Combinatorial Effects of Free and Nanoencapsulated Forms of Cabazitaxel and RAS-Selective Lethal 3 in Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines
2.3. Synthesis and Characterization of NPs
2.4. Evaluation of Cell Proliferation
2.5. Analysis of Combinatorial Effects
2.6. Cell Viability Assay
2.7. ROS Assay
2.8. Metabolic Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of NPs
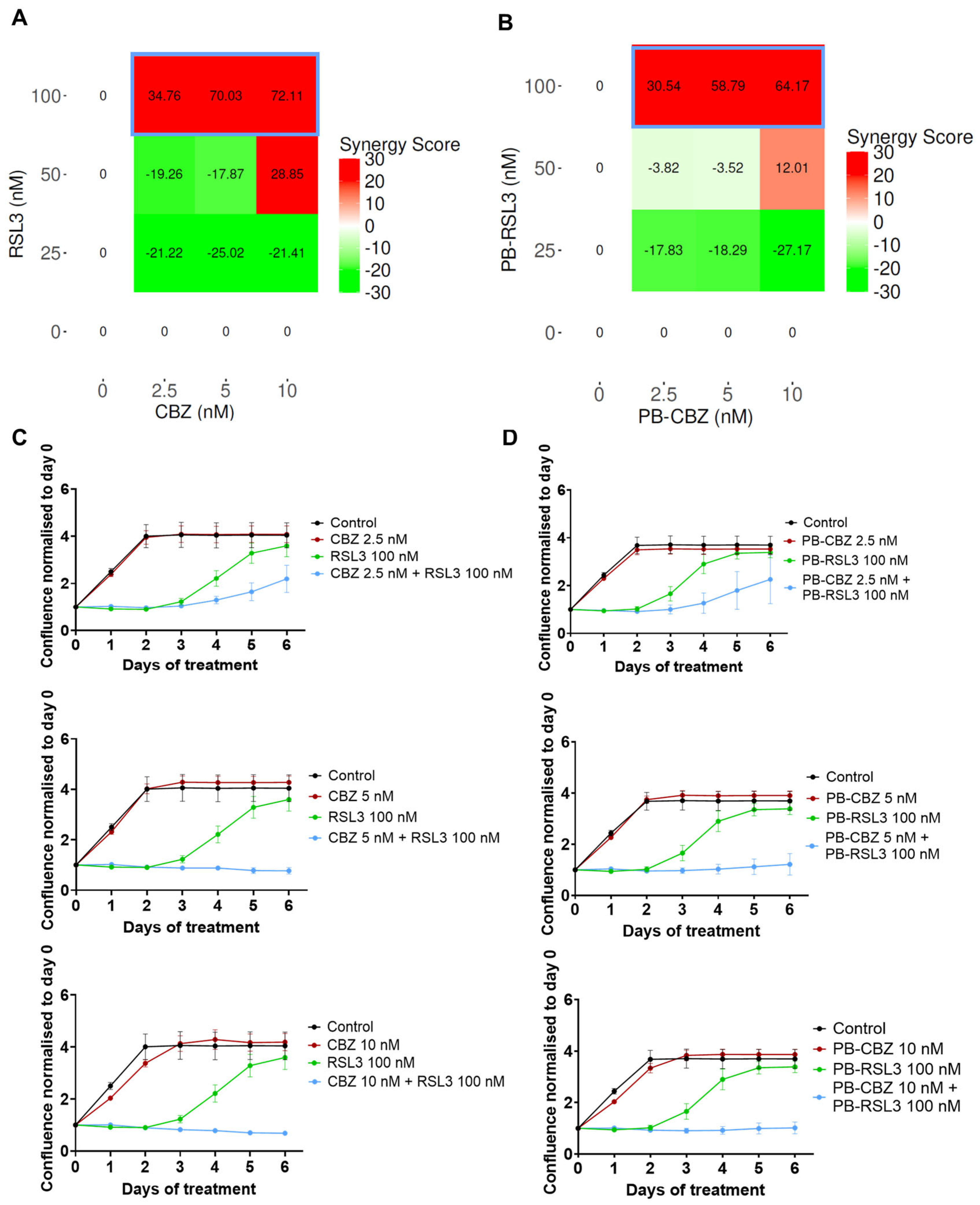
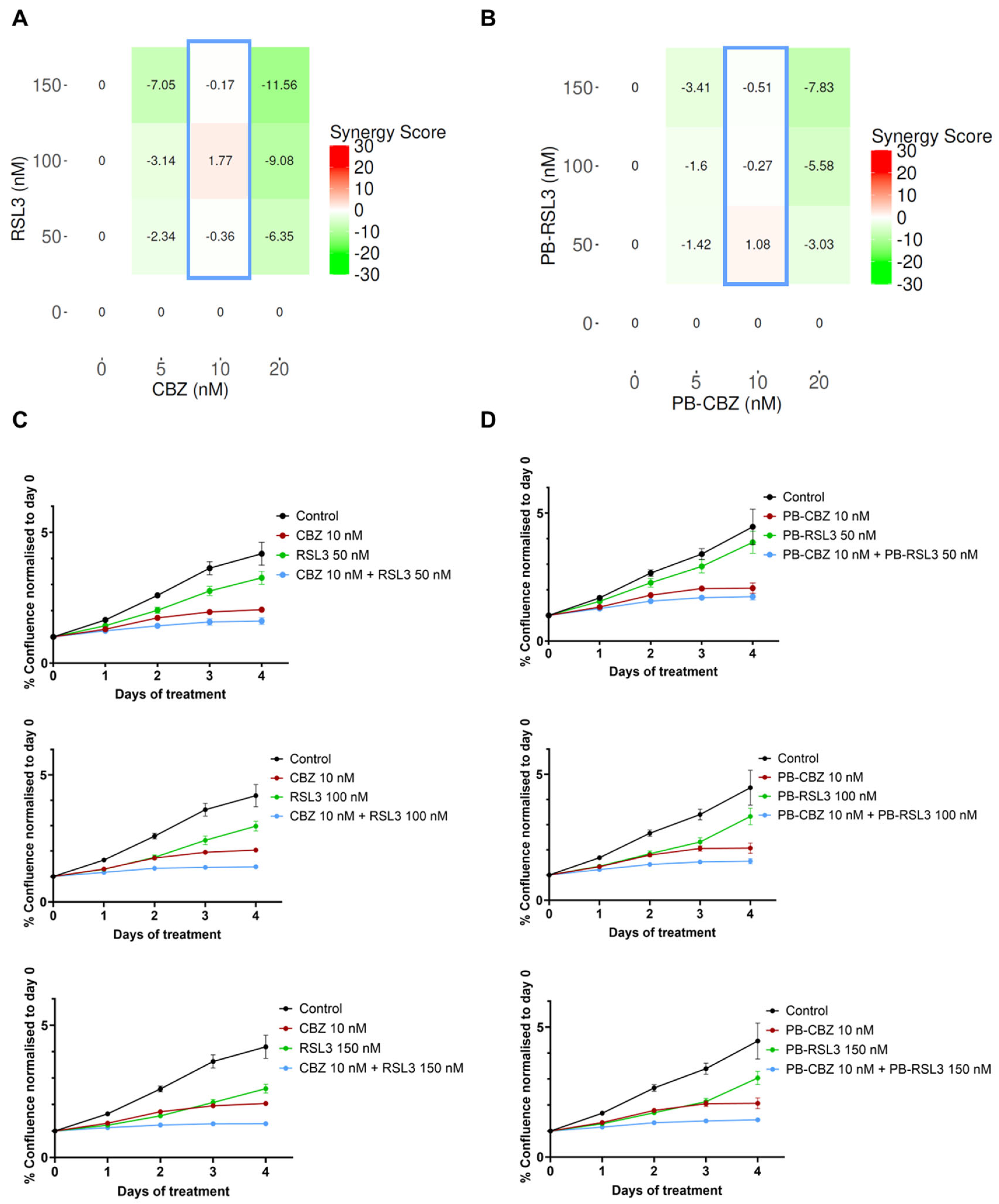
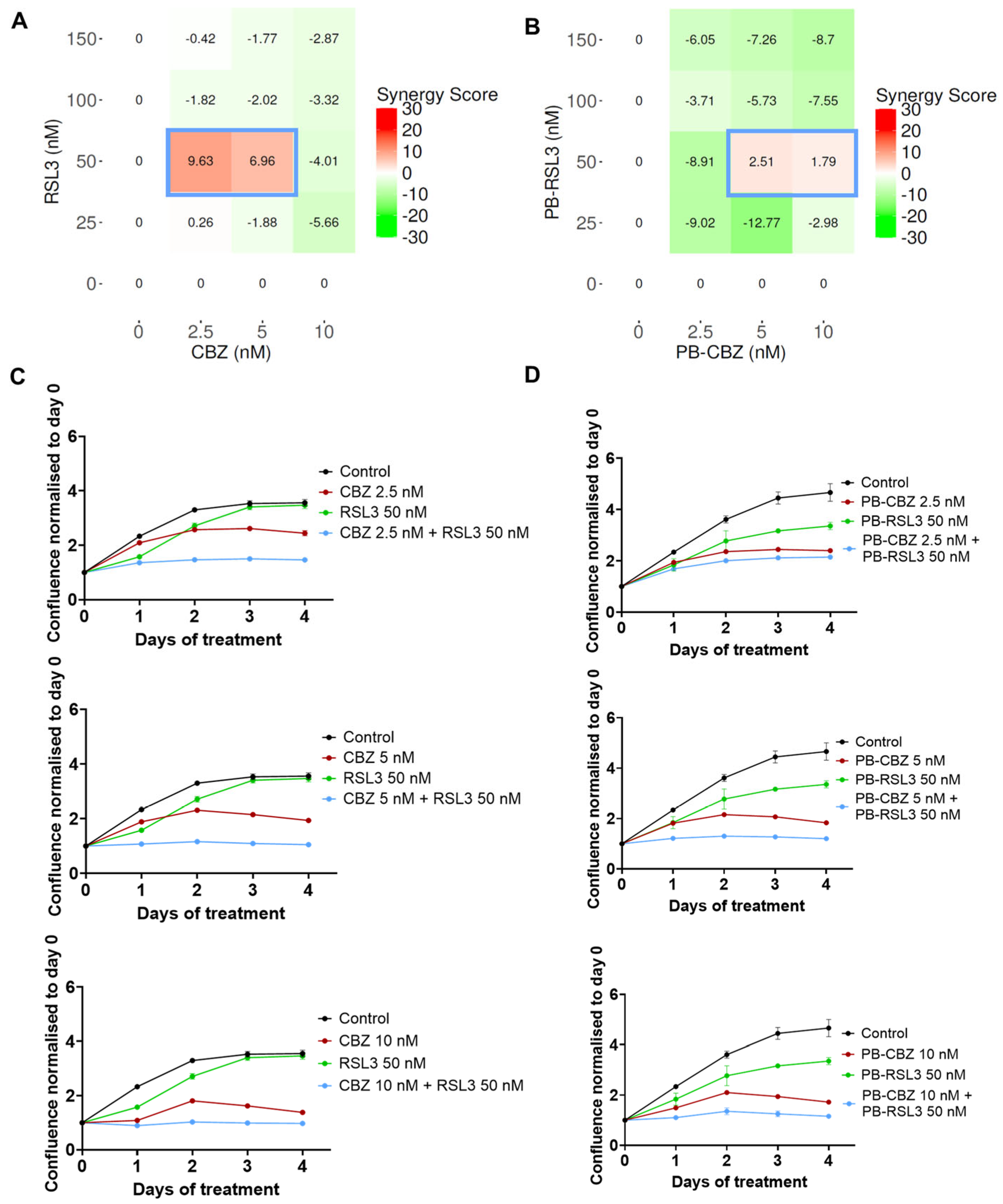
3.2. Combination of CBZ and RSL3 Improved Cytotoxic Effects Compared to Drugs Alone
3.3. In Vitro Combinatorial Effects Are Similar for Free Drugs and Encapsulated Drugs
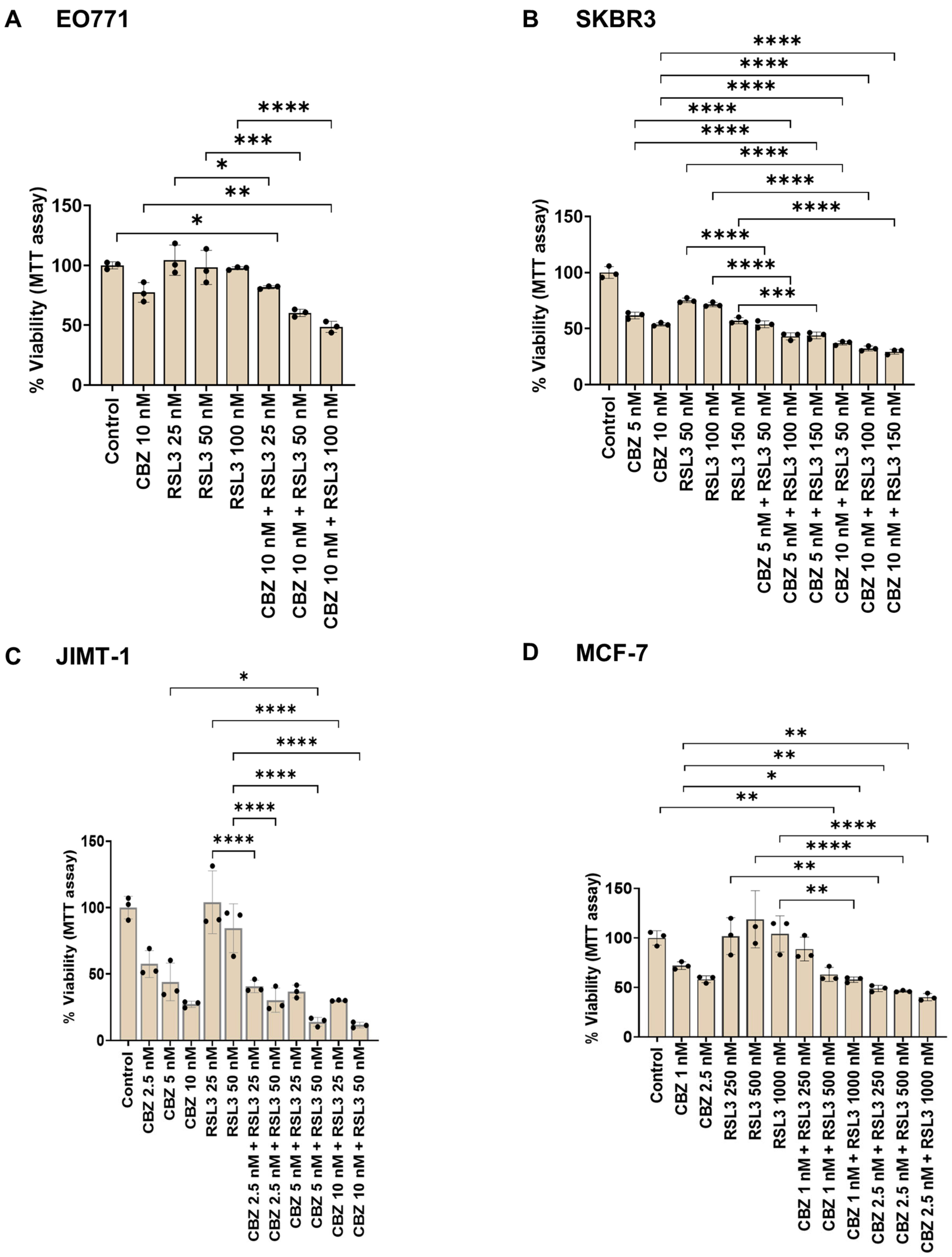
3.4. Combinatorial Effects Are Cell Type Dependent
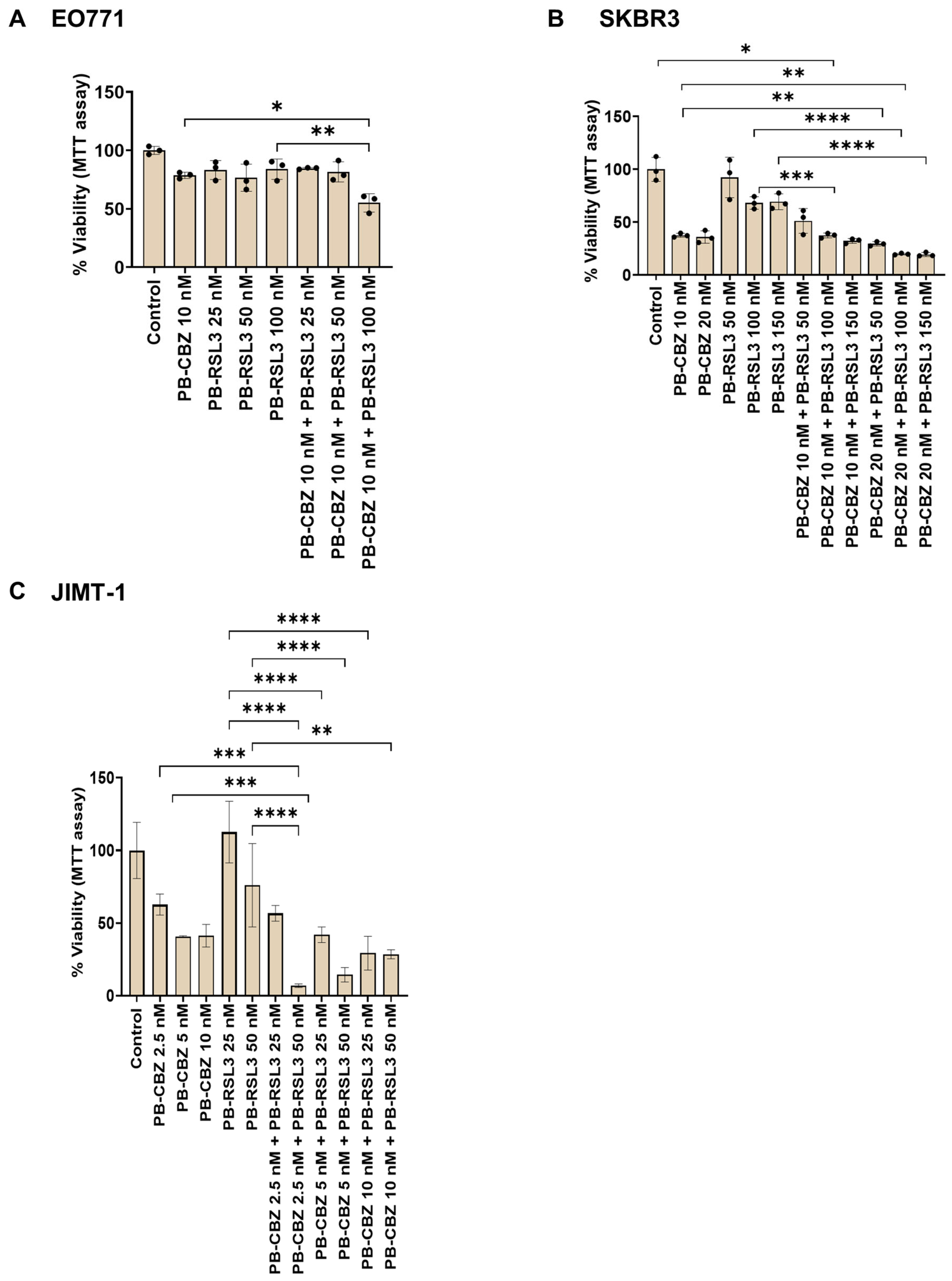
3.5. Cell Viability Assay Correlates with Cell Proliferation Assay
3.6. Combinatorial Treatment Did Not Cause Immediate Effects on ROS Levels
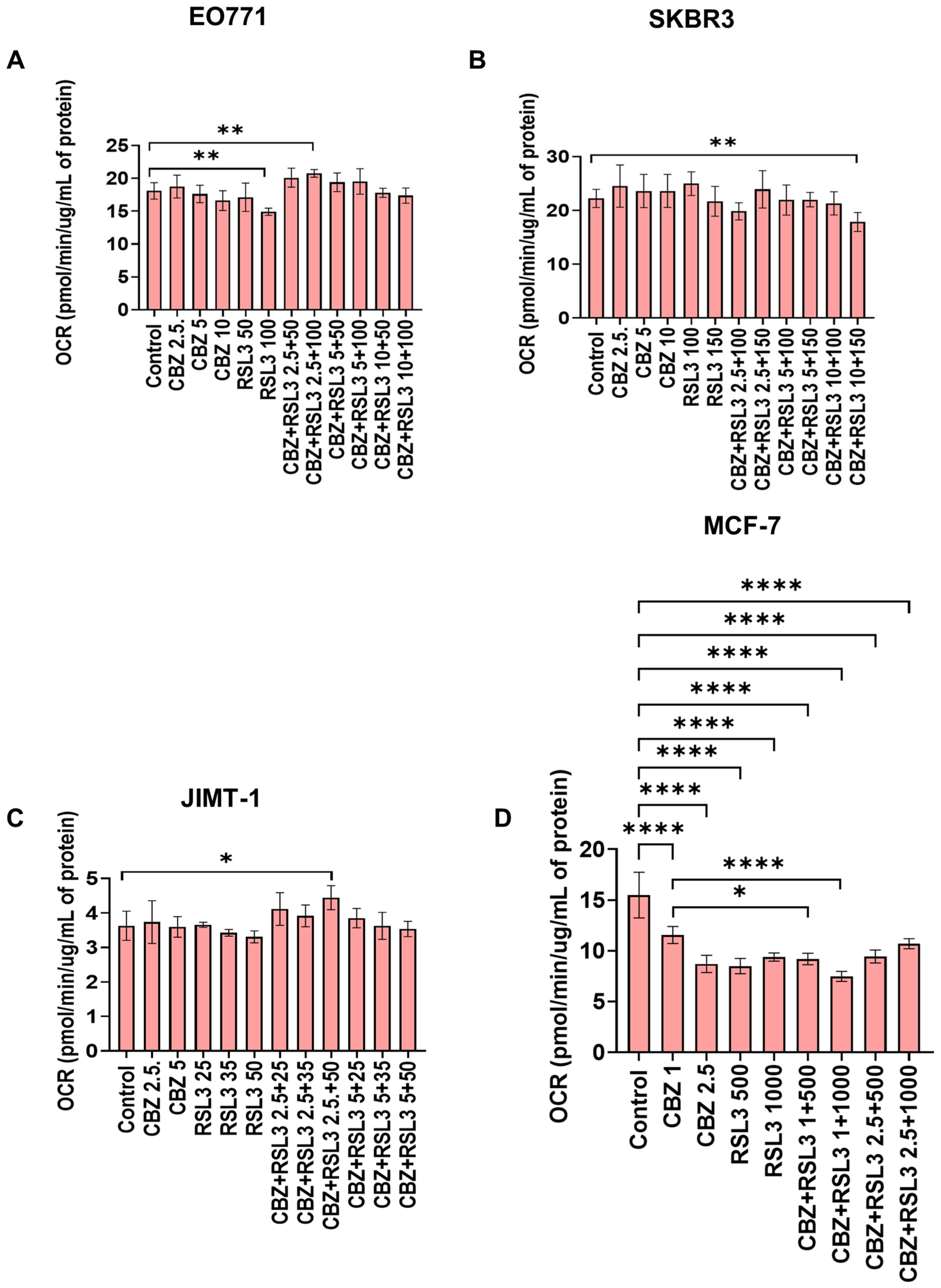
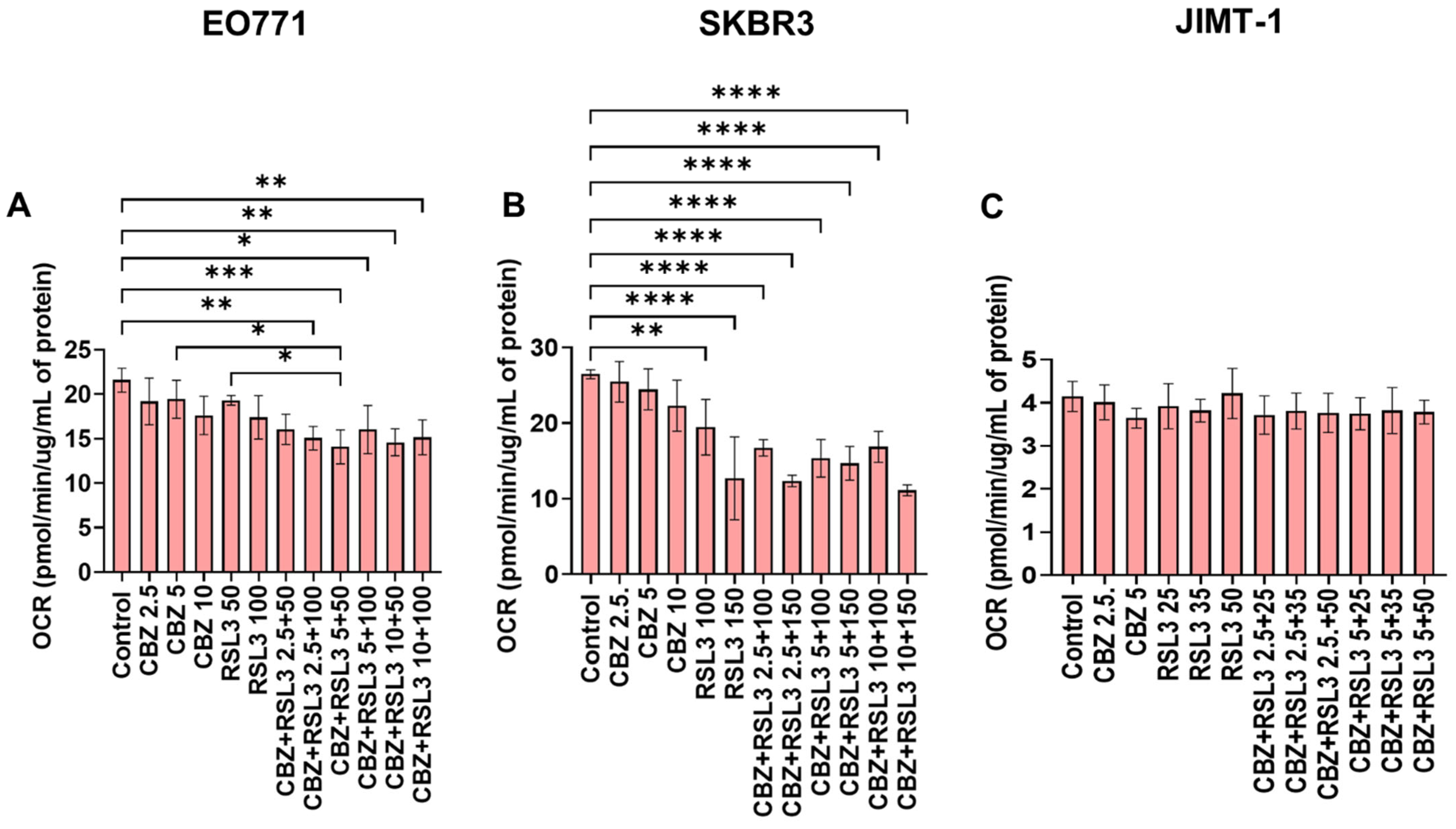
3.7. Combinatorial Effects Are Not Accompanied by Immediate Effects on Cell Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garner, H.; de Visser, K.E. Immune crosstalk in cancer progression and metastatic spread: A complex conversation. Nat. Rev. Immunol. 2020, 20, 483–497. [Google Scholar] [CrossRef]
- Nebert, D.W. Transcription factors and cancer: An overview. Toxicology 2002, 181, 131–141. [Google Scholar] [CrossRef]
- Slattery, M.L.; Fitzpatrick, F.A. Convergence of hormones, inflammation, and energy-related factors: A novel pathway of cancer etiology. Cancer Prev. Res. 2009, 2, 922–930. [Google Scholar] [CrossRef]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The challenge of drug resistance in cancer treatment: A current overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef]
