Voriconazole-Loaded Nanohydrogels Towards Optimized Antifungal Therapy for Cystic Fibrosis Patients
Abstract
1. Introduction
2. Materials and Methods
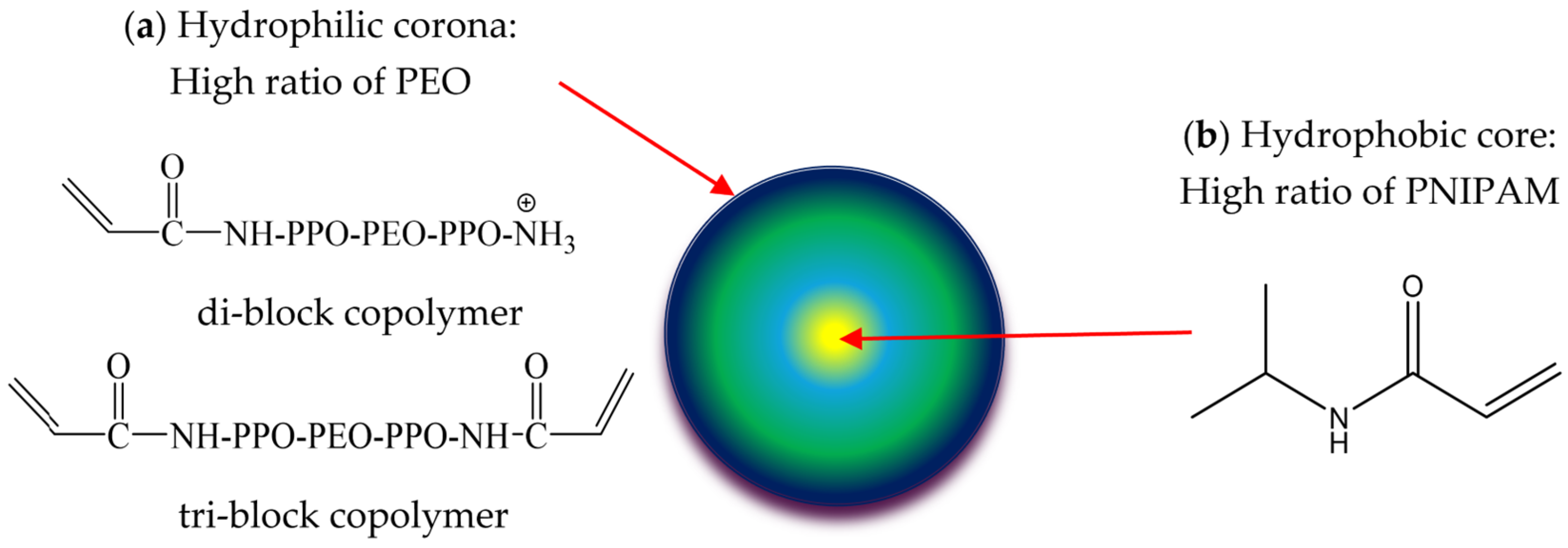
2.1. Synthesis of NHGs
2.2. DLS Analysis
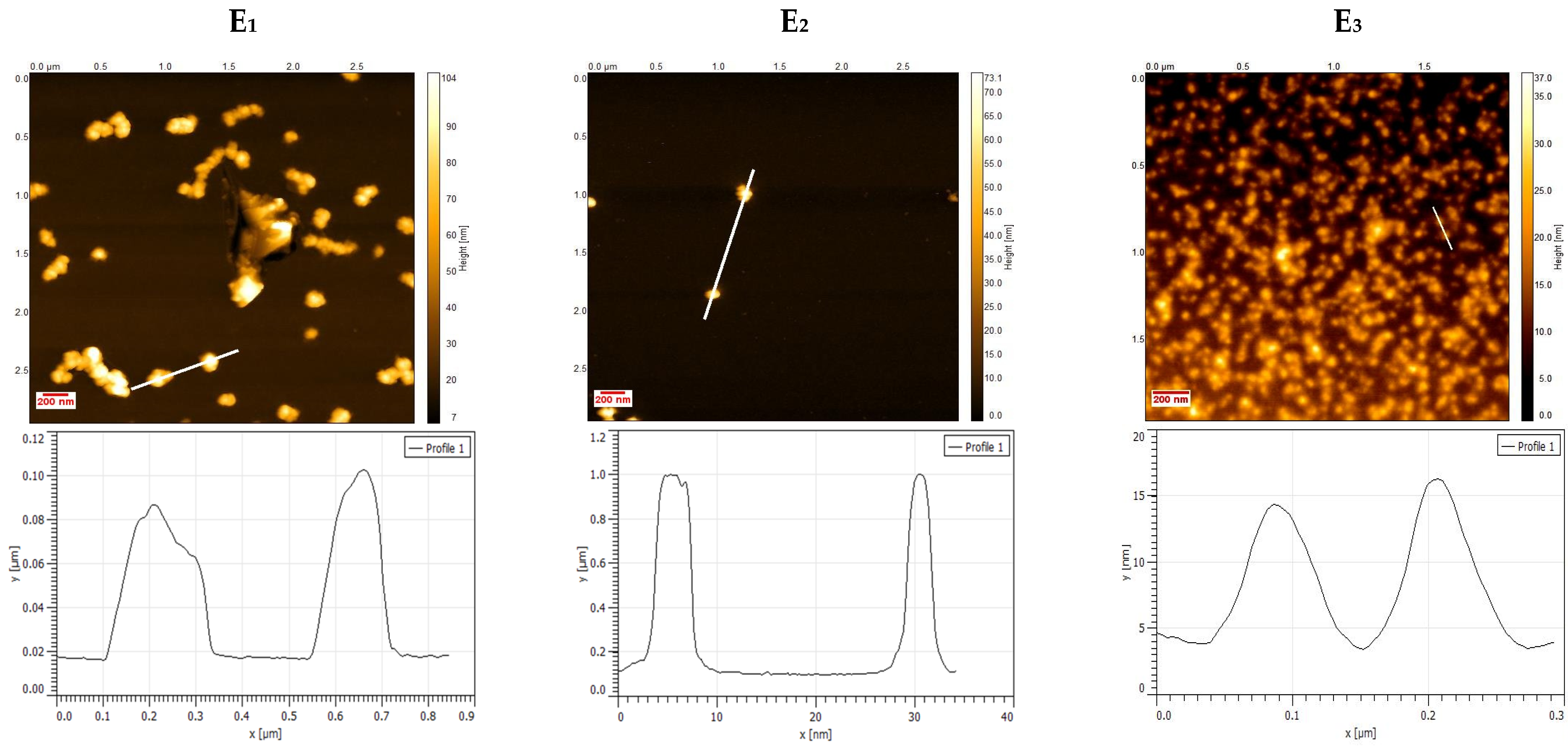
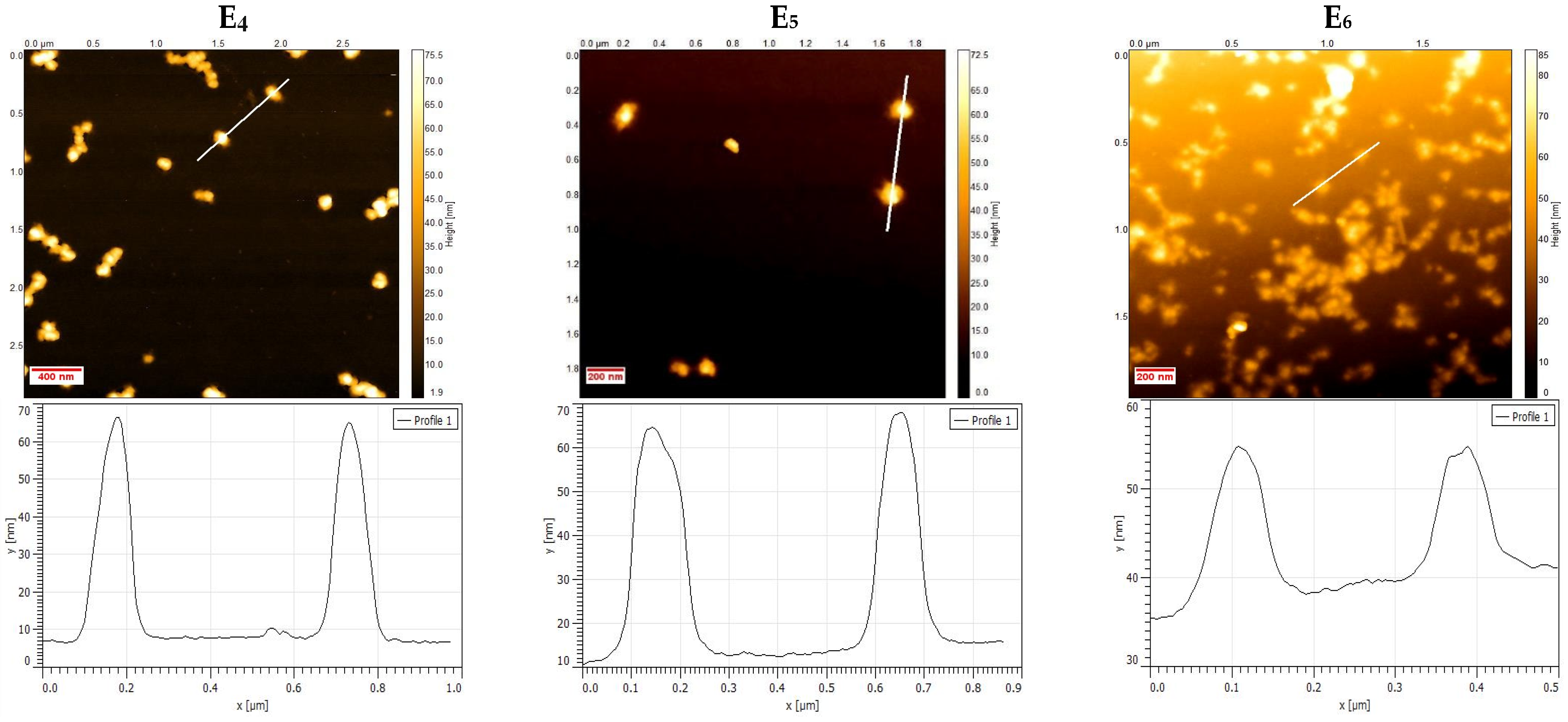
2.3. Atomic Force Microscopy Characterization
2.4. Concentration and Zeta Potential (ζ) by NTA
2.5. Mucus Penetration Assay for NHGs Monitored by NTA
2.6. Loading of VRC
2.7. In Vitro Release of VRC
2.8. Cell Culture
2.9. Cytotoxicity
2.10. Antifungal Susceptibility Testing
3. Results and Discussion
3.1. Physico-Chemical Characterization of NHGs and VRC-NHGs
3.2. Mucus Penetration Assay of NHGs Monitored by NTA
3.3. VRC Loading into NHGs
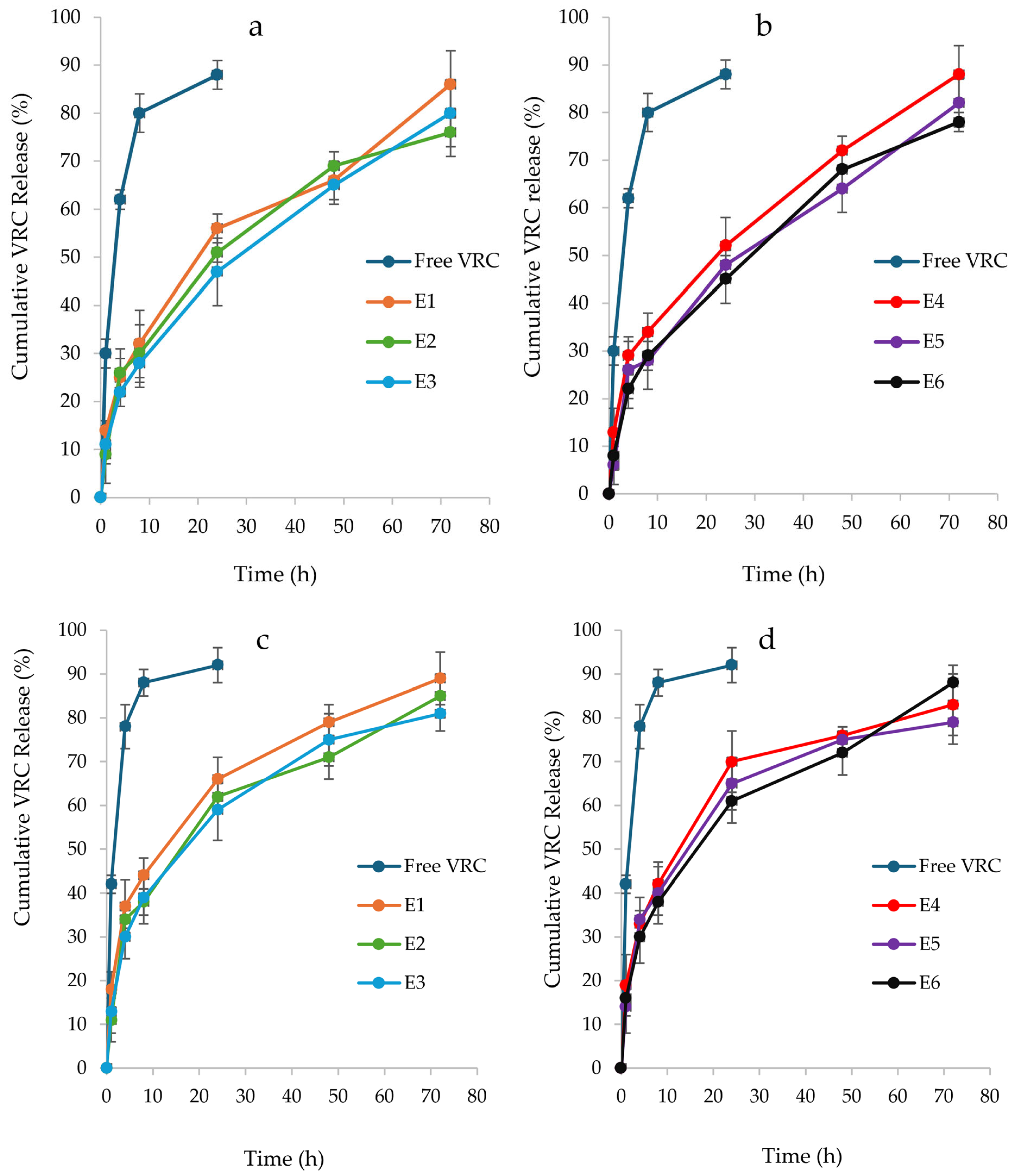
3.4. In Vitro Drug Release Study of VRC-NHGs
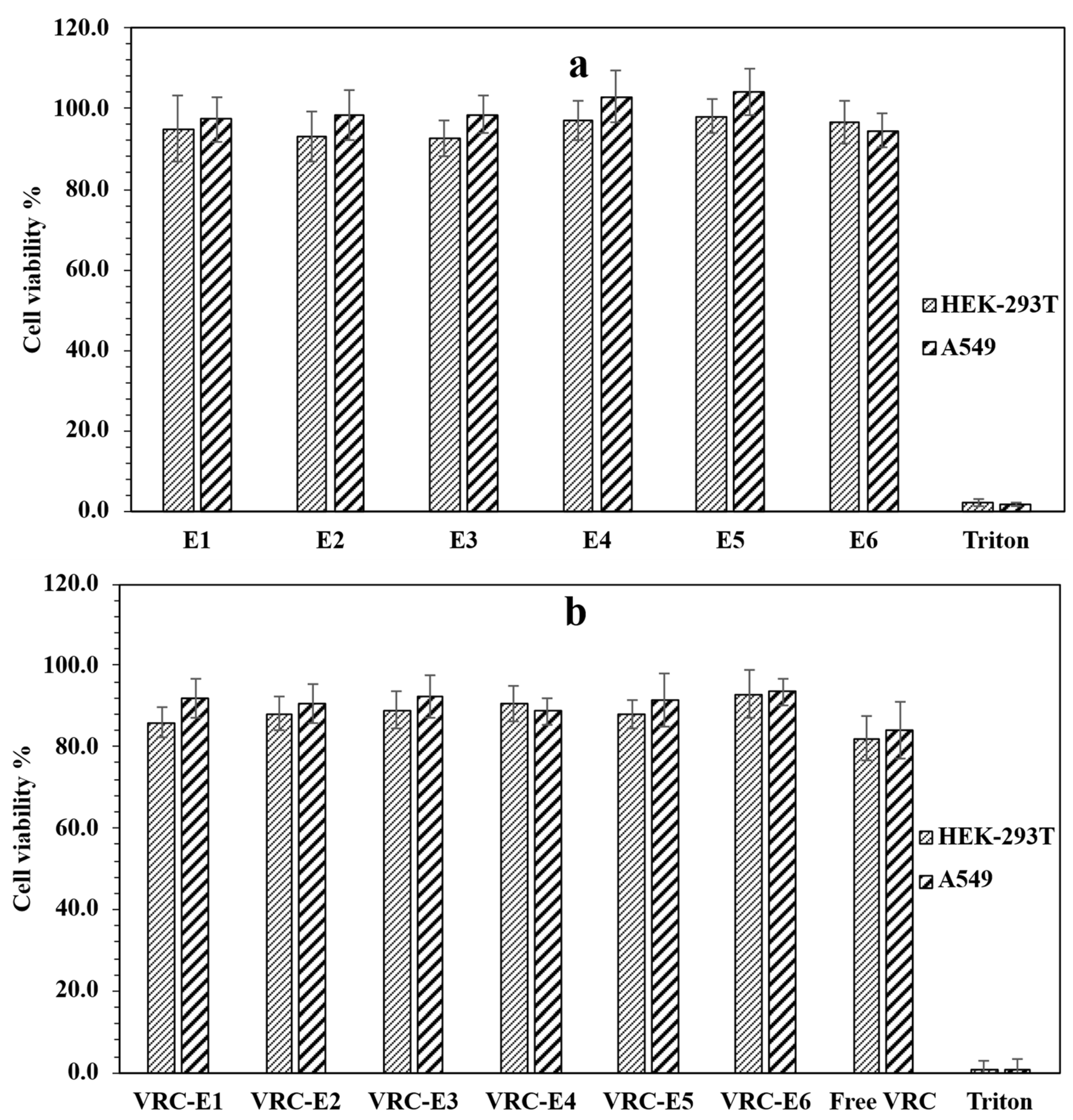
3.5. Cytotoxicity Studies of NHGs and VRC-NHGs
3.6. Antifungal Effect of VRC-NHGs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warris, A.; Bercusson, A.; Armstrong-James, D. Aspergillus colonization and antifungal immunity in cystic fibrosis patients. Med. Mycol. 2019, 57, S118–S126. [Google Scholar] [CrossRef] [PubMed]
- Farinha, C.M.; Callebaut, I. Molecular mechanisms of cystic fibrosis–how mutations lead to misfunction and guide therapy. Biosci. Rep. 2022, 42, BSR20212006. [Google Scholar] [CrossRef] [PubMed]
- Turcios, N.L. Cystic fibrosis lung disease: An overview. Respir. Care 2020, 65, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Lai, S.K.; Wang, Y.Y.; Ensign, L.M.; Zeitlin, P.L.; Boyle, M.P.; Hanes, J. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials 2009, 30, 2591–2597. [Google Scholar] [CrossRef]
- Yu, T.; Chisholm, J.; Choi, W.J.; Anonuevo, A.; Pulicare, S.; Zhong, W.; Chen, M.; Fridley, C.; Lai, S.K.; Ensign, L.M.; et al. Mucus-Penetrating Nanosuspensions for Enhanced Delivery of Poorly Soluble Drugs to Mucosal Surfaces. Adv. Healthc. Mater. 2016, 5, 2745–2750. [Google Scholar] [CrossRef]
- Hansson, G.C. Mucus and mucins in diseases of the intestinal and respiratory tracts. J. Intern. Med. 2019, 285, 479–490. [Google Scholar] [CrossRef]
- Fahy, J.V.; Dickey, B.F. Airway Mucus Function and Dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef]
