Negative Immune Checkpoint Inhibitors
Abstract
1. Introduction
2. CTLA-4
2.1. CTLA-4 Structure
2.2. CTLA-4’s Expression in Health and Disease
2.3. CTLA-4 as a Receptor
2.4. CTLA-4’s Inhibitors
2.4.1. Peptides
2.4.2. Small Molecules
3. PD-1
3.1. PD-1 Structure
3.2. PD-1’s Expression in Health and Disease
3.3. PD-1 as a Receptor
3.4. PD-1’s Inhibitors
3.4.1. Small Molecules
3.4.2. Peptides
3.4.3. PROTACs and LYTACs
4. VISTA
4.1. VISTA Structure
4.2. VISTA Expression in Health and Disease
4.3. VISTA as a Receptor and a Ligand
4.4. VISTA’s Inhibitors
4.4.1. Small Molecules
4.4.2. Peptides
4.4.3. PROTACs and LYTACs
5. BTLA
5.1. BTLA Structure
5.2. BTLA Expression in Health and Disease
5.3. BTLA as a Receptor
5.4. BTLA’s Inhibitors
Peptides
6. TIM-3
6.1. TIM-3 Structure
6.2. TIM-3 Expression in Health and Disease
6.3. TIM-3 as a Receptor
6.4. TIM-3’s Inhibitors
6.4.1. Small Molecules
6.4.2. Peptides
7. LAG-3
7.1. LAG-3 Structure
7.2. LAG-3 Expression in Health and Disease
7.3. LAG-3 as a Receptor
7.4. LAG-3’s Inhibitors
7.4.1. Small Molecules
7.4.2. Peptides
8. TIGIT
8.1. TIGIT Structure
8.2. TIGIT Expression in Health and Disease
8.3. TIGIT as a Receptor
8.4. TIGIT’s Inhibitors
8.4.1. Small Molecules
8.4.2. Peptides
9. Future Perspective—CD112R
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) Cells in Cancer: Can Treg Cells Be a New Therapeutic Target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Zang, X. 2018 Nobel Prize in Medicine Awarded to Cancer Immunotherapy: Immune Checkpoint Blockade—A Personal Account. Genes Dis. 2018, 5, 302–303. [Google Scholar] [CrossRef]
- Huang, P.-W.; Chang, J.W.-C. Immune Checkpoint Inhibitors Win the 2018 Nobel Prize. Biomed. J. 2019, 42, 299–306. [Google Scholar] [CrossRef]
- Shekari, N.; Shanehbandi, D.; Kazemi, T.; Zarredar, H.; Baradaran, B.; Jalali, S.A. VISTA and Its Ligands: The next Generation of Promising Therapeutic Targets in Immunotherapy. Cancer Cell Int. 2023, 23, 265. [Google Scholar] [CrossRef]
- Ning, Z.; Liu, K.; Xiong, H. Roles of BTLA in Immunity and Immune Disorders. Front. Immunol. 2021, 12, 654960. [Google Scholar] [CrossRef]
- Joller, N.; Anderson, A.C.; Kuchroo, V.K. LAG-3, TIM-3, and TIGIT: Distinct Functions in Immune Regulation. Immunity 2024, 57, 206–222. [Google Scholar] [CrossRef]
- Deuse, T.; Hu, X.; Agbor-Enoh, S.; Jang, M.K.; Alawi, M.; Saygi, C.; Gravina, A.; Tediashvili, G.; Nguyen, V.Q.; Liu, Y.; et al. The SIRPα–CD47 Immune Checkpoint in NK Cells. J. Exp. Med. 2021, 218, e20200839. [Google Scholar] [CrossRef]
- Thapa, B.; Kato, S.; Nishizaki, D.; Miyashita, H.; Lee, S.; Nesline, M.K.; Previs, R.A.; Conroy, J.M.; DePietro, P.; Pabla, S.; et al. OX40/OX40 Ligand and Its Role in Precision Immune Oncology. Cancer Metastasis Rev. 2024, 43, 1001–1013. [Google Scholar] [CrossRef]
- Sharma, P.; Goswami, S.; Raychaudhuri, D.; Siddiqui, B.A.; Singh, P.; Nagarajan, A.; Liu, J.; Subudhi, S.K.; Poon, C.; Gant, K.L.; et al. Immune Checkpoint Therapy—Current Perspectives and Future Directions. Cell 2023, 186, 1652–1669. [Google Scholar] [CrossRef]
- Ramagopal, U.A.; Liu, W.; Garrett-Thomson, S.C.; Bonanno, J.B.; Yan, Q.; Srinivasan, M.; Wong, S.C.; Bell, A.; Mankikar, S.; Rangan, V.S.; et al. Structural Basis for Cancer Immunotherapy by the First-in-Class Checkpoint Inhibitor Ipilimumab. Proc. Natl. Acad. Sci. USA 2017, 114, E4223–E4232. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, M.W.; Muroyama, Y.; Drake, C.G.; Sharpe, A.H. Inhibitors of the PD-1 Pathway in Tumor Therapy. J. Immunol. 2018, 200, 375–383. [Google Scholar] [CrossRef]
- Paik, J. Nivolumab Plus Relatlimab: First Approval. Drugs 2022, 82, 925–931. [Google Scholar] [CrossRef]
- Mahmood, I. Clinical Pharmacology of Antibody-Drug Conjugates. Antibodies 2021, 10, 20. [Google Scholar] [CrossRef]
- Wang, W.; Wang, E.; Balthasar, J. Monoclonal Antibody Pharmacokinetics and Pharmacodynamics. Clin. Pharmacol. Ther. 2008, 84, 548–558. [Google Scholar] [CrossRef]
- Goswami, S.; Wang, W.; Arakawa, T.; Ohtake, S. Developments and Challenges for MAb-Based Therapeutics. Antibodies 2013, 2, 452–500. [Google Scholar] [CrossRef]
- Singh, R.; Chandley, P.; Rohatgi, S. Recent Advances in the Development of Monoclonal Antibodies and Next-Generation Antibodies. Immunohorizons 2023, 7, 886–897. [Google Scholar] [CrossRef]
- Song, Q.; Yu, Z.; Lu, W.; Zhuo, Z.; Chang, L.; Mei, H.; Cui, Y.; Zhang, D. PD-1/PD-L1 Inhibitors Related Adverse Events: A Bibliometric Analysis from 2014 to 2024. Hum. Vaccines Immunother. 2025, 21, 2424611. [Google Scholar] [CrossRef]
- Zak, K.M.; Grudnik, P.; Magiera, K.; Dömling, A.; Dubin, G.; Holak, T.A. Structural Biology of the Immune Checkpoint Receptor PD-1 and Its Ligands PD-L1/PD-L2. Structure 2017, 25, 1163–1174. [Google Scholar] [CrossRef]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The Safety and Side Effects of Monoclonal Antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef]
- Kerr, W.G.; Chisholm, J.D. The Next Generation of Immunotherapy for Cancer: Small Molecules Could Make Big Waves. J. Immunol. 2019, 202, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Alejandra, W.-P.; Miriam Irene, J.-P.; Fabio Antonio, G.-S.; Patricia, R.-G.R.; Elizabeth, T.-A.; Aleman-Aguilar, J.P.; Rebeca, G.-V. Production of Monoclonal Antibodies for Therapeutic Purposes: A Review. Int. Immunopharmacol. 2023, 120, 110376. [Google Scholar] [CrossRef] [PubMed]
- Ezan, E. Pharmacokinetic Studies of Protein Drugs: Past, Present and Future. Adv. Drug Deliv. Rev. 2013, 65, 1065–1073. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide Therapeutics: Current Status and Future Directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric Molecules That Target Proteins to the Skp1–Cullin–F Box Complex for Ubiquitination and Degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef]
- Berkley, K.; Zalejski, J.; Sharma, N.; Sharma, A. Journey of PROTAC: From Bench to Clinical Trial and Beyond. Biochemistry 2025, 64, 563–580. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, M.; Yang, Y.; Du, C.; Zhou, H.; Liu, C.; Chen, Y.; Fan, L.; Ma, H.; Gong, Y.; et al. An Overview of PROTACs: A Promising Drug Discovery Paradigm. Mol. Biomed. 2022, 3, 46. [Google Scholar] [CrossRef]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC Targeted Protein Degraders: The Past Is Prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, G.; Luo, W.; Xu, M.; Peng, R.; Du, Z.; Liu, Y.; Bai, Z.; Xiao, X.; Qin, S. PROTAC Technology: From Drug Development to Probe Technology for Target Deconvolution. Eur. J. Med. Chem. 2024, 276, 116725. [Google Scholar] [CrossRef]
- Brunet, J.-F.; Denizot, F.; Luciani, M.-F.; Roux-Dosseto, M.; Suzan, M.; Mattei, M.-G.; Golstein, P. A New Member of the Immunoglobulin Superfamily—CTLA-4. Nature 1987, 328, 267–270. [Google Scholar] [CrossRef]
- Linsley, P.S.; Brady, W.; Urnes, M.; Grosmaire, L.S.; Damle, N.K.; Ledbetter, J.A. CTLA-4 Is a Second Receptor for the B Cell Activation Antigen B7. J. Exp. Med. 1991, 174, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Stamper, C.C.; Zhang, Y.; Tobin, J.F.; Erbe, D.V.; Ikemizu, S.; Davis, S.J.; Stahl, M.L.; Seehra, J.; Somers, W.S.; Mosyak, L. Crystal Structure of the B7-1/CTLA-4 Complex That Inhibits Human Immune Responses. Nature 2001, 410, 608–611. [Google Scholar] [CrossRef]
- Schwartz, J.-C.D.; Zhang, X.; Fedorov, A.A.; Nathenson, S.G.; Almo, S.C. Structural Basis for Co-Stimulation by the Human CTLA-4/B7-2 Complex. Nature 2001, 410, 604–608. [Google Scholar] [CrossRef]
- Xu, J. (Ed.) Regulation of Cancer Immune Checkpoints; Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; Volume 1248, ISBN 978-981-15-3265-8. [Google Scholar]
- Wang, S.; Liu, C.; Yang, C.; Jin, Y.; Cui, Q.; Wang, D.; Ge, T.; He, G.; Li, W.; Zhang, G.; et al. PI3K/AKT/MTOR and PD-1/CTLA-4/CD28 Pathways as Key Targets of Cancer Immunotherapy (Review). Oncol. Lett. 2024, 28, 567. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, P. Preserving the CTLA-4 Checkpoint for Safer and More Effective Cancer Immunotherapy. Trends Pharmacol. Sci. 2020, 41, 4–12. [Google Scholar] [CrossRef]
- Song, Z.; Wang, M.; Ge, Y.; Chen, X.-P.; Xu, Z.; Sun, Y.; Xiong, X.-F. Tyrosine Phosphatase SHP2 Inhibitors in Tumor-Targeted Therapies. Acta Pharm. Sin. B 2021, 11, 13–29. [Google Scholar] [CrossRef]
- Valk, E.; Rudd, C.E.; Schneider, H. CTLA-4 Trafficking and Surface Expression. Trends Immunol. 2008, 29, 272–279. [Google Scholar] [CrossRef]
- Burke, K.P.; Chaudhri, A.; Freeman, G.J.; Sharpe, A.H. The B7:CD28 Family and Friends: Unraveling Coinhibitory Interactions. Immunity 2024, 57, 223–244. [Google Scholar] [CrossRef]
- Guan, J.; Liu, H.; Chai, Y.; Yu, J.; Yao, J.; Wang, J.; Pan, Z.; Zhang, J.; Zhou, Y.; Liu, H.; et al. Characterization of the High-Affinity Anti-CTLA-4 Monoclonal Antibody JS007 for Immune Checkpoint Therapy of Cancer. MAbs 2023, 15, 2153409. [Google Scholar] [CrossRef]
- Lee, P.S.; MacDonald, K.G.; Massi, E.; Chew, P.V.; Bee, C.; Perkins, P.; Chau, B.; Thudium, K.; Lohre, J.; Nandi, P.; et al. Improved Therapeutic Index of an Acidic PH-Selective Antibody. MAbs 2022, 14, 2024642. [Google Scholar] [CrossRef]
- Gan, X.; Shan, Q.; Li, H.; Janssens, R.; Shen, Y.; He, Y.; Chen, F.; van Haperen, R.; Drabek, D.; Li, J.; et al. An Anti-CTLA-4 Heavy Chain–Only Antibody with Enhanced Treg Depletion Shows Excellent Preclinical Efficacy and Safety Profile. Proc. Natl. Acad. Sci. USA 2022, 119, e2200879119. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cai, H.; Liu, J.; Wang, X.; Zheng, P.; Devenport, M.; Xu, T.; Dou, F.; Liu, Y.; Zhou, A. Structure of CTLA-4 Complexed with a PH-Sensitive Cancer Immunotherapeutic Antibody. Cell Discov. 2020, 6, 79. [Google Scholar] [CrossRef]
- He, M.; Chai, Y.; Qi, J.; Zhang, C.W.H.; Tong, Z.; Shi, Y.; Yan, J.; Tan, S.; Gao, G.F. Remarkably Similar CTLA-4 Binding Properties of Therapeutic Ipilimumab and Tremelimumab Antibodies. Oncotarget 2017, 8, 67129–67139. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, H.T.; Shin, W.; Chae, J.; Choi, J.; Kim, S.H.; Lim, H.; Won Heo, T.; Park, K.Y.; Lee, Y.J.; et al. Structural Basis of Checkpoint Blockade by Monoclonal Antibodies in Cancer Immunotherapy. Nat. Commun. 2016, 7, 13354. [Google Scholar] [CrossRef]
- Yu, C.; Sonnen, A.F.-P.; George, R.; Dessailly, B.H.; Stagg, L.J.; Evans, E.J.; Orengo, C.A.; Stuart, D.I.; Ladbury, J.E.; Ikemizu, S.; et al. Rigid-Body Ligand Recognition Drives Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) Receptor Triggering. J. Biol. Chem. 2011, 286, 6685–6696. [Google Scholar] [CrossRef]
- Sonnen, A.F.-P.; Yu, C.; Evans, E.J.; Stuart, D.I.; Davis, S.J.; Gilbert, R.J.C. Domain Metastability: A Molecular Basis for Immunoglobulin Deposition? J. Mol. Biol. 2010, 399, 207–213. [Google Scholar] [CrossRef]
- Iiyama, M.; Numoto, N.; Ogawa, S.; Kuroda, M.; Morii, H.; Abe, R.; Ito, N.; Oda, M. Molecular Interactions of the CTLA-4 Cytoplasmic Region with the Phosphoinositide 3-Kinase SH2 Domains. Mol. Immunol. 2021, 131, 51–59. [Google Scholar] [CrossRef]
- Follows, E.R.; Mcpheat, J.C.; Minshull, C.; Moore, N.C.; Pauptit, R.A.; Rowsell, S.; Stacey, C.L.; Stanway, J.J.; Taylor, I.W.F.; Abbott, W.M. Study of the Interaction of the Medium Chain Μ2 Subunit of the Clathrin-Associated Adapter Protein Complex 2 with Cytotoxic T-Lymphocyte Antigen 4 and CD28. Biochem. J. 2001, 359, 427. [Google Scholar] [CrossRef]
- Baker, D.; Yang, W.; Hicks, D.R.; Ghosh, A.; Schwartze, T.A.; Coventry, B.; Goreshnik, I.; Allen, A.; Halabiya, S.; Kim, C.; et al. Design of High Affinity Binders to Convex Protein Target Sites. bioRxiv 2024. [Google Scholar] [CrossRef]
- Pioli, C.; Gatta, L.; Ubaldi, V.; Doria, G. Inhibition of IgG1 and IgE Production by Stimulation of the B Cell CTLA-4 Receptor. J. Immunol. 2000, 165, 5530–5536. [Google Scholar] [CrossRef]
- Muthana, M.M.; Du, X.; Liu, M.; Wang, X.; Wu, W.; Ai, C.; Su, L.; Zheng, P.; Liu, Y. CTLA-4 Antibody-Drug Conjugate Reveals Autologous Destruction of B-Lymphocytes Associated with Regulatory T Cell Impairment. eLife 2023, 12, RP87281. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Jeong, D.; Eum, H.H.; Kim, N.; Na, M.; Kang, H.; Lee, H. CTLA-4 Inhibition Facilitates Follicular T and B Cell Interaction and the Production of Tumor-specific Antibodies. Int. J. Cancer 2023, 152, 1964–1976. [Google Scholar] [CrossRef]
- Wang, X.-B.; Giscombe, R.; Yan, Z.; Heiden, T.; Xu, D.; Lefvert, A.K. Expression of CTLA-4 by Human Monocytes. Scand. J. Immunol. 2002, 55, 53–60. [Google Scholar] [CrossRef]
- Clavijo, P.E.; Moore, E.C.; Chen, J.; Davis, R.J.; Friedman, J.; Kim, Y.; Van Waes, C.; Chen, Z.; Allen, C.T. Resistance to CTLA-4 Checkpoint Inhibition Reversed Through Selective Elimination of Granulocytic Myeloid Cells. Oncotarget 2017, 8, 55804–55820. [Google Scholar] [CrossRef]
