Phytosynthesis and Characterization of Silver Nanoparticles from Antigonon leptopus: Assessment of Antibacterial and Cytotoxic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagenents
2.2. Synthesis of AgNPs with Antignon leptopus Ethanolic Extract (EXT-AL)
2.3. UV-Vis Spectroscopy Analysis
2.4. The Ferric Ion Reducing Antioxidant Power (FRAP) Assay
2.5. Kinetics Growth Curve
2.6. Dynamic Light Scattering (DLS)
2.7. Zeta Potential (ZP)
2.8. Transmission Electron Microscopy (TEM) and Atomic Force Microscopy (AFM)
2.9. Fourier-Transform Infrared Spectroscopy (FTIR)
2.10. Thermogravimetry Analysis (TGA)
2.11. X-Ray Diffractometry (XRD)
2.12. Stability Assay
2.13. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS)
2.14. Antibacterial Activity of AgNPs
2.15. Cytotoxicity Assays of AgNPs
2.16. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of AgNPs
3.1.1. Optimization of AgNPs Synthesis
3.1.2. Particle Size and Zeta Potential Measurements
3.2. Transmission Electron Microscopy (TEM) and Atomic Force Microscopy (AFM) Analyses
3.3. Kinetic Growth of AgNPs
3.4. X-Ray Diffraction (XRD)
3.5. Fourier Transform Infrared (FT-IR) Spectroscopy
3.6. Thermogravimetric Analysis (TGA)
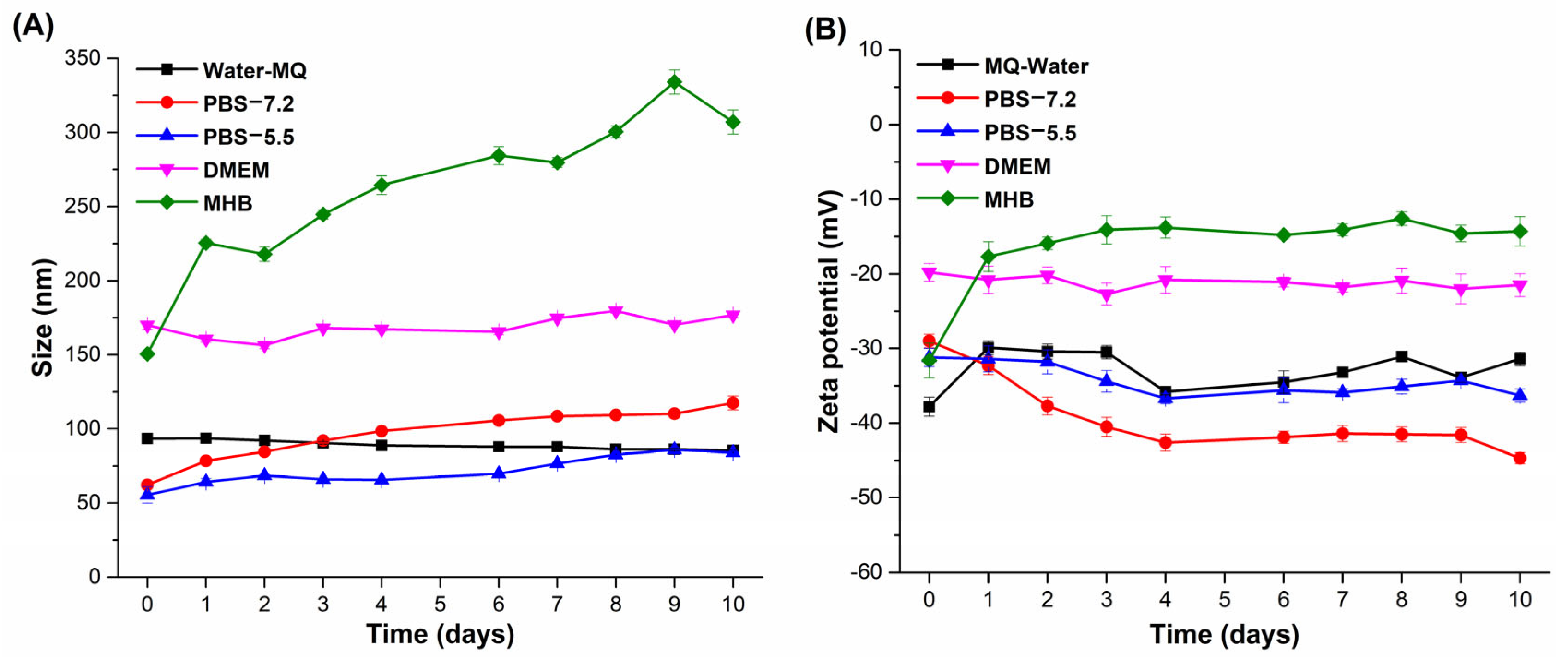
3.7. Stability of AgNPs
3.8. Ag+ Content in AgNPs by ICP-MS
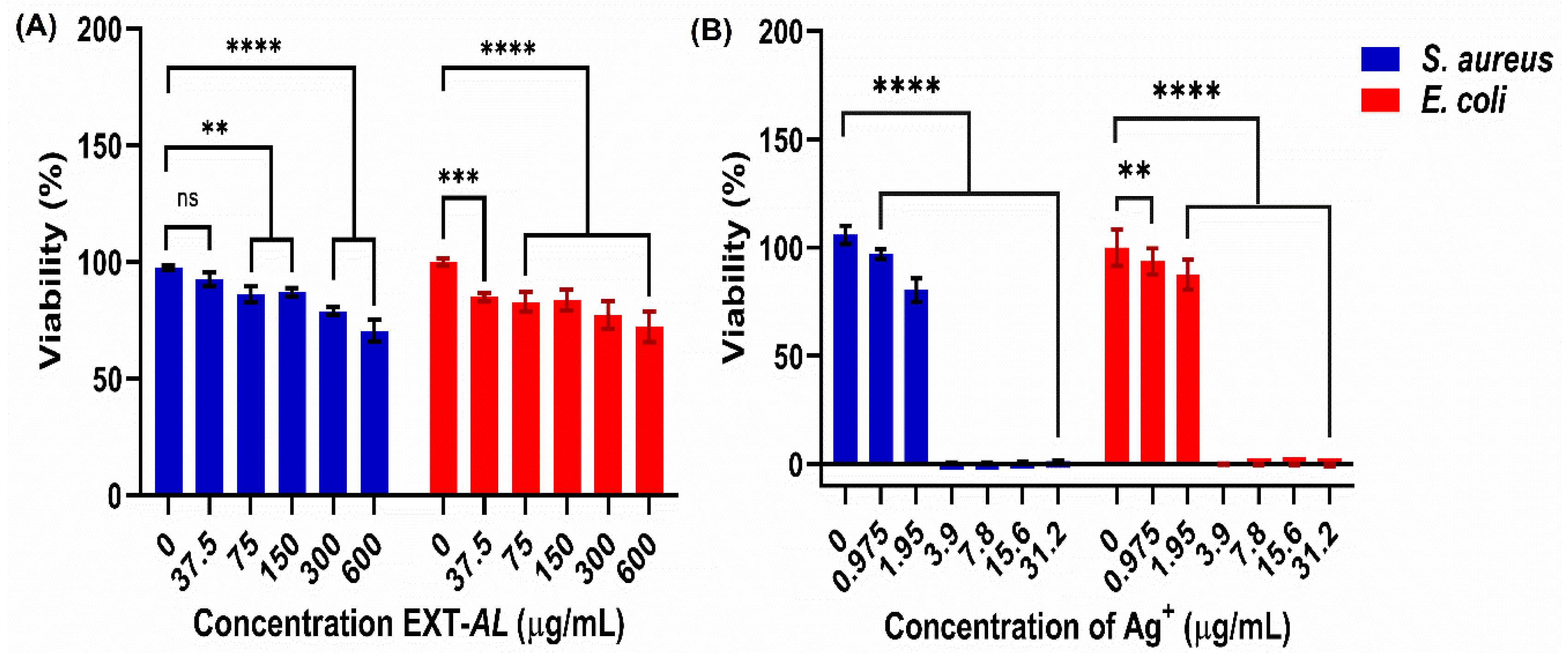
3.9. Antibacterial Activity of EXT-AL and AgNPs
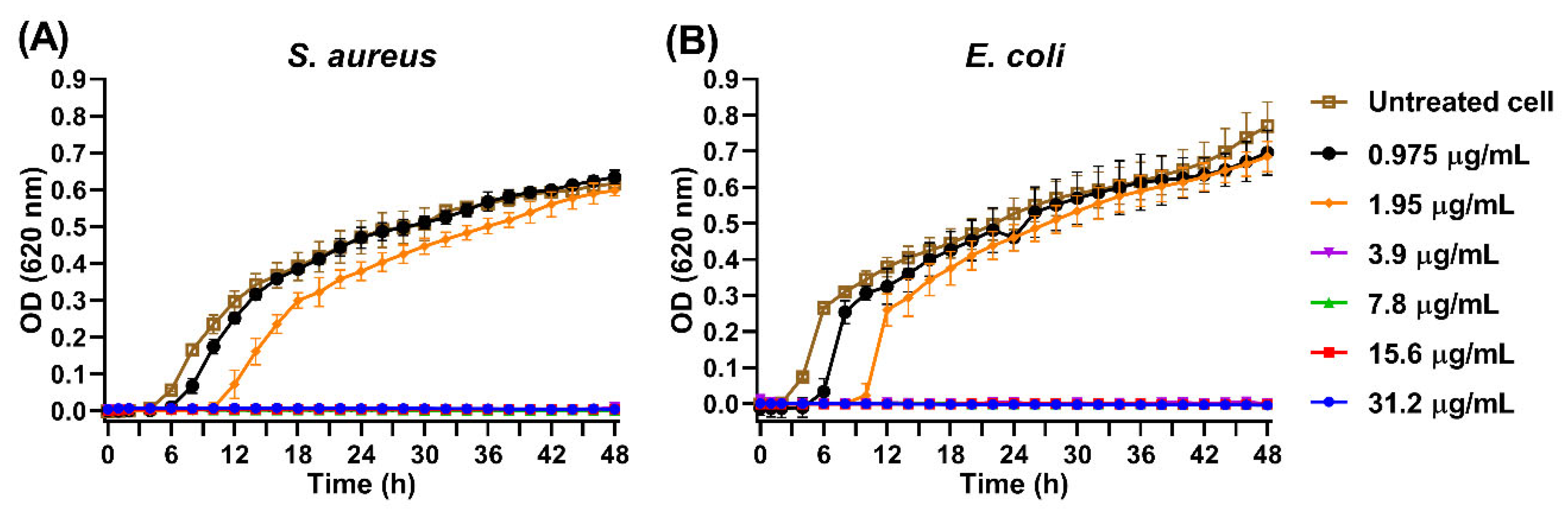
3.10. Inhibition of Bacterial Growth by AgNPs
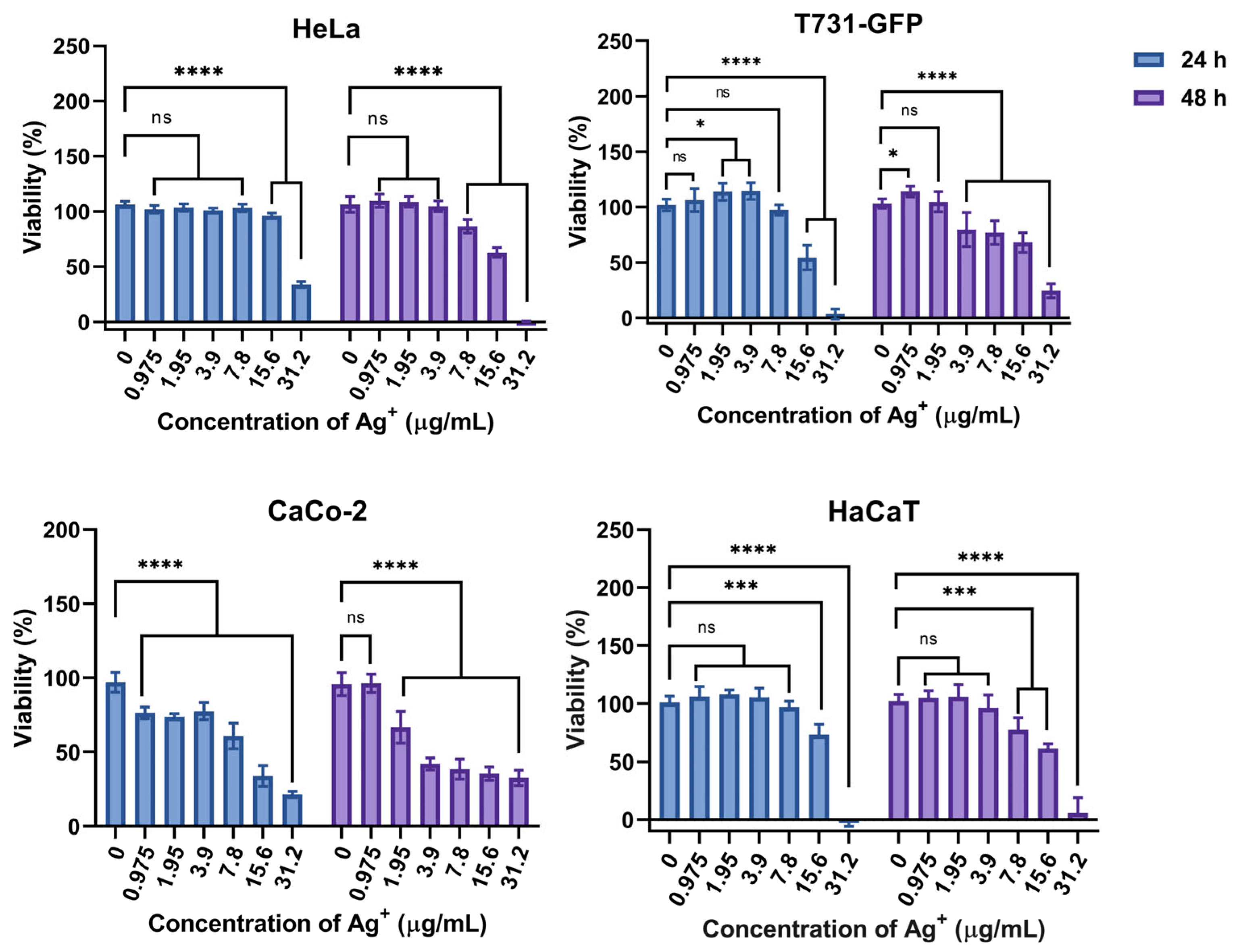
3.11. Cytotoxicity Effect of AgNPs on HeLa, T731-GFP, CaCo-2, and HaCaT Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| AgNPs | silver nanoparticles |
| AL | Antigonon leptopus |
| ATCC | American Type Culture Collection |
| CCK-8 | Cell Counting Kit-8 |
| CFU | colony forming unit |
| Dh | hydrodynamic diameter |
| DLS | dynamic light scattering |
| DMEM | Dulbecco’s modified eagle media |
| EXT-AL | ethanolic extracts from Antigonon leptopus leaves |
| FBS | fetal bovine serum |
| FRAP | ferric ion reducing antioxidant power |
| FTIR | Fourier-transform infrared spectroscopy |
| ICP-MS | inductively coupled plasma-mass spectrometry |
| LSPR | localized surface plasmon resonance |
| MHB | Mueller–Hinton broth |
| MIC | minimum inhibitory concentration |
| NPs | nanoparticles |
| PBS | Phosphate-buffered saline |
| TEM | transmission electron microscopy |
| TGA | thermogravimetry analysis |
| UV-Vis | ultraviolet-visible |
| v/v | volume/volume |
| XRD | X-ray diffraction |
| ZP | Zeta potential |
References
- Owen, R.; Crane, M.; Grieger, K.; Handy, R.; Linkov, I.; Depledge, M. Strategic Approaches for the Management of Environmental Risk Uncertainties Posed by Nanomaterials. In Nanomaterials: Risks and Benefits; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9781402094903. [Google Scholar]
