Abstract
Background/Objectives: Paediatric Obstructive Sleep Apnoea (OSA) is characterised by recurrent episodes of upper airway obstruction during sleep, manifesting as snoring, intermittent oxygen desaturation, and frequent nocturnal awakenings. Standard treatments include surgical interventions, pharmacological therapies, intranasal corticosteroids, and oral montelukast. However, significant variability exists across studies regarding dosage and outcome assessment. This literature review systematically evaluated clinical evidence regarding the efficacy and safety of intranasal corticosteroids and oral montelukast for treating sleep-disordered breathing and its primary underlying condition, adenoid hypertrophy, in otherwise healthy children. Methods: The MEDLINE (PubMed), Scopus, and Web of Science databases were systematically searched up to 13 February 2025, using tailored search terms combining keywords and synonyms related to paediatric OSA, adenoidal hypertrophy, corticosteroids, montelukast, and randomised controlled trials. Owing to variability in outcome measures, Fisher’s method for p-value combination was employed to enable a comprehensive comparison of drug effects. Results: Available evidence shows that intranasal corticosteroids (mometasone, beclometasone, budesonide, fluticasone, and flunisolide), either as monotherapy or in combination with other agents, consistently lead to clinical and instrumental improvements in adenoid hypertrophy and related respiratory symptoms, with a generally favourable safety profile. Combining montelukast with intranasal corticosteroids appears to offer superior benefits compared with monotherapy. Nevertheless, the reviewed studies varied widely in dosage, treatment duration, design, and sample size. The reported side effects are mostly mild; however, long-term studies are lacking to establish the complete safety of these treatments in children. Conclusions: Intranasal corticosteroids and oral montelukast effectively and safely manage adenoid hypertrophy and mild-to-moderate OSA symptoms in children. Nonetheless, the heterogeneity of study designs necessitates larger prospective trials with standardised protocols and more extended follow-up periods to draw more robust conclusions. Future studies should aim to stratify treatment outcomes based on OSA severity and duration to tailor therapeutic approaches better.
1. Introduction
Obstructive Sleep Apnoea (OSA) is a disorder characterised by recurrent episodes of upper airway obstruction during sleep, associated with snoring, noisy breathing, intermittent desaturation, and nocturnal awakenings [1]. OSA affects approximately 3% of preschool- and school-aged children [2]. The most common cause is adenotonsillar hypertrophy (ATH) [2]. Other risk factors include obesity, craniofacial abnormalities, and neuromuscular diseases, although they are less common [2]. Children with OSA may present with habitual snoring, restless sleep, mouth breathing, and attention and mood disorders more frequently [3].
The gold standard for diagnosis is overnight Polysomnography (PSG). However, owing to the limited availability of paediatric sleep laboratories, screening questionnaires have been developed to identify children at risk of OSA, such as the Paediatric Sleep Questionnaire (PSQ) and OSA-18 [4,5]. An anatomical evaluation of the upper airway is required to complete the diagnostic assessment. In particular, adenoid size is assessed through nasopharyngeal endoscopy and/or radiological imaging [6,7].
The treatment of paediatric OSA is selected based on the severity of the clinical polysomnographic profile and the underlying causes [8]. In moderate-to-severe forms, adenotonsillectomy is the first-line treatment for significant ATH [9]. Medical treatment is available in mild-to-moderate cases, or when surgery is contraindicated. Specifically, local and systemic anti-inflammatory therapies may have therapeutic roles in paediatric OSA [9,10]. Intranasal corticosteroids and leukotriene antagonists, such as oral montelukast, may improve symptoms and reduce polysomnographic severity indices [11].
Intranasal corticosteroids reduce nasal mucosal oedema and inflammatory mediator production (IL-6, IL-1β, and TNF-α) [12] and decrease adenoid volume [12]. Montelukast inhibits the cysLT1 receptor, reduces eosinophilic infiltration and oedema in lymphoid tissues [13], decreases the expression of COX-2 and 5-lipoxygenase, and reduces circulating levels of IL-8 and high-sensitivity CRP [14,15].
Literature from the past decade suggests that intranasal corticosteroids can improve symptoms related to adenoid hypertrophy (AH). A meta-analysis of eight RCTs demonstrated that mometasone significantly reduced nasal obstruction and the adenoid-to-choana ratio (A/C ratio) [16]. Another meta-analysis, which included five trials (mometasone furoate, fluticasone propionate, and budesonide), also conducted on adults, revealed a high risk of bias and considerable methodological heterogeneity [17].
Oral montelukast, evaluated in four RCTs involving children with mild to moderate OSA, significantly improved polysomnographic parameters [15]. However, a review of five trials, three on intranasal corticosteroids and two on montelukast, with the Apnoea–Hypopnea index (AHI) as the primary outcome, demonstrated insufficient evidence supporting corticosteroid efficacy and only short-term benefits for montelukast, without long-term safety data [9]. Another study involving children aged 1 to 16 years confirmed the short-term positive effects of intranasal corticosteroids and montelukast on desaturation indices and oxygen saturation [18].
The combined use of montelukast and intranasal corticosteroids, as well as montelukast monotherapy, also appears effective in the short-term management of paediatric OSA (reduction in AHI) [11] and in the treatment of AH [19]. In an analysis of 17 RCTs involving children aged 2–14 years with OSA, mometasone combined with montelukast, budesonide, and montelukast monotherapy showed superiority over placebo in improving AHI [20]. Similarly, combined treatment with mometasone and montelukast was more effective than mometasone alone in improving symptoms and adenoid-to-nasopharynx ratio (A/N ratio) [21].
A traditional meta-analysis approach may be complex or less informative because of the significant heterogeneity in the primary outcomes reported in previous studies. Fisher’s Combined Probability Test enables aggregating results based on p-values, avoiding methodological issues associated with data transformation into a single standard measure. Moreover, this method determines the overall significance of the association between the intervention and outcome [22,23].
2. Materials and Methods
2.1. Search Strategy and Study Selection
We searched three major databases—MEDLINE (PubMed), Scopus, and Web of Science—for English-language studies published up to 13 February 2025. MeSH terms and text words (and their combinations and truncated synonyms) were used. Customised search terms were adapted for each database, combining keywords and synonyms: (children OR pediatric) AND (obstructive sleep apnoea OR sleep-disordered breathing OR adenoidal hypertrophy) AND (corticosteroids OR montelukast) AND (randomised controlled study). The search terms used are presented in Appendix A.
Studies were eligible for inclusion if they were
- (1)
- The paediatric population.
- (2)
- Assessed the effects of intranasal corticosteroids and/or montelukast in treating Obstructive Sleep Apnoea (OSA), sleep disordered breathing (SDB), and adenoid hypertrophy (AH).
- (3)
- Employed a randomised controlled design.
- (4)
- Reported the clinical and/or instrumental outcomes.
We excluded studies that did not meet these criteria, non-English publications, animal studies, and studies involving surgical or non-pharmacological interventions. No restrictions were placed on geographical region, sex, or specific age ranges within the paediatric population. No particular outcome domains were predefined to allow the inclusion of all clinically relevant findings compatible with the scope of the review.
Duplicate articles were removed before screening the abstracts. Two authors (MZ and LN) reviewed the titles and abstracts independently, followed by full-text assessments. Discrepancies were resolved through discussion or adjudication by a third reviewer (AP). The reference lists of the included studies were screened for additional eligible articles. Ethical approval was not required because the study did not involve human participants. The systematic review followed the PRISMA guidelines [24].
2.2. Statistics
The AI tools (QUADAS-2 and QUADAS-C, ASReview, Abstrackr, RobotAnalyst, EPPI-Reviewer, and DistillerSR) are a promising innovation for systematic review practices [25,26]. During the revision process, Generative AI (ChatGPT 4 and 4o, Plus plan, OpenAI) [27,28] was used to explore appropriate statistical approaches for synthesising heterogeneous p-values derived from RCT.
