Phase-Inversion In Situ Systems: Problems and Prospects of Biomedical Application
Abstract
1. Introduction
2. Basic Characteristics of Phase-Inversion Systems
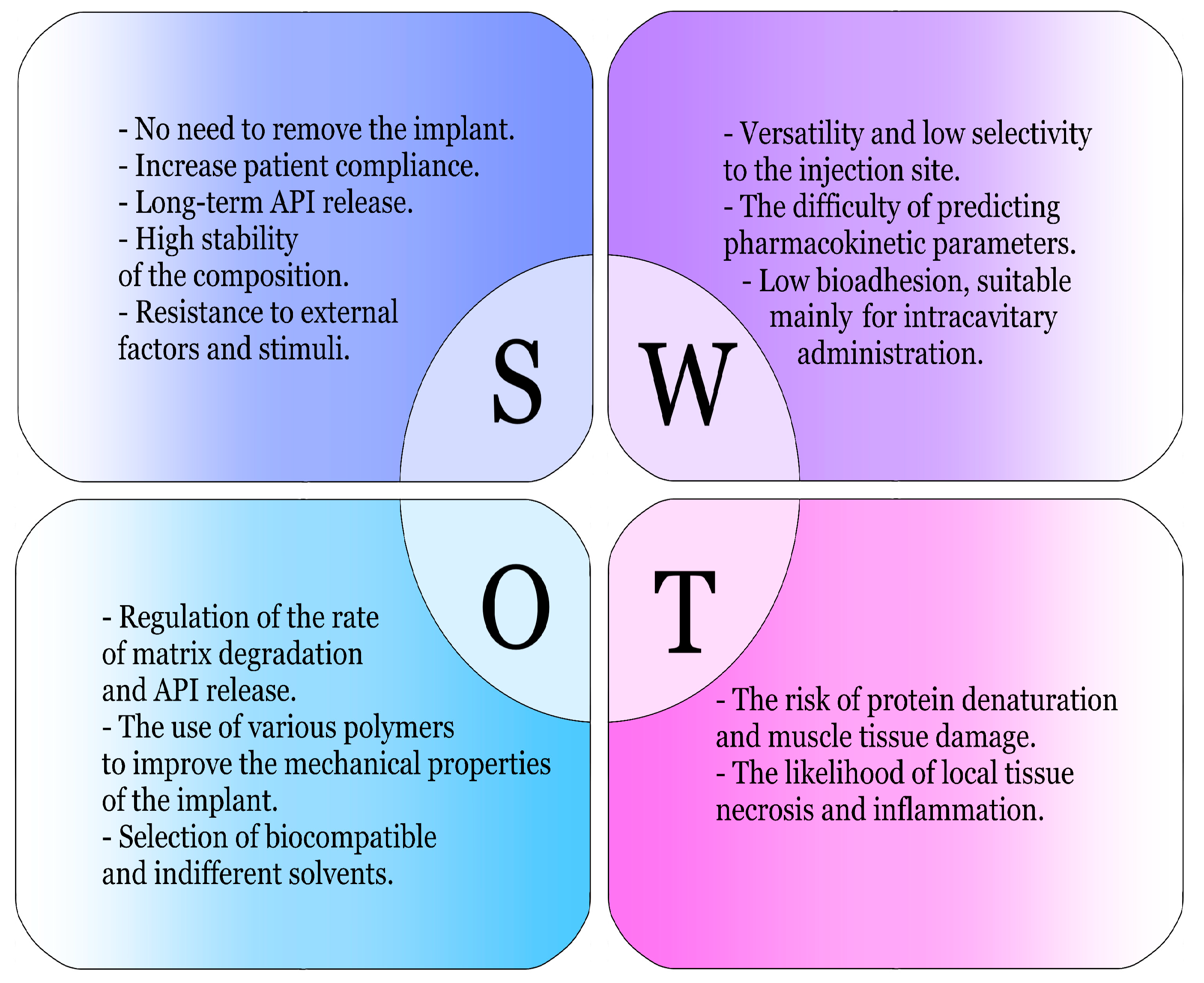
2.1. Advantages and Disadvantages
2.2. Main Matrix Formers
2.2.1. Lactic Acid Homopolymer
2.2.2. Polycaprolactone
2.2.3. N-Lauroyl-L-alanine Methyl Ester
2.2.4. Sucrose Acetate Isobutyrate
2.2.5. Borneol
2.2.6. Shellac
2.2.7. Polylactide-Co-Glycolide
2.3. Main Solvents
2.3.1. N-Methylpyrrolidone and 2-Pyrrolidone
2.3.2. Dimethyl Sulfoxide
2.3.3. Benzyl Benzoate and Benzyl Alcohol
2.3.4. Triacetin and Triethyl Citrate
2.3.5. Glycofurole
2.3.6. Ethyl Lactate
2.3.7. Propylene Carbonate
3. Application of Phase-Inversion Systems
3.1. Existing Preparations and Prospective Modifications
3.2. Application in Dental Practice
3.3. Oncology
3.4. Other Areas of Application
3.4.1. Therapy of Oropharyngeal Candidiasis
3.4.2. Musculoskeletal Diseases
3.4.3. Future Application Perspectives
3.5. The Main Screening Parameters
4. Discussion
4.1. Problems of Research Harmonization and Standardization
4.2. Regulatory Hurdles
4.3. Scale-Up Limitations
4.4. The Problem of Patient Acceptance
4.5. Future Directions and Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shende, P.; Basarkar, V. Recent Trends and Advances in Microbe-Based Drug Delivery Systems. DARU J. Pharm. Sci. 2019, 27, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Bakhrushina, E.O.; Mikhel, J.B.; Kondratieva, V.M.; Demina, N.B.; Grebennikova, T.V. In Situ Gels as a Modern Method of Intranasal Vaccine Delivery. Probl. Virol. 2022, 67, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Packhaeuser, C.B.; Schnieders, J.; Oster, C.G.; Kissel, T. In situ Forming Parenteral Drug Delivery Systems: An Overview. Eur. J. Pharm. Biopharm. 2004, 58, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Chandra, N.S.; Gorantla, S.; Priya, S.; Singhvi, G. Insight on Updates in Polysaccharides for Ocular Drug Delivery. Carbohydr. Polym. 2022, 297, 120014. [Google Scholar] [CrossRef]
- Fatima, G.N.; Maurya, P.; Nishtha; Saraf, S.K. In-Situ Gels for Brain Delivery: Breaching the Barriers. Curr. Pharm. Des. 2023, 29, 3240–3253. [Google Scholar] [CrossRef]
- Corazza, E.; Di Cagno, M.P.; Bauer-Brandl, A.; Abruzzo, A.; Cerchiara, T.; Bigucci, F.; Luppi, B. Drug Delivery to the Brain: In Situ Gelling Formulation Enhances Carbamazepine Diffusion Through Nasal Mucosa Models with Mucin. Eur. J. Pharm. Sci. 2022, 179, 106294. [Google Scholar] [CrossRef]
- Passos, J.S.; Apolinario, A.C.; Ishida, K.; Martins, T.S.; Lopes, L.B. Nanostructured Lipid Carriers Loaded into in situ Gels for Breast Cancer Local Treatment. Eur. J. Pharm. Sci. 2024, 192, 106638. [Google Scholar] [CrossRef]
- Garg, A.; Agrawal, R.; Singh Chauhan, C.; Deshmukh, R. In-Situ Gel: A Smart Carrier for Drug Delivery. Int. J. Pharm. 2024, 652, 123819. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Cook, M.T. In situ Gelling Drug Delivery Systems for Topical Drug Delivery. Eur. J. Pharm. Biopharm. 2023, 184, 36–49. [Google Scholar] [CrossRef]
- Park, S.H.; Ji, Y.B.; Park, J.Y.; Ju, H.J.; Lee, M.; Lee, S.; Kim, J.H.; Min, B.H.; Kim, M.S. Injectable In situ-Forming Hydrogels for Protein and Peptide Delivery. In Biomimicked Biomaterials; Chun, H.J., Reis, R.L., Motta, A., Khang, G., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; Volume 1250, pp. 35–48. ISBN 9789811532610. [Google Scholar]
- Zhang, J.; Hurren, C.; Lu, Z.; Wang, D. pH-Sensitive Alginate Hydrogel for Synergistic Anti-Infection. Int. J. Biol. Macromol. 2022, 222, 1723–1733. [Google Scholar] [CrossRef]
- Mfoafo, K.; Omidi, Y.; Omidian, H. Thermoresponsive Mucoadhesive Hybrid Gels in Advanced Drug Delivery Systems. Int. J. Pharm. 2023, 636, 122799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Wang, Y.; Xu, X.; Zhou, P. Myofibrillar Protein Can Form a Thermo-reversible Gel through Elaborate Deamidation Using Protein-glutaminase. J. Sci. Food Agric. 2023, 103, 3118–3128. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Shakushiro, K.; Sako, K. Ion-Responsive Drug Delivery Systems. CDT 2018, 19, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Chen, J.; Hu, Y.; Erturk, A. Photo-Responsive Hydrogel-Based Re-Programmable Metamaterials. Sci. Rep. 2022, 12, 13033. [Google Scholar] [CrossRef]
- Bakhrushina, E.O.; Sakharova, P.S.; Konogorova, P.D.; Pyzhov, V.S.; Kosenkova, S.I.; Bardakov, A.I.; Zubareva, I.M.; Krasnyuk, I.I.; Krasnyuk, I.I. Burst Release from In Situ Forming PLGA-Based Implants: 12 Effectors and Ways of Correction. Pharmaceutics 2024, 16, 115. [Google Scholar] [CrossRef]
- Bakhrushina, E.O.; Mikhel, I.B.; Buraya, L.M.; Moiseev, E.D.; Zubareva, I.M.; Belyatskaya, A.V.; Evzikov, G.Y.; Bondarenko, A.P.; Krasnyuk, I.I.; Krasnyuk, I.I. Implantation of In situ Gelling Systems for the Delivery of Chemotherapeutic Agents. Gels 2024, 10, 44. [Google Scholar] [CrossRef]
- Maulding, H.V. Prolonged Delivery of Peptides by Microcapsules. J. Control. Release 1987, 6, 167–176. [Google Scholar] [CrossRef]
- Gomaa, E.; Eissa, N.G.; Ibrahim, T.M.; El-Bassossy, H.M.; El-Nahas, H.M.; Ayoub, M.M. Development of Depot PLGA-Based in-Situ Implant of Linagliptin: Sustained Release and Glycemic Control. Saudi Pharm. J. 2023, 31, 499–509. [Google Scholar] [CrossRef]
- Giacalone, G.; Quaillet, M.; Huang, N.; Nicolas, V.; Boulogne, C.; Gillet, C.; Fattal, E.; Bochot, A.; Hillaireau, H. An Injectable, Nanostructured Implant for the Delivery of Adenosine Triphosphate: Towards Long-Acting Formulations of Small, Hydrophilic Drugs. Drug Deliv. Transl. Res. 2024, 14, 2146–2157. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, X.; Li, Q.; Zheng, R.; Chen, J.; Liu, Y.; Tong, X.; Wan, Z.; Gong, T. The Advances in Phospholipids-Based Phase Separation Gels for the Sustained Release of Peptides, Proteins, and Chemotherapeutics. Pharmaceutics 2024, 16, 875. [Google Scholar] [CrossRef]
- Hatefi, A.; Amsden, B. Biodegradable Injectable in situ Forming Drug Delivery Systems. J. Control. Release 2002, 80, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Rupenthal, I.D. Injectable Implants for the Sustained Release of Protein and Peptide Drugs. Drug Discov. Today 2013, 18, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Schädlich, A.; Kempe, S.; Mäder, K. Non-Invasive in vivo Characterization of Microclimate pH inside in situ Forming PLGA Implants Using Multispectral Fluorescence Imaging. J. Control. Release 2014, 179, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, J.; Liu, X.; Sun, F.; Zhou, Y.; Teng, L.; Li, Y. Parenteral Thermo-Sensitive Organogel for Schizophrenia Therapy, in vitro and in vivo Evaluation. Eur. J. Pharm. Sci. 2014, 60, 40–48. [Google Scholar] [CrossRef]
- Wersig, T.; Hacker, M.C.; Kressler, J.; Mäder, K. Poly(Glycerol Adipate)—Indomethacin Drug Conjugates—Synthesis and in vitro Characterization. Int. J. Pharm. 2017, 531, 225–234. [Google Scholar] [CrossRef]
- Senarat, S.; Pichayakorn, W.; Phaechamud, T.; Tuntarawongsa, S. Antisolvent Eudragit® Polymers Based in situ Forming Gel for Periodontal Controlled Drug Delivery. J. Drug Deliv. Sci. Technol. 2023, 82, 104361. [Google Scholar] [CrossRef]
- Sakharova, P.S.; Pyzhov, V.S.; Bakhrushina, E.O. Poly(l-Lactide-Co-Glycolide) and Shellac in the Development of Phase-Sensitive in situ Implants. Aspir. Vestn. Povolzhiya 2022, 22, 51–57. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Drugs@FDA: FDA-Approved Drugs. Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm (accessed on 19 November 2024).
- United States Pharmacopeial Convention, Usp. Usp36-Nf31; United States Pharmacopeia: Rockville, MA, USA, 2012; ISBN 978-1-936424-12-2. [Google Scholar]
- Council of Europe; European Pharmacopoeia Commission; European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia; Council of Europe: Strasbourg, France, 2010; ISBN 978-92-871-6960-0.
- Medicine and Medical Device Regulatory Science Foundation. Japanese Pharmacopoeia; Stationery Office: London, UK, 2017; ISBN 978-4-8408-1371-6. [Google Scholar]
- Pharmacopoeial Committee of the Eurasian Economic Union. The Pharmacopoeia of the Eurasian Economic Union; Eurasian Economic Commission: Moscow, Russia, 2023; ISBN 978-5-6049680-0-0. [Google Scholar]
- Nahm, N.J.; Conway, J.D. Resorbable Polylactide Membrane for the Treatment of Segmental Bone Defects. Injury 2022, 53, 376–380. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.; Xia, T.; Lin, N. Melt Compounding of Poly(Lactic Acid)-Based Composites: Blending Strategies, Process Conditions, and Mechanical Properties. Macromol. Rapid Commun. 2024, 45, 2400380. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Durairaju, P.; Bouarab, L.; Cottaz, A.; Planchon, S.; Oulahal, N.; Joly, C. Method for Assessing the Biodegradation of Poly(Lactic Acid) in vitro (on Agar Plates): Application Using PLA Oligomers and Bacillus Licheniformis Vegetative Cells or Spores. Polym. Test. 2024, 132, 108345. [Google Scholar] [CrossRef]
- Oksman, K.; Skrifvars, M.; Selin, J.-F. Natural Fibres as Reinforcement in Polylactic Acid (PLA) Composites. Compos. Sci. Technol. 2003, 63, 1317–1324. [Google Scholar] [CrossRef]
- Maduka, C.V.; Makela, A.V.; Tundo, A.; Ural, E.; Stivers, K.B.; Kuhnert, M.M.; Alhaj, M.; Hoque Apu, E.; Ashammakhi, N.; Hankenson, K.D.; et al. Regulating the Proinflammatory Response to Composite Biomaterials by Targeting Immunometabolism. Bioact. Mater. 2024, 40, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Hembram, K.C. Poly(Lactic Acid) (PLA) as Drug and Gene Delivery System for Tumor. In Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2024; pp. 143–177. ISBN 978-0-443-15401-0. [Google Scholar]
- Ouedraogo, S.; Grosjean, M.; Brigaud, I.; Carneiro, K.; Luchnikov, V.; Mathieu, N.; Garric, X.; Nottelet, B.; Anselme, K.; Pieuchot, L.; et al. Fabrication and Characterization of Thin Self-Rolling Film for Anti-Inflammatory Drug Delivery. Colloids Surf. B Biointerfaces 2024, 241, 114039. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Más Estellés, J.; Vidaurre, A.; Meseguer Dueñas, J.M.; Castilla Cortázar, I. Physical Characterization of Polycaprolactone Scaffolds. J. Mater. Sci. Mater. Med. 2008, 19, 189–195. [Google Scholar] [CrossRef]
- Baptista, C.; Azagury, A.; Shin, H.; Baker, C.M.; Ly, E.; Lee, R.; Mathiowitz, E. The Effect of Temperature and Pressure on Polycaprolactone Morphology. Polymer 2020, 191, 122227. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Youssef, S.H.; Kim, S.; Khetan, R.; Afinjuomo, F.; Song, Y.; Garg, S. The Development of 5-Fluorouracil Biodegradable Implants: A Comparative Study of PCL/PLGA Blends. J. Drug Deliv. Sci. Technol. 2023, 81, 104300. [Google Scholar] [CrossRef]
- Kweon, H.Y.; Yoo, M.K.; Park, I.K.; Kim, T.H.; Lee, H.C.; Lee, H.S.; Oh, J.S.; Akaike, T.; Cho, C.S. A Novel Degradable Polycaprolactone Networks for Tissue Engineering. Biomaterials 2003, 24, 801–808. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR Study of Polycaprolactone Chain Organization at Interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Mi, C.-H.; Qi, X.-Y.; Zhou, Y.-W.; Ding, Y.-W.; Wei, D.-X.; Wang, Y. Advances in Medical Polyesters for Vascular Tissue Engineering. Discov. Nano 2024, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Di Berardino, C.; Peserico, A.; Camerano Spelta Rapini, C.; Liverani, L.; Capacchietti, G.; Russo, V.; Berardinelli, P.; Unalan, I.; Damian-Buda, A.-I.; Boccaccini, A.R.; et al. Bioengineered 3D Ovarian Model for Long-Term Multiple Development of Preantral Follicle: Bridging the Gap for Poly(ε-Caprolactone) (PCL)-Based Scaffold Reproductive Applications. Reprod. Biol. Endocrinol. 2024, 22, 95. [Google Scholar] [CrossRef]
- James, B.D.; Medvedev, A.V.; Makarov, S.S.; Nelson, R.K.; Reddy, C.M.; Hahn, M.E. Moldable Plastics (Polycaprolactone) Can Be Acutely Toxic to Developing Zebrafish and Activate Nuclear Receptors in Mammalian Cells. ACS Biomater. Sci. Eng. 2024, 10, 5237–5251. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cao, J.; Li, H.; Liu, H.; Han, F.; Liu, Z.; Tong, C.; Li, S. Self-Assembled Drug Delivery System Based on Low-Molecular-Weight Bis-Amide Organogelator: Synthesis, Properties and In Vivo Evaluation. Drug Deliv. 2016, 23, 3168–3178. [Google Scholar] [CrossRef]
- Du, X.; Li, Z.; Xia, J.; Zhang, F.; Wang, Z. Facile Sonochemistry-Assisted Assembly of the Water-Loving Drug-Loaded Micro-Organogel with Thermo- and Redox-Sensitive Behavior. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 47–56. [Google Scholar] [CrossRef]
- Tan, C.; Zhu, Y.; Ahari, H.; Jafari, S.M.; Sun, B.; Wang, J. Sonochemistry: An Emerging Approach to Fabricate Biopolymer Cross-Linked Emulsions for the Delivery of Bioactive Compounds. Adv. Colloid Interface Sci. 2023, 311, 102825. [Google Scholar] [CrossRef]
- Jose, J.; Gopalan, K. Organogels: A Versatile Drug Delivery Tool in Pharmaceuticals. Res. J. Pharm. Technol. 2018, 11, 1242. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 9903964, n-Lauroyl-l-alanine Methyl Ester. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/n-Lauroyl-l-alanine-methyl-ester (accessed on 2 June 2025).