- Ahire, S.A.; Bachhav, A.A.; Pawar, T.B.; Jagdale, B.S.; Patil, A.V.; Koli, P.B. The Augmentation of nanotechnology era: A concise review on fundamental concepts of nanotechnology and applications in material science and technology. Results Chem. 2022, 4, 100633. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of nanotechnology in medical field: A brief review. Glob. Heal. J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Mohammed, N.; Nawar, S.; Etawy, M.; Nassar, G.; Hassabo, A. Nanotechnology and its Applications in Industry and Product Design. J. Text. Color. Polym. Sci. 2024, 21, 273–284. [Google Scholar] [CrossRef]
- Sahoo, M.; Panigrahi, C.; Vishwakarma, S.; Kumar, J. A Review on Nanotechnology: Applications in Food Industry, Future Opportunities, Challenges and Potential Risks. J. Nanotechnol. Nanomater. 2022, 3, 28–33. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Zeeshan, S.; Shah, A.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; et al. Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Sajid, M. Nanomaterials: Types, properties, recent advances, and toxicity concerns. Curr. Opin. Environ. Sci. Health 2022, 25, 100319. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Krishnan, S.; Hii, Y.S.; Pan, S.; Chan, Y.S.; Acquah, C.; Danquah, M.K.; Rodrigues, J. Synthesis approach-dependent antiviral properties of silver nanoparticles and nanocomposites. J. Nanostructure Chem. 2022, 12, 809–831. [Google Scholar] [CrossRef]
- Nair, G.M.; Sajini, T.; Mathew, B. Advanced green approaches for metal and metal oxide nanoparticles synthesis and their environmental applications. Talanta Open 2022, 5, 100080. [Google Scholar] [CrossRef]
- Crisan, C.M.; Mocan, T.; Manolea, M.; Lasca, L.I.; Tăbăran, F.A.; Mocan, L. Review on silver nanoparticles as a novel class of antibacterial solutions. Appl. Sci. 2021, 11, 1120. [Google Scholar] [CrossRef]
- Jaswal, T.; Gupta, J. A review on the toxicity of silver nanoparticles on human health. Mater. Today Proc. 2021, 81, 859–863. [Google Scholar] [CrossRef]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Abdullah, C.A.C.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver Nanoparticles: Review of Antiviral Properties, Mechanism of Action and Applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N.; et al. A review on silver nanoparticles: Classification, various methods of synthesis, and their potential roles in biomedical applications and water treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Khan, R.; Akter, M.; Kamaraj, C.; Malafaia, G.; Ragavendran, C.; Rahman, M. Environmental Nanotechnology, Monitoring & Management Green synthesis of silver nanoparticles with its bioactivity, toxicity and environmental applications: A comprehensive literature review. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100872. [Google Scholar] [CrossRef]
