Developing the Oxalate, Fumarate and Succinate Salts of Tetrabenazine: Solid-State Characterization and Solubility
Abstract
1. Introduction
2. Materials and Methods
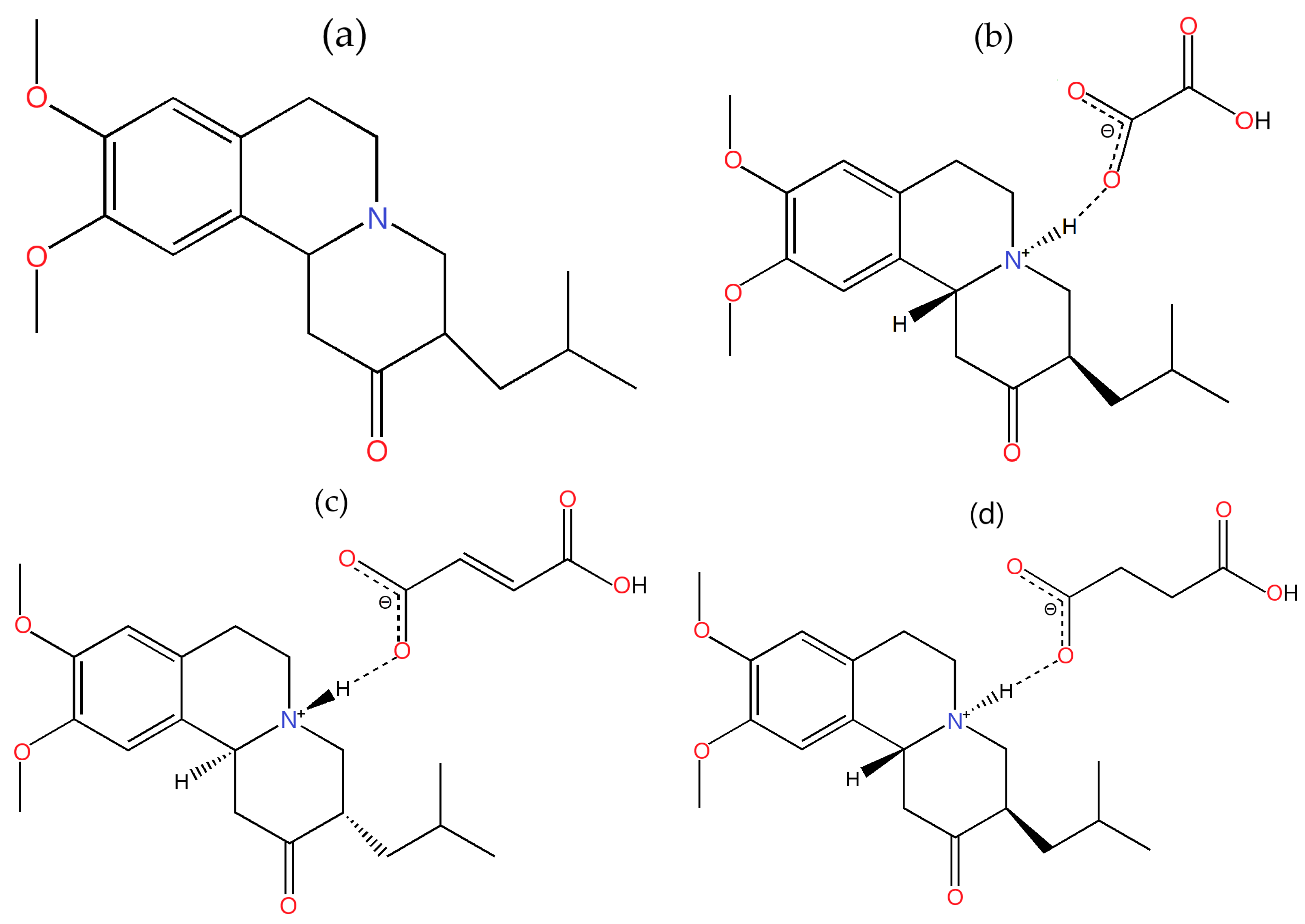
2.1. Tetrabenazine Salts Preparation and Single Crystal Growth
- (i)
- Tetrabenazine (denoted TBZ, Figure 1a);
- (ii)
- The oxalate salt of tetrabenazine (denoted TBZ&OX, Figure 1b);
- (iii)
- The fumarate salt of tetrabenazine (denoted TBZ&FUM, Figure 1c);
- (iv)
- The succinate salt of tetrabenazine (denoted TBZ&SUC, Figure 1d).