- Sivaramakrishna, D.; Thirupathi Reddy, S.; Nagaraju, T.; Swamy, M.J. Self-Assembly, Supramolecular Organization, and Phase Transitions of a Homologous Series of N-Acyl-l-Alanines (N = 8–20). Colloids Surf. A Physicochem. Eng. Asp. 2015, 471, 108–116. [Google Scholar] [CrossRef]
- Cheng, T.L.; Schindeler, A.; Little, D.G. BMP-2 Delivered via Sucrose Acetate Isobutyrate (SAIB) Improves Bone Repair in a Rat Open Fracture Model. J. Orthop. Res. 2016, 34, 1168–1176. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, M.; Han, X.; Men, X.; Oh, G.; Choi, S.-I.; Lee, O.-H. Improvement of Methods for Analysing Sucrose Acetate Isobutyrate (SAIB) in Soft Drinks Using GC-FID and Evaluating Antioxidant Effects in the SAIB Emulsion System. Food Addit. Contam. Part A 2022, 39, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 31339, Sucrose Acetate Isobutyrate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sucrose-Acetate-Isobutyrate (accessed on 2 June 2025).
- Dharani, S.; Sediri, K.; Cook, P.; Arunagiri, R.; Khan, M.A.; Rahman, Z. Preparation and Characterization of Stable Amorphous Glassy Solution of BCS II and IV Drugs. AAPS PharmSciTech 2021, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Oberhaus, E.L.; Wilson, K.M.; Camp, C.M.; Sones, J.L. Sucrose Acetate Isobutyrate (SAIB) as a Delivery Vehicle for Estradiol and Sulpiride: Evaluation of Endocrine Responses in Geldings and Ovarian Response in Seasonally Anovulatory Mares. J. Equine Vet. Sci. 2022, 112, 103896. [Google Scholar] [CrossRef] [PubMed]
- Harloff-Helleberg, S.; Fliervoet, L.A.L.; Fanø, M.; Schmitt, M.; Antopolski, M.; Urtti, A.; Nielsen, H.M. Exploring the Mucoadhesive Behavior of Sucrose Acetate Isobutyrate: A Novel Excipient for Oral Delivery of Biopharmaceuticals. Drug Deliv. 2019, 26, 532–541. [Google Scholar] [CrossRef]
- Bazraee, S.; Mobedi, H.; Mashak, A.; Jamshidi, A. Long-Lasting In situ Forming Implant Loaded with Bupivacaine: Investigationon Polymeric and Non-Polymeric Carrier and Solvent Effect. Curr. Drug Deliv. 2022, 19, 157–166. [Google Scholar] [CrossRef]
- Barakh Ali, S.F.; Dharani, S.; Afrooz, H.; Mohamed, E.M.; Cook, P.; Khan, M.A.; Rahman, Z. Development of Abuse-Deterrent Formulations Using Sucrose Acetate Isobutyrate. AAPS PharmSciTech 2020, 21, 99. [Google Scholar] [CrossRef]
- Yehia, S.A.; Halim, S.A.A.; Aziz, M.Y. Polymeric and Non Polymeric Injectable In-Situ Forming Implant Systems for Sustained Delivery of Lornoxicam: In vitro and In vivo Evaluation. Curr. Drug Deliv. 2018, 15, 1193–1203. [Google Scholar] [CrossRef]
- Dantas, M.G.B.; Reis, S.A.G.B.; Damasceno, C.M.D.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; Almeida, J.R.G.D.S. Development and Evaluation of Stability of a Gel Formulation Containing the Monoterpene Borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6552009, Borneol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Camphol (accessed on 2 June 2025).
- Horvathova, E.; Kozics, K.; Srancikova, A.; Hunakova, L.; Galova, E.; Sevcovicova, A.; Slamenova, D. Borneol Administration Protects Primary Rat Hepatocytes against Exogenous Oxidative DNA Damage. Mutagenesis 2012, 27, 581–588. [Google Scholar] [CrossRef]
- Slameňová, D.; Horváthová, E.; Wsólová, L.; Šramková, M.; Navarová, J. Investigation of Anti-Oxidative, Cytotoxic, DNA-Damaging and DNA-Protective Effects of Plant Volatiles Eugenol and Borneol in Human-Derived HepG2, Caco-2 and VH10 Cell Lines. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 677, 46–52. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.; Wang, H.; Bie, H. A Mucoadhesive, Thermoreversible in situ Nasal Gel of Geniposide for Neurodegenerative Diseases. PLoS ONE 2017, 12, e0189478. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Pan, X.; Zhang, P.; Yang, X.; Guan, H.; Dou, H.; Lu, Q. Defeating Melanoma Through a Nano-Enabled Revision of Hypoxic and Immunosuppressive Tumor Microenvironment. Int. J. Nanomed. 2023, 18, 3711–3725. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yan, Y.; Liu, W.; Liu, J.; Fan, T.; Deng, H.; Cai, Y. Advances and Perspectives on Pharmacological Activities and Mechanisms of the Monoterpene Borneol. Phytomedicine 2024, 132, 155848. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, C.; Chen, X.; Bu, F.; Li, G.; Zhang, P.; Wang, X. In situ Borneol-Modified Polyester with Antibacterial Adhesion and Long-Term Fungal-Repellent Properties. React. Funct. Polym. 2024, 202, 105993. [Google Scholar] [CrossRef]
- Lv, Y.; Yu, Z.; Li, C.; Zhou, J.; Lv, X.; Chen, J.; Wei, M.; Liu, J.; Yu, X.; Wang, C.; et al. Gelatin-Based Nanofiber Membranes Loaded with Curcumin and Borneol as a Sustainable Wound Dressing. Int. J. Biol. Macromol. 2022, 219, 1227–1236. [Google Scholar] [CrossRef]
- Lertsuphotvanit, N.; Tuntarawongsa, S.; Sirirak, J.; Phaechamud, T. Morphological and Physicochemical Behaviors of Borneol Precipitates. Mater. Today Proc. 2022, 65, 2315–2321. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, Y.; Chen, H.; Xiao, H.; Wang, Y.; Zhen, H.; Tan, C. Shellac-Based Delivery Systems for Food Bioactive Compounds. Int. J. Biol. Macromol. 2024, 271, 132623. [Google Scholar] [CrossRef]
- Wang, A.; Lenaghan, S.C.; Zhong, Q. Structures and Interactions Forming Stable Shellac-Casein Nanocomplexes with a pH-Cycle. Int. J. Biol. Macromol. 2024, 267, 131585. [Google Scholar] [CrossRef]
- Phaechamud, T.; Lertsuphotvanit, N.; Praphanwittaya, P. Viscoelastic and Thermal Properties of Doxycycline Hyclate-Loaded Bleached Shellac In Situ—Forming Gel and –Microparticle. J. Drug Deliv. Sci. Technol. 2018, 44, 448–456. [Google Scholar] [CrossRef]
- Yuan, Y.; He, N.; Xue, Q.; Guo, Q.; Dong, L.; Haruna, M.H.; Zhang, X.; Li, B.; Li, L. Shellac: A Promising Natural Polymer in the Food Industry. Trends Food Sci. Technol. 2021, 109, 139–153. [Google Scholar] [CrossRef]
- Ahuja, A.; Rastogi, V.K. Physicochemical and Thermal Characterization of the Edible Shellac Films Incorporated with Oleic Acid to Enhance Flexibility, Water Barrier and Retard Aging. Int. J. Biol. Macromol. 2024, 269, 132136. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, P.W.; Naicker, B.; Kalombo, L. Micronization, Characterization and in-Vitro Dissolution of Shellac from PGSS Supercritical CO2 Technique. Int. J. Pharm. 2016, 499, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Lamkhao, S.; Tandorn, S.; Thavornyutikarn, P.; Chokethawai, K.; Rujijanagul, G.; Thongkorn, K.; Jarupoom, P.; Randorn, C. Synergistic Amalgamation of Shellac with Self-Antibacterial Hydroxyapatite and Carboxymethyl Cellulose: An Interactive Wound Dressing for Ensuring Safety and Efficacy in Preliminary In Vivo Studies. Int. J. Biol. Macromol. 2023, 253, 126809. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.N.; Ulsh, J.B.; Gupta, R.; Tang, M.M.; Peredo, A.P.; Teinturier, T.D.; Mauck, R.L.; Gullbrand, S.; Hast, M.W. Characterization of Degradation Kinetics of Additively Manufactured PLGA under Variable Mechanical Loading Paradigms. J. Mech. Behav. Biomed. Mater. 2024, 153, 106457. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 36797, Plga. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Plga (accessed on 2 June 2025).
- Wachowiak, S.; Danede, F.; Willart, J.F.; Siepmann, F.; Siepmann, J.; Hamoudi, M. PLGA Implants for Controlled Dexamethasone Delivery: Impact of the Polymer Chemistry. J. Drug Deliv. Sci. Technol. 2023, 86, 104648. [Google Scholar] [CrossRef]
- Lehner, E.; Liebau, A.; Menzel, M.; Schmelzer, C.E.H.; Knolle, W.; Scheffler, J.; Binder, W.H.; Plontke, S.K.; Mäder, K. Characterization of PLGA versus PEG-PLGA Intracochlear Drug Delivery Implants: Degradation Kinetics, Morphological Changes, and pH Alterations. J. Drug Deliv. Sci. Technol. 2024, 99, 105972. [Google Scholar] [CrossRef]
- Hamoudi-Ben Yelles, M.C.; Tran Tan, V.; Danede, F.; Willart, J.F.; Siepmann, J. PLGA Implants: How Poloxamer/PEO Addition Slows Down or Accelerates Polymer Degradation and Drug Release. J. Control. Release 2017, 253, 19–29. [Google Scholar] [CrossRef]
- Bassand, C.; Freitag, J.; Benabed, L.; Verin, J.; Siepmann, F.; Siepmann, J. PLGA Implants for Controlled Drug Release: Impact of the Diameter. Eur. J. Pharm. Biopharm. 2022, 177, 50–60. [Google Scholar] [CrossRef]
- Schoenhammer, K.; Petersen, H.; Guethlein, F.; Goepferich, A. Injectable in situ Forming Depot Systems: PEG-DAE as Novel Solvent for Improved PLGA Storage Stability. Int. J. Pharm. 2009, 371, 33–39. [Google Scholar] [CrossRef]
- Retsa, P.; Sidhu, M.; Odeyemi, I.; Tombal, B.; Wex, J. PCN102 Economic Evaluation of Three Formulations of Leuprolide Acetate with Atrigel® in Androgen Deprivation Therapy for Advanced Prostate Cancer in Nine European Countries. Value Health 2011, 14, A452–A453. [Google Scholar] [CrossRef]
- Saliy, O.; Popova, M.; Tarasenko, H.; Getalo, O. Development Strategy of Novel Drug Formulations for the Delivery of Doxycycline in the Treatment of Wounds of Various Etiologies. Eur. J. Pharm. Sci. 2024, 195, 106636. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Yalkowsky, S.H. Solubilization of Poorly Soluble Compounds Using 2-Pyrrolidone. Int. J. Pharm. 2007, 342, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dampalla, C.S.; Kim, Y.; Zabiegala, A.; Howard, D.J.; Nguyen, H.N.; Madden, T.K.; Thurman, H.A.; Cooper, A.; Liu, L.; Battaile, K.P.; et al. Structure-Guided Design of Potent Coronavirus Inhibitors with a 2-Pyrrolidone Scaffold: Biochemical, Crystallographic, and Virological Studies. J. Med. Chem. 2024, 67, 11937–11956. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Ma, B.; Chen, G.-Q.; Zhang, X. Stereodivergent Synthesis of Enantioenriched 2-Pyrrolidones via Diastereoselective Hydrogenation of α,β-Unsaturated γ-Lactams. Org. Lett. 2023, 25, 2426–2431. [Google Scholar] [CrossRef]
- Cai, S.; Cai, T.; Liu, S.; Yang, Q.; He, J.; Chen, L.; Hu, J. Biodegradation of N-Methylpyrrolidone by Paracoccus sp. NMD-4 and Its Degradation Pathway. Int. Biodeterior. Biodegrad. 2014, 93, 70–77. [Google Scholar] [CrossRef]
- Poet, T.S.; Schlosser, P.M.; Rodriguez, C.E.; Parod, R.J.; Rodwell, D.E.; Kirman, C.R. Using Physiologically Based Pharmacokinetic Modeling and Benchmark Dose Methods to Derive an Occupational Exposure Limit for N-Methylpyrrolidone. Regul. Toxicol. Pharmacol. 2016, 76, 102–112. [Google Scholar] [CrossRef]
- Heidari, M.R. N-Methylpyrrolidone. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 588–593. ISBN 978-0-12-386455-0. [Google Scholar]
- Mohammadi, H.S.; Haghighi Asl, A.; Khajenoori, M. Production of Pharmaceutical Micro and Nano Particles by Subcritical Water Based Technologies: A Review. J. Drug Deliv. Sci. Technol. 2023, 85, 104621. [Google Scholar] [CrossRef]
- Hoelgaard, A.; Møllgaard, B.; Baker, E. Vehicle Effect on Topical Drug Delivery. IV. Effect of N-Methylpyrrolidone and Polar Lipids on Percutaneous Drug Transport. Int. J. Pharm. 1988, 43, 233–240. [Google Scholar] [CrossRef]
- Bonina, F.P.; Montenegro, L. Penetration Enhancer Effects on in vitro Percutaneous Absorption of Heparin Sodium Salt. Int. J. Pharm. 1992, 82, 171–177. [Google Scholar] [CrossRef]
- Ghayor, C.; Correro, R.M.; Lange, K.; Karfeld-Sulzer, L.S.; Grätz, K.W.; Weber, F.E. Inhibition of Osteoclast Differentiation and Bone Resorption by N-Methylpyrrolidone. J. Biol. Chem. 2011, 286, 24458–24466. [Google Scholar] [CrossRef]
- Öhlander, A.; Lüdtke, C.; Sahakjan, A.; Johnsson, R.E. N-Butylpyrrolidinone Is an Equally Good Solvent as N,N-dimethylformamide for Microwave Assisted Solid Phase Peptide Synthesis. J. Pept. Sci. 2024, 30, e3612. [Google Scholar] [CrossRef] [PubMed]
- Kirman, C.R.; Sonawane, B.R.; Seed, J.G.; Azu, N.O.; Barranco, W.T.; Hamilton, W.R.; Stedeford, T.J.; Hays, S.M. An Evaluation of Reproductive Toxicity Studies and Data Interpretation of N-Methylpyrrolidone for Risk Assessment: An Expert Panel Review. Regul. Toxicol. Pharmacol. 2023, 138, 105337. [Google Scholar] [CrossRef] [PubMed]
- Hass, U.; Lund, S.P.; Elsner, J. Effects of Prenatal Exposure to N-Methylpyrrolidone on Postnatal Development and Behavior in Rats. Neurotoxicology Teratol. 1994, 16, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Becci, P.; Knickerbocker, M.; Reagan, E.; Parent, R.; Burnette, L. Teratogenicity Study of N-Methylpyrrolidone after Dermal Application to Sprague-Dawley Rats. Fundam. Appl. Toxicol. 1982, 2, 73–76. [Google Scholar] [CrossRef]
- Mashreghi, N.; Bayrami, Z. 1-Methyl-2-Pyrrolidinone. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 351–357. ISBN 978-0-323-85434-4. [Google Scholar]
- Wendlinger, M.; Cardenas, A.F.M.; Figueredo De Siqueira, F.S.; Moreira, P.H.D.A.; Trovão, M.M.A.; Stape, T.H.S.; Tezvergil-Mutluay, A.; Loguercio, A.D. Does the Application of Dimethyl Sulfoxide Improve Resin Bonding to Eroded Dentine? Four-Year in vitro Evaluation. Dent. Mater. 2023, 39, 1051–1057. [Google Scholar] [CrossRef]
- Tunçer Çağlayan, S.; Gurbanov, R. Modulation of Bacterial Membranes and Cellular Macromolecules by Dimethyl Sulfoxide: A Dose-Dependent Study Providing Novel Insights. Int. J. Biol. Macromol. 2024, 267, 131581. [Google Scholar] [CrossRef]
