Agmatine Abrogates Tacrolimus-Induced Testicular Injury in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals and Drugs
2.3. Experimental Design
2.4. Semen Analysis
2.5. Assessment of Serum TT and LH Levels
2.6. Assessment of Oxidative Stress Status
2.7. Assessment of Bcl-2, Inerleukin-17 (IL-17), Hemeoxygenase-1 (HO-1), Sirtuin1 (SIRT1), and iNOS Expressions
2.8. Histopathological Analyses
2.9. Testicular Morphometry
2.10. Immunohistochemical Analyses
2.11. Statistics
3. Results
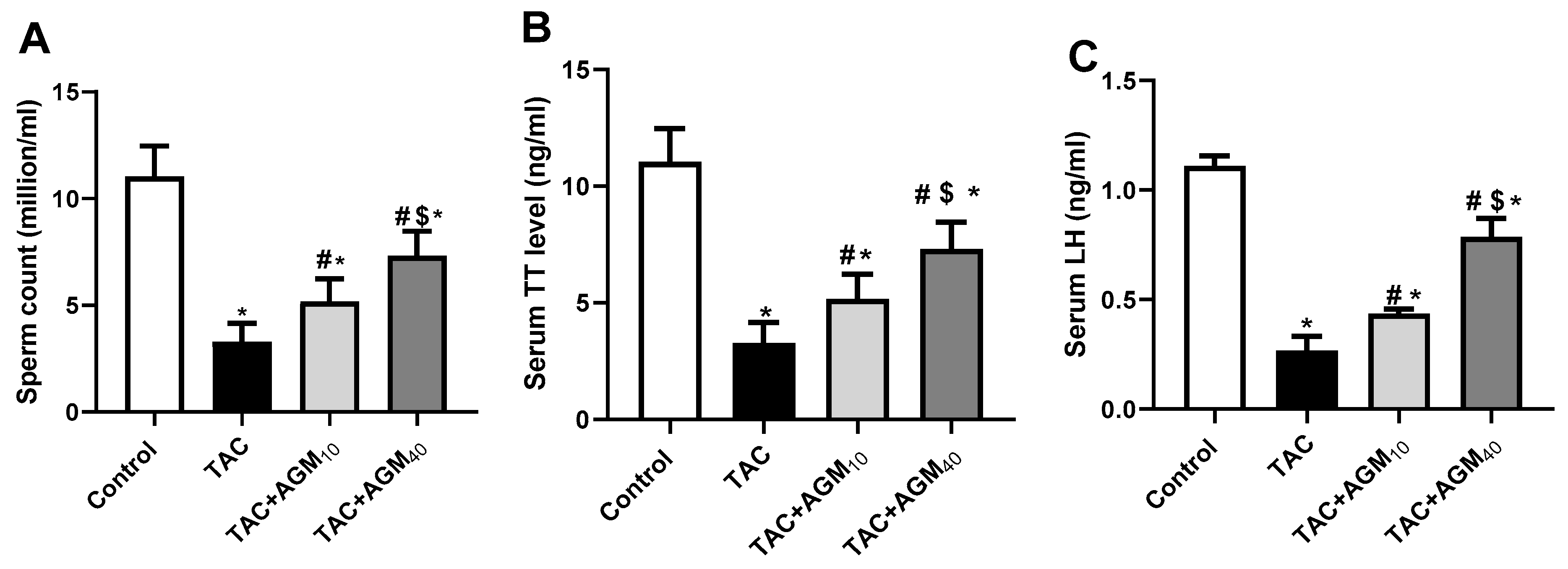
3.1. Impact of AGM10 and AGM40 on TAC-Induced Changes in Sperm Count, Sperm Viability, Serum TT, and LH Concentration
3.2. Impact of AGM10 and AGM40 on TAC-Induced Histopathological Changes
3.3. Impact of AGM10 and AGM40 on TAC-Induced Changes in Morphometry of the Seminiferous Tubules
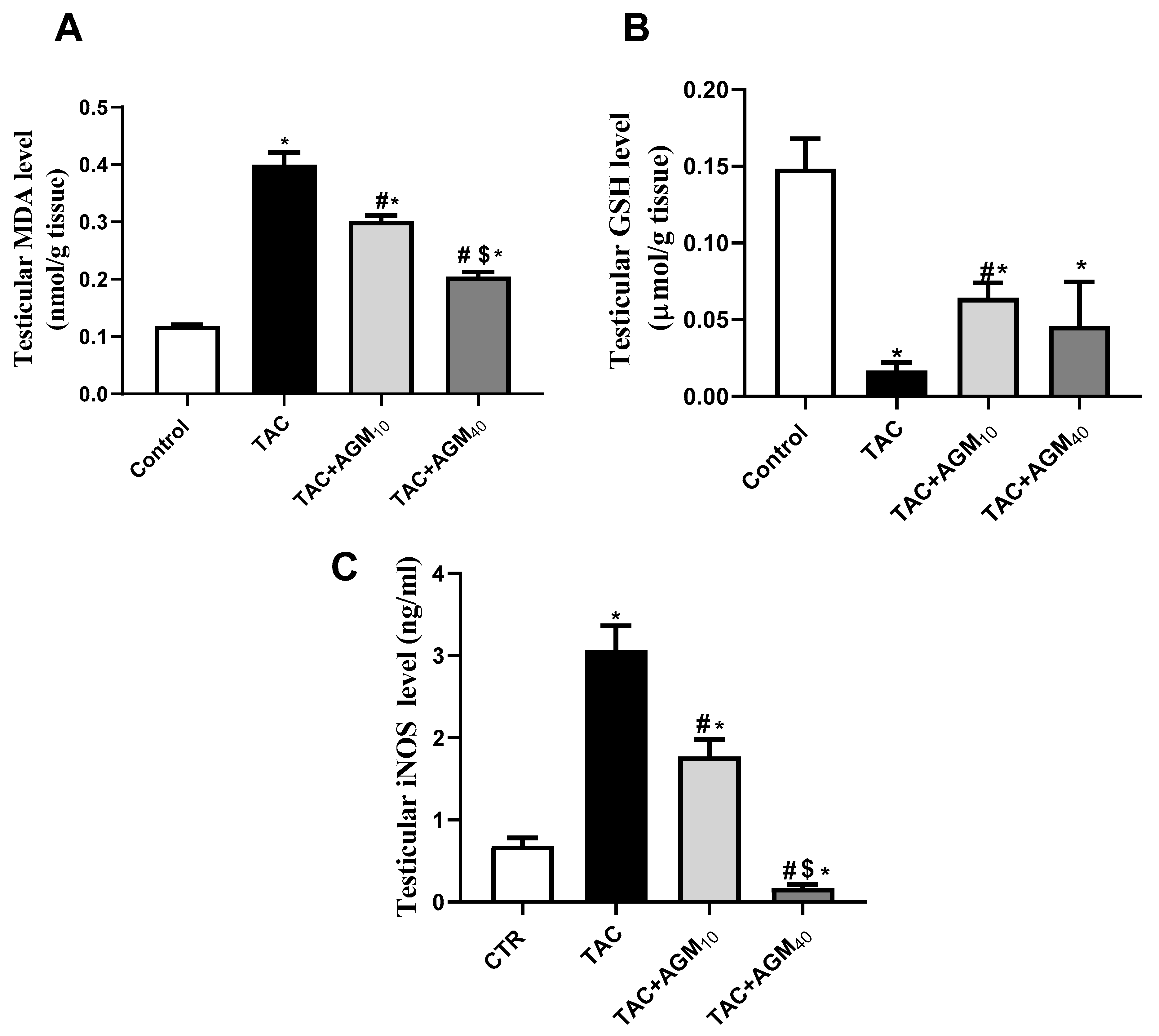
3.4. Impact of AGM10 and AGM40 on TAC-Induced Changes in Oxidative Stress Status
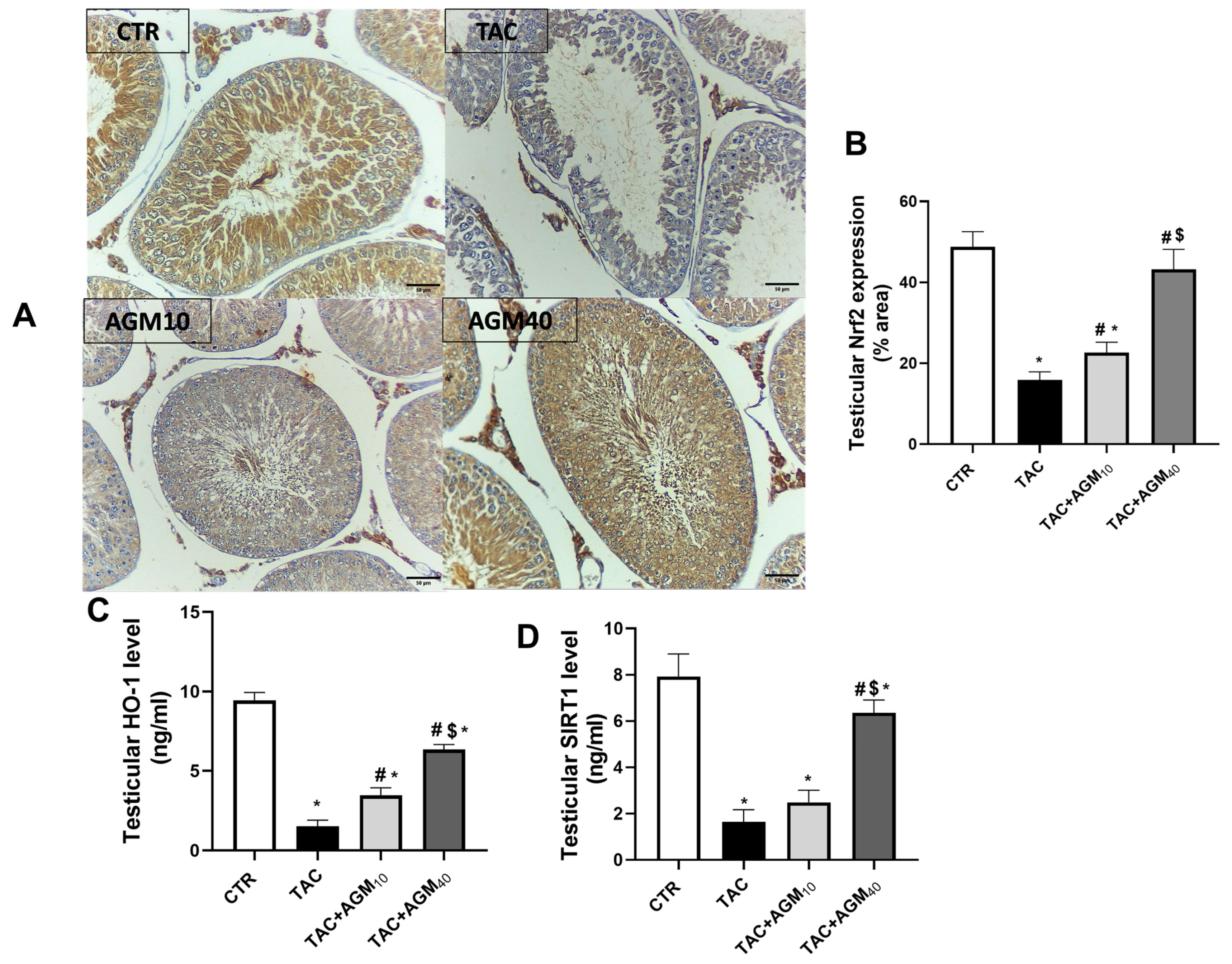
3.5. Impact of AGM10 and AGM40 on TAC-Induced Changes in Nrf2, HO-1, and SIRT1 Expression
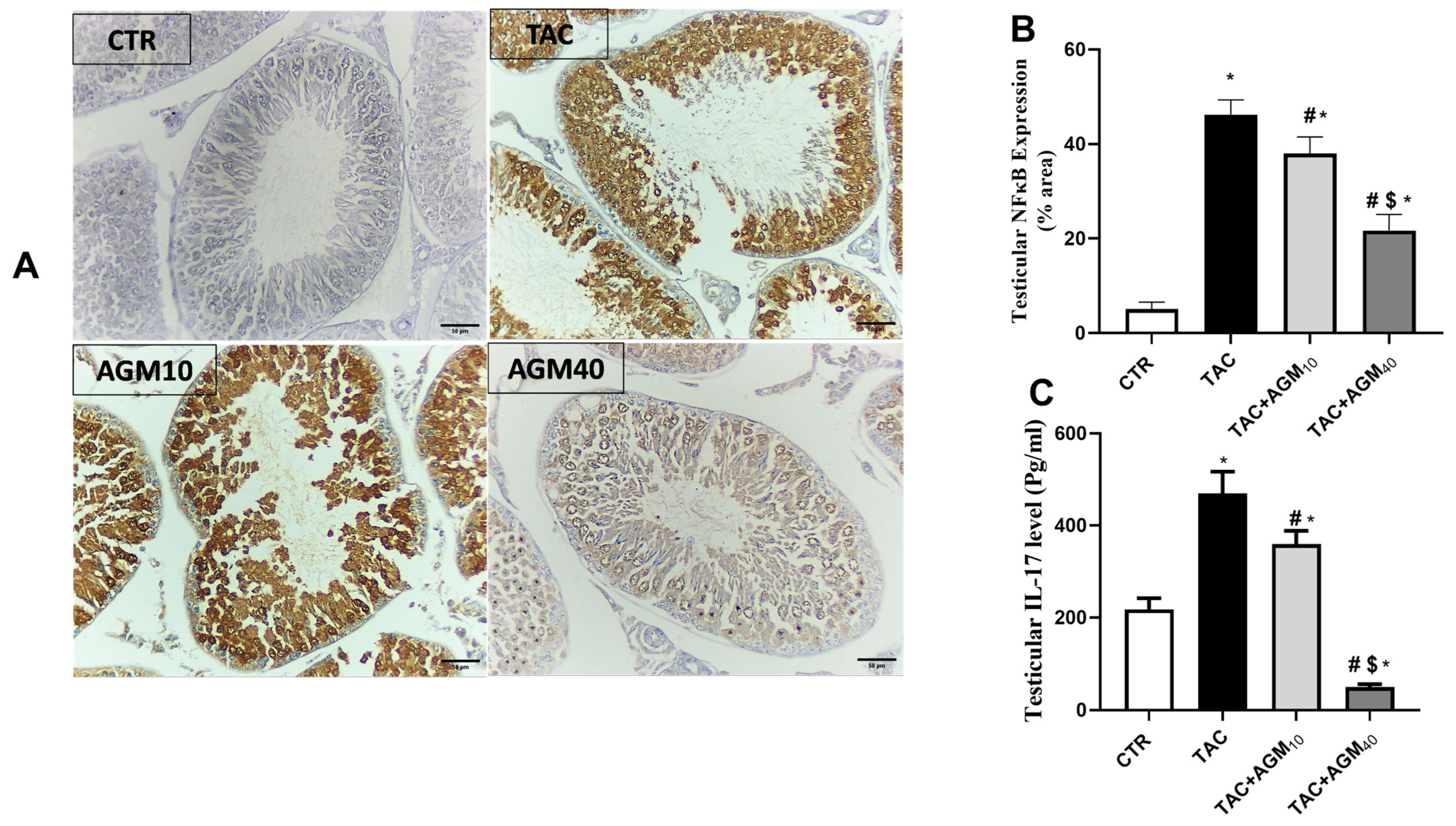
3.6. Impact of AGM10 and AGM40 on TAC-Induced Changes in NF-κB and IL-17 Expressions
3.7. Impact of AGM10 and AGM40 on TAC-Induced Changes in Caspase-3 and Bcl-2 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hart, A.; Lentine, K.; Smith, J.; Miller, J.; Skeans, M.; Prentice, M.; Robinson, A.; Foutz, J.; Booker, S.; Israni, A. OPTN/SRTR 2019 annual data report: Kidney. Am. J. Transplant. 2021, 21, 21–137. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, V.W.; Oellerich, M. New developments in the immunosuppressive drug monitoring of cyclosporine, tacrolimus, and azathioprine. Clin. Biochem. 2001, 34, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ferjani, H.; El Arem, A.; Bouraoui, A.; Achour, A.; Abid, S.; Bacha, H.; Boussema-Ayed, I. Protective effect of mycophenolate mofetil against nephrotoxicity and hepatotoxicity induced by tacrolimus in Wistar rats. J. Physiol. Biochem. 2016, 72, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Fujihira, S.; Ueno, H.; Kagawa, M.; Katsuoka, Y.; Mori, H. Ultrastructural study on cytotoxic effects of cyclosporine A in spermiogenesis in rats. Med. Electron. Microsc. 2003, 36, 183–191. [Google Scholar] [CrossRef]