- Kaufman, K.A. The CTLA-4 Gene Is Expressed in Placental Fibroblasts. Mol. Hum. Reprod. 1999, 5, 84–87. [Google Scholar] [CrossRef]
- Fraser, J.H.; Rincón, M.; McCoy, K.D.; Le Gros, G. CTLA4 Ligation Attenuates AP-1, NFAT and NF-ΚB Activity in Activated T Cells. Eur. J. Immunol. 1999, 29, 838–844. [Google Scholar] [CrossRef]
- Hossen, M.M.; Ma, Y.; Yin, Z.; Xia, Y.; Du, J.; Huang, J.Y.; Huang, J.J.; Zou, L.; Ye, Z.; Huang, Z. Current Understanding of CTLA-4: From Mechanism to Autoimmune Diseases. Front. Immunol. 2023, 14, 1198365. [Google Scholar] [CrossRef]
- Vendetti, S.; Riccomi, A.; Sacchi, A.; Gatta, L.; Pioli, C.; De Magistris, M.T. Cyclic Adenosine 5′-Monophosphate and Calcium Induce CD152 (CTLA-4) Up-Regulation in Resting CD4+ T Lymphocytes. J. Immunol. 2002, 169, 6231–6235. [Google Scholar] [CrossRef]
- Schneider, H.; Downey, J.; Smith, A.; Zinselmeyer, B.H.; Rush, C.; Brewer, J.M.; Wei, B.; Hogg, N.; Garside, P.; Rudd, C.E. Reversal of the TCR Stop Signal by CTLA-4. Science 2006, 313, 1972–1975. [Google Scholar] [CrossRef]
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 Have Opposing Effects on the Response of T Cells to Stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef]
- Sansom, D.M.; Manzotti, C.N.; Zheng, Y. What’s the Difference Between CD80 and CD86? Trends Immunol. 2003, 24, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Holt, M.P.; Punkosdy, G.A.; Glass, D.D.; Shevach, E.M. TCR Signaling and CD28/CTLA-4 Signaling Cooperatively Modulate T Regulatory Cell Homeostasis. J. Immunol. 2017, 198, 1503–1511. [Google Scholar] [CrossRef]
- Thompson, R.H.; Allison, J.P.; Kwon, E.D. Anti-Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) Immunotherapy for the Treatment of Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2006, 24, 442–447. [Google Scholar] [CrossRef]
- Lee, H.J.; Li, C.W.; Hammerstad, S.S.; Stefan, M.; Tomer, Y. Immunogenetics of Autoimmune Thyroid Diseases: A Comprehensive Review. J. Autoimmun. 2015, 64, 82–90. [Google Scholar] [CrossRef]
- Khan, U.; Ali, F.; Khurram, M.S.; Zaka, A.; Hadid, T. Immunotherapy-Associated Autoimmune Hemolytic Anemia. J. Immunother. Cancer 2017, 5, 15. [Google Scholar] [CrossRef]
- Egg, D.; Rump, I.C.; Mitsuiki, N.; Rojas-Restrepo, J.; Maccari, M.-E.; Schwab, C.; Gabrysch, A.; Warnatz, K.; Goldacker, S.; Patiño, V.; et al. Therapeutic Options for CTLA-4 Insufficiency. J. Allergy Clin. Immunol. 2022, 149, 736–746. [Google Scholar] [CrossRef]
- Schwab, C.; Gabrysch, A.; Olbrich, P.; Patiño, V.; Warnatz, K.; Wolff, D.; Hoshino, A.; Kobayashi, M.; Imai, K.; Takagi, M.; et al. Phenotype, Penetrance, and Treatment of 133 Cytotoxic T-Lymphocyte Antigen 4–Insufficient Subjects. J. Allergy Clin. Immunol. 2018, 142, 1932–1946. [Google Scholar] [CrossRef]
- Sojka, D.K.; Hughson, A.; Fowell, D.J. CTLA-4 Is Required by CD4+CD25+ Treg to Control CD4+ T-cell Lymphopenia-induced Proliferation. Eur. J. Immunol. 2009, 39, 1544–1551. [Google Scholar] [CrossRef]
- Mitsuiki, N.; Schwab, C.; Grimbacher, B. What Did We Learn from CTLA-4 Insufficiency on the Human Immune System? Immunol. Rev. 2019, 287, 33–49. [Google Scholar] [CrossRef]
- LaBelle, J.L.; Hanke, C.A.; Blazar, B.R.; Truitt, R.L. Negative Effect of CTLA-4 on Induction of T-Cell Immunity in Vivo to B7-1+, but Not B7-2+, Murine Myelogenous Leukemia. Blood 2002, 99, 2146–2153. [Google Scholar] [CrossRef]
- Goenka, A.; Khan, F.; Verma, B.; Sinha, P.; Dmello, C.C.; Jogalekar, M.P.; Gangadaran, P.; Ahn, B. Tumor Microenvironment Signaling and Therapeutics in Cancer Progression. Cancer Commun. 2023, 43, 525–561. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Ishino, T.; Ueda, Y.; Nagasaki, J.; Sadahira, T.; Dansako, H.; Araki, M.; Togashi, Y. Activated CTLA-4-independent Immunosuppression of Treg Cells Disturbs CTLA-4 Blockade-mediated Antitumor Immunity. Cancer Sci. 2023, 114, 1859–1870. [Google Scholar] [CrossRef]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A Moving Target in Immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Ceeraz, S.; Nowak, E.C.; Noelle, R.J. B7 Family Checkpoint Regulators in Immune Regulation and Disease. Trends Immunol. 2013, 34, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Witt, K.; Evans-Axelsson, S.; Lundqvist, A.; Johansson, M.; Bjartell, A.; Hellsten, R. Inhibition of STAT3 Augments Antitumor Efficacy of Anti-CTLA-4 Treatment against Prostate Cancer. Cancer Immunol. Immunother. 2021, 70, 3155–3166. [Google Scholar] [CrossRef]
- Small, E.J.; Tchekmedyian, N.S.; Rini, B.I.; Fong, L.; Lowy, I.; Allison, J.P. A Pilot Trial of CTLA-4 Blockade with Human Anti-CTLA-4 in Patients with Hormone-Refractory Prostate Cancer. Clin. Cancer Res. 2007, 13, 1810–1815. [Google Scholar] [CrossRef]
- Willsmore, Z.N.; Coumbe, B.G.T.; Crescioli, S.; Reci, S.; Gupta, A.; Harris, R.J.; Chenoweth, A.; Chauhan, J.; Bax, H.J.; McCraw, A.; et al. Combined Anti-PD-1 and Anti-CTLA-4 Checkpoint Blockade: Treatment of Melanoma and Immune Mechanisms of Action. Eur. J. Immunol. 2021, 51, 544–556. [Google Scholar] [CrossRef]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef]
- Campbell, K.M.; Amouzgar, M.; Pfeiffer, S.M.; Howes, T.R.; Medina, E.; Travers, M.; Steiner, G.; Weber, J.S.; Wolchok, J.D.; Larkin, J.; et al. Prior Anti-CTLA-4 Therapy Impacts Molecular Characteristics Associated with Anti-PD-1 Response in Advanced Melanoma. Cancer Cell 2023, 41, 791–806.e4. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Y.; Zhao, Y.; Zhao, H.; Zhou, N.; Zhang, Y.; Chen, L.; Zhou, T.; Chen, G.; Wu, T.; et al. QL1706 (Anti-PD-1 IgG4/CTLA-4 Antibody) Plus Chemotherapy with or Without Bevacizumab in Advanced Non-Small Cell Lung Cancer: A Multi-Cohort, Phase II Study. Signal Transduct. Target. Ther. 2024, 9, 23. [Google Scholar] [CrossRef]
- Shen, X.; Huang, S.; Xiao, H.; Zeng, S.; Liu, J.; Ran, Z.; Xiong, B. Efficacy and Safety of PD-1/PD-L1 plus CTLA-4 Antibodies ± Other Therapies in Lung Cancer: A Systematic Review and Meta-Analysis. Eur. J. Hosp. Pharm. 2023, 30, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Friese, C.; Harbst, K.; Borch, T.H.; Westergaard, M.C.W.; Pedersen, M.; Kverneland, A.; Jönsson, G.; Donia, M.; Svane, I.M.; Met, Ö. CTLA-4 Blockade Boosts the Expansion of Tumor-Reactive CD8+ Tumor-Infiltrating Lymphocytes in Ovarian Cancer. Sci. Rep. 2020, 10, 3914. [Google Scholar] [CrossRef]
- Chen, J.; Kang, S.; Wu, J.; Zhao, J.; Si, W.; Sun, H.; Li, Y. CTLA-4 Polymorphism Contributes to the Genetic Susceptibility of Epithelial Ovarian Cancer. J. Obstet. Gynaecol. Res. 2022, 48, 1240–1247. [Google Scholar] [CrossRef]
- Linsley, P.S.; Greene, J.L.; Brady, W.; Bajorath, J.; Ledbetter, J.A.; Peach, R. Human B7-1 (CD80) and B7-2 (CD86) Bind with Similar Avidities but Distinct Kinetics to CD28 and CTLA-4 Receptors. Immunity 1994, 1, 793–801. [Google Scholar] [CrossRef]
- Zhang, X.; Schwartz, J.-C.D.; Almo, S.C.; Nathenson, S.G. Crystal Structure of the Receptor-Binding Domain of Human B7-2: Insights into Organization and Signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 2586–2591. [Google Scholar] [CrossRef]
- Fukumoto, T.; Torigoe, N.; Kawabata, S.; Murakami, M.; Uede, T.; Nishi, T.; Ito, Y.; Sugimura, K. Peptide Mimics of the CTLA4-Binding Domain Stimulate T-Cell Proliferation. Nat. Biotechnol. 1998, 16, 267–270. [Google Scholar] [CrossRef]
- Maaß, F.; Wüstehube-Lausch, J.; Dickgießer, S.; Valldorf, B.; Reinwarth, M.; Schmoldt, H.; Daneschdar, M.; Avrutina, O.; Sahin, U.; Kolmar, H. Cystine-knot Peptides Targeting Cancer-relevant Human Cytotoxic T Lymphocyte-associated Antigen 4 (CTLA-4). J. Pept. Sci. 2015, 21, 651–660. [Google Scholar] [CrossRef]
- Ramanayake Mudiyanselage, T.M.R.; Michigami, M.; Ye, Z.; Uyeda, A.; Inoue, N.; Sugiura, K.; Fujii, I.; Fujiwara, D. An Immune-Stimulatory Helix–Loop–Helix Peptide: Selective Inhibition of CTLA-4–B7 Interaction. ACS Chem. Biol. 2020, 15, 360–368. [Google Scholar] [CrossRef]
- Thakkar, R.; Upreti, D.; Ishiguro, S.; Tamura, M.; Comer, J. Computational Design of a Cyclic Peptide That Inhibits the CTLA4 Immune Checkpoint. RSC Med. Chem. 2023, 14, 658–670. [Google Scholar] [CrossRef]
- Schönfeld, D.; Matschiner, G.; Chatwell, L.; Trentmann, S.; Gille, H.; Hülsmeyer, M.; Brown, N.; Kaye, P.M.; Schlehuber, S.; Hohlbaum, A.M.; et al. An Engineered Lipocalin Specific for CTLA-4 Reveals a Combining Site with Structural and Conformational Features Similar to Antibodies. Proc. Natl. Acad. Sci. USA 2009, 106, 8198–8203. [Google Scholar] [CrossRef]
- Schröder, S.K.; Gasterich, N.; Weiskirchen, S.; Weiskirchen, R. Lipocalin 2 Receptors: Facts, Fictions, and Myths. Front. Immunol. 2023, 14, 1229885. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, S.; Yuan, B.; Wu, Y.; Gao, Y.; Wan, G.; Li, G. A Novel CTLA-4 Affinity Peptide for Cancer Immunotherapy by Increasing the Integrin αvβ3 Targeting. Discov. Oncol. 2022, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Su, L.; Chen, Z.; Feng, D.; Wei, J.; Sun, J. Construction of Small Molecular CTLA4 Analogs with CD80-Binding Affinity. Biochem. Biophys. Res. Commun. 2019, 513, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Erbe, D.V.; Wang, S.; Xing, Y.; Tobin, J.F. Small Molecule Ligands Define a Binding Site on the Immune Regulatory Protein B7.1. J. Biol. Chem. 2002, 277, 7363–7368. [Google Scholar] [CrossRef]
- Huxley, P.; Sutton, D.H.; Debnam, P.; Matthews, I.R.; Brewer, J.E.; Rose, J.; Trickett, M.; Williams, D.D.; Andersen, T.B.; Classon, B.J. High-Affinity Small Molecule Inhibitors of T Cell Costimulation: Compounds for Immunotherapy. Chem. Biol. 2004, 11, 1651–1658. [Google Scholar] [CrossRef]
- Uvebrant, K.; Da Graça Thrige, D.; Rosén, A.; Åkesson, M.; Berg, H.; Walse, B.; Björk, P. Discovery of Selective Small-Molecule CD80 Inhibitors. SLAS Discov. 2007, 12, 464–472. [Google Scholar] [CrossRef]
- Green, N.J.; Xiang, J.; Chen, J.; Chen, L.; Davies, A.M.; Erbe, D.; Tam, S.; Tobin, J.F. Structure–Activity Studies of a Series of Dipyrazolo[3,4-b:3′,4′-d]Pyridin-3-Ones Binding to the Immune Regulatory Protein B7.1. Bioorg Med. Chem. 2003, 11, 2991–3013. [Google Scholar] [CrossRef]
- Haanstra, K.G.; Endell, J.; Estévâo, D.; Kondova, I.; Jonker, M. Blocking T Cell Co-Stimulation Using a CD80 Blocking Small Molecule Reduces Delayed Type Hypersensitivity Responses in Rhesus Monkeys. Clin. Exp. Immunol. 2009, 158, 91–98. [Google Scholar] [CrossRef]
- Sobhani, N.; Tardiel-Cyril, D.R.; Chai, D.; Generali, D.; Li, J.-R.; Vazquez-Perez, J.; Lim, J.M.; Morris, R.; Bullock, Z.N.; Davtyan, A.; et al. Artificial Intelligence-Powered Discovery of Small Molecules Inhibiting CTLA-4 in Cancer. BJC Rep. 2024, 2, 4. [Google Scholar] [CrossRef]
- Patsoukis, N.; Wang, Q.; Strauss, L.; Boussiotis, V.A. Revisiting the PD-1 Pathway. Sci. Adv. 2020, 6, eabd2712. [Google Scholar] [CrossRef]
- Agata, Y.; Kawasaki, A.; Nishimura, H.; Ishida, Y.; Tsubat, T.; Yagita, H.; Honjo, T. Expression of the PD-1 Antigen on the Surface of Stimulated Mouse T and B Lymphocytes. Int. Immunol. 1996, 8, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced Expression of PD-1, a Novel Member of the Immunoglobulin Gene Superfamily, upon Programmed Cell Death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Zhang, X.; Schwartz, J.-C.D.; Guo, X.; Bhatia, S.; Cao, E.; Chen, L.; Zhang, Z.-Y.; Edidin, M.A.; Nathenson, S.G.; Almo, S.C. Structural and Functional Analysis of the Costimulatory Receptor Programmed Death-1. Immunity 2004, 20, 337–347. [Google Scholar] [CrossRef]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef]
- Marasco, M.; Berteotti, A.; Weyershaeuser, J.; Thorausch, N.; Sikorska, J.; Krausze, J.; Brandt, H.J.; Kirkpatrick, J.; Rios, P.; Schamel, W.W.; et al. Molecular Mechanism of SHP2 Activation by PD-1 Stimulation. Sci. Adv. 2020, 6, eaay4458. [Google Scholar] [CrossRef]
- Riley, J.L. PD-1 Signaling in Primary T Cells. Immunol. Rev. 2009, 229, 114–125. [Google Scholar] [CrossRef]
- Lázár-Molnár, E.; Yan, Q.; Cao, E.; Ramagopal, U.; Nathenson, S.G.; Almo, S.C. Crystal Structure of the Complex Between Programmed Death-1 (PD-1) and Its Ligand PD-L2. Proc. Natl. Acad. Sci. USA 2008, 105, 10483–10488. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 Is a Second Ligand for PD-1 and Inhibits T Cell Activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef]
- Cheng, X.; Veverka, V.; Radhakrishnan, A.; Waters, L.C.; Muskett, F.W.; Morgan, S.H.; Huo, J.; Yu, C.; Evans, E.J.; Leslie, A.J.; et al. Structure and Interactions of the Human Programmed Cell Death 1 Receptor. J. Biol. Chem. 2013, 288, 11771–11785. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-Y.; Zhu, Y.; Shen, Y.-Y.; Xu, Q.-Y.; Tang, H.-Y.; Cui, N.-X.; Jiang, L.; Dai, X.-M.; Chen, W.-Q.; Lin, Q.; et al. The Role of PD-1 Signaling in Health and Immune-Related Diseases. Front. Immunol. 2023, 14, 1163633. [Google Scholar] [CrossRef] [PubMed]
