Wound Healing Properties of Plant-Based Hydrogel and Oleogel Formulations in a Rat Scald Burn Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants
- Boswellia serrata (BS),
- Ocimum basilicum (OB),
- Sambucus nigra flower (SNF),
- Sambucus nigra bark (SNB),
- Galium verum (GV),
- A combined extract containing equal parts by weight of the four plant extracts (BS_OB_SNF_GV).
- Commercially available tea preparations purchased from an online pharmacy (planteea.ro):
- ○
- Basil tea (Ceai de busuioc, Lot 1958, SC Stefmar Productie SRL, Ramnicu Valcea, Romania),
- ○
- Lady’s Bedstraw tea (Sânziene galbene, Lot 84941, Dacia Plant, Bod, Romania),
- ○
- Elderflower tea, (Soc flori, Lot 85053, Dacia Plant, Bod, Romania),
- Incense granules (100% Tămâie 100%, Lot 5819 LIFE Bio, SC Bionovativ SRL, Podu Olt, Romania),
- Harvested from wild-growing plants (bark from young branches of Sambucus nigra bushes of 2 m height, collected from the outer part of the tree).
2.2. Plant Extraction Procedure
2.3. Chemical Agents and Excipients
- An oleogel base {Olive oil (Ulei de măsline, Azelis SRL, Bucharest, Romania) 80% w/w OR sunflower oil (Ulei de floarea soarelui, Azelis SRL, Bucharest, Romania) 80% w/w OR Isopropyl myristate (Sigma-Aldrich, Taufkirchen, Germany) 80% w/w OR Diethylene glycol monoethyl ether (Thermo Fisher Scientific, Bremen, Germany) 80% w/w AND Glyceryl dibehenate (Compritol 888 ATO, Gattefossé, Saint-Priest Cedex France) 20% w/w},
- A hydrogel base {purified water 65% w/w, Poloxamer 407 (Kolliphor® P 407 Geismar, BASF Pharma, Hannover, Germany) 25% w/w, and glycerol 10% w/w}.
2.4. Preparation of Blank and Plant Extract-Loaded Oleogel Formulations
2.5. Preparation of Blank and Plant Extract-Loaded Hydrogels Based on Poloxamer 407
2.6. Animals
2.7. Anesthesia, Preoperative Preparation, and Pain Management
2.8. Burn Induction and Wound Standardization
2.9. Treatment Application and Dressing Protocol
2.10. Scald Wounds and Skin and Inflammation Monitoring
2.11. Skin Perfusion Measurement
2.12. Transepidermal Skin Biophysical Assessments
- Erythema index and melanin content (Mexameter® MX18),
- Trans-epidermal water loss (TEWL, Tewameter® TM300),
- Skin hydration (Corneometer® CM825),
- Sebum levels (Sebumeter®),
- Skin temperature (Temperature Probe).
2.13. Histological Evaluation of Burn Wounds
2.14. Euthanasia
2.15. Statistical Analysis
3. Results
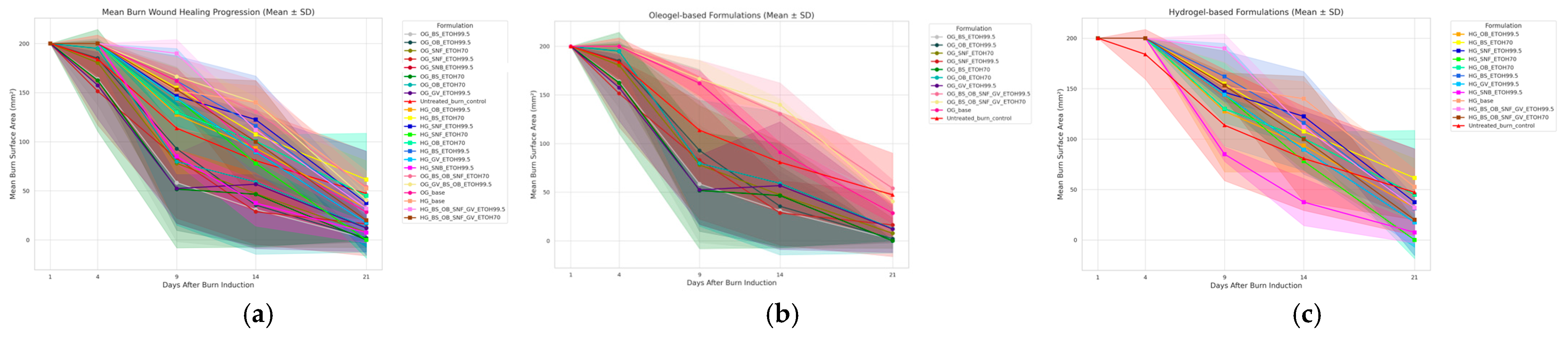
3.1. Wound Surface Area Reduction
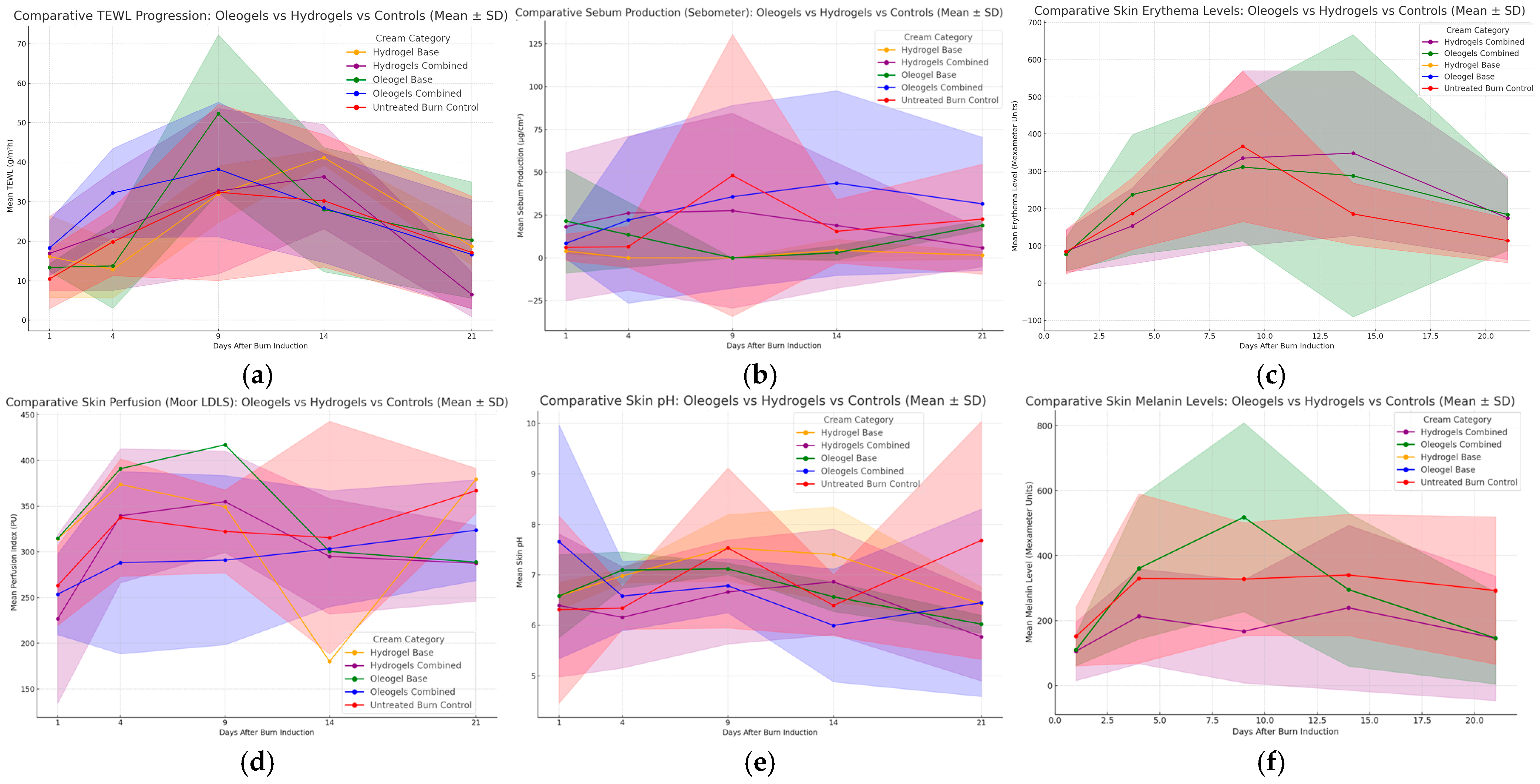
3.2. Transepidermal Water Loss (TEWL) and Skin Hydration
3.3. Sebum Production (Sebometer)