2.2. Single-Crystal X-Ray Diffraction
2.3. X-Ray Powder Diffraction
2.4. Differential Thermal Analysis
2.5. Fourier-Transform Infrared Spectroscopy
2.6. Stability
2.7. UV Measurements
3. Results
3.1. Analysis of Crystal Structures
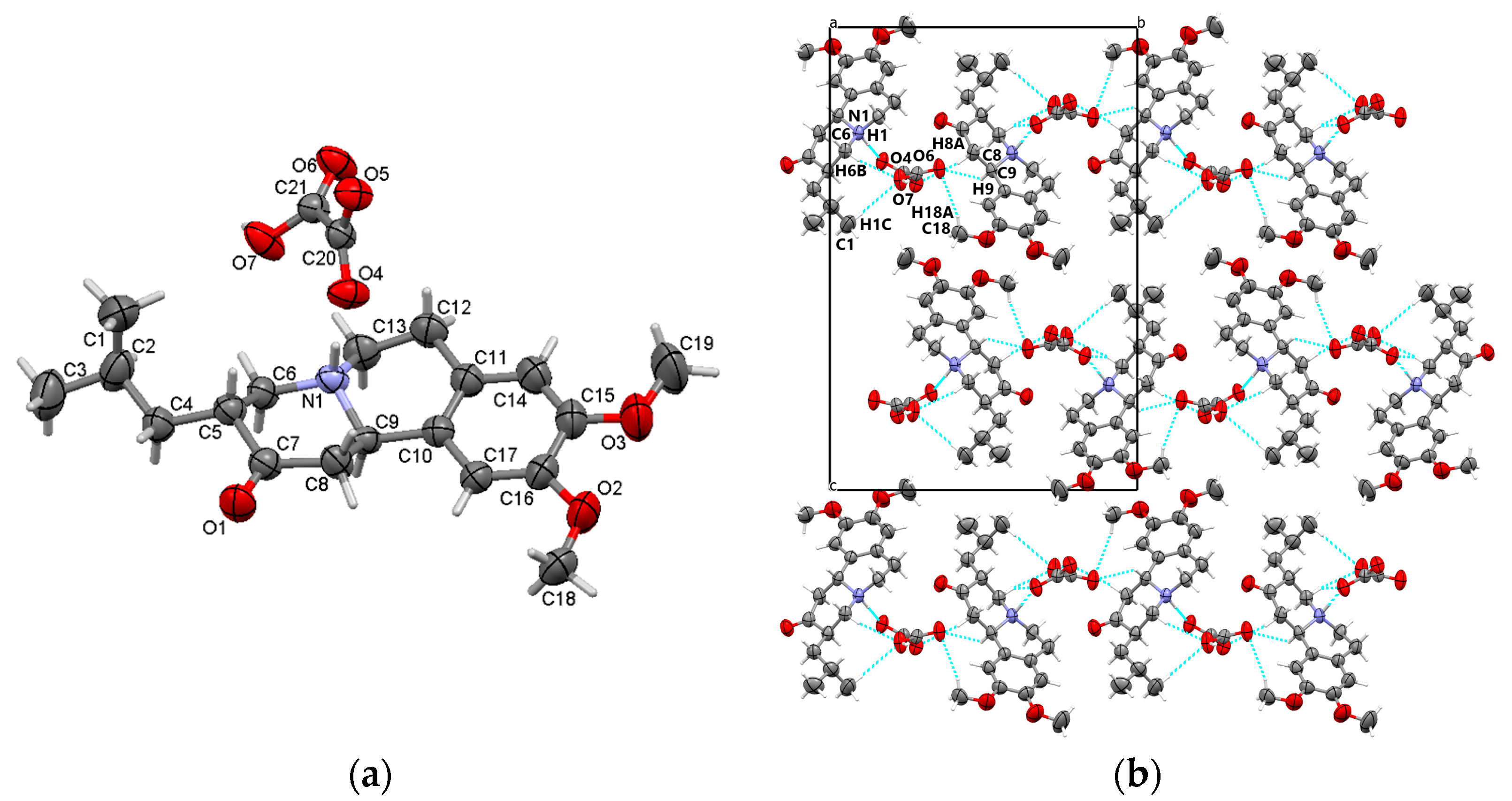
3.1.1. TBZ&OX
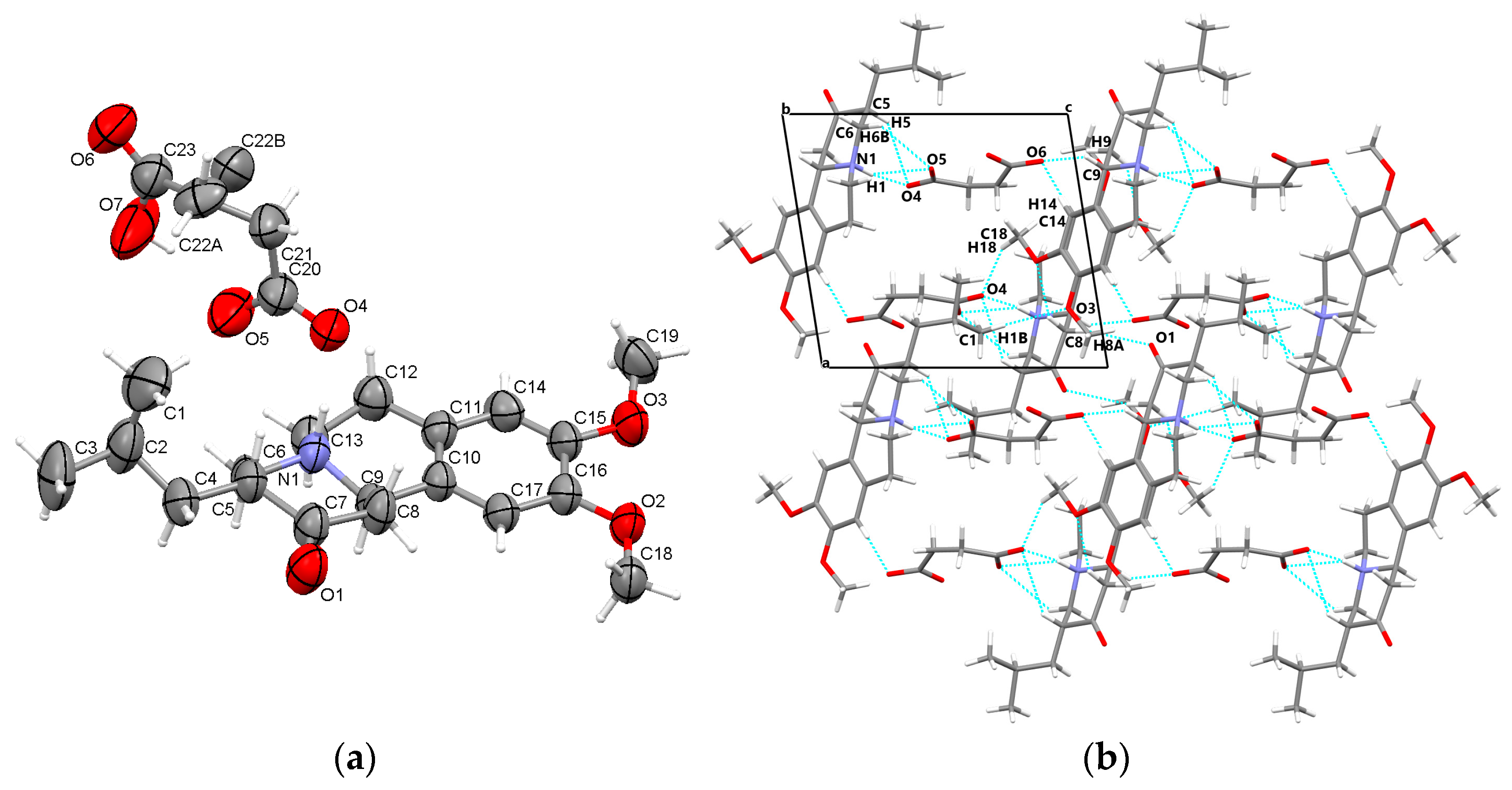
3.1.2. TBZ&FUM
3.1.3. TBZ&SUC
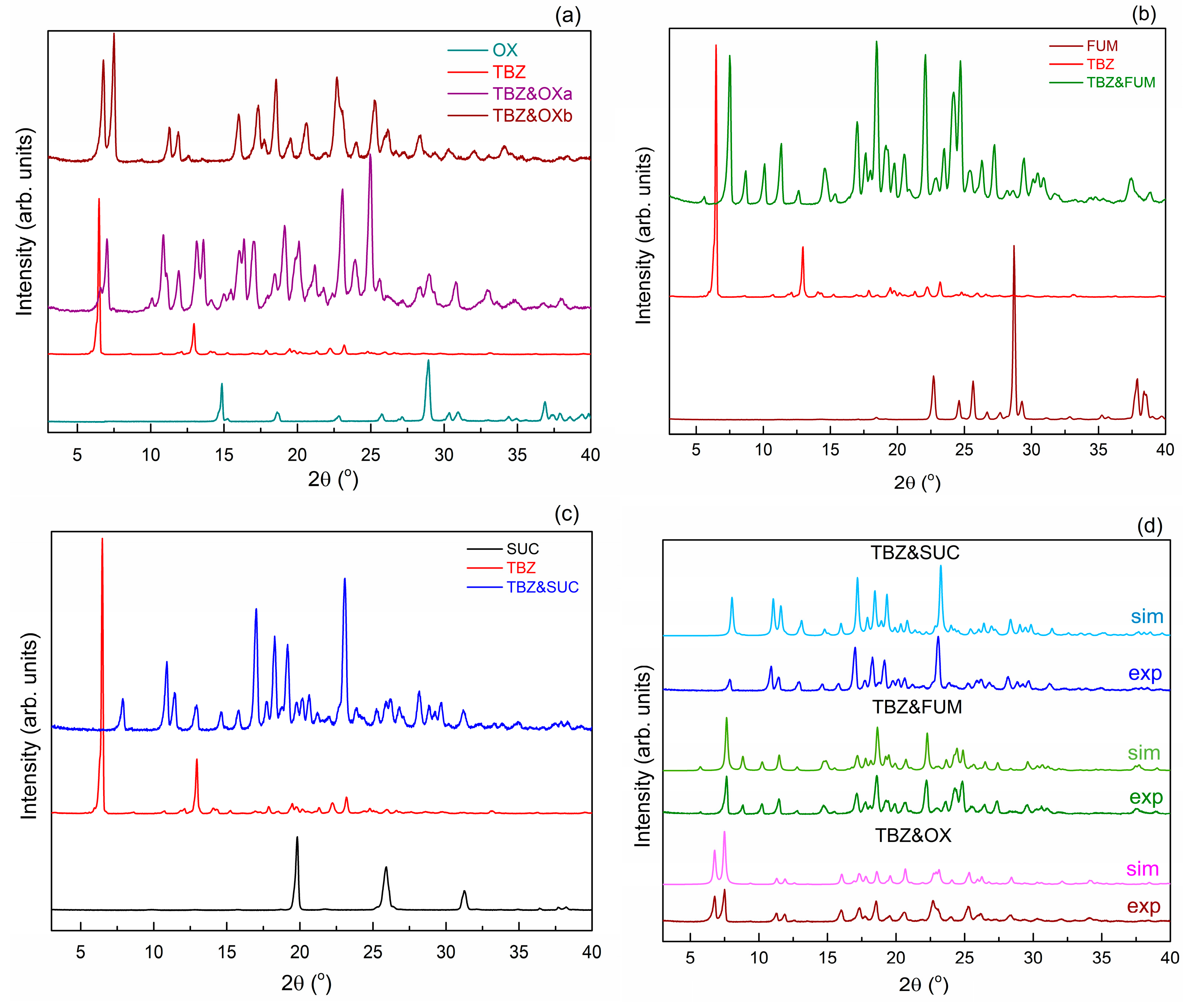
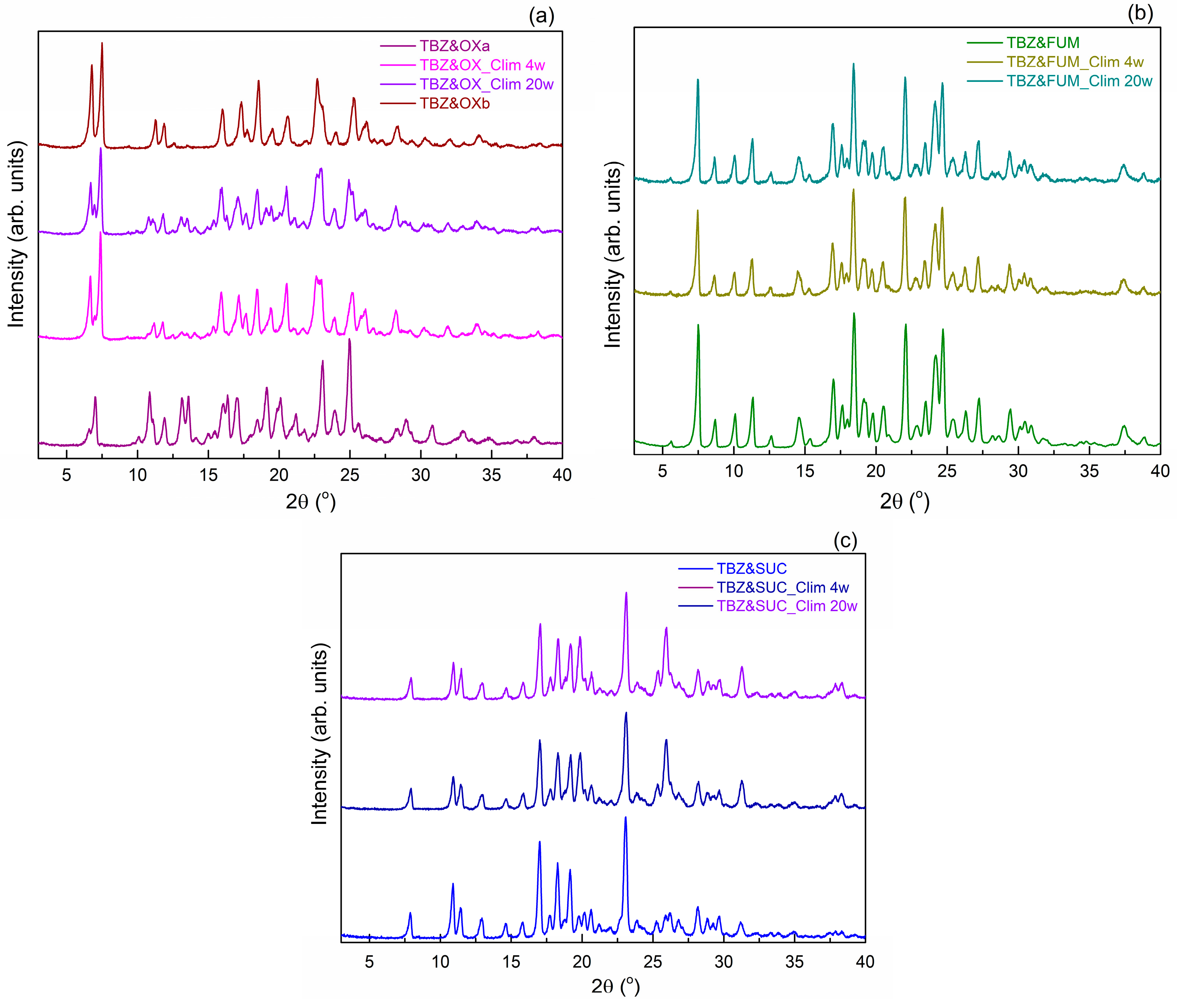
3.2. Powder X-Ray Diffraction Analysis
3.3. DSC Thermal Analysis
3.3.1. TBZ&OX
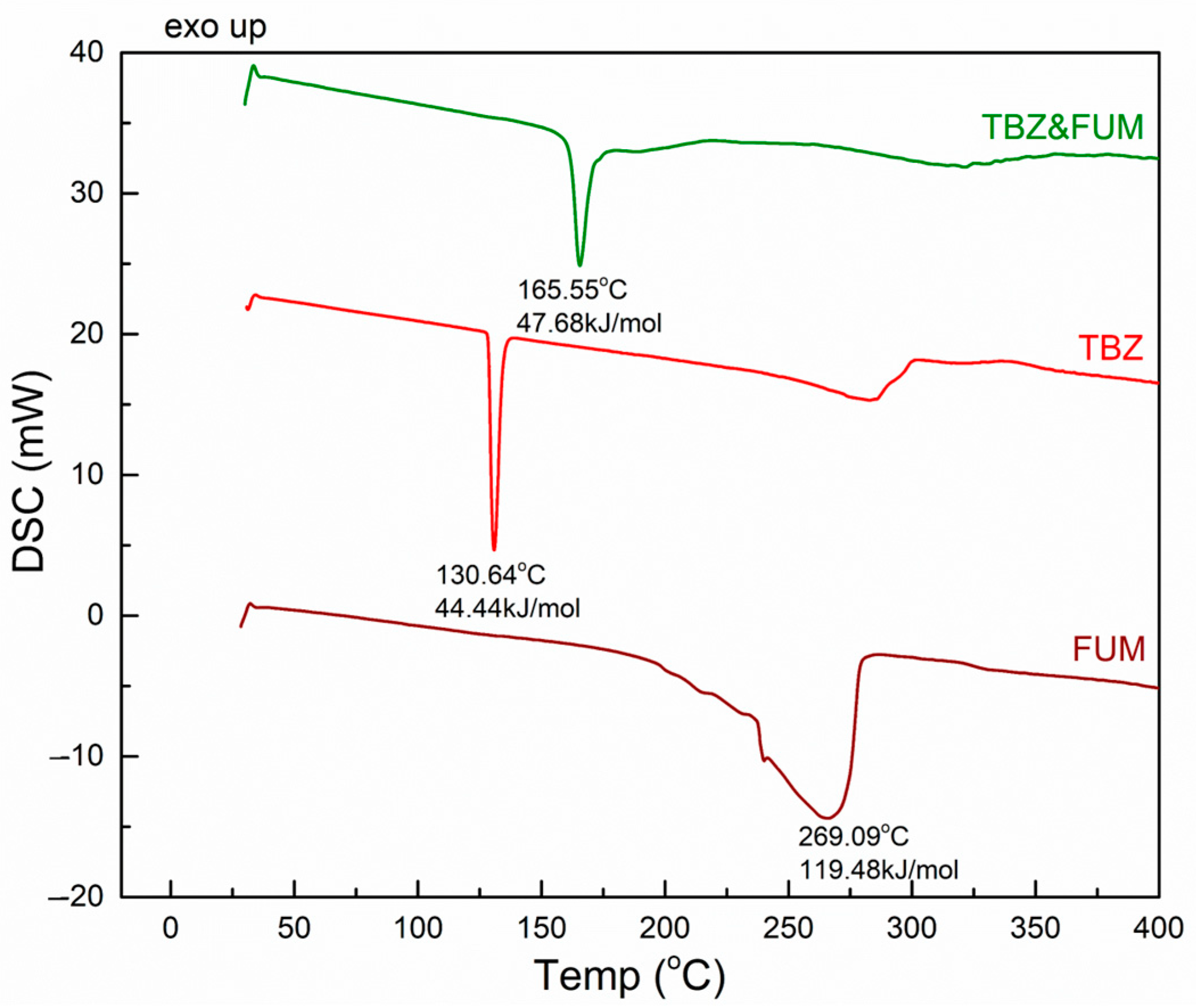
3.3.2. TBZ&FUM
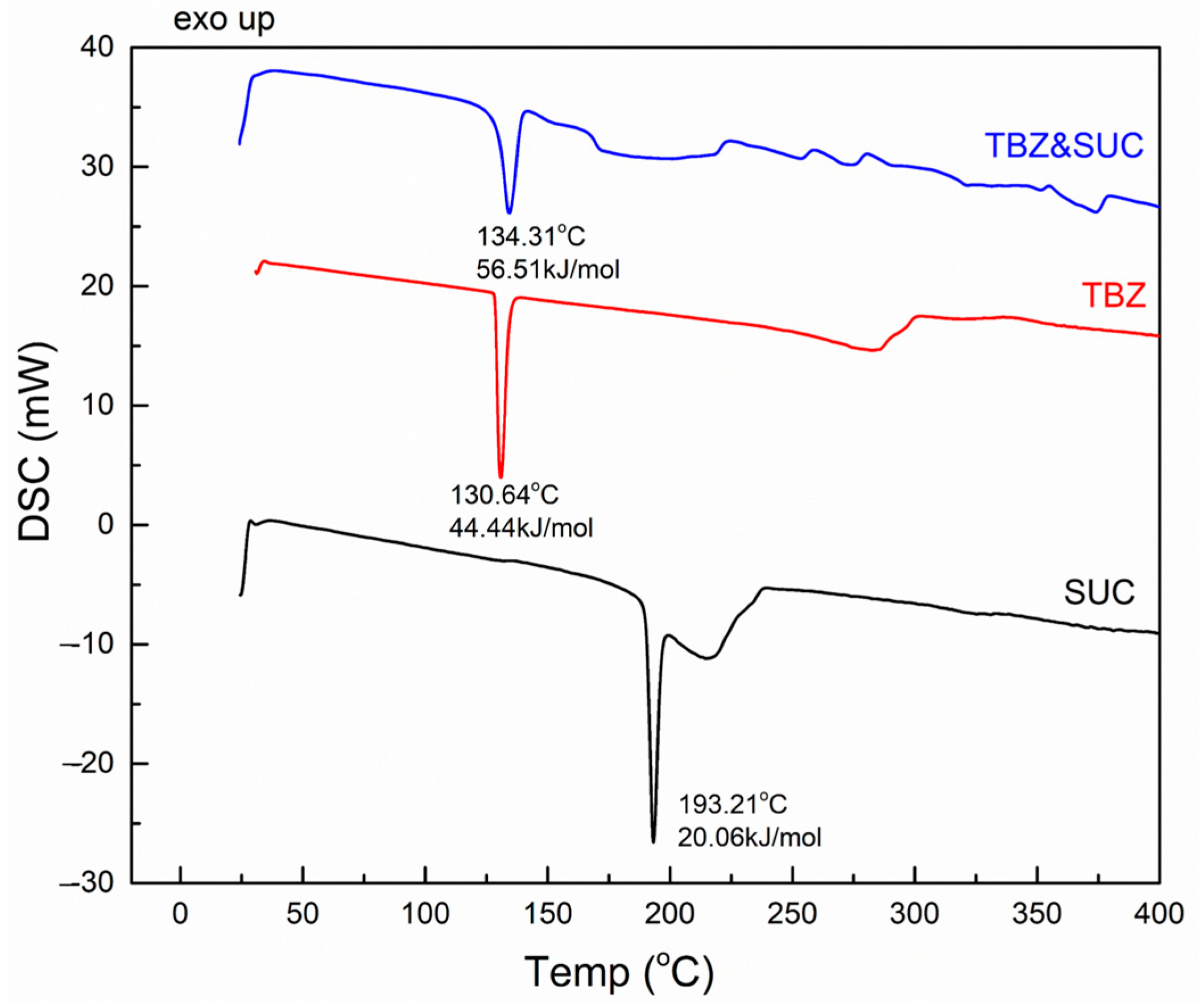
3.3.3. TBZ&SUC
3.4. Stability of the New Tetrabenazine-Based Salts
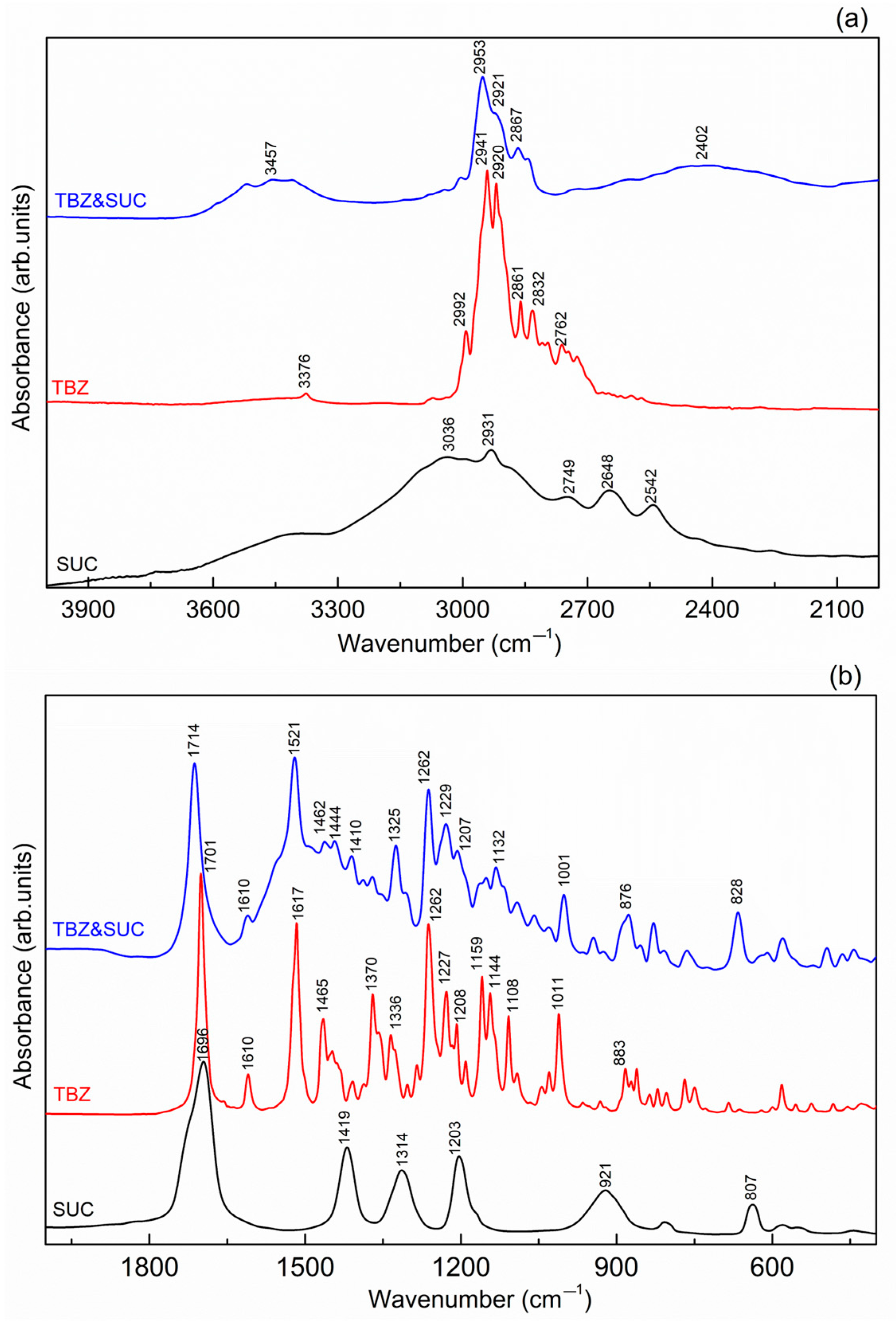
3.5. FTIR Spectroscopy
3.5.1. TBZ&OX
3.5.2. TBZ&FUM
3.5.3. TBZ&SUC
3.6. In Vitro Solubility Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Login, I.S.; Cronin, M.J.; Macleod, R.M. Tetrabenazine Has Properties of a Dopamine Receptor Antagonist. Ann. Neurol. 1982, 12, 257–262. [Google Scholar] [CrossRef]
- Leung, J.G.; Breden, E.L. Tetrabenazina Para El Tratamiento de Discinesia Tardía. Ann. Pharmacother. 2011, 45, 525–531. [Google Scholar] [CrossRef]
- Ray, P.C.; Pawar, Y.D.; Singare, D.T.; Deshpande, T.N.; Singh, G.P. Novel Process for Preparation of Tetrabenazine and Deutetrabenazine. Org. Process Res. Dev. 2018, 22, 520–526. [Google Scholar] [CrossRef]