- Im, S.J.; Obeng, R.C.; Nasti, T.H.; McManus, D.; Kamphorst, A.O.; Gunisetty, S.; Prokhnevska, N.; Carlisle, J.W.; Yu, K.; Sica, G.L.; et al. Characteristics and Anatomic Location of PD-1+TCF1+ Stem-like CD8 T Cells in Chronic Viral Infection and Cancer. Proc. Natl. Acad. Sci. USA 2023, 120, e2221985120. [Google Scholar] [CrossRef]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef]
- Wherry, E.J.; Ha, S.-J.; Kaech, S.M.; Haining, W.N.; Sarkar, S.; Kalia, V.; Subramaniam, S.; Blattman, J.N.; Barber, D.L.; Ahmed, R. Molecular Signature of CD8+ T Cell Exhaustion during Chronic Viral Infection. Immunity 2007, 27, 670–684. [Google Scholar] [CrossRef]
- Hashimoto, M.; Araki, K.; Cardenas, M.A.; Li, P.; Jadhav, R.R.; Kissick, H.T.; Hudson, W.H.; McGuire, D.J.; Obeng, R.C.; Wieland, A.; et al. PD-1 Combination Therapy with IL-2 Modifies CD8+ T Cell Exhaustion Program. Nature 2022, 610, 173–181. [Google Scholar] [CrossRef]
- Schönrich, G.; Raftery, M.J. The PD-1/PD-L1 Axis and Virus Infections: A Delicate Balance. Front. Cell Infect. Microbiol. 2019, 9, 207. [Google Scholar] [CrossRef]
- Ando, S.; Perkins, C.M.; Sajiki, Y.; Chastain, C.; Valanparambil, R.M.; Wieland, A.; Hudson, W.H.; Hashimoto, M.; Ramalingam, S.S.; Freeman, G.J.; et al. MTOR Regulates T Cell Exhaustion and PD-1–Targeted Immunotherapy Response during Chronic Viral Infection. J. Clin. Investig. 2023, 133, e160025. [Google Scholar] [CrossRef]
- Hudson, W.H.; Gensheimer, J.; Hashimoto, M.; Wieland, A.; Valanparambil, R.M.; Li, P.; Lin, J.-X.; Konieczny, B.T.; Im, S.J.; Freeman, G.J.; et al. Proliferating Transitory T Cells with an Effector-like Transcriptional Signature Emerge from PD-1+ Stem-like CD8+ T Cells during Chronic Infection. Immunity 2019, 51, 1043–1058.e4. [Google Scholar] [CrossRef]
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-cell Exhaustion in HIV Infection. Immunol. Rev. 2019, 292, 149–163. [Google Scholar] [CrossRef]
- Velu, V.; Shetty, R.D.; Larsson, M.; Shankar, E.M. Role of PD-1 Co-Inhibitory Pathway in HIV Infection and Potential Therapeutic Options. Retrovirology 2015, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Porichis, F.; Kaufmann, D.E. Role of PD-1 in HIV Pathogenesis and as Target for Therapy. Curr. HIV/AIDS Rep. 2012, 9, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, J.; Li, P.; Luo, J.; Qin, R.; Peng, Q.; Li, B.; Wei, X.; Wang, T.; Shi, H.; et al. Single-Cell Analysis Reveals HBV-Specific PD-1+CD8+ TRM Cells in Tumor Borders Are Associated with HBV-Related Hepatic Damage and Fibrosis in HCC Patients. J. Exp. Clin. Cancer Res. 2023, 42, 152. [Google Scholar] [CrossRef]
- Chua, C.; Salimzadeh, L.; Ma, A.T.; Adeyi, O.A.; Seo, H.; Boukhaled, G.M.; Mehrotra, A.; Patel, A.; Ferrando-Martinez, S.; Robbins, S.H.; et al. IL-2 Produced by HBV-Specific T Cells as a Biomarker of Viral Control and Predictor of Response to PD-1 Therapy across Clinical Phases of Chronic Hepatitis B. Hepatol. Commun. 2023, 7, e0337. [Google Scholar] [CrossRef]
- Féray, C.; López-Labrador, F.X. Is PD-1 Blockade a Potential Therapy for HBV? JHEP Rep. 2019, 1, 142–144. [Google Scholar] [CrossRef]
- Peña-Asensio, J.; Calvo, H.; Torralba, M.; Miquel, J.; Sanz-de-Villalobos, E.; Larrubia, J.-R. Gamma-Chain Receptor Cytokines & PD-1 Manipulation to Restore HCV-Specific CD8+ T Cell Response during Chronic Hepatitis C. Cells 2021, 10, 538. [Google Scholar] [CrossRef]
- Xiao, W.; Jiang, L.F.; Deng, X.Z.; Zhu, D.Y.; Pei, J.P.; Xu, M.L.; Li, B.J.; Wang, C.J.; Zhang, J.H.; Zhang, Q.; et al. PD-1/PD-L1 Signal Pathway Participates in HCV F Protein-Induced T Cell Dysfunction in Chronic HCV Infection. Immunol. Res. 2016, 64, 412–423. [Google Scholar] [CrossRef]
- Radziewicz, H.; Dunham, R.M.; Grakoui, A. PD-1 Tempers Tregs in Chronic HCV Infection. J. Clin. Investig. 2009, 119, 450–453. [Google Scholar] [CrossRef]
- Collier, J.L.; Pauken, K.E.; Lee, C.A.A.; Patterson, D.G.; Markson, S.C.; Conway, T.S.; Fung, M.E.; France, J.A.; Mucciarone, K.N.; Lian, C.G.; et al. Single-Cell Profiling Reveals Unique Features of Diabetogenic T Cells in Anti-PD-1-Induced Type 1 Diabetes Mice. J. Exp. Med. 2023, 220, e20221920. [Google Scholar] [CrossRef]
- Pipitone, R.M.; Lupo, G.; Zito, R.; Javed, A.; Petta, S.; Pennisi, G.; Grimaudo, S. The PD-1/PD-L1 Axis in the Biology of MASLD. Int. J. Mol. Sci. 2024, 25, 3671. [Google Scholar] [CrossRef]
- Rao, D.A.; Gurish, M.F.; Marshall, J.L.; Slowikowski, K.; Fonseka, C.Y.; Liu, Y.; Donlin, L.T.; Henderson, L.A.; Wei, K.; Mizoguchi, F.; et al. Pathologically Expanded Peripheral T Helper Cell Subset Drives B Cells in Rheumatoid Arthritis. Nature 2017, 542, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Canavan, M.; Floudas, A.; Veale, D.J.; Fearon, U. The PD-1:PD-L1 Axis in Inflammatory Arthritis. BMC Rheumatol. 2021, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Kleffel, S.; Posch, C.; Barthel, S.R.; Mueller, H.; Schlapbach, C.; Guenova, E.; Elco, C.P.; Lee, N.; Juneja, V.R.; Zhan, Q.; et al. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell 2015, 162, 1242–1256. [Google Scholar] [CrossRef] [PubMed]
- Huuhtanen, J.; Kasanen, H.; Peltola, K.; Lönnberg, T.; Glumoff, V.; Brück, O.; Dufva, O.; Peltonen, K.; Vikkula, J.; Jokinen, E.; et al. Single-Cell Characterization of Anti–LAG-3 and Anti–PD-1 Combination Treatment in Patients with Melanoma. J. Clin. Investig. 2023, 133, e164809. [Google Scholar] [CrossRef]
- Kwaśnik, P.; Zaleska, J.; Link-Lenczowska, D.; Zawada, M.; Wysogląd, H.; Ochrem, B.; Bober, G.; Wasilewska, E.; Hus, I.; Szarejko, M.; et al. High Level of CD8+PD-1+ Cells in Patients with Chronic Myeloid Leukemia Who Experienced Loss of MMR after Imatinib Discontinuation. Cells 2024, 13, 723. [Google Scholar] [CrossRef]
- Lee, M.Y.; Park, C.-J.; Cho, Y.-U.; You, E.; Jang, S.; Seol, C.A.; Seo, E.-J.; Choi, E.-J.; Lee, J.-H. Differences in PD-1 Expression on CD8+ T-Cells in Chronic Myeloid Leukemia Patients According to Disease Phase and TKI Medication. Cancer Immunol. Immunother. 2020, 69, 2223–2232. [Google Scholar] [CrossRef]
- Dumitru, A.; Dobrica, E.-C.; Croitoru, A.; Cretoiu, S.M.; Gaspar, B.S. Focus on PD-1/PD-L1 as a Therapeutic Target in Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 12067. [Google Scholar] [CrossRef]
- Wan, C.; Keany, M.P.; Dong, H.; Al-Alem, L.F.; Pandya, U.M.; Lazo, S.; Boehnke, K.; Lynch, K.N.; Xu, R.; Zarrella, D.T.; et al. Enhanced Efficacy of Simultaneous PD-1 and PD-L1 Immune Checkpoint Blockade in High-Grade Serous Ovarian Cancer. Cancer Res. 2021, 81, 158–173. [Google Scholar] [CrossRef]
- Thommen, D.S.; Koelzer, V.H.; Herzig, P.; Roller, A.; Trefny, M.; Dimeloe, S.; Kiialainen, A.; Hanhart, J.; Schill, C.; Hess, C.; et al. A Transcriptionally and Functionally Distinct PD-1+ CD8+ T Cell Pool with Predictive Potential in Non-Small-Cell Lung Cancer Treated with PD-1 Blockade. Nat. Med. 2018, 24, 994–1004. [Google Scholar] [CrossRef]
- Patil, N.S.; Nabet, B.Y.; Müller, S.; Koeppen, H.; Zou, W.; Giltnane, J.; Au-Yeung, A.; Srivats, S.; Cheng, J.H.; Takahashi, C.; et al. Intratumoral Plasma Cells Predict Outcomes to PD-L1 Blockade in Non-Small Cell Lung Cancer. Cancer Cell 2022, 40, 289–300.e4. [Google Scholar] [CrossRef]
- Rossi, C.; Casasnovas, R.-O. PD-1 Inhibitors in Patients with Hodgkin Lymphoma. Eur. J. Cancer 2022, 164, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Bie, L.; Ying, J. Cancer Cell-Intrinsic PD-1: Its Role in Malignant Progression and Immunotherapy. Biomed. Pharmacother. 2023, 167, 115514. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, R.; Leuci, V.; Donini, C.; Galvagno, F.; Massa, A.; De Santis, M.C.; Peirone, S.; Medico, G.; Sanlorenzo, M.; Vujic, I.; et al. Novel Lymphocyte-Independent Antitumor Activity by PD-1 Blocking Antibody against PD-1+ Chemoresistant Lung Cancer Cells. Clin. Cancer Res. 2023, 29, 621–634. [Google Scholar] [CrossRef]
- Ieranò, C.; Righelli, D.; D’Alterio, C.; Napolitano, M.; Portella, L.; Rea, G.; Auletta, F.; Santagata, S.; Trotta, A.M.; Guardascione, G.; et al. In PD-1+ Human Colon Cancer Cells NIVOLUMAB Promotes Survival and Could Protect Tumor Cells from Conventional Therapies. J. Immunother. Cancer 2022, 10, e004032. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Liu, S.; Guo, L.; Zhang, B.; Zhang, J.; Ye, Q. Programmed Cell Death-1 (PD-1) Checkpoint Blockade in Combination with a Mammalian Target of Rapamycin Inhibitor Restrains Hepatocellular Carcinoma Growth Induced by Hepatoma Cell–Intrinsic PD-1. Hepatology 2017, 66, 1920–1933. [Google Scholar] [CrossRef]
- Pu, N.; Gao, S.; Yin, H.; Li, J.; Wu, W.; Fang, Y.; Zhang, L.; Rong, Y.; Xu, X.; Wang, D.; et al. Cell-Intrinsic PD-1 Promotes Proliferation in Pancreatic Cancer by Targeting CYR61/CTGF via the Hippo Pathway. Cancer Lett. 2019, 460, 42–53. [Google Scholar] [CrossRef]
- Liotti, F.; Kumar, N.; Prevete, N.; Marotta, M.; Sorriento, D.; Ieranò, C.; Ronchi, A.; Marino, F.Z.; Moretti, S.; Colella, R.; et al. PD-1 Blockade Delays Tumor Growth by Inhibiting an Intrinsic SHP2/Ras/MAPK Signalling in Thyroid Cancer Cells. J. Exp. Clin. Cancer Res. 2021, 40, 22. [Google Scholar] [CrossRef]
- Mirzaei, R.; Gordon, A.; Zemp, F.J.; Kumar, M.; Sarkar, S.; Luchman, H.A.; Bellail, A.C.; Hao, C.; Mahoney, D.J.; Dunn, J.F.; et al. PD-1 Independent of PD-L1 Ligation Promotes Glioblastoma Growth Through the NFκB Pathway. Sci. Adv. 2021, 7, 2148. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Y.; Li, X.; Liu, H.; You, T.; Cai, T.; Yang, F. YB-1 Promotes Cell Proliferation and Metastasis by Targeting Cell-Intrinsic PD-1/PD-L1 Pathway in Breast Cancer. Int. J. Biochem. Cell Biol. 2022, 153, 106314. [Google Scholar] [CrossRef]
- Cao, Z.; Kon, N.; Liu, Y.; Xu, W.; Wen, J.; Yao, H.; Zhang, M.; Wu, Z.; Yan, X.; Zhu, W.-G.; et al. An Unexpected Role for P53 in Regulating Cancer Cell–Intrinsic PD-1 by Acetylation. Sci. Adv. 2021, 7, 4148–4179. [Google Scholar] [CrossRef]
- Liu, J.; Peng, X.; Yang, S.; Li, X.; Huang, M.; Wei, S.; Zhang, S.; He, G.; Zheng, H.; Fan, Q.; et al. Extracellular Vesicle PD-L1 in Reshaping Tumor Immune Microenvironment: Biological Function and Potential Therapy Strategies. Cell Commun. Signal. 2022, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. Co-Inhibitory Molecules of the B7–CD28 Family in the Control of T-Cell Immunity. Nat. Rev. Immunol. 2004, 4, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Ling, V.; Carreno, B.M. The B7 Family of Immune-Regulatory Ligands. Genome Biol. 2005, 6, 223. [Google Scholar] [CrossRef][Green Version]
- Carreno, B.M.; Collins, M. The B7 Family of Ligands and Its Receptors: New Pathways for Costimulation and Inhibition of Immune Responses. Annu. Rev. Immunol. 2002, 20, 29–53. [Google Scholar] [CrossRef]
- Youngnak, P.; Kozono, Y.; Kozono, H.; Iwai, H.; Otsuki, N.; Jin, H.; Omura, K.; Yagita, H.; Pardoll, D.M.; Chen, L.; et al. Differential Binding Properties of B7-H1 and B7-DC to Programmed Death-1. Biochem. Biophys. Res. Commun. 2003, 307, 672–677. [Google Scholar] [CrossRef]
- Pedoeem, A.; Azoulay-Alfaguter, I.; Strazza, M.; Silverman, G.J.; Mor, A. Programmed Death-1 Pathway in Cancer and Autoimmunity. Clin. Immunol. 2014, 153, 145–152. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, P.; Gao, F.; Cheng, H.; Qi, J.; Gao, G.F. A Dimeric Structure of PD-L1: Functional Units or Evolutionary Relics? Protein Cell 2010, 1, 153–160. [Google Scholar] [CrossRef]
- Wang, S.; Bajorath, J.; Flies, D.B.; Dong, H.; Honjo, T.; Chen, L. Molecular Modeling and Functional Mapping of B7-H1 and B7-DC Uncouple Costimulatory Function from PD-1 Interaction. J. Exp. Med. 2003, 197, 1083–1091. [Google Scholar] [CrossRef]
- Lin, D.Y.; Tanaka, Y.; Iwasaki, M.; Gittis, A.G.; Su, H.-P.; Mikami, B.; Okazaki, T.; Honjo, T.; Minato, N.; Garboczi, D.N. The PD-1/PD-L1 Complex Resembles the Antigen-Binding Fv Domains of Antibodies and T Cell Receptors. Proc. Natl. Acad. Sci. USA 2008, 105, 3011–3016. [Google Scholar] [CrossRef]
- Zarganes-Tzitzikas, T.; Konstantinidou, M.; Gao, Y.; Krzemien, D.; Zak, K.; Dubin, G.; Holak, T.A.; Dömling, A. Inhibitors of Programmed Cell Death 1 (PD-1): A Patent Review (2010–2015). Expert. Opin. Ther. Pat. 2016, 26, 973–977. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Butte, M.J.; Oyama, S. Modulators of Immunoinhibitory Receptor PD-1, and Methods of Use Thereof. U.S. Patent Application No. US13/519,621, 24 January 2013. [Google Scholar]
- Yeung, K.-S.; Connolly, T.P.; Frennesson, D.B.; Grant-Young, K.A.; Hewawasam, P.; Langley, D.R.; Meng, Z.; Mull, E.; Parcella, K.E.; Saulnier, M.G.; et al. Compounds Useful as Immunomodulators. U.S. Patent Application No. PCT/US2014/053695, 12 March 2015. [Google Scholar]
- Skalniak, L.; Zak, K.M.; Guzik, K.; Magiera, K.; Musielak, B.; Pachota, M.; Szelazek, B.; Kocik, J.; Grudnik, P.; Tomala, M.; et al. Small-Molecule Inhibitors of PD-1/PD-L1 Immune Checkpoint Alleviate the PD-L1-Induced Exhaustion of T-Cells. Oncotarget 2017, 8, 72167–72181. [Google Scholar] [CrossRef] [PubMed]
