Design of Tranilast-Loaded Cation-Type Contact Lens for Sustainable Ocular Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Measurement of TRA Content
2.4. Loading of TRA in the CLs
2.5. Characteristics of TRA Suspensions
2.6. In Vitro TRA Release from CLs
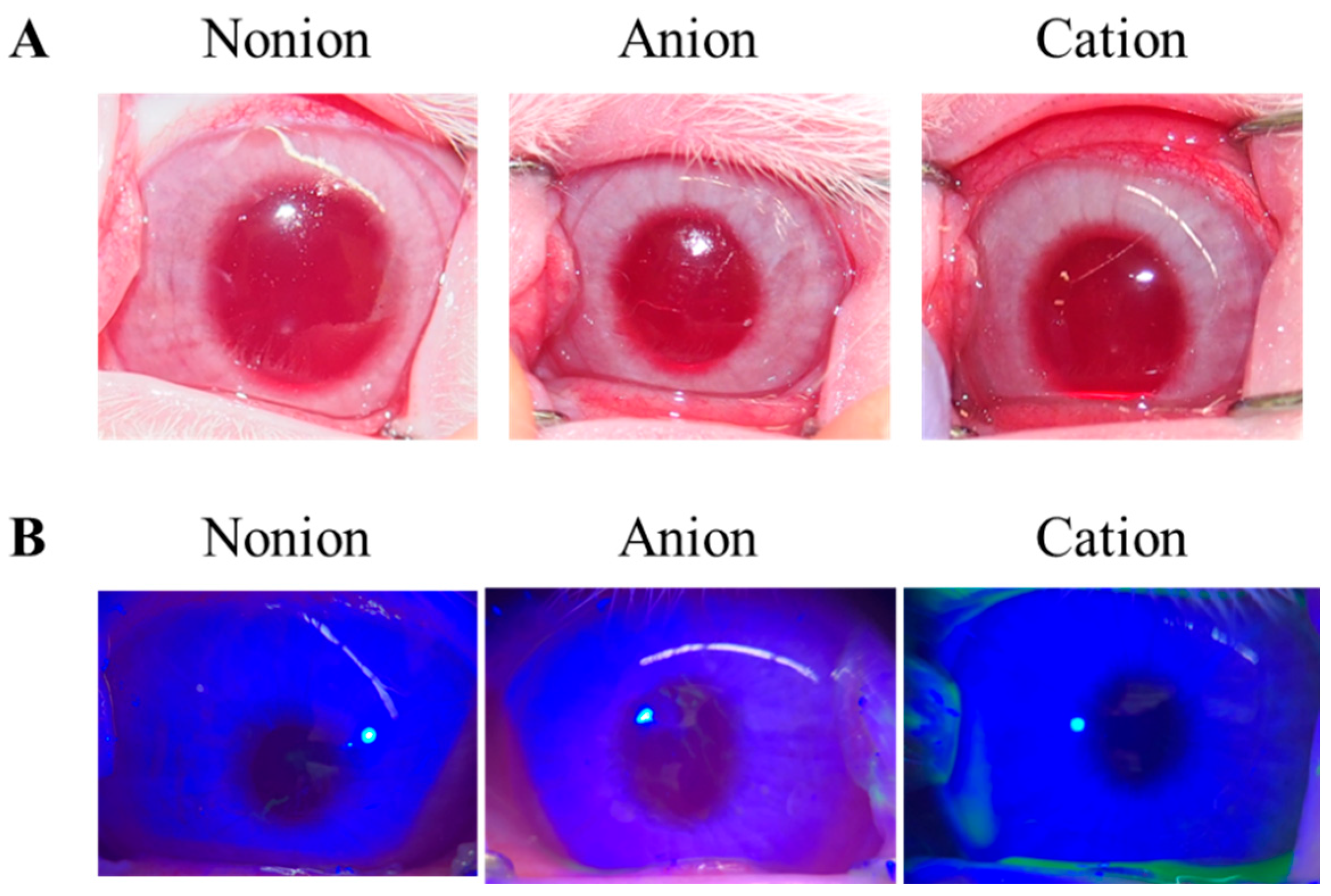
2.7. Image Analysis of Corneal Toxicity in Rabbits
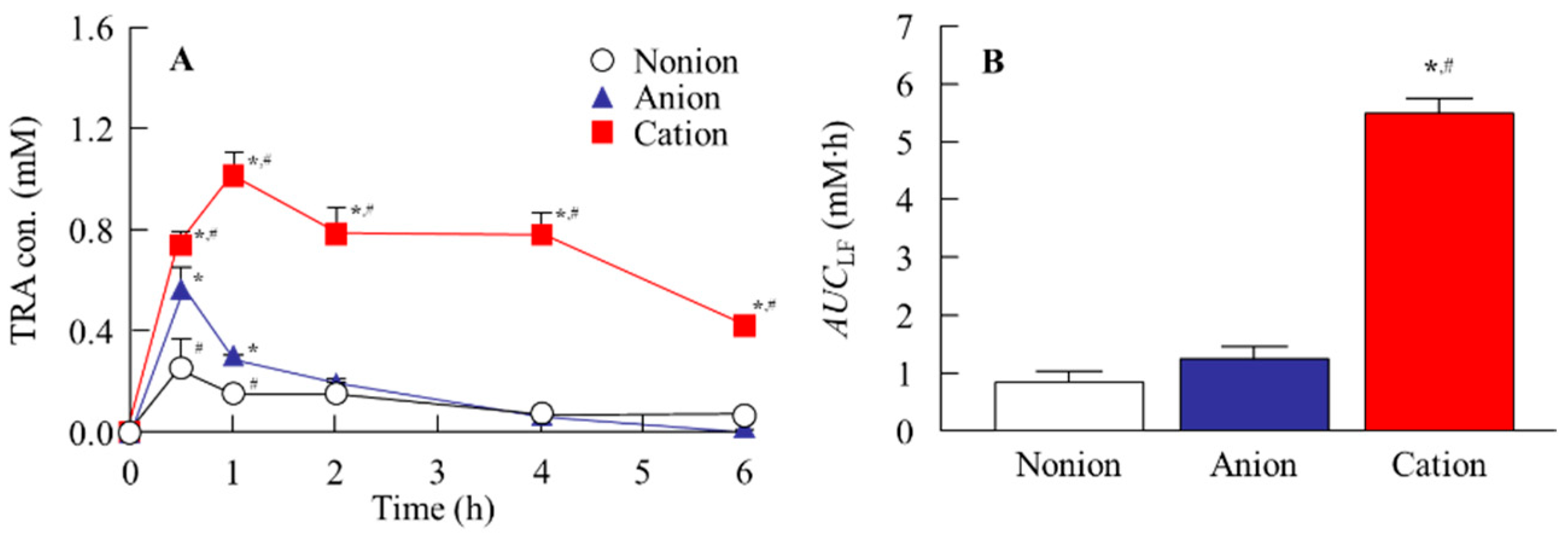
2.8. In Vivo TRA Release from CLs into Lacrimal Fluid
2.9. Statistical Analysis
3. Results
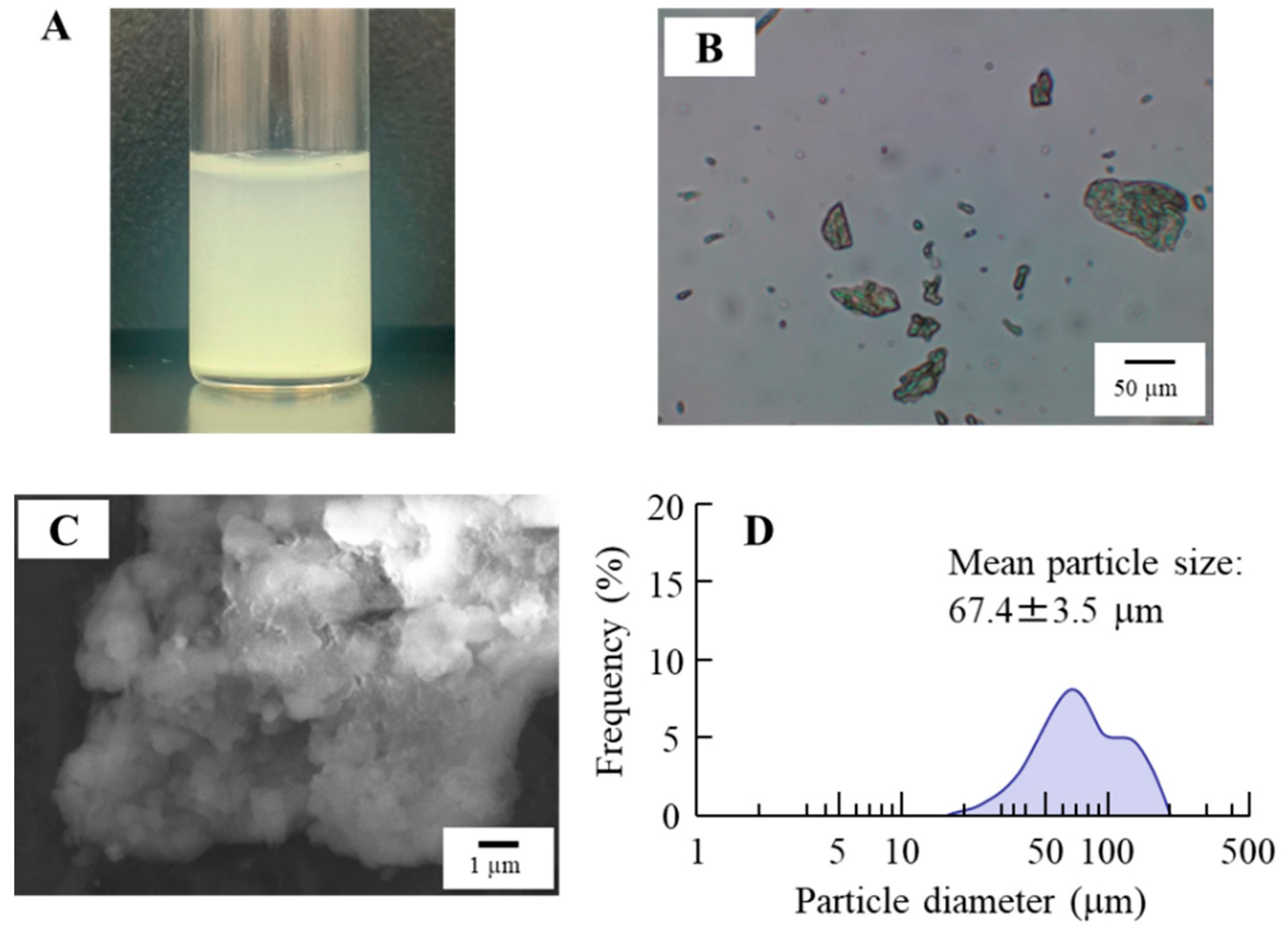
3.1. Design of the TRA-Loaded CLs and Evaluation of Their Characteristics
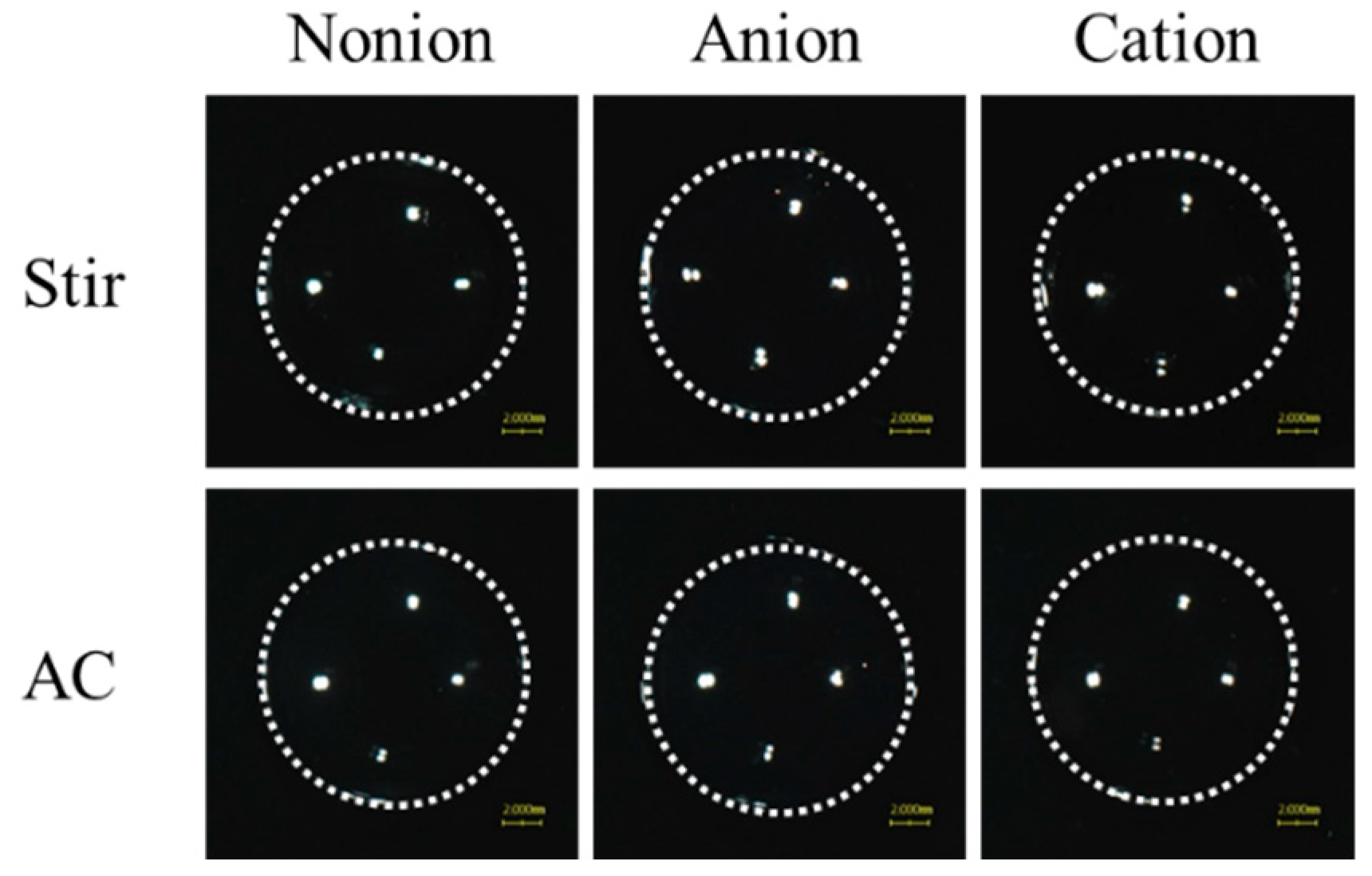
3.2. Drug Release from TRA-Loaded CLs to the Ocular Surface
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Komatsu, H.; Kojima, M.; Tsutsumi, N.; Hamano, S.; Kusama, H.; Ujiie, A.; Ikeda, S.; Nakazawa, M. Study of the mechanism of inhibitory action of tranilast on chemical mediator release. Jpn. J. Pharmacol. 1998, 46, 43–51. [Google Scholar] [CrossRef]
- Itoh, F.; Komatsu, Y.; Taya, F.; Isaji, M.; Momose, Y.; Suzawa, H.; Miyata, H.; Shibazaki, T. Effect of tranilast ophthalmic solution on allergic conjunctivitis in guinea pigs. Nihon Yakurigaku Zasshi 1993, 101, 27–32. [Google Scholar] [CrossRef]
- Kumar, A.; Malviya, R.; Sharma, P. Recent trends in ocular drug delivery: A short review. Am. J. Appl. Sci. 2011, 3, 86–92. [Google Scholar]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.H.; Toda, I.; Sakai, C.; Yoshida, A.; Tsubota, K. Punctal plugs for treatment of post-LASIK dry eye. Jpn. J. Ophthalmol. 2012, 56, 208–213. [Google Scholar] [CrossRef]
