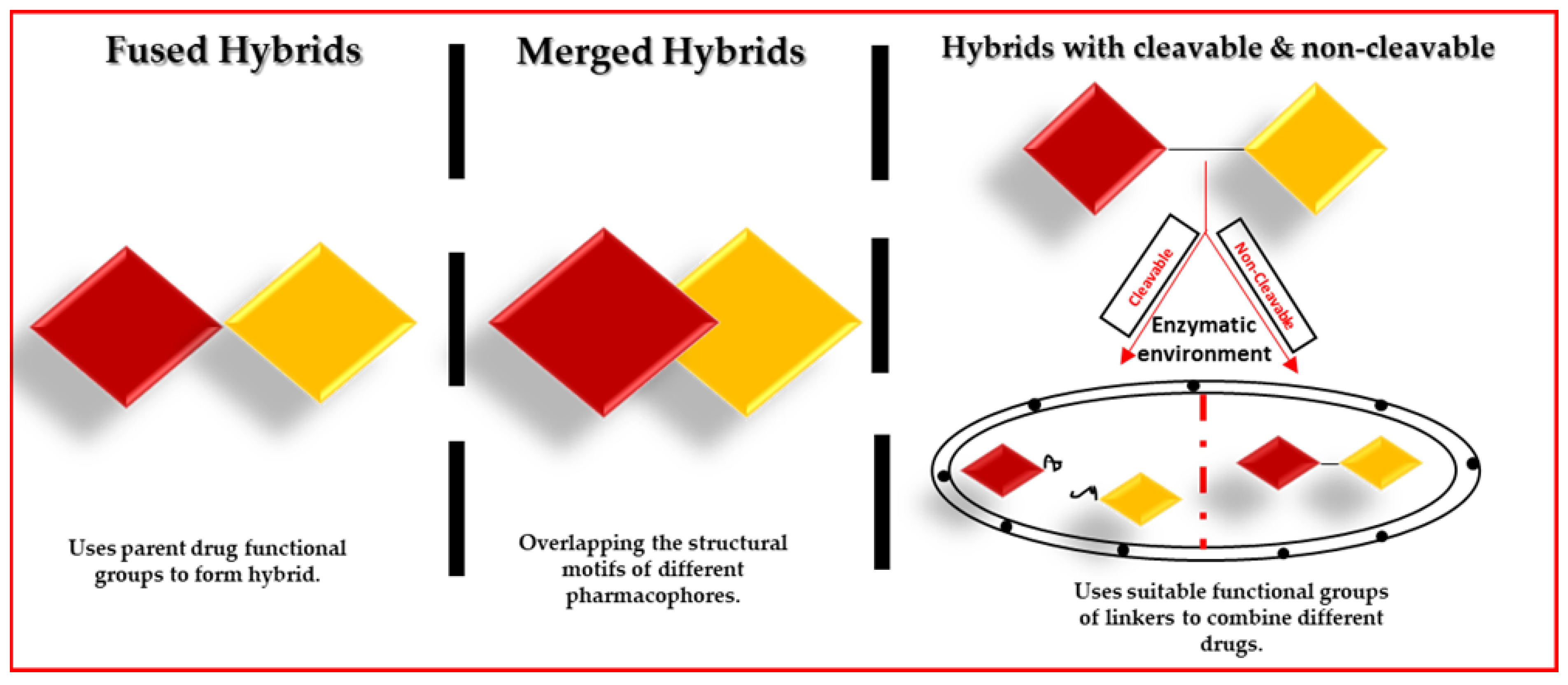
Ferrocene-Based Hybrid Drugs as Potential Anticancer and Antibacterial Therapeutic Agents for Incorporation into Nanocarriers: In Silico, In Vitro, Molecular Docking Evaluations
Abstract
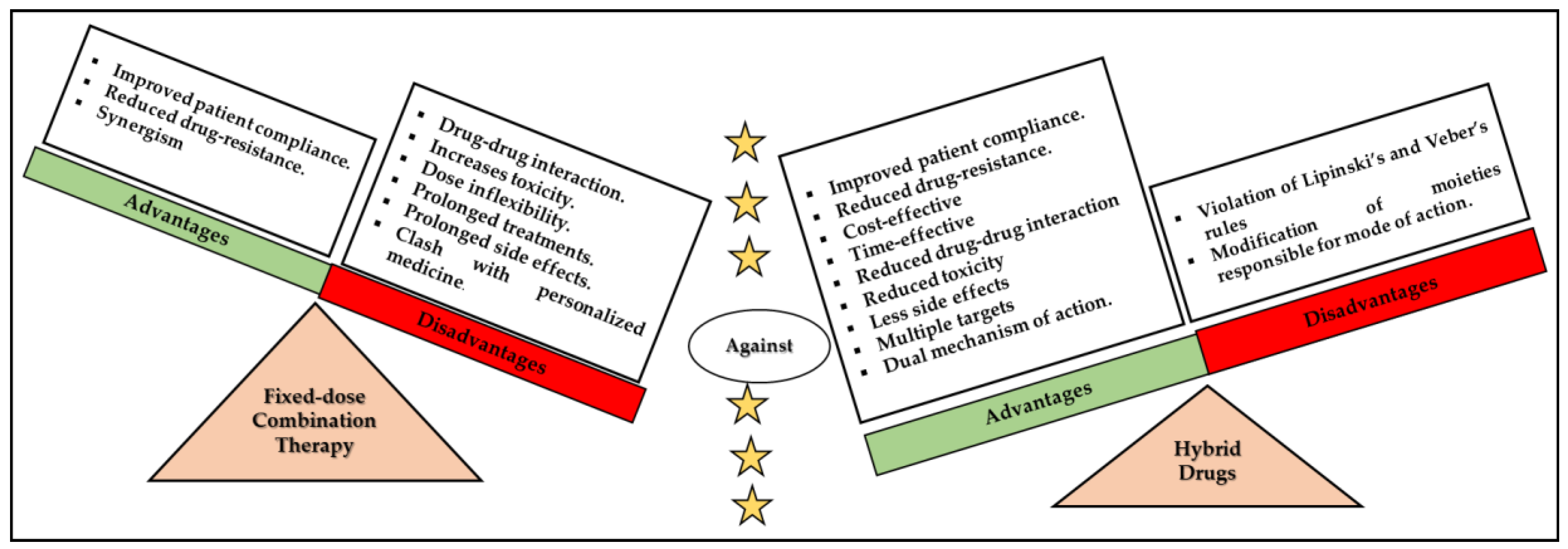
1. Introduction
2. Materials and Methods
2.1. Apparatus
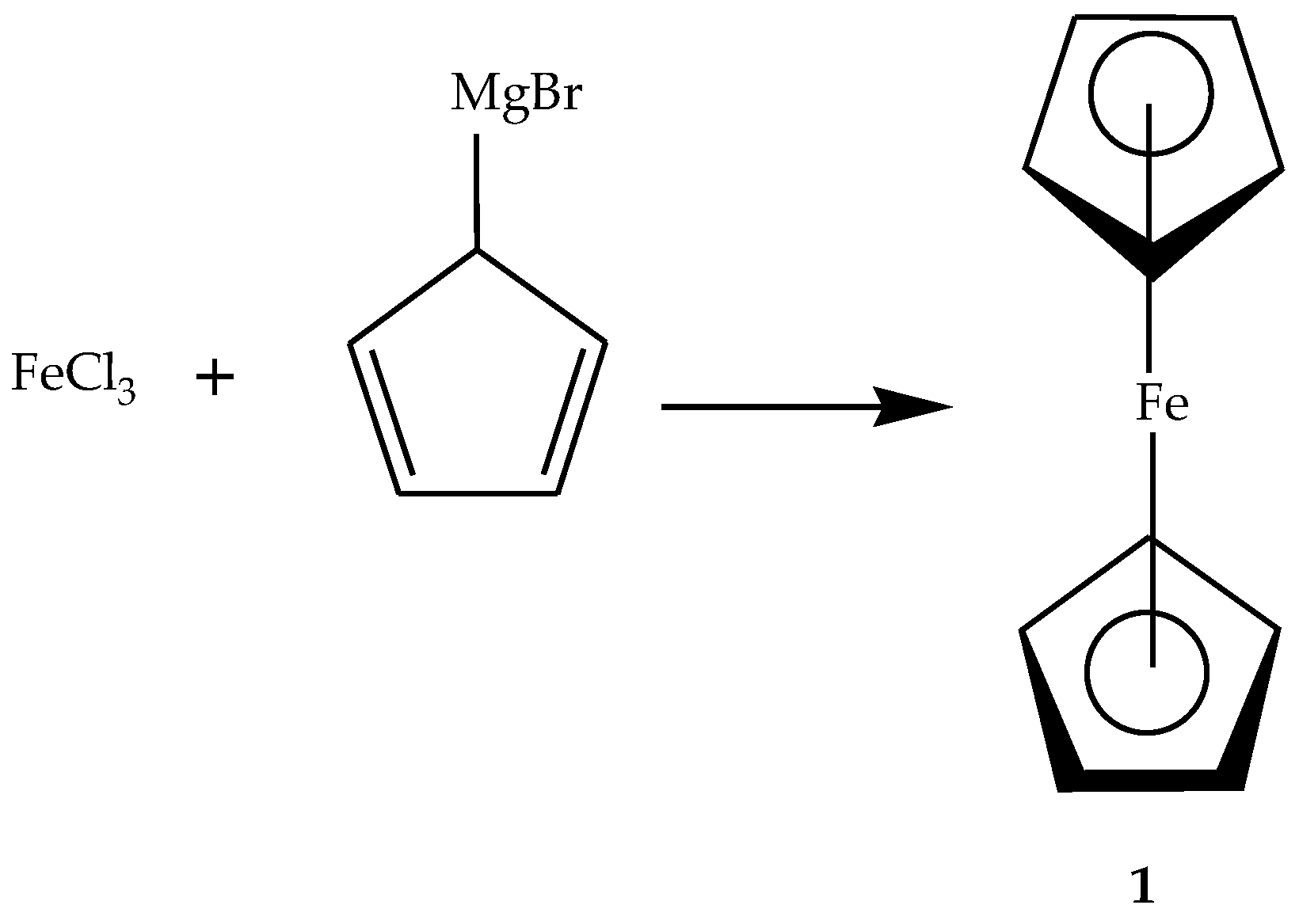
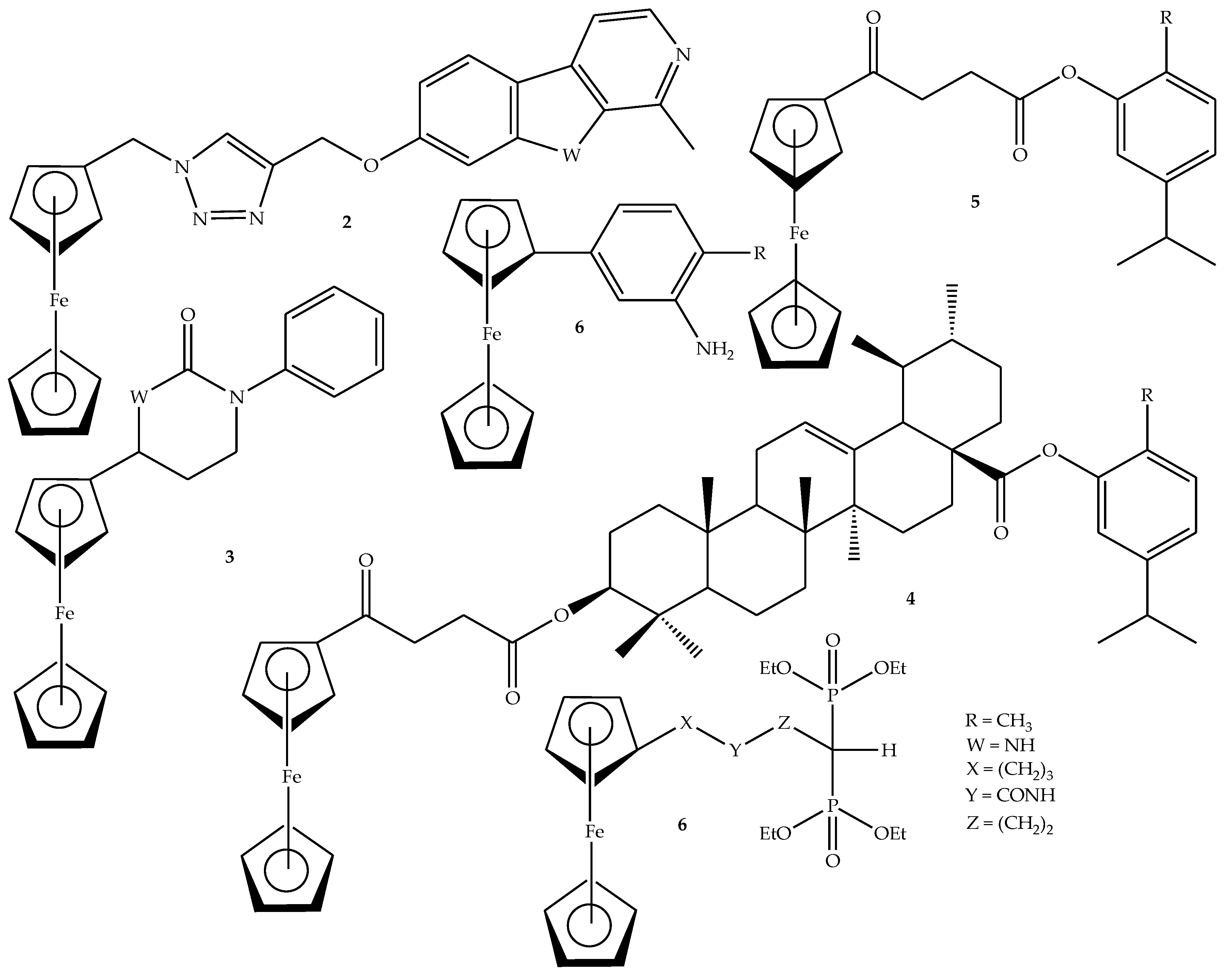
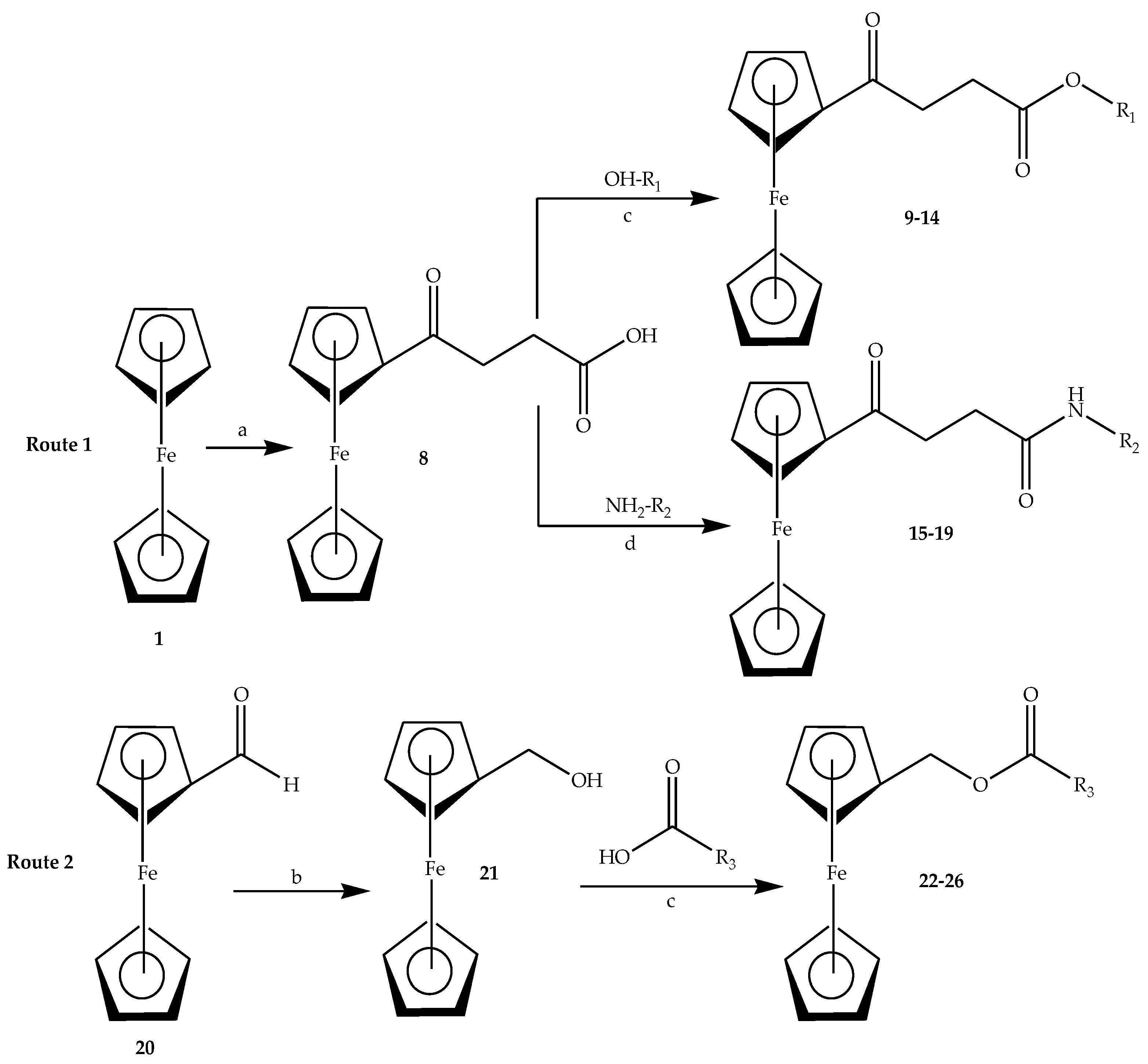
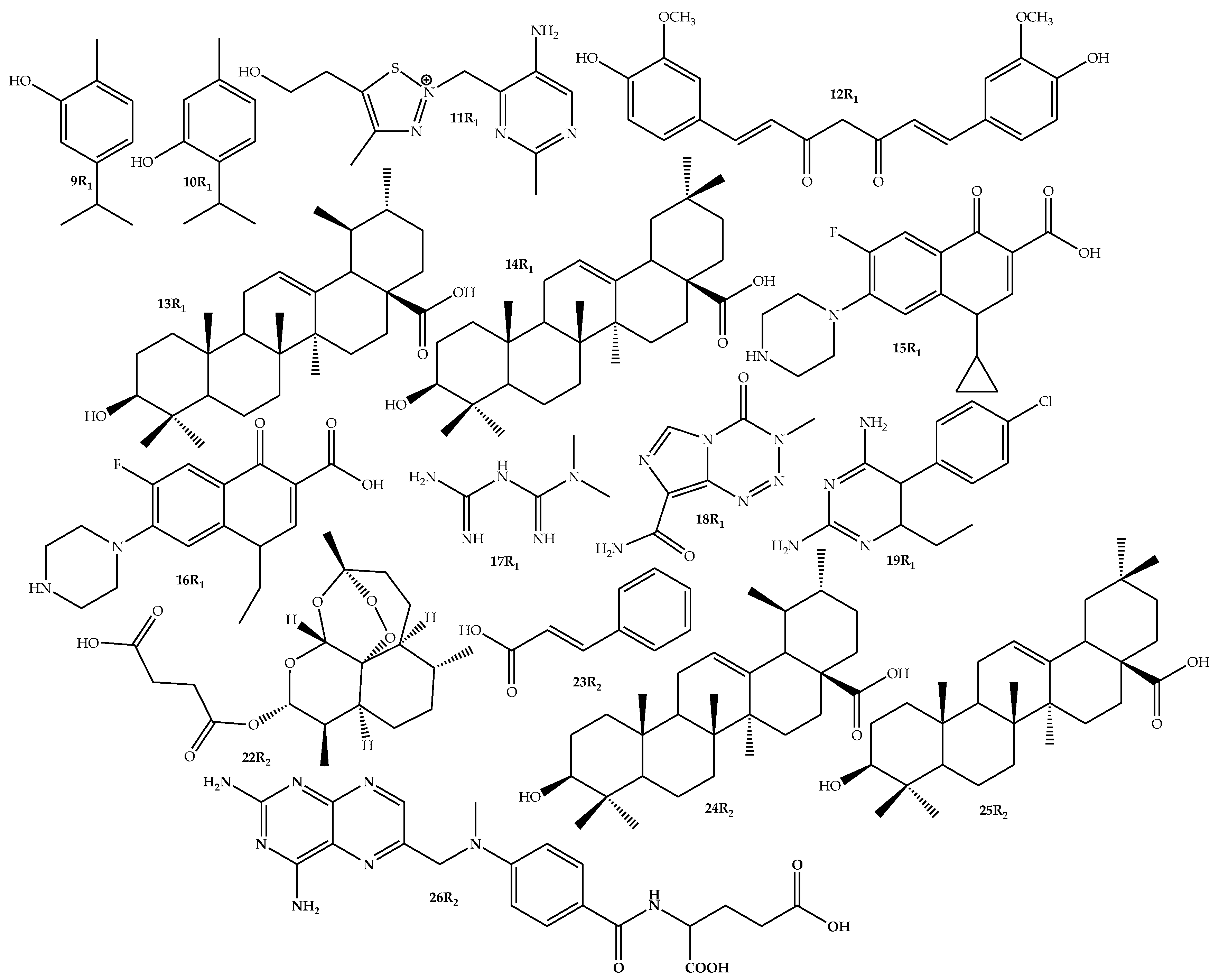
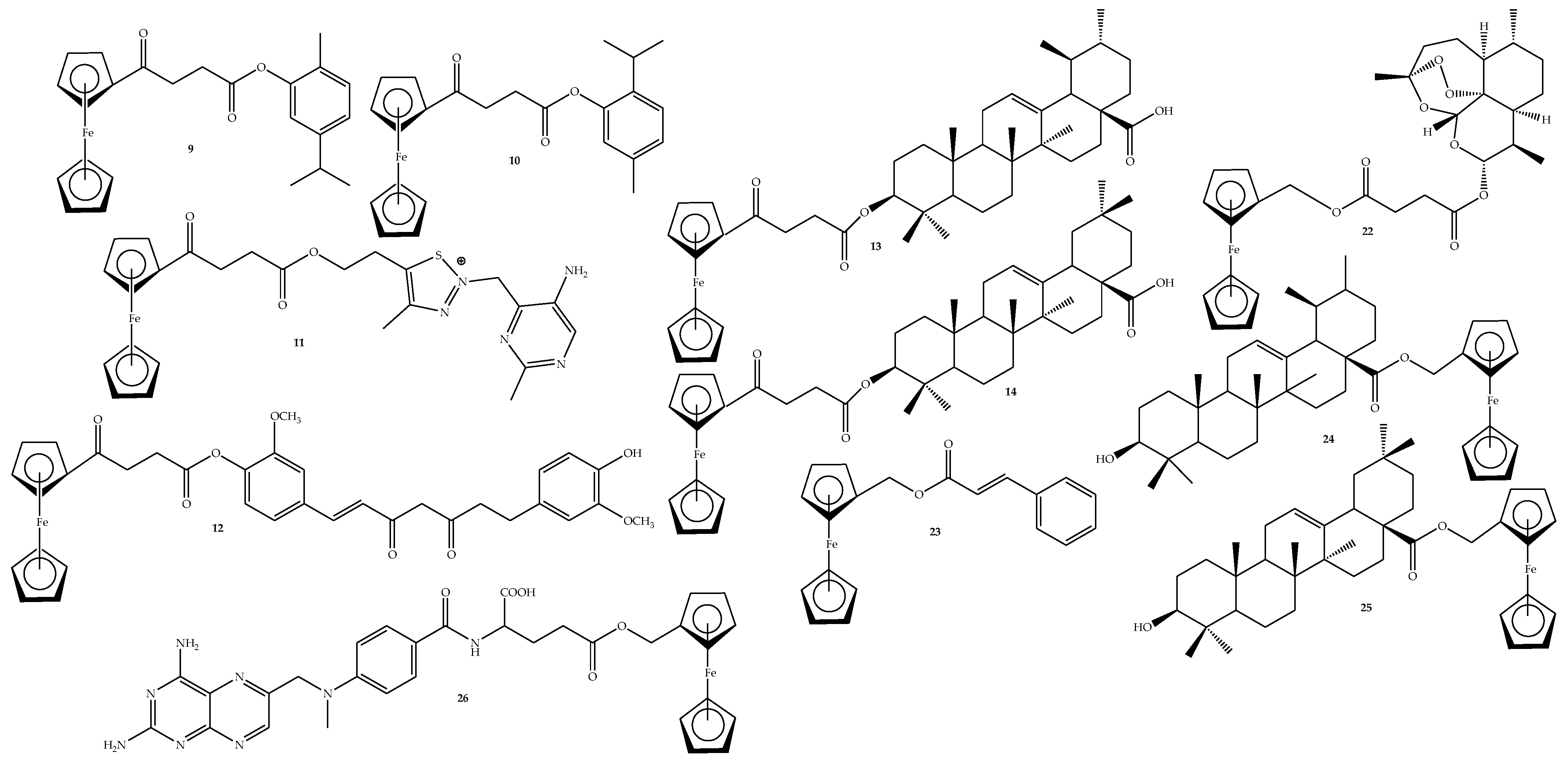
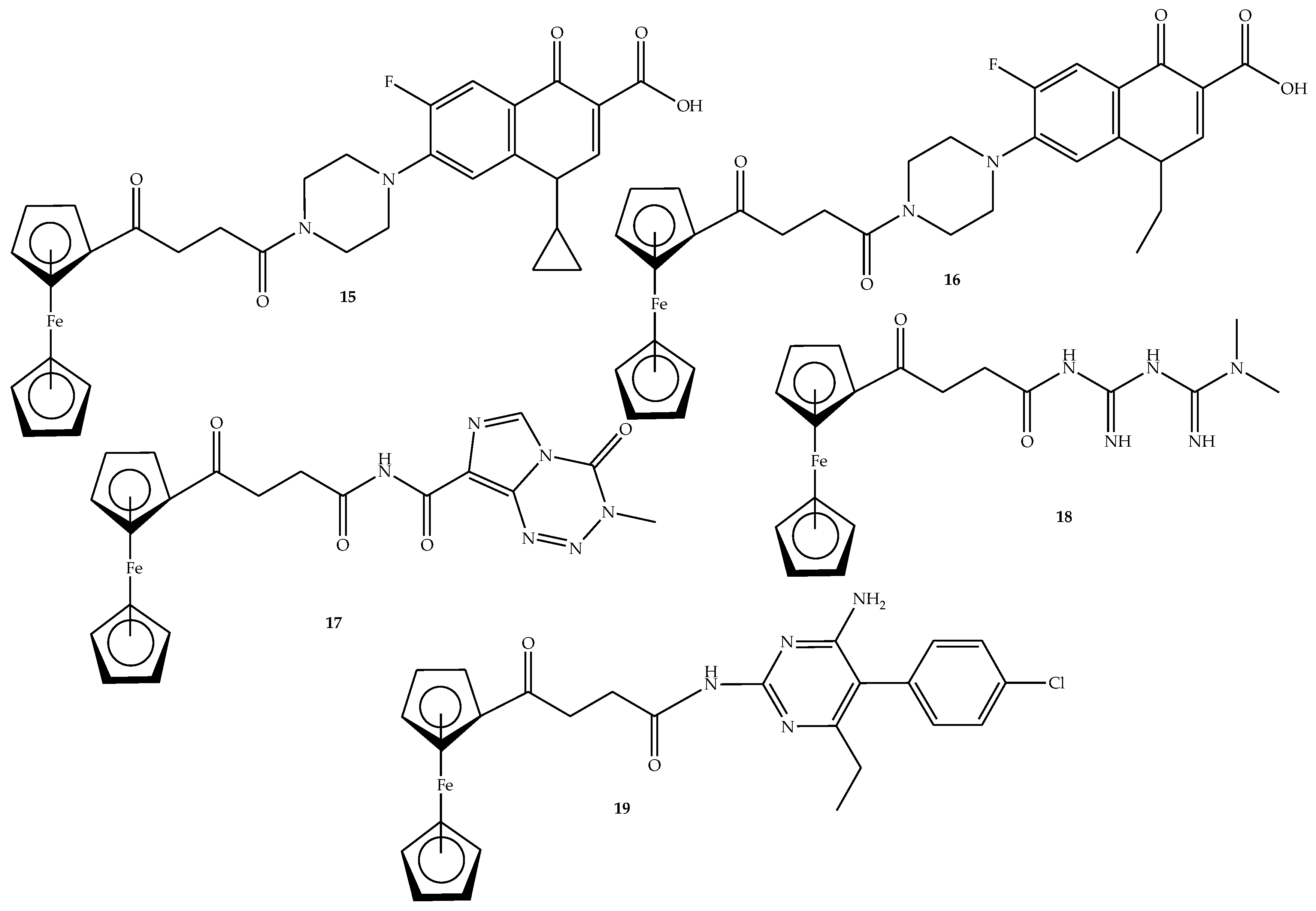
2.2. General Procedure for the Synthesis of Ferrocene-Based Hybrids, 9–26
2.3. General Synthesis of Amide-Linked Hybrids via Route 1 (15–19)
2.4. Biological Evaluation
2.4.1. In Vitro Antibacterial Evaluation
2.4.2. In Vitro Anticancer Evaluation
2.4.3. In Silico Studies
3. Results and Discussion
3.1. Characterization
3.2. In Vitro Antibacterial Activity
3.3. In Vitro Cytotoxicity Activity
3.4. Molecular Docking
3.5. Toxicity and Swiss ADME Predictions
4. Conclusions
4.1. Experimental Section
4.1.1. Compound 9
4.1.2. Compound 10
4.1.3. Compound 11
4.1.4. Compound 12
4.1.5. Compound 13
4.1.6. Compound 14
4.1.7. Compound 15
4.1.8. Compound 16
4.1.9. Compound 17
4.1.10. Compound 18
4.1.11. Compound 19
4.1.12. Compound 22
4.1.13. Compound 23
4.1.14. Compound 24
4.1.15. Compound 25
4.1.16. Compound 26
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rossi, R.; Ciofalo, M. An updated review on the synthesis and antibacterial activity of molecular hybrids and conjugates bearing imidazole moiety. Molecules 2020, 25, 5133. [Google Scholar] [CrossRef] [PubMed]
- Bizuayehu, H.M.; Dadi, A.F.; Hassen, T.A.; Ketema, D.B.; Ahmed, K.Y.; Kassa, Z.Y.; Amsalu, E.; Kibret, G.D.; Alemu, A.A.; Alebel, A.; et al. Global burden of 34 cancers among women in 2020 and projections to 2040: Population-based data from 185 countries/territories. Int. J. Cancer 2024, 154, 1377–1393. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Kitano, S.; Puah, G.R.Y.; Kittelmann, S.; Hwang, I.Y.; Chang, M.W. Microbiome and human health: Current understanding, engineering, and enabling technologies. Chem. Rev. 2023, 123, 31–72. [Google Scholar] [CrossRef] [PubMed]
- Barghady, N.; Chalkha, M.; Yamari, I.; Aflak, N.; Abchir, O.; Chebbac, K.; Nakkabi, A.; Chtita, S.; Chkirate, K.; Mague, J.T.; et al. Synthesis, characterization, mechanistic study, in-vitro and in-silico evaluation of antibacterial and antioxidant activities of novel pyrazole-pyrazoline hybrid systems. J. Mol. Struct. 2024, 1309, 138087. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, H.; Liu, M.; Tang, K.; Li, S.; Fang, Q.; Du, H.; Zhou, X.; Lin, X.; Yang, Y.; et al. Recent design strategies for boosting chemodynamic therapy of bacterial infections. Exploration 2023, 4, 20230087. [Google Scholar] [CrossRef]
- Mithuna, R.; Tharanyalakshmi, R.; Jain, I.; Singhal, S.; Sikarwar, D.; Das, S.; Ranjitha, J.; Ghosh, D.; Rahman, M.M.; Das, B. Emergence of antibiotic resistance due to the excessive use of antibiotics in medicines and feed additives: A global scenario with emphasis on the Indian perspective. Emerg. Contam. 2024, 10, 100389. [Google Scholar] [CrossRef]
- Shen, S.; Huang, Y.; Yuan, A.; Lv, F.; Liu, L.; Wang, S. Electrochemical regulation of antibacterial activity using ferrocene-containing antibiotics. CCS Chem. 2021, 3, 129–135. [Google Scholar] [CrossRef]
- Yang, X.; Li, C.; Wang, X.; Zheng, Z.; Sun, P.; Xu, C.; Chen, L.; Jiang, J.; Normark, S.; Normark, B.H.; et al. An update on the clinical pipelines of new antibacterial drugs developed in China. Engineering 2024, 38, 52–68. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, M.; Bhatt, S.; Saini, V.; Malik, A. Cancer causes and treatments. J. Int. Ilmu Farm. 2020, 11, 1–23. [Google Scholar]
- Yang, P.L.; Liu, L.X.; Li, E.M.; Xu, L.Y. Stat3, the Challenge for chemotherapeutic and radiotherapeutic efficacy. Cancers 2020, 12, 2459. [Google Scholar] [CrossRef]
- Gao, Q.; Feng, J.; Liu, W.; Wen, C.; Wu, Y.; Liao, Q.; Zou, L.; Sui, X.; Xie, T.; Zhang, J.; et al. Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Adv. Drug Deliv. Rev. 2022, 188, 114445. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Update 2023, 68, 100954. [Google Scholar] [CrossRef]
- Szumilak, M.; Wiktorowska-Owczarek, A.; Stanczak, A. Hybrid drugs-a strategy for overcoming anticancer drug resistance? Molecules 2021, 26, 2601. [Google Scholar] [CrossRef]
