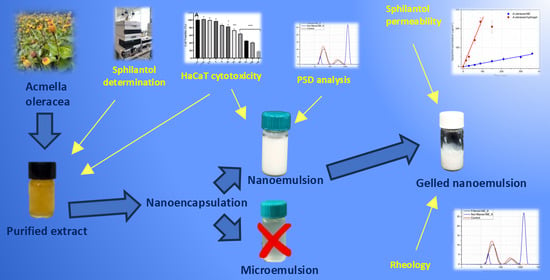
Hydrogels Powered by Nanoemulsion Technology for the Topical Delivery of Acmella oleracea Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Materials
2.3. Preparation of A. oleracea n-Hexane Extract
2.4. HPLC-DAD-MS Analysis of N-Alkylamides in A. oleracea Extract
2.5. Nanoemulsion (NE) Formulation
2.6. Microemulsion (ME) Development
2.7. Characterization of Micro- (MEs) and Nanoemulsions (NEs)
2.8. Preparation of A. oleracea Extract NE-Based Hydrogels
2.9. Rheological Characterization of A. oleracea Extract NE-Based Hydrogels
2.10. Permeability Studies Using Vertical Diffusion Cells
2.11. Cell Cytotoxicity Study on HaCaT Cell Line
3. Results
3.1. HPLC-MS-DAD Analysis of N-Alkylamides in A. oleracea Extract
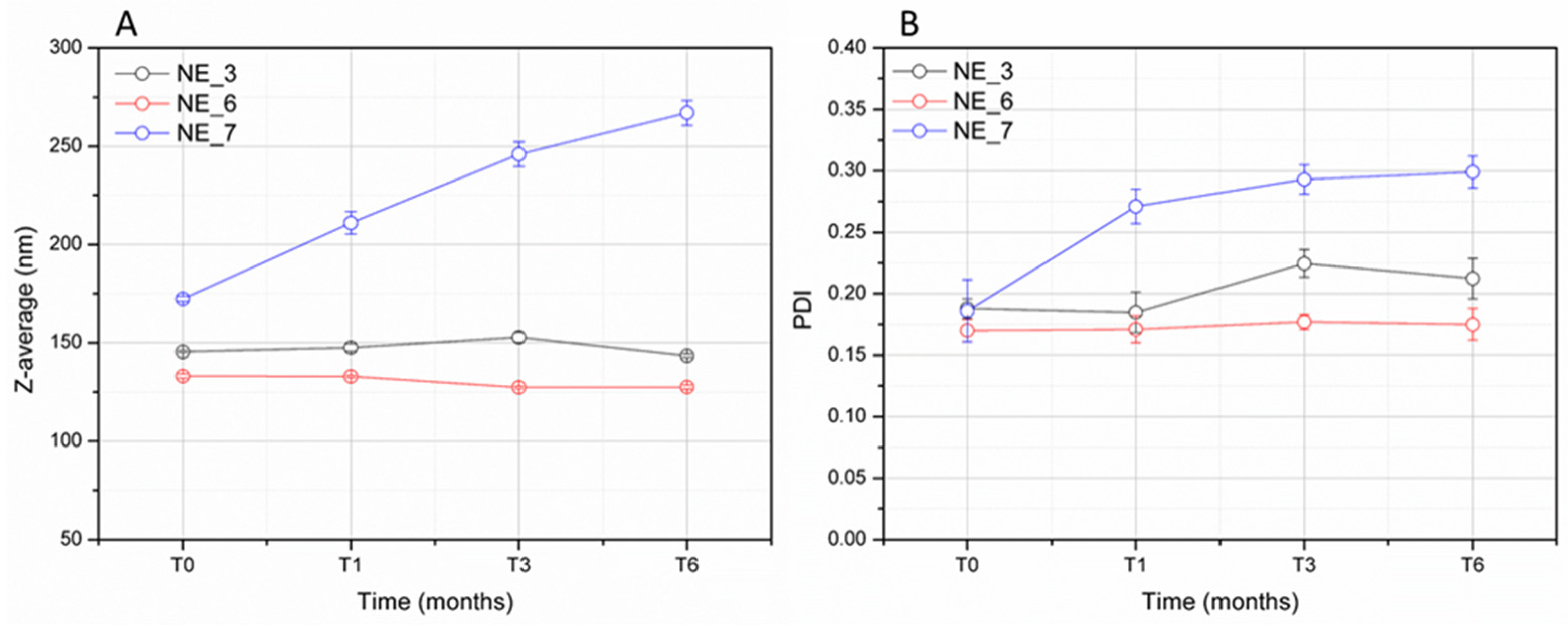
3.2. Formulation Study and Stability of Nanoemulsions (NEs)
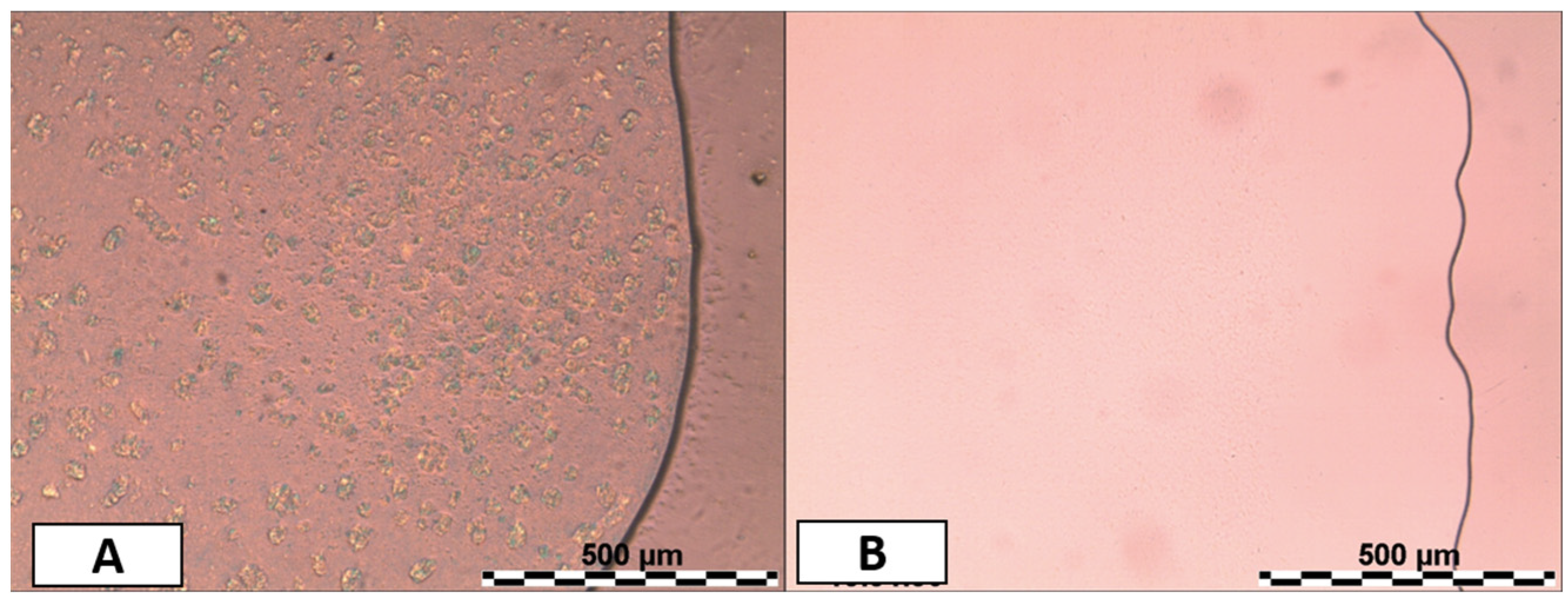
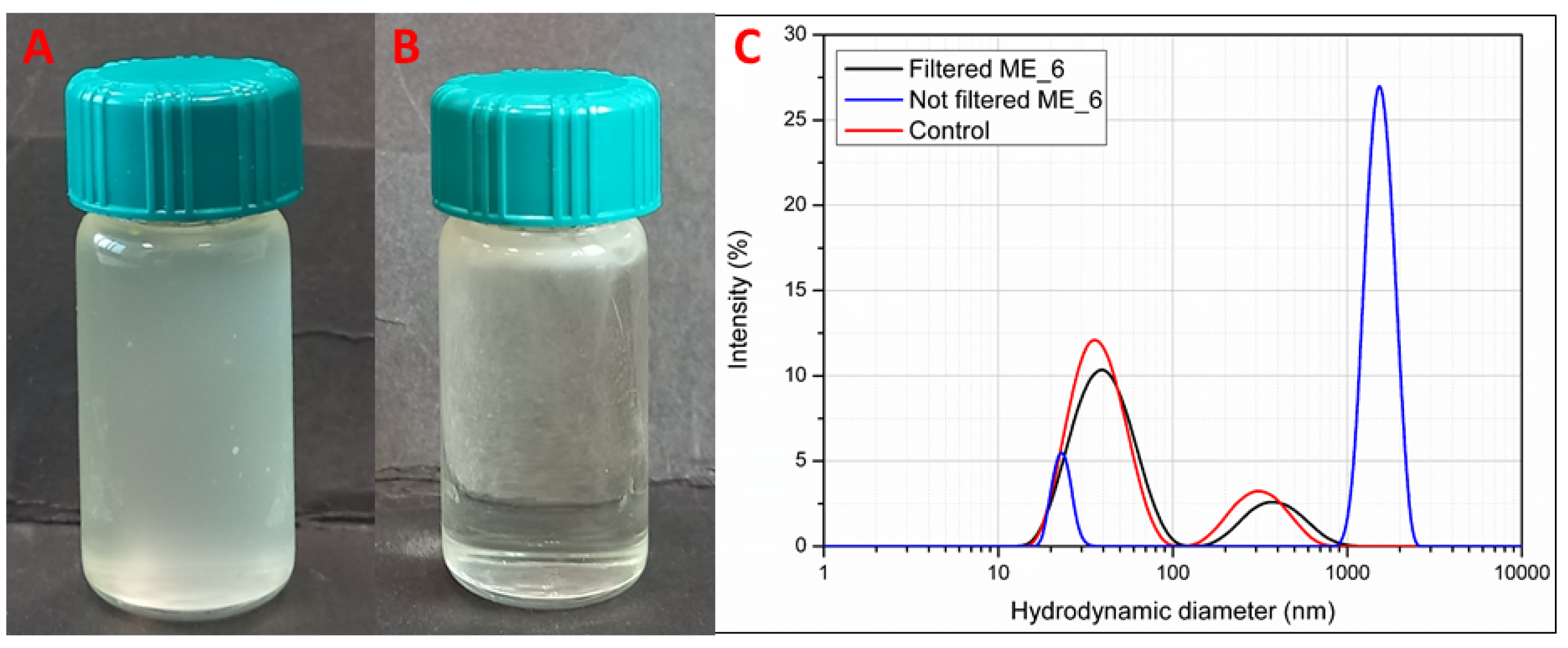
3.3. Formulation Study and Stability of Microemulsions (MEs)
3.4. Rheological Characterization of the A. oleracea NE-Based Hydrogels
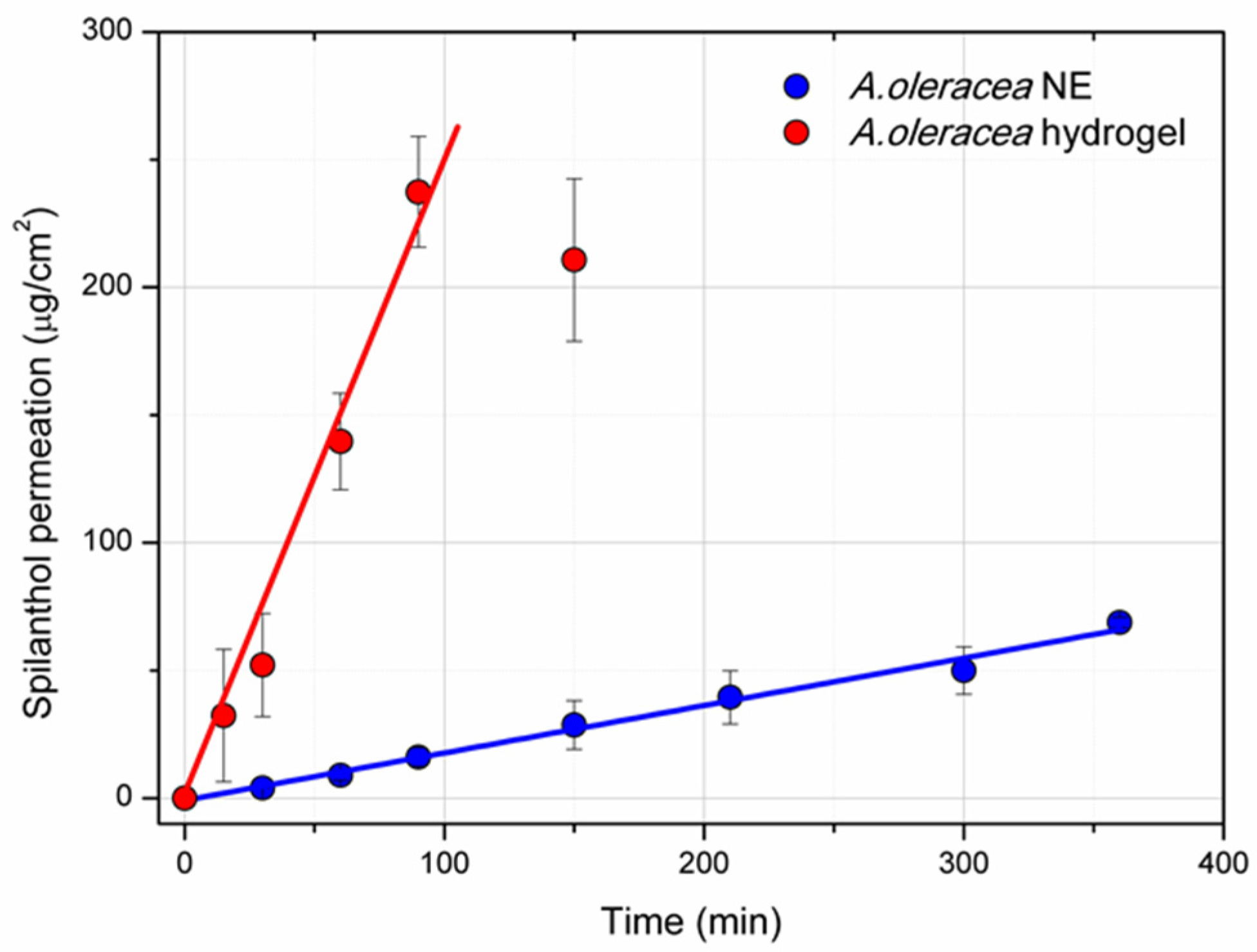
3.5. Permeation Study
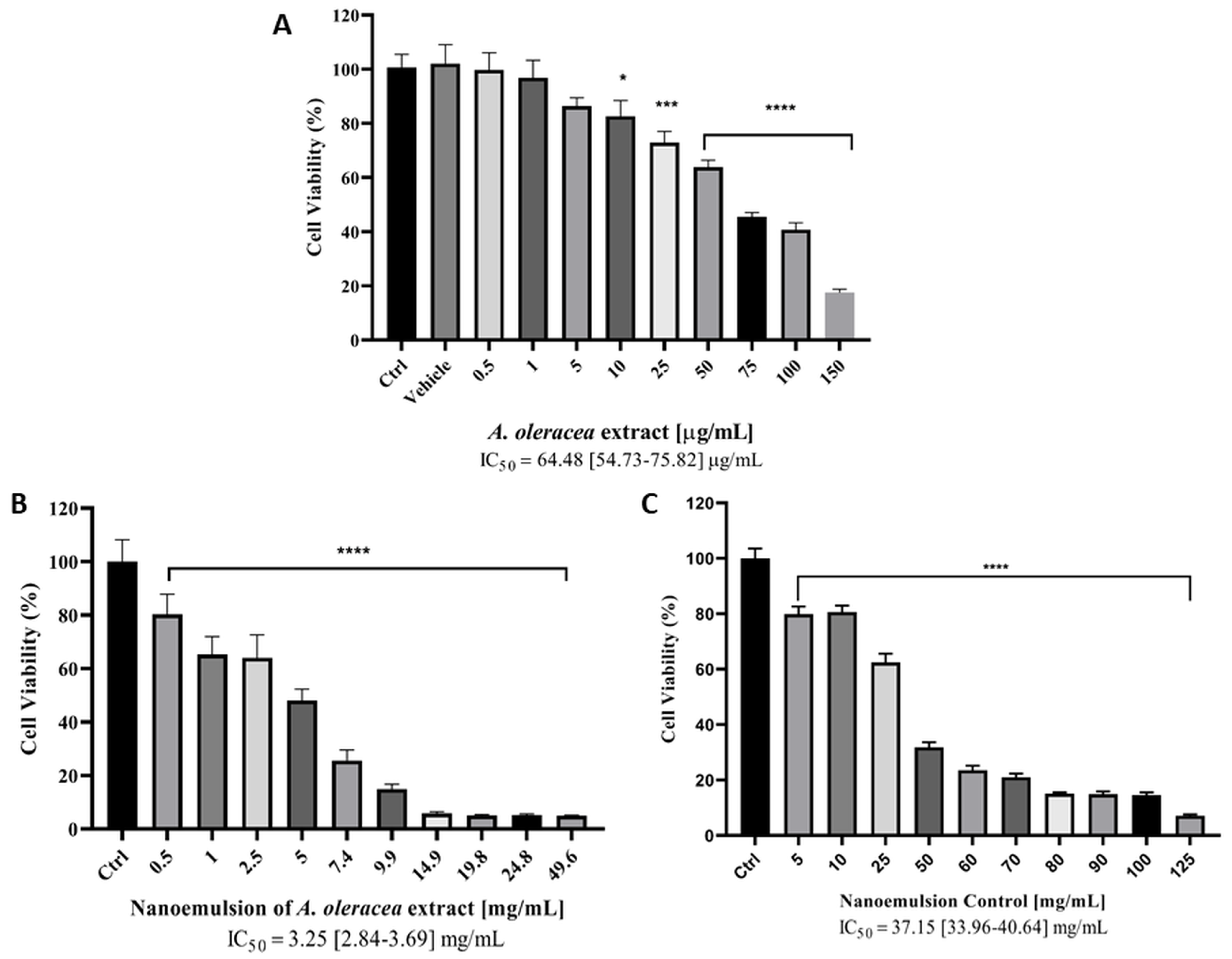
3.6. Cytotoxicity Study on HaCaT Cells
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dini, I.; Laneri, S. The New Challenge of Green Cosmetics: Natural Food Ingredients for Cosmetic Formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Alsalahat, I.; Daoud, S.; Abutayeh, R.F.; Mahmod, A.I. Plant-Derived Natural Products in Cancer Research: Extraction, Mechanism of Action, and Drug Formulation. Molecules 2020, 25, 5319. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Yadav, A.N. Natural Bioactive Products in Sustainable Agriculture; Springer Nature: Singapore, 2020; ISBN 9789811530241. [Google Scholar]
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A. Botanicals—Challenges abound, solutions in sight? Curr. Opin. Food Sci. 2020, 32, 144–148. [Google Scholar] [CrossRef]
- Vanti, G. Recent strategies in nanodelivery systems for natural products: A review. Environ. Chem. Lett. 2021, 19, 4311–4326. [Google Scholar] [CrossRef]