- Meldrum, O.W.; Chotirmall, S.H. Mucus, microbiomes and pulmonary disease. Biomedicines 2021, 9, 675. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Pearson, J.P.; Chater, P.I.; Wilcox, M.D. The properties of the mucus barrier, a unique gel-how can nanoparticles cross it? Ther. Deliv. 2016, 7, 229–244. [Google Scholar] [CrossRef]
- Murgia, X.; Loretz, B.; Hartwig, O.; Hittinger, M.; Lehr, C.M. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv. Drug Deliv. Rev. 2018, 124, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, H.H.; Kirch, J.; Lehr, C.M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. 2013, 453, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Boegh, M.; Nielsen, H.M. Mucus as a barrier to drug delivery-Understanding and mimicking the barrier properties. Basic Clin. Pharmacol. Toxicol. 2015, 116, 179–186. [Google Scholar] [CrossRef]
- King, J.; Brunel, S.F.; Warris, A. Aspergillus infections in cystic fibrosis. J. Infect. 2016, 72, S50–S55. [Google Scholar] [CrossRef]
- Chotirmall, S.H.; McElvaney, N.G. Fungi in the cystic fibrosis lung: Bystanders or pathogens? Int. J. Biochem. Cell Biol. 2014, 52, 161–173. [Google Scholar] [CrossRef]
- Martín-Gómez, M.T. Taking a look on fungi in cystic fibrosis: More questions than answers. Rev. Iberoam. Micol. 2020, 37, 17–23. [Google Scholar] [CrossRef]
- Montagna, M.T.; Barbuti, G.; Paglionico, F.; Lovero, G.; Iatta, R.; De Giglio, O.; Cuna, T.; Coretti, C.; Santostasi, T.; Polizzi, A.; et al. Retrospective analysis of microorganisms isolated from cystic fibrosis patients in Southern Italy, 2002–2010. J. Prev. Med. Hyg. 2011, 52, 209–214. [Google Scholar]
- Viñado, C.; Girón, R.M.; Ibáñez, E.; García-Ortega, A.; Pérez, I.; Polanco, D.; Pemán, J.; Solé, A. Filamentous fungi in the airway of patients with cystic fibrosis: Just spectators? Rev. Iberoam. Micol. 2021, 38, 168–174. [Google Scholar] [CrossRef]
- Seidel, D.; Meißner, A.; Lackner, M.; Piepenbrock, E.; Salmanton-García, J.; Stecher, M.; Mellinghoff, S.; Hamprecht, A.; Graeff, L.D.; Köhler, P.; et al. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScope®. Crit. Rev. Microbiol. 2019, 45, 1–21. [Google Scholar] [CrossRef]
- Pihet, M.; Carrere, J.; Cimon, B.; Chabasse, D.; Delhaes, L.; Symoens, F.; Bouchara, J.P. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis—A review. Med. Mycol. 2009, 47, 387–397. [Google Scholar] [CrossRef]