- Bennett, B.; Hanotaux, J.; Pasala, A.R.; Hasan, T.; Hassan, D.; Shor, R.; Allan, D.S.; Maganti, H.B. Impact of Lower Concentrations of Dimethyl Sulfoxide on Cryopreservation of Autologous Hematopoietic Stem Cells: A Systematic Review and Meta-Analysis of Controlled Clinical Studies. Cytotherapy 2024, 26, 482–489. [Google Scholar] [CrossRef]
- Mancías-Guerra, C.; Sánchez-García, S.A.; Carreño-Salcedo, S.A.; Gutiérrez-Aguirre, C.H. Dimethyl Sulfoxide Toxicity in Umbilical Cord Blood Transplantation in Patients Less than 4.5 Kilos of Weigh. Hematol. Transfus. Cell Ther. 2023, 45, 106–109. [Google Scholar] [CrossRef]
- Gilfanova, R.; Auclair, K.M.; Hui, A.; Norris, P.J.; Muench, M.O. Reduced Dimethyl Sulfoxide Concentrations Successfully Cryopreserve Human Hematopoietic Stem Cells with Multi-Lineage Long-Term Engraftment Ability in Mice. Cytotherapy 2021, 23, 1053–1059. [Google Scholar] [CrossRef]
- Tomoe, H. Dimethyl Sulfoxide: A Review of Pharmacology and Clinical Effect on Interstitial Cystitis/Bladder Pain Syndrome. Continence 2023, 8, 101058. [Google Scholar] [CrossRef]
- Zabeu, G.S.; Giacomini, M.C.; Scaffa, P.M.C.; Tjäderhane, L.; Mosquim, V.; Wang, L. Solvation Role of Dimethyl Sulfoxide on the Interaction with Dentin Bonding Systems after 30 Months. Dent. Mater. 2023, 39, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, Z.; Zhang, B.; Peng, W.; Guo, L. Effects of Dimethyl Sulfoxide Pretreatment on the Bonding Properties of Fluorotic Dentin of Different Severity: An In Vitro Study. J. Prosthet. Dent. 2024, 131, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Widmann, M.; Lieb, A.; Mutti, A.; Schwarzer, C. Dimethyl Sulfoxide’s Impact on Epileptiform Activity in a Mouse Model of Chronic Temporal Lobe Epilepsy. Epilepsy Res. 2023, 197, 107235. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Morimitsu, Y.; Nishi, R.; Yoshida, M.; Takei, T. Novel Hydrophobically Modified Agarose Cryogels Fabricated Using Dimethyl Sulfoxide. J. Biosci. Bioeng. 2022, 133, 390–395. [Google Scholar] [CrossRef]
- Sullivan, D.W.; Gad, S.E. Dimethyl Sulfoxide (DMSO). In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 805–809. ISBN 978-0-323-85434-4. [Google Scholar]
- Corcoran, G.B.; Ray, S.D. Benzyl Alcohol. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 429–432. ISBN 978-0-12-386455-0. [Google Scholar]
- Federico, S.A.P.; Hizon-Fradejas, A.B.; Grabato, J.R.H.; Mojica, E.-R.E. Benzyl Benzoate. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 23–26. ISBN 978-0-323-85434-4. [Google Scholar]
- Kim, J.-H.; Lee, D.; Lim, H.; Kim, T.; Suk, K.; Seo, J. Risk Assessment to Human Health: Consumer Exposure to Ingredients in Air Fresheners. Regul. Toxicol. Pharmacol. 2018, 98, 31–40. [Google Scholar] [CrossRef]
- Abdel-Baki, A.-A.S.; Ibrahium, S.M.; Aboelhadid, S.M.; Hassan, A.O.; Al-Quraishy, S.; Abdel-Tawab, H. Benzyl Alcohol, Benzyl Benzoate and Methyl Benzoate as Bio-Insecticides against Dried Bean Beetle Acanthoscelides Obtectus (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2024, 105, 102246. [Google Scholar] [CrossRef]
- Jörgensen, A.M.; Wibel, R.; Veider, F.; Hoyer, B.; Chamieh, J.; Cottet, H.; Bernkop-Schnürch, A. Self-Emulsifying Drug Delivery Systems (SEDDS): How Organic Solvent Release Governs the Fate of Their Cargo. Int. J. Pharm. 2023, 647, 123534. [Google Scholar] [CrossRef]
- Santiago, M.G.; Dohanik Da Silva, C.; De Souza, B.M.; Assis, B.R.D.; Pinto, P.N.; Keller, K.M.; Vilela, R.V.R.; De Oliveira, C.S.F.; Goulart, G.A.C. Topical Hydrophilic Gel with Itraconazole-Loaded Polymeric Nanomicelles Improves Wound Healing in the Treatment of Feline Sporotrichosis. Int. J. Pharm. 2023, 634, 122619. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Wu, C.-L.; Tseng, S.-H.; Kuo, P.-Y.; Tseng, S.-Y. In vitro Benzyl Alcohol Cytotoxicity: Implications for Intravitreal Use of Triamcinolone Acetonide. Exp. Eye Res. 2008, 86, 942–950. [Google Scholar] [CrossRef]
- Bassi, A.; Piccolo, V.; Mazzatenta, C. “One-Shot” Combined Therapy with Oral Ivermectin and Local Benzyl Benzoate: Is the Current Best Therapeutic Option in the Era of Permethrin Resistant Scabies? Travel Med. Infect. Dis. 2023, 53, 102585. [Google Scholar] [CrossRef]
- Hussar, D.A. New Drugs: Tapentadol Hydrochloride, Tolvaptan, and Benzyl Alcohol. J. Am. Pharm. Assoc. 2009, 49, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.A.; Miller, G.W. Benzyl Benzoate. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 433–434. ISBN 978-0-12-386455-0. [Google Scholar]
- Murase, J.E.; Heller, M.M.; Butler, D.C. Safety of Dermatologic Medications in Pregnancy and Lactation. J. Am. Acad. Dermatol. 2014, 70, 401.e1–401.e14. [Google Scholar] [CrossRef] [PubMed]
- Api, A.M.; Belmonte, F.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; et al. RIFM Fragrance Ingredient Safety Assessment, Benzyl Benzoate, CAS Registry Number 120-51-4. Food Chem. Toxicol. 2020, 144, 111500. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Yuan, S.; Bai, L.; Zhang, X.; Chen, P.; Zhang, W. Benzyl Alcohol Accelerates Recovery from Achilles Tendon Injury, Potentially via TGF-Β1/Smad2/3 Pathway. Injury 2020, 51, 1515–1521. [Google Scholar] [CrossRef]
- Wilson, L.; Martin, S. Benzyl Alcohol as an Alternative Local Anesthetic. Ann. Emerg. Med. 1999, 33, 495–499. [Google Scholar] [CrossRef]
- Milisavljevic, V.; Tran, L.P.; Batmalle, C.; Bootsma, H.J. Benzyl Alcohol and Ethanol Can Enhance the Pathogenic Potential of Clinical Staphylococcus Epidermidis Strains. Am. J. Infect. Control 2008, 36, 552–558. [Google Scholar] [CrossRef]
- Inada, M.; Kato, H.; Maruyama, H.; Okada, E.; Imai, F.; Inada, S. Toxic Benzyl Alcohol Inhalation: Altered Mental Status with Metabolic Acidosis and Hyperammonemia. Am. J. Emerg. Med. 2022, 57, 234.e3–234.e5. [Google Scholar] [CrossRef]
- Garner, J.; Skidmore, S.; Hadar, J.; Park, H.; Park, K.; Kuk Jhon, Y.; Qin, B.; Wang, Y. Analysis of Semi-Solvent Effects for PLGA Polymers. Int. J. Pharm. 2021, 602, 120627. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chen, K.-S.; Run-Chu, L. Organic Esters of Plasticizers Affecting the Water Absorption, Adhesive Property, Glass Transition Temperature and Plasticizer Permanence of Eudragit Acrylic Films. J. Control. Release 2000, 68, 343–350. [Google Scholar] [CrossRef]
- Liu, H.; Venkatraman, S.S. Cosolvent Effects on the Drug Release and Depot Swelling in Injectable In situ Depot-Forming Systems. J. Pharm. Sci. 2012, 101, 1783–1793. [Google Scholar] [CrossRef]
- Gutierrezrocca, J.; Mcginity, J. Influence of Water Soluble and Insoluble Plasticizers on the Physical and Mechanical Properties of Acrylic Resin Copolymers. Int. J. Pharm. 1994, 103, 293–301. [Google Scholar] [CrossRef]
- Šnejdrová, E.; Martiška, J.; Loskot, J.; Paraskevopoulos, G.; Kováčik, A.; Regdon, G., Jr.; Budai-Szűcs, M.; Palát, K.; Konečná, K. PLGA Based Film Forming Systems for Superficial Fungal Infections Treatment. Eur. J. Pharm. Sci. 2021, 163, 105855. [Google Scholar] [CrossRef]
- Lynch, J.W.; Bailey, J.W. Dietary intake of the short-chain triglyceride triacetin vs. long-chain triglycerides decreases adipocyte diameter and fat deposition in rats. J. Nutr. 1995, 125, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Karlstad, M.; Killeffer, J.; Bailey, J.; DeMichele, S. Parenteral Nutrition with Short- and Long-Chain Triglycerides: Triacetin Reduces Atrophy of Small and Large Bowel Mucosa and Improves Protein Metabolism in Burned Rats. Am. J. Clin. Nutr. 1992, 55, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.W.; Barker, R.L.; Karlstad, M.D. Total Parenteral Nutrition with Short- and Long-Chain Triglycerides: Triacetin Improves Nitrogen Balance in Rats. J. Nutr. 1992, 122, 1823–1829. [Google Scholar] [CrossRef]
- Bailey, J.W.; Heath, H.; Miles, J.M. Calcium, Magnesium, and Phosphorus Metabolism in Dogs given Intravenous Triacetin. Am. J. Clin. Nutr. 1989, 49, 385–388. [Google Scholar] [CrossRef]
- Mekala, J.R.; Kurappalli, R.K.; Ramalingam, P.; Moparthi, N.R. N-Acetyl l-Aspartate and Triacetin Modulate Tumor Suppressor MicroRNA and Class I and II HDAC Gene Expression Induce Apoptosis in Glioblastoma Cancer Cells in vitro. Life Sci. 2021, 286, 120024. [Google Scholar] [CrossRef]
- Quinn, M.J.; Ziolkowski, D. Wildlife Toxicity Assessment for Triacetin. In Wildlife Toxicity Assessments for Chemicals of Military Concern; Elsevier: Amsterdam, The Netherlands, 2015; pp. 291–301. ISBN 978-0-12-800020-5. [Google Scholar]
- Zhu, Y.; Shah, N.H.; Malick, A.W.; Infeld, M.H.; McGinity, J.W. Solid-State Plasticization of an Acrylic Polymer with Chlorpheniramine Maleate and Triethyl Citrate. Int. J. Pharm. 2002, 241, 301–310. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Y.; Zhu, J. Dry Powder Coated Osmotic Drug Delivery System. Eur. J. Pharm. Sci. 2018, 111, 383–392. [Google Scholar] [CrossRef]
- Gruetzmann, R.; Wagner, K.G. Quantification of the Leaching of Triethyl Citrate/Polysorbate 80 Mixtures from Eudragit® RS Films by Differential Scanning Calorimetry. Eur. J. Pharm. Biopharm. 2005, 60, 159–162. [Google Scholar] [CrossRef]
- Snejdrova, E.; Dittrich, M.; Drastik, M. Plasticized Branched Aliphatic Oligoesters as Potential Mucoadhesive Drug Carriers. Int. J. Pharm. 2013, 458, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xu, Q.; Zhang, W.; Zhang, D.; Liao, X.; Zhao, X.; Zhang, J.; Sun, T.; Weng, D. Plasticizer Acetyl Triethyl Citrate (ATEC) Induces Lipogenesis and Obesity. Toxicol. Appl. Pharmacol. 2024, 482, 116788. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, M.; Gold, H. Toxicology of the Citric Acid Esters: Tributyl Citrate, Acetyl Tributyl Citrate, Triethyl Citrate, and Acetyl Triethyl Citrate. Toxicol. Appl. Pharmacol. 1959, 1, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.B.; Guess, W.L.; Autian, J. Mechanistic Toxicology of Triethyl Citrate in Cultured Mammalian Cells by Cinematography. J. Pharm. Sci. 1968, 57, 293–299. [Google Scholar] [CrossRef]
- Rungseevijitprapa, W.; Brazeau, G.A.; Simkins, J.W.; Bodmeier, R. Myotoxicity Studies of O/W-in situ Forming Microparticle Systems. Eur. J. Pharm. Biopharm. 2008, 69, 126–133. [Google Scholar] [CrossRef]
- Bagger, M.A.; Nielsen, H.W.; Bechgaard, E. Nasal Bioavailability of Peptide T in Rabbits: Absorption Enhancement by Sodium Glycocholate and Glycofurol. Eur. J. Pharm. Sci. 2001, 14, 69–74. [Google Scholar] [CrossRef]
- Bechgaard, E.; Gizurarson, S.; Hjortkjær, R.K.; Sørensen, A.R. Intranasal Administration of Insulin to Rabbits Using Glycofurol as an Absorption Promoter. Int. J. Pharm. 1996, 128, 287–289. [Google Scholar] [CrossRef]
- Tran, M.-K.; Hassani, L.N.; Calvignac, B.; Beuvier, T.; Hindré, F.; Boury, F. Lysozyme Encapsulation within PLGA and CaCO3 Microparticles Using Supercritical CO2 Medium. J. Supercrit. Fluids 2013, 79, 159–169. [Google Scholar] [CrossRef]
- Mottu, F.; Gailloud, P.; Massuelle, D.; Rüfenacht, D.A.; Doelker, E. In vitro Assessment of New Embolic Liquids Prepared from Preformed Polymers and Water-Miscible Solvents for Aneurysm Treatment. Biomaterials 2000, 21, 803–811. [Google Scholar] [CrossRef]
- Boia, R.; Dias, P.A.N.; Martins, J.M.; Galindo-Romero, C.; Aires, I.D.; Vidal-Sanz, M.; Agudo-Barriuso, M.; De Sousa, H.C.; Ambrósio, A.F.; Braga, M.E.M.; et al. Porous Poly(ε-Caprolactone) Implants: A Novel Strategy for Efficient Intraocular Drug Delivery. J. Control. Release 2019, 316, 331–348. [Google Scholar] [CrossRef]
- Bœuf-Muraille, G.; Rigaux, G.; Callewaert, M.; Zambrano, N.; Van Gulick, L.; Roullin, V.G.; Terryn, C.; Andry, M.-C.; Chuburu, F.; Dukic, S.; et al. Evaluation of mTHPC-Loaded PLGA Nanoparticles for in vitro Photodynamic Therapy on C6 Glioma Cell Line. Photodiagnosis Photodyn. Ther. 2019, 25, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Viehof, A.; Javot, L.; Béduneau, A.; Pellequer, Y.; Lamprecht, A. Oral Insulin Delivery in Rats by Nanoparticles Prepared with Non-Toxic Solvents. Int. J. Pharm. 2013, 443, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Yasaka, W.J.; Sasame, H.A.; Saul, W.; Maling, H.M.; Gillette, J.R. Mechanisms in the Potentiation and Inhibition of Pharmacological Actions of Hexobarbital and Zoxazolamine by Glycofurol. Biochem. Pharmacol. 1978, 27, 2851–2858. [Google Scholar] [CrossRef]
- Punet, X.; Levato, R.; Bataille, I.; Letourneur, D.; Engel, E.; Mateos-Timoneda, M.A. Polylactic Acid Organogel as Versatile Scaffolding Technique. Polymer 2017, 113, 81–91. [Google Scholar] [CrossRef]
- Salerno, A.; Fanovich, M.A.; Pascual, C.D. The Effect of Ethyl-Lactate and Ethyl-Acetate Plasticizers on PCL and PCL–HA Composites Foamed with Supercritical CO2. J. Supercrit. Fluids 2014, 95, 394–406. [Google Scholar] [CrossRef]
- Dandia, A.; Jain, A.K.; Laxkar, A.K. Ethyl Lactate as a Promising Bio Based Green Solvent for the Synthesis of Spiro-Oxindole Derivatives via 1,3-Dipolar Cycloaddition Reaction. Tetrahedron Lett. 2013, 54, 3929–3932. [Google Scholar] [CrossRef]
- Álvarez, I.; Gutiérrez, C.; Rodríguez, J.F.; De Lucas, A.; García, M.T. Production of Drug-Releasing Biodegradable Microporous Scaffold Impregnated with Gemcitabine Using a CO2 Foaming Process. J. CO2 Util. 2020, 41, 101227. [Google Scholar] [CrossRef]
