Synergistic Cancer Therapies Enhanced by Nanoparticles: Advancing Nanomedicine Through Multimodal Strategies
Abstract
1. Introduction
2. Nanoparticle Classification in Cancer Therapy
3. Multimodal Nanoparticle-Enhanced Combination Therapies
3.1. Chemotherapy, EPR-Driven Delivery, and Tumor Vasculature
3.2. Photothermal and Photodynamic Therapies
3.2.1. Photothermal Therapy (PTT)
3.2.2. Photodynamic Therapy (PDT)
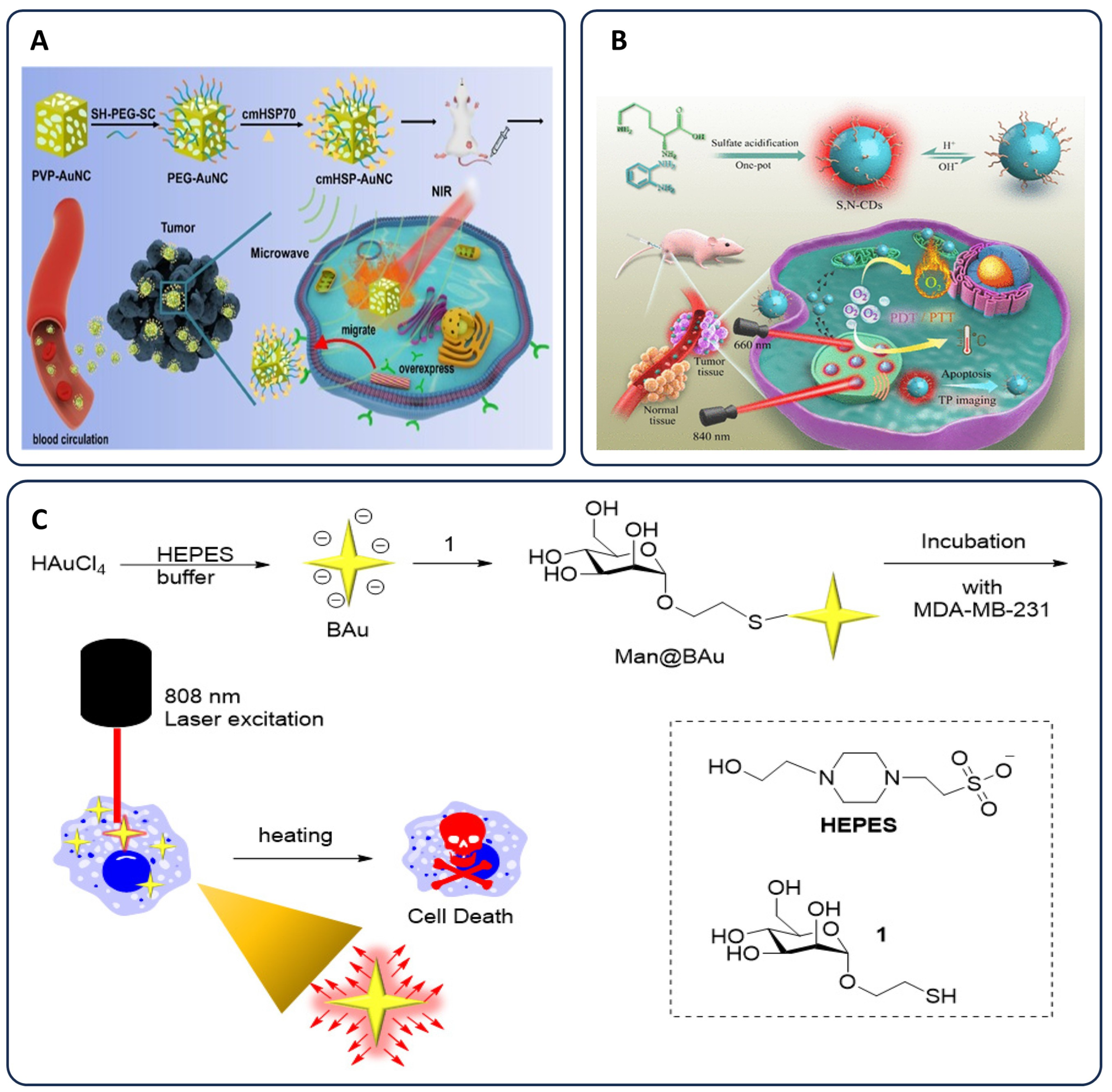
3.2.3. Chemo-Phototherapy
Synergistic Mechanisms and Drug Uptake Enhancement
Apoptosis Induction and Chemotherapy Sensitization
Nanoparticle Applications in Chemo-Phototherapy
Advanced Nanoparticle-Based Dual Therapy
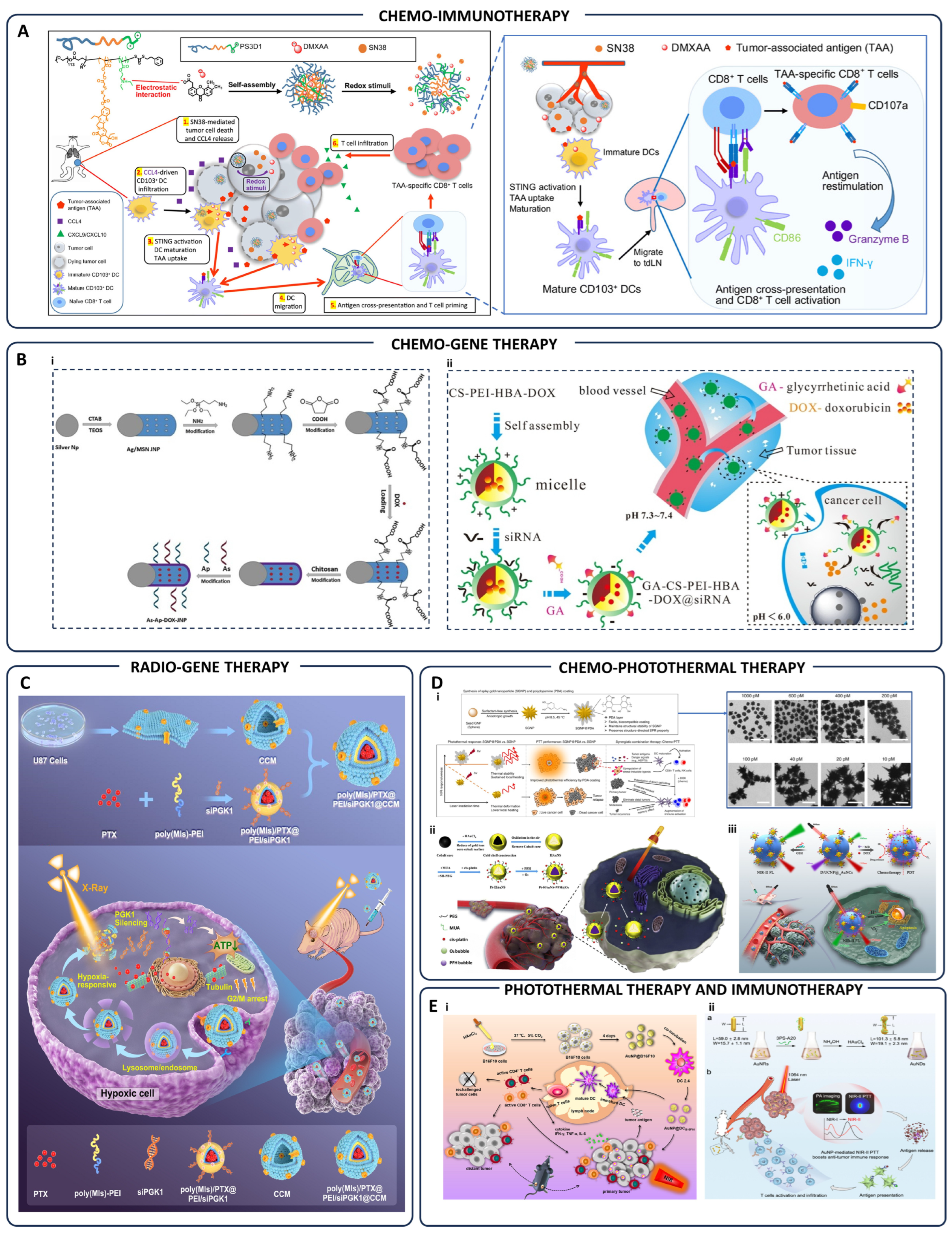
| Type of Therapy | Nanoparticle System | Mechanism of Action | Tumor Model/Samples | Ref. |
|---|---|---|---|---|
| Chemo-Immunotherapy | PS3D1@DMXAA nanoparticles | Redox-responsive release of SN38/DMXAA; DC maturation; CD8+ T-cell activation | Breast & melanoma tumors (4T1, B16F10) | [115] |
| Chemo-Gene Therapy | As-Ap-JNP nanoparticles | Aptamer/antisense targeted gene therapy; specific tumor targeting via functionalized surface | Tumor cells | [116] |
| GA-CS-PEI-HBA-DOX@siRNA micelles | pH-sensitive delivery of DOX and siRNA; targeted co-delivery and gene silencing | Tumor cells | [117] | |
| Radio-Gene Therapy | Hypoxia-responsive iRNA nanomedicine | PGK1 silencing under hypoxia; sensitization of glioblastoma to chemo-radiotherapy | Glioblastoma tumor cells | [118] |
| Chemo-Photothermal Therapy | PDA-coated spiky gold nanoparticles (SGNP@PDA) | Enhanced photothermal stability; tumor ablation; immunological memory induction | Primary & metastatic tumors | [119] |
| Pt-HAuNS-PFH@O2 nanoparticles | Chemo-photothermal synergistic therapy; controlled O2/drug release | Breast cancer cells | [120] | |
| D/UCNP@cgAuNCs nanoassemblies | Tumor-responsive NIR-II imaging; synergistic chemo-photodynamic therapy | Tumor cells | [121] | |
| Photothermal-Immunotherapy | AuNP@DCB16F10 nanoparticles | Photothermal tumor ablation; enhanced antitumor immune responses | Melanoma tumor cells | [122] |
| Gold nanotheranostics | NIR-II photothermal therapy; targeted immunotherapy enhancement | Tumor-bearing animal models | [123] | |
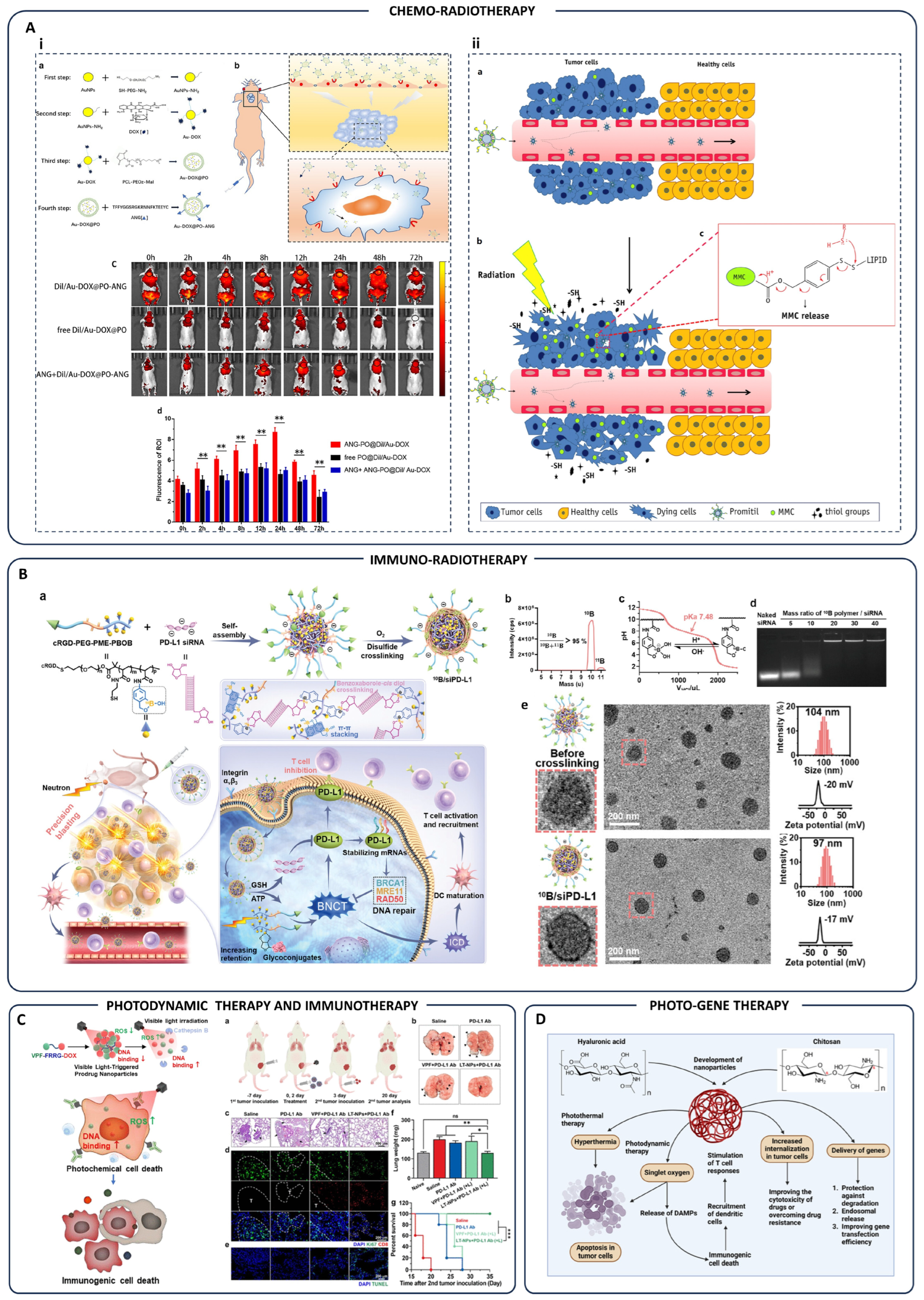
| Chemo-Radiotherapy | Au-DOX@PO-ANG polymersomes | Targeted drug delivery to glioblastoma; radiation-triggered drug release | Glioblastoma tumor-bearing mice | [124] |
| Promitil nanoparticles (MMC prodrug) | Radiation-induced thiol-mediated MMC activation and tumor-specific cytotoxicity | Tumor models | [125] | |
| Immuno-Radiotherapy | 10B/siPD-L1 nanoparticles | Combined boron neutron capture therapy and PD-L1-targeted immunotherapy | Tumor cells/tissues | [126] |
| Photodynamic-Immunotherapy | LT-NPs (VPF-FRRG-DOX conjugates) | Cathepsin B-sensitive ROS generation; immunogenic cell death; PD-L1 blockade | Tumor-bearing mice | [127] |
| Photo-Gene Therapy | Chitosan/hyaluronic acid nanoparticles | Hyperthermia & photodynamic therapy; gene delivery; immune activation | Tumor cells | [128] |
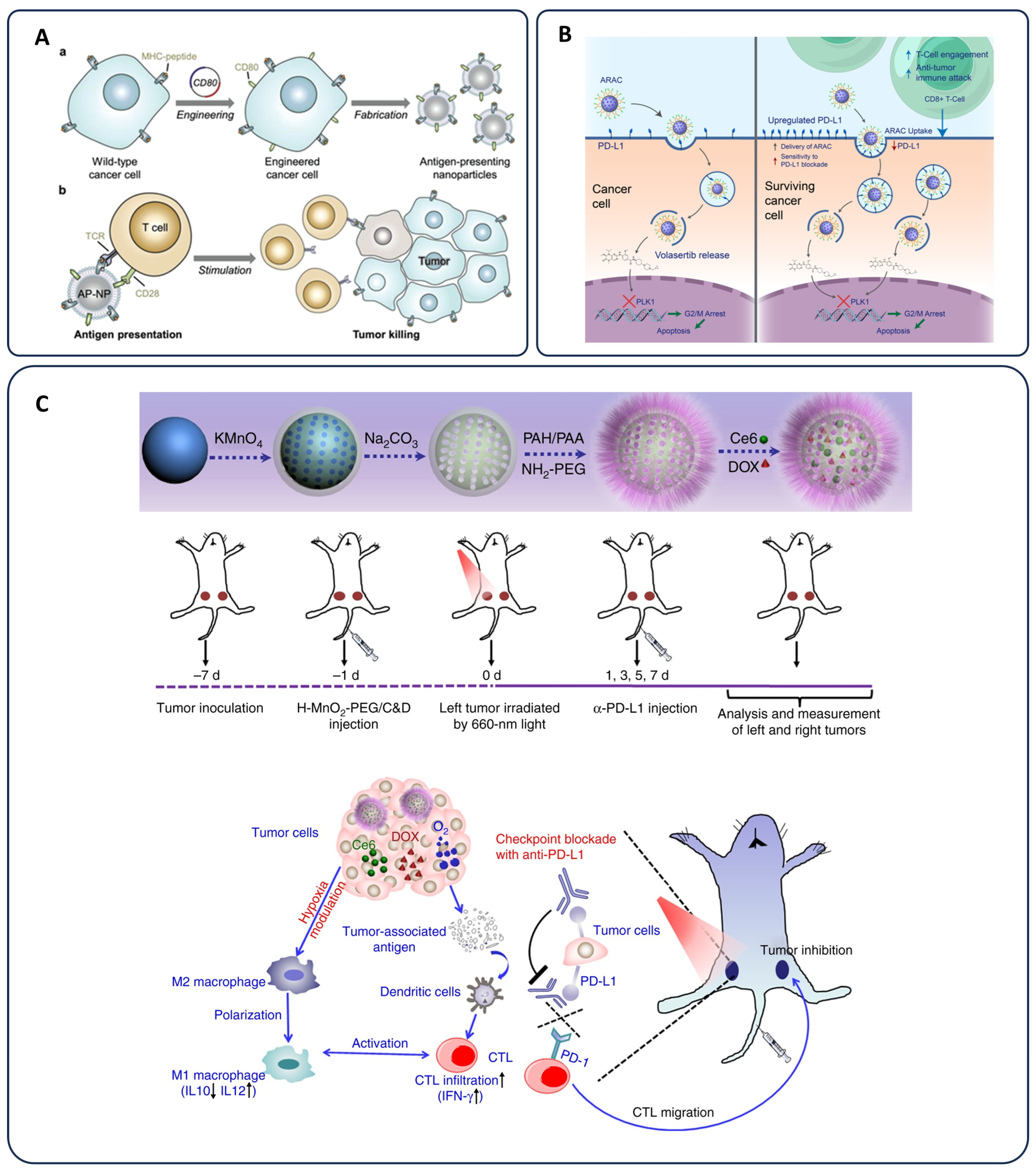
3.3. Immunotherapy in Cancer
3.3.1. Checkpoint Inhibitors and Overcoming Immunosuppression
3.3.2. Nanoparticle-Based Cancer Vaccines
3.3.3. Chemo-Immunotherapy and Immune Modulation
3.3.4. Advances in Nanoparticle-Based Immunotherapy
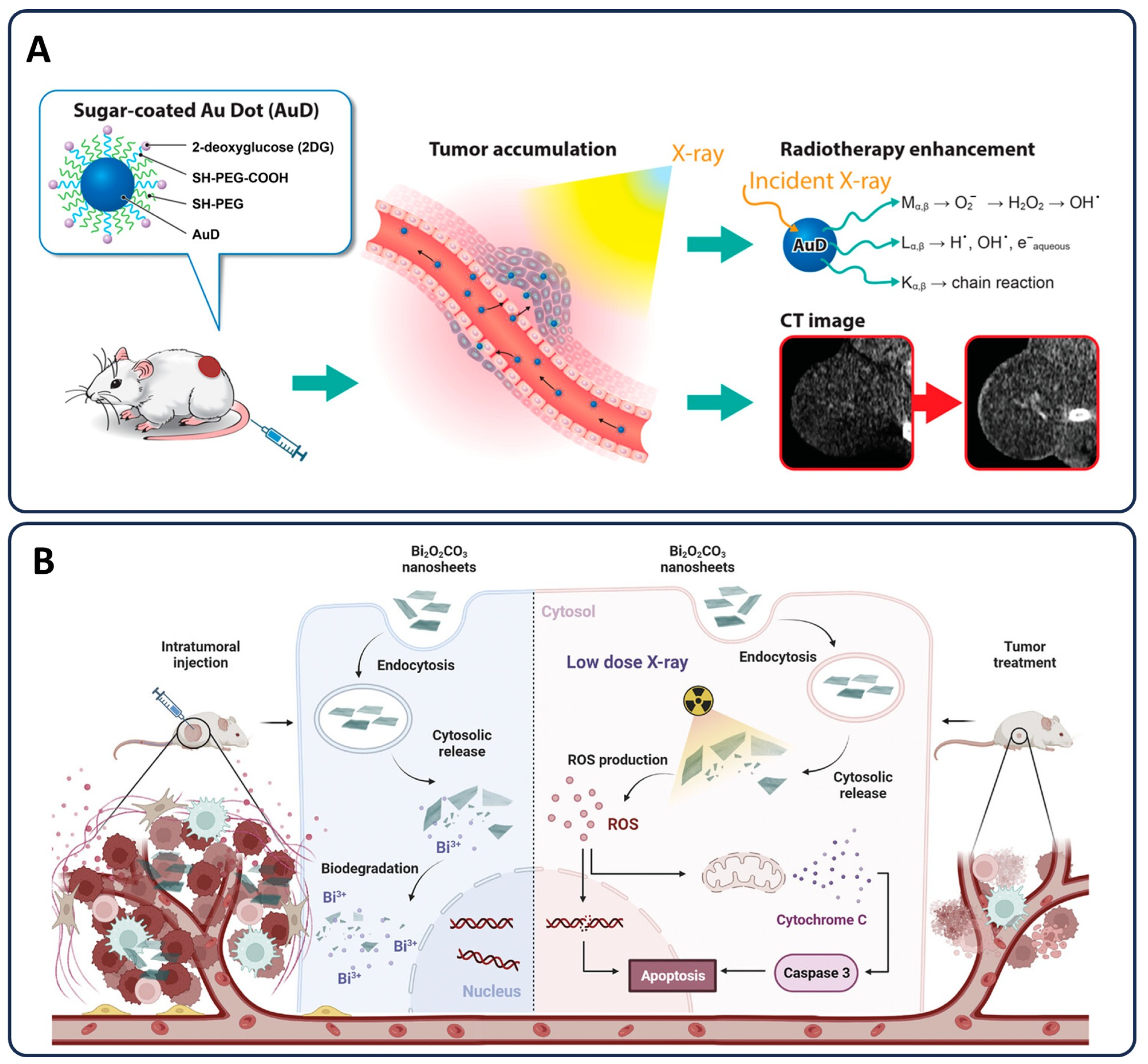
3.4. Radiotherapy Enhancements
3.4.1. Nanoparticles as Radiosensitizers and Protecting Healthy Tissue
3.4.2. Chemo-Radiotherapy: Synergistic Effects and DNA Damage Amplification
3.4.3. Advanced Nanoparticle Applications in Radiotherapy
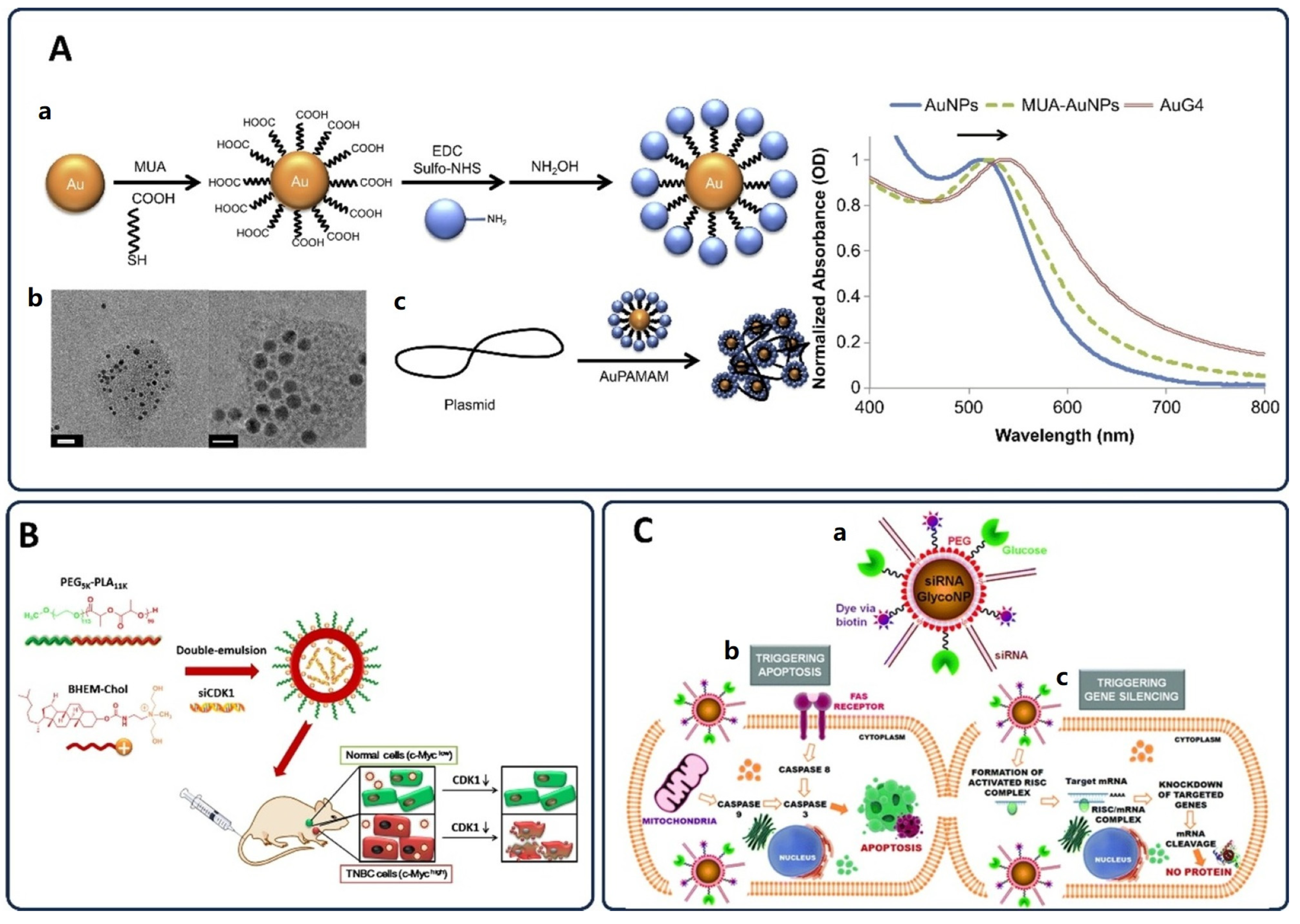
3.5. Gene Therapy Approaches in Cancer Treatment