- Cimon, B.; Carrère, J.; Vinatier, J.F.; Chazalette, J.P.; Chabasse, D.; Bouchara, J.P. Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Güngör, Ö.; Tamay, Z.; Güler, N.; Erturan, Z. Frequency of fungi in respiratory samples from Turkish cystic fibrosis patients. Mycoses 2013, 56, 123–129. [Google Scholar] [CrossRef]
- Ziesing, S.; Suerbaum, S.; Sedlacek, L. Fungal epidemiology and diversity in cystic fibrosis patients over a 5-year period in a national reference center. Med. Mycol. 2016, 54, 781–786. [Google Scholar] [CrossRef]
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue penetration of antifungal agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef]
- Lakhani, P.; Patil, A.; Majumdar, S. Challenges in the Polyene- and Azole-Based Pharmacotherapy of Ocular Fungal Infections. J. Ocul. Pharmacol. Ther. 2019, 35, 6–22. [Google Scholar] [CrossRef]
- Levine, M.T.; Chandrasekar, P.H. Adverse effects of voriconazole: Over a decade of use. Clin. Transplant. 2016, 30, 1377–1386. [Google Scholar] [CrossRef]
- Bentley, S.; Gupta, A.; Balfour-Lynn, I.M. Subtherapeutic itraconazole and voriconazole levels in children with cystic fibrosis. J. Cyst. Fibros. 2013, 12, 418–419. [Google Scholar] [CrossRef]
- Brunet, K.; Martellosio, J.P.; Tewes, F.; Marchand, S.; Rammaert, B. Inhaled Antifungal Agents for Treatment and Prophylaxis of Bronchopulmonary Invasive Mold Infections. Pharmaceutics 2022, 14, 641. [Google Scholar] [CrossRef]
- Sinha, B.; Mukherjee, B.; Pattnaik, G. Poly-lactide-co-glycolide nanoparticles containing voriconazole for pulmonary delivery: In vitro and in vivo study. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 94–104. [Google Scholar] [CrossRef]
- Beinborn, N.A.; Du, J.; Wiederhold, N.P.; Smyth, H.D.C.; Williams, R.O. Dry powder insufflation of crystalline and amorphous voriconazole formulations produced by thin film freezing to mice. Eur. J. Pharm. Biopharm. 2012, 81, 600–608. [Google Scholar] [CrossRef]
- Das, P.J.; Paul, P.; Mukherjee, B.; Mazumder, B.; Mondal, L.; Baishya, R.; Debnath, M.C.; Dey, K.S. Pulmonary Delivery of Voriconazole Loaded Nanoparticles Providing a Prolonged Drug Level in Lungs: A Promise for Treating Fungal Infection. Mol. Pharm. 2015, 12, 2651–2664. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Sengupta, S.; Mukherjee, B.; Shaw, T.K.; Gaonkar, R.H.; Debnath, M.C. Chitosan-coated nanoparticles enhanced lung pharmacokinetic profile of voriconazole upon pulmonary delivery in mice. Nanomedicine 2018, 13, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Yip, L.; Chow, M.Y.T.; Chow, S.F.; Chan, H.K.; Kwok, P.C.L.; Lam, J.K.W. Porous and highly dispersible voriconazole dry powders produced by spray freeze drying for pulmonary delivery with efficient lung deposition. Int. J. Pharm. 2019, 560, 144–154. [Google Scholar] [CrossRef]
- Wan, F.; Bohr, S.S.R.; Kłodzińska, S.N.; Jumaa, H.; Huang, Z.; Nylander, T.; Thygesen, M.B.; Sørensen, K.K.; Jensen, K.J.; Sternberg, C.; et al. Ultrasmall TPGS-PLGA Hybrid Nanoparticles for Site-Specific Delivery of Antibiotics into Pseudomonas aeruginosa Biofilms in Lungs. ACS Appl. Mater. Interfaces 2020, 12, 380–389. [Google Scholar] [CrossRef]
- Suk, J.S.; Kim, A.J.; Trehan, K.; Schneider, C.S.; Cebotaru, L.; Woodward, O.M.; Boylan, N.J.; Boyle, M.P.; Lai, S.K.; Guggino, W.B.; et al. Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J. Control. Release 2014, 178, 8–17. [Google Scholar] [CrossRef]
- Pacheco, D.P.; Butnarasu, C.S.; Vangosa, F.B.; Pastorino, L.; Visai, L.; Visentin, S.; Petrini, P. Disassembling the complexity of mucus barriers to develop a fast screening tool for early drug discovery. J. Mater. Chem. B 2019, 7, 4940–4952. [Google Scholar] [CrossRef]
- Cemal, S.D.; Kazimirsky, G.; Shadkchan, Y.; Eswaran, L.; Abramovitch, R.; Abudi, N.; Cuestas, M.L.; Osherov, N.; Byk, G. Biocompatible narrow size nanohydrogels for drug delivery. Nanomed. Nanotechnol. Biol. Med. 2025, 66, 102824. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Q.; Chen, W.; Zhao, Y.; Yong, T.; Gan, L.; Xu, H.; Yang, X. Hydrophilicity/hydrophobicity reversable and redox-sensitive nanogels for anticancer drug delivery. Mol. Pharm. 2015, 12, 1636–1647. [Google Scholar] [CrossRef]
- Palakkal, S.; Logviniuk, D.; Byk, G. Tuning the size and hydrophobicity of nanohydrogels exploiting a self-assembly assisted polymerization mechanism for controlled drug delivery. J. Nanoparticle Res. 2020, 22, 1–16. [Google Scholar] [CrossRef]
- Shi, F.; Ding, J.; Xiao, C.; Zhuang, X.; He, C.; Chen, L.; Chen, X. Intracellular microenvironment responsive PEGylated polypeptide nanogels with ionizable cores for efficient doxorubicin loading and triggered release. J. Mater. Chem. 2012, 22, 14168–14179. [Google Scholar] [CrossRef]
