Development of 3D-Printed Hydrogel Disks as Standardized Platform for Evaluating Excipient Impact on Metronidazole’s Antimicrobial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study Design
- Stage I—Optimization of MET content in disks for the assessment of its antimicrobial activity
- A series of hydrogel inks differing in MET concentration were prepared.
- From the obtained hydrogel inks, hydrogel matrices in the shape of microbiological disks were printed. MET-containing disks, which were assessed with the disk diffusion method, were obtained for content analysis and to determine at what content of the active substance the greatest changes in the growth inhibition zones of B. fragilis ATCC® 25285™ appear.
- On this basis, the optimal MET concentration was selected in the hydrogel ink and printed disk, guaranteeing the highest sensitivity of the method (large differences in the inhibition zones with small changes in MET concentration).
- Stage II—Assessment of the impact of plasticizers on MET activity
- Based on the results of Stage I, a series of hydrogel inks with a fixed, constant MET content was prepared, but differing in the type and/or the amount of plasticizers (polyethylene glycol 400 (PEG 400), propylene glycol (PG), diethylene glycol monoethyl ether (DEGEE), anhydrous glycerol (GL)).
- Hydrogel matrices in the shape of microbiological disks were printed from the obtained hydrogel inks.
- The final antimicrobial activity of MET-contained disks was assessed using the disk diffusion method (measuring the bacterial growth inhibition zones) and MET content analysis using the HPLC method. In this way, it was verified whether the presence of individual plasticizers affects the availability and activity of MET.
2.3. Preparation of Hydrogel Inks
2.4. Printing of Hydrogel Disks
2.4.1. Design of Microbiological Disks
2.4.2. Printing Parameters and Procedure
2.5. Determination of Metronidazole Content in Hydrogel Inks and Disks
2.5.1. Sample Preparation
2.5.2. HPLC Analysis Conditions
2.6. Microbiological Methods
Disk Diffusion Method
2.7. Statistical Analysis
3. Results
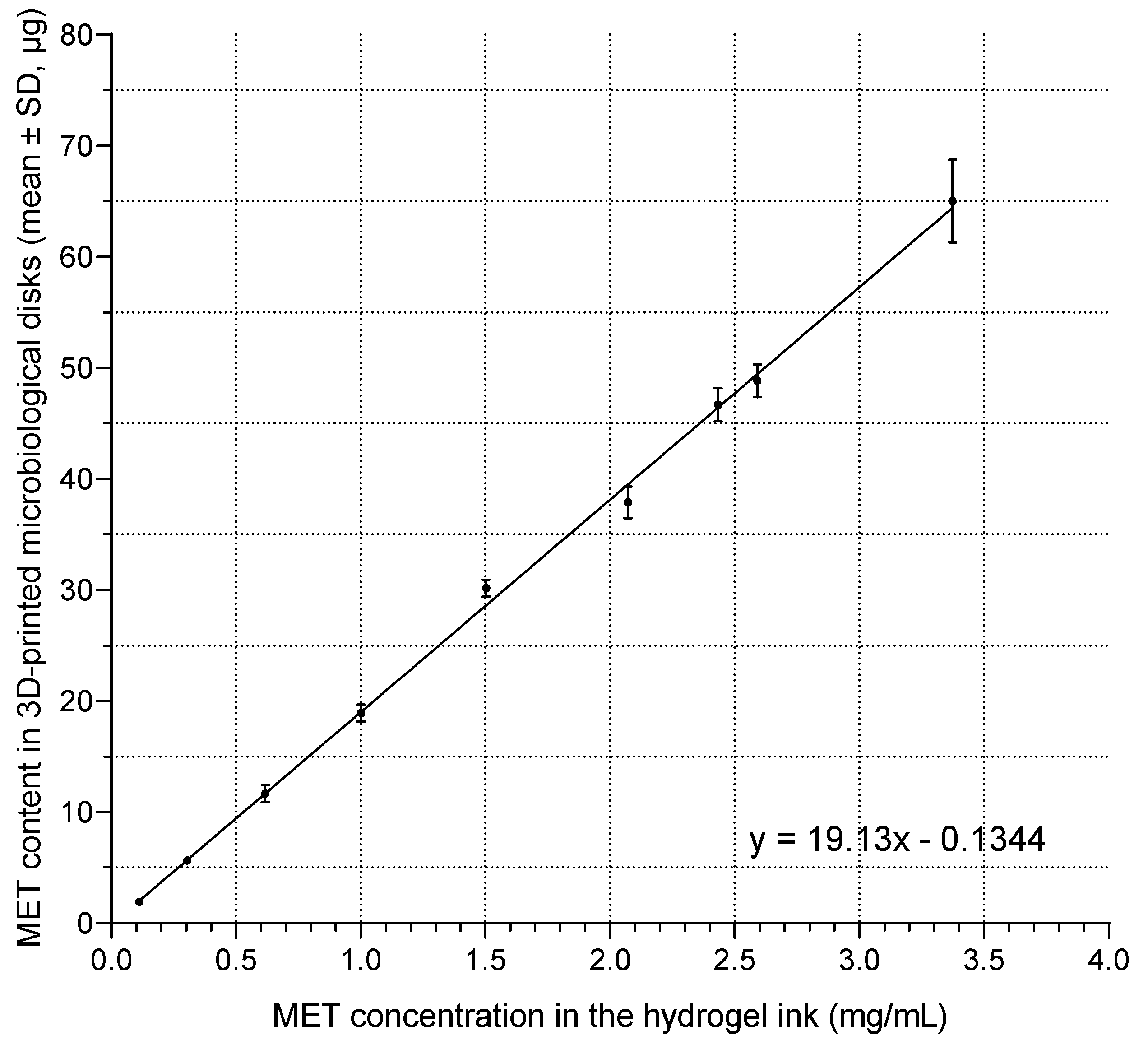