3.4. Mexameter (Melanin and Erythema)
3.5. Skin pH
3.6. Skin Perfusion (Moor LDLS)
3.7. Skin Hydration (Corneometer)
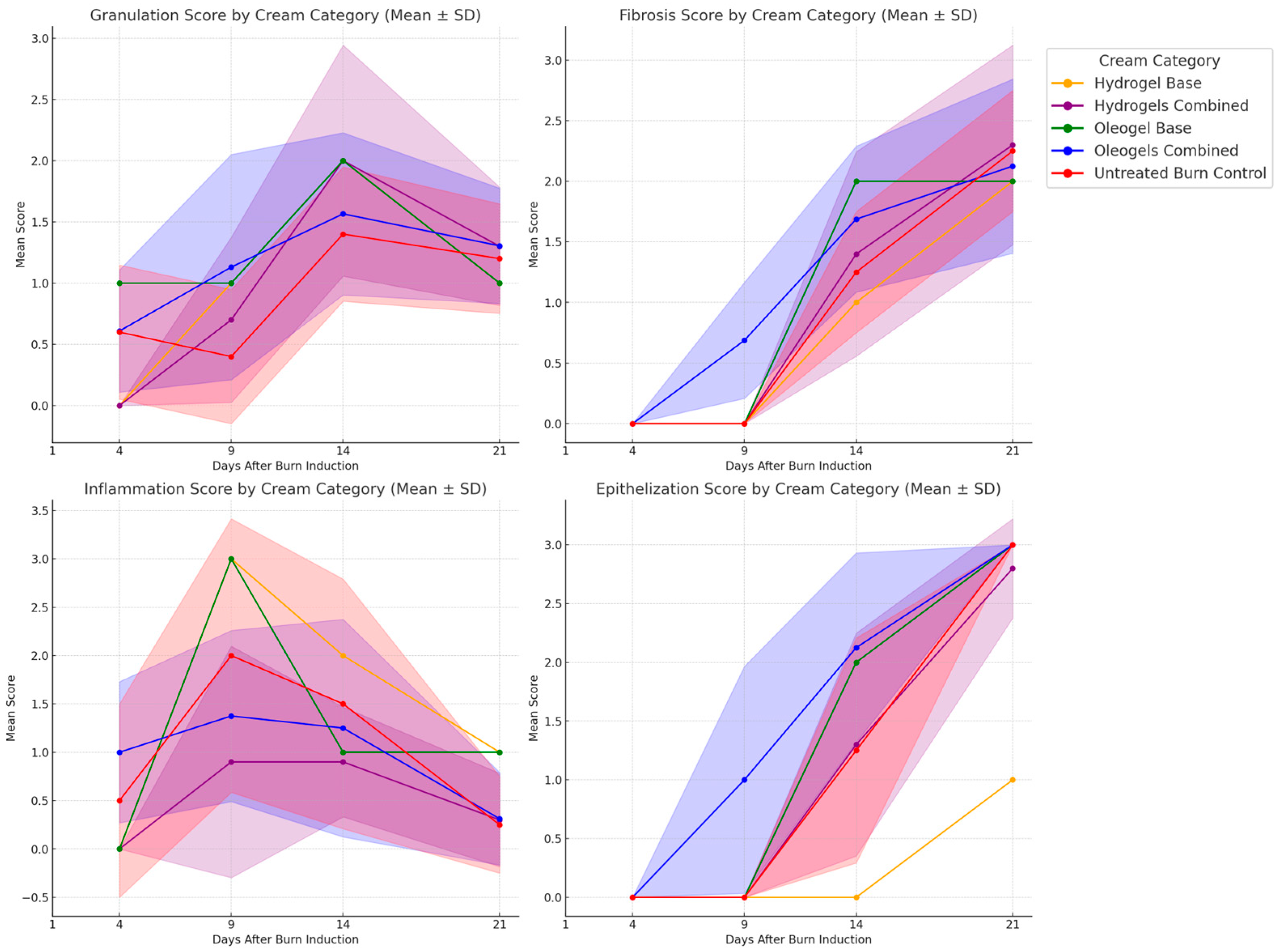
3.8. Histological Evaluation
4. Discussion
4.1. Overview of Standard Burn Care and Limitations of SSD
4.2. Comparative Analysis of Wound Healing Effects of Studied Plant Extracts
4.3. Plant-Derived Bioactive Hydrogels and Oleogels in Burn Wound Healing
4.4. Experimental Results on Wistar Rats with Scald Wounds Treated with Oleogels and Hydrogels
4.5. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OB | Ocimum basilicum |
| BS | Boswellia serrata |
| GV | Galium verum |
| SNF | Sambucus nigra flower |
| SNB | Sambucus nigra bark |
| OG | Oleogel |
| HG | Hydrogel |
| SSD | Silver sulfadiazine |
| RAPID-3D | Rat Printed Induction Device—3D |
| PVP-I | Liposome polyvinyl-pyrrolidone-iodine |
| TA | Topical antimicrobial |
| RCT | Randomized controlled trial |
References
- Chen, Z.; Zeng, Y.; Tian, F. Developing a Third-Degree Burn Model of Rats Using the Delphi Method. Sci. Rep. 2022, 12, 13852. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Moser, E.M.; Pfortmueller, C.A.; Olariu, R.; Lehmann, B.; Exadaktylos, A.K. Aetiology of Adult Burns Treated from 2000 to 2012 in a Swiss University Hospital. Burns 2016, 42, 919–925. [Google Scholar] [CrossRef]
- Salas, M.M.; Clifford, J.L.; Hayden, J.R.; Iadarola, M.J.; Averitt, D.L. Local Resiniferatoxin Induces Long-Lasting Analgesia in a Rat Model of Full Thickness Thermal Injury. Pain Med. 2017, 18, 2453–2465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Selçuk, C.T.; Durgun, M.; Tekin, R.; Yolbas, L.; Bozkurt, M.; Akçay, C.; Alabalk, U.; Basarali, M.K. Evaluation of the Effect of Thymo-Quinone Treatment on Wound Healing in a Rat Burn Model. J. Burn Care Res. 2013, 34, e274–e281. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, S.; Karim, S.; Asad, M.H.H.B.; Akram, M.R.; Khan, A.K.; Malik, A.; Chen, C.; Murtaza, G. Anti-Aging Potential of Phytoextract Loaded-Pharmaceutical Creams for Human Skin Cell Longevity. Oxid. Med. Cell. Longev. 2015, 2015, 709628. [Google Scholar] [CrossRef]
- Casetti, F.; Wölfle, U.; Gehring, W.; Schempp, C.M. Dermocosmetics for Dry Skin: A New Role for Botanical Extracts. Skin Pharmacol. Physiol. 2011, 24, 289–293. [Google Scholar] [CrossRef]
