Enhanced Ocular Drug Delivery of Dexamethasone Using a Chitosan-Coated Soluplus®-Based Mixed Micellar System
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
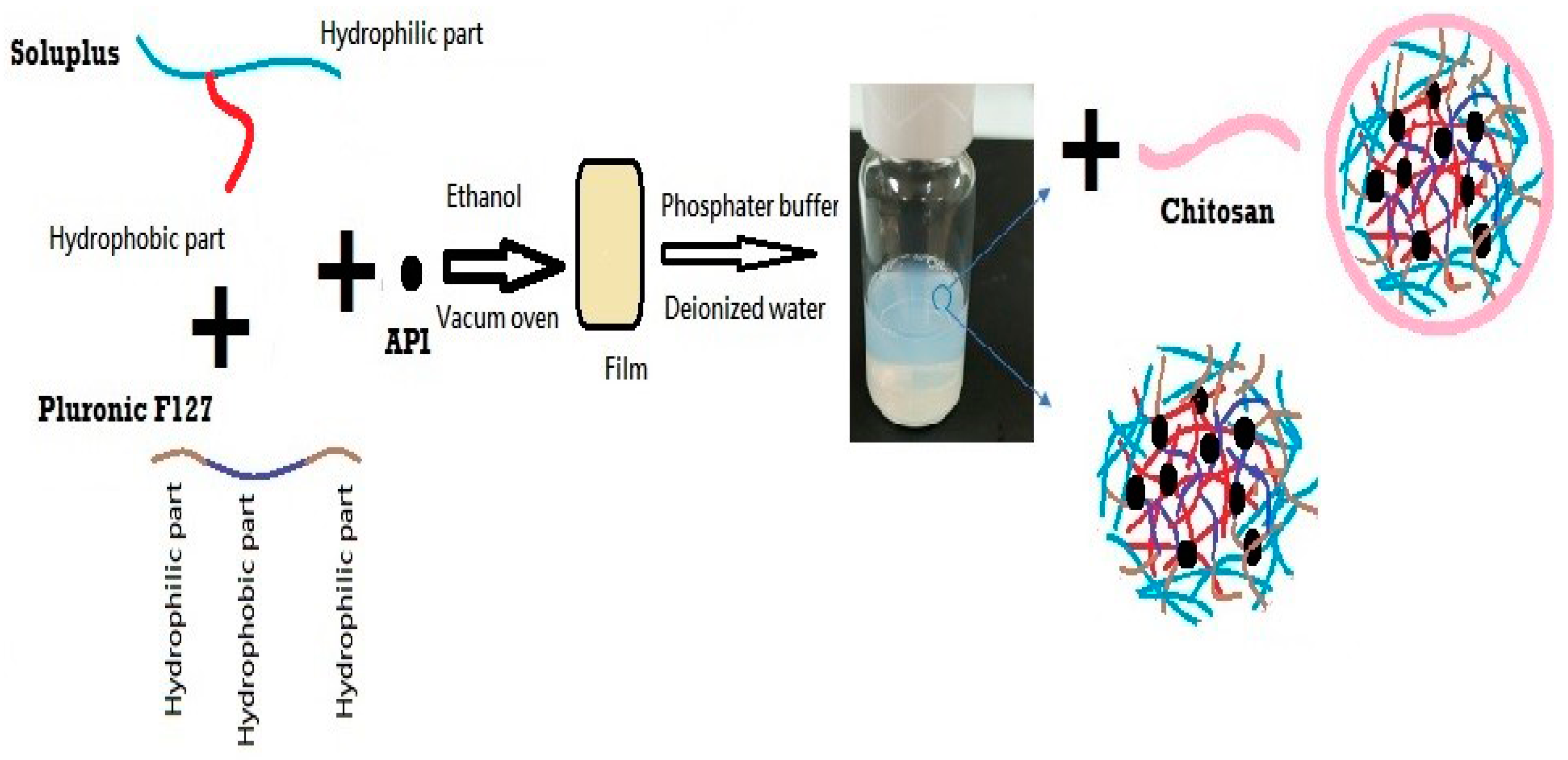
2.2.1. Preparation of DEX-MM and DEX-CMM
2.2.2. Characterization of DEX-MM and DEX-CMM
2.2.3. Analytical Method
2.2.4. Calculation of the Encapsulation Efficiency (%EE)
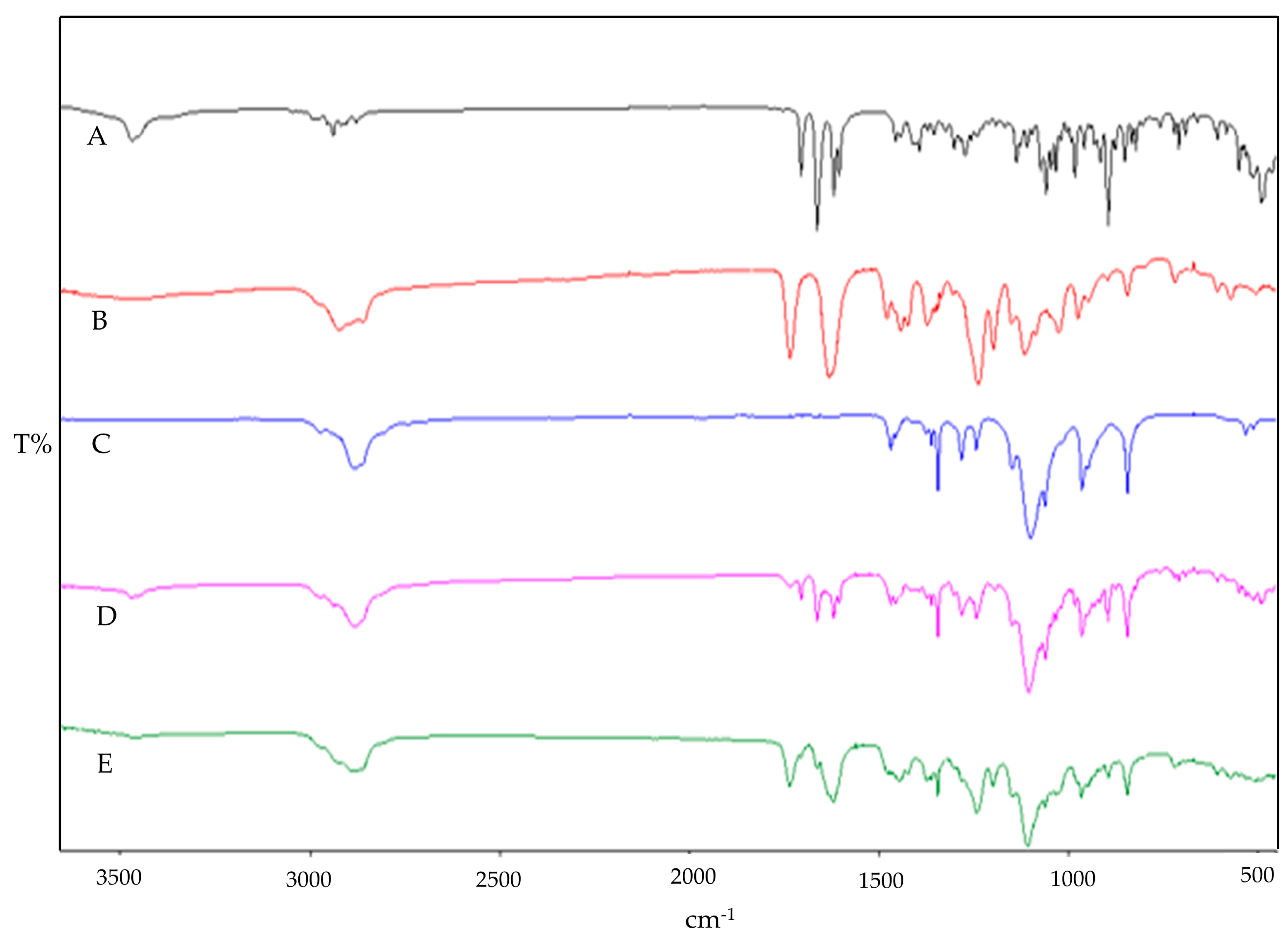
2.2.5. ATR-FTIR Studies
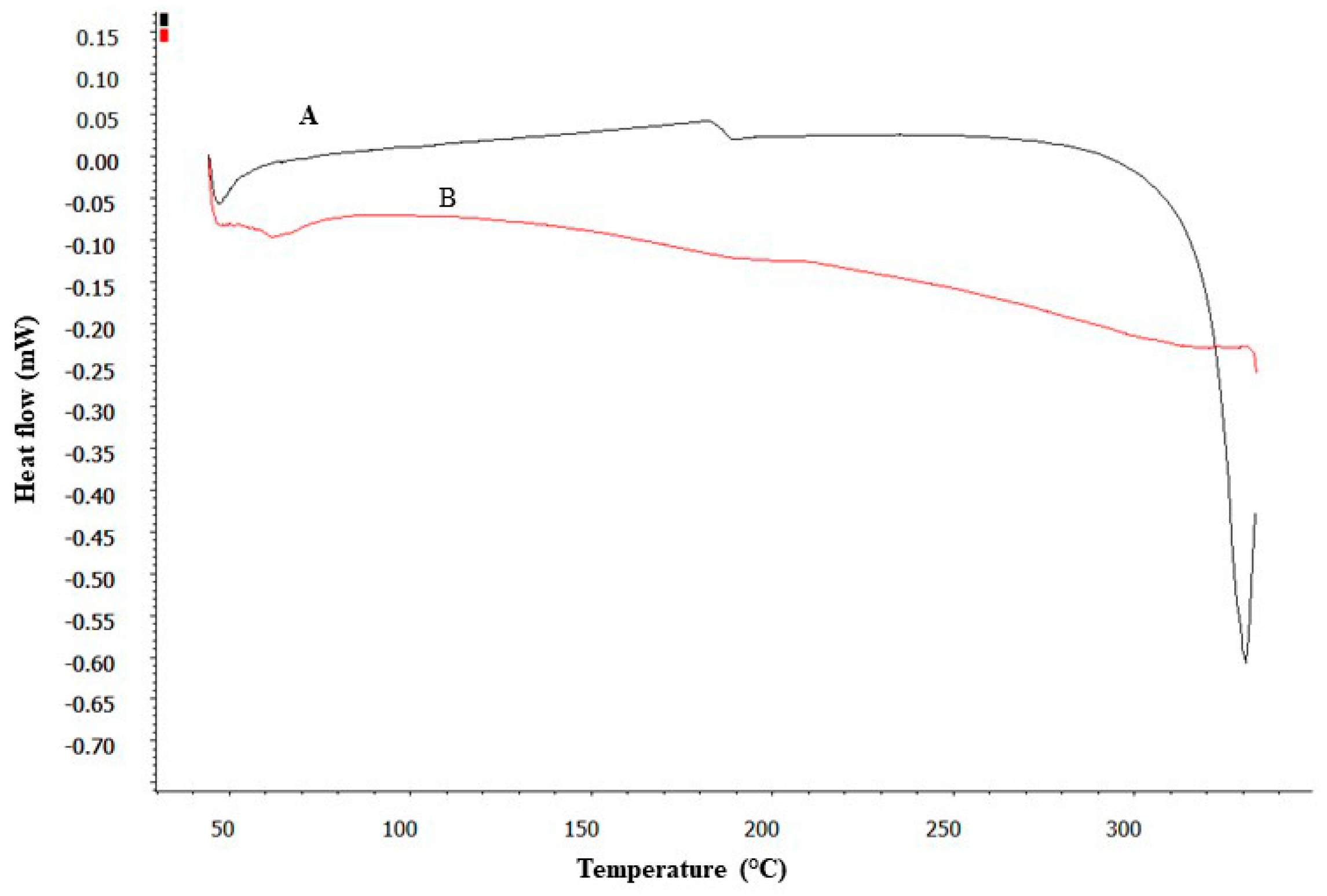
2.2.6. DSC Studies
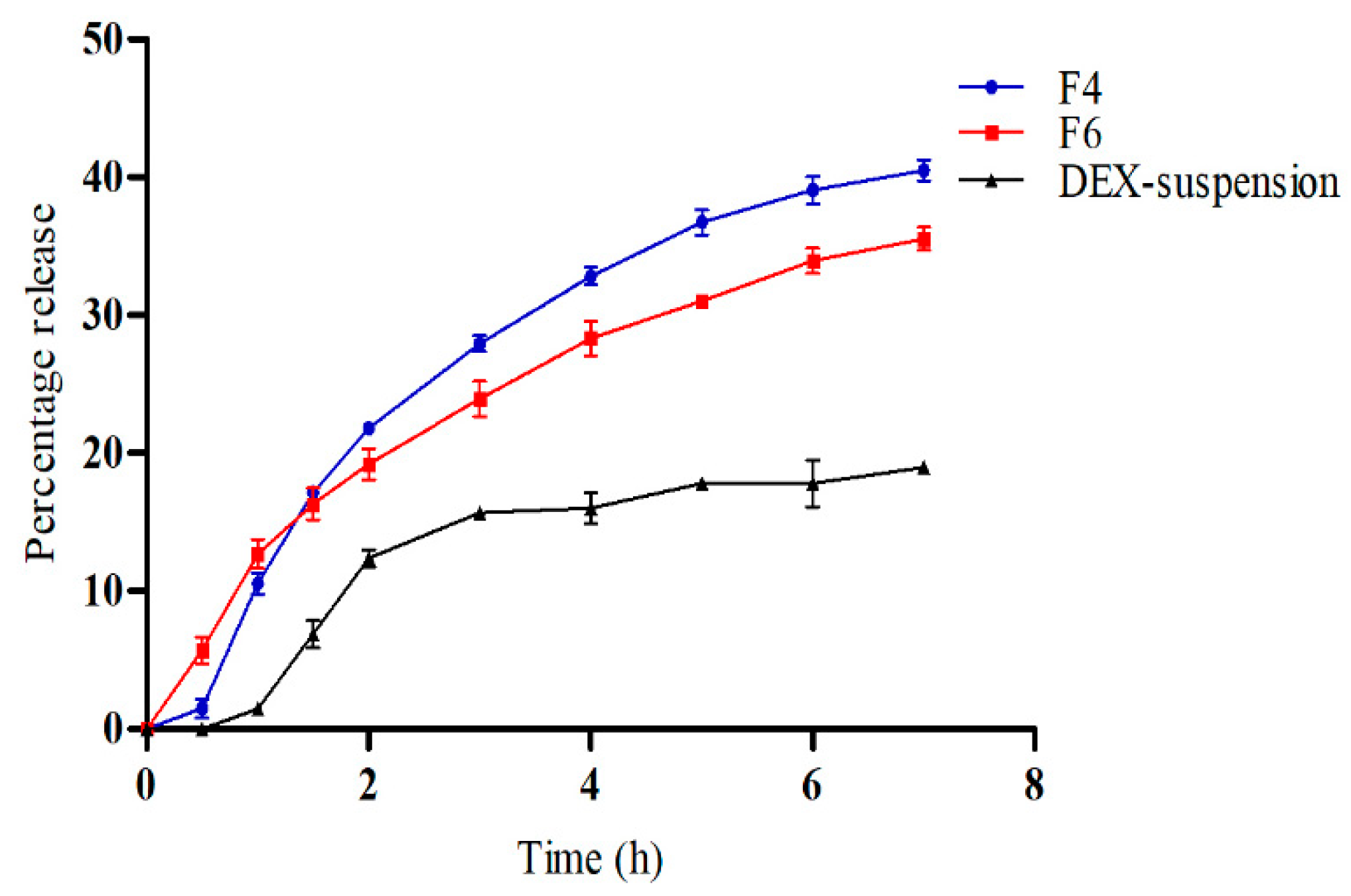
2.2.7. In Vitro Release Study
2.2.8. Ex Vivo Permeation Study
2.2.9. Morphology Observation by Transmission Electron Microscope (TEM)
2.2.10. Physical Stability Test
2.2.11. In Vitro Ocular Irritation Testing Using the Hen’s Egg Test–Chorioallantoic Membrane (HET-CAM)
2.2.12. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Physicochemical Characterization
3.2. ATR-FTIR Studies
3.3. DSC Studies
3.4. In Vitro Drug Release
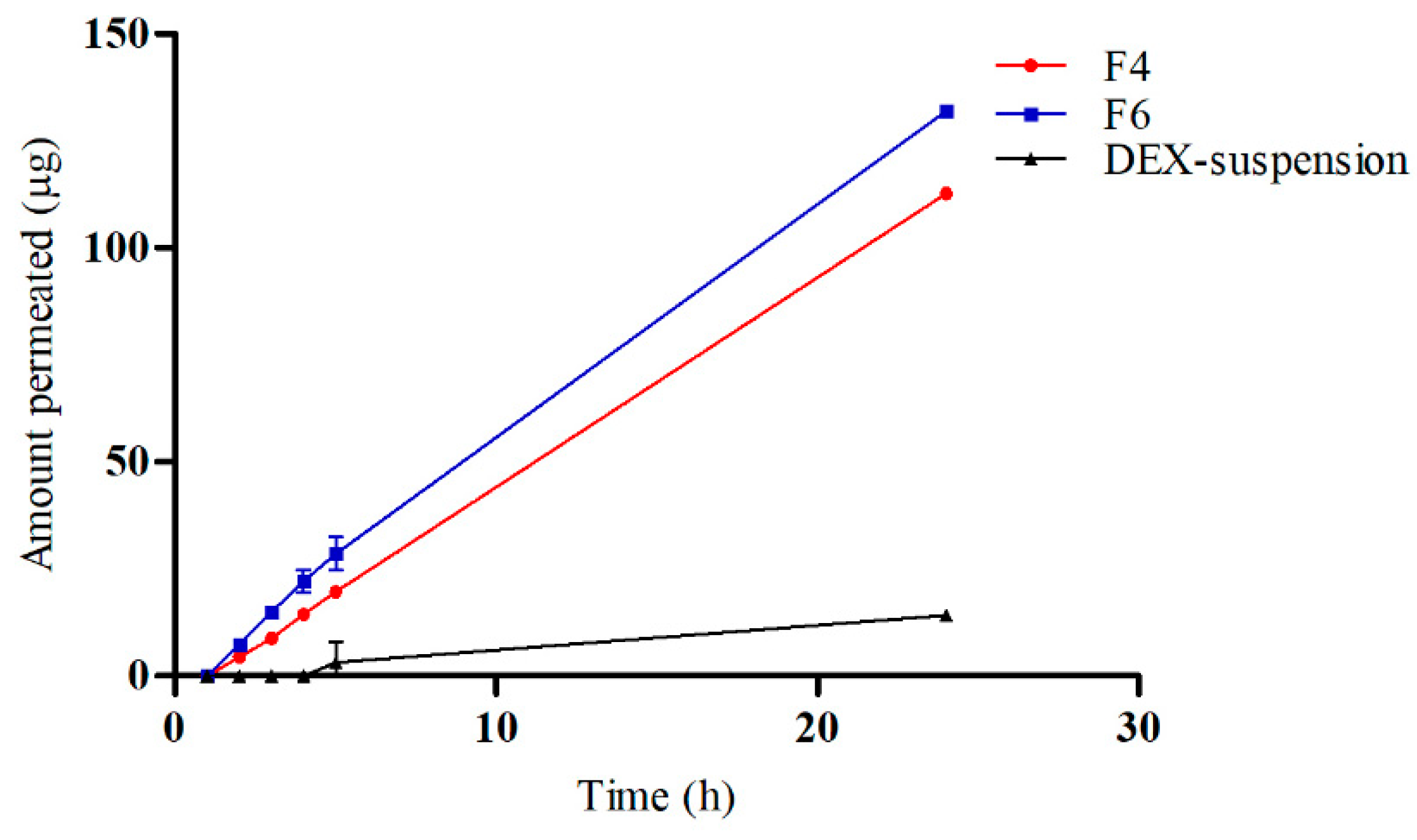
3.5. Ex Vivo Drug Permeation
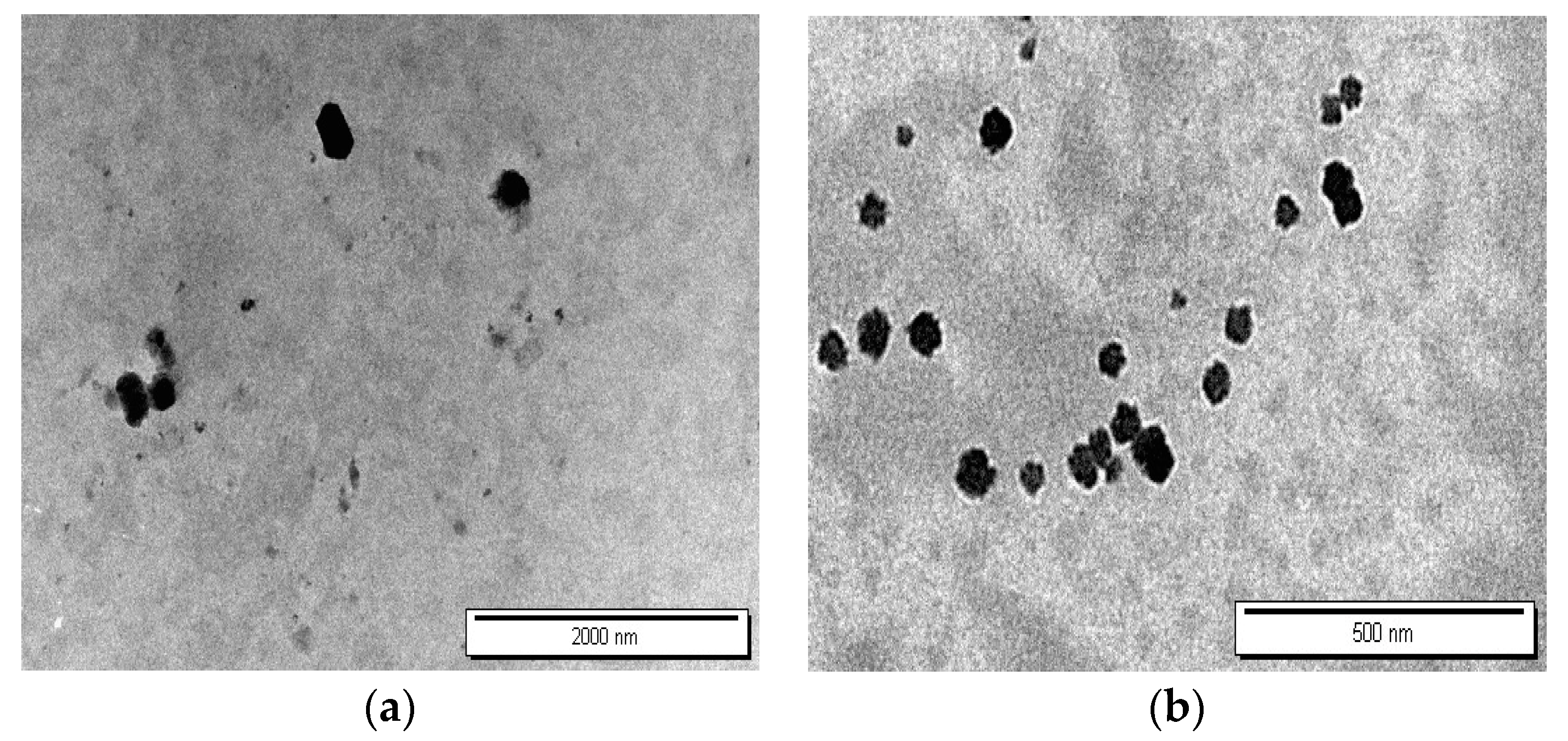
3.6. TEM Characterization of Nanoparticle Morphology
3.7. Physical Stability of DEX-CMM
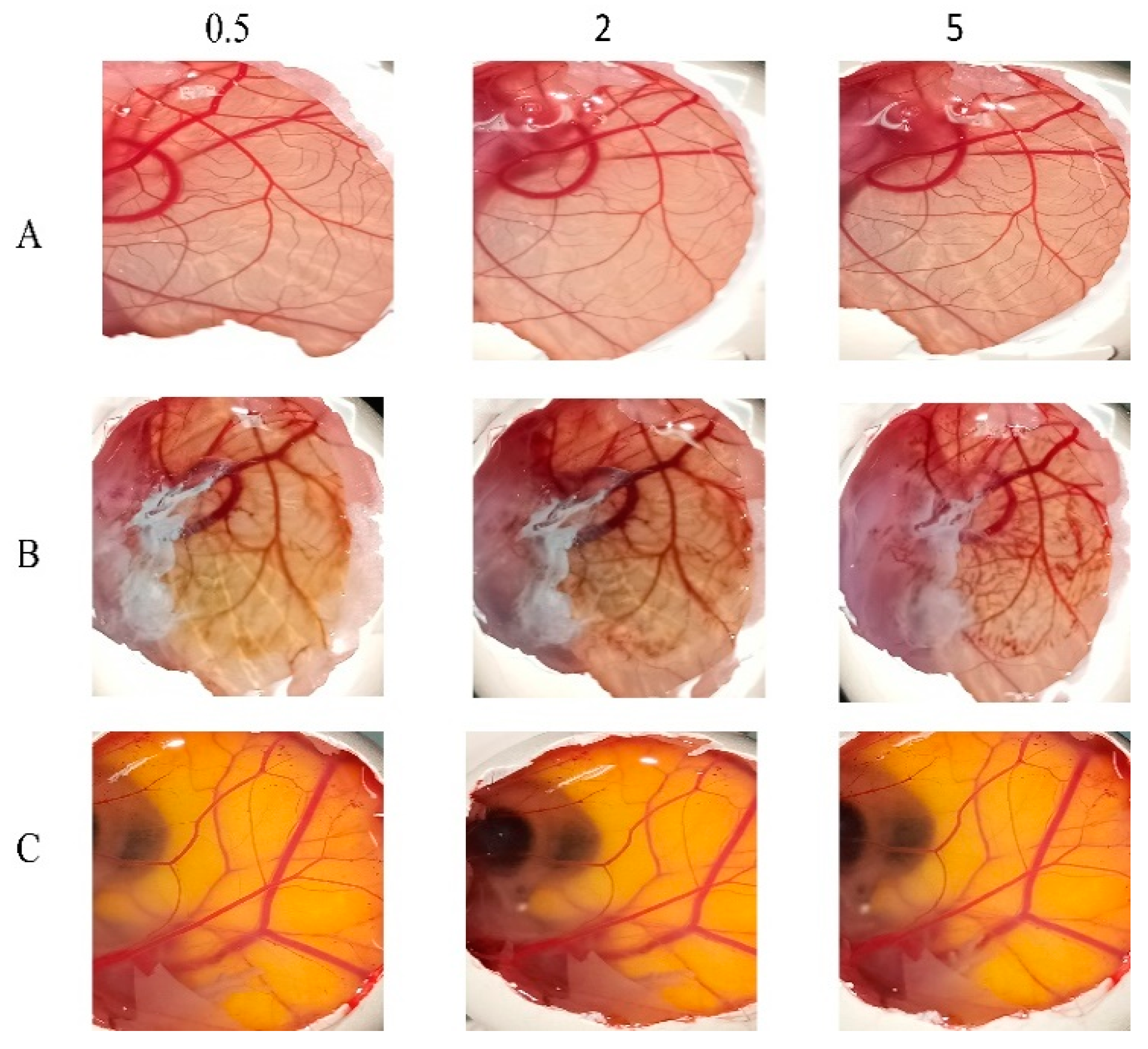
3.8. In Vitro Ocular Irritation Testing Using HET-CAM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hellinen, L.; Hongisto, H.; Ramsay, E.; Kaarniranta, K.; Vellonen, K.S.; Skottman, H.; Ruponen, M. Comparison of barrier properties of outer blood-retinal barrier models–Human stem cell-based models as a novel tool for ocular drug discovery. Eur. J. Pharm. Biopharm. 2023, 184, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Li, S.; Xu, M.; Zhong, Y.; Fan, W.; Xu, J.; Zhou, T.; Ji, J.; Ye, J.; Yao, K. Polymer-and lipid-based nanocarriers for ocular drug delivery: Current status and future perspectives. Adv. Drug Deliv. Rev. 2023, 196, 114770. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Navas, A.; López-Cano, J.J.; Johnson, M.; Sigen, A.; Vicario-De-La-Torre, M.; Andrés-Guerrero, V.; Tai, H.; Wang, W.; Bravo-Osuna, I.; Herrero-Vanrell, R. Smart biodegradable hydrogels: Drug-delivery platforms for treatment of chronic ophthalmic diseases affecting the back of the eye. Int. J. Pharm. 2024, 649, 123653. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.J.; González-Méijome, J.M.; Oliveira, M.E.C.R.; Carracedo, G.; Lúcio, M. Recent advances and strategies for nanocarrier-mediated topical therapy and theranostic for posterior eye disease. Adv. Drug Deliv. Rev. 2024, 210, 115321. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Bhardwaj, K.; Bansal, M. Polymeric Micelles as Drug Delivery System: Recent Advances, Approaches, Applications and Patents. Curr. Drug Saf. 2024, 19, 163–171. [Google Scholar] [CrossRef]
- Butt, F.; Devonport, H. Treatment of non-infectious posterior uveitis with dexamethasone intravitreal implants in a real-world setting. Clin. Ophthalmol. 2023, 17, 601–611. [Google Scholar] [CrossRef]
- Won, J.; Kang, J.; Kang, W. Comparative Ocular Pharmacokinetics of Dexamethasone Implants in Rabbits. J. Ocul. Pharmacol. Ther. 2024. [Google Scholar] [CrossRef]
- Gaballa, S.A.; Kompella, U.B.; Elgarhy, O.; Alqahtani, A.M.; Pierscionek, B.; Alany, R.G.; Abdelkader, H. Corticosteroids in ophthalmology: Drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv. Transl. Res. 2021, 11, 866–893. [Google Scholar] [CrossRef]
- Qi, Q.; Wei, Y.; Zhang, X.; Guan, J.; Mao, S. Challenges and strategies for ocular posterior diseases therapy via non-invasive advanced drug delivery. J. Control. Release 2023, 361, 191–211. [Google Scholar] [CrossRef]
- Valtari, A.; Posio, S.; Toropainen, E.; Balla, A.; Puranen, J.; Sadeghi, A.; Ruponen, M.; Ranta, V.-P.; Vellonen, K.-S.; Urtti, A.; et al. Comprehensive ocular and systemic pharmacokinetics of dexamethasone after subconjunctival and intravenous injections in rabbits. Eur. J. Pharm. Biopharm. 2024, 198, 114260. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Mohammadinejad, R.; Uzieliene, I.; Nabavi, N.; Dehshahri, A.; Garcia-Couce, J.; Tavakol, S.; Moghassemi, S.; Dadashzadeh, A.; Makvandi, P. Dexamethasone: Insights into pharmacological aspects, therapeutic mechanisms, and delivery systems. ACS Biomater. Sci. Eng. 2022, 8, 1763–1790. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Amin, M.M.; Sayed, S. Ocular drug delivery: A comprehensive review. AAPS PharmSciTech 2023, 24, 66. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; Omar, S.; Nasereddin, J.; Hamed, R.; Obeidat, R. Design of Colon-Targeted Drug Delivery of Dexamethasone: Formulation and in vitro characterization of Solid Dispersions. Heliyon 2024, 10, e34212. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Villanueva, J.; de la Villa, P.; Herrero-Vanrell, R.; Bravo-Osuna, I.; Guzmán-Navarro, M. Useful Role of a New Generation of Dexamethasone, Vitamin E and Human Serum Albumin Microparticles in the Prevention of Excitotoxicity Injury in Retinal Ocular Diseases. Pharmaceutics 2024, 16, 406. [Google Scholar] [CrossRef] [PubMed]
- Thareja, A.; Leigh, T.; Hakkarainen, J.J.; Hughes, H.; Alvarez-Lorenzo, C.; Fernandez-Trillo, F.; Blanch, R.J.; Ahmed, Z. Improving corneal permeability of dexamethasone using penetration enhancing agents: First step towards achieving topical drug delivery to the retina. Int. J. Pharm. 2024, 660, 124305. [Google Scholar] [CrossRef]
- Mishra, A.; Shaima, K.A.; Sindhu, R.K. Novel Drug Delivery System for Ocular Target. In Nanotechnology and Drug Delivery; Jenny Stanford Publishing: New Delhi, India, 2024; Volume 29, pp. 205–249. ISBN 9781003430407. [Google Scholar]
- Binkhathlan, Z.; Ali, R.; Alomrani, A.H.; Abul Kalam, M.; Alshamsan, A.; Lavasanifar, A. Role of polymeric micelles in Ocular Drug Delivery: An overview of decades of Research. Mol. Pharm. 2023, 20, 5359–5382. [Google Scholar] [CrossRef]
- Kaushal, N.; Kumar, M.; Singh, A.; Tiwari, A.; Tiwari, V.; Pahwa, R. A review on polymeric nanostructured micelles for the ocular inflammation-main emphasis on uveitis. Pharm. Nanotechnol. 2023, 11, 34–43. [Google Scholar] [CrossRef]
- Zheng, Q.; Ge, C.; Li, K.; Wang, L.; Xia, X.; Liu, X.; Mehmood, R.; Shen, J.; Nan, K.; Chen, W.; et al. Remote-controlled dexamethasone-duration on eye-surface with a micelle-magnetic nanoparticulate co-delivery system for dry eye disease. Acta Pharm. Sin. B 2024, 14, 205–249. [Google Scholar] [CrossRef]
- Paganini, V.; Chetoni, P.; Di Gangi, M.; Monti, D.; Tampucci, S.; Burgalassi, S. Nanomicellar eye drops: A review of recent advances. Expert Opin. Drug Deliv. 2024, 21, 381–397. [Google Scholar] [CrossRef]