- Seethalakshmi, L.; Flores, C.; Carboni, A.A.; Bala, R.A.M.; Diamond, D.A.; Menon, M. Cyclosporine: Its Effects on Testicular Function and Fertility in the Prepubertal Rat. J. Androl. 1990, 11, 17–24. [Google Scholar] [CrossRef]
- Hisatomi, A.; Fujihira, S.; Fujimoto, Y.; Fujii, T.; Mine, Y.; Ohara, K. Effect of Prograf (FK506) on spermatogenesis in rats. Toxicology 1996, 109, 75–83. [Google Scholar] [CrossRef]
- Walensky, L.D.; Dawson, T.M.; Steiner, J.P.; Sabatini, D.M.; Suarez, J.D.; Klinefelter, G.R.; Snyder, S.H. The 12 kD FK506 Binding Protein FKBP12 Is Released in the Male Reproductive Tract and Stimulates Sperm Motility. Mol. Med. 1998, 4, 502–514. [Google Scholar] [CrossRef]
- Annett, S.; Moore, G.; Robson, T. FK506 binding proteins and inflammation related signalling pathways; basic biology, current status and future prospects for pharmacological intervention. Pharmacol. Ther. 2020, 215, 107623. [Google Scholar] [CrossRef]
- Park, C.; Kwon, D.H.; Hwang, S.J.; Han, M.H.; Jeong, J.-W.; Hong, S.H.; Cha, H.-J.; Hong, S.-H.; Kim, G.-Y.; Lee, H.-J.; et al. Protective Effects of Nargenicin A1 against Tacrolimus-Induced Oxidative Stress in Hirame Natural Embryo Cells. Int. J. Environ. Res. Public Health 2019, 16, 1044. [Google Scholar] [CrossRef]
- El-Kashef, D.H.; El-Kenawi, A.E.; Rahim, M.A.; Suddek, G.M.; Salem, H.A. Agmatine improves renal function in gentamicin-induced nephrotoxicity in rats. Can. J. Physiol. Pharmacol. 2016, 94, 278–286. [Google Scholar] [CrossRef]
- Xu, W.; Gao, L.; Li, T.; Shao, A.; Zhang, J. Neuroprotective Role of Agmatine in Neurological Diseases. Curr. Neuropharmacol. 2018, 16, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, N.; Fujiwara, S. The therapeutic and nutraceutical potential of agmatine, and its enhanced production using Aspergillus oryzae. Amino Acids 2020, 52, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Sharawy, M.H.; Abdelrahman, R.S.; El-Kashef, D.H. Agmatine attenuates rhabdomyolysis-induced acute kidney injury in rats in a dose dependent manner. Life Sci. 2018, 208, 79–86. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbeeny, N.A.; Nader, M.A.; Attia, G.M.; Ateyya, H. Agmatine protects rat liver from nicotine-induced hepatic damage via antioxidative, antiapoptotic, and antifibrotic pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 1341–1351. [Google Scholar] [CrossRef]
