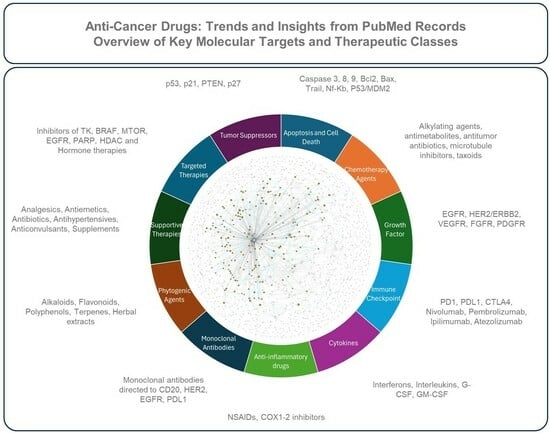
Anti-Cancer Drugs: Trends and Insights from PubMed Records
Abstract
1. Introduction
2. Materials and Methods
- Apoptosis and Cell Death: Proteins and mechanisms involved in programmed cell death (e.g., caspases, Bcl-2, p53), crucial in tumorigenesis and cancer treatment.
- Chemotherapy Agents: Cytotoxic drugs that damage DNA, inhibit nucleotide synthesis, or interfere with cell division (e.g., alkylating agents, antimetabolites, platinum compounds).
- Growth Factor Receptors: Characterized primarily by their direct modulation of oncogenic signaling pathways controlling angiogenesis (e.g., VEGF/VEGFR-2/KDR), cell proliferation, differentiation, and survival (e.g., EGFR, ERBB2/HER2, IGF-I, FGF, TGF-β).
- Immune Checkpoint: Defined specifically by their immunomodulatory action, reversing tumor-induced immune suppression through the targeted inhibition of checkpoint molecules (e.g., PD-1/PD-L1 axis, CTLA-4), thus restoring antitumor T-cell activity and significantly improving patient outcomes in various tumor types.
- Cytokines: Immune-modulating cytokines (e.g., interferons, interleukins) used as adjuvants to enhance immune response against cancer.
- Anti-inflammatory Drugs: NSAIDs and COX inhibitors (e.g., aspirin, celecoxib) with potential roles in cancer prevention and treatment through inflammation modulation.
- Monoclonal Antibodies: Distinguished by their high antigen specificity, representing a therapeutic platform engineered to selectively bind and neutralize defined tumor-associated antigens across diverse malignancies (e.g., CD20, CD19) and advanced antibody formats (e.g., immunoconjugates, immunotoxins, bispecific antibodies).
- Phytogenic Agents: Plant-derived compounds (e.g., alkaloids, flavonoids) with anti-cancer potential.
- Supportive Therapies: Non-anti-cancer drugs (e.g., analgesics, antiemetics) that manage cancer-related symptoms and improve patient quality of life.
- Targeted Therapies: Molecules targeting key signaling pathways (e.g., tyrosine kinase inhibitors, mTOR inhibitors), offering more precise treatments than chemotherapy.
- Tumor Suppressors: Proteins (e.g., p53, PTEN) that regulate cell growth, often inactivated in cancer, with research focused on reactivating or targeting these pathways.Detailed information on the topics in each class is available in Appendix A.1, and an overview of the drugs is shown in Figure 1.
3. Results
3.1. General Trend in Scientific Production (1962–2024)
3.2. Geographical Scientific Contribution in Publication
