Herbal Medicine: Enhancing the Anticancer Potential of Natural Products in Hepatocellular Carcinoma Therapy Through Advanced Drug Delivery Systems
Abstract
1. Introduction
2. Methodology
3. Liver and Its Disorders
4. Hepatoprotective Natural Products
4.1. Shikonin
4.2. Fucoidan
4.3. Nigella sativa
4.4. Curcumin
4.5. Green Tea Catechins
| Natural Product | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Shikonin |
|
| [47,66,67] |
| Fucoidan |
|
| [68,69] |
| Nigella sativa |
|
| [70,71,72] |
| Curcumin |
|
| [62,73] |
| Green Tea Catechins |
|
| [74] |
5. Challenges of Using Nature Products as Therapeutic Agents for HCC
6. Role of Nanotechnology in HCC Treatment
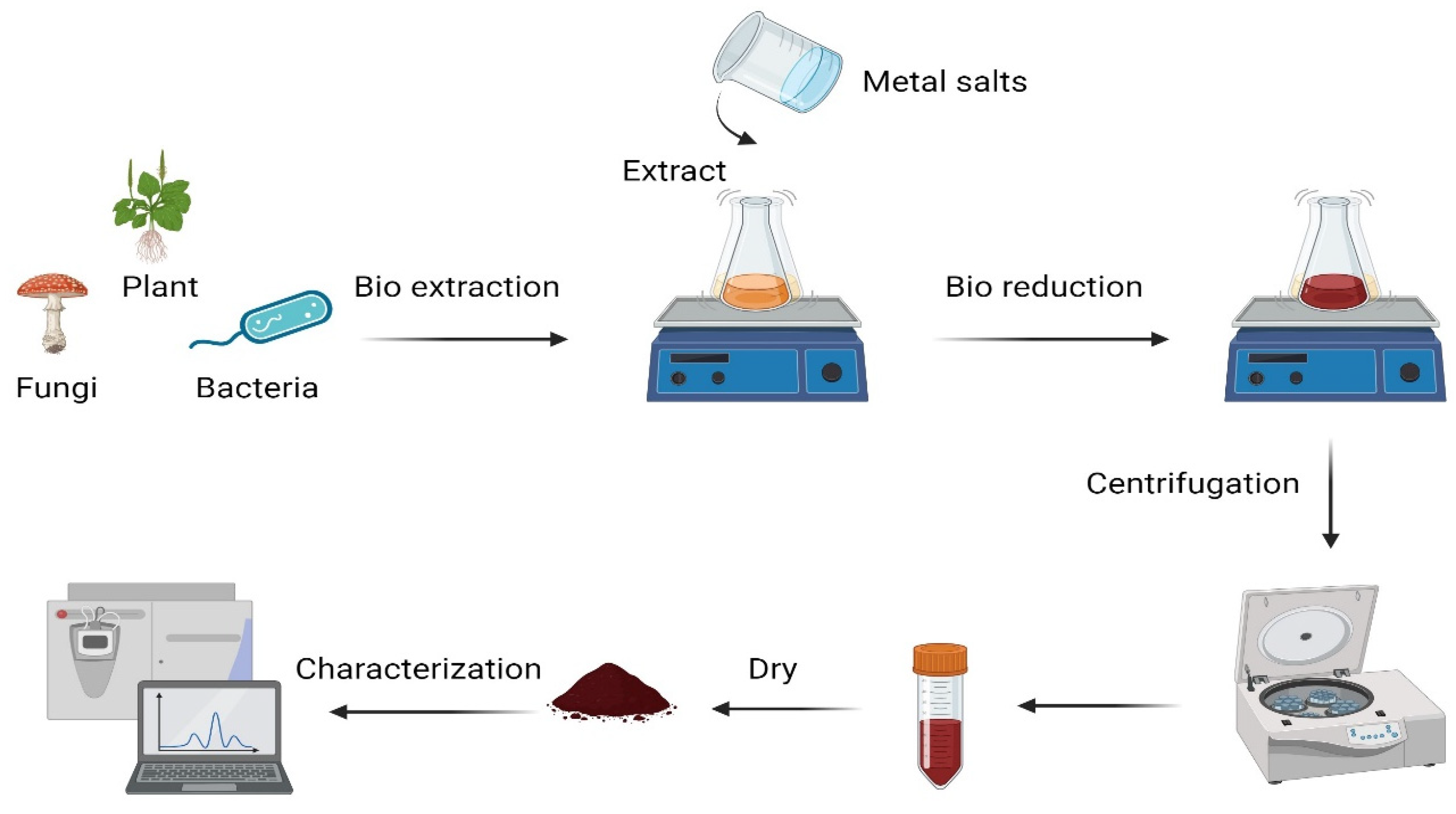
7. Green Synthesis of Nanoparticles
| Nanoparticle | Natural Product | Particle Size (nm) | Shape | IC50 | The Effect on HCC | Reference |
|---|---|---|---|---|---|---|
| AuNP | Dendrobium officinale extract | 30 nm | Spherical | 200 μg/mL | Anticancer and immune regulation. | [130] |
| AuNP | C. militaris extract | 5–25 nm | Face-center-cubic | 10–12.5 µg/mL | Activating the gene expression of Bax, Bid, Caspase-3 and Caspase-9. | [131] |
| AgNP | Nigella sativa | 10–20 nm | Spherical | 7.16 μg/mL | Growth inhibition without harming healthy cells. | [132] |
| CuONP | Momordica cochinchinensis (Lour.) extract | 40–80 nm | Cubic | 75 μg/mL | Induce apoptosis and ROS. | [133] |
| AgNP | Podophyllum hexandrum Royal leaf extract | 14 nm | Spherical | - | Apoptotic effect. | [134] |
| AgNP | Amla extract | 188 nm | Spherical and cubic | - | Apoptotic effect. | [134] |
| AuNP | Ziziphus spina-christi leaves | 31.26–58.06 nm | Spherical | - | Induce apoptosis in cancer cells. | [135] |
| AuNP | Cordia myxa L. leaves | 56.49–89.38 nm | Spherical | - | Induce apoptosis in cancer cells. | [135] |
| AgNP | Leucus aspera | 40.67–58.17 nm | Spherical | 158 µg/mL | Induce apoptosis in cancer cells. | [136] |
8. Nanoparticles Used for HCC
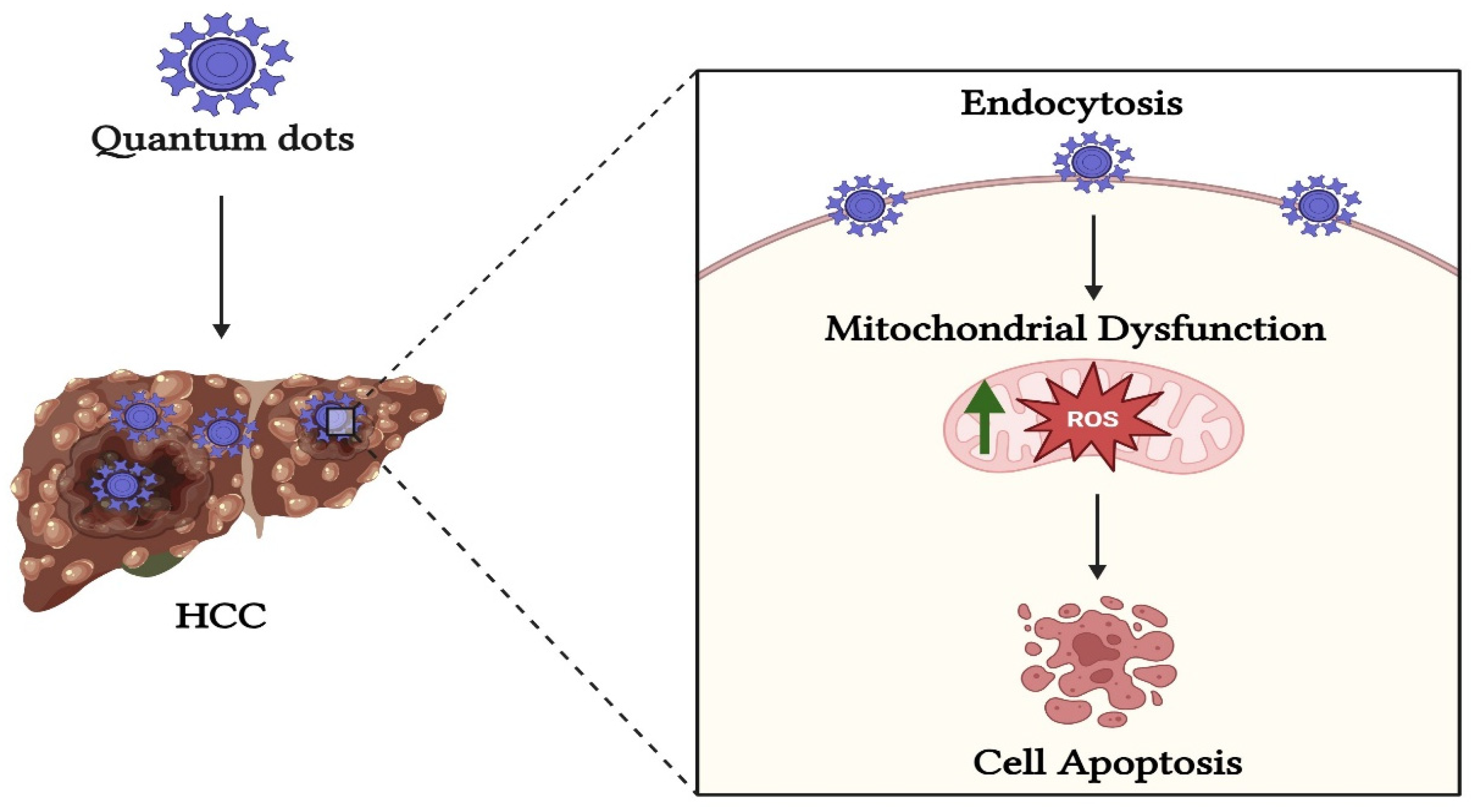
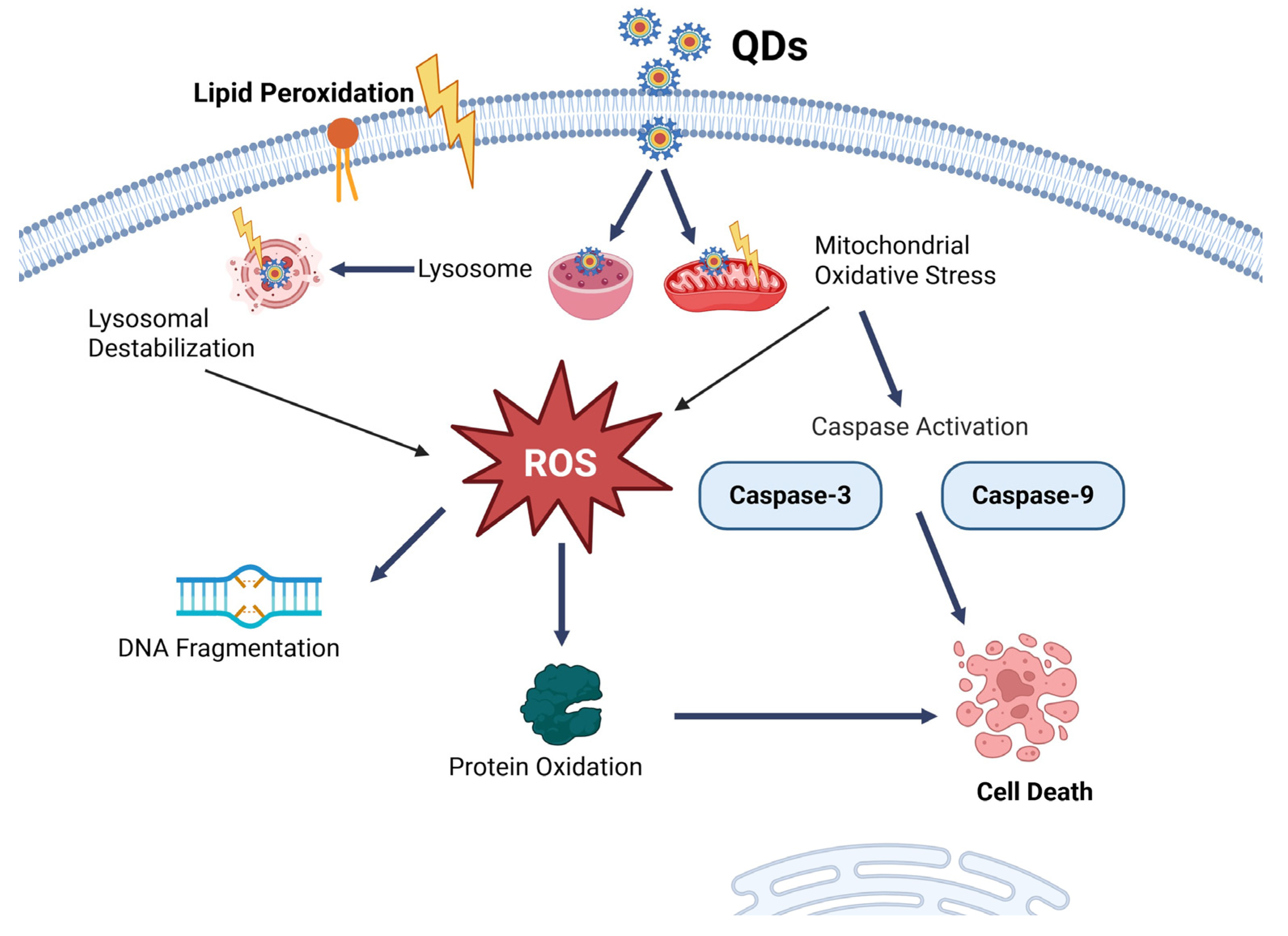
8.1. Quantum Dots NP
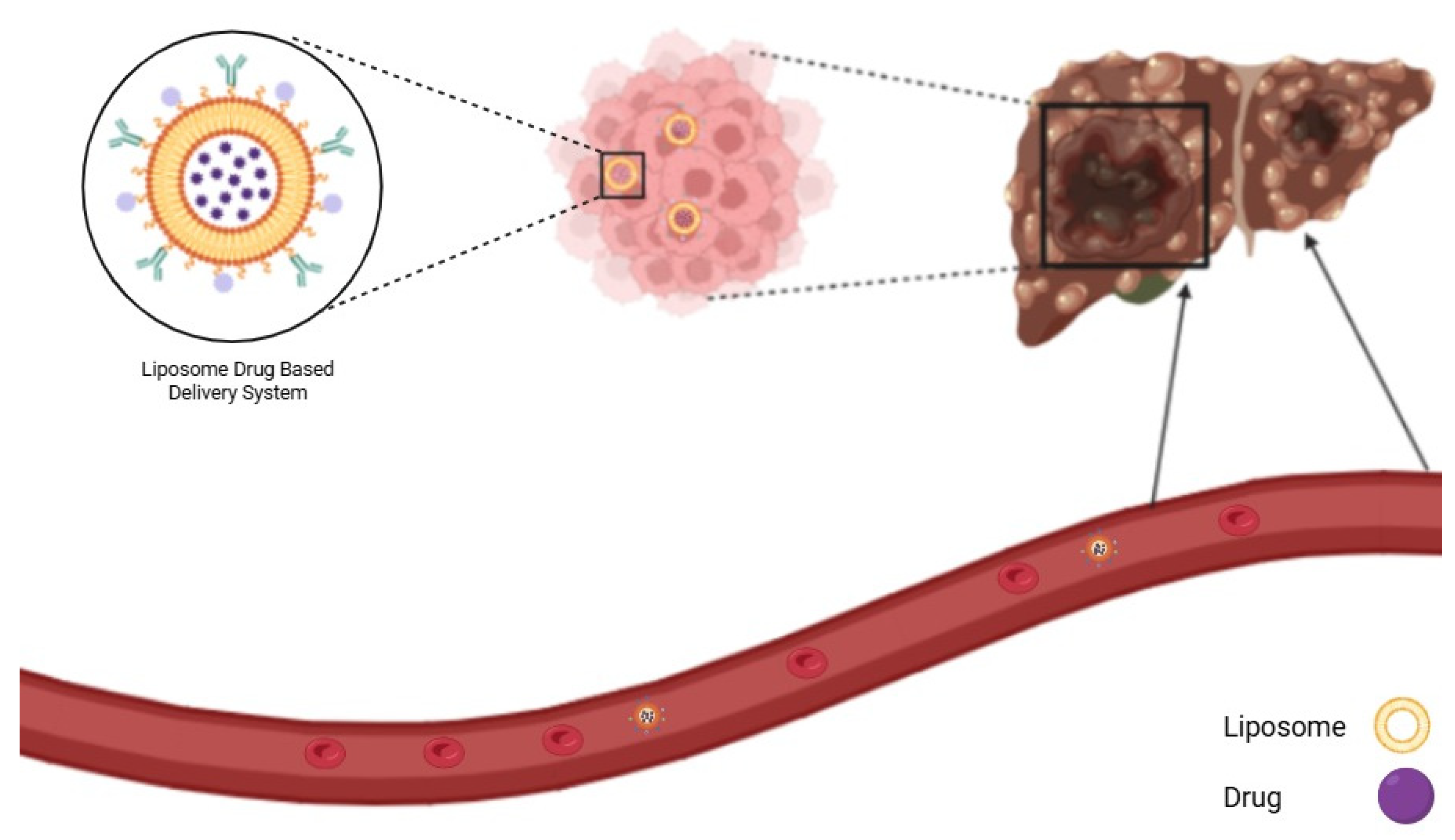
8.2. Liposome NP
8.3. Polymeric NP
8.4. Nanozyme
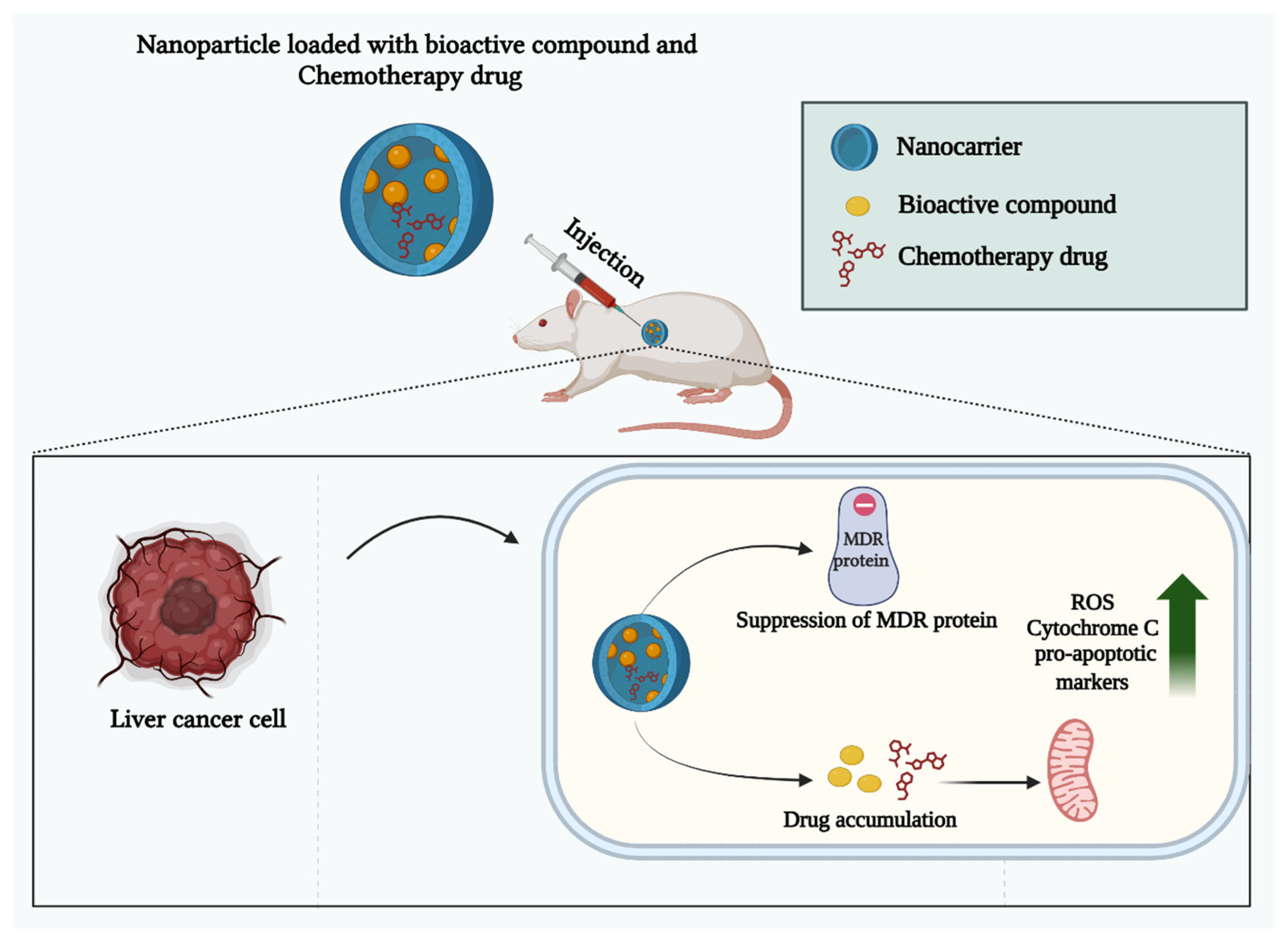
9. Overcoming Drug Resistance in HCC Using Natural Product-Nanoparticle Combinations
10. Natural Product Delivery Systems for HCC
11. Nano-Enabled Personalized Medicine Approaches in HCC
12. Limitations in Herbal Medication Based on Nanoparticles
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| PKM2 | Pyruvate kinase type M2 |
| Hep-G2 | Hepatic cancer cell line |
| PYCR1 | Pyroline-5-carboxylate reductase 1 |
| ROS | Reactive oxygen species |
| GTCs | Green Tea Catechins |
| EGCG | Epigallocatechin-3-gallate |
| AFP | Alpha-fetoprotien |
| MRI | Magnetic resonance imaging |
| CT | Computed tomography |
| MDR | Multidrug resistance |
| ICG | Indocyanine Green |
| NPs | Nanoparticles |
| TKIs | Tyrosine kinase inhibitors |
| SFN | Sorafenib |
| QDs | Quantum dots |
| TQ | Thymoquinone |
| VEGF | Vascular endothelial growth factor |
| CdSe QDs | Cadmium-selenium quantum dots |
| PNPs | Polymeric nanoparticles |
| PLA | Polylactic acid |
| CVD | Chemical vapor deposition |
| PVA | Polyvinyl alcohol |
| NARNPs | Naringenin nanoparticles |
| QC | Quercetin |
| C-GPL | Galactose-modified PEGylated liposomes |
| MLNPs | Morus nigra L.-derived lipid nanoparticles |
| PLGA | Poly(lactic-co-glycolic acid) |
References
- Center, M.M.; Jemal, A. Data from International Trends in Liver Cancer Incidence Rates; American Association for Cancer Research: Philadelphia, PA, USA, 2023. [Google Scholar]
- Wang, R.H.; Hu, M.; Yang, Z.Y.; Niu, Z.Y.; Chen, H.S.; Zhou, X.; Cao, G.W. Global liver cancer incidence and mortality and future trends from 2000 to 2020: GLOBOCAN data analysis. Zhonghua Gan Zang Bing Za Zhi (Chin. J. Hepatol.) 2023, 31, 271–280. [Google Scholar] [CrossRef]
- Serraino, D.; Fratino, L.; Piselli, P. Epidemiological Aspects of Hepatocellular Carcinoma. In Hepatocellular Carcinoma; Ettorre, G.M., Ed.; Updates in Surgery; Springer International Publishing: Cham, Switzerland, 2023; pp. 3–9. ISBN 978-3-031-09370-8. [Google Scholar]
- Islami, F.; Ward, E.M.; Sung, H.; Cronin, K.A.; Tangka, F.K.L.; Sherman, R.L.; Zhao, J.; Anderson, R.N.; Henley, S.J.; Yabroff, K.R.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Ong, S.C.; Li, F.; Shen, Y.; Weng, Z.; Zhao, K.; Jiang, Z.; Wang, M. Cost-Effectiveness of Immune Checkpoint Inhibitors as a First-Line Therapy for Advanced Hepatocellular Carcinoma: A Systematic Review. Health Econ. Rev. 2024, 14, 48. [Google Scholar] [CrossRef]
- Wang, W.; Wei, C. Advances in the Early Diagnosis of Hepatocellular Carcinoma. Genes. Dis. 2020, 7, 308–319. [Google Scholar] [CrossRef]
- Lurje, I.; Czigany, Z.; Bednarsch, J.; Roderburg, C.; Isfort, P.; Neumann, U.P.; Lurje, G. Treatment Strategies for Hepatocellular Carcinoma—A Multidisciplinary Approach. Int. J. Mol. Sci. 2019, 20, 1465. [Google Scholar] [CrossRef]
- Petrowsky, H.; Fritsch, R.; Guckenberger, M.; De Oliveira, M.L.; Dutkowski, P.; Clavien, P.-A. Modern Therapeutic Approaches for the Treatment of Malignant Liver Tumours. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Luo, C.; Yan, M.; Zhao, G.; Ma, L.; Gao, W. Treatment for Liver Cancer: From Sorafenib to Natural Products. Eur. J. Med. Chem. 2021, 224, 113690. [Google Scholar] [CrossRef]
- Doustmihan, A.; Fathi, M.; Mazloomi, M.; Salemi, A.; Hamblin, M.R.; Jahanban-Esfahlan, R. Molecular Targets, Therapeutic Agents and Multitasking Nanoparticles to Deal with Cancer Stem Cells: A Narrative Review. J. Control. Release 2023, 363, 57–83. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting Drug Chemo-Resistance in Cancer Using Natural Products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Njiki, S.; Mbemi, A.; Yedjou, C.G.; Tchounwou, P.B. Pharmacological Effects of Cisplatin Combination with Natural Products in Cancer Chemotherapy. Int. J. Mol. Sci. 2022, 23, 1532. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Hou, Y.; Sun, W.; Yu, J.; Liu, X.; Niu, Y.; Lu, J.; Chen, X. Natural Products to Prevent Drug Resistance in Cancer Chemotherapy: A Review. Ann. N. Y. Acad. Sci. 2017, 1401, 19–27. [Google Scholar] [CrossRef]
- Roy, N.K.; Tewari, D.; Esposito, M.T. Editorial: Chemosensitizing Effect of Natural Products against Cancers: Applications in Enhancing Chemotherapy and Immunotherapy. Front. Pharmacol. 2022, 13, 988226. [Google Scholar] [CrossRef]
- Scaria, B.; Sood, S.; Raad, C.; Khanafer, J.; Jayachandiran, R.; Pupulin, A.; Grewal, S.; Okoko, M.; Arora, M.; Miles, L.; et al. Natural Health Products (NHP’s) and Natural Compounds as Therapeutic Agents for the Treatment of Cancer; Mechanisms of Anti-Cancer Activity of Natural Compounds and Overall Trends. Int. J. Mol. Sci. 2020, 21, 8480. [Google Scholar] [CrossRef] [PubMed]