- Ommati, M.M.; Farshad, O.; Mousavi, K.; Taghavi, R.; Farajvajari, S.; Azarpira, N.; Moezi, L.; Heidari, R. Agmatine alleviates hepatic and renal injury in a rat model of obstructive jaundice. PharmaNutrition 2020, 13, 100212. [Google Scholar] [CrossRef]
- Battaglia, V.; Rossi, C.A.; Colombatto, S.; Grillo, M.A.; Toninello, A. Different behavior of agmatine in liver mitochondria: Inducer of oxidative stress or scavenger of reactive oxygen species? Biochim. Biophys. Acta 2007, 1768, 1147–1153. [Google Scholar] [CrossRef]
- El-Agamy, D.S.; Makled, M.N.; Gamil, N.M. Protective effects of agmatine against D-galactosamine and lipopolysaccharide-induced fulminant hepatic failure in mice. Inflammopharmacology 2014, 22, 187–194. [Google Scholar] [CrossRef]
- Dejanovic, B.; Stevanovic, I.; Ninkovic, M.; Stojanovic, I.; Lavrnja, I.; Radicevic, T. Protective effects of agmatine against chlorpromazine-induced toxicity in the liver of wistar rats. Acta Fac. Med. Naissensis 2016, 33, 13. [Google Scholar] [CrossRef]
- Sari, Z.A.S. Protective effects of agmatine against gastric mucosal injury induced by nonsteroidal anti-inflammatory drugs: Role of nitric oxide and oxidative stress. J. Gastroenterol. Hepatol. 2020, 35, 1036–1044. [Google Scholar]
- Gonzalez, A.M. Agmatine protects against oxidative stress and inflammation in a model of neurodegeneration. J. Neuroinflammation 2018, 15, 239. [Google Scholar]
- Hisatomi, A.; Sakuma, S.; Fujiwara, M.; Seki, J. Effect of tacrolimus on the cauda epididymis in rats: Analysis of epididymal biochemical markers or antioxidant defense enzymes. Toxicology 2008, 243, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, T.; Tajima, S.; Fu, R.; Zhang, M.; Itoyama, Y.; Tsuchimoto, A.; Egashira, N.; Ieiri, I. The mTOR inhibitor everolimus attenuates tacrolimus-induced renal interstitial fibrosis in rats. Life Sci. 2022, 288, 120150. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.H.G.F.; Oliveira, G.A.; Dias, F.G.F.; Gomes, R.D.P.X.; Tambara Filho, R.; Fraga, R.D. Effects of immunosuppression with tacrolimus and mycophenolate mofetil on renal histology and function in single kidney rats submitted to ischemia and reperfusion. Acta Cir. Bras. 2015, 30, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kempinas, W.; Lamano-Carvalho, T.L. A method for estimating the concentration of spermatozoa in the rat cauda epididymidis. Lab. Anim. 1988, 22, 154–156. [Google Scholar] [CrossRef]
- Björndahl, L.; Söderlund, I.; Kvist, U. Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Hum. Reprod. 2003, 18, 813–816. [Google Scholar] [CrossRef]
- Masuda, T.; Oikawa, H.; Sato, S.; Satodate, R.; Suzuki, K.; Sato, S. Distinguishing small lymph vessels in the portal tracts of human liver from portal veins by immunohistochemistry for α smooth muscle actin. Int. Hepatol. Commun. 1996, 4, 277–282. [Google Scholar] [CrossRef]
- Perez-Garcia, L.F.; Dolhain, R.J.E.M.; Vorstenbosch, S.; Bramer, W.; van Puijenbroek, E.; Hazes, J.M.W.; Te Winkel, B. The effect of paternal exposure to immunosuppressive drugs on sexual function, reproductive hormones, fertility, pregnancy and offspring outcomes: A systematic review. Hum. Reprod. Update 2020, 26, 961–1001. [Google Scholar] [CrossRef]
- Sasaki, J.C.; Chapin, R.E.; Hall, D.G.; Breslin, W.; Moffit, J.; Saldutti, L.; Enright, B.; Seger, M.; Jarvi, K.; Hixon, M.; et al. Incidence and nature of testicular toxicity findings in pharmaceutical development. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2011, 92, 511–525. [Google Scholar] [CrossRef]
- Zheng, H.-L.; Zhang, H.-Y.; Zhu, C.-L.; Li, H.-Y.; Cui, S.; Jin, J.; Piao, S.-G.; Jiang, Y.-J.; Xuan, M.-Y.; Jin, J.-Z. L-Carnitine protects against tacrolimus-induced renal injury by attenuating programmed cell death via PI3K/AKT/PTEN signaling. Acta Pharmacol. Sin. 2021, 42, 77–87. [Google Scholar] [CrossRef]
- Takeshima, T.; Usui, K.; Mori, K.; Asai, T.; Yasuda, K.; Kuroda, S.; Yumura, Y. Oxidative stress and male infertility. Reprod. Med. Biol. 2021, 20, 41–52. [Google Scholar] [CrossRef]