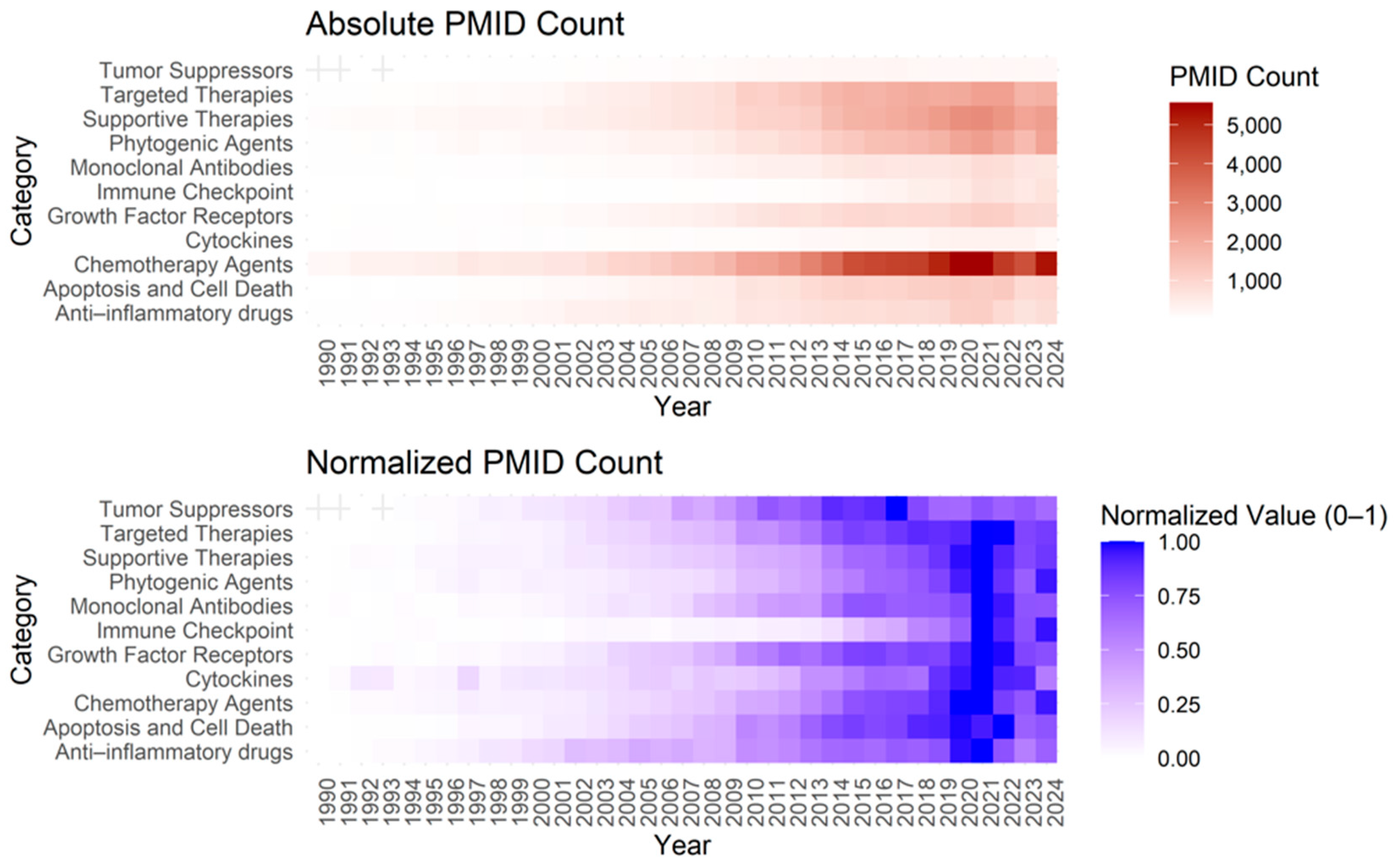
3.3. Anti-Cancer Drug Classification and Grouped Analysis
- Quantitative tracking: Measured the frequency of cited elements, revealing their temporal evolution and role in shaping scientific discourse.
- Link analysis: Examined relationships between elements, uncovering dynamics within and across research themes.
- Article classification: Categorized articles into general topics and niche areas, providing insight into the research landscape.
3.4. COVID-19 Pandemic Impact on Research Directions
- 2020 (Surge +24.64%): Publications increased sharply to 3197 articles, with a deviation of +335.9%, indicating a major shift in research activity.
- 2021 (Peak +8.20%): Publications rose to 3459 articles, reaching a maximum deviation of +534.8%, marking the peak of pandemic-driven research.
- 2022–2023 (Plateau 0.37–0.72%): Growth slowed but remained high, with a deviation above +375%, indicating a new steady-state.
- 2024 (Stabilization −0.97%): Publications slightly declined to 3463, but still remained above historical trends, suggesting a structural shift.
3.5. Publication Trends
3.5.1. Immune Checkpoint
3.5.2. Cancer Vaccine Therapies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Classification, Key Observations, and Notable Substances
- Reactive Oxygen Species (ROS) play a role in inducing oxidative stress and apoptosis in cancer cells.
- Caspases (e.g., caspase 3, 8, 9) are key executors of apoptosis.
- BCL Family Proteins regulate mitochondrial apoptosis pathways. Proteins like BAX promote apoptosis, while BCL2 and BCL-X can block it.
- Fas/FasL are involved in the extrinsic apoptosis pathway.
- Inhibitors of Apoptosis (IAP) proteins help tumor cells evade apoptosis.
- TNF and TNF-Alpha cytokines can induce cell death or survival depending on context.
- Survivin (BIRC5) and Mcl-1 are overexpressed in cancers to prevent apoptosis.
- NF-κB regulates apoptosis, often associated with inflammation and cancer survival.
- HIF1A and Hypoxia-Inducible Factor 1 influence apoptosis under hypoxic conditions in tumors.
- Heat-shock proteins (HSP70, HSP90) protect cells from stress-induced apoptosis.
- c-Myc and MDM2 modulate apoptosis, with MDM2 negatively regulating p53.
- DNA-Damaging Agents include alkylating agents, organoplatinum compounds like cisplatin, oxaliplatin, and carboplatin, along with fluorouracil, methotrexate, gemcitabine, capecitabine, and cytarabine.
- Topoisomerase Inhibitors such as etoposide, doxorubicin, camptothecin, irinotecan, and topotecan target cancer DNA.