- Itin, C.; Komargodski, R.; Domb, A.J.; Hoffman, A. Controlled Delivery of Apomorphine Through Buccal Mucosa, Towards a Noninvasive Administration Method in Parkinson’s Disease: A Preclinical Mechanistic Study. J. Pharm. Sci. 2020, 109, 2729–2734. [Google Scholar] [CrossRef]
- Álvarez, I.; Gutiérrez, C.; Rodríguez, J.F.; De Lucas, A.; García, M.T. Production of Biodegradable PLGA Foams Processed with High Pressure CO2. J. Supercrit. Fluids 2020, 164, 104886. [Google Scholar] [CrossRef]
- Álvarez, I.; Gutiérrez, C.; De Lucas, A.; Rodríguez, J.F.; García, M.T. Measurement, Correlation and Modelling of High-Pressure Phase Equilibrium of PLGA Solutions in CO2. J. Supercrit. Fluids 2020, 155, 104637. [Google Scholar] [CrossRef]
- Grizić, D.; Lamprecht, A. Predictability of Drug Encapsulation and Release from Propylene Carbonate/PLGA Microparticles. Int. J. Pharm. 2020, 586, 119601. [Google Scholar] [CrossRef] [PubMed]
- Grune, C.; Thamm, J.; Werz, O.; Fischer, D. CyreneTM as an Alternative Sustainable Solvent for the Preparation of Poly(Lactic-Co-Glycolic Acid) Nanoparticles. J. Pharm. Sci. 2021, 110, 959–964. [Google Scholar] [CrossRef]
- Matschke, C. Sustained-Release Injectables Formed in situ and Their Potential Use for Veterinary Products. J. Control. Release 2002, 85, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Avachat, A.M.; Kapure, S.S. Asenapine Maleate in situ Forming Biodegradable Implant: An Approach to Enhance Bioavailability. Int. J. Pharm. 2014, 477, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Kamali, H.; Khodaverdi, E.; Hadizadeh, F.; Mohajeri, S.A. In-Vitro, Ex-Vivo, and in-Vivo Evaluation of Buprenorphine HCl Release from an in situ Forming Gel of PLGA-PEG-PLGA Using N-methyl-2-pyrrolidone as Solvent. Mater. Sci. Eng. C 2019, 96, 561–575. [Google Scholar] [CrossRef]
- Jain, A.; Kunduru, K.R.; Basu, A.; Mizrahi, B.; Domb, A.J.; Khan, W. Injectable Formulations of Poly(Lactic Acid) and Its Copolymers in Clinical Use. Adv. Drug Deliv. Rev. 2016, 107, 213–227. [Google Scholar] [CrossRef]
- Nkanga, C.I.; Fisch, A.; Rad-Malekshahi, M.; Romic, M.D.; Kittel, B.; Ullrich, T.; Wang, J.; Krause, R.W.M.; Adler, S.; Lammers, T.; et al. Clinically Established Biodegradable Long Acting Injectables: An Industry Perspective. Adv. Drug Deliv. Rev. 2020, 167, 19–46. [Google Scholar] [CrossRef]
- Kalsi, R.; Vandana, K.; Prakash, S. Effect of Local Drug Delivery in Chronic Periodontitis Patients: A Meta-Analysis. J. Indian Soc. Periodontol. 2011, 15, 304. [Google Scholar] [CrossRef]
- Tomasi, C.; Wennström, J.L. Locally Delivered Doxycycline Improves the Healing Following Non-surgical Periodontal Therapy in Smokers. J. Clin. Periodontol. 2004, 31, 589–595. [Google Scholar] [CrossRef]
- Salvi, G.E.; Mombelli, A.; Mayfield, L.; Rutar, A.; Suvan, J.; Garrett, S.; Lang, N.P. Local Antimicrobial Therapy after Initial Periodontal Treatment: A Randomized Clinical Trial Comparing Three Biodegradable Sustained Release Polymers. J. Clin. Periodontol. 2002, 29, 540–550. [Google Scholar] [CrossRef]
- Kanwar, N.; Sinha, V.R. In situ Forming Depot as Sustained-Release Drug Delivery Systems. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 93–136. [Google Scholar] [CrossRef] [PubMed]
- Abulateefeh, S.R. Long-Acting Injectable PLGA/PLA Depots for Leuprolide Acetate: Successful Translation from Bench to Clinic. Drug Deliv. Transl. Res. 2023, 13, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Malek, R.; Wu, S.-T.; Serrano, D.; Tho, T.; Umbas, R.; Teoh, J.; Lojanapiwat, B.; Ong, T.A.; On, W.K.; Thai, S.M.; et al. ELIGANT: A Phase 4, Interventional, Safety Study of Leuprorelin Acetate (ELIGARD®) in Asian Men with Prostate Cancer. Transl. Androl. Urol. 2022, 11, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.S.M.D.; Soares, A.N. Efficacy of Leuprorelide Acetate (Eligard®) in Daily Practice in Brazil: A Retrospective Study with Depot Formulations in Patients with Prostate Cancer. Int. Braz. J. Urol. 2020, 46, 383–389. [Google Scholar] [CrossRef]
- Ohlmann, C.-H.; Gross-Langenhoff, M. Efficacy and Tolerability of Leuprorelin Acetate (Eligard®) in Daily Practice in Germany: Pooled Data from 2 Prospective, Non-Interventional Studies with 3- or 6-Month Depot Formulations in Patients with Advanced Prostate Cancer. Urol. Int. 2018, 100, 66–71. [Google Scholar] [CrossRef]
- Cox, M.C.; Scripture, C.D.; Figg, W.D. Leuprolide Acetate given by a Subcutaneous Extended-Release Injection: Less of a Pain? Expert Rev. Anticancer. Ther. 2005, 5, 605–611. [Google Scholar] [CrossRef]
- Braeckman, J.; Michielsen, D. Efficacy and Tolerability of 1- and 3-Month Leuprorelin Acetate Depot Formulations (Eligard®/Depo-Eligard®) for Advanced Prostate Cancer in Daily Practice: A Belgian Prospective Non-Interventional Study. Arch. Med. Sci. 2014, 3, 477–483. [Google Scholar] [CrossRef]
- Alphandéry, E.; Grand-Dewyse, P.; Lefèvre, R.; Mandawala, C.; Durand-Dubief, M. Cancer Therapy Using Nanoformulated Substances: Scientific, Regulatory and Financial Aspects. Expert Rev. Anticancer. Ther. 2015, 15, 1233–1255. [Google Scholar] [CrossRef]
- Sartor, O. Eligard: Leuprolide Acetate in a Novel Sustained-Release Delivery System. Urology 2003, 61, 25–31. [Google Scholar] [CrossRef]
- Merseburger, A.S.; Roesch, M.C. Advanced Delivery of Leuprorelin Acetate for the Treatment of Prostatic Cancer. Expert Rev. Anticancer. Ther. 2022, 22, 703–715. [Google Scholar] [CrossRef]
- Berges, R.; Bello, U. Effect of a New Leuprorelin Formulation on Testosterone Levels in Patients with Advanced Prostate Cancer. Curr. Med. Res. Opin. 2006, 22, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Gonella, A.; Grizot, S.; Liu, F.; López Noriega, A.; Richard, J. Long-Acting Injectable Formulation Technologies: Challenges and Opportunities for the Delivery of Fragile Molecules. Expert Opin. Drug Deliv. 2022, 19, 927–944. [Google Scholar] [CrossRef] [PubMed]
- Roberge, C.; Cros, J.-M.; Serindoux, J.; Cagnon, M.-E.; Samuel, R.; Vrlinic, T.; Berto, P.; Rech, A.; Richard, J.; Lopez-Noriega, A. BEPO®: Bioresorbable Diblock mPEG-PDLLA and Triblock PDLLA-PEG-PDLLA Based in situ Forming Depots with Flexible Drug Delivery Kinetics Modulation. J. Control. Release 2020, 319, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Brolin, C.; Lim, E.W.K.; Grizot, S.; Olsen, C.H.; Yavari, N.; Krag, T.O.; Nielsen, P.E. Approaches for Systemic Delivery of Dystrophin Antisense Peptide Nucleic Acid in the Mdx Mouse Model. Nucleic Acid Ther. 2021, 31, 208–219. [Google Scholar] [CrossRef]
- Ng, F.; Nicoulin, V.; Peloso, C.; Curia, S.; Richard, J.; Lopez-Noriega, A. In vitro and In vivo Hydrolytic Degradation Behaviors of a Drug-Delivery System Based on the Blend of PEG and PLA Copolymers. ACS Appl. Mater. Interfaces 2023, 15, 55495–55509. [Google Scholar] [CrossRef]
- Peloso, C.; Trichet, A.-P.; Descotes, J.; Richard, J.; Roberge, C.; Lopez-Noriega, A. Evaluation of Loco-Regional Skin Toxicity Induced by an In Situ Forming Depot After a Single Subcutaneous Injection at Different Volumes and Flow Rates in Göttingen Minipigs. Int. J. Mol. Sci. 2021, 22, 9250. [Google Scholar] [CrossRef]
- Pooda, S.H.; Moiroux, N.; Porciani, A.; Courjaud, A.-L.; Roberge, C.; Gaudriault, G.; Sidibé, I.; Belem, A.M.G.; Rayaissé, J.-B.; Dabiré, R.K.; et al. Proof-of-Concept Study for a Long-Acting Formulation of Ivermectin Injected in Cattle as a Complementary Malaria Vector Control Tool. Parasites Vectors 2023, 16, 66. [Google Scholar] [CrossRef]
- Kamali, H.; Karimi, M.; Abbaspour, M.; Nadim, A.; Hadizadeh, F.; Khodaverdi, E.; Eisvand, F. Comparison of Lipid Liquid Crystal Formulation and Vivitrol® for Sustained Release of Naltrexone: In vitro Evaluation and Pharmacokinetics in Rats. Int. J. Pharm. 2022, 611, 121275. [Google Scholar] [CrossRef]
- Albayaty, M.; Linden, M.; Olsson, H.; Johnsson, M.; Strandgården, K.; Tiberg, F. Pharmacokinetic Evaluation of Once-Weekly and Once-Monthly Buprenorphine Subcutaneous Injection Depots (CAM2038) Versus Intravenous and Sublingual Buprenorphine in Healthy Volunteers Under Naltrexone Blockade: An Open-Label Phase 1 Study. Adv. Ther. 2017, 34, 560–575. [Google Scholar] [CrossRef]
- Liu, T.; Gobburu, J.V.S. A Physiologically Based Pharmacokinetic Modeling Approach to Predict Drug-Drug Interactions of Buprenorphine After Subcutaneous Administration of CAM2038 with Perpetrators of CYP3A4. J. Pharm. Sci. 2018, 107, 942–948. [Google Scholar] [CrossRef]
- Haasen, C.; Linden, M.; Tiberg, F. Pharmacokinetics and Pharmacodynamics of a Buprenorphine Subcutaneous Depot Formulation (CAM2038) for Once-Weekly Dosing in Patients with Opioid Use Disorder. J. Subst. Abus. Treat. 2017, 78, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Tiberg, F.; Roberts, J.; Cervin, C.; Johnsson, M.; Sarp, S.; Tripathi, A.P.; Linden, M. Octreotide s.c. Depot Provides Sustained Octreotide Bioavailability and Similar IGF-1 Suppression to Octreotide LAR in Healthy Volunteers. Br. J. Clin. Pharmacol. 2015, 80, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, M.; Pedroncelli, A.M.; Hansson, A.; Tiberg, F. Pharmacokinetics and Pharmacodynamics of a Pasireotide Subcutaneous Depot (CAM4071) and Comparison with Immediate and Long-Acting Release Pasireotide. Endocrine 2024, 84, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- A Multicenter, Open-Label, Single Ascending-Dose Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Depot Buprenorphine (RBP-6000) in Opioid-Dependent Subjects. ClinicalTrials.gov ID NCT03002961. Available online: https://clinicaltrials.gov/study/NCT03002961?term=NCT03002961&rank=1 (accessed on 6 May 2025).
- A Single-Dose, Open-Label Study of Depot Buprenorphine (RBP-6000) in Opioid-Dependent Individuals. ClinicalTrials.gov ID NCT02765867. Available online: https://clinicaltrials.gov/study/NCT02765867?term=NCT02765867&rank=1 (accessed on 6 May 2025).
- A Single-Center, Randomized, Open-Label, Single-Dose Study to Evaluate the Pharmacokinetics, Safety, and Tolerability of Depot Buprenorphine (RBP-6000) Using Poly(DL-lactide-co-glycolide) Polymer of Two Different Molecular Weights (Low and High Molecular Weights as Test Treatments) in Comparison to Intermediate Molecular Weight (Reference Treatment) in Treatment-Seeking Subjects with Opioid Use Disorder. ClinicalTrials.gov ID NCT02559973. Available online: https://clinicaltrials.gov/study/NCT02559973?term=NCT02559973&rank=1 (accessed on 6 May 2025).
- A Multiple-Dose Study of Blockade of Subjective Opioid Effects, Plasma Levels, and Safety of Subcutaneous Injections of Depot Buprenorphine (RBP-6000) in Subjects with Opioid Use Disorder. ClinicalTrials.gov ID NCT02044094. Available online: https://clinicaltrials.gov/study/NCT02044094?term=NCT02044094&rank=1 (accessed on 6 May 2025).
- Nasser, A.F.; Greenwald, M.K.; Vince, B.; Fudala, P.J.; Twumasi-Ankrah, P.; Liu, Y.; Jones, J.; Heidbreder, C. Sustained-Release Buprenorphine (RBP-6000) Blocks the Effects of Opioid Challenge with Hydromorphone in Subjects with Opioid Use Disorder. J. Clin. Psychopharmacol. 2016, 36, 18–26. [Google Scholar] [CrossRef]
- Open-Label, Multicenter, Multiple Dose Study of Safety, Tolerability, Pharmacokinetics, Efficacy Markers, and Opioid Receptor Availability of Subcutaneous Injections of Depot Buprenorphine in Treatment Seeking Opioid-Dependent Subjects. ClinicalTrials.gov ID NCT01738503. Available online: https://clinicaltrials.gov/study/NCT01738503?term=NCT01738503&rank=1 (accessed on 6 May 2025).
- An Open-Label, Long-Term Safety and Tolerability Study of Depot Buprenorphine (RBP-6000) in Treatment-Seeking Subjects with Opioid Use Disorder. ClinicalTrials.gov ID NCT02510014. Available online: https://clinicaltrials.gov/study/NCT02510014?term=NCT02510014&rank=1 (accessed on 6 May 2025).
- Jones, A.K.; Ngaimisi, E.; Gopalakrishnan, M.; Young, M.A.; Laffont, C.M. Population Pharmacokinetics of a Monthly Buprenorphine Depot Injection for the Treatment of Opioid Use Disorder: A Combined Analysis of Phase II and Phase III Trials. Clin. Pharmacokinet. 2021, 60, 527–540. [Google Scholar] [CrossRef]
- Andorn, A.C.; Haight, B.R.; Shinde, S.; Fudala, P.J.; Zhao, Y.; Heidbreder, C.; Learned, S.M.; Fox, N.L.; Nadipelli, V.R.; Hassman, D.; et al. Treating Opioid Use Disorder with a Monthly Subcutaneous Buprenorphine Depot Injection: 12-Month Safety, Tolerability, and Efficacy Analysis. J. Clin. Psychopharmacol. 2020, 40, 231–239. [Google Scholar] [CrossRef]
- An Open-Label, Depot Buprenorphine (RBP-6000) Treatment Extension Study in Subjects with Opioid Use Disorder. ClinicalTrials.gov ID NCT02896296. Available online: https://clinicaltrials.gov/study/NCT02896296?term=NCT02896296&rank=1 (accessed on 6 May 2025).
- Rutrick, D.; Learned, S.M.; Boyett, B.; Hassman, D.; Shinde, S.; Zhao, Y. 18-Month Efficacy and Safety Analysis of Monthly Subcutaneous Buprenorphine Injection for Opioid Use Disorder: Integrated Analysis of Phase 3 Studies. J. Subst. Use Addict. Treat. 2023, 154, 209155. [Google Scholar] [CrossRef]
- An Open-label, Multicentre, Single-arm Trial of Monthly Injections of Depot Buprenorphine in People with Opioid Dependence. ClinicalTrials.gov ID NCT03809143. Available online: https://clinicaltrials.gov/study/NCT03809143?term=NCT03809143&rank=1 (accessed on 6 May 2025).
