Augmented Marshmallow Extract Lipid Nanoparticles with Clove Oil Embedded in Collagen Sponge for Ultimate Antimicrobial Healing of Diabetic Mouth Ulcer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material and Extract Preparation
2.3. Metabolic Profiling Using LC/MS/MS
2.3.1. Sample Preparation
2.3.2. LC/MS/MS Analysis
2.3.3. Mass Spectrophotometry
2.4. Experimental Design Construction of M-LNs
2.5. Preparation of Marshmallow–Clove Oil Loaded Solid Lipid Nanoparticles
2.6. Investigation of Marshmallow–Clove Oil Loaded Solid Lipid Nanoparticles
2.6.1. Measurement of Particle Size, Polydispersity Index, and Zeta Potential
2.6.2. Percentage of Encapsulation Efficiency (EE%)
2.6.3. Optimization of Marshmallow–Clove Oil Loaded Solid Lipid Nanoparticles
2.7. Improvement of Optimized M-CO-SLNs
2.7.1. Transmission Electron Microscopy (TEM) Analysis
2.7.2. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
2.7.3. Analysis of Differential Scanning Calorimeters (DSCs)
2.7.4. In Vitro Release Study
2.7.5. Evaluation of Stability
2.8. Preparation of M-CO-SLNs Sponges
2.8.1. Characterization of M-CO-SLNs Sponges
Morphological Characterization of M-CO-SLNs Sponges
Fourier Transform Infrared (FTIR) Spectroscopy Analysis
Analysis of Differential Scanning Calorimeters (DSCs)
Porosity Assessment
Swelling Ratio
Mechanical Characterization
2.9. Antimicrobial Study
2.9.1. Diffusion Agar Method
2.9.2. Assessment of the Minimum Inhibitory Concentration (MIC)
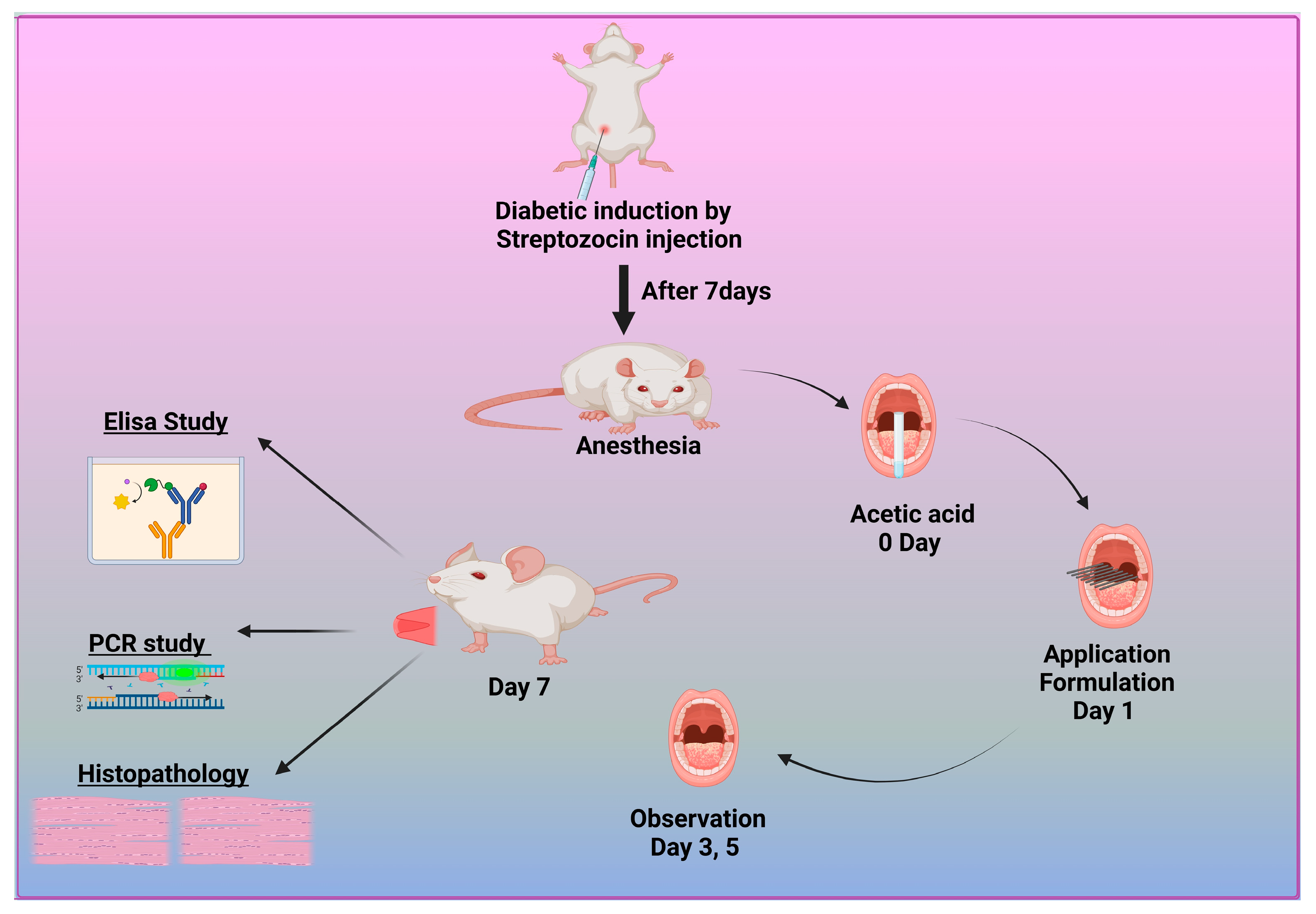
2.10. In Vivo Study
2.10.1. Induction of Type 1 Diabetic Rat Model
2.10.2. Induction of Diabetic Oral Ulcers
2.10.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10.4. RNA Extraction and Real-Time PCR Method
RNA Extraction and Reverse Transcription
The qRT-PCR Gene Expression Assay
2.11. Histopathological Examination
2.12. Statical Analysis
3. Results and Discussion
3.1. LC/MS/MS Analysis of Marshmallow (M.) Leaf Extract
3.2. Optimization of Factorial Design
3.2.1. The Influence of Formulation Parameters on Particle Size (P.S)
3.2.2. Impact of Formulation Variables on Polydispersity Index (PDI)
3.2.3. The Effect of Formulation Variables on Zeta Potential (ZP)
3.2.4. The Impact of Formulation Variables on Entrapment Efficiency (EE%)
3.2.5. Identifying the Optimized M-CO-SLNs
3.3. Improvement of Optimized M-CO-SLNs
3.3.1. Transmission Electron Microscopy (TEM) Analysis
3.3.2. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
3.3.3. Analysis of Differential Scanning Calorimeters (DSCs)
3.3.4. In Vitro Release Study of Solid Lipid Nanoparticles
3.3.5. Evaluation of Stability
3.4. Characterization of M-CO-SLN Sponge
3.4.1. Morphological Characterization of M-CO-SLNs Sponge
3.4.2. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
3.4.3. Analysis of Differential Scanning Calorimeters (DSCs)
3.4.4. In Vitro Release Study of Sponges
3.4.5. Porosity Assessment
3.4.6. Swelling Ratio
3.4.7. Mechanical Characterization
3.5. Antimicrobial Study
3.5.1. Diffusion Agar Method
3.5.2. Assessment of the Minimum Inhibitory Concentration (MIC)
3.6. In Vivo Study
3.6.1. Therapeutic Efficacy of Different Formulations in Diabetic Oral Ulcers
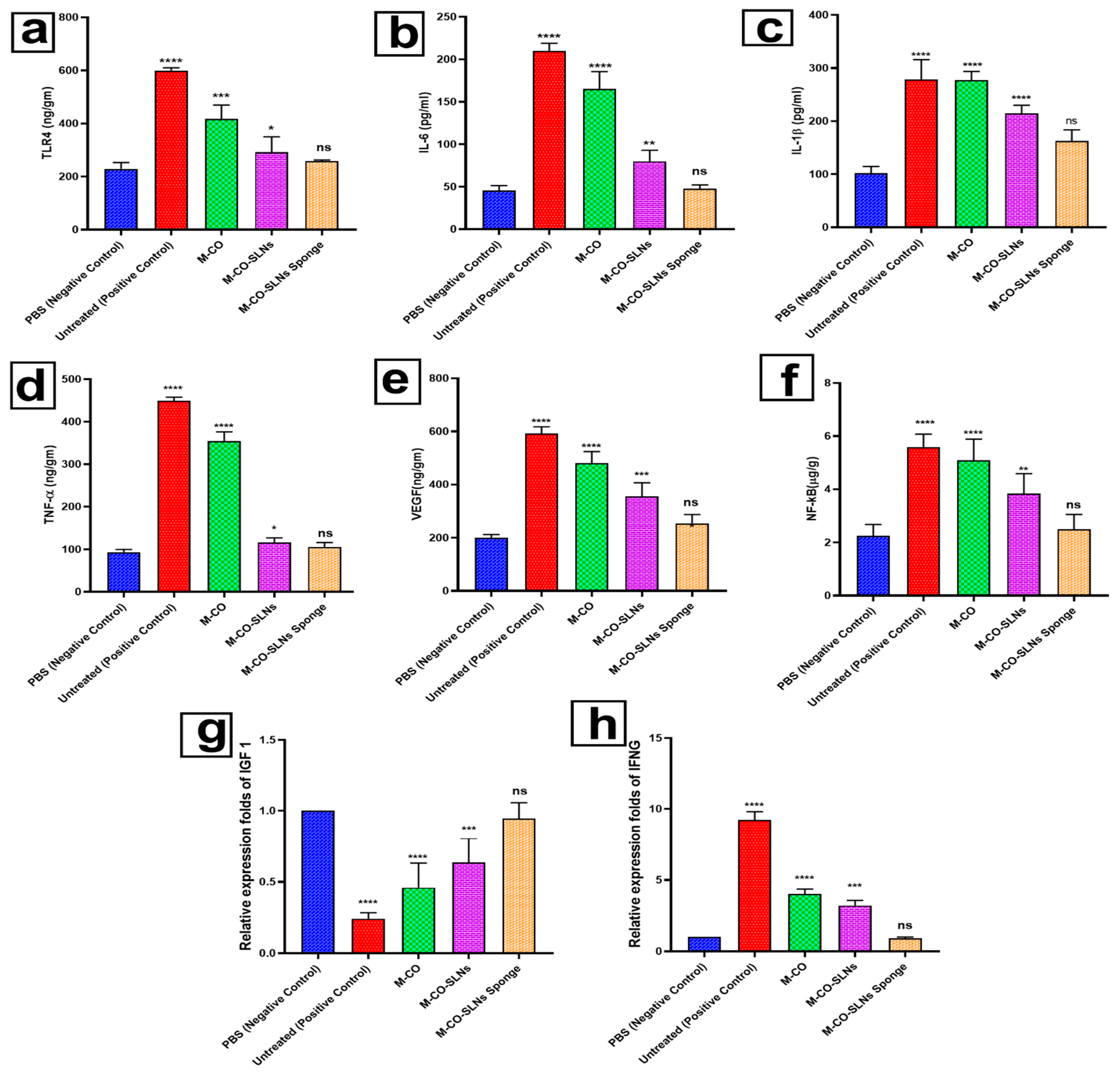
3.6.2. Enzyme-Linked Immunosorbent Assay (ELISA)
3.6.3. Effect of RNA Extraction and Real-Time PCR on Transcription Levels of IGF1 and IFNG in Oral Ulcer Treatment
3.7. Histopathological Examination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| M-CO | Marshmallow–clove oil |
| CO | Clove oil |
| SLNs | Solid lipid nanoparticles |
| GV | Gelatin Variant |
| NUB | Nahda University in Beni-Suef |
| FTIR | Fourier Transform Infrared Spectroscopy |
| SEM | Scanning Electron Microscopy |
| PBS | Phosphate-Buffered Saline |
| CFU | Colony-Forming Unit |
| TEM | Transmission electron microscopy |
| DLS | Dynamic light scattering |
| PDI | Polydispersity index |
| ZP | Zeta potential |
| ROS | Reactive oxygen species |
| TGF-β | Transforming Growth Factor Beta |
| VEGF | Vascular Endothelial Growth Factor |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor Necrosis Factor Alpha |
| MDA | Malondialdehyde |
| RT-PCR | Real-time polymerase chain reaction |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| DNA | Deoxyribonucleic Acid |
| MMP | Matrix Metalloproteinases |
| H&E | Hematoxylin and Eosin (staining) |
| RNA | Ribonucleic Acid |
| EE | Entrapment efficiency percent |
| SAA | Surface Active Agent |
| P.S | Particle size |
| IFNG | Interferon Gamma |
| IGF-1 | Insulin-like growth factors |
References
- Haghighatseir, N.; Mozafari, N.; Shadvand, E.; Ashrafi, H.; Daneshamouz, S.; Azadi, A. Mixed-Micelle in Situ Gel as a Candidate for Oral Inflammatory Ulcerative Diseases. AAPS PharmSciTech 2024, 25, 144. [Google Scholar] [CrossRef]