3.1. Determination of the Optimal Metronidazole Content in Microbiological Disks
3.2. Determination of Metronidazole Concentration in Hydrogel Ink for Printing Microbiological Disks
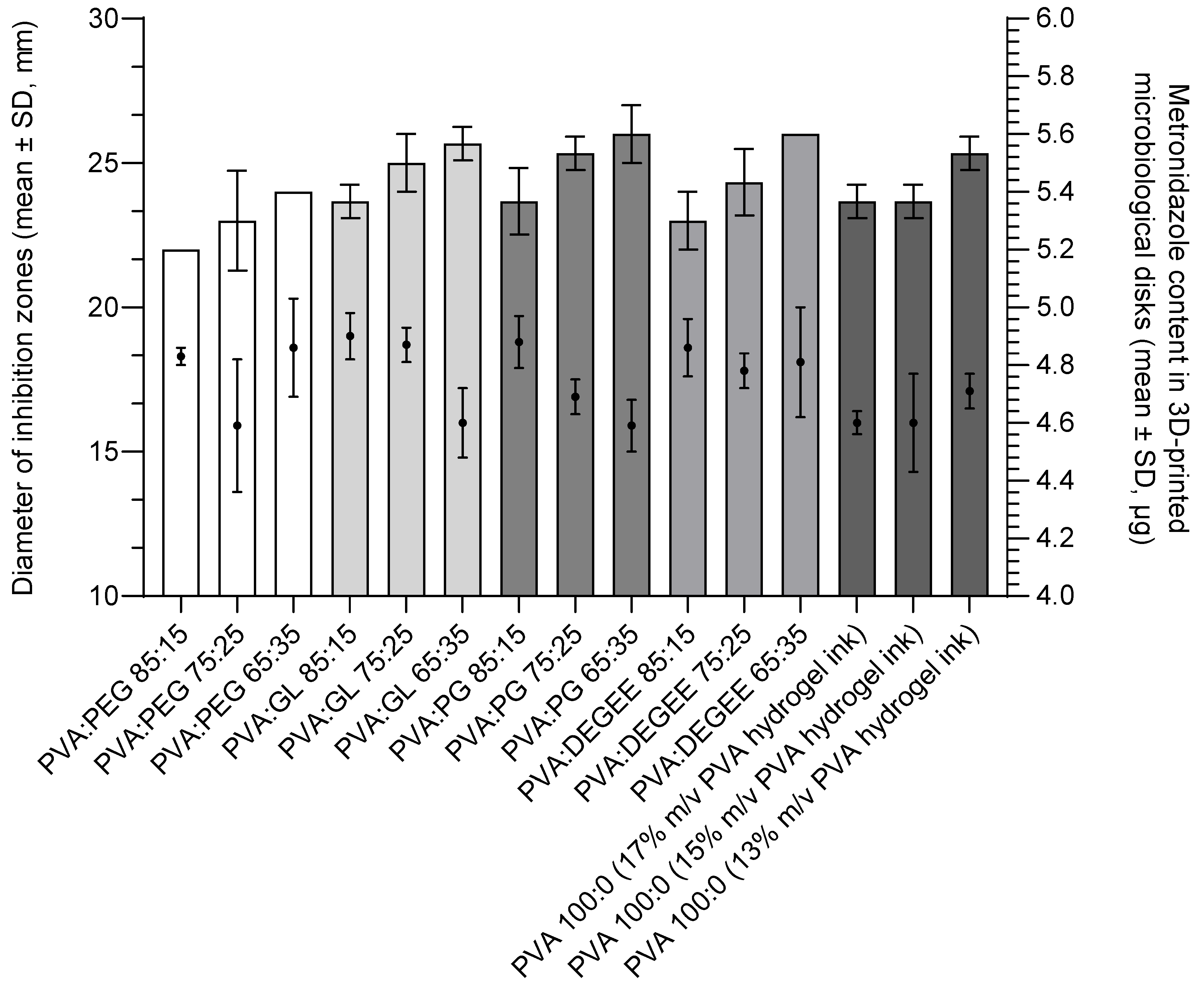
3.3. Effect of 3D-Printed Hydrogel Matrices Composition on Antimicrobial Activity Against Bacteroides fragilis ATCC® 25285™
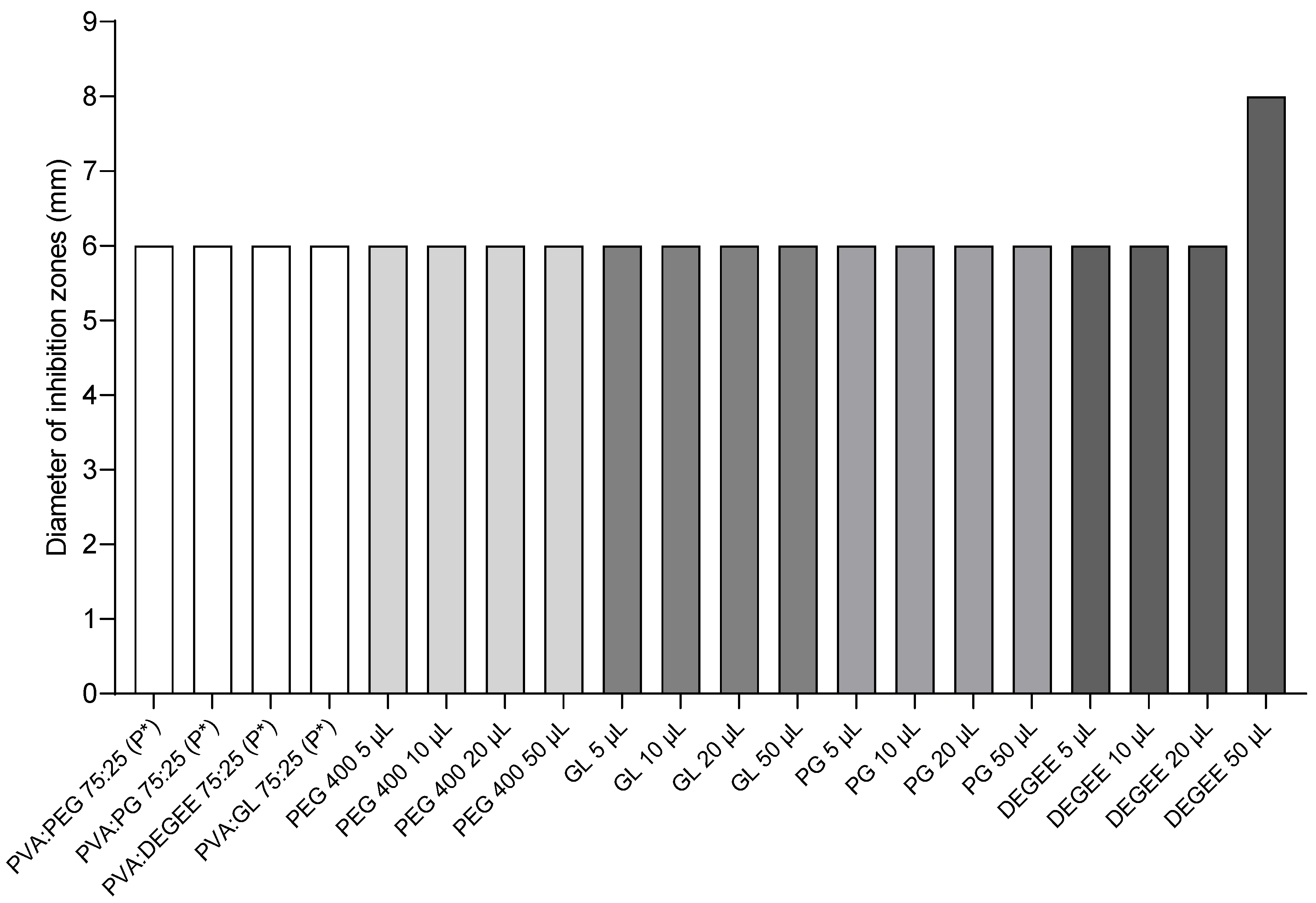
3.4. Activity of Placebo and Individual Components of the Hydrogel Matrix
4. Discussion
4.1. Precision and Repeatability of Metronidazole Content in 3D-Printed Microbiological Disks
4.2. Manufacturing of Microbiological Disks Using 3D Printing: A Standardized Platform
4.3. Effect of Plasticizers on Metronidazole Activity and Potential Underlying Mechanisms
- Modification of matrix structure and crystallinity: Plasticizers, such as those used in this study, are known to modify the structure of PVA-based hydrogels. They can reduce the degree of crystallinity of PVA matrices and alter thermal properties like the glass transition temperature (Tg) and melting temperature of crystallites, often by increasing polymer chain mobility, disrupting polymer chain interactions, or destroying hydrogen bonds between macromolecules [33,61]. For instance, Mohammed and El-Sayed demonstrated that the addition of PEG (Mw 4000) significantly decreased the crystallinity (Xc) from 31.24% to 25.45% and lowered the Tg of PVA films from 89.2 to 60.6 °C [62]. Similarly, Panova et al. reported that the addition of glycerol to PVA films led to a significant decrease in Tg (from 94 °C for pure PVA to 42 °C for PVA with 20 wt% glycerol) and a reduction in the degree of crystallinity (from 49% for pure PVA to 42% for PVA with 20 wt% glycerol) [61]. A less ordered, more amorphous polymer structure can facilitate drug diffusion through the matrix and counteract the retarding effect of high crystallinity on drug release, as observed for MET by Mallapragada et al. [54].
