Beyond ADME: The Endogenous Functions of Drug Transporters and Its Impact on Human Disease
Abstract
1. Introduction
2. Overview of Drug Transporters
| Transporter | Gene | Endogenous Substrates | Primary Tissues | Citations |
|---|---|---|---|---|
| P-gp | ABCB1 | Steroids (e.g., cortisol), neurotoxins (e.g., beta-amyloid), lipids (e.g., Phosphatidylcholine), endocannabinoids (e.g., anandamide) | Brain (BBB), intestine, liver, kidney | [28,29,30,31] |
| BCRP | ABCG2 | Uric acid, bile acids (e.g., glycocholic acid), porphyrins (e.g., protoporphyrin IX), sulfated estrogens (e.g., estrone sulfate), neurotoxins (e.g., beta-amyloid) | Intestine, placenta, brain, liver | [32,33,34,35] |
| OATP1B1 | SLCO1B1 | Bilirubin, bile acids (e.g., taurocholate, glycochenodeoxycholate sulfate, glycodeoxycholic acid sulfate), hormones and steroid conjugates (e.g., estradiol-17β-glucuronide, Estrone-3-sulfate), Porphyrins (e.g., coproporphyrin I) | Liver | [36,37,38,39,40] |
| OATP1B3 | SLCO1B3 | Bilirubin, bile acids (e.g., taurocholate, glycochenodeoxycholate sulfate, glycodeoxycholic acid sulfate), hormones and steriod conjugates (e.g., estradiol-17β-glucuronide, estrone-3-sulfate), Porphyrins (e.g., protoporphyrin IX), Peptide hormones (e.g., cholecystokinin octapeptide (CCK-8)) | Liver | [39,41,42,43] |
| OAT1 | SLC22A6 | Uremic toxins (e.g., indoxyl sulfate, p-cresyl sulfate), prostaglandins (e.g., PGE2), Tryptophan metabolites (e.g., kynurenine, xanthurenic acid), Vitamins (e.g., nicotinate (niacin), 4-pyridoxic acid (vitamin B6 metabolite), pantothenic acid), taurine, creatinine | Kidney | [44,45,46,47] |
| OAT3 | SLC22A8 | Uremic toxins (e.g., indoxyl sulfate, p-cresyl sulfate, CMPF, kynurenic acid), prostaglandins (e.g., PGE2), cyclic nucleotides (e.g., cAMP, cGMP), kynurenic acid, xanthurenic acid, estrone sulfatetaurine, N-acetylaspartate | Kidney | [20,47,48,49,50] |
| OCT2 | SLC22A2 | Uremic toxins (e.g., creatinine, guanidine, methylguanidine, guanidinosuccinic acid), Neurotransmitters and neuromodulators (e.g., dopamine, serotonin, histamine), Vitamins and derivatives (e.g., 1-methylnicotinamide), tryptophan | Kidney | [51,52,53,54] |
| MATE1 | SLC47A1 | Vitamins and cofactors (e.g., N-methylnicotinamide, thiamine, carnitine), Neurotransmitters and derivatives (e.g., histamine, serotonin), Organic cations and uremic solutes (e.g., creatinine, guanidine, TMAO), Anionic conjugates (e.g., estrone sulfate) | Kidney, liver | [24,53,55,56,57] |