- Fathy Elhabal, S.; El-Nabarawi, M.A.; Abdelaal, N.; Elrefai, M.F.M.; Ghaffar, S.A.; Khalifa, M.M.; Mohie, P.M.; Waggas, D.S.; Hamdan, A.M.E.; Alshawwa, S.Z.; et al. Development of Canagliflozin Nanocrystals Sublingual Tablets in the Presence of Sodium Caprate Permeability Enhancer: Formulation Optimization, Characterization, in-Vitro, in Silico, and in-Vivo Study. Drug Deliv. 2023, 30, 2241665. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, Z.; Yin, M.; Ru, J.; Liu, X.; Chen, Z.; Liu, Y.; Sun, W.; Zheng, L.; Zhao, X.; et al. Polyphenol-Modified Mg−Zn Layered Hydroxide-Contained Microneedle Patch Enhance Mucosal Repair by Remolding Diabetic Oral Microenvironment. Adv. Healthc. Mater. 2025, 14, 2403883. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Lin, L.; Tang, R.; Xu, Z.; Kong, S.; Lv, D.; Bai, D.; Liu, Y.; Li, H. Preparation and Characterization of Mussel-Inspired Chitosan/Polydopamine Films and Their Feasibility for Oral Mucosa Application. Int. J. Biol. Macromol. 2024, 279, 135179. [Google Scholar] [CrossRef]
- Isei, T.; Abe, M.; Ikegami, R.; Kato, H.; Sakurai, E.; Tanizaki, H.; Nakanishi, T.; Matsuo, K.; Yamasaki, O.; Asai, J.; et al. Wound, Pressure Ulcer, and Burn Guidelines - 3: Guidelines for the Diagnosis and Treatment of Diabetic Ulcers and Gangrene, Second Edition. J. Dermatol. 2025. [Google Scholar] [CrossRef]
- Salcan, I.; Dilber, M.; Suleyman, Z.; Yucel, N.; Salcan, S.; Kesan, S.; Yazici, G.N.; Celik, F.; Koseturk, M.; Alcan Alp, N.; et al. Protective Effect of Adenosine Triphosphate against Cisplatin-Induced Necrotic and Degenerative Oral Mucositis in Rats. J. Appl. Oral. Sci. 2025, 33, e20250007. [Google Scholar] [CrossRef] [PubMed]
- Vales-Villamarín, C.; de Dios, O.; Pérez-Nadador, I.; Gavela-Pérez, T.; Soriano-Guillén, L.; Garcés, C. PPARγ2 Pro12Ala Polymorphism Is Associated in Children With Traits Related to Susceptibility to Type 2 Diabetes. Front. Pharmacol. 2021, 12, 763853. [Google Scholar] [CrossRef]
- Song, J.; Geng, Z.; Luan, X.; Zhang, D.; Wang, Q.; Pan, L.; Yu, X.; Dong, W.; Wu, D.; You, S. Construction of Natural Hydrogels Consisting of Oxidized Dextran, Quaternized Chitosan and Cuttlefish Ink Nanoparticles for Treating Diabetic Oral Ulcers. Int. J. Biol. Macromol. 2024, 283, 137737. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhuang, Z.; Zhou, R.; Zheng, L.; Li, C.; Zhou, R.; Gao, Y.; Zhang, L.; Zheng, Y.; Zhao, L.; et al. Next-Generation Oral Ulcer Management: Integrating Cold Atmospheric Plasma (CAP) with Nanogel-Based Pharmaceuticals for Inflammation Regulation. Adv. Healthc. Mater. 2025, 14, e2403223. [Google Scholar] [CrossRef]
- ELhabal, S.F.; El-Nabarawi, M.A.; Hassanin, S.O.; Hassan, F.E.; Abbas, S.S.; Gebril, S.M.; Albash, R. Transdermal Fluocinolone Acetonide Loaded Decorated Hyalurosomes Cellulose Acetate/Polycaprolactone Nanofibers Mitigated Freund’s Adjuvant-Induced Rheumatoid Arthritis in Rats. J. Pharm. Investig. 2024, 55, 113–132. [Google Scholar] [CrossRef]
- Ozdemir, E.; Kavakli, O. Risk Factors for Oral Mucosal Pressure Injury Associated with Endotracheal Tubes in Intensive Care Unit Patients: A Single-Centre Longitudinal Study with Brief Follow-Up. Nurs. Crit. Care 2025, 30, e70009. [Google Scholar] [CrossRef] [PubMed]
- Elhabal, S.F.; Al-Zuhairy, S.A.S.; El-Nabarawi, M.; Elrefai, M.F.M.; Shoela, M.S.; Hababeh, S.; Nelson, J.; Khalek, M.A.A.; Fady, M.; Elzohairy, N.A.; et al. Enhancing Photothermal Therapy for Antibiofilm Wound Healing: Insights from Graphene Oxide-Cranberry Nanosheet Loaded Hydrogel in Vitro, in Silico, and in Vivo Evaluation. Int. J. Nanomedicine 2024, 19, 12999–13027. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.H.H.; Hamed, A.N.E.; Elhabal, S.F.; Mokhtar, F.A.; Abdelmohsen, U.R.; Fouad, M.A.; Kamel, M.S. Chemical Composition and Anti-Proliferative Activities of Hyophorbe Lagenicaulis Aerial Parts and Their Biogenic Nanoparticles Supported by Network Pharmacology Study. S. Afr. J. Bot. 2023, 156, 398–410. [Google Scholar] [CrossRef]
- Mohammed, M.H.H.; Hamed, A.N.E.; Elhabal, S.F.; Mokhtar, F.A.; Abdelmohsen, U.R.; Fouad, M.A.; Kamel, M.S. Metabolic Profiling and Cytotoxic Activities of Ethanol Extract of Dypsis Leptocheilos Aerial Parts and Its Green Synthesized Silver Nanoparticles Supported by Network Pharmacology Analysis. S. Afr. J. Bot. 2023, 161, 648–665. [Google Scholar] [CrossRef]
- Kucharzewski, M.; Spyrka, K.; Rojczyk, E.; Brela, J. Topical Application of Manuka Honey for the Treatment of Non-Healing Venous Leg Ulcers. Pharmaceuticals 2025, 18, 149. [Google Scholar] [CrossRef]
- Xu, J.; Chang, L.; Xiong, Y.; Peng, Q. Chitosan-Based Hydrogels as Antibacterial/Antioxidant/Anti-Inflammation Multifunctional Dressings for Chronic Wound Healing. Adv. Healthc. Mater. 2024, 13, e2401490. [Google Scholar] [CrossRef]