- Faur, A.; Watz, C.; Moacă, E.-A.; Avram, Ş.; Borcan, F.; Pinzaru, I.; Iftode, A.; Nicolov, M.; Popovici, R.A.; Raica, M.; et al. Correlations on Phenolic Screening Related to In Vitro and In Ovo Assessment of Ocimum basilicum L. Hydro-Alcoholic Extracts Used as Skin Active Ingredient. Molecules 2020, 25, 5442. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Churpek, M.M.; Zadravecz, F.J.; Winslow, C.; Howell, M.D.; Edelson, D.P. Incidence and Prognostic Value of the Systemic Inflammatory Response Syndrome and Organ Dysfunctions in Ward Patients. Am. J. Respir. Crit. Care Med. 2015, 192, 958–964. [Google Scholar] [CrossRef]
- Pham, T.N.; Cancio, L.C.; Gibran, N.S. American Burn Association Practice Guidelines Burn Shock Resuscitation. J. Burn Care Res. 2008, 29, 257–266. [Google Scholar] [CrossRef]
- Colohan, S.M. Predicting Prognosis in Thermal Burns with Associated Inhalational Injury: A Systematic Review of Prognostic Factors in Adult Burn Victims. J. Burn Care Res. 2010, 31, 529–539. [Google Scholar] [CrossRef]
- Rosen, J.; Landriscina, A.; Kutner, A.; Adler, B.L.; Krausz, A.E.; Nosanchuk, J.D.; Friedman, A.J. Silver Sulfadiazine Retards Wound Healing in Mice via Alterations in Cytokine Expression. J. Investig. Dermatol. 2015, 135, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.; Christie, J.; Liu, Z.; Westby, M.J.; Jefferies, J.M.; Hudson, T.; Edwards, J.; Mohapatra, D.P.; Hassan, I.A.; Dumville, J.C. Antiseptics for Burns. Cochrane Database Syst. Rev. 2017, 7, CD011821. [Google Scholar] [CrossRef] [PubMed]
- Boonkaew, B.; Kempf, M.; Kimble, R.; Cuttle, L. Cytotoxicity Testing of Silver-Containing Burn Treatments Using Primary and Immortal Skin Cells. Burns 2014, 40, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Raymond, S.L.; Zecevic, A.; Larson, S.D.; Ruzic, A.; Islam, S. Delayed Healing Associated with Silver Sulfadiazine Use for Partial Thickness Scald Burns in Children. Am. Surg. 2018, 84, 836–840. [Google Scholar] [CrossRef]
- Edger-Lacoursière, Z.; Zhu, M.; Jean, S.; Marois-Pagé, E.; Nedelec, B. Evidence Supporting Conservative Scar Management Interventions Following Burn Injury: A Review Article. J. Burn Care Res. 2024, irae204. [Google Scholar] [CrossRef]
- Shu, W.; Wang, Y.; Zhang, X.; Li, C.; Le, H.; Chang, F. Functional Hydrogel Dressings for Treatment of Burn Wounds. Front. Bioeng. Biotechnol. 2021, 9, 788461. [Google Scholar] [CrossRef]
- Tan, S.; Dosan, R. Lessons from Epithelialization: The Reason Behind Moist Wound Environment. Open Dermatol. J. 2019, 13, 34. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, J.; Liu, Y.; Ben, C.; Li, H.; Cheng, D.; Sun, Y.; Guang-Yi, W.; Zhu, S. Analysis of Povidone Iodine, Chlorhexidine Acetate and Polyhexamethylene Biguanide as Wound Disinfectants: In Vitro Cytotoxicity and Antibacterial Activity. BMJ Nutr. Prev. Health 2023, 6, 21–27. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, A.; Yuan, C.; Chen, X.; Liu, Y. Recent Trends on Burn Wound Care: Hydrogel Dressings and Scaffolds. Biomater. Sci. 2021, 9, 4523–4540. [Google Scholar] [CrossRef]