- Patel, R.R.; Singh, S.K.; Singh, M. Materials Advances biological applications, delivery and toxicity. Mater. Adv. 2023, 4, 1831–1849. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Zhang, Y.; Seerangaraj, S. Applications of Green Synthesized Metal Nanoparticles—A Review. Biol. Trace Elem. Res. 2024, 202, 360–386. [Google Scholar] [CrossRef]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green Synthesis of Silver Nanoparticles: A Review. Sci. Res. 2016, 6, 34–56. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Chemico-Biological Interactions Mechanism of plant-mediated synthesis of silver nanoparticles e A review on biomolecules involved, characterisation and antibacterial activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef]
- Malik, M.; Aamir, M.; Iqbal, Y.; Malik, M.; Bakhsh, S.; Irfan, S.; Ahmad, R.; Pham, P.V. Biosynthesis of silver nanoparticles for biomedical applications: A mini review. Inorg. Chem. Commun. 2022, 145, 109980. [Google Scholar] [CrossRef]
- Sivakumar, S.; Subban, M.; Chinnasamy, R. Green synthesized silver nanoparticles using Andrographis macrobotrys Nees leaf extract and its potential to antibacterial, antioxidant, anti-inflammatory and lung cancer cells cytotoxicity effects D). Inorg. Chem. Commun. 2023, 153, 110787. [Google Scholar] [CrossRef]
- Patini, R.; Mangino, G.; Martellacci, L.; Quaranta, G.; Masucci, L.; Gallenzi, P. The effect of different antibiotic regimens on bacterial resistance: A systematic review. Antibiotics 2020, 9, 22. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Rodrigues, L.; Tavares, A.H.; Tom, G.; Caetano, M.; Chaves, C.; Afreixo, V. Analysis of Potential Risk Factors for Multidrug-Resistance at a Burn Unit. Eur. Burn. J. 2023, 4, 9–17. [Google Scholar] [CrossRef]
- Slocker-barrio, M.; Bustinza-arriort, A.; Fres, E. Increase in Incidence Rates and Risk Factors for Multidrug Resistant Bacteria in Septic Children: A Nationwide Spanish. Antibiotics 2023, 33, 1626. [Google Scholar] [CrossRef]
- Vandebroek, I.; Picking, D.; Aiken, S.; Lewis, P.A.; Oberli, A.; Mitchell, S.; Boom, B. A Review of Coralilla (Antigonon leptopus): An Invasive and Popular Urban Bush Medicine in Jamaica. Econ. Bot. 2018, 72, 229–245. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Liang, Z.; Fan, S.; Gao, X.; Jia, H.; Li, B.; Shi, L.; Zhai, A.; Wu, C. Epidemiology and prediction of multidrug-resistant bacteria based on hospital level. J. Glob. Antimicrob. Resist. 2022, 29, 155–162. [Google Scholar] [CrossRef]
- Long, Y.; Zheng, Y.; Li, C.; Guo, Z.; Li, P.; Zhang, F.; Liu, W.; Wang, Y. Respiratory pathogenic microbial infections: A narrative review. Int. J. Med. Sci. 2024, 21, 826–836. [Google Scholar] [CrossRef]
- Diniz, L.; Ant, A.; De Moraes, B.; Gal, P.; Paulo, S.; Maria, C.; Costa, L.; Cruz, J.N.; Helena, E.; Andrade, D.A.; et al. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; De Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2022, 11, 46. [Google Scholar] [CrossRef]
- Wanda, M. Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
