Design and Evaluation of a Zingiber officinale–Kaolinite–Maltodextrin Delivery System: Antioxidant, Antimicrobial, and Cytotoxic Activity Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Plant Material
2.2. Cell Lines
2.3. Bacterial Strains
2.4. Plant Sample Preparation for Chemical Screening
2.5. GC–MS Analysis
2.6. MS Analysis
2.7. Spray-Drying Process
2.8. Phytocarrier (ZO–Kaolinite) System Preparation
2.9. Preparation of the Maltodextrin–ZO (MZO) Carrier
2.10. Preparation of the Maltodextrin–ZO–Kaolinite (MZO–Kaolinite) System
2.11. Characterization of Carrier Systems
2.11.1. FTIR Spectroscopy
2.11.2. XRD Analysis
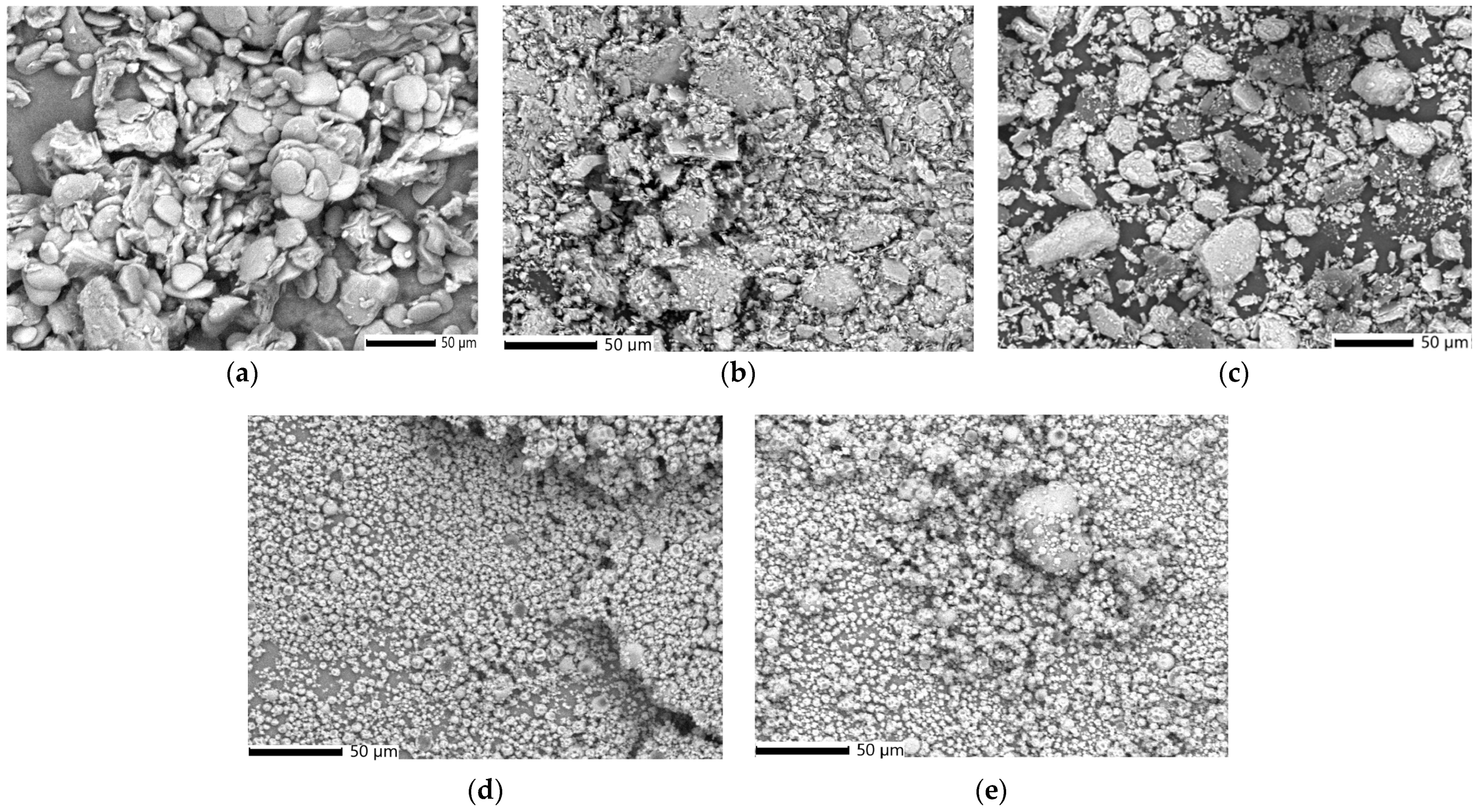
2.11.3. SEM Analysis
2.11.4. DLS Particle Size Distribution Analysis
2.11.5. Encapsulation Efficiency, Loading Capacity, and Encapsulation Yield
2.12. Thermal Analysis
2.13. Estimation of Total Phenolic Content and Antioxidant Activity
2.13.1. Sample Preparation Procedure
2.13.2. TPC Assay
2.13.3. FRAP Assay
2.13.4. DPPH Radical Scavenging Assay
2.13.5. Phosphomolybdate Assay (Total Antioxidant Capacity)
2.14. Antimicrobial Activity
2.15. Cell Culture Procedure
2.15.1. Cell Culture and Treatment
2.15.2. MTT Assay
2.16. Statistical Analysis
3. Results
3.1. GC–MS Analysis
3.2. MS Analysis
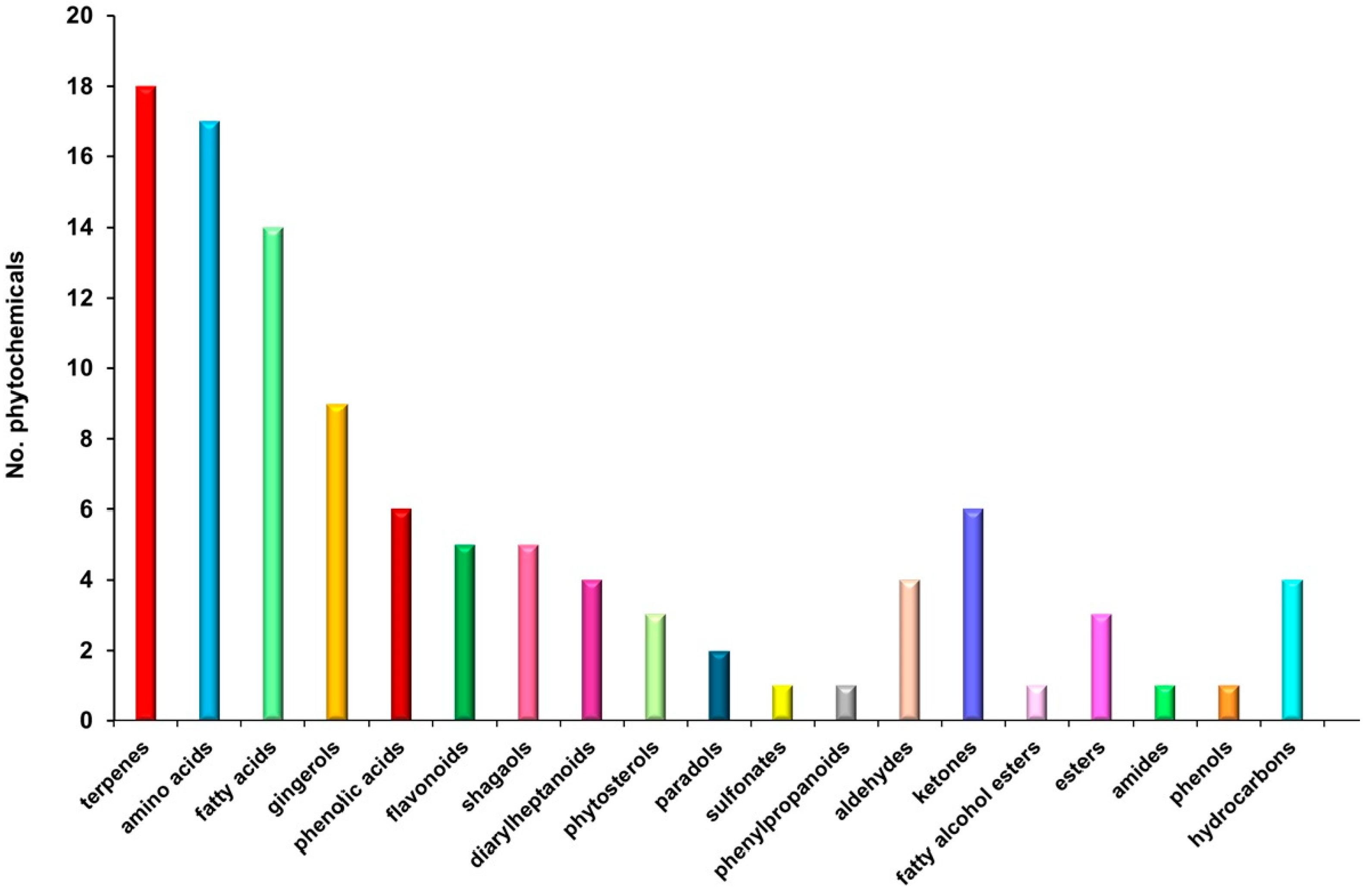
3.3. Chemical Screening
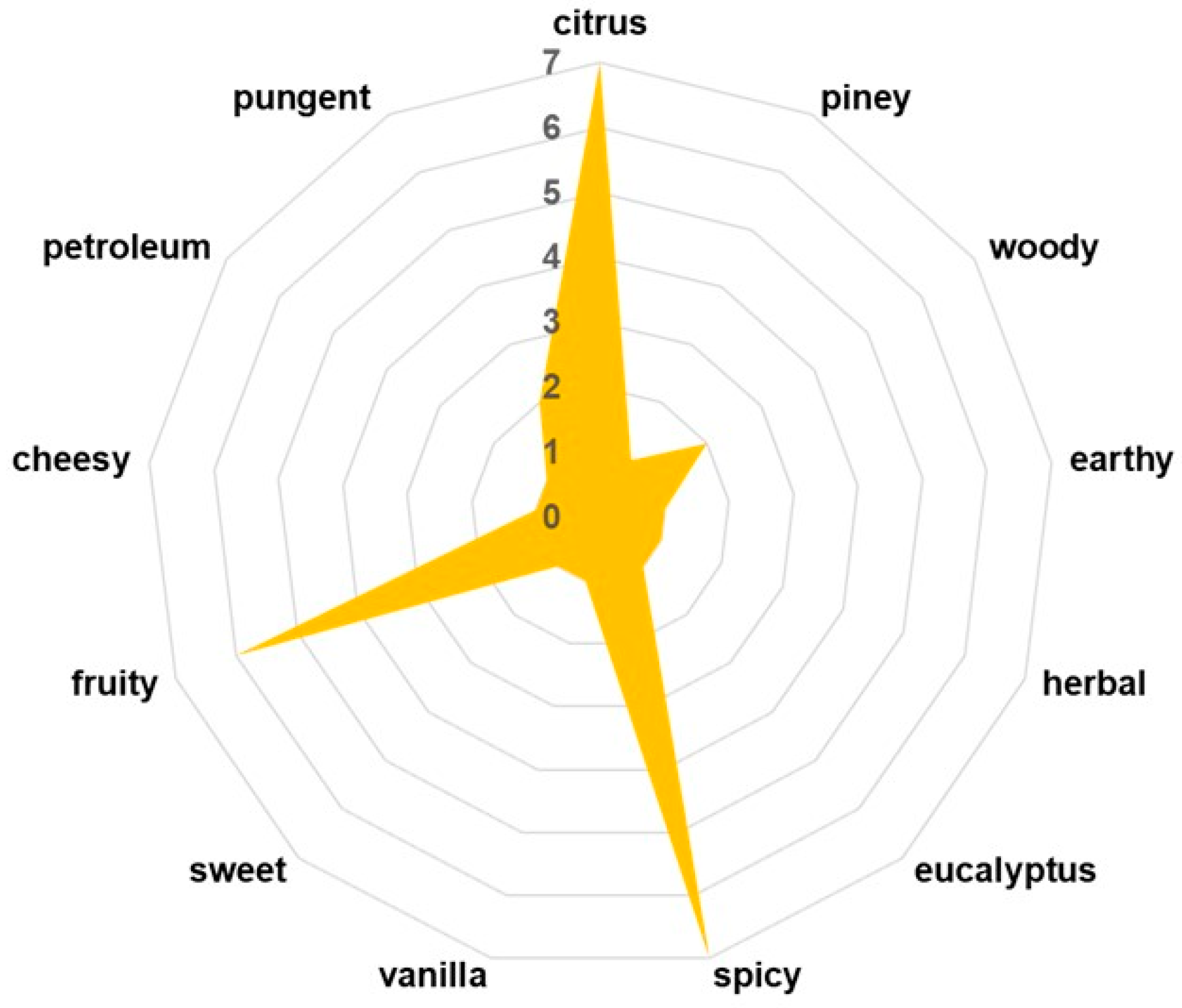
3.4. Key Aroma-Active Compounds and Their Contribution to the Flavor Profile
3.5. Phytocarrier System
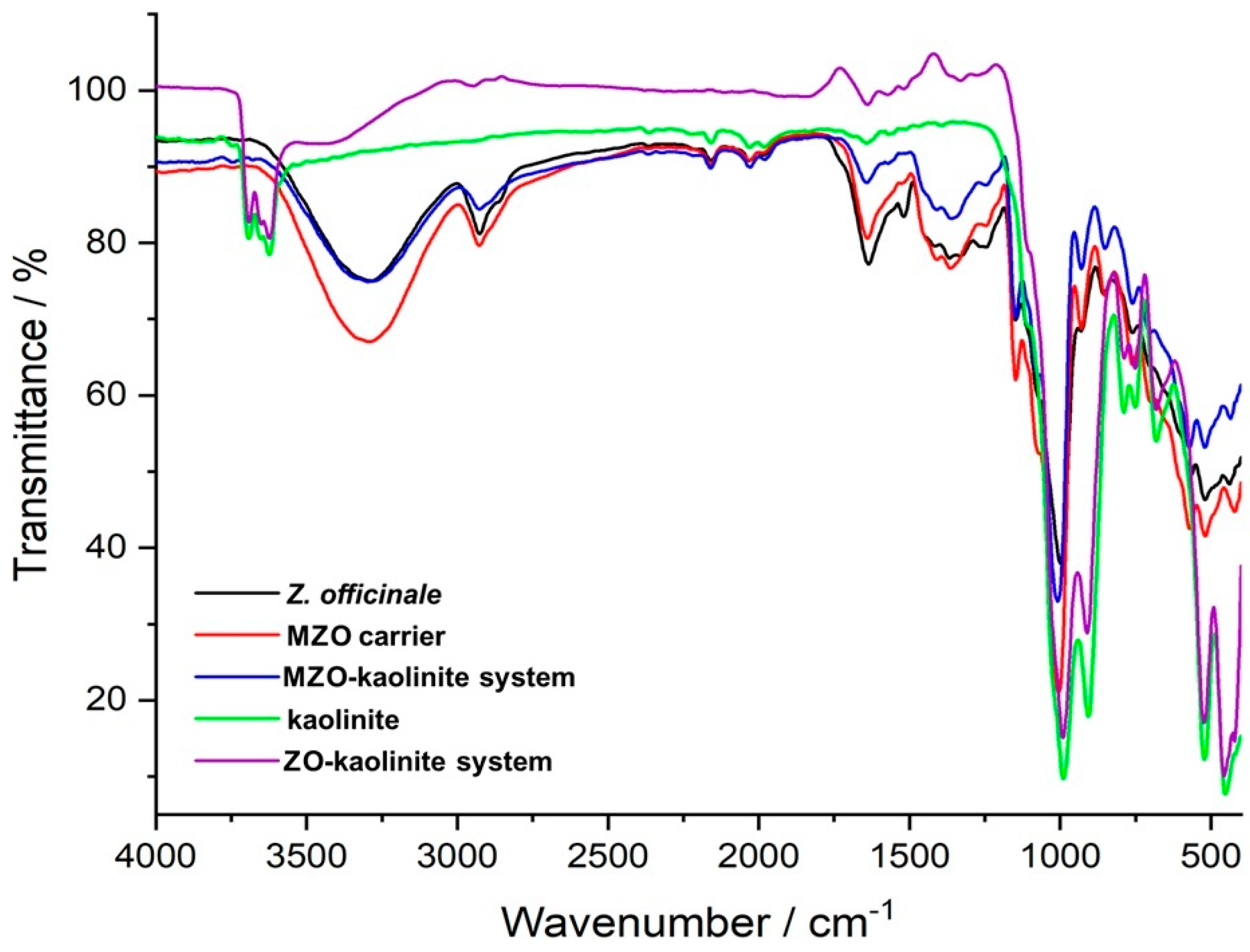
3.5.1. FTIR Analysis
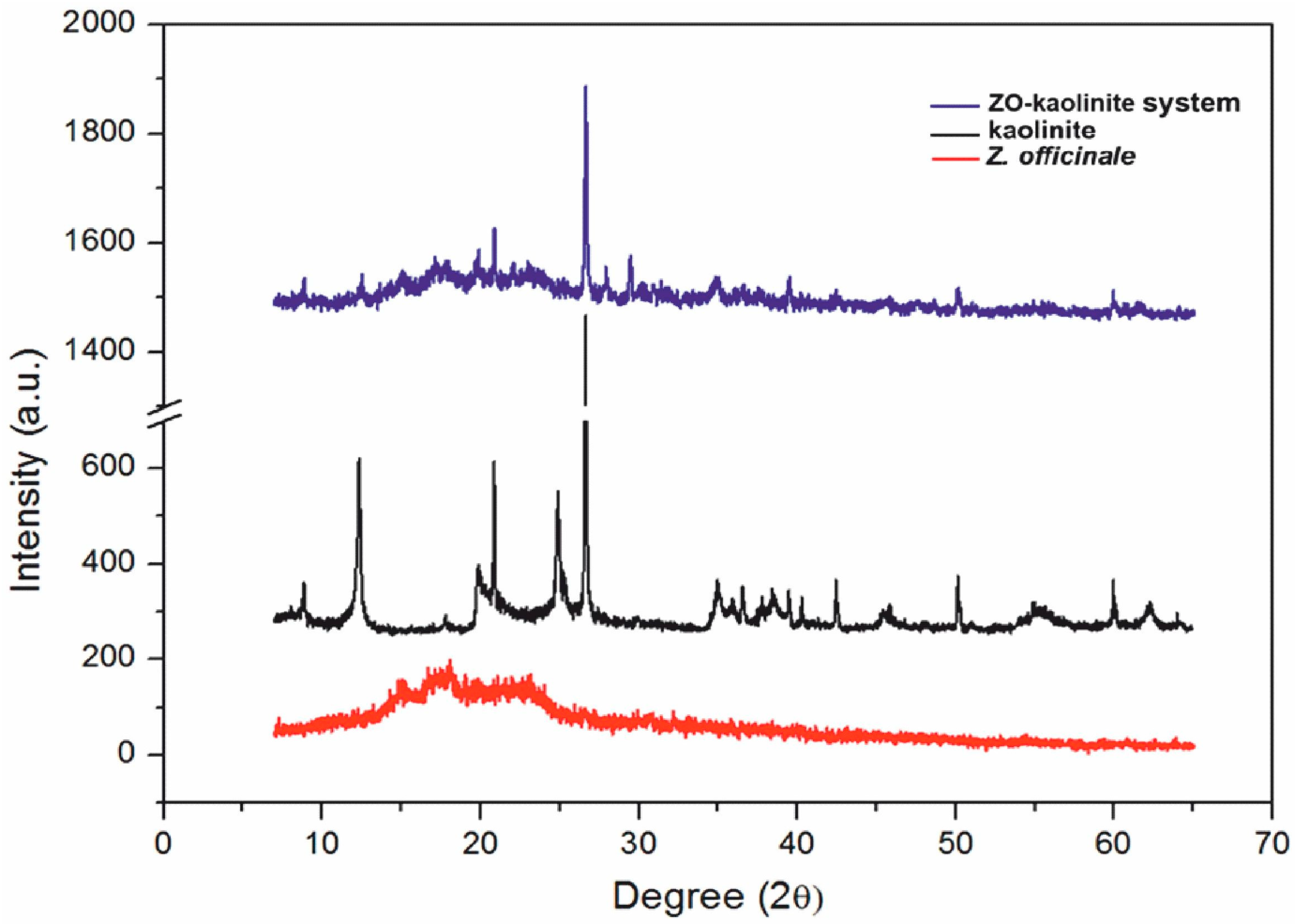
3.5.2. XRD Analysis
3.5.3. SEM–EDX Analysis
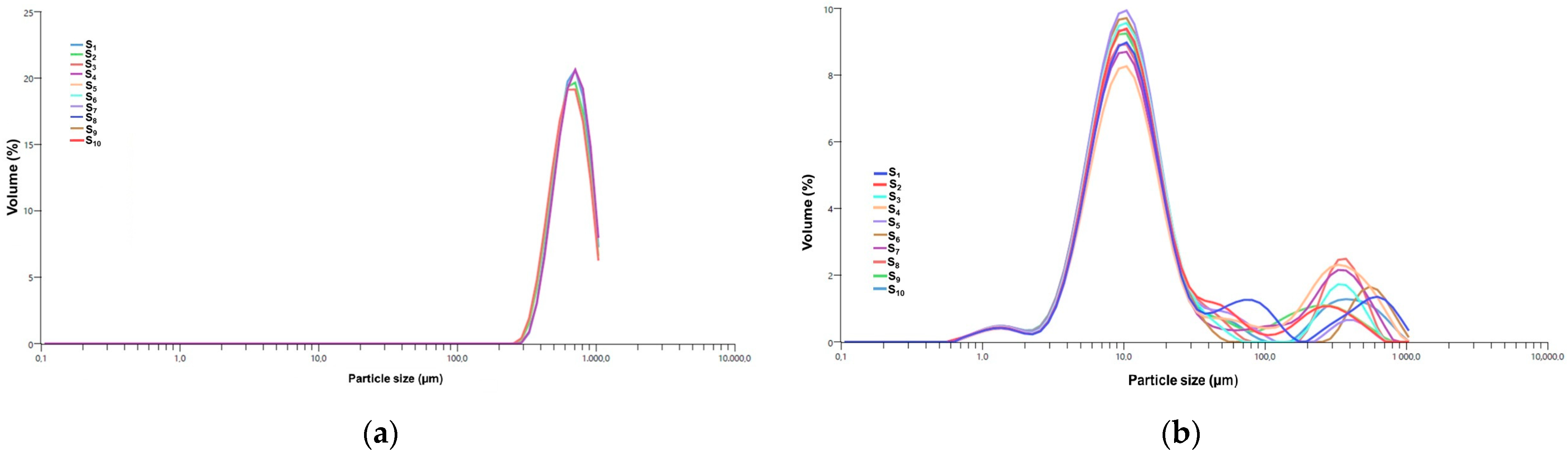
3.5.4. DLS Analysis
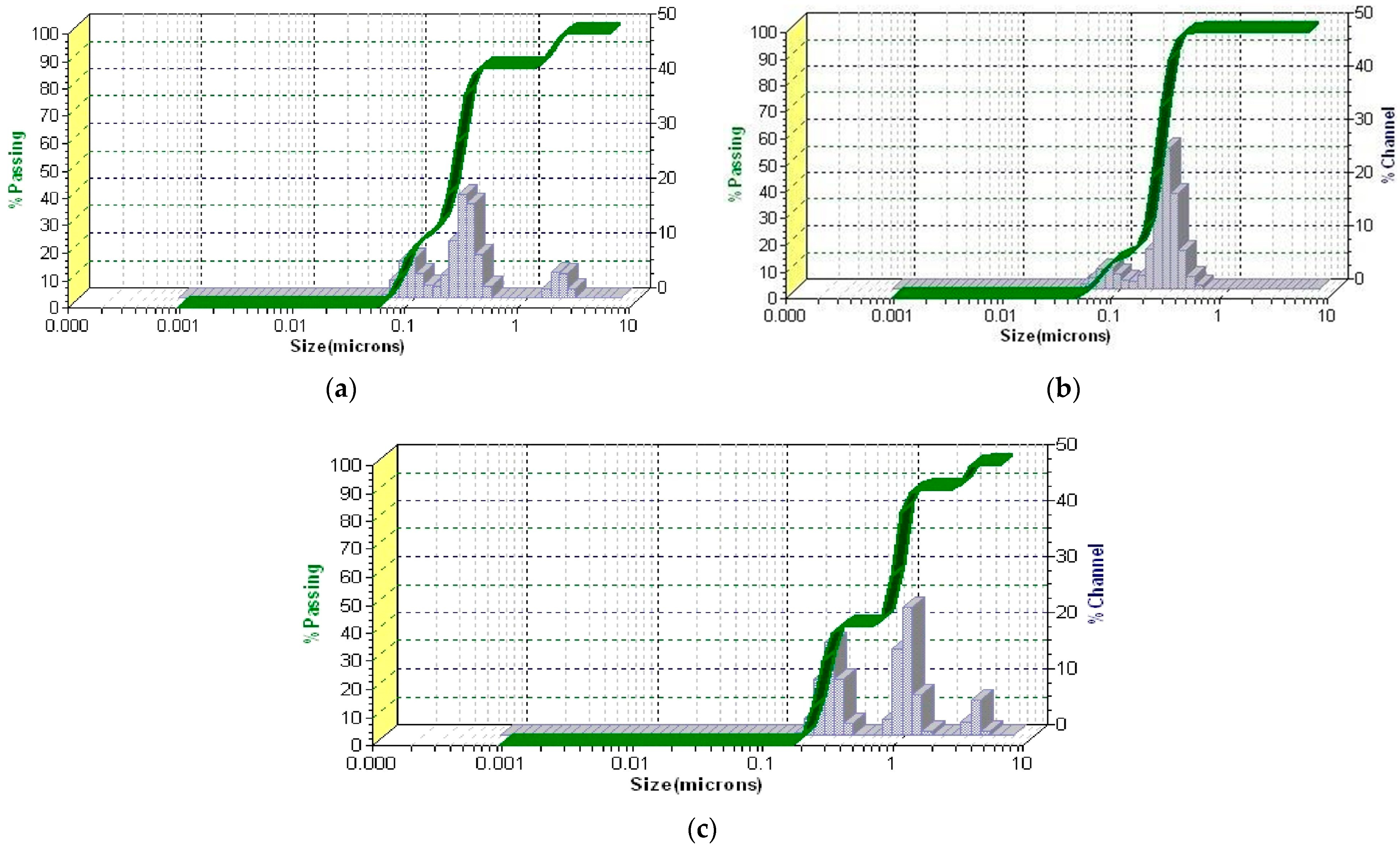
3.5.5. PSD Analysis by Laser Diffraction
3.5.6. Encapsulation Efficiency, Loading Capacity, and Encapsulation Yield
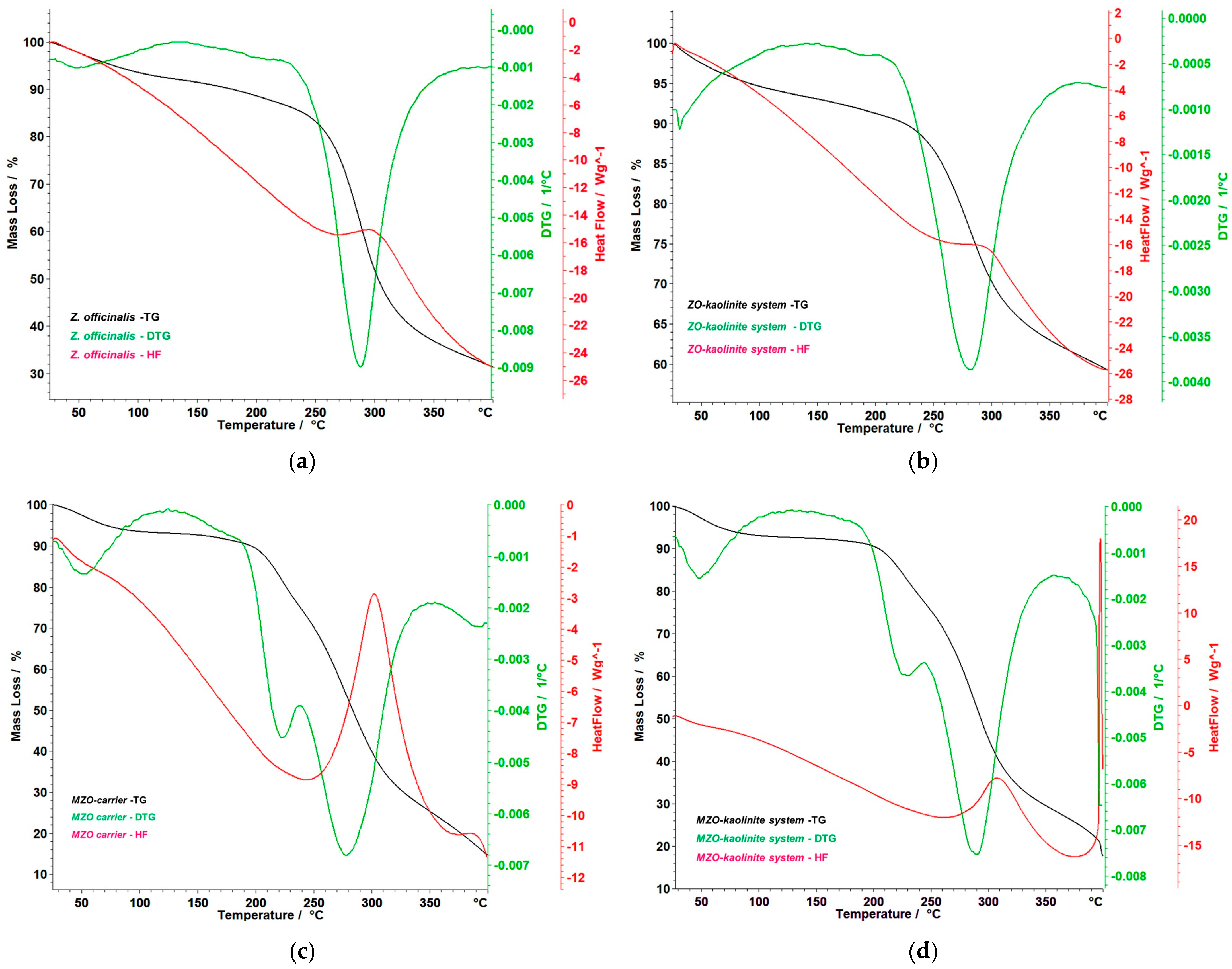
3.6. Thermal Behavior
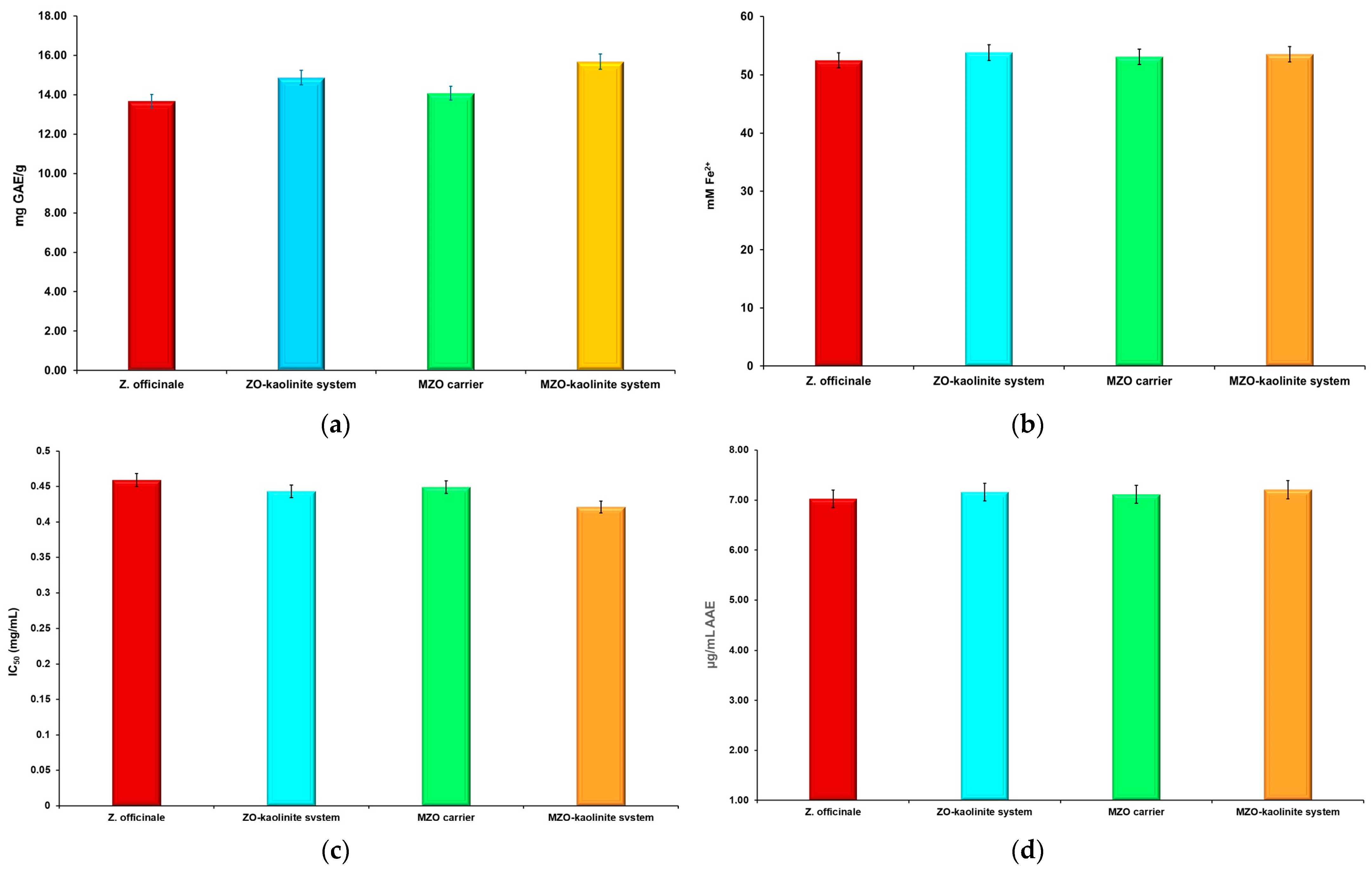
3.7. TPC and Estimation of Antioxidant Potential
3.8. Antimicrobial Screening
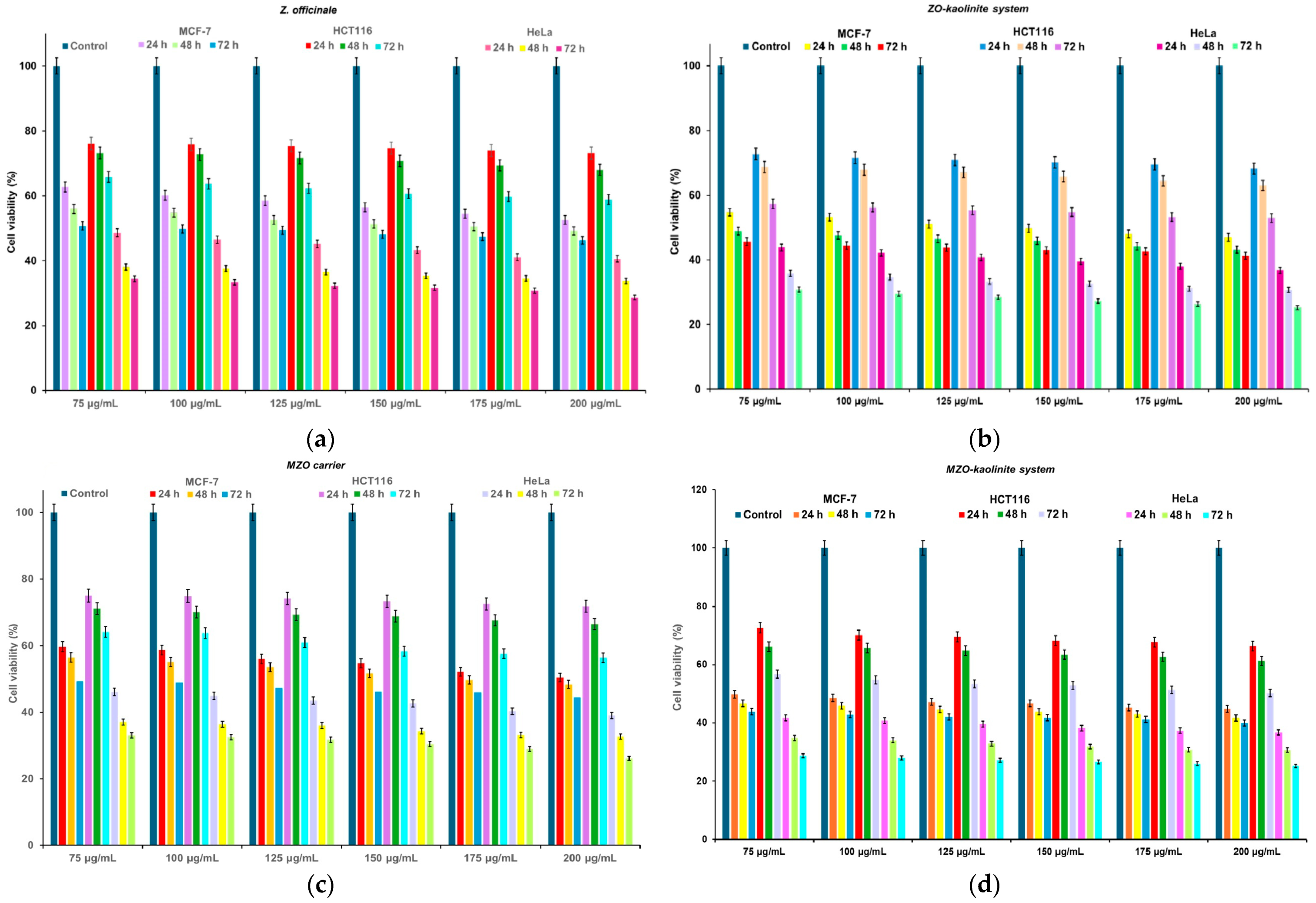
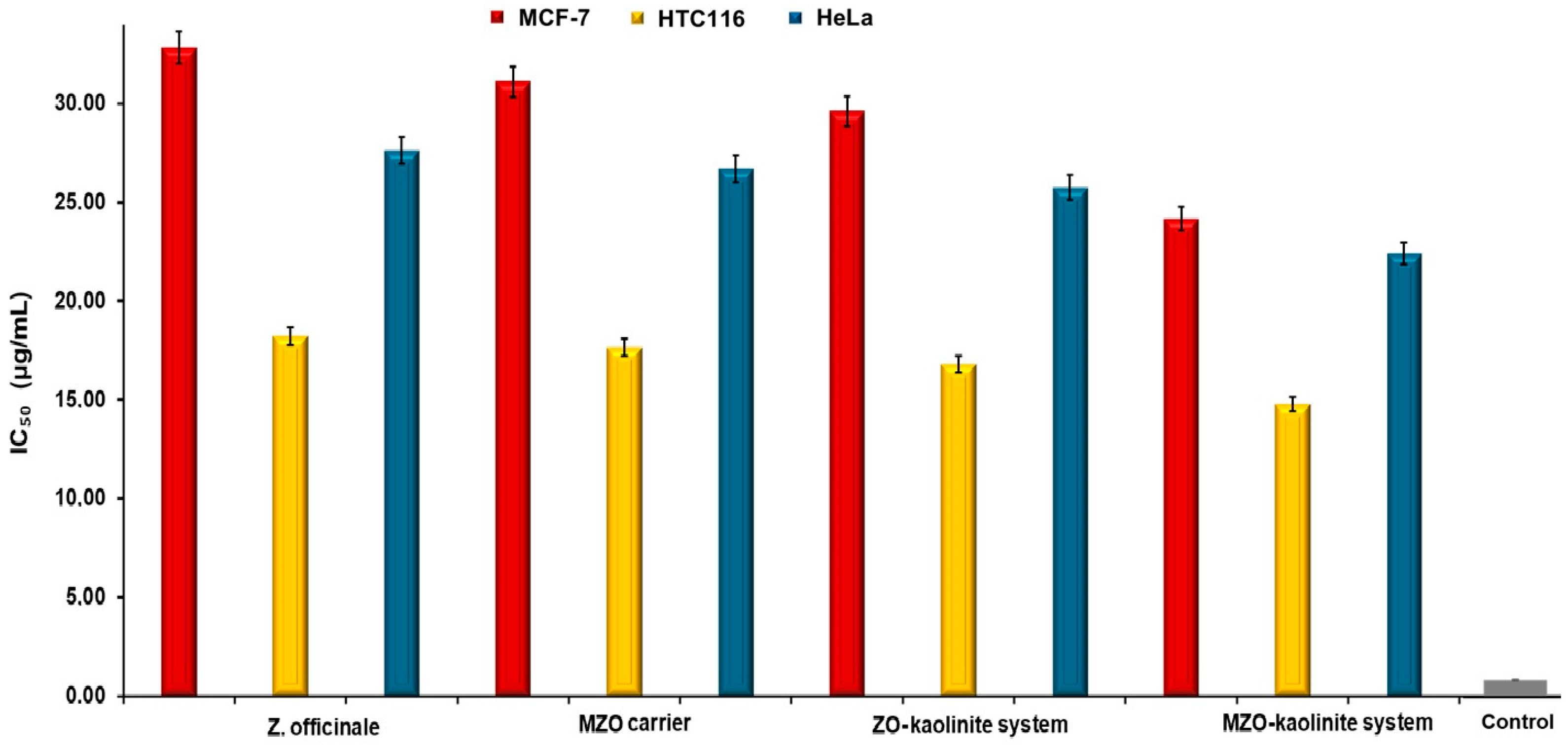
3.9. Cell Viability Assay
4. Discussion
4.1. Phytochemical Screening
4.2. Antioxidant Potential
4.3. Antimicrobial Screening
4.4. Cytotoxic Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAEs | Ascorbic acid equivalents |
| Akt/mTOR | Protein kinase B/mammalian target of rapamycin |
| AMR | Antimicrobial resistance |
| ANOVA | Analysis of variance |
| ASTM | American Society for Testing and Materials |
| ATCC | American Type Culture Collection |
| ATR | Attenuated total reflectance |
| CFU | Colony-forming units |
| CRKP | Carbapenem-resistant K. pneumoniae |
| DDS | Drug delivery system |
| ΔH | Enthalpy change |
| DLS | Dynamic light scattering |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DTA | Differential thermal analysis |
| DTG | Derivative thermogravimetry |
| EC% | Loading capacity |
| EDS | Energy-dispersive X-ray (EDX) spectroscopy |
| EE% | Encapsulation efficiency |
| ESI–QTOF–MS | Electro-spray ionization–quadrupole time-of-flight–mass spectrometry |
| EHEC | Enterohemorrhagic E. coli |
| ETEC | Enterotoxigenic E. coli |
| EY% | Encapsulation yield |
| FBS | Fetal bovine serum |
| FEG | Field emission gun |
| FRAP | Ferric reducing antioxidant power |
| FTIR | Fourier-transform infrared |
| GAE | Gallic acid equivalents |
| GC–MS | Gas chromatography–mass spectrometry |
| GRAS | Generally recognized as safe |
| HF | Heat flow |
| HT | High temperature |
| IC50 | Half-maximal inhibitory concentration |
| ICDD | International Centre for Diffraction Data |
| IL-6 | Interleukin-6 |
| IS | Internal standard |
| IZ | Inhibition zone |
| MBC | Minimum bactericidal concentration |
| MIC | Minimum inhibitory concentration |
| MMP-9 | Matrix metalloproteinase-9 |
| MRSA | Methicillin-resistant S. aureus |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| MZO | Maltodextrin–Zingiber officinale |
| NBS | National Bureau of Standards |
| NF-κB | Nuclear factor-kappa B |
| NIST | National Institute of Standards and Technology |
| PDI | Polydispersity index |
| PSD | Particle size distribution |
| ROS | Reactive oxygen species |
| RT | Room temperature |
| SD | Standard deviation |
| SEM | Scanning electron microscopy |
| TAC | Total antioxidant capacity |
| TG | Thermogravimetry |
| TNF-α | Tumor necrosis factor-alpha |
| TPC | Total phenolic content |
| UAE | Ultrasound-assisted extraction |
| UPEC | Uropathogenic E. coli |
| UTI | Urinary tract infection |
| UV–Vis | Ultraviolet–visible |
| VOC | Volatile organic compound |
| VRE | Vancomycin resistance |
| WPPF | Whole powder profile fitting |
| XRD | X-ray diffraction |
| ZO | Zingiber officinale |
References
- Szymczak, J.; Grygiel-Górniak, B.; Cielecka-Piontek, J. Zingiber officinale Roscoe: The Antiarthritic Potential of a Popular Spice—Preclinical and Clinical Evidence. Nutrients 2024, 16, 741. [Google Scholar] [CrossRef]
- Banerjee, S.; Banerjee, J.; Mullick, H.I.; Ghosh, A.K. Zingiber officinale: A natural gold. Int. J. Pharm. Biosci. 2011, 2, 283–294. [Google Scholar]
- Stan, D.; Enciu, A.M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural Compounds with Antimicrobial and Antiviral Effect and Nanocarriers Used for Their Transportation. Front. Pharmacol. 2021, 12, 723233. [Google Scholar] [CrossRef]
- Fadaki, F.; Modaresi, M.; Sajjadian, I. The Effects of Ginger Extract and Diazepam on Anxiety Reduction in Animal Model. Indian J. Pharm. Educ. Res. 2017, 51, S159–S162. [Google Scholar] [CrossRef]
- Bartels, E.M.; Folmer, V.N.; Bliddal, H.; Altman, R.D.; Juhl, C.; Tarp, S.; Zhang, W.; Christensen, R. Efficacy and safety of ginger in osteoarthritis patients: A meta-analysis of randomized placebo-controlled trials. Osteoarthritis Cartil. 2015, 23, 13–21. [Google Scholar] [CrossRef]
- Crichton, M.; Marshall, S.; Marx, W.; McCarthy, A.L.; Isenring, E. Efficacy of Ginger (Zingiber officinale) in Ameliorating Chemotherapy-Induced Nausea and Vomiting and Chemotherapy-Related Outcomes: A Systematic Review Update and Meta-Analysis. J. Acad. Nutr. Diet. 2019, 119, 2055–2068. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Balogun, F.O.; Tayo AdeyeOluwa, E.; Ashafa, A.O.T. Pharmacological potentials of ginger. In Ginger Cultivation and Its Antimicrobial and Pharmacological Potentials, 1st ed.; Wang, H., Ed.; IntechOpen: London, UK, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 546–556. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, R.; Wang, D.; Wang, L.; Zhang, Q.; Wei, S.; Lu, F.; Peng, W.; Wu, C. Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health beneficial agents. Phytother. Res. 2020, 35, 711–742. [Google Scholar] [CrossRef]
- López, E.I.C.; Balcázar, M.F.H.; Mendoza, J.M.R.; Ortiz, A.D.R.; Melo, M.T.O.; Parrales, R.S.; Delgado, T.H. Antimicrobial Activity of Essential Oil of Zingiber officinale Roscoe (Zingiberaceae). Am. J. Plant Sci. 2017, 8, 1511–1524. [Google Scholar] [CrossRef]