- Pimenta, A.F.R.; Serro, A.P.; Colaço, R.; Chauhan, A. Drug delivery to the eye anterior chamber by intraocular lenses: An in vivo concentration estimation model. Eur. J. Pharm. Biopharm. 2018, 133, 63–69. [Google Scholar] [CrossRef]
- Lobo, A.-M.; Sobrin, L.; Papaliodis, G.N. Drug delivery options for the treatment of ocular inflammation. Semin. Ophthalmol. 2010, 25, 283–288. [Google Scholar] [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Kakisu, K.; Matsunaga, T.; Kobayakawa, S.; Sato, T.; Tochikubo, T. Development and efficacy of a drug-releasing soft contact lens. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2551–2561. [Google Scholar] [CrossRef]
- Costa, V.P.; Braga, M.E.M.; Duarte, C.M.M.; Alvarez-Lorenzo, C.; Concheiro, A.; Gil, M.H.; De Sousa, H.C. Anti-glaucoma drug-loaded contact lenses prepared using supercritical solvent impregnation. J. Supercrit. Fluids 2010, 53, 165–173. [Google Scholar] [CrossRef]
- Ciolino, J.B.; Stefanescu, C.F.; Ross, A.E.; Salvador-Culla, B.; Cortez, P.; Ford, E.M.; Wymbs, K.A.; Sprague, S.L.; Mascoop, D.R.; Rudina, S.S.; et al. In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials 2014, 35, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.; Figueiras, A.; Veiga, F. Improvements in topical ocular drug delivery systems: Hydrogels and contact lenses. J. Pharm. Pharm. Sci. 2015, 18, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Cho, S.; Park, H.S.; Kwon, I. Ocular drug delivery through pHEMA-hydrogel contact lenses co-loaded with lipophilic vitamins. Sci. Rep. 2016, 6, 34194. [Google Scholar] [CrossRef]
