Ocular Drug Delivery: Emerging Approaches and Advances
Abstract
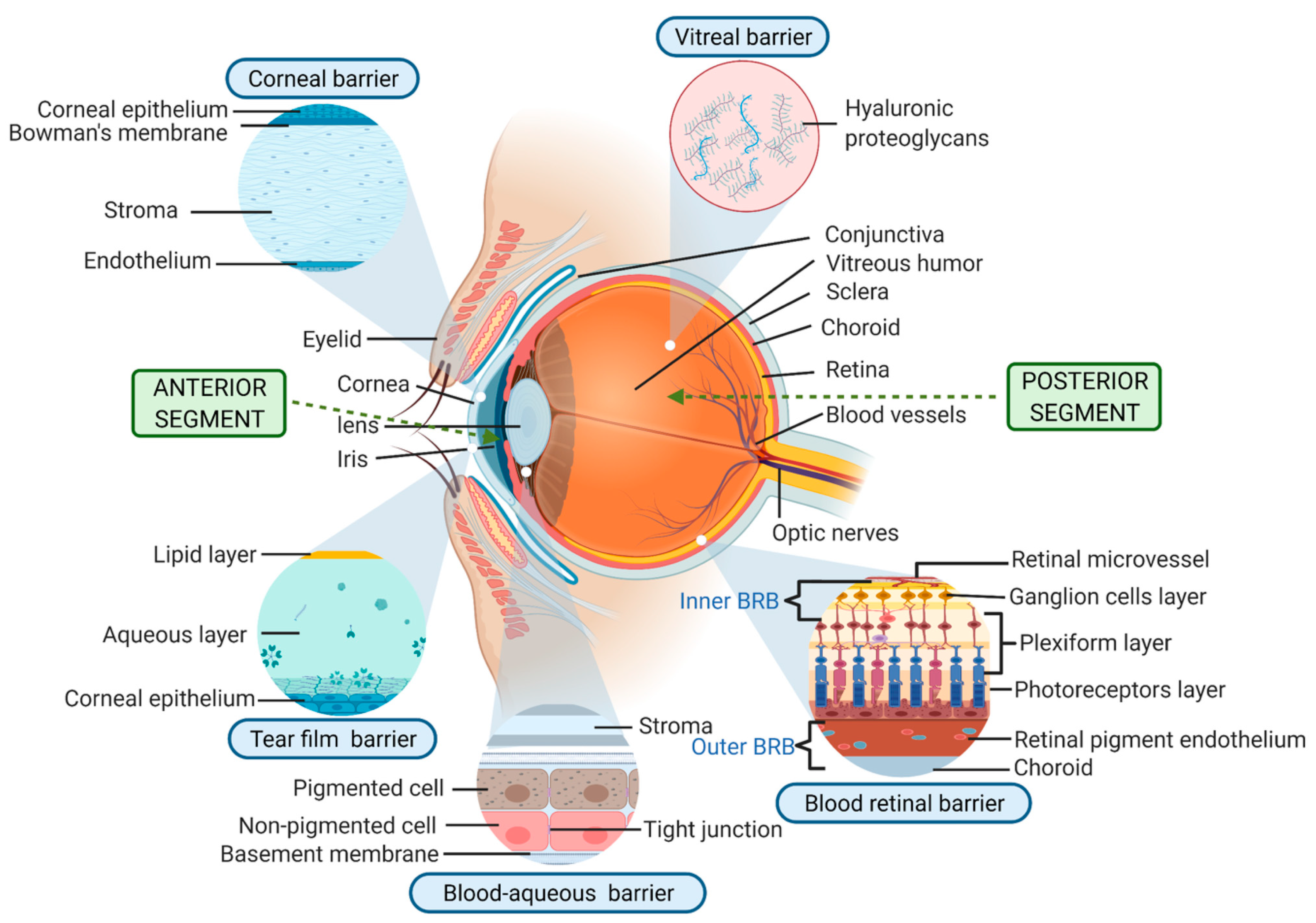
1. Introduction
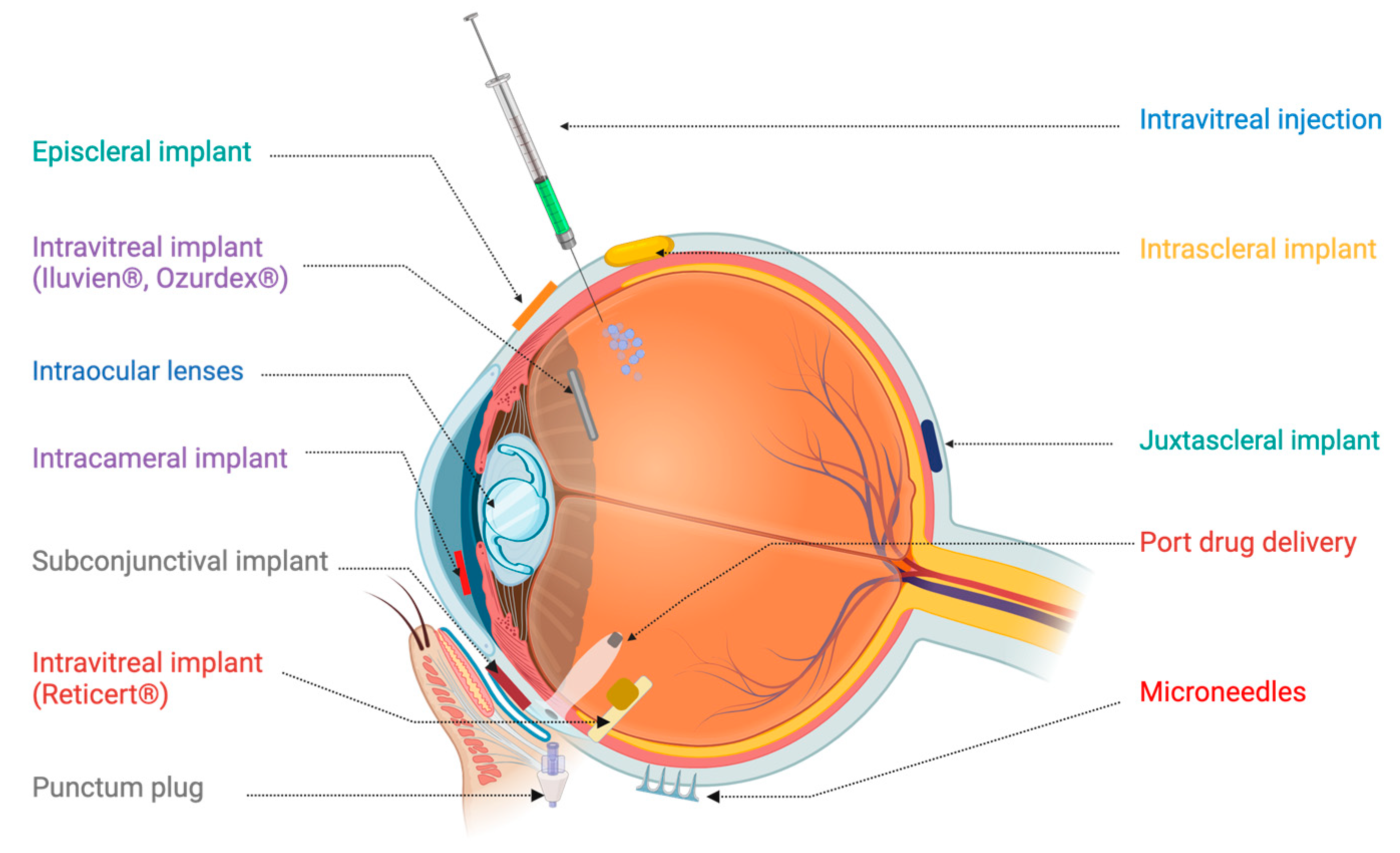
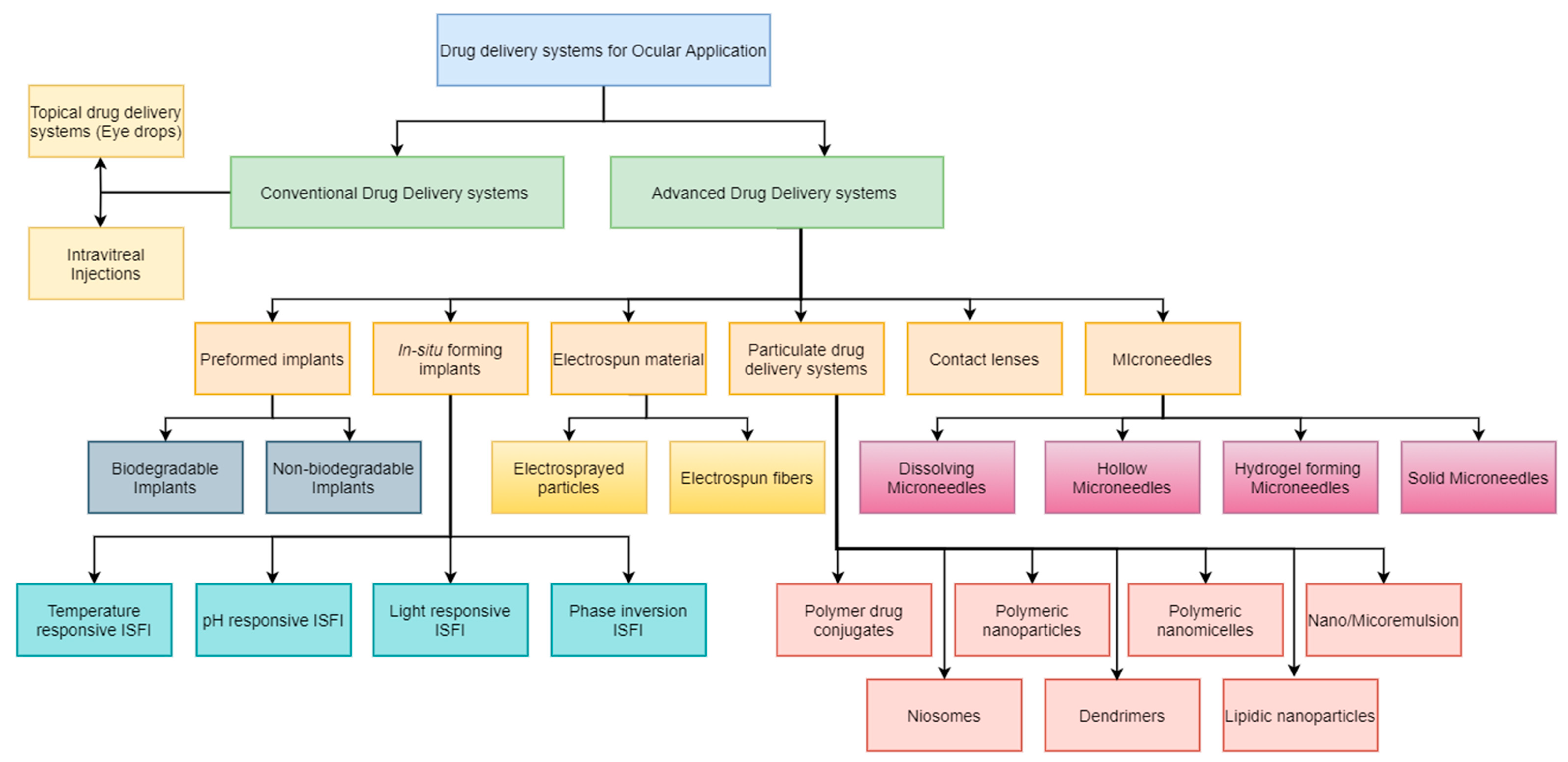
2. Advanced Drug Delivery Systems for Ocular Disorders
2.1. Preformed Ocular Implants
Biodegradable Preformed Implants
2.2. Nonbiodegradable Preformed Implants
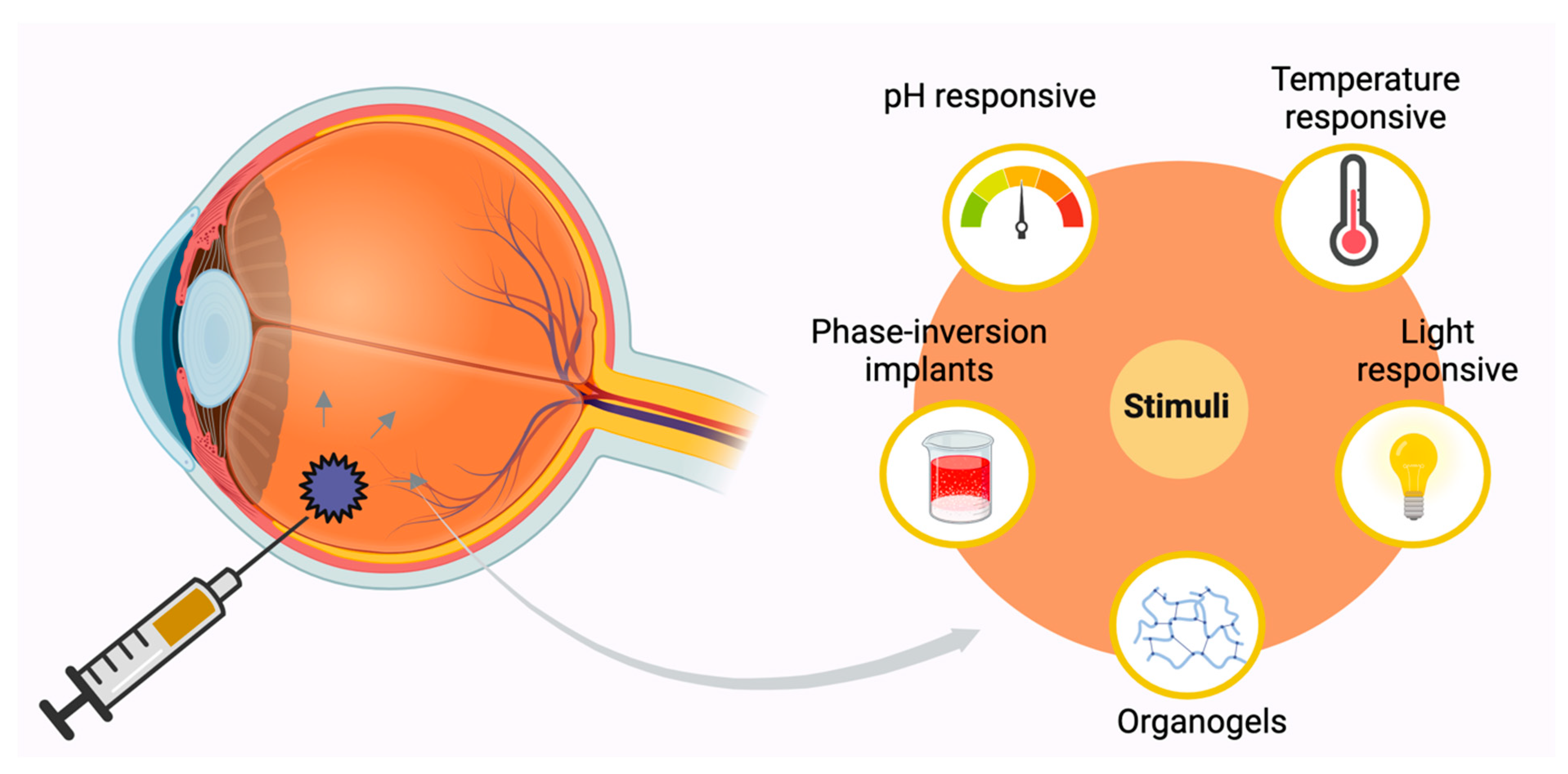
2.3. In Situ Forming Implants (ISFI)
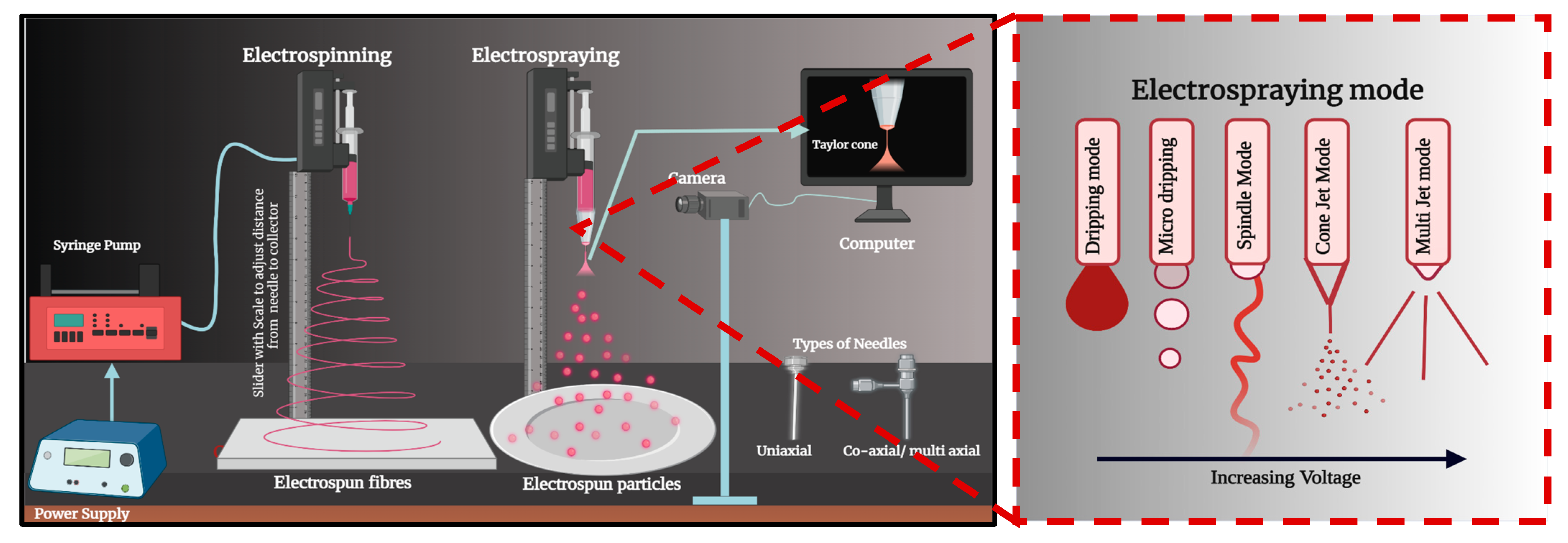
2.4. Electrospun Patches
2.5. Particulate Systems in the Management of Ocular Diseases
| Product Name | Drug and Drug Loading | Indication | Type of Formulation | Polymer(s)/Lipids | PK/PD Findings | Route of Administration | Phase of Approval |
|---|---|---|---|---|---|---|---|
| Restasis® | Cyclosporin A | Dry eye syndrome | Nanoemulsion | Castor oil | AUC0–72 h 14,333.2 ng/g.h, corneal clearance of 1.4 g/h [96] | Topical | Approved |
| Cyclokat® | Cyclosporin A | Dry eye syndrome | Cationic nanoemulsion | Castor oil | AUC0–72 h 26,477 ng/g.h Systematic absorption below LOD (0.1 ng/mL) [97] | Topical | Approved |
| Cequa® | Cyclosporin A | keratocon- junctivitis sicca | Nano micellar solution | Octoxynol-40, polyoxyl 40 hydrogenated castor oil | AUC0–1 h 828.25 ± 53.2 ng/g.h [98] | Topical | Approved |
| Visudyne® | Verteporfin | Choroidal neovascularization | Liposomes | EPG and DMPC (3:5 molar ratio) | AUC0-t (µg.h/mL) 1.62, clearance 99.6 [99] | injection | Approved |
| Lacrisek® | Vitamins A and E | Dry eye syndrome | Liposomal spray | Hydrogenated phospholipids | The tear blink intervals were more with Lacrisek® compared to Artelac Rebalance® [100] | Topical | Approved |
| Artelac Rebalance® | Vitamin B12 | Dry eye syndrome | Liposomal eye drops | Hyaluronic acid, polyethylene glycol 8000 | The aqueous-based Artelac Rebalance® was found to improve TBUT [100] | Topical | Approved |