- Microtubule-Targeting Drugs like paclitaxel, docetaxel, vincristine, and vinblastine inhibit cell division.
- Others include bleomycin, mitomycin, mitoxantrone, and arsenic trioxide.
- ERBB receptors (951), including EGFR and HER2/ErbB2, are involved in breast, lung, and other cancers.
- EGF/VEGFR play critical roles in angiogenesis.
- IGF and FGF families are also implicated in tumor growth.
- Immune checkpoint inhibitors (ICIs) target PD-1, PD-L1, and CTLA-4.
- PD-1/PD-L1 pathway includes PD-1, PD-L1, Nivolumab, Pembrolizumab, and Atezolizumab.
- CTLA-4 pathway includes Ipilimumab.
- Recombinant Fusion Proteins are being explored for blocking immune checkpoints.
- NSAIDs: Common non-steroidal anti-inflammatory drugs like aspirin, ibuprofen, and celecoxib.
- COX Enzymes: COX-2 and COX-1 are targets for inflammation control.
- Prostaglandins and Arachidonic Acid are involved in inflammatory pathways.
- Interleukin-2 also plays a role in certain immunotherapies.
- Trastuzumab and bevacizumab are key in targeting HER2 and VEGF, respectively.
- Cetuximab, rituximab, and panitumumab target EGFR and CD20.
- Bispecific antibodies and immunoconjugates are newer research directions.
- Interferons (e.g., IFN-γ, IFN-α) help boost immune responses against tumors.
- Granulocyte Colony-Stimulating Factor and GM-CSF support immune cell activity.
- Cancer vaccines aim to elicit immune responses against tumor antigens.
- Opioids, Analgesics, Antiemetics: Manage pain, nausea, and other side effects in cancer patients.
- Diphosphonates help manage bone metastases.
- Chinese herbal (1638) and plant extracts (895) are extensively researched for potential anti-cancer properties.
- Curcumin, Resveratrol, and Quercetin are among the most studied compounds.
- Protein Kinase Inhibitors (1305) and Enzyme Inhibitors (930) remain a focus.
- PARP Inhibitors and Proteasome Inhibitors (e.g., Bortezomib) are also prominent.
- p53 is the most studied tumor suppressor, with p21 and p27 playing key roles in cell cycle regulation.
Appendix A.2. Shifting Patterns in Scientific Collaboration: Trends in Authorship Count
