Extracellular Vesicles as Precision Delivery Systems for Biopharmaceuticals: Innovations, Challenges, and Therapeutic Potential
Abstract
1. Introduction
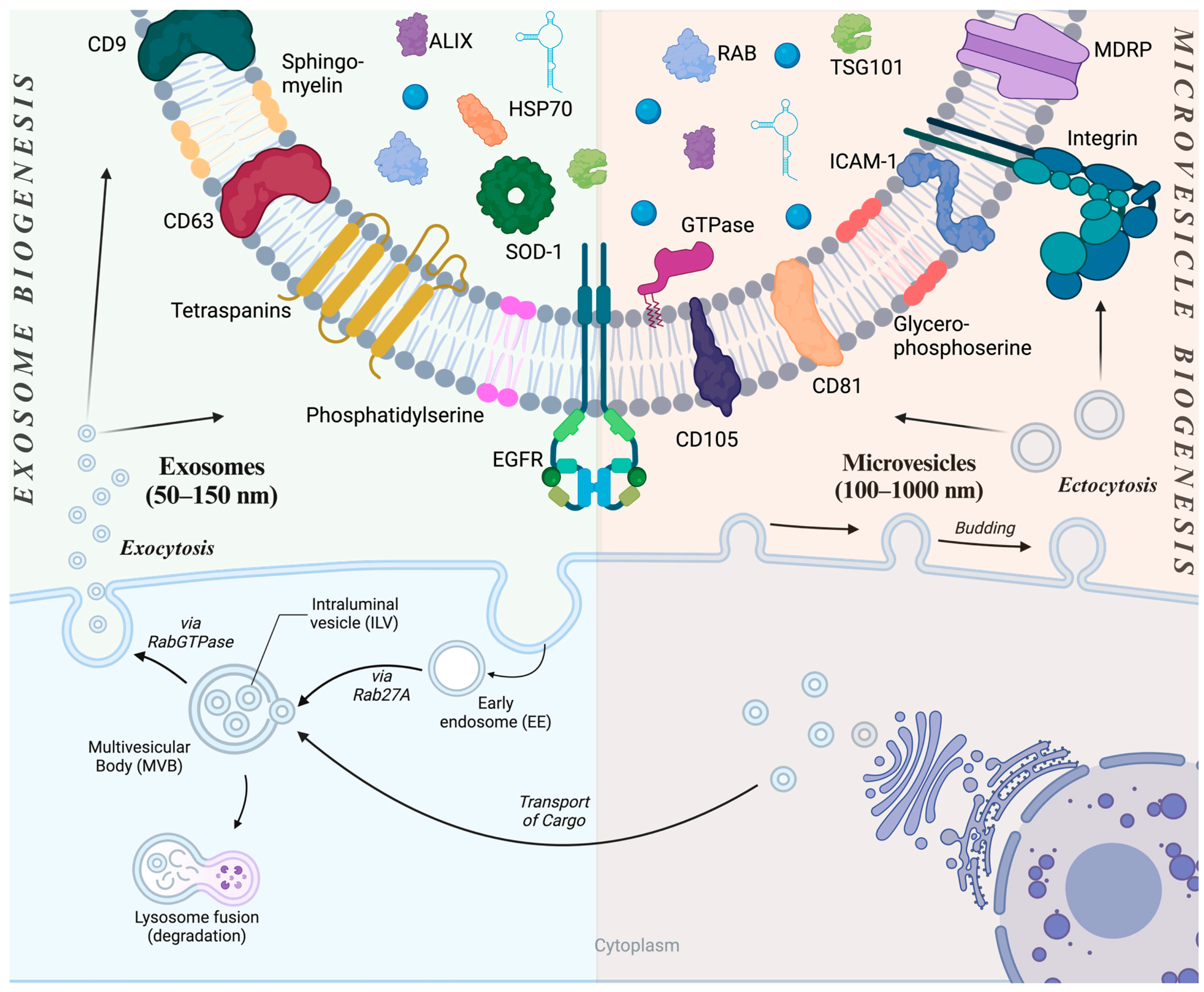
2. Extracellular Vesicles
3. Preparation of EVs for Therapeutic Purposes
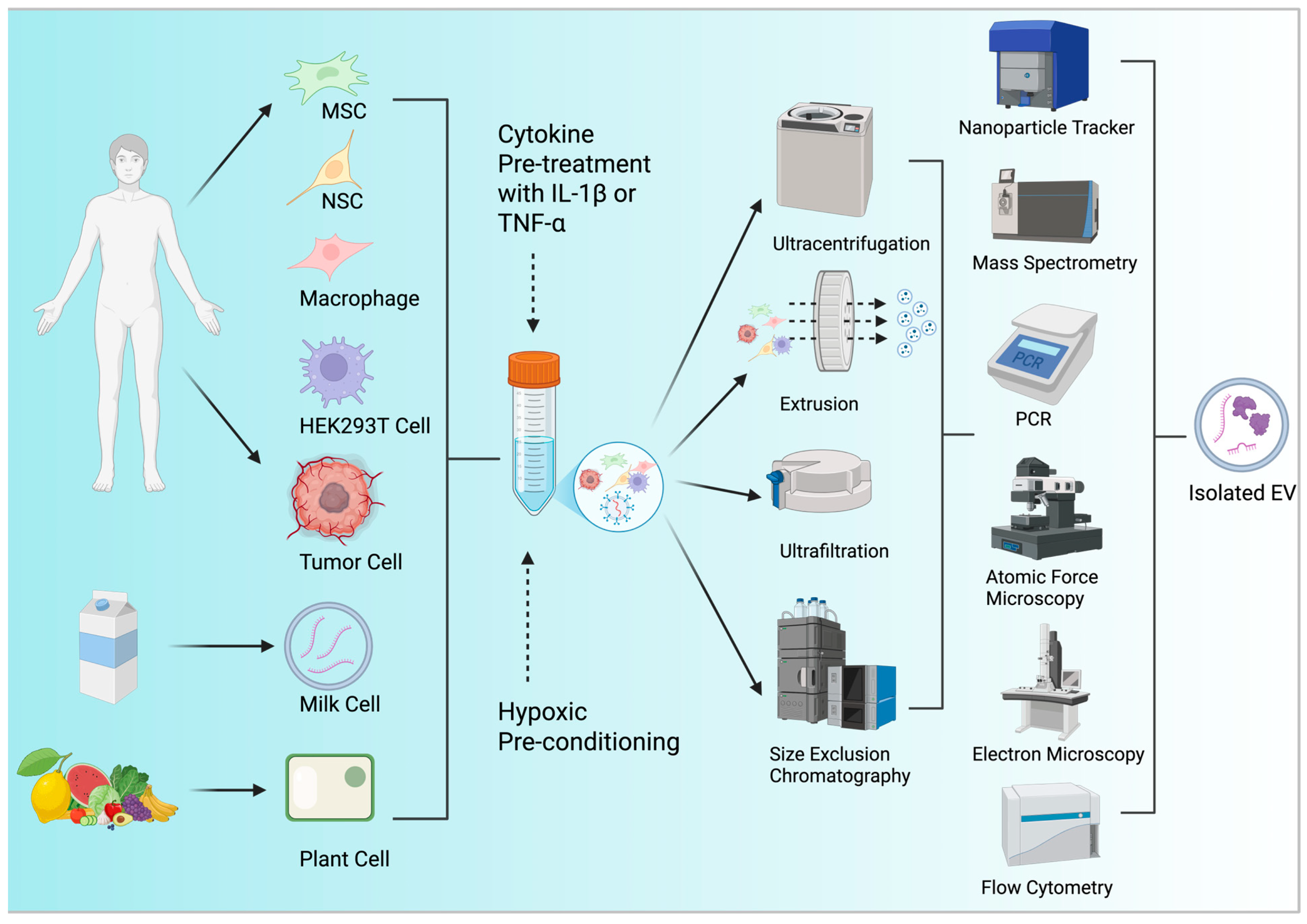
3.1. Resourcing EVs
3.2. EV Yield Optimization
3.3. EV Characterization Techniques
| EV Processing Stage | Method | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| EV Isolation | Ultracentrifugation (UC) |
|
| [79,80,81] |
| Extrusion |
|
| [82,83] | |
| Ultrafiltration (UF) |
|
| [84] | |
| Size Exclusion Chromatography (SEC) |
|
| [85,86,87] | |
| EV Characterization by Total Number of Exosomes | Nanoparticle Tracking Analysis (NTA) |
|
| [99,100] |
| EV Characterization by Surface Markers and Protein Numbers | Mass Spectrometry |
|
| [101] |
| ELISA |
|
| [90] | |
| EV Characterization by Lipid Content | Raman Spectroscopy |
|
| [102] |
| EV Characterization by DNA/RNA Content | Next Generation Sequencing (NGS) |
|
| [93] |
| Polymerase Chain Reaction (PCR) |
|
| [93] | |
| EV Characterization by Structure | Atomic Force Microscopy (AFM) |
|
| [95] |
| Scanning Electron Microscopy (SEM) |
|
| [103] | |
| EV Characterization by Size | Flow Cytometry |
|
| [104] |
| Electron Microscopy |
|
| [105] | |
| EV Characterization by Chemical Composition | Raman Spectroscopy |
|
| [102] |
| EV Characterization by Topology | Atomic Force Microscopy |
|
| [95] |
3.4. EV Cargo Loading Strategies
3.5. EV Surface Modifications
4. EVs as Delivery Vehicles for Biopharmaceuticals
| EV Source | Cargo | Disease Application | EV Surface Modifications | Therapeutic Effect | Reference |
|---|---|---|---|---|---|
| Mesenchymal Stem Cell (MSC)-derived exosomes | miR-26a | Hepatocellular Carcinoma | Lamp2b fused with anti-GPC3 single-chain variable fragment (scFv) for targeted delivery to GPC3-positive HCC cells | Downregulation of Cyclin D2 and Cyclin E2 expression, leading to inhibited tumor cell proliferation and suppressed tumor growth in vivo | [145] |
| Cancer-derived exosomes | CRISPR/Cas9 plasmid targeting PARP-1 | Ovarian Cancer | None; utilized inherent tumor tropism of cancer-derived exosome | Suppression of PARP-1 expression, leading to apoptosis in ovarian cancer cells and enhanced sensitivity to cisplatin chemotherapy | [150] |
| Dendritic cell-derived exosomes | siRNA targeting BACE1 | Alzheimer’s Disease | Lamp2b fused with rabies virus glycoprotein (RVG) peptide for neuron-specific targeting | Achieved 60% mRNA and 62% protein knockdown of BACE1 in the mouse brain, demonstrating effective gene silencing in neurons, microglia, and oligodendrocytes following systemic administration | [40] |
| Genetically engineered exosomes | Neprilysin variant | Alzheimer’s Disease | Display of RVG peptide for targeting α7 nicotinic acetylcholine receptors (α7-nAChR) | Enhanced degradation of amyloid-beta (Aβ) peptides, reduction of pro-inflammatory cytokines (IL-1α, TNF-α, NF-κB), and increased anti-inflammatory cytokine (IL-10) expression in the hippocampus | [151] |
| Macrophage-derived exosomes | Catalase enzyme | Parkinson’s Disease | None; utilized natural exosome properties for delivery | Intranasal administration of catalase-loaded exosomes (exoCAT) in a Parkinson’s disease mouse model led to significant neuroprotective effects, including reduced oxidative stress and inflammation, and improved neuronal survival | [127] |