- Ashour, M.A.; Fatima, W.; Imran, M.; Ghoneim, M.M.; Alshehri, S.; Shakeel, F. A review on the main phytoconstituents, traditional uses, inventions, and patent literature of gum Arabic emphasizing Acacia seyal. Molecules 2022, 27, 1171. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, F.; Borgmann, H.; Struck, J.P.; Salem, J.; Kuru, T.H. Antioxidant Supplementation on Male Fertility-A Systematic Review. Antioxidants 2023, 12, 836. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.; Agarwal, A.; Majzoub, A.; Panner Selvam, M.K.; Baskaran, S.; Henkel, R.; Elbardisi, H. Efficacy of Antioxidant Supplementation on Conventional and Advanced Sperm Function Tests in Patients with Idiopathic Male Infertility. Antioxidants 2020, 9, 219. [Google Scholar] [CrossRef]
- Gonzales, H.M.; McGillicuddy, J.W.; Rohan, V.; Chandler, J.L.; Nadig, S.N.; Dubay, D.A.; Taber, D.J. A comprehensive review of the impact of tacrolimus intrapatient variability on clinical outcomes in kidney transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2020, 20, 1969–1983. [Google Scholar] [CrossRef]
- Fatima, N.; Sheikh, N.; Satoskar, A.R.; Akhtar, T.; Tayyeb, A.; Ashfaq, I.; Ryan, N.; Ambreen, S.; Jha, B.K.; Oghumu, S. Effect of Short-Term Tacrolimus Exposure on Rat Liver: An Insight into Serum Antioxidant Status, Liver Lipid Peroxidation, and Inflammation. Mediat. Inflamm. 2021, 2021, 6613786. [Google Scholar] [CrossRef]
- Al-Harbi, N.O.; Imam, F.; Al-Harbi, M.M.; Iqbal, M.; Nadeem, A.; Sayed-Ahmed, M.M.; Alabidy, A.D.; Almukhallafi, A.F. Olmesartan attenuates tacrolimus-induced biochemical and ultrastructural changes in rat kidney tissue. BioMed Res. Int. 2014, 2014, 607246. [Google Scholar] [CrossRef]
- Park, Y.; Lee, H.; Eum, S.H.; Ko, E.J.; Min, J.W.; Yoon, S.-H.; Hwang, W.-M.; Yun, S.-R.; Yang, C.W.; Shin, J.; et al. Combined impact of the inter and intra-patient variability of tacrolimus blood level on allograft outcomes in kidney transplantation. Front. Immunol. 2022, 13, 1037566. [Google Scholar] [CrossRef]
- El-Awady, M.S.; Nader, M.A.; Sharawy, M.H. The inhibition of inducible nitric oxide synthase and oxidative stress by agmatine attenuates vascular dysfunction in rat acute endotoxemic model. Environ. Toxicol. Pharmacol. 2017, 55, 74–80. [Google Scholar] [CrossRef]
- Ferents, I.V.; Brodyak, I.V.; Lyuta, M.Y.; Burda, V.A.; Gavrylyshyn, G.S.; Sybirna, N.O. Effect of agmatine on the blood system parameters of rats under the condition of experimental diabetes mellitus. Stud. Biol. 2012, 6, 65–72. [Google Scholar] [CrossRef]