- Li, S.; Kennedy, M.; Payne, S.; Kennedy, K.; Seewaldt, V.L.; Pizzo, S.V.; Bachelder, R.E. Model of tumor dormancy/recurrence after short-term chemotherapy. PLoS ONE 2014, 9, e98021. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022. [Google Scholar] [CrossRef]
- Gotwals, P.; Cameron, S.; Cipolletta, D.; Cremasco, V.; Crystal, A.; Hewes, B.; Mueller, B.; Quaratino, S.; Sabatos-Peyton, C.; Petruzzelli, L. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer 2017, 17, 286–301. [Google Scholar] [CrossRef]
- Palmer, A.C.; Sorger, P.K. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell 2017, 171, 1678–1691.e1613. [Google Scholar] [CrossRef]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunol. Immunother. 2022, 71, 507–526. [Google Scholar] [CrossRef]
- Zahler, S.; Ghazi, N.G.; Singh, A.D. Principles and complications of chemotherapy. In Clinical Ophthalmic Oncology; Springer: Cham, Switzerland, 2019; pp. 129–142. [Google Scholar]
- Gilad, Y.; Gellerman, G.; Lonard, D.M.; O’malley, B.W. Drug combination in cancer treatment—From cocktails to conjugated combinations. Cancers 2021, 13, 669. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Berman, D.M.; Aznar, M.A.; Korman, A.J.; Gracia, J.L.P.; Haanen, J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat. Rev. Cancer 2015, 15, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.D.; Sun, G.; Li, J.; Xu, J.; Wang, X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019, 452, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Antonarakis, E.S. Cabazitaxel: A novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Des. Dev. Ther. 2011, 5, 117–124. [Google Scholar]
- Koutras, A.; Zagouri, F.; Koliou, G.-A.; Psoma, E.; Chryssogonidis, I.; Lazaridis, G.; Tryfonopoulos, D.; Kotsakis, A.; Kentepozidis, N.K.; Razis, E. Phase 2 study of Cabazitaxel as second-line treatment in patients with HER2-negative metastatic breast cancer previously treated with taxanes—A Hellenic Cooperative Oncology Group (HeCOG) Trial. Br. J. Cancer 2020, 123, 355–361. [Google Scholar] [CrossRef]