| HEK293T-derived exosomes | Plasmid DNA encoding shRNA targeting α-synuclein | Parkinson’s Disease | Lamp2b fused with RVG peptide for targeting neurons via nicotinic acetylcholine receptors | Significant reduction of α-synuclein mRNA and protein levels in the enteric nervous system and spinal cord following intravenous administration | [146] |
| MSC-derived exosomes | mRNA encoding ZFP362-DNMT3A fusion protein (ZPAMt) | Human Immunodeficiency Virus Type 1 Infection | None; utilized natural tropism of MSC-derived exosomes | Induced stable epigenetic repression of HIV-1 by promoting DNA methylation of the viral promoter, leading to sustained suppression of viral replication in humanized mouse models and increased CD4⁺ T-cell counts | [149] |
| Small EVs from HEK293T cells | Antiviral siRNA targeting Zika virus (ZIKV) | Zika Virus Infection and Microcephaly | Surface display of RVG peptide for targeting neurons via nicotinic acetylcholine receptors | Selective delivery of siRNA to fetal brain, resulting in inhibition of ZIKV infection and mitigation of ZIKV-induced microcephaly in a mouse model | [147] |
| HEK293T cell-derived EVs | mRNA encoding low-density lipoprotein receptor (LDLR) | Familial Hypercholesterolemia (FH) | Surface functionalization with an ApoB100-derived peptide for targeted delivery to hepatocytes | Restoration of LDLR expression in hepatocytes, leading to enhanced clearance of low-density lipoprotein cholesterol (LDL-C) and amelioration of hypercholesterolemia in FH mouse models | [148] |
| Engineered exosomes displaying E7 peptide | Kartogenin (KGN) | Degenerative Joint Disease | Fusion of MSC-targeting E7 peptide with exosomal membrane protein Lamp2b | Enhanced chondrogenic differentiation of synovial fluid-derived mesenchymal stem cells (SF-MSCs) and improved cartilage regeneration in vivo | [153] |
5. Clinical Trials of EVs as Delivery Vehicles of Biopharmaceuticals
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amir, E.; Miller, N.; Geddie, W.; Freedman, O.; Kassam, F.; Simmons, C.; Oldfield, M.; Dranitsaris, G.; Tomlinson, G.; Laupacis, A.; et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J. Clin. Oncol. 2012, 30, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, S.; Zhang, J.; Zhang, L.; Chen, Y.; Wang, L.; Jin, L.; Hu, Y.; Qi, X.; Huang, H.; et al. Preimplantation Genetic Diagnosis of Multiple Endocrine Neoplasia Type 2A Using Informative Markers Identified by Targeted Sequencing. Thyroid 2018, 28, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Ying, J.; Yang, L.; Fang, W.; Han, W.; Hu, H.; Zhang, S.; Yuan, Y. Dual targeted therapy with pyrotinib and trastuzumab for HER2-positive advanced colorectal cancer: A phase 2 trial. Cancer Sci. 2023, 114, 1067–1074. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. What Are “Biologics” Questions and Answers. Available online: https://www.fda.gov/about-fda/center-biologics-evaluation-and-research-cber/what-are-biologics-questions-and-answers (accessed on 6 February 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 70678557, Insulin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Insulin (accessed on 28 January 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 2244, Aspirin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Aspirin (accessed on 28 January 2025).