- Gupta, V.; Ahsan, F. Influence of PEI as a core modifying agent on PLGA microspheres of PGE 1, a pulmonary selective vasodilator. Int. J. Pharm. 2011, 413, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Costabile, G.; D’Angelo, I.; Rampioni, G.; Bondì, R.; Pompili, B.; Ascenzioni, F.; Mitidieri, E.; D’Emmanuele Di Villa Bianca, R.; Sorrentino, R.; Miro, A.; et al. Toward Repositioning Niclosamide for Antivirulence Therapy of Pseudomonas aeruginosa Lung Infections: Development of Inhalable Formulations through Nanosuspension Technology. Mol. Pharm. 2015, 12, 2604–2617. [Google Scholar] [CrossRef]
- Pellosi, D.S.; d’Angelo, I.; Maiolino, S.; Mitidieri, E.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Quaglia, F.; Ungaro, F. In vitro/in vivo investigation on the potential of Pluronic® mixed micelles for pulmonary drug delivery. Eur. J. Pharm. Biopharm. 2018, 130, 30–38. [Google Scholar] [CrossRef]
- Gulati, N.; Rastogi, R.; Dinda, A.K.; Saxena, R.; Koul, V. Characterization and cell material interactions of PEGylated PNIPAAM nanoparticles. Colloids Surf. B Biointerfaces 2010, 79, 164–173. [Google Scholar] [CrossRef]
- Devoto, T.B.; Hermida-Alva, K.; Posse, G.; Finquelievich, J.L.; García-Effrón, G.; Cuestas, M.L. Antifungal susceptibility patterns for Aspergillus, Scedosporium, and Exophiala isolates recovered from cystic fibrosis patients against amphotericin B, and three triazoles and their impact after long-term therapies. Med. Mycol. 2023, 61, myad089. [Google Scholar] [CrossRef]
- CLSI M38; Clinical Laboratory Standard Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Clinical Laboratory Standard Institute: Wayne, PA, USA, 2017.
- Khandadash, R.; Machtey, V.; Shainer, I.; Gottlieb, H.E.; Gothilf, Y.; Ebenstein, Y.; Weiss, A.; Byk, G. Novel biocompatible hydrogel nanoparticles: Generation and size-tuning of nanoparticles by the formation of micelle templates obtained from thermo-responsive monomers mixtures. J. Nanoparticle Res. 2014, 16, 2796. [Google Scholar] [CrossRef]
- Eswaran, L.; Kazimirsky, G.; Byk, G. New Biocompatible Nanohydrogels of Predefined Sizes for Complexing Nucleic Acids. Pharmaceutics 2023, 15, 332. [Google Scholar] [CrossRef]
- Yang, S.C.; Paik, S.Y.R.; Ryu, J.; Choi, K.O.; Kang, T.S.; Lee, J.K.; Song, C.W.; Ko, S. Dynamic light scattering-based method to determine primary particle size of iron oxide nanoparticles in simulated gastrointestinal fluid. Food Chem. 2014, 161, 185–191. [Google Scholar] [CrossRef]