- Yardley, D.A.; Hart, L.L.; Ward, P.J.; Wright, G.L.; Shastry, M.; Finney, L.; DeBusk, L.M.; Hainsworth, J.D. Cabazitaxel Plus Lapatinib as Therapy for HER2 Metastatic Breast Cancer With Intracranial Metastases: Results of a Dose-finding Study. Clin. Breast Cancer 2018, 18, E781–E787. [Google Scholar] [CrossRef]
- Villanueva, C.; Awada, A.; Campone, M.; Machiels, J.P.; Besse, T.; Magherini, E.; Dubin, F.; Semiond, D.; Pivot, X. A multicentre dose-escalating study of cabazitaxel (XRP6258) in combination with capecitabine in patients with metastatic breast cancer progressing after anthracycline and taxane treatment: A phase I/II study. Eur. J. Cancer 2011, 47, 1037–1045. [Google Scholar] [CrossRef]
- Duran, G.E.; Derdau, V.; Weitz, D.; Philippe, N.; Blankenstein, J.; Atzrodt, J.; Semiond, D.; Gianolio, D.A.; Mace, S.; Sikic, B.I. Cabazitaxel is more active than first-generation taxanes in ABCB1(+) cell lines due to its reduced affinity for P-glycoprotein. Cancer Chemother. Pharmacol. 2018, 81, 1095–1103. [Google Scholar] [CrossRef]
- Sun, B.; Lovell, J.F.; Zhang, Y. Current development of cabazitaxel drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1854. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, R.; Liu, S.; Duan, T.; Zhai, L.; Zhang, M.; Han, X.; Xiang, Y.; Huang, X.; Lin, H.; et al. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front. Pharmacol. 2018, 9, 1371. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, C.; Jiang, C.; Liu, N.; Yang, Z.; Xing, H. RSL3 induces ferroptosis by activating the NF-κB signalling pathway to enhance the chemosensitivity of triple-negative breast cancer cells to paclitaxel. Sci. Rep. 2025, 15, 1654. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yan, S.; Zhu, J.; Lu, R.; Kang, C.; Tang, K.; Zeng, J.; Ding, M.; Guo, Z.; Lai, X.; et al. Combination RSL3 Treatment Sensitizes Ferroptosis- and EGFR-Inhibition-Resistant HNSCCs to Cetuximab. Int. J. Mol. Sci. 2022, 23, 9014. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Wang, X.; Wei, X.; Wang, D.; Liu, X.; Xu, L.; Batu, W.; Li, Y.; Guo, B. RSL3 enhances the antitumor effect of cisplatin on prostate cancer cells via causing glycolysis dysfunction. Biochem. Pharmacol. 2021, 192, 114741. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Y.; Hou, F.; Du, J.; Tong, X. Rapamycin enhances inhibitory effect of RSL3 on proliferation, invasion and migration of testicular cancer I-10 cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao 2023, 43, 2145–2151. [Google Scholar]
- Peng, S.; Chen, G.; Yu, K.; Feng, Y.; Zhao, L.; Yang, M.; Cao, W.; Almahi, W.A.A.; Sun, M.; Xu, Y. Synergism of non-thermal plasma and low concentration RSL3 triggers ferroptosis via promoting xCT lysosomal degradation through ROS/AMPK/mTOR axis in lung cancer cells. Cell Commun. Signal. 2024, 22, 112. [Google Scholar] [CrossRef] [PubMed]
- Fusser, M.; Overbye, A.; Pandya, A.D.; Morch, Y.; Borgos, S.E.; Kildal, W.; Snipstad, S.; Sulheim, E.; Fleten, K.G.; Askautrud, H.A.; et al. Cabazitaxel-loaded Poly(2-ethylbutyl cyanoacrylate) nanoparticles improve treatment efficacy in a patient derived breast cancer xenograft. J. Control. Release 2019, 293, 183–192. [Google Scholar] [CrossRef]
- Øverbye, A.; Torgersen, M.L.; Sønstevold, T.; Iversen, T.G.; Mørch, Ý.; Skotland, T.; Sandvig, K. Cabazitaxel-loaded poly (alkyl cyanoacrylate) nanoparticles: Toxicity and changes in the proteome of breast, colon and prostate cancer cells. Nanotoxicology 2021, 15, 865–884. [Google Scholar] [CrossRef]
- Valsalakumari, R.; Pandya, A.D.; Prasmickaite, L.; Kvalvaag, A.; Myrann, A.G.; Aslund, A.K.O.; Kjos, M.S.; Fontecha-Cuenca, C.; Haroon, H.B.; Ribeiro, A.R.S.; et al. Preclinical Efficacy of Cabazitaxel Loaded Poly(2-alkyl cyanoacrylate) Nanoparticle Variants. Int. J. Nanomed. 2024, 19, 3009–3029. [Google Scholar] [CrossRef]