- Saffran, M.; Kumar, G.S.; Savariar, C.; Burnham, J.C.; Williams, F.; Neckers, D.C. A new approach to the oral administration of insulin and other peptide drugs. Science 1986, 233, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Schilling, R.J.; Mitra, A.K. Degradation of insulin by trypsin and alpha-chymotrypsin. Pharm. Res. 1991, 8, 721–727. [Google Scholar] [CrossRef]
- Boyes, R.N.; Scott, D.B.; Jebson, P.J.; Godman, M.J.; Julian, D.G. Pharmacokinetics of lidocaine in man. Clin. Pharmacol. Ther. 1971, 12, 105–116. [Google Scholar] [CrossRef]
- Fincke, A.; Winter, J.; Bunte, T.; Olbrich, C. Thermally induced degradation pathways of three different antibody-based drug development candidates. Eur. J. Pharm. Sci. 2014, 62, 148–160. [Google Scholar] [CrossRef]
- Back, J.F.; Oakenfull, D.; Smith, M.B. Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry 1979, 18, 5191–5196. [Google Scholar] [CrossRef]
- Tobio, M.; Sanchez, A.; Vila, A.; Soriano, I.I.; Evora, C.; Vila-Jato, J.L.; Alonso, M.J. The role of PEG on the stability in digestive fluids and in vivo fate of PEG-PLA nanoparticles following oral administration. Colloids Surf. B Biointerfaces 2000, 18, 315–323. [Google Scholar] [CrossRef]
- Csaba, N.; Sanchez, A.; Alonso, M.J. PLGA:poloxamer and PLGA:poloxamine blend nanostructures as carriers for nasal gene delivery. J. Control. Release 2006, 113, 164–172. [Google Scholar] [CrossRef]
- de Souza, G.A.; Godoy, L.M.; Mann, M. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol. 2006, 7, R72. [Google Scholar] [CrossRef]
- Lee, V.H.; Chien, D.S.; Sasaki, H. Ocular ketone reductase distribution and its role in the metabolism of ocularly applied levobunolol in the pigmented rabbit. J. Pharmacol. Exp. Ther. 1988, 246, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Ma, J.; Li, Q.; Li, Y.; Zhou, X.; Zhao, D.; Song, H.; Chen, Q.; Zhu, X. Light/magnetic hyperthermia triggered drug released from multi-functional thermo-sensitive magnetoliposomes for precise cancer synergetic theranostics. J. Control. Release 2018, 272, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Vroman, L. Effect of absorbed proteins on the wettability of hydrophilic and hydrophobic solids. Nature 1962, 196, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Xu, C.; Wang, Y.; Zhou, X.; Fang, L.; Cao, F. Multifunctional Nanocomposites Based on Liposomes and Layered Double Hydroxides Conjugated with Glycylsarcosine for Efficient Topical Drug Delivery to the Posterior Segment of the Eye. Mol. Pharm. 2019, 16, 2845–2857. [Google Scholar] [CrossRef]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of Wood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filen, J.J.; Lahesmaa, R.; Norman, M.; Neve, E.P.; Scheynius, A.; Gabrielsson, S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kanai-Azuma, M.; Akimoto, Y.; Kawakami, H.; Yanoshita, R. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol. Pharm. Bull. 2008, 31, 1059–1062. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef]
- Pan, B.T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985, 101, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Katzmann, D.J.; Babst, M.; Emr, S.D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 2001, 106, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Raymond, C.K.; Howald-Stevenson, I.; Vater, C.A.; Stevens, T.H. Morphological classification of the yeast vacuolar protein sorting mutants: Evidence for a prevacuolar compartment in class E vps mutants. Mol. Biol. Cell 1992, 3, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Babst, M.; Sato, T.K.; Banta, L.M.; Emr, S.D. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 1997, 16, 1820–1831. [Google Scholar] [CrossRef]
- Escola, J.M.; Kleijmeer, M.J.; Stoorvogel, W.; Griffith, J.M.; Yoshie, O.; Geuze, H.J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 1998, 273, 20121–20127. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Regnault, A.; Garin, J.; Wolfers, J.; Zitvogel, L.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 1999, 147, 599–610. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Febbraio, M.A. Exosome-dependent trafficking of HSP70: A novel secretory pathway for cellular stress proteins. J. Biol. Chem. 2005, 280, 23349–23355. [Google Scholar] [CrossRef]
- Nabhan, J.F.; Hu, R.; Oh, R.S.; Cohen, S.N.; Lu, Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. USA 2012, 109, 4146–4151. [Google Scholar] [CrossRef]
- Taylor, J.; Azimi, I.; Monteith, G.; Bebawy, M. Ca2+ mediates extracellular vesicle biogenesis through alternate pathways in malignancy. J. Extracell. Vesicles 2020, 9, 1734326. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 2009, 19, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, A.; Majkowska, I.; Nagase, H.; Di Liegro, I.; Troeberg, L. Microvesicles shed by oligodendroglioma cells and rheumatoid synovial fibroblasts contain aggrecanase activity. Matrix Biol. 2012, 31, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, C.; Wang, H.; Wang, L.; Xiao, F.; Guo, Z.; Zhang, H. Surface Phosphatidylserine Is Responsible for the Internalization on Microvesicles Derived from Hypoxia-Induced Human Bone Marrow Mesenchymal Stem Cells into Human Endothelial Cells. PLoS ONE 2016, 11, e0147360. [Google Scholar] [CrossRef] [PubMed]
- Kanada, M.; Bachmann, M.H.; Hardy, J.W.; Frimannson, D.O.; Bronsart, L.; Wang, A.; Sylvester, M.D.; Schmidt, T.L.; Kaspar, R.L.; Butte, M.J.; et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Natl. Acad. Sci. USA 2015, 112, E1433–E1442. [Google Scholar] [CrossRef]
- Tian, T.; Zhu, Y.L.; Hu, F.H.; Wang, Y.Y.; Huang, N.P.; Xiao, Z.D. Dynamics of exosome internalization and trafficking. J. Cell Physiol. 2013, 228, 1487–1495. [Google Scholar] [CrossRef]
- Somiya, M.; Kuroda, S.i. Verification of extracellular vesicle-mediated functional mRNA delivery via RNA editing. bioRxiv 2022. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Huang, W.; Qu, M.; Li, L.; Liu, T.; Lin, M.; Yu, X. SiRNA in MSC-derived exosomes silences CTGF gene for locomotor recovery in spinal cord injury rats. Stem Cell Res. Ther. 2021, 12, 334. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hong, Y.; Bae, Y.R.; Goo, J.; Kim, S.A.; Choi, Y.; Nam, G.H.; Kwon, M.; Yun, S.G.; Lee, G.; et al. Advantage of extracellular vesicles in hindering the CD47 signal for cancer immunotherapy. J. Control. Release 2022, 351, 727–738. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Fliervoet, L.A.L.; van der Meel, R.; Fens, M.; Heijnen, H.F.G.; van Bergen En Henegouwen, P.M.P.; Vader, P.; Schiffelers, R.M. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Control. Release 2016, 224, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Graf, I.; Kuang, Y.; Zheng, X.; Haupt, M.; Majid, A.; Kilic, E.; Hermann, D.M.; Psychogios, M.N.; Weber, M.S.; et al. Neural Progenitor Cell-Derived Extracellular Vesicles Enhance Blood-Brain Barrier Integrity by NF-κB (Nuclear Factor-kappaB)-Dependent Regulation of ABCB1 (ATP-Binding Cassette Transporter B1) in Stroke Mice. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1127–1145. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wu, W.; Xu, X.; Liao, L.; Zheng, F.; Messinger, S.; Sun, X.; Chen, J.; Yang, S.; Cai, J.; et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: A randomized controlled trial. JAMA 2012, 307, 1169–1177. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Bruno, S.; Chatterjee, D.; Quesenberry, P.J.; Tetta, C.; Camussi, G. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int. J. Mol. Med. 2014, 33, 1055–1063. [Google Scholar] [CrossRef]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef]