- Enhancement of diffusion channels and swelling: Hydrophilic plasticizers can enhance the hydration of the hydrogel matrix, potentially leading to the enlargement of diffusion channels within the polymer network when in contact with the aqueous environment of the agar [56]. This increased free volume and matrix porosity can accelerate the drug release rate. For example, PEG has been used as a porogen to improve the permeability and mass transfer capability of PVA hydrogels; its addition led to the formation of pores within the gel, providing channels for diffusion, with larger pores forming at higher PEG molecular weights or concentrations [63]. Studies by Abdel-Mottaleb et al. on PVA hydrogels also showed that the addition of PEG (including PEG 400) significantly increased the release rate of fluconazole [64].
- Co-solvency or improved drug dispersion: Plasticizers can act as co-solvents or improve the dispersion of the API within the hydrogel matrix [41,58]. The plasticizers used in this study (DEGEE, glycerol, PEG 400, and propylene glycol) are known for their solvent properties. DEGEE is recognized as a powerful solubilizing agent and penetration enhancer [44,59], and PG has been shown to increase the solubility of metronidazole in topical formulations [58,59]. For example, Lee et al. demonstrated that PEG 400 was an excellent solvent for lifitegrast, significantly increasing its solubility compared to water, and these solvents (DEGEE, GL, PEG 400, PG) are generally recognized as “green solvents” [65]. Enhanced solubilization or finer dispersion of MET within the PVA matrix due to the presence of these plasticizers could lead to a higher effective concentration gradient at the disk–agar interface, thereby promoting more efficient drug transport into the agar, in accordance with the observations of Orienti et al. regarding the effect of solubilization on drug release [66]. Cai et al. also observed that glycerol accelerated insulin release, especially in the initial phase from PVA hydrogels [56]. Studies on the release of ciprofloxacin from PVA matrices also showed that the addition of PEG 6000 significantly increased the release efficiency, with proposed mechanisms including an increase in microchannel size and number and a weakening of PVA–drug interactions [67].