3.5.1. Gene Silencing and Gene Editing in Cancer Therapy
3.5.2. Chemo-Gene Therapy: Combining Genetic and Chemotherapeutic Approaches
3.5.3. Nanoparticles for Gene Therapy and Drug Delivery
3.5.4. Advances in Targeted Gene Editing and Future Perspectives
4. Challenges & Limitations
4.1. Toxicity & Safety Concerns
4.2. Manufacturing, Quality Control, and Scalability
4.3. Regulatory and Ethical Challenges
4.4. Biological Barriers to Nanoparticle Therapy
5. Future Perspectives
5.1. Personalized Nanomedicine and Biomarker Integration
5.2. Emerging Nanotechnologies in Medicine
5.3. Interdisciplinary Collaborations in Nanomedicine
5.4. Translational Pathways: Public-Private Partnerships and Clinical Advancements
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| APCs | Antigen-Presenting Cells |
| AuNPs | Gold Nanoparticles |
| BiNPs | Bismuth Nanoparticles |
| CAR-T | Chimeric Antigen Receptor T Cells |
| CRISPR/Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9 |
| CTLs | Cytotoxic T Lymphocytes |
| CTLA-4 | Cytotoxic T-lymphocyte–associated Antigen 4 |
| DAMPs | Damage-Associated Molecular Patterns |
| DCs | Dendritic Cells |
| DLS | Dynamic Light Scattering |
| EGFR | Epidermal Growth Factor Receptor |
| EMA | European Medicines Agency |
| EPR | Enhanced Permeability and Retention |
| FDA | U.S. Food and Drug Administration |
| Fe3O4 | Iron(II,III) Oxide |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| HMGB1 | High-Mobility Group Box 1 |
| ICD | Immunogenic Cell Death |
| ICIs | Immune Checkpoint Inhibitors |
| IFN-γ | Interferon Gamma |
| IL-2 | Interleukin-2 |
| LNPs | Lipid Nanoparticles |
| LPHNPs | Lipid-Polymer Hybrid Nanoparticles |
| MAPK | Mitogen-Activated Protein Kinase |
| MDR | Multidrug Resistance |
| MDSCs | Myeloid-Derived Suppressor Cells |
| MOFs | Metal-Organic Frameworks |
| MRI | Magnetic Resonance Imaging |
| NF-κB | Nuclear Factor kappa B |
| NHEJ | Non-Homologous End Joining |
| NP(s) | Nanoparticle(s) |
| PEG | Polyethylene Glycol |
| PEI | Polyethylenimine |
| PET | Positron Emission Tomography |
| PLGA | Poly(lactic-co-glycolic acid) |
| PDT | Photodynamic Therapy |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PTT | Photothermal Therapy |
| PVP | Polyvinylpyrrolidone |
| QbD | Quality-by-Design |
| QDs | Quantum Dots |
| ROS | Reactive Oxygen Species |
| shRNA | Short Hairpin RNA |
| siRNA | Small Interfering RNA |
| SPR | Surface Plasmon Resonance |
| SPIONs | Superparamagnetic Iron Oxide Nanoparticles |
| TAAs | Tumor-Associated Antigens |
| TEM | Transmission Electron Microscopy |
| TLR | Toll-Like Receptor |
| TME | Tumor Microenvironment |
| UCNPs | Up-Conversion Nanoparticles |
| VEGF | Vascular Endothelial Growth Factor |
References
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Cooper, G.; Hausman, R. The development and causes of cancer. Cell Mol. Approach 2000, 2, 725–766. [Google Scholar]
- Torok, J.A.; Wu, Y.; Chino, J.; Prosnitz, L.R.; Beaven, A.W.; Kim, G.J.; Kelsey, C.R. Chemotherapy or combined modality therapy for early-stage Hodgkin lymphoma. Anticancer Res. 2018, 38, 2875–2881. [Google Scholar]
- Cote, D.J.; Dubois, H.M.; Karhade, A.V.; Smith, T.R. (Eds.) Venous thromboembolism in patients undergoing craniotomy for brain tumors: A US nationwide analysis. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers: New York, NY, USA, 2016. [Google Scholar]
- Senders, J.T.; Goldhaber, N.H.; Cote, D.J.; Muskens, I.S.; Dawood, H.Y.; De Vos, F.Y.; Gormley, W.B.; Smith, T.R.; Broekman, M.L. Venous thromboembolism and intracranial hemorrhage after craniotomy for primary malignant brain tumors: A National Surgical Quality Improvement Program analysis. J. Neuro-Oncol. 2018, 136, 135–145. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K.; Arihiro, K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006, 66, 5527–5536. [Google Scholar] [CrossRef]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef]