- Paroha, S.; Dewangan, R.P.; Sahoo, P.K. Pharmaceutical Technology for Improving the Bioavailability of Natural Products. In Sustainable Agriculture Reviews 43; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–32. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Nabavi, S.; Behzad, S. Herbal Drugs and Natural Products in the light of Nanotechnology and Nanomedicine for Developing Drug Formulations. Mini Rev. Med. Chem. 2021, 21, 302–313. [Google Scholar] [CrossRef]
- Altay, Ö.; Köprüalan, Ö.; İlter, I.; Koç, M.; Ertekin, F.K.; Jafari, S.M. Spray drying encapsulation of essential oils; process efficiency, formulation strategies, and applications. Crit. Rev. Food Sci. Nutr. 2022, 64, 1139–1157. [Google Scholar] [CrossRef]
- De Luca, I.; Pedram, P.; Moeini, A.; Cerruti, P.; Peluso, G.; Di Salle, A.; Germann, N. Nanotechnology Development for Formulating Essential Oils in Wound Dressing Materials to Promote the Wound-Healing Process: A Review. Appl. Sci. 2021, 11, 1713. [Google Scholar] [CrossRef]
- Xia, Q.; Liang, T.; Zhou, Y.; Liu, J.; Tang, Y.; Liu, F. Recent Advances in Biomedical Nanotechnology Related to Natural Products. Curr. Pharm. Biotechnol. 2023, 24, 944–961. [Google Scholar] [CrossRef]
- Weisany, W.; Yousefi, S.; Tahir, N.A.R.; Golestanehzadeh, N.; McClements, D.J.; Adhikari, B.; Ghasemlou, M. Targeted delivery and controlled released of essential oils using nanoencapsulation: A review. Adv. Colloid Interface Sci. 2022, 303, 102655. [Google Scholar] [CrossRef]
- Mushtaq, A.; Mohd Wani, S.; Malik, A.R.; Gull, A.; Ramniwas, S.; Ahmad Nayik, G.; Ercisli, S.; Alina Marc, R.; Ullah, R.; Bari, A. Recent insights into Nanoemulsions: Their preparation, properties and applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T.F.; Anton, N.; Vandamme, T.F. Nano-emulsions and Micro-emulsions: Clarifications of the Critical Differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Formation of flavor oil microemulsions, nanoemulsions and emulsions: Influence of composition and preparation method. J. Agric. Food Chem. 2011, 59, 5026–5035. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An overview of micro-and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef]
- Pavoni, L.; Pavela, R.; Cespi, M.; Bonacucina, G.; Maggi, F.; Zeni, V.; Canale, A.; Lucchi, A.; Bruschi, F.; Benelli, G. Green Micro- and Nanoemulsions for Managing Parasites, Vectors and Pests. Nanomaterials 2019, 9, 1285. [Google Scholar] [CrossRef]