- Mahaling, B.; Katti, D.S. Understanding the influence of surface properties of nanoparticles and penetration enhancers for improving bioavailability in eye tissues in vivo. Int. J. Pharm. 2016, 501, 1–9. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Saju, A.; Cheerla, K.D.; Gade, S.K.; Garg, P.; Venuganti, V.V.K. Corneal delivery of besifloxacin using rapidly dissolving polymeric microneedles. Drug Deliv. Transl. Res. 2018, 8, 473–483. [Google Scholar] [CrossRef]
- Roy, G.; Galigama, R.D.; Thorat, V.S.; Mallela, L.S.; Roy, S.; Garg, P.; Venuganti, V.V.K. Amphotericin B containing microneedle ocular patch for effective treatment of fungal keratitis. Int. J. Pharm. 2019, 572, 118808. [Google Scholar] [CrossRef]
- Gilger, B.C.; Mandal, A.; Shah, S.; Mitra, A.K. Episcleral, intrascleral, and suprachoroidal routes of ocular drug delivery—Recent research advances and patents. Recent Pat. Drug Deliv. Formul. 2014, 8, 81–91. [Google Scholar] [CrossRef]
- Costa, J.R.; Silva, N.C.; Sarmento, B.; Pintado, M. Potential chitosan-coated alginate nanoparticles for ocular delivery of daptomycin. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Destruel, P.-L.; Zeng, N.; Maury, M.; Mignet, N.; Boudy, V. In vitro and in vivo evaluation of in situ gelling systems for sustained topical ophthalmic delivery: State of the art and beyond. Drug Discov. Today 2017, 22, 638–651. [Google Scholar] [CrossRef]
- Eljarrat-Binstock, E.; Domb, A.J. Iontophoresis: A non-invasive ocular drug delivery. J. Control. Release 2006, 110, 479–489. [Google Scholar] [CrossRef]
- Guzman-Aranguez, A.; Colligris, B.; Pintor, J. Contact lenses: Promising devices for ocular drug delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 189–199. [Google Scholar] [CrossRef]