- Nirmal, J.; Sathe, P.; Kailasam, V.; Sankar, S.; Hiremath, M.S.; Kumara, B.N.; Prasad, K.S.; Das, D. Biocompatible nanomicelles to improve the therapeutic outcome of water insoluble drugs to treat anterior segment diseases. Investig. Ophthalmol. Vis. Sci. 2024, 65, 3987. [Google Scholar]
- Attia, M.S.; Elshahat, A.; Hamdy, A.; Fathi, A.M.; Emad-Eldin, M.; Ghazy, F.-E.S.; Chopra, H.; Ibrahim, T.M. Soluplus® as a solubilizing excipient for poorly water-soluble drugs: Recent advances in formulation strategies and pharmaceutical product features. J. Drug Deliv. Sci. Technol. 2023, 84, 104519. [Google Scholar] [CrossRef]
- Pignatello, R.; Corsaro, R.; Bonaccorso, A.; Zingale, E.; Carbone, C.; Musumeci, T. Soluplus® polymeric nanomicelles improve solubility of BCS-class II drugs. Drug Deliv. Transl. Res. 2022, 12, 1991–2006. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Sousa Lobo, J.M. Applications of poloxamers in ophthalmic pharmaceutical formulations: An overview. Expert Opin. Drug Deliv. 2013, 10, 1223–1237. [Google Scholar] [CrossRef]
- Wang, T.J.; Rethi, L.; Ku, M.Y.; Nguyen, H.T.; Chuang, A.E.Y. A review on revolutionizing ophthalmic therapy: Unveiling the potential of chitosan, hyaluronic acid, cellulose, cyclodextrin, and poloxamer in eye disease treatments. Int. J. Biol. Macromol. 2024, 273, 132700. [Google Scholar] [CrossRef]
- Albarqi, H.A.; Garg, A.; Ahmad, M.Z.; Alqahtani, A.A.; Walbi, I.A.; Ahmad, J. Recent Progress in Chitosan-Based Nanomedicine for Its Ocular Application in Glaucoma. Pharmaceutics 2023, 15, 681. [Google Scholar] [CrossRef] [PubMed]
- Dmour, I. Absorption enhancement strategies in chitosan-based nanosystems and hydrogels intended for ocular delivery: Latest advances for optimization of drug permeation. Carbohydr. Polym. 2024, 343, 122486. [Google Scholar] [CrossRef]
- Alkholief, M.; Kalam, M.A.; Raish, M.; Ansari, M.A.; Alsaleh, N.B.; Almomen, A.; Ali, R.; Alshamsan, A. Topical Sustained-Release Dexamethasone-Loaded Chitosan Nanoparticles: Assessment of Drug Delivery Efficiency in a Rabbit Model of Endotoxin-Induced Uveitis. Pharmaceutics 2023, 15, 2273. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, A.R.N.; Detsi, A. Nanoparticles for ocular drug delivery: Modified and non-modified chitosan as a promising biocompatible carrier. Nanomedicine 2019, 14, 1889–1909. [Google Scholar] [CrossRef] [PubMed]