To comprehensively compare the effect of the drug versus control across these studies, considering the variety of outcome measures and the predominant availability of p-values, we employed the probability combination method known as Fisher’s [22,23].
Using Fisher’s method, the statistic , where is the p-value from the ith test, and k is the total number of combined tests. The resulting variables X2 approximately follow a chi-square distribution with 2k degrees of freedom, calculated as twice the number of studies included. By comparing this X2 value against critical values from the chi-square distribution with 2k degrees of freedom (as found in the chi-square distribution tables), an overall combined p-value was obtained [29].
The AI tool also assisted in designing a datasheet for applying Fisher’s method of p-value combinations. The detailed procedure for calculating the combined p-values using Fisher’s method in Microsoft® Excel® for Microsoft 365 MSO (Version 2503) is provided in Appendix B, Table A1.
The main p-values reported by each study were selected by identifying the value(s) representing the primary endpoint or the most directly comparable outcome across the trials. For each study, at least one p-value for the difference between the treatment and control groups was considered. This approach allowed for an overall estimation of treatment effectiveness, even when individual studies reported distinct p-values without sufficiently homogeneous data. The authors critically reviewed and approved all the analyses, decisions, and interpretations.
3. Results
In total, 170 articles were identified, of which 46 were duplicates. Consequently, 124 studies were included (Figure 1). After the selection process, 40 articles were included in the final analysis. After full-text analysis, an additional 10 studies were examined and excluded. Overall, 30 studies met the inclusion criteria.
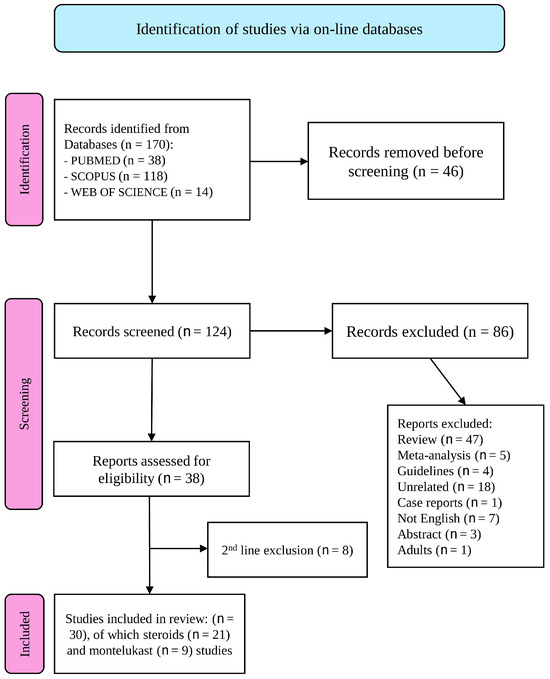
Figure 1.
The PRISMA flow diagram [24] visually represents the study selection process and number of studies included at each stage. For more information, visit: http://www.prisma-statement.org/ (accessed on 16 February 2025).
Table 1 summarises the controlled clinical trials, including randomised [30,31,32,33], double-blind (placebo-controlled) [30,31,32], crossover [34], and multi-centre studies [31]. Some studies included larger sample sizes (n > 200) [31], whereas others enrolled fewer than 30 participants [34]. Inclusion criteria were clearly defined, targeting children with AH [30,34,35,36] associated with obstructive respiratory symptoms [31,32] and otitis media with effusion (OME) [35,36].

Table 1.
Clinical studies on treating adenoid hypertrophy and sleep-disordered breathing symptoms with mometasone furoate (MF).
The exclusion criteria were recent steroid use [30,31,32,33,34,35,36], craniofacial anomalies or malformations [30,31,32,33,35,36], and allergic or immunodeficiency conditions [30,33,34,36].
All studies measured at least one objective parameter (e.g., adenoid volume reduction via endoscopy, radiography, A/C ratio), obstructive AHI—oAHI—on PSG (etc.), and subjective symptoms (snoring and mouth breathing). Many studies have employed validated tools such as the PedsQL or the Glasgow Children’s Benefit Inventory [31]. One study extensively analysed polysomnographic parameters (AHI and ODI) [32]. Studies with greater methodological robustness (multi-centre, large sample size, double-blind) and others that were smaller [34] or shorter in duration [36].
The dosage of mometasone furoate typically ranges from 50 to 100 µg per nostril per day (100–200 µg/day total), administered once or twice daily. Some protocols begin with two puffs per nostril per day [35], others with a single puff [30], occasionally followed by alternate-day maintenance or reduced dosing [30,35]. The treatment duration varied from 6 weeks [36] to 3–4 months [30], 6 months [35], or a one-year follow-up [33]. Some studies have reported reduced sample sizes and significant dropout rates [32]. Most comparisons were between mometasone furoate and placebo (saline solution) [30,31,32,33,34,35].
The most significant studies [30] showed decreased endoscopic or radiological obstruction (A/C ratio). One study did not find statistically significant differences in adenoid volume, but noted symptomatic improvement [34]. Most studies reported reduced severity scores for symptoms (apnoea, snoring, and nasal obstruction) in the mometasone group compared to controls [30,32,35,36]. One study documented a significant reduction in polysomnographic parameters (oAHI and ODI) [32]. In cases of OME, some studies demonstrated quicker radiological and clinical resolution/improvement in patients treated with mometasone [35,36]. Other studies reported decreased symptom severity scores and reduced perceived need for adenoidectomy [31]. Relevant adverse events were rare; one study noted mild epistaxis [30], but mometasone use was generally tolerated.
Considering the statistical analysis of p-values reported, due to the very small individual p-values (except for one study with p = 0.51) [33], the sum of log-transformed p-values was extremely low. The resulting χ2 statistic far exceeded the critical threshold of a χ2 distribution with 2k degrees of freedom. Notably, one study reporting a non-significant p-value for the ‘resolution of SDB symptoms’ (p = 0.51) still indicated significant differences in other outcomes, such as quality of life scores (p = 0.001) [33].
The studies presented in Table 2 [37,38,39] aimed to evaluate the effectiveness of nasal beclomethasone spray in reducing AH and associated obstructive symptoms such as nasal obstruction, snoring, and related issues (e.g., OME). All studies were randomised and double-blind, with some including a crossover study component.

Table 2.
Clinical studies on the treatment of adenoid hypertrophy and sleep-related breathing disorder symptoms with beclometasone.
Despite some differences in the study design (duration, dosage, and outcome measures), there is a general trend toward moderate methodological quality. Specifically, the enrolled sample sizes were relatively small, ranging from a minimum of 20 children [39] to a maximum of 60 children [38], with notable dropout rates during follow-up [39].
Regarding therapeutic protocols, all three studies used nasal beclomethasone (generally 200–400 µg/day) for periods ranging from 8 weeks [37] to 24 weeks [38]. One study began with a higher initial dose, followed by a maintenance phase at a reduced dose [38]. The follow-up durations varied from 8 weeks [37] to 24 weeks [39]. In one case, the authors followed the children for up to 100 weeks [38]. However, only a part of the protocol was double-blind and controlled.
Concerning efficacy, one study reported modest and sometimes non-significant reductions in adenoid size documented through instrumental examinations [37]. Another study demonstrated a 29% reduction in the adenoid area after 24 weeks of therapy [39], significantly improving obstructive symptoms.
In the study by Lepcha et al. [37], p-values ranged from 0.71 to 1.00 for most variables, with the lowest value of 0.07 for endoscopy; thus, a p-value of 0.07 was selected for the endoscopic endpoint. Criscuoli et al. [38] reported p-values of 0.71 (nasal obstruction), 0.30 (snoring), and 0.39 (nasal discharge), along with values greater than 0.05 for endoscopic and radiographic findings. Since all values exceeded 0.05, a representative p-value of 0.30 (snoring) was selected. Demain and Goetz [39] reported significantly lower p-values for the A/C ratio reduction (p = 0.0002 right, p = 0.0006 left) and around 0.05 for symptomatic scores. The smallest and most statistically significant p-value for adenoid reduction was 0.0002.