- Farrell, M.; Shahbazi, J.; Byrne, M.; Grebely, J.; Lintzeris, N.; Chambers, M.; Larance, B.; Ali, R.; Nielsen, S.; Dunlop, A.; et al. Outcomes of a Single-Arm Implementation Trial of Extended-Release Subcutaneous Buprenorphine Depot Injections in People with Opioid Dependence. Int. J. Drug Policy 2022, 100, 103492. [Google Scholar] [CrossRef]
- A Multicenter, Randomized, Open-Label, Single-Dose Study to Evaluate the Pharmacokinetics, Safety, and Tolerability of RBP-7000 Using Poly(DL-lactide-co-glycolide) Polymer of Two Different Molecular Weights (Low and High Molecular Weights as Test Treatments) Compared to Intermediate Molecular Weight (Reference Treatment) Polymer in Subjects with Schizophrenia. ClinicalTrials.gov ID NCT02687984. Available online: https://clinicaltrials.gov/study/NCT02687984?term=NCT02687984&rank=1 (accessed on 6 May 2025).
- A Phase 2A, Open-Label, Multiple Ascending Dose Study of the Safety, Tolerability, Pharmacokinetics, and Primary Pharmacodynamic Markers of Efficacy of 60mg, 90mg, and 120mg of Risperidone in RBP-7000 Subcutaneous Injections in Subjects with Clinically Stable Schizophrenia. ClinicalTrials.gov ID NCT01677377. Available online: https://clinicaltrials.gov/study/NCT01677377?term=NCT01677377&rank=1 (accessed on 6 May 2025).
- Laffont, C.M.; Gomeni, R.; Zheng, B.; Heidbreder, C.; Fudala, P.J.; Nasser, A.F. Population Pharmacokinetics and Prediction of Dopamine D2 Receptor Occupancy After Multiple Doses of RBP-7000, a New Sustained-Release Formulation of Risperidone, in Schizophrenia Patients on Stable Oral Risperidone Treatment. Clin. Pharmacokinet. 2014, 53, 533–543. [Google Scholar] [CrossRef]
- A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Efficacy, Safety and Tolerability of RBP-7000 as a Treatment in Subjects with Acute Schizophrenia Over 8 Weeks (2 Subcutaneous Doses). ClinicalTrials.gov ID NCT02109562. Available online: https://clinicaltrials.gov/study/NCT02109562?term=NCT02109562&rank=1 (accessed on 6 May 2025).
- Le Moigne, A.; Csernansky, J.; Leadbetter, R.A.; Andorn, A.C.; Graham, J.A.; Heath, A.T.; Walling, D.P.; Newcomer, J.W.; Marder, S.R. PANSS Individual Item and Marder Dimension Analyses from a Pivotal Trial of RBP-7000 (Monthly Extended-Release Risperidone) in Schizophrenia Patients. J. Clin. Psychiatry 2021, 82, 37024. [Google Scholar] [CrossRef]
- An Open-Label, Long-Term Safety and Tolerability Study of RBP-7000 in the Treatment of Subjects with Schizophrenia. ClinicalTrials.gov ID NCT02203838. Available online: https://clinicaltrials.gov/study/NCT02203838?term=NCT02203838&rank=1 (accessed on 6 May 2025).
- Andorn, A.; Graham, J.; Csernansky, J.; Newcomer, J.W.; Shinde, S.; Muma, G.; Heidbreder, C.; Fava, M. Monthly Extended-Release Risperidone (RBP-7000) in the Treatment of Schizophrenia: Results From the Phase 3 Program. J. Clin. Psychopharmacol. 2019, 39, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Effect of Three Periodontal Therapies in Current Smokers and Non-Smokers. ClinicalTrials.gov ID NCT00066066. Available online: https://clinicaltrials.gov/study/NCT00066066?term=NCT00066066&rank=1 (accessed on 6 May 2025).
- Prospective Multicenter Observational Program for Evaluation of Efficacy and Tolerability of the 6-Month Depot Eligard 45 mg in Patients with Advanced Prostate Carcinoma in Routine Clinical Practice of Uro-Oncologists in the Russian Federation. ClinicalTrials.gov ID NCT02128334. Available online: https://clinicaltrials.gov/study/NCT02128334?term=NCT02128334&rank=1 (accessed on 6 May 2025).
- Matveev, V.B.; Markova, A.S. Final Results of the Prospective Multicenter Observational Program RU-EGD-NI-001 for Evaluation of Efficacy and Tolerability of the 6-Month Depot Eligard 45 Mg in Patients with Advanced Prostate Carcinoma in Routine Clinical Practice of Uro-Oncologists in the Russian Federation. Cancer Urol. 2017, 13, 79–86. [Google Scholar] [CrossRef]
- A Phase IV Interventional Safety Study of ELIGARD® in Prostate Cancer Patients in Asia (ELIGANT). ClinicalTrials.gov ID NCT03035032. Available online: https://clinicaltrials.gov/study/NCT03035032?term=NCT03035032&rank=1 (accessed on 6 May 2025).
- A Phase 2, Randomized, Single-Blind, Active-Control, Parallel Group Study to Evaluate Safety and Activity of a Single Administration of F14 for Management of Postoperative Pain in Participants Undergoing Unilateral Total Knee Replacement. ClinicalTrials.gov ID NCT03541655. Available online: https://clinicaltrials.gov/study/NCT03541655?term=NCT03541655&rank=1 (accessed on 6 May 2025).
- A Phase 3, Multicenter, Randomized, Double-Blind, Parallel Group Study of the Efficacy and Safety of a Single Administration of F14 for Postoperative Analgesia in Patients Undergoing Unilateral Total Knee Replacement. ClinicalTrials.gov ID NCT05603832. Available online: https://clinicaltrials.gov/study/NCT05603832?term=NCT05603832&rank=1 (accessed on 6 May 2025).
- An Open Label Safety Study of a Single Administration of F14 in Patients Undergoing Unilateral Total Knee Replacement. ClinicalTrials.gov ID NCT04860635. Available online: https://clinicaltrials.gov/study/NCT04860635?term=NCT04860635&rank=1 (accessed on 6 May 2025).
- A Multiple Dose Opioid Challenge Study to Assess Blockade of Subjective Opioid Effects of CAM2038 q1w (Buprenorphine FluidCrystal® Subcutaneous Injection Depots) In Adults with Opioid Use Disorder. ClinicalTrials.gov ID NCT02611752. Available online: https://clinicaltrials.gov/study/NCT02611752?term=NCT02611752&rank=1 (accessed on 6 May 2025).
- An Open-Label Multicenter Study Assessing the Long-Term Safety of a Once-Weekly and Once-Monthly, Long-Acting Subcutaneous Injection Depot of Buprenorphine (CAM2038) in Adult Outpatients with Opioid Use Disorder. ClinicalTrials.gov ID NCT02672111. Available online: https://clinicaltrials.gov/study/NCT02672111?term=NCT02672111&rank=1 (accessed on 6 May 2025).
- Frost, M.; Bailey, G.L.; Lintzeris, N.; Strang, J.; Dunlop, A.; Nunes, E.V.; Jansen, J.B.; Frey, L.C.; Weber, B.; Haber, P.; et al. Long-term Safety of a Weekly and Monthly Subcutaneous Buprenorphine Depot (CAM2038) in the Treatment of Adult Out-Patients with Opioid Use Disorder. Addiction 2019, 114, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- A Phase III, Randomized, Double-Blind, Active-Controlled, Parallel Group, Multi-Center Trial Assessing the Efficacy and Safety of a Once-Weekly and Once-Monthly, Long-Acting Subcutaneous Injectable Depot of Buprenorphine (CAM2038) in Treatment of Adult Outpatients with Opioid Use Disorder. ClinicalTrials.gov ID NCT02651584. Available online: https://clinicaltrials.gov/study/NCT02651584?term=NCT02651584&rank=1 (accessed on 6 May 2025).
- Lofwall, M.R.; Walsh, S.L.; Nunes, E.V.; Bailey, G.L.; Sigmon, S.C.; Kampman, K.M.; Frost, M.; Tiberg, F.; Linden, M.; Sheldon, B.; et al. Weekly and Monthly Subcutaneous Buprenorphine Depot Formulations vs Daily Sublingual Buprenorphine with Naloxone for Treatment of Opioid Use Disorder: A Randomized Clinical Trial. JAMA Intern. Med. 2018, 178, 764. [Google Scholar] [CrossRef]
- A Randomized, Placebo-Controlled, Double-Blind, Multi-Center Trial to Assess Efficacy and Safety of Octreotide Subcutaneous Depot (CAM2029) in Patients with Symptomatic Polycystic Liver Disease. ClinicalTrials.gov ID NCT05281328. Available online: https://clinicaltrials.gov/study/NCT05281328?term=NCT05281328&rank=1 (accessed on 6 May 2025).
- A Phase II, Open-label, Multicentre, Randomised Study of the Pharmacokinetics, Pharmacodynamics, Efficacy, and Safety of CAM2029 in Patients With Acromegaly and Neuroendocrine Tumours (NETs) Previously Treated with Sandostatin® LAR®. ClinicalTrials.gov ID NCT02299089. Available online: https://clinicaltrials.gov/study/NCT02299089?term=NCT02299089&rank=1 (accessed on 6 May 2025).
- Pavel, M.; Borson-Chazot, F.; Cailleux, A.; Hörsch, D.; Lahner, H.; Pivonello, R.; Tauchmanova, L.; Darstein, C.; Olsson, H.; Tiberg, F.; et al. Octreotide SC Depot in Patients with Acromegaly and Functioning Neuroendocrine Tumors: A Phase 2, Multicenter Study. Cancer Chemother. Pharmacol. 2019, 83, 375–385. [Google Scholar] [CrossRef]
- A Phase II, Open Label, Active Control, Multi-National, Multi-Centre, Randomized, Parallel Group Study Assessing Pharmacokinetics, Pharmacodynamics, Efficacy and Safety of CAM2032 (Leuprolide Acetate FluidCrystal® Injection Depot Once Monthly) After Repeat Doses of 3.75 mg and 7.5 mg of Leuprolide Acetate vs. Eligard® 7.5 mg in Patients with Prostate Cancer. ClinicalTrials.gov ID NCT02212197. Available online: https://clinicaltrials.gov/study/NCT02212197?term=NCT02212197&rank=1 (accessed on 6 May 2025).
- American Dental Association. Periodontitis Treatment. J. Am. Dent. Assoc. 2001, 132, 75. [Google Scholar] [CrossRef]
- Batool, F.; Agossa, K.; Lizambard, M.; Petit, C.; Bugueno, I.M.; Delcourt-Debruyne, E.; Benkirane-Jessel, N.; Tenenbaum, H.; Siepmann, J.; Siepmann, F.; et al. In-Situ Forming Implants Loaded with Chlorhexidine and Ibuprofen for Periodontal Treatment: Proof of Concept Study In Vivo. Int. J. Pharm. 2019, 569, 118564. [Google Scholar] [CrossRef]
- Phaechamud, T.; Setthajindalert, O. Antimicrobial In-Situ Forming Gels Based on Bleached Shellac and Different Solvents. J. Drug Deliv. Sci. Technol. 2018, 46, 285–293. [Google Scholar] [CrossRef]
- Rein, S.M.T.; Lwin, W.W.; Tuntarawongsa, S.; Phaechamud, T. Meloxicam-Loaded Solvent Exchange-Induced in situ Forming Beta-Cyclodextrin Gel and Microparticle for Periodontal Pocket Delivery. Mater. Sci. Eng. C 2020, 117, 111275. [Google Scholar] [CrossRef]
- Phaechamud, T.; Thurein, S.M.; Chantadee, T. Role of Clove Oil in Solvent Exchange-Induced Doxycycline Hyclate-Loaded Eudragit RS in situ Forming Gel. Asian J. Pharm. Sci. 2018, 13, 131–142. [Google Scholar] [CrossRef]
- Do, M.P.; Neut, C.; Delcourt, E.; Seixas Certo, T.; Siepmann, J.; Siepmann, F. In situ Forming Implants for Periodontitis Treatment with Improved Adhesive Properties. Eur. J. Pharm. Biopharm. 2014, 88, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.; Willart, J.-F.; Neut, C.; Agossa, K.; Siepmann, J.; Siepmann, F. In-Situ Forming PLGA Implants: Towards Less Toxic Solvents. Int. J. Pharm. 2024, 657, 124121. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Deng, Y.; Ma, S.; Ran, M.; Jia, Y.; Meng, J.; Han, F.; Gou, J.; Yin, T.; He, H.; et al. Local Drug Delivery Systems as Therapeutic Strategies against Periodontitis: A Systematic Review. J. Control. Release 2021, 333, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Khaing, E.M.; Intaraphairot, T.; Chuenbarn, T.; Chantadee, T.; Phaechamud, T. Natural Resin-Based Solvent Exchange Induced in-Situ Forming Gel for Vancomycin HCl Delivery to Periodontal Pocket. Mater. Today Proc. 2021, 47, 3585–3593. [Google Scholar] [CrossRef]
- Lizambard, M.; Menu, T.; Fossart, M.; Bassand, C.; Agossa, K.; Huck, O.; Neut, C.; Siepmann, F. In-Situ Forming Implants for the Treatment of Periodontal Diseases: Simultaneous Controlled Release of an Antiseptic and an Anti-Inflammatory Drug. Int. J. Pharm. 2019, 572, 118833. [Google Scholar] [CrossRef]
- Agossa, K.; Delepierre, A.; Lizambard, M.; Delcourt-Debruyne, E.; Siepmann, J.; Siepmann, F.; Neut, C. In-Situ Forming Implants for Dual Controlled Release of Chlorhexidine and Ibuprofen for Periodontitis Treatment: Microbiological and Mechanical Key Properties. J. Drug Deliv. Sci. Technol. 2020, 60, 101956. [Google Scholar] [CrossRef]
- Rajeshwari, H.R.; Dhamecha, D.; Jagwani, S.; Rao, M.; Jadhav, K.; Shaikh, S.; Puzhankara, L.; Jalalpure, S. Local Drug Delivery Systems in the Management of Periodontitis: A Scientific Review. J. Control. Release 2019, 307, 393–409. [Google Scholar] [CrossRef]