- Alieva, M.; Margarido, A.S.; Wieles, T.; Abels, E.R.; Colak, B.; Boquetale, C.; Jan Noordmans, H.; Snijders, T.J.; Broekman, M.L.; Van Rheenen, J. Preventing inflammation inhibits biopsy-mediated changes in tumor cell behavior. Sci. Rep. 2017, 7, 7529. [Google Scholar] [CrossRef]
- Tagliabue, E.; Agresti, R.; Carcangiu, M.L.; Ghirelli, C.; Morelli, D.; Campiglio, M.; Martel, M.; Giovanazzi, R.; Greco, M.; Balsari, A. Role of HER2 in wound-induced breast carcinoma proliferation. Lancet 2003, 362, 527–533. [Google Scholar] [CrossRef]
- Snyder, G.; Greenberg, S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br. J. Anaesth. 2010, 105, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Takagi, Y.; Aoki, S.; Futamura, M.; Saji, S. Significant detection of circulating cancer cells in the blood by reverse transcriptase–polymerase chain reaction during colorectal cancer resection. Ann. Surg. 2000, 232, 58–65. [Google Scholar] [CrossRef]
- Jiao, Y.; Lv, Q. Does primary tumor resection induce accelerated metastasis in breast cancer? A Review. J. Surg. Res. 2023, 283, 1005–1017. [Google Scholar] [CrossRef]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for cancer: A trigger for metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef]
- Zeien, J.; Qiu, W.; Triay, M.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kaye, A.D. Clinical implications of chemotherapeutic agent organ toxicity on perioperative care. Biomed. Pharmacother. 2022, 146, 112503. [Google Scholar] [CrossRef]
- Livshits, Z.; Rao, R.B.; Smith, S.W. An approach to chemotherapy-associated toxicity. Emerg. Med. Clin. 2014, 32, 167–203. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, L.; Shi, Y.; Gu, W.; Ding, W.; Zheng, X.; Liu, Y.; Jiang, J.; Zheng, Z. Efficacy and safety of bispecific antibodies vs. immune checkpoint blockade combination therapy in cancer: A real-world comparison. Mol. Cancer 2024, 23, 77. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Konnerth, D.; Gaasch, A.; Westphalen, C.B.; Heinrich, K.; Niyazi, M.; Eze, C.; Rogowski, P.; Marschner, S.; Zinn, A.; Belka, C. Targeted RT study: Results on early toxicity of targeted therapies and radiotherapy. Radiat. Oncol. 2024, 19, 113. [Google Scholar] [CrossRef]
- Wang, K.; Tepper, J.E. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef]
- Abraham, J.; Staffurth, J. Hormonal therapy for cancer. Medicine 2016, 44, 30–33. [Google Scholar] [CrossRef]
- Ke, W.; Crist, R.M.; Clogston, J.D.; Stern, S.T.; Dobrovolskaia, M.A.; Grodzinski, P.; Jensen, M.A. Trends and patterns in cancer nanotechnology research: A survey of NCI’s caNanoLab and nanotechnology characterization laboratory. Adv. Drug Deliv. Rev. 2022, 191, 114591. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. Cancer Immunotherapy; American Association for the Advancement of Science: Washington, DC, USA, 2013. [Google Scholar]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef]
- Juarranz, Á.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Tampa, M.; Sarbu, M.-I.; Matei, C.; Mitran, C.-I.; Mitran, M.-I.; Caruntu, C.; Constantin, C.; Neagu, M.; Georgescu, S.-R. Photodynamic therapy: A hot topic in dermato-oncology. Oncol. Lett. 2019, 17, 4085–4093. [Google Scholar] [CrossRef]
- National Cancer Institute. Photodynamic Therapy to Treat Cancer Was Originally Published by the National Cancer Institute. 2021. Available online: https://www.cancer.gov/about-cancer/treatment/types/photodynamic-therapy (accessed on 15 May 2025).