- Alkhzem, A.H.; Woodman, T.J.; Blagbrough, I.S. Design and synthesis of hybrid compounds as novel drugs and medicines. RSC Adv. 2022, 12, 19470–19484. [Google Scholar] [CrossRef]
- Peter, S.; Sotondoshe, N.; Aderibigbe, B.A. Carvacrol and thymol hybrids: Potential anticancer and antibacterial therapeutics. Molecules 2024, 29, 2277. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Paliwal, H.; Thareja, S.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.H.; et al. Concept of hybrid drugs and recent advancements in anticancer hybrids. Pharmaceuticals 2022, 15, 1071. [Google Scholar] [CrossRef]
- Wilkins, C.A.; Hamman, H.; Hamman, J.H.; Steenekamp, J.H. Fixed-dose combination formulations in solid oral drug therapy: Advantages, limitations, and design features. Pharmaceutics 2024, 16, 178. [Google Scholar] [CrossRef]
- Sampath Kumar, H.M.; Herrmann, L.; Tsogoeva, S.B. Structural hybridization as a facile approach to new drug candidates. Bioorg. Med. Chem. Lett. 2020, 30, 127514. [Google Scholar] [CrossRef]
- Duarte, D.; Rêma, A.; Amorim, I.; Vale, N. Drug combinations: A new strategy to extend drug repurposing and epithelial-mesenchymal transition in breast and colon cancer cells. Biomolecules 2022, 12, 190. [Google Scholar] [CrossRef]
- Sharma, A.; Rana, R.; Kumar, N.; gulati, H.K.; Jyoti; Khanna, A.; Sharma, S.; Pooja; Singh, J.V.; Bedi, P.M.S. Ferrocene-based compounds: Promising anticancer and antimalarial agents in modern therapeutics. Med. Chem. Res. 2025, 34, 1177–1199. [Google Scholar] [CrossRef]
- Marchesi, E.; Perrone, D.; Navacchia, M.L. Molecular Hybridization as a Strategy for Developing Artemisinin-Derived Anticancer Candidates. Pharmaceutics 2023, 15, 2185. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, J.; Radomska, D.; Czarnomysy, R.; Marciniec, K. Recent Development of Fluoroquinolone Derivatives as Anticancer Agents. Molecules 2024, 29, 3538. [Google Scholar] [CrossRef] [PubMed]
- Similie, D.; Minda, D.; Bora, L.; Kroškins, V.; Lugiņina, J.; Turks, M.; Dehelean, C.A.; Danciu, C. An Update on Pentacyclic Triterpenoids Ursolic and Oleanolic Acids and Related Derivatives as Anticancer Candidates. Antioxidants 2024, 13, 952. [Google Scholar] [CrossRef]
- Micale, N.; Molonia, M.S.; Citarella, A.; Cimino, F.; Saija, A.; Cristani, M.; Speciale, A. Natural Product-Based Hybrids as Potential Candidates for the Treatment of Cancer: Focus on Curcumin and Resveratrol. Molecules 2021, 26, 4665. [Google Scholar] [CrossRef]
- Leriche, G.; Chisholm, L.; Wagner, A. Cleavable linkers in chemical biology. Bioorg. Med. Chem. 2012, 20, 571–582. [Google Scholar] [CrossRef]
- Sheyi, R.; de la Torre, B.G.; Albericio, F. Linkers: An assurance for controlled delivery of antibody-drug conjugate. Pharmaceutics 2022, 14, 396. [Google Scholar] [CrossRef]
- Das, A.P.; Agarwal, S.M. Recent advances in the area of plant-based anticancer drug discovery using computational approaches. Mol. Divers. 2024, 28, 901–925. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Panda, S.R.; Meher, A.; Prusty, G.; Behera, S.; Prasad, B.R. Antibacterial properties and therapeutic potential of few medicinal plants: Current insights and challenges. Discov. Plants 2025, 2, 21. [Google Scholar] [CrossRef]
- Ali, S.S.; Al-Tohamy, R.; Al-Zahrani, M.; Badr, A.; Sun, J. Essential oils and plant-derived bioactive compounds: A comprehensive review of their therapeutic potential, mechanisms of action, and advances in extraction technologies. Phytochem. Rev. 2025, 1–49. [Google Scholar] [CrossRef]
- Cybulski, M.; Michalak, O.; Buchowicz, W.; Mazur, M. Ansa–ferrocene derivatives as potential therapeutics. Molecules 2024, 29, 4903. [Google Scholar] [CrossRef] [PubMed]
- Kaverin, M.V.; Telegina, L.N.; Rodionov, A.N.; Volodin, A.D.; Borisov, Y.A.; Kiselev, S.S.; Snegur, L.V. Ferrocene-based amides, amines and alcohols as a platform for the design and synthesis of redox-active hybrids: Synthesis, electrochemical and computational studies. J. Mol. Struct. 2024, 1312, 138584. [Google Scholar] [CrossRef]
- Poje, G.; Marinović, M.; Pavić, K.; Mioč, M.; Kralj, M.; de Carvalho, L.P.; Held, J.; Perković, I.; Rajić, Z. Harmicens, Novel harmine and ferrocene hybrids: Design, synthesis and biological activity. Int. J. Mol. Sci. 2022, 23, 9315. [Google Scholar] [CrossRef]