- Barraj, E. Phytomedicine Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Adnan, S.; Shah, A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Choudhary, M.K.; Kataria, J.; Sharma, S. Evaluation of the kinetic and catalytic properties of biogenically synthesized silver nanoparticles. J. Clean. Prod. 2018, 198, 882–890. [Google Scholar] [CrossRef]
- Rolim, W.R.; Pelegrino, M.T.; de Araújo Lima, B.; Ferraz, L.S.; Costa, F.N.; Bernardes, J.S.; Rodigues, T.; Brocchi, M.; Seabra, A.B. Green tea extract mediated biogenic synthesis of silver nanoparticles: Characterization, cytotoxicity evaluation and antibacterial activity. Appl. Surf. Sci. 2019, 463, 66–74. [Google Scholar] [CrossRef]
- Garibo, D.; Nuñez, H.A.B.; De León, J.N.D.; Mendoza, E.G.; Estrada, I.; Magaña, Y.T.; Tiznado, H.; Marroquin, M.O.; Ramos, A.G.S.; Blanco, A.; et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef]
- Reddy, N.V.; Li, H.; Hou, T.; Bethu, M.S.; Ren, Z.; Zhang, Z. Phytosynthesis of Silver Nanoparticles Using Perilla frutescens Leaf Extract: Characterization and Evaluation of Antibacterial, Antioxidant, and Anticancer Activities. Int. J. Nanomed. 2021, 16, 15–29. [Google Scholar] [CrossRef]
- Fareed, N.; Nisa, S.; Bibi, Y.; Fareed, A.; Ahmed, W.; Sabir, M.; Alam, S.; Sajjad, A.; Kumar, S.; Hussain, M.; et al. Green synthesized silver nanoparticles using carrot extract exhibited strong antibacterial activity against multidrug resistant bacteria. J. King Saud Univ. 2023, 35, 102477. [Google Scholar] [CrossRef]
- Vanisree, M.; Alexander-Lindo, R.L.; DeWitt, D.L.; Nair, M.G. Functional food components of Antigonon leptopus tea. Food Chem. 2008, 106, 487–492. [Google Scholar] [CrossRef]
- Prashith Kekuda, T.R.; Raghavendra, H.L. Medicinal Uses, Phytochemistry and Pharmacological Activities of Antigonon leptopus Hook. and Arn. (Polygonaceae): A Review. J. Chem. Pharm. Res. 2018, 10, 103–110. [Google Scholar]
- Balasubramani, G.; Ramkumar, R.; Krishnaveni, N.; Pazhanimuthu, A.; Natarajan, T.; Sowmiya, R.; Perumal, P. Structural characterization, antioxidant and anticancer properties of gold nanoparticles synthesized from leaf extract (decoction) of Antigonon leptopus Hook. & Arn. J. Trace Elem. Med. Biol. 2015, 30, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Bollapragada, M.; Shantaram, M. Comparative Phytochemical Profiles and Antioxidant Properties of Antigonon leptopus, Artabotrys hexapetalus and Allamanda blanchetii Leaf Extracts. Int. J. Pharmacogn. Phytochem. Res. 2018, 10, 327–333. [Google Scholar]
- Ganaie, S.U.; Abbasi, T.; Abbasi, S.A. Low-cost, environment-friendly synthesis of palladium nanoparticles by utilizing a terrestrial weed Antigonon leptopus. Part. Sci. Technol. 2016, 34, 201–208. [Google Scholar] [CrossRef]
- Ganaie, S.U.; Abbasi, T.; Abbasi, S.A. Rapid and green synthesis of bimetallic Au–Ag nanoparticles using an otherwise worthless weed Antigonon leptopus. J. Exp. Nanosci. 2016, 11, 395–417. [Google Scholar] [CrossRef]