| Ikervis® | Cyclosporin A | Keratitis in dry eye disease | Cationic nanoemulsion | Medium-chain triglycerides, glycerol, cetalkonium chloride, poloxamer, tyloxapol | AUC0–72 h 26,703.0 ng/g.h, corneal clearance of 0.8 h/h [96] | Topical | Approved |
| OCS-01 | Dexamethasone and cyclodextrin | Postoperative corneal inflammation | Nanoparticles | Cyclodextrin | 51% of patients (post cataract surgery) experienced absence of anterior inflammation vs. 19.6% with vehicle control and 72.5% vs. 54.9% with no pain with OCS-01 and vehicle control, respectively [101,102]. | Topical | Phase II |
| SeeQ | CdSe Nanoparticle | Retinitis pigmentosa (RP) | Nanoparticles | Cadmium selenium | BCVA was decreased in patients with RP at least 6 lines in 1 h after IVT [103]. | Intravitreal injections | NA |
| LE-MPP | Loteprednol etabonate | Postoperative inflammation and pain | MPP (Mucus penetrating particles) | Pluronic F127 | AUC0–12 h of LE-MPP in cornea and conjunctiva was 1.5-fold higher than lotemax 0.5% eye drops [104] | Topical | Phase III |
2.6. Drug-Eluting Contact Lenses
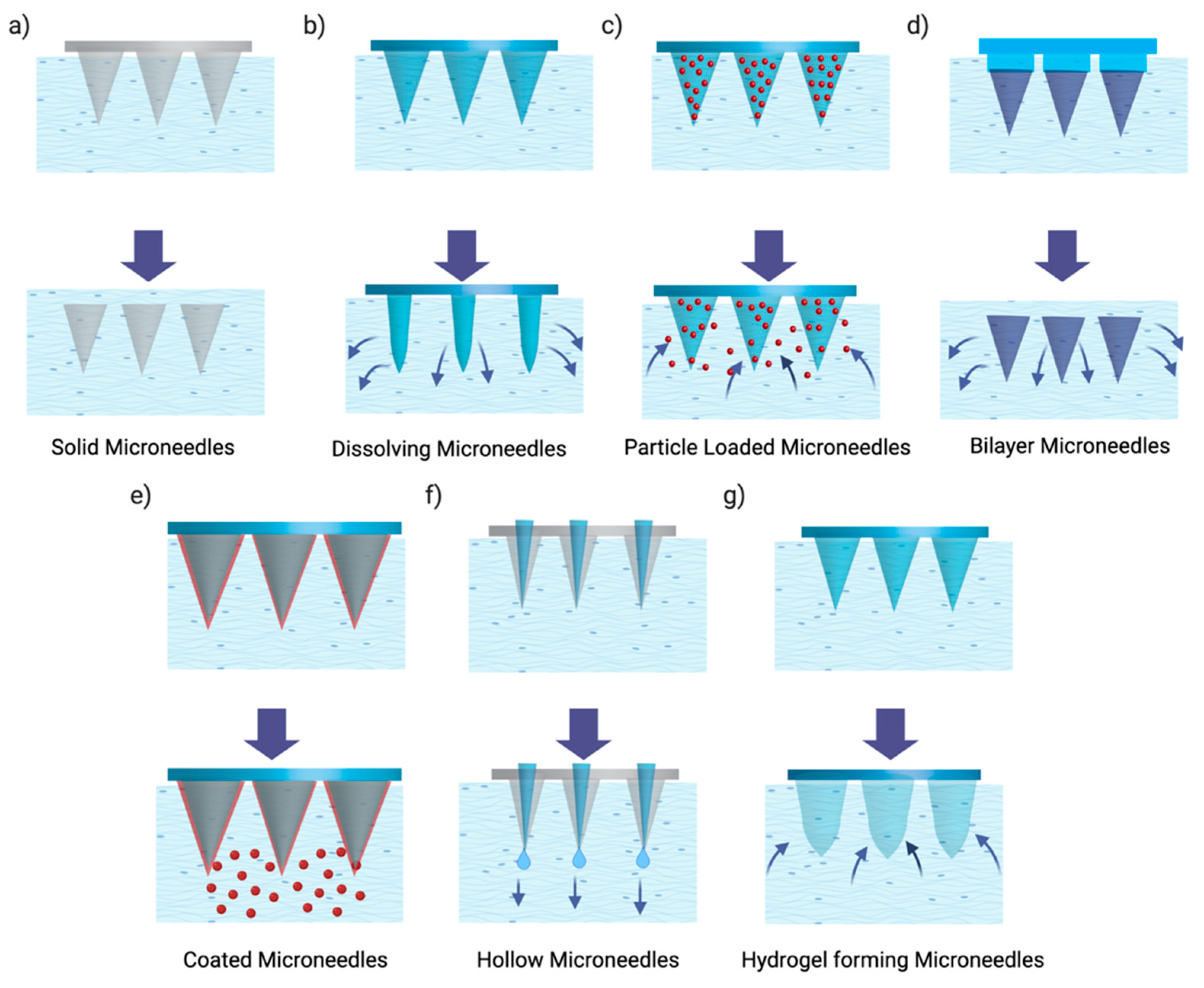
2.7. Microneedles
3. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Cholkar, K.; Dasari, S.R.; Pal, D.; Mitra, A.K. 1—Eye: Anatomy, Physiology and Barriers to Drug Delivery. In Ocular Transporters and Receptors; Mitra, A.K., Ed.; Woodhead Publishing Series in Biomedicine; Woodhead Publishing: Southampton, UK, 2013; pp. 1–36. [Google Scholar] [CrossRef]
- Mishra, D.; Gade, S.; Glover, K.; Sheshala, R.; Singh, T.R.R. Vitreous Humor: Composition, Characteristics and Implication on Intravitreal Drug Delivery. Curr. Eye Res. 2023, 48, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.H.; Michels, S.; Rodrigues, E.B.; Hager, A.; Mennel, S.; Schmidt, J.C.; Helb, H.M.; Farah, M.E. Incidence of rhegmatogenous retinal detachments after intravitreal antivascular endothelial factor injections. Acta Ophthalmol. 