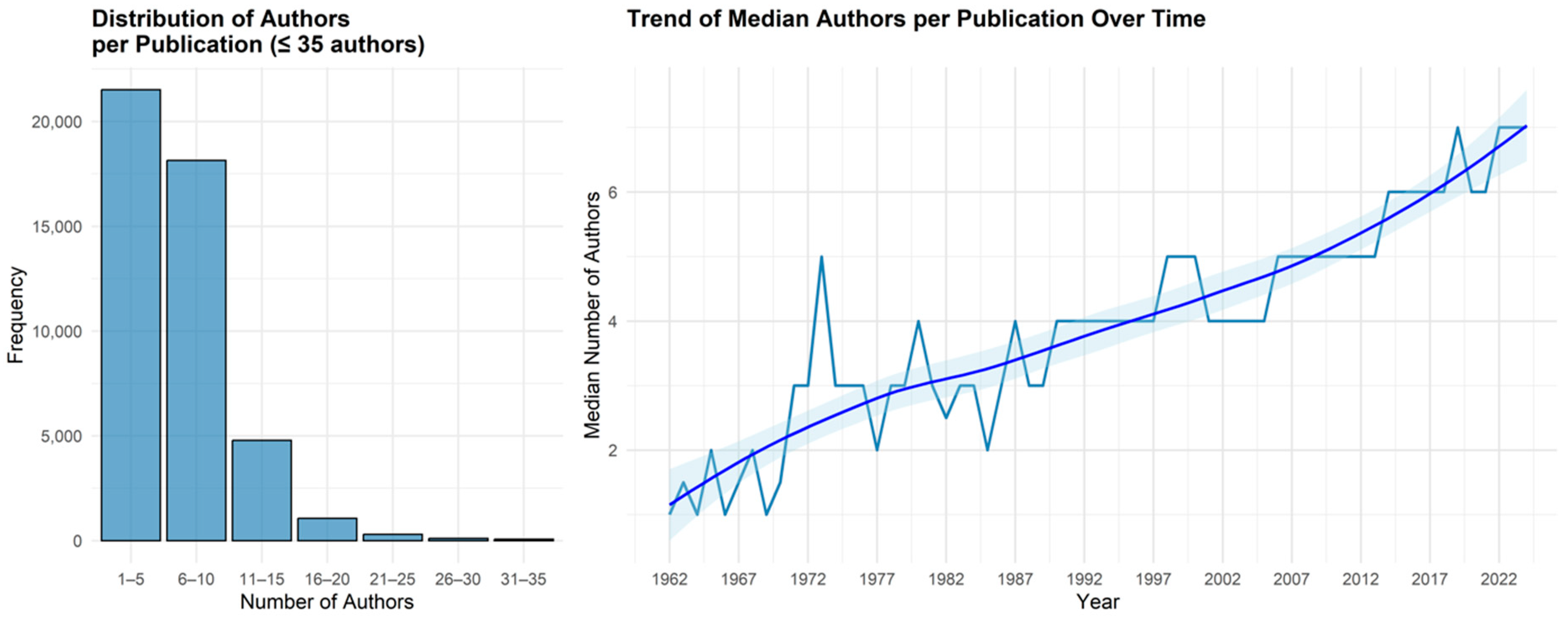
| Year | N. Articles | Mean Authors | Median Authors | Min Authors | Max Authors | Q1 | Q3 |
|---|---|---|---|---|---|---|---|
| 2000 | 459 | 5 | 5 | 1 | 24 | 2 | 7 |
| 2001 | 505 | 5 | 4 | 1 | 21 | 2 | 6 |
| 2002 | 661 | 5 | 4 | 1 | 40 | 2 | 7 |
| 2003 | 662 | 5 | 4 | 1 | 24 | 2 | 6 |
| 2004 | 706 | 5 | 4 | 1 | 25 | 3 | 7 |
| 2005 | 818 | 5 | 4 | 1 | 33 | 3 | 7 |
| 2006 | 839 | 5 | 5 | 1 | 25 | 3 | 7 |
| 2007 | 830 | 5 | 5 | 1 | 25 | 3 | 7 |
| 2008 | 880 | 5 | 5 | 1 | 28 | 3 | 7 |
| 2009 | 1016 | 6 | 5 | 1 | 23 | 3 | 8 |
| 2010 | 1182 | 6 | 5 | 1 | 24 | 3 | 8 |
| 2011 | 1216 | 6 | 5 | 1 | 26 | 3 | 8 |
| 2012 | 1333 | 6 | 5 | 1 | 21 | 3 | 8 |
| 2013 | 1468 | 6 | 5 | 1 | 34 | 3 | 8 |
| 2014 | 1752 | 6 | 6 | 1 | 35 | 4 | 9 |
| 2015 | 1974 | 7 | 6 | 1 | 71 | 4 | 9 |
| 2016 | 2111 | 9 | 6 | 1 | 2440 | 4 | 9 |
| 2017 | 2144 | 7 | 6 | 1 | 40 | 4 | 9 |
| 2018 | 2425 | 7 | 6 | 1 | 38 | 4 | 9 |
| 2019 | 2564 | 7 | 7 | 1 | 44 | 4 | 9 |
| 2020 | 3190 | 7 | 6 | 1 | 57 | 4 | 9 |
| 2021 | 3453 | 7 | 6 | 1 | 76 | 4 | 9 |
| 2022 | 3471 | 8 | 7 | 1 | 80 | 4 | 10 |
| 2023 | 3490 | 8 | 7 | 1 | 228 | 5 | 10 |
| 2024 | 3449 | 8 | 7 | 1 | 112 | 5 | 10 |
Appendix A.3. Anti-Cancer Drug Classification Insights
Appendix A.4. COVID-19 Pandemic Impact on Scientific Publication
- Absolute Deviation from Baseline (SpikeHeight): The difference between the observed publications and the baseline in a given year.
- Percent Deviation from Baseline (PercentVariation): The deviation as a percentage relative to the historical average.
- Outlier Detection via Standard Deviation: A year was classified as a statistical anomaly if it exceeded μ + 2σ\mu + 2\sigmaμ + 2σ, where σ\sigmaσ is the standard deviation of the historical data.
- Peak Area Calculation: The area under the curve between the observed publication count and the baseline was computed to quantify the total excess publications during the pandemic period.
- Identification of the Maximum Peak: The year with the highest deviation was extracted, marking the peak of the increase in scientific output.