- Gene Therapy. 2024. Available online: https://www.mayoclinic.org/tests-procedures/gene-therapy/about/pac-20384619 (accessed on 23 April 2024).
- Cleveland Clinic. Cardiac Care—Spring 2024; Cleveland Clinic: Weston, FL, USA, 2024; Available online: https://my.clevelandclinic.org/-/scassets/files/org/florida/hvti/ccf-cardiac-care-spring-2024.pdf (accessed on 10 May 2025).
- Chu, D.-T.; Nguyen, T.T.; Tien, N.L.B.; Tran, D.-K.; Jeong, J.-H.; Anh, P.G.; Thanh, V.V.; Truong, D.T.; Dinh, T.C. Recent Progress of Stem Cell Therapy in Cancer Treatment: Molecular Mechanisms and Potential Applications. Cells 2020, 9, 563. [Google Scholar] [CrossRef]
- Vasievich, E.A.; Huang, L. The Suppressive Tumor Microenvironment: A Challenge in Cancer Immunotherapy. Mol. Pharm. 2011, 8, 635–641. [Google Scholar] [CrossRef]
- García-Martínez, J.-M.; Collar, E.P. Organic–inorganic hybrid materials. Polymers 2020, 13, 86. [Google Scholar] [CrossRef]
- Parveen, S.; Gupta, P.; Kumar, S.; Banerjee, M. Lipid polymer hybrid nanoparticles as potent vehicles for drug delivery in cancer therapeutics. Med. Drug Discov. 2023, 20, 100165. [Google Scholar] [CrossRef]
- Iqbal, H.; Yang, T.; Li, T.; Zhang, M.; Ke, H.; Ding, D.; Deng, Y.; Chen, H. Serum protein-based nanoparticles for cancer diagnosis and treatment. J. Control. Release 2021, 329, 997–1022. [Google Scholar] [CrossRef]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed Res. Int. 2014, 2014, 180549. [Google Scholar] [CrossRef]
- Sandra, F.; Khaliq, N.U.; Sunna, A.; Care, A. Developing protein-based nanoparticles as versatile delivery systems for cancer therapy and imaging. Nanomaterials 2019, 9, 1329. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic nanoparticles in cancer therapy. Pharm. Res. 2011, 28, 237–259. [Google Scholar] [CrossRef]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic nanoparticles for cancer therapy: A transition from lab to clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Cheng, J.; Yuan, Z. Recent advances on inorganic nanoparticle-based cancer therapeutic agents. Int. J. Environ. Res. Public Health 2016, 13, 1182. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Rayhan, M.T.; Mobarak, M.H.; Rimon, M.I.H.; Hossain, N.; Islam, S.; Al Kafi, S.A. Advances and significances of gold nanoparticles in cancer treatment: A comprehensive review. Results Chem. 2024, 8, 101559. [Google Scholar] [CrossRef]
- Peng, X.-H.; Qian, X.; Mao, H.; Wang, A.Y.; Chen, Z.; Nie, S.; Shin, D.M. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int. J. Nanomed. 2008, 3, 311–321. [Google Scholar]
- Li, X.; Salzano, G.; Qiu, J.; Menard, M.; Berg, K.; Theodossiou, T.; Ladavière, C.; Gref, R. Drug-loaded lipid-coated hybrid organic-inorganic “stealth” nanoparticles for cancer therapy. Front. Bioeng. Biotechnol. 2020, 8, 1027. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in clinical translation for cancer therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.S.; Okano, T. Polymeric micelles as new drug carriers. Adv. Drug Deliv. Rev. 1996, 21, 107–116. [Google Scholar] [CrossRef]
- Djordjevic, J.; Del Rosario, L.S.; Wang, J.; Uhrich, K.E. Amphiphilic scorpion-like macromolecules as micellar nanocarriers. J. Bioact. Compat. Polym. 2008, 23, 532–551. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Deivayanai, V.; Thamarai, P.; Karishma, S.; Saravanan, A.; Yaashikaa, P.; Vickram, A.; Hemavathy, R.; Rohith Kumar, R.; Rishikeshavan, S.; Shruthi, S. A comprehensive review on advances in nanoparticle-mediated cancer therapeutics: Current research and future perspectives. Cancer Pathog. Ther. 2024, 2, E01–E16. [Google Scholar] [CrossRef]
- Kesharwani, P.; Chadar, R.; Sheikh, A.; Rizg, W.Y.; Safhi, A.Y. CD44-targeted nanocarrier for cancer therapy. Front. Pharmacol. 2022, 12, 800481. [Google Scholar] [CrossRef]
- Prajapati, A.; Rangra, S.; Patil, R.; Desai, N.; Jyothi, V.G.S.; Salave, S.; Amate, P.; Benival, D.; Kommineni, N. Receptor-targeted nanomedicine for cancer therapy. Receptors 2024, 3, 323–361. [Google Scholar] [CrossRef]
- Li, J.; Kataoka, K. Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, H.; Waqar, A.; Yaseen, F.; Shahid, M.; Sultana, M.; Tariq, M.; Bashir, M.K.; Tahseen, H.; Raza, T.; Ahmad, F. Role of nanoparticles in enhancing chemotherapy efficacy for cancer treatment. Next Mater. 2024, 2, 100128. [Google Scholar] [CrossRef]
- Lee, H.; Hoang, B.; Fonge, H.; Reilly, R.M.; Allen, C. In vivo distribution of polymeric nanoparticles at the whole-body, tumor, and cellular levels. Pharm. Res. 2010, 27, 2343–2355. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252, IN26–IN27. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef]
- Devita, V.T., Jr.; Young, R.C.; Canellos, G.P. Combination versus single agent chemotherapy: A review of the basis for selection of drug treatment of cancer. Cancer 1975, 35, 98–110. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhang, X.; Wei, X.; Tang, Z.; Zhou, S. Precise polymerization of a highly tumor microenvironment-responsive nanoplatform for strongly enhanced intracellular drug release. ACS Appl. Mater. Interfaces 2016, 8, 5833–5846. [Google Scholar] [CrossRef]
- Li, J.; Huo, M.; Wang, J.; Zhou, J.; Mohammad, J.M.; Zhang, Y.; Zhu, Q.; Waddad, A.Y.; Zhang, Q. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials 2012, 33, 2310–2320. [Google Scholar] [CrossRef]
- Qi, S.-S.; Sun, J.-H.; Yu, H.-H.; Yu, S.-Q. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 2017, 24, 1909–1926. [Google Scholar] [CrossRef]
- Gad, A.; Kydd, J.; Piel, B.; Rai, P. Targeting cancer using polymeric nanoparticle mediated combination chemotherapy. Int. J. Nanomed. Nanosurg. 2016, 2, 10–16966. [Google Scholar] [CrossRef]
- Yan, C.; Wang, C.; Shao, X.; Shu, Q.; Hu, X.; Guan, P.; Teng, Y.; Cheng, Y. Dual-targeted carbon-dot-drugs nanoassemblies for modulating Alzheimer’s related amyloid-β aggregation and inhibiting fungal infection. Mater. Today Bio 2021, 12, 100167. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, N.; Hu, Y.; Jia, C.; Chou, T.; Du, H.; Wang, H. Gold nanoparticle-enhanced photodynamic therapy: Effects of surface charge and mitochondrial targeting. Ther. Deliv. 2015, 6, 307–321. [Google Scholar] [CrossRef]
- Deng, X.; Shao, Z.; Zhao, Y. Solutions to the drawbacks of photothermal and photodynamic cancer therapy. Adv. Sci. 2021, 8, 2002504. [Google Scholar] [CrossRef]
- Lee, D.; Kwon, S.; Jang, S.-y.; Park, E.; Lee, Y.; Koo, H. Overcoming the obstacles of current photodynamic therapy in tumors using nanoparticles. Bioact. Mater. 2022, 8, 20–34. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; O’Hagan, J.; Zhang, S.; Donnelly, R.F. Photothermal therapy. J. Control. Release 2020, 325, 52–71. [Google Scholar] [CrossRef]
- Khazamipour, N.; Souri, A.; Babaee, O.; Dadashnia, B.; Soltan-Khamsi, P.; Mousavi, S.; Mohajerzadeh, S. Linker-free Functionalization of Phosphorene Nanosheets by Sialic Acid Biomolecules. Langmuir 2024, 40, 7067–7077. [Google Scholar] [CrossRef]
- Choi, W.I.; Kim, J.-Y.; Kang, C.; Byeon, C.C.; Kim, Y.H.; Tae, G. Tumor Regression In Vivo by Photothermal Therapy Based on Gold-Nanorod-Loaded, Functional Nanocarriers. ACS Nano 2011, 5, 1995–2003. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Dynlacht, J.R.; Dikomey, E. Mechanism of radiosensitization by hyperthermia (43°C) as derived from studies with DNA repair defective mutant cell lines. Int. J. Hyperth. 2004, 20, 131–139. [Google Scholar] [CrossRef]
- Hannon, G.; Tansi, F.L.; Hilger, I.; Prina-Mello, A. The Effects of Localized Heat on the Hallmarks of Cancer. Adv. Ther. 2021, 4, 2000267. [Google Scholar] [CrossRef]
- Bettaieb, A.; Averill-Bates, D.A. Thermotolerance induced at a mild temperature of 40°C alleviates heat shock-induced ER stress and apoptosis in HeLa cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, Y.; Jin, F.; Hu, K.; Lv, M.; Zhou, Y.; Zhao, W.; Hu, Y.; Wu, J.; Yang, Y.; et al. Multifunctional nanoparticles potentiate in-situ tumor vaccines via reversing insufficient Photothermal therapy by disrupting tumor vasculature. J. Control. Release 2024, 376, 842–860. [Google Scholar] [CrossRef] [PubMed]
- Bashiru, M.; Macchi, S.; Forson, M.; Khan, A.; Ishtiaq, A.; Oyebade, A.; Jalihal, A.; Ali, N.; Griffin, R.J.; Oyelere, A.K. Doxorubicin-Based Ionic Nanomedicines for Combined Chemo-Phototherapy of Cancer. ACS Appl. Nano Mater. 2024, 7, 2176–2189. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, R.; Yao, C.; Li, X.; Wang, C.; Zhang, X.; Xu, C.; Zeng, A.; Zhao, D.; Zhang, F. Single-band upconversion nanoprobes for multiplexed simultaneous in situ molecular mapping of cancer biomarkers. Nat. Commun. 2015, 6, 6938. [Google Scholar] [CrossRef]
- Cheng, Y.; Bao, D.; Chen, X.; Wu, Y.; Wei, Y.; Wu, Z.; Li, F.; Piao, J.-G. Microwave-triggered/HSP-targeted gold nano-system for triple-negative breast cancer photothermal therapy. Int. J. Pharm. 2021, 593, 120162. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-C.; Hsu, K.-F.; Lai, C.-L.; Wu, T.-C.; Chen, H.-F.; Lai, C.-H. Mannoside-modified branched gold nanoparticles for photothermal therapy to MDA-MB-231 cells. Molecules 2020, 25, 1853. [Google Scholar] [CrossRef]
- Chang, X.; Tang, X.; Liu, J.; Zhu, Z.; Mu, W.; Tang, W.; Zhang, Y.; Chen, X. Precise Starving Therapy via Physiologically Dependent Photothermal Conversion Promoted Mitochondrial Calcification Based on Multi-Functional Gold Nanoparticles for Effective Tumor Treatment. Adv. Funct. Mater. 2023, 33, 2303596. [Google Scholar] [CrossRef]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef]