- Li, C.C.; Chauhan, A. Ocular transport model for ophthalmic delivery of timolol through p-HEMA contact lenses. J. Drug Deliv. Sci. Technol. 2007, 17, 69–79. [Google Scholar] [CrossRef]
- Kim, J.; Chauhan, A. Dexamethasone transport and ocular delivery from poly(hydroxyethyl methacrylate) gels. Int. J. Pharm. 2008, 53, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Hehl, E.M.; Beck, R.; Luthard, K.; Guthoff, R.; Drewelow, B. Improved penetration of aminoglycosides and fluorozuinolones into the aqueous humour of patients by means of Acuvue contact lenses. Eur. J. Clin. Pharmacol. 1999, 55, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ruben, M.; Watkins, R. Pilocarpine dispensation for the soft hydrophilic contact lens. Br. J. Ophthalmol. 1975, 59, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Veiga, F.; Santos, D.; Torres-Labandeira, J.J.; Concheiro, A.; Alvarez-Lorenzo, C. Hydrophilic acrylic hydrogels with builtin or pendant cyclodextrins for delivery of anti-glaucoma drugs. Carbohydr. Polym. 2012, 88, 977–985. [Google Scholar] [CrossRef]
- Jones, L.; Powell, C.H. Uptake and release phenomena in contact lens care by silicone hydrogel lenses. Eye Contact Lens 2013, 39, 29–36. [Google Scholar] [CrossRef]
- Schultz, C.L.; Poling, T.R.; Mint, J.O. A medical device/drug delivery system for treatment of glaucoma. Clin. Exp. Optom. 2009, 92, 343–348. [Google Scholar] [CrossRef]
- Peng, C.-C.; Kim, J.; Chanhan, A. Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing vitamin E diffusion barriers. Biomaterials 2010, 31, 4032–4047. [Google Scholar] [CrossRef]