- Ettouati, L.; Senta-Loys, Z.; Bourgeois, S.; Fenet, B.; Le Borgne, M.; Fessi, H. Behaviour of Tetrabenazine in Acid Medium: Reassessment and Impact on Formulation. Pharmaceutics 2019, 11, 44. [Google Scholar] [CrossRef]
- Schneider, F.; Stamler, D.; Bradbury, M.; Loupe, P.S.; Hellriegel, E.; Cox, D.S.; Savola, J.M.; Gordon, M.F.; Rabinovich-Guilatt, L. Pharmacokinetics of Deutetrabenazine and Tetrabenazine: Dose Proportionality and Food Effect. Clin. Pharmacol. Drug Dev. 2021, 10, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Soares-Weiser, K.; Fernandez, H.H. Tardive Dyskinesia. Semin. Neurol. 2007, 27, 159–169. [Google Scholar] [CrossRef]
- Davey, R.J. Polymorphism in Molecular Crystals Joel Bernstein. Oxford University Press, New York, 2002. Cryst. Growth Des. 2002, 2, 675–676. [Google Scholar] [CrossRef]
- Turza, A.; Borodi, G.; Miclaus, M.; Muresan-Pop, M. Exploring the Polymorphism of Selective Androgen Receptor Modulator YK11. J. Mol. Struct. 2023, 1273, 134281. [Google Scholar] [CrossRef]
- Chistyakov, D.; Sergeev, G. The Polymorphism of Drugs: New Approaches to the Synthesis of Nanostructured Polymorphs. Pharmaceutics 2020, 12, 34. [Google Scholar] [CrossRef]
- Loftsson, T. Drug Solubilization by Complexation. Int. J. Pharm. 2017, 531, 276–280. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and Their Pharmaceutical Applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Pop, M.; Kacso, I.; Grosu, I.G.; Miclǎuş, M.; Vodnar, D.; Lung, I.; Filip, G.A.; Olteanu, E.D.; Moldovan, R.; et al. Ketoconazole- p-Aminobenzoic Acid Cocrystal: Revival of an Old Drug by Crystal Engineering. Mol. Pharm. 