- Pinto, T.C.; Martins, A.J.; Pastrana, L.; Pereira, M.C.; Cerqueira, M.A. Oleogel-Based Systems for the Delivery of Bioactive Compounds in Foods. Gels 2021, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Cai, K.; Zhang, B.; Tang, S.; Zhang, W.; Liu, W. Antibacterial Polysaccharide-Based Hydrogel Dressing Containing Plant Essential Oil for Burn Wound Healing. Burn. Trauma 2021, 9, tkab041. [Google Scholar] [CrossRef]
- Antonescu, I.A.; Antonescu, A.; Miere, F.; Fritea, L.; Teușdea, A.C.; Vicaș, L.; Vicaș, S.I.; Brihan, I.; Domuța, M.; Zdrinca, M.; et al. Evaluation of Wound Healing Potential of Novel Hydrogel Based on Ocimum basilicum and Trifolium pratense Extracts. Processes 2021, 9, 2096. [Google Scholar] [CrossRef]
- Skowrońska, W.; Granica, S.; Piwowarski, J.P.; Jakupović, L.; Zovko Končić, M.; Bazylko, A. Wound Healing Potential of Extract from Sambucus nigra L. Leaves and Its Fractions. J. Ethnopharmacol. 2024, 320, 117423. [Google Scholar] [CrossRef] [PubMed]
- Pengzong, Z.; Yuanmin, L.; Xiaoming, X.; Shang, D.; Wei, X.; Zhigang, L.; Dongzhou, D.; Wenjing, Y.; Jianbiao, Y.; Yang, X.; et al. Wound Healing Potential of the Standardized Extract of Boswellia serrata on Experimental Diabetic Foot Ulcer via Inhibition of Inflammatory, Angiogenetic and Apoptotic Markers. Planta Med. 2019, 85, 657–669. [Google Scholar] [CrossRef]
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef] [PubMed]
- Roșca, O.-J.; Nistor, A.; Brandabur, C.; Heredea, R.E.; Hoinoiu, B.; Șoica, C. Rat 3D Printed Induction Device (RAPID-3D): A 3D-Printed Device for Uniform and Reproducible Scald Burn Induction in Rats with Histological and Microvascular Validation. Biology 2025, 14, 378. [Google Scholar] [CrossRef]
- Hu, X.; Wang, X.; Hong, X.; Fan, H.; Zhang, X.; Chen, A.; Wang, G.; Jin, J.; Xia, Z. Modification and Utility of a Rat Burn Wound Model. Wound Repair Regen. 2020, 28, 797–811. [Google Scholar] [CrossRef]
- Ali Khan, B.; Ullah, S.; Khan, M.K.; Alshahrani, S.M.; Braga, V.A. Formulation and Evaluation of Ocimum basilicum-Based Emulgel for Wound Healing Using Animal Model. Saudi Pharm. J. 2020, 28, 1842–1850. [Google Scholar] [CrossRef]
- Dubey, G.; Pathak, A.K. Wound Healing Activity of Hydro-Alcoholic Extract of Ocimum basilicum Linn. Aerial Parts in Wistar Rats. Int. J. Indig. Herb. Drug 2017, II, 11–13. Available online: https://saapjournals.org/index.php/herbsanddrugs/article/view/35 (accessed on 5 January 2025).