| Year | Publications (N) | Percent Change (Δ%) | Deviation from Baseline | Spike Height (N in Excess Compared to Baseline) | Peak Area (Cumulative N in Excess Compared to Baseline) |
|---|---|---|---|---|---|
| 2019 | 2565 | 5.64% | +249.7% | 0 | 0 |
| 2020 | 3197 | 24.64% | +335.9% | 452 | 452 |
| 2021 | 3459 | 8.20% | +371.6% | 534 | 987 |
| 2022 | 3484 | 0.72% | +375.0% | 380 | 1367 |
| 2023 | 3497 | 0.37% | +376.8% | 213 | 1581 |
| 2024 | 3463 | −0.97% | +372.1% | 0.0 | 1581 |
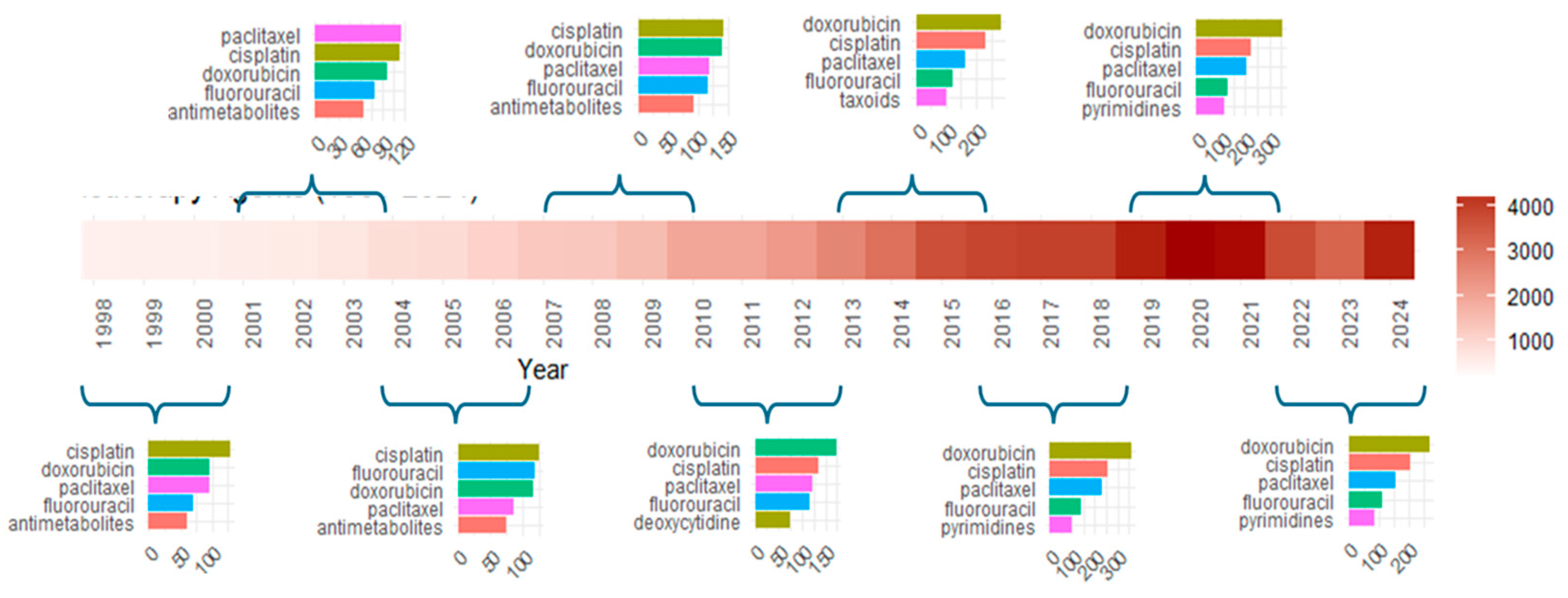
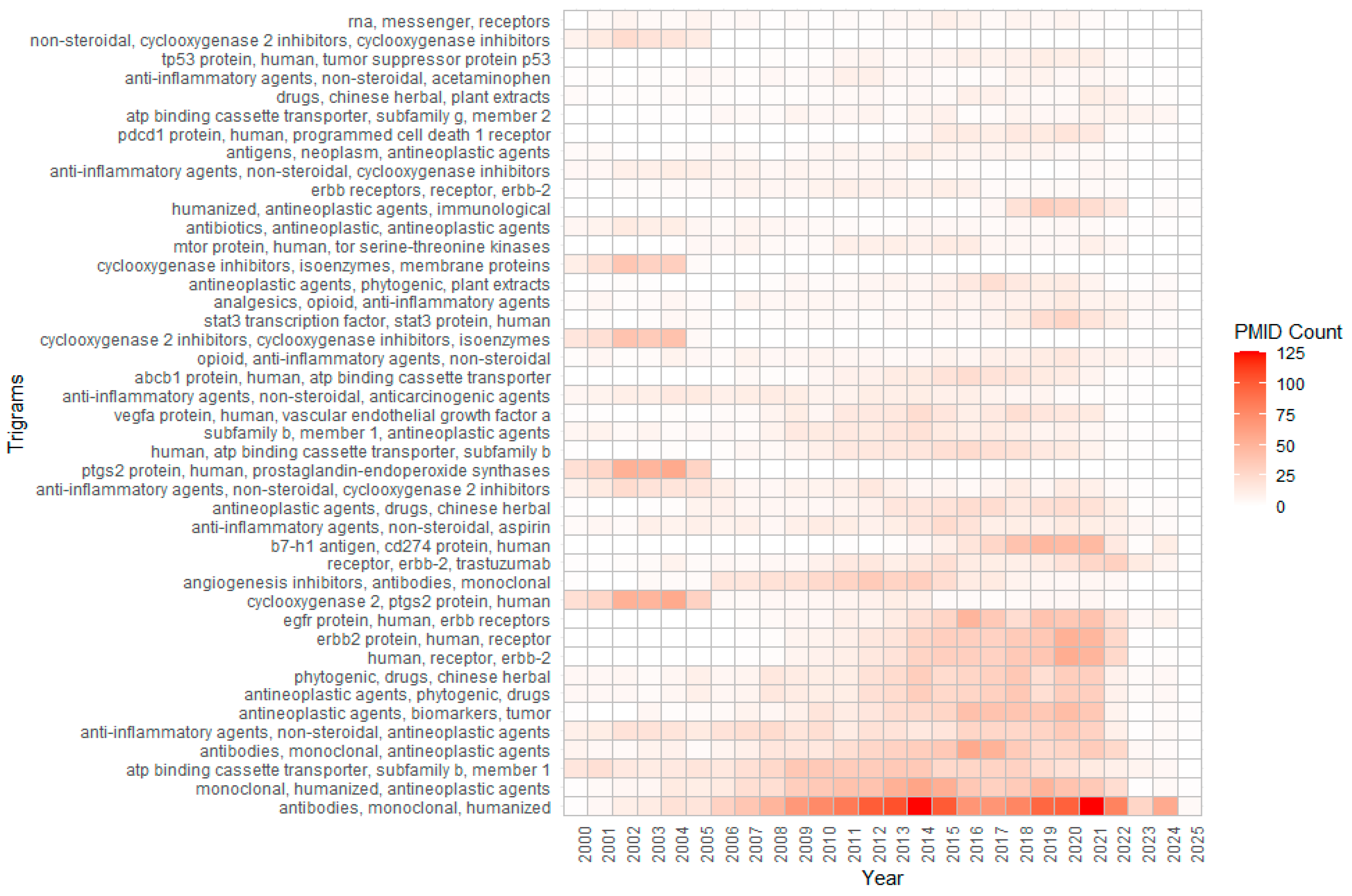
Appendix A.5. Substance-Related Trigrams and Research Trends
- Synergistic Therapies: Combining apoptosis-inducing strategies with checkpoint blockers or growth factor receptor inhibitors.
- Personalized Biomarkers: Multi-omics panels to enable the precise use of treatments and monitor minimal residual disease.
- Advanced Formulations: Liposomal- or nanoparticle-based systems for targeted delivery.
- Expanded Immuno-Oncology: Incorporating next-gen immune checkpoints, adoptive T-cell therapies, and vaccine-based approaches.
- Phytogenic and Integrative Treatments: Integrating plant-derived agents with modern therapies.
References
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Johnston, P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef] [PubMed]
- McLeod, H.L. Cancer pharmacogenomics: Early promise, but concerted effort needed. Science 2013, 339, 1563–1566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221, Erratum in Nature 2018, 555, 402. https://doi.org/10.1038/nature25145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Y.; Wei, Y.; Tian, X.; Wei, X. Cancer vaccines: Current status and future directions. J. Hematol. Oncol. 2025, 18, 18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, A.; Torchilin, V. Intracellular delivery of nanocarriers and targeting to subcellular organelles. Expert. Opin. Drug Deliv. 2016, 13, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MEDLINE® PubMed® XML Element Descriptions and their Attributes. Available online: https://wayback.archive-it.org/org-350/20240220194809/https://www.nlm.nih.gov/bsd/licensee/elements_descriptions.html (accessed on 15 January 2025).
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, S.; Bergstrom, C.T.; Börner, K.; Evans, J.A.; Helbing, D.; Milojević, S.; Petersen, A.M.; Radicchi, F.; Sinatra, R.; Uzzi, B.; et al. Science of science. Science 2018, 359, eaao0185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Upadhaya, S.; Yu, J.X.; Oliva, C.; Hooton, M.; Hodge, J.; Hubbard-Lucey, V.M. Impact of COVID-19 on oncology clinical trials. Nat. Rev. Drug Discov. 2020, 19, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.P.; Papenburg, J.; Desjardins, M.; Kanjilal, S.; Quach, C.; Libman, M.; Dittrich, S.; Yansouni, C.P. Diagnostic Testing for Severe Acute Respiratory Syndrome-Related Coronavirus 2: A Narrative Review. Ann. Intern. Med. 2020, 172, 726–734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sathian, B.; Asim, M.; Banerjee, I.; Pizarro, A.B.; Roy, B.; van Teijlingen, E.R.; do Nascimento, I.J.B.; Alhamad, H.K. Impact of COVID-19 on clinical trials and clinical research: A systematic review. Nepal. J. Epidemiol. 