- Pavoni, L.; Maggi, F.; Mancianti, F.; Nardoni, S.; Ebani, V.V.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. Microemulsions: An effective encapsulation tool to enhance the antimicrobial activity of selected EOs. J. Drug Deliv. Sci. Technol. 2019, 53, 101101. [Google Scholar] [CrossRef]
- Uthpala, T.G.G.; Navaratne, S.B. Acmella oleracea Plant; Identification, Applications and Use as an Emerging Food Source—Review. Food Rev. Int. 2021, 37, 399–414. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Suphapong, S.; Worachartcheewan, A.; Lawung, R.; Ruchirawat, S.; Prachayasittikul, V. Bioactive metabolites from Spilanthes acmella Murr. Molecules 2009, 14, 850–867. [Google Scholar] [CrossRef]
- Spinozzi, E.; Ferrati, M.; Baldassarri, C.; Cappellacci, L.; Marmugi, M.; Caselli, A.; Benelli, G.; Maggi, F.; Petrelli, R. A Review of the Chemistry and Biological Activities of Acmella oleracea (“jambù”, Asteraceae), with a View to the Development of Bioinsecticides and Acaricides. Plants 2022, 11, 2721. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, J.; Govindarajan, R.; Palpu, P. The genus spilanthes ethnopharmacology, phytochemistry, and pharmacological properties: A review. Adv. Pharmacol. Sci. 2013, 2013, 510298. [Google Scholar] [CrossRef]
- Cheng, Y.B.; Liu, R.H.; Ho, M.C.; Wu, T.Y.; Chen, C.Y.; Lo, I.W.; Hou, M.F.; Yuan, S.S.; Wu, Y.C.; Chang, F.R. Alkylamides of Acmella oleracea. Molecules 2015, 20, 6970–6977. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Blanco, V.S.; Franz-Montan, M.; Groppo, F.C.; De Carvalho, J.E.; Figueira, G.M.; Serpe, L.; Sousa, I.M.O.; Damasio, V.A.G.; Yamane, L.T.; Paula, E.; et al. Development and Evaluation of a Novel Mucoadhesive Film Containing Acmella oleracea Extract for Oral Mucosa Topical Anesthesia. PLoS ONE 2016, 11, e0162850. [Google Scholar] [CrossRef]
- Rondanelli, M.; Fossari, F.; Vecchio, V.; Braschi, V.; Riva, A.; Allegrini, P.; Petrangolini, G.; Iannello, G.; Faliva, M.A.; Peroni, G.; et al. Acmella oleracea for pain management. Fitoterapia 2020, 140, 104419. [Google Scholar] [CrossRef]
- Nomura, E.C.O.; Rodrigues, M.R.A.; Da Silva, C.F.; Hamm, L.A.; Nascimento, A.M.; De Souza, L.M.; Cipriani, T.R.; Baggio, C.H.; De Paula Werner, M.F. Antinociceptive effects of ethanolic extract from the flowers of Acmella oleracea (L.) R.K. Jansen in mice. J. Ethnopharmacol. 2013, 150, 583–589. [Google Scholar] [CrossRef]