- Phan, C.-M.; Subbaraman, L.N.; Jones, L. In vitro uptake and release of natamycin from conventional and silicone hydrogel contact lens materials. Eye Contact Lens 2013, 39, 162–168. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Contact Lenses as Ophthalmic Drug Delivery Systems: A Review. Polymers 2021, 13, 1102. [Google Scholar] [CrossRef] [PubMed]
- Rykowska, I.; Nowak, I.; Nowak, R. Soft Contact Lenses as Drug Delivery Systems: A Review. Molecules 2021, 26, 5577. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Minami, M.; Otake, H.; Nakazawa, Y.; Okamoto, N.; Yamamoto, N.; Sasaki, H.; Nagai, N. Balance of drug residence and diffusion in lacrimal fluid determine ocular bioavailability in in situ gels incorporating tranilast nanoparticles. Pharmaceutics 2021, 13, 1425. [Google Scholar] [CrossRef]
- Nagai, N.; Minami, M.; Deguchi, S.; Otake, H.; Sasaki, H.; Yamamoto, N. An in situ gelling system based on methylcellulose and tranilast solid nanoparticles enhances ocular residence time and drug absorption into the cornea and conjunctiva. Front. Bioeng. Biotechnol. 2020, 8, 764. [Google Scholar] [CrossRef]
- Otake, H.; Goto, R.; Ogata, F.; Isaka, T.; Kawasaki, N.; Kobayakawa, S.; Matsunaga, T.; Nagai, N. Fixed-combination eye drops based on fluorometholone nanoparticles and bromfenac/levofloxacin solution improve drug corneal penetration. Int. J. Nanomed. 2021, 16, 5343–5356. [Google Scholar] [CrossRef]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A comprehensive review on contact lens for ophthalmic drug delivery. J. Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef]