- Gennai, S.; Monsel, A.; Hao, Q.; Park, J.; Matthay, M.A.; Lee, J.W. Microvesicles Derived From Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. Am. J. Transplant. 2015, 15, 2404–2412. [Google Scholar] [CrossRef]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef]
- Tieu, A.; Stewart, D.J.; Chwastek, D.; Lansdell, C.; Burger, D.; Lalu, M.M. Biodistribution of mesenchymal stromal cell-derived extracellular vesicles administered during acute lung injury. Stem Cell Res. Ther. 2023, 14, 250. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Wang, J.; Wang, P.; Liu, X.; Liu, P.; Cheng, X.; Cao, L.; Wu, H.; Chen, J.; Zhou, L. Neural stem cell-derived exosomes and regeneration: Cell-free therapeutic strategies for traumatic brain injury. Stem Cell Res. Ther. 2023, 14, 198. [Google Scholar] [CrossRef]
- Qian, C.; Wang, Y.; Ji, Y.; Chen, D.; Wang, C.; Zhang, G.; Wang, Y. Neural stem cell-derived exosomes transfer miR-124-3p into cells to inhibit glioma growth by targeting FLOT2. Int. J. Oncol. 2022, 61, 115. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Joshi, B.S.; Zuhorn, I.S. Heparan sulfate proteoglycan-mediated dynamin-dependent transport of neural stem cell exosomes in an in vitro blood-brain barrier model. Eur. J. Neurosci. 2021, 53, 706–719. [Google Scholar] [CrossRef]
- Graham, F.L.; Smiley, J.; Russell, W.C.; Nairn, R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977, 36, 59–74. [Google Scholar] [CrossRef]
- Lin, Y.C.; Boone, M.; Meuris, L.; Lemmens, I.; Van Roy, N.; Soete, A.; Reumers, J.; Moisse, M.; Plaisance, S.; Drmanac, R.; et al. Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nat. Commun. 2014, 5, 4767. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Yi, J.; Liu, Y.; Li, D.; Wang, J.; Hou, D.; Jiang, X.; Zhang, J.; Wang, J.; et al. Identification and Characterization of 293T Cell-Derived Exosomes by Profiling the Protein, mRNA and MicroRNA Components. PLoS ONE 2016, 11, e0163043. [Google Scholar] [CrossRef]
- Dellgren, C.; Nehlin, J.O.; Barington, T. Cell surface expression level variation between two common Human Leukocyte Antigen alleles, HLA-A2 and HLA-B8, is dependent on the structure of the C terminal part of the alpha 2 and the alpha 3 domains. PLoS ONE 2015, 10, e0135385. [Google Scholar] [CrossRef]
- Giles, J.R.; Globig, A.M.; Kaech, S.M.; Wherry, E.J. CD8+ T cells in the cancer-immunity cycle. Immunity 2023, 56, 2231–2253. [Google Scholar] [CrossRef]
- Betker, J.L.; Angle, B.M.; Graner, M.W.; Anchordoquy, T.J. The Potential of Exosomes from Cow Milk for Oral Delivery. J. Pharm. Sci. 2019, 108, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.; Chisanga, D.; Liem, M.; Keerthikumar, S.; Anand, S.; Ang, C.S.; Adda, C.G.; Versteegen, E.; Jois, M.; Mathivanan, S. Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth. Sci. Rep. 2017, 7, 5933. [Google Scholar] [CrossRef] [PubMed]
- Quan, S.; Du, C.; Wang, K.; Nan, X.; Xiong, B. Different Diets Change Milk Extracellular Vesicle-Protein Profile in Lactating Cows. Agriculture 2022, 12, 1234. [Google Scholar] [CrossRef]
- Castellani, S.; Basirico, L.; Maggiolino, A.; Lecchi, C.; De Palo, P.; Bernabucci, U. Effects of milk extracellular vesicles from Holstein Friesian and Brown Swiss heat-stressed dairy cows on bovine mammary epithelial cells. J. Dairy. Sci. 2025, 108, 1978–1991. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhao, W.; Wu, C.; Wang, X.; Chen, J.; Shi, X.; Sha, S.; Li, J.; Liang, X.; Yang, Y.; et al. Lemon-Derived Extracellular Vesicles Nanodrugs Enable to Efficiently Overcome Cancer Multidrug Resistance by Endocytosis-Triggered Energy Dissipation and Energy Production Reduction. Adv. Sci. 2022, 9, e2105274. [Google Scholar] [CrossRef]
- Luo, L.; Wu, Z.; Wang, Y.; Li, H. Regulating the production and biological function of small extracellular vesicles: Current strategies, applications and prospects. J. Nanobiotechnol. 2021, 19, 422. [Google Scholar] [CrossRef]
- Kojima, R.; Bojar, D.; Rizzi, G.; Hamri, G.C.; El-Baba, M.D.; Saxena, P.; Auslander, S.; Tan, K.R.; Fussenegger, M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018, 9, 1305. [Google Scholar] [CrossRef]
- Xiong, Y.; Tang, R.; Xu, J.; Jiang, W.; Gong, Z.; Zhang, L.; Li, X.; Ning, Y.; Huang, P.; Xu, J.; et al. Sequential transplantation of exosomes and mesenchymal stem cells pretreated with a combination of hypoxia and Tongxinluo efficiently facilitates cardiac repair. Stem Cell Res. Ther. 2022, 13, 63. [Google Scholar] [CrossRef]
- Kumar, A.; Deep, G. Hypoxia in tumor microenvironment regulates exosome biogenesis: Molecular mechanisms and translational opportunities. Cancer Lett. 2020, 479, 23–30. [Google Scholar] [CrossRef]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef]
- Gupta, S.; Rawat, S.; Krishnakumar, V.; Rao, E.P.; Mohanty, S. Hypoxia preconditioning elicit differential response in tissue-specific MSCs via immunomodulation and exosomal secretion. Cell Tissue Res. 2022, 388, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Dorayappan, K.D.P.; Wanner, R.; Wallbillich, J.J.; Saini, U.; Zingarelli, R.; Suarez, A.A.; Cohn, D.E.; Selvendiran, K. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: A novel mechanism linking STAT3/Rab proteins. Oncogene 2018, 37, 3806–3821. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dou, H.; Li, X.; Zhao, X.; Li, Y.; Liu, D.; Ji, J.; Liu, F.; Ding, L.; Ni, Y.; et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1beta-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells 2017, 35, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shin, D.I.; Choi, B.H.; Min, B.H. Exosomes from IL-1beta-Primed Mesenchymal Stem Cells Inhibited IL-1beta- and TNF-alpha-Mediated Inflammatory Responses in Osteoarthritic SW982 Cells. Tissue Eng. Regen. Med. 2021, 18, 525–536. [Google Scholar] [CrossRef]
- Nakao, Y.; Fukuda, T.; Zhang, Q.; Sanui, T.; Shinjo, T.; Kou, X.; Chen, C.; Liu, D.; Watanabe, Y.; Hayashi, C.; et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021, 122, 306–324. [Google Scholar] [CrossRef]
- Coughlan, C.; Bruce, K.D.; Burgy, O.; Boyd, T.D.; Michel, C.R.; Garcia-Perez, J.E.; Adame, V.; Anton, P.; Bettcher, B.M.; Chial, H.J.; et al. Exosome Isolation by Ultracentrifugation and Precipitation and Techniques for Downstream Analyses. Curr. Protoc. Cell Biol. 2020, 88, e110. [Google Scholar] [CrossRef]