5. Study Limitations and Challenges
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| API | active pharmaceutical ingredient |
| ATCC | American Type Culture Collection |
| DEGEE | diethylene glycol monoethyl ether (Transcutol® P) |
| DSC | differential scanning calorimetry |
| DV | declared value |
| FTIR | Fourier-transform infrared spectroscopy |
| GL | Anhydrous glycerol |
| HFE | hydrogel-forming extrusion |
| HPLC | high-performance liquid chromatography |
| MET | metronidazole |
| PEG 400 | polyethylene glycol 400 |
| PG | propylene glycol |
| Ph. Eur. | European Pharmacopoeia |
| PVA | polyvinyl alcohol 31,000–50,000, 98–99% hydrolyzed |
| R2 | coefficient of determination |
| RSD | relative standard deviation |
| SD | standard deviation |
| SSE | semi-solid extrusion |
References
- ICH Harmonised Tripartite Guideline. Q8(R2) Pharmaceutical Development. Available online: https://database.ich.org/sites/default/files/Q8%28R2%29%20Guideline.pdf (accessed on 25 May 2025).
- Flohr, C.; Hay, R. Putting the Burden of Skin Diseases on the Global Map. Br. J. Dermatol. 2021, 184, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.C.; Pieper, B.A. Topical Metronidazole for the Treatment of Wound Odor: A Review of the Literature. Ostomy Wound Manage. 2008, 54, 18–27, quiz 28–29. [Google Scholar] [PubMed]
- Bale, S.; Tebble, N.; Price, P. A Topical Metronidazole Gel Used to Treat Malodorous Wounds. Br. J. Nurs. 2004, 13, S4–S11. [Google Scholar] [CrossRef]
- Brayfield, A. Martindale: The Complete Drug Reference, 38th ed.; Pharmaceutical Press: London, UK, 2014; ISBN 978-0-85711-139-5. [Google Scholar]
- Schaechter, M.; Engleberg, N.C.; DiRita, V.J.; Dermody, T. (Eds.) Schaechter’s Mechanisms of Microbial Disease, 5th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; ISBN 978-0-7817-8744-4. [Google Scholar]
- Cooley, L.; Teng, J. Anaerobic Resistance: Should We Be Worried? Curr. Opin. Infect. Dis. 2019, 32, 523–530. [Google Scholar] [CrossRef]
- Newman, V.; Allwood, M.; Oakes, R. The Use of Metronidazole Gel to Control the Smell of Malodorous Lesions. Palliat. Med. 1989, 3, 303–305. [Google Scholar] [CrossRef]
- Witkowski, J.A.; Parish, L.C. Topical Metronidazole Gel.: The Bacteriology of Decubitus Ulcers. Int. J. Dermatol. 1991, 30, 660–661. [Google Scholar] [CrossRef]
- Bower, M.; Stein, R.; Evans, T.R.J.; Hedley, A.; Pert, P.; Coombes, R.C. A Double-Blind Study of the Efficacy of Metronidazole Gel in the Treatment of Malodorous Fungating Tumours. Eur. J. Cancer 1992, 28, 888–889. [Google Scholar] [CrossRef]
- Finlay, I.G.; Bowszyc, J.; Ramlau, C.; Gwiezdzinski, Z. The Effect of Topical 0.75% Metronidazole Gel on Malodorous Cutaneous Ulcers. J. Pain Symptom Manag. 1996, 11, 158–162. [Google Scholar] [CrossRef]
- Watanabe, K.; Shimo, A.; Tsugawa, K.; Tokuda, Y.; Yamauchi, H.; Miyai, E.; Takemura, K.; Ikoma, A.; Nakamura, S. Safe and Effective Deodorization of Malodorous Fungating Tumors Using Topical Metronidazole 0.75% Gel (GK567): A Multicenter, Open-Label, Phase III Study (RDT.07.SRE.27013). Support. Care Cancer 2016, 24, 2583–2590. [Google Scholar] [CrossRef]
- Peng, L.; Dai, Y. Effect of Metronidazole Combined with Autolytic Debridement for the Management of Malignant Wound Malodor. J. Int. Med. Res. 2020, 48, 030006051988974. [Google Scholar] [CrossRef]
- Wu, Y.; Fassihi, R. Stability of Metronidazole, Tetracycline HCl and Famotidine Alone and in Combination. Int. J. Pharm. 2005, 290, 1–13. [Google Scholar] [CrossRef] [PubMed]
- George, R.; Prasoona, T.S.; Kandasamy, R.; Cherian, R.; Celine, T.; Jeba, J.; Murali, S.; Mathew, D. Improving Malodour Management in Advanced Cancer: A 10-Year Retrospective Study of Topical, Oral and Maintenance Metronidazole. BMJ Support. Palliat. Care 2017, 7, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Bom, S.; Ribeiro, R.; Ribeiro, H.M.; Santos, C.; Marto, J. On the Progress of Hydrogel-Based 3D Printing: Correlating Rheological Properties with Printing Behaviour. Int. J. Pharm. 2022, 615, 121506. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Torres, E.; Suárez-González, J.; Monzón-Rodríguez, C.N.; Santoveña-Estévez, A.; Fariña, J.B. Characterization and Validation of a New 3D Printing Ink for Reducing Therapeutic Gap in Pediatrics through Individualized Medicines. Pharmaceutics 2023, 15, 1642. [Google Scholar] [CrossRef]