Although two studies reported non-statistically significant results for the primary endpoints [37,38], the pronounced effect observed by Demain and Goetz [39] (p = 0.0002) strongly influenced Fisher’s combined analysis, resulting in a highly significant global p-value (p < 0.001). Fisher’s combined p-value analysis suggested an overall benefit of beclometasone compared to controls (p < 0.001). However, discrepancies among studies (two showing no significant differences [37,38]), one demonstrating significance [39], and the variability of outcomes warrant caution in clinical interpretation.
The studies presented in Table 3 evaluate the effects of beclometasone on AH and symptoms related to SDB [40,41,42]. These studies exhibited good methodological quality: prospective, randomised, placebo-controlled, and double blind. Inclusion and exclusion criteria were clearly defined, with sufficiently large populations ranging from 60 [40] to 100 children [41]. However, not all studies have provided long-term follow-up [40,42].

Table 3.
Clinical studies on treating adenoid hypertrophy and sleep-related breathing disorder symptoms with budesonide.
Budesonide was administered at dosages between 64 µg [41] and 128 µg [40] per day via nasal spraying [41] or transnasal nebulisation. The treatment duration ranged from six weeks [40] to approximately 14 weeks [41]. One study included additional observation weeks to monitor potential recurrence [41]. In one case, a two-week double-blind phase (with nebulised budesonide or placebo) was followed by a twelve-week open-label phase using a nasal spray [41].
Clinically, the results confirmed significant improvement in SDB, including reduced nasal obstruction and snoring [40] and decreased AHI scores in mild-to-moderate OSA cases [42]. Positive impacts on the quality of life of patients and their families have also been reported [40]. Additionally, some studies have documented a reduction in the adenoid-to-nasopharynx ratio [42].
From a safety and tolerability perspective, the adverse events reported were mild and clinically insignificant. The study by Gudnadottir et al. included various outcome measures, such as OSA-18, sleep disturbances, caregiver concerns, and quality of life, reporting a p-value of 0.0014 for the OSA-18 score (considered the most comprehensive variable) [40].
Hong et al. reported p-values < 0.001 for the nasal obstruction index (NOI), snoring, and nasal discharge, while the adenoidectomy rate had a p-value of 0.002 [41]. Considering NOI as the primary endpoint, we assumed an indicative p value of 0.0005 (between 0.0001 and 0.001, representing p < 0.001). Kheirandish-Gozal and Gozal reported p-values of <0.0001 for oAHI, 0.001 for the arousal index (RAI), 0.004 for Nadir SpO2, and again <0.0001 for the adenoid/nasopharynx (N/P) ratio. Typically, oAHI is the primary endpoint in OSA studies; hence, a reference value of p = 0.0001 (equivalent to p < 0.0001) was selected for calculations [42]. For a χ2 distribution with 6 degrees of freedom, a value of approximately 46.76 corresponds to a p-value of <0.0001, favouring budesonide efficacy compared to placebo in the considered studies.
The three clinical trials presented in Table 4 investigate the treatment of AH and related symptoms of SDB using fluticasone [43,44,45]. Each of these studies follows an RCT methodology, but differs in design (open-label [43] vs. blinded [44]).

Table 4.
Clinical studies on the treatment of adenoid hypertrophy and sleep breathing disorder symptoms with fluticasone.
In the study by Esteitie et al., conducted on 24 children aged 2 to 12 years diagnosed with OSA (AHI ≥ 5/hour), nasal spray fluticasone furoate (55 µg per nostril, once daily) was administered for only two weeks compared to an untreated control group [43]. Although prospective, this study was not blinded, introducing potential observer bias. The primary outcome was evaluating an inflammatory marker (IL-6) in adenoid tissue. Children treated with steroids showed a significant reduction in IL-6 compared to untreated controls, while other mediators (IL-10, TGF-β, TNF, etc.) showed no relevant changes [43].
The study by Brouillette et al. is a triple-blind randomised trial involving 25 children with mild-to-moderate OSA [44]. Treatment consisted of nasal fluticasone at 200 µg/day for one week, reduced to 100 µg/day for the subsequent five weeks, compared to a placebo. Results showed a significant reduction in AHI and ODI in the steroid-treated group [44].
Demirhan et al. evaluated the efficacy of fluticasone propionate (400 µg/day) nasal drops for eight weeks compared to saline solution in 45 children aged 4 to 16 years with indications for adenoidectomy [45]. The primary objective parameter was the A/C ratio measured endoscopically and an obstructive symptom index. After eight weeks, the steroid-treated group showed substantial improvement in the A/C ratio and symptoms, with three-quarters of patients avoiding surgery compared to 80% progressing to surgery in the control group.
Overall, methodological quality appears heterogeneous. Sample sizes were modest [43,44], and observation periods were relatively brief [43]. However, the rigorous triple-blind design of Brouillette’s study lends credibility to its findings [44]. Demirhan’s study supports the potential utility of intranasal steroids in quickly reducing AH [45]. Esteitie’s conclusions are limited, focusing on local inflammatory markers rather than long-term clinical effects [43].
Esteitie et al. reported p-values for various inflammatory markers (IL-6, IL-10, etc.), with the lowest, potentially significant value being p = 0.05 for IL-6. Despite being marginally significant, this value is chosen as a reference [43]. Brouillette et al. reported severity indices for OSA with p-values: AHI (p = 0.04), ODI (p = 0.03), arousal index (p = 0.05), and desaturation frequency (p = 0.030). As AHI is typically the primary endpoint in OSA studies, p = 0.04 was selected [44].
Demirhan et al. reported p < 0.05 for total symptom reduction and A/C ratio improvement without exact values provided. For calculation purposes, a conservative intermediate value of p = 0.02 was assumed [45]. A χ2 value of approximately 20.25 with 6 degrees of freedom corresponds to p ≈ 0.002. Thus, Fisher’s combined analysis indicates a significant overall effect (p < 0.01) favouring fluticasone treatment compared to control.
The two studies mentioned in Table 5 [46,47], while sharing the goal of evaluating the effectiveness of intranasal flunisolide in reducing AH and related obstructive symptoms, present differing methodological features.

Table 5.
Clinical studies on treating adenoid hypertrophy and sleep-related breathing disorder symptoms with flunisolide.
The study by Ciprandi et al. involved a sample of 178 children aged between 3 and 6 years, divided into a larger group treated with flunisolide (n = 139) and a control group receiving saline solution (n = 39) [46]. The treatment duration was eight weeks, with flunisolide dosage based on body weight, specifically 0.5 drops × kg per nostril, twice daily, administered via an aerosolisation device (Rinowash).
More than 70% of subjects in the treatment arm showed a significant reduction of adenoid tissue, compared to approximately 30% in the placebo group. Many children with grade IV AH improved to lower levels, thus avoiding surgery. However, randomisation appeared imbalanced, and it was unclear whether a genuine blinding procedure was applied [46].
The study by Varricchio et al., for which only the abstract is available, refers to a sample of 178 children with grade III or IV AH. The duration of flunisolide treatment was also eight weeks, with a longer follow-up (at 6 and 12 months post-treatment). Reported data indicate a statistically significant reduction in AH severity by the end of treatment (p < 0.01) [47]. The maintenance of benefit was more stable in allergic children (p < 0.05).
Each study reported multiple outcomes. We selected p1 = 0.02 [46] and p2 = 0.01 [47]. For a χ2 distribution with 4 degrees of freedom, a value of 17.03 corresponds to p < 0.001. The combination of these p-values derived from the two studies indicates a highly significant effect of flunisolide in reducing AH compared to controls [46,47].
Several clinical studies have focused on the treatment of AH and SDB with steroids in combination with other drugs (Table A2).