- Guzik, K.; Zak, K.M.; Grudnik, P.; Magiera, K.; Musielak, B.; Törner, R.; Skalniak, L.; Dömling, A.; Dubin, G.; Holak, T.A. Small-Molecule Inhibitors of the Programmed Cell Death-1/Programmed Death-Ligand 1 (PD-1/PD-L1) Interaction via Transiently Induced Protein States and Dimerization of PD-L1. J. Med. Chem. 2017, 60, 5857–5867. [Google Scholar] [CrossRef] [PubMed]
- Zak, K.M.; Grudnik, P.; Guzik, K.; Zieba, B.J.; Musielak, B.; Dömling, A.; Dubin, G.; Holak, T.A. Structural Basis for Small Molecule Targeting of the Programmed Death Ligand 1 (PD-L1). Oncotarget 2016, 7, 30323–30335. [Google Scholar] [CrossRef]
- Sasikumar, P.G.; Ramachandra, M. Small Molecule Agents Targeting PD-1 Checkpoint Pathway for Cancer Immunotherapy: Mechanisms of Action and Other Considerations for Their Advanced Development. Front. Immunol. 2022, 13, 752065. [Google Scholar] [CrossRef]
- Sasmal, P.; Prabitha, P.; Prashantha Kumar, B.R.; Swetha, B.R.; Babasahib, S.K.; Raghavendra, N.M. Beyond Peptides: Unveiling the Design Strategies, Structure Activity Correlations and Protein-Ligand Interactions of Small Molecule Inhibitors against PD-1/PD-L1. Bioorg. Chem. 2025, 154, 108036. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, X.; Liu, X.; Ouyang, Y.; Xu, C.; Shi, Y. Development of Small Molecule Drugs Targeting Immune Checkpoints. Cancer Biol. Med. 2024, 21, 382–399. [Google Scholar] [CrossRef]
- Jiang, J.; Zou, X.; Liu, Y.; Liu, X.; Dong, K.; Yao, X.; Feng, Z.; Chen, X.; Sheng, L.; Li, Y. Simultaneous Determination of a Novel PD-L1 Inhibitor, IMMH-010, and Its Active Metabolite, YPD-29B, in Rat Biological Matrices by Polarity-Switching Liquid Chromatography-Tandem Mass Spectrometry: Application to ADME Studies. Front. Pharmacol. 2021, 12, 677120. [Google Scholar] [CrossRef]
- Koblish, H.K.; Wu, L.; Wang, L.-C.S.; Liu, P.C.C.; Wynn, R.; Rios-Doria, J.; Spitz, S.; Liu, H.; Volgina, A.; Zolotarjova, N.; et al. Characterization of INCB086550: A Potent and Novel Small-Molecule PD-L1 Inhibitor. Cancer Discov. 2022, 12, 1482–1499. [Google Scholar] [CrossRef]
- Park, J.-J.; Thi, E.P.; Carpio, V.H.; Bi, Y.; Cole, A.G.; Dorsey, B.D.; Fan, K.; Harasym, T.; Iott, C.L.; Kadhim, S.; et al. Checkpoint Inhibition Through Small Molecule-Induced Internalization of Programmed Death-Ligand 1. Nat. Commun. 2021, 12, 1222. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.; Chen, H.; Feng, Z. Design, Synthesis, Evaluation, and SAR of 4-Phenylindoline Derivatives, a Novel Class of Small-Molecule Inhibitors of the Programmed Cell Death-1/Programmed Cell Death-Ligand 1 (PD-1/PD-L1) Interaction. Eur. J. Med. Chem. 2021, 211, 113001. [Google Scholar] [CrossRef]
- Xu, Y.; Du, H.; Guo, W.; Liu, B.; Yan, W.; Zhang, C.; Qin, L.; Huang, J.; Wang, H.; Wu, S.; et al. Discovery of Highly Potent Small-Molecule PD-1/PD-L1 Inhibitors with a Novel Scaffold for Cancer Immunotherapy. J. Med. Chem. 2024, 67, 4083–4099. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, P.G.N.; Ramachandra, M.; Naremaddepalli, S.S.S. 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Derivatives AS Immunomodulators. U.S. Patent Application No. PCT/IB2014/064281, 12 March 2015. [Google Scholar]
- Musielak, B.; Kocik, J.; Skalniak, L.; Magiera-Mularz, K.; Sala, D.; Czub, M.; Stec, M.; Siedlar, M.; Holak, T.A.; Plewka, J. CA-170—A Potent Small-Molecule PD-L1 Inhibitor or Not? Molecules 2019, 24, 2804. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.M.; Mapelli, C.; Allen, M.P.; Bowsher, M.S.; Boy, K.M.; Gillis, E.P.; Langley, D.R.; Mull, E.; Poirier, M.A.; Sanghvi, N.; et al. Macrocyclic Inhibitors of the PD-1/PD-L1 and CD80(B7-1)/PD-L1 Protein/Protein Interactions. U.S. Patent Application No. US14/201,977, 12 April 2016. [Google Scholar]
- Sasikumar, P.G.; Ramachandra, M. Small-Molecule Immune Checkpoint Inhibitors Targeting PD-1/PD-L1 and Other Emerging Checkpoint Pathways. BioDrugs 2018, 32, 481–497. [Google Scholar] [CrossRef]
- Sasikumar, P.G.; Ramachandra, M. Peptide and Peptide-Inspired Checkpoint Inhibitors: Protein Fragments to Cancer Immunotherapy. Med. Drug Discov. 2020, 8, 100073. [Google Scholar] [CrossRef]
- Shindo, Y.; McDonough, J.S.; Chang, K.C.; Ramachandra, M.; Sasikumar, P.G.; Hotchkiss, R.S. Anti-PD-L1 Peptide Improves Survival in Sepsis. J. Surg. Res. 2017, 208, 33–39. [Google Scholar] [CrossRef]
- Boohaker, R.J.; Sambandam, V.; Segura, I.; Miller, J.; Suto, M.; Xu, B. Rational Design and Development of a Peptide Inhibitor for the PD-1/PD-L1 Interaction. Cancer Lett. 2018, 434, 11–21. [Google Scholar] [CrossRef]
- Miller, M.M.; Mapelli, C.; Allen, M.P.; Bowsher, M.S.; Boy, K.M.; Gillis, E.P.; Langley, D.R.; Mull, E.; Poirier, M.A.; Sanghvi, N.; et al. Macrocyclic Inhibitors of The PD-1/PD-L1 And Cd80(B7-1)/Pd-L1 Protein/Protein Interactions. U.S. Patent Application No. PCT/US2014/026138, 25 September 2014. [Google Scholar]
- Miller, M.M.; Allen, M.P.; Bowsher, M.S.; Boy, K.M.; Gillis, E.P.; Langley, D.R.; Mull, E.; Sun, L.-Q.; Yeung, K.-S.; Reid, P.C.; et al. Macrocyclic Inhibitors of the PD-1/PD-L1 and CD80(B7-1)/PD-L1 Protein/Protein Interactions. U.S. Patent Application No. US15/204,627, January 2018. [Google Scholar]
- Magiera-Mularz, K.; Skalniak, L.; Zak, K.M.; Musielak, B.; Rudzinska-Szostak, E.; Berlicki, Ł.; Kocik, J.; Grudnik, P.; Sala, D.; Zarganes-Tzitzikas, T.; et al. Bioactive Macrocyclic Inhibitors of the PD-1/PD-L1 Immune Checkpoint. Angew. Chem. Int. Ed. 2017, 56, 13732–13735. [Google Scholar] [CrossRef]
- Rodriguez, I.; Kocik-Krol, J.; Skalniak, L.; Musielak, B.; Wisniewska, A.; Ciesiołkiewicz, A.; Berlicki, Ł.; Plewka, J.; Grudnik, P.; Stec, M.; et al. Structural and Biological Characterization of PAC65, a Macrocyclic Peptide That Blocks PD-L1 with Equivalent Potency to the FDA-Approved Antibodies. Mol. Cancer 2023, 22, 150. [Google Scholar] [CrossRef]
- Miao, Q.; Zhang, W.; Zhang, K.; Li, H.; Zhu, J.; Jiang, S. Rational Design of a Potent Macrocyclic Peptide Inhibitor Targeting the PD-1/PD-L1 Protein–Protein Interaction. RSC Adv. 2021, 11, 23270–23279. [Google Scholar] [CrossRef]
- Bhardwaj, G.; Mulligan, V.K.; Bahl, C.D.; Gilmore, J.M.; Harvey, P.J.; Cheneval, O.; Buchko, G.W.; Pulavarti, S.V.S.R.K.; Kaas, Q.; Eletsky, A.; et al. Accurate de Novo Design of Hyperstable Constrained Peptides. Nature 2016, 538, 329–335. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, X.; Huang, Y.-H.; King, G.J.; Collins, B.M.; Gao, Y.; Craik, D.J.; Wang, C.K. Rational Design of Potent Peptide Inhibitors of the PD-1:PD-L1 Interaction for Cancer Immunotherapy. J. Am. Chem. Soc. 2021, 143, 18536–18547. [Google Scholar] [CrossRef] [PubMed]
- Pascolutti, R.; Sun, X.; Kao, J.; Maute, R.L.; Ring, A.M.; Bowman, G.R.; Kruse, A.C. Structure and Dynamics of PD-L1 and an Ultra-High-Affinity PD-1 Receptor Mutant. Structure 2016, 24, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Ciesiołkiewicz, A.; Lizandra Perez, J.; Skalniak, L.; Noceń, P.; Berlicki, Ł. Miniprotein Engineering for Inhibition of PD-1/PD-L1 Interaction. Protein Sci. 2024, 33, e5106. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Z.; Zhang, L.; Li, Y.; Jain, A.; Barve, A.; Jin, W.; Liu, Y.; Fetse, J.; Cheng, K. Discovery of Low-Molecular Weight Anti-PD-L1 Peptides for Cancer Immunotherapy. J. Immunother. Cancer 2019, 7, 270. [Google Scholar] [CrossRef]
- Li, C.; Zhang, N.; Zhou, J.; Ding, C.; Jin, Y.; Cui, X.; Pu, K.; Zhu, Y. Peptide Blocking of PD-1/PD-L1 Interaction for Cancer Immunotherapy. Cancer Immunol. Res. 2018, 6, 178–188. [Google Scholar] [CrossRef]
- Qin, Y.; Meng, X.; Li, L.; Liu, C.; Gao, F.; Yuan, X.; Huang, Y.; Zhu, Y. Develop a PD-1-Blockade Peptide to Reinvigorate T-Cell Activity and Inhibit Tumor Progress. Eur. J. Pharmacol. 2023, 960, 176144. [Google Scholar] [CrossRef]
- Yu, W.; He, X.; Yang, Z.; Yang, X.; Xiao, W.; Liu, R.; Xie, R.; Qin, L.; Gao, H. Sequentially Responsive Biomimetic Nanoparticles with Optimal Size in Combination with Checkpoint Blockade for Cascade Synergetic Treatment of Breast Cancer and Lung Metastasis. Biomaterials 2019, 217, 119309. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Shi, K.; Xiao, Y.; Liu, Q.; Han, R.; Wei, X.; Qian, Z. Rationally Designed Peptide-Conjugated Gold/Platinum Nanosystem with Active Tumor-Targeting for Enhancing Tumor Photothermal-Immunotherapy. J. Control. Release 2019, 308, 29–43. [Google Scholar] [CrossRef]
- Li, Q.; Quan, L.; Lyu, J.; He, Z.; Wang, X.; Meng, J.; Zhao, Z.; Zhu, L.; Liu, X.; Li, H. Discovery of Peptide Inhibitors Targeting Human Programmed Death 1 (PD-1) Receptor. Oncotarget 2016, 7, 64967–64976. [Google Scholar] [CrossRef]
- Cheng, B.; Ren, Y.; Cao, H.; Chen, J. Discovery of Novel Resorcinol Diphenyl Ether-Based PROTAC-like Molecules as Dual Inhibitors and Degraders of PD-L1. Eur. J. Med. Chem. 2020, 199, 112377. [Google Scholar] [CrossRef]
- Banik, S.M.; Pedram, K.; Wisnovsky, S.; Ahn, G.; Riley, N.M.; Bertozzi, C.R. Lysosome-Targeting Chimaeras for Degradation of Extracellular Proteins. Nature 2020, 584, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Qi, Z.; Guo, J.; Shen, G.; Ni, X.; Jiang, S.; Zhang, K.; Wang, T.; Zhang, X. Discovery of Small Molecules for Autophagy-Lysosome Degradation of Immune Checkpoint Proteins. Eur. J. Med. Chem. 2024, 280, 116958. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.-Y.; Shi, Y.-Y.; Wang, A.-J.; Liu, X.-L.; Liu, M.; Cai, H.-B. High-Potency PD-1/PD-L1 Degradation Induced by Peptide-PROTAC in Human Cancer Cells. Cell Death Dis. 2022, 13, 924. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rubinstein, R.; Lines, J.L.; Wasiuk, A.; Ahonen, C.; Guo, Y.; Lu, L.-F.; Gondek, D.; Wang, Y.; Fava, R.A.; et al. VISTA, a Novel Mouse Ig Superfamily Ligand That Negatively Regulates T Cell Responses. J. Exp. Med. 2011, 208, 577–592. [Google Scholar] [CrossRef]
- Xie, S.; Huang, J.; Qiao, Q.; Zang, W.; Hong, S.; Tan, H.; Dong, C.; Yang, Z.; Ni, L. Expression of the Inhibitory B7 Family Molecule VISTA in Human Colorectal Carcinoma Tumors. Cancer Immunol. Immunother. 2018, 67, 1685–1694. [Google Scholar] [CrossRef]
- Flies, D.B.; Wang, S.; Xu, H.; Chen, L. Cutting Edge: A Monoclonal Antibody Specific for the Programmed Death-1 Homolog Prevents Graft-versus-Host Disease in Mouse Models. J. Immunol. 2011, 187, 1537–1541. [Google Scholar] [CrossRef]
- Yoon, K.W.; Byun, S.; Kwon, E.; Hwang, S.-Y.; Chu, K.; Hiraki, M.; Jo, S.-H.; Weins, A.; Hakroush, S.; Cebulla, A.; et al. Control of Signaling-Mediated Clearance of Apoptotic Cells by the Tumor Suppressor P53. Science 2015, 349, 1261669. [Google Scholar] [CrossRef]
- Sakr, M.A.; Takino, T.; Domoto, T.; Nakano, H.; Wong, R.W.; Sasaki, M.; Nakanuma, Y.; Sato, H. GI24 Enhances Tumor Invasiveness by Regulating Cell Surface Membrane-Type 1 Matrix Metalloproteinase. Cancer Sci. 2010, 101, 2368–2374. [Google Scholar] [CrossRef]
- Aloia, L.; Parisi, S.; Fusco, L.; Pastore, L.; Russo, T. Differentiation of Embryonic Stem Cells 1 (Dies1) Is a Component of Bone Morphogenetic Protein 4 (BMP4) Signaling Pathway Required for Proper Differentiation of Mouse Embryonic Stem Cells. J. Biol. Chem. 2010, 285, 7776–7783. [Google Scholar] [CrossRef]
- Yang, Y.-S. Sisp-1, A Novel P53 Target Gene and Use Thereof 2007. U.S. Patent Application No. US11/679,803, 27 February 2007. [Google Scholar]
- Emaldi, M.; Alamillo-Maeso, P.; Rey-Iborra, E.; Mosteiro, L.; Lecumberri, D.; Pulido, R.; López, J.I.; Nunes-Xavier, C.E. A Functional Role for Glycosylated B7-H5/VISTA Immune Checkpoint Protein in Metastatic Clear Cell Renal Cell Carcinoma. iScience 2024, 27, 110587. [Google Scholar] [CrossRef]
- Slater, B.T.; Han, X.; Chen, L.; Xiong, Y. Structural Insight into T Cell Coinhibition by PD-1H (VISTA). Proc. Natl. Acad. Sci. USA 2020, 117, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Thisted, T.; Smith, F.D.; Mukherjee, A.; Kleschenko, Y.; Feng, F.; Jiang, Z.-G.; Eitas, T.; Malhotra, K.; Biesova, Z.; Onumajuru, A.; et al. VISTA Checkpoint Inhibition by PH-Selective Antibody SNS-101 with Optimized Safety and Pharmacokinetic Profiles Enhances PD-1 Response. Nat. Commun. 2024, 15, 2917. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.J.; Su, L.J.; Pinckney, J.; Critton, D.; Boyer, E.; Krishnakumar, A.; Corbett, M.; Rankin, A.L.; Dibella, R.; Campbell, L.; et al. VISTA Is an Acidic PH-Selective Ligand for PSGL-1. Nature 2019, 574, 565–570. [Google Scholar] [CrossRef]
- Mehta, N.; Maddineni, S.; Mathews, I.I.; Andres Parra Sperberg, R.; Huang, P.-S.; Cochran, J.R. Structure and Functional Binding Epitope of V-Domain Ig Suppressor of T Cell Activation. Cell Rep. 2019, 28, 2509–2516.e5. [Google Scholar] [CrossRef]
- Nowak, E.C.; Lines, J.L.; Varn, F.S.; Deng, J.; Sarde, A.; Mabaera, R.; Kuta, A.; Le Mercier, I.; Cheng, C.; Noelle, R.J. Immunoregulatory Functions of VISTA. Immunol. Rev. 2017, 276, 66–79. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Freeman, G.J. Acidity Changes Immunology: A New VISTA Pathway. Nat. Immunol. 2020, 21, 13–16. [Google Scholar] [CrossRef]