- Linares, R.; Tan, S.; Gounou, C.; Arraud, N.; Brisson, A.R. High-speed centrifugation induces aggregation of extracellular vesicles. J. Extracell. Vesicles 2015, 4, 29509. [Google Scholar] [CrossRef]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef]
- Guo, P.; Busatto, S.; Huang, J.; Morad, G.; Moses, M.A. A facile magnetic extrusion method for preparing endosome-derived vesicles for cancer drug delivery. Adv. Funct. Mater. 2021, 31, 2008326. [Google Scholar] [CrossRef]
- Wen, Y.; Fu, Q.; Soliwoda, A.; Zhang, S.; Zheng, M.; Mao, W.; Wan, Y. Cell-derived nanovesicles prepared by membrane extrusion are good substitutes for natural extracellular vesicles. Extracell. Vesicle 2022, 1, 100004. [Google Scholar] [CrossRef]
- Shu, S.; Yang, Y.; Allen, C.L.; Hurley, E.; Tung, K.H.; Minderman, H.; Wu, Y.; Ernstoff, M.S. Purity and yield of melanoma exosomes are dependent on isolation method. J. Extracell. Vesicles 2020, 9, 1692401. [Google Scholar] [CrossRef] [PubMed]
- Gamez-Valero, A.; Monguio-Tortajada, M.; Carreras-Planella, L.; Franquesa, M.; Beyer, K.; Borras, F.E. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents. Sci. Rep. 2016, 6, 33641. [Google Scholar] [CrossRef] [PubMed]
- Welton, J.L.; Webber, J.P.; Botos, L.A.; Jones, M.; Clayton, A. Ready-made chromatography columns for extracellular vesicle isolation from plasma. J. Extracell. Vesicles 2015, 4, 27269. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Mager, I.; Lee, Y.; Gorgens, A.; Bultema, J.; Giebel, B.; Wood, M.J.A.; Nordin, J.Z.; Andaloussi, S.E. Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci. Rep. 2017, 7, 11561. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.; Hole, P.; Carr, B.; Redman, C.W.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 2011, 7, 780–788. [Google Scholar] [CrossRef]
- Wang, L.; Skotland, T.; Berge, V.; Sandvig, K.; Llorente, A. Exosomal proteins as prostate cancer biomarkers in urine: From mass spectrometry discovery to immunoassay-based validation. Eur. J. Pharm. Sci. 2017, 98, 80–85. [Google Scholar] [CrossRef]
- Yokoyama, S.; Takeuchi, A.; Yamaguchi, S.; Mitani, Y.; Watanabe, T.; Matsuda, K.; Hotta, T.; Shively, J.E.; Yamaue, H. Clinical implications of carcinoembryonic antigen distribution in serum exosomal fraction-Measurement by ELISA. PLoS ONE 2017, 12, e0183337. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Cheng, L.; Sharples, R.A.; Scicluna, B.J.; Hill, A.F. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J. Extracell. Vesicles 2014, 3, 23743. [Google Scholar] [CrossRef]
- Khodakov, D.; Wang, C.; Zhang, D.Y. Diagnostics based on nucleic acid sequence variant profiling: PCR, hybridization, and NGS approaches. Adv. Drug Deliv. Rev. 2016, 105, 3–19. [Google Scholar] [CrossRef]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef] [PubMed]
- Parisse, P.; Rago, I.; Ulloa Severino, L.; Perissinotto, F.; Ambrosetti, E.; Paoletti, P.; Ricci, M.; Beltrami, A.P.; Cesselli, D.; Casalis, L. Atomic force microscopy analysis of extracellular vesicles. Eur. Biophys. J. 2017, 46, 813–820. [Google Scholar] [CrossRef]
- Sharma, S.; Rasool, H.I.; Palanisamy, V.; Mathisen, C.; Schmidt, M.; Wong, D.T.; Gimzewski, J.K. Structural-mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopy. ACS Nano 2010, 4, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Mun, J.Y. Sample Preparation and Imaging of Exosomes by Transmission Electron Microscopy. J. Vis. Exp. 2018, e56482. [Google Scholar] [CrossRef]
- Morales-Kastresana, A.; Jones, J.C. Flow Cytometric Analysis of Extracellular Vesicles. Methods Mol. Biol. 2017, 1545, 215–225. [Google Scholar] [CrossRef]
- Kanwar, S.S.; Dunlay, C.J.; Simeone, D.M.; Nagrath, S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip 2014, 14, 1891–1900. [Google Scholar] [CrossRef]
- Yang, F.; Liao, X.; Tian, Y.; Li, G. Exosome separation using microfluidic systems: Size-based, immunoaffinity-based and dynamic methodologies. Biotechnol. J. 2017, 12, 1600699. [Google Scholar] [CrossRef]
- Makawita, S.; Diamandis, E.P. The bottleneck in the cancer biomarker pipeline and protein quantification through mass spectrometry-based approaches: Current strategies for candidate verification. Clin. Chem. 2010, 56, 212–222. [Google Scholar] [CrossRef]
- Liangsupree, T.; Multia, E.; Saarinen, J.; Ruiz-Jimenez, J.; Kemell, M.; Riekkola, M.L. Raman spectroscopy combined with comprehensive gas chromatography for label-free characterization of plasma-derived extracellular vesicle subpopulations. Anal. Biochem. 2022, 647, 114672. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, W.; Klinke, D.J., II. Exosomes: Improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst 2015, 140, 6631–6642. [Google Scholar] [CrossRef]
- Varga, Z.; Yuana, Y.; Grootemaat, A.E.; van der Pol, E.; Gollwitzer, C.; Krumrey, M.; Nieuwland, R. Towards traceable size determination of extracellular vesicles. J. Extracell. Vesicles 2014, 3, 23298. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, S.; Haag, P.; Viktorsson, K.; Krozer, A.; Fogel, K.; Lewensohn, R.; Linnros, J.; Dev, A. Comparison and optimization of nanoscale extracellular vesicle imaging by scanning electron microscopy for accurate size-based profiling and morphological analysis. Nanoscale Adv. 2021, 3, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jones, T.W.; Dutta, S.; Zhu, Y.; Wang, X.; Narayanan, S.P.; Fagan, S.C.; Zhang, D. Overview and Update on Methods for Cargo Loading into Extracellular Vesicles. Processes 2021, 9, 356. [Google Scholar] [CrossRef]
- Xu, C.; Zhai, Z.; Ying, H.; Lu, L.; Zhang, J.; Zeng, Y. Curcumin primed ADMSCs derived small extracellular vesicle exert enhanced protective effects on osteoarthritis by inhibiting oxidative stress and chondrocyte apoptosis. J. Nanobiotechnol. 2022, 20, 123. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Li, G.; Chen, J.; Yang, Y.; Bian, L.; Zhou, J.; Wu, Y.; Chen, Y. Enhanced Therapeutic Potential of Hybrid Exosomes Loaded with Paclitaxel for Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 3645. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, C.; Zhang, H.; Shi, H.; Mao, F.; Qian, H.; Xu, W.; Wang, D.; Pan, J.; Fang, X.; et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci. Adv. 2022, 8, eabj8207. [Google Scholar] [CrossRef]
- Benchimol, M.J.; Hsu, M.J.; Schutt, C.E.; Hall, D.J.; Mattrey, R.F.; Esener, S.C. Phospholipid/Carbocyanine Dye-Shelled Microbubbles as Ultrasound-Modulated Fluorescent Contrast Agents. Soft Matter 2013, 9, 2384–2388. [Google Scholar] [CrossRef]
- Schutt, C.E.; Ibsen, S.; Benchimol, M.; Hsu, M.; Esener, S. Optical detection of harmonic oscillations in fluorescent dye-loaded microbubbles ensonified by ultrasound. Opt. Lett. 2015, 40, 2834–2837. [Google Scholar] [CrossRef]