- Sanguansajapong, V.; Sakdiset, P.; Puttarak, P. Development of Oral Microemulsion Spray Containing Pentacyclic Triterpenes-Rich Centella Asiatica (L.) Urb. Extract for Healing Mouth Ulcers. Pharmaceutics 2022, 14, 2531. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zeng, H.; Ouyang, J.; Wen, S.; Zhou, F.; Jiang, R.; Zhang, X.; Wang, Z.; Huang, J.; Liu, Z. Eurotium-Cristatum Fermented Black Tea Alleviates Ulcerative Colitis through the PPARγ-NF-ΚB Signaling Axis. Food Res. Int. 2025, 200, 115436. [Google Scholar] [CrossRef]
- da Silva, L.M.; Pezzini, B.C.; Somensi, L.B.; Bolda Mariano, L.N.; Mariott, M.; Boeing, T.; dos Santos, A.C.; Longo, B.; Cechinel-Filho, V.; de Souza, P.; et al. Hesperidin, a Citrus Flavanone Glycoside, Accelerates the Gastric Healing Process of Acetic Acid-Induced Ulcer in Rats. Chem. Biol. Interact. 2019, 308, 45–50. [Google Scholar] [CrossRef]
- Santin, J.R.; Lemos, M.; Klein-Júnior, L.C.; Machado, I.D.; Costa, P.; De Oliveira, A.P.; Tilia, C.; De Souza, J.P.; De Sousa, J.P.B.; Bastos, J.K.; et al. Gastroprotective Activity of Essential Oil of the Syzygium Aromaticum and Its Major Component Eugenol in Different Animal Models. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 149–158. [Google Scholar] [CrossRef]
- Al-Zuhairy, S.A.K.S.; Elhabal, S.F.; Mohamed Elrefai, M.F.; Hababeh, S.; Nelson, J.; Fady, M.; Elzohairy, N.A.; Ewedah, T.M.; Mousa, I.S.; Hamdan, A.M.E. Polylactic-Co-Glycolic Acid/Alginate/Neem Oil-Reduced Graphene Oxide as a PH-Sensitive Nanocarrier for Hesperidin Drug Delivery: Antimicrobial and Acute Otitis Media Assessments. Pharmaceuticals 2025, 18, 381. [Google Scholar] [CrossRef]
- Zeshan, M.Q.; Ashraf, M.; Omer, M.O.; Anjum, A.A.; Ali, M.A.; Najeeb, M.; Majeed, J. Antimicrobial Activity of Essential Oils of Curcuma Longa and Syzygium Aromaticum against Multiple Drug-Resistant Pathogenic Bacteria. Trop. Biomed. 2023, 40, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Somensi, L.B.; Costa, P.; Boeing, T.; Bolda Mariano, L.N.; de Gregório, E.; E Silva, A.T.M.; Longo, B.; Locatelli, C.; de Souza, P.; Magalhães, C.G.; et al. Lupeol Stearate Accelerates Healing and Prevents Recurrence of Gastric Ulcer in Rodents. Evid. Based Complement. Alternat Med. 2022, 2022, 6134128. [Google Scholar] [CrossRef] [PubMed]
- Sutovska, M.; Capek, P.; Franova, S.; Joskova, M.; Sutovsky, J.; Marcinek, J.; Kalman, M. Antitussive Activity of Althaea Officinalis L. Polysaccharide Rhamnogalacturonan and Its Changes in Guinea Pigs with Ovalbumine-Induced Airways Inflammation. Bratisl. Med. J. 2011, 112, 670–675. [Google Scholar]
- Bhagat, N.; Nalawala, Z.; Patel, J.; Das, D.; Baldha, R.; Sarolia, J.; Rathod, S. Self-Assembled Systems for Nose-to-Brain Delivery of Temozolamide (TMZ) in Brain Tumor Therapy. Int. J. Pharm. 2025, 675, 125540. [Google Scholar] [CrossRef]
- Kapoor, D.U.; Prajapati, B.G.; Bhattacharya, S.; Singhai, N.J.; Maheshwari, R. Advances in Parenteral Nanocarriers and Delivery Devices: A Comprehensive Review. Curr. Pharm. Des. 2025, 31, 1844–1865. [Google Scholar] [CrossRef]
- Bandiwadekar, A.; Jose, J.; Gopan, G.; Augustin, V.; Ashtekar, H.; Khot, K.B. Transdermal Delivery of Resveratrol Loaded Solid Lipid Nanoparticle as a Microneedle Patch: A Novel Approach for the Treatment of Parkinson’s Disease. Drug Deliv. Transl. Res. 2025, 15, 1043–1073. [Google Scholar] [CrossRef]
- Bombana, H.S.; Meirelles, G.d.P.; de Oliveira, R.A.; Leyton, V.; Yonamine, M. Synthetic Cannabinoids Receptor Agonists in Oral Fluid: Development of a Dispersive Liquid-Liquid Microextraction (DLLME) Method with Liquid Chromatography-Mass Spectrometry Detection. J. Anal. Toxicol. 2025, bkaf027. [Google Scholar] [CrossRef]
- Imran, A.; Ye, S.; Li, J.A.; Ajaj, R.; Rauf, A.; Ahmad, Z.; Hemeg, H.A.; Al-Awthan, Y.S.M.; Bahattab, O.S.; Quradha, M.M.; et al. LC-ESI-QTOF-MS/MS Characterization of Phenolic Compounds in the Stem, Roots, and Leaves of Syzygium Cumini and Their Antioxidant Potential. Food Sci. Nutr. 2025, 13, e70112. [Google Scholar] [CrossRef]
- Rezaei, M.; Dadgar, Z.; Noori-Zadeh, A.; Mesbah-Namin, S.A.; Pakzad, I.; Davodian, E. Evaluation of the Antibacterial Activity of the Althaea Officinalis L. Leaf Extract and Its Wound Healing Potency in the Rat Model of Excision Wound Creation. Avicenna J. Phytomed 2015, 5, 105. [Google Scholar]
- Zhang, X.; Wang, Z.; Li, Y.; Zhou, Z.; Wei, B.; Dong, T.; Zhao, Y.; Ye, C.; Li, J.; Cui, J.; et al. Sea Buckthorn Leaves and Gallic Acid Inhibit C48/80-Induced Pseudo-Allergic Reaction via the PLC/IP3 Signaling Pathway Both in Vitro and in Vivo. Int. Immunopharmacol. 2025, 154, 114563. [Google Scholar] [CrossRef]