- Rowe, R.C. (Ed.) Handbook of Pharmaceutical Excipients, 6th ed.; APhA: Washington, DC, USA; Pharmaceutical Press: London, UK, 2009; ISBN 978-0-85369-792-3. [Google Scholar]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A Review on Polymeric Hydrogel Membranes for Wound Dressing Applications: PVA-Based Hydrogel Dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Juang, J.H.; Bonner-Weir, S.; Ogawa, Y.; Vacanti, J.P.; Weir, G.C. Outcome of Subcutaneous Islet Transplantation Improved by Polymer Device. Transplantation 1996, 61, 1557–1561. [Google Scholar] [CrossRef]
- Akbari, E.; Imani, R.; Shokrollahi, P.; Heidari Keshel, S. Preparation of Nanoparticle-Containing Ring-Implanted Poly(Vinyl Alcohol) Contact Lens for Sustained Release of Hyaluronic Acid. Macromol. Biosci. 2021, 21, e2100043. [Google Scholar] [CrossRef]
- Rivera-Hernández, G.; Antunes-Ricardo, M.; Martínez-Morales, P.; Sánchez, M.L. Polyvinyl Alcohol Based-Drug Delivery Systems for Cancer Treatment. Int. J. Pharm. 2021, 600, 120478. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and Applications of Poly(Vinyl Alcohol) Hydrogels Produced by Conventional Crosslinking or by Freezing/Thawing Methods. In Biopolymers PVA Hydrogels, Anionic Polymerisation Nanocomposites; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2000; Volume 153, pp. 37–65. ISBN 978-3-540-67313-2. [Google Scholar]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(Vinyl Alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Rumon, M.M.H. Synthesis of PVA-Based Hydrogels for Biomedical Applications: Recent Trends and Advances. Gels 2025, 11, 88. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-Scavenging Hydrogel to Promote Healing of Bacteria Infected Diabetic Wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Wang, Y.; Cai, R.; Chang, H.; Song, K.; Zuo, H.; Zhao, P.; Xia, Q.; He, H. Design and Performance of Sericin/Poly(Vinyl Alcohol) Hydrogel as a Drug Delivery Carrier for Potential Wound Dressing Application. Mater. Sci. Eng. C 2019, 101, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-H.; Ko, S.-C.; Oh, G.-W.; Heo, S.-J.; Kang, D.-H.; Bae, S.-Y.; Jung, W.-K. Fabrication and Characterization of Phlorotannins/Poly (Vinyl Alcohol) Hydrogel for Wound Healing Application. J. Biomater. Sci. Polym. Ed. 2018, 29, 972–983. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Taher, M.; Mandal, U.K.; Jaffri, J.M.; Susanti, D.; Mahmood, S.; Zakaria, Z.A. Pharmacological Properties of Centella Asiatica Hydrogel in Accelerating Wound Healing in Rabbits. BMC Complement. Altern. Med. 2019, 19, 213. [Google Scholar] [CrossRef]
- Gutha, Y.; Pathak, J.L.; Zhang, W.; Zhang, Y.; Jiao, X. Antibacterial and Wound Healing Properties of Chitosan/Poly(Vinyl Alcohol)/Zinc Oxide Beads (CS/PVA/ZnO). Int. J. Biol. Macromol. 2017, 103, 234–241. [Google Scholar] [CrossRef]
- Chandika, P.; Kim, M.-S.; Khan, F.; Kim, Y.-M.; Heo, S.-Y.; Oh, G.-W.; Kim, N.G.; Jung, W.-K. Wound Healing Properties of Triple Cross-Linked Poly (Vinyl Alcohol)/Methacrylate Kappa-Carrageenan/Chitooligosaccharide Hydrogel. Carbohydr. Polym. 2021, 269, 118272. [Google Scholar] [CrossRef]
- Hubner, P.; Marcilio, N.R.; Tessaro, I.C. Gelatin/Poly(Vinyl Alcohol) Based Hydrogel Film—A Potential Biomaterial for Wound Dressing: Experimental Design and Optimization Followed by Rotatable Central Composite Design. J. Biomater. Appl. 2021, 36, 682–700. [Google Scholar] [CrossRef]
- Lim, L.Y.; Wan, L.S.C. The Effect of Plasticizers on the Properties of Polyvinyl Alcohol Films. Drug Dev. Ind. Pharm. 1994, 20, 1007–1020. [Google Scholar] [CrossRef]
- Jones, D.S.; Muldoon, B.C.O.; Woolfson, A.D.; Andrews, G.P.; Sanderson, F.D. Physicochemical Characterization of Bioactive Polyacrylic Acid Organogels as Potential Antimicrobial Implants for the Buccal Cavity. Biomacromolecules 2008, 9, 624–633. [Google Scholar] [CrossRef]
- Darabian, B.; Bagheri, H.; Mohammadi, S. Improvement in Mechanical Properties and Biodegradability of PLA Using Poly(Ethylene Glycol) and Triacetin for Antibacterial Wound Dressing Applications. Prog. Biomater. 2020, 9, 45–64. [Google Scholar] [CrossRef]