- Yang, F.H.; Zhang, Q.; Liang, Q.Y.; Wang, S.Q.; Zhao, B.X.; Wang, Y.T.; Cai, Y.; Li, G.F. Bioavailability enhancement of paclitaxel via a novel oral drug delivery system: Paclitaxel-loaded glycyrrhizic acid micelles. Molecules 2015, 20, 4337–4356. [Google Scholar] [CrossRef]
- Ensign, L.M.; Schneider, C.; Suk, J.S.; Cone, R.; Hanes, J. Mucus penetrating nanoparticles: Biophysical tool and method of drug and gene delivery. Adv. Mater. 2012, 24, 3887–3894. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Ye, J.; Chen, Y.; Wang, R.; Jin, D.; Liu, Y. Mechanism Study on Nanoparticle Negative Surface Charge Modification by Ascorbyl Palmitate and Its Improvement of Tumor Targeting Ability. Molecules 2022, 27, 4408. [Google Scholar] [CrossRef] [PubMed]
- Gumustas, M.; Sengel-Turk, C.T.; Gumustas, A.; Ozkan, S.A.; Uslu, B. Effect of Polymer-Based Nanoparticles on the Assay of Antimicrobial Drug Delivery Systems. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–108. [Google Scholar] [CrossRef]
- Blackburn, W.H.; Lyon, L.A. Size-controlled synthesis of monodisperse core/shell nanogels. Colloid Polym. Sci. 2008, 286, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Mukherjee, M.; Sen, A.K. Swelling, thermal and mechanical properties of NIPAM-based terpolymeric hydrogel. Polym. Bull. 2016, 73, 125–145. [Google Scholar] [CrossRef]
- Costa, M.C.M.; Silva, S.M.C.; Antunes, F.E. Adjusting the low critical solution temperature of poly(N-isopropyl acrylamide) solutions by salts, ionic surfactants and solvents: A rheological study. J. Mol. Liq. 2015, 210, 113–118. [Google Scholar] [CrossRef]
- Cu, Y.; Saltzman, W.M. Controlled surface modification with poly(ethylene)glycol enhances diffusion of PLGA nanoparticles in human cervical Mucus. Mol. Pharm. 2009, 6, 173–181. [Google Scholar] [CrossRef]
- Murgia, X.; Pawelzyk, P.; Schaefer, U.F.; Wagner, C.; Willenbacher, N.; Lehr, C.M. Size-Limited Penetration of Nanoparticles into Porcine Respiratory Mucus after Aerosol Deposition. Biomacromolecules 2016, 17, 1536–1542. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress; current status; prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef]