| MATE2-K | SLC47A2 | Uremic toxins and nitrogenous wastes (e.g., Creatinine, guanidine), Vitamin metabolites and cofactors (e.g., N-methylnicotinamide (NMN), thiamine (Vitamin B1), Steroid conjugates (Estrone sulfate) | Kidney | [24,55,56] |
3. Endogenous Roles of Drug Transporters in Physiology
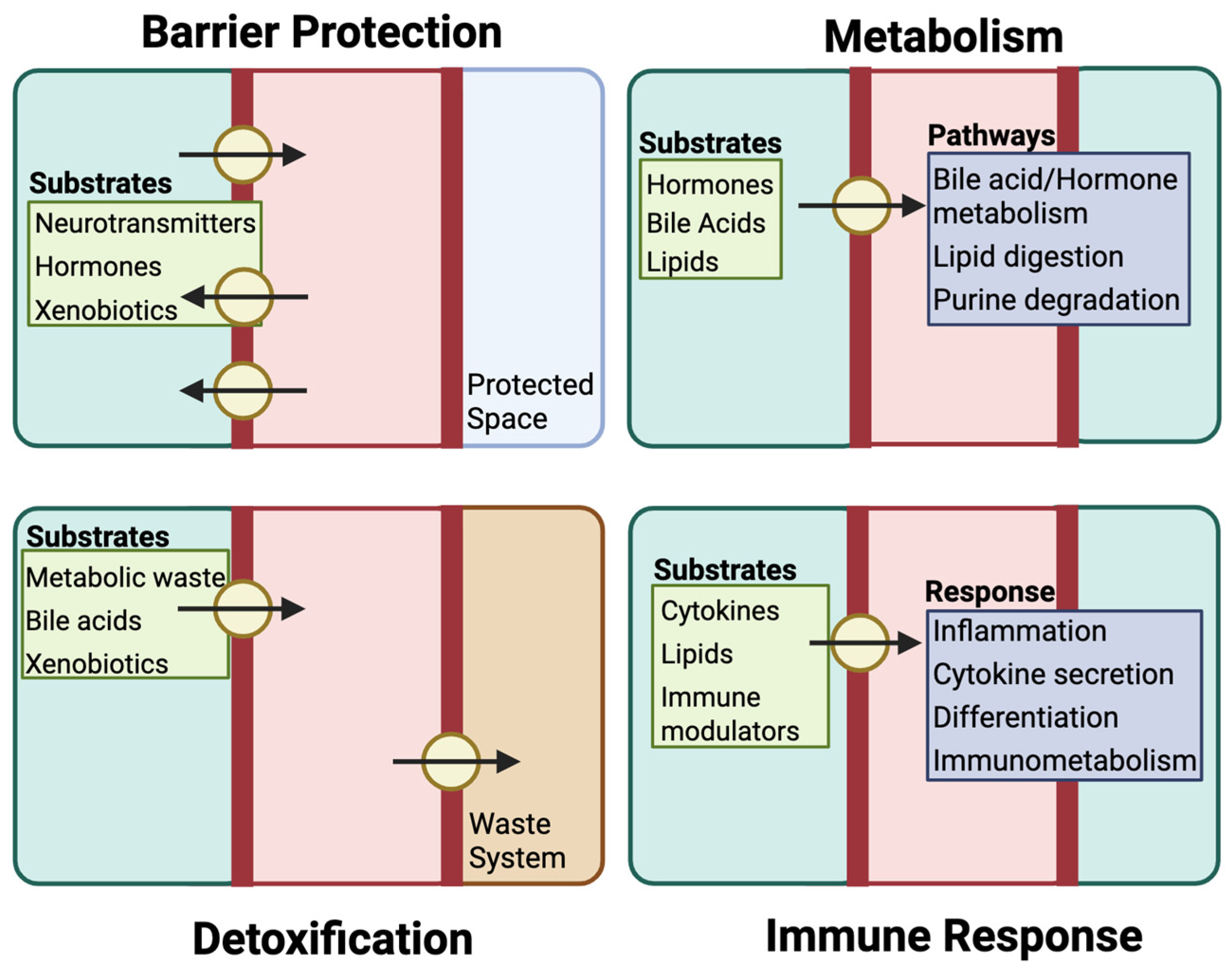
3.1. Barrier Function and Protection
3.2. Metabolic Regulation
3.3. Detoxification
3.4. Immune Modulation
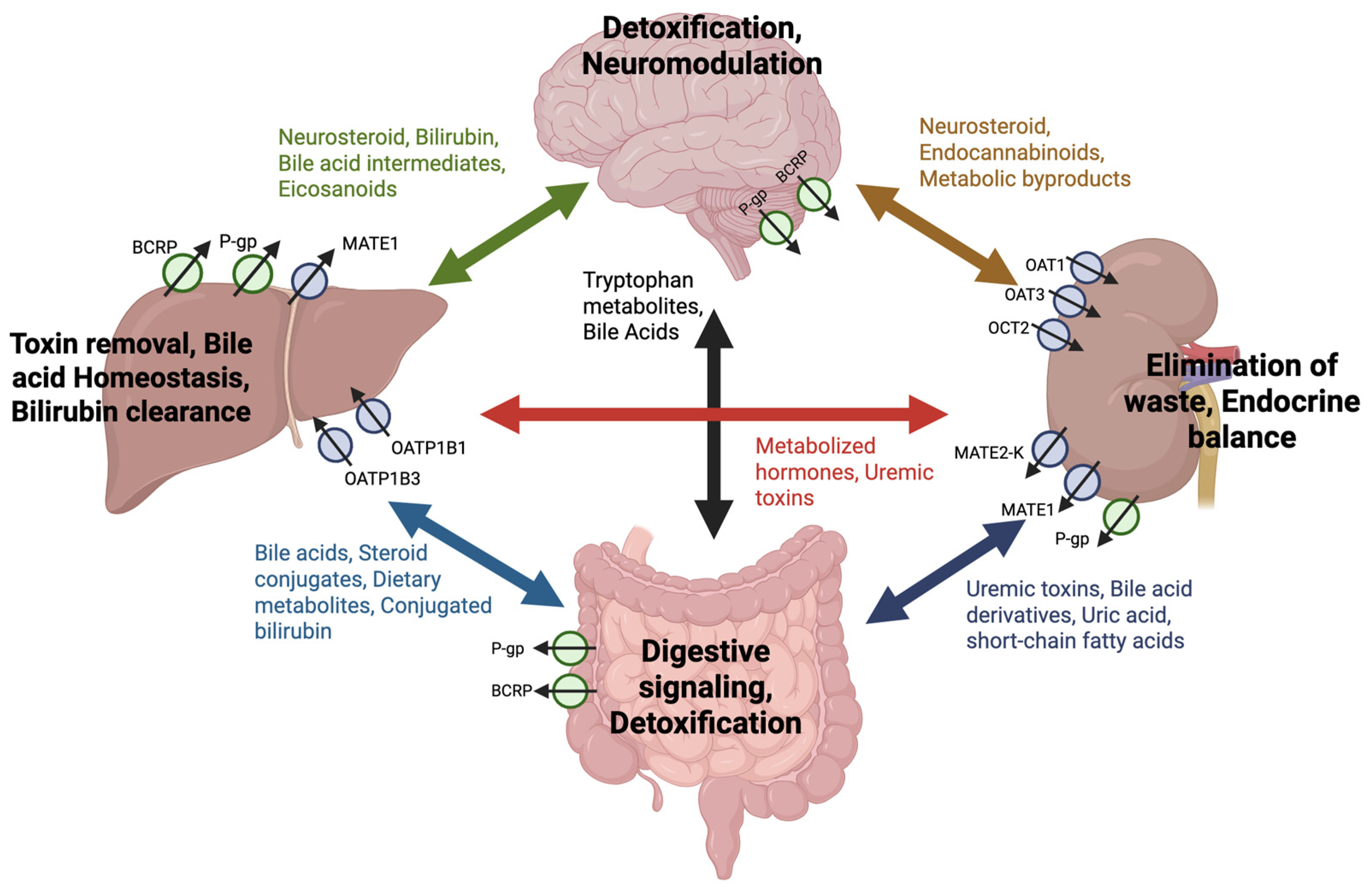
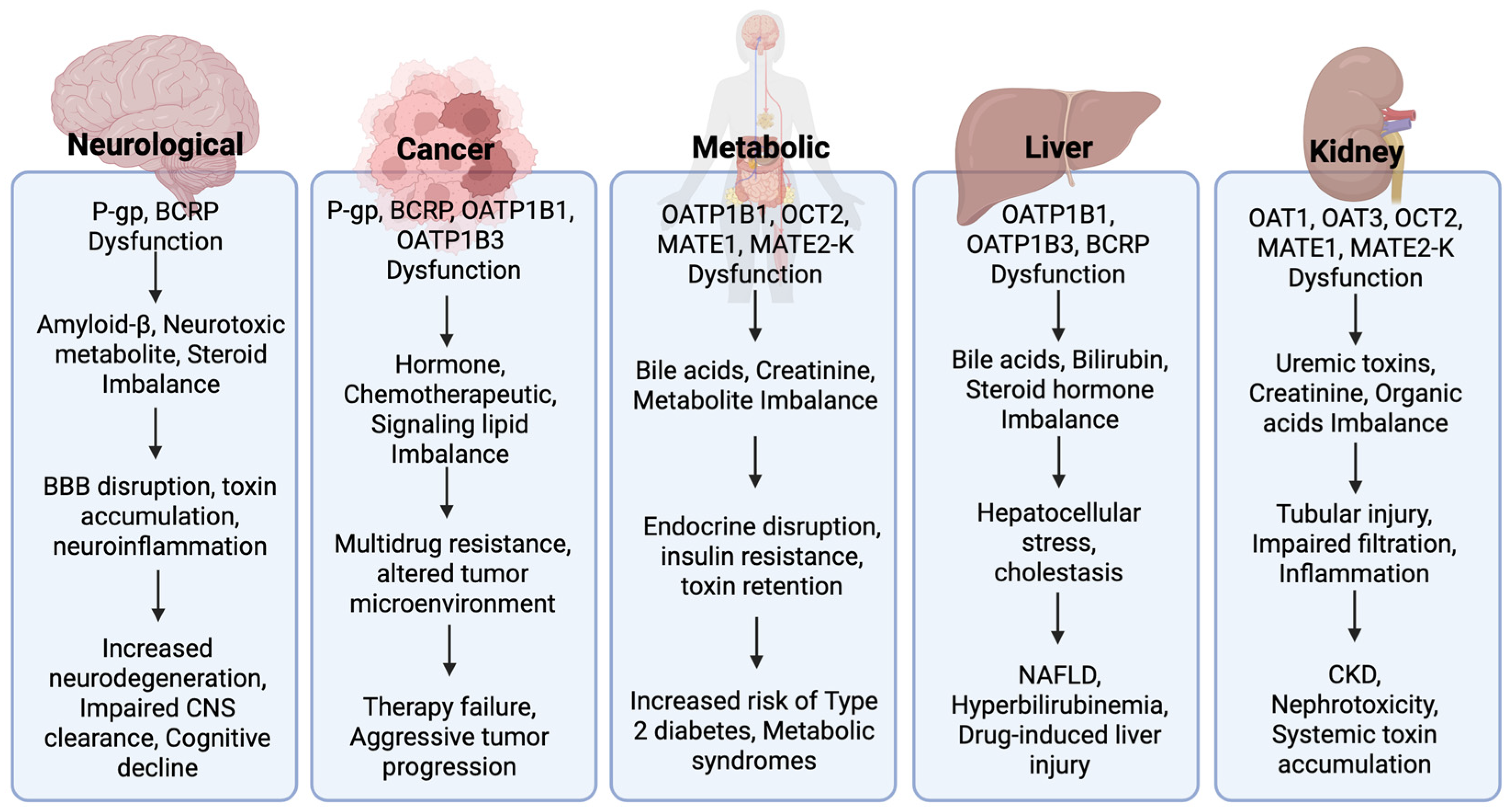
4. Dysregulation of Drug Transporters in Disease Pathophysiology
4.1. Neurological Disorders
4.2. Cancer
4.3. Metabolic Disorders
4.4. Kidney Disease
4.5. Hepatic Conditions
5. Research Gaps and Challenges
6. Future Research Directions
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.; Zhou, Z.; Zhou, J.; Chen, S.-Q. Pharmacogenomics of drug metabolizing enzymes and transporters: Relevance to precision medicine. Genom. Proteom. Bioinform. 2016, 14, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Rendic, S.P. Metabolism and interactions of Ivermectin with human cytochrome P450 enzymes and drug transporters, possible adverse and toxic effects. Arch. Toxicol. 2021, 95, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Volpe, D.A.; Balimane, P.V. Application of in vitro CYP and transporter assays to predict clinical drug-drug interactions. Bioanalysis 2018, 10, 619–623. [Google Scholar] [CrossRef]
- Rodieux, F.; Gotta, V.; Pfister, M.; Anker, J.N. Causes and Consequences of Variability in Drug Transporter Activity in Pediatric Drug Therapy. J. Clin. Pharmacol. 2016, 56, S173–S192. [Google Scholar] [CrossRef]
- Saito, Y.; Itagaki, S.; Otsuka, Y.; Kobayashi, Y.; Okumura, H.; Kobayashi, M.; Hirano, T.; Iseki, K. Substrate specificity of the nateglinide/H(+) cotransport system for phenolic acids. J. Agric. Food Chem. 2005, 53, 6100–6104. [Google Scholar] [CrossRef]
- Keogh, J.P. Membrane transporters in drug development. Adv. Pharmacol. 2012, 63, 1–42. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). In Vitro Metabolism- and Transporter-Mediated Drug-Drug Interaction Studies: Guidance for Industry. 2017. Available online: https://www.fda.gov/files/drugs/published/In-Vitro-Metabolism--and-Transporter--Mediated-Drug-Drug-Interaction-Studies-Guidance-for-Industry.pdf (accessed on 15 January 2024).
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). In Vitro Drug Interaction Studies—Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions: Guidance for Industry. 2020. Available online: https://digirepo.nlm.nih.gov/master/borndig/101767646/download.pdf (accessed on 15 January 2024).