2011, 89, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Chang-Lin, J.E.; Attar, M.; Acheampong, A.A.; Robinson, M.R.; Whitcup, S.M.; Kuppermann, B.D.; Welty, D. Pharmacokinetics and Pharmacodynamics of a Sustained-Release Dexamethasone Intravitreal Implant. Investig. Ophthalmol. Vis. Sci. 2011, 52, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.R.; Kompella, U.B. Intravitreal, Subretinal, and Suprachoroidal Injections: Evolution of Microneedles for Drug Delivery. J. Ocul. Pharmacol. Ther. 2018, 34, 141. [Google Scholar] [CrossRef]
- Adrianto, M.F.; Annuryanti, F.; Wilson, C.G.; Sheshala, R.; Thakur, R.R.S. In Vitro Dissolution Testing Models of Ocular Implants for Posterior Segment Drug Delivery. Drug Deliv. Transl. Res. 2022, 12, 1355–1375. [Google Scholar] [CrossRef]
- Li, L.; Guo, D.; Guo, J.; Song, J.; Wu, Q.; Liu, D.; Bi, H.; Xie, X. Thermosensitive in-situ forming gels for ophthalmic delivery of tea polyphenols. J. Drug Deliv. Sci. Technol. 2018, 46, 243–250. [Google Scholar] [CrossRef]
- Rohde, F.; Walther, M.; Wächter, J.; Knetzger, N.; Lotz, C.; Windbergs, M. In-situ tear fluid dissolving nanofibers enable prolonged viscosity-enhanced dual drug delivery to the eye. Int. J. Pharm. 2022, 616, 121513. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Ang, B.C.; Andriyana, A.; Afifi, A.M. A review on fabrication of nanofibers via electrospinning and their applications. SN Appl. Sci. 2019, 1, 1248. [Google Scholar] [CrossRef]
- Vora, L.K.; Sabri, A.H.; Naser, Y.; Himawan, A.; Hutton, A.R.J.; Anjani, Q.K.; Volpe-Zanutto, F.; Mishra, D.; Li, M.; Rodgers, A.M.; et al. Long-acting microneedle formulations. Adv. Drug Deliv. Rev. 2023, 201, 115055. [Google Scholar] [CrossRef]