- Jančić, A.M.; Katanić Stanković, J.S.; Srećković, N.; Mihailović, V.; Komatina, D.I.; Stevanović, D. Ferrocene-containing tetrahydropyrimidin-2(1h)-ones: Antioxidant and antimicrobial activity. J. Organomet. Chem. 2022, 967, 122335. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Oselusi, S.O.; Morifi, E.; Nwamadi, M.; Tantoh Ndinteh, D.; Ramushu, P.; Matsebatlela, T.; Aderibigbe, B.A. Synthesis of ester-linked ursolic acid-based hybrid compounds: Potential antibacterial and anticancer agents. Chem. Biodivers. 2023, 20, e202300034. [Google Scholar] [CrossRef]
- Mbese, Z.; Nell, M.; Fonkui, Y.T.; Ndinteh, D.T.; Steenkamp, V.; Aderibigbe, B.A. Hybrid compounds containing carvacrol scaffold: In vitro antibacterial and cytotoxicity evaluation. Recent. Adv. Anti-Infect. Drug Discov. 2022, 17, 54–68. [Google Scholar] [CrossRef]
- Asghar, F.; Munir, S.; Fatima, S.; Murtaza, B.; Patujo, J.; Badshah, A.; Butler, I.; Taj, M.; Tahir, M. Ferrocene-functionalized anilines as potent anticancer and antidiabetic agents: Synthesis, spectroscopic elucidation, and DFT calculations. J. Mol. Struct. 2021, 1249, 131632. [Google Scholar] [CrossRef]
- Anusionwu, C.G.; Fonkui, T.Y.; Oselusi, S.O.; Egieyeh, S.A.; Aderibigbe, B.A.; Mbianda, X.Y. Ferrocene-bisphosphonates hybrid drug molecules: In vitro antibacterial and antifungal, in silico ADME, drug-likeness, and molecular docking studies. Results Chem. 2024, 7, 101278. [Google Scholar] [CrossRef]
- Rai, S.; Raj, U.; Tichkule, S.; Kumar, H.; Mishra, S.; Sharma, N. Recent trends in in-silico drug discovery. IJCB 2016, 5, 57–76. [Google Scholar] [CrossRef]
- Roney, M.; Mohd Aluwi, M.F.F. The Importance of in silico studies in drug discovery. Intell. Pharm. 2024, 2, 578–579. [Google Scholar] [CrossRef]
- Alkan, A.; Thomi, L.; Gleede, T.; Wurm, F.R. Vinyl Ferrocenyl glycidyl ether: An unprotected orthogonal ferrocene monomer for anionic and radical polymerization. Polym. Chem. 2015, 6, 3617–3624. [Google Scholar] [CrossRef]
- Peter, S.; Morifi, E.; Aderibigbe, B.A. Hybrid compounds containing a ferrocene scaffold as potential antimalarials. ChemistrySelect 2021, 6, 1756–1763. [Google Scholar] [CrossRef]
- Singh, A.; Lumb, I.; Mehra, V.; Kumar, V. Ferrocene-appended pharmacophores: An exciting approach for modulating the biological potential of organic scaffolds. Dalton Trans. 2019, 48, 2840–2860. [Google Scholar] [CrossRef]
- Fonkui, T.Y.; Ikhile, M.I.; Muganza, F.M.; Fotsing, M.C.D.; Arderne, C.; Siwe-Noundou, X.; Krause, R.W.M.; Ndinteh, D.T.; Njobeh, P.B. Synthesis, characterization and biological applications of novel schiff bases of 2-(trifluoromethoxy)aniline. J. Chin. Pharm. Sci. 2018, 27, 307–323. [Google Scholar]
- Waziri, I.; Wahab, O.O.; Mala, G.A.; Oselusi, S.O.; Egieyeh, E.; Nasir, H. Zinc(ii) complex of (z)-4-((4-nitrophenyl)amino)pent-3-en-2-one, a potential antimicrobial agent: Synthesis, characterization, antimicrobial screening, DFT calculation and docking study. Chem. Soc. Ethiop. 2023, 37, 633–651. [Google Scholar] [CrossRef]
- Rappie, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A., III; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Phys. Chem. 1992, 5806, 10024–10035. [Google Scholar] [CrossRef]
- Chen, T.; Shu, X.; Zhou, H.; Beckford, F.A.; Misir, M. Algorithm selection for protein–ligand docking: Strategies and analysis on ACE. Sci. Rep. 2023, 13, 8219. [Google Scholar] [CrossRef]
- Jadhav, J.; Das, R.; Kamble, S.; Chowdhury, M.G.; Kapoor, S.; Gupta, A.; Vyas, H.; Shard, A. Ferrocene-based modulators of cancer-associated tumor pyruvate kinase M2. J. Organomet. Chem. 2022, 968–969, 122338. [Google Scholar] [CrossRef]
- Anusionwu, C.G.; Aderibigbe, B.A.; Adeyemi, S.A.; Ubanako, P.; Oselusi, S.O.; Choonara, Y.E.; Mbianda, Y.X. Novel ferrocenylbisphosphonate hybrid compounds: Synthesis, characterization and potent activity against cancer cell lines. Bioorg. Med. Chem. 2022, 58, 116652. [Google Scholar] [CrossRef]
- Türkyılmaz, O.; Darcan, C. Resistance mechanism of Escherichia coli strains with different ampicillin resistance levels. Appl. Microbiol. Biotechnol. 2024, 108, 5. [Google Scholar] [CrossRef] [PubMed]
- Maturano, D.; Omar, C.; López, O.; María, F.; De López, C. Removal of ampicillin with nitrifying cultures in a SBR reactor. Appl. Biochem. Biotechnol. 2025, 197, 2624–2638. [Google Scholar] [CrossRef] [PubMed]