2020, 10, 878–887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahin, U.; Türeci, Ö. Personalized vaccines for cancer immunotherapy. Science 2018, 359, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M.; et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018, 362, eaar3593, Erratum in Science 2019, 363, eaax1384. https://doi.org/10.1126/science.aax1384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015, 27, 450–461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olver, I.; Keefe, D.; Herrstedt, J.; Warr, D.; Roila, F.; Ripamonti, C.I. Supportive care in cancer-a MASCC perspective. Support. Care Cancer. 2020, 28, 3467–3475. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, A.; Socinski, M.A.; Villaruz, L.C. Immune Checkpoint Blockade in Oncogene-Driven Non-Small-Cell Lung Cancer. Drugs 2020, 80, 883–892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Presti, M.; Westergaard, M.C.W.; Draghi, A.; Chamberlain, C.A.; Gokuldass, A.; Svane, I.M.; Donia, M. The effects of targeted immune-regulatory strategies on tumor-specific T-cell responses in vitro. Cancer Immunol. Immunother. 2021, 70, 1771–1776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Hu, H.; Yuan, X.; Fan, X.; Zhang, C. Advances in Immune Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 896752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hao, L.; Li, S.; Ye, F.; Wang, H.; Zhong, Y.; Zhang, X.; Hu, X.; Huang, X. The current status and future of targeted-immune combination for hepatocellular carcinoma. Front. Immunol. 2024, 15, 1418965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, S.; Peng, L.; Liu, Y.; Till, B.G.; Yan, X.; Zhang, J.; Zhu, L.; Wang, H.; Zhang, S.; Li, H.; et al. Low-dose anlotinib confers improved survival in combination with immune checkpoint inhibitor in advanced non-small cell lung cancer patients. Cancer Immunol. Immunother. 2023, 72, 437–448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, Y.; Fu, Y.; Xie, Q.; Zhu, B.; Wang, J.; Zhang, B. Anti-angiogenic Agents in Combination with Immune Checkpoint Inhibitors: A Promising Strategy for Cancer Treatment. Front. Immunol. 2020, 11, 1956. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, C.; Wang, Y.; Li, Y. Research Progress of Histone Deacetylase Inhibitor Combined with Immune Checkpoint Inhibitor in the Treatment of Tumor. Zhongguo Fei Ai Za Zhi. 2021, 24, 204–211. (In Chinese) [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maes, K.; Mondino, A.; Lasarte, J.J.; Agirre, X.; Vanderkerken, K.; Prosper, F.; Breckpot, K. Epigenetic Modifiers: Anti-Neoplastic Drugs With Immunomodulating Potential. Front. Immunol. 2021, 12, 652160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, J.H.; Woo, I.S. Metronomic chemotherapy as a potential partner of immune checkpoint inhibitors for metastatic colorectal cancer treatment. Cancer Lett. 2023, 565, 216236. [Google Scholar] [CrossRef] [PubMed]