- Bronisz, A.; Wang, Y.; Nowicki, M.O.; Peruzzi, P.; Ansari, K.; Ogawa, D.; Balaj, L.; De Rienzo, G.; Mineo, M.; Nakano, I.; et al. Extracellular vesicles modulate the glioblastoma microenvironment via a tumor suppression signaling network directed by miR-1. Cancer Res. 2014, 74, 738–750. [Google Scholar] [CrossRef]
- Li, D.; Huang, S.; Zhu, J.; Hu, T.; Han, Z.; Zhang, S.; Zhao, J.; Chen, F.; Lei, P. Exosomes from MiR-21-5p-Increased Neurons Play a Role in Neuroprotection by Suppressing Rab11a-Mediated Neuronal Autophagy In Vitro After Traumatic Brain Injury. Med. Sci. Monit. 2019, 25, 1871–1885. [Google Scholar] [CrossRef]
- Monfared, H.; Jahangard, Y.; Nikkhah, M.; Mirnajafi-Zadeh, J.; Mowla, S.J. Potential Therapeutic Effects of Exosomes Packed With a miR-21-Sponge Construct in a Rat Model of Glioblastoma. Front. Oncol. 2019, 9, 782. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.L.; Yao, J.L.; Wang, K.; Ai, H. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Endometrial Cancer Cell Proliferation and Migration through Delivery of Exogenous miR-302a. Stem Cells Int. 2019, 2019, 8108576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Bai, M.; Wang, J.; Zhu, K.; Liu, R.; Ge, S.; Li, J.; Ning, T.; Deng, T.; et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018, 109, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Sun, J.; Qiu, J.; Chen, G.; Wang, X.; Mu, Y.; Li, K.; Wang, W. Antitumor Activity of Cabazitaxel and MSC-TRAIL Derived Extracellular Vesicles in Drug-Resistant Oral Squamous Cell Carcinoma. Cancer Manag. Res. 2020, 12, 10809–10820. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Zhao, Y.; Jin, Y.S.; Li, S.M.; Bago, J.R.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Macrophage-Derived Extracellular Vesicles as Drug Delivery Systems for Triple Negative Breast Cancer (TNBC) Therapy. J. Neuroimmune Pharmacol. 2020, 15, 487–500. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Mamand, D.R.; Mohammad, D.K.; Zheng, W.; Jawad Wiklander, R.; Sych, T.; Zickler, A.M.; Liang, X.; Sharma, H.; Lavado, A.; et al. Antibody-displaying extracellular vesicles for targeted cancer therapy. Nat. Biomed. Eng. 2024, 8, 1453–1468. [Google Scholar] [CrossRef]
- Danilushkina, A.A.; Emene, C.C.; Barlev, N.A.; Gomzikova, M.O. Strategies for Engineering of Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 13247. [Google Scholar] [CrossRef]
- Guo, W.; Shu, Q.; Gao, L.; Gao, N.; Wang, Z.; Wei, W.; Zhang, Y.; Huyan, T.; Li, Q. A bibliometric analysis of extracellular vesicles as drug delivery vehicles in disease treatment (2010–2024). Extracell. Vesicle 2024, 4, 100051. [Google Scholar] [CrossRef]
- Wang, Z.; Rich, J.; Hao, N.; Gu, Y.; Chen, C.; Yang, S.; Zhang, P.; Huang, T.J. Acoustofluidics for simultaneous nanoparticle-based drug loading and exosome encapsulation. Microsyst. Nanoeng. 2022, 8, 45. [Google Scholar] [CrossRef]
- Abas, B.I.; Demirbolat, G.M.; Cevik, O. Wharton jelly-derived mesenchymal stem cell exosomes induce apoptosis and suppress EMT signaling in cervical cancer cells as an effective drug carrier system of paclitaxel. PLoS ONE 2022, 17, e0274607. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Harrison, E.B.; Zhao, Y.; Kabanov, A.V.; Batrakova, E.V. TPP1 Delivery to Lysosomes with Extracellular Vesicles and their Enhanced Brain Distribution in the Animal Model of Batten Disease. Adv. Healthc. Mater. 2019, 8, e1801271. [Google Scholar] [CrossRef] [PubMed]
- Yerneni, S.S.; Yalcintas, E.P.; Smith, J.D.; Averick, S.; Campbell, P.G.; Ozdoganlar, O.B. Skin-targeted delivery of extracellular vesicle-encapsulated curcumin using dissolvable microneedle arrays. Acta Biomater. 2022, 149, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh Gharehdaghi, E.; Amani, A.; Khoshayand, M.R.; Banan, M.; Esmaeilzadeh Gharehdaghi, E.; Amini, M.A.; Faramarzi, M.A. Chitosan nanoparticles for siRNA delivery: Optimization of processing/formulation parameters. Nucleic Acid. Ther. 2014, 24, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Liang, S.; Xu, H.; Ye, B.C. Membrane-Decorated Exosomes for Combination Drug Delivery and Improved Glioma Therapy. Langmuir 2022, 38, 299–308. [Google Scholar] [CrossRef]
- Zhou, S.; Hu, T.; Zhang, F.; Tang, D.; Li, D.; Cao, J.; Wei, W.; Wu, Y.; Liu, S. Integrated Microfluidic Device for Accurate Extracellular Vesicle Quantification and Protein Markers Analysis Directly from Human Whole Blood. Anal. Chem. 2020, 92, 1574–1581. [Google Scholar] [CrossRef]
- Han, B.H.; Kim, S.; Seo, G.; Heo, Y.; Chung, S.; Kang, J.Y. Isolation of extracellular vesicles from small volumes of plasma using a microfluidic aqueous two-phase system. Lab Chip 2020, 20, 3552–3559. [Google Scholar] [CrossRef]
- Wiklander, O.P.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mager, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- Luo, H.; Chen, D.; Li, R.; Li, R.; Teng, Y.; Cao, Y.; Zou, X.; Wang, W.; Zhou, C. Genetically engineered CXCR4-modified exosomes for delivery of miR-126 mimics to macrophages alleviate periodontitis. J. Nanobiotechnol. 2023, 21, 116. [Google Scholar] [CrossRef]
- Mukerjee, N.; Maitra, S.; Kaur, M.; Rekha, M.M.; Soothwal, P.; Arora, I.; Thorat, N.D.; Sharma, P.K.; Kaushik, A. Click chemistry-based modified exosomes: Towards enhancing precision in cancer theranostics. Chem. Eng. J. 2025, 512, 160915. [Google Scholar] [CrossRef]
- Song, S.; Shim, M.K.; Lim, S.; Moon, Y.; Yang, S.; Kim, J.; Hong, Y.; Yoon, H.Y.; Kim, I.S.; Hwang, K.Y.; et al. In Situ One-Step Fluorescence Labeling Strategy of Exosomes via Bioorthogonal Click Chemistry for Real-Time Exosome Tracking In Vitro and In Vivo. Bioconjug. Chem. 2020, 31, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, P.; Li, D.; Guo, S.; Wang, E. Effect of freeze-thawing on lipid bilayer-protected gold nanoparticles. Langmuir 2008, 24, 3407–3411. [Google Scholar] [CrossRef] [PubMed]
- Missirlis, D.; Krogstad, D.V.; Tirrell, M. Internalization of p53(14–29) peptide amphiphiles and subsequent endosomal disruption results in SJSA-1 cell death. Mol. Pharm. 2010, 7, 2173–2184. [Google Scholar] [CrossRef] [PubMed]
- Rubel, D.; Boulanger, J.; Craciun, F.; Xu, E.Y.; Zhang, Y.; Phillips, L.; Callahan, M.; Weber, W.; Song, W.; Ngai, N.; et al. Anti-microRNA-21 Therapy on Top of ACE Inhibition Delays Renal Failure in Alport Syndrome Mouse Models. Cells 2022, 11, 594. [Google Scholar] [CrossRef]
- Mendell, J.R.; Rodino-Klapac, L.R.; Sahenk, Z.; Roush, K.; Bird, L.; Lowes, L.P.; Alfano, L.; Gomez, A.M.; Lewis, S.; Kota, J.; et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann. Neurol. 2013, 74, 637–647. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: First Regulatory Approvals for CRISPR-Cas9 Therapeutic Gene Editing for Sickle Cell Disease and Transfusion-Dependent beta-Thalassemia. Med. Sci. Monit. 2024, 30, e944204. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA Approves First-of-Its Kind Targeted RNA-Based Therapy to Treat a Rare Disease. 2018. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-targeted-rna-based-therapy-treat-rare-disease (accessed on 3 March 2025).