- Das Gupta, V. Quantitative Determination of Piperazine Citrate in Piperazine Citrate Syrup USP. Am. J. Hosp. Pharm. 1976, 33, 283–284. [Google Scholar]
- Hamza, A.A.; Heeba, G.H.; Hamza, S.; Abdalla, A.; Amin, A. Standardized Extract of Ginger Ameliorates Liver Cancer by Reducing Proliferation and Inducing Apoptosis through Inhibition Oxidative Stress/Inflammation Pathway. Biomed. Pharmacother. 2021, 134, 111102. [Google Scholar] [CrossRef]
- Li, J.; Wei, H.; Liu, Y.; Li, Q.; Guo, H.; Guo, Y.; Chang, Z. Curcumin Inhibits Hepatocellular Carcinoma via Regulating miR-21/TIMP3 Axis. Evid.-Based Complement. Altern. Med. 2020, 2020, 2892917. [Google Scholar] [CrossRef]
- Zheng, Y.; Jia, R.; Li, J.; Tian, X.; Qian, Y. Curcumin- and Resveratrol-Co-Loaded Nanoparticles in Synergistic Treatment of Hepatocellular Carcinoma. J. Nanobiotechnol. 2022, 20, 339. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Chen, L.; Wu, L.; Zhang, L.; Sun, B.; Wu, W.; Lei, Y.; Zhu, L.; Sun, T.; Liang, B.; Zhao, H.; et al. Effect of Metformin on Hepatocellular Carcinoma Patients with Type II Diabetes Receiving Transarterial Chemoembolization: A Multicenter Retrospective Cohort Study. Int. J. Surg. 2025, 111, 828–838. [Google Scholar] [CrossRef]
- Zheng, P.; Xu, D.; Cai, Y.; Zhu, L.; Xiao, Q.; Peng, W.; Chen, B. A Multi-Omic Analysis Reveals That Gamabufotalin Exerts Anti-Hepatocellular Carcinoma Effects by Regulating Amino Acid Metabolism through Targeting STAMBPL1. Phytomedicine 2024, 135, 156094. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, H.; Zheng, Z.; Liu, Z.; Hu, H.; Wu, W.; Wang, T. Advances in the Study of Bioactive Nanoparticles for the Treatment of HCC and Its Postoperative Residual Cancer. Int. J. Nanomed. 2023, 18, 2721–2735. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Jiang, Z. Platinum–Iron Nanoparticles for Oxygen-Enhanced Sonodynamic Tumor Cell Suppression. Inorganics 2024, 12, 331. [Google Scholar] [CrossRef]
- Mansour, W.; El Fedawy, S.F.; Atta, S.A.; Zarie, R.M.; Fouad, N.T.A.; Maher, S.; Hussein, T.M.; Abdel Aziz, D.M.; Kamel, M. Targeted Therapy for HCC Using Dumbbell-like Nanoparticles Conjugated to Monoclonal Antibodies against VEGF and Cancer Stem Cell Receptors in Mice. Cancer Nanotechnol. 2023, 14, 14. [Google Scholar] [CrossRef]
- Clemons, T.D.; Singh, R.; Sorolla, A.; Chaudhari, N.; Hubbard, A.; Iyer, K.S. Distinction Between Active and Passive Targeting of Nanoparticles Dictate Their Overall Therapeutic Efficacy. Langmuir 2018, 34, 15343–15349. [Google Scholar] [CrossRef]
- Khafaga, D.S.R.; Eid, M.M.; Mohamed, M.H.; Abdelmaksoud, M.D.E.; Afify, M.; El-Khawaga, A.M.; Abdelhakim, H.K. Enhanced Anticancer Activity of Silver Doped Zinc Oxide Magnetic Nanocarrier Loaded with Sorafenib for Hepatocellular Carcinoma Treatment. Sci. Rep. 2024, 14, 15538. [Google Scholar] [CrossRef]
- Zeng, M.; Guo, D.; Fernández-Varo, G.; Zhang, X.; Fu, S.; Ju, S.; Yang, H.; Liu, X.; Wang, Y.-C.; Zeng, Y.; et al. The Integration of Nanomedicine with Traditional Chinese Medicine: Drug Delivery of Natural Products and Other Opportunities. Mol. Pharm. 2023, 20, 886–904. [Google Scholar] [CrossRef]
- Basu, A.; Namporn, T.; Ruenraroengsak, P. Critical Review in Designing Plant-Based Anticancer Nanoparticles against Hepatocellular Carcinoma. Pharmaceutics 2023, 15, 1611. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Zhang, S.; Zhao, Y.; Gao, D.; Xing, J.; Cao, Y.; Xu, G. Purslane (Portulaca oleracea L.) Polysaccharide Attenuates Carbon Tetrachloride-Induced Acute Liver Injury by Modulating the Gut Microbiota in Mice. Genomics 2025, 117, 110983. [Google Scholar] [CrossRef]
- Tan, Q.; Chu, H.; Wei, J.; Yan, S.; Sun, X.; Wang, J.; Zhu, L.; Yang, F. Astaxanthin Alleviates Hepatic Lipid Metabolic Dysregulation Induced by Microcystin-LR. Toxins 2024, 16, 401. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Tang, R.; Yang, P.; Zhuo, Y.; Jiang, X.; Che, L.; Lin, Y.; Xu, S.; Li, J.; et al. Bile Acids Metabolism in the Gut-Liver Axis Mediates Liver Injury during Lactation. Life Sci. 2024, 338, 122380. [Google Scholar] [CrossRef]
- Campbell, C.; Wang, T.; McNaughton, A.L.; Barnes, E.; Matthews, P.C. Risk Factors for the Development of Hepatocellular Carcinoma (HCC) in Chronic Hepatitis B Virus (HBV) Infection: A Systematic Review and Meta-Analysis. J. Viral Hepat. 2021, 28, 493–507. [Google Scholar] [CrossRef]
- Gao, S.; Jiang, X.; Wang, L.; Jiang, S.; Luo, H.; Chen, Y.; Peng, C. The Pathogenesis of Liver Cancer and the Therapeutic Potential of Bioactive Substances. Front. Pharmacol. 2022, 13, 1029601. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yan, W.; Duan, H.; Wang, D.; Zhou, Y.; Feng, D.; Zheng, Y.; Zhou, S.; Liu, G.; Qin, X. Therapeutic Effects of Natural Products on Liver Cancer and Their Potential Mechanisms. Nutrients 2024, 16, 1642. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, X.; Nie, S.; Liu, J.; Wang, S. Disorders of Cancer Metabolism: The Therapeutic Potential of Cannabinoids. Biomed. Pharmacother. 2023, 157, 113993. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, S.; Wang, K.; Bao, X.; Liu, Y.; Zhou, S.; Liu, H.; Qiu, Y.; Wang, T.; Yu, H. Alkaloids from Traditional Chinese Medicine against Hepatocellular Carcinoma. Biomed. Pharmacother. 2019, 120, 109543. [Google Scholar] [CrossRef]
- Bailly, C. Molecular and Cellular Basis of the Anticancer Activity of the Prenylated Flavonoid Icaritin in Hepatocellular Carcinoma. Chem. Biol. Interact. 2020, 325, 109124. [Google Scholar] [CrossRef]
- Jiang, C.-H.; Sun, T.-L.; Xiang, D.-X.; Wei, S.-S.; Li, W.-Q. Anticancer Activity and Mechanism of Xanthohumol: A Prenylated Flavonoid From Hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Zhao, L.; Yang, L.; Lou, C. Recent Advances in Natural Polysaccharides against Hepatocellular Carcinoma: A Review. Int. J. Biol. Macromol. 2023, 253, 126766. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; He, J.; Song, X.; Tan, L.; Wang, M.; Jiang, P.; Li, Y.; Cao, Z.; Peng, C. Pharmacological Properties and Derivatives of Shikonin—A Review in Recent Years. Pharmacol. Res. 2019, 149, 104463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhu, Y.; Hu, J.; Jiang, L.; Li, L.; Jia, S.; Zen, K. Shikonin Inhibits Tumor Growth in Mice by Suppressing Pyruvate Kinase M2-Mediated Aerobic Glycolysis. Sci. Rep. 2018, 8, 14517. [Google Scholar] [CrossRef]
- Boulos, J.C.; Rahama, M.; Hegazy, M.-E.F.; Efferth, T. Shikonin Derivatives for Cancer Prevention and Therapy. Cancer Lett. 2019, 459, 248–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shang, L.; Jiang, W.; Wu, W. Shikonin Induces Apoptosis and Autophagy via Downregulation of Pyrroline-5-Carboxylate Reductase1 in Hepatocellular Carcinoma Cells. Bioengineered 2022, 13, 7904–7918. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Cheng, W.-C.; Wang, S.-C.; Fang, S.-H.; Tu, H.-P.; Su, C.-C.; Hung, Y.-L.; Liu, P.-L.; Chen, C.-S.; et al. RNA-Seq Transcriptome Analysis of Breast Cancer Cell Lines under Shikonin Treatment. Sci. Rep. 2018, 8, 2672. [Google Scholar] [CrossRef]