2020, 17, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Vippagunta, S.R.; Brittain, H.G.; Grant, D.J.W. Crystalline Solids. Adv. Drug Deliv. Rev. 2001, 48, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Z.; Li, X.; Tang, J. A Concise Synthesis of Tetrabenazine and Its Crystal Structure. Mol. Cryst. Liq. Cryst. 2012, 557, 39–49. [Google Scholar] [CrossRef]
- Paek, S.M.; Kim, N.J.; Shin, D.; Jung, J.K.; Jung, J.W.; Chang, D.J.; Moon, H.; Suh, Y.G. A Concise Total Synthesis of (+)-Tetrabenazine and (+)-α-Dihydrotetrabenazine. Chem. A Eur. J. 2010, 16, 4623–4628. [Google Scholar] [CrossRef]
- Yu, Q.; Luo, W.; Deschamps, J.; Holloway, H.W.; Kopajtic, T.; Katz, J.L.; Brossi, A.; Greig, N.H. Preparation and Characterization of Tetrabenazine Enantiomers against Vesicular Monoamine Transporter 2. ACS Med. Chem. Lett. 2010, 1, 105–109. [Google Scholar] [CrossRef]
- Kukor, A.J.; Depner, N.; Cai, I.; Tucker, J.L.; Culhane, J.C.; Hein, J.E. Enantioselective Synthesis of (−)-Tetrabenazine via Continuous Crystallization-Induced Diastereomer Transformation. Chem. Sci. 2022, 13, 10765–10772. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Z.; Li, X.; Tang, J.; Qin, X. (+)-9-Benzyloxy-α-Dihydrotetrabenazine as an Important Intermediate for the VMAT2 Imaging Agents: Absolute Configuration and Chiral Recognition. Chirality 2013, 25, 215–223. [Google Scholar] [CrossRef]