- Azad, A.; Zafar, H.; Raza, F.; Sulaiman, M. Factors Influencing the Green Synthesis of Metallic Nanoparticles Using Plant Extracts: A Comprehensive Review. Pharm. Front. 2023, 5, e117–e131. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Rao, H.; Ahmad, S.; Aati, H.Y.; Basit, A.; Ahmad, I.; Ahmad Ghalloo, B.; Nadeem Shehzad, M.; Nazar, R.; Zeeshan, M.; Nasim, M.J.; et al. Phytochemical screening, biological evaluation, and molecular docking studies of aerial parts of Trigonella hamosa (branched Fenugreek). Arab. J. Chem. 2023, 16, 104795. [Google Scholar] [CrossRef]
- Provencher, S.W.; Štěpánek, P. Global analysis of dynamic light scattering autocorrelation functions. Part. Part. Syst. Charact. 1996, 13, 291–294. [Google Scholar] [CrossRef]
- CLSI. M07-A10 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow; CLSI: Wayne, PA, USA, 2015; ISBN 1562389874. [Google Scholar]
- Leyu, A.M.; Debebe, S.E.; Bachheti, A.; Rawat, Y.S.; Bachheti, R.K. Green Synthesis of Gold and Silver Nanoparticles Using Invasive Alien Plant Parthenium hysterophorus and Their Antimicrobial and Antioxidant Activities. Sustainability 2023, 15, 9456. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Stoica, A.E.; Dima-Bălcescu, M.Ș.; Chircov, C.; Gharbia, S.; Baltă, C.; Roșu, M.; Herman, H.; Holban, A.M.; Ficai, A.; et al. Electrospun polyethylene terephthalate nanofibers loaded with silver nanoparticles: Novel approach in anti-infective therapy. J. Clin. Med. 2019, 8, 1039. [Google Scholar] [CrossRef]
- Vanaraj, S.; Keerthana, B.B.; Preethi, K. Biosynthesis, Characterization of Silver Nanoparticles Using Quercetin from Biosynthesis, Characterization of Silver Nanoparticles Using Quercetin from Clitoria ternatea L to Enhance Toxicity Against Bacterial Biofilm. J. Inorg. Organomet. Polym. Mater. 2017, 027, 775–781. [Google Scholar] [CrossRef]
- Hani Ramli, R.; Soon, C.F.; Mohd Rus, A.Z. Characterisation of silver nanoparticles produced by three different methods based on Borohydride reducing agent. MATEC Web Conf. 2016, 78, 01032. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M.; Esmail, G.A.; Al-Dhabi, N.A.; Arasu, M.V. Green synthesis and characterization of silver nanoparticles from Moringa oleifera flower and assessment of antimicrobial and sensing properties. J. Photochem. Photobiol. B Biol. 2020, 205, 111836. [Google Scholar] [CrossRef]
- Shashiraj, K.N.; Nayaka, S.; Kumar, R.S.; Kantli, G.B.; Basavarajappa, D.S.; Gunagambhire, P.V.; Almansour, A.I.; Perumal, K. Rotheca serrata Flower Bud Extract Mediated Bio-Friendly Preparation of Silver Nanoparticles: Their Characterizations, Anticancer, and Apoptosis Inducing Ability against Pancreatic Ductal Adenocarcinoma Cell Line. Processes 2023, 11, 893. [Google Scholar] [CrossRef]
- Balan, K.; Qing, W.; Wang, Y.; Liu, X.; Palvannan, T.; Wang, Y.; Zhang, Y. Antidiabetic activity of silver nanoparticles from green synthesis using Lonicera japonica leaf extract. RSC Adv. 2016, 6, 40162–40168. [Google Scholar] [CrossRef]