One study utilised a double-blind, double-dummy design involving 240 children, initially divided into two groups: mometasone furoate vs. placebo. Subsequently, non-responders were assigned to four combination groups with or without oxymetazoline. The results provide evidence of a significant reduction in the A/C ratio and an improvement in symptom scores (snoring, nasal congestion, rhinorrhoea) [48]. The efficacy appears to be enhanced in the presence of the nasal vasoconstrictor.
Another study compared the use of fluticasone spray with a repeated course of azithromycin in 39 children with AH and OSA symptoms [49]. Although a placebo group was lacking, the study design included two randomised active treatments. Overall efficacy was similar between the two treatments, with a slight advantage for the fluticasone-treated group in residual apnoea parameters (p = 0.020) [49]. However, the study had a small sample size (n = 39) and an open-label design. Therefore, while the data are encouraging, they should be interpreted cautiously.
Finally, Evangelisti et al. evaluated the effect of combined therapy with oral betamethasone and intranasal beclomethasone for one week, compared to intranasal beclomethasone alone in 28 children with severe OSAS [50]. The results showed significant differences in the improvement of oxygenation (mean and minimum SpO2) and acute symptoms, favouring the group receiving systemic steroids [50]. However, the absence of a placebo group reduces the experimental rigour.
The studies listed in Table 6 focus on the use of montelukast in children with AH and/or mild-to-moderate OSAS. These studies predominantly follow a randomised, placebo-controlled methodological design [13,51,52], sometimes with a double-blind approach [13,14,52,53]. The recruited samples are not always large [14,53], and the follow-up duration varies from one month [53] to three to four months [13,14,51,52].

Table 6.
Clinical studies on treating adenoid hypertrophy and sleep-related breathing disorder symptoms with montelukast.
Some research focuses on reducing AH and nasal obstruction symptoms [51,52]. Other studies have conducted polysomnographic assessments: AHI and ODI were decreased significantly in children treated with montelukast [13,14]. However, in Goldbart et al.’s study, the reduction in AHI did not reach full statistical significance (p = 0.07) [14], while the OAI and adenoid size showed significant improvement (p = 0.01). Conversely, in Kheirandish-Gozal et al.’s study [13], montelukast was associated with a marked reduction in AHI, with p < 0.0001, along with benefits in tonsillar and adenoidal size [13].
The double-blind, placebo-controlled study by Wang et al., conducted over four weeks, histopathologically assessed adenoid tissue. The group treated with montelukast showed reduced inflammatory infiltration. The limitations of these studies mainly lie in the small sample sizes (treated patients ranged from n = 23 to n = 30) [14,53] and relatively short follow-up periods (minimum four weeks and maximum three months) [14,52,53].
Naqi et al.’s study included several outcomes (endoscopy, radiography, snoring, mouth breathing, etc.) with p ≤ 0.0001 [51]. We select p = 0.0001 (endoscopy score or radiography). Goldbart et al.’s study reported p < 0.001 for the A/N ratio (the lowest) and p < 0.01 for OAI, while AHI, desaturation, and SaO2 showed p > 0.05 [14]. We choose p = 0.001 (A/N ratio). Kheirandish-Gozal et al.’s study reported p < 0.0001 for AHI and SpO2 nadir, p = 0.001 for ODI3%, etc. [13]. We take the most classical endpoint (AHI) with p = 0.0001. Shokouhi et al.’s study reported p < 0.0001 for total symptoms, mouth breathing, and sleep discomfort and p = 0.007 for snoring [52]. We use p = 0.0001 (total symptom score). Wang et al.’s study reported p = 0.029 (number of germinal centres), p = 0.024 (cystic cavities), and p = 0.040 (inflammatory infiltration) [53]. We select the lowest value, p = 0.024 (cystic cavities). With 10 degrees of freedom (2 × 5 studies), a χ2 value of 76.55 corresponds to a p-value < 0.0001.
The data suggest that, in paediatric settings (AH, OSAS, OME, etc.), the use of montelukast is associated with significant improvements in various parameters compared to placebo or control treatment.
The studies in Table 7 focus on administering montelukast with mometasone furoate, compared with using each drug alone or with a placebo. The studies exhibit some heterogeneity regarding experimental design, sample size, and the evaluation tools [54,55,56,57].

Table 7.
Clinical studies on treating adenoid hypertrophy and sleep-related breathing disorder symptoms with montelukast and mometasone.
Yang et al.’s study involved 195 children with mild-to-moderate OSAS, who were randomised into three groups: montelukast, mometasone, or a combination of both [54]. The study shows that after 12 weeks, all treated groups experienced a significant reduction in AHI and an improvement in minimum SpO2, with the most important benefit observed in the combination therapy group.
In the prospective randomised study by Ras et al., the researchers divided 100 patients into montelukast plus mometasone and mometasone alone [55]. The study did not include a placebo group. After three months of treatment and an additional three-month follow-up, the montelukast–mometasone combination resulted in a more marked reduction in adenoid volume than nasal steroid treatment alone.
The studies by TuhanıOğLu and Erkan and Ras et al., which assessed AH control through symptom scores, reported similar findings [55,57]. The combination of montelukast and mometasone significantly reduced adenoid volume and associated symptoms (snoring, obstruction, mouth breathing) compared to mometasone alone. TuhanıOğLu and Erkan also included groups treated with montelukast alone or receiving no treatment. Their findings indicate that any therapeutic option provides benefits compared to the absence of treatment [56].
Most studies propose a treatment duration of at least 8–12 weeks [54,55,56,57], sometimes extending follow-up to six months [55]. In Jafari et al.’s study, the analysis did not detect a statistically significant difference between the montelukast + mometasone group and the mometasone-alone group [56].
Yang et al.’s study reported p < 0.01 (AHI), p < 0.05 (Min SaO2, A/N ratio, mouth breathing), and p < 0.01 (snoring). We select p = 0.01 [54]. Ras et al.’s study reported improvements with p = 0.001, p = 0.008, p = 0.019, and up to p < 0.001 for the A/N ratio [55]. We take the lowest value, p = 0.0001, as a conservative estimate of p < 0.001. Jafari et al. showed no significant differences between montelukast + mometasone and Mometasone alone (clinical score: p = 0.117; A/N ratio: p = 0.161) [56]. We select p = 0.117. TuhanıOğLu and Erkan reported p < 0.05 for all treatments (montelukast, mometasone, and combination) vs. control [57]. Since they did not provide a precise p-value, we adopt p = 0.05 as a reasonable estimate. For a χ2 distribution with 10 degrees of freedom, approximately 42.83 corresponds to a p-value far below 0.0001.
From a statistical perspective, the combined results of the five studies strongly suggest that montelukast, mometasone, or their combination have a significant positive effect compared to controls. The presence of one study with a non-significant p-value (p = 0.117) does not invalidate the evidence provided by the other four, which report p < 0.05, some well below 0.001.
The studies reported in Table A3 differ from the previous ones in several aspects, making their inclusion in the study incompatible. Gelardi et al.’s study focuses on saline/iodine aerosol therapy [58]. In contrast, Tracy’s study (1998) evaluates the role of antibiotics in combination with beclometasone (or placebo) for the treatment of OME rather than AH [59].
Hood et al.’s study involves populations with sickle cell anaemia [60], where the primary endpoint is improving cognitive performance rather than reducing adenoidal obstruction or OSAS. Sobhy et al.’s study is dedicated to children who have already undergone adenoidectomy and focuses on the prevention of post-surgical recurrences [33].
The publications by Bilgili et al. lack a proper control group, and their primary focus is on eustachian tube dysfunction associated with adenoids rather than simple AH or OSAS [61]. Wang et al.’s study consists of retrospective or descriptive analyses of steroid use [62].
4. Discussion
The results of the study converge in demonstrating that intranasal steroid therapy (mometasone, beclometasone, budesonide, fluticasone, flunisolide), whether as monotherapy or in combination with other drugs, leads to clinical and instrumental improvement in AH and related symptoms compared to the control population. The safety profile of the studies is generally reasonable.