- Xu, W.; Hiếu, T.; Malarkannan, S.; Wang, L. The Structure, Expression, and Multifaceted Role of Immune-Checkpoint Protein VISTA as a Critical Regulator of Anti-Tumor Immunity, Autoimmunity, and Inflammation. Cell. Mol. Immunol. 2018, 15, 438–446. [Google Scholar] [CrossRef]
- Le Mercier, I.; Lines, J.L.; Noelle, R.J. Beyond CTLA-4 and PD-1, the Generation Z of Negative Checkpoint Regulators. Front. Immunol. 2015, 6, 418. [Google Scholar] [CrossRef]
- Borggrewe, M.; Grit, C.; Den Dunnen, W.F.A.; Burm, S.M.; Bajramovic, J.J.; Noelle, R.J.; Eggen, B.J.L.; Laman, J.D. VISTA Expression by Microglia Decreases during Inflammation and Is Differentially Regulated in CNS Diseases. Glia 2018, 66, 2645–2658. [Google Scholar] [CrossRef]
- Ren, R.; Chang, X.; Chen, C.; Yu, H.; Han, L. VISTA as a Prospective Immune Checkpoint in Gynecological Malignant Tumors: A Review of the Literature. Open Medicine 2023, 18, 20230866. [Google Scholar] [CrossRef]
- Lines, J.L.; Pantazi, E.; Mak, J.; Sempere, L.F.; Wang, L.; O’Connell, S.; Ceeraz, S.; Suriawinata, A.A.; Yan, S.; Ernstoff, M.S.; et al. VISTA Is an Immune Checkpoint Molecule for Human T Cells. Cancer Res. 2014, 74, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Bharaj, P.; Chahar, H.S.; Alozie, O.K.; Rodarte, L.; Bansal, A.; Goepfert, P.A.; Dwivedi, A.; Manjunath, N.; Shankar, P. Characterization of Programmed Death-1 Homologue-1 (PD-1H) Expression and Function in Normal and HIV Infected Individuals. PLoS ONE 2014, 9, e109103. [Google Scholar] [CrossRef] [PubMed]
- Prodeus, A.; Abdul-Wahid, A.; Sparkes, A.; Fischer, N.W.; Cydzik, M.; Chiang, N.; Alwash, M.; Ferzoco, A.; Vacaresse, N.; Julius, M.; et al. VISTA.COMP—An Engineered Checkpoint Receptor Agonist That Potently Suppresses T Cell–Mediated Immune Responses. JCI Insight 2017, 2, e94308. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Le Mercier, I.; Kuta, A.; Noelle, R.J. A New VISTA on Combination Therapy for Negative Checkpoint Regulator Blockade. J. Immunother. Cancer 2016, 4, 86. [Google Scholar] [CrossRef]
- Gray, C.C.; Biron-Girard, B.; Wakeley, M.E.; Chung, C.-S.; Chen, Y.; Quiles-Ramirez, Y.; Tolbert, J.D.; Ayala, A. Negative Immune Checkpoint Protein, VISTA, Regulates the CD4+ Treg Population During Sepsis Progression to Promote Acute Sepsis Recovery and Survival. Front. Immunol. 2022, 13, 861670. [Google Scholar] [CrossRef]
- Hid Cadena, R.; Reitsema, R.D.; Huitema, M.G.; van Sleen, Y.; van der Geest, K.S.M.; Heeringa, P.; Boots, A.M.H.; Abdulahad, W.H.; Brouwer, E. Decreased Expression of Negative Immune Checkpoint VISTA by CD4+ T Cells Facilitates T Helper 1, T Helper 17, and T Follicular Helper Lineage Differentiation in GCA. Front. Immunol. 2019, 10, 1638. [Google Scholar] [CrossRef]
- Jlassi, A.; Manai, M.; Morjen, M.; Sahraoui, G.; Elasmi Allal, M.; ELBini-Dhouib, I.; Naija, L.; Charfi, L.; Rejaibi, R.; Ben Ahmed, M.; et al. VISTA+/CD8+ Status Correlates with Favorable Prognosis in Epithelial Ovarian Cancer. PLoS ONE 2023, 18, e0278849. [Google Scholar] [CrossRef]
- Le Mercier, I.; Chen, W.; Lines, J.L.; Day, M.; Li, J.; Sergent, P.; Noelle, R.J.; Wang, L. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014, 74, 1933–1944. [Google Scholar] [CrossRef]
- Yum, J.-E.I.; Hong, Y.-K. Terminating Cancer by Blocking VISTA as a Novel Immunotherapy: Hasta La Vista, Baby. Front. Oncol. 2021, 11, 658488. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, Y.; Liu, W.; He, X.; Chen, W.; Liu, L.; Yang, L.; Wang, Y.; Wu, Y.; Liu, J. Transcriptome Profiling Reveals Transcriptional Regulation of VISTA in T Cell Activation. Mol. Immunol. 2023, 157, 101–111. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Liu, J.; Wei, Y.; Wang, X.; Fang, H.; Du, H.; Huang, J.; Li, Q.; Ren, G.; et al. Aldehyde Dehydrogenase 2-Mediated Aldehyde Metabolism Promotes Tumor Immune Evasion by Regulating the NOD/VISTA Axis. J. Immunother. Cancer 2023, 11, e007487. [Google Scholar] [CrossRef] [PubMed]
- ElTanbouly, M.A.; Croteau, W.; Noelle, R.J.; Lines, J.L. VISTA: A Novel Immunotherapy Target for Normalizing Innate and Adaptive Immunity. Semin. Immunol. 2019, 42, 101308. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, J.; Sarde, A.; Lines, J.L.; Lee, Y.-C.; Qian, D.C.; Pechenick, D.A.; Manivanh, R.; Le Mercier, I.; Lowrey, C.H.; et al. Hypoxia-Induced VISTA Promotes the Suppressive Function of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Cancer Immunol. Res. 2019, 7, 1079–1090. [Google Scholar] [CrossRef]
- Rosenbaum, S.R.; Knecht, M.; Mollaee, M.; Zhong, Z.; Erkes, D.A.; McCue, P.A.; Chervoneva, I.; Berger, A.C.; Lo, J.A.; Fisher, D.E.; et al. FOXD3 Regulates VISTA Expression in Melanoma. Cell Rep. 2020, 30, 510–524.e6. [Google Scholar] [CrossRef]
- Martin, A.S.; Molloy, M.; Ugolkov, A.; von Roemeling, R.W.; Noelle, R.J.; Lewis, L.D.; Johnson, M.; Radvanyi, L.; Martell, R.E. VISTA Expression and Patient Selection for Immune-Based Anticancer Therapy. Front. Immunol. 2023, 14, 1086102. [Google Scholar] [CrossRef]
- Mutsaers, P.; Balcioglu, H.E.; Kuiper, R.; Hammerl, D.; Wijers, R.; van Duin, M.; van der Holt, B.; Broijl, A.; Gregory, W.; Zweegman, S.; et al. V-Domain Ig Suppressor of T Cell Activation (VISTA) Expression Is an Independent Prognostic Factor in Multiple Myeloma. Cancers 2021, 13, 2219. [Google Scholar] [CrossRef]
- ElTanbouly, M.A.; Zhao, Y.; Nowak, E.; Li, J.; Schaafsma, E.; Le Mercier, I.; Ceeraz, S.; Lines, J.L.; Peng, C.; Carriere, C.; et al. VISTA Is a Checkpoint Regulator for Naïve T Cell Quiescence and Peripheral Tolerance. Science 2020, 367, eaay0524. [Google Scholar] [CrossRef]
- Sergent, P.; Plummer, S.; Pettus, J.; Mabaera, R.; DeLong, J.; Pechenick, D.; Burns, C.; Noelle, R.; Ceeraz, S. Blocking the VISTA Pathway Enhances Disease Progression in (NZB × NZW) F1 Female Mice. Lupus 2018, 27, 210–216. [Google Scholar] [CrossRef]
- Borggrewe, M.; Kooistra, S.M.; Wesseling, E.M.; Gierschek, F.L.; Brummer, M.L.; Nowak, E.C.; Medeiros-Furquim, T.; Otto, T.A.; Lee, S.W.; Noelle, R.J.; et al. VISTA Regulates Microglia Homeostasis and Myelin Phagocytosis, and Is Associated with MS Lesion Pathology. Acta Neuropathol. Commun. 2021, 9, 91. [Google Scholar] [CrossRef]
- Derakhshani, A.; Asadzadeh, Z.; Baradaran, B.; Safarpour, H.; Rahmani, S.; Leone, P.; Abdoli Shadbad, M.; Hosseinkhani, N.; Ghasemigol, M.; Ayromlou, H.; et al. The Expression Pattern of VISTA in the PBMCs of Relapsing-Remitting Multiple Sclerosis Patients: A Single-Cell RNA Sequencing-Based Study. Biomed. Pharmacother. 2022, 148, 112725. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Ji, K.; Jiang, S.; Dong, Y.; Sun, L.; Wang, J.; Hu, G.; Chen, D.; Chen, K.; et al. CD39 Inhibition and VISTA Blockade May Overcome Radiotherapy Resistance by Targeting Exhausted CD8+ T Cells and Immunosuppressive Myeloid Cells. Cell Rep. Med. 2023, 4, 101151. [Google Scholar] [CrossRef] [PubMed]
- Borggrewe, M.; Kooistra, S.M.; Noelle, R.J.; Eggen, B.J.L.; Laman, J.D. Exploring the VISTA of Microglia: Immune Checkpoints in CNS Inflammation. J. Mol. Med. 2020, 98, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Z.; Jin, K.; Shao, F.; Zeng, H.; Wang, Y.; Zhu, Y.; Xu, L.; Wang, Z.; Chang, Y.; et al. Immune Inactivation by VISTA Predicts Clinical Outcome and Therapeutic Benefit in Muscle-Invasive Bladder Cancer. BMC Cancer 2023, 23, 661. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, X.; Li, E.; Zhang, G.; Wang, X.; Tang, T.; Bai, X.; Liang, T. VISTA: An Immune Regulatory Protein Checking Tumor and Immune Cells in Cancer Immunotherapy. J. Hematol. Oncol. 2020, 13, 83. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, K.; Zhang, Z.; Gu, Y.; Gu, Y.; Li, W.; Zhang, W.; Shen, Z.; Xu, J.; Qin, J. Blockade of V-domain Immunoglobulin Suppressor of T-cell Activation Reprograms Tumour-associated Macrophages and Improves Efficacy of PD-1 Inhibitor in Gastric Cancer. Clin. Transl. Med. 2024, 14, e1578. [Google Scholar] [CrossRef]
- Liao, H.; Zhu, H.; Liu, S.; Wang, H. Expression of V-domain Immunoglobulin Suppressor of T Cell Activation Is Associated with the Advanced Stage and Presence of Lymph Node Metastasis in Ovarian Cancer. Oncol. Lett. 2018, 16, 3465–3472. [Google Scholar] [CrossRef]
- Mulati, K.; Hamanishi, J.; Matsumura, N.; Chamoto, K.; Mise, N.; Abiko, K.; Baba, T.; Yamaguchi, K.; Horikawa, N.; Murakami, R.; et al. VISTA Expressed in Tumour Cells Regulates T Cell Function. Br. J. Cancer 2019, 120, 115–127. [Google Scholar] [CrossRef]
- Zong, L.; Zhou, Y.; Zhang, M.; Chen, J.; Xiang, Y. VISTA Expression Is Associated with a Favorable Prognosis in Patients with High-Grade Serous Ovarian Cancer. Cancer Immunol. Immunother. 2020, 69, 33–42. [Google Scholar] [CrossRef]
- Jlassi, A.; Rejaibi, R.; Manai, M.; Sahraoui, G.; Guerfali, F.Z.; Charfi, L.; Mezlini, A.; Manai, M.; Mrad, K.; Doghri, R. VISTA/CTLA4/PD1 Coexpression on Tumor Cells Confers a Favorable Immune Microenvironment and Better Prognosis in High-Grade Serous Ovarian Carcinoma. Front. Oncol. 2024, 14, 1352053. [Google Scholar] [CrossRef]
- Kakavand, H.; Jackett, L.A.; Menzies, A.M.; Gide, T.N.; Carlino, M.S.; Saw, R.P.M.; Thompson, J.F.; Wilmott, J.S.; Long, G.V.; Scolyer, R.A. Negative Immune Checkpoint Regulation by VISTA: A Mechanism of Acquired Resistance to Anti-PD-1 Therapy in Metastatic Melanoma Patients. Mod. Pathol. 2017, 30, 1666–1676. [Google Scholar] [CrossRef]
- Duval, K.E.A.; Tavakkoli, A.D.; Kheirollah, A.; Soderholm, H.E.; Demidenko, E.; Lines, J.L.; Croteau, W.; Zhang, S.C.; Wagner, R.J.; Aulwes, E.; et al. Enhancement of Radiation Therapy Through Blockade of the Immune Checkpoint, V-Domain Ig Suppressor of T Cell Activation (VISTA), in Melanoma and Adenocarcinoma Murine Models. Int. J. Mol. Sci. 2023, 24, 13742. [Google Scholar] [CrossRef] [PubMed]
- Blando, J.; Sharma, A.; Higa, M.G.; Zhao, H.; Vence, L.; Yadav, S.S.; Kim, J.; Sepulveda, A.M.; Sharp, M.; Maitra, A.; et al. Comparison of Immune Infiltrates in Melanoma and Pancreatic Cancer Highlights VISTA as a Potential Target in Pancreatic Cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Pan, Y.; Fei, Q.; Lin, Y.; Zhou, Y.; Liu, Y.; Guan, H.; Yu, X.; Lin, X.; Lu, F.; et al. Prognostic Significance and Therapeutic Potential of the Immune Checkpoint VISTA in Pancreatic Cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Popp, F.; Capino, I.; Bartels, J.; Damanakis, A.; Li, J.; Datta, R.; Löser, H.; Zhao, Y.; Quaas, A.; Lohneis, P.; et al. Expression of Immune Checkpoint Regulators IDO, VISTA, LAG3, and TIM3 in Resected Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 2689. [Google Scholar] [CrossRef]
- Zong, L.; Mo, S.; Yu, S.; Zhou, Y.; Zhang, M.; Chen, J.; Xiang, Y. Expression of the Immune Checkpoint VISTA in Breast Cancer. Cancer Immunol. Immunother. 2020, 69, 1437–1446. [Google Scholar] [CrossRef]
- Olbromski, M.; Mrozowska, M.; Piotrowska, A.; Smolarz, B.; Romanowicz, H. The VISTA/VSIG3/PSGL-1 Axis: Crosstalk Between Immune Effector Cells and Cancer Cells in Invasive Ductal Breast Carcinoma. Cancer Immunol. Immunother. 2024, 73, 136. [Google Scholar] [CrossRef]
- Pilones, K.A.; Hensler, M.; Daviaud, C.; Kraynak, J.; Fucikova, J.; Galluzzi, L.; Demaria, S.; Formenti, S.C. Converging Focal Radiation and Immunotherapy in a Preclinical Model of Triple Negative Breast Cancer: Contribution of VISTA Blockade. Oncoimmunology 2020, 9, 1830524. [Google Scholar] [CrossRef]
- Muñoz Perez, N.; Pensabene, J.M.; Galbo, P.M.; Sadeghipour, N.; Xiu, J.; Moziak, K.; Yazejian, R.M.; Welch, R.L.; Bell, W.R.; Sengupta, S.; et al. VISTA Emerges as a Promising Target against Immune Evasion Mechanisms in Medulloblastoma. Cancers 2024, 16, 2629. [Google Scholar] [CrossRef]
- Nishizaki, D.; Kurzrock, R.; Miyashita, H.; Adashek, J.J.; Lee, S.; Nikanjam, M.; Eskander, R.N.; Patel, H.; Botta, G.P.; Nesline, M.K.; et al. Viewing the Immune Checkpoint VISTA: Landscape and Outcomes across Cancers. ESMO Open 2024, 9, 102942. [Google Scholar] [CrossRef]
- ElTanbouly, M.A.; Schaafsma, E.; Noelle, R.J.; Lines, J.L. VISTA: Coming of Age as a Multi-Lineage Immune Checkpoint. Clin. Exp. Immunol. 2020, 200, 120–130. [Google Scholar] [CrossRef]
- Im, E.; Sim, D.Y.; Lee, H.-J.; Park, J.E.; Park, W.Y.; Ko, S.; Kim, B.; Shim, B.S.; Kim, S.-H. Immune Functions as a Ligand or a Receptor, Cancer Prognosis Potential, Clinical Implication of VISTA in Cancer Immunotherapy. Semin. Cancer Biol. 2022, 86, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Molberg, K.; Carrick, K.; Niu, S.; Rivera Colon, G.; Gwin, K.; Lewis, C.; Lea, J.; Panwar, V.; Zheng, W.; et al. Expression and Prognostic Significance of LAG-3, TIGIT, VISTA, and IDO1 in Endometrial Serous Carcinoma. Mod. Pathol. 2024, 37, 100532. [Google Scholar] [CrossRef] [PubMed]