- Valsalam, S.; Paul, A.; Arasu, M.V.; Ravindran, B.; Chang, S.W. Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B: Biol. 2018, 191, 65–74. [Google Scholar] [CrossRef]
- Gonfa, Y.H.; Gelagle, A.A.; Hailegnaw, B.; Kabeto, S.A.; Workeneh, G.A.; Tessema, F.B.; Tadesse, M.G.; Wabaidur, S.M.; Dahlous, K.A.; Fayssal, S.A.; et al. Optimization, Characterization, and Biological Applications of Silver Nanoparticles Synthesized Using Essential Oil of Aerial Part of Laggera tomentosa. Sustainability 2023, 15, 797. [Google Scholar] [CrossRef]
- Renuka, R.; Devi, K.R.; Sivakami, M.; Thilagavathi, T.; Uthrakumar, R. Biocatalysis and Agricultural Biotechnology Biosynthesis of silver nanoparticles using phyllanthus emblica fruit extract for antimicrobial application. Biocatal. Agric. Biotechnol. 2020, 24, 101567. [Google Scholar] [CrossRef]
- Aty, A.M.A.; Barakat, A.Z.; Bassuiny, R.I.; Mohamed, S.A. Statistical optimization, characterization, antioxidant and antibacterial properties of silver nanoparticle biosynthesized by saw palmetto seed phenolic extract. Sci. Rep. 2023, 13, 15605. [Google Scholar] [CrossRef]
- Tan, T.; Thi, N.; Huong, T.; Dinh, T.; Thanh, N.; Quoc, V.; Tran, P.; Nguyen, N. Biogenic one-step synthesis of silver nanoparticles using Quisqualis indica linn flower extract: Characterization, molecular docking, and DFT studies. Inorg. Chem. Commun. 2023, 158, 111469. [Google Scholar] [CrossRef]
- Sibiya, N.P.; Moloto, M.J. Shape control of silver selenide nanoparticles using green capping molecules. Green Process. Synth. 2017, 6, 183–188. [Google Scholar] [CrossRef]
- Labulo, A.H.; David, O.A.; Terna, A.D. Green synthesis and characterization of silver nanoparticles using Morinda lucida leaf extract and evaluation of its antioxidant and antimicrobial activity. Chem. Pap. 2022, 76, 7313–7325. [Google Scholar] [CrossRef]
- Pinto, V.V.; José, M.; Silva, R.; Santos, H.A.; Silva, F.; Pereira, C.M. Colloids and Surfaces A: Physicochemical and Engineering Aspects Long time effect on the stability of silver nanoparticles in aqueous medium: Effect of the synthesis and storage conditions. Colloids Surf. Physicochem. Eng. Asp. 2010, 364, 19–25. [Google Scholar] [CrossRef]
- Martin, M.N.; Allen, A.J.; Maccuspie, R.I.; Hackley, V.A. Dissolution, agglomerate morphology, and stability limits of protein-coated silver nanoparticles. Langmuir 2014, 30, 11442–11452. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, N. Oridonin inhibits Hela cell proliferation via downregulation of glutathione metabolism: A new insight from metabolomics. J. Pharm. Pharmacol. 2023, 75, 837–845. [Google Scholar] [CrossRef]
- Pascale, R.M.; Calvisi, D.F.; Simile, M.M.; Feo, C.F.; Feo, F. The warburg effect 97 years after its discovery. Cancers 2020, 12, 2819. [Google Scholar] [CrossRef]
- Sánchez-Arribas, N.; Martínez-Negro, M.; Villar, E.M.; Pérez, L.; Barcina, J.O.; Aicart, E.; Taboada, P.; Guerrero-Martínez, A.; Junquera, E. Protein expression knockdown in cancer cells induced by a gemini cationic lipid nanovector with histidine-based polar heads. Pharmaceutics 2020, 12, 791. [Google Scholar] [CrossRef]