- Hasa, D.; Voinovich, D.; Perissutti, B.; Grassi, M.; Bonifacio, A.; Sergo, V.; Cepek, C.; Chierotti, M.R.; Gobetto, R.; Dall’Acqua, S.; et al. Enhanced Oral Bioavailability of Vinpocetine through Mechanochemical Salt Formation: Physico-Chemical Characterization and in Vivo Studies. Pharm. Res. 2011, 28, 1870–1883. [Google Scholar] [CrossRef]
- Fischer, F.; Heidrich, A.; Greiser, S.; Benemann, S.; Rademann, K.; Emmerling, F. Polymorphism of Mechanochemically Synthesized Cocrystals: A Case Study. Cryst. Growth Des. 2016, 16, 1701–1707. [Google Scholar] [CrossRef]
- Hasa, D.; Perissutti, B.; Cepek, C.; Bhardwaj, S.; Carlino, E.; Grassi, M.; Invernizzi, S.; Voinovich, D. Drug Salt Formation via Mechanochemistry: The Case Study of Vincamine. Mol. Pharm. 2013, 10, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Mureşan-Pop, M.; Simon, S.; Bodoki, E.; Simon, V.; Turza, A.; Todea, M.; Vulpoi, A.; Magyari, K.; Iacob, B.C.; Bărăian, A.I.; et al. Mechanochemical Synthesis of New Praziquantel Cocrystals: Solid-State Characterization and Solubility. Cryst. Growth Des. 2024, 24, 4668–4681. [Google Scholar] [CrossRef]
- Muresan-Pop, M.; Vulpoi, A.; Simon, V.; Todea, M.; Magyari, K.; Pap, Z.; Simion, A.; Filip, C.; Simon, S. Co-Crystals of Etravirine by Mechanochemical Activation. J. Pharm. Sci. 2022, 111, 1178–1186. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- CrysAlisPro. Data Collection and Processing Software for Agilent X-Ray Diffractometers User Manual; revision 5.2; Read the Main Diffractometer User Manual, in Particular the Health and Safety Information, before Operating CrysAlisPro with the Diffractometer; Agilent Technologies, XRD Products: Yarnton, UK, 2013. [Google Scholar]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Sulistyowaty, M.I.; Setyawan, D.; Prameswari, P.P.M.; Susilo, R.J.K.; Amrillah, T.; Zaini, E.; Zidan, S.A.H. A Comparison Study between Green Synthesis of Microwave Irradiation and Solvent Evaporation Methods in The Formation of P-Methoxycinnamic Acid-Succinic Acid Cocrystals. Sci. Technol. Indones. 2024, 9, 629–636. [Google Scholar] [CrossRef]
- Buddhadev, S.S.; Garala, K.C. Pharmaceutical Cocrystals—A Review. Proceedings 2020, 62, 14. [Google Scholar] [CrossRef]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical Cocrystals: A Review of Preparations, Physicochemical Properties and Applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef]
- Nijhawan, M.; Godugu, M.; Saxena, T.; Farheen, T.; Dwivedi, K. Pharmaceutical Co-Crystals of Posaconazole for Improvement of Physicochemical Properties. Braz. J. Pharm. Sci. 2022, 58, e191024. [Google Scholar] [CrossRef]
- Sommer, A.; Zhang, C.; Carter, J.; Bradbury, M.; Gant, T.; Shahbaz, M. Formulations Pharmacokinetics of Deuterated Benzoquinoline Inhibitors of Vesicular Monoamine Transporter 2. Patent EP2897615A4, 27 April 2016. [Google Scholar]