- Dąbrowski, J.M. Chapter Nine—Reactive Oxygen Species in Photodynamic Therapy: Mechanisms of Their Generation and Potentiation. In Advances in Inorganic Chemistry; van Eldik, R., Hubbard, C.D., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 70, pp. 343–394. [Google Scholar]
- Mallidi, S.; Anbil, S.; Bulin, A.-L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the barriers of light penetration: Strategies, perspectives and possibilities for photodynamic therapy. Theranostics 2016, 6, 2458. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, J.; Wang, S.; Lin, T.; Ye, F.; Zhao, S. Carbon Dots with Absorption Red-Shifting for Two-Photon Fluorescence Imaging of Tumor Tissue pH and Synergistic Phototherapy. ACS Appl. Mater. Interfaces 2021, 13, 35365–35375. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, H.T.; Pham, T.T.; Choi, J.Y.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Development of a Graphene Oxide Nanocarrier for Dual-Drug Chemo-phototherapy to Overcome Drug Resistance in Cancer. ACS Appl. Mater. Interfaces 2015, 7, 28647–28655. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Shen, N.; Sun, J.; Tang, Z.; Chen, X. Destruction of tumor vasculature by vascular disrupting agents in overcoming the limitation of EPR effect. Adv. Drug Deliv. Rev. 2022, 183, 114138. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.-H.; Childs, C.J.H.; Sibata, C.H. Photosensitizers in clinical PDT. Photodiagn. Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Sharifi, M.; Attar, F.; Saboury, A.A.; Akhtari, K.; Hooshmand, N.; Hasan, A.; El-Sayed, M.A.; Falahati, M. Plasmonic gold nanoparticles: Optical manipulation, imaging, drug delivery and therapy. J. Control. Release 2019, 311, 170–189. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, L.; Zhao, Y.; Meng, D.; Li, D.; Li, H.; Zhang, B.; Shi, J.; Zhang, H.; Zhang, Z. Targeted imaging and chemo-phototherapy of brain cancer by a multifunctional drug delivery system. Macromol. Biosci. 2015, 15, 1571–1585. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Simón, M.; Jørgensen, J.T.; Norregaard, K.; Henriksen, J.R.; Clergeaud, G.; Andresen, T.L.; Hansen, A.E.; Kjaer, A. Neoadjuvant Gold Nanoshell-Based Photothermal Therapy Combined with Liposomal Doxorubicin in a Mouse Model of Colorectal Cancer. Int. J. Nanomed. 2023, 18, 829–841. [Google Scholar] [CrossRef]
- Liao, S.; Cai, M.; Zhu, R.; Fu, T.; Du, Y.; Kong, J.; Zhang, Y.; Qu, C.; Dong, X.; Ni, J. Antitumor effect of photodynamic therapy/sonodynamic therapy/sono-photodynamic therapy of chlorin e6 and other applications. Mol. Pharm. 2023, 20, 875–885. [Google Scholar] [CrossRef]
- Panikkanvalappil, S.R.; Hooshmand, N.; El-Sayed, M.A. Intracellular assembly of nuclear-targeted gold nanosphere enables selective plasmonic photothermal therapy of cancer by shifting their absorption wavelength toward near-infrared region. Bioconjug. Chem. 2017, 28, 2452–2460. [Google Scholar] [CrossRef] [PubMed]
- Macchi, S.; Jalihal, A.; Hooshmand, N.; Zubair, M.; Jenkins, S.; Alwan, N.; El-Sayed, M.; Ali, N.; Griffin, R.J.; Siraj, N. Enhanced photothermal heating and combination therapy of NIR dye via conversion to self-assembled ionic nanomaterials. J. Mater. Chem. B 2022, 10, 806–816. [Google Scholar] [CrossRef]
- Deinavizadeh, M.; Kiasat, A.R.; Shafiei, M.; Sabaeian, M.; Mirzajani, R.; Zahraei, S.M.; Khalili, F.; Shao, M.; Wu, A.; Makvandi, P. Synergistic chemo-photothermal therapy using gold nanorods supported on thiol-functionalized mesoporous silica for lung cancer treatment. Sci. Rep. 2024, 14, 4373. [Google Scholar] [CrossRef]
- Deinavizadeh, M.; Kiasat, A.R.; Hooshmand, N.; Shafiei, M.; Sabaeian, M.; Mirzajani, R.; Zahraei, S.M.; Makvandi, P.; El-Sayed, M.A. NIR/pH dual-responsive DOX-loaded AuNRs@S-β-CD nanocomposite for highly effective chemo-photothermal synergistic therapy against lung cancer cells. J. Phys. Chem. C 2022, 126, 18754–18766. [Google Scholar] [CrossRef]
- Falahati, M.; Attar, F.; Sharifi, M.; Saboury, A.A.; Salihi, A.; Aziz, F.M.; Kostova, I.; Burda, C.; Priecel, P.; Lopez-Sanchez, J.A. Gold nanomaterials as key suppliers in biological and chemical sensing, catalysis, and medicine. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2020, 1864, 129435. [Google Scholar] [CrossRef]
- Liao, S.; Yue, W.; Cai, S.; Tang, Q.; Lu, W.; Huang, L.; Qi, T.; Liao, J. Improvement of gold nanorods in photothermal therapy: Recent progress and perspective. Front. Pharmacol. 2021, 12, 664123. [Google Scholar] [CrossRef]
- Deinavizadeh, M.; Kiasat, A.; Hooshmand, N.; Shafiei, M.; Sabaeian, M.; Mirzajani, R.; Zahraei, S.M.; Labouta, H.I.; El-Sayed, M.A. Smart NIR-light and pH responsive doxorubicin-loaded GNRs@ SBA-15-SH nanocomposite for chemo-photothermal therapy of cancer. Nanophotonics 2021, 10, 3303–3319. [Google Scholar] [CrossRef]
- Deinavizadeh, M.; Kiasat, A.R.; Hooshmand, N.; Labouta, H.I.; Shafiei, M.; Sabaeian, M.; Mirzajani, R.; Zahraei, S.M.; Makvandi, P.; El-Sayed, M.A. Near-infrared/pH dual-responsive nanosponges encapsulating gold nanorods for synergistic chemo-phototherapy of lung cancer. ACS Appl. Nano Mater. 2023, 6, 16332–16342. [Google Scholar] [CrossRef]
- Haase, M.; Schäfer, H. Upconverting nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef]
- Liang, L.; Lu, Y.; Zhang, R.; Care, A.; Ortega, T.A.; Deyev, S.M.; Qian, Y.; Zvyagin, A.V. Deep-penetrating photodynamic therapy with KillerRed mediated by upconversion nanoparticles. Acta Biomater. 2017, 51, 461–470. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ohulchanskyy, T.Y.; Kumar, R.; Ågren, H.; Prasad, P.N. Ultrasmall Monodisperse NaYF4:Yb3+/Tm3+ Nanocrystals with Enhanced Near-Infrared to Near-Infrared Upconversion Photoluminescence. ACS Nano 2010, 4, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, H.; Hu, H.; Yu, M.; Li, F.; Zhang, Q.; Zhou, Z.; Yi, T.; Huang, C. Versatile synthesis strategy for carboxylic acid− functionalized upconverting nanophosphors as biological labels. J. Am. Chem. Soc. 2008, 130, 3023–3029. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, N.; Vetrone, F.; Ozin, G.A.; Capobianco, J.A. Synthesis of Ligand-Free Colloidally Stable Water Dispersible Brightly Luminescent Lanthanide-Doped Upconverting Nanoparticles. Nano Lett. 2011, 11, 835–840. [Google Scholar] [CrossRef]
- Bagheri, A.; Arandiyan, H.; Boyer, C.; Lim, M. Lanthanide-Doped upconversion nanoparticles: Emerging intelligent light-activated drug delivery systems. Adv. Sci. 2016, 3, 1500437. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef]
- Shan, X.; Zhao, Z.; Wang, C.; Sun, J.; He, Z.; Luo, C.; Zhang, S. Emerging Prodrug-Engineered nanomedicines for synergistic Chemo-Phototherapy. Chem. Eng. J. 2022, 442, 136383. [Google Scholar] [CrossRef]
- Christofi, T.; Baritaki, S.; Falzone, L.; Libra, M.; Zaravinos, A. Current Perspectives in Cancer Immunotherapy. Cancers 2019, 11, 1472. [Google Scholar] [CrossRef]
- Liang, J.; Wang, H.; Ding, W.; Huang, J.; Zhou, X.; Wang, H.; Dong, X.; Li, G.; Chen, E.; Zhou, F.; et al. Nanoparticle-enhanced chemo-immunotherapy to trigger robust antitumor immunity. Sci. Adv. 2020, 6, eabc3646. [Google Scholar] [CrossRef]
- Gharehbaba, A.M.; Omidi, Y.; Barar, J.; Eskandani, M.; Adibkia, K. Synergistic pH-responsive MUC-1 aptamer-conjugated Ag/MSN Janus nanoparticles for targeted chemotherapy, photothermal therapy, and gene therapy in breast cancer. Biomater. Adv. 2025, 166, 214081. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, S.; Hui, W.; He, J.; Liu, Z.; Cheng, J. Chitosan based pH-responsive polymeric prodrug vector for enhanced tumor targeted co-delivery of doxorubicin and siRNA. Carbohydr. Polym. 2020, 250, 116781. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, X.-L.; Zhao, M.-J.; Zhang, Y.-D.; Xiao, Y.; Liu, Y.-Y.; Qian, C.-F.; Xie, Y.-D.; Liu, Y.; Zou, Y.-J.; et al. Biomimetic hypoxia-triggered RNAi nanomedicine for synergistically mediating chemo/radiotherapy of glioblastoma. J. Nanobiotechnol. 2023, 21, 210. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Son, S.; Ochyl, L.J.; Kuai, R.; Schwendeman, A.; Moon, J.J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun. 2018, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; He, P.; Yu, L.; Wen, C.; Xie, X.; Yao, G. Oxygen self-enriched nanoplatform combined with US imaging and chemo/photothermal therapy for breast cancer. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102238. [Google Scholar] [CrossRef]
- Hu, S.; Huang, L.; Zhou, L.; Wu, T.; Zhao, S.; Zhang, L. Single-Excitation Triple-Emission Down-/Up-Conversion Nanoassemblies for Tumor Microenvironment-Enhanced Ratiometric NIR-II Fluorescence Imaging and Chemo-/Photodynamic Combination Therapy. Anal. Chem. 2023, 95, 3830–3839. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, T.; Qin, X.; Qiao, Q.; Shang, L.; Song, Q.; Yang, C.; Zhang, Z. Intracellularly Generated Immunological Gold Nanoparticles for Combinatorial Photothermal Therapy and Immunotherapy against Tumor. Nano Lett. 2019, 19, 6635–6646. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, T.; Feng, T.; Wan, Y.; Blum, N.T.; Liu, C.; Zheng, C.; Zhao, Z.; Jiang, T.; Wang, J.; et al. Plasmonic modulation of gold nanotheranostics for targeted NIR-II photothermal-augmented immunotherapy. Nano Today 2020, 35, 100987. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.; Ding, Y.; Xue, K.; Wang, X.; Yang, R.; An, Y.; Liu, D.; Hu, C.; Tang, Q. LRP1-mediated pH-sensitive polymersomes facilitate combination therapy of glioblastoma in vitro and in vivo. J. Nanobiotechnol. 2021, 19, 29. [Google Scholar] [CrossRef]