An alternative to steroids for paediatric OSA is oral montelukast. Furthermore, combining montelukast and intranasal corticosteroids offers more significant benefits than treating mild OSA alone. The studies also report mild side effects; however, long-term studies are lacking to verify the safety of these treatments in paediatric patients. Overall, the variety of experimental designs and sample sizes suggests a degree of caution in the clinical interpretation of the results.
Although heterogeneity in study design, population characteristics, and outcome measures limits definitive conclusions, the available evidence suggests that intranasal corticosteroids and montelukast are particularly effective in cases of mild-to-moderate OSA [32]. Similarly, montelukast demonstrated efficacy in studies focusing on mild-to-moderate cases [13,14,51,52,53]. Furthermore, combination therapy with mometasone and montelukast appears particularly beneficial in this subgroup [54,55], although not all trials confirmed superiority over monotherapy [56]. In terms of treatment duration, studies with longer follow-up (≥12 weeks) tend to report more substantial clinical and instrumental improvements with mometasone therapy [63]. Similarly, positive outcomes were documented following 12–16 weeks of montelukast treatment [13,52,54,55]. These trends underscore the potential influence of baseline disease severity and treatment duration on therapeutic outcomes.
It is essential to highlight the variability in clinical outcomes across the different studies. In particular, the reduction in adenoidal volume assessed endoscopically or radiologically, exhibited a variable range. In trials involving mometasone furoate, the A/C ratio decreased from 80% to 40% over six weeks in one study [36], whereas other studies reported more modest or non-significant reductions [34]. Similarly, the improvement in the AHI varied considerably. In patients treated with budesonide, the AHI decreased from 3.7 to 1.3 [42], while in studies involving montelukast, reductions ranged from 9.2 to 4.2 [13] or were not statistically significant (e.g., from 6.0 to 3.6; p = 0.07) [14]. In addition, symptom scores also demonstrated considerable heterogeneity. In one study with mometasone furoate, the total symptom score decreased from 11 to 3 [30], whereas in other cases, the reduction was more limited (e.g., from 6.5 to 5.3) [34]. Finally, in some studies, the combination of mometasone and oral montelukast was associated with a more pronounced improvement than monotherapy, showing greater reductions in AHI and endoscopic obstruction [54,55]. However, other studies did not observe significant differences [56]. Although a formal meta-analysis was not performed due to the considerable variability in outcome measures, treatment durations, and study designs, we have extracted and summarised the effect sizes from key studies in Figure A1 shown in Appendix C.
These findings underscore clinical heterogeneity across studies. Therefore, clinical interpretation of results requires caution, and further standardised trials are needed to clarify the actual benefits in different paediatric subpopulations.
Intranasal steroids, treatment duration, and sample size.
The available studies on mometasone furoate have treatment durations ranging from a few weeks [31,36] to one year of follow-up [33]. Typical findings include a significant reduction in endoscopic or radiological obstruction (A/C ratio) [30,36], improvement in polysomnographic and clinical parameters (snoring and apnoea) [30,31,34,36], and a positive effect on patients’ quality of life [35]. Statistical analysis reveals highly significant results.
Studies on beclometasone [37,38,39] are generally double-blind but involve small samples (20–60 children) [38,39]. The treatment duration can extend up to 24 weeks [39]. Two studies did not report statistically significant differences in AH [37,38], while one showed a marked reduction [39]. However, the overall statistical analysis result is substantial.
Similar results emerge from studies on budesonide [40,41,42], with sample sizes ranging from 60 [40] to 100 subjects [41] and follow-up durations of 6 [40,42] to 14 weeks [41]. These studies confirm improvements in respiratory symptoms [40,41] and a reduction in AHI [41].
For fluticasone [43,44,45], a reduction in the A/C ratio [42], a decrease in AHI [44], and an improvement in local inflammation [43] have been observed. The combination of p-values highlights a significantly positive overall effect.
For flunisolide [46,47], the results confirm a significant reduction in adenoid volume [46,47]. The available data indicate robust statistical significance (p < 0.001).
4.1. Intranasal Steroids and Dosages
The available studies show significant variability in the dosages used for intranasal corticosteroids in treating paediatric SDB. Mometasone furoate ranges from 40 µg/day [33] to 200 µg/day [32,34,35], beclometasone from 200 µg/day [37] to 400 µg/day [38], budesonide from 64 µg/day [42] to 1 mg/2 mL nebulised [41], fluticasone from 55 µg/day [43] to 400 µg/day [45], while flunisolide is administered based on body weight or through unspecified dosing regimens [46,47].
This heterogeneity limits the identification of an optimal therapeutic regimen and complicates efficacy comparisons between studies [44], particularly given differences in the study populations (age, severity, comorbidities). Furthermore, chronic use of intranasal steroids raises safety concerns, especially in young children. High-dose treatments require long-term monitoring to identify potential adverse effects [45], such as hypothalamic–pituitary–adrenal axis suppression or reduced growth. Therefore, a personalised therapeutic approach may be appropriate, tailored to the patient’s age [34] and clinical severity, potentially including systemic steroid therapy in more severe cases [50].
Steroids in combination with other drugs (oxymetazoline, azithromycin, betamethasone).
Some studies have explored the use of steroids in combination with other drugs, such as oxymetazoline (mometasone vs. placebo) [48], azithromycin (comparison between fluticasone spray and a macrolide) [49], or oral betamethasone combined with intranasal beclometasone [50]. The addition of a nasal vasoconstrictor (oxymetazoline) or systemic therapy (betamethasone) is associated with an enhancement of the steroid effect.
Statistical analyses from individual studies confirm a significant advantage compared to control groups. However, while these studies provide encouraging preliminary data, methodological limitations—including the absence of placebo groups in two studies and small sample sizes—necessitate caution in interpreting the results.
4.2. Montelukast
The studies reviewed on montelukast, both as monotherapy [13,14,51,52,53] and in combination with mometasone [54,55,56], collectively present a positive outlook for treating mild-to-moderate AH and OSAS. Double-blind, placebo-controlled studies [13,51,52,53] offer high methodological evidence. However, one study did not demonstrate improvement across all variables [14]. The effects on specific parameters (e.g., AHI) may vary depending on the clinical presentation’s initial complexity and severity.
The combination of montelukast and mometasone appears particularly beneficial, addressing both inflammatory/leukotriene mechanisms and obstructive components. Studies comparing monotherapy (either montelukast or mometasone) with their combination typically indicate superior outcomes with the combined therapy [54,55,57]. However, one study found no significant differences between mometasone monotherapy and combination treatment [56]. This suggests that synergistic effectiveness may depend on factors such as treatment duration and AH severity. Additionally, a significant proportion of the analysed studies involved small to moderate sample sizes [14,53], potentially limiting statistical power. Furthermore, follow-up durations often did not exceed three to four months [54,55,56,57]. More extended monitoring periods would allow a better assessment of the long-term clinical benefits.
Figure 2 shows a flowchart that outlines the pharmacologic treatment approach for paediatric OSA, particularly in cases of mild-to-moderate severity or when surgical intervention is not feasible. It integrates current evidence on using intranasal corticosteroids, oral montelukast, and their combination. The approach emphasises initial evaluation, stratified treatment selection, and reassessment after 8–12 weeks.