- Cho, Y.; Yoon, J.-H.; Yoo, J.; Lee, M.; Lee, D.H.; Cho, E.J.; Lee, J.-H.; Yu, S.J.; Kim, Y.J.; Kim, C.Y. Fucoidan Protects Hepatocytes from Apoptosis and Inhibits Invasion of Hepatocellular Carcinoma by Up-Regulating P42/44 MAPK-Dependent NDRG-1/CAP43. Acta Pharm. Sin. B 2015, 5, 544–553. [Google Scholar] [CrossRef]
- Ma, D.; Wei, J.; Chen, S.; Wang, H.; Ning, L.; Luo, S.-H.; Liu, C.-L.; Song, G.; Yao, Q. Fucoidan Inhibits the Progression of Hepatocellular Carcinoma via Causing lncRNA LINC00261 Overexpression. Front. Oncol. 2021, 11, 653902. [Google Scholar] [CrossRef]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The Anti-Cancer Effects of Fucoidan: A Review of Both In Vivo and In Vitro Investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef]
- Rashwan, H.K.; Mahgoub, S.; Abuelezz, N.Z.; Amin, H.K. Black Cumin Seed (Nigella sativa) in Inflammatory Disorders: Therapeutic Potential and Promising Molecular Mechanisms. Drugs Drug Candidates 2023, 2, 516–537. [Google Scholar] [CrossRef]
- Abd-Rabou, A.A.; Edris, A.E. Cytotoxic, Apoptotic, and Genetic Evaluations of Nigella sativa Essential Oil Nanoemulsion against Human Hepatocellular Carcinoma Cell Lines. Cancer Nanotechnol. 2021, 12, 28. [Google Scholar] [CrossRef]
- Sadeghi, E.; Imenshahidi, M.; Hosseinzadeh, H. Molecular Mechanisms and Signaling Pathways of Black Cumin (Nigella sativa) and Its Active Constituent, Thymoquinone: A Review. Mol. Biol. Rep. 2023, 50, 5439–5454. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Chen, Z.; Shin, D.M. Application of Nanotechnology in Cancer Therapy and Imaging. CA Cancer J. Clin. 2008, 58, 97–110. [Google Scholar] [CrossRef]
- Jehan, S.; Zhong, C.; Li, G.; Zulqarnain Bakhtiar, S.; Li, D.; Sui, G. Thymoquinone Selectively Induces Hepatocellular Carcinoma Cell Apoptosis in Synergism with Clinical Therapeutics and Dependence of P53 Status. Front. Pharmacol. 2020, 11, 555283. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Li, Z.; Wan, D.; Pan, J. Targeted Drug Delivery Strategies for the Treatment of Hepatocellular Carcinoma. Molecules 2024, 29, 4405. [Google Scholar] [CrossRef]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Nigella sativa: A Comprehensive Review of Its Therapeutic Potential, Pharmacological Properties, and Clinical Applications. Int. J. Mol. Sci. 2024, 25, 13410. [Google Scholar] [CrossRef]
- Elshnoudy, I.A.; Elkhouly, A.M.; Masoud, M.; Rabea, H.A.; Mansour, F.R. Medicinal Plants Cultivated in Egypt with Anticancer Potential; a Systematic Review. Phytochem. Rev. 2024, 24, 527–583. [Google Scholar] [CrossRef]
- Shao, J.; Shi, C.-J.; Li, Y.; Zhang, F.; Pan, F.; Fu, W.; Zhang, J. LincROR Mediates the Suppressive Effects of Curcumin on Hepatocellular Carcinoma Through Inactivating Wnt/β-Catenin Signaling. Front. Pharmacol. 2020, 11, 847. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Wang, X.; Wang, Z.; Xing, E.; Li, J.; Wang, D. Curcumin Inhibits Proliferation of Hepatocellular Carcinoma Cells by Blocking PTPN1 and PTPN11 Expression. Oncol. Lett. 2023, 26, 307. [Google Scholar] [CrossRef]
- Gull, N.; Arshad, F.; Naikoo, G.A.; Hassan, I.U.; Pedram, M.Z.; Ahmad, A.; Aljabali, A.A.A.; Mishra, V.; Satija, S.; Charbe, N.; et al. Recent Advances in Anticancer Activity of Novel Plant Extracts and Compounds from Curcuma Longa in Hepatocellular Carcinoma. J. Gastrointest. Canc 2023, 54, 368–390. [Google Scholar] [CrossRef]
- Oh, J.-W.; Muthu, M.; Pushparaj, S.S.C.; Gopal, J. Anticancer Therapeutic Effects of Green Tea Catechins (GTCs) When Integrated with Antioxidant Natural Components. Molecules 2023, 28, 2151. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A Review on Anti-Cancer Effect of Green Tea Catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Du, D.; Liu, C.; Qin, M.; Zhang, X.; Xi, T.; Yuan, S.; Hao, H.; Xiong, J. Metabolic Dysregulation and Emerging Therapeutical Targets for Hepatocellular Carcinoma. Acta Pharm. Sin. B 2022, 12, 558–580. [Google Scholar] [CrossRef]
- Iranzadeh, S.; Dalil, D.; Kohansal, S.; Isakhani, M. Shikonin in Breast Cancer Treatment: A Comprehensive Review of Molecular Pathways and Innovative Strategies. J. Pharm. Pharmacol. 2024, 76, 967–982. [Google Scholar] [CrossRef]
- Kiselevskiy, M.V.; Anisimova, N.Y.; Ustyuzhanina, N.E.; Vinnitskiy, D.Z.; Tokatly, A.I.; Reshetnikova, V.V.; Chikileva, I.O.; Shubina, I.Z.H.; Kirgizov, K.I.; Nifantiev, N.E. Perspectives for the Use of Fucoidans in Clinical Oncology. Int. J. Mol. Sci. 2022, 23, 11821. [Google Scholar] [CrossRef]
- Duan, Y.; Li, J.; Jing, X.; Ding, X.; Yu, Y.; Zhao, Q. Fucoidan Induces Apoptosis and Inhibits Proliferation of Hepatocellular Carcinoma via the P38 MAPK/ERK and PI3K/Akt Signal Pathways. Cancer Manag. Res. 2020, 12, 1713–1723. [Google Scholar] [CrossRef]
- Adam, S.H.; Mohd Nasri, N.; Kashim, M.I.A.M.; Abd Latib, E.H.; Ahmad Juhari, M.A.A.; Mokhtar, M.H. Potential Health Benefits of Nigella sativa on Diabetes Mellitus and Its Complications: A Review from Laboratory Studies to Clinical Trials. Front. Nutr. 2022, 9, 1057825. [Google Scholar] [CrossRef]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (Black Cumin): A Promising Natural Remedy for Wide Range of Illnesses. Evid.-Based Complement. Altern. Med. 2019, 2019, 1528635. [Google Scholar] [CrossRef]
- Berköz, M. Nigella sativa L. In Novel Drug Targets with Traditional Herbal Medicines: Scientific and Clinical Evidence; Gürağaç Dereli, F.T., Ilhan, M., Belwal, T., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 445–461. ISBN 978-3-031-07753-1. [Google Scholar]
- Wang, X.; Tian, Y.; Lin, H.; Cao, X.; Zhang, Z. Curcumin Induces Apoptosis in Human Hepatocellular Carcinoma Cells by Decreasing the Expression of STAT3/VEGF/HIF-1α Signaling. Open Life Sci. 2023, 18, 20220618. [Google Scholar] [CrossRef]
- Farhan, M. Green Tea Catechins: Nature’s Way of Preventing and Treating Cancer. Int. J. Mol. Sci. 2022, 23, 10713. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, S.V.; Kumar, R.; Kumar, S.; Senapati, S.; Pandey, A.K. Current Therapeutic Modalities and Chemopreventive Role of Natural Products in Liver Cancer: Progress and Promise. World J. Hepatol. 2023, 15, 1–18. [Google Scholar] [CrossRef]
- Andreani, T.; Cheng, R.; Elbadri, K.; Ferro, C.; Menezes, T.; Dos Santos, M.R.; Pereira, C.M.; Santos, H.A. Natural Compounds-Based Nanomedicines for Cancer Treatment: Future Directions and Challenges. Drug Deliv. Transl. Res. 2024, 14, 2845–2916. [Google Scholar] [CrossRef]
- Gupta, M.; Sarwat, M. Protective Effects of Plant-Derived Natural Products against Hepatocellular Carcinoma. In Herbal Medicines; Elsevier: Amsterdam, The Netherlands, 2022; pp. 609–627. ISBN 978-0-323-90572-5. [Google Scholar]