- Momoh, J.O.; Olaleye, O.N. Evaluation of Secondary Metabolites Profiling of Ginger (Zingiber officinale Roscoe) Rhizome using GC–MS and Its Antibacterial Potential on Staphylococcus aureus and Escherichia coli. Microbiol. Res. J. Int. 2022, 32, 7–31. [Google Scholar] [CrossRef]
- Aleem, M.; Imran Khan, M.; Shakshaz, F.A.; Akbari, N.; Anwar, D. Botany, phytochemistry and antimicrobial activity of ginger (Zingiber officinale): A review. Int. J. Herb. Med. 2020, 8, 36–49. [Google Scholar] [CrossRef]
- Nalbantsoy, A.; Ayyildiz Tamiş, D.; Akgün, I.H.; Öztürk Yalçin, T.; Deliloğlu Gürhan, I.; Karaboz, İ. Antimicrobial and Cytotoxic Activities of Zingiber officinalis Extracts. FABAD J. Pharm. Sci. 2008, 33, 77–86. [Google Scholar]
- Nath, R.; Roy, R.; Barai, G.; Bairagi, S.; Manna, S.; Chakraborty, R. Modern Developments of Nano Based Drug Delivery System by Combined with Phytochemicals—Presenting New Aspects. Int. J. Sci. Res. Sci. Technol. 2021, 8, 107–129. [Google Scholar] [CrossRef]
- Aparicio-Blanco, J.; Vishwakarma, N.; Lehr, C.M.; Prestidge, C.A.; Thomas, N.; Roberts, R.J.; Thorn, C.R.; Melero, A. Antibiotic resistance and tolerance: What can drug delivery do against this global threat? Drug Deliv. Transl. Res. 2024, 14, 1725–1734. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Publ. Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Singh, I.P.; Ahmad, F.; Chatterjee, D.; Bajpai, R.; Sengar, N. Natural products: Drug discovery and development. In Drug Discovery and Development: From Targets and Molecules to Medicines, 1st ed.; Poduri, R., Ed.; Springer Nature: Singapore, 2021; pp. 11–65. [Google Scholar] [CrossRef]
- Wu, Q.; Liao, J.; Yang, H. Recent Advances in Kaolinite Nanoclay as Drug Carrier for Bioapplications: A Review. Adv. Sci. (Weinh.) 2023, 10, 2300672. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.R.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- Mei, H.; Cai, S.; Huang, D.; Gao, H.; Cao, J.; He, B. Carrier-free nanodrugs with efficient drug delivery and release for cancer therapy: From intrinsic physicochemical properties to external modification. Bioact. Mater. 2022, 8, 220–240. [Google Scholar] [CrossRef]
- Lee, Y. Cytotoxicity Evaluation of Essential Oil and Its Component from Zingiber officinale Roscoe. Toxicol. Res. 2016, 32, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Roshan, S.; Hammad, S.; Pandohee, J.; Hu, X.; Zengin, G. Ginger and its active compounds in cancer therapy: From folk uses to nano-therapeutic applications. Semin. Cancer Biol. 2019, 69, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Tao, Q.; Wu, X.; Dou, H.; Spencer, S.; Mang, C.; Xu, L.; Sun, L.; Zhao, Y.; Li, H.; et al. Cytotoxic, cytoprotective and antioxidant effects of isolated phenolic compounds from fresh ginger. Fitoterapia 2012, 83, 568–585. [Google Scholar] [CrossRef] [PubMed]
- Lechner, J.F.; Stoner, G.D. Gingers and Their Purified Components as Cancer Chemopreventative Agents. Molecules 2019, 24, 2859. [Google Scholar] [CrossRef]
- Mustafa, I.; Chin, N.L. Antioxidant Properties of Dried Ginger (Zingiber officinale Roscoe) var. Bentong. Foods 2023, 12, 178. [Google Scholar] [CrossRef]
- Mošovská, S.; Nováková, D.; Kaliňák, M. Antioxidant activity of ginger extract and identification of its active components. Acta Chim. Slovaca 2015, 8, 115–119. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Grozescu, I.; Sfirloaga, P. The influence of extraction process parameters of some biomaterials precursors from Helianthus annuus. Dig. J. Nanomater. Biostruct. 2013, 8, 1423–1433. [Google Scholar]
- Popescu, C.; Fitigau, F.; Segneanu, A.E.; Balcu, I.; Martagiu, R.; Vaszilcsin, C.G. Separation and characterization of anthocyanins by analytical and electrochemical methods. Environ. Eng. Manag. J. 2011, 10, 697–701. [Google Scholar] [CrossRef]
- Vaszilcsin, C.G.; Segneanu, A.E.; Balcu, I.; Pop, R.; Fitigău, F.; Mirica, M.C. Eco-friendly extraction and separation methods of capsaicines. Environ. Eng. Manag. J. 2010, 9, 971–976. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Damian, D.; Hulka, I.; Grozescu, I.; Salifoglou, A. A simple and rapid method for calixarene-based selective extraction of bioactive molecules from natural products. Amino Acids 2016, 48, 849–858. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Dabici, A.; Zuguang, Y.; Jianrong, L.; Grozescu, I. Comparative analytical study of active compounds from Zingiber officinale. Optoelectron. Adv. Mater. 2012, 6, 652–655. [Google Scholar]
- Bejenaru, L.E.; Radu, A.; Segneanu, A.-E.; Biţă, A.; Manda, C.-V.; Mogoşanu, G.D.; Bejenaru, C. Innovative Strategies for Upcycling Agricultural Residues and Their Various Pharmaceutical Applications. Plants 2024, 13, 2133. [Google Scholar] [CrossRef]
- Dewi, M.K.; Chaerunisaa, A.Y.; Muhaimin, M.; Joni, I.M. Improved Activity of Herbal Medicines through Nanotechnology. Nanomaterials 2022, 12, 4073. [Google Scholar] [CrossRef]
- Stojiljković, S.T.; Stojiljković, M.S. Application of bentonite clay for human use. In Proceedings of the 4th Advanced Ceramics and Applications Conference, Belgrade, Serbia, 21–23 September 2015. [Google Scholar]
- Williams, L.B. Natural Antibacterial Clays: Historical Uses and Modern Advances. Clays Clay Miner. 2019, 67, 7–24. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Riela, S. The Use of Some Clay Minerals as Natural Resources for Drug Carrier Applications. J. Funct. Biomater. 2018, 9, 58. [Google Scholar] [CrossRef]
- Rebitski, E.P.; Darder, M.; Sainz-Diaz, C.I.; Carraro, R.; Aranda, P.; Ruiz-Hitzky, E. Theoretical and experimental investigation on the intercalation of metformin into layered clay minerals. Appl. Clay Sci. 2020, 186, 105418. [Google Scholar] [CrossRef]
- Behroozian, S.; Zlosnik, J.E.A.; Xu, W.; Li, L.Y.; Davies, J.E. Antibacterial Activity of a Natural Clay Mineral against Burkholderia cepacia Complex and Other Bacterial Pathogens Isolated from People with Cystic Fibrosis. Microorganisms 2023, 11, 150. [Google Scholar] [CrossRef]
- Martsouka, F.; Papagiannopoulos, K.; Hatziantoniou, S.; Barlog, M.; Lagiopoulos, G.; Tatoulis, T.; Tekerlekopoulou, A.G.; Lampropoulou, P.; Papoulis, D. The Antimicrobial Properties of Modified Pharmaceutical Bentonite with Zinc and Copper. Pharmaceutics 2021, 13, 1190. [Google Scholar] [CrossRef]
- Awad, M.E.; López-Galindo, A.; Setti, M.; El-Rahmany, M.M.; Iborra, C.V. Kaolinite in pharmaceutics and biomedicine. Int. J. Pharm. 2017, 533, 34–48. [Google Scholar] [CrossRef]
- Awad, M.E.; López-Galindo, A.; El-Rahmany, M.M.; El-Desoky, H.M.; Viseras, C. Characterization of Egyptian kaolins for health-care uses. Appl. Clay. Sci. 2017, 135, 176–189. [Google Scholar] [CrossRef]
- Saad, H.; Ayed, A.; Srasra, M.; Attia, S.; Srasra, E.; Charrier-El Bouhtoury, F.; Tabbene, O. New trends in clay-based nanohybrid applications: Essential oil encapsulation strategies to improve their biological activity. In Nanoclay—Recent Advances, New Perspectives and Applications, 1st ed.; Oueslati, W., Ed.; IntechOpen: London, UK, 2022; pp. 1–26. [Google Scholar] [CrossRef]