- Singh, V.; Ubaid, S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation 2020, 43, 1589–1598. [Google Scholar] [CrossRef]
- Luo, J.; Nikolaev, A.Y.; Imai, S.-I.; Chen, D.; Su, F.; Shiloh, A.; Guarente, L.; Gu, W. Negative Control of p53 by Sir2α Promotes Cell Survival under Stress. Cell 2001, 107, 137–148. [Google Scholar] [CrossRef]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer. Int. J. Mol. Sci. 2019, 20, 3153. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef]
- Lamiri, W.M. Correlation Between Semen Interleukin-17 and Seminal Parameters in Infertile Males. ALEXMED ePosters 2021, 3, 23–24. [Google Scholar] [CrossRef]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef]
- de Gregorio, E.; Colell, A.; Morales, A.; Marí, M. Relevance of SIRT1-NF-κB Axis as Therapeutic Target to Ameliorate Inflammation in Liver Disease. Int. J. Mol. Sci. 2020, 21, 3858. [Google Scholar] [CrossRef]
- Borgia, F.; Li Pomi, F.; Vaccaro, M.; Alessandrello, C.; Papa, V.; Gangemi, S. Oxidative stress and phototherapy in atopic dermatitis: Mechanisms, role, and future perspectives. Biomolecules 2022, 12, 1904. [Google Scholar] [CrossRef]
- Nady, M.E.; El-Raouf, O.M.A.; El-Sayed, E.-S.M. Linagliptin ameliorates tacrolimus-induced renal injury: Role of Nrf2/HO-1 and HIF-1α/CTGF/PAI-1. Mol. Biol. Rep. 2024, 51, 608. [Google Scholar] [CrossRef]
- Yang, W.; Huang, J.; Xiao, B.; Liu, Y.; Zhu, Y.; Wang, F.; Sun, S. Taurine Protects Mouse Spermatocytes from Ionizing Radiation-Induced Damage Through Activation of Nrf2/HO-1 Signaling. Cell. Physiol. Biochem. 2017, 44, 1629–1639. [Google Scholar] [CrossRef]
- Khawar, M.B.; Sohail, A.M.; Li, W. SIRT1: A Key Player in Male Reproduction. Life 2022, 12, 318. [Google Scholar] [CrossRef]
- He, G.; Karin, M. NF-κB and STAT3—Key players in liver inflammation and cancer. Cell Res. 2011, 21, 159–168. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Rafael-Vidal, C.; Pérez, N.; Altabás, I.; Garcia, S.; Pego-Reigosa, J.M. Blocking IL-17: A Promising Strategy in the Treatment of Systemic Rheumatic Diseases. Int. J. Mol. Sci. 2020, 21, 7100. [Google Scholar] [CrossRef]