- Lenze, M.; Benedetti, M.D.; Roco, J.; Ramírez, P.; Blanco, R.; Yaceszen, S.; Corrales, C.; Wikinski, S.; Gutiérrez, M. Advancing ocular safety research: A comprehensive examination of benzocaine acute exposure without animal testing. Toxicol. Lett. 2024, 394, 138–145. [Google Scholar] [CrossRef]
- Fine-Shamir, N.; Dahan, A. Ethanol-based solubility-enabling oral drug formulation development: Accounting for the solubility-permeability interplay. Int. J. Pharm. 2024, 653, 123893. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Sun, X. Ethanol injection method for liposome preparation. In Liposomes: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2023; pp. 65–70. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, C.; Wei, F.; Lv, H. Improved solubility and oral bioavailability of apigenin via Soluplus/Pluronic F127 binary mixed micelles system. Drug Dev. Ind. Pharm. 2017, 43, 1276–1282. [Google Scholar] [CrossRef]
- Klemetsrud, T.; Kjøniksen, A.L.; Hiorth, M.; Jacobsen, J.; Smistad, G. Polymer coated liposomes for use in the oral cavity–a study of the in vitro toxicity, effect on cell permeability and interaction with mucin. J. Liposome Res. 2018, 28, 62–73. [Google Scholar] [CrossRef]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Fathi-Karkan, S.; Ramsheh, N.A.; Arkaban, H.; Narooie-Noori, F.; Mirinejad, S.; Roostaee, M.; Sargazi, S.; Barani, M.; Shadman, S.M.; Althomali, R.H.; et al. Nanosuspensions in ophthalmology: Overcoming challenges and enhancing drug delivery for eye diseases. Int. J. Pharm. 2024, 658, 124226. [Google Scholar] [CrossRef] [PubMed]
- Pepić, I.; Filipović-Grčić, J.; Jalšenjak, I. Interactions in a nonionic surfactant and chitosan mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2008, 327, 95–102. [Google Scholar] [CrossRef]
- Pepić, I.; Hafner, A.; Lovrić, J.; Pirkić, B.; Filipović-Grcčić, J. A Nonionic Surfactant/Chitosan Micelle System in an Innovative Eye Drop Formulation. J. Pharm. Sci. 2010, 99, 4317–4325. [Google Scholar] [CrossRef]
- Chougale, R.; Patil, K.; Disouza, J.; Hajare, A.; Jadhav, N.; Kumbhar, P. Development of docetaxel-loaded (Soluplus®–PF108) mixed micelles vacuum foam—Dried product for improved stability and melanoma treatment by QbD approach. Future J. Pharm. Sci. 2024, 10, 54. [Google Scholar] [CrossRef]