- Laureanti, E.J.G.; Paiva, T.S.; de Matos Jorge, L.M.; Jorge, R.M.M. Microencapsulation of bioactive compound extracts using maltodextrin and gum Arabic by spray and freeze-drying techniques. Int. J. Biol. Macromol. 2023, 253, 126969. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, X.; Zheng, H.; Li, J.; Wu, X.; Xu, J.; Zhen, Z.; Du, C. The application of encapsulation technology in the food industry: Classifications, recent advances, and perspectives. Food Chem. X 2024, 21, 101240. [Google Scholar] [CrossRef]
- Bejenaru, C.; Radu, A.; Segneanu, A.-E.; Biţă, A.; Ciocîlteu, M.V.; Mogoşanu, G.D.; Bradu, I.A.; Vlase, T.; Vlase, G.; Bejenaru, L.E. Pharmaceutical Applications of Biomass Polymers: Review of Current Research and Perspectives. Polymers 2024, 16, 1182. [Google Scholar] [CrossRef]
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A retention index calculator simplifies identification of plant volatile organic compounds. Phytochem. Anal. 2009, 20, 378–384. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. (Eds.) Compendium of Chemical Terminology: IUPAC Recommendations, 2nd ed.; International Union of Pure and Applied Chemistry (IUPAC) Gold Book, Online Version (2019) Created by S.J. Chalk; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar] [CrossRef]
- Vargas, V.; Saldarriaga, S.; Sánchez, F.S.; Cuellar, L.N.; Paladines, G.M. Effects of the spray-drying process using maltodextrin on bioactive compounds and antioxidant activity of the pulp of the tropical fruit açai (Euterpe oleracea Mart.). Heliyon 2024, 10, e33544. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Tsimidou, M.Z. Properties of encapsulated saffron extracts in maltodextrin using the Büchi B-90 nano spray-dryer. Food Chem. 2018, 266, 458–465. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Vlase, G.; Vlase, T.; Sicoe, C.A.; Ciocalteu, M.V.; Herea, D.D.; Ghirlea, O.-F.; Grozescu, I.; Nanescu, V. Wild-Grown Romanian Helleborus purpurascens Approach to Novel Chitosan Phyto-Nanocarriers—Metabolite Profile and Antioxidant Properties. Plants 2023, 12, 3479. [Google Scholar] [CrossRef]
- Vlase, G.; Segneanu, A.-E.; Bejenaru, L.E.; Bradu, I.A.; Sicoe, C.; Vlase, T.; Mogoşanu, G.D.; Buema, G.; Herea, D.-D.; Ciocîlteu, M.V.; et al. Wild-Grown Romanian Eupatorium cannabinum: Advancing Phyto-Nanocarriers via Maltodextrin Micro-Spray Encapsulation—Metabolite Profiling, Antioxidant, Antimicrobial, and Cytotoxicity Insights. Polymers 2025, 17, 482. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Vlase, G.; Vlase, T.; Bejenaru, L.E.; Mogoşanu, G.D.; Buema, G.; Herea, D.-D.; Ciocîlteu, M.V.; Bejenaru, C. Insight into Romanian Wild-Grown Heracleum sphondylium: Development of a New Phytocarrier Based on Silver Nanoparticles with Antioxidant, Antimicrobial and Cytotoxicity Potential. Antibiotics 2024, 13, 911. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Vlase, G.; Marin, C.N.; Vlase, T.; Sicoe, C.; Herea, D.D.; Ciocîlteu, M.V.; Bejenaru, L.E.; Minuti, A.E.; Zară, C.M.; et al. Wild grown Portulaca oleracea as a novel magnetite based carrier with in vitro antioxidant and cytotoxicity potential. Sci. Rep. 2025, 15, 8694. [Google Scholar] [CrossRef] [PubMed]
- Segneanu, A.-E.; Vlase, G.; Vlase, T.; Ciocalteu, M.-V.; Bejenaru, C.; Buema, G.; Bejenaru, L.E.; Boia, E.R.; Dumitru, A.; Boia, S. Romanian Wild-Growing Chelidonium majus—An Emerging Approach to a Potential Antimicrobial Engineering Carrier System Based on AuNPs: In Vitro Investigation and Evaluation. Plants 2024, 13, 734. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Vlase, G.; Chirigiu, L.; Herea, D.D.; Pricop, M.-A.; Saracin, P.-A.; Tanasie, Ş.E. Romanian Wild-Growing Armoracia rusticana L.—Untargeted Low-Molecular Metabolomic Approach to a Potential Antitumoral Phyto-Carrier System Based on Kaolinite. Antioxidants 2023, 12, 1268. [Google Scholar] [CrossRef]
- Kavaz, D.; Faraj, R.E.K.E. Investigation of composition, antioxidant, antimicrobial and cytotoxic characteristics from Juniperus sabina and Ferula communis extracts. Sci. Rep. 2023, 13, 7193. [Google Scholar] [CrossRef]
- Hossain, M.L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. A Review of Commonly Used Methodologies for Assessing the Antibacterial Activity of Honey and Honey Products. Antibiotics 2022, 11, 975. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Adil, M.; Filimban, F.Z.; Ambrin; Quddoos, A.; Sher, A.A.; Naseer, M. Phytochemical screening, HPLC analysis, antimicrobial and antioxidant effect of Euphorbia parviflora L. (Euphorbiaceae Juss.). Sci. Rep. 2024, 14, 5627. [Google Scholar] [CrossRef]
- Kar, S.; Sengupta, D.; Deb, M.; Shilpi, A.; Parbin, S.; Rath, S.K.; Pradhan, N.; Rakshit, M.; Patra, S.K. Expression profiling of DNA methylation-mediated epigenetic gene-silencing factors in breast cancer. Clin. Epigenetics 2014, 6, 20. [Google Scholar] [CrossRef]
- Babushok, V.I.; Zenkevich, I.G. Retention Indices for Most Frequently Reported Essential Oil Compounds in GC. Chromatographia 2009, 69, 257–269. [Google Scholar] [CrossRef]
- Shareef, H.K.; Muhammed, H.J.; Hussein, H.M.; Hameed, I.H. Antibacterial effect of ginger (Zingiber officinale) Roscoe and bioactive chemical analysis using gas chromatography mass spectrum. Orient. J. Chem. 2016, 32, 817–837. [Google Scholar] [CrossRef]
- Nishidono, Y.; Saifudin, A.; Deevanhxay, P.; Tanaka, K. Metabolite Profiling of Ginger (Zingiber officinale Roscoe) Using GC–MS and Multivariate Statistical Analysis. J. Asia Jpn. Res. Inst. Ritsumeikan Univ. 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Zhengkui, L.; Yingfang, H. Chemical constituents of the essential oil from Zingiber officinale rose of Sichuan. Chin. J. Org. Chem. 1987, 7, 444–448. [Google Scholar]
- Edo, G.I.; Igbuku, U.A.; Makia, R.S.; Isoje, E.F.; Gaaz, T.S.; Yousif, E.; Jikah, A.N.; Zainulabdeen, K.; Akpoghelie, P.O.; Opiti, R.A.; et al. Phytochemical profile, therapeutic potentials, nutritional composition, and food applications of ginger: A comprehensive review. Discov. Food 2025, 5, 25. [Google Scholar] [CrossRef]
- Styawan, A.A.; Susidarti, R.A.; Purwanto; Windarsih, A.; Rahmawati, N.; Sholikhah, I.K.M.; Rohman, A. Review on ginger (Zingiber officinale Roscoe): Phytochemical composition, biological activities and authentication analysis. Food Res. 2022, 6, 443–454. [Google Scholar] [CrossRef]
- Oforma, C.C.; Udourioh, G.A.; Ojinnaka, C.M. Characterization of Essential Oils and Fatty Acids Composition of Stored Ginger (Zingiber officinale Roscoe). J. Appl. Sci. Environ. Manag. 2019, 23, 2231–2238. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Yu, M.; Luo, D.; Chen, S.; Liu, W.; Zhang, Y.; Zhang, L.; Zhao, T. Essential oils of Zingiber officinale: Chemical composition, in vivo alleviation effects on TPA induced ear swelling in mice and in vitro bioactivities. Front. Nutr. 2022, 9, 1043175. [Google Scholar] [CrossRef]