- Sulheim, E.; Iversen, T.G.; To Nakstad, V.; Klinkenberg, G.; Sletta, H.; Schmid, R.; Hatletveit, A.R.; Wagbo, A.M.; Sundan, A.; Skotland, T.; et al. Cytotoxicity of Poly(Alkyl Cyanoacrylate) Nanoparticles. Int. J. Mol. Sci. 2017, 18, 2454. [Google Scholar] [CrossRef]
- Lyles, R.D.Z.; Martinez, M.J.; Sherman, B.; Schürer, S.; Burnstein, K.L. Automation, live-cell imaging, and endpoint cell viability for prostate cancer drug screens. PLoS ONE 2023, 18, e0287126. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, W.; Aldahdooh, J.; Malyutina, A.; Shadbahr, T.; Tanoli, Z.; Pessia, A.; Tang, J. SynergyFinder plus: Toward better interpretation and annotation of drug combination screening datasets. Genom. Proteom. Bioinform. 2022, 20, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, M.C. What is synergy? Pharmacol. Rev. 1989, 41, 93–141. [Google Scholar] [CrossRef]
- Bliss, C.I. The toxicity of poisons applied jointly 1. Ann. Appl. Biol. 1939, 26, 585–615. [Google Scholar] [CrossRef]
- Loewe, S. The problem of synergism and antagonism of combined drugs. Arzneimittel-Forschung 1953, 3, 285–290. [Google Scholar]
- Yadav, B.; Wennerberg, K.; Aittokallio, T.; Tang, J. Searching for drug synergy in complex dose–response landscapes using an interaction potency model. Comput. Struct. Biotechnol. J. 2015, 13, 504–513. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q. Using seahorse machine to measure OCR and ECAR in cancer cells. Cancer Metab. Methods Protoc. 2019, 1928, 353–363. [Google Scholar]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef]
- Kemp, J.A.; Shim, M.S.; Heo, C.Y.; Kwon, Y.J. “Combo” nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 3–18. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Craft of co-encapsulation in nanomedicine: A struggle to achieve synergy through reciprocity. ACS Pharmacol. Transl. Sci. 2022, 5, 278–298. [Google Scholar] [CrossRef]