- von Vietinghoff, S.; Ley, K. Interleukin 17 in vascular inflammation. Cytokine Growth Factor Rev. 2010, 21, 463–469. [Google Scholar] [CrossRef]
- Bahrami-Asl, Z.; Farzadi, L.; Fattahi, A.; Yousefi, M.; Quinonero, A.; Hakimi, P.; Latifi, Z.; Nejabati, H.R.; Ghasemnejad, T.; Sadigh, A.R.; et al. Tacrolimus Improves the Implantation Rate in Patients with Elevated Th1/2 Helper Cell Ratio and Repeated Implantation Failure (RIF). Geburtshilfe Frauenheilkd. 2020, 80, 851–862. [Google Scholar] [CrossRef]
- Li, Y.; Guptill, J.T.; Russo, M.A.; Massey, J.M.; Juel, V.C.; Hobson-Webb, L.D.; Howard, J.F.; Chopra, M.; Liu, W.; Yi, J.S. Tacrolimus inhibits Th1 and Th17 responses in MuSK-antibody positive myasthenia gravis patients. Exp. Neurol. 2019, 312, 43–50. [Google Scholar] [CrossRef]
- Kim, M.G.; Kim, D.H.; Lee, H.R.; Lee, J.S.; Jin, S.J.; Lee, H.T. Sirtuin inhibition leads to autophagy and apoptosis in porcine preimplantation blastocysts. Biochem. Biophys. Res. Commun. 2017, 488, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Kozako, T.; Aikawa, A.; Shoji, T.; Fujimoto, T.; Yoshimitsu, M.; Shirasawa, S.; Tanaka, H.; Honda, S.i.; Shimeno, H.; Arima, N.; et al. High expression of the longevity gene product SIRT1 and apoptosis induction by sirtinol in adult T-cell leukemia cells. Int. J. Cancer 2012, 131, 2044–2055. [Google Scholar] [CrossRef]
- Coussens, M.; Maresh, J.G.; Yanagimachi, R.; Maeda, G.; Allsopp, R. Sirt1 deficiency attenuates spermatogenesis and germ cell function. PLoS ONE 2008, 3, e1571. [Google Scholar] [CrossRef]
- Chung, Y.W.; Chung, M.W.; Choi, S.K.; Choi, S.J.; Choi, S.J.N.; Chung, S.Y. Tacrolimus-Induced Apoptosis is Mediated by Endoplasmic Reticulum–derived Calcium-dependent Caspases-3,-12 in Jurkat Cells. Transplant. Proc. 2018, 50, 1172–1177. [Google Scholar] [CrossRef]
- Chai, J.; Luo, L.; Hou, F.; Fan, X.; Yu, J.; Ma, W.; Tang, W.; Yang, X.; Zhu, J.; Kang, W.; et al. Agmatine Reduces Lipopolysaccharide-Mediated Oxidant Response via Activating PI3K/Akt Pathway and Up-Regulating Nrf2 and HO-1 Expression in Macrophages. PLoS ONE 2016, 11, e0163634. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.E.; Egea, J.; Buendía, I.; Navarro, E.; Rada, P.; Cuadrado, A.; Rodrigues, A.L.S.; López, M.G. Agmatine induces Nrf2 and protects against corticosterone effects in hippocampal neuronal cell line. Mol. Neurobiol. 2015, 51, 1504–1519. [Google Scholar] [CrossRef] [PubMed]