- Noelle, R.J.; Lines, J.L.; Lewis, L.D.; Martell, R.E.; Guillaudeux, T.; Lee, S.W.; Mahoney, K.M.; Vesely, M.D.; Boyd-Kirkup, J.; Nambiar, D.K.; et al. Clinical and Research Updates on the VISTA Immune Checkpoint: Immuno-Oncology Themes and Highlights. Front. Oncol. 2023, 13, 1225081. [Google Scholar] [CrossRef]
- Chmiel, P.; Gęca, K.; Michalski, A.; Kłosińska, M.; Kaczyńska, A.; Polkowski, W.P.; Pelc, Z.; Skórzewska, M. Vista of the Future: Novel Immunotherapy Based on the Human V-Set Immunoregulatory Receptor for Digestive System Tumors. Int. J. Mol. Sci. 2023, 24, 9945. [Google Scholar] [CrossRef]
- Tagliamento, M.; Bironzo, P.; Novello, S. New Emerging Targets in Cancer Immunotherapy: The Role of VISTA. ESMO Open 2019, 4, e000683. [Google Scholar] [CrossRef]
- Mostböck, S.; Wu, H.H.; Fenn, T.; Riegler, B.; Strahlhofer, S.; Huang, Y.; Hansen, G.; Kroe-Barrett, R.; Tirapu, I.; Vogt, A.B. Distinct Immune Stimulatory Effects of Anti-Human VISTA Antibodies Are Determined by Fc-Receptor Interaction. Front. Immunol. 2022, 13, 862757. [Google Scholar] [CrossRef]
- Ta, H.M.; Roy, D.; Zhang, K.; Alban, T.; Juric, I.; Dong, J.; Parthasarathy, P.B.; Patnaik, S.; Delaney, E.; Gilmour, C.; et al. LRIG1 Engages Ligand VISTA and Impairs Tumor-Specific CD8+ T Cell Responses. Sci. Immunol. 2024, 9, eadi7418. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Xiong, Y.-L.; Shi, X.-G.; Zhao, Y.-B.; Shi, A.-P.; Zheng, K.-F.; Liu, Y.-J.; Jiang, T.; Ma, N.; Zhao, J.-B. IGSF11 and VISTA: A Pair of Promising Immune Checkpoints in Tumor Immunotherapy. Biomark. Res. 2022, 10, 49. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Chen, W.; Jiang, J.; Wang, L.; Li, T.; Sun, H.; Liu, J. Structural Basis of VSIG3: The Ligand for VISTA. Front. Immunol. 2021, 12, 625808. [Google Scholar] [CrossRef]
- Abadier, M.; Ley, K. P-Selectin Glycoprotein Ligand-1 in T Cells. Curr. Opin. Hematol. 2017, 24, 265–273. [Google Scholar] [CrossRef]
- Carlow, D.A.; Gossens, K.; Naus, S.; Veerman, K.M.; Seo, W.; Ziltener, H.J. PSGL-1 Function in Immunity and Steady State Homeostasis. Immunol. Rev. 2009, 230, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, G.; Manick, B.; Hernandez, V.; Renelt, M.; Erickson, C.; Guan, J.; Singh, R.; Rollins, S.; Solorz, A.; et al. VSIG-3 as a Ligand of VISTA Inhibits Human T-cell Function. Immunology 2019, 156, 74–85. [Google Scholar] [CrossRef] [PubMed]
- ElTanbouly, M.A.; Zhao, Y.; Schaafsma, E.; Burns, C.M.; Mabaera, R.; Cheng, C.; Noelle, R.J. VISTA: A Target to Manage the Innate Cytokine Storm. Front. Immunol. 2021, 11, 595950. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, D.; Paliwal, S.; Dharmadhikari, B.; Guan, S.; Liu, L.; Kar, S.; Tulsian, N.K.; Gruber, J.J.; DiMascio, L.; Paszkiewicz, K.H.; et al. Rationally Targeted Anti-VISTA Antibody That Blockades the C-C′ Loop Region Can Reverse VISTA Immune Suppression and Remodel the Immune Microenvironment to Potently Inhibit Tumor Growth in an Fc Independent Manner. J. Immunother. Cancer 2022, 10, e003382. [Google Scholar] [CrossRef]
- Moore, K.L. Structure and Function of P-Selectin Glycoprotein Ligand-1. Leuk. Lymphoma 1998, 29, 1–15. [Google Scholar] [CrossRef]
- Baïsse, B.; Galisson, F.; Giraud, S.; Schapira, M.; Spertini, O. Evolutionary Conservation of P-Selectin Glycoprotein Ligand-1 Primary Structure and Function. BMC Evol. Biol. 2007, 7, 166. [Google Scholar] [CrossRef]
- Tinoco, R.; Otero, D.C.; Takahashi, A.A.; Bradley, L.M. PSGL-1: A New Player in the Immune Checkpoint Landscape. Trends Immunol. 2017, 38, 323–335. [Google Scholar] [CrossRef]
- Díaz-García, E.; García-Sánchez, A.; Alfaro, E.; López-Fernández, C.; Mañas, E.; Cano-Pumarega, I.; López-Collazo, E.; García-Río, F.; Cubillos-Zapata, C. PSGL-1: A Novel Immune Checkpoint Driving T-Cell Dysfunction in Obstructive Sleep Apnea. Front. Immunol. 2023, 14, 1277551. [Google Scholar] [CrossRef]
- Pan, J.; Chen, Y.; Zhang, Q.; Khatun, A.; Palen, K.; Xin, G.; Wang, L.; Yang, C.; Johnson, B.D.; Myers, C.R.; et al. Inhibition of Lung Tumorigenesis by a Small Molecule CA170 Targeting the Immune Checkpoint Protein VISTA. Commun. Biol. 2021, 4, 906. [Google Scholar] [CrossRef]
- Sasikumar, P.G.; Sudarshan, N.S.; Adurthi, S.; Ramachandra, R.K.; Samiulla, D.S.; Lakshminarasimhan, A.; Ramanathan, A.; Chandrasekhar, T.; Dhudashiya, A.A.; Talapati, S.R.; et al. PD-1 Derived CA-170 Is an Oral Immune Checkpoint Inhibitor That Exhibits Preclinical Anti-Tumor Efficacy. Commun. Biol. 2021, 4, 699. [Google Scholar] [CrossRef]
- Gabr, M.T.; Gambhir, S.S. Discovery and Optimization of Small-Molecule Ligands for V-Domain Ig Suppressor of T-Cell Activation (VISTA). J. Am. Chem. Soc. 2020, 142, 16194–16198. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, Y.; Yang, H.; Wang, X.; Zeng, X.; Zhuang, R.; Du, J.; Zhang, X.; Guo, Z. Small-Molecule Radiotracers for Visualization of V-Domain Immunoglobulin Suppressor of T Cell Activation. J. Med. Chem. 2024, 67, 17690–17700. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, K.; Zhang, Y.; Zhang, K.; Cai, S.; Jiang, S.; Xiao, Y.; Zhang, X. Novel Benzimidazoles as Potent Small-Molecule Inhibitors and Degraders of V-Domain Ig Suppressor of T-Cell Activation (VISTA). J. Med. Chem. 2023, 66, 11881–11892. [Google Scholar] [CrossRef]
- Li, T.; Jiang, J.; Qie, C.; Xuan, C.; Hu, X.; Liu, W.; Chen, W.; Liu, J. Identification of Active Small-Molecule Modulators Targeting the Novel Immune Checkpoint VISTA. BMC Immunol. 2021, 22, 55. [Google Scholar] [CrossRef]
- Sun, C.; He, Y.; Wang, G.; Zhang, G.; Zhang, Y.; Shen, H.; Hu, L.; Sun, Y.; Jiang, B.; Wang, X.; et al. Design, Synthesis, and Antitumor Activity Evaluation of Novel VISTA Small Molecule Inhibitors. J. Med. Chem. 2024, 67, 3590–3605. [Google Scholar] [CrossRef]
- Shahab, M.; Al-Madhagi, H.; Zheng, G.; Zeb, A.; Alasmari, A.F.; Alharbi, M.; Alasmari, F.; Khan, M.Q.; Khan, M.; Wadood, A. Structure Based Virtual Screening and Molecular Simulation Study of FDA-Approved Drugs to Inhibit Human HDAC6 and VISTA as Dual Cancer Immunotherapy. Sci. Rep. 2023, 13, 14466. [Google Scholar] [CrossRef]
- Muneer, I.; Ahmad, S.; Naz, A.; Abbasi, S.W.; Alblihy, A.; Aloliqi, A.A.; Aba Alkhayl, F.F.; Alrumaihi, F.; Ahmad, S.; El Bakri, Y.; et al. Discovery of Novel Inhibitors from Medicinal Plants for V-Domain Ig Suppressor of T-Cell Activation. Front. Mol. Biosci. 2021, 8, 716735. [Google Scholar] [CrossRef]
- Hu, X.; Qie, C.; Jiang, J.; Xie, X.; Chen, W.; Liu, W.; Liu, J. M351-0056 Is a Novel Low MW Compound Modulating the Actions of the Immune-checkpoint Protein VISTA. Br. J. Pharmacol. 2021, 178, 1445–1458. [Google Scholar] [CrossRef]
- Niu, X.; Wu, M.; Li, G.; Zhou, X.; Cao, W.; Zhai, W.; Wu, A.; Zhou, X.; Jin, S.; Chen, G.; et al. Identification and Optimization of Peptide Inhibitors to Block VISTA/PSGL-1 Interaction for Cancer Immunotherapy. Acta Pharm. Sin. B 2023, 13, 4511–4522. [Google Scholar] [CrossRef]
- Chemnitz, J.M.; Lanfranco, A.R.; Braunstein, I.; Riley, J.L. B and T Lymphocyte Attenuator-Mediated Signal Transduction Provides a Potent Inhibitory Signal to Primary Human CD4 T Cells That Can Be Initiated by Multiple Phosphotyrosine Motifs. J. Immunol. 2006, 176, 6603–6614. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Liang, J.; Zhang, W.; Tanaka, Y.; Sugiyama, H. Immunotherapies: The Blockade of Inhibitory Signals. Int. J. Biol. Sci. 2012, 8, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Bernard, D.; Hansen, J.; Dupasquier, L.; Lefranc, M.; Benmansour, A.; Boudinot, P. Costimulatory Receptors in Jawed Vertebrates: Conserved CD28, Odd CTLA4 and Multiple BTLAs. Dev. Comp. Immunol. 2007, 31, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.C.; Loyet, K.M.; Calemine-Fenaux, J.; Chauhan, V.; Wranik, B.; Ouyang, W.; Eaton, D.L. A Coreceptor Interaction Between the CD28 and TNF Receptor Family Members B and T Lymphocyte Attenuator and Herpesvirus Entry Mediator. Proc. Natl. Acad. Sci. USA 2005, 102, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Gavrieli, M.; Sedy, J.R.; Yang, J.; Fallarino, F.; Loftin, S.K.; Hurchla, M.A.; Zimmerman, N.; Sim, J.; Zang, X.; et al. BTLA Is a Lymphocyte Inhibitory Receptor with Similarities to CTLA-4 and PD-1. Nat. Immunol. 2003, 4, 670–679. [Google Scholar] [CrossRef]
- Tao, R.; Wang, L.; Han, R.; Wang, T.; Ye, Q.; Honjo, T.; Murphy, T.L.; Murphy, K.M.; Hancock, W.W. Differential Effects of B and T Lymphocyte Attenuator and Programmed Death-1 on Acceptance of Partially versus Fully MHC-Mismatched Cardiac Allografts. J. Immunol. 2005, 175, 5774–5782. [Google Scholar] [CrossRef]
- Truong, W.; Plester, J.C.; Hancock, W.W.; Merani, S.; Murphy, T.L.; Murphy, K.M.; Kaye, J.; Anderson, C.C.; Shapiro, A.M.J. Combined Coinhibitory and Costimulatory Modulation with Anti-BTLA and CTLA4Ig Facilitates Tolerance in Murine Islet Allografts. Am. J. Transplant. 2007, 7, 2663–2674. [Google Scholar] [CrossRef]
- Andrzejczak, A.; Karabon, L. BTLA Biology in Cancer: From Bench Discoveries to Clinical Potentials. Biomark. Res. 2024, 12, 8. [Google Scholar] [CrossRef]
- Thibult, M.-L.; Rivals, J.-P.; Mamessier, E.; Gertner-Dardenne, J.; Pastor, S.; Speiser, D.E.; Derré, L.; Olive, D. CpG-ODN-Induced Sustained Expression of BTLA Mediating Selective Inhibition of Human B Cells. J. Mol. Med. 2013, 91, 195–205. [Google Scholar] [CrossRef]
- Mittal, R.; Chen, C.-W.; Lyons, J.D.; Margoles, L.M.; Liang, Z.; Coopersmith, C.M.; Ford, M.L. Murine Lung Cancer Induces Generalized T-Cell Exhaustion. J. Surg. Res. 2015, 195, 541–549. [Google Scholar] [CrossRef]
- Derré, L.; Rivals, J.-P.; Jandus, C.; Pastor, S.; Rimoldi, D.; Romero, P.; Michielin, O.; Olive, D.; Speiser, D.E. BTLA Mediates Inhibition of Human Tumor-Specific CD8+ T Cells That Can Be Partially Reversed by Vaccination. J. Clin. Investig. 2010, 120, 157–167. [Google Scholar] [CrossRef]
- M’Hidi, H.; Thibult, M.L.; Chetaille, B.; Rey, F.; Bouadallah, R.; Nicollas, R.; Olive, D.; Xerri, L. High Expression of the Inhibitory Receptor BTLA in T-Follicular Helper Cells and in B-Cell Small Lymphocytic Lymphoma/Chronic Lymphocytic Leukemia. Am. J. Clin. Pathol. 2009, 132, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Krieg, C.; Boyman, O.; Fu, Y.-X.; Kaye, J. B and T Lymphocyte Attenuator Regulates CD8+ T Cell–Intrinsic Homeostasis and Memory Cell Generation. Nat. Immunol. 2007, 8, 162–171. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.E.; Cao, X. Co-Stimulatory and Co-Inhibitory Pathways in Cancer Immunotherapy. In Advances in Cancer Research; Academic Press Inc.: Cambridge, MA, USA, 2019; Volume 143, pp. 145–194. [Google Scholar]
- Chen, Y.-L.; Lin, H.-W.; Chien, C.-L.; Lai, Y.-L.; Sun, W.-Z.; Chen, C.-A.; Cheng, W.-F. BTLA Blockade Enhances Cancer Therapy by Inhibiting IL-6/IL-10-Induced CD19high B Lymphocytes. J. Immunother. Cancer 2019, 7, 313. [Google Scholar] [CrossRef]
- Gavrieli, M.; Sedy, J.; Nelson, C.A.; Murphy, K.M. BTLA and HVEM Cross Talk Regulates Inhibition and Costimulation. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2006; Volume 92, pp. 157–185. [Google Scholar]
- Thommen, D.S.; Schreiner, J.; Müller, P.; Herzig, P.; Roller, A.; Belousov, A.; Umana, P.; Pisa, P.; Klein, C.; Bacac, M.; et al. Progression of Lung Cancer Is Associated with Increased Dysfunction of T Cells Defined by Coexpression of Multiple Inhibitory Receptors. Cancer Immunol. Res. 2015, 3, 1344–1355. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Cui, G.; Yu, L.; Zhang, X. BTLA Expression in Stage I–III Non–Small-Cell Lung Cancer and Its Correlation with PD-1/PD-L1 and Clinical Outcomes. OncoTargets Ther. 2020, 13, 215–224. [Google Scholar] [CrossRef]
- Murphy, K.M.; Nelson, C.A.; Šedý, J.R. Balancing Co-Stimulation and Inhibition with BTLA and HVEM. Nat. Rev. Immunol. 2006, 6, 671–681. [Google Scholar] [CrossRef]
- Spodzieja, M.; Kuncewicz, K.; Sieradzan, A.; Karczyńska, A.; Iwaszkiewicz, J.; Cesson, V.; Węgrzyn, K.; Zhukov, I.; Maszota-Zieleniak, M.; Michielin, O.; et al. Disulfide-Linked Peptides for Blocking BTLA/HVEM Binding. Int. J. Mol. Sci. 2020, 21, 636. [Google Scholar] [CrossRef]
- Wojciechowicz, K.; Kuncewicz, K.; Lisowska, K.A.; Wardowska, A.; Spodzieja, M. Peptides Targeting the BTLA-HVEM Complex Can Modulate T Cell Immune Response. Eur. J. Pharm. Sci. 2024, 193, 106677. [Google Scholar] [CrossRef]
- Wojciechowicz, K.; Spodzieja, M.; Wardowska, A. The BTLA-HVEM Complex—The Future of Cancer Immunotherapy. Eur. J. Med. Chem. 2024, 268, 116231. [Google Scholar] [CrossRef]
- Fuchs, N.; Zhang, L.; Calvo-Barreiro, L.; Kuncewicz, K.; Gabr, M. Inhibitors of Immune Checkpoints: Small Molecule- and Peptide-Based Approaches. J. Pers. Med. 2024, 14, 68. [Google Scholar] [CrossRef]
- Sauer, N.; Janicka, N.; Szlasa, W.; Skinderowicz, B.; Kołodzińska, K.; Dwernicka, W.; Oślizło, M.; Kulbacka, J.; Novickij, V.; Karłowicz-Bodalska, K. TIM-3 as a Promising Target for Cancer Immunotherapy in a Wide Range of Tumors. Cancer Immunol. Immunother. 2023, 72, 3405–3425. [Google Scholar] [CrossRef] [PubMed]