- Bonten, M.; Johnson, J.R.; Van Den Biggelaar, A.H.J.; Georgalis, L.; Geurtsen, J.; De Palacios, P.I.; Gravenstein, S.; Verstraeten, T.; Hermans, P.; Poolman, J.T. Epidemiology of Escherichia coli bacteremia: A systematic literature review. Clin. Infect. Dis. 2021, 72, 1211–1219. [Google Scholar] [CrossRef]
- Yan, J.; Yue, K.; Fan, X.; Xu, X.; Wang, J.; Qin, M.; Zhang, Q.; Hou, X.; Li, X.; Wang, Y. Synthesis and bioactivity evaluation of ferrocene-based hydroxamic acids as selective histone deacetylase 6 inhibitors. Eur. J. Med. Chem. 2023, 246, 115004. [Google Scholar] [CrossRef]
- Nagaraju, P.; Reddy, P.N.; Padmaja, P.; Ugale, V.G. Synthesis, antiproliferative activity and molecular docking studies of novel benzo[a]pyrano-[2,3-c]phenazine derivatives. Chem. Data Collect. 2020, 30, 100541. [Google Scholar] [CrossRef]
- Skoupilova, H.; Rak, V.; Pinkas, J.; Karban, J.; Hrstka, R. The cytotoxic effect of newly synthesized ferrocenes against cervical carcinoma cells alone and in combination with radiotherapy. Appl. Sci. 2020, 10, 3728. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights into protein–ligand interactions: Mechanisms, models, and methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Li, S.; Yang, T.; Liu, L.; Zhao, J.; Liu, H. Prediction of HERG potassium channel blockage using ensemble learning methods and molecular fingerprints. Toxicol. Lett. 2020, 332, 88–96. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Mbese, Z.; Choene, M.; Morifi, E.; Nwamadi, M.; Adeyemi, S.; Kolawole, A.; Adeyinka, A.S.; George, B.; Aderibigbe, B.A. Hybrid molecules containing methotrexate, vitamin D, and platinum derivatives: Synthesis, characterization, in vitro cytotoxicity, in silico ADME docking, molecular docking and dynamics. Chem. Biodivers. 2024, 22, e202400373. [Google Scholar] [CrossRef]
- Asiri, A.M.; Ali, A.; Al, A.; Abu-alghayth, M.H. Targeting breast cancer with dasatinib derivatives: A multi-parameter strategy to uncover potent lead compounds. Int. J. Pharm. Investig. 2024, 14, 1260–1272. [Google Scholar] [CrossRef]
- Kralj, S.; Jukič, M.; Bren, U. Molecular filters in medicinal chemistry. Encyclopedia 2023, 3, 501–511. [Google Scholar] [CrossRef]
- Oselusi, S.O.; Sibuyi, N.R.S.; Meyer, M.; Madiehe, A.M. Ehretia species phytoconstituents as potential lead compounds against Klebsiella pneumoniae carbapenemase: A computational approach. Biomed. Res. Int. 2023, 2023, 8022356. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or evade multidrug resistance P-glycoprotein in cancer treatment. J. Med. Chem. B 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Abuhelwa, A.Y.; Williams, D.B.; Upton, R.N.; Foster, D.J.R. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharm. Biopharm. 2017, 112, 234–248. [Google Scholar] [CrossRef]
- Bashiardes, S.; Christodoulou, C. Orally administered drugs and their complicated relationship with our gastrointestinal tract. Microorganisms 2024, 12, 242. [Google Scholar] [CrossRef]
- He, Y.; Zheng, Y.; Zhu, C.; Lei, P.; Yu, J.; Tang, C.; Chen, H.; Diao, X. Radioactive ADME demonstrates ARV-110’s high druggability despite low oral bioavailability. J. Med. Chem. 2024, 67, 14277–14291. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood-Brain Barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 7, 69. [Google Scholar] [CrossRef]
- Archie, S.R.; Al Shoyaib, A.; Cucullo, L. Blood-Brain Barrier dysfunction in CNS disorders and putative therapeutic targets: An overview. Pharmaceutics 2021, 13, 1779. [Google Scholar] [CrossRef]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The bioavailability of drugs-the current state of knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef]
- Oomariyah, N.; van Dijk, G. The bioavailability prediction and screening of phytochemicals Sansevieria Trifasciata leaves extract. MATEC Web Conf. 2022, 372, 02003. [Google Scholar] [CrossRef]
- Riyadi, P.H.; Sari, I.D.; Kurniasih, R.A.; Agustini, T.W.; Swastawati, F.; Herawati, V.E.; Tanod, W.A. SwissADME predictions of pharmacokinetics and drug-likeness properties of small molecules present in Spirulina platensis. IOP Conf. Ser. Earth Environ. Sci. 2021, 890, 012021. [Google Scholar] [CrossRef]


| Type of the Linker | Examples | Advantages | Disadvantages |
|---|---|---|---|
| Cleavable |
|
|
|
| Non-cleavable |
|
|
|
| Hybrids | Minimum Inhibitory Concentration (MIC, µg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-Positive | Gram-Negative | |||||||||||