- Mallik, A.; Goupale, D.; Dhongade, H.; Nayak, S. Evaluation of Boswellia serrata Oleo-Gum Resin for Wound Healing Activity. Der Pharm. Lett. 2010, 2, 457–463. Available online: https://www.scholarsresearchlibrary.com/articles/evaluation-of-boswellia-serrata-oleogum-resin-for-wound-healing-activity.pdf (accessed on 5 January 2025).
- Schmolka, I.R. Artificial Skin I. Preparation and Properties of Pluronic F-127 Gels for Treatment of Burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef]
- Albrecht, M.; Henke, J.; Tacke, S.; Markert, M.; Guth, B. Influence of Repeated Anaesthesia on Physiological Parameters in Male Wistar Rats: A Telemetric Study about Isoflurane, Ketamine-Xylazine and a Combination of Medetomidine, Midazolam and Fentanyl. BMC Vet. Res. 2014, 10, 310. [Google Scholar] [CrossRef]
- NC3Rs. Revision of the ARRIVE Guidelines. Available online: https://nc3rs.org.uk/our-portfolio/revision-arrive-guidelines (accessed on 1 January 2025).
- Lanham, J.S.; Nelson, N.K.; Hendren, B.; Jordan, T.S. Outpatient Burn Care: Prevention and Treatment. Am. Fam. Physician 2020, 101, 463–470. [Google Scholar] [PubMed]
- Zhang, Y.; Wu, B.M. Current Advances in Stimuli-Responsive Hydrogels as Smart Drug Delivery Carriers. Gels 2023, 9, 838. [Google Scholar] [CrossRef]
- Goertz, O.; Abels, C.; Knie, U.; May, T.; Hirsch, T.; Daigeler, A.; Steinau, H.U.; Langer, S. Clinical Safety and Efficacy of a Novel Thermoreversible Polyhexanide-Preserved Wound Covering Gel. Eur. Surg. Res. 2010, 44, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Holbert, M.D.; Kimble, R.M.; Chatfield, M.; Griffin, B.R. Effectiveness of a Hydrogel Dressing as an Analgesic Adjunct to First Aid for the Treatment of Acute Paediatric Burn Injuries: A Prospective Randomised Controlled Trial. BMJ Open 2021, 11, e039981. [Google Scholar] [CrossRef]
- Homann, H.H.; Rosbach, O.; Moll, W.; Vogt, P.M.; Germann, G.; Hopp, M.; Langer-Brauburger, B.; Reimer, K.; Steinau, H.U. A Liposome Hydrogel with Polyvinyl-Pyrrolidone Iodine in the Local Treatment of Partial-Thickness Burn Wounds. Ann. Plast. Surg. 2007, 59, 423–427. [Google Scholar] [CrossRef]
- Maghsoudi, H.; Monshizadeh, S.; Mesgari, M. A Comparative Study of the Burn Wound Healing Properties of Saline-Soaked Dressing and Silver Sulfadiazine in Rats. Indian J. Surg. 2011, 73, 24–27. [Google Scholar] [CrossRef]
- Genuino, G.A.; Baluyut-Angeles, K.V.; Espiritu, A.P.; Lapitan, M.C.; Buckley, B.S. Topical Petrolatum Gel Alone versus Topical Silver Sulfadiazine with Standard Gauze Dressings for the Treatment of Superficial Partial Thickness Burns in Adults: A Randomized Controlled Trial. Burns 2014, 40, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Frew, Q.; Rennekampff, H.O.; Dziewulski, P.; Moiemen, N.; BBW-11 Study Group; Zahn, T.; Hartmann, B. Betulin Wound Gel Accelerated Healing of Superficial Partial Thickness Burns: Results of a Randomized, Intra-Individually Controlled, Phase III Trial with 12-Months Follow-Up. Burns 2019, 45, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, M.M.; Zangeneh, A.; Seydi, N.; Moradi, R. Evaluation of Cutaneous Wound Healing Activity of Ocimum basilicum Aqueous Extract Ointment in Rats. Comp. Clin. Pathol. 2019, 28, 1447–1454. [Google Scholar] [CrossRef]
- Mota, A.H.; Duarte, N.; Serra, A.T.; Ferreira, A.; Bronze, M.R.; Custódio, L.; Gaspar, M.M.; Simões, S.; Rijo, P.; Ascensão, L.; et al. Further Evidence of Possible Therapeutic Uses of Sambucus nigra L. Extracts by the Assessment of the In Vitro and In Vivo Anti-Inflammatory Properties of Its PLGA and PCL-Based Nanoformulations. Pharmaceutics 2020, 12, 1181. [Google Scholar] [CrossRef]