- Tzogani, K.; Penttila, K.; Lapvetelainen, T.; Hemmings, R.; Koenig, J.; Freire, J.; Marcia, S.; Cole, S.; Coppola, P.; Flores, B.; et al. EMA Review of Daunorubicin and Cytarabine Encapsulated in Liposomes (Vyxeos, CPX-351) for the Treatment of Adults with Newly Diagnosed, Therapy-Related Acute Myeloid Leukemia or Acute Myeloid Leukemia with Myelodysplasia-Related Changes. Oncologist 2020, 25, e1414–e1420. [Google Scholar] [CrossRef] [PubMed]
- Wiernicki, B.; Dubois, H.; Tyurina, Y.Y.; Hassannia, B.; Bayir, H.; Kagan, V.E.; Vandenabeele, P.; Wullaert, A.; Vanden Berghe, T. Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes including pyroptosis. Cell Death Dis. 2020, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Panzilius, E.; Holstein, F.; Dehairs, J.; Planque, M.; von Toerne, C.; Koenig, A.-C.; Doll, S.; Bannier-Hélaouët, M.; Ganz, H.M.; Hauck, S.M. Cell density-dependent ferroptosis in breast cancer is induced by accumulation of polyunsaturated fatty acid-enriched triacylglycerides. BioRxiv 2018, preprint. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Sennoune, S.R.; Nelius, T.; Jarvis, C.; Pruitt, K.; Kottapalli, K.R.; Filleur, S. The Wnt non-canonical signaling modulates cabazitaxel sensitivity in prostate cancer cells. PLoS ONE 2020, 15, e0234078. [Google Scholar] [CrossRef]
- Machioka, K.; Izumi, K.; Kadono, Y.; Iwamoto, H.; Naito, R.; Makino, T.; Kadomoto, S.; Natsagdorj, A.; Keller, E.T.; Zhang, J. Establishment and characterization of two cabazitaxel-resistant prostate cancer cell lines. Oncotarget 2018, 9, 16185. [Google Scholar] [CrossRef]
- Huang, W.; Guo, Y.; Qian, Y.; Liu, X.; Li, G.; Wang, J.; Yang, X.; Wu, M.; Fan, Y.; Luo, H.; et al. Ferroptosis-inducing compounds synergize with docetaxel to overcome chemoresistance in docetaxel-resistant non-small cell lung cancer cells. Eur. J. Med. Chem. 2024, 276, 116670. [Google Scholar] [CrossRef]