- Sánchez-Arribas, N.; Martínez-Negro, M.; Villar, E.M.; Pérez, L.; Aicart, E.; Taboada, P.; Guerrero-Martínez, A.; Junquera, E. Biocompatible Nanovector of siRNA Consisting of Arginine-Based Cationic Lipid for Gene Knockdown in Cancer Cells. ACS Appl. Mater. Interfaces 2020, 12, 34536–34547. [Google Scholar] [CrossRef]
- Zhu, W.; Cremonini, E.; Oteiza, P.I. Changes in redox network expression during Caco-2 cell differentiation into enterocytes. Redox Experimental Medicine 2024, 1, A1–A12. [Google Scholar] [CrossRef]
- Mi, R.; Fu, Z.; Jiang, J.; Gao, S.; Guan, X.; Wang, X.; Zhou, Z. Sea Cucumber Viscera Processed by Protease Hydrolysis Combined with Cordyceps militaris Fermentation Protect Caco-2 Cells against Oxidative Damage via Enhancing Antioxidant Capacity, Activating Nrf2/HO-1 Pathway and Improving Cell Metabolism. Antioxidants 2024, 13, 988. [Google Scholar] [CrossRef]
- Zhou, X.; Fei, F.; Song, W.; Ma, H.; Xu, Z.; Yue, J.; Cao, B.; Sun, R.; Zhao, Y.; Yang, Y.; et al. Metabolomics analysis reveals cytotoxic effects of ouabain towards psoriatic keratinocytes via impairment of glutathione metabolism. Mol. Genet. Genomics 2023, 298, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Lesnierowski, G.; Cegielska-Radziejewska, R.; Kijowski, J. Thermally and chemical-thermally modified lysozyme and its bacteriostatic activity. Worlds. Poult. Sci. J. 2004, 60, 303–310. [Google Scholar] [CrossRef]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef]
- Friedman, M.; Ju, H.S. Effect of pH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Mehta, S.K.; Chaudhary, S.; Gradzielski, M. Time dependence of nucleation and growth of silver nanoparticles generated by sugar reduction in micellar media. J. Colloid Interface Sci. 2010, 343, 447–453. [Google Scholar] [CrossRef]
- Maccuspie, R.I. Colloidal stability of silver nanoparticles in biologically relevant conditions. J. Nanoparticle Res. 2011, 13, 2893–2908. [Google Scholar] [CrossRef]
- Barabadi, H.; Mojab, F.; Vahidi, H.; Marashi, B.; Talank, N. Green synthesis, characterization, antibacterial and biofilm inhibitory activity of silver nanoparticles compared to commercial silver nanoparticles. Inorg. Chem. Commun. 2021, 129, 108647. [Google Scholar] [CrossRef]
- Barabadi, H.; Hosseini, O.; Jounaki, K.; Sadeghian-abadi, S.; Ashouri, F.; Mostafa, A.; Alrikabi, A.; Vahidi, H.; Amidi, S.; Mojab, F.; et al. Bioinspired green-synthesized silver nanoparticles: In vitro physicochemical, antibacterial, biofilm inhibitory, genotoxicity, antidiabetic, antioxidant, and anticoagulant performance. Mater. Adv. 2023, 4, 3037–3054. [Google Scholar] [CrossRef]
- Riaz, M.; Sharafat, U.; Zahid, N.; Ismail, M.; Park, J.; Ahmad, B.; Rashid, N.; Fahim, M.; Imran, M.; Tabassum, A. Synthesis of Biogenic Silver Nanocatalyst and their Antibacterial and Organic Pollutants Reduction Ability. ACS Omega 2022, 7, 14723–14734. [Google Scholar] [CrossRef]