- Celik, N.; Khoramnia, R.; Auffarth, G.U.; Sel, S.; Mayer, C.S. Complications of dexamethasone implants: Risk factors, prevention, and clinical management. Int. J. Ophthalmol. 2020, 13, 1612. [Google Scholar] [CrossRef]
- Lee, J.-H.; Pidaparti, R.M.; Atkinson, G.M.; Moorthy, R.S. Design of an Implantable Device for Ocular Drug Delivery. J. Drug Deliv. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Vora, L.K.; McMillian, H.; Mishra, D.; Jones, D.; Thakur, R.R.S. In-situ forming solvent-induced phase inversion (SIPI) implants for controlled drug delivery: Role of hydrophilic polymers. J. Pharm. Sci. 2025, 114, 103717, . [Google Scholar] [CrossRef]
- So, Y.H.; Mishra, D.; Gite, S.; Sonawane, R.; Waite, D.; Shaikh, R.; Vora, L.K.; Thakur, R.R.S. Emerging Trends in Long-Acting Sustained Drug Delivery for Glaucoma Management. Drug Deliv. Transl. Res. 2025. [CrossRef] [PubMed]
- Mishra, D.; Gade, S.; Pathak, V.; Vora, L.K.; Mcloughlin, K.; Medina, R.; Donnelly, R.F.; Thakur, R.R.S. Ocular Application of Electrospun Materials for Drug Delivery and Cellular Therapies. Drug Discov. Today 2023, 28, 103676, . [Google Scholar] [CrossRef]
- Loewenstein, A.; Quiroz-Mercado, H.; Guerrero-Naranjo, J.L.; Rojas, S.; Santos, A.; Altamirano, J.C.; Morales, Y.; Schiffman, R. Interim results of a dose escalation study of NT-503 encapsulated cell therapy for the treatment of choroidal neovascularization in AMD. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2277. [Google Scholar]
- Barar, J.; Aghanejad, A.; Fathi, M.; Omidi, Y. Advanced drug delivery and targeting technologies for the ocular diseases. BioImpacts 2016, 6, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef] [PubMed]
- NCT00692614, A Study of MK0140 in Diabetic Patients with Macular Edema (0140-001). 2008. Available online: https://Clinicaltrials.Gov/Show/NCT00692614 (accessed on 21 February 2025).