- Dong, F.; Zheng, L.; Zhang, X. Alpha-Boswellic Acid Accelerates Acute Wound Healing via NF-κB Signaling Pathway. PLoS ONE 2024, 19, e0308028. [Google Scholar] [CrossRef]
- Turcov, D.; Barna, A.S.; Trifan, A.; Blaga, A.C.; Tanasă, A.M.; Suteu, D. Antioxidants from Galium verum as Ingredients for the Design of New Dermatocosmetic Products. Plants 2022, 11, 2454. [Google Scholar] [CrossRef]
- Vuletić, M.; Jakovljević, V.; Živanović, S.; Papić, M.; Papić, M.; Mladenović, R.; Živković, V.; Srejović, I.; Jeremić, J.; Anđić, M.; et al. The Evaluation of Healing Properties of Galium verum-Based Oral Gel in Aphthous Stomatitis in Rats. Molecules 2022, 27, 4680. [Google Scholar] [CrossRef]


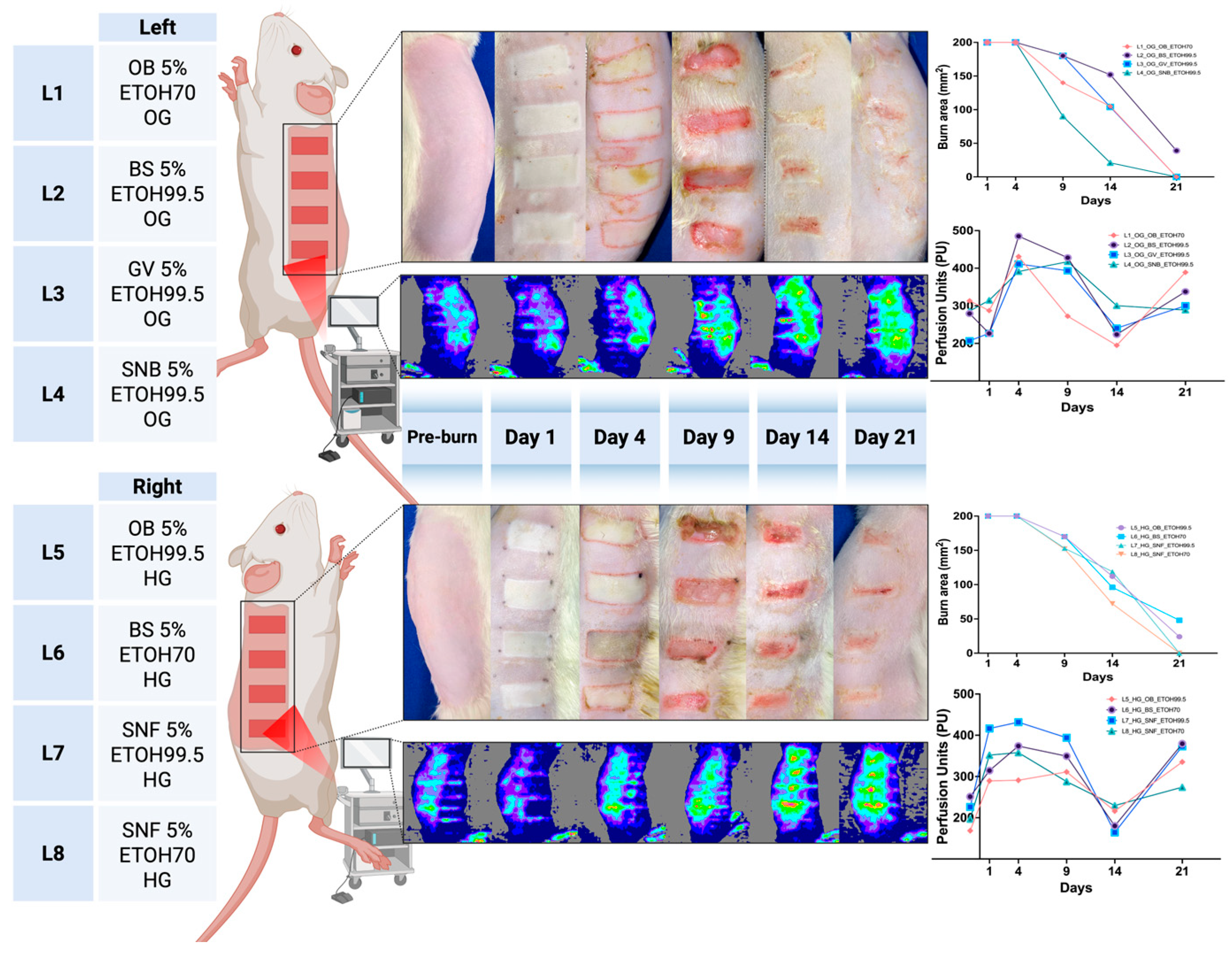

| Formulation | Extraction Method | Extract and Concentration (w/w) | Gel Components | Unique Name | Description |
|---|---|---|---|---|---|
| OG | ETOH99.5 | OB 5% | Sunflower oil 80% Glyceryl dibehenate 15% | OG_OB_ETOH99.5 | Oleogel with basil extract in absolute ethanol, based on glyceryl dibehenate and sunflower oil |
| OG | ETOH99.5 | BS 5% | Sunflower oil 80% Glyceryl dibehenate 15% | OG_BS_ETOH99.5 | Oleogel with frankincense extract in absolute ethanol, based on glyceryl dibehenate and sunflower oil |
| OG | ETOH99.5 | SNF 5% | Olive oil 80% Glyceryl dibehenate 15% | OG_SNF_ETOH99.5 | Oleogel with elderflower extract in absolute ethanol, based on glyceryl dibehenate and olive oil |
| OG | ETOH99.5 | SNB 5% | Sunflower oil 80% Glyceryl dibehenate 15% | OG_SNB_ETOH99.5 | Oleogel with elder bark extract in absolute ethanol, based on glyceryl dibehenate and sunflower oil |
| OG | ETOH99.5 | GV 5% | Olive oil 80% Glyceryl dibehenate 15% | OG_GV_ETOH99.5 | Oleogel with Galium verum extract in absolute ethanol, based on glyceryl dibehenate and olive oil |
| OG | ETOH70 | OB 5% | Isopropyl myristate 80% Glyceryl dibehenate 15% | OG_OB_ETOH70 | Oleogel with basil extract in 70% ethanol, based on glyceryl dibehenate and isopropyl myristate |
| OG | ETOH70 | BS 5% | Sunflower oil 80% Glyceryl dibehenate 15% | OG_BS_ETOH70 | Oleogel with frankincense extract in 70% ethanol, based on glyceryl dibehenate and sunflower oil |
| OG | ETOH70 | SNF 5% | Diethylene glycol monoethyl ether 80% Glyceryl dibehenate 15% | OG_SNF_ETOH70 | Oleogel with elderflower extract in 70% ethanol, based on glyceryl dibehenate and diethylene glycol monoethyl ether |
| OG | ETOH99.5 | BS_OB_SNF_GV 1.25:1.25:1.25:1.25% | Sunflower oil 20% Olive oil 20% Isopropyl myristate 20% Diethylene glycol monoethyl ether 20% Glyceryl dibehenate 15% | OG_BS_OB_SNF_GV_ETOH99.5 | Oleogel with 4 plant extracts in absolute ethanol |
| OG | ETOH70 | BS_OB_SNF_GV 1.25:1.25:1.25:1.25% | Sunflower oil 20% Olive oil 20% Isopropyl myristate 20% Diethylene glycol monoethyl ether 20% Glyceryl dibehenate 15% | OG_BS_OB_SNF_GV_ETOH70 | Oleogel with 4 plant extracts in 70% ethanol |
| HG | ETOH99.5 | OB 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_OB_ETOH99.5 | Hydrogel with basil extract in absolute ethanol |
| HG | ETOH99.5 | BS 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_BS_ETOH99.5 | Hydrogel with frankincense extract in absolute ethanol |
| HG | ETOH99.5 | SNF 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_SNF_ETOH99.5 | Hydrogel with elderflower extract in absolute ethanol |
| HG | ETOH99.5 | GV 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_GV_ETOH99.5 | Hydrogel with Galium verum extract in absolute ethanol |