- Wibowo, D.P.; Mariani, R.; Hasanah, S.U.; Aulifa, D.L. Chemical Constituents, Antibacterial Activity and Mode of Action of Elephant Ginger (Zingiber officinale var. officinale) and Emprit Ginger Rhizome (Zingiber officinale var. amarum) Essential Oils. Pharmacogn. J. 2020, 12, 404–409. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Król, M.; Minkiewicz, J.; Mozgawa, W. IR spectroscopy studies of zeolites in geopolymeric materials derived from kaolinite. J. Mol. Struct. 2016, 1126, 200–206. [Google Scholar] [CrossRef]
- Vargas-Muñoz, D.P.; Kurozawa, L.E. Influence of combined hydrolyzed collagen and maltodextrin as carrier agents in spray drying of cocona pulp. Braz. J. Food Technol. 2020, 23, e2019254. [Google Scholar] [CrossRef]
- Simon-Brown, K.; Mis Solval, K.; Chotiko, A.; Alfaro, L.; Reyes, V.; Liu, C.; Dzandu, B.; Kyereh, E.; Goldson Barnaby, A.; Thompson, I.; et al. Microencapsulation of ginger (Zingiber officinale) extract by spray drying technology. LWT 2016, 70, 119–125. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Marin, C.N.; Herea, D.D.; Stanusoiu, I.; Muntean, C.; Grozescu, I. Romanian Viscum album L.—Untargeted Low-Molecular Metabolomic Approach to Engineered Viscum–AuNPs Carrier Assembly. Plants 2022, 11, 1820. [Google Scholar] [CrossRef]
- Sachan, A.; Penumadu, D. Identification of Microfabric of Kaolinite Clay Mineral Using X-ray Diffraction Technique. Geotech. Geol. Eng. 2007, 25, 603–616. [Google Scholar] [CrossRef]
- Edraki, M.; Moghaddampour, I.M.; Alinia-Ahandani, E.; Keivani, M.B.; Sheydaei, M. Ginger intercalated sodium montmorillonite nano clay: Assembly, characterization, and investigation antimicrobial properties. Chem. Rev. Lett. 2021, 4, 120–129. [Google Scholar] [CrossRef]
- Silva, S.; Alves, N.; Silva, P.; Vieira, T.; Maciel, P.; Castellano, L.R.; Bonan, P.; Velozo, C.; Albuquerque, D. Antibacterial Activity of Rosmarinus officinalis, Zingiber officinale, Citrus aurantium bergamia, and Copaifera officinalis Alone and in Combination with Calcium Hydroxide against Enterococcus faecalis. Biomed Res. Int. 2019, 2019, 8129439. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Silva, A.M.; Laveriano-Santos, E.P.; Lozano-Castellón, J.; Lamuela-Raventós, R.M.; Švarc-Gajíc, J.; Delerue-Matos, C.; Estevinho, B.N.; Costa, P.C.; Rodrigues, F. Development and characterization of spray-drying microparticles loaded with chestnut shells extract: New insights for oral mucositis therapy. Powder Technol. 2024, 446, 120182. [Google Scholar] [CrossRef]
- Johnson, J.B.; Batley, R.J.; Mani, J.S.; Naiker, M. How Low Can It Go? ATR–FTIR Characterization of Compounds Isolated from Ginger at the Nanogram Level. Eng. Proc. 2023, 56, 80. [Google Scholar] [CrossRef]
- Karthickeyan, V. Effect of nature based antioxidant from Zingiber officinale Rosc. on the oxidation stability, engine performance and emission characteristics with neem oil methyl ester. Heat Mass Transf. 2018, 54, 3409–3420. [Google Scholar] [CrossRef]
- Alzarieni, K.Z.; Bani Amer, A.R.; El-Elimat, T.; Kenttämaa, H.I. Characterization of Natural Cellulosic Fiber Obtained from the Flower Heads of Milk Thistle (Silybum marianum) as a Potential Polymer Reinforcement Material. J. Nat. Fibers 2023, 20, 2211289. [Google Scholar] [CrossRef]
- Dash, D.K.; Tyagi, C.K.; Sahu, A.K.; Tripathi, V. Revisiting the medicinal value of terpenes and terpenoids. In Revisiting Plant Biostimulants, 1st ed.; Meena, V.S., Parewa, H.P., Meena, S.K., Eds.; IntechOpen: London, UK, 2022; pp. 1–17. [Google Scholar] [CrossRef]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Pinzon-Guzman, C.; Barbul, A. Arginine—Dual roles as an onconutrient and immunonutrient. J. Surg. Oncol. 2017, 115, 273–280. [Google Scholar] [CrossRef]
- Kim, S.H.; Roszik, J.; Grimm, E.A.; Ekmekcioglu, S. Impact of L-Arginine Metabolism on Immune Response and Anticancer Immunotherapy. Front. Oncol. 2018, 8, 67. [Google Scholar] [CrossRef]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Coniglio, S.; Shumskaya, M.; Vassiliou, E. Unsaturated Fatty Acids and Their Immunomodulatory Properties. Biology 2023, 12, 279. [Google Scholar] [CrossRef]
- Vezza, T.; Canet, F.; de Marañón, A.M.; Bañuls, C.; Rocha, M.; Víctor, V.M. Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders. Antioxidants 2020, 9, 1266. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I.I.; Gheorghiţă, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Sun, D.J.; Zhu, L.J.; Zhao, Y.Q.; Zhen, Y.Q.; Zhang, L.; Lin, C.C.; Chen, L.X. Diarylheptanoid: A privileged structure in drug discovery. Fitoterapia 2020, 142, 104490. [Google Scholar] [CrossRef]
- Yu, T.-J.; Tang, J.-Y.; Shiau, J.-P.; Hou, M.-F.; Yen, C.-H.; Ou-Yang, F.; Chen, C.-Y.; Chang, H.-W. Gingerenone A Induces Antiproliferation and Senescence of Breast Cancer Cells. Antioxidants 2022, 11, 587. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, S.; Zhang, Q.; Zhang, H.; Fan, Y.; Qiu, F.; Kang, N. Biological and pharmacological effects of hexahydrocurcumin, a metabolite of curcumin. Arch. Biochem. Biophys. 2018, 646, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Polaquini, C.R.; Morão, L.G.; Nazaré, A.C.; Torrezan, G.S.; Dilarri, G.; Cavalca, L.B.; Campos, D.L.; Silva, I.C.; Pereira, J.A.; Scheffers, D.J.; et al. Antibacterial activity of 3,3’-dihydroxycurcumin (DHC) is associated with membrane perturbation. Bioorg. Chem. 2019, 90, 103031. [Google Scholar] [CrossRef]
- Xi, H.; Weng, Y.; Zheng, Y.; Wu, L.; Han, D. Diacetoxy-6-gingerdiol protects the extracellular matrix of nucleus pulposus cells and ameliorates intervertebral disc degeneration by inhibiting the IL-1β-mediated NLRP3 pathway. Heliyon 2024, 10, e37877. [Google Scholar] [CrossRef] [PubMed]
- Alberti, Á.; Riethmüller, E.; Béni, S. Characterization of diarylheptanoids: An emerging class of bioactive natural products. J. Pharm. Biomed. Anal. 2018, 147, 13–34. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Al Shabrmi, F.M.; Aly, S.M. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int. J. Physiol. Pathophysiol. Pharmacol. 2014, 6, 125–136. [Google Scholar]
- Imm, J.; Zhang, G.; Chan, L.Y.; Nitteranon, V.; Parkin, K.L. [6]-Dehydroshogaol, a minor component in ginger rhizome, exhibits quinone reductase inducing and anti-inflammatory activities that rival those of curcumin. Food Res. Int. 2010, 43, 2208–2213. [Google Scholar] [CrossRef]
- Li, F.; Nitteranon, V.; Tang, X.; Liang, J.; Zhang, G.; Parkin, K.L.; Hu, Q. In vitro antioxidant and anti-inflammatory activities of 1-dehydro-[6]-gingerdione, 6-shogaol, 6-dehydroshogaol and hexahydrocurcumin. Food Chem. 2012, 135, 332–337. [Google Scholar] [CrossRef]
- Pandey, V.K.; Srivastava, S.; Ashish; Dash, K.K.; Singh, R.; Dar, A.H.; Singh, T.; Farooqui, A.; Shaikh, A.M.; Kovacs, B. Bioactive properties of clove (Syzygium aromaticum) essential oil nanoemulsion: A comprehensive review. Heliyon 2024, 10, e22437. [Google Scholar] [CrossRef]
- Hamdy, A.E.; Abdel-Aziz, H.F.; El-khamissi, H.; AlJwaizea, N.I.; El-Yazied, A.A.; Selim, S.; Tawfik, M.M.; AlHarbi, K.; Ali, M.S.M.; Elkelish, A. Kaolin Improves Photosynthetic Pigments, and Antioxidant Content, and Decreases Sunburn of Mangoes: Field Study. Agronomy 2022, 12, 1535. [Google Scholar] [CrossRef]