| BS | EF | SE | SA | MS | EC | PV | KO | PA | PM | E. coli | KP | |
| 8 | 15.625 | 31.25 | 250 | 15.625 | 15.625 | 62.5 | 15.625 | 125 | 15.625 | 15.625 | 15.625 | 250 |
| 9 | 15.625 | 62.5 | 250 | 125 | 31.25 | 31.25 | 15.625 | 125 | 31.25 | 15.625 | 15.625 | 250 |
| 10 | 15.625 | 15.625 | 250 | 31.25 | 15.625 | 15.625 | 15.625 | 31.25 | 15.625 | 15.625 | 15.625 | 250 |
| 11 | 15.625 | 15.625 | 250 | 31.5 | 62.5 | 62.5 | 15.625 | 125 | 15.625 | 15.625 | 15.625 | 250 |
| 12 | 125 | 250 | 250 | 15.625 | 62.5 | 15.625 | 15.625 | 125 | 125 | 125 | 15.625 | 250 |
| 13 | 250 | 125 | 250 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 15.625 | 250 |
| 14 | 250 | 250 | 250 | 15.625 | 15.625 | 15.625 | 250 | 62.5 | 250 | 250 | 250 | 250 |
| 15 | 15.625 | 15.625 | 250 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 250 |
| 16 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 125 |
| 17 | 15.625 | 15.625 | 250 | 15.625 | 62.5 | 15.625 | 125 | 31.25 | 15.625 | 15.625 | 15.625 | 250 |
| 18 | 15.625 | 15.625 | 250 | 15.625 | 31.25 | 15.625 | 15.625 | 125 | 62.5 | 15.625 | 15.625 | 250 |
| 19 | 15.625 | 15.625 | 62.5 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 15.625 | 250 |
| 22 | 7.8125 | 7.8125 | 250 | 500 | 250 | 7.8125 | 7.8125 | 7.8125 | 7.8125 | 250 | 250 | 250 |
| 23 | 7.8125 | 7.8125 | 250 | 500 | 250 | 7.8125 | 7.8125 | 7.8125 | 7.8125 | 250 | 250 | 250 |
| 24 | 7.8125 | 7.8125 | 250 | 7.8125 | 250 | 7.8125 | 7.8125 | 7.8125 | 7.8125 | 31.25 | 250 | 250 |
| 25 | 7.8125 | 7.8125 | 500 | 125 | 250 | 7.8125 | 7.8125 | 7.8125 | 7.8125 | 125 | 250 | 250 |
| 26 | 7.8125 | 7.8125 | 500 | 500 | 250 | 7.8125 | 7.8125 | 7.8125 | 7.8125 | 250 | 250 | 250 |
| Ampicillin | 26 | 26 | 26 | 26 | 26 | 26 | 416 | 26 | 64 | 26 | 26 | 26 |
| Compounds | IC50 Values (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HeLa Cancer Cells | CHO Cells | Vero Normal Cells | |||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| 8 | >100 | >100 | >100 | NT | NT | NT | NT | NT | NT |
| POA | 55.78 | 0.0243 | 5.9360 | NT | NT | NT | NT | NT | NT |
| 9 | >100 | >100 | >100 | NT | NT | NT | NT | NT | NT |
| 10 | 42.42 | 44.94 | 45.37 | 73.37 | 50.64 | 54.61 | 86.22 | 83.76 | 85.70 |
| 11 | >100 | >100 | >100 | NT | NT | NT | NT | NT | NT |
| 12 | >100 | >100 | >100 | NT | NT | NT | NT | NT | NT |
| 13 | >100 | >100 | >100 | 94.00 | 68.63 | 64.63 | >100 | >100 | >100 |
| 14 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 15 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 16 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 17 | >100 | >100 | >100 | NT | NT | NT | NT | NT | NT |
| 18 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 19 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 22 | >100 | >100 | >100 | NT | NT | NT | NT | NT | NT |
| 23 | >100 | >100 | >100 | NT | NT | NT | NT | NT | NT |
| 24 | 68.49 | 75.52 | 83.97 | >100 | >100 | >100 | >100 | >100 | >100 |
| 25 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 26 | >100 | >100 | >100 | NT | NT | NT | NT | NT | NT |
| Hybrids | Docking Score (kcal/mol) | Hydrogen Bond | Hydrophobic (Alkyl and pi-Sigma Bonds) |
|---|---|---|---|
| 3eqm_10 | −8.7 | Cys437, Ala438 | Ile132, Ile133, Phe148, Ala306, Val370, Val373. |
| 3eqm_24 | −8.8 | Cys437, Ala438, Gly439 | Ile132, Ile133, Phe148, Met303, Ala306, Ala438 |
| 4qtb_10 | −7.3 | Thr223, Asn316 | Ala191, Lys224, Pro315 |
| 4qtb_24 | −9.5 | Ser301 | Leu261 Lys317 |
| 2w3l_10 | −7.4 | None | Phe63, Tyr67, Phe71 Leu96, Ala108 |
| 2w3l_24 | −9.3 | Glu73, Leu96 | Tyr67, Leu96, Ala108 |
| 3poz_10 | −9.2 | Thr854 | Leu718, Val726, Ala743, Lys745, Cys755, Met766, Leu777, Leu788, Leu792, Leu844, Phe856 |
| 3poz_24 | −11.0 | None | Lys745, Met766, Leu777, Leu788, Phe856 |
| Hybrids | Binary Prediction | Confiability% | AD | ||
|---|---|---|---|---|---|
| Blocker | Non-Blocker | Inside | Outside | ||
| 9 | X | 80.31 | X | ||
| 10 | X | 60.60 | X | ||
| 11 | X | 51.09 | X | ||
| 12 | X | 89.09 | X | ||
| 13 | X | 71.87 | X | ||
| 14 | X | 54.00 | X | ||
| 15 | X | 75.02 | X | ||
| 16 | X | 61.20 | X | ||
| 17 | X | 50.36 | X | ||
| 18 | X | 78.65 | X | ||
| 19 | X | 80.20 | X | ||
| 22 | X | 81.80 | X | ||
| 23 | X | 98.30 | X | ||
| 24 | X | 87.33 | X | ||
| 25 | X | 86.97 | X | ||
| 26 | X | 59.39 | X | ||
| Hybrids | Molecular Weight (g/mol) | Bioavailability Score | TPSA (Å2) |
|---|---|---|---|
| 9 | 418.1231 | 0.55 | 43.37 |