- Rondanelli, M.; Riva, A.; Allegrini, P.; Faliva, M.A.; Naso, M.; Peroni, G.; Nichetti, M.; Gasparri, C.; Spadaccini, D.; Iannello, G.; et al. The use of a new food-grade lecithin formulation of highly standardized ginger (Zingiber officinale) and acmella oleracea extracts for the treatment of pain and inflammation in a group of subjects with moderate knee osteoarthritis. J. Pain Res. 2020, 13, 761–770. [Google Scholar] [CrossRef]
- de Freitas Blanco, V.S.; Michalak, B.; Zelioli, Í.A.M.; da Silva Santos de Oliveira, A.; Rodrigues, M.V.N.; Ferreira, A.G.; Garcia, V.L.; Cabral, F.A.; Kiss, A.K.; Rodrigues, R.A.F. Isolation of spilanthol from Acmella oleracea based on Green Chemistry and evaluation of its in vitro anti-inflammatory activity. J. Supercrit. Fluids 2018, 140, 372–379. [Google Scholar] [CrossRef]
- Dubey, S.; Maity, S.; Singh, M.; Saraf, S.A.; Saha, S. Phytochemistry, pharmacology and toxicology of spilanthes acmella: A review. Adv. Pharmacol. Sci. 2013, 2013, 423750. [Google Scholar] [CrossRef]
- Moro, S.D.d.S.; de Oliveira Fujii, L.; Teodoro, L.F.R.; Frauz, K.; Mazoni, A.F.; Esquisatto, M.A.M.; Rodrigues, R.A.F.; Pimentel, E.R.; de Aro, A.A. Acmella oleracea extract increases collagen content and organization in partially transected tendons. Microsc. Res. Tech. 2021, 84, 2588–2597. [Google Scholar] [CrossRef]
- Demarne, F.; Passaro, G. Use of an Acmella oleracea Extract for the Botox-like Effect Thereof in an Anti-Wrinkle Cosmetic Composition. United States US 2008.00699 12A1, 20 March 2008. [Google Scholar]
- Barbosa, A.F.; Silva, K.C.B.; de Oliveira, M.C.C.; de Carvalho, M.G.; Sabaa Srur, A.U.O. Effects of Acmella oleracea methanolic extract and fractions on the tyrosinase enzyme. Rev. Bras. Farmacogn. 2016, 26, 321–325. [Google Scholar] [CrossRef]
- Carvalho, J.C.T.; De Souza, G.C.; Da Silva, I.D.R.; Viana, M.D.; De Melo, N.C.; Sánchez-Ortiz, B.L.; Oliveira, M.M.R.D.; Barbosa, W.R.; Ferreira, I.M. Acute Toxicity of the Hydroethanolic Extract of the Flowers of Acmella oleracea L. in Zebrafish (Danio rerio): Behavioral and Histopathological Studies. Pharmaceuticals 2019, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Savic, S.M.; Cekic, N.D.; Savic, S.R.; Ilic, T.M.; Savic, S.D. ‘All-natural’ anti-wrinkle emulsion serum with Acmella oleracea extract: A design of experiments (DoE) formulation approach, rheology and in vivo skin performance/efficacy evaluation. Int. J. Cosmet. Sci. 2021, 43, 530–546. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, E.; Pavela, R.; Bonacucina, G.; Perinelli, D.R.; Cespi, M.; Petrelli, R.; Cappellacci, L.; Fiorini, D.; Scortichini, S.; Garzoli, S.; et al. Spilanthol-rich essential oil obtained by microwave-assisted extraction from Acmella oleracea (L.) R.K. Jansen and its nanoemulsion: Insecticidal, cytotoxic and anti-inflammatory activities. Ind. Crops Prod. 2021, 172, 114027. [Google Scholar] [CrossRef]
- Spinozzi, E. Medicinal and Food Plants as Sources of Biopesticides and Biologically Active Compounds: A Focus on Carlina Acaulis and Acmella oleracea. Ph.D. Thesis, School of Advances Studies, University of Camerino, Camerino, Italy, 2023. [Google Scholar]
- Boukouvala, M.C.; Kavallieratos, N.G.; Maggi, F.; Angeloni, S.; Ricciutelli, M.; Spinozzi, E.; Ferrati, M.; Petrelli, R.; Canale, A.; Benelli, G. Being exposed to Acmella oleracea-based insecticide extract reduces mobility and mating success in Prostephanus truncatus, the major pest of maize in storages. J. Stored Prod. Res. 2023, 104, 102151. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Ciacciarelli, A.; Quassinti, L.; Bramucci, M.; Miano, A.; Casettari, L.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. Properties and stability of nanoemulsions: How relevant is the type of surfactant? J. Drug Deliv. Sci. Technol. 2020, 58, 101772. [Google Scholar] [CrossRef]
- Cespi, M.; Quassinti, L.; Perinelli, D.R.; Bramucci, M.; Iannarelli, R.; Papa, F.; Ricciutelli, M.; Bonacucina, G.; Palmieri, G.F.; Maggi, F. Microemulsions enhance the shelf-life and processability of Smyrnium olusatrum L. essential oil. Flavour Fragr. J. 2017, 32, 159–164. [Google Scholar] [CrossRef]
- Lapasin, R. Rheological characterization of hydrogels-Chapter 3. In Polysaccharide Hydrogels: Characterization and Biomedical Applications; Alhaique, F., Matricardi, P., Coviello, T., Eds.; Pan Stanford Publishing: Singapore, 2016. [Google Scholar]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Cespi, M.; Bonacucina, G.; Tiboni, M.; Casettari, L.; Cambriani, A.; Fini, F.; Perinelli, D.R.; Palmieri, G.F. Insights in the rheological properties of PLGA-PEG-PLGA aqueous dispersions: Structural properties and temperature-dependent behaviour. Polymer 2021, 213, 123216. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Razavi, S.M.A.; Mikkonen, K.S. Novel nanobiocomposite hydrogels based on sage seed gum-laponite: Physico-chemical and rheological characterization. Carbohydr. Polym. 2018, 192, 282–290. [Google Scholar] [CrossRef]