- King-Smith, P.E.; Fink, B.A.; Hill, R.M.; Koelling, K.W.; Tiffany, J.M. The thickness of the tear film. Curr. Eye Res. 2004, 29, 357–368. [Google Scholar] [CrossRef]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int. J. Nanomed. 2019, 14, 1213–1227. [Google Scholar] [CrossRef]


| AUC0–6 h | TRA Release (µmol∙h/Lens) | ||
|---|---|---|---|
| Non-Ion | Anion | Cation | |
| Pre-lens | 60.3 ± 4.1 # | 30.0 ± 2.8 * | 179.3 ± 8.1 *,# |
| Post-lens | 63.4 ± 4.9 # | 34.6 ± 3.7 * | 179.1 ± 8.5 *,# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsunaga, T.; Kuwamura, R.; Hino, S.; Ogata, F.; Otake, H.; Kawasaki, N.; Kobayakawa, S.; Nagai, N. Design of Tranilast-Loaded Cation-Type Contact Lens for Sustainable Ocular Drug Delivery. Pharmaceutics 2025, 17, 712. https://doi.org/10.3390/pharmaceutics17060712
Matsunaga T, Kuwamura R, Hino S, Ogata F, Otake H, Kawasaki N, Kobayakawa S, Nagai N. Design of Tranilast-Loaded Cation-Type Contact Lens for Sustainable Ocular Drug Delivery. Pharmaceutics. 2025; 17(6):712. https://doi.org/10.3390/pharmaceutics17060712
Chicago/Turabian StyleMatsunaga, Toru, Ryotaro Kuwamura, Shiori Hino, Fumihiko Ogata, Hiroko Otake, Naohito Kawasaki, Shinichiro Kobayakawa, and Noriaki Nagai. 2025. "Design of Tranilast-Loaded Cation-Type Contact Lens for Sustainable Ocular Drug Delivery" Pharmaceutics 17, no. 6: 712. https://doi.org/10.3390/pharmaceutics17060712
APA StyleMatsunaga, T., Kuwamura, R., Hino, S., Ogata, F., Otake, H., Kawasaki, N., Kobayakawa, S., & Nagai, N. (2025). Design of Tranilast-Loaded Cation-Type Contact Lens for Sustainable Ocular Drug Delivery. Pharmaceutics, 17(6), 712. https://doi.org/10.3390/pharmaceutics17060712