- Evaristo, J.; de Laia, E.; Tavares, B.; Mendonça, E.; Grisostenes, L.; Rodrigues, C.; do Nascimento, W.; Garcia, C.; Guterres, S.; Nogueira, F.; et al. Identification of Bioactive Metabolites of Capirona Macrophylla by Metabolomic Analysis, Molecular Docking, and In Vitro Antiparasitic Assays. Metabolites 2025, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Pan, X.; Wang, Y.; Li, Z.; Li, W. Prediction of Potential Active Ingredients and Mechanism of Forsythia Suspense Leaf Green Tea Extract in Treating COPD Mice Based on UPLC-MS and Network Pharmacology. J. Sci. Food Agric. 2025. Early View. [Google Scholar] [CrossRef]
- El Gendy, S.N.; Elmotayam, A.K.; Samir, R.; Ezzat, M.I.; Abo-Elfadl, M.T.; El Sayed, A.M. Biotransformation of Quercetin by Bacillus Subtilis and Anticancer Activity Evaluation: In Vitro and in Silico. AMB Express 2025, 15, 58. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Thi Nguyen, H.T.; Thi Nguyen, D.C.; Lu, P.T.; Dang, T.H.; Ma, T.T.H.; Huynh, T.A.H.; Do, T.V.M.C. Advancing UPLC-MS/MS for Mapping the Chemical Fingerprint of Bioactive Compounds in Lotus Leaves (Folium Nelumbinis). J. Pharm. Biomed. Anal. 2025, 261, 116840. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, G.; Alamer, C.; Sherif, S.A.; Alamer, S.A.; Sherif, F. El Characterizing the Role of Moringa Oleifera Lam (MO) Leaves and Root Extracts on Dictyostelium Discoideum Cell Behavior. Biology 2025, 14, 284. [Google Scholar] [CrossRef]
- Elhabal, S.F.; El-Nabarawi, M.; Elrefai, M.F.M.; Teaima, M.H.; Shoela, M.S.; Khamis, G.M.; Faheem, A.M.; kholeif, N.A.; Sanad, M.T. Nano-Spanlastics-Loaded Dissolving Microneedle Patches for Ketotifen Fumarate: Advanced Strategies for Allergic Conjunctivitis Treatment and Molecular Insights. Drug Deliv. Transl. Res. 2025, 15, 1–24. [Google Scholar] [CrossRef]
- El-Nawawy, T.M.; Adel, Y.A.; Teaima, M.; Nassar, N.N.; Nemr, A.; Al-Samadi, I.; El-Nabarawi, M.A.; Elhabal, S.F. Intranasal Bilosomes in Thermosensitive Hydrogel: Advancing Desvenlafaxine Succinate Delivery for Depression Management. Pharm. Dev. Technol. 2024, 29, 663–674. [Google Scholar] [CrossRef]
- Shoman, N.A.; Saady, M.; Teaima, M.; Abdelmonem, R.; El-Nabarawi, M.A.; Elhabal, S.F. Merging Konjac Glucomannan with Other Copolymeric Hydrogels as a Cutting-Edge Liquid Raft System for Dual Delivery of Etoricoxib and Famotidine. Drug Deliv. 2023, 30, 2189630. [Google Scholar] [CrossRef]
- Al-Shoubki, A.A.; Teaima, M.H.; Abdelmonem, R.; El-Nabarawi, M.A.; Elhabal, S.F. Potential Application of Sucrose Acetate Isobutyrate, and Glyceryl Monooleate for Nanonization and Bioavailability Enhancement of Rivaroxaban Tablets. Pharm. Sci. Adv. 2024, 2, 100015. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Abdelmonem, R.; El Nashar, R.M.; Elrefai, M.F.M.; Hamdan, A.M.E.; Safwat, N.A.; Shoela, M.S.; Hassan, F.E.; Rizk, A.; Kabil, S.L.; et al. Enhanced Antibacterial Activity of Clindamycin Using Molecularly Imprinted Polymer Nanoparticles Loaded with Polyurethane Nanofibrous Scaffolds for the Treatment of Acne Vulgaris. Pharmaceutics 2024, 16, 947. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, L.; Chen, Z.; Mao, G.; Gao, Z.; Lai, X.; Zhu, X.; Zhu, J. Nanostructured Lipid Carriers, Solid Lipid Nanoparticles, and Polymeric Nanoparticles: Which Kind of Drug Delivery System Is Better for Glioblastoma Chemotherapy? Drug Deliv. 2016, 23, 3408–3416. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, C.; Jia, M.; Chen, M. Enhanced Dermal Delivery of Nanoparticulate Formulation of Cutibacterium Acnes Using Sponge Spicules for Atopic Dermatitis Treatment. Int. J. Nanomedicine 2025, 20, 3235–3249. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Collard, D.; Thomann, J.-S.; Duday, D. Antimicrobial Sponge: A Polyvinyl Alcohol, Tannic Acid and Curcumin-Loaded Nanolignin Hydrogel Composite Scaffold. Gels 2025, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Pal, N.; Banerjee, S.; Roy, P.; Pal, K. Cellulose Nanocrystals-silver Nanoparticles-Reduced Graphene Oxide Based Hybrid PVA Nanocomposites and Its Antimicrobial Properties. Int. J. Biol. Macromol. 2021, 191, 445–456. [Google Scholar] [CrossRef]
- Aminoroaya, N.; Ehtesabi, H.; Monfared-Hajishirkiaee, R.; Rezaei, A.; Latifi, H.; Ahadian, M.M. Investigating the Absorbent Properties of Carboxymethyl Cellulose-Chitosan-carbon Dots Nanocomposite Sponge Using Photoacoustic Microscopy. Int. J. Biol. Macromol. 2025, 308, 142517. [Google Scholar] [CrossRef]
- Abedi, M.; Arbabi, M.; Gholampour, R.; Amini, J.; Barandeh, Z.; Hosseini, S.; Abedi, A.; Gholibegloo, E.; Zomorrodian, H.; Raoufi, M. Zinc Oxide Nanoparticle-Embedded Tannic Acid/Chitosan-Based Sponge: A Highly Absorbent Hemostatic Agent with Enhanced Antimicrobial Activity. Int. J. Biol. Macromol. 2025, 300, 140337. [Google Scholar] [CrossRef]