- Shah, T.J.; Conway, M.D.; Peyman, G.A. Intracameral dexamethasone injection in the treatment of cataract surgery induced inflammation: Design, development, and place in therapy. Clin. Ophthalmol. 2018, 12, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Dias, J.R.; Nunes, R.P.; Goldhardt, R. New Drugs and New Posterior Delivery Methods in CME. Curr. Ophthalmol. Rep. 2017, 5, 160–168. [Google Scholar] [CrossRef]
- Yasukawa, T.; Ogura, Y.; Sakurai, E.; Tabata, Y.; Kimura, H. Intraocular sustained drug delivery using implantable polymeric devices. Adv. Drug Deliv. Rev. 2005, 57, 2033–2046. [Google Scholar] [CrossRef]
- Shukla, S.; Robey, R.W.; Bates, S.E.; Ambudkar, S.V. Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2. Drug. Metab. Dispos. 2009, 37, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Ogura, Y. Biodegradable polymers for ocular drug delivery. Ophthalmologica 2001, 215, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; George, S.; Yu, H.; Damoiseaux, R.; France, B.; Ng, K.W.; Loo, J.S.C. Size influences the cytotoxicity of poly (lactic-co-glycolic acid) (PLGA) and titanium dioxide (TiO2) nanoparticles. Arch. Toxicol. 2013, 87, 1075–1086. [Google Scholar] [CrossRef]
- Massa, H.; Georgoudis, P.; Panos, G.D. Dexamethasone intravitreal implant (OZURDEX®) for macular edema secondary to noninfectious uveitis: A review of the literature. Ther. Deliv. 2019, 10, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, A.; Joshi, M.; Christoforidis, J. Drug delivery implants in the treatment of vitreous inflammation. Mediat. Inflamm. 2013, 2013, 780634. [Google Scholar] [CrossRef]
- Bhagat, R.; Zhang, J.; Farooq, S.; Li, X.Y. Comparison of the release profile and pharmacokinetics of intact and fragmented dexamethasone intravitreal implants in rabbit eyes. J. Ocul. Pharmacol. Ther. 2014, 30, 854–858. [Google Scholar] [CrossRef]
- Robinson, M.R.; Whitcup, S.M. Pharmacologic and clinical profile of dexamethasone intravitreal implant. Expert Rev. Clin. Pharmacol. 2014, 5, 629–647. [Google Scholar] [CrossRef] [PubMed]
- Costello, M.A.; Liu, J.; Wang, Y.; Qin, B.; Xu, X.; Li, Q.; Lynd, N.A.; Zhang, F. Reverse engineering the Ozurdex dexamethasone intravitreal implant. Int. J. Pharm. 2023, 634, 122625. [Google Scholar] [CrossRef]
- Vagiakis, I.; Papadopoulou, E.P.; Amaxilati, E.; Tsiropoulos, G.N.; Konstas, A.G.; Panos, G.D. Bimatoprost Intracameral Implant (Durysta®): A New Era in Glaucoma Management Through Sustained-Release Innovation. Drug Des. Dev. Ther. 2025, 19, 703–714. [Google Scholar] [CrossRef]
- Okabe, J.; Kimura, H.; Kunou, N.; Okabe, K.; Kato, A.; Ogura, Y. Biodegradable intrascleral implant for sustained intraocular delivery of betamethasone phosphate. Investig. Ophthalmol. Vis. Sci. 2003, 44, 740–744. [Google Scholar] [CrossRef]
- Ambati, J.; Canakis, C.S.; Miller, J.W.; Gragoudas, E.S.; Edwards, A.; Weissgold, D.J.; Kim, I.; Delori, F.C.; Adamis, A.P. Diffusion of High Molecular Weight Compounds through Sclera. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1181–1185. [Google Scholar] [PubMed]
- Ambati, J.; Gragoudas, E.S.; Miller, J.W.; You, T.T.; Miyamoto, K.; Delori, F.C.; Adamis, A.P. Transscleral Delivery of Bioactive Protein to the Choroid and Retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1186–1191. [Google Scholar] [PubMed]
- Del Amo, E.M.; Urtti, A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov. Today 2008, 13, 135–143. [Google Scholar] [CrossRef]
- Shen, J.; Lu, G.W.; Hughes, P. Targeted Ocular Drug Delivery with Pharmacokinetic/Pharmacodynamic Considerations. Pharm. Res. 2018, 35, 217. [Google Scholar] [CrossRef]
- Leinonen, S.; Immonen, I.; Kotaniemi, K. Fluocinolone acetonide intravitreal implant (Retisert®) in the treatment of sight threatening macular oedema of juvenile idiopathic arthritis-related uveitis. Acta Ophthalmol. 2018, 96, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Kane, F.E.; Burdan, J.; Cutino, A.; Green, K.E. IluvienTM: A new sustained delivery technology for posterior eye disease. Expert. Opin. Drug Deliv. 2008, 5, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Marcus, D.M.; Awh, C.C.; Regillo, C.; Adamis, A.P.; Bantseev, V.; Chiang, Y.; Ehrlich, J.S.; Erickson, S.; Hanley, W.D.; et al. The Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration: Results from the Randomized Phase 2 Ladder Clinical Trial. Ophthalmology 2019, 126, 1141–1154. [Google Scholar] [CrossRef]
- Holekamp, N.M.; Campochiaro, P.A.; Chang, M.; Miller, D.; Pieramici, D.; Adamis, A.P.; Brittain, C.; Evans, E.; Kaufman, D.; Maass, K.F.; et al. Archway Randomized Phase 3 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2021, 129, 295–307. [Google Scholar] [CrossRef]
- Lee, S.S.; Hughes, P.; Ross, A.D.; Robinson, M.R. Biodegradable Implants for Sustained Drug Release in the Eye. Pharm. Res. 2010, 27, 2043–2053. [Google Scholar] [CrossRef]
- Tsujinaka, H.; Fu, J.; Shen, J.; Yu, Y.; Hafiz, Z.; Kays, J.; McKenzie, D.; Cardona, D.; Culp, D.; Peterson, W.; et al. Sustained treatment of retinal vascular diseases with self-aggregating sunitinib microparticles. Nat. Commun. 2020, 11, 694. [Google Scholar] [CrossRef]
- Turturro, S.B.; Guthrie, M.J.; Appel, A.A.; Drapala, P.W.; Brey, E.M.; Pérez-Luna, V.H.; Mieler, W.F.; Kang-Mieler, J.J. The effects of cross-linked thermo-responsive PNIPAAm-based hydrogel injection on retinal function. Biomaterials 2011, 32, 3620–3626. [Google Scholar] [CrossRef] [PubMed]
- Derwent, J.J.K. Thermoresponsive Hydrogels as a New Ocular Drug Delivery Platform To the eye. Trans. Am. Ophthalmol. Soc. 2008, 106, 206–214. [Google Scholar]
- Seuring, J.; Bayer, F.M.; Huber, K.; Agarwal, S. Upper Critical Solution Temperature of Poly(N-acryloyl glycinamide) in Water: A Concealed Property. Macromolecules 2012, 45, 374–384. [Google Scholar] [CrossRef]
- Sponchioni, M.; Bassam, P.R.; Moscatelli, D.; Arosio, P.; Palmiero, U.C. Biodegradable zwitterionic nanoparticles with tunable UCST-type phase separation under physiological conditions. Nanoscale 2019, 11, 16582–16591. [Google Scholar] [CrossRef]
- Niskanen, J.; Vapaavuori, J.; Pellerin, C.; Winnik, F.M.; Tenhu, H. Polysulfobetaine-surfactant solutions and their use in stabilizing hydrophobic compounds in saline solution. Polymer 2017, 127, 77–87. [Google Scholar] [CrossRef]
- Kurniawansyah, I.S.; Rahmi, F.; Sopyan, I. pH Triggered In-situ Gelling Ophthalmic Drug Delivery System. Int. J. Drug Deliv. Technol. 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Allam, A.; El-Mokhtar, M.A.; Elsabahy, M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation. J. Pharm. Pharmacol. 2019, 71, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Allam, A.; Elsabahy, M.; el Badry, M.; Eleraky, N.E. Betaxolol-loaded niosomes integrated within pH-sensitive in situ forming gel for management of glaucoma. Int. J. Pharm. 2021, 598, 120380. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Manjappa, A.; Nanjwade, B.; Manvi, F.V.; Rayasa, M. Sustained Ophthalmic In Situ Gel of Ketorolac Tromethamine: Rheology and In Vivo Studies. Drug Dev. Res. 2009, 70, 417–424. [Google Scholar] [CrossRef]