- Jadhav, K.; Deore, S.; Dhamecha, D.; Hr, R.; Jagwani, S.; Jalalpure, S.; Bohara, R. Phytosynthesis of Silver Nanoparticles: Characterization, Biocompatibility Studies, and Anticancer Activity. ACS Biomater. Sci. Eng. 2018, 4, 892–899. [Google Scholar] [CrossRef]
- Kumar, D.; Gurrala, P.; Rao, M. Biogenic synthesis of silver nanoparticles from the leaf extract of Erythroxylum Monogynum Roxb: Evaluation of antibacterial and anticancer effects. Nano-Struct. Nano-Objects 2024, 39, 101222. [Google Scholar] [CrossRef]
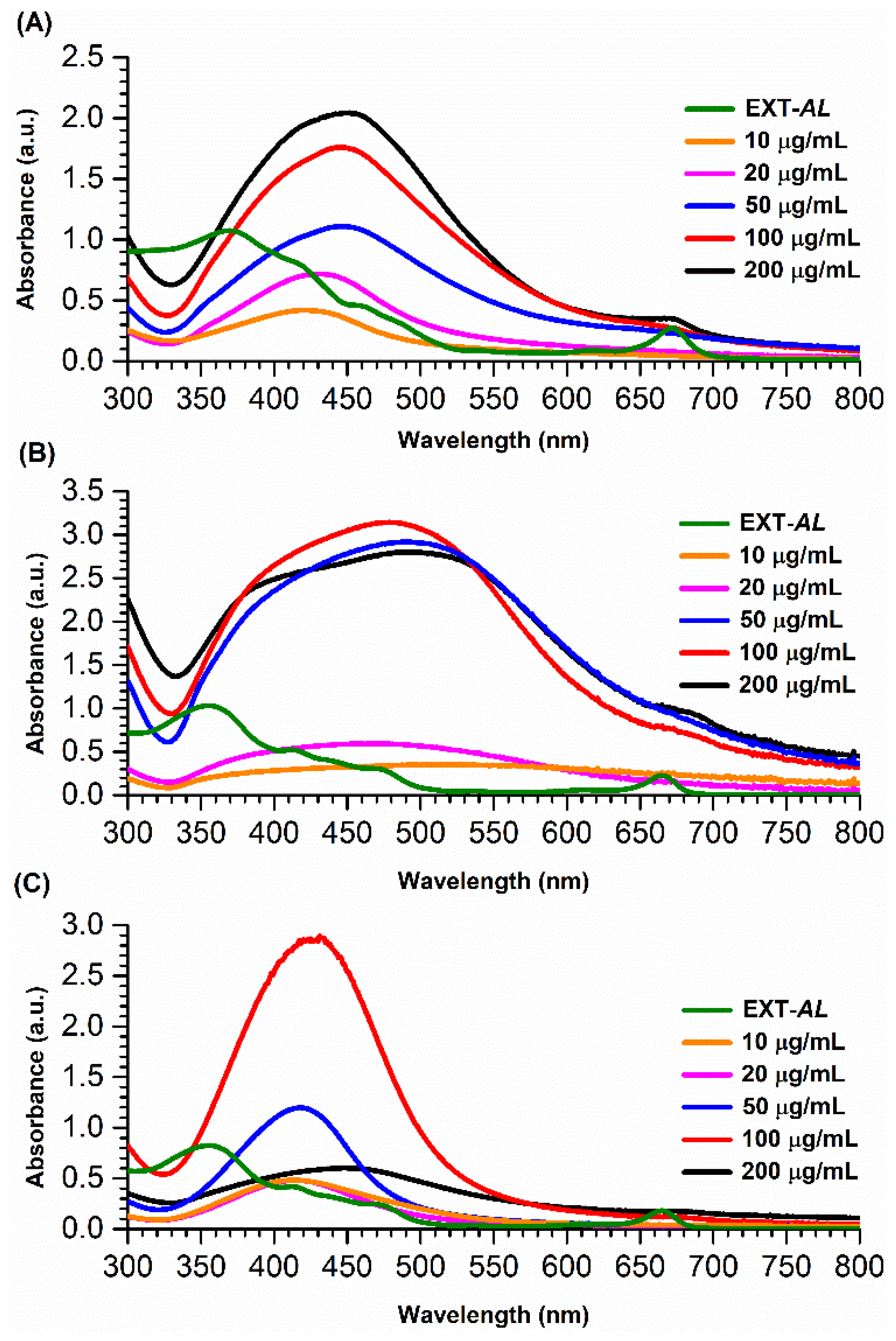
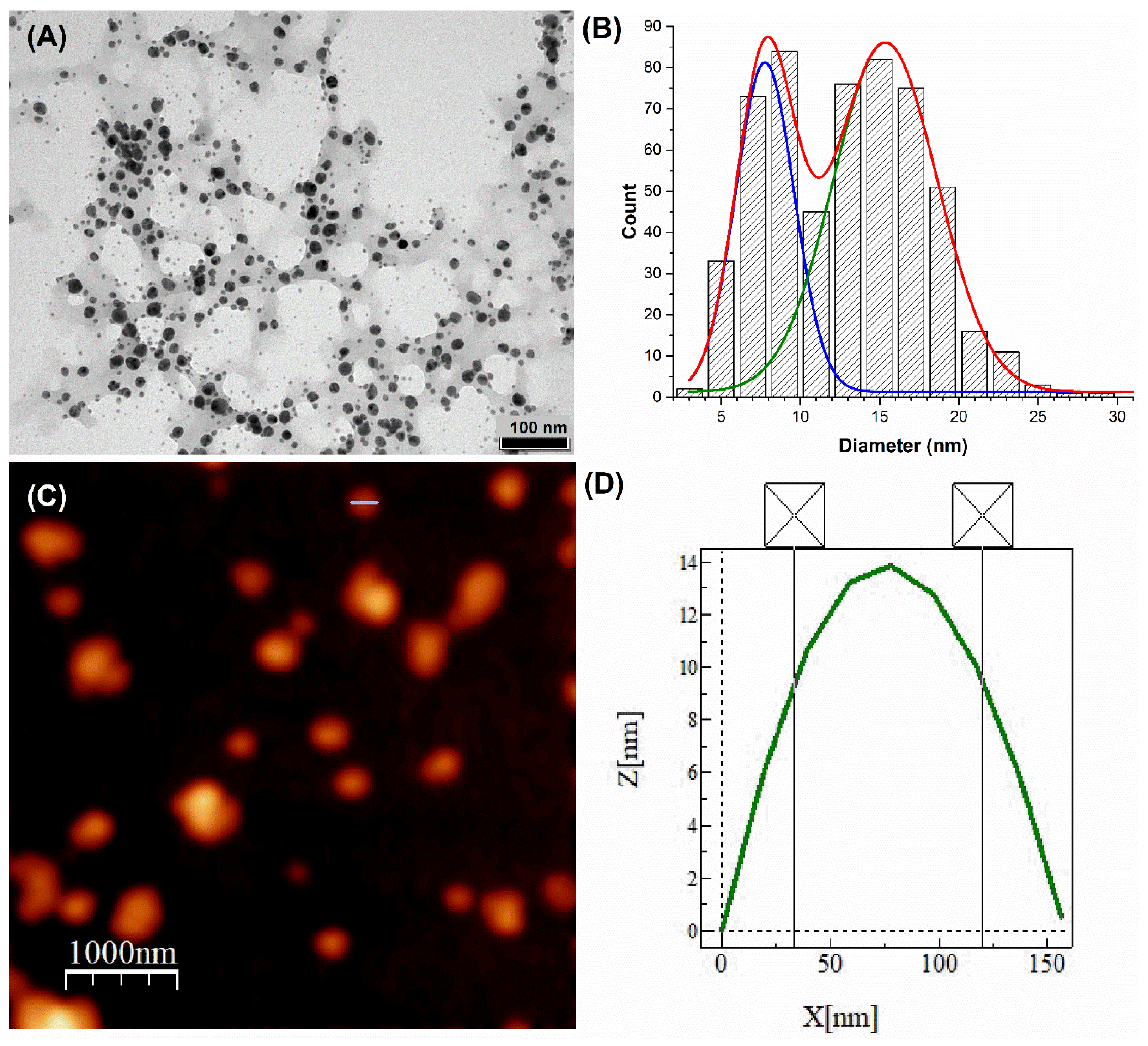


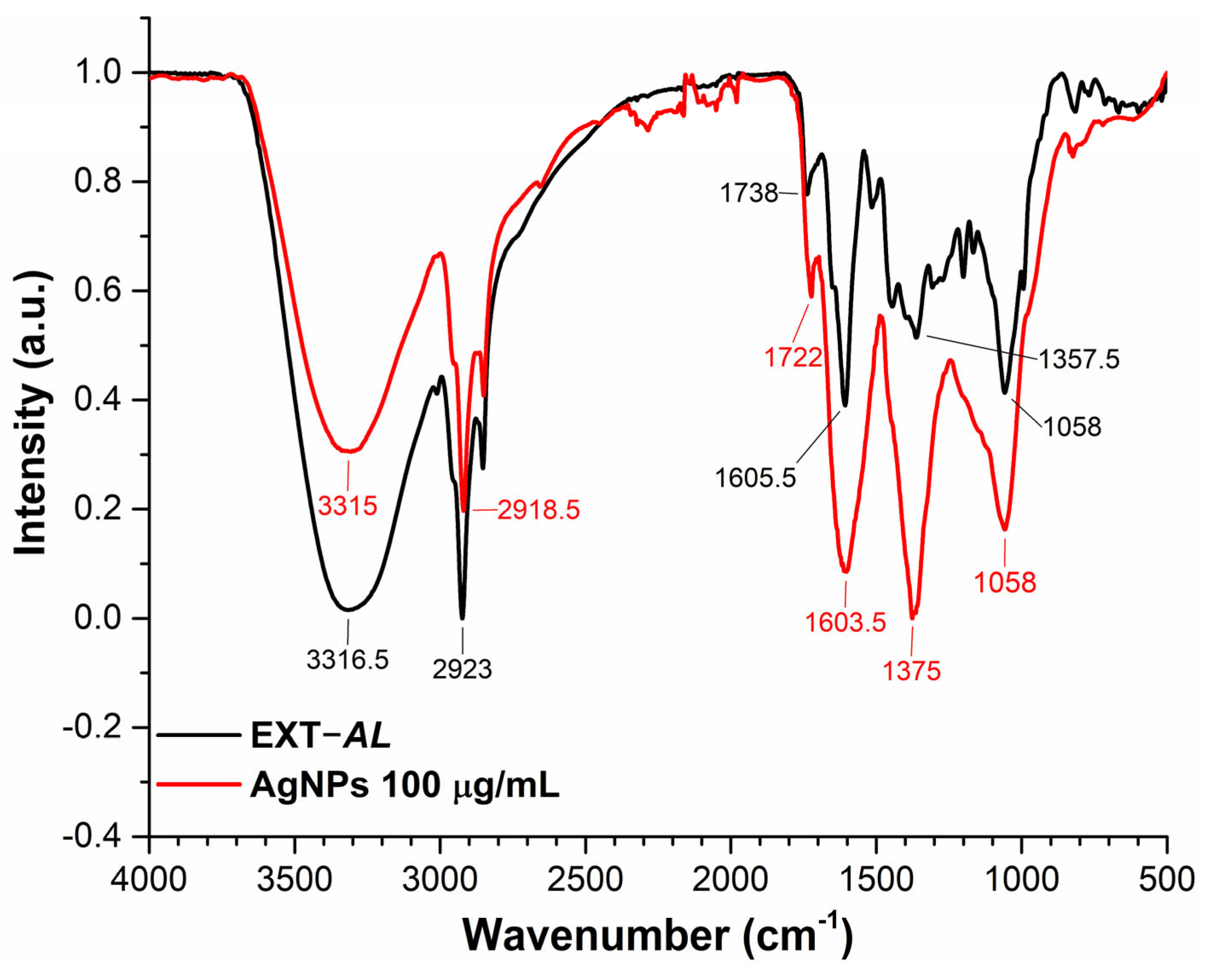
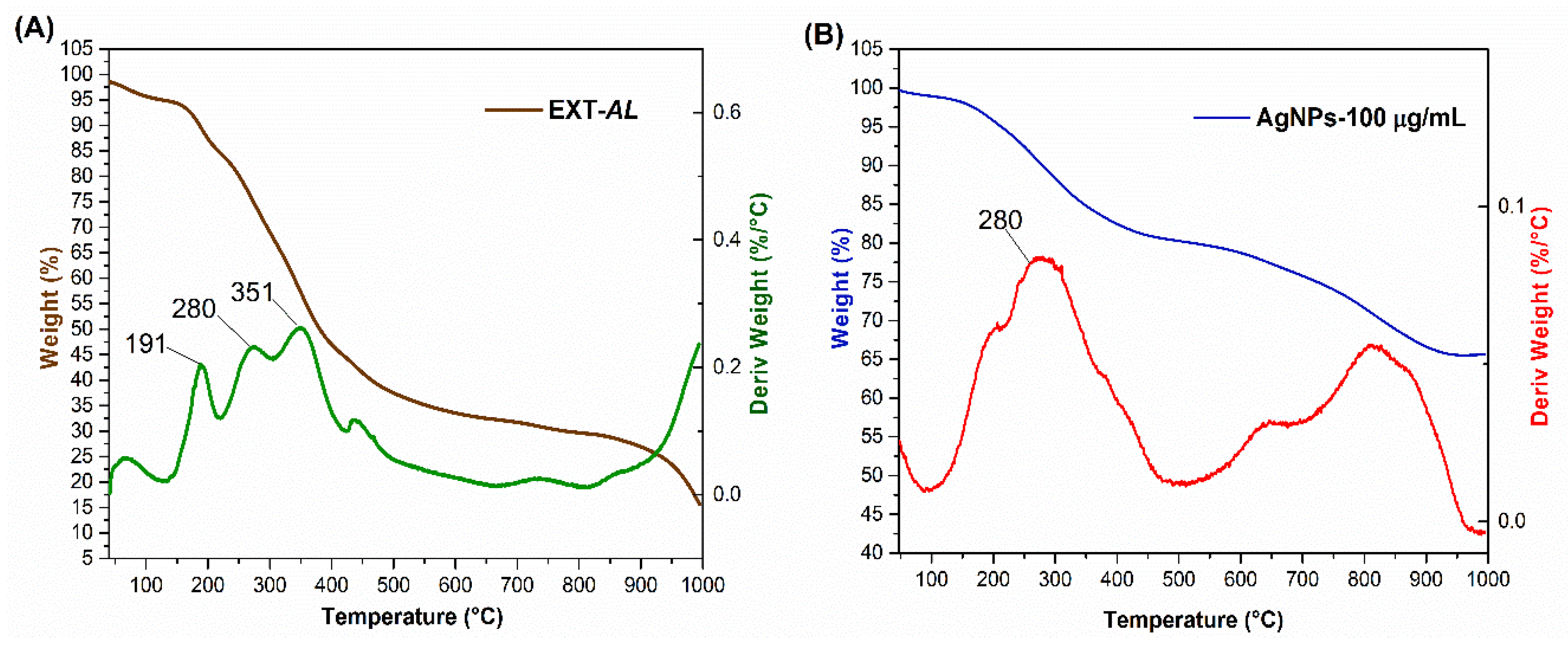
| Concentration of EXT-AL | Dh (nm) | PDI | ZP (mV) |
|---|---|---|---|
| 10 µg/mL | 95.10 ± 1.30 b | 0.34 ± 0.01 c | −27.88 ± 1.88 b |
| 20 µg/mL | 120.10 ± 2.12 c | 0.30 ± 0.10 c | −31.90 ± 1.91 b |
| 50 µg/mL | 130.90 ± 1.30 d | 0.30 ± 0.02 b | −30.50 ± 1.31 c |
| 100 µg/mL | 93.48 ± 1.88 e | 0.25 ± 0.01 a | −37.80 ± 1.28 d |
| 200 µg/mL | 267.90 ± 7.92 a | 0.37 ± 0.10 ab | −26.60 ± 0.90 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gastelum-Cabrera, M.; Mendez-Pfeiffer, P.; Ballesteros-Monrreal, M.G.; Velasco-Rodríguez, B.; Martínez-Flores, P.D.; Silva-Bea, S.; Domínguez-Arca, V.; Prieto, G.; Barbosa, S.; Otero, A.; et al. Phytosynthesis and Characterization of Silver Nanoparticles from Antigonon leptopus: Assessment of Antibacterial and Cytotoxic Properties. Pharmaceutics 2025, 17, 672. https://doi.org/10.3390/pharmaceutics17050672
Gastelum-Cabrera M, Mendez-Pfeiffer P, Ballesteros-Monrreal MG, Velasco-Rodríguez B, Martínez-Flores PD, Silva-Bea S, Domínguez-Arca V, Prieto G, Barbosa S, Otero A, et al. Phytosynthesis and Characterization of Silver Nanoparticles from Antigonon leptopus: Assessment of Antibacterial and Cytotoxic Properties. Pharmaceutics. 2025; 17(5):672. https://doi.org/10.3390/pharmaceutics17050672
Chicago/Turabian StyleGastelum-Cabrera, Marisol, Pablo Mendez-Pfeiffer, Manuel G. Ballesteros-Monrreal, Brenda Velasco-Rodríguez, Patricia D. Martínez-Flores, Sergio Silva-Bea, Vicente Domínguez-Arca, Gerardo Prieto, Silvia Barbosa, Ana Otero, and et al. 2025. "Phytosynthesis and Characterization of Silver Nanoparticles from Antigonon leptopus: Assessment of Antibacterial and Cytotoxic Properties" Pharmaceutics 17, no. 5: 672. https://doi.org/10.3390/pharmaceutics17050672
APA StyleGastelum-Cabrera, M., Mendez-Pfeiffer, P., Ballesteros-Monrreal, M. G., Velasco-Rodríguez, B., Martínez-Flores, P. D., Silva-Bea, S., Domínguez-Arca, V., Prieto, G., Barbosa, S., Otero, A., Taboada, P., & Juárez, J. (2025). Phytosynthesis and Characterization of Silver Nanoparticles from Antigonon leptopus: Assessment of Antibacterial and Cytotoxic Properties. Pharmaceutics, 17(5), 672. https://doi.org/10.3390/pharmaceutics17050672