- Ewedah, T.M.; Abdalla, A.; Hagag, R.S.; Elhabal, S.F.; Teaima, M.H.; El-Nabarawi, M.A.; Schlatter, G.; Shoueir, K.R. Enhancing Cellular Affinity for Skin Disorders: Electrospun Polyurethane/Collagen Nanofiber Mats Coated with Phytoceramides. Int. J. Pharm. 2024, 663, 124541. [Google Scholar] [CrossRef]
- Cheng, H.; Tian, G.; Liu, H.; Bai, D.; Zhang, Y.; Wang, Q.; Zhao, M.; Cao, S.; Deng, D.; Wang, X. A Molybdenum Sulfide Based Nitric Oxide Controlled Release Oral Gel for Rapid Healing of Oral Mucosal Ulcers. J. Colloid. Interface Sci. 2025, 678, 560–571. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Li, K.; Chen, Z.; Zhang, J.; Zhao, G.; Sun, F.; Xiao, P.; Yang, Y. Periplaneta Americana Extract Improves Recurrent Oral Ulcers through Regulation of TLR4/NF-ΚB and Nrf2/HO-1 Pathways. Sci. Rep. 2025, 15, 8578. [Google Scholar] [CrossRef]
- Qian, S.; Wang, F.; Liao, W.; Liu, J. The Role of HMGA2 in Activating the IGFBP2 Expression to Promote Angiogenesis and LUAD Metastasis via the PI3K/AKT/VEGFA Signaling Pathway. Neoplasma 2024, 71, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, L.; Liu, Y.; Zheng, T.; Chen, N.; Du, P.; Ye, H. Exploration of Key Genes Associated with Oxidative Stress in Polycystic Ovary Syndrome and Experimental Validation. Front. Med. 2025, 12, 1493771. [Google Scholar] [CrossRef]
- Cao, C.; Xu, W.; Lei, J.; Zheng, Y.; Zhang, A.; Xu, A.; Lin, F.; Zhou, M. The IL-6 Autocrine Loop Promoting IFN-γ-Induced Fibroblast Senescence Is Involved in Psychological Stress-Mediated Exacerbation of Vitiligo. Inflamm Res 2025, 74, 72. [Google Scholar] [CrossRef] [PubMed]
- Paredes, S.D.; Hernández-Cortés, J.; Falahat, F.; Rancan, L.; Arias-Díaz, J.; Vara, E. Somatostatin Mitigates Gastric Mucosal Damage Induced by LPS in a Male Wistar Rat Model of Sepsis. Biomolecules 2025, 15, 508. [Google Scholar] [CrossRef]
- Ashraf, M.U.; Iqbal, M.A.; Barkat, K.; Anjum, I.; Mushtaq, M.N.; Gul, R.; Aamir, M.; Ibenmoussa, S.; Salamatullah, A.M.; Bourhia, M.; et al. Exploring the Potential of a PH-Sensitive Hydrogel Sponge: Interpenetrating Network of Tragacanth and Pectin for Controlled Delivery of Levosulpiride. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 398, 5579–5592. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Zhou, L.; Shi, L.; Liu, T.; Guo, Y.; Chen, H.; Luo, H.; Ma, J.; Zhang, H.; Xiong, P.; et al. Quercetin-Crosslinked Chitosan Nanoparticles: A Potential Treatment for Allergic Rhinitis. Sci. Rep. 2024, 14, 4021. [Google Scholar] [CrossRef]
- Lambuk, L.; Suhaimi, N.A.A.; Sadikan, M.Z.; Jafri, A.J.A.; Ahmad, S.; Nasir, N.A.A.; Uskoković, V.; Kadir, R.; Mohamud, R. Nanoparticles for the Treatment of Glaucoma-Associated Neuroinflammation. Eye Vis. 2022, 9, 26. [Google Scholar] [CrossRef]
- Keyvan Rad, J.; Alinejad, Z.; Khoei, S.; Mahdavian, A.R. Controlled Release and Photothermal Behavior of Multipurpose Nanocomposite Particles Containing Encapsulated Gold-Decorated Magnetite and 5-FU in Poly(Lactide- Co-Glycolide). ACS Biomater. Sci. Eng. 2019, 5, 4425–4434. [Google Scholar] [CrossRef]
- Zarif Attalla, K.; Hassan, D.H.; Teaima, M.H.; Yousry, C.; El-Nabarawi, M.A.; Said, M.A.; Elhabal, S.F. Enhanced Intranasal Delivery of Atorvastatin via Superparamagnetic Iron-Oxide-Loaded Nanocarriers: Cytotoxicity and Inflammation Evaluation and In Vivo, In Silico, and Network Pharmacology Study for Targeting Glioblastoma Management. Pharmaceuticals 2025, 18, 421. [Google Scholar] [CrossRef]
- Yurtdaş Kirimlioğlu, G.; Öztürk, A.A. Levocetirizine Dihydrochloride-Loaded Chitosan Nanoparticles: Formulation and In Vitro Evaluation. Turk. J. Pharm. Sci. 2020, 17, 27–35. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Ge, X.X.; Qi, X.; Xiang, Y.; Shi, Y.; Li, Y.; Pan, Y.; Wang, Y.; Ru, Y.; et al. Ferric Iron/Shikonin Nanoparticle-Embedded Hydrogels with Robust Adhesion and Healing Functions for Treating Oral Ulcers in Diabetes. Adv. Sci. 2024, 11. [Google Scholar] [CrossRef] [PubMed]
- Gönüllü, Ü.; Üner, M.; Yener, G.; Karaman, E.F.; Aydoʇmuş, Z. Formulation and Characterization of Solid Lipid Nanoparticles, Nanostructured Lipid Carriers and Nanoemulsion of Lornoxicam for Transdermal Delivery. Acta Pharm. 2015, 65, 1–13. [Google Scholar] [CrossRef]