- Alnajjar, A.; Elsiesy, H. Natural Products and Hepatocellular Carcinoma: A Review. Hepatoma Res. 2015, 1, 119. [Google Scholar] [CrossRef][Green Version]
- Rodriguez, S.; Skeet, K.; Mehmetoglu-Gurbuz, T.; Goldfarb, M.; Karri, S.; Rocha, J.; Shahinian, M.; Yazadi, A.; Poudel, S.; Subramani, R. Phytochemicals as an Alternative or Integrative Option, in Conjunction with Conventional Treatments for Hepatocellular Carcinoma. Cancers 2021, 13, 5753. [Google Scholar] [CrossRef]
- Sayed, N.; Khurana, A.; Godugu, C. Pharmaceutical Perspective on the Translational Hurdles of Phytoconstituents and Strategies to Overcome. J. Drug Deliv. Sci. Technol. 2019, 53, 101201. [Google Scholar] [CrossRef]
- Yang, J.; Li, K.; He, D.; Gu, J.; Xu, J.; Xie, J.; Zhang, M.; Liu, Y.; Tan, Q.; Zhang, J. Toward a Better Understanding of Metabolic and Pharmacokinetic Characteristics of Low-Solubility, Low-Permeability Natural Medicines. Drug Metab. Rev. 2020, 52, 19–43. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, W.; Zhang, H.; Guo, Q.; Yang, W.; Li, B.; Sun, Z.; Gao, S.; Cui, R. Modulation of Multiple Signaling Pathways of the Plant-Derived Natural Products in Cancer. Front. Oncol. 2019, 9, 1153. [Google Scholar] [CrossRef]
- SR Khafaga, D.; Ewies, E. Drug Delivery Systems Designed to Maximize the Therapeutic Efficacy of Herbal Medication: A Review Article. Egypt. J. Chem. 2023, 66, 477–485. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, Y.; Chen, H.; Meng, X.; Ma, W.; Yu, M.; Li, Z.; Li, C.; Liu, H.; Zhang, X.; et al. Illuminating Platinum Transportation While Maximizing Therapeutic Efficacy by Gold Nanoclusters via Simultaneous Near-Infrared-I/II Imaging and Glutathione Scavenging. ACS Nano 2020, 14, 13536–13547. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Chen, Q.; Xu, W.; Yu, M.; Yang, Y.; Zou, B.; Zhang, Y.S.; Ding, J.; Yu, Z. Self-Targeting Visualizable Hyaluronate Nanogel for Synchronized Intracellular Release of Doxorubicin and Cisplatin in Combating Multidrug-Resistant Breast Cancer. Nano Res. 2021, 14, 846–857. [Google Scholar] [CrossRef]
- Jin, D.; Zhu, Y.; Liu, M.; Yu, W.; Yu, J.; Zheng, X.; Wang, L.; Wu, Y.; Wei, K.; Cheng, J.; et al. A Leaking-Proof Theranostic Nanoplatform for Tumor-Targeted and Dual-Modality Imaging-Guided Photodynamic Therapy. BME Front. 2023, 4, 0015. [Google Scholar] [CrossRef]
- Yang, J.D.; Heimbach, J.K. New Advances in the Diagnosis and Management of Hepatocellular Carcinoma. BMJ 2020, 371, m3544. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, M.; Liang, L.; Xing, H.; Zhang, C.; Shen, F.; Huang, D.; Yang, T. Nanotechnology for Hepatocellular Carcinoma: From Surveillance, Diagnosis to Management. Small 2021, 17, 2005236. [Google Scholar] [CrossRef]
- Xu, M.; Yang, L.; Lin, Y.; Lu, Y.; Bi, X.; Jiang, T.; Deng, W.; Zhang, L.; Yi, W.; Xie, Y.; et al. Emerging Nanobiotechnology for Precise Theranostics of Hepatocellular Carcinoma. J. Nanobiotechnol. 2022, 20, 427. [Google Scholar] [CrossRef]
- Leung, T.W.T.; Yu, S.; Johnson, P.J.; Geschwind, J.; Vogl, T.J.; Engelmann, K.; Gores, G.J.; Giovannini, M.; O’Grady, J.; Heneghan, M.; et al. Phase II Study of the Efficacy and Safety of Cisplatin-Epinephrine Injectable Gel Administered to Patients With Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2003, 21, 652–658. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, X.; Zeng, L.; Liu, J.; Zhang, Z. A Randomized Multicenter Phase II Clinical Trial of Mitoxantrone-Loaded Nanoparticles in the Treatment of 108 Patients with Unresected Hepatocellular Carcinoma. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 419–423. [Google Scholar] [CrossRef]
- Chi, X.; Liu, K.; Luo, X.; Yin, Z.; Lin, H.; Gao, J. Recent Advances of Nanomedicines for Liver Cancer Therapy. J. Mater. Chem. B 2020, 8, 3747–3771. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Wang, L.; Ye, W.; Guo, S.; Bao, X.; Wang, Y.; Xia, Z.; Chen, R.; Liu, C.; Lin, X.; et al. CCAT2 Contributes to Hepatocellular Carcinoma Progression via Inhibiting miR-145 Maturation to Induce MDM2 Expression. J. Cell. Physiol. 2020, 235, 6307–6320. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, M.; Rahimi, F.; Rezayan, A.H.; Shahbazi, M.-A.; Witzigmann, D.; Huwyler, J. Combined Cerium Oxide Nanocapping and Layer-by-Layer Coating of Porous Silicon Containers for Controlled Drug Release. J. Mater. Sci. 2018, 53, 14975–14988. [Google Scholar] [CrossRef]
- He, M.; Yu, L.; Yang, Y.; Zou, B.; Ma, W.; Yu, M.; Lu, J.; Xiong, G.; Yu, Z.; Li, A. Delivery of Triptolide with Reduction-Sensitive Polymer Nanoparticles for Liver Cancer Therapy on Patient-Derived Xenografts Models. Chin. Chem. Lett. 2020, 31, 3178–3182. [Google Scholar] [CrossRef]
- Zhu, J.; Chu, C.; Li, D.; Zhang, Y.; Cheng, Y.; Lin, H.; Wang, X.; Liu, J.; Pang, X.; Cheng, J.; et al. Superior Fluorescent Nanoemulsion Illuminates Hepatocellular Carcinoma for Surgical Navigation. Front. Bioeng. Biotechnol. 2022, 10, 890668. [Google Scholar] [CrossRef]
- Lai, X.; Zhao, Y.; Shi, Z.; Xing, L.; Li, X.; Jia, L.; Lin, K. Plant-Derived Paclitaxel-Loaded Ultra-Small Fe3O4 Nanoparticles for MR Imaging-Mediated Antitumor Therapy. Ind. Crops Prod. 2025, 228, 120902. [Google Scholar] [CrossRef]
- Herranz-Blanco, B.; Shahbazi, M.; Correia, A.R.; Balasubramanian, V.; Kohout, T.; Hirvonen, J.; Santos, H.A. pH-Switch Nanoprecipitation of Polymeric Nanoparticles for Multimodal Cancer Targeting and Intracellular Triggered Delivery of Doxorubicin. Adv. Healthc. Mater. 2016, 5, 1904–1916. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Almeida, P.V.; Correia, A.; Herranz-Blanco, B.; Shrestha, N.; Mäkilä, E.; Salonen, J.; Hirvonen, J.; Santos, H.A. Intracellular Responsive Dual Delivery by Endosomolytic Polyplexes Carrying DNA Anchored Porous Silicon Nanoparticles. J. Control. Release 2017, 249, 111–122. [Google Scholar] [CrossRef]
- Sedighi, M.; Sieber, S.; Rahimi, F.; Shahbazi, M.-A.; Rezayan, A.H.; Huwyler, J.; Witzigmann, D. Rapid Optimization of Liposome Characteristics Using a Combined Microfluidics and Design-of-Experiment Approach. Drug Deliv. Transl. Res. 2019, 9, 404–413. [Google Scholar] [CrossRef]
- Zhang, D.; Song, J.; Jing, Z.; Qin, H.; Wu, Y.; Zhou, J.; Zang, X. Stimulus Responsive Nanocarrier for Enhanced Antitumor Responses Against Hepatocellular Carcinoma. Int. J. Nanomed. 2024, 19, 13339–13355. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Shrestha, N.; Mäkilä, E.; Araújo, F.; Correia, A.; Ramos, T.; Sarmento, B.; Salonen, J.; Hirvonen, J.; Santos, H.A. A Prospective Cancer Chemo-Immunotherapy Approach Mediated by Synergistic CD326 Targeted Porous Silicon Nanovectors. Nano Res. 2015, 8, 1505–1521. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Sedighi, M.; Bauleth-Ramos, T.; Kant, K.; Correia, A.; Poursina, N.; Sarmento, B.; Hirvonen, J.; Santos, H.A. Targeted Reinforcement of Macrophage Reprogramming Toward M2 Polarization by IL-4-Loaded Hyaluronic Acid Particles. ACS Omega 2018, 3, 18444–18455. [Google Scholar] [CrossRef]