- Mubeen, I.; Abbas, G.; Shah, S.; Assiri, A.A. Conjugated Linoleic Acid–Carboxymethyl Chitosan Polymeric Micelles to Improve the Solubility and Oral Bioavailability of Paclitaxel. Pharmaceutics. 2024, 16, 342. [Google Scholar] [CrossRef]
- Arshad, A.; Arshad, S.; Alamgeer; Mahmood, A.; Asim, M.H.; Ijaz, M.; Irfan, H.M.; Rubab, M.; Ali, S.; Hashmi, A.R. Zeta potential changing self-nanoemulsifying drug delivery systems: A newfangled approach for enhancing oral bioavailability of poorly soluble drugs. Int. J. Pharm. 2024, 655, 123998. [Google Scholar] [CrossRef]
- Santos, W.M.; Nóbrega, F.P.; Andrade, J.C.; Almeida, L.F.; Conceição, M.M.; Medeiros, A.C.D.; Medeiros, F.D. Pharmaceutical compatibility of dexamethasone with excipients commonly used in solid oral dosage forms. J. Therm. Anal. Calorim. 2021, 145, 361–378. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Al-Naji, I.; Adwan, S.; Al-Remawi, M.; Shubair, M. Enhancement of curcumin solubility using a novel solubilizing polymer Soluplus®. J. Pharm. Innov. 2020, 16, 558–565. [Google Scholar] [CrossRef]
- Branca, C.; Khouzami, K.; Wanderlingh, U.; D’Angelo, G. Effect of intercalated chitosan/clay nanostructures on concentrated pluronic F127 solution: A FTIR-ATR, DSC and rheological study. J. Colloid Interface Sci. 2018, 517, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Racaniello, G.F.; Balenzano, G.; Arduino, I.; Iacobazzi, R.M.; Lopalco, A.; Lopedota, A.A.; Sigurdsson, H.H.; Denora, N. Chitosan and Anionic Solubility Enhancer Sulfobutylether-β-Cyclodextrin-Based Nanoparticles as Dexamethasone Ophthalmic Delivery System for Anti-Inflammatory Therapy. Pharmaceutics 2024, 16, 277. [Google Scholar] [CrossRef] [PubMed]
- Sutariya, V.; Tur, J.; Kelly, S.; Halasz, K.; Chapalamadugu, K.C.; Nimbalkar, R.; Pathak, Y.V.; Weigel, R.; Daviau, T.; Webb, T.; et al. Nanodrug delivery platform for glucocorticoid use in skeletal muscle injury. Can. J. Physiol. Pharmacol. 2018, 96, 681–689. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Wilson, B.K.; Sinko, P.J.; Prud’homme, R.K. Encapsulation and controlled release of a camptothecin prodrug from nanocarriers and microgels: Tuning release rate with nanocarrier excipient composition. Mol. Pharm. 2021, 18, 1093–1101. [Google Scholar] [CrossRef]