- Lertsuphotvanit, N.; Santimaleeworagun, W.; Narakornwit, W.; Chuenbarn, T.; Mahadlek, J.; Chantadee, T.; Phaechamud, T. Borneol-Based Antisolvent-Induced In Situ Forming Matrix for Crevicular Pocket Delivery of Vancomycin Hydrochloride. Int. J. Pharm. 2022, 617, 121603. [Google Scholar] [CrossRef]
- Tuntarawongsa, S.; Mahadlek, J.; Senarat, S.; Phaechamud, T. Eudragit® RL in 2-Pyrrolidone as Antisolvent-Based in-Situ Forming Matrix. Mater. Today Proc. 2022, 52, 2534–2538. [Google Scholar] [CrossRef]
- Juvekar, S.; Kathpalia, H. Solvent Removal Precipitation Based in situ Forming Implant for Controlled Drug Delivery in Periodontitis. J. Control. Release 2017, 251, 75–81. [Google Scholar] [CrossRef]
- Do, M.P.; Neut, C.; Metz, H.; Delcourt, E.; Siepmann, J.; Mäder, K.; Siepmann, F. Mechanistic Analysis of PLGA/HPMC-Based in-Situ Forming Implants for Periodontitis Treatment. Eur. J. Pharm. Biopharm. 2015, 94, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Chantadee, T.; Thammasut, W.; Phaechamud, T. Fluid Properties and Phase Transition of Antimicrobial Eudragit RS/Clove Oil In Situ Forming Depot. Mater. Today Proc. 2022, 65, 2296–2302. [Google Scholar] [CrossRef]
- Saklani, R.; Yadav, P.K.; Nengroo, M.A.; Gawali, S.L.; Hassan, P.A.; Datta, D.; Mishra, D.P.; Dierking, I.; Chourasia, M.K. An Injectable In Situ Depot-Forming Lipidic Lyotropic Liquid Crystal System for Localized Intratumoral Drug Delivery. Mol. Pharm. 2022, 19, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Lozza, I.; Martín-Sabroso, C.; Torres-Suárez, A.I.; Fraguas-Sánchez, A.I. In situ Forming PLA and PLGA Implants for the Parenteral Administration of Cannabidiol. Int. J. Pharm. 2024, 661, 124468. [Google Scholar] [CrossRef]
- Gao, L.; Yuan, Z.; Ma, N.; Zhou, X.; Huang, X.; Chen, W.; Qiao, H. Nitric Oxide Polymersome-Immobilized Hydrogels for Prevention of Post-Surgical Tumor Recurrence and Metastasis. Chem. Eng. J. 2024, 485, 149688. [Google Scholar] [CrossRef]
- Long, D.; Gong, T.; Zhang, Z.; Ding, R.; Fu, Y. Preparation and Evaluation of a Phospholipid-Based Injectable Gel for the Long Term Delivery of Leuprolide Acetate. Acta Pharm. Sin. B 2016, 6, 329–335. [Google Scholar] [CrossRef]
- Breitsamer, M.; Winter, G. Vesicular Phospholipid Gels as Drug Delivery Systems for Small Molecular Weight Drugs, Peptides and Proteins: State of the Art Review. Int. J. Pharm. 2019, 557, 1–8. [Google Scholar] [CrossRef]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, Promising Drug Delivery Systems: An Update of State-of-the-Art and Recent Applications. J. Control. Release 2018, 271, 1–20. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Zak, J.K. Biomaterial-Based Regional Chemotherapy: Local Anticancer Drug Delivery to Enhance Chemotherapy and Minimize Its Side-Effects. Mater. Sci. Eng. C 2016, 62, 927–942. [Google Scholar] [CrossRef]
- Fakhari, A.; Anand Subramony, J. Engineered In-Situ Depot-Forming Hydrogels for Intratumoral Drug Delivery. J. Control. Release 2015, 220, 465–475. [Google Scholar] [CrossRef]
- Hope, A.; Wade, S.J.; Aghmesheh, M.; Vine, K.L. Localized Delivery of Immunotherapy via Implantable Scaffolds for Breast Cancer Treatment. J. Control. Release 2022, 341, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Jeganathan, S.; Budziszewski, E.; Bielecki, P.; Kolios, M.C.; Exner, A.A. In Situ Forming Implants Exposed to Ultrasound Enhance Therapeutic Efficacy in Subcutaneous Murine Tumors. J. Control. Release 2020, 324, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Mahadlek, J.; Rein, S.M.T.; Chinpaisal, C.; Phaechamud, T. Physical Properties and Bioactivity of Clove Oil-Loaded Solvent Exchange-Induced in situ Forming Gel. Mater. Today Proc. 2021, 47, 3509–3516. [Google Scholar] [CrossRef]
- Khaing, E.M.; Toungsuwan, P.; Chantadee, T.; Rein, S.M.T.; Phaechamud, T.; Charoenteeraboon, J.; Mahadlek, J. Clotrimazole-Incorporated Fatty Acid-Based in situ Forming Film Containing Pressure Sensitive Adhesive. Mater. Today Proc. 2022, 65, 2277–2283. [Google Scholar] [CrossRef]
- Mahadlek, J.; Myo Thurein, S.; Thammasut, W.; Phaechamud, T. Clotrimazole-Loaded Fatty Acid-Based in situ Forming Film Oral Spray. Mater. Today Proc. 2022, 52, 2479–2484. [Google Scholar] [CrossRef]
- Karp, F.; Turino, L.N.; Helbling, I.M.; Islan, G.A.; Luna, J.A.; Estenoz, D.A. In Situ Formed Implants, Based on PLGA and Eudragit Blends, for Novel Florfenicol Controlled Release Formulations. J. Pharm. Sci. 2021, 110, 1270–1278. [Google Scholar] [CrossRef]
- Damiri, F.; Fatimi, A.; Magdalena Musuc, A.; Paiva Santos, A.C.; Paszkiewicz, S.; Igwe Idumah, C.; Singh, S.; Varma, R.S.; Berrada, M. Nano-Hydroxyapatite (nHAp) Scaffolds for Bone Regeneration: Preparation, Characterization and Biological Applications. J. Drug Deliv. Sci. Technol. 2024, 95, 105601. [Google Scholar] [CrossRef]
- Khattab, A.; Abouhussein, D.M.N.; Mohammad, F.E. Development of Injectable Tenoxicam in situ Forming Microparticles Based on Sesame Oil and Poly-DL-Lactide: Characterization, Efficacy and Acute Toxicity. J. Drug Deliv. Sci. Technol. 2019, 51, 682–694. [Google Scholar] [CrossRef]
- Śmiga-Matuszowicz, M.; Korytkowska-Wałach, A.; Nowak, B.; Pilawka, R.; Lesiak, M.; Sieroń, A.L. Poly(Isosorbide Succinate)-Based in situ Forming Implants as Potential Systems for Local Drug Delivery: Preliminary Studies. Mater. Sci. Eng. C 2018, 91, 311–317. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, L.; Sheng, L.; Wang, H.; Li, C.; Lin, X.; Yang, P. Design of an Injectable Sustained Release In-Situ Forming Depot of Meloxicam for Pain Relief. J. Drug Deliv. Sci. Technol. 2024, 93, 105460. [Google Scholar] [CrossRef]
- Elder, S.H.; Ross, M.K.; Nicaise, A.J.; Miller, I.N.; Breland, A.N.; Hood, A.R.S. Development of in situ Forming Implants for Controlled Delivery of Punicalagin. Int. J. Pharm. 2024, 652, 123842. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Gad, S.F.; Chobisa, D.; Li, Y.; Yeo, Y. Local Drug Delivery Systems for Inflammatory Diseases: Status Quo, Challenges, and Opportunities. J. Control. Release 2021, 330, 438–460. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Shan, X.; Li, X.; Luo, Z.; Yu, X.; Liu, H.; Wang, S.; Zhao, X.; Zhu, Y.; Zhou, H.; et al. Solvent Exchange-Motivated and Tunable in situ Forming Implants Sustaining Triamcinolone Acetonide Release for Arthritis Treatment. Int. J. Pharm. 2023, 645, 123383. [Google Scholar] [CrossRef] [PubMed]
- Niloy, K.K.; Lowe, T.L. Injectable Systems for Long-Lasting Insulin Therapy. Adv. Drug Deliv. Rev. 2023, 203, 115121. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A.; Zhao, X.; Liu, X.; Wang, D.; Sun, F.; Li, Y. Design of a Long-Term Antipsychotic In Situ Forming Implant and Its Release Control Method and Mechanism. Int. J. Pharm. 2012, 427, 284–292. [Google Scholar] [CrossRef]
- Zhang, T.; Luo, J.; Peng, Q.; Dong, J.; Wang, Y.; Gong, T.; Zhang, Z. Injectable and Biodegradable Phospholipid-Based Phase Separation Gel for Sustained Delivery of Insulin. Colloids Surf. B Biointerfaces 2019, 176, 194–201. [Google Scholar] [CrossRef]
- Kaurav, H.; Sharma, A.; Upadhyay, N.K.; Kapoor, D.N. Long Term Delivery of Glibenclamide from in situ Forming Microparticles for the Treatment of Ischemic Stroke. J. Drug Deliv. Sci. Technol. 2021, 66, 102860. [Google Scholar] [CrossRef]
- Cao, Z.; Tang, X.; Zhang, Y.; Yin, T.; Gou, J.; Wang, Y.; He, H. Novel Injectable Progesterone-Loaded Nanoparticles Embedded in SAIB-PLGA in situ Depot System for Sustained Drug Release. Int. J. Pharm. 2021, 607, 121021. [Google Scholar] [CrossRef]
- Battaglia, L.S.; Dorati, R.; Maestrelli, F.; Conti, B.; Gabriele, M.; Di Cesare Mannelli, L.; Selmin, F.; Cosco, D. Repurposing of Parenterally Administered Active Substances Used to Treat Pain Both Systemically and Locally. Drug Discov. Today 2022, 27, 103321. [Google Scholar] [CrossRef]
- Chen, S.; Singh, J. Controlled Delivery of Testosterone from Smart Polymer Solution Based Systems: In Vitro Evaluation. Int. J. Pharm. 2005, 295, 183–190. [Google Scholar] [CrossRef]
- Bode, C.; Kranz, H.; Siepmann, F.; Siepmann, J. In-Situ Forming PLGA Implants for Intraocular Dexamethasone Delivery. Int. J. Pharm. 2018, 548, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Abrishami, M.; Farzadnia, M.; Kamali, H.; Malaekeh-Nikouei, B. In-Situ Forming Biodegradable Implants for Sustained Fluocinolone Acetonide Release to the Posterior Eye: In-Vitro and In-Vivo Investigations in Rabbits. Int. J. Pharm. 2024, 654, 123973. [Google Scholar] [CrossRef] [PubMed]
- Sarode, I.M.; Jindal, A.B. Current Status of Dolutegravir Delivery Systems for the Treatment of HIV-1 Infection. J. Drug Deliv. Sci. Technol. 2022, 76, 103802. [Google Scholar] [CrossRef]
- Shiadeh, S.N.R.; Khodaverdi, E.; Maleki, M.F.; Eisvand, F.; Nazari, A.; Zarqi, J.; Hadizadeh, F.; Kamali, H. A Sustain-Release Lipid-Liquid Crystal Containing Risperidone Based on Glycerol Monooleate, Glycerol Dioleate, and Glycerol Trioleate: In-Vitro Evaluation and Pharmacokinetics in Rabbits. J. Drug Deliv. Sci. Technol. 2022, 70, 103257. [Google Scholar] [CrossRef]
- Sharma, R.; Yadav, S.; Yadav, V.; Akhtar, J.; Katari, O.; Kuche, K.; Jain, S. Recent Advances in Lipid-Based Long-Acting Injectable Depot Formulations. Adv. Drug Deliv. Rev. 2023, 199, 114901. [Google Scholar] [CrossRef]
- Thalhauser, S.; Peterhoff, D.; Wagner, R.; Breunig, M. Silica Particles Incorporated into PLGA-Based in situ-Forming Implants Exploit the Dual Advantage of Sustained Release and Particulate Delivery. Eur. J. Pharm. Biopharm. 2020, 156, 1–10. [Google Scholar] [CrossRef]
- Lin, X.; Al Zouabi, N.N.; Ward, L.E.; Zhen, Z.; Darji, M.; Masese, F.K.; Hargrove, D.; O’Reilly Beringhs, A.; Kasi, R.M.; Li, Q.; et al. Implant Dynamics, Inner Structure, and Their Impact on Drug Release of In Situ Forming Implants Uncovered Through CT Imaging. J. Control. Release 2024, 375, 802–811. [Google Scholar] [CrossRef]
- Senarat, S.; Tuntarawongsa, S.; Lertsuphotvanit, N.; Rojviriya, C.; Phaechamud, T.; Chantadee, T. Levofloxacin HCl-Loaded Eudragit L-Based Solvent Exchange-Induced In Situ Forming Gel Using Monopropylene Glycol as a Solvent for Periodontitis Treatment. Gels 2023, 9, 583. [Google Scholar] [CrossRef]
- Phaechamud, T.; Mahadlek, J.; Tuntarawongsa, S. Peppermint Oil/Doxycycline Hyclate-Loaded Eudragit RS in situ Forming Gel for Periodontitis Treatment. J. Pharm. Investig. 2018, 48, 451–464. [Google Scholar] [CrossRef]
- Phaechamud, T.; Setthajindalert, O. Cholesterol in situ Forming Gel Loaded with Doxycycline Hyclate for Intra-Periodontal Pocket Delivery. Eur. J. Pharm. Sci. 2017, 99, 258–265. [Google Scholar] [CrossRef]
- Phaechamud, T.; Senarat, S.; Puyathorn, N.; Praphanwittaya, P. Solvent Exchange and Drug Release Characteristics of Doxycycline Hyclate-Loaded Bleached Shellac in situ-Forming Gel and -Microparticle. Int. J. Biol. Macromol. 2019, 135, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Palvinskiy, A.G.; Bakhrushina, E.O.; Kholina, P.A.; Krasnyuk, I.I. Biopharmaceutical Study of Berberine Bisulphate Dental Gel. Probl. Biol. Med. Pharm. Chem. 2022, 25, 10–14. [Google Scholar] [CrossRef]
- Tipton, A.J.; Fujita, S.M.; Dunn, R.L. Biodegradable in Situ Forming Film Dressing. US5792469A, 11 August 1998. Available online: https://patents.google.com/patent/US5792469A/en?oq=US5792469A (accessed on 6 May 2025).
- Su, D.; Ashton, P.; Chen, J. In Situ Gelling Drug Delivery System. US9566336B2, 14 February 2017. Available online: https://patents.google.com/patent/US9566336B2/en?oq=US9566336B2 (accessed on 6 May 2025).
- Gong, T.; Song, X.; Zhang, Z.R.; Zhang, Y.; Wei, G.F.; Hu, M.; Chen, T.J.; Sun, X.; Fu, Y. A Kind of Small Molecule Drug in Situ Phase Change Gel Sustained Release System and Preparation Method Thereof. CN107049932B, 6 November 2020. Available online: https://patents.google.com/patent/CN107049932B/en?oq=CN107049932B (accessed on 6 May 2025).