- Górska, A.; Baran, E.; Knapik-Kowalczuk, J.; Szafraniec-Szczęsny, J.; Paluch, M.; Kulinowski, P.; Mendyk, A. Physically Cross-Linked PVA Hydrogels as Potential Wound Dressings: How Freezing Conditions and Formulation Composition Define Cryogel Structure and Performance. Pharmaceutics 2024, 16, 1388. [Google Scholar] [CrossRef] [PubMed]
- Bialik-Wąs, K.; Pluta, K.; Malina, D.; Barczewski, M.; Malarz, K.; Mrozek-Wilczkiewicz, A. The Effect of Glycerin Content in Sodium Alginate/Poly(Vinyl Alcohol)-Based Hydrogels for Wound Dressing Application. Int. J. Mol. Sci. 2021, 22, 12022. [Google Scholar] [CrossRef] [PubMed]
- Leppiniemi, J.; Lahtinen, P.; Paajanen, A.; Mahlberg, R.; Metsä-Kortelainen, S.; Pinomaa, T.; Pajari, H.; Vikholm-Lundin, I.; Pursula, P.; Hytönen, V.P. 3D-Printable Bioactivated Nanocellulose–Alginate Hydrogels. ACS Appl. Mater. Interfaces 2017, 9, 21959–21970. [Google Scholar] [CrossRef] [PubMed]
- Chitrattha, S.; Phaechamud, T. Porous Poly(DL-Lactic Acid) Matrix Film with Antimicrobial Activities for Wound Dressing Application. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 1122–1130. [Google Scholar] [CrossRef]
- Panraksa, P.; Tipduangta, P.; Jantanasakulwong, K.; Jantrawut, P. Formulation of Orally Disintegrating Films as an Amorphous Solid Solution of a Poorly Water-Soluble Drug. Membranes 2020, 10, 376. [Google Scholar] [CrossRef]
- Bashyal, S.; Shin, C.Y.; Hyun, S.M.; Jang, S.W.; Lee, S. Preparation, Characterization, and In Vivo Pharmacokinetic Evaluation of Polyvinyl Alcohol and Polyvinyl Pyrrolidone Blended Hydrogels for Transdermal Delivery of Donepezil HCl. Pharmaceutics 2020, 12, 270. [Google Scholar] [CrossRef]
- Kovtun, G.; Casas, D.; Cuberes, T. Influence of Glycerol on the Surface Morphology and Crystallinity of Polyvinyl Alcohol Films. Polymers 2024, 16, 2421. [Google Scholar] [CrossRef]
- Nagakawa, Y.; Kato, M.; Suye, S.-I.; Fujita, S. Fabrication of Tough, Anisotropic, Chemical-Crosslinker-Free Poly(Vinyl Alcohol) Nanofibrous Cryogels via Electrospinning. RSC Adv. 2020, 10, 38045–38054. [Google Scholar] [CrossRef]
- Osborne, D.W. Diethylene Glycol Monoethyl Ether: An Emerging Solvent in Topical Dermatology Products. J. Cosmet. Dermatol. 2011, 10, 324–329. [Google Scholar] [CrossRef]
- Zidan, A.; Alayoubi, A.; Coburn, J.; Asfari, S.; Ghammraoui, B.; Cruz, C.N.; Ashraf, M. Extrudability Analysis of Drug Loaded Pastes for 3D Printing of Modified Release Tablets. Int. J. Pharm. 2019, 554, 292–301. [Google Scholar] [CrossRef]
- Naseri, E.; Cartmell, C.; Saab, M.; Kerr, R.G.; Ahmadi, A. Development of N,O-Carboxymethyl Chitosan-Starch Biomaterial Inks for 3D Printed Wound Dressing Applications. Macromol. Biosci. 2021, 21, 2100368. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Davila, S.; Potel-Alvarellos, C.; Carballo, R.; González-Rodríguez, L.; López-Álvarez, M.; Serra, J.; Díaz-Rodríguez, P.; Landín, M.; González, P. Vancomycin-Loaded 3D-Printed Polylactic Acid-Hydroxyapatite Scaffolds for Bone Tissue Engineering. Polymers 2023, 15, 4250. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.; Averianov, I.; Gofman, I.; Shevchenko, N.; Rubinstein, A.; Egorova, T.; Trulioff, A.; Nashchekina, Y.; Kudryavtsev, I.; Demyanova, E.; et al. Drug Loaded 3D-Printed Poly(ε-Caprolactone) Scaffolds for Local Antibacterial or Anti-Inflammatory Treatment in Bone Regeneration. Polymers 2023, 15, 3957. [Google Scholar] [CrossRef]
- Mirek, A.; Belaid, H.; Barranger, F.; Grzeczkowicz, M.; Bouden, Y.; Cavaillès, V.; Lewińska, D.; Bechelany, M. Development of a New 3D Bioprinted Antibiotic Delivery System Based on a Cross-Linked Gelatin-Alginate Hydrogel. J. Mater. Chem. B 2022, 10, 8862–8874. [Google Scholar] [CrossRef]
- Wu, Z.; Hong, Y. Combination of the Silver–Ethylene Interaction and 3D Printing to Develop Antibacterial Superporous Hydrogels for Wound Management. ACS Appl. Mater. Interfaces 2019, 11, 33734–33747. [Google Scholar] [CrossRef]
- Chug, M.K.; Bachtiar, E.; Narwold, N.; Gall, K.; Brisbois, E.J. Tailoring Nitric Oxide Release with Additive Manufacturing to Create Antimicrobial Surfaces. Biomater. Sci. 2021, 9, 3100–3111. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, C.; Xu, Z.; Chen, Y.; Gan, Y.; Zhou, M.; Li, L.; Duan, Q.; Huang, T.; Lin, J. Mussel-Inspired HA@TA-CS/SA Biomimetic 3D Printed Scaffolds with Antibacterial Activity for Bone Repair. Front. Bioeng. Biotechnol. 2023, 11, 1193605. [Google Scholar] [CrossRef]