- Tian, X.; Warner, S.B.; Wagner, K.T.; Caster, J.M.; Zhang, T.; Ohana, P.; Gabizon, A.A.; Wang, A.Z. Preclinical Evaluation of Promitil, a Radiation-Responsive Liposomal Formulation of Mitomycin C Prodrug, in Chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 547–555. [Google Scholar] [CrossRef]
- Deng, S.; Hu, L.; Chen, G.; Ye, J.; Xiao, Z.; Guan, T.; Guo, S.; Xia, W.; Cheng, D.; Wan, X.; et al. A PD-L1 siRNA-Loaded Boron Nanoparticle for Targeted Cancer Radiotherapy and Immunotherapy. Adv. Mater. 2025, 37, 2419418. [Google Scholar] [CrossRef]
- Choi, J.; Shim, M.K.; Yang, S.; Hwang, H.S.; Cho, H.; Kim, J.; Yun, W.S.; Moon, Y.; Kim, J.; Yoon, H.Y.; et al. Visible-Light-Triggered Prodrug Nanoparticles Combine Chemotherapy and Photodynamic Therapy to Potentiate Checkpoint Blockade Cancer Immunotherapy. ACS Nano 2021, 15, 12086–12098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pang, S.; Liu, X.; Dong, Z.; Tian, Y.; Ashrafizadeh, M.; Rabiee, N.; Ertas, Y.N.; Mao, Y. Chitosan- and hyaluronic acid-based nanoarchitectures in phototherapy: Combination cancer chemotherapy, immunotherapy and gene therapy. Int. J. Biol. Macromol. 2024, 273, 132579. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef]
- de Miguel, M.; Calvo, E. Clinical challenges of immune checkpoint inhibitors. Cancer Cell 2020, 38, 326–333. [Google Scholar] [CrossRef]
- Overchuk, M.; Zheng, G. Overcoming obstacles in the tumor microenvironment: Recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials 2018, 156, 217–237. [Google Scholar] [CrossRef]
- Wen, R.; Umeano, A.C.; Kou, Y.; Xu, J.; Farooqi, A.A. Nanoparticle Systems for Cancer Vaccine. Nanomedicine 2019, 14, 627–648. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Zhang, Y. Present and future of cancer nano-immunotherapy: Opportunities, obstacles and challenges. Mol. Cancer 2025, 24, 26. [Google Scholar] [CrossRef]
- Lu, Q.; Kou, D.; Lou, S.; Ashrafizadeh, M.; Aref, A.R.; Canadas, I.; Tian, Y.; Niu, X.; Wang, Y.; Torabian, P.; et al. Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy. J. Hematol. Oncol. 2024, 17, 16. [Google Scholar] [CrossRef]
- Reda, M.; Ngamcherdtrakul, W.; Nelson, M.A.; Siriwon, N.; Wang, R.; Zaidan, H.Y.; Bejan, D.S.; Reda, S.; Hoang, N.H.; Crumrine, N.A.; et al. Development of a nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat. Commun. 2022, 13, 4261. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Chao, Y.; Xu, J.; Sun, X.; Wu, Y.; Peng, R.; Liu, Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, H.; Wang, D.; Li, Y. Improving cancer vaccine efficiency by nanomedicine. Adv. Biosyst. 2019, 3, 1800287. [Google Scholar] [CrossRef]
- Rabiee, N.; Kiani, M.; Bagherzadeh, M.; Rabiee, M.; Ahmadi, S. Nanoparticle (NP)-Based Delivery Vehicles; Morgan & Claypool Publishers: San Rafael, CA, USA, 2019. [Google Scholar]
- Zhu, G.; Zhang, F.; Ni, Q.; Niu, G.; Chen, X. Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano 2017, 11, 2387–2392. [Google Scholar] [CrossRef]
- Das, A.; Ali, N. Nanovaccine: An emerging strategy. Expert Rev. Vaccines 2021, 20, 1273–1290. [Google Scholar] [CrossRef]
- Sokolovska, A.; Hem, S.L.; HogenEsch, H. Activation of dendritic cells and induction of CD4+ T cell differentiation by aluminum-containing adjuvants. Vaccine 2007, 25, 4575–4585. [Google Scholar] [CrossRef]
- Lu, F.; Mencia, A.; Bi, L.; Taylor, A.; Yao, Y.; HogenEsch, H. Dendrimer-like alpha-d-glucan nanoparticles activate dendritic cells and are effective vaccine adjuvants. J. Control. Release 2015, 204, 51–59. [Google Scholar] [CrossRef]
- Aikins, M.E.; Xu, C.; Moon, J.J. Engineered nanoparticles for cancer vaccination and immunotherapy. Acc. Chem. Res. 2020, 53, 2094–2105. [Google Scholar] [CrossRef]
- Jiang, Y.; Krishnan, N.; Zhou, J.; Chekuri, S.; Wei, X.; Kroll, A.V.; Yu, C.L.; Duan, Y.; Gao, W.; Fang, R.H.; et al. Engineered Cell-Membrane-Coated Nanoparticles Directly Present Tumor Antigens to Promote Anticancer Immunity. Adv. Mater. 2020, 32, 2001808. [Google Scholar] [CrossRef]
- Antonia, S.J.; Mirza, N.; Fricke, I.; Chiappori, A.; Thompson, P.; Williams, N.; Bepler, G.; Simon, G.; Janssen, W.; Lee, J.-H.; et al. Combination of p53 Cancer Vaccine with Chemotherapy in Patients with Extensive Stage Small Cell Lung Cancer. Clin. Cancer Res. 2006, 12, 878–887. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, L.; Luo, C.; Jiang, M. Emerging strategies in cancer therapy combining chemotherapy with immunotherapy. Cancer Lett. 2019, 454, 191–203. [Google Scholar] [CrossRef]
- Gebremeskel, S.; Johnston, B. Concepts and mechanisms underlying chemotherapy induced immunogenic cell death: Impact on clinical studies and considerations for combined therapies. Oncotarget 2015, 6, 41600. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Kraehenbuehl, L.; Weng, C.-H.; Eghbali, S.; Wolchok, J.D.; Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2022, 19, 37–50. [Google Scholar] [CrossRef]
- Da Silva, C.G.; Camps, M.G.M.; Li, T.M.W.Y.; Chan, A.B.; Ossendorp, F.; Cruz, L.J. Co-delivery of immunomodulators in biodegradable nanoparticles improves therapeutic efficacy of cancer vaccines. Biomaterials 2019, 220, 119417. [Google Scholar] [CrossRef]
- Zhou, L.; Zou, M.; Xu, Y.; Lin, P.; Lei, C.; Xia, X. Nano drug delivery system for tumor immunotherapy: Next-generation therapeutics. Front. Oncol. 2022, 12, 864301. [Google Scholar] [CrossRef]
- Garbuzenko, O.B.; Sapiezynski, J.; Girda, E.; Rodriguez-Rodriguez, L.; Minko, T. Personalized Versus Precision Nanomedicine for Treatment of Ovarian Cancer. Small 2024, 20, 2307462. [Google Scholar] [CrossRef]
- Sorrentino, C.; Ciummo, S.L.; Fieni, C.; Di Carlo, E. Nanomedicine for cancer patient-centered care. MedComm 2024, 5, e767. [Google Scholar] [CrossRef]
- Grosser, R.; Cherkassky, L.; Chintala, N.; Adusumilli, P.S. Combination Immunotherapy with CAR T Cells and Checkpoint Blockade for the Treatment of Solid Tumors. Cancer Cell 2019, 36, 471–482. [Google Scholar] [CrossRef]
- Zhou, Y.; Mu, W.; Wang, C.; Zhuo, Z.; Xin, Y.; Li, H.; Wang, C. Ray of dawn: Anti-PD-1 immunotherapy enhances the chimeric antigen receptor T-cell therapy in Lymphoma patients. BMC Cancer 2023, 23, 1019. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008, 60, 977–985. [Google Scholar] [CrossRef]
- Cheng, S.; Xu, C.; Jin, Y.; Li, Y.; Zhong, C.; Ma, J.; Yang, J.; Zhang, N.; Li, Y.; Wang, C.; et al. Artificial Mini Dendritic Cells Boost T Cell–Based Immunotherapy for Ovarian Cancer. Adv. Sci. 2020, 7, 1903301. [Google Scholar] [CrossRef] [PubMed]
- Kempson, I. Mechanisms of nanoparticle radiosensitization. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1656. [Google Scholar] [CrossRef]
- Duwa, R.; Pokhrel, R.H.; Banstola, A.; Pandit, M.; Shrestha, P.; Jeong, J.-H.; Chang, J.-H.; Yook, S. T-cell engaging poly(lactic-co-glycolic acid) nanoparticles as a modular platform to induce a potent cytotoxic immunogenic response against PD-L1 overexpressing cancer. Biomaterials 2022, 291, 121911. [Google Scholar] [CrossRef]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar]
- Lawrence, T.S.; Blackstock, A.W.; McGinn, C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin. Radiat. Oncol. 2003, 13, 13–21. [Google Scholar] [CrossRef]
- He, M.; Chen, S.; Yu, H.; Fan, X.; Wu, H.; Wang, Y.; Wang, H.; Yin, X. Advances in nanoparticle-based radiotherapy for cancer treatment. iScience 2025, 28, 111602. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Zeng, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Prostate-specific membrane antigen targeted gold nanoparticles for prostate cancer radiotherapy: Does size matter for targeted particles? Chem. Sci. 2019, 10, 8119–8128. [Google Scholar] [CrossRef]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830–4838. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Zalba, S.; ten Hagen, T.L.M.; Burgui, C.; Garrido, M.J. Stealth nanoparticles in oncology: Facing the PEG dilemma. J. Control. Release 2022, 351, 22–36. [Google Scholar] [CrossRef]
- Sivasubramanian, M.; Chu, C.-H.; Hsia, Y.; Chen, N.-T.; Cai, M.-T.; Tew, L.S.; Chuang, Y.-C.; Chen, C.-T.; Aydogan, B.; Liao, L.-D.; et al. Illuminating and Radiosensitizing Tumors with 2DG-Bound Gold-Based Nanomedicine for Targeted CT Imaging and Therapy. Nanomaterials 2023, 13, 1790. [Google Scholar] [CrossRef] [PubMed]
- Yasui, H.; Takeuchi, R.; Nagane, M.; Meike, S.; Nakamura, Y.; Yamamori, T.; Ikenaka, Y.; Kon, Y.; Murotani, H.; Oishi, M.; et al. Radiosensitization of tumor cells through endoplasmic reticulum stress induced by PEGylated nanogel containing gold nanoparticles. Cancer Lett. 2014, 347, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Li, X.; Zhang, Y.; Huang, H.; Liu, J.; Zhang, J.; Wang, Z.; Niu, H.; Zhang, Y.; Mei, Q. Ultrathin and Biodegradable Bismuth Oxycarbonate Nanosheets with Massive Oxygen Vacancies for Highly Efficient Tumor Therapy. Small 2024, 20, 2307974. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, R.; Dong, X.; Zhu, S.; Zhou, R.; Yan, L.; Zhang, C.; Wang, Q.; Gu, Z.; Zhao, Y. BiO2−x Nanosheets as Radiosensitizers with Catalase-Like Activity for Hypoxia Alleviation and Enhancement of the Radiotherapy of Tumors. Inorg. Chem. 2020, 59, 3482–3493. [Google Scholar] [CrossRef]
- Hennequin, C.; Favaudon, V. Biological basis for chemo-radiotherapy interactions. Eur. J. Cancer 2002, 38, 223–230. [Google Scholar] [CrossRef]
- Froelich, J.J.; Schneller, F.R.G.; Zahn, R.K. The Influence of Radiation and Chemotherapy-Related DNA Strand Breaks on Carcinogenesis: An Evaluation. Clin. Chem. Lab. Med. 1999, 37, 403–408. [Google Scholar] [CrossRef]