- Goodwin, J.; Huges, R.W. Chapter 4. Linear Viscoelasticity I: Phenomenological Approach. In Rheology for Chemists; Royal Society of Chemistry: Cambridge, UK, 2008; pp. 92–134. [Google Scholar]
- Ferrati, M.; Spinozzi, E.; Baldassarri, C.; Rossi, P.; Favia, G.; Fiorini, D.; De Zordi, N.; Drenaggi, E.; De Fazi, L.; Benelli, G.; et al. Green purification of Acmella oleracea extract by wiped-film short path molecular distillation boosts the insecticidal activity on mosquito larvae. Ind. Crops Prod. 2024, 218, 118818. [Google Scholar] [CrossRef]
- Nie, Y.; Pan, Y.; Jiang, Y.; Xu, D.; Yuan, R.; Zhu, Y.; Zhang, Z. Stability and bioactivity evaluation of black pepper essential oil nanoemulsion. Heliyon 2023, 9, 2405–8440. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.R.; Pulikkal, A.K. Preparation, stability and biological activity of essential oil-based nano emulsions: A comprehensive review. OpenNano 2022, 8, 100066. [Google Scholar] [CrossRef]
- Moura, J.d.S.; Gemaque, E.d.M.; Bahule, C.E.; Martins, L.H.d.S.; Chisté, R.C.; Lopes, A.S. Bioactive Compounds of Jambu (Acmella oleracea (L.) R. K. Jansen) as Potential Components of Biodegradable Food Packing: A Review. Sustainability 2023, 15, 15231. [Google Scholar] [CrossRef]
- Abeysiri, G.R.P.I.; Dharmadasa, R.M.; Abeysinghe, D.C.; Samarasinghe, K. Screening of phytochemical, physico-chemical and bioactivity of different parts of Acmella oleraceae Murr. (Asteraceae), a natural remedy for toothache. Ind. Crops Prod. 2013, 50, 852–856. [Google Scholar] [CrossRef]
- Batista, L.L.; Koga, R.d.C.R.; Teixeira, A.V.T.d.L.; Teixeira, T.A.; de Melo, E.L.; Carvalho, J.C.T. Clinical Safety of a Pharmaceutical Formulation Containing an Extract of Acmella oleracea (L.) in Patients With Premature Ejaculation: A Pilot Study. Am. J. Mens. Health 2023, 17, 15579883231167819. [Google Scholar] [CrossRef]
- Afzal, A.; Shah, S.N.H.; Javed, H.; Mumtaz, A.; Saeed, J.; Rasheed, H.M.; Arshad, R.; Ansari, S.A.; Alkahtani, H.M.; Ansari, I.A. Spilanthes acmella Extract-Based Natural Oils Loaded Emulgel for Anti-Microbial Action against Dermatitis. Gels 2023, 9, 832. [Google Scholar] [CrossRef]