| 10 | 418.1231 | 0.55 | 43.37 |
| 11 | 534.1257 | 0.55 | 140.18 |
| 12 | 636.1603 | 0.55 | 116.20 |
| 13 | 724.3790 | 0.56 | 80.67 |
| 14 | 724.3790 | 0.56 | 80.67 |
| 15 | 598.1566 | 0.56 | 94.99 |
| 16 | 586.1566 | 0.56 | 94.99 |
| 17 | 462.0739 | 0.55 | 128.32 |
| 18 | 398.1235 | 0.55 | 109.14 |
| 19 | 516.1015 | 0.55 | 97.97 |
| 22 | 582.1916 | 0.55 | 89.52 |
| 23 | 346.0656 | 0.55 | 26.30 |
| 24 | 654.3735 | 0.17 | 45.43 |
| 25 | 654.3735 | 0.17 | 46.53 |
| 26 | 652.1845 | 0.11 | 199.54 |
| Hybrids | Classification | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ Toxicity | Toxicity Endpoints | |||||||||||
| Hepatoxicity | Cardiotoxicity | Carcinogenicity | Immunotoxicity | Mutagenicity | Cytotoxicity | |||||||
| Prediction | Probability | Prediction | Probability | Prediction | Probability | Prediction | Probability | Prediction | Probability | Prediction | Probability | |
| 9 | Active | 0.59 | Inactive | 0.63 | Inactive | 0.69 | Inactive | 0.88 | Inactive | 0.77 | Inactive | 0.65 |
| 10 | Active | 0.59 | Inactive | 0.63 | Inactive | 0.69 | Inactive | 0.98 | Inactive | 0.77 | Inactive | 0.65 |
| 11 | Inactive | 0.61 | Inactive | 0.66 | Inactive | 0.50 | Inactive | 0.97 | Inactive | 0.51 | Inactive | 0.60 |
| 12 | Active | 0.50 | Inactive | 0.53 | Inactive | 0.68 | Active | 0.98 | Inactive | 0.80 | Inactive | 0.75 |
| 13 | Inactive | 0.70 | Active | 0.74 | Inactive | 0.58 | Active | 0.99 | Inactive | 0.95 | Inactive | 0.61 |
| 14 | Inactive | 0.70 | Active | 0.74 | Inactive | 0.58 | Active | 0.99 | Inactive | 0.95 | Inactive | 0.61 |
| 15 | Inactive | 0.70 | Inactive | 0.77 | Inactive | 0.58 | Active | 0.70 | Inactive | 0.60 | Inactive | 0.56 |
| 16 | Inactive | 0.71 | Inactive | 0.77 | Inactive | 0.59 | Active | 0.78 | Inactive | 0.64 | Inactive | 0.55 |
| 17 | Inactive | 0.50 | Inactive | 0.85 | Inactive | 0.53 | Inactive | 0.99 | Inactive | 0.53 | Inactive | 0.73 |
| 18 | Inactive | 0.69 | Inactive | 0.58 | Inactive | 0.60 | Inactive | 0.98 | Inactive | 0.66 | Inactive | 0.67 |
| 19 | Inactive | 0.57 | Inactive | 0.77 | Inactive | 0.61 | Inactive | 0.98 | Inactive | 0.66 | Inactive | 0.74 |
| 22 | Inactive | 0.83 | Active | 0.91 | Inactive | 0.66 | Active | 0.98 | Inactive | 0.69 | Inactive | 0.63 |
| 23 | Inactive | 0.60 | Inactive | 0.73 | Inactive | 0.61 | Inactive | 0.88 | Inactive | 0.66 | Inactive | 0.82 |
| 24 | Inactive | 0.65 | Active | 0.82 | Inactive | 0.57 | Active | 0.99 | Inactive | 0.91 | Inactive | 0.92 |
| 25 | Inactive | 0.65 | Active | 0.82 | Inactive | 0.57 | Active | 0.99 | Inactive | 0.91 | Inactive | 0.92 |
| 26 | Inactive | 0.59 | Inactive | 0.59 | Inactive | 0.51 | Inactive | 0.97 | Inactive | 0.79 | Inactive | 0.66 |
| Hybrids | Rat IP | Rat IV | Rat Oral | Rat SC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LD50 (mg/kg) | Toxicity Class | LD50 (mg/kg) | Toxicity Class | LD50 (mg/kg) | Toxicity Class | LD50 (mg/kg) | Toxicity Class | |||||||||
| Class | In AD | Out of AD | Class | In AD | Out of AD | Class | In AD | Out of AD | Class | In AD | Out of AD | |||||
| 9 | 150,200 | 4 | X | 25,770 | 3 | X | 673,100 | 4 | X | 381,500 | 4 | X | ||||
| 10 | 277,900 | 4 | X | 23,740 | 3 | X | 808,800 | 4 | X | 569,600 | 4 | X | ||||
| 11 | 745,000 | 5 | X | 111,700 | 4 | X | 1,085,000 | 4 | X | 195,500 | 4 | X | ||||
| 12 | 283,600 | 4 | X | 53,990 | 4 | X | 197,100 | 3 | X | 134,800 | 3 | X | ||||
| 13 | 1,013,000 | 5 | X | 11,900 | 3 | X | 1,981,000 | 4 | X | 57,810 | 3 | X | ||||
| 14 | 1,032,000 | 5 | X | 10,540 | 3 | X | 967,200 | 4 | X | 47,510 | 3 | X | ||||
| 15 | 524,600 | 5 | X | 51,810 | 4 | X | 627,800 | 4 | X | 82,050 | 3 | X | ||||
| 16 | 608,900 | 5 | X | 47,190 | 4 | X | 423,000 | 3 | X | 80,010 | 3 | X | ||||
| 17 | 523,000 | 5 | X | 178,900 | 4 | X | 497,400 | 3 | X | 84,390 | 3 | X | ||||
| 18 | 460,300 | 4 | X | 81,900 | 4 | X | 664,900 | 4 | X | 480,000 | 4 | X | ||||
| 19 | 416,700 | 4 | X | 60,120 | 4 | X | 983,600 | 4 | X | 410,000 | 4 | X | ||||
| 22 | 730,200 | 5 | X | 17,410 | 3 | X | 1,360,000 | 4 | X | 547,100 | 4 | X | ||||
| 23 | 371,000 | 4 | X | 39,230 | 3 | X | 1,503,000 | 4 | X | 639,700 | 4 | X | ||||
| 24 | 741,400 | 5 | X | 8015 | 3 | X | 862,400 | 4 | X | 34,730 | 3 | X | ||||
| 25 | 925,200 | 5 | X | 6589 | 2 | X | 608,600 | 4 | X | 39,920 | 3 | X | ||||
| 26 | 859,700 | 5 | X | 239,800 | 4 | X | 3,421,000 | 5 | X | 496,200 | 4 | X | ||||