- Bakhrushina, E.O.; Afonina, A.M.; Mikhel, I.B.; Demina, N.B.; Plakhotnaya, O.N.; Belyatskaya, A.V.; Krasnyuk, I.I., Jr.; Krasnyuk, I.I. Role of Sterilization on In situ Gel-Forming Polymer Stability. Polymers 2024, 16, 2943. [Google Scholar] [CrossRef] [PubMed]
- Titova, S.A.; Kruglova, M.P.; Stupin, V.A.; Manturova, N.E.; Achar, R.R.; Deshpande, G.; Parfenov, V.A.; Silina, E.V. Excipients for Cerium Dioxide Nanoparticle Stabilization in the Perspective of Biomedical Applications. Molecules 2025, 30, 1210. [Google Scholar] [CrossRef]
- Long, L.; Ji, D.; Hu, C.; Yang, L.; Tang, S.; Wang, Y. Microneedles for in Situ Tissue Regeneration. Mater. Today Bio. 2023, 19, 100579. [Google Scholar] [CrossRef]
- C., D.K.; Raghavendra Naveen, N.; Goudanavar, P.S.; Ramesh, B.; Kiran Kumar, G.B. Prospection of Fabrication Techniques and Material Selection of Microneedles for Transdermal Drug Delivery: An Update on Clinical Trials. Mater. Today Proc. 2022, 69, 187–192. [Google Scholar] [CrossRef]
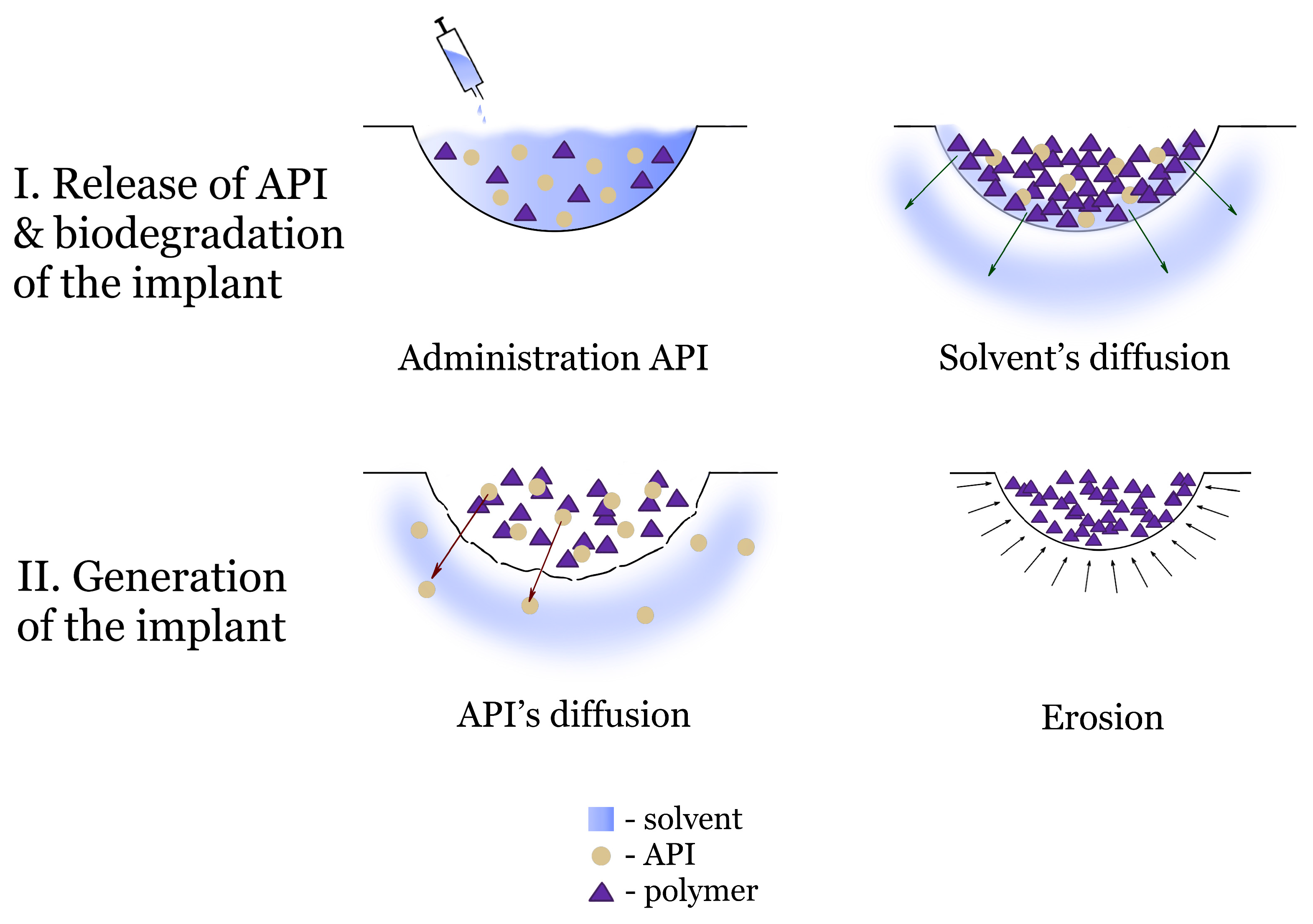

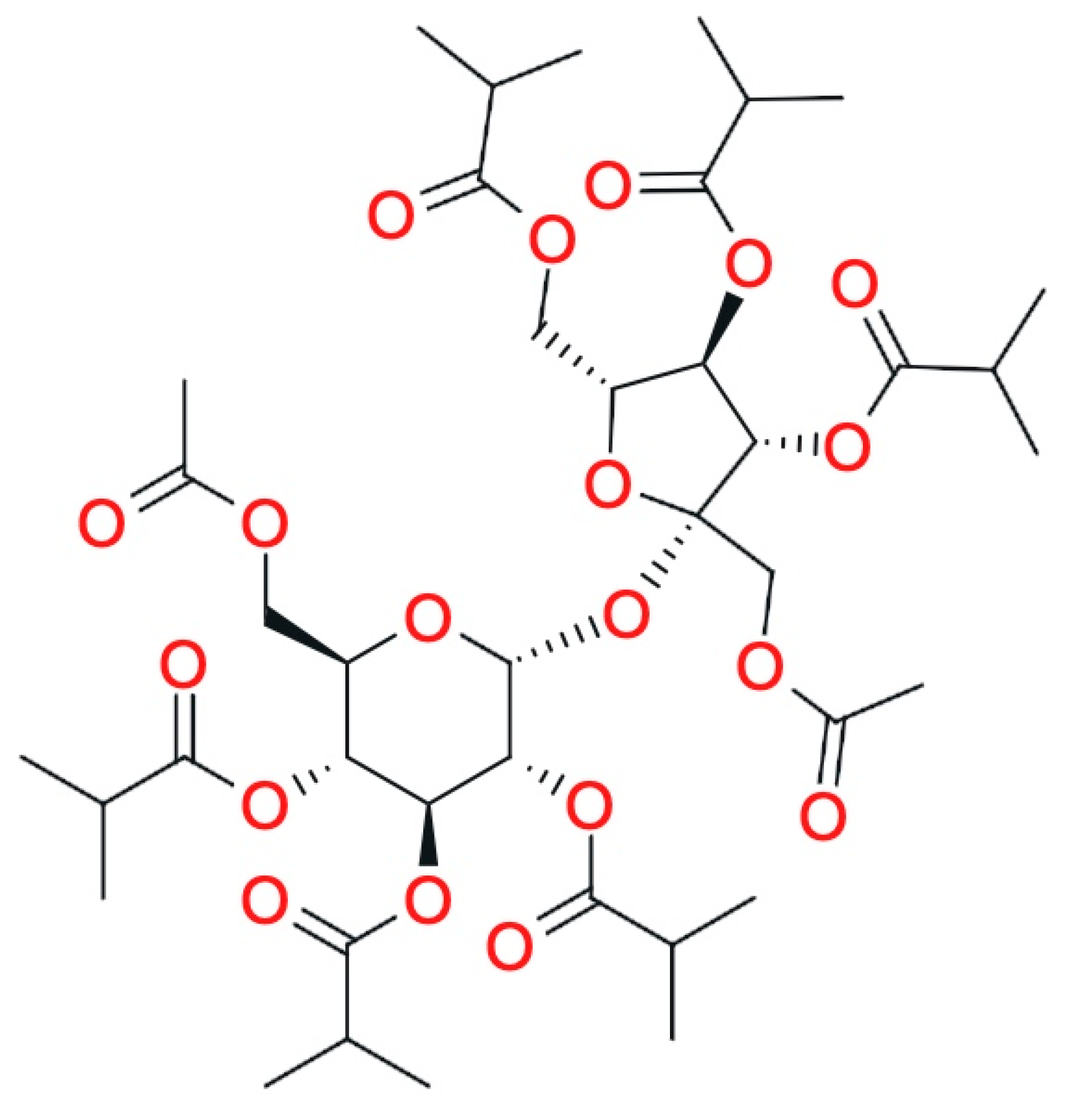
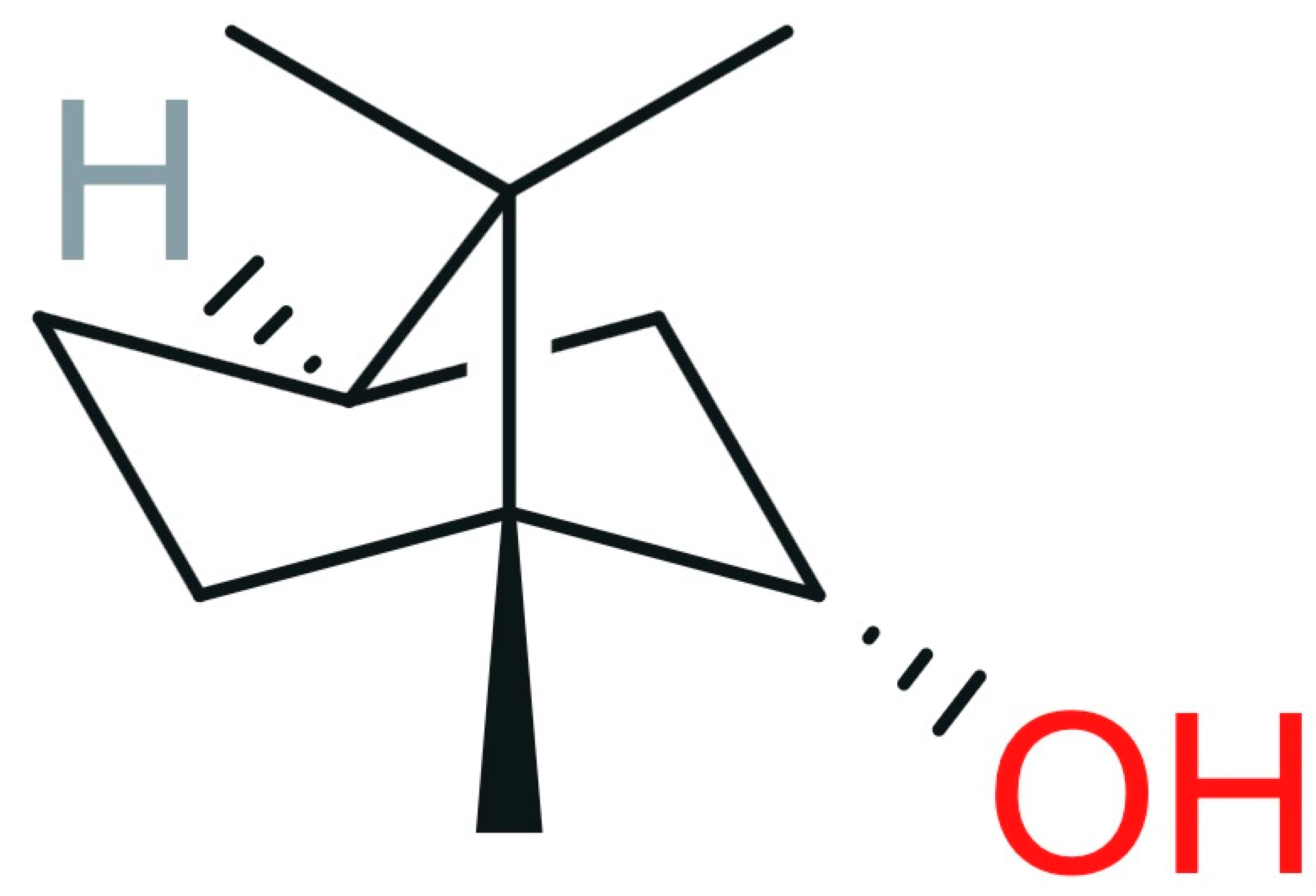
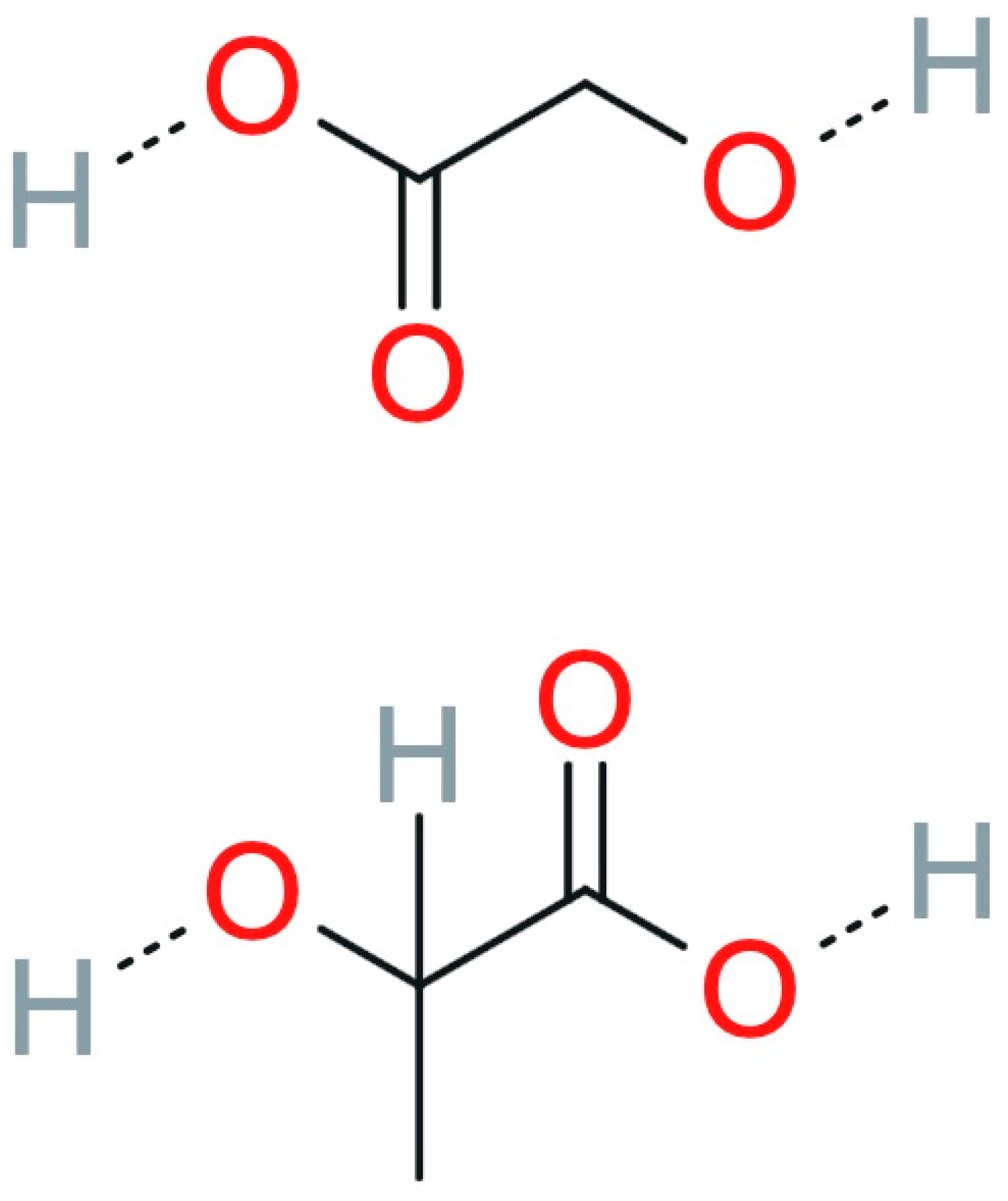
| Availability Data | FDA [29] | USP [30] | EP [31] | JP [32] | EAEU Pharmacopeia [33] | |
|---|---|---|---|---|---|---|
| Matrix Former | ||||||
| lactic acid homopolymer | Approved | Mentioned | No data | Mentioned | Mentioned | |
| polycaprolactone | No data | No data | No data | Mentioned | No data | |
| N-lauroyl-L-alanine methyl ether | No data | No data | No data | No data | No data | |
| sucrose acetate isobutyrate | Approved | No data | No data | No data | No data | |
| borneol | Approved | No data | No data | Mentioned | Mentioned | |
| shellac | Approved | Mentioned | Mentioned | Mentioned | No data | |
| PLGA | Approved | No data | No data | Mentioned | No data | |
| Availability Data | FDA [29] | USP [30] | EP [31] | JP [32] | EAEU Pharmacopeia [33] | |
|---|---|---|---|---|---|---|
| Solvent | ||||||
| N-methylpyrrolidone | Approved | Mentioned | Mentioned | Mentioned | Mentioned | |
| dimethyl sulfoxide | Approved | No data | Mentioned | Mentioned | Mentioned | |
| benzyl benzoate | No data | No data | Mentioned | Mentioned | No data | |
| benzyl alcohol | Approved | No data | Mentioned | Mentioned | No data | |
| triacetin | Approved | No data | Mentioned | Mentioned | Mentioned | |
| triethyl citrate | Approved | Mentioned | Mentioned | Mentioned | No data | |
| glycofurol | Approved | No data | No data | No data | No data | |
| ethyl lactate | Approved | No data | No data | No data | No data | |
| propylene carbonate | Approved | Mentioned | No data | No data | No data | |
| Technology | API | Comparator Drug | Drug Administration | Blood Plasma Concentrations, ng/mL or Population Pharmacokinetic Model Data | Side Effects | Efficiency | Phase | Study Type | The Number of Participants | Date | Sources |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atrigel® | BPN | No comparison drug was available | Subcutaneous injection | - | - | - | 1 | A multicenter, open-label, single ascending dose study | 48 | 2012–2013 | [202] |
| No comparison drug was available | Subcutaneous injection | - | - | - | 1 | An open-label, single-center, first-in-human study | 18 | 2010–2011 | [203] | ||
| No comparison drug was available | Subcutaneous injection | - | - | - | 1 | A single-center, randomized, open-label, single-dose study | 47 | 2015–2016 | [204] | ||
| No comparison drug was available | Subcutaneous injection | After the first injection: 2; before the second injection: 1.8–1.9; After the second injection: 3 | 64.1%; mostly constipation (30.8%), injection site reactions (79.5%) | therapeutic concentration was maintained for 28 days | 2 | A multiple-dose, single-center study | 39 | 2013–2014 | [205,206] | ||
| No comparison drug was available | Subcutaneous injection | - | - | - | 2 | An open-label, multicenter, multiple dose study | 124 | 2012–2014 | [207] | ||
| No comparison drug was available | Subcutaneous injection | After the first injection: 2; monthly injection: 5–10 | - | - | 3 | An open-label, long-term study | 775 | 2015–2017 | [208,209] | ||
| Placebo | Subcutaneous injection | - | For 1–6 months: 62.5–76.8% For 7–12 months: 38.1–58.0% Serious side effects: 2.7–4.4% | the percentage of participants abstinent from opioids: For 6 months: 65.5–66.3% For 12 months: 47.1–53.9% | 3 | An open-label, multicenter study | 669 for safety study; 901 for efficiency study | 2015–2017 | [208,210] | ||
| Placebo | Subcutaneous injection | - | For 1–6 months: 72.5% For 7–12 months: 55.6% For 13–18 months: 31.8% Serious side effects: For 2–6 months: 0.0–0.9% For 7–12 months: 5.4–7.8% For 13–18 months: 6.6–7.8% Severe side effects: For 2–6 months: 0.7–1.6% For 7–12 months: 0.0–0.5% For 13–18 months: 0.3–0.6% | the percentage of participants abstinent from opioids: For 18 months: 80–92.7% | 3 | An open-label, multicenter study | 208 | 2016–2017 | [211,212] | ||
| No comparison drug was available | Subcutaneous injection | - | Total number of adverse events: 91% Number of people with a treatment-related adverse event: 45% Total number of serious adverse events: 26% Number of people with a serious adverse event over the treatment period: 16% | Treatment retention: For 12 months: 75% For 6 months: 86% | 3 | An open-label, multicenter, single-arm study | 100 | 2019–2021 | [213,214] | ||
| Atrigel® | RPD | No comparison drug was available | Subcutaneous injection | - | - | - | 1 | A multicenter, randomized, open-label, single-dose study | 44 | 2016 | [215] |
| Atrigel® | No comparison drug was available | Subcutaneous injection | Oral risperidone model: (1) rate constant for the absorption: 3.64; (2) rate constant for conversion to 9-hydroxyrisperidone: 0.0990; (3) rate constant for elimination by other processes: 0.0344; (4) elimination rate constant for 9-hydroxyrisperidone: 0.0782; (5) volume of distribution of the central compartment: 63.8; Atrigel®RPD model: (1) rate constant for the rapid absorption: 0.0266; (2) rate constant for the slow absorption: 0.0185, (3) transit rate constant: 0.0247; (4) rate constant for elimination by other processes: 0.011; (5) rate constant for conversion to 9-hydroxyrisperidone: 0.102; (6) exchange rates between the central and the peripheral compartments of risperidone: 0.572 and 0.0114; (7) volume of distribution of the central compartment: 171; | The results of Monte Carlo simulation analyses suggest a potentially safer profile of Atrigel®RPD compared to oral risperidone in relation to any extrapyramidal symptoms | - | 2 | An open-label, multiple ascending dose study | 45 | 2012–2013 | [216,217] | |
| Atrigel® | Placebo | Subcutaneous injection | - | (1) Any side effects: 90 mg: 70.4% 120 mg: 77.8% Placebo: 68.6% (2) Any serious side effects: 90 mg: -% 120 mg: 0.9% Placebo: 0.9% (3) Any extrapyramidal symptoms: 90 mg: 7.8% 120 mg: 11.1% Placebo: 9.3% | Both Atrigel®RPD groups showed significant improvement with respect to positive symptoms. A significant improvement with respect to negative symptoms was found only in the 120 mg group | 3 | A randomized, double-blind, placebo-controlled, multicenter study | 354 | 2014 | [218,219] | |
| Atrigel® | Placebo | Subcutaneous injection | - | A total of 73.4% of participants reported 1 or more side effects. Only 4.8% of participants reported one or more severe side effects. The most common side effects, associated with Atrigel®RPD, were injection-site pain (12.8%), weight increase (11.4%) and akathisia (5.8%). | Mean changes on the Positive and Negative Syndrome Scale: (1) the Positive Scale score: Placebo: −7.8 90 mg: −3.7 120 mg −3.7 (among rollover participants); Among de novo participants mean changes were −1.3; (2) the Negative Scale score: Placebo: −4.0 90 mg: −4.1 120 mg −0.9 (among rollover participants); Among de novo participants mean changes were −1.3; (3) the General Psychopathology Scale score: Placebo: −8.3 90 mg: −4.7 120 mg −6.4 (among rollover participants); Among de novo participants mean changes were −0.4; | 3 | An open-label, long-term safety and tolerability study | 500 | 2014–2016 | [220,221] | |
| Atrigel® | ADX | Scaling and root planning; Metronidazole | - | - | - | - | 2 | A randomized interventional study | 146 | 2003–2009 | [222] |
| Atrigel® | LPA | No comparison drug was available | Subcutaneous injections | - | The rate of adverse events was 1.41%. The most common adverse events were asthenia, headache, arterial hypertension and nausea. | Average plasma PSA level decreased by 81.7% Average plasma testosterone level decreased by 88.0%; target testosterone level was achieved in 88.0–97.0% of patients | - | A prospective, multicenter, observational study | 645 | 2013–2016 | [223,224] |
| No comparison drug was available | Subcutaneous injections | - | The incidence of side effects was 7.7%. The most common side effects were sexual impotence (6.2%) and hot flashes (2.5%). Severe toxicity was observed in 0.9% of cases | Mean plasma PSA levels decreased by 99.7% after 12 months | - | A retrospective study | 932 | 2007–2018 | [182] | ||
| No comparison drug was available | Subcutaneous injections | - | 75.5% of patients experienced at least one side effect. For example, the most common side effects were PSA level increase (17%), cough (9.4%) and hot flashes (7.5%). Severe toxicity was observed in 0.9% of cases | Average plasma PSA level decreased by 68.5–81.5% Average plasma testosterone level decreased by 51.4–80.0%; | 4 | An interventional study | 107 | 2017–2019 | [181,225] | ||
| BEPO® | CLX | Bupivacaine Hydrochloride | - | - | - | - | 2 | A randomized, single-blind, active-control, parallel group study | 20 | 2018–2020 | [226] |
| Bupivacaine Hydrochloride+Acetaminophen+Methocarbamol | - | - | - | - | 3 | A multicenter, randomized, double-blind, parallel group study | 151 | 2022–2024 | [227] | ||
| - | - | - | - | - | 2–3 | An open-label safety study | 100 | 2025–2027 | [228] | ||
| FluidCrystal® | BPN | Sublingual tablet Subutex®, infusion solution Temgesic® | Subcutaneous injection | C (trough): (1) 24 h after dosing for infusion solution: 0.042 (2) Sublingual tablet (2.1) First dose 8 mg: 0.52 16 mg: 1.1 24 mg: 1.47 (2.2) Seventh dose 8 mg: 1.52 16 mg: 3.81 24 mg: 4.11 (3) FluidCrystal® (3.1) weekly 16 mg: First dose 0.29 Fourth dose 0.42 (3.2) monthly 64 mg: 0.14 96 mg: 0.15 128 mg: 0.27 192 mg: 0.39 | The most commonly reported side effects after FluidCrystal® BPN administration were nausea (63%) and dizziness (54%). The number of side effects was smallest for the FluidCrystal® formulations, followed by sublingual tablets and infusion solution | - | 1 | An open label, randomized controlled study | 87 for safety study, and 75 subjects for pharmacokinetics study | 2014–2016 | [197] |