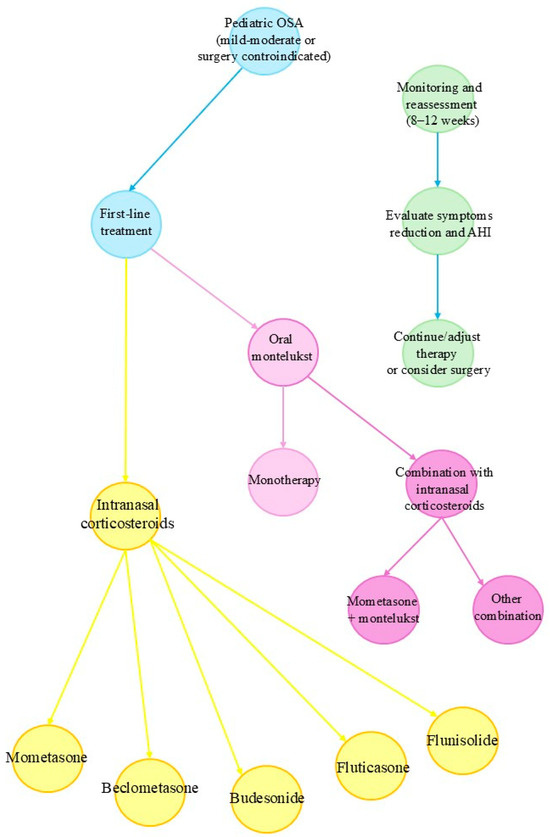
Figure 2.
Clinical decision-making flowchart for pharmacologic treatment of paediatric OSA.
Given the reports of neuropsychiatric adverse effects, including agitation, depression, and suicidal thoughts, the U.S. Food and Drug Administration (FDA; 4 March 2020) has issued a boxed warning regarding the use of montelukast, particularly in children. Other regulatory agencies have echoed similar warnings (GOV.UK; 29 April 2024). Therefore, careful consideration is warranted when prescribing montelukast, with a preference for alternative therapies when appropriate. If used, close monitoring of neuropsychiatric symptoms is recommended [64].
4.3. Study Limitations
The presence of trials with and without placebo groups and heterogeneous or sometimes absent control groups complicates direct comparisons across studies. Inclusion criteria were not consistently homogeneous. Some studies targeted children with mild OSAS, others included more moderate forms, while others focused on AH associated with diverse clinical scenarios (allergic rhinitis, recurrent otitis media with effusion, etc.). Such variability complicates the interpretation and practical clinical applicability of the findings.
To address this heterogeneity, we employed Fisher’s combined probability test. Although this test does not offer quantitative effect estimates such as risk ratios or mean differences, it indicates the overall statistical significance of the available evidence. However, a more accurate clinical and quantitative evaluation would require meta-analytic analyses based on more homogeneous data, standardised outcomes, measurement units, and known variances. The available literature, however, demonstrates substantial heterogeneity among studies in terms of outcome measures and sample sizes, restricting the possibility of rigorous synthesis.
Another limitation of the current literature is the lack of stratification by OSA severity and treatment duration. Our review highlights that several studies suggest greater efficacy in cases of mild-to-moderate OSA and with longer treatment courses. However, these findings are not consistently reported or statistically stratified, which limits the ability to draw definitive conclusions regarding optimal treatment regimens and clinical indications.
5. Conclusions
Findings from this review confirm that intranasal corticosteroid therapy, whether as monotherapy or in combination with other medications, is an effective and safe option for managing AH and mild-to-moderate OSAS symptoms in children. Oral montelukast is validated as an alternative therapeutic option, demonstrating particular benefit when combined with intranasal corticosteroids. Nevertheless, variability in trial designs—including the use or absence of a placebo and the heterogeneity or lack of control groups—complicates direct comparisons across studies. Future prospective studies on larger scales, with longer follow-ups and standardised protocols, are essential to evaluate the effectiveness of different treatment durations and identify patient subgroups most likely to benefit from specific therapeutic strategies. Finally, future studies should adopt consistent criteria to classify OSA severity and evaluate these interventions’ time-dependent efficacy.
Author Contributions
Conceptualisation, M.Z. and A.P.; methodology, M.Z.; software, M.Z.; validation, L.N., F.A. and R.F.; formal analysis, M.Z.; investigation, M.Z.; resources, A.P. and G.P.; data curation, R.F.; writing—original draft preparation, M.Z.; writing—review and editing, F.A.; visualisation, R.F.; supervision, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data derived from public domain resources.
Acknowledgments
During the preparation of this manuscript, the authors reviewed the protocol development process and employed OpenAI’s ChatGPT (versions GPT-4 and GPT-4o, OpenAI, March 2024) to support the identification of a suitable statistical method and to assist in structuring a spreadsheet for the calculation of combined p-values. The authors have reviewed, edited, and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AH | Adenoid Hypertrophy |
| AHI | Apnoea–Hypopnea Index |
| ATH | Adenotonsillar Hypertrophy |
| ADI | Oxygen Desaturation Index |
| A/N ratio | Adenoid-to-Nasopharynx ratio |
| A/C ratio | Adenoid-to-Choana ratio |
| oAHI | Obstructive Apnoea–Hypopnea Index |
| OME | Otitis Media with Effusion |
| OSA | Obstructive Sleep Apnoea |
| PSQ | Paediatric Sleep Questionnaire |
| PSG | Polysomnography |
| RCS | Randomised Controlled Study |
| RCT | Randomised Controlled Trial |
| SDB | Sleep Disordered Breathing |
Appendix A
Customised and database-specific search terms (MEDLINE (PubMed), Scopus, and Web of Science), combining keywords and synonyms.
PUBMED: (“children” OR “child” OR “infants” OR “infant” OR “pediatric” OR “paediatric”) AND (“sleep disordered breathing” OR “sleep-disordered breathing” OR “sleep related breathing disorder” OR “SDB” OR “upper airway resistance syndrome” OR “UARS” OR “obstructive sleep apnea” OR “obstructive sleep apnoea” OR “OSA” OR “obstructive sleep apnea syndrome” OR “OSAS” OR “sleep apnea syndrome” OR “sleep apnoea” OR “adenoid hypertrophy” OR “adenoidal hypertrophy” OR “adenoid enlargement” OR “tonsillar hypertrophy” OR “tonsil hypertrophy” OR “tonsillar enlargement” OR “snoring” OR “snore”) AND (“corticosteroid” OR “corticosteroids” OR “steroid” OR “steroids” OR “intranasal corticosteroid” OR “nasal corticosteroid” OR “mometasone” OR “fluticasone” OR “beclometasone” OR “budesonide” OR “prednisone” OR “prednisolone” OR “montelukast” OR “leukotriene receptor antagonist”) AND (“Randomised, double-blind, placebo-controlled” OR “randomised controlled study” OR “randomised controlled trial” OR “randomised controlled study” OR “randomised controlled trial” OR “RCT” OR “randomised clinical trial” OR “randomised clinical trial”) NOT review.
SCOPUS: TITLE-ABS-KEY((“children” OR “child” OR “infants” OR “infant” OR “pediatric” OR “paediatric”)) AND TITLE-ABS-KEY((“sleep disordered breathing” OR “sleep-disordered breathing” OR “sleep related breathing disorder” OR “SDB” OR “upper airway resistance syndrome” OR “UARS” OR “obstructive sleep apnea” OR “obstructive sleep apnoea” OR “OSA” OR “obstructive sleep apnea syndrome” OR “OSAS” OR “sleep apnea syndrome” OR “sleep apnoea” OR “adenoid hypertrophy” OR “adenoidal hypertrophy” OR “adenoid enlargement” OR “tonsillar hypertrophy” OR “tonsil hypertrophy” OR “tonsillar enlargement” OR “snoring” OR “snore”)) AND TITLE-ABS-KEY((“corticosteroid” OR “corticosteroids” OR “steroid” OR “steroids” OR “intranasal corticosteroid” OR “nasal corticosteroid” OR “mometasone” OR “fluticasone” OR “beclometasone” OR “budesonide” OR “prednisone” OR “prednisolone” OR “montelukast” OR “leukotriene receptor antagonist”)) AND TITLE-ABS-KEY((“Randomised, double-blind, placebo-controlled” OR “randomised controlled study” OR “randomised controlled trial” OR “randomised controlled study” OR “randomised controlled trial” OR “RCT” OR “randomised clinical trial” OR “randomised clinical trial”)) AND NOT (DOCTYPE(review))
WEB-OF-SCIENCE: TS = ((“children” OR “child” OR “infants” OR “infant” OR “pediatric” OR “paediatric”) AND (“sleep disordered breathing” OR “sleep-disordered breathing” OR “sleep related breathing disorder” OR “SDB” OR “upper airway resistance syndrome” OR “UARS” OR “obstructive sleep apnea” OR “obstructive sleep apnoea” OR “OSA” OR “obstructive sleep apnea syndrome” OR “OSAS” OR “sleep apnea syndrome” OR “sleep apnoea” OR “adenoid hypertrophy” OR “adenoidal hypertrophy” OR “adenoid enlargement” OR “tonsillar hypertrophy” OR “tonsil hypertrophy” OR “tonsillar enlargement” OR “snoring” OR “snore”) AND (“corticosteroid” OR “corticosteroids” OR “steroid” OR “steroids” OR “intranasal corticosteroid” OR “nasal corticosteroid” OR “mometasone” OR “fluticasone” OR “beclometasone” OR “budesonide” OR “prednisone” OR “prednisolone” OR “montelukast” OR “leukotriene receptor antagonist”) AND (“Randomised, double-blind, placebo-controlled” OR “randomised controlled study” OR “randomised controlled trial” OR “randomised controlled study” OR “randomised controlled trial” OR “RCT” OR “randomised clinical trial” OR “randomised clinical trial”)) NOT DT = (Review).