- Onija, O.; Borodi, G.; Kacso, I.; Pop, M.N.; Dadarlat, D.; Bratu, I.; Jumate, N. Preparation and Characterization of Urea-Oxalic Acid Solid Form. In Proceedings of the AIP Conference Proceedings, Cluj Napoca, Romania, 29 September–1 October 2011; Volume 1425, pp. 35–38. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies; John Wiley and Sons, Ltd.: Chichester, UK, 2004; ISBN 978-0-470-09307-8. [Google Scholar]




| Crystal | TBZ&OX | TBZ&FUM | TBZ&SUC |
|---|---|---|---|
| Formula | C19H28NO3+●C2HO4− | C19H28NO3+●C4H3O4− | C19H28NO3+●C4H5O4− |
| Dcalc./g cm−3 | 1.290 | 1.268 | 1.259 |
| µ/mm−1 | 0.802 | 0.773 | 0.766 |
| Formula Weight | 407.45 | 433.49 | 434.66 |
| T/K | 293(2) | 293(2) | 293(2) |
| Crystal System | monoclinic | triclinic | triclinic |
| Space Group | P21/c | P-1 | P-1 |
| a/Å | 5.6753(4) | 6.3043(7) | 10.0954(18) |
| b/Å | 15.6643(8) | 12.0998(10) | 10.399(2) |
| c/Å | 23.6427(17) | 15.8475(12) | 11.1625(15) |
| α/° | 90 | 78.934(7) | 87.063(15) |
| β/° | 93.583(7) | 79.640(8) | 80.658(13) |
| γ/° | 90 | 75.039(8) | 82.728(17) |
| V/Å3 | 2097.7(2) | 1135.30(18) | 1146.5(4) |
| Z | 4 | 2 | 2 |
| Z’ | 1 | 1 | 1 |
| Wavelength/Å | 1.54184 | 1.54184 | 1.54184 |
| Radiation type | Cu Kα | Cu Kα | Cu Kα |
| ϴmin/° | 3.387 | 2.868 | 4.015 |
| ϴmax/° | 71.333 | 71.067 | 71.146 |
| Measured Refl. | 13,425 | 7172 | 7084 |
| Independent Refl. | 4027 | 4289 | 4315 |
| Reflections with I > 2(I) | 2603 | 2360 | 2479 |