- Datta, D.; Bal, T.; Swain, S.; Goswami, S. Fabrication and Evaluation of Physically Crosslinked Stimuli Sensitive Polymeric Blend of Pva-Gelatin as Drug Delivery System by Freeze Thaw Cycles: Crosslinked Stimuli Sensitive Polymeric Blend by Freeze Thaw Cycles. Iran. J. Pharm. Sci. 2018, 14, 117–144. [Google Scholar] [CrossRef]
- Mallapragada, S.K.; Peppas, N.A.; Colombo, P. Crystal Dissolution-Controlled Release Systems. II. Metronidazole Release from Semicrystalline Poly(Vinyl Alcohol) Systems. J. Biomed. Mater. Res. 1997, 36, 125–130. [Google Scholar] [CrossRef]
- Björklund, S.; Engblom, J.; Thuresson, K.; Sparr, E. Glycerol and urea can be used to increase skin permeability in reduced hydration conditions. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 50, 638–645. [Google Scholar] [CrossRef]
- Cai, Y.; Che, J.; Yuan, M.; Shi, X.; Chen, W.; Yuan, W.-E. Effect of Glycerol on Sustained Insulin Release from PVA Hydrogels and Its Application in Diabetes Therapy. Exp. Ther. Med. 2016, 12, 2039–2044. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, G.; Yadav, S.K.; Patel, R.R.; Nath, G.; Bansal, M.; Mishra, B. Development and Evaluation of Biodegradable Chitosan Films of Metronidazole and Levofloxacin for the Management of Periodontitis. AAPS PharmSciTech 2016, 17, 1312–1325. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.-S.; Hamudi, F.F.; Khalil, E.A. Effect of Ethylcellulose and Propylene Glycol on the Controlled-Release Performance of Glyceryl Monooleate-Mertronidazole Periodontal Gel. Pharm. Dev. Technol. 2015, 20, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Dahlizar, S.; Futaki, M.; Okada, A.; Yatomi, C.; Todo, H.; Sugibayashi, K. Combined Use of N-Palmitoyl-Glycine-Histidine Gel and Several Penetration Enhancers on the Skin Permeation and Concentration of Metronidazole. Pharmaceutics 2018, 10, 163. [Google Scholar] [CrossRef]
- Alzainy, A.; Boateng, J. Novel Mucoadhesive Wafers for Treating Local Vaginal Infections. Biomedicines 2022, 10, 3060. [Google Scholar] [CrossRef]
- Panova, T.V.; Efimova, A.A.; Berkovich, A.K.; Efimov, A.V. Plasticity Control of Poly(Vinyl Alcohol)-Graphene Oxide Nanocomposites. RSC Adv. 2020, 10, 24027–24036. [Google Scholar] [CrossRef]
- Mohammed, M.I.; El-Sayed, F. PEG’s Impact as a Plasticizer on the PVA Polymer’s Structural, Thermal, Mechanical, Optical, and Dielectric Characteristics. Opt. Quantum Electron. 2023, 55, 1141. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, L. Improvement of Permeability of Poly(Vinyl Alcohol) Hydrogel by Using Poly(Ethylene Glycol) as Porogen. Polym. Plast. Technol. Eng. 2011, 50, 776–782. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.A.; Mortada, N.D.; El-Shamy, A.A.; Awad, G.A.S. Physically Cross-Linked Polyvinyl Alcohol for the Topical Delivery of Fluconazole. Drug Dev. Ind. Pharm. 2009, 35, 311–320. [Google Scholar] [CrossRef]
- Lee, S.-K.; Ha, E.-S.; Park, H.; Jeong, J.-S.; Ryu, H.-J.; Pyo, Y.-J.; Choi, D.H.; Kim, M.-S. Measurement and Correlation of Solubility of Lifitegrast in Four Mixtures of (Diethylene Glycol Monoethyl Ether, Glycerol, PEG 400, and Propylene Glycol + Water) from 288.15 K to 308.15 K. J. Mol. Liq. 2021, 340, 117181. [Google Scholar] [CrossRef]
- Orienti, I.; Bigucci, F.; Gentilomi, G.; Zecchi, V. Self-assembling Poly(Vinyl Alcohol) Derivatives, Interactions with Drugs and Control of Release. J. Pharm. Sci. 2001, 90, 1435–1444. [Google Scholar] [CrossRef]
- Zhou, X.-H.; Wei, D.-X.; Ye, H.-M.; Zhang, X.; Meng, X.; Zhou, Q. Development of Poly(Vinyl Alcohol) Porous Scaffold with High Strength and Well Ciprofloxacin Release Efficiency. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 326–335. [Google Scholar] [CrossRef]




| Formulation | MET Content in Hydrogel (mg) | MET Content in Disk (mean ± SD, µg) | RSD of MET Content in Disk (%) |
|---|---|---|---|
| 1 | 0 | 0.00 | 0 |
| 2 | 5.62 | 1.93 ± 0.08 | 4.1 |
| 3 | 15.22 | 5.66 ± 0.30 | 5.4 |
| 4 | 30.85 | 11.68 ± 0.77 | 6.6 |
| 5 | 50.07 | 18.94 ± 0.78 | 4.1 |
| 6 | 75.18 | 30.17 ± 0.78 | 2.6 |
| 7 | 103.6 | 37.89 ± 1.42 | 3.8 |
| 8 | 121.65 | 46.67 ± 1.51 | 3.2 |