- Almeida, S.D.; Ramesh, S.H.; Radhakrishna, G.K.; Sireesha, G.; Ramesh, S.; Kumar, B.S.; Hosur Dinesh, B.G.; Ganjipete, S.; Nagaraj, S.; Theivendren, P.; et al. Development and Evaluation of S-Carboxymethyl-L-Cystine-Loaded Solid Lipid Nanoparticles for Parkinson’s Disease in Murine and Zebrafish Models. Sci. Rep. 2025, 15, 10885. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, S.-H.; Lee, K.-A. A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of Nanosuspensions in Drug Delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Sangnim, T.; Limmatvapirat, S.; Nunthanid, J.; Sriamornsak, P.; Sittikijyothin, W.; Wannachaiyasit, S.; Huanbutta, K. Design and Characterization of Clindamycin-Loaded Nanofiber Patches Composed of Polyvinyl Alcohol and Tamarind Seed Gum and Fabricated by Electrohydrodynamic Atomization. Asian J. Pharm. Sci. 2018, 13, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Al-Shoubki, A.A.; Teaima, M.H.; Abdelmonem, R.; El-Nabarawi, M.A.; Elhabal, S.F. Sucrose Acetate Isobutyrate (SAIB) and Glyceryl Monooleate (GMO) Hybrid Nanoparticles for Bioavailability Enhancement of Rivaroxaban: An Optimization Study. Pharm. Dev. Technol. 2023, 28, 928–938. [Google Scholar] [CrossRef]
- Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Krueger, C.G.; Reed, J.D. Antimicrobial Proanthocyanidin-Chitosan Composite Nanoparticles Loaded with Gentamicin. Int. J. Biol. Macromol. 2020, 162, 1500–1508. [Google Scholar] [CrossRef]
- Chelminiak-Dudkiewicz, D.; Wujak, M.; Mlynarczyk, D.T.; Dlugaszewska, J.; Mylkie, K.; Smolarkiewicz-Wyczachowski, A.; Ziegler-Borowska, M. Enhancing the Porosity of Chitosan Sponges with CBD by Adding Antimicrobial Violacein. Heliyon 2024, 10, e35389. [Google Scholar] [CrossRef]
- Giani, M.; Valentino, C.; Vigani, B.; Ruggeri, M.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Falabella, P.; Sandri, G.; Rossi, S. Hermetia Illucens-Derived Chitosan as a Promising Sustainable Biomaterial for Wound Healing Applications: Development of Sponge-like Scaffolds. Int. J. Biol. Macromol. 2025, 304, 140903. [Google Scholar] [CrossRef]
- Qi, H.; Yang, L.; Ma, R.; Xiang, Y.; Dai, Y.; Ren, J.; Xu, B.B.; El-Bahy, Z.M.; Thabet, H.K.; Huang, Z.; et al. Amoxicillin-Laded Sodium Alginate/Cellulose Nanocrystals/Polyvinyl Alcohol Composite Nanonetwork Sponges with Enhanced Wound Healing and Antibacterial Performance. Int. J. Biol. Macromol. 2024, 280, 135701. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.; Amaral, G.O.; do Espirito Santo, G.; Dos Santos Jorge Sousa, K.; Martignago, C.C.S.; Souza E Silva, L.C.; de Lima, L.E.; Vitor de Souza, D.; Cruz, M.A.; Ribeiro, D.A.; et al. 3D Printed Skin Dressings Manufactured with Spongin-like Collagen from Marine Sponges: Physicochemical Properties and in Vitro Biological Analysis. Biomed. Mater. 2025, 20, 025016. [Google Scholar] [CrossRef]
- Chen, G.; Yang, C.; Xu, X.; Yang, L.; Zhang, Y.; Cai, C.; Muhitdinov, B.; Turaev, A.; Qiu, H.; Huang, S.; et al. Multifunctional Hydrogel Dressing Composed of Trichosanthes Polysaccharide and Carboxymethyl Chitosan Accelerates Cachectic Wound Healing and Reduces Scar Hyperplasia. Carbohydr. Polym. 2025, 357, 123378. [Google Scholar] [CrossRef]
- Xu, Q.; Su, W.; Huang, C.; Zhong, H.; Huo, L.; Cai, J.; Li, P. Multifunctional Polysaccharide Self-Healing Wound Dressing: NIR-Responsive Carboxymethyl Chitosan/Quercetin Hydrogel. Adv. Healthc. Mater. 2025, 14, e2403267. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Li, M.; Li, Z.; Wang, J.; Li, J.; Guo, B. Polymer Applied in Hydrogel Wound Dressing for Wound Healing: Modification/Functionalization Method and Design Strategies. ACS Biomater. Sci. Eng. 2025, 11, 1921–1944. [Google Scholar] [CrossRef]
- Di Giulio, M.; Zappacosta, R.; Di Lodovico, S.; Di Campli, E.; Siani, G.; Fontana, A.; Cellini, L. Antimicrobial and Antibiofilm Efficacy of Graphene Oxide against Chronic Wound Microorganisms. Antimicrob. Agents Chemother. 2018, 62, e00547-18. [Google Scholar] [CrossRef]
- Borehalli Mayegowda, S.; Roy, A.; Manjula, N.G.; Pandit, S.; Alghamdi, S.; Almehmadi, M.; Allahyani, M.; Awwad, N.S.; Sharma, R. Eco-Friendly Synthesized Nanoparticles as Antimicrobial Agents: An Updated Review. Front. Cell Infect. Microbiol. 2023, 13, 1224778. [Google Scholar] [CrossRef] [PubMed]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Brink, I.; Šipailienė, A.; Leskauskaitė, D. Antimicrobial Properties of Chitosan and Whey Protein Films Applied on Fresh Cut Turkey Pieces. Int. J. Biol. Macromol. 2019, 130, 810–817. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis Investigations of Terpenoid, Alkaloid, and Flavonoid Antimicrobial Agents Derived from Medicinal Plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, D.N.; Moon, J.H.; Park, H.N.; Ko, W.K.; Bae, M.S.; Lee, J.B.; Park, S.W.; Kim, E.C.; Lee, C.H.; et al. Chitosan/Polyurethane Blended Fiber Sheets Containing Silver Sulfadiazine for Use as an Antimicrobial Wound Dressing. J. Nanosci. Nanotechnol. 2014, 14, 7488–7494. [Google Scholar] [CrossRef] [PubMed]
- Bang, F.S.; Leeberg, V.; Ding, M.; Dreyer, C.H. The Effect of VEGF Stimulation in Diabetic Foot Ulcers: A Systematic Review. Wound Repair. Regen. 2024, 32, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiang, Y.; Chen, Y.; Wang, Y.; Dong, W.; Liu, Y.; Qi, X.; Shen, J. A Natural Eumelanin-Assisted Pullulan/Chitosan Hydrogel for the Management of Diabetic Oral Ulcers. Macromol Biosci 2024, 25, 2400526. [Google Scholar] [CrossRef]