- Keogh, J.; Hagenbuch, B.; Rynn, C.; Stieger, B.; Nicholls, G. Drug Transporters: Volume 1: Role and Importance in ADME and Drug Development. R. Soc. Chem. 2016, 1, 151–183. [Google Scholar]
- Giacomini, K.M.; Huang, S.-M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.R.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [CrossRef]
- Yano, K.; Seto, S.; Kamioka, H.; Mizoi, K.; Ogihara, T. Testosterone and androstenedione are endogenous substrates of P-glycoprotein. Biochem. Biophys. Res. Commun. 2019, 520, 166–170. [Google Scholar] [CrossRef]
- Kimura, A.; Kagawa, T.; Takei, H.; Maruo, Y.; Sakugawa, H.; Sasaki, T.; Murai, T.; Naritaka, N.; Takikawa, H.; Nittono, H. Rotor syndrome: Glucuronidated bile acidemia from defective reuptake by hepatocytes. Hepatol. Commun. 2021, 5, 629–633. [Google Scholar] [CrossRef]
- Steinbüchel, M.; Menne, J.; Schröter, R.; Neugebauer, U.; Schlatter, E.; Ciarimboli, G. Regulation of Transporters for Organic Cations by High Glucose. Int. J. Mol. Sci. 2023, 24, 14051. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, K.M.; Galetin, A.; Huang, S.-M. The International Transporter Consortium: Summarizing Advances in the Role of Transporters in Drug Development. Clin. Pharmacol. Ther. 2018, 104, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Zamek-Gliszczynski, M.J.; Sangha, V.; Shen, H.; Feng, B.; Wittwer, M.B.; Varma, M.V.S.; Liang, X.; Sugiyama, Y.; Zhang, L.; Bendayan, R. Transporters in Drug Development: International Transporter Consortium Update on Emerging Transporters of Clinical Importance. Clin. Pharmacol. Ther. 2022, 112, 485–500. [Google Scholar] [CrossRef]
- Staud, F.; Ceckova, M.; Micuda, S.; Pavek, P. Expression and function of p-glycoprotein in normal tissues: Effect on pharmacokinetics. Methods Mol. Biol. 2010, 596, 199–222. [Google Scholar] [CrossRef]
- Chiou, W.J. In vitro OATP1B1 and OATP1B3 inhibition is associated with observations of benign clinical unconjugated hyperbilirubinemia. Xenobiotica 2014, 44, 276–282. [Google Scholar] [CrossRef]
- Ciută, A.D.; Nosol, K.; Kowal, J. Structure of human drug transporters OATP1B1 and OATP1B3. Nat. Commun. 2023, 14, 5774. [Google Scholar] [CrossRef]
- Vávra, J.; Mančíková, A.; Pavelcová, K.; Hasíková, L.; Bohatá, J.; Stibůrková, B. Functional characterization of rare variants in OAT1/SLC22A6 and OAT3/SLC22A8 urate transporters identified in a gout and hyperuricemia cohort. Cells 2022, 11, 1063. [Google Scholar] [CrossRef]
- Bush, K.T.; Wu, W.; Lun, C.; Nigam, S.K. The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut–liver–kidney axis. J. Biol. Chem. 2017, 292, 15789–15803. [Google Scholar] [CrossRef]
- Granados, J.C.; Nigam, A.K.; Bush, K.T.; Jamshidi, N.; Nigam, S.K. A key role for the transporter OAT1 in systemic lipid metabolism. J. Biol. Chem. 2021, 296, 0021–9258. [Google Scholar] [CrossRef]
- Jamshidi, N.; Nigam, S.K. Drug Transporters OAT1 and OAT3 Have Specific Effects on Multiple Organs and Gut Microbiome as Revealed by Contextualized Metabolic Network Reconstructions. Sci. Rep. 2022, 12, 21091. [Google Scholar] [CrossRef]
- Puri, N.M.; Romano, G.R.; Lin, T.Y.; Mai, Q.N.; Irannejad, R. The organic cation transporter 2 regulates dopamine D1 receptor signaling at the Golgi apparatus. eLife 2022, 25, e75468. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, Y.; Masuda, S.; Sato, T.; Katsura, T.; Ogawa, O.; Inui, K. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H+-organic cation antiporters. Biochem. Pharmacol. 2007, 74, 933–945. [Google Scholar] [CrossRef]
- Astorga, B.; Ekins, S.; Morales, M.; Wright, S.H. Molecular determinants of ligand selectivity for the human multidrug and toxin extruder proteins MATE1 and MATE2-K. J. Pharmacol. Exp. Ther. 2012, 341, 829–838. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, J.; Long, F.; Kang, R.; Kroemer, G.; Tang, D.; Yang, M. The lipid flippase SLC47A1 blocks metabolic vulnerability to ferroptosis. Nat. Commun. 2022, 13, 7965. [Google Scholar] [CrossRef]
- Liu, H.; Doke, T.; Guo, D. Epigenomic and transcriptomic analyses define core cell types, genes and targetable mechanisms for kidney disease. Nat. Genet. 2022, 54, 950–962. [Google Scholar] [CrossRef]
- Ueda, K.; Okamura, N.; Hirai, M.; Tanigawara, Y.; Saeki, T.; Kioka, N.; Komano, T.; Hori, R. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J. Biol. Chem. 1992, 267, 24248–24252. [Google Scholar] [CrossRef]
- Wang, W.; Bodles-Brakhop, A.M.; Barger, S.W. A role for P-glycoprotein in clearance of Alzheimer amyloid-β peptide from the brain. Curr. Alzheimer Res. 2016, 13, 615–620. [Google Scholar] [CrossRef]
- Bosch, I.; Dunussi-Joannopoulos, K.; Wu, R.L.; Furlong, S.T.; Croop, J. Phosphatidylcholine and phosphatidylethanolamine behave as substrates of the human MDR1 P-glycoprotein. Biochemistry 1997, 36, 5685–5694. [Google Scholar] [CrossRef]
- Szabady, R.L.; Louissaint, C.; Lubben, A.; Xie, B.; Reeksting, S.; Tuohy, C.; Demma, Z.; Foley, S.E.; Faherty, C.S.; Llanos-Chea, A.; et al. Intestinal P-glycoprotein exports endocannabinoids to prevent inflammation and maintain homeostasis. J. Clin. Investig. 2018, 128, 4044–4056. [Google Scholar] [CrossRef]
- Hosomi, A.; Nakanishi, T.; Fujita, T.; Tamai, I. Extra-renal elimination of uric acid via intestinal efflux transporter BCRP/ABCG2. PLoS ONE 2012, 7, e30456. [Google Scholar] [CrossRef]
- Zattoni, I.F.; Kronenberger, T.; Kita, D.H.; Guanaes, L.D.; Guimaraes, M.M.; de Oliveira Prado, L.; Ziasch, M.; Vesga, L.C.; de Moraes Rego, F.G.; Picheth, G. A new porphyrin as selective substrate-based inhibitor of breast cancer resistance protein (BCRP/ABCG2). Chem.-Biol. Interact. 2022, 351, 109718. [Google Scholar] [CrossRef]
- Imai, Y.; Asada, S.; Tsukahara, S.; Ishikawa, E.; Tsuruo, T.; Sugimoto, Y. Breast cancer resistance protein exports sulfated estrogens but not free estrogens. Mol. Pharmacol. 2003, 64, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Do, T.M.; Noel-Hudson, M.-S.; Ribes, S.; Besengez, C.; Smirnova, M.; Cisternino, S.; Buyse, M.; Calon, F.; Chimini, G.; Chacun, H. ABCG2-and ABCG4-mediated efflux of amyloid-β peptide 1-40 at the mouse blood-brain barrier. J. Alzheimer’s Dis. 2012, 30, 155–166. [Google Scholar] [CrossRef]
- Hagenbuch, B. Cellular entry of thyroid hormones by organic anion transporting polypeptides. Best. Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 209–221. [Google Scholar] [CrossRef]
- Mori, D.; Kashihara, Y.; Yoshikado, T.; Kimura, M.; Hirota, T.; Matsuki, S.; Maeda, K.; Irie, S.; Ieiri, I.; Sugiyama, Y.; et al. Effect of OATP1B1 genotypes on plasma concentrations of endogenous OATP1B1 substrates and drugs, and their association in healthy volunteers. Drug Metab. Pharmacokinet. 2019, 34, 78–86. [Google Scholar] [CrossRef]
- Eckhardt, U.; Schroeder, A.; Stieger, B.; Höchli, M.; Landmann, L.; Tynes, R.E.; Meier, P.J.; Hagenbuch, B. Polyspecific substrate uptake by the hepatic organic anion transporter Oatp1 in stably transfected CHO cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 1999, 276, G1037–G1042. [Google Scholar] [CrossRef]
- Orozco, C.C.; Neuvonen, M.; Bi, Y.-A.; Cerny, M.A.; Mathialagan, S.; Tylaska, L.; Rago, B.; Costales, C.; King-Ahmad, A.; Niemi, M. Characterization of bile acid sulfate conjugates as substrates of human organic anion transporting polypeptides. Mol. Pharm. 2023, 20, 3020–3032. [Google Scholar] [CrossRef]
- Chan, G.; Houle, R.; Katwaru, R. Evaluation of the selectivity of several OATP1B biomarkers using relative activity factor method. Drug Metab. Dispos. 2023, 51, 522–533. [Google Scholar] [CrossRef]