| Rint | 0.0433 | 0.0498 | 0.0530 |
| Parameters | 272 | 293 | 295 |
| Restraints | 0 | 3 | 0 |
| Largest Peak | 0.152 | 0.308 | 0.365 |
| Deepest Hole | −0.171 | −0.294 | −0.332 |
| GooF | 1.182 | 1.030 | 1.047 |
| wR2 (all data) | 0.2186 | 0.2611 | 0.2791 |
| wR2 | 0.1987 | 0.1954 | 0.2227 |
| R1 (all data) | 0.1013 | 0.1160 | 0.1196 |
| R1 | 0.0624 | 0.0680 | 0.0821 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muresan-Pop, M.; Simon, V.; Borodi, G.; Turza, A. Developing the Oxalate, Fumarate and Succinate Salts of Tetrabenazine: Solid-State Characterization and Solubility. Pharmaceutics 2025, 17, 670. https://doi.org/10.3390/pharmaceutics17050670
Muresan-Pop M, Simon V, Borodi G, Turza A. Developing the Oxalate, Fumarate and Succinate Salts of Tetrabenazine: Solid-State Characterization and Solubility. Pharmaceutics. 2025; 17(5):670. https://doi.org/10.3390/pharmaceutics17050670
Chicago/Turabian StyleMuresan-Pop, Marieta, Viorica Simon, Gheorghe Borodi, and Alexandru Turza. 2025. "Developing the Oxalate, Fumarate and Succinate Salts of Tetrabenazine: Solid-State Characterization and Solubility" Pharmaceutics 17, no. 5: 670. https://doi.org/10.3390/pharmaceutics17050670
APA StyleMuresan-Pop, M., Simon, V., Borodi, G., & Turza, A. (2025). Developing the Oxalate, Fumarate and Succinate Salts of Tetrabenazine: Solid-State Characterization and Solubility. Pharmaceutics, 17(5), 670. https://doi.org/10.3390/pharmaceutics17050670









