y.; Kim, H.G.; Chun, H.K.; Chung, Y.C.; Jeong, H.G. Inhibitory effect of 3-caffeoyl-4-dicaffeoylquinic acid from Salicornia herbacea against phorbol ester-induced cyclooxygenase-2 expression in macrophages. Chem. Biol. Interact. 2010, 183, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Morante-Palacios, O.; Fondelli, F.; Ballestar, E.; Martinez-Caceres, E.M. Tolerogenic Dendritic Cells in Autoimmunity and Inflammatory Diseases. Trends Immunol. 2021, 42, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Ravetch, J.V.; Lanier, L.L. Immune inhibitory receptors. Science 2000, 290, 84–89. [Google Scholar] [CrossRef]
- Suciu-Foca, N.; Cortesini, R. Central role of ILT3 in the T suppressor cell cascade. Cell Immunol. 2007, 248, 59–67. [Google Scholar] [CrossRef]
- Brenk, M.; Scheler, M.; Koch, S.; Neumann, J.; Takikawa, O.; Hacker, G.; Bieber, T.; von Bubnoff, D. Tryptophan deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic cells favoring the induction of human CD4+CD25+ Foxp3+ T regulatory cells. J. Immunol. 2009, 183, 145–154. [Google Scholar] [CrossRef]
- Fallarino, F.; Grohmann, U.; Hwang, K.W.; Orabona, C.; Vacca, C.; Bianchi, R.; Belladonna, M.L.; Fioretti, M.C.; Alegre, M.L.; Puccetti, P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003, 4, 1206–1212. [Google Scholar] [CrossRef]
- Shevyrev, D.; Tereshchenko, V. Treg Heterogeneity, Function, and Homeostasis. Front. Immunol. 2019, 10, 3100. [Google Scholar] [CrossRef]
- Freeborn, R.A.; Strubbe, S.; Roncarolo, M.G. Type 1 regulatory T cell-mediated tolerance in health and disease. Front. Immunol. 2022, 13, 1032575. [Google Scholar] [CrossRef]
- Ye, C.; Yano, H.; Workman, C.J.; Vignali, D.A.A. Interleukin-35: Structure, Function and Its Impact on Immune-Related Diseases. J. Interferon Cytokine Res. 2021, 41, 391–406. [Google Scholar] [CrossRef]
- Sawant, D.V.; Yano, H.; Chikina, M.; Zhang, Q.; Liao, M.; Liu, C.; Callahan, D.J.; Sun, Z.; Sun, T.; Tabib, T.; et al. Adaptive plasticity of IL-10(+) and IL-35(+) T(reg) cells cooperatively promotes tumor T cell exhaustion. Nat. Immunol. 2019, 20, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, F.; Rostami-Nejad, M.; Amani, D.; Sadeghi, A.; Moradi, A.; Aghamohammadi, E.; Sahebkar, A.; Zali, M.R. Expression of tolerogenic dendritic cells in the small intestinal tissue of patients with celiac disease. Heliyon 2022, 8, e12273. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, W.; Yin, S.; Zhou, Y.; Chen, T.; Qian, J.; Su, R.; Hong, L.; Lu, H.; Zhang, F.; et al. Blocking Triggering Receptor Expressed on Myeloid Cells-1-Positive Tumor-Associated Macrophages Induced by Hypoxia Reverses Immunosuppression and Anti-Programmed Cell Death Ligand 1 Resistance in Liver Cancer. Hepatology 2019, 70, 198–214. [Google Scholar] [CrossRef]
- Tran, C.W.; Gold, M.J.; Garcia-Batres, C.; Tai, K.; Elford, A.R.; Himmel, M.E.; Elia, A.J.; Ohashi, P.S. Hypoxia-inducible factor 1 alpha limits dendritic cell stimulation of CD8 T cell immunity. PLoS ONE 2020, 15, e0244366. [Google Scholar] [CrossRef]
- Li, Y.; Gu, Y.; Yang, P.; Wang, Y.; Yu, X.; Li, Y.; Jin, Z.; Xu, L. CD69 is a Promising Immunotherapy and Prognosis Prediction Target in Cancer. Immunotargets Ther. 2024, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.Y.; Hayashizaki, K.; Tokoyoda, K.; Takamura, S.; Motohashi, S.; Nakayama, T. Crucial role for CD69 in allergic inflammatory responses: CD69-Myl9 system in the pathogenesis of airway inflammation. Immunol. Rev. 2017, 278, 87–100. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Bai, Y.; Hu, M.; Chen, Z.; Wei, J.; Du, H. Single-Cell Transcriptome Analysis Reveals RGS1 as a New Marker and Promoting Factor for T-Cell Exhaustion in Multiple Cancers. Front. Immunol. 2021, 12, 767070. [Google Scholar] [CrossRef]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef]
- Miggelbrink, A.M.; Jackson, J.D.; Lorrey, S.J.; Srinivasan, E.S.; Waibl-Polania, J.; Wilkinson, D.S.; Fecci, P.E. CD4 T-Cell Exhaustion: Does It Exist and What Are Its Roles in Cancer? Clin. Cancer Res. 2021, 27, 5742–5752. [Google Scholar] [CrossRef]
- Mita, Y.; Kimura, M.Y.; Hayashizaki, K.; Koyama-Nasu, R.; Ito, T.; Motohashi, S.; Okamoto, Y.; Nakayama, T. Crucial role of CD69 in anti-tumor immunity through regulating the exhaustion of tumor-infiltrating T cells. Int. Immunol. 2018, 30, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Cenerenti, M.; Saillard, M.; Romero, P.; Jandus, C. The Era of Cytotoxic CD4 T Cells. Front. Immunol. 2022, 13, 867189. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čolić, M.; Tomić, S.; Bekić, M.; Dubovina, A.; Häberlein, H.; Rademaekers, A.; Mašić, S.; Bokonjić, D. Ivy Leaf Dry Extract EA 575® Is a Potent Immunomodulator Acting on Dendritic Cells. Pharmaceutics 2025, 17, 773. https://doi.org/10.3390/pharmaceutics17060773
Čolić M, Tomić S, Bekić M, Dubovina A, Häberlein H, Rademaekers A, Mašić S, Bokonjić D. Ivy Leaf Dry Extract EA 575® Is a Potent Immunomodulator Acting on Dendritic Cells. Pharmaceutics. 2025; 17(6):773. https://doi.org/10.3390/pharmaceutics17060773
Chicago/Turabian StyleČolić, Miodrag, Sergej Tomić, Marina Bekić, Anđela Dubovina, Hanns Häberlein, André Rademaekers, Srđan Mašić, and Dejan Bokonjić. 2025. "Ivy Leaf Dry Extract EA 575® Is a Potent Immunomodulator Acting on Dendritic Cells" Pharmaceutics 17, no. 6: 773. https://doi.org/10.3390/pharmaceutics17060773
APA StyleČolić, M., Tomić, S., Bekić, M., Dubovina, A., Häberlein, H., Rademaekers, A., Mašić, S., & Bokonjić, D. (2025). Ivy Leaf Dry Extract EA 575® Is a Potent Immunomodulator Acting on Dendritic Cells. Pharmaceutics, 17(6), 773. https://doi.org/10.3390/pharmaceutics17060773