| 9 | 129.54 | 48.85 ± 1.48 | 3.0 |
| 10 | 168.68 | 65.02 ± 3.71 | 5.7 |
| Formulation | Concentration of Substances in the Hydrogel Inks for 3D Printing | ||||||
|---|---|---|---|---|---|---|---|
| PVA–Plasticizer Ratio | PVA (% m/v) | PEG 400 (% m/v) | PG (% m/v) | DEGEE (% m/v) | GL (% m/v) | MET (% m/v) | |
| PVA–PEG 85:15 | 85:15 | 17.0 | 3.0 | – | – | – | 0.027 |
| PVA–PEG 75:25 | 75:25 | 15.0 | 5.0 | – | – | – | 0.027 |
| PVA–PEG 65:35 | 65:35 | 13.0 | 7.0 | – | – | – | 0.027 |
| PVA–GL 85:15 | 85:15 | 17.0 | – | – | – | 3.0 | 0.027 |
| PVA–GL 75:25 | 75:25 | 15.0 | – | – | – | 5.0 | 0.027 |
| PVA–GL 65:35 | 65:35 | 13.0 | – | – | – | 7.0 | 0.027 |
| PVA–PG 85:15 | 85:15 | 17.0 | – | 3.0 | – | – | 0.027 |
| PVA–PG 75:25 | 75:25 | 15.0 | – | 5.0 | – | – | 0.027 |
| PVA–PG 65:35 | 65:35 | 13.0 | – | 7.0 | – | – | 0.027 |
| PVA–DEGEE 85:15 | 85:15 | 17.0 | – | – | 3.0 | – | 0.027 |
| PVA–DEGEE 75:25 | 75:25 | 15.0 | – | – | 5.0 | – | 0.027 |
| PVA–DEGEE 65:35 | 65:35 | 13.0 | – | – | 7.0 | – | 0.027 |
| PVA 100:0 (17% m/v PVA hydrogel ink) | 100:0 | 17.0 | – | – | – | – | 0.027 |
| PVA 100:0 (15% m/v PVA hydrogel ink) | 100:0 | 15.0 | – | – | – | – | 0.027 |
| PVA 100:0 (13% m/v PVA hydrogel ink) | 100:0 | 13.0 | – | – | – | – | 0.027 |
| PVA–PEG 75:25 (P *) | 75:25 | 15.0 | 5.0 | – | – | – | – |
| PVA–PG 75:25 (P *) | 75:25 | 15.0 | – | 5.0 | – | – | – |
| PVA–DEGEE 75:25 (P *) | 75:25 | 15.0 | – | – | 5.0 | – | – |
| PVA–GL 75:25 (P *) | 75:25 | 15.0 | – | – | – | 5.0 | – |
| Parameter | Value |
|---|---|
| Filament diameter | 4 mm |
| Layer count | 1 |
| Layer height | 0.1 mm |
| Path width | 0.3 mm |
| Travel speed | 120 mm/s |
| Print speed | 10 mm/s |
| Extrusion order | Nearest (proximity) |
| Retraction | Enabled |
| Retraction height | 2 mm |
| Retraction amount | 0 mm |
| Retraction speed (Z axis) | 50 mm/s |
| Retraction speed (extrusion) | 50 mm/s |
| Retraction minimum distance | 10 mm |
| Extra length on restart | 0 mm |
| Needle size | Diameter: 1.2 mm, length: 5 mm, flat-cut |
| Build platform temperature | Room temperature |
| Drying time and conditions | 24 h, room temperature |
| Airflow | Disabled |
| Formulation | MET Content (mean ± SD, µg) | RSD (%) | % DV (mean ± SD, %) |
|---|---|---|---|
| PVA–PEG 85:15 | 4.83 ± 0.03 | 0.62 | 96.6 ± 0.6 |
| PVA–PEG 75:25 | 4.59 ± 0.23 | 5.01 | 91.8 ± 4.6 |
| PVA–PEG 65:35 | 4.86 ± 0.17 | 3.50 | 97.2 ± 3.4 |
| PVA–GL 85:15 | 4.90 ± 0.08 | 1.63 | 98.0 ± 1.6 |
| PVA–GL 75:25 | 4.87 ± 0.06 | 1.23 | 97.4 ± 1.2 |
| PVA–GL 65:35 | 4.60 ± 0.12 | 2.61 | 92.0 ± 2.4 |
| PVA–PG 85:15 | 4.88 ± 0.09 | 1.84 | 97.6 ± 1.8 |
| PVA–PG 75:25 | 4.69 ± 0.06 | 1.28 | 93.8 ± 1.2 |
| PVA–PG 65:35 | 4.59 ± 0.09 | 1.96 | 91.8 ± 1.8 |
| PVA–DEGEE 85:15 | 4.86 ± 0.10 | 2.06 | 97.2 ± 2.0 |
| PVA–DEGEE 75:25 | 4.78 ± 0.06 | 1.26 | 95.6 ± 1.2 |
| PVA–DEGEE 65:35 | 4.81 ± 0.19 | 3.95 | 96.2 ± 3.8 |
| PVA 100:0 (17% m/v PVA hydrogel ink) | 4.60 ± 0.04 | 0.87 | 92.0 ± 0.8 |
| PVA 100:0 (15% m/v PVA hydrogel ink) | 4.60 ± 0.17 | 3.70 | 92.0 ± 3.4 |
| PVA 100:0 (13% m/v PVA hydrogel ink) | 4.71 ± 0.06 | 1.27 | 94.2 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnatowski, T.; Kwiecińska-Piróg, J.; Bogiel, T. Development of 3D-Printed Hydrogel Disks as Standardized Platform for Evaluating Excipient Impact on Metronidazole’s Antimicrobial Activity. Pharmaceutics 2025, 17, 749. https://doi.org/10.3390/pharmaceutics17060749
Gnatowski T, Kwiecińska-Piróg J, Bogiel T. Development of 3D-Printed Hydrogel Disks as Standardized Platform for Evaluating Excipient Impact on Metronidazole’s Antimicrobial Activity. Pharmaceutics. 2025; 17(6):749. https://doi.org/10.3390/pharmaceutics17060749
Chicago/Turabian StyleGnatowski, Tomasz, Joanna Kwiecińska-Piróg, and Tomasz Bogiel. 2025. "Development of 3D-Printed Hydrogel Disks as Standardized Platform for Evaluating Excipient Impact on Metronidazole’s Antimicrobial Activity" Pharmaceutics 17, no. 6: 749. https://doi.org/10.3390/pharmaceutics17060749
APA StyleGnatowski, T., Kwiecińska-Piróg, J., & Bogiel, T. (2025). Development of 3D-Printed Hydrogel Disks as Standardized Platform for Evaluating Excipient Impact on Metronidazole’s Antimicrobial Activity. Pharmaceutics, 17(6), 749. https://doi.org/10.3390/pharmaceutics17060749