| Hybrid | Lipinski | Ghose | Veber | Egan | Muegge |
|---|---|---|---|---|---|
| 9 | Yes; 1 violation: MLOGP > 4.15 | No; 1 violation: WLOGP > 5.6 | Yes | Yes | No; 1 violation: XLOGP3 > 5 |
| 10 | Yes; 1 violation: MLOGP > 4.15 | No; 1 violation: WLOGP > 5.6 | Yes | Yes | No; 1 violation: XLOGP3 > 5 |
| 11 | Yes; 1 violation: MW > 500 | No; 2 violations: MW > 480, MR > 130 | No; 2 violations: Rotors > 10, TPSA > 140 | No; 1 violation: TPSA > 131.6 | Yes |
| 12 | Yes; 1 violation: MW > 500 | No; 4 violations: MW > 480, WLOGP > 5.6, MR > 130, #atoms > 70 | No; 1 violation: Rotors > 10 | No; 1 violation: WLOGP > 5.88 | No; 3 violations: MW > 600, XLOGP3 > 5, Rotors > 15 |
| 13 | No; 2 violations: MW > 500, MLOGP > 4.15 | No; 4 violations: MW > 480, WLOGP > 5.6, MR > 130, #atoms > 70 | Yes | No; 1 violation: WLOGP > 5.88 | No; 2 violations: MW > 600, XLOGP3 > 5 |
| 14 | No; 2 violations: MW > 500, MLOGP > 4.15 | No; 4 violations: MW > 480, WLOGP > 5.6, MR > 130, #atoms > 70 | Yes | No; 1 violation: WLOGP > 5.88 | No; 2 violations: MW > 600, XLOGP3 > 5 |
| 15 | Yes; 1 violation: MW > 500 | No; 3 violations: MW > 480, MR > 130, #atoms > 70 | Yes | Yes | No; 1 violation: XLOGP3 > 5 |
| 16 | Yes; 1 violation: MW > 500 | No; 3 violations: MW > 480, MR > 130, #atoms > 70 | Yes | Yes | No; 1 violation: XLOGP3 > 5 |
| 17 | Yes; 0 violation | Yes | Yes | Yes | Yes |
| 18 | Yes; 0 violation | Yes | No; 1 violation: Rotors > 10 | Yes | Yes |
| 19 | Yes; 1 violation: MW > 500 | No; 2 violations: MW > 480, MR > 130 | Yes | Yes | Yes |
| 22 | Yes; 1 violation: MW > 500 | No; 3 violations: MW > 480, MR > 130, #atoms > 70 | Yes | Yes | No; 1 violation: XLOGP3 > 5 |
| 23 | Yes; 0 violation | Yes | Yes | Yes | Yes |
| 24 | No; 2 violations: MW > 500, MLOGP > 4.15 | No; 4 violations: MW > 480, WLOGP > 5.6, MR > 130, #atoms > 70 | Yes | No; 1 violation: WLOGP > 5.88 | No; 2 violations: MW > 600, XLOGP3 > 5 |
| 25 | No; 2 violations: MW > 500, MLOGP > 4.15 | No; 4 violations: MW > 480, WLOGP > 5.6, MR > 130, #atoms > 70 | Yes | No; 1 violation: WLOGP > 5.88 | No; 2 violations: MW > 600, XLOGP3 > 5 |
| 26 | No; 2 violations: MW > 500, NorO > 10 | No; 3 violations: MW > 480, MR > 130, #atoms > 70 | No; 2 violations: Rotors > 10, TPSA > 140 | No; 1 violation: TPSA > 131.6 | No; 2 violations: MW > 600, TPSA > 150 |
| Hybrids | GI Absorption | BBB Permeant | P-gp Substrate | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor | Log Kp (cm/s) |
|---|---|---|---|---|---|---|---|---|---|
| 9 | High | Yes | Yes | No | Yes | Yes | No | Yes | −5.07 |
| 10 | High | Yes | Yes | No | Yes | Yes | No | Yes | −5.07 |
| 11 | Low | No | Yes | No | Yes | Yes | No | No | −6.67 |
| 12 | Low | No | Yes | No | No | Yes | No | No | −6.46 |
| 13 | Low | No | Yes | No | No | No | No | No | −3.57 |
| 14 | Low | No | Yes | No | No | No | No | No | −3.47 |
| 15 | High | No | Yes | No | No | Yes | No | Yes | −6.35 |
| 16 | High | No | Yes | No | No | Yes | No | Yes | −6.31 |
| 17 | High | No | No | No | No | No | No | No | −7.72 |
| 18 | High | No | No | No | No | No | No | No | −7.43 |
| 19 | High | No | Yes | Yes | No | Yes | No | Yes | −6.19 |
| 22 | High | No | Yes | No | No | No | No | No | −6.25 |
| 23 | High | Yes | No | No | Yes | Yes | No | No | −5.27 |
| 24 | Low | No | Yes | No | No | No | No | No | −3.26 |
| 25 | Low | No | Yes | No | No | No | No | No | −3.17 |
| 26 | Low | No | No | No | No | No | No | Yes | −8.79 |
| Hybrids | Water Solubility | ||||||||
| Log S (ESOL) | Solubility (mg/mL; mol/L) | Class | Log S (Ali) | Solubility (mg/mL; mol/L) | Class | Log S (SILICOS-IT) | Solubility (mg/mL; mol/L) | Class | |
| 9 | −5.35 | 1.87 × 10−3; 4.47 × 10−6 | Moderately soluble | −5.98 | 4.35 × 10−4; 1.04 × 10−6 | Moderately soluble | −4.82 | 6.40 × 10−3; 1.53 × 10−5 | Moderately soluble |
| 10 | −5.35 | 1.87 × 10−3; 4.47 × 10−6 | Moderately soluble | −5.98 | 4.35 × 10−4; 1.04 × 10−6 | Moderately soluble | −4.82 | 6.40 × 10−3; 1.53 × 10−5 | Moderately soluble |
| 11 | −5.16 | 3.71 × 10−3; 6.95 × 10−6 | Moderately soluble | −6.72 | 1.02 × 10−4; 1.91 × 10−7 | Poorly soluble | −4.58 | 1.39 × 10−2; 2.60 × 10−5 | Moderately soluble |
| 12 | −6.19 | 4.10 × 10−4; 6.42 × 10−7 | Poorly soluble | −7.45 | 2.27 × 10−5; 3.55 × 10−8 | Poorly soluble | −6.74 | 1.15 × 10−4; 1.81 × 10−7 | Poorly soluble |
| 13 | −10.08 | 5.98 × 10−8; 8.25 × 10−11 | Insoluble | −11.69 | 1.46 × 10−9; 2.02 × 10−12 | Insoluble | −7.20 | 4.58 × 10−5; 6.32 × 10−8 | Poorly soluble |