| HG | ETOH70 | OB 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_OB_ETOH70 | Hydrogel with basil extract in 70% ethanol |
| HG | ETOH70 | BS 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_BS_ETOH70 | Hydrogel with frankincense extract in 70% ethanol |
| HG | ETOH70 | SNF 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_SNF_ETOH70 | Hydrogel with elderflower extract in 70% ethanol |
| HG | ETOH99.5 | SNB 5% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_SNB_ETOH99.5 | Hydrogel with elder bark extract in absolute ethanol |
| HG | ETOH70 | BS_OB_SNF_GV 1.25:1.25:1.25:1.25% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_BS_OB_SNF_GV_ETOH70 | Hydrogel with 4 plant extracts in 70% ethanol |
| HG | ETOH99.5 | BS_OB_SNF_GV 1.25:1.25:1.25:1.25% | Purified water 60% Poloxamer 407 25% Glycerol 10% | HG_BS_OB_SNF_GV_ETOH99.5 | Hydrogel with 4 plant extracts in absolute ethanol |
| Pre-burn | N/A | Baseline | N/A | Pre-burn_baseline | Control for the healthy skin |
| Untreated_ Burn | N/A | Untreated_Burn | N/A | Untreated_burn_control | Control for the untreated burn |
| OG | N/A | OG_base | Sunflower oil 21.5% Olive oil 21.5% Isopropyl myristate 21.5% Diethylene glycol monoethyl ether 21.5% Glyceryl dibehenate 15% | OG_base | Control oleogel (without bioactive component) |
| HG | N/A | HG_base | Purified water 65% Poloxamer 407 25% Glycerol 10% | HG_base | Control hydrogel (without bioactive component), based on Poloxamer 407 and glycerol, purified water |
| Paper | Plant (Extract Concentration) | Solvent Used | Formulation | Base Gel Component and Concentrations (w/w) | Wound Induction Method | Outcomes | Inflammatory Response |
|---|---|---|---|---|---|---|---|
| Mallik et al., 2010 [31] | Boswellia serrata (5%, 10%, 15%) | Ethanol | Cream | Base (N/A%) | In vivo Excision wound model | Boswellia serrata 15% w/w extract showed superior wound healing compared to control group. Wound contraction day 16: 98.02% vs. 64.13% in control. | 5% Boswellia serrata: Moderate inflammation reduction. Day 8: 10% and 15% formulations—significant inflammatory marker inhibition. Day 12: 15% formulation nearly eliminated inflammatory response. Day 16: 15% formulation had 98.02% wound contraction, complete resolution of inflammation. |
| Antonescu et al., 2021 [23] | Ocimum basilicum (5%) Trifolium pratense (5%) | Hydro- alcoholic 70% | Hydrogel | Distilled water 78.0% Ethanol 10.0% Glycerin 10.0% Carbopol 940 1.0% Triethanolamine 1.0% | In vivo Burn wound model with metallic device | Days 13–16: Near-complete wound closure (100%) in hydrogel group; control had slower healing and incomplete remodeling. | EOT-based hydrogel—significant reduction in inflammation vs. control group. Day 13—complete wound healing in EOT gel group vs. partial healing in control. |
| Vuletic et al., 2022 [47] | Galium verum (20%) | Ethanol 70% | Mucoadhesive oral gel | Triethanolamine 0.7% Carbomer 934 (N/A%) Propylene glycol (N/A%) Sodium benzoate (N/A%) Purified water (N/A%) | In vivo Glacial acetic acid for oral ulcer | Days 3 and 6: Marked increase in collagen deposition, improved tissue remodeling. Day 6: Significantly lower COX-2 expression. Day 10: Complete ulcer closure with remodeled connective tissue. | GVL Gel—significantly higher wound contraction across all time points compared to control. Day 13—GVL Gel 95.1% healing vs. 81% in control |