- Pan, Q.; Zhu, G.; Xu, Z.; Zhu, J.; Ouyang, J.; Tong, Y.; Zhao, N.; Zhang, X.; Cheng, Y.; Zhang, L.; et al. Organic anion transporting polypeptide (OATP) 1B3 is a significant transporter for hepatic uptake of conjugated bile acids in humans. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 223–242. [Google Scholar] [CrossRef]
- Schnegelberger, R.D.; Steiert, B.; Sandoval, P.J.; Hagenbuch, B. Using a competitive counterflow assay to identify novel cationic substrates of OATP1B1 and OATP1B3. Front. Physiol. 2022, 13, 969363. [Google Scholar] [CrossRef]
- DeGorter, M.K.; Ho, R.H.; Leake, B.F.; Tirona, R.G.; Kim, R.B. Interaction of three regiospecific amino acid residues is required for OATP1B1 gain of OATP1B3 substrate specificity. Mol. Pharm. 2012, 9, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Nagle, M.; Kouznetsova, V.L. Untargeted metabolomics identifies enterobiome metabolites and putative uremic toxins as substrates of organic anion transporter 1 (Oat1). J. Proteome Res. 2011, 10, 2842–2851. [Google Scholar] [CrossRef]
- Granados, J.C.; Richelle, A.; Gutierrez, J.M. Coordinate regulation of systemic and kidney tryptophan metabolism by the drug transporters OAT1 and OAT3. J. Biol. Chem. 2021, 296, 100575. [Google Scholar] [CrossRef]
- Wu, W.; Bush, K.T.; Nigam, S.K. Key role for the organic anion transporters, OAT1 and OAT3, in the in vivo handling of uremic toxins and solutes. Sci. Rep. 2017, 7, 4939. [Google Scholar] [CrossRef]
- Tsuruya, Y.; Kato, K.; Sano, Y.; Imamura, Y.; Maeda, K.; Kumagai, Y.; Sugiyama, Y.; Kusuhara, H. Investigation of endogenous compounds applicable to drug-drug interaction studies involving the renal organic anion transporters, OAT1 and OAT3, in humans. Drug Metab. Dispos. 2016, 44, 1925–1933. [Google Scholar] [CrossRef]
- Nilwarangkoon, S.; Anzai, N.; Shiraya, K.; Yu, E.; Islam, R.; Cha, S.H.; Onozato, M.L.; Miura, D.; Jutabha, P.; Tojo, A. Role of mouse organic anion transporter 3 (mOat3) as a basolateral prostaglandin E2 transport pathway. J. Pharmacol. Sci. 2007, 103, 48–55. [Google Scholar] [CrossRef]
- Sweet, D.H.; Bush, K.T.; Nigam, S.K. The organic anion transporter family: From physiology to ontogeny and the clinic. Am. J. Physiol. Ren. Physiol. 2001, 281, F197–F205. [Google Scholar] [CrossRef]
- Ma, Y.; Ran, F.-L.; Xin, M.-J. Albumin-bound kynurenic acid is an appropriate endogenous biomarker for assessment of the renal tubular OATs-MRP4 channel. J. Pharm. Anal. 2023, 13, 526–538. [Google Scholar] [CrossRef]
- De Deyn, P.P.; D’hooge, R.; Van Bogaert, P.-P.; Marescau, B. Endogenous guanidino compounds as uremic neurotoxins. Kidney Int. 2001, 59, S77–S83. [Google Scholar] [CrossRef]
- Gründemann, D.; Liebich, G.; Kiefer, N. Selective substrates for non-neuronal monoamine transporters. Mol. Pharmacol. 1999, 56, 1–10. [Google Scholar] [CrossRef]
- Tuerk, D.; Müller, F.; Fromm, M.F.; Selzer, D.; Dallmann, R.; Lehr, T. Renal transporter–mediated drug–biomarker interactions of the endogenous substrates creatinine and N1-methylnicotinamide: A PBPK modeling approach. Clin. Pharmacol. Ther. 2022, 111, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Song, I.S.; Lee, D.Y.; Shin, M. Pharmacogenetics meets metabolomics: Discovery of tryptophan as a new endogenous OCT2 substrate. PLoS ONE 2012, 7, e36637. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kusuhara, H.; Kumagai, Y.; Moriyama, Y.; Inoue, K.; Kondo, T.; Nakayama, H.; Horita, S.; Tanabe, K.; Yuasa, H.; et al. N-Methylnicotinamide is an endogenous probe for evaluation of drug–drug interactions involving multidrug and toxin extrusions (MATE1 and MATE2-K). Clin. Pharmacol. Ther. 2012, 92, 635–641. [Google Scholar] [CrossRef]
- Kato, K.; Mori, H.; Kito, T.; Yokochi, M.; Ito, S.; Inoue, K.; Yonezawa, A.; Katsura, T.; Kumagai, Y.; Yuasa, H.; et al. Investigation of endogenous compounds for assessing the drug interactions in the urinary excretion involving multidrug and toxin extrusion proteins. Pharm. Res. 2014, 31, 398–411. [Google Scholar] [CrossRef]
- Gessner, A.; König, J.; Fromm, M.F. Contribution of multidrug and toxin extrusion protein 1 (MATE1) to renal secretion of trimethylamine-N-oxide (TMAO). Sci. Rep. 2018, 8, 5790. [Google Scholar] [CrossRef]
- Lentzas, A.; Gooijer, M.C.; Zuidema, S. ATP-binding cassette transporter inhibitor potency and substrate drug affinity are critical determinants of successful drug delivery enhancement to the brain. Fluids Barriers CNS 2024, 21, 62. [Google Scholar] [CrossRef]
- Korszun-Karbowniczak, J.; Krysiak, Z.J.; Saluk, J. The Progress in Molecular Transport and Therapeutic Development in Human Blood–Brain Barrier Models in Neurological Disorders. Cell. Mol. Neurobiol. 2024, 44, 34. [Google Scholar] [CrossRef]
- Patel, K.J.; Tannock, I.F. The influence of P-glycoprotein expression and its inhibitors on the distribution of doxorubicin in breast tumors. BMC Cancer 2009, 9, 356. [Google Scholar] [CrossRef]
- Agarwal, S.; Hartz, A.M.; Elmquist, W.F.; Bauer, B. Breast cancer resistance protein and P-glycoprotein in brain cancer: Two gatekeepers team up. Curr. Pharm. Des. 2011, 17, 2793–2802. [Google Scholar] [CrossRef]
- Iorio, A.L.; Md, R.; Fantappiè, O.; Lucchesi, M.; Facchini, L.; Stival, A.; Becciani, S.; Guidi, M.; Favre, C.; Md, M.; et al. Blood-Brain Barrier and Breast Cancer Resistance Protein: A Limit to the Therapy of CNS Tumors and Neurodegenerative Diseases. Anti-Cancer Agents Med. Chem. 2016, 16, 810–815. [Google Scholar] [CrossRef]
- Sunakawa, H.; Mizoi, K.; Takahashi, R.; Takahashi, S.; Ogihara, T. Impact of P-Glycoprotein-Mediated Drug-Endogenous Substrate Interactions on Androgen and Blood-Brain Barrier Permeability. J. Pharm. Sci. 2024, 113, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Hindle, S.J.; Munji, R.N.; Dolghih, E.; Gaskins, G.; Orng, S.; Ishimoto, H.; Soung, A.; DeSalvo, M.; Kitamoto, T.; Keiser, M.J. Evolutionarily conserved roles for blood-brain barrier xenobiotic transporters in endogenous steroid partitioning and behavior. Cell Rep. 2017, 21, 1304–1316. [Google Scholar] [CrossRef]
- Hochman, J.H.; Chiba, M.; Audus, K.L. Intestinal secretion of drugs: The role of P-glycoprotein and related drug efflux systems in limiting oral drug absorption. Adv. Drug Deliv. Rev. 1994, 14, 183–192. [Google Scholar] [CrossRef]
- Oude Elferink, R.P.J.; Waart, R. Transporters in the intestine limiting drug and toxin absorption. J. Physiol. Biochem. 2007, 63, 75–81. [Google Scholar] [CrossRef]
- Han, L.W.; Gao, C.; Mao, Q. An update on expression and function of P-gp/ABCB1 and BCRP/ABCG2 in the placenta and fetus. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 817–829. [Google Scholar] [CrossRef]
- Szatmári, P.; Ducza, E. Changes in Expression and Function of Placental and Intestinal P-gp and BCRP Transporters during Pregnancy. Int. J. Mol. Sci. 2023, 24, 13089. [Google Scholar] [CrossRef]
- Kozlosky, D.; Barrett, E.; Aleksunes, L.M. Regulation of placental efflux transporters during pregnancy complications. Drug Metab. Dispos. 2022, 50, 1364–1375. [Google Scholar] [CrossRef]
- St-Pierre, M.V.; Hagenbuch, B.; Ugele, B.; Meier, P.J.; Stallmach, T. Characterization of an organic anion-transporting polypeptide (OATP-B) in human placenta. J. Clin. Endocrinol. Metab. 2002, 87, 1856–1863. [Google Scholar] [CrossRef]
- Mathias, A.A.; Hitti, J.; Unadkat, J.D.; Hebert, M.F. Placental ABC transporters: Biological impact and pharmaceutical significance. Pharm. Res. 2017, 34, 1769–1791. [Google Scholar]
- Yamashita, M.; Markert, U.R. Overview of drug transporters in human placenta. Int. J. Mol. Sci. 2021, 22, 13149. [Google Scholar] [CrossRef]