- Najem, R.S. Antimicrobial activity of ginger (Zingiber officinale) extracts against Klebsiella pneumoniae and Pseudomonas aeruginosa. J. Biol. Nat. 2015, 4, 193–199. [Google Scholar]
- Dharmapala, K.P.; Amarakoon, R. An Evaluation of Antimicrobial Activity of Common Zingiber officinale Cultivars Grown in Sri Lanka. Eur. J. Agric. Food Sci. 2024, 6, 33–38. [Google Scholar] [CrossRef]
- Sebiomo, A.; Awofodu, A.D.; Awosanya, A.O.; Awotona, F.E.; Ajayi, A.J. Comparative studies of antibacterial effect of some antibiotics and ginger (Zingiber officinale) on two pathogenic bacteria. J. Microbiol. Antimicrob. 2011, 3, 18–22. [Google Scholar]
- Shehu, Z.; Lamayi, D.W.; Sabo, M.A.; Shafiu, M.M. Synthesis, Characterization and Antibacterial Activity of Kaolin/Gum Arabic Nanocomposite on Escherichia coli and Pseudomonas aeruginosa. Res. J. Nanosci. Eng. 2018, 2, 23–29. [Google Scholar] [CrossRef]
- Isichei-ukah, O.B.; Ofie, O.G. Antimicrobial Activity of Clay against Some Skin Infection Isolates. Niger J. Microbiol. 2023, 37, 6683–6690. [Google Scholar]
- Karaubayeva, A.A.; Bekezhanova, T.; Zhaparkulova, K.; Susniak, K.; Sobczynski, J.; Kazimierczak, P.; Przekora, A.; Skalicka-Wozniak, K.; Kulinowski, Ł.; Glowniak-Lipa, A.; et al. Antimicrobial Mixture Based on Micronized Kaolinite and Ziziphora Essential Oil as a Promising Formulation for the Management of Infected Wounds. Int. J. Mol. Sci. 2024, 25, 13192. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, E.K.; Bava, S.V.; Narayanan, S.S.; Nath, L.R.; Thulasidasan, A.K.T.; Soniya, E.V.; Anto, R.J. [6]-Gingerol Induces Caspase-Dependent Apoptosis and Prevents PMA-Induced Proliferation in Colon Cancer Cells by Inhibiting MAPK/AP-1 Signaling. PLoS ONE 2014, 9, e104401. [Google Scholar] [CrossRef]
- Zivarpour, P.; Nikkhah, E.; Maleki Dana, P.; Asemi, Z.; Hallajzadeh, J. Molecular and biological functions of gingerol as a natural effective therapeutic drug for cervical cancer. J. Ovarian Res. 2021, 14, 43. [Google Scholar] [CrossRef]
- Choi, N.R.; Choi, W.G.; Kwon, M.J.; Woo, J.H.; Kim, B.J. [6]-Gingerol Induces Caspase-Dependent Apoptosis in Bladder Cancer Cells via MAPK and ROS Signaling. Int. J. Med. Sci. 2022, 19, 1093–1102. [Google Scholar] [CrossRef]
- Sarami, S.; Dadmanesh, M.; Hassan, Z.M.; Ghorban, K. Study on the Effect of Ethanol Ginger Extract on Cell Viability and p53 Level in Breast and Pancreatic Cancer. Arch. Pharm. Pract. 2020, 11, 115–121. [Google Scholar]
- Malmir, S.; Ebrahimi, A.; Mahjoubi, F. Effect of ginger extracts on colorectal cancer HCT-116 cell line in the expression of MMP-2 and KRAS. Gene Rep. 2020, 21, 100824. [Google Scholar] [CrossRef]
- Yao, G.L.; Li, H.; Teng, L.; Fan, Y.; Huang, W. Green decorated of gold nanoparticles over kaolin mediated by plant extract and investigation of its anti-bile duct cancer properties in the in vitro condition. Arab. J. Chem. 2024, 17, 105893. [Google Scholar] [CrossRef]
- Gianni, E.; Avgoustakis, K.; Papoulis, D. Kaolinite group minerals: Applications in cancer diagnosis and treatment. Eur. J. Pharm. Biopharm. 2020, 154, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.D.; Nasser, N.; AlZahrani, S.A.; Allam, A.A.; Abukhadra, M.R. Characterization of Kaolinite Single Methoxy Nano-Sheets as Potential Carriers of Oxaliplatin Drug of Enhanced Loading, Release, and Cytotoxicity Properties During the Treatment of Colorectal Cancer. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2111–2126. [Google Scholar] [CrossRef]
- Tian, L.; Abukhadra, M.R.; Mohamed, A.S.; Nadeem, A.; Ahmad, S.F.; Ibrahim, K.E. Insight into the Loading and Release Properties of an Exfoliated Kaolinite/Cellulose Fiber (EXK/CF) Composite as a Carrier for Oxaliplatin Drug: Cytotoxicity and Release Kinetics. ACS Omega 2020, 5, 19165–19173. [Google Scholar] [CrossRef] [PubMed]
- Rozhina, E.; Batasheva, S.; Miftakhova, R.; Yan, X.; Vikulina, A.; Volodkin, D.; Fakhrullin, R. Comparative cytotoxicity of kaolinite, halloysite, multiwalled carbon nanotubes and graphene oxide. Appl. Clay Sci. 2021, 205, 106041. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Vlase, G.; Vlase, T.; Bita, A.; Bejenaru, C.; Buema, G.; Bejenaru, L.E.; Dumitru, A.; Boia, E.R. An Innovative Approach to a Potential Neuroprotective Sideritis scardica–Clinoptilolite Phyto-Nanocarrier: In Vitro Investigation and Evaluation. Int. J. Mol. Sci. 2024, 25, 1712. [Google Scholar] [CrossRef]
- Salem, M.A.I.; Marzouk, M.I.; El-Kazak, A.M. Synthesis and Characterization of Some New Coumarins with In Vitro Antitumor and Antioxidant Activity and High Protective Effects against DNA Damage. Molecules 2016, 21, 249. [Google Scholar] [CrossRef]
- Al Hafiz, S.S.; Syofyan, S.; Alen, Y.; Hamidi, D. FT–IR Fingerprinting Analysis for Classification of West Sumatra Small Ginger (Zingiber officinale Roscoe) Essential Oil and its Antioxidant Activity. Trop. J. Nat. Prod. Res. 2024, 8, 6081–6086. [Google Scholar] [CrossRef]
- Gaikwad, D.D.; Shinde Sachin, K.; Kawade Ashwini, V.; Jadhav, S.J.; Gadhave, M.V. Isolation and standardization of gingerol from ginger rhizome by using TLC, HPLC, and identification tests. Pharma. Innov. J. 2017, 6, 179–182. [Google Scholar]
- Singh, V.; Pandey, H.; Misra, V.; Singh, D. Biocompatible herbal polymeric nano-formulation of [6]-gingerol: Development, optimisation, and characterization. Ecol. Environ. Conserv. 2022, 28, 1473–1477. [Google Scholar] [CrossRef]
- Scarsini, M.; Thurotte, A.; Veidl, B.; Amiard, F.; Niepceron, F.; Badawi, M.; Lagarde, F.; Schoefs, B.; Marchand, J. Metabolite Quantification by Fourier Transform Infrared Spectroscopy in Diatoms: Proof of Concept on Phaeodactylum tricornutum. Front. Plant Sci. 2021, 12, 756421. [Google Scholar] [CrossRef]



| No. | tR (min) | RI Determined | Kováts Index | Compound | Formula | Molecular Weight (g/mol) | Area (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.78 | 998 | 1000 | octanal | C8H16O | 128.21 | 1.71 | [64,65] |
| 2 | 5.43 | 2444 | 2445 | isooctyl phthalate | C24H38O4 | 390.56 | 1.83 | [64,65] |
| 3 | 6.62 | 1174 | 1175 | decanal | C10H20O | 156.26 | 2.08 | [64,65] |
| 4 | 7.09 | 1341 | 1343 | eugenol | C10H12O2 | 164.20 | 1.11 | [64,65] |
| 5 | 8.22 | 796 | 798 | hexanal | C6H12O | 100.16 | 1.23 | [64,65] |
| 6 | 8.88 | 1036 | 1039 | cineole | C10H18O | 154.25 | 1.83 | [64,65] |
| 7 | 9.54 | 2656 | 2657 | squalene | C30H50 | 410.7 | 1.77 | [64,65] |
| 8 | 10.01 | 1408 | 1410 | vanillin | C8H8O3 | 152.15 | 1.42 | [64,65] |
| 9 | 10.94 | 1177 | 1179 | α-terpinene | C10H16 | 136.23 | 1.57 | [64,65,66,67] |
| 10 | 11.15 | 1238 | 1240 | citral | C10H16O | 152.23 | 2.62 | [64,65,66,67] |
| 11 | 12.09 | 1493 | 1496 | zingiberene | C15H24 | 204.35 | 10.18 | [67] |
| 12 | 13.67 | 1656 | 1657 | zingiberol | C15H26O | 222.37 | 5.07 | [64,65,66,67] |
| 13 | 14.91 | 1323 | 1385 | linalool | C10H18O | 154.25 | 3.39 | [64,65] |
| 14 | 15.73 | 1387 | 1389 | 2-nonanone | C9H18O | 142.24 | 3.12 | [64,65] |
| 15 | 16.42 | 981 | 983 | methylheptenone | C8H14O | 126.20 | 2.62 | [64,65] |