| Mota et al., 2020 [44] | Sambucus nigra flower extract | Methanol | 1. Placebo Gel 2. Free Plant Extract (0.15%) 3. PLGA Nanoparticle-Loaded Plant Extract (0.21%) 4. PCL Nanoparticle-Loaded Plant Extract (0.165%) | Carbopol 940 0.5% Methyl-4-hydroxybenzoate 0.2% Propyl-4-hydroxybenzoate 0.02% NaOH 0.2% Distilled water QS | In vivo Carrageenan-induced paw edema model | Moderate inflammation, slightly reduced edema compared to the untreated group. Mild fibroblast activation. Healing slightly better than control, no statistical significance. | 41.2% paw edema reduction. Reduced inflammation, less strong effect compared to nanoformulated extracts or diclofenac. |
| Ali Khan et al., 2020 [29] | Ocimum basilicum leaves (5%) | Methanol 70% | Emulgel (5%) | Carbopol-934: 0.25%; Triethanolamine (TEA) Tween 80 0.30% Span 80 0.45% Liquid paraffin 3.75 Propylene glycol: 3.50 Methyl paraben: 0.01% Distilled water 50% | In vivo Carrageenan-induced paw edema model | Day 4: Severe inflammation, necrosis present. Days 8–12: Reduced inflammation, fibroblast proliferation, collagen remodeling. Day 16: Complete re-epithelialization, minimal inflammation, organized tissue. | 75% wound contraction at day 8 with emuglel, similar to SSD; 25–30% lower TNF-α, IL-6 vs. untreated group. Reduced inflammation, enhanced fibroblast proliferation, and improved collagen deposition. |
| Dubey et al., 2017 [30] | Ocimum basilicum aerial parts (5%) | Hydro- alcoholic | Ointment | Lipophilic base ointment (N/A%) | In vivo Carrageenan-induced paw edema model | Day 8: Inflammation decreased, and fibroblast activation began in the extract-treated group. Day 12: Collagen deposition increased, while the control still showed inflammation. Day 16: The extract-treated wounds had organized collagen fibers and complete epithelialization, whereas healing was slower in the control group. | Extract-based cream significantly reduced inflammation vs. control (simple vehicle). Faster wound contraction (97.97% vs. 88.17% control), shorter epithelialization (18 vs. 22 days control), lower inflammatory infiltrates in study group. Plant extract modulates the inflammatory response, accelerating tissue regeneration. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roșca, O.J.; Nistor, A.; Coneac, G.H.; Olariu, I.V.; Cotan, A.-M.; Racoviceanu, R.; Heredea, E.R.; Ciudoiu, A.; Didea, G.; Lupou, C.M.; et al. Wound Healing Properties of Plant-Based Hydrogel and Oleogel Formulations in a Rat Scald Burn Model. Pharmaceutics 2025, 17, 597. https://doi.org/10.3390/pharmaceutics17050597
Roșca OJ, Nistor A, Coneac GH, Olariu IV, Cotan A-M, Racoviceanu R, Heredea ER, Ciudoiu A, Didea G, Lupou CM, et al. Wound Healing Properties of Plant-Based Hydrogel and Oleogel Formulations in a Rat Scald Burn Model. Pharmaceutics. 2025; 17(5):597. https://doi.org/10.3390/pharmaceutics17050597
Chicago/Turabian StyleRoșca, Oana Janina, Alexandru Nistor, Georgeta Hermina Coneac, Ioana Viorica Olariu, Ana-Maria Cotan, Roxana Racoviceanu, Elena Rodica Heredea, Adelin Ciudoiu, Gabriela Didea, Camelia Mihaela Lupou, and et al. 2025. "Wound Healing Properties of Plant-Based Hydrogel and Oleogel Formulations in a Rat Scald Burn Model" Pharmaceutics 17, no. 5: 597. https://doi.org/10.3390/pharmaceutics17050597
APA StyleRoșca, O. J., Nistor, A., Coneac, G. H., Olariu, I. V., Cotan, A.-M., Racoviceanu, R., Heredea, E. R., Ciudoiu, A., Didea, G., Lupou, C. M., Borcan, F., Hoinoiu, T., Dehelean, C. A., Vlaia, L. L., & Șoica, C. M. (2025). Wound Healing Properties of Plant-Based Hydrogel and Oleogel Formulations in a Rat Scald Burn Model. Pharmaceutics, 17(5), 597. https://doi.org/10.3390/pharmaceutics17050597








.jpg)