| 1 | Control group | Rats received vehicle only for 14 days |
| 2 | AGM10 group | Rats received AGM (10 mg/kg/day, oral) for 14 days [13] |
| 3 | AGM40 group | Rats received AGM (40 mg/kg/day, oral) for 14 days [13] |
| 4 | TAC group | Rats received TAC (5 mg/kg/day, i.p.) for 14 days |
| 5 | TAC + AGM10 group | Rats received TAC (5 mg/kg/day, i.p.) and AGM (10 mg/kg/day, oral) for 14 days simultaneously [13] |
| 6 | TAC + AGM40 group | received TAC (5 mg/kg/day, i.p.) and AGM (40 mg/kg/day, oral) for 14 days simultaneously [13] |
| Sperm Viability % | |||
|---|---|---|---|
| After 1 h | After 2 h | After 3 h | |
| Control | 52.50 ± 2.50 | 47.50 ± 2.50 | 42.50 ± 2.50 |
| TAC | 28.75 ± 4.15 * | 23.75 ± 4.15 * | 24.25 ± 2.44 * |
| TAC + AGM10 | 34.29 ± 4.32 | 29.00 ± 4.18 | 29.00 ± 9.62 |
| TAC + AGM40 | 44.50 ± 3.71 # | 39.00 ± 4.18 # | 34.00 ± 4.18 # |
| The Height of the Seminiferous Epithelium (µm) | The Diameter of the Seminiferous Tubules (µm) | |
|---|---|---|
| Control | 70.6 ± 12.3 | 244.4 ± 16.8 |
| TAC | 42.0 ± 2.0 * | 182.2 ± 6.8 * |
| TAC + AGM10 | 59.4 ± 6.7 # | 198.4 ± 8.1 |
| TAC + AGM40 | 65.4 ± 4.6 # | 212.6 ± 3.6 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, N.; Nour, O.; Makled, M.N.; Nader, M. Agmatine Abrogates Tacrolimus-Induced Testicular Injury in Rats. Pharmaceutics 2025, 17, 703. https://doi.org/10.3390/pharmaceutics17060703
Alharbi N, Nour O, Makled MN, Nader M. Agmatine Abrogates Tacrolimus-Induced Testicular Injury in Rats. Pharmaceutics. 2025; 17(6):703. https://doi.org/10.3390/pharmaceutics17060703
Chicago/Turabian StyleAlharbi, Naif, Omnia Nour, Mirhan N. Makled, and Manar Nader. 2025. "Agmatine Abrogates Tacrolimus-Induced Testicular Injury in Rats" Pharmaceutics 17, no. 6: 703. https://doi.org/10.3390/pharmaceutics17060703
APA StyleAlharbi, N., Nour, O., Makled, M. N., & Nader, M. (2025). Agmatine Abrogates Tacrolimus-Induced Testicular Injury in Rats. Pharmaceutics, 17(6), 703. https://doi.org/10.3390/pharmaceutics17060703