- Wang, Q.; Sun, Y.; Zhang, Z.; Duan, Y. Targeted polymeric therapeutic nanoparticles: Design and interactions with hepatocellular carcinoma. Biomaterials 2015, 56, 229–240. [Google Scholar] [CrossRef]
- Guan, J.; Shen, Q.; Zhang, Z.; Jiang, Z.; Yang, Y.; Lou, M.; Qian, J.; Lu, W.; Zhan, C. Enhanced immunocompatibility of ligand-targeted liposomes by attenuating natural IgM absorption. Nat. Commun. 2018, 9, 2982. [Google Scholar] [CrossRef]
- Tzeng, A.; Kwan, B.H.; Opel, C.F.; Navaratna, T.; Wittrup, K.D. Antigen specificity can be irrelevant to immunocytokine efficacy and biodistribution. Proc. Natl. Acad. Sci. USA 2015, 112, 3320–3325. [Google Scholar] [CrossRef]
- Mahati, S.; Fu, X.; Ma, X.; Zhang, H.; Xiao, L. Delivery of miR-26a Using an Exosomes-Based Nanosystem Inhibited Proliferation of Hepatocellular Carcinoma. Front. Mol. Biosci. 2021, 8, 738219. [Google Scholar] [CrossRef] [PubMed]
- Izco, M.; Schleef, M.; Schmeer, M.; Carlos, E.; Verona, G.; Alvarez-Erviti, L. Targeted Extracellular Vesicle Gene Therapy for Modulating Alpha-Synuclein Expression in Gut and Spinal Cord. Pharmaceutics 2023, 15, 1230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Fu, Y.; Cheng, M.; Ma, W.; Zheng, N.; Wang, Y.; Wu, Z. sEVs(RVG) selectively delivers antiviral siRNA to fetus brain, inhibits ZIKV infection and mitigates ZIKV-induced microcephaly in mouse model. Mol. Ther. 2022, 30, 2078–2091. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ji, P.; Li, Z.; Zhang, R.; Wei, M.; Yang, Y.; Yuan, L.; Han, Y.; Yang, G. Improved extracellular vesicle-based mRNA delivery for familial hypercholesterolemia treatment. Theranostics 2023, 13, 3467–3479. [Google Scholar] [CrossRef]
- Shrivastava, S.; Ray, R.M.; Holguin, L.; Echavarria, L.; Grepo, N.; Scott, T.A.; Burnett, J.; Morris, K.V. Exosome-mediated stable epigenetic repression of HIV-1. Nat. Commun. 2021, 12, 5541. [Google Scholar] [CrossRef]
- Kim, S.M.; Yang, Y.; Oh, S.J.; Hong, Y.; Seo, M.; Jang, M. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J. Control. Release 2017, 266, 8–16. [Google Scholar] [CrossRef]
- Yu, Y.; Li, W.; Mao, L.; Peng, W.; Long, D.; Li, D.; Zhou, R.; Dang, X. Genetically engineered exosomes display RVG peptide and selectively enrich a neprilysin variant: A potential formulation for the treatment of Alzheimer’s disease. J. Drug Target. 2021, 29, 1128–1138. [Google Scholar] [CrossRef]
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A.; et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005, 3, 9. [Google Scholar] [CrossRef]
- Xu, X.; Liang, Y.; Li, X.; Ouyang, K.; Wang, M.; Cao, T.; Li, W.; Liu, J.; Xiong, J.; Li, B.; et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials 2021, 269, 120539. [Google Scholar] [CrossRef]
- Escudier, B.; Dorval, T.; Chaput, N.; Andre, F.; Caby, M.P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef]
- Vansteenkiste, J.F.; Cho, B.C.; Vanakesa, T.; De Pas, T.; Zielinski, M.; Kim, M.S.; Jassem, J.; Yoshimura, M.; Dahabreh, J.; Nakayama, H.; et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016, 17, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ren, Z.; Zhao, J.; Zhu, Y.; Huang, B.; Xiao, C.; Zhang, Y.; Deng, J.; Mao, L.; Tang, L.; et al. Global analysis of HLA-A2 restricted MAGE-A3 tumor antigen epitopes and corresponding TCRs in non-small cell lung cancer. Theranostics 2023, 13, 4449–4468. [Google Scholar] [CrossRef] [PubMed]
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A.; et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2016, 5, e1071008. [Google Scholar] [CrossRef] [PubMed]
- A First-in-Human Study of CDK-002 (exoSTING) in Subjects with Advanced/Metastatic, Recurrent, Injectable Solid Tumors, with Emphasis on Squamous Cell Carcinoma of the Head and Neck, Triple Negative Breast Cancer, Anaplastic Thyroid Carcinoma, and Cutaneous Squamous Cell Carcinoma. 2020. Available online: https://clinicaltrials.gov/study/NCT04592484 (accessed on 3 November 2024).
- Phase I Study of Mesenchymal Stromal Cells-Derived Exosomes with KrasG12D SiRNA for Metastatic Pancreas Cancer Patients Harboring KrasG12D Mutation. 2018. Available online: https://clinicaltrials.gov/study/NCT03608631 (accessed on 3 November 2024).
- Phase 1 Study in Humans Evaluating the Safety of Rectus Sheath Implantation of Diffusion Chambers Encapsulating Autologous Malignant Glioma Cells Treated With Insulin-Like Growth Factor Receptor-1 Antisense Oligodeoxynucleotide in 12 Patients with Recurrent Malignant Glioma. 2012. Available online: https://clinicaltrials.gov/study/NCT01550523 (accessed on 3 November 2024).
- Maris, C.; D’Haene, N.; Trepant, A.L.; Le Mercier, M.; Sauvage, S.; Allard, J.; Rorive, S.; Demetter, P.; Decaestecker, C.; Salmon, I. IGF-IR: A new prognostic biomarker for human glioblastoma. Br. J. Cancer 2015, 113, 729–737. [Google Scholar] [CrossRef]
- Judy, K.D.; Andrews, D.W.; Harshyne, L.; Kenyon, L.; Talekar, K.; Atsina, K.-B.; Kim, L.; Shi, W.; Werner-Wasik, M.; Kean, R.; et al. Abstract B71: Phase 1b/2 prospective randomized trial of four autologous cell vaccine dose cohorts for initial treatment of glioblastoma. Cancer Immunol. Res. 2020, 8, B71. [Google Scholar] [CrossRef]
- James Graham Brown Cancer Center. Preliminary Clinical Trial Investigating the Ability of Plant Exosomes to Abrogate Oral Mucositis Induced by Combined Chemotherapy and Radiation in Head and Neck Cancer Patients. 2012. Available online: https://clinicaltrials.gov/study/NCT01668849 (accessed on 3 November 2024).
- Farina, E.; Daghero, H.; Bollati-Fogolin, M.; Boido, E.; Cantero, J.; Moncada-Basualto, M.; Olea-Azar, C.; Polticelli, F.; Paulino, M. Antioxidant Capacity and NF-kB-Mediated Anti-Inflammatory Activity of Six Red Uruguayan Grape Pomaces. Molecules 2023, 28, 3909. [Google Scholar] [CrossRef]
- Mandic, A.I.; Đilas, S.M.; Ćetković, G.S.; Čanadanović-Brunet, J.M.; Tumbas, V.T. Polyphenolic Composition and Antioxidant Activities of Grape Seed Extract. Int. J. Food Prop. 2008, 11, 713–726. [Google Scholar] [CrossRef]
- Grigoropoulos, I.; Tsioulos, G.; Kastrissianakis, A.; Shapira, S.; Green, O.; Rapti, V.; Tsakona, M.; Konstantinos, T.; Savva, A.; Kavatha, D.; et al. The safety and potential efficacy of exosomes overexpressing CD24 (EXO-CD24) in mild-moderate COVID-19 related ARDS. Respir. Res. 2024, 25, 151. [Google Scholar] [CrossRef]
- Phase I Clinical Trial Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Malignant Colon Tissue. 2011. Available online: https://clinicaltrials.gov/study/NCT01294072 (accessed on 3 November 2024).
- Aegle Therapeutics Corp. Dystrophic Epidermolysis Bullosa. 2025. Available online: https://aegletherapeutics.com/pipeline/ (accessed on 10 March 2025).
- BioSpace. Aegle Therapeutics Corp. Announces Positive Data for the First Patient in a Phase 1/2a Clinical Trial Dosed With AGLE-102™, a Novel Extracellular Vesicle Therapy. 2024. Available online: https://www.biospace.com/aegle-therapeutics-corp-announces-positive-data-for-the-first-patient-in-a-phase-1-2a-clinical-trial-dosed-with-agle-102-a-novel-extracellular-vesicle-therapy (accessed on 28 March 2025).
- Tan, S.T.; Aisyah, P.B.; Firmansyah, Y.; Nathasia, N.; Budi, E.; Hendrawan, S. Effectiveness of Secretome from Human Umbilical Cord Mesenchymal Stem Cells in Gel (10% SM-hUCMSC Gel) for Chronic Wounds (Diabetic and Trophic Ulcer). J. Multidiscip. Healthc. 2023, 16, 1763–1777. [Google Scholar] [CrossRef]
- Air Force Military Medical University, China. Exosome-Based Nanoplatform for Ldlr mRNA Delivery in Familial Hypercholesterolemia. 2021. Available online: https://clinicaltrials.gov/study/NCT05043181 (accessed on 3 November 2024).
- Harshyne, L.A.; Hooper, K.M.; Andrews, E.G.; Nasca, B.J.; Kenyon, L.C.; Andrews, D.W.; Hooper, D.C. Glioblastoma exosomes and IGF-1R/AS-ODN are immunogenic stimuli in a translational research immunotherapy paradigm. Cancer Immunol. Immunother. 2015, 64, 299–309. [Google Scholar] [CrossRef]
- Phase II Trial of a Vaccination with Tumor Antigen-Loaded Dendritic Cell-derived Exosomes on Patients with Unresectable Non Small Cell Lung Cancer Responding to Induction Chemotherapy. 2010. Available online: https://clinicaltrials.gov/study/NCT01159288 (accessed on 3 November 2024).