- Abouelmagd, S.A.; Sun, B.; Chang, A.C.; Ku, Y.J.; Yeo, Y. Release kinetics study of poorly water-soluble drugs from nanoparticles: Are we doing it right? Mol. Pharm. 2015, 12, 997–1003. [Google Scholar] [CrossRef]
- Patil, J.S.; Sarasija, S. Pulmonary drug delivery strategies: A concise, systematic review. Lung India 2012, 29, 44–49. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Moazeni, E.; Gilani, K.; Najafabadi, A.R.; Rouini, M.R.; Mohajel, N.; Amini, M.; Barghi, M.A. Preparation and evaluation of inhalable itraconazole chitosan based polymeric micelles. DARU J. Pharm. Sci. 2012, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.B.; Alava, K.H.; Bernabeu, E.; Fuentes, P.; Devoto, T.B.; Höcht, C.; Chiappetta, D.A.; Cuestas, M.L.; Moretton, M.A. Highly effective inhalable voriconazole-loaded nanomicelles for fungal infections in cystic fibrosis patients: A promising therapeutic strategy for allergic bronchopulmonary aspergillosis. J. Drug Deliv. Sci. Technol. 2024, 100, 106126. [Google Scholar] [CrossRef]
- Czech, M.; Stock, F.; Aneke, C.; Lionakis, M.; Cuellar-Rodriguez, J.; Seyedmousavi, A. 2758. Clinical Significance and Antifungal Susceptibility Profile of 103 Clinical Scedosporium Species Complex and Lomentospora prolificans Isolated from NIH Hospitalized Patients. Open Forum Infect. Dis. 2023, 10, ofad500.2369. [Google Scholar] [CrossRef]
- Gülmez, D.; Doğan, Ö.; Boral, B.; Döğen, A.; İlkit, M.; de Hoog, G.S.; Arikan-Akdagli, S. In vitro activities of antifungal drugs against environmental Exophiala isolates and review of the literature. Mycoses 2018, 61, 561–569. [Google Scholar] [CrossRef]
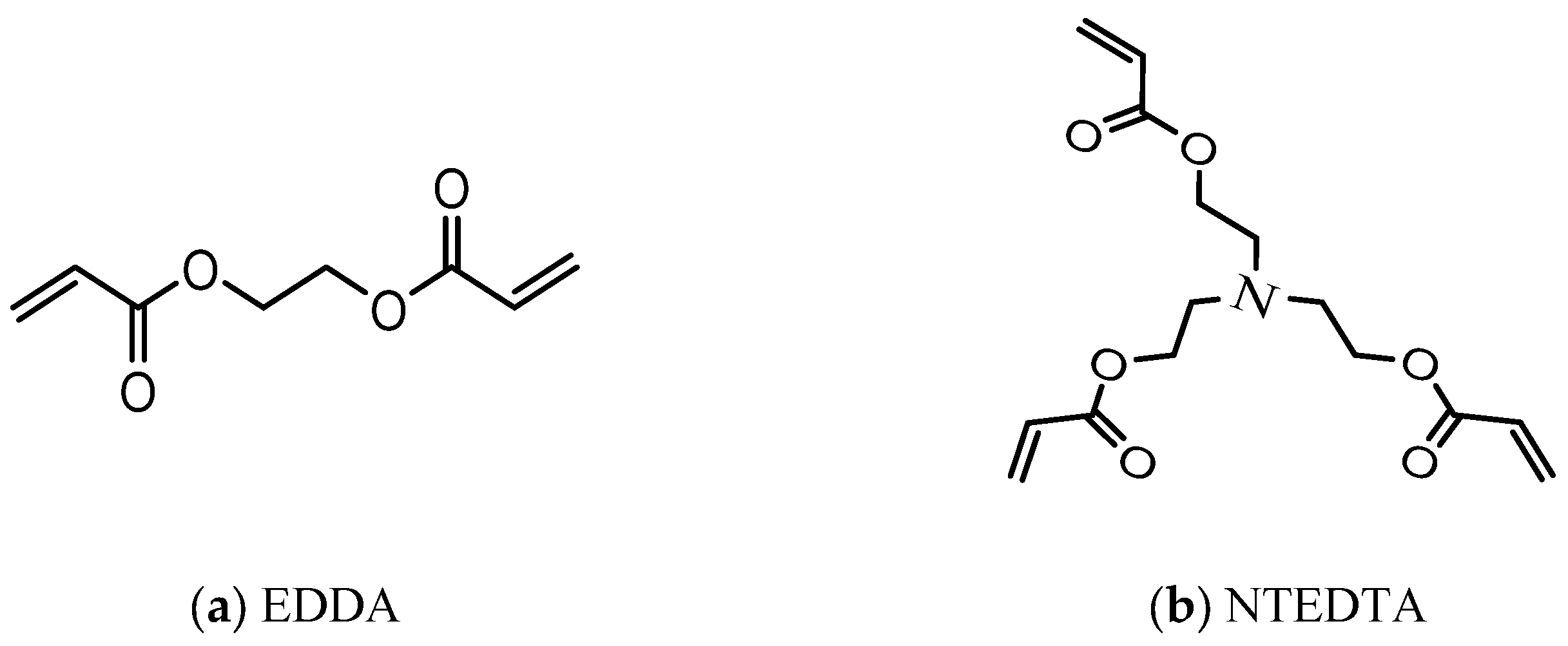

| # | (Acr)1.1Jeffamine1900 | NIPAM | EDDA | NTEDTA |
|---|---|---|---|---|
| E1 | 130 | 120 | 15 | - |
| E2 | 260 | 120 | 15 | - |
| E3 | 320 | 120 | 15 | - |
| E4 | 200 | 188 | - | 19 |
| E5 | 300 | 188 | - | 19 |
| E6 | 400 | 188 | - | 19 |
| # | NHGs | VRC-NHGs | ||||||
|---|---|---|---|---|---|---|---|---|
| Dh [nm] | PDI | ζ [mV] | NPs/mL | Dh [nm] | PDI | ζ [mV] | NPs/mL | |
| E1 | 410 ± 4 | 0.17 | −27.9 | 5.7 × 1012 | 424 ± 2 | 0.12 | −21.7 | 1.4 × 1012 |
| E2 | 242 ± 8 | 0.28 | −28.8 | 7.4 × 1012 | 251 ± 6 | 0.19 | −9.2 | 4.8 × 1012 |
| E3 | 136 ± 5 | 0.17 | −23.3 | 3.7 × 1012 | 145 ± 5 | 0.22 | −27.5 | 5.2 × 1012 |
| E4 | 392 ± 2 | 0.08 | −27.1 | 1.3 × 1012 | 407 ± 4 | 0.14 | −26.7 | 1.6 × 1012 |
| E5 | 198 ± 6 | 0.15 | −27.3 | 2.4 × 1012 | 220 ± 5 | 0.23 | −23.5 | 6.7 × 1012 |