- Mugarza, E.; van Maldegem, F.; Boumelha, J.; Moore, C.; Rana, S.; Llorian Sopena, M.; East, P.; Ambler, R.; Anastasiou, P.; Romero-Clavijo, P.; et al. Therapeutic KRASG12C inhibition drives effective interferon-mediated antitumor immunity in immunogenic lung cancers. Sci Adv. 2022, 8, eabm8780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, Y.; Yu, T.; Zhang, S.; Song, G.; Meng, T.; Yuan, H.; Hu, F. Combination of tumor vessel normalization and immune checkpoint blockade for breast cancer treatment via multifunctional nanocomplexes. Biomater. Sci. 2022, 10, 4140–4155. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, B.; Qanash, H.; Binsaleh, N.K.; Alharthi, S.; Elasbali, A.M.; Gharekhan, C.H.; Mahmoud, M.; Lioudakis, E.; O’Leary, J.J.; Doherty, D.G.; et al. Proof of concept nanotechnological approach to in vitro targeting of malignant melanoma for enhanced immune checkpoint inhibition. Sci. Rep. 2023, 13, 7462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer. 2021, 21, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Brugiapaglia, S.; Spagnolo, F.; Intonti, S.; Novelli, F.; Curcio, C. Fighting Pancreatic Cancer with a Vaccine-Based Winning Combination: Hope or Reality? Cells 2024, 13, 1558. [Google Scholar] [CrossRef]
- Roviello, G.; MolinaCerrillo, J.; Massari, F.; Cerbone, L.; Fiala, O.; Fornarini, G.; Monteiro, F.S.M.; Cattrini, C.; Landmesser, J.; Messina, C.; et al. First-line immune-based combinations or sunitinib in favorable-risk metastatic renal cell carcinoma: A real-world retrospective comparison from the ARON-1 study. Cancer Immunol. Immunother. 2025, 74, 65. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, S.; Cheng, C.-S.; Chen, L. Lenvatinib and immune-checkpoint inhibitors in hepatocellular carcinoma: Mechanistic insights, clinical efficacy, and future perspectives. J. Hematol. Oncol. 2024, 17, 130. [Google Scholar] [CrossRef]
- Chan, S.W.S.; Al Maqrashi, Z.A.A.; Young, J.; Kim, D.-H.; Pond, G.; Meyers, B.M.; Kartolo, A. Concurrent immunotherapy and radiation in cisplatin-ineligible patients with HNSCC: A systematic review & meta-analysis. Immunotherapy 2024, 16, 1227–1233. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, M.; Tang, J.; Zou, D.; Liu, F.; Shi, Q.; Gao, T.; Li, C.; Zhu, G. Efficacy and safety of PD-1 monoclonal antibody combined with interferon-alpha 1b and anlotinib hydrochloride as the second-line therapy in patients with unresectable advanced melanoma: A retrospective study. Cancer Med. 2024, 13, e70087. [Google Scholar] [CrossRef]
- Bei, W.; Dong, S.; Liu, G.; Lin, L.; Jiang, Y.; Lu, N.; Li, W.; Liang, H.; Xiang, Y.; Xia, W. Efficacy and Safety of Re-Challenging PD-1 Inhibitors in Second-Line Treatment in Metastatic Nasopharyngeal Carcinoma Previously Treated with Chemotherapy and PD-1 Inhibitors. Cancer Manag. Res. 2024, 16, 771–780. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, K.H.; Park, E.Y.; Kim, E.T.; Kim, E.J.; Tan, D.S.P.; Lee, J.-Y.; Park, S.-Y.; Fotopoulou, C.; Lim, M.C. Efficacy of immune-checkpoint inhibitors combined with cytotoxic chemotherapy in advanced or recurrent endometrial cancer: A systematic review and meta-analysis. Gynecol Oncol. 2024, 187, 85–91. [Google Scholar] [CrossRef]