- Figueiredo, P.; Balasubramanian, V.; Shahbazi, M.-A.; Correia, A.; Wu, D.; Palivan, C.G.; Hirvonen, J.T.; Santos, H.A. Angiopep2-Functionalized Polymersomes for Targeted Doxorubicin Delivery to Glioblastoma Cells. Int. J. Pharm. 2016, 511, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Baboci, L.; Capolla, S.; Di Cintio, F.; Colombo, F.; Mauro, P.; Dal Bo, M.; Argenziano, M.; Cavalli, R.; Toffoli, G.; Macor, P. The Dual Role of the Liver in Nanomedicine as an Actor in the Elimination of Nanostructures or a Therapeutic Target. J. Oncol. 2020, 2020, 4638192. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, Y.; Zhang, H. CCND1 Silencing Suppresses Liver Cancer Stem Cell Differentiation and Overcomes 5-Fluorouracil Resistance in Hepatocellular Carcinoma. J. Pharmacol. Sci. 2020, 143, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kinoh, H.; Shibasaki, H.; Liu, X.; Yamasoba, T.; Cabral, H.; Kataoka, K. Nanomedicines Blocking Adaptive Signals in Cancer Cells Overcome Tumor TKI Resistance. J. Control. Release 2020, 321, 132–144. [Google Scholar] [CrossRef]
- Sedighi, M.; Rahimi, F.; Shahbazi, M.-A.; Rezayan, A.H.; Kettiger, H.; Einfalt, T.; Huwyler, J.; Witzigmann, D. Controlled Tyrosine Kinase Inhibitor Delivery to Liver Cancer Cells by Gate-Capped Mesoporous Silica Nanoparticles. ACS Appl. Bio Mater. 2020, 3, 239–251. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of Nanomaterials Using Various Top-down and Bottom-Up Approaches, Influencing Factors, Advantages, and Disadvantages: A Review. Adv. Colloid. Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Anu Mary Ealia, S.; Saravanakumar, M.P. A Review on the Classification, Characterisation, Synthesis of Nanoparticles and Their Application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Kasi Viswanath, I.V.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y.L.N. Review on Nanomaterials: Synthesis and Applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail Review on Chemical, Physical and Green Synthesis, Classification, Characterizations and Applications of Nanoparticles. Green. Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Alhamad, A.A.; Zeghoud, S.; Ben Amor, I.; Zaater, A.; Ben Amor, A.; Aouadif, A.; Hemmami, A.; Chile Nleonu, E. A Short Review of Nanomaterials: Synthesis Methods, Properties, and Applications. Alger. J. Chem. Eng. 2023, 1, 01–07. [Google Scholar] [CrossRef]
- Rashwan, D.; Nagy, R.; El-deen, M.A.; Elhakim, H.; Mohamed, M.; Afify, M.; Abd El Hamed, M.; Mohamed, A.E.R. Green Synthesis of Zinc Oxide Nanocomposite Using Fusarium oxysporum and Evaluation of the Anticancer Effect on Hepatocellular Carcinoma. Egypt. J. Chem. 2022, 65, 197–207. [Google Scholar] [CrossRef]
- Roy, S.; Ghosh, C.K.; Sarkar, C.K. (Eds.) Nanotechnology: Synthesis to Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-11673-0. [Google Scholar]
- Yashveer, S.; Singh, V.; Kaswan, V.; Kaushik, A.; Tokas, J. Green Biotechnology, Nanotechnology and Bio-Fortification: Perspectives on Novel Environment-Friendly Crop Improvement Strategies. Biotechnol. Genet. Eng. Rev. 2014, 30, 113–126. [Google Scholar] [CrossRef]
- Musa, I.O.; Isibor, P.O.; Samuel, J.O.; Mustapha, A.; Mustapha, A.; Akande, S.; Oyewole, O.A.; Kayode-Edwards, I.I.; Adeniji, H. Introduction to Nanotoxicology. In Environmental Nanotoxicology; Isibor, P.O., Devi, G., Enuneku, A.A., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 1–22. ISBN 978-3-031-54153-7. [Google Scholar]
- Parthiban, E.; Manivannan, N.; Ramanibai, R.; Mathivanan, N. Green Synthesis of Silver-Nanoparticles from Annona Reticulata Leaves Aqueous Extract and Its Mosquito Larvicidal and Anti-Microbial Activity on Human Pathogens. Biotechnol. Rep. 2019, 21, e00297. [Google Scholar] [CrossRef]
- Yao, S.; Jin, B.; Liu, Z.; Shao, C.; Zhao, R.; Wang, X.; Tang, R. Biomineralization: From Material Tactics to Biological Strategy. Adv. Mater. 2017, 29, 1605903. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, Y.; Deveaux, J.G.; Masoud, M.A.; Chandra, F.S.; Chen, H.; Zhang, D.; Feng, L. Biomineralization Forming Process and Bio-Inspired Nanomaterials for Biomedical Application: A Review. Minerals 2019, 9, 68. [Google Scholar] [CrossRef]
- Faivre, D.; Schüler, D. Magnetotactic Bacteria and Magnetosomes. Chem. Rev. 2008, 108, 4875–4898. [Google Scholar] [CrossRef]
- Kotakadi, S.M.; Borelli, D.P.R.; Nannepaga, J.S. Therapeutic Applications of Magnetotactic Bacteria and Magnetosomes: A Review Emphasizing on the Cancer Treatment. Front. Bioeng. Biotechnol. 2022, 10, 789016. [Google Scholar] [CrossRef]
- Pucci, C.; Degl’Innocenti, A.; Belenli Gümüş, M.; Ciofani, G. Superparamagnetic Iron Oxide Nanoparticles for Magnetic Hyperthermia: Recent Advancements, Molecular Effects, and Future Directions in the Omics Era. Biomater. Sci. 2022, 10, 2103–2121. [Google Scholar] [CrossRef]
- He, S.; Guo, Z.; Zhang, Y.; Zhang, S.; Wang, J.; Gu, N. Biosynthesis of Gold Nanoparticles Using the Bacteria Rhodopseudomonas capsulata. Mater. Lett. 2007, 61, 3984–3987. [Google Scholar] [CrossRef]
- Schlüter, M.; Hentzel, T.; Suarez, C.; Koch, M.; Lorenz, W.G.; Böhm, L.; Düring, R.-A.; Koinig, K.A.; Bunge, M. Synthesis of Novel Palladium(0) Nanocatalysts by Microorganisms from Heavy-Metal-Influenced High-Alpine Sites for Dehalogenation of Polychlorinated Dioxins. Chemosphere 2014, 117, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, G.; Arockiamary, J.S.; Kumar, V.G.; Govindaraju, K. A Novel Extracellular Synthesis of Monodisperse Gold Nanoparticles Using Marine Alga, Sargassum wightii Greville. Colloids Surf. B Biointerfaces 2007, 57, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, J.; Zhong, C.; Zhang, X.; Bao, Y. Green Synthesis of Gold Nanoparticles from Dendrobium officinale and Its Anticancer Effect on Liver Cancer. Drug Deliv. 2021, 28, 985–994. [Google Scholar] [CrossRef]
- Ji, Y.; Cao, Y.; Song, Y. Green Synthesis of Gold Nanoparticles Using a Cordyceps militaris Extract and Their Antiproliferative Effect in Liver Cancer Cells (HepG2). Artif. Cells Nanomed. Biotechnol. 2019, 47, 2737–2745. [Google Scholar] [CrossRef]
- Usmani, A.; Mishra, A.; Jafri, A.; Arshad, M.; Siddiqui, M.A. Green Synthesis of Silver Nanocomposites of Nigella sativa Seeds Extract for Hepatocellular Carcinoma. Curr. Nanomater. 2019, 4, 191–200. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Z.; Jiang, O.; Li, Y.; Xu, Q.; Jiang, L.; Yu, J.; Xu, D. Green Synthesis of CuO NPs, Characterization and Their Toxicity Potential against HepG2 Cells. Mater. Res. Express 2021, 8, 015011. [Google Scholar] [CrossRef]
- Rawat, M. Green Synthesis of Silver Nanoparticles via Various Plant Extracts for Anti-Cancer Applications. Glob. J. Nanomed. 2017, 2, 555590. [Google Scholar] [CrossRef]
- Abed, A.S.; Khalaf, Y.H.; Mohammed, A.M. Green Synthesis of Gold Nanoparticles as an Effective Opportunity for Cancer Treatment. Results Chem. 2023, 5, 100848. [Google Scholar] [CrossRef]