- Schinkel, A.H. The physiological function of drug-transporting P-glycoproteins. Semin. Cancer Biol. 1997, 8, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Tetro, N.; Moushaev, S.; Rubinchik-Stern, M.; Eyal, S. The placental barrier: The gate and the fate in drug distribution. Pharm. Res. 2018, 35, 71. [Google Scholar] [CrossRef]
- Álvarez-Fernández, L.; Blanco-Paniagua, E.; Merino, G. ABCG2 Transports the Flukicide Nitroxynil and Affects Its Biodistribution and Secretion into Milk. Pharmaceutics 2024, 16, 558. [Google Scholar] [CrossRef]
- Kato, K.; Moriyama, C.; Ito, N.; Zhang, X.; Hachiuma, K.; Hagima, N.; Iwata, K.; Yamaguchi, J.-i.; Maeda, K.; Ito, K. Involvement of organic cation transporters in the clearance and milk secretion of thiamine in mice. Pharm. Res. 2015, 32, 2192–2204. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bush, K.T.; Martovetsky, G.; Ahn, S.Y.; Liu, H.C.; Richard, E.; Bhatnagar, V.; Wu, W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 2015, 95, 83–123. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.L.; Wu, Y.W.; Bian, H.G.; Yang, H.; Wang, H.; Meng, X.M.; Jin, J. Function of Uric Acid Transporters and Their Inhibitors in Hyperuricaemia. Front. Pharmacol. 2021, 12, 667753. [Google Scholar] [CrossRef]
- Motohashi, H.; Inui, K. Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS J. 2013, 15, 581–588. [Google Scholar] [CrossRef]
- Pou Casellas, C.; Jansen, K.; Rookmaaker, M.B.; Clevers, H.; Verhaar, M.C.; Masereeuw, R. Regulation of solute carriers oct2 and OAT1/3 in the kidney: A phylogenetic, ontogenetic, and cell dynamic perspective. Physiol. Rev. 2022, 102, 993–1024. [Google Scholar] [CrossRef]
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef]
- Garrigues, A.; Escargueil, A.E.; Orlowski, S. The multidrug transporter, P-glycoprotein, actively mediates cholesterol redistribution in the cell membrane. Proc. Natl. Acad. Sci. USA 2002, 99, 10347–10352. [Google Scholar] [CrossRef]
- Song, W.; Li, D.; Tao, L.; Luo, Q.; Chen, L. Solute carrier transporters: The metabolic gatekeepers of immune cells. Acta Pharm. Sin. B 2020, 10, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bischof, S.; Röllinghoff, M.; Lohoff, M. Demonstration of organic anion transport in T lymphocytes. L-lactate and fluo-3 are target molecules. J. Immunol. 1994, 15, 3523–3532. [Google Scholar] [CrossRef]
- Taki, K.; Nakamura, S.; Miglinas, M.; Enomoto, A.; Niwa, T. Accumulation of Indoxyl Sulfate in OAT1/3-Positive Tubular Cells in Kidneys of Patients With Chronic Renal Failure. J. Ren. Nutr. 2006, 16, 199–203. [Google Scholar] [CrossRef]
- Lai, Q.; Zhu, X.; Zhang, L.; Kou, J.; Liu, F.; Yu, B.; Li, F. Inhibition of OAT1/3 and CMPF uptake attenuates myocardial ischemia-induced chronic heart failure via decreasing fatty acid oxidation and the therapeutic effects of ruscogenin. Transl. Res. 2023, 261, 1–15. [Google Scholar] [CrossRef]
- Łapczuk-Romańska, J.; Droździk, M.; Oswald, S.; Droździk, M. Kidney Drug Transporters in Pharmacotherapy. Int. J. Mol. Sci. 2023, 24, 2856. [Google Scholar] [CrossRef]
- Drozdzik, M.; Drozdzik, M.; Oswald, S. Membrane Carriers and Transporters in Kidney Physiology and Disease. Biomedicines 2021, 9, 426. [Google Scholar] [CrossRef]
- van de Steeg, E.; Wagenaar, E.; van der Kruijssen, C.M.; Burggraaff, J.E.; de Waart, D.R.; Oude Elferink, R.P.; Kenworthy, K.E.; Schinkel, A.H. Organic anion transporting polypeptide 1a/1b–knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J. Clin. Investig. 2010, 120, 2942–2952. [Google Scholar] [CrossRef]
- de Lange, E.C. Potential role of ABC transporters as a detoxification system at the blood-CSF barrier. Adv. Drug Deliv. Rev. 2004, 56, 1793–1809. [Google Scholar] [CrossRef]
- Gedeon, C.; Anger, G.; Piquette-Miller, M.; Koren, G. Breast cancer resistance protein: Mediating the trans-placental transfer of glyburide across the human placenta. Placenta 2008, 29, 39–43. [Google Scholar] [CrossRef]
- Dietrich, C.; Geier, A.; Oude Elferink, R. ABC of oral bioavailability: Transporters as gatekeepers in the gut. Gut 2003, 52, 1788–1795. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Sundrud, M.S. ATP-dependent transporters: Emerging players at the crossroads of immunity and metabolism. Front. Immunol. 2023, 14, 1286696. [Google Scholar] [CrossRef] [PubMed]
- Bleier, B.S.; Koss, M.A.; Albano, G.D.; Cohen, N.A. P-glycoprotein functions as an immunomodulator in healthy human primary nasal epithelial cells. Int. Forum Allergy Rhinol. 2013, 3, 433–438. [Google Scholar] [CrossRef]
- Kooij, G.; Backer, R.; Koning, J.J.; Reijerkerk, A.; Horssen, J.; Pol, S.M.; Drexhage, J.A.; Schinkel, A.H.; Dijkstra, C.D.; Het Hof, B. P-glycoprotein acts as an immunomodulator during neuroinflammation. PLoS ONE 2009, 4, 8212. [Google Scholar] [CrossRef]
- Kawase, S.; Ishikawa, T.; Ohta, Y.; Takahashi, N.; Sato, T.; Ohashi, N.; Isobe, K.; Sato, K. Distinct roles of HMGB1 in the regulation of P-glycoprotein expression in kidney macrophages during lipopolysaccharide-induced acute kidney injury. Sci. Rep. 2022, 12, 342. [Google Scholar] [CrossRef]
- Berg, T.; Hegelund-Myrbäck, T.; Öckinger, J.; Zhou, X.-H.; Brännström, M.; Hagemann-Jensen, M.; Werkström, V.; Seidegård, J.; Grunewald, J.; Nord, M. Expression of MATE1, P-gp, OCTN1 and OCTN2, in epithelial and immune cells in the lung of COPD and healthy individuals. Respir. Res. 2018, 19, 68. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, F.; Chen, Q. Chronic inflammation up-regulates P-gp in peripheral mononuclear blood cells via the STAT3/Nf-κb pathway in 2,4,6-trinitrobenzene sulfonic acid-induced colitis mice. Sci. Rep. 2015, 5, 13558. [Google Scholar] [CrossRef]
- Kawase, A.; Irie, K.; Matsuda, N.; Takai, Y.; Shimada, H.; Iwaki, M. Distinct roles of HMGB1 in the regulation of P-glycoprotein expression in the liver and kidney of mice with lipopolysaccharide-induced inflammation. Mol. Med. Rep. 2022, 26, 342. [Google Scholar] [CrossRef]
- Panwala, C.M.; Jones, J.C.; Viney, J.L. A Novel Model of Inflammatory Bowel Disease: Mice Deficient for the Multiple Drug Resistance Gene, mdr1a, Spontaneously Develop Colitis. J. Immunol. 1998, 161, 5733–5744. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef]
- van de Ven, R.; Oerlemans, R.; van der Heijden, J.W.; Scheffer, G.L.; de Gruijl, T.D.; Jansen, G.; Scheper, R.J. ABC drug transporters and immunity: Novel therapeutic targets in autoimmunity and cancer. J. Leukoc. Biol. 2009, 86, 1075–1087. [Google Scholar] [CrossRef]
- Storelli, F.; Billington, S.; Kumar, A.R.; Unadkat, J.D. Abundance of P-glycoprotein and other drug transporters at the human blood-brain barrier in Alzheimer’s disease: A quantitative targeted proteomic study. Clin. Pharmacol. Ther. 2021, 109, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, X. Contributions of Drug Transporters to Blood-Brain Barriers. Adv. Exp. Med. Biol. 2019, 1141, 407–466. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martins, E.; Barbosa, D.J.; Silva, V.; Remião, F.; Silva, R. Dysfunction of ABC transporters at the blood-brain barrier: Role in neurological disorders. Pharmacol. Ther. 2020, 213, 107554. [Google Scholar] [CrossRef]
- McCormick, J.W.; McCormick, L.A.; Chen, G.; Vogel, P.D.; Wise, J.G. Transport of Alzheimer’s associated amyloid-β catalyzed by P-glycoprotein. PLoS ONE 2021, 16, e0250371. [Google Scholar] [CrossRef]
- Chai, A.B.; Leung, G.K.F.; Callaghan, R.; Gelissen, I.C. P-glycoprotein: A role in the export of amyloid-β in Alzheimer’s disease? FEBS J. 2020, 287, 612–625. [Google Scholar] [CrossRef]