Appendix B

Table A1.
Procedure (Fisher’s combined probability test) for calculating the combination of probabilities (p-values) in Microsoft Excel for Windows.
Table A1.
Procedure (Fisher’s combined probability test) for calculating the combination of probabilities (p-values) in Microsoft Excel for Windows.
| A | B |
|---|---|
| p-values | LN(p-value) |
| A2: p-value1 | =LN(A2) |
| A3: p-value2 | =LN(A3) |
| ... | ... |
| A11: p-value10 | =LN(A11) |
| SUM: | =SUM(B2:B11) |
| X2 = −2 × SUM: | =−2 × B12 |
| combined p-value: | =CHISQ.DIST.RT(B13, 2 × COUNT(A2:A11)) |
Legend: LN, natural logarithm; LN(A2), natural logarithmic conversion; SUM(B2:B11), sum of logarithms; (=−2 × B12), Chi-square statistic calculation; 2 × COUNT(A2:A11), degrees of freedom; CHISQ.DIST.RT(B13, 2 × COUNT(A2:A11)), combined p-value calculation.

Table A2.
Clinical studies on the treatment of adenoid hypertrophy and sleep-related breathing disorder symptoms with steroids in combination with other drugs.
Table A2.
Clinical studies on the treatment of adenoid hypertrophy and sleep-related breathing disorder symptoms with steroids in combination with other drugs.
| Reference | Design | Pop (Age) | N | In/Ex | Tx vs. Ctrl | Dur | O1 | O2 | Out (Tx) | Out (Ctrl) | Stat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liu (2017) [48] | Two-stage, parallel, Rnd, DB, DD | Child. 6–12 yrs AH + AR | 240 tot (MF vs. Placebo; Stage 2: 4 groups) | In: AH ≥ 75% rhinoph., sympt. ≥ 12 mo, positive allergy tests Ex: Seasonal rhinitis, craniofacial anomalies, recent ster./ATB | Stage 1 (6 wks): MF 50 µg/nostril/day vs placebo Stage 2 (8 wks, non-responders): MF + Oxymet vs. MF + Placebo vs. Placebo + Oxymet vs. Placebo + Placebo | 6 wks + 8 wks | A/C reduction, increased nasal volume | Symptom reduction (congestion, snoring, etc.) | A/C: 87.2→27.3; Nasal vol. 11.2→16.8; TSS: 16.5→4.8 | A/C: 85.1→83.4; Nasal vol. 11.1→11.2; TSS: 15.3→14.9 | p < 0.05 (A/C, nasal vol., TSS, congestion, snoring) |
| Hashemi Jazi (2011) [49] | Prosp., Rnd, longitudinal | Child. 2–10 yrs AH | 39 tot (20 Flutic. vs. 19 Azithro) | In: AH + OSA sympt. (apnoea, snoring, hypernasality) Ex: Craniofacial, neuromusc. anomalies, recurrent infections, ster./ATB <4 wks | Nasal Fluticasone (1 puff/nostril × 2/day ×1 wk, then 1/day × 5 wks) vs Azithromycin (12 mg/kg × 5 days in cycles ×6 wks) | 6 wks | AH severity reduction, OSA sympt. reduction | Reduced nasal obstr., snoring, mouth breathing | Obstr. 51–100%: 70%→40%; Snoring: 85%→10%; Mouth Breathing: 60%→10%; Apnoea: 10%→5% | Obstr. 51–100%: 79%→47%; Snoring: 95%→5%; Mouth Breathing: 68%→5%; Apnoea: 15%→0% | Obstr. p = 0.004; Snoring/Mouth Breathing p < 0.001; Apnoea p = 0.02 |
| Evangelisti (2022) [50] | Unblinded, open-label | Child. 3–10 yrs Severe OSAS (AHI > 10) | 28 tot (15 vs. 13) | In: Severe OSAS (PSG), awaiting A-T Ex: Chronic cardiopulm., craniofacial, genetic disorders, epilepsy, severe obesity | Oral Betamethasone (0.1 mg/kg/day × 7 days) + Nasal Beclometasone vs. Nasal Beclometasone alone | 7 days | Improved SpO2 (mean, min.), SCR reduction | ODI reduction, time < 90% SpO2 | SCR: 12.6→8.3; Mean SpO2: 95.3→97.0; Min SpO2: 78.8→89.2; ODI: 11.7→3.0; <90% time: 1.75→0.0 | SCR: 12.2→12.3; Mean SpO2: 95.6→95.5; Min SpO2: 82.5→77.8; ODI: 12.3→11.3; <90% time: 1.35→2.0 | p = 0.0001 (SCR, Mean SpO2), p = 0.001 (Min SpO2), p < 0.0001 (ODI, desaturation <90% time) |
Legend: A/C: Adenoid/Choana ratio; AHI: Apnoea–Hypopnea Index; AH: Adenoid Hypertrophy; A-T: Adenotonsillectomy; ATB: Antibiotics; Azithro: Azithromycin; DB: Double-Blind; DD: Double Dummy; desatur.: Desaturation; Flutic.: Fluticasone; In/Ex: Inclusion/Exclusion criteria; Mom.: Mometasone; mo: months; Nasal vol.: Nasal Volume; Obstr.: Obstruction; ODI: Oxygen Desaturation Index; OAI: Obstructive Apnoea Index; OSA: Obstructive Sleep Apnoea; OSAS: Obstructive Sleep Apnoea Syndrome; PSG: Polysomnography; prosp.: Prospective; Rnd: Randomised; SCR: Sleep-Related Complaints; SpO2: Oxygen Saturation; sympt.: Symptoms; TSS: Total Symptom Score; tot: total; Tx vs. Ctrl: Treatment vs. Control; wks: weeks; yrs: years.

Table A3.
Clinical studies that were excluded from the review after a comprehensive assessment of the full manuscript.
Table A3.