| min | MAX | mean | median | SD | variance |
| 1 | 3497 | 733.5079 | 245 | 1037.403 | 1,076,204 |
| Q1 | Q3 | IQR | range | skewness | kurtosis |
| 15 | 948.5 | 933.5 | 3496 | 1.515994 | 1.13817 |
| NameOf Substance | Descriptor Name | Keywords | |
|---|---|---|---|
| TotalPMIDs | 37,521 | 39,237 | 23,330 |
| Total Unique Elements | 14,456 | 13,105 | 47,107 |
| Median Elements Per PMID | 5 | 14 | 5 |
| Max Elements | 39 | 54 | 216 |
| PMIDs With 1 Element | 3280 | 5 | 17 |
| PMIDs With 2 Elements | 4030 | 26 | 88 |
| PMIDs With 3 Elements | 4796 | 118 | 1066 |
| PMIDs With > 3 Elements | 25,415 | 39,088 | 22,159 |
| Category | Example of Combination with ICIs | Rationale |
|---|---|---|
| Drug Resistance | ATP-binding cassette transporters | Chronic immune evasion can coincide with increased expression of drug-resistance genes. Tumors that acquire resistance to classical agents may still respond to immunotherapies targeting the PD-1/PD-L1 or CTLA-4 pathways |
| Anti-inflammatory | COX-2 inhibitor | Anti-inflammatory milieu might enhance or occasionally reduce the efficacy of checkpoint inhibitors |
| Phytogenic agents/ Chinese Herbal | flavonoids, terpenoids | Enhance dendritic cell function or modulate T regulatory cells |
| Growth Factor Receptors | EGFR, VEGF/VEGFR | Normalize the tumor vasculature and improve immune cell access |
| Targeted Therapies | BRAF, EGFR, or mTOR inhibitors | Boost tumor antigen release and create a favorable setting for T-cell priming |
| Chemotherapy Agents | chemotherapy | Chemotherapy-induced cell death can generate antigenic debris, prompting dendritic cell activation, ICIs support T-cell-mediated clearance of residual tumor cells |
| Tumor Suppressors | BCL-2, BAX, TP53 | Combined modulation of cell death pathways and immune checkpoints can lead to robust tumor regression as consequence of Fas ligand and TNF-related apoptosis |
| Nucleic Acids/ Gene Therapies | siRNA, mRNA vaccines | Enhance T-cell-mediated killing |
| Drug Delivery/ Advanced Formulations | liposomes, nanoparticles | Improved release of drugs and off-target effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spagnolo, F.; Brugiapaglia, S.; Perin, M.; Intonti, S.; Curcio, C. Anti-Cancer Drugs: Trends and Insights from PubMed Records. Pharmaceutics 2025, 17, 610. https://doi.org/10.3390/pharmaceutics17050610
Spagnolo F, Brugiapaglia S, Perin M, Intonti S, Curcio C. Anti-Cancer Drugs: Trends and Insights from PubMed Records. Pharmaceutics. 2025; 17(5):610. https://doi.org/10.3390/pharmaceutics17050610
Chicago/Turabian StyleSpagnolo, Ferdinando, Silvia Brugiapaglia, Martina Perin, Simona Intonti, and Claudia Curcio. 2025. "Anti-Cancer Drugs: Trends and Insights from PubMed Records" Pharmaceutics 17, no. 5: 610. https://doi.org/10.3390/pharmaceutics17050610
APA StyleSpagnolo, F., Brugiapaglia, S., Perin, M., Intonti, S., & Curcio, C. (2025). Anti-Cancer Drugs: Trends and Insights from PubMed Records. Pharmaceutics, 17(5), 610. https://doi.org/10.3390/pharmaceutics17050610