- Zhang, H.; Li, T.; Luo, W.; Peng, G.X.; Xiong, J. Green Synthesis of Ag Nanoparticles from Leucus Aspera and Its Application in Anticancer Activity Against Alveolar Cancer. J. Exp. Nanosci. 2022, 17, 47–60. [Google Scholar] [CrossRef]
- Mansur, H.S. Quantum Dots and Nanocomposites. WIREs Nanomed. Nanobiotechnol 2010, 2, 113–129. [Google Scholar] [CrossRef]
- Rempel, A.A.; Ovchinnikov, O.V.; Weinstein, I.A.; Rempel, S.V.; Kuznetsova, Y.V.; Naumov, A.V.; Smirnov, M.S.; Eremchev, I.U.; Vokhmintsev, A.S.; Savchenko, S.S. Quantum Dots: Modern Methods of Synthesis and Optical Properties. Russ. Chem. Rev. 2024, 93, RCR5114. [Google Scholar] [CrossRef]
- Agarwal, K.; Rai, H.; Mondal, S. Quantum Dots: An Overview of Synthesis, Properties, and Applications. Mater. Res. Express 2023, 10, 062001. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon Quantum Dots: Synthesis, Properties and Applications. J. Mater. Chem. C 2014, 2, 6921. [Google Scholar] [CrossRef]
- Abtahi, M.S.; Fotouhi, A.; Rezaei, N.; Akalin, H.; Ozkul, Y.; Hossein-Khannazer, N.; Vosough, M. Nano-Based Drug Delivery Systems in Hepatocellular Carcinoma. J. Drug Target. 2024, 32, 977–995. [Google Scholar] [CrossRef]
- Xing, L.; Chen, Y.; Zheng, T. Research Progress of Nanoparticles in Diagnosis and Treatment of Hepatocellular Carcinoma. Open Life Sci. 2024, 19, 20220932. [Google Scholar] [CrossRef]
- Li, K.; Xia, C.; Wang, B.; Chen, H.; Wang, T.; He, Q.; Cao, H.; Wang, Y. Effects of Quantum Dots on the ROS Amount of Liver Cancer Stem Cells. Colloids Surf. B Biointerfaces 2017, 155, 193–199. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, D.; Ma, Y.; Cao, Y.; Pang, Y.; Tang, M.; Pu, Y.; Zhang, T. Intracellular Reactive Oxygen Species Trigger Mitochondrial Dysfunction and Apoptosis in Cadmium Telluride Quantum Dots-Induced Liver Damage. NanoImpact 2022, 25, 100392. [Google Scholar] [CrossRef]
- Rahman, M.M.; Opo, F.A.D.M.; Asiri, A.M. Cytotoxicity Study of Cadmium-Selenium Quantum Dots (Cdse QDs) for Destroying the Human HepG2 Liver Cancer Cell. J. Biomed. Nanotechnol. 2021, 17, 2153–2164. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Tian, J.; Wang, G.; Luo, W.; Huang, Z.; Huang, Y.; Li, N.; Guo, M.; Fan, X. Tryptophan-Sorbitol Based Carbon Quantum Dots for Theranostics against Hepatocellular Carcinoma. J. Nanobiotechnol. 2022, 20, 78. [Google Scholar] [CrossRef]
- Luo, G.; Long, J.; Zhang, B.; Liu, C.; Ji, S.; Xu, J.; Yu, X.; Ni, Q. Quantum Dots in Cancer Therapy. Expert Opin. Drug Deliv. 2012, 9, 47–58. [Google Scholar] [CrossRef]
- Mazahir, F.; Sharma, R.; Yadav, A.K. Bioinspired Theranostic Quantum Dots: Paving the Road to a New Paradigm for Cancer Diagnosis and Therapeutics. Drug Discov. Today 2023, 28, 103822. [Google Scholar] [CrossRef]
- Jovanović, S.; Marković, Z.; Budimir, M.; Prekodravac, J.; Zmejkoski, D.; Kepić, D.; Bonasera, A.; Marković, B.T. Lights and Dots toward Therapy—Carbon-Based Quantum Dots as New Agents for Photodynamic Therapy. Pharmaceutics 2023, 15, 1170. [Google Scholar] [CrossRef]
- Reshma, V.G.; Mohanan, P.V. Assessment of Immunotoxicity and Oxidative Stress Induced by Zinc Selenium/Zinc Sulphide Quantum Dots. Front. Nanotechnol. 2021, 2, 597382. [Google Scholar] [CrossRef]
- Chan, W.-H.; Shiao, N.-H.; Lu, P.-Z. CdSe Quantum Dots Induce Apoptosis in Human Neuroblastoma Cells via Mitochondrial-Dependent Pathways and Inhibition of Survival Signals. Toxicol. Lett. 2006, 167, 191–200. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome Composition in Drug Delivery Design, Synthesis, Characterization, and Clinical Application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Liu, Y.; Castro Bravo, K.M.; Liu, J. Targeted Liposomal Drug Delivery: A Nanoscience and Biophysical Perspective. Nanoscale Horiz. 2021, 6, 78–94. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, P.; Wu, S.; Yang, T.; Chen, Y.; Zhang, X.; He, C.; Zheng, C.; Li, K.; Ma, X.; et al. Cisplatin and Curcumin Co-Loaded Nano-Liposomes for the Treatment of Hepatocellular Carcinoma. Int. J. Pharm. 2018, 545, 261–273. [Google Scholar] [CrossRef]
- Xu, F.; Liao, J.; Xiang, G.; Zhao, P.; Ye, F.; Zhao, Q.; He, X. MiR-101 and Doxorubicin Codelivered by Liposomes Suppressing Malignant Properties of Hepatocellular Carcinoma. Cancer Med. 2017, 6, 651–661. [Google Scholar] [CrossRef]
- Wei, X.; Yang, D.; Xing, Z.; Cai, J.; Wang, L.; Zhao, C.; Wei, X.; Jiang, M.; Sun, H.; Zhou, L.; et al. Hepatocyte-Targeted Delivery Using Oleanolic Acid-Loaded Liposomes for Enhanced Hepatocellular Carcinoma Therapy. Biomater. Sci. 2023, 11, 3952–3964. [Google Scholar] [CrossRef]
- Samaei, S.S.; Daryab, M.; Gholami, S.; Rezaee, A.; Fatehi, N.; Roshannia, R.; Hashemi, S.; Javani, N.; Rahmanian, P.; Amani-Beni, R.; et al. Multifunctional and Stimuli-Responsive Liposomes in Hepatocellular Carcinoma Diagnosis and Therapy. Transl. Oncol. 2024, 45, 101975. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Xu, Z.; Wang, Z. Advances in Nanoliposomes for the Diagnosis and Treatment of Liver Cancer. Int. J. Nanomed. 2022, 17, 909–925. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, R.; Yao, W.; Pang, X.; Wang, N.; Lai, S.; Wei, X.; Yuan, Y.; Jiang, X.; Yang, R. iRGD-Mediated Liposomal Nanoplatforms for Improving Hepatocellular Carcinoma Targeted Combination Immunotherapy and Monitoring Tumor Response via IVIM-MRI. J. Mater. Chem. B 2024, 12, 9963–9978. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Delfi, M.; Zarrabi, A.; Bigham, A.; Sharifi, E.; Rabiee, N.; Paiva-Santos, A.C.; Kumar, A.P.; Tan, S.C.; Hushmandi, K.; et al. Stimuli-Responsive Liposomal Nanoformulations in Cancer Therapy: Pre-Clinical & Clinical Approaches. J. Control. Release 2022, 351, 50–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, X.; Xiang, D. Nanoparticle Drug Delivery Systems: An Excellent Carrier for Tumor Peptide Vaccines. Drug Deliv. 2018, 25, 1319–1327. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef]
- Rahman, M.; Almalki, W.H.; Alrobaian, M.; Iqbal, J.; Alghamdi, S.; Alharbi, K.S.; Alruwaili, N.K.; Hafeez, A.; Shaharyar, A.; Singh, T.; et al. Nanocarriers-Loaded with Natural Actives as Newer Therapeutic Interventions for Treatment of Hepatocellular Carcinoma. Expert Opin. Drug Deliv. 2021, 18, 489–513. [Google Scholar] [CrossRef] [PubMed]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of Enhanced Permeability and Retention Effect (EPR): Nanoparticle-Based Precision Tools for Targeting of Therapeutic and Diagnostic Agent in Cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Chen, M.; Quan, G.; Sun, Y.; Yang, D.; Pan, X.; Wu, C. Nanoparticles-Encapsulated Polymeric Microneedles for Transdermal Drug Delivery. J. Control. Release 2020, 325, 163–175. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, L.; Liao, H.; Ren, H.; Niu, H.; Li, Z.; Zhang, X.; Huang, X.; Liu, Y.; Zhou, Z.; et al. Nanoenzyme-Based Sensors for the Detection of Anti-Tumor Drugs. Microchim. Acta 2025, 192, 103. [Google Scholar] [CrossRef]