| 16 | 17.93 | 1354 | 1356 | citronellyl acetate | C12H22O2 | 198.30 | 3.23 | [64,65] |
| 17 | 19.08 | 1093 | 1095 | camphor | C10H16O | 152.23 | 1.62 | [64,65] |
| 18 | 19.62 | 1152 | 1154 | myrtenal | C10H14O | 150.22 | 1.16 | [64,65] |
| 19 | 20.17 | 2182 | 2184 | gingerol | C17H26O4 | 294.4 | 5.44 | [64,65] |
| 20 | 20.96 | 972 | 974 | 2-heptanone | C7H14O | 114.19 | 2.03 | [64,65] |
| 21 | 21.36 | 1485 | 1487 | curcumene | C15H22 | 202.33 | 2.57 | [64,65,66,67] |
| 22 | 22.21 | 1276 | 1277 | bornyl acetate | C12H20O2 | 196.29 | 4.36 | [64,65] |
| 23 | 23.07 | 1345 | 1347 | ethyl cinnamate | C11H12O2 | 176.21 | 1.73 | [64,65] |
| 24 | 24.03 | 1279 | 1281 | ascaridole | C10H16O2 | 168.23 | 2.17 | [64,65] |
| 25 | 24.77 | 597 | 599 | ethyl acetate | C4H8O2 | 88.11 | 1.78 | [64,65,66] |
| 26 | 25.68 | 1650 | 1651 | zingerone | C11H14O3 | 194.23 | 5.42 | [64,65,66,67] |
| 27 | 26.93 | 2085 | 2087 | 4-shogaol | C15H20O3 | 248.32 | 4.26 | [67] |
| 28 | 27.76 | 2234 | 2235 | 6-paradol | C17H26O3 | 278.40 | 3.51 | [67] |
| 29 | 29.11 | 1167 | 1168 | decanone | C10H20O | 156.26 | 1.53 | [64,65] |
| 30 | 30.81 | 1498 | 1451 | α-cadinene | C15H26 | 204.35 | 1.49 | [64,65] |
| 31 | 32.22 | 1296 | 1297 | 2-undecanone | C11H22O | 170.29 | 2.06 | [64,65] |
| 32 | 34.52 | 1579 | 1581 | spathulenol | C15H24O | 220.35 | 1.23 | [64,65] |
| 33 | 38.86 | 1732 | 1734 | zerumbone | C15H22O | 218.33 | 3.12 | [67] |
| No. | m/z Detected | Theoretic m/z | Formula | Tentative Identification | Category | Ref. |
|---|---|---|---|---|---|---|
| 1 | 76.07 | 75.07 | C2H5NO2 | glycine | amino acids | [7,10,68,69] |
| 2 | 90.09 | 89.09 | C3H7NO2 | alanine | amino acids | [7,10,68,69] |
| 3 | 106.09 | 105.09 | C3H7NO3 | serine | amino acids | [7,10,68,69] |
| 4 | 116.14 | 115.13 | C5H9NO2 | proline | amino acids | [7,10,68,69] |
| 5 | 118.15 | 117.15 | C5H11NO2 | valine | amino acids | [7,10,68,69] |
| 6 | 120.12 | 119.12 | C4H9NO3 | threonine | amino acids | [7,10,68,69] |
| 7 | 122.16 | 121.16 | C3H7NO2S | cysteine | amino acids | [7,10,68,69] |
| 8 | 132.17 | 131.17 | C6H13NO2 | leucine | amino acids | [7,10,68,69] |
| 9 | 134.11 | 133.10 | C4H7NO4 | aspartic acid | amino acids | [7,10,26,68,69] |
| 10 | 147.11 | 146.12 | C5H8NO4 | glutamate | amino acids | [7,10,68,69] |
| 11 | 147.18 | 146.19 | C6H14N2O2 | lysine | amino acids | [7,10,68,69] |
| 12 | 150.22 | 149.21 | C5H11NO2S | methionine | amino acids | [7,10,68,69] |
| 13 | 156.14 | 155.15 | C6H9N3O2 | histidine | amino acids | [7,10,68,69] |
| 14 | 166.18 | 165.19 | C9H11NO2 | phenylalanine | amino acids | [7,10,68,69] |
| 15 | 175.21 | 174.20 | C6H14N4O2 | arginine | amino acids | [7,10,68,69] |
| 16 | 182.18 | 181.19 | C9H11NO3 | tyrosine | amino acids | [7,10,68,69] |
| 17 | 205.23 | 204.22 | C11H12N2O2 | tryptophan | amino acids | [7,10,68,69] |
| 18 | 207.25 | 206.24 | C12H14O3 | eugenyl acetate | phenylpropanoids | [7,10,68,69] |
| 19 | 357.41 | 356.40 | C21H24O5 | gingerenone A | diarylheptanoids | [7,10,14,23,24,25,26,27,28,68,69] |
| 20 | 375.39 | 374.40 | C21H26O6 | hexahydrocurcumin | diarylheptanoids | [7,10,14,23,24,25,26,27,28,68,69] |
| 21 | 381.49 | 380.48 | C21H32O6 | diacetoxy-6-gingerdiol | diarylheptanoids | [7,10,14,23,24,25,26,27,28,68,69] |
| 22 | 401.41 | 400.40 | C21H20O8 | dihydroxy-curcumin | diarylheptanoids | [7,10,14,23,24,25,26,27,28,68,69] |
| 23 | 291.39 | 290.40 | C17H22O4 | 6-dehydro-gingerdione | gingerols (polyphenols) | [7,10,14,23,24,25,26,27,28,68,69] |
| 24 | 145.22 | 144.21 | C8H16O2 | caprylic acid | fatty acids | [7,10,68,69,70] |
| 25 | 173.26 | 172.26 | C10H20O2 | capric acid | fatty acids | [7,10,68,69,70] |
| 26 | 201.31 | 200.32 | C12H24O2 | lauric acid | fatty acids | [7,10,68,69,70] |
| 27 | 229.37 | 228.37 | C14H28O2 | myristic acid | fatty acids | [7,10,68,69,70] |
| 28 | 255.41 | 254.41 | C16H30O2 | palmitoleic acid | fatty acids | [7,10,68,69,70] |
| 29 | 271.49 | 270.50 | C17H34O2 | margaric acid | fatty acids | [7,10,68,69,70] |
| 30 | 285.51 | 284.50 | C18H36O2 | stearic acid | fatty acids | [7,10,68,69,70] |
| 31 | 313.49 | 312.50 | C20H40O2 | arachidic acid | fatty acids | [7,10,68,69,70] |
| 32 | 341.59 | 340.60 | C22H44O2 | behenic acid | fatty acids | [7,10,68,69,70] |
| 33 | 369.61 | 368.60 | C24H48O2 | lignoceric acid | fatty acids | [7,10,68,69,70] |
| 34 | 187.29 | 186.29 | C11H22O2 | undecanoic acid | fatty acids | [7,10,68,69,70] |
| 35 | 257.43 | 256.42 | C16H32O2 | palmitic acid | fatty acids | [7,10,68,69,70] |
| 36 | 281.39 | 280.40 | C18H32O2 | linoleic acid | fatty acids | [7,10,68,69,70] |
| 37 | 283.51 | 282.50 | C18H34O2 | oleic acid | fatty acids | [7,10,68,69,70] |
| 38 | 271.25 | 270.24 | C15H10O5 | apigenin | flavonoids | [7,10,25,68,69] |
| 39 | 287.23 | 286.24 | C15H10O6 | kaempferol | flavonoids | [7,10,25,68,69] |
| 40 | 291.27 | 290.27 | C15H14O6 | catechin | flavonoids | [7,10,25,68,69] |
| 41 | 303.24 | 302.23 | C15H10O7 | quercetin | flavonoids | [7,10,25,68,69] |
| 42 | 611.49 | 610.50 | C27H30O16 | rutin | flavonoids | [7,10,25,68,69] |
| 43 | 171.11 | 170.12 | C7H6O5 | gallic acid | phenolic acids | [7,10,25,68,69] |
| 44 | 195.17 | 194.18 | C10H10O4 | ferulic acid | phenolic acids | [7,10,25,68,69] |
| 45 | 149.17 | 148.16 | C9H8O2 | cinnamic acid | phenolic acids | [7,10,25,68,69] |
| 46 | 139.13 | 138.12 | C7H6O3 | salicylic acid | phenolic acids | [7,10,25,68,69] |
| 47 | 361.31 | 360.30 | C18H16O8 | rosmarinic acid | phenolic acids | [7,10,25,68,69] |
| 48 | 155.12 | 154.12 | C7H6O4 | protocatechuic acid | phenolic acids | [7,10,25,68,69] |
| 49 | 413.69 | 412.70 | C29H48O | stigmasterol | sterols | [7,10,68,69] |
| 50 | 415.71 | 414.70 | C29H50O | β-sitosterol | sterols | [7,10,68,69] |
| 51 | 577.81 | 576.80 | C35H60O6 | daucosterol | sterols | [7,10,68,69] |
| 52 | 137.23 | 136.23 | C10H16 | α-terpinene | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 53 | 151.21 | 150.22 | C10H14O | myrtenal | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 54 | 153.23 | 152.23 | C10H16O | citral | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 55 | 155.25 | 154.25 | C10H18O | cineole | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 56 | 155.25 | 154.25 | C10H18O | linalool | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 57 | 165.19 | 164.20 | C10H12O2 | eugenol | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 58 | 169.23 | 168.23 | C10H16O2 | ascaridole | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 59 | 171.24 | 170.25 | C10H18O2 | 8-hydroxy geraniol | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 60 | 197.29 | 196.29 | C12H20O2 | bornyl acetate | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 61 | 199.31 | 198.30 | C12H22O2 | citronellyl acetate | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 62 | 203.32 | 202.33 | C15H22 | α-curcumene | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 63 | 205.35 | 204.35 | C15H24 | zingiberene | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 64 | 207.36 | 206.37 | C15H26 | cadinene | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 65 | 219.34 | 218.33 | C15H22O | zerumbone | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 66 | 221.35 | 220.35 | C15H24O | spathulenol | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 67 | 223.37 | 222.37 | C15H26O | zingiberol | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 68 | 409.51 | 408.50 | C22H32O7 | ingol-12-acetate | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 69 | 411.69 | 410.70 | C30H50 | squalene | terpenes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 70 | 151.18 | 150.17 | C9H10O2 | 4-vinylguaiacol | phenols | [7,10,68,69] |