- Battaglia, L.; Ugazio, E. Lipid nano- and microparticles: An overview of patent-related research. J. Nanomater. 2019, 2019, 2834941. [Google Scholar] [CrossRef]
- Wennerström, H.; Balogh, J.; Olsson, U. Interfacial tensions in microemulsions. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 291, 69–77. [Google Scholar] [CrossRef]
- Boonen, J.; Baert, B.; Roche, N.; Burvenich, C.; De Spiegeleer, B. Transdermal behaviour of the N-alkylamide spilanthol (affinin) from Spilanthes acmella (Compositae) extracts. J. Ethnopharmacol. 2010, 127, 77–84. [Google Scholar] [CrossRef]
- Yang, D.; Li, W.; Fang, L.; Liu, C. Investigation of Controlled Release Molecular Mechanism of Oil Phase in Spilanthol Emulsion: Development and In Vitro, In Vivo Characterization. AAPS PharmSciTech 2019, 20, 227. [Google Scholar] [CrossRef] [PubMed]
- Torlak, C.; Güleli, M.; Kızılok, Ş.; Kartop, R.A.; Çalışkan, C.; Kurşun Baysak, F. Comparison of permeability of topical Cream drug through polymer synthetic membranes of different structures using Franz Cell diffusion test. J. Dispers. Sci. Technol. 2024, 1–9. [Google Scholar] [CrossRef]
- Ng, S.F.; Rouse, J.J.; Sanderson, F.D.; Meidan, V.; Eccleston, G.M. Validation of a static Franz diffusion cell system for in vitro permeation studies. AAPS PharmSciTech 2010, 11, 1432–1441. [Google Scholar] [CrossRef] [PubMed]

| Sample | Extract (%) | Ethyl Oleate (%) | Polysorbate 80 (%) | H2O (%) |
|---|---|---|---|---|
| NE_1 | 1 | - | 0.50 | 98.50 |
| NE_2 | 1 | 0.5 | 0.50 | 98.00 |
| NE_3 | 1 | 1 | 0.50 | 97.50 |
| NE_4 | 1 | 1 | 0.25 | 97.75 |
| NE_5 | 1 | 1 | 0.75 | 97.25 |
| NE_6 | 1 | 2 | 0.75 | 96.25 |
| NE_7 | 2 | 2 | 1.00 | 95.00 |
| Sample | A. oleracea Extract (%) | Ethyl Oleate (%) | Polysorbate 80 (%) | Alcoholic Mixture (%) | H2O (%) |
|---|---|---|---|---|---|
| ME_1 | 1 | 1 | 10 | 35 | 53 |
| ME_2 | 1 | 1 | 13 | 35 | 50 |
| ME_3 | 1 | 1 | 16 | 35 | 47 |
| ME_4 | 0.5 | 0.5 | 10 | 35 | 54 |
| ME_5 | 0.5 | 0.5 | 13 | 35 | 51 |
| ME_6 | 0.5 | 0.5 | 16 | 35 | 48 |
| ME_7 | 1 | 1 | 10 | 0 | 88 |
| ME_8 | 1 | 1 | 13 | 0 | 85 |
| ME_9 | 1 | 1 | 16 | 0 | 82 |
| ME_10 | 0.5 | 0.5 | 10 | 0 | 89 |
| ME_11 | 0.5 | 0.5 | 13 | 0 | 86 |
| ME_12 | 0.5 | 0.5 | 16 | 0 | 83 |
| N-Alkylamide | [MH]+ (m/z) | [M + NH4]+ (m/z) | [M + Na]+ (m/z) | RE a | PE e | |||