- Holyavka, M.G.; Goncharova, S.S.; Redko, Y.A.; Lavlinskaya, M.S.; Sorokin, A.V.; Artyukhov, V.G. Novel Biocatalysts Based on Enzymes in Complexes with Nano- and Micromaterials. Biophys. Rev. 2023, 15, 1127–1158. [Google Scholar] [CrossRef]
- Wen, Y.; Jing, N.; Zhang, M.; Huo, F.; Li, Z.; Yin, C. A Space-Dependent ‘Enzyme-Substrate’ Type Probe Based on ‘Carboxylesterase-Amide Group’ for Ultrafast Fluorescent Imaging Orthotopic Hepatocellular Carcinoma. Adv. Sci. 2023, 10, 2206681. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, N.; Zhang, C.; Li, M.; He, X.; Yin, C.; Tu, Q.; Shen, X.; Zhang, L.; Lv, J.; et al. Fructose-1,6-Bisphosphate Aldolase B Depletion Promotes Hepatocellular Carcinogenesis Through Activating Insulin Receptor Signaling and Lipogenesis. Hepatology 2021, 74, 3037–3055. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Altea-Manzano, P.; Demicco, M.; Doglioni, G.; Bornes, L.; Fukano, M.; Vandekeere, A.; Cuadros, A.M.; Fernández-García, J.; Riera-Domingo, C.; et al. PHGDH Heterogeneity Potentiates Cancer Cell Dissemination and Metastasis. Nature 2022, 605, 747–753. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Han, A.; Tang, C.; Xu, R.; Feng, L.; Yang, Y.; Chen, L.; Lin, Z. The NQO1/P53/SREBP1 Axis Promotes Hepatocellular Carcinoma Progression and Metastasis by Regulating Snail Stability. Oncogene 2022, 41, 5107–5120. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Tang, X.; Deng, H.; Yang, X.; Tao, J.; Xu, F.; Liu, Y.; Wu, K.; Wang, K.; Mei, Z.; et al. Metabolic Enzyme SLC27A5 Regulates PIP4K2A pre-mRNA Splicing as a Noncanonical Mechanism to Suppress Hepatocellular Carcinoma Metastasis. Adv. Sci. 2024, 11, 2305374. [Google Scholar] [CrossRef]
- Shi, X.; Liu, J.; Wang, G. A Peroxidase-like Magneto-Gold Nanozyme AuNC@Fe3O4 with Photothermal Effect for Induced Cell Apoptosis of Hepatocellular Carcinoma Cells In Vitro. Front. Bioeng. Biotechnol. 2023, 11, 1168750. [Google Scholar] [CrossRef]
- Jiang, B.; Yan, L.; Zhang, J.; Zhou, M.; Shi, G.; Tian, X.; Fan, K.; Hao, C.; Yan, X. Biomineralization Synthesis of the Cobalt Nanozyme in SP94-Ferritin Nanocages for Prognostic Diagnosis of Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2019, 11, 9747–9755. [Google Scholar] [CrossRef]
- Luo, B.; Zhou, J.; Zhan, X.; Ying, B.; Lan, F.; Wu, Y. Visual and Colorimetric Detection of microRNA in Clinical Samples Based on Strand Displacement Amplification and Nanozyme-Mediated CRISPR-Cas12a System. Talanta 2024, 277, 126310. [Google Scholar] [CrossRef]
- Wen, L.; Liang, C.; Chen, E.; Chen, W.; Liang, F.; Zhi, X.; Wei, T.; Xue, F.; Li, G.; Yang, Q.; et al. Regulation of Multi-Drug Resistance in Hepatocellular Carcinoma Cells Is TRPC6/Calcium Dependent. Sci. Rep. 2016, 6, 23269. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Huang, C.; Bai, J.; Zhang, Y.L.; Wang, X.; Yang, J.; Li, J. Multidrug Resistance in Hepatocellular Carcinoma. In Hepatocellular Carcinoma; Tirnitz-Parker, J.E.E., Ed.; Codon Publications: Singapore, 2019; pp. 141–158. ISBN 978-0-9944381-8-8. [Google Scholar]
- Majidinia, M.; Mirza-Aghazadeh-Attari, M.; Rahimi, M.; Mihanfar, A.; Karimian, A.; Safa, A.; Yousefi, B. Overcoming Multidrug Resistance in Cancer: Recent Progress in Nanotechnology and New Horizons. IUBMB Life 2020, 72, 855–871. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Sharma, P.; Ansari, M.S.; Gorain, B. Nanocarrier-Based Co-Delivery Approaches of Chemotherapeutics with Natural P-Glycoprotein Inhibitors in the Improvement of Multidrug Resistance Cancer Therapy. J. Drug Target. 2022, 30, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Yu, M.; Xu, J.; Li, B.-Y.; Zhao, Y.; Kankala, R.K. Overcoming Cancer Multi-Drug Resistance (MDR): Reasons, Mechanisms, Nanotherapeutic Solutions, and Challenges. Biomed. Pharmacother. 2023, 162, 114643. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, Y.; Kim, J.; Cho, H.; Kim, K. Overcoming Cancer Drug Resistance with Nanoparticle Strategies for Key Protein Inhibition. Molecules 2024, 29, 3994. [Google Scholar] [CrossRef] [PubMed]
- Lepeltier, E.; Rijo, P.; Rizzolio, F.; Popovtzer, R.; Petrikaite, V.; Assaraf, Y.G.; Passirani, C. Nanomedicine to Target Multidrug Resistant Tumors. Drug Resist. Updates 2020, 52, 100704. [Google Scholar] [CrossRef]
- Stan, D.; Enciu, A.-M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural Compounds With Antimicrobial and Antiviral Effect and Nanocarriers Used for Their Transportation. Front. Pharmacol. 2021, 12, 723233. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, J.; Guo, M.; Zhou, H.; Jin, J.; Liu, J.; Liu, Y.; Zhang, Z.; Chen, C. Synergistic Combination Chemotherapy Using Carrier-Free Celastrol and Doxorubicin Nanocrystals for Overcoming Drug Resistance. Nanoscale 2018, 10, 12639–12649. [Google Scholar] [CrossRef]
- Colone, M.; Calcabrini, A.; Stringaro, A. Drug Delivery Systems of Natural Products in Oncology. Molecules 2020, 25, 4560. [Google Scholar] [CrossRef]
- Kong, F.-H.; Ye, Q.-F.; Miao, X.-Y.; Liu, X.; Huang, S.-Q.; Xiong, L.; Wen, Y.; Zhang, Z.-J. Current Status of Sorafenib Nanoparticle Delivery Systems in the Treatment of Hepatocellular Carcinoma. Theranostics 2021, 11, 5464–5490. [Google Scholar] [CrossRef]
- Khafaga, D.S.R.; El-Khawaga, A.M.; Elfattah Mohammed, R.A.; Abdelhakim, H.K. Green Synthesis of Nano-Based Drug Delivery Systems Developed for Hepatocellular Carcinoma Treatment: A Review. Mol. Biol. Rep. 2023, 50, 10351–10364. [Google Scholar] [CrossRef] [PubMed]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.J.S.; Shim, Y.Y.; Reaney, M.J.T.; Cho, J.Y. Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef]
- Gao, Q.; Chen, N.; Li, B.; Zu, M.; Ma, Y.; Xu, H.; Zhu, Z.; Reis, R.L.; Kundu, S.C.; Xiao, B. Natural Lipid Nanoparticles Extracted from Morus nigra L. Leaves for Targeted Treatment of Hepatocellular Carcinoma via the Oral Route. J. Nanobiotechnol. 2024, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, X.; Hu, J.; Qiu, Z.; Yuan, M.; Zheng, G. Celastrol-Loaded Galactosylated Liposomes Effectively Inhibit AKT/c-Met-Triggered Rapid Hepatocarcinogenesis in Mice. Mol. Pharm. 2020, 17, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Elwan, A.G.; Mohamed, T.M.; Beltagy, D.M.; El Gamal, D.M. The Therapeutic Role of Naringenin Nanoparticles on Hepatocellular Carcinoma. BMC Pharmacol. Toxicol. 2025, 26, 3. [Google Scholar] [CrossRef]
- Khaled, A.M.; Othman, M.S.; Obeidat, S.T.; Aleid, G.M.; Aboelnaga, S.M.; Fehaid, A.; Hathout, H.M.R.; Bakkar, A.A.; Moneim, A.E.A.; El-Garawani, I.M.; et al. Green-Synthesized Silver and Selenium Nanoparticles Using Berberine: A Comparative Assessment of In Vitro Anticancer Potential on Human Hepatocellular Carcinoma Cell Line (HepG2). Cells 2024, 13, 287. [Google Scholar] [CrossRef]