- El-Awady, R.A.; Semreen, M.H.; Saber-Ayad, M.M.; Cyprian, F.; Menon, V.; Al-Tel, T.H. Modulation of DNA damage response and induction of apoptosis mediates synergism between doxorubicin and a new imidazopyridine derivative in breast and lung cancer cells. DNA Repair 2016, 37, 1–11. [Google Scholar] [CrossRef]
- Li, W.B.; Stangl, S.; Klapproth, A.; Shevtsov, M.; Hernandez, A.; Kimm, M.A.; Schuemann, J.; Qiu, R.; Michalke, B.; Bernal, M.A. Application of high-z gold nanoparticles in targeted cancer radiotherapy—Pharmacokinetic modeling, monte carlo simulation and radiobiological effect modeling. Cancers 2021, 13, 5370. [Google Scholar] [CrossRef]
- Chen, Q.; Fang, C.; Xia, F.; Wang, Q.; Li, F.; Ling, D. Metal nanoparticles for cancer therapy: Precision targeting of DNA damage. Acta Pharm. Sin. B 2024, 14, 1132–1149. [Google Scholar] [CrossRef]
- Slade, D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef]
- Wen, S.; Ovais, M.; Li, X.; Ren, J.; Liu, T.; Wang, Z.; Cai, R.; Chen, C. Tailoring bismuth-based nanoparticles for enhanced radiosensitivity in cancer therapy. Nanoscale 2022, 14, 8245–8254. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Han, J.; Cui, Y.; Shang, D.; Xiang, H. Prospect of Gold Nanoparticles in Pancreatic Cancer. Pharmaceutics 2024, 16, 806. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, C.; Silva, W.F.; Garcia, J.; Zaragosa, G.P.; Ilem, C.N.D.; Sales, T.O.; Santos, H.D.; Conde, B.I.C.; Barbosa, H.P.; Malik, S. Nanoparticles based image-guided thermal therapy and temperature feedback. J. Mater. Chem. B 2025, 13, 54–102. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.H. Gene therapy for cancer: Present status and future perspective. Mol. Cell. Ther. 2014, 2, 27. [Google Scholar] [CrossRef]
- Huang, W.-S.; Wang, J.-P.; Wang, T.; Fang, J.-Y.; Lan, P.; Ma, J.-P. ShRNA-mediated gene silencing of β-catenin inhibits growth of human colon cancer cells. World J. Gastroenterol. WJG 2007, 13, 6581. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Collins, M.A.; Pasca di Magliano, M. Kras as a key oncogene and therapeutic target in pancreatic cancer. Front. Physiol. 2014, 4, 407. [Google Scholar] [CrossRef]
- Sánchez-Rivera, F.J.; Jacks, T. Applications of the CRISPR–Cas9 system in cancer biology. Nat. Rev. Cancer 2015, 15, 387–393. [Google Scholar] [CrossRef]
- Mirgayazova, R.; Khadiullina, R.; Chasov, V.; Mingaleeva, R.; Miftakhova, R.; Rizvanov, A.; Bulatov, E. Therapeutic Editing of the TP53 Gene: Is CRISPR/Cas9 an Option? Genes 2020, 11, 704. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.-H.; Mao, C.-Q.; Dou, S.; Shen, S.; Tan, Z.-B.; Wang, J. Triple negative breast cancer therapy with CDK1 siRNA delivered by cationic lipid assisted PEG-PLA nanoparticles. J. Control. Release 2014, 192, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.J.; Farmer, M.R.; Atienza, C., Jr.; Stilwell, A.; Dong, Y.B.; Yang, H.L.; Wong, S.L.; McMasters, K.M. E2F-1 Gene Therapy Induces Apoptosis and Increases Chemosensitivity in Human Pancreatic Carcinoma Cells. Tumor Biol. 2002, 23, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Bentires-Alj, M.; Barbu, V.; Fillet, M.; Chariot, A.; Relic, B.; Jacobs, N.; Gielen, J.; Merville, M.-P.; Bours, V. NF-κB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene 2003, 22, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Abbasi Dezfouli, S.; Michailides, M.E.; Uludag, H. Delivery Aspects for Implementing siRNA Therapeutics for Blood Diseases. Biochemistry 2024, 63, 3059–3077. [Google Scholar] [CrossRef]
- Li, Y.; Gao, X.; Yu, Z.; Liu, B.; Pan, W.; Li, N.; Tang, B. Reversing multidrug resistance by multiplexed gene silencing for enhanced breast cancer chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 15461–15466. [Google Scholar] [CrossRef]
- Zhuang, Z.; Lu, J.; Lonser, R.R.; Kovach, J.S. Enhancement of cancer chemotherapy by simultaneously altering cell cycle progression and DNA-damage defenses through global modification of the serine/threonine phospho-proteome. Cell Cycle 2009, 8, 3303–3306. [Google Scholar] [CrossRef]
- Milella, M.; Estrov, Z.; Kornblau, S.M.; Carter, B.Z.; Konopleva, M.; Tari, A.; Schober, W.D.; Harris, D.; Leysath, C.E.; Lopez-Berestein, G.; et al. Synergistic induction of apoptosis by simultaneous disruption of the Bcl-2 and MEK/MAPK pathways in acute myelogenous leukemia. Blood 2002, 99, 3461–3464. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, Y.; Wang, Y.; Gao, Y.; Song, Y. Harnessing Gene Editing Technology for Tumor Microenvironment Modulation: An Emerging Anticancer Strategy. Chem.–Eur. J. 2024, 30, e202402485. [Google Scholar] [CrossRef]
- Huang, W.; Liang, Y.; Sang, C.; Mei, C.; Li, X.; Chen, T. Therapeutic nanosystems co-deliver anticancer drugs and oncogene SiRNA to achieve synergetic precise cancer chemo-gene therapy. J. Mater. Chem. B 2018, 6, 3013–3022. [Google Scholar] [CrossRef]
- Conde, J.; Tian, F.; Hernandez, Y.; Bao, C.; Baptista, P.V.; Cui, D.; Stoeger, T.; de la Fuente, J.M. RNAi-based glyconanoparticles trigger apoptotic pathways for in vitro and in vivo enhanced cancer-cell killing. Nanoscale 2015, 7, 9083–9091. [Google Scholar] [CrossRef]
- Luo, R.; Le, H.; Wu, Q.; Gong, C. Nanoplatform-Based In Vivo Gene Delivery Systems for Cancer Therapy. Small 2024, 20, 2312153. [Google Scholar] [CrossRef] [PubMed]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Liyanage, W.; Kannan, G.R.; Kannan, S.; Kannan, R.M. Efficient intracellular delivery of CRISPR-Cas9 ribonucleoproteins using dendrimer nanoparticles for robust genomic editing. Nano Today 2025, 61, 102654. [Google Scholar] [CrossRef]
- Vidal, F.; Guzman, L. Dendrimer nanocarriers drug action: Perspective for neuronal pharmacology. Neural Regen. Res. 2015, 10, 1029–1031. [Google Scholar]
- Gonçalves, G.A.R.; Paiva, R.d.M.A. Terapia gênica: Avanços, desafios e perspectivas. Einstein 2017, 15, 369–375. [Google Scholar] [CrossRef]
- Miraghaie, S.H.; Zandi, A.; Davari, Z.; Mousavi-kiasary, M.S.; Saghafi, Z.; Gilani, A.; Kordehlachin, Y.; Shojaeian, F.; Mamdouh, A.; Heydari, Z. Targeted delivery of anticancer drug loaded charged PLGA polymeric nanoparticles using electrostatic field. Macromol. Biosci. 2023, 23, 2300181. [Google Scholar] [CrossRef]
- Kumar, K.; Rani, V.; Mishra, M.; Chawla, R. New paradigm in combination therapy of siRNA with chemotherapeutic drugs for effective cancer therapy. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100103. [Google Scholar] [CrossRef]
- Yong, S.-B.; Ramishetti, S.; Goldsmith, M.; Diesendruck, Y.; Hazan-Halevy, I.; Chatterjee, S.; Somu Naidu, G.; Ezra, A.; Peer, D. Dual-Targeted Lipid Nanotherapeutic Boost for Chemo-Immunotherapy of Cancer. Adv. Mater. 2022, 34, 2106350. [Google Scholar] [CrossRef]
- Dechbumroong, P.; Hu, R.; Keaswejjareansuk, W.; Namdee, K.; Liang, X.-J. Recent advanced lipid-based nanomedicines for overcoming cancer resistance. Cancer Drug Resist. 2024, 7, 24. [Google Scholar] [CrossRef]
- Liu, X.; Liu, G.; Mao, Y.; Luo, J.; Cao, Y.; Tan, W.; Li, W.; Yu, H.; Jia, X.; Li, H. Engineering Extracellular Vesicles Mimetics for Targeted Chemotherapy of Drug-Resistant Ovary Cancer. Nanomedicine 2024, 19, 25–41. [Google Scholar] [CrossRef]
- Packeiser, E.-M.; Engels, L.; Nolte, I.; Goericke-Pesch, S.; Murua Escobar, H. MDR1 Inhibition Reverses Doxorubicin-Resistance in Six Doxorubicin-Resistant Canine Prostate and Bladder Cancer Cell Lines. Int. J. Mol. Sci. 2023, 24, 8136. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, E.R.; Lin, A.Y.; Yan, J.; Luo, L.; Foster, A.E.; Drezek, R.A. Optimization of PAMAM-gold nanoparticle conjugation for gene therapy. Biomaterials 2014, 35, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Paus, C.; van der Voort, R.; Cambi, A. Nanomedicine in cancer therapy: Promises and hurdles of polymeric nanoparticles. Explor. Med. 2021, 2, 167–185. [Google Scholar] [CrossRef]
- Zheng, C.; Li, M.; Ding, J. Challenges and opportunities of nanomedicines in clinical translation. Bio Integr. 2021, 2, 57. [Google Scholar] [CrossRef]
- Kus-Liśkiewicz, M.; Fickers, P.; Ben Tahar, I. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. Int. J. Mol. Sci. 2021, 22, 10952. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, C.; Wu, L.; Bai, X.; Zhai, S. Cytotoxicity-related bioeffects induced by nanoparticles: The role of surface chemistry. Front. Bioeng. Biotechnol. 2019, 7, 414. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.-K.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef]
- Jakic, K.; Selc, M.; Razga, F.; Nemethova, V.; Mazancova, P.; Havel, F.; Sramek, M.; Zarska, M.; Proska, J.; Masanova, V. Long-Term Accumulation, Biological Effects and Toxicity of BSA-Coated Gold Nanoparticles in the Mouse Liver, Spleen, and Kidneys. Int. J. Nanomed. 2024, 19, 4103–4120. [Google Scholar] [CrossRef]
- Fadeel, B.; Farcal, L.; Hardy, B.; Vázquez-Campos, S.; Hristozov, D.; Marcomini, A.; Lynch, I.; Valsami-Jones, E.; Alenius, H.; Savolainen, K. Advanced tools for the safety assessment of nanomaterials. Nat. Nanotechnol. 2018, 13, 537–543. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Khanna, P.; Ong, C.; Bay, B.H.; Baeg, G.H. Nanotoxicity: An interplay of oxidative stress, inflammation and cell death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, N.; Maitra, S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef]
- Fadeel, B. The right stuff: On the future of nanotoxicology. Front. Toxicol. 2019, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Olesen, P.; Hougaard, M.; Dang, D.A.; Hoffmann, H.J.; Autrup, H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol. Lett. 2009, 190, 156–162. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, D.Y. Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J. Appl. Toxicol. 2013, 33, 78–89. [Google Scholar] [CrossRef]