- Thambi, T.; Phan, V.H.G.; Lee, D.S. Stimuli-Sensitive Injectable Hydrogels Based on Polysaccharides and Their Biomedical Applications. Macromol. Rapid Commun. 2016, 37, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.K.; Das, M.; Jain, S. In situ gel systems as “smart” carriers for sustained ocular drug delivery. Expert. Opin. Drug Deliv. 2012, 9, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2011, 63, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K. UV radiation and the Eye. Optician 2009, 237, 26–33. [Google Scholar]
- Glickman, R.D. Ultraviolet Phototoxicity to the Retina. Eye Contact Lens 2011, 37, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Huu, V.A.N.; Luo, J.; Zhu, J.; Zhu, J.; Patel, S.; Boone, A.; Mahmoud, E.; McFearin, C.; Olejniczak, J.; de Gracia Lux, C.; et al. Light-responsive nanoparticle depot to control release of a small molecule angiogenesis inhibitor in the posterior segment of the eye. J. Control. Release 2015, 200, 71–77. [Google Scholar] [CrossRef]
- Griffin, D.R.; Kasko, A.M. Photodegradable macromers and hydrogels for live cell encapsulation and release. J. Am. Chem. Soc. 2012, 134, 13103–13107. [Google Scholar] [CrossRef]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef]
- Evan Dijk, H.C.; van Rijssen, T.J.; Subhi, Y.; Boon, C.J.F. Photodynamic Therapy for Chorioretinal Diseases: A Practical Approach. Ophthalmol. Ther. 2020, 9, 329–342. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, T.; Zhang, Y.; Dang, G. Anti-VEGF monotherapy versus photodynamic therapy and anti-VEGF combination treatment for neovascular age-related macular degeneration: A meta-analysis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4307–4317. [Google Scholar] [CrossRef]
- Armbrecht, A.M.; Aspinall, P.A.; Dhillon, B. A prospective study of visual function and quality of life following PDT in patients with wet age related macular degeneration. Br. J. Ophthalmol. 2004, 88, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Belin, M.W.; Lim, L.; Rajpal, R.K.; Hafezi, F.; Gomes, J.A.P.; Cochener, B. Corneal cross-linking: Current USA status report from the cornea society. Cornea 2018, 37, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Bisht, R.; Jaiswal, J.K.; Oliver, V.F.; Eurtivong, C.; Reynisson, J.; Rupenthal, I.D. Preparation and evaluation of PLGA nanoparticle-loaded biodegradable light-responsive injectable implants as a promising platform for intravitreal drug delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 142–156. [Google Scholar] [CrossRef]
- Tyagi, P.; Barros, M.; Stansbury, J.W.; Kompella, U.B. Light-activated, in situ forming gel for sustained suprachoroidal delivery of bevacizumab. Mol. Pharm. 2013, 10, 2858–2867. [Google Scholar] [CrossRef]
- Dunn, R.L.; English, J.P.; Cowsar, D.R.; Vanderbilt, D.P. Biodegradable In-Situ Forming Implants and Methods of Producing the Same. US Patent 4,938,763, 3 July 1990. [Google Scholar]
- Thakur, R.R.S.; McMillan, H.L.; Jones, D.S. Solvent induced phase inversion-based in situ forming controlled release drug delivery implants. J. Control. Release 2014, 176, 8–23. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) Acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Kranz, H.; Bodmeier, R. Structure formation and characterization of injectable drug loaded biodegradable devices: In situ implants versus in situ microparticles. Eur. J. Pharm. Sci. 2008, 34, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Singh, J. Controlled delivery of aspirin: Effect of aspirin on polymer degradation and in vitro release from PLGA based phase sensitive systems. Int. J. Pharm. 2008, 357, 119–125. [Google Scholar] [CrossRef]
- Schoenhammer, K.; Petersen, H.; Guethlein, F.; Goepferich, A. Poly(ethyleneglycol) 500 dimethylether as novel solvent for injectable in situ forming depots. Pharm. Res. 2009, 26, 2568–2577. [Google Scholar] [CrossRef]
- Hasbiyani, N.A.F.; Hikmawati, D. Siswanto. Electrospun Collagen-based Scaffold as Therapeutic Agent for Ocular Chemical Injury. J. Phys. Conf. Ser. 2020, 1445, 12022. [Google Scholar] [CrossRef]
- Guo, Q.; Mather, J.P.; Yang, P.; Boden, M.; Mather, P.T. Fabrication of Polymeric Coatings with Controlled Microtopographies Using an Electrospraying Technique. PLoS ONE 2015, 10, e0129960. [Google Scholar] [CrossRef]
- Pawar, A.; Thakkar, S.; Misra, M. A bird’s eye view of nanoparticles prepared by electrospraying: Advancements in drug delivery field. J. Control. Release 2018, 286, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Andreadis, I.I.; Karavasili, C.; Thomas, A.; Komnenou, A.; Tzimtzimis, M.; Tzetzis, D.; Andreadis, D.; Bouropoulos, N.; Fatouros, D.G. In Situ Gelling Electrospun Ocular Films Sustain the Intraocular Pressure-Lowering Effect of Timolol Maleate: In Vitro, Ex Vivo, and Pharmacodynamic Assessment. Mol. Pharm. 2022, 19, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Mirzaeei, S.; Taghe, S.; Asare-Addo, K.; Nokhodchi, A. Polyvinyl Alcohol/Chitosan Single-Layered and Polyvinyl Alcohol/Chitosan/Eudragit RL100 Multi-layered Electrospun Nanofibers as an Ocular Matrix for the Controlled Release of Ofloxacin: An In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2021, 22, 170. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ng, W.J.; Lee, L.Y.; Wang, C.-H. Encapsulation of protein drugs in biodegradable microparticles by co-axial electrospray. J. Colloid Interface Sci. 2008, 317, 469–476. [Google Scholar] [CrossRef]
- Angkawinitwong, U.; Awwad, S.; Khaw, P.T.; Brocchini, S.; Williams, G.R. Electrospun formulations of bevacizumab for sustained release in the eye. Acta Biomater. 2017, 64, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ni, C.; Chase, D.B.; Rabolt, J.F. Preparation of Multilayer Biodegradable Nanofibers by Triaxial Electrospinning. ACS Macro Lett. 2013, 2, 466–468. [Google Scholar] [CrossRef]
- Yu, D.-G.; Li, X.-Y.; Wang, X.; Yang, J.-H.; Bligh, S.W.A.; Williams, G.R. Nanofibers Fabricated Using Triaxial Electrospinning as Zero Order Drug Delivery Systems. ACS Appl. Mater. Interfaces 2015, 7, 18891–18897. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wu, H.; Wang, Y.; Lin, J.; Chen, Q.; Zhu, X. Preparation and ocular pharmacokinetics of hyaluronan acid-modified mucoadhesive liposomes. Drug Deliv. 2016, 23, 1144–1151. [Google Scholar] [CrossRef]
- Battaglia, L.; Serpe, L.; Foglietta, F.; Muntoni, E.; Gallarate, M.; Del Pozo Rodriguez, A.; Solinis, M.A. Application of lipid nanoparticles to ocular drug delivery. Expert. Opin. Drug Deliv. 2016, 13, 1743–1757. [Google Scholar] [CrossRef]
- Tsoplaktsoglou, M.; Spyratou, E.; Droulias, A.; Zachou, M.-E.; Efstathopoulos, E.P. The Contribution of Nanomedicine in Ocular Oncology. Cancers 2025, 17, 1186 . [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Xu, Y.N.; Zheng, H.H.; Huan, Y.R.; Zheng, N.X.; Lin, L.; Zhang, W.Z.; Xu, W. Carboplatin-loaded Surface Modified-PLGA Nanoparticles Confer Sustained Inhibitory Effect against Retinoblastoma Cell In Vitro. Arq. Bras. Oftalmol. 2022, 85, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Buitrago, E.; Winter, U.; Williams, G.; Asprea, M.; Chantada, G.; Schaiquevich, P. Pharmacokinetics of Melphalan after Intravitreal Injection in a Rabbit Model. J. Ocul. Pharmacol. Ther. 2016, 32, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Kudelkina, V.V.; Gerasimov, A.D.; Kosyreva, A.M.; Alekseeva, A.I.; Makarova, O.V. PLGA Polymers and Doxorubicin for the Treatment of Malignant Gliomas in Adults: An Overview. Open Med. Chem. J. 2025, 19. [Google Scholar] [CrossRef]