- Ye, J.; Jiang, X.; Dong, Z.; Hu, S.; Xiao, M. Low-concentration PTX and RSL3 inhibits tumor cell growth synergistically by inducing ferroptosis in mutant p53 hypopharyngeal squamous carcinoma. Cancer Manag. Res. 2019, 11, 9783–9792. [Google Scholar] [CrossRef]
- Nozhat, Z.; Khalaji, M.S.; Hedayati, M.; Kia, S.K. Different methods for cell viability and proliferation assay: Essential tools in pharmaceutical studies. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2022, 22, 703–712. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Eryilmaz, I.E.; Egeli, U.; Cecener, G. Association between the apoptotic effect of Cabazitaxel and its pro-oxidant efficacy on the redox adaptation mechanisms in prostate cancer cells with different resistance phenotypes. Cancer Biol. Ther. 2024, 25, 2329368. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, T.; Hongo, H.; Miyazaki, Y.; Nishimoto, K.; Miyajima, A.; Oya, M. Reactive oxygen species induction by cabazitaxel through inhibiting Sestrin-3 in castration resistant prostate cancer. Oncotarget 2017, 8, 87675–87683. [Google Scholar] [CrossRef] [PubMed]
- Szwed, M.; Sønstevold, T.; Øverbye, A.; Engedal, N.; Grallert, B.; Mørch, Ý.; Sulheim, E.; Iversen, T.-G.; Skotland, T.; Sandvig, K. Small variations in nanoparticle structure dictate differential cellular stress responses and mode of cell death. Nanotoxicology 2019, 13, 761–782. [Google Scholar] [CrossRef] [PubMed]
- Feith, M.; Das Sajib, S.; Myrann, A.G.; Høgset, A.; Garrido, P.; Martinez, A.; Knutsen, E.; Sandvig, K.; Skotland, T.; Maelandsmo, G.M.; et al. Induction of Cell Death by Combined Treatment with Photosensitizer-Chitosan Nanoparticles and the Ferroptosis Inducer RSL3 in Breast Cancer Cell Lines. Adv. NanoBiomed Res. 2025, 5, 2400208. [Google Scholar] [CrossRef]