| No comparison drug was available | Subcutaneous injection | C trough: 24 mg: First dose 1.81 Second dose 2.29 32 mg: First dose 2.24 Second dose 3.05 | A total of 64% of participants with 24 mg and 96% of participants with 32 mg dose experienced 1 or more side effects. Adverse events suspected to be related to FluidCrystal® BPN were reported by 57.4%, including constipation (19%) and injection-site pain (9%), with most rated as mild severity. | Opioid withdrawal was completely suppressed on day 1 after FluidCrystal® BPN injection and remained suppressed for the study duration | 2 | A multi-site, randomized, double-blind, repeat-dose study | 47 | 2015–2016 | [229] | ||
| No comparison drug was available | Subcutaneous injection | - | In total, 56.4% of side effects were mild or moderate in intensity: injection-site pain (15.4%), swelling (11.9%), erythema (9.3%) and headache (7.9%). 6.6% of side effects were severe intensity | For new-to-treatment participants, the composite of illicit opioid-negative urine samples and self-reports was 63.0%. For other participants, the percentage negative for illicit opioids was 82.8% | 3 | An open-label, multicenter study | 228 | 2015–2017 | [230,231] | ||
| Placebo+buprenorphine/naloxone | Subcutaneous injection | - | The most common adverse events (regardless of study medication) were injection-site pain (8.4%), headache (7.7%), constipation (7.5%) and nausea (7.5%). All injection-site adverse events were mild (73.9%) or moderate (26.1%) in intensity. In total, 4.2% of patients experienced at least 1 nonfatal serious adverse event | Treatment difference between increased from 5.9% to 8.5% relative to the comparison group during 3 months | 3 | A randomized, double-blind, active-controlled, parallel group, multicenter trial | 428 | 2015–2016 | [232,233] | ||
| OCT | Long-acting octreotide | Subcutaneous injection | Ctrough FluidCrystal® OCT: 10 mg: 0.24 20 mg: 0.52–0.53 30 mg: 0.82–0.86 Ctrough long acting octreotide 30 mg: 0.61 | (1) Total number of side effects: 10 mg: 93.3–100% 20 mg: 85.7–100% 30 mg: 100% long acting octreotide 30 mg: 71.4% (2) Diarrhea: 10 mg: 80–88.2% 20 mg: 78.6–81.3% 30 mg: 76.5–80% long acting octreotide 30 mg: 35.7% (3) Headache: 10 mg: 39.5–41.9% 20 mg: 39.4–44.2% 30 mg: 41.2–53.3% long acting octreotide 30 mg: 49.2% (4) Abdominal pain: 10 mg: 33.3–41.2% 20 mg: 28.6–37.5% 30 mg: 26.7–52.9% long acting octreotide 30 mg: 34.4% (5) Injection site pain: 10 mg: 11.8–20% 20 mg: 21.4–25% 30 mg: 21.4–29.4% long acting octreotide 30 mg: 22.1% | Maximum inhibition of IGF-1 concentration for FluidCrystal® OCT: (1) First injection 10 mg: 39.5–41.9% 20 mg: 39.4–44.2% 30 mg: 42.8–43.8% maximum inhibition of IGF-1 concentration for long acting octreotide 30 mg: 35.7% (2) Second injection 10 mg: 36.3–37.2% 20 mg: 37.5–40.0% 30 mg: 39.1–36.2% maximum inhibition of IGF-1 concentration for long acting octreotide 30 mg: 41.2% (3) Third injection 10 mg: 36.3–35.6% 20 mg: 33.5–45.4% 30 mg: 39.3–44.8% maximum inhibition of IGF-1 concentration for long acting octreotide 30 mg: 41.4% | 1 | A randomized, open-label parallel group study | 122 | - | [200] | |
| Placebo | Subcutaneous injection | - | - | - | 2–3 | A randomized, placebo-controlled, double-blind, multicenter trial | 71 | 2022–2027 | [234] | ||
| Long-acting octreotide | Subcutaneous injection | (1) Acromegaly Ctrough FluidCrystal® OCT (day 56): 10 mg: 1.0 20 mg: 1.0 Ctrough long acting octreotide (day 28): 10 mg: 0.2 30 mg: 1.2 (2) neuroendocrine tumors Ctrough FluidCrystal® OCT (day 56): 10 mg: 1.3 20 mg: 1.7 Ctrough long acting octreotide (day 28): 20 mg: 0.9 30 mg: 1.3 | Total number of side effects: 50–67%. The most commonly reported side effects: diarrhea (25%), injection site pain (25%), nausea (8.3%) and headache (16.7%) | (1) Acromegaly: IGF-1 maintenance (2) Neuroendocrine tumors: Maintaining symptom control or improving symptoms of carcinoid. | 2 | An open-label, multicenter, randomized study | 12 | 2015–2016 | [235,236] | ||
| LPA | Eligard® | Subcutaneous injection | - | - | - | 2 | An open-label, multicenter, randomized study | 51 | 2014–2016 | [237] | |
| PST | Pasireotide immediate release and pasireotide long-acting release | Subcutaneous injection | Ctrough (1) pasireotide immediate release: 600 μg: 1.12 900 μg: 2.06 (2) pasireotide long-acting release 60 mg: 7.85 (3) FluidCrystal® PST thigh: 5 mg: 0.30 10 mg: 0.51 20 mg: 0.85 40 mg: 2.17 80 mg: 4.09 buttock: 20 mg: 0.96 | (1) Pasireotide immediate release (900 μg): Total number of side effects: 100% Injection site pain: 0% Diarrhea: 60.0% Nausea: 50.0% (2) pasireotide long-acting release (60 mg): Total number of side effects: 80.0% Injection site pain: 10.0% Diarrhea: 40.0% Nausea: 40.0% (3) FluidCrystal® PST thigh: 5 mg: Total number of side effects: 50.0% Injection site pain: 16.7% Diarrhea: 0% Nausea: 0% 10 mg: Total number of side effects: 83.3% Injection site pain: 25.0% Diarrhea: 0% Nausea: 0% 20 mg: Total number of side effects: 91.7% Injection site pain: 50.0% Diarrhea: 0% Nausea: 0% 40 mg: Total number of side effects: 100% Injection site pain: 41.7% Diarrhea: 25.0% Nausea: 16.7% 80 mg: Total number of side effects: 100% Injection site pain: 66.7% Diarrhea: 100% Nausea: 16.7% buttock: 20 mg: Total number of side effects: 50.0% Injection site pain: 14.3% Diarrhea: 0% Nausea: 7.1% | Maximum inhibition of IGF-1 concentration relative to baseline: (1) pasireotide immediate release: 600 μg: −24.41 900 μg: −52.40 (2) pasireotide long-acting release 60 mg: −46.76 (3) FluidCrystal® PST thigh: 5 mg: −24.64 10 mg: −36.32 20 mg: −27.50 40 mg: −50.67 80 mg: −58.87 buttock: 20 mg: −35.22 | 1 | A randomized, open-label study | 94 | - | [201] |
| Matrix | Solvent | Degradability | Gelation Time | Burst Release Tendency | Compatibility with Drugs | Cytotoxicity | The Force of Syringeability, N | Sources |
|---|---|---|---|---|---|---|---|---|
| Shellac | NMP | 41.18–63.42% | The speed of this transformation was as follows: DMSO > NMP > 2-Pyrrolidone > eutectic mixture | 54.01% (168 h) | doxycycline hyclate | 29.63–40.14 | [240] | |
| 75.00% | - | 45.00% (15 h); 68.00% (168 h) | doxycycline hyclate | - | [293] | |||
| DMSO | 32.40–62.49% | - | 61.72% (168 h) | doxycycline hyclate | - | 31.00–40.14 | [240] | |
| 71.00% | - | 60.00% (15 h); 78% (168 h) | doxycycline hyclate | - | [293] | |||
| 2-Pyrrolidone | 80.05–99.98% | - | 43.91% (168 h) | doxycycline hyclate | - | 34.77–48.66 | [240] | |
| 92.00% | - | 20.00% (15 h); 82% (168 h) | doxycycline hyclate | - | [293] | |||
| Eutectic mixture of menthol and camphor | 64.81–92.90% | - | 33.67% (168 h) | doxycycline hyclate | - | 86.81 ± 8.26 | [240] | |
| β-cyclodextrin | DMSO | 78.54–93.32% | 5–140 min | 23.60–78.60% (4 h) | meloxicam | - | 5.00–20.00 | [240] |
| Eudragit® | NMP | - | Immediately; 0.5 min | 55–90% (16.67 h) | doxycycline hyclate | - | 3.00–66.00 | [242] |
| - | 0.5 min | - | doxycycline hyclate | - | F remaining/F max deformation ≤ 0.1 | [254] | ||
| 59.65–63.12% | 1 min | dialysis method: 20–35% (16.67 h) membrane-less method:65–78% (16.67 h) | doxycycline hyclate | - | 24.72–40.37 | [291] | ||
| 2-Pyrrolidone | - | 1 min | 64.18–83.60% (10 h); 75.36–87.94% (24 h) | doxycycline hyclate | - | - | [251] | |
| monopropylene glycol | 100% | 1 min | 38.00–50.00% (24 h) | levofloxacin hydrochloride | - | 1.02–30.87 | [290] | |
| PLGA | NMP | - | - | 25.00–50.00% (24 h) | doxycycline hyclate | - | F remaining/F max deformation ≤0.2 | [243] |
| - | - | - | chlorhexidine dihydrochloride; ibuprofen | - | 0.22–0.27 | [248] | ||
| - | Immediately | 71.00–75.00% (24 h) | cannabidiol | The signs of toxicity in ovo | Predominantly inadequate syringeability | [256] | ||
| DMSO | - | Immediately | 26.00–61.00% (24 h) | cannabidiol | no | Predominantly inadequate syringeability | [256] | |
| Poly (DL-lactide) | NMP | - | Immediately | 32.80- 58.45% (24 h) | tenoxicam | Moderate inflammatory cellular infiltration after 7 days in vivo | 13.50–127.00 | [270] |
| Benzoin | DMSO | - | 1 min | 23.00% (24 h) | vancomycin hydrochloride | - | 0.40–0.60 | [246] |
| Propolis | DMSO | - | 1 min | 40.00% (24 h) | vancomycin hydrochloride | - | 0.10–0.13 | [246] |
| Rosin | DMSO | - | 1 min | 58.00% (24 h) | vancomycin hydrochloride | - | 0.33–0.45 | [246] |
| Borneol | DMSO | 58–75% | 5 min | 45.00% (24 h) | vancomycin hydrochloride | - | - | [250] |
| Lauric acid | NMP | - | 1 min | - | clove oil | - | 0.783–0.936 | [265] |
| Poly (isosorbide succinate) | NMP | - | - | 17.00% (24 h) | doxycycline hyclate | No cytotoxicity was observed in the experiment with fibroblasts and aortic smooth muscle cells | - | [271] |
| cholesterol | NMP, menthol and benzyl benzoate | 70.78–99.50% | 10 min | 70.00–80.00% (16.67 h) | doxycycline hyclate | - | 1.08–2.03 | [292] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhrushina, E.O.; Titova, S.A.; Sakharova, P.S.; Plakhotnaya, O.N.; Grikh, V.V.; Patalova, A.R.; Gorbacheva, A.V.; Krasnyuk, I.I., Jr.; Krasnyuk, I.I. Phase-Inversion In Situ Systems: Problems and Prospects of Biomedical Application. Pharmaceutics 2025, 17, 750. https://doi.org/10.3390/pharmaceutics17060750
Bakhrushina EO, Titova SA, Sakharova PS, Plakhotnaya ON, Grikh VV, Patalova AR, Gorbacheva AV, Krasnyuk II Jr., Krasnyuk II. Phase-Inversion In Situ Systems: Problems and Prospects of Biomedical Application. Pharmaceutics. 2025; 17(6):750. https://doi.org/10.3390/pharmaceutics17060750
Chicago/Turabian StyleBakhrushina, Elena O., Svetlana A. Titova, Polina S. Sakharova, Olga N. Plakhotnaya, Viktoriya V. Grikh, Alla R. Patalova, Anna V. Gorbacheva, Ivan I. Krasnyuk, Jr., and Ivan I. Krasnyuk. 2025. "Phase-Inversion In Situ Systems: Problems and Prospects of Biomedical Application" Pharmaceutics 17, no. 6: 750. https://doi.org/10.3390/pharmaceutics17060750
APA StyleBakhrushina, E. O., Titova, S. A., Sakharova, P. S., Plakhotnaya, O. N., Grikh, V. V., Patalova, A. R., Gorbacheva, A. V., Krasnyuk, I. I., Jr., & Krasnyuk, I. I. (2025). Phase-Inversion In Situ Systems: Problems and Prospects of Biomedical Application. Pharmaceutics, 17(6), 750. https://doi.org/10.3390/pharmaceutics17060750