- Gharge, V.; Pawar, P. Recent trends in chitosan based nanotechnology: A reference to ocular drug delivery system. Int. J. Ophthalmol. Visual Sci. 2017, 2, 98–105. [Google Scholar] [CrossRef]
- Zoe, L.H.; David, S.R.; Rajabalaya, R. Chitosan nanoparticle toxicity: A comprehensive literature review of in vivo and in vitro assessments for medical applications. Toxicol. Rep. 2023, 11, 83–106. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its derivatives for ocular delivery formulations: Recent advances and developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef]
- Bulatao, B.P.; Nalinratana, N.; Jantaratana, P.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Lutein-loaded chitosan/alginate-coated Fe3O4 nanoparticles as effective targeted carriers for breast cancer treatment. Int. J. Biol. Macromol. 2023, 242, 124673. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Karati, D.; Singh, S.; Prajapati, B.G. Chitosan-based nanomedicine in the management of age-related macular degeneration: A review. Curr. Nanomed. 2024, 14, 13–27. [Google Scholar] [CrossRef]
- Chary, P.S.; Bansode, A.; Rajana, N.; Bhavana, V.; Singothu, S.; Sharma, A.; Guru, S.K.; Bhandari, V.; Mehra, N.K. Enhancing breast cancer treatment: Comprehensive study of gefitinib-loaded poloxamer 407/TPGS mixed micelles through design, development, in-silico modelling, In-Vitro testing, and Ex-Vivo characterization. Int. J. Pharm. 2024, 657, 124109. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, Y.; Wang, J.; Liu, H.; Wei, G.; Lu, W.; Liu, Y. Combination of PEGylation and Cationization on Phospholipid-Coated Cyclosporine Nanosuspensions for Enhanced Ocular Drug Delivery. In ACS Applied Materials & Interfaces; ACS Publications: Washington, DC, USA, 2024. [Google Scholar] [CrossRef]
- Alany, R.G.; Rades, T.; Nicoll, J.; Tucker, I.G.; Davies, N.M. W/O microemulsions for ocular delivery: Evaluation of ocular irritation and precorneal retention. J. Control. Release 2006, 111, 145–152. [Google Scholar] [CrossRef]