- Vulin, M.; Zhong, Y.; Maloney, B.J. Proteasome inhibition protects blood-brain barrier P-glycoprotein and lowers Aβ brain levels in an Alzheimer’s disease model. Fluids Barriers CNS 2023, 20, 70. [Google Scholar] [CrossRef]
- Arduino, I.; Iacobazzi, R.M.; Riganti, C.; Lopedota, A.A.; Perrone, M.G.; Lopalco, A.; Cutrignelli, A.; Cantore, M.; Laquintana, V.; Franco, M.; et al. Induced expression of P-gp and BCRP transporters on brain endothelial cells using transferrin functionalized nanostructured lipid carriers: A first step of a potential strategy for the treatment of Alzheimer’s disease. Int. J. Pharm. 2020, 591, 120011. [Google Scholar] [CrossRef]
- Westerlund, M.; Belin, A.C.; Anvret, A.; Håkansson, A.; Nissbrandt, H.; Lind, C.; Sydow, O.; Olson, L.; Galter, D. Association of a polymorphism in the ABCB1 gene with Parkinson’s disease. Park. Relat. Disord. 2009, 15, 422–424. [Google Scholar] [CrossRef]
- Tan, E.K.; Pavanni, R.; Chandran, V. Effect of MDR1 haplotype on risk of Parkinson disease. Neurology 2005, 64, 460–464. [Google Scholar] [CrossRef]
- Im, W.; Ban, J.-J.; Chung, J.-Y.; Lee, S.-T.; Chu, K.; Kim, M. Multidrug resistance protein 1 reduces the aggregation of mutant huntingtin in neuronal cells derived from the Huntington’s disease R6/2 model. Sci. Rep. 2015, 5, 16887. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Romanescu, C.; Popescu, B.O. The blood–brain barrier—A key player in multiple sclerosis disease mechanisms. Biomolecules 2022, 12, 538. [Google Scholar] [CrossRef] [PubMed]
- van de Geer, W.S.; Mathijssen, R.H.; van Riet, J.; Steeghs, N.; Labots, M.; van Herpen, C.; Devriese, L.A.; Tjan-Heijnen, V.C.; Voest, E.E.; Sleijfer, S. Identifying somatic changes in drug transporters using whole genome and transcriptome sequencing data of advanced tumors. Biomed. Pharmacother. 2023, 159, 114210. [Google Scholar] [CrossRef] [PubMed]
- Zappe, K.; Cichna-Markl, M. Aberrant DNA methylation of ABC transporters in cancer. Cells 2020, 9, 2281. [Google Scholar] [CrossRef]
- Buxhofer-Ausch, V.; Secky, L.; Wlcek, K.; Svoboda, M.; Kounnis, V.; Briasoulis, E.; Tzakos, A.G.; Jaeger, W.; Thalhammer, T. Tumor-specific expression of organic anion-transporting polypeptides: Transporters as novel targets for cancer therapy. J. Drug Deliv. 2013, 2013, 863539. [Google Scholar] [CrossRef]
- Cho, E.; Montgomery, R.B.; Mostaghel, E.A. Minireview: SLCO and ABC transporters: A role for steroid transport in prostate cancer progression. Endocrinology 2014, 155, 4124–4132. [Google Scholar] [CrossRef]
- Sissung, T.M.; Ley, A.M.; Strope, J.D.; McCrea, E.M.; Beedie, S.; Peer, C.J.; Shukla, S.; Van Velkinburgh, J.; Reece, K.; Troutman, S. Differential expression of OATP1B3 mediates unconjugated testosterone influx. Mol. Cancer Res. 2017, 15, 1096–1105. [Google Scholar] [CrossRef]
- Gao, H.; Pang, Z.; He, W. Editorial of special issue on tumor microenvironment and drug delivery. Acta Pharm. Sin. B 2020, 10, 2016–2017. [Google Scholar] [CrossRef]
- Domenichini, A.; Adamska, A.; Falasca, M. ABC transporters as cancer drivers: Potential functions in cancer development. Biochim. Biophys. Acta (BBA) Gen. Subj. 2019, 1863, 52–60. [Google Scholar] [CrossRef]
- Muriithi, W.; Macharia, L.W.; Heming, C.P.; Echevarria, J.L.; Nyachieo, A.; Niemeyer Filho, P.; Neto, V.M. ABC transporters and the hallmarks of cancer: Roles in cancer aggressiveness beyond multidrug resistance. Cancer Biol. Med. 2020, 17, 253–269. [Google Scholar] [CrossRef]
- Fan, J.; To, K.K.W.; Chen, Z.S.; Fu, L. ABC transporters affect tumor immune microenvironment to regulate cancer immunotherapy and multidrug resistance. Drug Resist. Updates 2023, 66, 100905. [Google Scholar] [CrossRef]
- Nakanishi, T.; Ross, D.D. Breast cancer resistance protein (BCRP/ABCG2): Its role in multidrug resistance and regulation of its gene expression. Chin. J. Cancer 2012, 31, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Kashi, Z.; Masoumi, P.; Mahrooz, A.; Hashemi-Soteh, M.B.; Bahar, A.; Alizadeh, A. The variant organic cation transporter 2 (OCT2)-T201M contributes to changes in insulin resistance in patients with type 2 diabetes treated with metformin. Diabetes Res. Clin. Pract. 2015, 108, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Schorn, S.; Dicke, A.-K.; Neugebauer, U.; Schröter, R.; Friedrich, M.; Reuter, S.; Ciarimboli, G. Expression and function of organic cation transporter 2 in pancreas. Front. Cell Dev. Biol. 2021, 9, 688885. [Google Scholar] [CrossRef]
- Schwabedissen, H.E.M.z.; Ware, J.A.; Finkelstein, D.; Chaudhry, A.S.; Mansell, S.; Leon-Ponte, M.; Strom, S.C.; Zaher, H.; Schwarz, U.I.; Freeman, D.J. Hepatic organic anion transporting polypeptide transporter and thyroid hormone receptor interplay determines cholesterol and glucose homeostasis. Hepatology 2011, 54, 644–654. [Google Scholar] [CrossRef]
- Zhang, L.L.; Lu, L.; Jin, S. Tissue-specific alterations in expression and function of P-glycoprotein in streptozotocin-induced diabetic rats. Acta Pharmacol. Sin. 2011, 32, 956–966. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Li, J.; Cheng, Y.; Chen, J.; Shen, M.; Zhang, S.; Wei, H. Insulin resistance contributes to multidrug resistance in HepG2 cells via activation of the PERK signaling pathway and upregulation of Bcl-2 and P-gp. Oncol. Rep. 2016, 35, 3018–3024. [Google Scholar] [CrossRef]
- Choudhuri, S.; Klaassen, C.D. Elucidation of OATP1B1 and 1B3 transporter function using transgenic rodent models and commonly known single nucleotide polymorphisms. Toxicol. Appl. Pharmacol. 2020, 399, 115039. [Google Scholar] [CrossRef]
- Kayesh, R.; Tambe, V.; Xu, C.; Yue, W. Differential preincubation effects of nicardipine on OATP1B1- and OATP1B3-mediated transport in the presence and absence of protein: Implications in assessing OATP1B1- and OATP1B3-mediated drug–drug interactions. Pharmaceutics 2023, 15, 1020. [Google Scholar] [CrossRef]
- Mishra, J.; Simonsen, R.; Kumar, N. Intestinal breast cancer resistance protein (BCRP) requires Janus kinase 3 activity for drug efflux and barrier functions in obesity. J. Biol. Chem. 2019, 294, 18337–18348. [Google Scholar] [CrossRef]
- Bush, K.T.; Singh, P.; Nigam, S.K. Gut-derived uremic toxin handling in vivo requires OAT-mediated tubular secretion in chronic kidney disease. JCI Insight 2020, 5, e133817. [Google Scholar] [CrossRef]
- Tan, S.P.F.; Scotcher, D.; Rostami-Hodjegan, A.; Galetin, A. Effect of chronic kidney disease on the renal secretion via organic anion transporters 1/3: Implications for physiologically-based pharmacokinetic modeling and dose adjustment. Clin. Pharmacol. Ther. 2022, 112, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yonezawa, A.; Hashimoto, S.; Katsura, T.; Inui, K.-i. Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem. Pharmacol. 2010, 80, 1762–1767. [Google Scholar] [CrossRef]
- Tohyama, K.; Chisaki, I.; Takai, Y.; Handa, Y.; Miyamoto, M.; Amano, N. Relationship of MATE1 inhibition and cytotoxicity in nephrotoxicity: Application for safety evaluation in early drug discovery. Toxicol. Sci. 2019, 170, 223–233. [Google Scholar] [CrossRef]
- Chang, C.; Hu, Y.; Hogan, S.L.; Mercke, N.; Gomez, M.; O’Bryant, C.; Bowles, D.W.; George, B.; Wen, X.; Aleksunes, L.M. Pharmacogenomic variants may influence the urinary excretion of novel kidney injury biomarkers in patients receiving cisplatin. Int. J. Mol. Sci. 2017, 18, 1333. [Google Scholar] [CrossRef]
- Hussain, M.G.; Saif, R.; Younus, A.; Zahid, M.; Jabeen, S.; Younas, H. Genetic Association of SLC47A1 Gene Variant (17: 19571562C> T) and Bioinformatics Analyses of MATE1 Protein in Chronic Kidney Disease Patients of Pakistani Origin. bioRxiv 2023. [Google Scholar] [CrossRef]