- Merkel, M.; Goebel, B.; Boll, M.; Adhikari, A.; Maurer, V.; Steinhilber, D.; Culmsee, C. Mitochondrial Reactive Oxygen Species Formation Determines ACSL4/LPCAT2-Mediated Ferroptosis. Antioxidants 2023, 12, 1590. [Google Scholar] [CrossRef]
- Liang, H.; Remmen, H.V.; Frohlich, V.; Lechleiter, J.; Richardson, A.; Ran, Q. Gpx4 protects mitochondrial ATP generation against oxidative damage. Biochem. Biophys. Res. Commun. 2007, 356, 893–898. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valsalakumari, R.; Feith, M.; Pettersen, S.; Åslund, A.K.O.; Mørch, Ý.; Skotland, T.; Sandvig, K.; Mælandsmo, G.M.; Iversen, T.-G. Combinatorial Effects of Free and Nanoencapsulated Forms of Cabazitaxel and RAS-Selective Lethal 3 in Breast Cancer Cells. Pharmaceutics 2025, 17, 657. https://doi.org/10.3390/pharmaceutics17050657
Valsalakumari R, Feith M, Pettersen S, Åslund AKO, Mørch Ý, Skotland T, Sandvig K, Mælandsmo GM, Iversen T-G. Combinatorial Effects of Free and Nanoencapsulated Forms of Cabazitaxel and RAS-Selective Lethal 3 in Breast Cancer Cells. Pharmaceutics. 2025; 17(5):657. https://doi.org/10.3390/pharmaceutics17050657
Chicago/Turabian StyleValsalakumari, Remya, Marek Feith, Solveig Pettersen, Andreas K. O. Åslund, Ýrr Mørch, Tore Skotland, Kirsten Sandvig, Gunhild Mari Mælandsmo, and Tore-Geir Iversen. 2025. "Combinatorial Effects of Free and Nanoencapsulated Forms of Cabazitaxel and RAS-Selective Lethal 3 in Breast Cancer Cells" Pharmaceutics 17, no. 5: 657. https://doi.org/10.3390/pharmaceutics17050657
APA StyleValsalakumari, R., Feith, M., Pettersen, S., Åslund, A. K. O., Mørch, Ý., Skotland, T., Sandvig, K., Mælandsmo, G. M., & Iversen, T.-G. (2025). Combinatorial Effects of Free and Nanoencapsulated Forms of Cabazitaxel and RAS-Selective Lethal 3 in Breast Cancer Cells. Pharmaceutics, 17(5), 657. https://doi.org/10.3390/pharmaceutics17050657