- Ocaña-Guzman, R.; Torre-Bouscoulet, L.; Sada-Ovalle, I. TIM-3 Regulates Distinct Functions in Macrophages. Front. Immunol. 2016, 7, 229. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Rangachari, M.; Kuchroo, V.K. Tim-3: A Co-Receptor with Diverse Roles in T Cell Exhaustion and Tolerance. Semin. Immunol. 2019, 42, 101302. [Google Scholar] [CrossRef]
- Wang, S.; Sun, F.; Li, M.; Qian, J.; Chen, C.; Wang, M.; Zang, X.; Li, D.; Yu, M.; Du, M. The Appropriate Frequency and Function of Decidual Tim-3+CTLA-4+CD8+ T Cells Are Important in Maintaining Normal Pregnancy. Cell Death Dis. 2019, 10, 407. [Google Scholar] [CrossRef]
- Sabins, N.C.; Chornoguz, O.; Leander, K.; Kaplan, F.; Carter, R.; Kinder, M.; Bachman, K.; Verona, R.; Shen, S.; Bhargava, V.; et al. TIM-3 Engagement Promotes Effector Memory T Cell Differentiation of Human Antigen-Specific CD8 T Cells by Activating MTORC1. J. Immunol. 2017, 199, 4091–4102. [Google Scholar] [CrossRef]
- Banerjee, H.; Kane, L.P. Immune Regulation by Tim-3. F1000Research 2018, 7, 316. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Fu, J.; Wang, X.; Feng, X.; Pan, X. Tim-3 Regulates Inflammatory Cytokine Expression and Th17 Cell Response Induced by Monocytes from Patients with Chronic Hepatitis B. Scand. J. Immunol. 2019, 89, e12755. [Google Scholar] [CrossRef]
- Acharya, N.; Sabatos-Peyton, C.; Anderson, A.C. Tim-3 Finds Its Place in the Cancer Immunotherapy Landscape. J. Immunother. Cancer 2020, 8, e000911. [Google Scholar] [CrossRef]
- Chiba, S.; Baghdadi, M.; Akiba, H.; Yoshiyama, H.; Kinoshita, I.; Dosaka-Akita, H.; Fujioka, Y.; Ohba, Y.; Gorman, J.V.; Colgan, J.D.; et al. Tumor-Infiltrating DCs Suppress Nucleic Acid–Mediated Innate Immune Responses Through Interactions Between the Receptor TIM-3 and the Alarmin HMGB1. Nat. Immunol. 2012, 13, 832–842. [Google Scholar] [CrossRef]
- Ju, Y.; Hou, N.; Zhang, X.; Zhao, D.; Liu, Y.; Wang, J.; Luan, F.; Shi, W.; Zhu, F.; Sun, W.; et al. Blockade of Tim-3 Pathway Ameliorates Interferon-γ Production from Hepatic CD8+ T Cells in a Mouse Model of Hepatitis B Virus Infection. Cell. Mol. Immunol. 2009, 6, 35–43. [Google Scholar] [CrossRef]
- Rangachari, M.; Zhu, C.; Sakuishi, K.; Xiao, S.; Karman, J.; Chen, A.; Angin, M.; Wakeham, A.; Greenfield, E.A.; Sobel, R.A.; et al. Bat3 Promotes T Cell Responses and Autoimmunity by Repressing Tim-3–Mediated Cell Death and Exhaustion. Nat. Med. 2012, 18, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Li, A.; Krishnamurthy, N.; Lemmon, M.A. Phosphatidylserine Binding Directly Regulates TIM-3 Function. Biochem. J. 2021, 478, 3331–3349. [Google Scholar] [CrossRef] [PubMed]
- Proba, K.; Honegger, A.; Plückthun, A. A Natural Antibody Missing a Cysteine in VH: Consequences for Thermodynamic Stability and Folding. J. Mol. Biol. 1997, 265, 161–172. [Google Scholar] [CrossRef]
- Abdel-Rahman, S.A.; Ovchinnikov, V.; Gabr, M.T. Structure-Based Rational Design of Constrained Peptides as TIM-3 Inhibitors. ACS Med. Chem. Lett. 2024, 15, 806–813. [Google Scholar] [CrossRef]
- Cao, E.; Zang, X.; Ramagopal, U.A.; Mukhopadhaya, A.; Fedorov, A.; Fedorov, E.; Zencheck, W.D.; Lary, J.W.; Cole, J.L.; Deng, H.; et al. T Cell Immunoglobulin Mucin-3 Crystal Structure Reveals a Galectin-9-Independent Ligand-Binding Surface. Immunity 2007, 26, 311–321. [Google Scholar] [CrossRef]
- Lu, C.; Tan, Y. Promising Immunotherapy Targets: TIM3, LAG3, and TIGIT Joined the Party. Mol. Ther. Oncol. 2024, 32, 200773. [Google Scholar] [CrossRef]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 Pathways to Reverse T Cell Exhaustion and Restore Anti-Tumor Immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, Y.; Li, G.; Huang, H.; Zhang, G.; Wang, F.; Sun, J.; Yang, Q.; Zhang, X.; Lu, B. TIM-3 Expression Characterizes Regulatory T Cells in Tumor Tissues and Is Associated with Lung Cancer Progression. PLoS ONE 2012, 7, e30676. [Google Scholar] [CrossRef]
- Guo, Z.; Cheng, D.; Xia, Z.; Luan, M.; Wu, L.; Wang, G.; Zhang, S. Combined TIM-3 Blockade and CD137 Activation Affords the Long-Term Protection in a Murine Model of Ovarian Cancer. J. Transl. Med. 2013, 11, 215. [Google Scholar] [CrossRef]
- Carlino, M.S.; Larkin, J.; Long, G. V Immune Checkpoint Inhibitors in Melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive Resistance to Therapeutic PD-1 Blockade Is Associated with Upregulation of Alternative Immune Checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, A.; Zhang, X.; Li, Y.; Li, B.; Jin, S.; Dong, Q.; Niu, X.; Zhang, L.; Zhou, X.; et al. Identification of a Novel Small-Molecule Inhibitor Targeting TIM-3 for Cancer Immunotherapy. Biochem. Pharmacol. 2023, 212, 115583. [Google Scholar] [CrossRef]
- Zhong, T.; Zhao, C.; Wang, S.; Tao, D.; Ma, S.; Shou, C. The Biologically Functional Identification of a Novel TIM3-Binding Peptide P26 in Vitro and in Vivo. Cancer Chemother. Pharmacol. 2020, 86, 783–792. [Google Scholar] [CrossRef]
- Odstrcil, R.E.; Dutta, P.; Liu, J. Prediction of the Peptide–TIM3 Binding Site in Inhibiting TIM3–Galectin 9 Binding Pathways. J. Chem. Theory Comput. 2023, 19, 6500–6509. [Google Scholar] [CrossRef]
- Workman, C.J.; Rice, D.S.; Dugger, K.J.; Kurschner, C.; Vignali, D.A.A. Phenotypic Analysis of the Murine CD4-Related Glycoprotein, CD223 (LAG-3). Eur. J. Immunol. 2002, 32, 2255. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Zuazo, M.; Arasanz, H.; Bocanegra, A.; Fernández-Rubio, L.; Morente, P.; Fernández-Hinojal, G.; Echaide, M.; Garnica, M.; et al. Understanding LAG-3 Signaling. Int. J. Mol. Sci. 2021, 22, 5282. [Google Scholar] [CrossRef]
- Long, L.; Zhang, X.; Chen, F.; Pan, Q.; Phiphatwatchara, P.; Zeng, Y.; Chen, H. The Promising Immune Checkpoint LAG-3: From Tumor Microenvironment to Cancer Immunotherapy. Genes. Cancer 2018, 9, 176–189. [Google Scholar] [CrossRef]
- Workman, C.J.; Vignali, D.A.A. The CD4-related Molecule, LAG-3 (CD223), Regulates the Expansion of Activated T Cells. Eur. J. Immunol. 2003, 33, 970–979. [Google Scholar] [CrossRef]
- Kuhns, M.S.; Davis, M.M.; Garcia, K.C. Deconstructing the Form and Function of the TCR/CD3 Complex. Immunity 2006, 24, 133–139. [Google Scholar] [CrossRef]
- Guy, C.; Mitrea, D.M.; Chou, P.-C.; Temirov, J.; Vignali, K.M.; Liu, X.; Zhang, H.; Kriwacki, R.; Bruchez, M.P.; Watkins, S.C.; et al. LAG3 Associates with TCR–CD3 Complexes and Suppresses Signaling by Driving Co-Receptor–Lck Dissociation. Nat. Immunol. 2022, 23, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.J.; Dugger, K.J.; Vignali, D.A.A. Cutting Edge: Molecular Analysis of the Negative Regulatory Function of Lymphocyte Activation Gene-3. J. Immunol. 2002, 169, 5392–5395. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a Cancer Immunotherapy Target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Okonogi, N.; Nakano, T. Rationale of Combination of Anti-PD-1/PD-L1 Antibody Therapy and Radiotherapy for Cancer Treatment. Int. J. Clin. Oncol. 2020, 25, 801–809. [Google Scholar] [CrossRef]
- Camisaschi, C.; Casati, C.; Rini, F.; Perego, M.; De Filippo, A.; Triebel, F.; Parmiani, G.; Belli, F.; Rivoltini, L.; Castelli, C. LAG-3 Expression Defines a Subset of CD4+CD25highFoxp3+ Regulatory T Cells That Are Expanded at Tumor Sites. J. Immunol. 2010, 184, 6545–6551. [Google Scholar] [CrossRef]
- Baitsch, L.; Baumgaertner, P.; Devêvre, E.; Raghav, S.K.; Legat, A.; Barba, L.; Wieckowski, S.; Bouzourene, H.; Deplancke, B.; Romero, P.; et al. Exhaustion of Tumor-Specific CD8+ T Cells in Metastases from Melanoma Patients. J. Clin. Investig. 2011, 121, 2350–2360. [Google Scholar] [CrossRef]
- Cai, L.; Li, Y.; Tan, J.; Xu, L.; Li, Y. Targeting LAG-3, TIM-3, and TIGIT for Cancer Immunotherapy. J. Hematol. Oncol. 2023, 16, 101. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination Strategies with PD-1/PD-L1 Blockade: Current Advances and Future Directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, A.; Qiu, C.; Wang, W.; Yang, Y.; Qiu, C.; Liu, A.; Zhu, L.; Yuan, S.; Hu, H.; et al. The Upregulation of LAG-3 on T Cells Defines a Subpopulation with Functional Exhaustion and Correlates with Disease Progression in HIV-Infected Subjects. J. Immunol. 2015, 194, 3873–3882. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Xie, T.; Huang, L.; Hu, Y.; Wen, B.; Tang, P.; Guo, F.; Jin, K.; Zhang, P.; et al. Elevated LAG-3 on CD4+ T Cells Negatively Correlates with Neutralizing Antibody Response during HCV Infection. Immunol. Lett. 2019, 212, 46–52. [Google Scholar] [CrossRef]
- Okamura, T.; Yamamoto, K.; Fujio, K. Early Growth Response Gene 2-Expressing CD4+LAG3+ Regulatory T Cells: The Therapeutic Potential for Treating Autoimmune Diseases. Front. Immunol. 2018, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Zhao, M.; Wang, R.; Li, H. Fibrinogen-like Protein 1 (FGL1): The next Immune Checkpoint Target. J. Hematol. Oncol. 2021, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 2019, 176, 334–347.e12. [Google Scholar] [CrossRef]
- Ren, K.; Hamdy, H.; Meyiah, A.; Elkord, E. Lymphocyte-Activation Gene 3 in Cancer Immunotherapy: Function, Prognostic Biomarker and Therapeutic Potentials. Front. Immunol. 2024, 15, 1501613. [Google Scholar] [CrossRef]
- Xu, F.; Liu, J.; Liu, D.; Liu, B.; Wang, M.; Hu, Z.; Du, X.; Tang, L.; He, F. LSECtin Expressed on Melanoma Cells Promotes Tumor Progression by Inhibiting Antitumor T-Cell Responses. Cancer Res. 2014, 74, 3418–3428. [Google Scholar] [CrossRef]
- Hannier, S.; Tournier, M.; Bismuth, G.; Triebel, F. CD3/TCR Complex-Associated Lymphocyte Activation Gene-3 Molecules Inhibit CD3/TCR Signaling. J. Immunol. 1998, 161, 4058–4065. [Google Scholar] [CrossRef]
- Huo, J.-L.; Wang, Y.-T.; Fu, W.-J.; Lu, N.; Liu, Z.-S. The Promising Immune Checkpoint LAG-3 in Cancer Immunotherapy: From Basic Research to Clinical Application. Front. Immunol. 2022, 13, 956090. [Google Scholar] [CrossRef]
- Ma, Q.-Y.; Huang, D.-Y.; Zhang, H.-J.; Wang, S.; Chen, X.-F. Function and Regulation of LAG3 on CD4+CD25− T Cells in Non-Small Cell Lung Cancer. Exp. Cell Res. 2017, 360, 358–364. [Google Scholar] [CrossRef]
- Maruhashi, T.; Sugiura, D.; Okazaki, I.; Shimizu, K.; Maeda, T.K.; Ikubo, J.; Yoshikawa, H.; Maenaka, K.; Ishimaru, N.; Kosako, H.; et al. Binding of LAG-3 to Stable Peptide-MHC Class II Limits T Cell Function and Suppresses Autoimmunity and Anti-Cancer Immunity. Immunity 2022, 55, 912–924.e8. [Google Scholar] [CrossRef]
- Ibrahim, R.; Saleh, K.; Chahine, C.; Khoury, R.; Khalife, N.; Cesne, A. Le LAG-3 Inhibitors: Novel Immune Checkpoint Inhibitors Changing the Landscape of Immunotherapy. Biomedicines 2023, 11, 1878. [Google Scholar] [CrossRef]
- Chavanton, A.; Mialhe, F.; Abrey, J.; Baeza Garcia, A.; Garrido, C. LAG-3: Recent Developments in Combinational Therapies in Cancer. Cancer Sci. 2024, 115, 2494–2505. [Google Scholar] [CrossRef] [PubMed]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An Open-Ended Story. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2006, 1760, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Sun, Y.; Shi, P.; Zhou, X.; Zhang, Q.; Dong, Q.; Jin, S.; Qiu, L.; Niu, X.; Zhou, X.; et al. Development of LAG-3/FGL1 Blocking Peptide and Combination with Radiotherapy for Cancer Immunotherapy. Acta Pharm. Sin. B 2024, 14, 1150–1165. [Google Scholar] [CrossRef]
- Abdel-Rahman, S.A.; Rehman, A.U.; Gabr, M.T. Discovery of First-in-Class Small Molecule Inhibitors of Lymphocyte Activation Gene 3 (LAG-3). ACS Med. Chem. Lett. 2023, 14, 629–635. [Google Scholar] [CrossRef]
- Calvo-Barreiro, L.; Zhang, L.; Ali, Y.; Rehman, A.U.; Gabr, M. Design and Biophysical Characterization of Second-Generation Cyclic Peptide LAG-3 Inhibitors for Cancer Immunotherapy. Bioorganic Med. Chem. Lett. 2024, 113, 129979. [Google Scholar] [CrossRef]
- Boles, K.S.; Vermi, W.; Facchetti, F.; Fuchs, A.; Wilson, T.J.; Diacovo, T.G.; Cella, M.; Colonna, M. A Novel Molecular Interaction for the Adhesion of Follicular CD4 T Cells to Follicular DC. Eur. J. Immunol. 2009, 39, 695–703. [Google Scholar] [CrossRef]
- Levin, S.D.; Taft, D.W.; Brandt, C.S.; Bucher, C.; Howard, E.D.; Chadwick, E.M.; Johnston, J.; Hammond, A.; Bontadelli, K.; Ardourel, D.; et al. Vstm3 Is a Member of the CD28 Family and an Important Modulator of T-cell Function. Eur. J. Immunol. 2011, 41, 902–915. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Wang, Q.; Zhang, X.; Zhang, Q.; Zhou, X.; Shi, R.; Wu, Y.; Zhai, W.; Chen, Z.; et al. Dual Targeting of TIGIT and PD-1 with a Novel Small Molecule for Cancer Immunotherapy. Biochem. Pharmacol. 2024, 223, 116162. [Google Scholar] [CrossRef]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The Surface Protein TIGIT Suppresses T Cell Activation by Promoting the Generation of Mature Immunoregulatory Dendritic Cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef]