| 71 | 295.41 | 294.40 | C17H26O4 | gingerol | gingerols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 72 | 291.39 | 290.40 | C17H22O4 | dehydrogingerdione | gingerols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72,73] |
| 73 | 295.41 | 294.40 | C17H26O4 | 6-gingerol | gingerols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 74 | 309.39 | 308.40 | C18H28O4 | methyl-6-gingerol | gingerols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 75 | 323.45 | 322.44 | C19H30O4 | 8-gingerol | gingerols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 76 | 351.49 | 350.50 | C21H34O4 | 10-gingerol | gingerols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 77 | 381.51 | 380.50 | C21H32O6 | diacetoxy-6-gingerdiol | gingerols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 78 | 393.61 | 392.60 | C24H40O4 | methyl-12-gingerol | gingerols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 79 | 359.49 | 358.50 | C17H26O6S | 6-gingesulfonic acid | sulfonates | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 80 | 297.39 | 296.40 | C17H28O4 | gingerdiol | fatty alcohol esters | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 81 | 249.33 | 248.32 | C15H20O3 | 4-shogaol | shogaols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 82 | 275.35 | 274.35 | C17H22O3 | 6-dehydroshogaol | shogaols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72,73] |
| 83 | 277.39 | 276.40 | C17H24O3 | shogaol | shogaols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 84 | 333.51 | 332.50 | C21H32O3 | 10-shogaol | shogaols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 85 | 361.54 | 360.53 | C23H36O3 | 12-shogaol | gingerols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 86 | 279.39 | 278.40 | C17H26O3 | paradol | paradols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 87 | 307.45 | 306.44 | C19H30O3 | 8-paradol | paradols (polyphenols) | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 88 | 89.12 | 88.11 | C4H8O2 | ethyl acetate | esters | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 89 | 177.21 | 176.21 | C11H12O2 | ethyl cinnamate | esters | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 90 | 391.61 | 390.60 | C24H38O4 | isooctyl phthalate | esters | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 91 | 338.59 | 337.60 | C22H43NO | 13-docosamide | amides | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 92 | 153.15 | 152.15 | C8H8O3 | vanillin | aldehydes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 93 | 157.25 | 156.26 | C10H20O | decanal | aldehydes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 94 | 101.17 | 100.16 | C6H12O | hexanal | aldehydes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 95 | 129.21 | 128.21 | C8H16O | octanal | aldehydes | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 96 | 115.19 | 114.19 | C7H14O | 2-heptanone | ketones | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 97 | 127.21 | 126.20 | C8H14O | methylheptenone | ketones | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 98 | 143.25 | 142.24 | C9H18O | 2-nonanone | ketones | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 99 | 157.25 | 156.26 | C10H20O | decanone | ketones | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 100 | 171.29 | 170.29 | C11H22O | 2-undecanone | ketones | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 101 | 195.23 | 194.23 | C11H14O3 | zingerone | ketones | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 102 | 43.09 | 42.08 | C3H6 | cyclopropane | hydrocarbons | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 103 | 85.17 | 84.16 | C6H12 | cyclohexane | hydrocarbons | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 104 | 349.59 | 348.60 | C25H48 | 1-pentadecyl-decahydronaphthalene | hydrocarbons | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| 105 | 367.71 | 366.70 | C26H54 | hexacosane | hydrocarbons | [7,10,12,13,14,23,24,25,26,27,28,68,69,70,71,72] |
| VOC | Odor Profile |
|---|---|
| α-terpinene | woody |
| myrtenal | spicy |
| citral | citrus |
| cineole | eucalyptus |
| eugenol | spicy |
| ascaridole | pungent |
| 8-hydroxy geraniol | citrus |
| bornyl acetate | piney |
| citronellyl acetate | fruity |
| α-curcumene | spicy |
| zingiberene | spicy |
| cadinene | citrus |
| zerumbone | pungent |
| spathulenol | earthy |
| myrtenal | spicy |
| linalool | floral, woody |
| 4-vinylguaiacol | spicy |
| ethyl acetate | fruity |
| ethyl cinnamate | fruity |
| vanillin | vanilla |
| decanal | citrus |
| hexanal | herbal |
| octanal | citrus |
| 2-heptanone | fruity |
| methylheptenone | fruity |
| 2-nonanone | cheesy |
| decanone | citrus |
| 2-undecanone | fruity |
| zingerone | spicy |
| cyclopropane | petroleum |
| cyclohexane | sweet |
| Phytochemicals Category | Wavenumber (cm−1) | Ref. |
|---|---|---|
| terpenoids | 2925; 2349; 1738; 1249; 1090; 998; 815 | [7,74] |
| alkaloids | 3388; 1652; 1639; 1602; 1569; 1405; 753; 738; 663 | [7,74] |
| coumarins | 3430; 1727; 1607; 1265; 1132; 1252; 900–603 | [7,75] |
| flavonoids | 3405–3100; 1526; 1460; 1438; 1363; 1270 | [7,58] |
| phenolic acids | 1364; 1250; 1240; 1171–1100; 1034 | [57,58,74] |
| fatty acids | 2925; 1351; 1247; 1089; 742; 720 | [7,58,60] |
| phytosterols | 1465; 1378; 1059; 741 | [7,58,60] |
| phenylpropanoids | 3190; 3003; 1635; 1505; 1451; 1246 | [7,76,77] |
| phytoecdysteroids | 3303; 1651 | [7,76,77] |
| diarylheptanoids | 2102–2549; 1621–1742 | [77] |
| gingerols | 3398; 2838; 1665; 1623; 1518; 1368; 1274; 1234; 1037; 962; 812 | [7,76,77,78,79] |
| shogaols | 2834; 1732; 1597; 1481; 1246; 1182; 815 | [7,76,77,78,79] |
| amino acids | 3401; 3328–3132; 2531–2758; 2128; 1725–1756; 1690; 1673; 1661; 1650; 1643; 1634; 1619; 1609; 1612–1658; 1502–1602 | [58,59,61] |
| paradols | 3402; 2970; 1601; 1298; 778 | [7,76,77,78,79] |
| fatty acid esters | 1745; 1310; 3450; 2972; 1469; 813 | [80,81] |
| Sample | Particle Size Diameter (μm) | Volume Diameter (μm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| D [3,2] | D [4,3] | d10 | d50 | d90 | |||||
| MZO carrier | 771.21 ± 0.004 | 806.12 ± 0.002 | 431.13 ± 0.008 | 628.19 ± 0.012 | 879.23 ± 0.019 | ||||
| MZO–kaolinite system | Peak 1 | Peak 2 | Overall | Peak 1 | Peak 2 | Overall | 4.14 ± 0.002 | 10.11 ± 0.013 | 90.41 ± 0.007 |
| 8.42 ± 0.003 | 203.04 ± 0.002 | 34.27 ± 0.006 | 10.89 ± 0.005 | 251.73 ± 0.005 | 64.45 ± 0.004 | ||||
| Sample | EE% | EC% | EY% |
|---|---|---|---|