- Han, C.; Zheng, J.; Wang, F.; Lu, Q.; Chen, Q.; Hu, A.; Visentin, M.; Kullak-Ublick, G.A.; Gai, Z.; Chu, L. The role of NF-kB in the downregulation of organic cation transporter 2 expression and renal cation secretion in kidney disease. Front. Med. 2022, 8, 800421. [Google Scholar] [CrossRef]
- Cheung, K.W.K.; Hsueh, C.-H.; Zhao, P.; Meyer, T.W.; Zhang, L.; Huang, S.-M.; Giacomini, K.M. The effect of uremic solutes on the organic cation transporter 2. J. Pharm. Sci. 2017, 106, 2551–2557. [Google Scholar] [CrossRef]
- Schophuizen, C.M.; Wilmer, M.J.; Jansen, J.; Gustavsson, L.; Hilgendorf, C.; Hoenderop, J.G.; van den Heuvel, L.P.; Masereeuw, R. Cationic uremic toxins affect human renal proximal tubule cell functioning through interaction with the organic cation transporter. Pflüg. Arch. Eur. J. Physiol. 2013, 465, 1701–1714. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, Y.; Huang, B.; Lin, H.; Zhou, Q.; Wang, Y.; Cai, Z.; Liu, M. The system profile of renal drug transporters in tubulointerstitial fibrosis model and consequent effect on pharmacokinetics. Molecules 2022, 27, 704. [Google Scholar] [CrossRef]
- Tsang, Y.P.; Hao, T.; Mao, Q.; Kelly, E.J.; Unadkat, J.D. Dysregulation of the mRNA expression of human renal drug transporters by proinflammatory cytokines in primary human proximal tubular epithelial cells. Pharmaceutics 2024, 16, 285. [Google Scholar] [CrossRef]
- Zager, R.A. P glycoprotein-mediated cholesterol cycling determines proximal tubular cell viability. Kidney Int. 2001, 60, 944–956. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Komazawa, H.; Yamaguchi, H.; Hidaka, K.; Ogura, J.; Kobayashi, M.; Iseki, K. Renal uptake of substrates for organic anion transporters Oat1 and Oat3 and organic cation transporters Oct1 and Oct2 is altered in rats with adenine-induced chronic renal failure. J. Pharm. Sci. 2013, 102, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- del Moral, R.G.; Olmo, A.; Aguilar, M.; O’Valle, F. P glycoprotein: A new mechanism to control drug-induced nephrotoxicity. Exp. Nephrol. 1998, 6, 89–97. [Google Scholar] [CrossRef]
- Pippa, L.F.; Vieira, C.P.; Caris, J.A.; Rocha, A.; Marques, M.P.; Garcia, C.P.; Rezende, R.E.F.; Lanchote, V.L. Effect of chronic hepatitis C on the activity of the membrane transporters P-gp and OATP1B1/BCRP on patients with different stages of hepatic fibrosis. Clin. Pharmacol. Ther. 2023, 114, 173–181. [Google Scholar] [CrossRef]
- Wagner, M.; Zollner, G.; Trauner, M. Nuclear receptor regulation of the adaptive response of bile acid transporters in cholestasis. Semin. Liver Dis. 2010, 30, 160–177. [Google Scholar] [CrossRef]
- Zollner, G.; Fickert, P.; Silbert, D.; Fuchsbichler, A.; Marschall, H.U.; Zatloukal, K.; Denk, H.; Trauner, M. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J. Hepatol. 2003, 38, 717–727. [Google Scholar] [CrossRef]
- Marin, J.J.; Cives-Losada, C.; Macias, R.I.; Romero, M.R.; Marijuan, R.P.; Hortelano-Hernandez, N.; Delgado-Calvo, K.; Villar, C.; Gonzalez-Santiago, J.M.; Monte, M.J. Impact of liver diseases and pharmacological interactions on the transportome involved in hepatic drug disposition. Biochem. Pharmacol. 2024, 228, 116166. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.; Xue, Y.; Li, H.; Zeng, C.; Chen, M. The Role of Solute Carrier Family Transporters in Hepatic Steatosis and Hepatic Fibrosis. J. Clin. Transl. Hepatol. 2025, 13, 233. [Google Scholar] [CrossRef]
- Taniguchi, T.; Zanetti-Yabur, A.; Wang, P.; Usyk, M.; Burk, R.D.; Wolkoff, A.W. Interindividual diversity in expression of organic anion uptake transporters in normal and cirrhotic human liver. Hepatol. Commun. 2020, 4, 739–752. [Google Scholar] [CrossRef]
- Jetter, A.; Kullak-Ublick, G.A. Drugs and hepatic transporters: A review. Pharmacol. Res. 2020, 154, 104234. [Google Scholar] [CrossRef]
- Porteous, C.M.; Menon, D.K.; Aigbirhio, F.I.; Smith, R.A.; Murphy, M.P. P-glycoprotein (Mdr1a/1b) and breast cancer resistance protein (Bcrp) decrease the uptake of hydrophobic alkyl triphenylphosphonium cations by the brain. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 3458–3465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, M.; Cui, Y.; Xiang, Q. Metabolism of hepatic stellate cells in chronic liver diseases: Emerging molecular and therapeutic interventions. Theranostics 2025, 15, 1715. [Google Scholar] [CrossRef] [PubMed]
- Foucaud-Vignault, M.; Soayfane, Z.; Ménez, C.; Bertrand-Michel, J.; Martin, P.G.P.; Guillou, H.; Collet, X.; Lespine, A. P-glycoprotein dysfunction contributes to hepatic steatosis and obesity in mice. PLoS ONE 2011, 6, e23614. [Google Scholar] [CrossRef]
- Ghanem, C.I.; Manautou, J.E. Role and regulation of hepatobiliary ATP-binding cassette transporters during chemical-induced liver injury. Drug Metab. Dispos. 2022, 50, 1376–1388. [Google Scholar] [CrossRef]
- Billington, S.; Ray, A.S.; Salphati, L.; Xiao, G.; Chu, X.; Humphreys, W.G.; Liao, M.; Lee, C.A.; Mathias, A.; Hop, C.E. Transporter expression in noncancerous and cancerous liver tissue from donors with hepatocellular carcinoma and chronic hepatitis C infection quantified by LC-MS/MS proteomics. Drug Metab. Dispos. 2018, 46, 189–196. [Google Scholar] [CrossRef]
- Liang, Y.; Li, S.; Chen, L. The physiological role of drug transporters. Protein Cell 2015, 6, 334–350. [Google Scholar] [CrossRef]
- Nigam, S.K. What do drug transporters really do? Nat. Rev. Drug Discov. 2015, 14, 29–44. [Google Scholar] [CrossRef]
- Kell, D. Implications of endogenous roles of transporters for drug discovery: Hitchhiking and metabolite-likeness. Nat. Rev. Drug Discov. 2016, 15, 143. [Google Scholar] [CrossRef]
- Dong, F.; Lojko, P.; Bazzone, A.; Bernhard, F.; Borodina, I. Transporter function characterization via continuous-exchange cell-free synthesis and solid supported membrane-based electrophysiology. Bioelectrochemistry 2024, 159, 108732. [Google Scholar] [CrossRef]
- Gebauer, L.; Jensen, O.; Neif, M.; Brockmöller, J.; Dücker, C. Overlap and specificity in the substrate spectra of human monoamine transporters and organic cation transporters 1, 2, and 3. Int. J. Mol. Sci. 2021, 22, 12816. [Google Scholar] [CrossRef]
- Aronica, E.; Sisodiya, S.M.; Gorter, J.A. Cerebral expression of drug transporters in epilepsy. Adv. Drug Deliv. Rev. 2012, 64, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, L.; Suo, B.; Wei, Y.; Xu, Y.; Jiang, M.; Dong, J.; Li, X.; Song, Z.; Liu, D. Distribution Characteristics and Impacting Factors of Drug CYP Enzymes and Transporters in the Gastrointestinal Tract of Chinese Healthy Subjects. Clin. Pharmacol. Ther. 2024, 117, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Mu, H.; Zhang, X.; Li, W.; Wang, R. Progress in Epigenetic Modification Regulating Drug Transporters in the Hypoxic Environment. Curr. Drug Deliv. 2024; in press. [Google Scholar] [CrossRef]
- Thakur, A.; Subash, S.; Ahire, D.; Prasad, B. Developmental Expression of Drug Transporters and Conjugating Enzymes Involved in Enterohepatic Recycling: Implication for Pediatric Drug Dosing. Clin. Pharmacol. Ther. 2024, 116, 1615–1626. [Google Scholar] [CrossRef]
- Francis, L.; Ogungbenro, K.; Bruyn, T.; Houston, B.H.; Hallifax, D. Exploring the Boundaries for In Vitro–In Vivo Extrapolation: Use of Isolated Rat Hepatocytes in Co-culture and Impact of Albumin Binding Properties in the Prediction of Clearance of Various Drug Types. Drug Metab. Dispos. 2023, 51, 1463–1473. [Google Scholar] [CrossRef]