- Borgeaud, M.; Sandoval, J.; Obeid, M.; Banna, G.; Michielin, O.; Addeo, A.; Friedlaender, A. Novel Targets for Immune-Checkpoint Inhibition in Cancer. Cancer Treat. Rev. 2023, 120, 102614. [Google Scholar] [CrossRef]
- Chauvin, J.-M.; Zarour, H.M. TIGIT in Cancer Immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef] [PubMed]
- Stengel, K.F.; Harden-Bowles, K.; Yu, X.; Rouge, L.; Yin, J.; Comps-Agrar, L.; Wiesmann, C.; Bazan, J.F.; Eaton, D.L.; Grogan, J.L. Structure of TIGIT Immunoreceptor Bound to Poliovirus Receptor Reveals a Cell–Cell Adhesion and Signaling Mechanism That Requires Cis-Trans Receptor Clustering. Proc. Natl. Acad. Sci. USA 2012, 109, 5399–5404. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.-S.; Nam, H.; Lee, J.; Park, H.-Y.; Cho, K.J.; Sheen, J.H.; Song, E.; Oh, M.; Lee, S.; Choi, H.; et al. Structural and Functional Characterization of a Monoclonal Antibody Blocking TIGIT. MAbs 2022, 14, 2013750. [Google Scholar] [CrossRef]
- Deuss, F.A.; Gully, B.S.; Rossjohn, J.; Berry, R. Recognition of Nectin-2 by the Natural Killer Cell Receptor T Cell Immunoglobulin and ITIM Domain (TIGIT). J. Biol. Chem. 2017, 292, 11413–11422. [Google Scholar] [CrossRef]
- Stanietsky, N.; Rovis, T.L.; Glasner, A.; Seidel, E.; Tsukerman, P.; Yamin, R.; Enk, J.; Jonjic, S.; Mandelboim, O. Mouse TIGIT Inhibits NK-cell Cytotoxicity upon Interaction with PVR. Eur. J. Immunol. 2013, 43, 2138–2150. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, W.; Yang, Y. TIGIT: A Potential Immunotherapy Target for Gynecological Cancers. Pathol. Res. Pract. 2024, 255, 155202. [Google Scholar] [CrossRef]
- Manieri, N.A.; Chiang, E.Y.; Grogan, J.L. TIGIT: A Key Inhibitor of the Cancer Immunity Cycle. Trends Immunol. 2017, 38, 20–28. [Google Scholar] [CrossRef]
- Zhou, X.; Khan, S.; Huang, D.; Li, L. V-Set and Immunoglobulin Domain Containing (VSIG) Proteins as Emerging Immune Checkpoint Targets for Cancer Immunotherapy. Front. Immunol. 2022, 13, 938470. [Google Scholar] [CrossRef]
- Wang, G.; Tai, R.; Wu, Y.; Yang, S.; Wang, J.; Yu, X.; Lei, L.; Shan, Z.; Li, N. The Expression and Immunoregulation of Immune Checkpoint Molecule VISTA in Autoimmune Diseases and Cancers. Cytokine Growth Factor. Rev. 2020, 52, 1–14. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Cheng, Y.; Wei, Y.; Wei, X. f Small-Molecule Inhibitors for Cancer Treatment. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2019, 1871, 199–224. [Google Scholar] [CrossRef]
- Lee, D.J. The Relationship Between TIGIT+ Regulatory T Cells and Autoimmune Disease. Int. Immunopharmacol. 2020, 83, 106378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, L.; Yin, H.; Feng, X.; Lu, Q. TIGIT: An Emerging Immune Checkpoint Target for Immunotherapy in Autoimmune Disease and Cancer. Int. Immunopharmacol. 2023, 120, 110358. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, B.; Li, J.; Wu, T.; Jin, X.; Yuan, R.; Shi, P.; Zhou, Y.; Li, L.; Yu, F. Dysregulated T Cell Activation and Aberrant Cytokine Expression Profile in Systemic Lupus Erythematosus. Mediat. Inflamm. 2019, 2019, 8450947. [Google Scholar] [CrossRef]
- Anaparti, V.; Tanner, S.; Zhang, C.; O’Neil, L.; Smolik, I.; Meng, X.; Marshall, A.J.; El-Gabalawy, H. Increased Frequency of TIGIT+ CD4 T Cell Subset in Autoantibody-Positive First-Degree Relatives of Patients with Rheumatoid Arthritis. Front. Immunol. 2022, 13, 932627. [Google Scholar] [CrossRef]
- Varghese, J.F.; Kaskow, B.J.; von Glehn, F.; Case, J.; Li, Z.; Julé, A.M.; Berdan, E.; Ho Sui, S.J.; Hu, Y.; Krishnan, R.; et al. Human Regulatory Memory B Cells Defined by Expression of TIM-1 and TIGIT Are Dysfunctional in Multiple Sclerosis. Front. Immunol. 2024, 15, 1360219. [Google Scholar] [CrossRef]
- McTaggart, T.; Lim, J.X.; Smith, K.J.; Heaney, B.; McDonald, D.; Hulme, G.; Hussain, R.; Coxhead, J.; Degnan, A.E.A.; Isaacs, J.; et al. Deep Phenotyping of T Regulatory Cells in Psoriatic Arthritis Highlights Targetable Mechanisms of Disease. J. Biol. Chem. 2025, 301, 108059. [Google Scholar] [CrossRef]
- Trivedi, P.; Jhala, G.; De George, D.J.; Chiu, C.; Selck, C.; Ge, T.; Catterall, T.; Elkerbout, L.; Boon, L.; Joller, N.; et al. TIGIT Acts as an Immune Checkpoint upon Inhibition of PD1 Signaling in Autoimmune Diabetes. Front. Immunol. 2024, 15, 1370907. [Google Scholar] [CrossRef]
- Holder, K.A.; Grant, M.D. TIGIT Blockade: A Multipronged Approach to Target the HIV Reservoir. Front. Cell Infect. Microbiol. 2020, 10, 175. [Google Scholar] [CrossRef]
- Freed-Pastor, W.A.; Lambert, L.J.; Ely, Z.A.; Pattada, N.B.; Bhutkar, A.; Eng, G.; Mercer, K.L.; Garcia, A.P.; Lin, L.; Rideout, W.M.; et al. The CD155/TIGIT Axis Promotes and Maintains Immune Evasion in Neoantigen-Expressing Pancreatic Cancer. Cancer Cell 2021, 39, 1342–1360.e14. [Google Scholar] [CrossRef]
- Paolini, R.; Molfetta, R. CD155 and Its Receptors as Targets for Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 12958. [Google Scholar] [CrossRef]
- Bevelacqua, V.; Bevelacqua, Y.; Candido, S.; Skarmoutsou, E.; Amoroso, A.; Guarneri, C.; Strazzanti, A.; Gangemi, P.; Mazzarino, M.C.; D’Amico, F.; et al. Nectin Like-5 Overexpression Correlates with the Malignant Phenotype in Cutaneous Melanoma. Oncotarget 2012, 3, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Ravens, I.; Seth, S.; Förster, R.; Bernhardt, G. Characterization and Identification of Tage4 as the Murine Orthologue of Human Poliovirus Receptor/CD155. Biochem. Biophys. Res. Commun. 2003, 312, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Miyata, M.; Shiotani, H.; Kameyama, T.; Takai, Y. Nectin-2 in General and in the Brain. Mol. Cell Biochem. 2022, 477, 167–180. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Byrnes, J.R.; Kirkemo, L.L.; Driks, H.; Belair, C.D.; Aguilar, O.A.; Lanier, L.L.; Wells, J.A.; Fong, L.; et al. PVRL2 Suppresses Antitumor Immunity Through PVRIG- and TIGIT-Independent Pathways. Cancer Immunol. Res. 2024, 12, 575–591. [Google Scholar] [CrossRef]
- Devilard, E.; Xerri, L.; Dubreuil, P.; Lopez, M.; Reymond, N. Nectin-3 (CD113) Interacts with Nectin-2 (CD112) to Promote Lymphocyte Transendothelial Migration. PLoS ONE 2013, 8, e77424. [Google Scholar] [CrossRef]
- Chatterjee, S.; Sinha, S.; Kundu, C.N. Nectin Cell Adhesion Molecule-4 (NECTIN-4): A Potential Target for Cancer Therapy. Eur. J. Pharmacol. 2021, 911, 174516. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Wang, H.; Qi, Q.; Wang, X.; Lu, H. Targeted Therapeutic Strategies for Nectin-4 in Breast Cancer: Recent Advances and Future Prospects. Breast 2025, 79, 103838. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium Nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Xue, L.; Cheng, L.; Zhang, J.; Chen, X.; Shen, Z.; Li, K.; Wang, L.; Huang, C.; et al. Structural Insights into the Unique PH-Responsive Characteristics of the Anti-TIGIT Therapeutic Antibody Ociperlimab. Structure 2024, 32, 550–561.e5. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; Ribatti, D. Update in TIGIT Immune-Checkpoint Role in Cancer. Front. Oncol. 2022, 12, 871085. [Google Scholar] [CrossRef]
- Zeng, T.; Cao, Y.; Jin, T.; Tian, Y.; Dai, C.; Xu, F. The CD112R/CD112 Axis: A Breakthrough in Cancer Immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 285. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, L.F.; Smyth, M.J.; Martinet, L. DNAM-1 Control of Natural Killer Cells Functions Through Nectin and Nectin-like Proteins. Immunol. Cell Biol. 2014, 92, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Runge, P.; Haghayegh Jahromi, N.; Naboth, H.; Landtwing, A.; Montecchi, R.; Leicht, N.; Hunter, M.C.; Takai, Y.; Halin, C. CD112 Regulates Angiogenesis and T Cell Entry into the Spleen. Cells 2021, 10, 169. [Google Scholar] [CrossRef]
- Solomon, B.L.; Garrido-Laguna, I. TIGIT: A Novel Immunotherapy Target Moving from Bench to Bedside. Cancer Immunol. Immunother. 2018, 67, 1659–1667. [Google Scholar] [CrossRef]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel Immune Checkpoint Targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef]
- Zhou, X.; Jiao, L.; Qian, Y.; Dong, Q.; Sun, Y.; Zheng, W.V.; Zhao, W.; Zhai, W.; Qiu, L.; Wu, Y.; et al. Repositioning Azelnidipine as a Dual Inhibitor Targeting CD47/SIRPα and TIGIT/PVR Pathways for Cancer Immuno-Therapy. Biomolecules 2021, 11, 706. [Google Scholar] [CrossRef]
- Zhou, X.; Du, J.; Wang, H.; Chen, C.; Jiao, L.; Cheng, X.; Zhou, X.; Chen, S.; Gou, S.; Zhao, W.; et al. Repositioning Liothyronine for Cancer Immunotherapy by Blocking the Interaction of Immune Checkpoint TIGIT/PVR. Cell Commun. Signal. 2020, 18, 142. [Google Scholar] [CrossRef]
- Wellington, K.; Scott, L.J. Azelnidipine. Drugs 2003, 63, 2613–2621. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Zhang, X.; Li, B.; Jin, S.; Wu, M.; Zhou, X.; Dong, Q.; Du, J.; Zhai, W.; et al. Hemin Blocks TIGIT/PVR Interaction and Induces Ferroptosis to Elicit Synergistic Effects of Cancer Immunotherapy. Sci. China Life Sci. 2024, 67, 996–1009. [Google Scholar] [CrossRef]
- Lü, X.; Wei, X.; Wang, C.; Tang, M.; Jin, Y.; Fan, S.; Yang, Z. Identification of the Therapeutic Potential of Novel TIGIT/PVR Interaction Blockers Based Advanced Computational Techniques and Experimental Validation. Biophys. Chem. 2025, 318, 107383. [Google Scholar] [CrossRef]
- Xiong, F.; Yu, M.; Xu, H.; Zhong, Z.; Li, Z.; Guo, Y.; Zhang, T.; Zeng, Z.; Jin, F.; He, X. Discovery of TIGIT Inhibitors Based on DEL and Machine Learning. Front. Chem. 2022, 10, 982539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zuo, C.; Li, W.; Shi, W.; Zhou, X.; Wang, H.; Chen, S.; Du, J.; Chen, G.; Zhai, W.; et al. A Novel D-Peptide Identified by Mirror-Image Phage Display Blocks TIGIT/PVR for Cancer Immunotherapy. Angew. Chem. Int. Ed. 2020, 59, 15114–15118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Paniccia, A.; Schulick, A.C.; Chen, W.; Koenig, M.R.; Byers, J.T.; Yao, S.; Bevers, S.; Edil, B.H. Identification of CD112R as a Novel Checkpoint for Human T Cells. J. Exp. Med. 2016, 213, 167–176. [Google Scholar] [CrossRef]
- Hu, S.; Han, P.; Wang, M.; Cao, X.; Liu, H.; Zhang, S.; Zhang, S.; Liu, J.; Han, Y.; Xiao, J.; et al. Structural Basis for the Immune Recognition and Selectivity of the Immune Receptor PVRIG for Ligand Nectin-2. Structure 2024, 32, 918–929.e4. [Google Scholar] [CrossRef]
- Jo, Y.; Sim, H.-I.; Yun, B.; Park, Y.; Jin, H. Revisiting T-Cell Adhesion Molecules as Potential Targets for Cancer Immunotherapy: CD226 and CD2. Exp. Mol. Med. 2024, 56, 2113–2126. [Google Scholar] [CrossRef]
- Jin, H.; Park, Y. Hitting the Complexity of the TIGIT-CD96-CD112R-CD226 Axis for next-Generation Cancer Immunotherapy. BMB Rep. 2021, 54, 2–11. [Google Scholar] [CrossRef]
- Yang, C.; Mandelkow, T.; Bady, E.; Raedler, J.B.; Simon, R.; Sauter, G.; Lennartz, M.; Büscheck, F.; Luebke, A.M.; Dum, D.; et al. Nonredundant Upregulation of CD112R (PVRIG) and PD-1 on Cytotoxic T Lymphocytes Located in T Cell Nests of Colorectal Cancer. Mod. Pathol. 2023, 36, 100089. [Google Scholar] [CrossRef]
- Whelan, S.; Ophir, E.; Kotturi, M.F.; Levy, O.; Ganguly, S.; Leung, L.; Vaknin, I.; Kumar, S.; Dassa, L.; Hansen, K.; et al. PVRIG and PVRL2 Are Induced in Cancer and Inhibit CD8+ T-Cell Function. Cancer Immunol. Res. 2019, 7, 257–268. [Google Scholar] [CrossRef]
- Singh, S.; Julia, E.; Kalita, P.; Mason, C.; Ming, Q.; Lee-Sam, A.; Gordon, S.; Buitrago, M.E.; Leung, D.W.; Hwu, P.; et al. Structure-Guided Engineering of CD112 Receptor Variants for Optimized Immunotherapy. Mol. Ther. 2025, 8. [Google Scholar] [CrossRef]
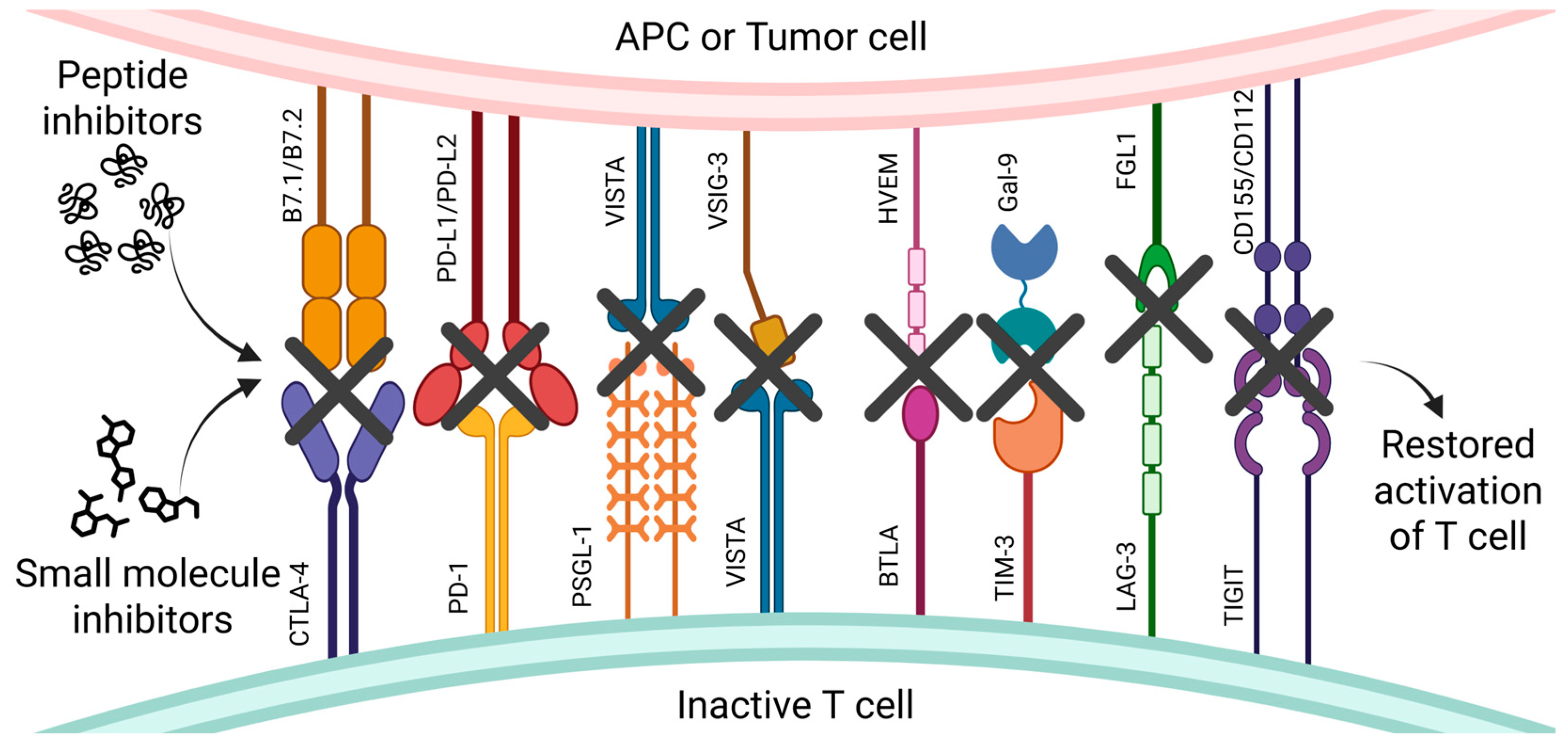
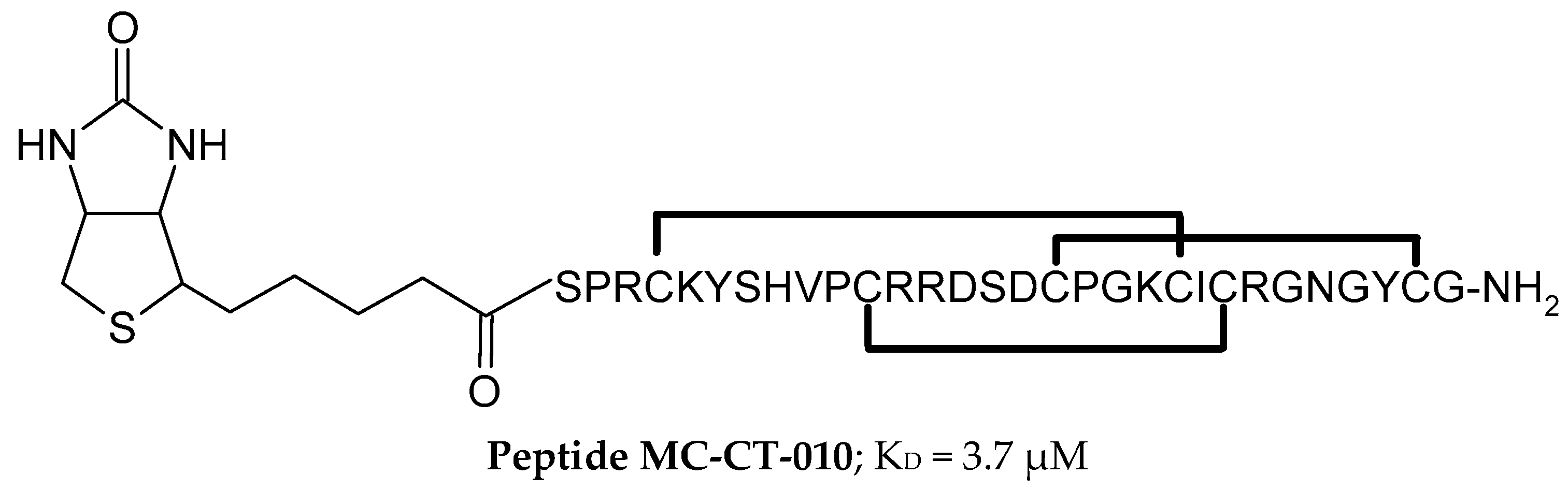
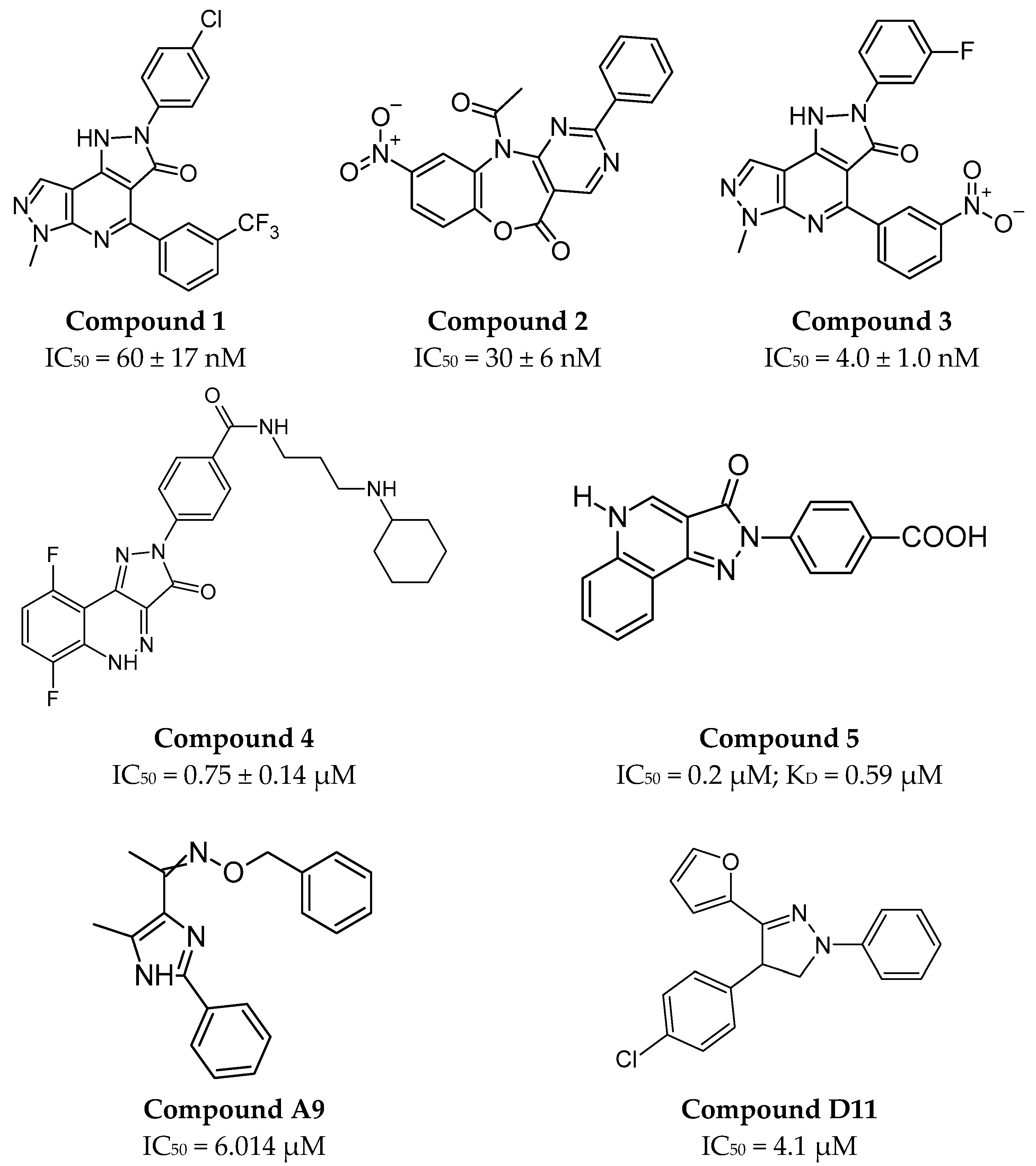
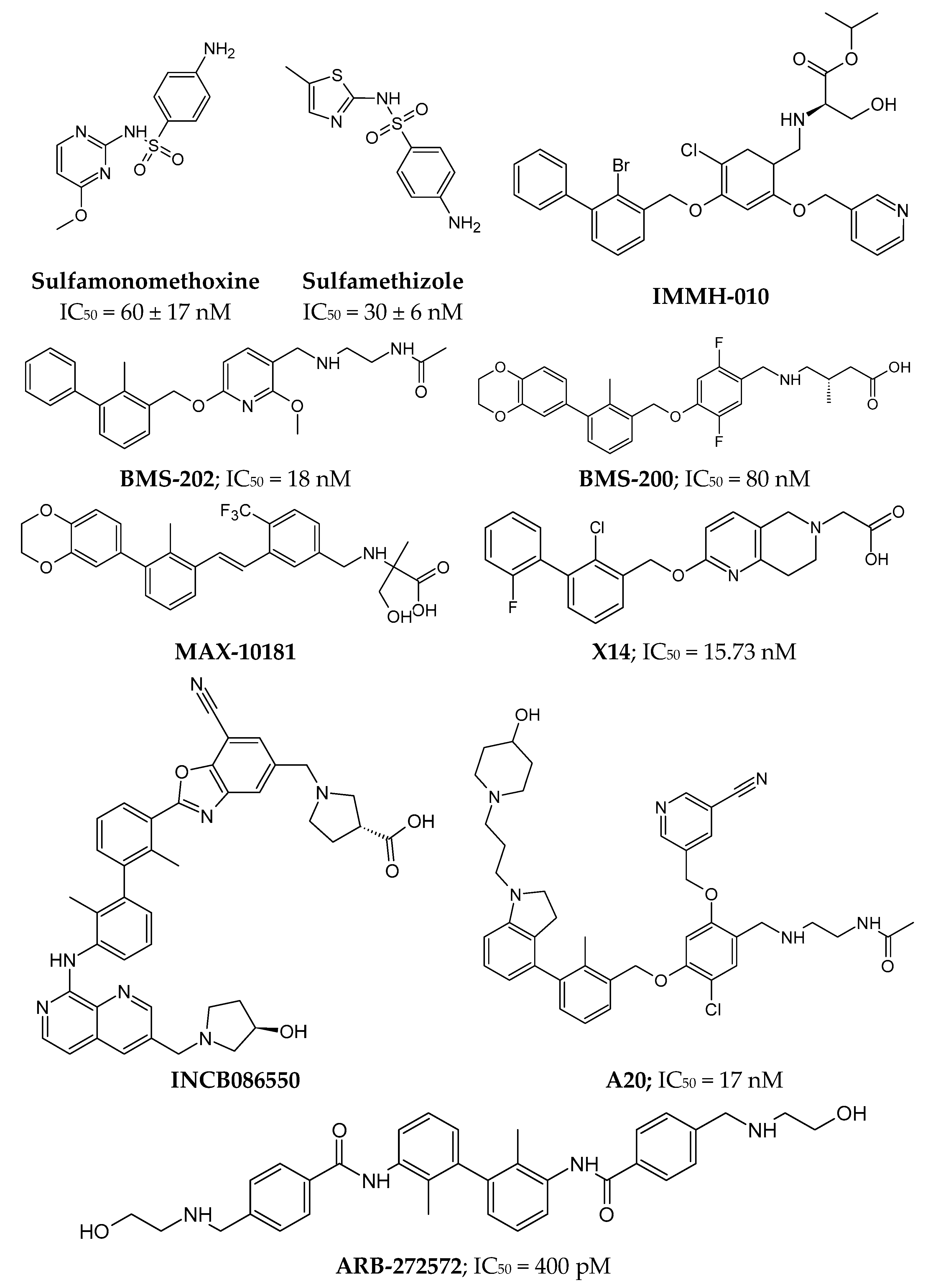
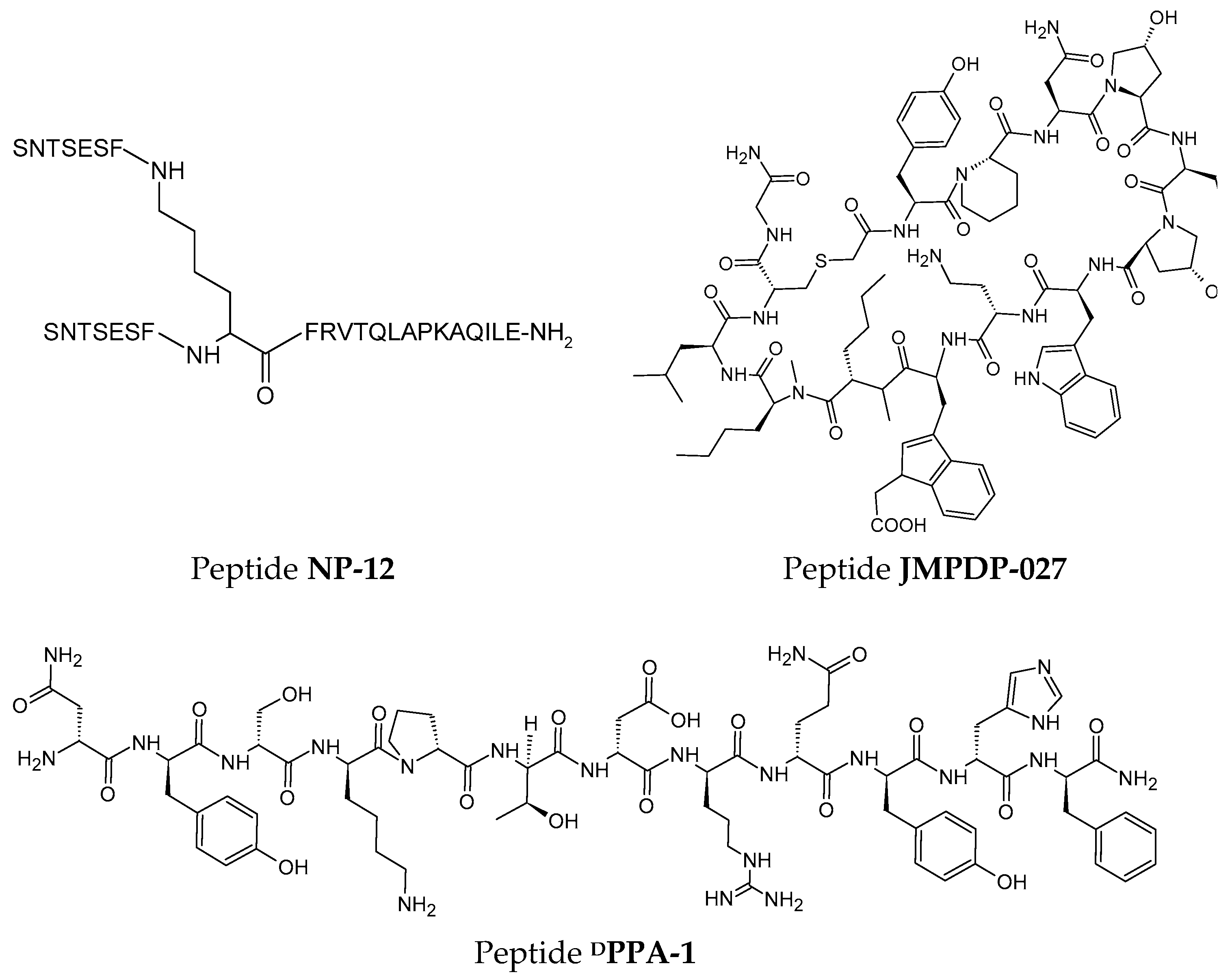
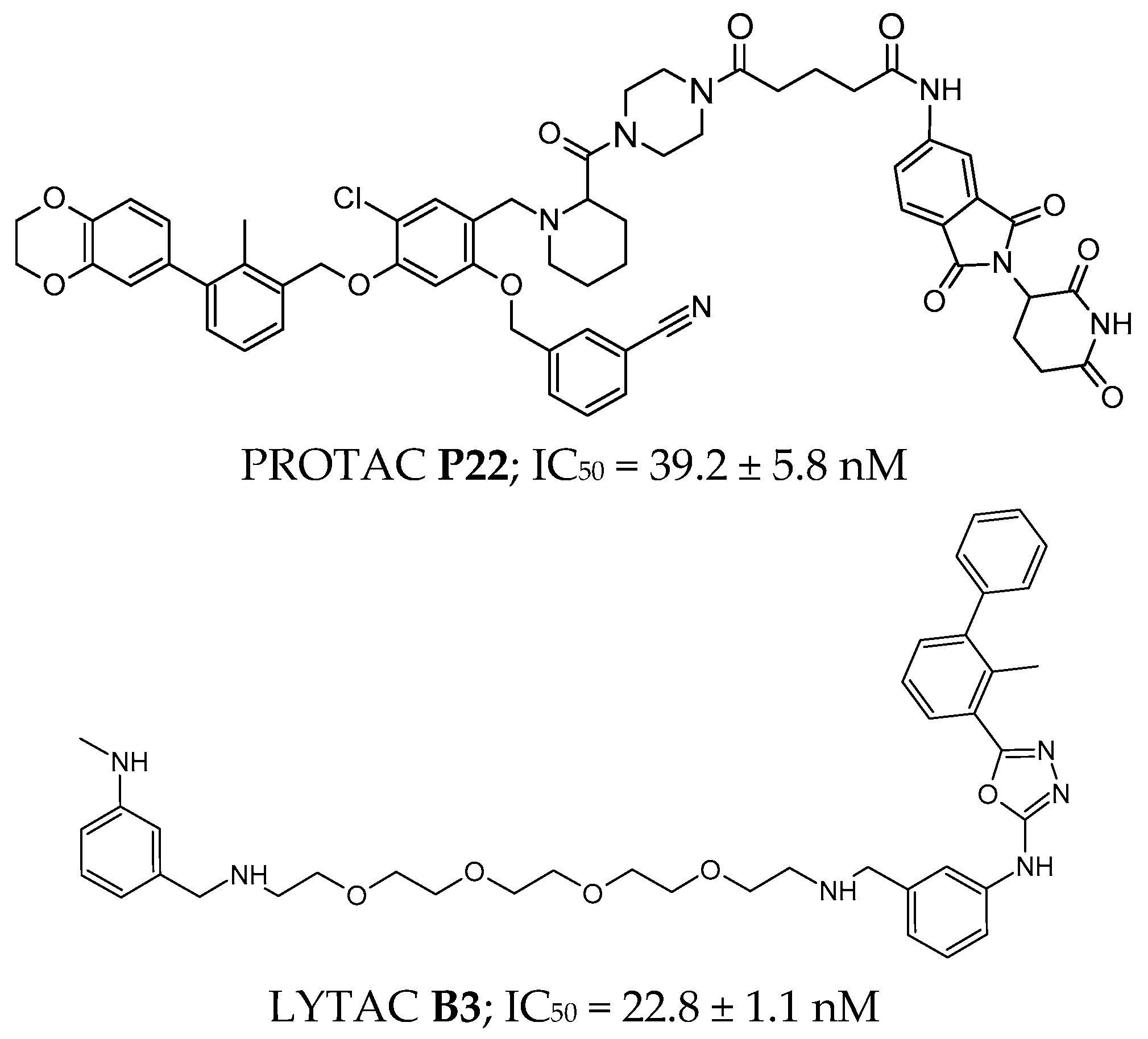
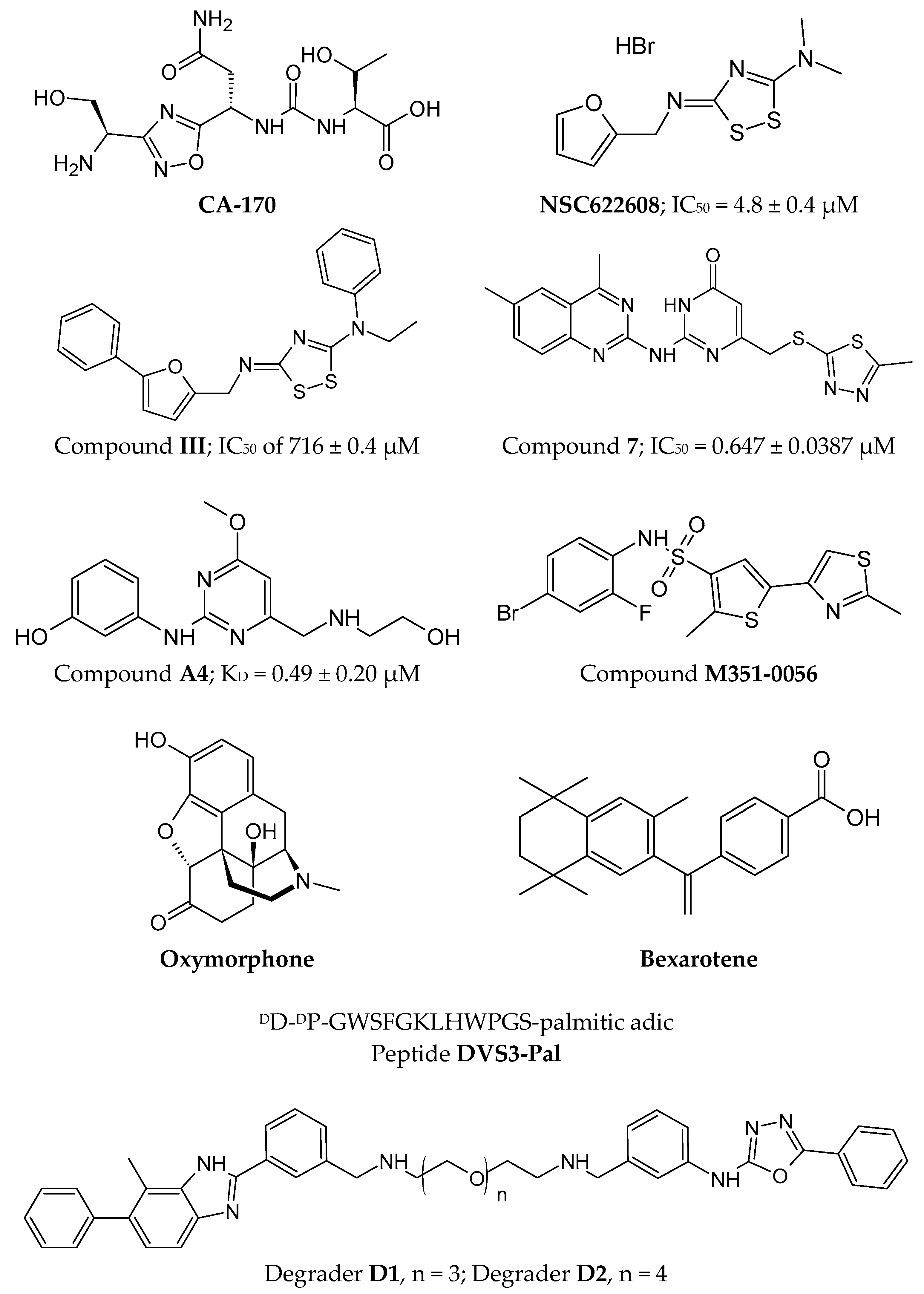
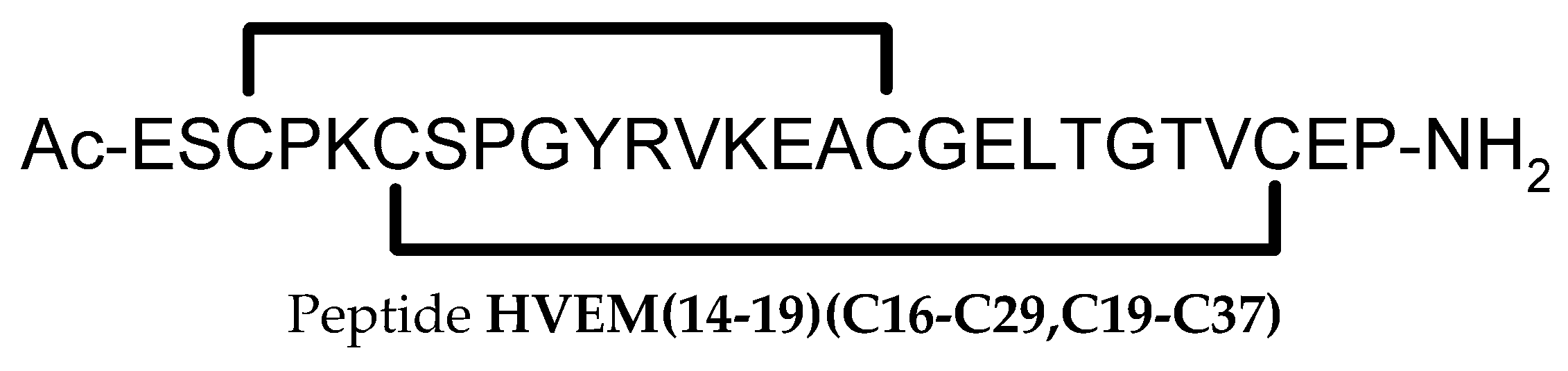
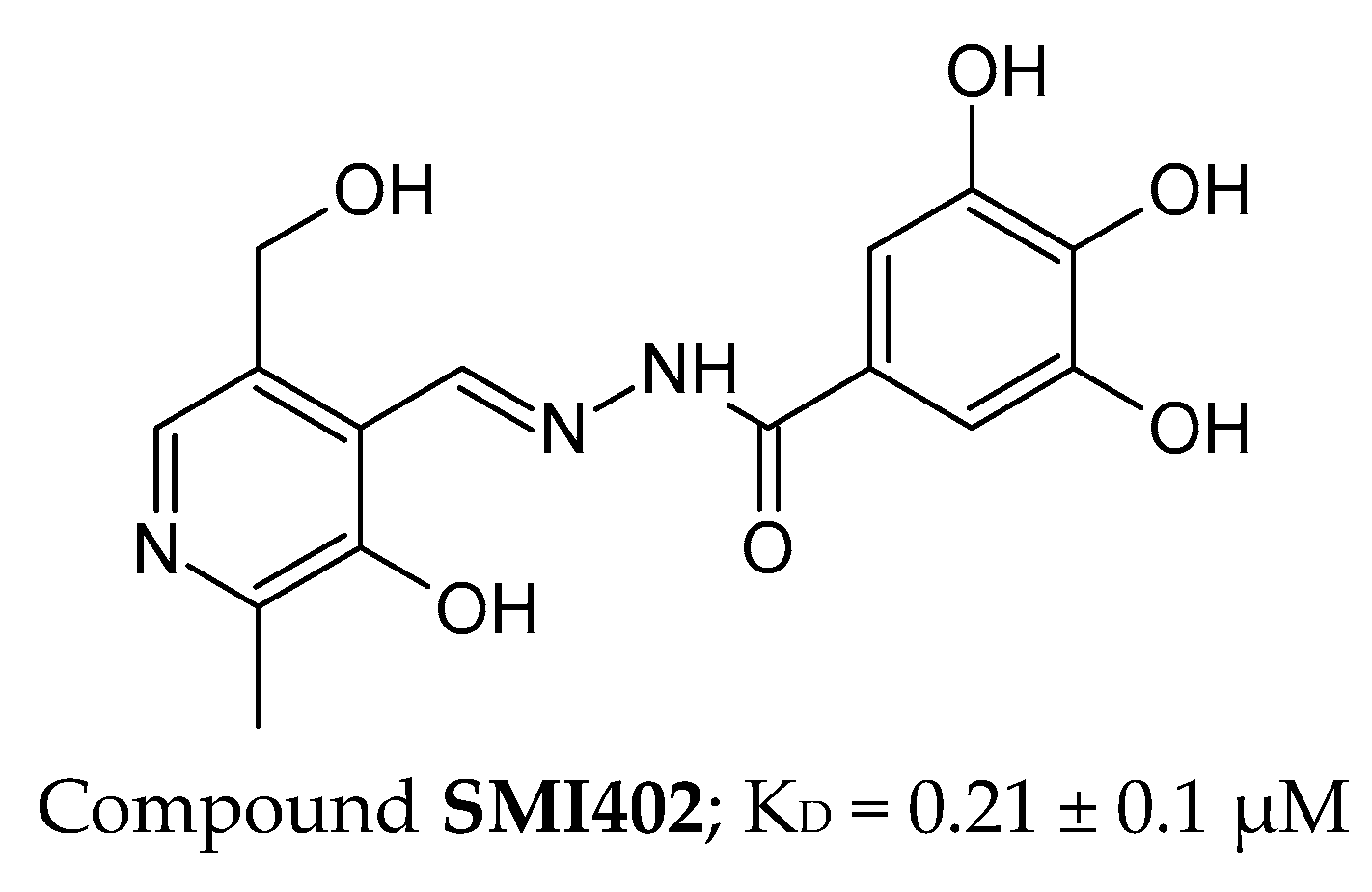
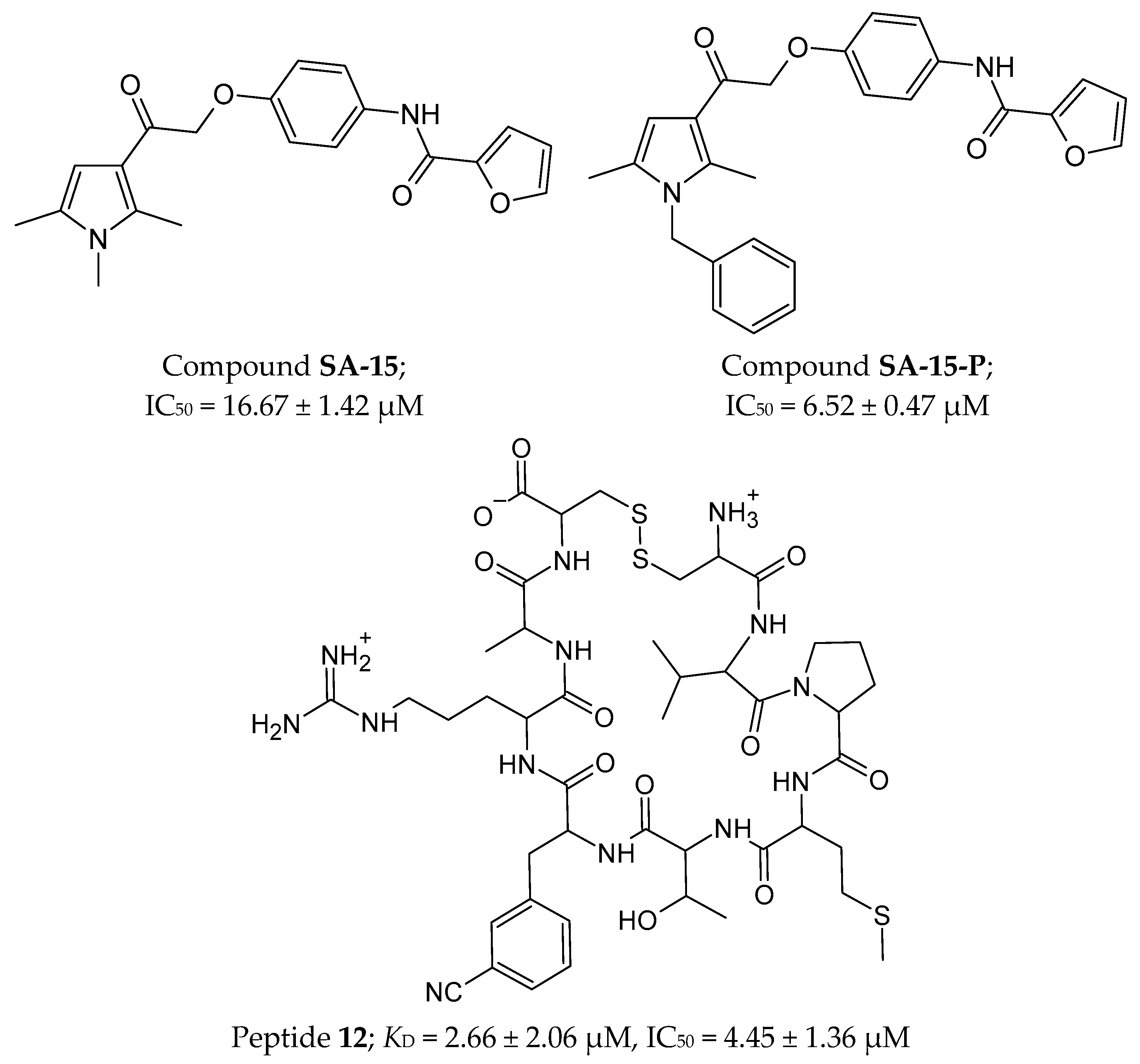
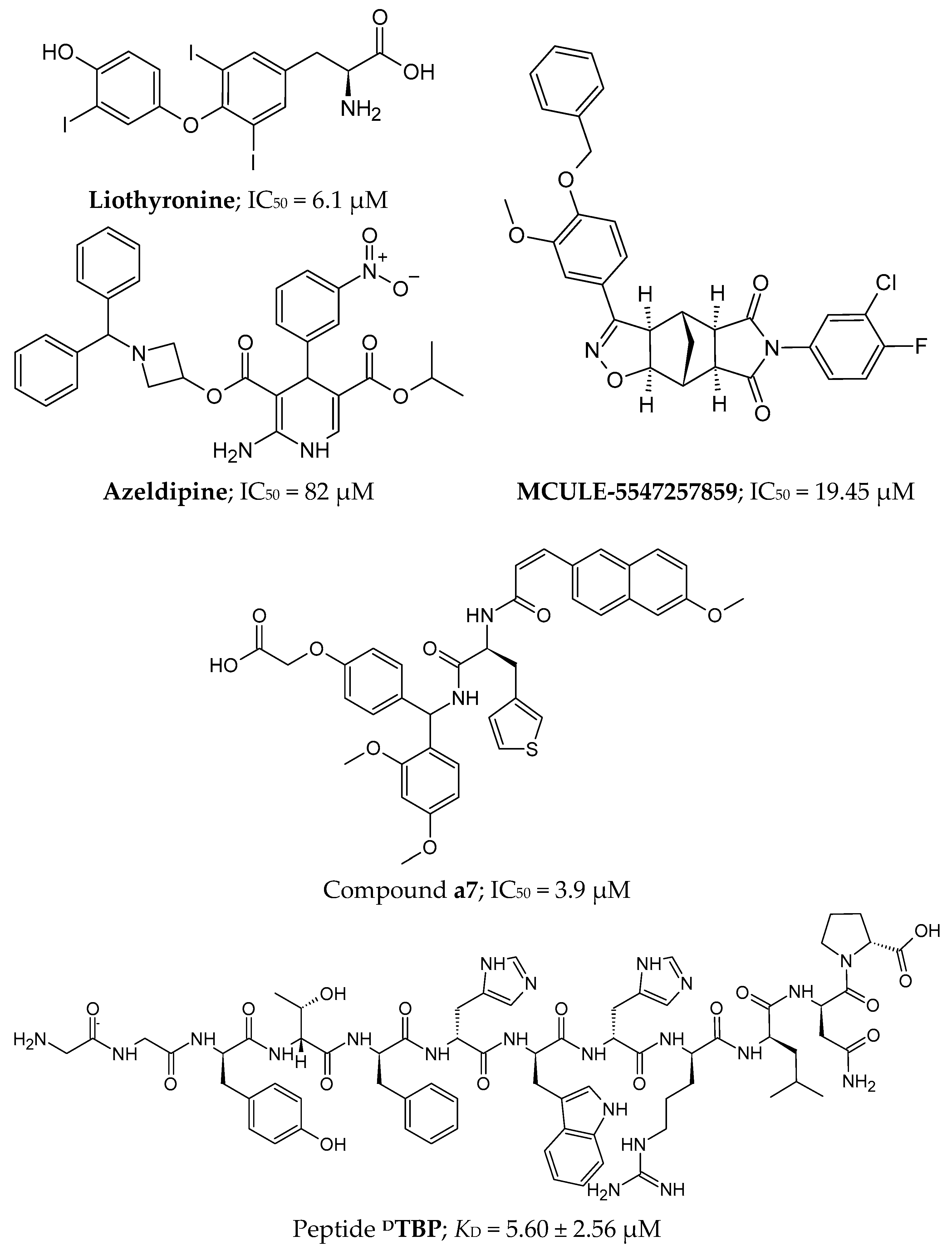
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drewniak-Świtalska, M.; Fortuna, P.; Krzystek-Korpacka, M. Negative Immune Checkpoint Inhibitors. Pharmaceutics 2025, 17, 713. https://doi.org/10.3390/pharmaceutics17060713
Drewniak-Świtalska M, Fortuna P, Krzystek-Korpacka M. Negative Immune Checkpoint Inhibitors. Pharmaceutics. 2025; 17(6):713. https://doi.org/10.3390/pharmaceutics17060713
Chicago/Turabian StyleDrewniak-Świtalska, Magda, Paulina Fortuna, and Małgorzata Krzystek-Korpacka. 2025. "Negative Immune Checkpoint Inhibitors" Pharmaceutics 17, no. 6: 713. https://doi.org/10.3390/pharmaceutics17060713
APA StyleDrewniak-Świtalska, M., Fortuna, P., & Krzystek-Korpacka, M. (2025). Negative Immune Checkpoint Inhibitors. Pharmaceutics, 17(6), 713. https://doi.org/10.3390/pharmaceutics17060713