- Congressionally Directed Medical Research Programs. A Pilot Safety Study of Mesenchymal Stem Cell Derived Extracellular Vesicles for the Treatment of Burn Wounds. 2021. Available online: https://clinicaltrials.gov/study/NCT05078385 (accessed on 3 November 2024).
- Stem Cell and Cancer Institute, Kalbe Farma Tbk; PT Pharma Metric Labs. Therapeutic Potential of Stem Cell Conditioned Medium on Chronic Ulcer Wounds: Pilot Study in Human. 2019. Available online: https://clinicaltrials.gov/study/NCT04134676 (accessed on 3 November 2024).
- Costa, M.H.G.; Costa, M.S.; Painho, B.; Sousa, C.D.; Carrondo, I.; Oltra, E.; Pelacho, B.; Prosper, F.; Isidro, I.A.; Alves, P.; et al. Enhanced bioprocess control to advance the manufacture of mesenchymal stromal cell-derived extracellular vesicles in stirred-tank bioreactors. Biotechnol. Bioeng. 2023, 120, 2725–2741. [Google Scholar] [CrossRef] [PubMed]
- Visan, K.S.; Lobb, R.J.; Ham, S.; Lima, L.G.; Palma, C.; Edna, C.P.Z.; Wu, L.Y.; Gowda, H.; Datta, K.K.; Hartel, G.; et al. Comparative analysis of tangential flow filtration and ultracentrifugation, both combined with subsequent size exclusion chromatography, for the isolation of small extracellular vesicles. J. Extracell. Vesicles 2022, 11, e12266. [Google Scholar] [CrossRef] [PubMed]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.L.; Dickson, D.W.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 2018, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Dobnik, D.; Kogovsek, P.; Jakomin, T.; Kosir, N.; Tusek Znidaric, M.; Leskovec, M.; Kaminsky, S.M.; Mostrom, J.; Lee, H.; Ravnikar, M. Accurate Quantification and Characterization of Adeno-Associated Viral Vectors. Front. Microbiol. 2019, 10, 1570. [Google Scholar] [CrossRef]
- Man, K.; Barroso, I.A.; Brunet, M.Y.; Peacock, B.; Federici, A.S.; Hoey, D.A.; Cox, S.C. Controlled Release of Epigenetically-Enhanced Extracellular Vesicles from a GelMA/Nanoclay Composite Hydrogel to Promote Bone Repair. Int. J. Mol. Sci. 2022, 23, 832. [Google Scholar] [CrossRef]
- Patel, N.; Avery, E.; Chung, E.J. Supramolecular hydrogels for sustained extracellular vesicle delivery. MRS Commun. 2024, 14, 1037–1044. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, G.; Zhang, A.; Xu, L.X.; He, X. Preferential vitrification of water in small alginate microcapsules significantly augments cell cryopreservation by vitrification. Biomed. Microdevices 2010, 12, 89–96. [Google Scholar] [CrossRef]
- Peinado, H.; Aleckovic, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Umezu, T.; Tadokoro, H.; Azuma, K.; Yoshizawa, S.; Ohyashiki, K.; Ohyashiki, J.H. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 2014, 124, 3748–3757. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Kandimalla, R.; Wallen, M.; Tyagi, N.; Wilcher, S.; Yan, J.; Schultz, D.J.; Spencer, W.; et al. Exosome-mediated delivery of RNA and DNA for gene therapy. Cancer Lett. 2021, 505, 58–72. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, S.; Liu, J.; Liu, F.; Du, F.; Li, M.; Chen, A.T.; Bao, Y.; Suh, H.W.; Avery, J.; et al. Targeted Drug Delivery to Stroke via Chemotactic Recruitment of Nanoparticles Coated with Membrane of Engineered Neural Stem Cells. Small 2019, 15, e1902011. [Google Scholar] [CrossRef]
- Molinaro, R.; Corbo, C.; Martinez, J.O.; Taraballi, F.; Evangelopoulos, M.; Minardi, S.; Yazdi, I.K.; Zhao, P.; De Rosa, E.; Sherman, M.B.; et al. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater. 2016, 15, 1037–1046. [Google Scholar] [CrossRef]

| Body System | Clinical Trial ID and Phase | EV Source | EV Cargo | Purpose | Reference |
|---|---|---|---|---|---|
| Cardiovascular (Homozygous Familial Hypercholesterolemia—HoFH) | NCT05043181—Phase 1 (n = 30) | Bone Marrow Mesenchymal Stromal Cell-EVs | Low-density lipoprotein (LDL) mRNA | Safety and effectiveness of exosome-mRNA therapy in HoFH | [171] |
| Central Nervous System (Recurrent Glioblastoma) | NCT01550523 *—Phase 1 (n = 13) | Autologous tumor cells | Antisense oligodeoxynucleotides (IGF-1R AS ODN) | Stimulate immune response in glioblastoma | [160,172] |
| Gastrointestinal (Colon) | NCT01294072—Phase 1 (n = 35) | Plant-derived exosomes | Curcumin | Plant exosomes for curcumin delivery to colon tissue | [167] |
| Gastrointestinal (Metastatic Pancreatic Ductal Adenocarcinoma) | NCT03608631—Interventional (n = 15) | Mesenchymal stromal cells | KrasG12D siRNA | MSC exosomes for Kras mutation in pancreatic cancer | [159] |
| Immunological (Unresectable Non-Small Cell Lung Cancer) | NCT01159288 *—Phase 2 (n = 41) | Dendritic cell-derived exosomes | Tumor antigens (MAGE-3 DP04, MAGE-1 A2, MAGE-3 A2, NY-ESO-1, MART-1 A2) | Assess exosome vaccines in NSCLC | [173] |
| Integumentary (Skin; Severe Second-Degree Burns) | NCT05078385 *—Phase 1/2a (n = 1) | Allogeneic Mesenchymal Stem Cells | AGLE-102 (COL7A1 mRNA) | MSC exosomes for severe burns | [174] |
| Oral/Dental (Prevention of Chemoradiation-associated Oral Mucositis) | NCT01668849 *—Phase 1 (n = 60) | Edible plant-derived exosomes | Grape exosomes | Effects of plant exosomes on chemoradiation-associated oral mucositis | [163] |
| Respiratory (COVID-19) | NCT04902183—Phase 2 (n = 90) | Human Embryonic Kidney (HEK)-293 cells | CD24 overexpressed exosomes | CD24 exosomes for inflammation in COVID-19 | [166] |
| Skin (Chronic Ulcer Wounds) | NCT04134676 *—Phase 1 (n = 38) | Wharton’s Jelly MSCs | Proangiogenic and wound healing promoting factors (TGF-B, VEGF, IGF-1, IL-6, IL-8) | Conditioned medium for chronic wound healing | [175] |
| Various Advanced/Metastatic, Recurrent, Injectable Solid Tumors | NCT04592484 *—Phase 1/2 (n = 27) | HEK-293 cells | Intratumoral injection of CDK-002 (STING agonist) | Intratumoral injection for solid tumors | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohak, S.; Fabian, Z. Extracellular Vesicles as Precision Delivery Systems for Biopharmaceuticals: Innovations, Challenges, and Therapeutic Potential. Pharmaceutics 2025, 17, 641. https://doi.org/10.3390/pharmaceutics17050641
Mohak S, Fabian Z. Extracellular Vesicles as Precision Delivery Systems for Biopharmaceuticals: Innovations, Challenges, and Therapeutic Potential. Pharmaceutics. 2025; 17(5):641. https://doi.org/10.3390/pharmaceutics17050641
Chicago/Turabian StyleMohak, Sidhesh, and Zsolt Fabian. 2025. "Extracellular Vesicles as Precision Delivery Systems for Biopharmaceuticals: Innovations, Challenges, and Therapeutic Potential" Pharmaceutics 17, no. 5: 641. https://doi.org/10.3390/pharmaceutics17050641
APA StyleMohak, S., & Fabian, Z. (2025). Extracellular Vesicles as Precision Delivery Systems for Biopharmaceuticals: Innovations, Challenges, and Therapeutic Potential. Pharmaceutics, 17(5), 641. https://doi.org/10.3390/pharmaceutics17050641