- Prijic, S.; Sersa, G. Magnetic nanoparticles as targeted delivery systems in oncology. Radiol Oncol. 2011, 45, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Meng, T.; Chen, Q.; Zhou, K.; Shao, Y.; Matlock, G.; Ma, X.; Wu, W.; Du, Y.; Wang, X.; et al. Fenofibrate-Loaded Biodegradable Nanoparticles for the Treatment of Experimental Diabetic Retinopathy and Neovascular Age-Related Macular Degeneration. Mol. Pharm. 2019, 16, 1958–1970. [Google Scholar] [CrossRef]
- Di Tommaso, C.; Bourges, J.L.; Valamanesh, F.; Trubitsyn, G.; Torriglia, A.; Jeanny, J.C.; Behar-Cohen, F.; Gurny, R.; Möller, M. Novel micelle carriers for cyclosporin A topical ocular delivery: In vivo cornea penetration, ocular distribution and efficacy studies. Eur. J. Pharm. Biopharm. 2012, 81, 257–264. [Google Scholar] [CrossRef]
- Emad Eldeeb, A.; Salah, S.; Ghorab, M. Proniosomal gel-derived niosomes: An approach to sustain and improve the ocular delivery of brimonidine tartrate; formulation, in-vitro characterization, and in-vivo pharmacodynamic study. Drug Deliv. 2019, 26, 509–521. [Google Scholar] [CrossRef]
- Iezzi, R.; Guru, B.R.; Glybina, I.V.; Mishra, M.K.; Kennedy, A.; Kannan, R.M. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Biomaterials 2012, 33, 979–988. [Google Scholar] [CrossRef]
- Spataro, G.; Malecaze, F.; Turrin, C.O.; Soler, V.; Duhayon, C.; Elena, P.P.; Majoral, J.P.; Caminade, A.M. Designing dendrimers for ocular drug delivery. Eur. J. Med. Chem. 2010, 45, 326–334. [Google Scholar] [CrossRef]
- Lin, L.; Fan, Y.; Gao, F.; Jin, L.; Li, D.; Sun, W.; Li, F.; Qin, P.; Shi, Q.; Shi, X.; et al. UTMD-Promoted Co-Delivery of Gemcitabine and miR-21 Inhibitor by Dendrimer-Entrapped Gold Nanoparticles for Pancreatic Cancer Therapy. Theranostics 2018, 8, 1923–1939. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.G.; Dias, K.; Silva, S.A.M.; De Rezende, L.C.D.; Rocha, E.M.; Emery, F.S.; Lopez, R.F.V. Transcorneal iontophoresis of dendrimers: PAMAM corneal penetration and dexamethasone delivery. J. Control. Release 2015, 200, 115–124. [Google Scholar] [CrossRef]
- Lallemand, F.; Schmitt, M.; Bourges, J.-L.; Gurny, R.; Benita, S.; Garrigue, J.-S. Cyclosporine A delivery to the eye: A comprehensive review of academic and industrial efforts. Eur. J. Pharm. Biopharm. 2017, 117, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.-S. Successfully improving ocular drug delivery using the cationic nanoemulsion, novasorb. J. Drug Deliv. 2012, 2012, 604204. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Gote, V.; Pal, D.; Mitra, A.K.; Ogundele, A.; Mitra, A.K. Ocular Pharmacokinetics of a Topical Ophthalmic Nanomicellar Solution of Cyclosporine (Cequa®) for Dry Eye Disease. Pharm. Res. 2019, 36, 36. [Google Scholar] [CrossRef] [PubMed]
- Houle, J.-M.; Strong, A. Clinical Pharmacokinetics of Verteporfin. J. Clin. Pharmacol. 2002, 42, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Garrigue, J.-S.; Amrane, M.; Faure, M.-O.; Holopainen, J.M.; Tong, L. Relevance of Lipid-Based Products in the Management of Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2017, 33, 647–661. [Google Scholar] [CrossRef]
- Wong, C.W.; Metselaar, J.M.; Storm, G.; Wong, T.T. A review of the clinical applications of drug delivery systems for the treatment of ocular anterior segment inflammation. Br. J. Ophthalmol. 2021, 105, 1617–1622. [Google Scholar] [CrossRef]
- Papangkorn, K.; Truett, K.R.; Vitale, A.T.; Jhaveri, C.; Scales, D.K.; Foster, C.S.; Montieth, A.; Higuchi, J.W.; Brar, B.; Higuchi, W.I. Novel dexamethasone sodium phosphate treatment (DSP-Visulex) for noninfectious anterior uveitis: A randomized phase I/II clinical trial. Curr. Eye Res. 2019, 44, 185–193. [Google Scholar] [CrossRef]
- Jackson, T.L.; Mandava, N.; Quiroz-Mercado, H.; Benage, M.; Garcia-Aguirre, G.; Morales-Canton, V.; Wilbur, L.; Olson, J. Intravitreal quantum dots for retinitis pigmentosa: A first-in-human safety study. Nanomedicine 2021, 16, 617–626. [Google Scholar] [CrossRef]
- Schopf, L.; Enlow, E.; Popov, A.; Bourassa, J.; Chen, H. Ocular Pharmacokinetics of a Novel Loteprednol Etabonate 0.4% Ophthalmic Formulation. Ophthalmol. Ther. 2014, 3, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Rykowska, I.; Nowak, I.; Nowak, R. Soft contact lenses as drug delivery systems: A review. Molecules 2021, 26, 5577. [Google Scholar] [CrossRef] [PubMed]
- Holgado, M.A.; Anguiano-Domínguez, A.; Martín-Banderas, L. Contact lenses as drug-delivery systems: A promising therapeutic tool. Arch. Soc. Española Oftalmol. Engl. Ed. 2020, 95, 24–33. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular drug delivery: Present innovations and future challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Morrison, P.W.J.; Khutoryanskiy, V.V. Advances in ophthalmic drug delivery. Ther. Deliv. 2014, 5, 1297–1315. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, C.S.A.; Fang, F. Contact lens materials: A materials science perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Bhamra, T.S.; Tighe, B.J. Mechanical properties of contact lenses: The contribution of measurement techniques and clinical feedback to 50 years of materials development. Contact Lens Anterior Eye 2017, 40, 70–81. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Sun, F. In vitro and in vivo evaluation of ketotifen fumarate-loaded silicone hydrogel contact lenses for ocular drug delivery. Drug Deliv. 2011, 18, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Iwatsu, M.; Kanai, A. Disposable 1-day Acuvue contact lenses for the delivery of lomefloxacin to rabbits’ eyes. CLAO J. 2001, 27, 212–215. [Google Scholar]
- Pereira-da-Mota, A.F.; Vivero-Lopez, M.; Topete, A.; Serro, A.P.; Concheiro, A.; Alvarez-Lorenzo, C. Atorvastatin-eluting contact lenses: Effects of molecular imprinting and sterilization on drug loading and release. Pharmaceutics 2021, 13, 606. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Wang, K.; Wang, L.; Yang, X.; Zhu, S. Cyclodextrin-containing hydrogels as an intraocular lens for sustained drug release. PLoS ONE 2017, 12, e0189778. [Google Scholar] [CrossRef] [PubMed]
- Bouledjouidja, A.; Masmoudi, Y.; Sergent, M.; Trivedi, V.; Meniai, A.; Badens, E. Drug loading of foldable commercial intraocular lenses using supercritical impregnation. Int. J. Pharm. 2016, 500, 85–99. [Google Scholar] [CrossRef]