- Murata, Y.; Neuhoff, S.; Rostami-Hodjegan, A.; Takita, H.; Al-Majdoub, Z.M.; Ogungbenro, K. In Vitro to In Vivo Extrapolation Linked to Physiologically Based Pharmacokinetic Models for Assessing the Brain Drug Disposition. APPS J. 2022, 24, 28. [Google Scholar] [CrossRef]
- Planz, V.; Lehr, C.-M.; Windbergs, M. In vitro models for evaluating safety and efficacy of novel technologies for skin drug delivery. J. Control. Release 2016, 242, 89–104. [Google Scholar] [CrossRef]
- Yang, Z.; Kirschke, C.P.; Cai, Y.; Huang, L. A double knockout for zinc transporter 8 and somatostatin in mice reveals their distinct roles in regulation of insulin secretion and obesity. Genes. Nutr. 2024, 19, 24. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Z.; Cui, H.; Yang, J.; Liu, A. Biological characteristics and immune responses of NK Cells in commonly used experimental mouse models. Front. Immunol. 2024, 15, 1478323. [Google Scholar] [CrossRef]
- Vijaywargi, G.; Kollipara, S.; Ahmed, T.; Chachad, S. Predicting transporter mediated drug-drug interactions via static and dynamic physiologically based pharmacokinetic modeling: A comprehensive insight on where we are now and the way forward. Biopharm. Drug Dispos. 2023, 44, 195–220. [Google Scholar] [CrossRef]
- Choi, H.J.; Madari, S.; Huang, F. Utilising Endogenous Biomarkers in Drug Development to Streamline the Assessment of Drug-Drug Interactions Mediated by Renal Transporters: A Pharmaceutical Industry Perspective. Clin. Pharmacokinet. 2024, 63, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Seithel, A.; Glaeser, H.; Fromm, M.F.; König, J. The functional consequences of genetic variations in transporter genes encoding human organic anion-transporting polypeptide family members. Expert. Opin. Drug Metab. Toxicol. 2008, 4, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Bleasby, K.; Hall, L.A.; Perry, J.L.; Mohrenweiser, H.W.; Pritchard, J.B. Functional consequences of single nucleotide polymorphisms in the human organic anion transporter hOAT1 (SLC22A6). J. Pharmacol. Exp. Ther. 2005, 314, 923–931. [Google Scholar] [CrossRef]
- Saviano, A.; Henderson, N.C.; Baumert, T.F. Single-cell genomics and spatial transcriptomics: Discovery of novel cell states and cellular interactions in liver physiology and disease biology. J. Hepatol. 2020, 73, 1219–1230. [Google Scholar] [CrossRef]
- Watson, B.R.; Paul, B.; Rahman, R.U. Spatial transcriptomics of healthy and fibrotic human liver at single-cell resolution. Nat. Commun. 2025, 16, 319. [Google Scholar] [CrossRef]
- Shang, L.; Wu, P.; Zhou, X. Statistical identification of cell type-specific spatially variable genes in spatial transcriptomics. Nat. Commun. 2025, 16, 1059. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Sabo, A.R.; Winfree, S.; Collins, K.S.; Janosevic, D.; Gulbronson, C.J.; Cheng, Y.-H.; Casbon, L.; Barwinska, D.; Ferkowicz, M.J. Integration of spatial and single-cell transcriptomics localizes epithelial cell–immune cross-talk in kidney injury. JCI Insight 2021, 6, e147703. [Google Scholar] [CrossRef]
- Granados, J.C.; Watrous, J.D.; Long, T.; Rosenthal, S.B.; Cheng, S.; Jain, M.P.; Nigam, S.K. Regulation of Human Endogenous Metabolites by Drug Transporters and Drug Metabolizing Enzymes: An Analysis of Targeted SNP-Metabolite Associations. Metabolites 2023, 13, 171. [Google Scholar] [CrossRef]
- Nigam, A.K.; Li, J.G.; Lall, K.; Shi, D.; Bush, K.T.; Bhatnagar, V.; Abagyan, R.; Nigam, S.K. Unique Metabolite Preferences of the Drug Transporters OAT1 and OAT3 Analyzed by Machine Learning. J. Biol. Chem. 2020, 295, 1802–1816. [Google Scholar] [CrossRef]
- Ganguly, S.; Finkelstein, D.; Shaw, T.I.; Michalek, R.D.; Zorn, K.M.; Ekins, S.; Yasuda, K.; Fukuda, Y.; Schuetz, J.D.; Mukherjee, K. Metabolomic and transcriptomic analysis reveals endogenous substrates and metabolic adaptation in rats lacking Abcg2 and Abcb1a transporters. PLoS ONE 2021, 16, e0253852. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Zhang, Z.; Shen, H.; Tang, X.; Wu, D.; Bao, X.; Xu, G.; Chen, S. Application of omics in the diagnosis, prognosis, and treatment of acute myeloid leukemia. Biomark. Res. 2024, 12, 45. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, P.; Ren, Q.; Wang, J.; Lin, H.; Huang, Y.; Wang, W. Integrative multi-omic and machine learning approach for prognostic stratification and therapeutic targeting in lung squamous cell carcinoma. Biofactors 2024, 50, 1123–1140. [Google Scholar] [CrossRef] [PubMed]
- Girardi, E.; César-Razquin, A.; Lindinger, S.; Papakostas, K.; Konecka, J.; Hemmerich, J.; Kickinger, S.; Kartnig, F.; Gürtl, B.; Klavins, K. A widespread role for SLC transmembrane transporters in resistance to cytotoxic drugs. Nat. Chem. Biol. 2020, 16, 469–478. [Google Scholar] [CrossRef]
- Rebsamen, M.; Girardi, E.; Sedlyarov, V.; Scorzoni, S.; Papakostas, K.; Vollert, M.; Konecka, J.; Guertl, B.; Klavins, K.; Wiedmer, T.; et al. Gain-of-function genetic screens in human cells identify SLC transporters overcoming environmental nutrient restrictions. Life Sci. Alliance 2022, 5, e202201404. [Google Scholar] [CrossRef]
- Chidley, C.; Darnell, A.M.; Gaudio, B.L. A CRISPRi/a screening platform to study cellular nutrient transport in diverse microenvironments. Nat. Cell Biol. 2024, 26, 825–838. [Google Scholar] [CrossRef]
- Wolf, G.; Craigon, C.; Teoh, S.T.; Essletzbichler, P.; Onstein, S.; Cassidy, D.; Uijttewaal, E.C.H.; Dvorak, V.; Cao, Y.; Bensimon, A.; et al. The efflux pump ABCC1/MRP1 constitutively restricts PROTAC sensitivity in cancer cells. Cell Chem. Biol. 2024, 32, 291–306.e6. [Google Scholar] [CrossRef]
- Aceves, J.O.; Heja, S.; Kobayashi, K. 3D proximal tubule-on-chip model derived from kidney organoids with improved drug uptake. Sci. Rep. 2022, 12, 14997. [Google Scholar] [CrossRef]
- Ma, C.; Banan Sadeghian, R.; Negoro, R.; Fujimoto, K.; Araoka, T.; Ishiguro, N.; Takasato, M.; Yokokawa, R. Efficient proximal tubule-on-chip model from hiPSC-derived kidney organoids for functional analysis of renal transporters. iScience 2024, 19, 110760. [Google Scholar] [CrossRef]
- Vriend, J.; Nieskens, T.T.G.; Vormann, M.K. Screening of Drug-Transporter Interactions in a 3D Microfluidic Renal Proximal Tubule on a Chip. AAPS J. 2018, 20, 87. [Google Scholar] [CrossRef]
- Musah, S.; Bhattacharya, R.; Himmelfarb, J. Kidney Disease Modeling with Organoids and Organs-on-Chips. Annu. Rev. Biomed. Eng. 2024, 26, 383–414. [Google Scholar] [CrossRef]
- Susa, K.; Kobayashi, K.; Galichon, P.; Matsumoto, T.; Tamura, A.; Hiratsuka, K.; Gupta, N.R.; Yazdi, I.K.; Bonventre, J.V.; Morizane, R. ATP/ADP biosensor organoids for drug nephrotoxicity assessment. Front. Cell Dev. Biol. 2023, 11, 1138504. [Google Scholar] [CrossRef]
- Matsui, T.; Shinozawa, T. Human organoids for predictive toxicology research and drug development. Front. Genet. 2021, 12, 767621. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaze, C.; Shu, Y. Beyond ADME: The Endogenous Functions of Drug Transporters and Its Impact on Human Disease. Pharmaceutics 2025, 17, 685. https://doi.org/10.3390/pharmaceutics17060685
Blaze C, Shu Y. Beyond ADME: The Endogenous Functions of Drug Transporters and Its Impact on Human Disease. Pharmaceutics. 2025; 17(6):685. https://doi.org/10.3390/pharmaceutics17060685
Chicago/Turabian StyleBlaze, Christine, and Yan Shu. 2025. "Beyond ADME: The Endogenous Functions of Drug Transporters and Its Impact on Human Disease" Pharmaceutics 17, no. 6: 685. https://doi.org/10.3390/pharmaceutics17060685
APA StyleBlaze, C., & Shu, Y. (2025). Beyond ADME: The Endogenous Functions of Drug Transporters and Its Impact on Human Disease. Pharmaceutics, 17(6), 685. https://doi.org/10.3390/pharmaceutics17060685