| 14 | −10.17 | 4.88 × 10−8; 6.73 × 10−11 | Insoluble | −11.84 | 1.05 × 10−9; 1.44 × 10−12 | Insoluble | −7.65 | 1.63 × 10−5; 2.24 × 10−8 | Poorly soluble |
| 15 | −6.19 | 3.84 × 10−4; 6.42 × 10−7 | Poorly soluble | −6.81 | 9.33 × 10−5; 1.56 × 10−7 | Poorly soluble | −4.21 | 3.68 × 10−2; 6.15 × 10−5 | Moderately soluble |
| 16 | −6.10 | 4.70 × 10−4; 8.02 × 10−7 | Poorly soluble | −6.77 | 1.01 × 10−4; 1.72 × 10−7 | Poorly soluble | −4.42 | 2.23 × 10−2; 3.80 × 10−5 | Moderately soluble |
| 17 | −3.57 | 1.25 × 10−1; 2.71 × 10−4 | Soluble | −4.29 | 2.37 × 10−2; 5.13 × 10−5 | Moderately soluble | −1.86 | 6.35 × 10+0; 1.37× 10−2 | Soluble |
| 18 | −2.72 | 7.51 × 10−1; 1.89 × 10−3 | Soluble | −3.73 | 7.37 × 10−2; 1.85 × 10−4 | Moderate soluble | −1.77 | 6.79 × 10+0; 1.71 × 10−2 | Soluble |
| 19 | −5.54 | 1.48 × 10−3; 2.86 × 10−6 | Moderately soluble | −6.38 | 2.14 × 10−4; 4.15 × 10−7 | Poorly soluble | −6.63 | 1.20 × 10−4; 2.32 × 10−7 | Poorly soluble |
| 22 | −5.99 | 5.94 × 10−4; 1.02 × 10−6 | Moderately soluble | −6.70 | 1.16 × 10−4; 1.98 × 10−7 | Poorly soluble | −3.09 | 4.78 × 10−1; 8.20 × 10−4 | Soluble |
| 23 | −4.51 | 1.07 × 10−2; 3.10 × 10−5 | Moderately soluble | −4.70 | 6.90 × 10−3; 1.99 × 10−5 | Moderately soluble | −3.40 | 1.38 × 10−1; 4.00 × 10−4 | Soluble |
| 24 | −9.74 | 1.19 × 10−7; 1.82 × 10−10 | Poorly soluble | −10.80 | 7.03 × 10−8; 1.58 × 10−11 | Insoluble | −7.00 | 6.55 × 10−5; 1.00 × 10−7 | Poorly soluble |
| 25 | −9.83 | 9.72 × 10−8; 1.48 × 10−10 | Poorly soluble | −10.95 | 7.40 × 10−9; 1.13 × 10−11 | Insoluble | −7.45 | 2.32 × 10−5; 3.55 × 10−8 | Poorly soluble |
| 26 | −4.48 | 2.15 × 10−2; 3.30 × 10−5 | Moderately soluble | −5.92 | 7.83 × 10−4; 1.20 × 10−6 | Moderately soluble | −5.32 | 3.14 × 10−3; 4.82 × 10−6 | Moderately soluble |
| Hybrid | Log Po/w (iLOGP) | Log Po/w (XLOGP3) | Log Po/w (WLOGP) | Log Po/w (MLOGP) | Log Po/w (SILICOS-IT) | Consensus Log Po/w |
|---|---|---|---|---|---|---|
| 9 | 0.00 | 5.32 | 5.65 | 4.30 | 3.81 | 3.82 |
| 10 | 0.00 | 5.32 | 5.65 | 4.30 | 3.81 | 3.82 |
| 11 | 0.00 | 4.07 | 3.19 | 1.46 | 2.81 | 2.31 |
| 12 | 0.00 | 5.26 | 6.01 | 2.73 | 5.24 | 3.85 |
| 13 | 0.00 | 10.07 | 10.27 | 6.91 | 6.37 | 6.73 |
| 14 | 0.00 | 10.21 | 10.42 | 6.91 | 6.77 | 6.86 |
| 15 | 0.00 | 5.07 | 4.40 | 3.14 | 2.72 | 3.07 |
| 16 | 0.00 | 5.03 | 4.46 | 2.95 | 2.71 | 3.03 |
| 17 | 0.00 | 1.97 | 0.71 | 2.11 | −1.40 | 0.68 |
| 18 | 0.00 | 1.82 | 1.75 | 1.52 | −0.76 | 0.87 |
| 19 | 0.00 | 4.60 | 5.33 | 3.15 | 3.14 | 3.24 |
| 22 | 0.00 | 5.08 | 5.34 | 3.82 | 1.93 | 3.23 |
| 23 | 0.00 | 4.43 | 4.42 | 4.05 | 2.41 | 3.06 |
| 24 | 0.00 | 9.90 | 9.93 | 7.44 | 6.02 | 6.64 |
| 25 | 0.00 | 10.04 | 9.97 | 7.44 | 6.42 | 6.77 |
| 26 | 0.00 | 2.10 | 2.87 | 1.25 | −0.12 | 1.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peter, S.; Morifi, E.; Nwamadi, M.; Oselusi, S.O.; Tantoh, A.L.; Fonkui, T.Y.; Ndinteh, D.T.; Aderibigbe, B.A. Ferrocene-Based Hybrid Drugs as Potential Anticancer and Antibacterial Therapeutic Agents for Incorporation into Nanocarriers: In Silico, In Vitro, Molecular Docking Evaluations. Pharmaceutics 2025, 17, 722. https://doi.org/10.3390/pharmaceutics17060722
Peter S, Morifi E, Nwamadi M, Oselusi SO, Tantoh AL, Fonkui TY, Ndinteh DT, Aderibigbe BA. Ferrocene-Based Hybrid Drugs as Potential Anticancer and Antibacterial Therapeutic Agents for Incorporation into Nanocarriers: In Silico, In Vitro, Molecular Docking Evaluations. Pharmaceutics. 2025; 17(6):722. https://doi.org/10.3390/pharmaceutics17060722
Chicago/Turabian StylePeter, Sijongesonke, Eric Morifi, Mutshinyalo Nwamadi, Samson Olaitan Oselusi, Asongwe Lioniel Tantoh, Thierry Youmbi Fonkui, Derek Tantoh Ndinteh, and Blessing Atim Aderibigbe. 2025. "Ferrocene-Based Hybrid Drugs as Potential Anticancer and Antibacterial Therapeutic Agents for Incorporation into Nanocarriers: In Silico, In Vitro, Molecular Docking Evaluations" Pharmaceutics 17, no. 6: 722. https://doi.org/10.3390/pharmaceutics17060722
APA StylePeter, S., Morifi, E., Nwamadi, M., Oselusi, S. O., Tantoh, A. L., Fonkui, T. Y., Ndinteh, D. T., & Aderibigbe, B. A. (2025). Ferrocene-Based Hybrid Drugs as Potential Anticancer and Antibacterial Therapeutic Agents for Incorporation into Nanocarriers: In Silico, In Vitro, Molecular Docking Evaluations. Pharmaceutics, 17(6), 722. https://doi.org/10.3390/pharmaceutics17060722