- Roquito, T.; Colaço, M.; Costa, J.P.; Borges, O. Curcumin-Encapsulated Glucan Nanoparticles as an Oxidative Stress Modulator against Human Hepatic Cancer Cells. Colloids Surf. B Biointerfaces 2025, 245, 114326. [Google Scholar] [CrossRef]
- Asl, A.M.; Kalaee, M.; Abdouss, M.; Homami, S.S. Novel Targeted Delivery of Quercetin for Human Hepatocellular Carcinoma Using Starch/Polyvinyl Alcohol Nanocarriers Based Hydrogel Containing Fe2O3 Nanoparticles. Int. J. Biol. Macromol. 2024, 257, 128626. [Google Scholar] [CrossRef]
- Leng, M.; Jiang, H.; Zhang, S.; Bao, Y. Green Synthesis of Gold Nanoparticles from Polygahatous Polysaccharides and Their Anticancer Effect on Hepatic Carcinoma through Immunoregulation. ACS Omega 2024, 9, 21144–21151. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q. Green Synthesis of AuNPs and Their Effect on Cell Apoptosis of HepG2 Cells for Applications in Treatment of Hepatocellular Carcinoma in Nursing Care. Plasmonics 2024, 19, 1553–1563. [Google Scholar] [CrossRef]
- Fakhar-E-Alam, M.; Shafiq, Z.; Mahmood, A.; Atif, M.; Anwar, H.; Hanif, A.; Yaqub, N.; Farooq, W.A.; Fatehmulla, A.; Ahmad, S.; et al. Assessment of Green and Chemically Synthesized Copper Oxide Nanoparticles against Hepatocellular Carcinoma. J. King Saud. Univ. Sci. 2021, 33, 101669. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Mustafa, M.A.; Kumar, A.; Pramanik, A.; Sharma, R.; Mohammed, F.; Jawad, I.A.; Mohammed, I.J.; Alshahrani, M.Y.; Ali Khalil, N.A.M.; et al. Exploiting the Immune System in Hepatic Tumor Targeting: Unleashing the Potential of Drugs, Natural Products, and Nanoparticles. Pathol. Res. Pract. 2024, 256, 155266. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, D.S.; Mandlik, S.K. Herbal and Natural Dietary Products: Upcoming Therapeutic Approach for Prevention and Treatment of Hepatocellular Carcinoma. Nutr. Cancer 2021, 73, 2130–2154. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Gavini, E.; Rassu, G.; Maestri, M.; Giunchedi, P. Chitosan Nanoparticles for Therapy and Theranostics of Hepatocellular Carcinoma (HCC) and Liver-Targeting. Nanomaterials 2020, 10, 870. [Google Scholar] [CrossRef]
- Karimi, K.; Mojtabavi, S.; Tehrany, P.M.; Nejad, M.M.; Rezaee, A.; Mohtashamian, S.; Hamedi, E.; Yousefi, F.; Salmani, F.; Zandieh, M.A.; et al. Chitosan-Based Nanoscale Delivery Systems in Hepatocellular Carcinoma: Versatile Bio-Platform with Theranostic Application. Int. J. Biol. Macromol. 2023, 242, 124935. [Google Scholar] [CrossRef]
- Lee, H.D.; Yuan, L.-Y. Nano-Revolution in Hepatocellular Carcinoma: A Multidisciplinary Odyssey—Are We There Yet? World J. Hepatol. 2024, 16, 684–687. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, W.; Yang, Y.; He, M.; Li, A.; Bai, L.; Yu, B.; Yu, Z. Cancer Nanotechnology: Enhancing Tumor Cell Response to Chemotherapy for Hepatocellular Carcinoma Therapy. Asian J. Pharm. Sci. 2019, 14, 581–594. [Google Scholar] [CrossRef]
- Ren, X.; Su, D.; Shi, D.; Xiang, X. The Improving Strategies and Applications of Nanotechnology-Based Drugs in Hepatocellular Carcinoma Treatment. Front. Bioeng. Biotechnol. 2023, 11, 1272850. [Google Scholar] [CrossRef]
- Liu, M.; Yan, C.; Han, J.; Guo, Z.; Wu, Y.; Huang, J.; Xiao, Z.; Zhu, W.-H. Engineering Photo-Controllable Fragrance Release with Flash Nanoprecipitation. Green. Chem. Eng. 2021, 2, 301–308. [Google Scholar] [CrossRef]
- Chavda, V.P.; Balar, P.C.; Patel, S.B. Interventional Nanotheranostics in Hepatocellular Carcinoma. Nanotheranostics 2023, 7, 128–141. [Google Scholar] [CrossRef]
- Ray, P.; Haideri, N.; Haque, I.; Mohammed, O.; Chakraborty, S.; Banerjee, S.; Quadir, M.; Brinker, A.; Banerjee, S. The Impact of Nanoparticles on the Immune System: A Gray Zone of Nanomedicine. J. Immunol. Sci. 2021, 5, 19–33. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Pang, Y.; Zhou, H. The Interaction between Nanoparticles and Immune System: Application in the Treatment of Inflammatory Diseases. J. Nanobiotechnol. 2022, 20, 127. [Google Scholar] [CrossRef] [PubMed]
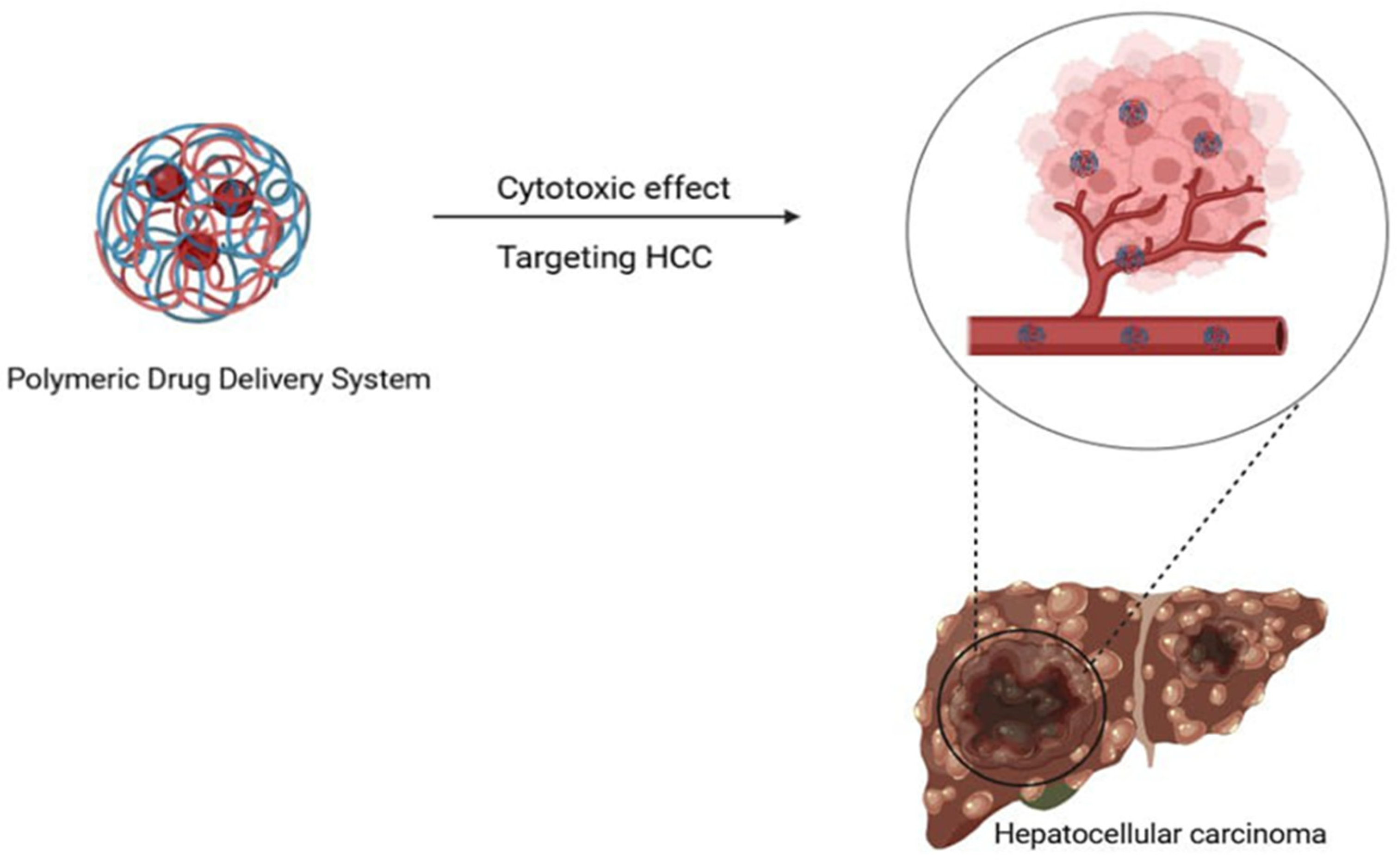
| Nanoparticle | Natural Product | Size of Nanocomposite | Characterization Techniques | Model | Advantages | Reference |
|---|---|---|---|---|---|---|
| Liposome of biocompatible matrix DSPE-PEG2000 decorated with short peptide ligand SP94 | Curcumin and resveratrol | <200 nm | TEM, DLS, and UV–VIS | In vivo and in vitro |
| [21] |
| Morus nigra L.-derived lipid nanoparticles (MLNPs) | Fresh leaves of Morus nigra L. | Approximately 100 nm Hydrodynamic size 162.1 nm | AFM, DLS, and TEM | In vivo and In vitro |
| [190] |
| Galactose-modified PEGylated liposomes (C-GPL) | Celastrol | 139.4 ± 2.7 nm | TEM, and DLS | In vivo and In vitro |
| [191] |
| Naringenin nanoparticles (NARNPs) | Naringenin | 54.96 ± 18.6 nm TEM 31.79 ± 6.8 nm SEM | SEM, TEM, FT-IR, and XRD | In vitro |
| [192] |
| SeNPs and AgNPs | Berberine | 171.5 ± 4.2 nm | DLS | In vitro |
| [193] |
| Glucan nanoparticles | Curcumin | 111.0 ± 49.0 nm | DLS, ELS, and Cryo-SEM | In vitro |
| [194] |
| Fe2O3/Starch/Polyvinyl alcohol nanocarrier (Fe2O3/S/PVA NC) | Quercetin (QC) | 240–340 nm | FTIR, XRD, FE-SEM, DLS, and VSM | In vitro |
| [195] |
| AuNPs | Polystyrene polysaccharide extracted from Polygonatum | 91–459 nm | UV–VIS, TEM, AFM, FTIR, DLS, and EDX | In vitro and In vivo |
| [196] |
| AuNPs | Panicum maximum leaf extract | 45∼50 nm | TEM, and UV–Vis | In vitro |
| [197] |
| CuONPs | Neem leaves extract Azadirachta indica | 15∼16 nm | XRD, FTIR, and UV–VIS | In vitro |
| [198] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muteeb, G.; El-Morsy, M.T.; Abo-Taleb, M.A.; Mohamed, S.K.; Khafaga, D.S.R. Herbal Medicine: Enhancing the Anticancer Potential of Natural Products in Hepatocellular Carcinoma Therapy Through Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 673. https://doi.org/10.3390/pharmaceutics17050673
Muteeb G, El-Morsy MT, Abo-Taleb MA, Mohamed SK, Khafaga DSR. Herbal Medicine: Enhancing the Anticancer Potential of Natural Products in Hepatocellular Carcinoma Therapy Through Advanced Drug Delivery Systems. Pharmaceutics. 2025; 17(5):673. https://doi.org/10.3390/pharmaceutics17050673
Chicago/Turabian StyleMuteeb, Ghazala, Manar T. El-Morsy, Mustafa Ali Abo-Taleb, Salma K. Mohamed, and Doaa S. R. Khafaga. 2025. "Herbal Medicine: Enhancing the Anticancer Potential of Natural Products in Hepatocellular Carcinoma Therapy Through Advanced Drug Delivery Systems" Pharmaceutics 17, no. 5: 673. https://doi.org/10.3390/pharmaceutics17050673
APA StyleMuteeb, G., El-Morsy, M. T., Abo-Taleb, M. A., Mohamed, S. K., & Khafaga, D. S. R. (2025). Herbal Medicine: Enhancing the Anticancer Potential of Natural Products in Hepatocellular Carcinoma Therapy Through Advanced Drug Delivery Systems. Pharmaceutics, 17(5), 673. https://doi.org/10.3390/pharmaceutics17050673