- Bariya, S.H.; Gohel, M.C.; Mehta, T.A.; Sharma, O.P. Microneedles: An emerging transdermal drug delivery system. J. Pharm. Pharmacol. 2012, 64, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, S.; Ling, P.; Hashmi, G.; Reed, M.; Gaugler, R.; Trimmer, W. Genetic transformation of nematodes using arrays of micromechanical piercing structures. Biotechniques 1995, 19, 766–770. [Google Scholar] [PubMed]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.R.S.; Tekko, I.A.; Al-Shammari, F.; Ali, A.A.; McCarthy, H.; Donnelly, R.F. Rapidly dissolving polymeric microneedles for minimally invasive intraocular drug delivery. Drug Deliv. Transl. Res. 2016, 6, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Grossniklaus, H.E.; Edelhauser, H.F.; Prausnitz, M.R. Intrastromal Delivery of Bevacizumab Using Microneedles to Treat Corneal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2014, 5, 7376–7386. [Google Scholar] [CrossRef]
- Patel, S.R.; Lin, A.S.P.; Edelhauser, H.F.; Prausnitz, M.R. Suprachoroidal Drug Delivery to the Back of the Eye Using Hollow Microneedles. Pharm. Res. 2011, 28, 166–176. [Google Scholar] [CrossRef]
- Owens, C.D.; Gasper, W.J.; Walker, J.P.; Alley, H.F.; Conte, M.S.; Grenon, S.M. Safety and feasibility of adjunctive dexamethasone infusion into the adventitia of the femoropopliteal artery following endovascular revascularization. J. Vasc. Surg. 2014, 59, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Traverso, G.; Schoellhammer, C.M.; Schroeder, A.; Maa, R.; Lauwers, G.Y.; Polat, B.E.; Anderson, D.G.; Blankschtein, D.; Langer, R. Microneedles for Drug Delivery via the Gastrointestinal Tract. J. Pharm. Sci. 2015, 104, 362–367. [Google Scholar] [CrossRef]
- Paderni, C.; Compilato, D.; Giannola, L.I.; Campisi, G. Oral local drug delivery and new perspectives in oral drug formulation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e25–e34. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, J.; Huang, K.; Ye, Y.; Su, T.; Qiao, L.; Hensley, M.T.; Caranasos, T.G.; Zhang, J.; Gu, Z.; et al. Cardiac cell–integrated microneedle patch for treating myocardial infarction. Sci. Adv. 2018, 4, eaat9365. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Morde, R.S.; Mariani, S.; La Mattina, A.A.; Vignali, E.; Yang, C.; Barillaro, G.; Lee, H. 4D Printing of a Bioinspired Microneedle Array with Backward-Facing Barbs for Enhanced Tissue Adhesion Adv. Funct. Mater. 2020, 30, 1909197. [Google Scholar] [CrossRef]
- Wu, Y.; Vora, L.K.; Donnelly, R.F.; Singh, T.R.R. Rapidly dissolving bilayer microneedles enabling minimally invasive and efficient protein delivery to the posterior segment of the eye, Drug Deliv. Transl. Res. 2023, 13, 2142–2158. [Google Scholar]
- Wu, Y.; Vora, L.K.; Mishra, D.; Adrianto, M.F.; Gade, S.; Paredes, A.J.; Donnelly, R.F.; Singh, T.R.R. Nanosuspension-Loaded Dissolving Bilayer Microneedles for Hydrophobic Drug Delivery to the Posterior Segment of the Eye. Biomater. Adv. 2022, 137, 212767. [Google Scholar] [CrossRef]
- Wu, Y.; Vora, L.K.; Wang, Y.; Adrianto, M.F.; Tekko, I.A.; Waite, D.; Donnelly, R.F.; Thakur, R.R.S. Long-acting nanoparticle-loaded bilayer microneedles for protein delivery to the posterior segment of the eye. Eur. J. Pharm. Biopharm. 2021, 165, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Faizi, H.S.; Nasiri, M.I.; Wu, Y.; Mishra, D.; Donnelly, R.F.; Minhas, M.U.; Vora, L.K.; Thakur, R.R.S. Deferasirox Nanosuspension Loaded Dissolving Microneedles for Ocular Drug Delivery. Int. J. Pharm. 2024, 664, 124614, . [Google Scholar] [CrossRef]
- Glover, K.; Mishra, D.; Gade, S.; Vora, L.K.; Wu, Y.; Paredes, A.J.; Donnelly, R.F.; Singh, T.R.R. Microneedles for advanced ocular drug delivery. Adv. Drug Deliv. Rev. 2023, 201, 115082, . [Google Scholar] [CrossRef]
- Song, H.B.; Lee, K.J.; Seo, I.H.; Lee, J.Y.; Lee, S.-M.; Kim, J.H.; Kim, J.H.; Ryu, W. Impact insertion of transfer-molded microneedle for localized and minimally invasive ocular drug delivery. J. Control. Release 2015, 209, 272–279. [Google Scholar] [CrossRef]
- Jiang, J.; Gill, H.S.; Ghate, D.; McCarey, B.E.; Patel, S.R.; Edelhauser, H.F.; Prausnitz, M.R. Coated Microneedles for Drug Delivery to the Eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4038–4043. [Google Scholar] [CrossRef]
- Lee, Y.; Park, S.; Kim, S.I.; Lee, K.; Ryu, W. Rapidly Detachable Microneedles Using Porous Water-Soluble Layer for Ocular Drug Delivery. Adv. Mater. Technol. 2020, 5, 1901145 . [Google Scholar] [CrossRef]
- Amer, M.; Chen, R.K. Self-Adhesive Microneedles with Interlocking Features for Sustained Ocular Drug Delivery. Macromol. Biosci. 2020, 20, 2000089 . [Google Scholar] [CrossRef] [PubMed]
- Gade, S.G.; Glover, K.; Mishra, D.; Sharma, S.; Guy, O.; Donnelly, R.F.; Vora, L.K.; Thakur, R.R.S. Hollow microneedles for ocular drug delivery. J. Control. Release 2024, 371, 43–66,. [Google Scholar] [CrossRef] [PubMed]

| Device | Medication | Therapeutic Use | Dose | Delivery Route | Release Duration | Composition | Category | Approval Year | Company |
|---|---|---|---|---|---|---|---|---|---|
| Retisert® | Fluocinolone acetonide | Non-infectious posterior uveitis | 0.59 mg | Intravitreal | 30 months | Silicone and polyvinyl alcohol | Non-degradable | 2005 | Bausch + Lomb (Bridgewater, NJ, USA) |
| Ozurdex® | Dexamethasone | Diabetic macular edema, RVO, uveitis | 0.7 mg | Intravitreal | Approximately 6 months | PLGA | Biodegradable | 2009 | AbbVie (Chicago, IL, USA; Berkshire, UK) |
| Iluvien® | Fluocinolone acetonide | Chronic diabetic macular edema | 0.19 mg | Intravitreal | 36 months | Polyimide tube with PVA and silicone | Non-degradable | 2011 | Alimera Sciences, Inc. (Alpharetta, GA, USA; Dublin, Ireland) |
| Yutiq® | Fluocinolone acetonide | Non-infectious posterior uveitis | 0.18 mg | Intravitreal | 36 months | Polyimide | Non-degradable | 2018 | EyePoint Pharmaceuticals, Inc. (Watertown, MA, USA) |
| Durysta® | Bimatoprost | Reduction of intraocular pressure | 10 mcg | Intracameral | Several months | PLGA combined with PDLA, PDLLA, and PEG3350 | Biodegradable | 2020 | AbbVie (Chicago, IL, USA) |
| Susvimo® | Ranibizumab | AMD | 2 mg | Intravitreal | Approximately 6 months | Port delivery system | Non-degradable | 2021 | Roche (Atlanta, GA, USA) |
| iDose TR® | Travoprost | Reduction of intraocular pressure | 75 mcg | Intracameral | 3 months | Titanium reservoir with a semipermeable membrane | Non-degradable | 2023 | Glaukos Corporation (San Clemente, CA, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gade, S.; So, Y.; Mishra, D.; Baviskar, S.M.; Assiri, A.A.; Glover, K.; Sheshala, R.; Vora, L.K.; Thakur, R.R.S. Ocular Drug Delivery: Emerging Approaches and Advances. Pharmaceutics 2025, 17, 599. https://doi.org/10.3390/pharmaceutics17050599
Gade S, So Y, Mishra D, Baviskar SM, Assiri AA, Glover K, Sheshala R, Vora LK, Thakur RRS. Ocular Drug Delivery: Emerging Approaches and Advances. Pharmaceutics. 2025; 17(5):599. https://doi.org/10.3390/pharmaceutics17050599
Chicago/Turabian StyleGade, Shilpkala, Yin So, Deepakkumar Mishra, Shubhamkumar M. Baviskar, Ahmad A. Assiri, Katie Glover, Ravi Sheshala, Lalitkumar K. Vora, and Raghu Raj Singh Thakur. 2025. "Ocular Drug Delivery: Emerging Approaches and Advances" Pharmaceutics 17, no. 5: 599. https://doi.org/10.3390/pharmaceutics17050599
APA StyleGade, S., So, Y., Mishra, D., Baviskar, S. M., Assiri, A. A., Glover, K., Sheshala, R., Vora, L. K., & Thakur, R. R. S. (2025). Ocular Drug Delivery: Emerging Approaches and Advances. Pharmaceutics, 17(5), 599. https://doi.org/10.3390/pharmaceutics17050599