- Fang, X.; Chen, X.; Dong, W.; Ye, F. A Poly(Tannic Acid) Particle-Supported β-Glucan/Chitosan Hydrogel for Managing Oral Ulcers in Diabetes. Int. J. Biol. Macromol. 2025, 306, 141609. [Google Scholar] [CrossRef]
- Chen, X.; Cao, X.; Zhang, J.; Jiang, C.; Yu, Y.; Chen, H. Enhancing Skin Wound Healing in Diabetic Mice Using SIKVAV-Modified Chitosan Hydrogels. Molecules 2024, 29, 5374. [Google Scholar] [CrossRef]
- Abd-Eldayem, A.M.; Makram, S.M.; Messiha, B.A.S.; Abd-Elhafeez, H.H.; Abdel-Reheim, M.A. Cyclosporine-Induced Kidney Damage Was Halted by Sitagliptin and Hesperidin via Increasing Nrf2 and Suppressing TNF-α, NF-ΚB, and Bax. Sci. Rep. 2024, 14, 7434. [Google Scholar] [CrossRef]
- Rodrigues, F.F.; Lino, C.I.; Oliveira, V.L.S.; Zaidan, I.; Melo, I.S.F.; Braga, A.V.; Costa, S.O.A.M.; Morais, M.I.; Barbosa, B.C.M.; da Costa, Y.F.G.; et al. A Clindamycin Acetylated Derivative with Reduced Antibacterial Activity Inhibits Articular Hyperalgesia and Edema by Attenuating Neutrophil Recruitment, NF-ΚB Activation and Tumor Necrosis Factor-α Production. Int. Immunopharmacol. 2023, 122, 110609. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.Y.; Shao, L. The Effect of IGF-1 on Cartilage Injury in Bone Marrow Mesenchymal Stem Cells through the BMP2-Smad1/5 Signaling Pathway. In Vitro Cell Dev. Biol. Anim. 2025, 61, 340–356. [Google Scholar] [CrossRef]
- Huang, Z.H.; Li, S.Q.; Kou, Y.; Huang, L.; Yu, T.; Hu, A. Risk Factors for the Recurrence of Diabetic Foot Ulcers among Diabetic Patients: A Meta-Analysis. Int. Wound J. 2019, 16, 1373–1382. [Google Scholar] [CrossRef]
- Longo, B.; Sommerfeld, E.P.; da Silva, R.; Somensi, L.B.; Mariano, L.N.B.; Boeing, T.; de Andrade, S.F.; de Souza, P.; da Silva, L.M. Dual Role of Eugenol on Chronic Gastric Ulcer in Rats: Low-Dose Healing Efficacy and the Worsening Gastric Lesion in High Doses. Chem. Biol. Interact. 2021, 333, 109335. [Google Scholar] [CrossRef]







| No. | Rt (min.) | Possible Compound | Class | Molecular Formula | Mol. Ion m/z [M − H]⁻ | MS2 Fragments (m/z) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 0.96 | Gallic acid | Phenolic acid | C7H6O5 | 169.01 | 125, 79 | [31] |
| 2 | 1.61 | Chlorogenic acid | Phenolic acid | C16H18O9 | 353.09 | 191, 179, 173 | [32] |
| 3 | 2.36 | Rutin (Quercetin-3-rutinoside) | Flavonoid | C27H30O16 | 609.14 | 301 (Quercetin), 179 | [33] |
| 4 | 3.65 | Isoquercitrin (Quercetin-3-glucoside) | Flavonoid | C21H20O12 | 463.09 | 301 (Quercetin), 179, 151 | [34] |
| 5 | 5.83 | Quercetin | Flavonoid | C15H10O7 | 301.03 | 179, 151 | [35] |
| 6 | 8.03 | Apigenin | Flavonoid | C15H10O5 | 269.04 | 117, 151 | [36] |
| Factors (Independent Variables) | Levels | ||
|---|---|---|---|
| A: Lipid Conc (%) | 1 | 3 | 5 |
| B: SAA Conc (%) | 0.5 | 2 | 4 |
| C: Lipid Type | Capric Acid | Stearic Acid | Linoleic Acid |
| D: SAA Type | Poloxamer 407® | Poloxamer 188® | Span 60® |
| Responses (Dependent variables) | Constraints | ||
| Y1: P.S (nm) | Minimize | ||
| Y2: PDI | Minimize | ||
| Y3: EE % | Maximize | ||
| Y4: ZP | Maximize (absolute value) | ||
| Gene | Primer Direction | Primer Sequence (5′-3′) | Reference |
|---|---|---|---|
| Insulin-like growth factors (IGF-1) | Forward | TTGCTCTCAACATCTCCCATCT | [37] |
| Reverse | TGCATCTTCACCTTCAAGAAAT | ||
| INFG | Forward | CGGCACAGTCATTGAAAGCCTA | [52] |
| Reverse | GTTGCTGATGGCCTGATTGTC | ||
| β-actin | Forward | TGACAGGATGCAGAAGGAGA | [53] |
| Reverse | TAGAGCCACCAATCCACACA |
| Factors | Responses | |||||||
|---|---|---|---|---|---|---|---|---|
| Run M.CO-SLNs | A: Lipid Conc(%) | B: SAA Conc(%) | C: Lipid Type | D: SAA Type | Y1: P.S (nm) | Y2: PDI | Y3: ZP (mV) | Y4: EE (%) |
| 1 | 3 | 2 | Capric Acid | Poloxamer 407 | 120 ± 0.21 | 0.21 ± 0.01 | −25 ± 0.34 | 82 ± 0.41 |
| 2 | 3 | 2 | Stearic Acid | Poloxamer 188 | 250 ± 0.31 | 0.34 ± 0.03 | −30 ± 0.41 | 70 ± 0.43 |
| 3 | 5 | 4 | Stearic Acid | Span 60 | 280 ± 0.42 | 0.4 ± 0.04 | −28 ± 0.95 | 76 ± 0.61 |
| 4 | 5 | 2 | Linoleic Acid | Poloxamer 188 | 140 ± 0.41 | 0.22 ± 0.02 | −26 ± 0.42 | 85 ± 0.34 |
| 5 | 1 | 4 | Stearic Acid | Span 60 | 300 ± 0.32 | 0.45 ± 0.12 | −27 ± 0.51 | 68 ± 0.51 |
| 6 | 5 | 2 | Capric Acid | Span 60 | 130 ± 0.52 | 0.24 ± 0.09 | −22 ± 0.43 | 86 ± 0.31 |
| 7 | 1 | 4 | Linoleic Acid | Poloxamer 407 | 150 ± 0.43 | 0.24 ± 0.05 | −24 ± 0.51 | 81 ± 0.35 |
| 8 | 1 | 4 | Stearic Acid | Poloxamer 188 | 270 ± 0.53 | 0.38 ± 0.05 | −29 ± 0.45 | 75 ± 0.33 |
| 9 | 1 | 0.5 | Stearic Acid | Poloxamer 188 | 320 ± 0.43 | 0.45 ± 0.03 | −30 ± 0.65 | 60 ± 0.29 |
| 10 | 3 | 2 | Capric Acid | Poloxamer 407 | 115 ± 0.56 | 0.19 ± 0.04 | −26 ± 0.72 | 88 ± 0.36 |