- Zavisova, V.; Koneracka, M.; Kovac, J.; Kubovcikova, M.; Antal, I.; Kopcansky, P.; Bednarikova, M.; Muckova, M. The cytotoxicity of iron oxide nanoparticles with different modifications evaluated in vitro. J. Magn. Magn. Mater. 2015, 380, 85–89. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.; Bhatia, S.N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Shukla, R.K.; Saxena, N.; Parmar, D.; Das, M.; Dhawan, A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol. Lett. 2009, 185, 211–218. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Owens III, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [CrossRef]
- Guo, C.; Yuan, H.; Wang, Y.; Feng, Y.; Zhang, Y.; Yin, T.; He, H.; Gou, J.; Tang, X. The interplay between PEGylated nanoparticles and blood immune system. Adv. Drug Deliv. Rev. 2023, 200, 115044. [Google Scholar] [CrossRef]
- Neto, F.N.S.; Morais, L.A.; Gorup, L.F.; Ribeiro, L.S.; Martins, T.J.; Hosida, T.Y.; Francatto, P.; Barbosa, D.B.; Camargo, E.R.; Delbem, A.C. Facile synthesis of PVP-Coated silver nanoparticles and evaluation of their physicochemical, antimicrobial and toxic activity. Colloids Interfaces 2023, 7, 66. [Google Scholar] [CrossRef]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef]
- Vinnacombe-Willson, G.A.; Conti, Y.; Stefancu, A.; Weiss, P.S.; Cortés, E.; Scarabelli, L. Direct Bottom-Up In Situ Growth: A Paradigm Shift for Studies in Wet-Chemical Synthesis of Gold Nanoparticles. Chem. Rev. 2023, 123, 8488–8529. [Google Scholar] [CrossRef]
- Ramos, T.I.; Villacis-Aguirre, C.A.; López-Aguilar, K.V.; Santiago Padilla, L.; Altamirano, C.; Toledo, J.R.; Santiago Vispo, N. The Hitchhiker’s Guide to Human Therapeutic Nanoparticle Development. Pharmaceutics 2022, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Wasti, S.; Lee, I.H.; Kim, S.; Lee, J.-H.; Kim, H. Ethical and legal challenges in nanomedical innovations: A scoping review. Front. Genet. 2023, 14, 1163392. [Google Scholar] [CrossRef] [PubMed]
- Hunckler, M.D.; Levine, A.D. Navigating ethical challenges in the development and translation of biomaterials research. Front. Bioeng. Biotechnol. 2022, 10, 949280. [Google Scholar] [CrossRef]
- Omenogor, C.E.; Adeniran, A.A. Advancing Precision Healthcare: The Integration of Nanotechnology, Millimeter Wave Sensing, Laser Technology, Fibre Bragg Grating, and Deep Learning Models. Int. J. Res. Publ. Rev. 2024, 5, 639–657. [Google Scholar] [CrossRef]
- Hanif, M.I. Innovative Applications of Nanotechnology in Orthopaedics: A Paradigm Shift in Healing and Patient Care. EC Orthop. 2023, 14, 54–60. [Google Scholar]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Ernst, L.M.; Casals, E.; Italiani, P.; Boraschi, D.; Puntes, V. The Interactions between Nanoparticles and the Innate Immune System from a Nanotechnologist Perspective. Nanomaterials 2021, 11, 2991. [Google Scholar] [CrossRef]
- Dorney, K.M.; Shropshire, N.S.; Adams, D.G.; Zandi, A.; Baker, J.; Brittle, S.; Kanel, S.; Hooshmand, N.; Pavel, I.E. Ecofriendly Filtration of Silver Nanoparticles for Ultrasensitive Surface-Enhanced (Resonance) Raman Spectroscopy-Based Detection. J. Phys. Chem. C 2024, 128, 16563–16575. [Google Scholar] [CrossRef]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef]
- Zhang, A.; Miao, K.; Sun, H.; Deng, C.-X. Tumor heterogeneity reshapes the tumor microenvironment to influence drug resistance. Int. J. Biol. Sci. 2022, 18, 3019. [Google Scholar] [CrossRef]
- Maldonado-Ortega, D.A.; Navarro-Tovar, G.; Martínez-Castañón, G.; Gonzalez, C. Effect of gold nanoparticles (AuNPs) on isolated rat tracheal segments. Toxicol. Rep. 2021, 8, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhan, Y.; Ding, J.; Song, Z.; Zhang, Y.; Li, J.; Su, T. Biomimetic Cell Membrane-Coated Nanoparticles for Cancer Theranostics. ChemMedChem 2024, 19, e202400410. [Google Scholar] [CrossRef]
- Arvejeh, P.M. Nanobiomaterials & nanomedicine. J. Transl. Med. 2024, 22, 1154. [Google Scholar]
- Buljan, M.; Wick, P. Tailoring Design of Nanomaterials and Systems to Individualize Patient Treatments. Chimia 2022, 76, 236–241. [Google Scholar] [CrossRef]
- Olawade, D.B.; Ige, A.O.; Olaremu, A.G.; Ijiwade, J.O.; Adeola, A.O. The synergy of artificial intelligence and nanotechnology towards advancing innovation and sustainability—A mini-review. Nano Trends 2024, 8, 100052. [Google Scholar] [CrossRef]
- Zhu, L.; Staley, C.; Kooby, D.; El-Rays, B.; Mao, H.; Yang, L. Current status of biomarker and targeted nanoparticle development: The precision oncology approach for pancreatic cancer therapy. Cancer Lett. 2017, 388, 139–148. [Google Scholar] [CrossRef]
- Zandi, A.; Sh, Z.D.; Shojaeian, F.; Mousavi-Kiasary, S.S.; Abbasvandi, F.; Zandi, A.; Gilani, A.; Saghafi, Z.; Kordehlachin, Y.; Mamdouh, A. The design and fabrication of nanoengineered platinum needles with laser welded carbon nanotubes (CNTs) for the electrochemical biosensing of cancer lymph nodes. Biomater. Sci. 2021, 9, 6214–6226. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Multifunctional lipid-based nanoparticles for codelivery of anticancer drugs and siRNA for treatment of non-small cell lung cancer with different level of resistance and EGFR mutations. Pharmaceutics 2021, 13, 1063. [Google Scholar] [CrossRef]
- Wu, F.; Qiu, F.; Wai-Keong, S.A.; Diao, Y. The smart dual-stimuli responsive nanoparticles for controlled anti-tumor drug release and cancer therapy. Anti-Cancer Agents Med. Chem. 2021, 21, 1202–1215. [Google Scholar] [CrossRef]
- Aliakbarzadeh, S.; Abdouss, M.; Fathi-karkan, S.; Rahdar, A.; Zarbanooei, P.; Kang, M.; Pandey, S. Micro-Surgeons and Nano-Pharmacists: The Future of Healthcare with Medical Nanorobots. J. Drug Deliv. Sci. Technol. 2024, 103, 106410. [Google Scholar] [CrossRef]
- Kong, X.; Gao, P.; Wang, J.; Fang, Y.; Hwang, K.C. Advances of medical nanorobots for future cancer treatments. J. Hematol. Oncol. 2023, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.E.; Wang, L.; Gautam, A.; Newton, I.G.; Steinmetz, N.F. Combining nanomedicine and immune checkpoint therapy for cancer immunotherapy. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1739. [Google Scholar] [CrossRef]
- Kang, X.; Mita, N.; Zhou, L.; Wu, S.; Yue, Z.; Babu, R.J.; Chen, P. Nanotechnology in Advancing Chimeric Antigen Receptor T Cell Therapy for Cancer Treatment. Pharmaceutics 2024, 16, 1228. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Adir, O.; Poley, M.; Chen, G.; Froim, S.; Krinsky, N.; Shklover, J.; Shainsky-Roitman, J.; Lammers, T.; Schroeder, A. Integrating Artificial Intelligence and Nanotechnology for Precision Cancer Medicine. Adv. Mater. 2020, 32, 1901989. [Google Scholar] [CrossRef]
- Woodson, T.S. Public private partnerships and emerging technologies: A look at nanomedicine for diseases of poverty. Res. Policy 2016, 45, 1410–1418. [Google Scholar] [CrossRef]
- Zingg, R.; Fischer, M. The rise of private–public collaboration in nanotechnology. Nano Today 2019, 25, 7–9. [Google Scholar] [CrossRef]
- Linton, J.D.; Xu, W. Understanding and Managing the Biotechnology Valley of Death. Trends Biotechnol. 2021, 39, 107–110. [Google Scholar] [CrossRef]
- Witten, J.; Hu, Y.; Langer, R.; Anderson, D.G. Recent advances in nanoparticulate RNA delivery systems. Proc. Natl. Acad. Sci. USA 2024, 121, e2307798120. [Google Scholar] [CrossRef]

| Nanoparticle Type | Toxicity Effects | Affected Systems | Notes on Mitigation | Ref. |
|---|---|---|---|---|
| AuNPs | ROS generation, DNA damage, pro-inflammatory effects | Liver, kidney, and blood cells | PEGylation reduces immune clearance and inflammation | [221,222] |
| AgNPs | High oxidative stress, mitochondrial damage, genotoxicity | Liver, lungs, immune cells | PVP coating reduces cytotoxicity | [223,224] |
| Fe3O4 | Iron overload, oxidative stress, ROS | Brain, liver, macrophages | Dextran or PEG coating enhances biocompatibility | [225,226] |
| QDs | Heavy metal ion release (e.g., Cd2+), long-term toxicity | Liver, spleen, reproductive organs | Silica/polymer coating prevents leaching | [227,228] |
| Zinc Oxide (ZnO NPs) | High ROS production, inflammatory response | Lung, liver, skin | Smaller size + surface modification lowers toxicity | [229,230] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi-Kiasary, S.M.S.; Senabreh, A.; Zandi, A.; Pena, R.; Cruz, F.; Adibi, A.; Hooshmand, N. Synergistic Cancer Therapies Enhanced by Nanoparticles: Advancing Nanomedicine Through Multimodal Strategies. Pharmaceutics 2025, 17, 682. https://doi.org/10.3390/pharmaceutics17060682
Mousavi-Kiasary SMS, Senabreh A, Zandi A, Pena R, Cruz F, Adibi A, Hooshmand N. Synergistic Cancer Therapies Enhanced by Nanoparticles: Advancing Nanomedicine Through Multimodal Strategies. Pharmaceutics. 2025; 17(6):682. https://doi.org/10.3390/pharmaceutics17060682
Chicago/Turabian StyleMousavi-Kiasary, Seyed Mohamad Sadegh, Ahmood Senabreh, Ashkan Zandi, Rogelio Pena, Frances Cruz, Ali Adibi, and Nasrin Hooshmand. 2025. "Synergistic Cancer Therapies Enhanced by Nanoparticles: Advancing Nanomedicine Through Multimodal Strategies" Pharmaceutics 17, no. 6: 682. https://doi.org/10.3390/pharmaceutics17060682
APA StyleMousavi-Kiasary, S. M. S., Senabreh, A., Zandi, A., Pena, R., Cruz, F., Adibi, A., & Hooshmand, N. (2025). Synergistic Cancer Therapies Enhanced by Nanoparticles: Advancing Nanomedicine Through Multimodal Strategies. Pharmaceutics, 17(6), 682. https://doi.org/10.3390/pharmaceutics17060682