- Ozdemir, S.; Uner, B. Prolonged release niosomes for ocular delivery of loteprednol: Ocular distribution assessment on dry eye disease induced rabbit model. AAPS PharmSciTech 2024, 25, 119. [Google Scholar] [CrossRef]
| Formulation | Soluplus® | PF127 | CS | DEX | Medium |
|---|---|---|---|---|---|
| F1 | 10 | 25 | 0 | 1 | PBS |
| F2 | 10 | 25 | 0.5 | 1 | PBS |
| F3 | 10 | 25 | 1 | 1 | PBS |
| F4 | 10 | 25 | 0 | 1 | DDW |
| F5 | 10 | 25 | 0.5 | 1 | DDW |
| F6 | 10 | 25 | 1 | 1 | DDW |
| Code | Size (nm) | PDI | Zeta Potential (mV) | %EE |
|---|---|---|---|---|
| F1 | 76.9 ± 0.8 | 0.160 ± 0.004 | −3.17 ± 0.74 | 81.32 ± 1.96 |
| F2 | 92.2 ± 0.2 | 0.139 ± 0.007 | −0.59 ± 1.34 | 75.62 ± 2.93 |
| F3 | 107 ± 0.1 | 0.125 ± 0.007 | 3.20 ± 1.87 | 76.90 ± 1.02 |
| F4 | 69.1 ± 0.6 | 0.053 ± 0.007 | −4.37 ± 0.57 | 89.44 ± 1.37 |
| F5 | 118.5 ± 1.5 | 0.157 ± 0.004 | 0.26 ± 0.36 | 85.50 ± 1.90 |
| F6 | 151.9 ± 1 | 0.168 ± 0.003 | 35.96 ± 2.13 | 91.95 ± 0.46 |
| Time (d) | 1 | 3 | 7 | 10 | 15 |
|---|---|---|---|---|---|
| Size (nm) | 151.9 ± 1 | 148.4 ± 1.2 | 151.4 ± 1.7 | 149.2 ± 0.5 | 145.9 ± 3.1 |
| PDI | 0.168 ± 0.003 | 0.166 ± 0.001 | 0.187 ± 0.007 | 0.182 ± 0.008 | 0.197 ± 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adwan, S.; Al-Akayleh, F.; Qasmieh, M.; Obeidi, T. Enhanced Ocular Drug Delivery of Dexamethasone Using a Chitosan-Coated Soluplus®-Based Mixed Micellar System. Pharmaceutics 2024, 16, 1390. https://doi.org/10.3390/pharmaceutics16111390
Adwan S, Al-Akayleh F, Qasmieh M, Obeidi T. Enhanced Ocular Drug Delivery of Dexamethasone Using a Chitosan-Coated Soluplus®-Based Mixed Micellar System. Pharmaceutics. 2024; 16(11):1390. https://doi.org/10.3390/pharmaceutics16111390
Chicago/Turabian StyleAdwan, Samer, Faisal Al-Akayleh, Madeiha Qasmieh, and Teiba Obeidi. 2024. "Enhanced Ocular Drug Delivery of Dexamethasone Using a Chitosan-Coated Soluplus®-Based Mixed Micellar System" Pharmaceutics 16, no. 11: 1390. https://doi.org/10.3390/pharmaceutics16111390
APA StyleAdwan, S., Al-Akayleh, F., Qasmieh, M., & Obeidi, T. (2024). Enhanced Ocular Drug Delivery of Dexamethasone Using a Chitosan-Coated Soluplus®-Based Mixed Micellar System. Pharmaceutics, 16(11), 1390. https://doi.org/10.3390/pharmaceutics16111390