| E6 | 121 ± 7 | 0.24 | −11.9 | 6.4 × 1012 | 134 ± 3 | 0.19 | −20.0 | 5.6 × 1012 |
| % Particles Trespassing Mucus Barrier | ||||||
|---|---|---|---|---|---|---|
| Time [h] | E1 | E2 | E3 | E4 | E5 | E6 |
| 4 | 7.2 | 3.8 | 1.5 | 3.8 | 1.8 | 0.3 |
| 24 | 20.1 | 15.1 | 12 | 7.8 | 2.8 | 0.4 |
| # | Loaded VRC [µg] | DLE [%] | * DLC [%] |
|---|---|---|---|
| E1 | 325 ± 8 | 16.2 | 3.2 |
| E2 | 276 ± 9 | 13.8 | 2.8 |
| E3 | 208 ± 5 | 10.4 | 2.1 |
| E4 | 312 ± 10 | 15.6 | 3.1 |
| E5 | 245 ± 7 | 12.2 | 2.4 |
| E6 | 198 ± 6 | 9.9 | 2.0 |
| Fungal Species | VRC-DMSO | VRC Susp. | VRC-E1 | VRC-E2 | VRC-E3 | VRC-E4 | VRC-E5 | VRC-E6 |
|---|---|---|---|---|---|---|---|---|
| A. fumigatus | 0.25 | 2 | 0.5 | 1 | 1 | 0.5 | 1 | 2 |
| * A. fumigatus | 0.25 | 2 | 0.5 | 1 | 1 | 0.5 | 1 | 2 |
| A. terreus | 1 | 2 | 0.5 | 1 | 2 | 0.5 | 1 | 2 |
| A. flavus | 0.5 | 2 | 0.5 | 1 | 2 | 0.5 | 1 | 2 |
| A. niger | 0.5 | 2 | 0.5 | 1 | 2 | 0.5 | 1 | 2 |
| A. nidulans | 0.5 | 2 | 0.5 | 1 | 1 | 0.25 | 1 | 2 |
| E. dermatitidis | 0.5 | 2 | 0.25 | 1 | 1 | 0.25 | 1 | 2 |
| S. auarantiacum | 0.5 | 2 | 0.5 | 1 | 2 | 0.5 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cemal, S.D.; Ladetto, M.F.; Alava, K.H.; Kazimirsky, G.; Cucher, M.; Glisoni, R.J.; Cuestas, M.L.; Byk, G. Voriconazole-Loaded Nanohydrogels Towards Optimized Antifungal Therapy for Cystic Fibrosis Patients. Pharmaceutics 2025, 17, 725. https://doi.org/10.3390/pharmaceutics17060725
Cemal SD, Ladetto MF, Alava KH, Kazimirsky G, Cucher M, Glisoni RJ, Cuestas ML, Byk G. Voriconazole-Loaded Nanohydrogels Towards Optimized Antifungal Therapy for Cystic Fibrosis Patients. Pharmaceutics. 2025; 17(6):725. https://doi.org/10.3390/pharmaceutics17060725
Chicago/Turabian StyleCemal, Shaul D., María F. Ladetto, Katherine Hermida Alava, Gila Kazimirsky, Marcela Cucher, Romina J. Glisoni, María L. Cuestas, and Gerardo Byk. 2025. "Voriconazole-Loaded Nanohydrogels Towards Optimized Antifungal Therapy for Cystic Fibrosis Patients" Pharmaceutics 17, no. 6: 725. https://doi.org/10.3390/pharmaceutics17060725
APA StyleCemal, S. D., Ladetto, M. F., Alava, K. H., Kazimirsky, G., Cucher, M., Glisoni, R. J., Cuestas, M. L., & Byk, G. (2025). Voriconazole-Loaded Nanohydrogels Towards Optimized Antifungal Therapy for Cystic Fibrosis Patients. Pharmaceutics, 17(6), 725. https://doi.org/10.3390/pharmaceutics17060725