|---|---|---|---|---|---|---|---|---|
| Concentration (g/100 g RE) b ± SD c | RSD % d | Concentration (g/100 g PE) ± SD | RSD % | |||||
| A1 | (2Z-N-isobutyl-2-nonene-6,8-diynamide | 204.7 | 222.7 | - | 0.1 ± 0.0 | 6.3 | 0.1 ± 0.0 | 9.9 |
| A2 | (2E)-N-isobutyl-2-undecene-8,10-diynamide | 232.6 | - | 254.6 | tr f | 5.7 | tr | 12.8 |
| A3 | (2E,6Z,8E)-N-isobutyl-2,6,8-decatrienamide (spilanthol) | 222.5 | - | 244.5 | 12.8 ± 0.2 | 1.7 | 8.2 ± 0.0 | 0.5 |
| A4 | (2E,7Z)-N-isobutyl-2,7-decadienamide | 224.6 | - | 246.6 | 0.1 ± 0.0 | 1.8 | 0.1 ± 0.0 | 20.3 |
| A5 | (2E)-N-(2-methylbutyl)-2-undecene-8,10-diynamide | 268.6 | - | 268.6 | ||||
| A6 | (2E,6Z,8E)-N-(2-methylbutyl)-2,6,8-decatrienamide | 236.6 | - | 258.6 | 0.9 ± 0.0 | 2.9 | 0.6 ± 0.0 | 0.2 |
| Total | - | - | - | 13.9 ± 0.2 | 1.8 | 8.9 ± 0.0 | 0.4 | |
| Z-Average (nm) | PDI | |
|---|---|---|
| NE_3 | 148.7 ± 0.6 | 0.178 ± 0.015 |
| NE_4 | 216.5 ± 1.6 | 0.274 ± 0.002 |
| NE_5 | 121.1 ± 0.7 | 0.198 ± 0.014 |
| NE_6 | 133.2 ± 1.1 | 0.170 ± 0.009 |
| NE_7 | 172.4 ± 1.2 | 0.186 ± 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinozzi, E.; Cespi, M.; Ferrati, M.; Petrelli, R.; Maggi, F.; Wang, J.; Alimi, S.S.; Perinelli, D.R.; Bonacucina, G. Hydrogels Powered by Nanoemulsion Technology for the Topical Delivery of Acmella oleracea Extract. Pharmaceutics 2025, 17, 625. https://doi.org/10.3390/pharmaceutics17050625
Spinozzi E, Cespi M, Ferrati M, Petrelli R, Maggi F, Wang J, Alimi SS, Perinelli DR, Bonacucina G. Hydrogels Powered by Nanoemulsion Technology for the Topical Delivery of Acmella oleracea Extract. Pharmaceutics. 2025; 17(5):625. https://doi.org/10.3390/pharmaceutics17050625
Chicago/Turabian StyleSpinozzi, Eleonora, Marco Cespi, Marta Ferrati, Riccardo Petrelli, Filippo Maggi, Junbiao Wang, Sunday Segun Alimi, Diego Romano Perinelli, and Giulia Bonacucina. 2025. "Hydrogels Powered by Nanoemulsion Technology for the Topical Delivery of Acmella oleracea Extract" Pharmaceutics 17, no. 5: 625. https://doi.org/10.3390/pharmaceutics17050625
APA StyleSpinozzi, E., Cespi, M., Ferrati, M., Petrelli, R., Maggi, F., Wang, J., Alimi, S. S., Perinelli, D. R., & Bonacucina, G. (2025). Hydrogels Powered by Nanoemulsion Technology for the Topical Delivery of Acmella oleracea Extract. Pharmaceutics, 17(5), 625. https://doi.org/10.3390/pharmaceutics17050625