| MZO carrier | 64.54 ± 0.12 | 62.23 ± 0.19 | 63.61 ± 0.17 |
| MZO–kaolinite system | 62.61 ± 0.09 | 60.05 ± 0.22 | 62.35 ± 0.34 |
| Pathogenic Microorganism | Sample | Inhibition Zone Diameter (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample Concentration (μg/mL) | Positive Control (Gentamicin, 100 μg/mL) | Negative Control (DMSO) | ||||||
| 100 | 125 | 150 | 175 | 200 | ||||
| Staphylococcus aureus | Z. officinale | 24.02 ± 0.18 | 35.72 ± 0.57 | 43.13 ± 0.25 | 49.73 ± 0.33 | 56.84 ± 0.33 | 22.18 ± 0.22 | 0 |
| Kaolinite | 12.35 ± 0.18 | 14.78 ± 0.23 | 16.17 ± 0.21 | 18.54 ± 0.36 | 20.87 ± 0.43 | |||
| ZO–kaolinite | 31.17 ± 0.31 | 43.32 ± 0.25 | 59.67 ± 0.34 | 67.73 ± 0.23 | 73.33 ± 0.41 | |||
| MZO carrier | 26.42 ± 0.16 | 37.93 ± 0.51 | 45.31 ± 0.26 | 51.34 ± 0.18 | 58.15 ± 0.63 | |||
| MZO–kaolinite system | 41.15 ± 0.41 | 47.68 ± 0.16 | 62.25 ± 0.17 | 72.31 ± 0.61 | 77.26 ± 0.58 | |||
| Enterococcus faecalis | Z. officinale | 14.29 ± 0.13 | 16.55 ± 0.21 | 19.31 ± 0.32 | 21.07 ± 0.18 | 23.73 ± 0.08 | 24.31 ± 0.21 | 0 |
| Kaolinite | 11.04 ± 0.15 | 12.89 ± 0.24 | 14.18 ± 0.31 | 16.27 ± 0.13 | 19.05 ± 0.42 | |||
| ZO–kaolinite | 18.70 ± 0.21 | 22.07 ± 0.32 | 29.44 ± 0.45 | 34.08 ± 0.22 | 40.13 ± 0.32 | |||
| MZO carrier | 16.77 ± 0.41 | 18.62 ± 0.17 | 21.19 ± 0.18 | 24.06 ± 0.22 | 27.05 ± 0.34 | |||
| MZO–kaolinite system | 27.36 ± 0.19 | 32.04 ± 0.13 | 38.22 ± 0.33 | 42.87 ± 0.54 | 45.47 ± 0.43 | |||
| Bacillus cereus | Z. officinale | 13.27 ± 0.19 | 17.01 ± 0.08 | 21.32 ± 0.42 | 26.03 ± 0.33 | 29.85 ± 0.51 | 18.22 ± 0.16 | 0 |
| Kaolinite | 10.49 ± 0.31 | 13.58 ± 0.28 | 17.05 ± 0.09 | 19.98 ± 0.17 | 22.04 ± 0.31 | |||
| ZO–kaolinite | 19.23 ± 0.16 | 20.61 ± 0.07 | 24.18 ± 0.22 | 34.91 ± 0.46 | 44.77 ± 0.33 | |||
| MZO carrier | 15.18 ± 0.28 | 19.13 ± 0.11 | 22.86 ± 0.08 | 28.54 ± 0.42 | 31.39 ± 0.47 | |||
| MZO–kaolinite system | 24.38 ± 0.11 | 28.03 ± 0.63 | 32.68 ± 0.35 | 39.89 ± 0.17 | 48.06 ± 0.27 | |||
| Pseudomonas aeruginosa | Z. officinale | 30.03 ± 0.15 | 35.12 ± 0.21 | 41.14 ± 0.35 | 49.26 ± 0.07 | 53.47 ± 0.32 | 30.54 ± 0.21 | 0 |
| Kaolinite | 13.08 ± 0.09 | 19.22 ± 0.12 | 24.66 ± 0.23 | 29.88 ± 0.48 | 35.62 ± 0.51 | |||
| ZO–kaolinite | 35.25 ± 0.21 | 49.87 ± 0.36 | 57.28 ± 0.51 | 62.82 ± 0.08 | 69.02 ± 0.38 | |||
| MZO carrier | 32.18 ± 0.11 | 37.14 ± 0.23 | 43.32 ± 0.34 | 51.18 ± 0.22 | 55.02 ± 0.41 | |||
| MZO–kaolinite system | 40.29 ± 0.63 | 56.37 ± 0.42 | 62.87 ± 0.17 | 69.27 ± 0.26 | 74.95 ± 0.14 | |||
| Klebsiella pneumoniae | Z. officinale | 40.02 ± 0.31 | 48.65 ± 0.23 | 54.22 ± 0.27 | 59.32 ± 0.24 | 66.47 ± 0.18 | 15.75 ± 0.23 | 0 |
| Kaolinite | 10.51 ± 0.06 | 13.23 ± 0.22 | 16.99 ± 0.13 | 19.89 ± 0.42 | 21.78 ± 0.33 | |||
| ZO–kaolinite | 42.34 ± 0.09 | 50.76 ± 0.34 | 56.43 ± 0.23 | 70.12 ± 0.17 | 78.72 ± 0.42 | |||
| MZO carrier | 49.67 ± 0.34 | 50.52 ± 0.51 | 56.23 ± 0.61 | 61.85 ± 0.17 | 68.75 ± 0.26 | |||
| MZO–kaolinite system | 58.89 ± 0.16 | 59.45 ± 0.35 | 65.27 ± 0.42 | 73.78 ± 0.23 | 82.03 ± 0.32 | |||
| Escherichia coli | Z. officinale | 13.25 ± 0.31 | 15.61 ± 0.12 | 17.82 ± 0.27 | 19.94 ± 0.33 | 21.12 ± 0.07 | 20.59 ± 0.32 | 0 |
| Kaolinite | 15.01 ± 0.67 | 18.89 ± 0.52 | 22.18 ± 0.27 | 26.87 ± 0.35 | 31.22 ± 0.18 | |||
| ZO–kaolinite | 21.26 ± 0.31 | 28.76 ± 0.23 | 30.66 ± 0.37 | 39.98 ± 0.42 | 43.02 ± 0.23 | |||
| MZO carrier | 15.75 ± 0.22 | 17.64 ± 0.41 | 19.78 ± 0.53 | 21.58 ± 0.33 | 23.85 ± 0.16 | |||
| MZO–kaolinite system | 26.72 ± 0.13 | 35.54 ± 0.32 | 40.16 ± 0.23 | 47.21 ± 0.43 | 50.63 ± 0.52 | |||
| Pathogenic Microorganism | Sample | MIC (μg/mL) | MBC (μg/mL) | Gentamicin | |
|---|---|---|---|---|---|
| MIC (μg/mL) | MBC (μg/mL) | ||||
| Staphylococcus aureus | Z. officinale | 0.73 ± 0.09 | 0.74 ± 0.11 | 0.62 ± 0.45 | 0.62 ± 0.49 |
| Kaolinite | 1.22 ± 0.26 | 1.41 ± 0.09 | |||
| ZO–kaolinite | 0.43 ± 0.05 | 0.45 ± 0.19 | |||
| MZO carrier | 0.65 ± 0.01 | 0.66 ± 0.13 | |||
| MZO–kaolinite system | 0.35 ± 0.07 | 0.32 ± 0.03 | |||
| Enterococcus faecalis | Z. officinale | 0.57 ± 0.19 | 0.56 ± 0.11 | 0.49 ± 0.14 | 0.45 ± 0.19 |
| Kaolinite | 0.71 ± 0.07 | 0.69 ± 0.13 | |||
| ZO–kaolinite | 0.42 ± 0.09 | 0.43 ± 0.04 | |||
| MZO carrier | 0.51 ± 0.07 | 0.52 ± 0.15 | |||
| MZO–kaolinite system | 0.29 ± 0.05 | 0.30 ± 0.11 | |||
| Bacillus cereus | Z. officinale | 1.32 ± 0.11 | 1.34 ± 0.17 | 1.29 ± 0.05 | 1.28 ± 0.03 |
| Kaolinite | 0.87 ± 0.31 | 0.89 ± 0.29 | |||
| ZO–kaolinite | 0.29 ± 0.24 | 0.28 ± 0.41 | |||
| MZO carrier | 1.28 ± 0.22 | 1.27 ± 0.11 | |||
| MZO–kaolinite system | 0.26 ± 0.09 | 0.25 ± 0.05 | |||
| Pseudomonas aeruginosa | Z. officinale | 1.73 ± 0.08 | 1.75 ± 0.53 | 1.97 ± 0.32 | 1.96 ± 0.26 |
| Kaolinite | 1.91 ± 0.21 | 1.90 ± 0.35 | |||
| ZO–kaolinite | 1.34 ± 0.12 | 1.41 ± 0.43 | |||
| MZO carrier | 1.71 ± 0.02 | 1.72 ± 0.09 | |||
| MZO–kaolinite system | 1.32 ± 0.13 | 1.31 ± 0.16 | |||
| Klebsiella pneumoniae | Z. officinale | 0.79 ± 0.12 | 0.79 ± 0.17 | 0.82 ± 0.19 | 0.82 ± 0.17 |
| Kaolinite | 1.02 ± 0.14 | 1.01 ± 0.33 | |||
| ZO–kaolinite | 0.66 ± 0.23 | 0.66 ± 0.21 | |||
| MZO carrier | 0.69 ± 0.17 | 0.71 ± 0.15 | |||
| MZO–kaolinite system | 0.61 ± 0.08 | 0.63 ± 0.03 | |||
| Escherichia coli | Z. officinale | 1.14 ± 0.13 | 1.15 ± 0.08 | 1.11 ± 0.25 | 1.11 ± 0.31 |
| Kaolinite | 1.38 ± 0.65 | 1.61 ± 0.42 | |||
| ZO–kaolinite | 0.98 ± 0.31 | 0.96 ± 0.07 | |||
| MZO carrier | 1.12 ± 0.04 | 1.12 ± 0.13 | |||
| MZO–kaolinite system | 0.83 ± 0.21 | 0.85 ± 0.03 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segneanu, A.-E.; Bradu, I.A.; Vlase, G.; Vlase, T.; Bejenaru, C.; Bejenaru, L.E.; Mogoşanu, G.D.; Ciocîlteu, M.V.; Herea, D.-D.; Boia, E.R. Design and Evaluation of a Zingiber officinale–Kaolinite–Maltodextrin Delivery System: Antioxidant, Antimicrobial, and Cytotoxic Activity Assessment. Pharmaceutics 2025, 17, 751. https://doi.org/10.3390/pharmaceutics17060751
Segneanu A-E, Bradu IA, Vlase G, Vlase T, Bejenaru C, Bejenaru LE, Mogoşanu GD, Ciocîlteu MV, Herea D-D, Boia ER. Design and Evaluation of a Zingiber officinale–Kaolinite–Maltodextrin Delivery System: Antioxidant, Antimicrobial, and Cytotoxic Activity Assessment. Pharmaceutics. 2025; 17(6):751. https://doi.org/10.3390/pharmaceutics17060751
Chicago/Turabian StyleSegneanu, Adina-Elena, Ionela Amalia Bradu, Gabriela Vlase, Titus Vlase, Cornelia Bejenaru, Ludovic Everard Bejenaru, George Dan Mogoşanu, Maria Viorica Ciocîlteu, Dumitru-Daniel Herea, and Eugen Radu Boia. 2025. "Design and Evaluation of a Zingiber officinale–Kaolinite–Maltodextrin Delivery System: Antioxidant, Antimicrobial, and Cytotoxic Activity Assessment" Pharmaceutics 17, no. 6: 751. https://doi.org/10.3390/pharmaceutics17060751
APA StyleSegneanu, A.-E., Bradu, I. A., Vlase, G., Vlase, T., Bejenaru, C., Bejenaru, L. E., Mogoşanu, G. D., Ciocîlteu, M. V., Herea, D.-D., & Boia, E. R. (2025). Design and Evaluation of a Zingiber officinale–Kaolinite–Maltodextrin Delivery System: Antioxidant, Antimicrobial, and Cytotoxic Activity Assessment. Pharmaceutics, 17(6), 751. https://doi.org/10.3390/pharmaceutics17060751