| 11 | 1 | 2 | Capric Acid | Poloxamer 188 | 180 ± 0.44 | 0.26 ± 0.05 | −22 ± 0.45 | 79 ± 0.37 |
| 12 | 1 | 0.5 | Linoleic Acid | Poloxamer 188 | 250 ± 0.49 | 0.35 ± 0.04 | −25 ± 0.65 | 74 ± 0.58 |
| 13 | 1 | 0.5 | Stearic Acid | Poloxamer 407 | 290 ± 0.43 | 0.41 ± 0.02 | −28 ± 0.34 | 65 ± 0.52 |
| 14 | 3 | 0.5 | Capric Acid | Span 60 | 140 ± 0.87 | 0.22 ± 0.03 | −21 ± 0.51 | 84 ± 0.42 |
| 15 | 3 | 2 | Stearic Acid | Span 60 | 260 ± 0.89 | 0.37 ± 0.09 | −27 ± 0.91 | 72 ± 0.41 |
| 16 | 5 | 4 | Capric Acid | Poloxamer 188 | 110 ± 0.76 | 0.18 ± 0.05 | −24 ± 0.32 | 90 ± 0.65 |
| 17 | 3 | 4 | Linoleic Acid | Span 60 | 135 ± 0.23 | 0.23 ± 0.04 | −23 ± 0.41 | 87 ± 0.61 |
| 18 | 1 | 4 | Linoleic Acid | Poloxamer 188 | 160 ± 0.45 | 0.27 ± 0.03 | −26 ± 0.34 | 78 ± 0.45 |
| 19 | 1 | 2 | Linoleic Acid | Span 60 | 170 ± 0.34 | 0.25 ± 0.02 | −25 ± 0.53 | 80 ± 0.22 |
| 20 | 3 | 2 | Stearic Acid | Span 60 | 255 ± 0.65 | 0.36 ± 0.01 | −29 ± 0.51 | 73 ± 0.41 |
| 21 | 1 | 4 | Capric Acid | Span 60 | 145 ± 0.91 | 0.21 ± 0.04 | −22 ± 0.43 | 85 ± 0.34 |
| 22 | 5 | 4 | Linoleic Acid | Poloxamer 407 | 130 ± 0.34 | 0.72 ± 0.03 | −25 ± 0.23 | 89 ± 0.47 |
| 23 | 5 | 0.5 | Linoleic Acid | Span 60 | 195 ± 0.65 | 0.29 ± 0.02 | −24 ± 0.13 | 83 ± 0.43 |
| 24 | 3 | 0.5 | Linoleic Acid | Poloxamer 407 | 180 ± 0.77 | 0.28 ± 0.01 | −27 ± 0.34 | 82 ± 0.82 |
| 25 | 5 | 0.5 | Stearic Acid | Poloxamer 407 | 230 ± 0.65 | 0.32 ± 0.04 | −28 ± 0.21 | 77 ± 0.48 |
| 26 | 3 | 4 | Stearic Acid | Poloxamer 407 | 200 ± 0.72 | 0.53 ± 0.05 | −26 ± 0.45 | 79 ± 0.32 |
| Responses | Y1: PS (nm) | Y2: PDI | Y3: ZP (mV) | Y4: EE (%) |
|---|---|---|---|---|
| p-Value | p ˂ 0.0001 | p ˂ 0.0001 | p ˂ 0.0001 | p ˂ 0.0001 |
| R2 | 0.9785 | 0.9886 | 0.9775 | 0.9858 |
| Adj. R-squared | 0.9732 | 0.9843 | 0.9721 | 0.9812 |
| Pre. R-squared | 0.9658 | 0.9765 | 0.9634 | 0.9740 |
| Adequate precision | 27.42 | 30.58 | 26.87 | 28.91 |
| Significant factors of the optimized (M-CO-SLNs) | A, B, C, D | |||
| The predicated value of the optimized (M-CO-SLNs) | 116.43 | 0.18 | −22.17 | 88.17 |
| The observed value of the optimized (M-CO-SLNs) | 110 | 0.18 | −24 | 90 |
| Formulations | P.S (nm) | PDI | ZP (mV) | EE (%) |
|---|---|---|---|---|
| M-CO-SLNs 16 Freshly Prepared | 110 ± 0.76 | 0.18 ± 0.05 | −24 ± 0.32 | 90 ± 0.65 |
| M-CO-SLNs 16 After Three Months of Storage at 4 °C | 107 ± 0.32 | 0.18 ± 0.63 | −24 ± 0.65 | 89 ± 0.39 |
| M-CO-SLNs 16 After Three Months of Storage at 25 °C | 108 ± 0.76 | 0.19 ± 0.24 | −23 ± 0.11 | 88 ± 0.19 |
| M-CO-SLNs 16 After Six Months of Storage at 4 °C | 103 ± 0.76 | 0.21 ± 0.87 | −21 ± 0.87 | 86 ± 0.98 |
| M-CO-SLNs 16 After Six Months of Storage at 25 °C | 104 ± 0.76 | 0.20 ± 0.09 | −21 ± 0.56 | 85 ± 0.75 |
| Strain | Zone Inhibition (mm) | MIC (µg/mL) | ||||
|---|---|---|---|---|---|---|
| Control | M-CO | M-CO-SLNs | Control | M-CO | M-CO-SLNs | |
| Pseudomonas aeruginosa | 27 ± 0.57 | 13 ± 0.91 | 10 ± 0.98 | 400 ± 12.71 | 500 ± 17.11 | 1000 ± 29.76 |
| Escherichia coli | 30 ± 0.50 | 11.96 ± 0.19 | 13.99 ± 0.72 | 10 ± 0.12 | 10 ± 0.04 | 10 ± 0,02 |
| Candida albicans | 20.0 ± 0.86 | 20.98 ± 0.94 | 22.56 ± 0.13 | 13 ± 0.05 | 31.25 ± 0.13 | 15.63 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhabal, S.F.; Faheem, A.M.; Hababeh, S.; Nelson, J.; Elzohairy, N.A.; Ibrahim, Y.F.; Ewedah, T.M.; Mousa, I.S.; Allam, K.M.; Hamdan, A.M.E. Augmented Marshmallow Extract Lipid Nanoparticles with Clove Oil Embedded in Collagen Sponge for Ultimate Antimicrobial Healing of Diabetic Mouth Ulcer. Pharmaceutics 2025, 17, 611. https://doi.org/10.3390/pharmaceutics17050611
Elhabal SF, Faheem AM, Hababeh S, Nelson J, Elzohairy NA, Ibrahim YF, Ewedah TM, Mousa IS, Allam KM, Hamdan AME. Augmented Marshmallow Extract Lipid Nanoparticles with Clove Oil Embedded in Collagen Sponge for Ultimate Antimicrobial Healing of Diabetic Mouth Ulcer. Pharmaceutics. 2025; 17(5):611. https://doi.org/10.3390/pharmaceutics17050611
Chicago/Turabian StyleElhabal, Sammar Fathy, Ahmed Mohsen Faheem, Sandra Hababeh, Jakline Nelson, Nahla A. Elzohairy, Yasmine F. Ibrahim, Tassneim M. Ewedah, Ibrahim S. Mousa, Khaled M. Allam, and Ahmed Mohsen Elsaid Hamdan. 2025. "Augmented Marshmallow Extract Lipid Nanoparticles with Clove Oil Embedded in Collagen Sponge for Ultimate Antimicrobial Healing of Diabetic Mouth Ulcer" Pharmaceutics 17, no. 5: 611. https://doi.org/10.3390/pharmaceutics17050611
APA StyleElhabal, S. F., Faheem, A. M., Hababeh, S., Nelson, J., Elzohairy, N. A., Ibrahim, Y. F., Ewedah, T. M., Mousa, I. S., Allam, K. M., & Hamdan, A. M. E. (2025). Augmented Marshmallow Extract Lipid Nanoparticles with Clove Oil Embedded in Collagen Sponge for Ultimate Antimicrobial Healing of Diabetic Mouth Ulcer. Pharmaceutics, 17(5), 611. https://doi.org/10.3390/pharmaceutics17050611