Clinical studies that were excluded from the review after a comprehensive assessment of the full manuscript.
| Reference | Design | Pop (Age) | N | In/Ex | Tx vs. Ctrl | Dur | O1 | O2 | Out (Tx) | Out (Ctrl) | Stat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gelardi (2013) [58] | Rnd, blinded, PC study | Child. 4–12 yrs AH/sub-obstr. tonsils | 45 tot (27 vs. 18) | In: AH/sub-obstr. tonsils, SDB/OME > 6 mo, no immunosuppr. < 3 mo Ex: Cardiac, bronch. disorders, iodine allergy, TB | Aerosal® (micronized NaCl + iodine) 10 sessions vs. Placebo (no aerosol) | ~2 wks (10 sessions) + 3-mo follow-up | ≥25% hypertrophy reduction (clinical-endoscopic) | Hearing (≥10 dB), tympanograms, SpO2, nasal cytology | ≥25% reduction: 44.4%; Hearing +5 dB; Tymp. improvement 29.6% | ≥25% reduction: 22.2%; Hearing 0 dB; Tymp. 5.6% | p = 0.204 (hypertrophy), p = 0.018 (hearing), p = 0.064 (tymp.) |
| Hood (2021) [60] | MC, DB, Rnd, CT | Child. 3–7.99 yrs with SCA + SDB | Expected 200 tot | In: SCA (HbSS/HbSβ0), SDB, no current mont. Ex: Adverse mont. reactions, developmental disorders, participation in other trials | Montelukast 4 mg/day vs. Placebo | 12 wks | Primary: ↑ cognitive processing speed (secondary: AH reduction) | Executive function, cerebral perfusion, SDB parameters | Mont. (n = 100): Processing speed +8 pts; A/N ratio 0.81→0.57 | Ctrl (n = 100): No change; A/N ratio unchanged | Power 90% for Δ = 8 pts cognitive; α = 0.025; ITT analysis |
| Tracy (1998) [59] | DB-PC-Rnd | Child. 3–11 yrs Chronic OME | 61 tot (20 vs. 19 vs. 20) (3 groups) | In: OME > 3 mo, >3 AOM episodes in 6 mo Ex: Systemic ster. <6 mo, tubes, beclom. allergy, nasal spray < 2 wks | 1) ATB (amoxic./sulf.) 2) ATB + nasal beclom. 336 µg/day 3) ATB + nasal placebo | 12 wks | Middle ear effusion resolution (tympanometry/otoscopy) | Ear pain, hearing, tympanic mobility | G2 (ATB+bec.): Faster effusion resolution, improved tympanic/otoscopy scores (p ≤ 0.05 at 4–8 wks) | G1/G3: Slower improvement, “catch-up” at 12 wks | p ≤ 0.01 (right tymp. pressure), p ≤ 0.004 (symptoms), p ≤ 0.05 (effusion) |
| Wang (2017) [62] | Cross-sect. | Child. <18 yrs with OME | Data on 1.94 × 109 visits | In: OME diagnosis Ex: Acute otitis media, ATB use, recent surgery | Intranasal steroids (various) vs. No use | Retrosp. 2005–2012 | Intranasal steroid prescription frequency in OME | QoC implications, appropriateness | Steroids 10.0% (95% CI 6.3–15.5) OR = 3.58 | Steroids 3.5% (95% CI 3.1–3.9) | p = 0.002 (OR), p < 0.001 (risk diff.) |
| Sobhy (2013) [33] | Rnd prosp. parallel | Child. 3–13 yrs Post-adenoidectomy | 200 tot (100 vs. 100) | In: Adenoidectomy, residual symptoms, no ster. < 1 yr Ex: Epistaxis, immunodef., genetic/neuromusc. disorders | Mom. furoate 40 µg/nostril/day × 12 wks vs. Saline | 12 mo | Prevention of nasal obstr., rhinorrhea, post-surg. snoring recurrence | QoL, re-surgery reduction | Nasal obstr.: 2.31→0.73; Discharge: 2.16→0.67; Snoring: 2.27→0.79 | Nasal obstr.: 2.33→1.49; Discharge: 2.12→1.53; Snoring: 2.25→1.44 | OR = 2.89 p = 0.001 (obstr.), OR = 3.21 p = 0.0001 (discharge), OR = 2.95 p = 0.0001 (snore) |
| Zhao (2023) [65] | Prosp. Rnd CT | Child. 2–9 yrs AH | 93 tot (31 AAT, 32 Xiaoxian + AAT, 30 Montel.) | In: AH + nasal congestion, mouth br., snoring, high OSA-18 Ex: Prev. adenoidectomy, polyps, septal dev., recent ster. | AAT vs. Xiaoxian + AAT vs Mont. 4–5 mg/day | 1 mo + 6-mo follow-up | Improvement in congestion, mouth br., snoring | QoL, recurrence rate | OSA-18: 69.56→53.47; Congestion: 1.63→0.50; Mouth br.: 1.56→0.47; Snoring: 1.88→0.38; Sec. sympt.: 3.0→0.72 | OSA-18: 67.43→58.50; Congestion: 1.67→0.57; Snoring: 1.87→1.0; Sec. sympt.: 2.87→1.32 | OSA-18: p = 0.004, Open-mouth p = 0.006, Snore p = 0.008, Sec. sympt. p = 0.0014 |
| Bilgili (2023b) [61] | Prosp. Rnd longit. | Child. 4–14 yrs ET dysfunction + AH | 100 tot | In: AH, ETD, snoring, mouth br., no prev. surgery Ex: Acute nas./otol. infections, craniofacial disorders, recent ster./ATB | Azelastine + Flutic. dipr. 2×/day (548 µg + 200 µg total/day) | 3 mo, No Ctrl | A/C reduction (endoscopic), ETS-7, tubomanometry | OME resolution, nasal symptoms | A/C 82→37%, ETS-7: 6.36→9.72, OME resolved 66% | N/A (no ctrl) | p < 0.01 (A/C), p = 0.001 (ETS-7, adenoid volume) |
| Bilgili (2023a) [66] | Prosp. Rnd longit. | Child. 4–13 yrs AH | 65 tot No Ctrl | In: AH > 6 mo, no ster. < 4 wks, no allergies/atopy Ex: Prev. surgery, craniofacial disorders | Azelastine+Flutic. (MP-AzeFlu) 2×/day (548 µg azel. + 200 µg flutic.) | 24 wks, No Ctrl | A/C reduction, symptom improvement (obstr., snoring, apnoea, etc.) | Need for surgery, QoL | A/C: 3.57→1.74, Total sympt.: 15.63→2.31, Snoring: 2.82→0.62, Apnoea: 2.52→0.22 | N/A (no ctrl) | p < 0.01– < 0.001 (A/C, all symptoms) |
Legend: ↑: increased; A/C ratio: Adenoid/Choana ratio; AH: Adenoid Hypertrophy; ATB: Antibiotics; Azel.: Azelastine; CI: Confidence Interval; Cross-sect.: Cross-Sectional; CT: Controlled Trial; DB: Double-Blind; Δ: Delta (Change); Endosc.: Endoscopy; ETD: Eustachian Tube Dysfunction; ETS-7: Eustachian Tube Score-7; Flutic.: Fluticasone; Immunsoppr.: Immunosuppression; In/Ex: Inclusion/Exclusion criteria; Longit.: Longitudinal; MC: Multi-Centre; Mom.: Mometasone; Mont.: Montelukast; MP-AzeFlu: Combination of Azelastine + Fluticasone; NaCl: Sodium Chloride (Saline); N/A: not available; NS: not significant; O1: Primary Outcome; O2: Secondary Outcome; OME: Otitis Media with Effusion; OR: Odds Ratio; OSA: Obstructive Sleep Apnoea; OSA-18: Paediatric Sleep Questionnaire for OSA; p: p-value (statistical significance); PC: Placebo-Controlled; Prosp.: Prospective; QoL: Quality of Life; Rnd: Randomised; RR: Relative Risk; SDB: Sleep-Disordered Breathing; Ster.: Steroids; tot: Total; TSS: Total Symptom Score; Tx vs. Ctrl: Treatment vs. Control; Tymp.: Tympanometry; VAS: Visual Analogue Scale; wks: Weeks; yrs: Years.
Appendix C
The descriptive forest plot illustrates the effect of intranasal corticosteroids and/or oral montelukast in paediatric patients with OSA or AH. As reported in individual studies, the horizontal bars represent the magnitude and direction of change in selected clinical outcomes (e.g., AHI, A/C, symptom scores). Negative values indicate improvement (i.e., reduction from baseline or compared to control).
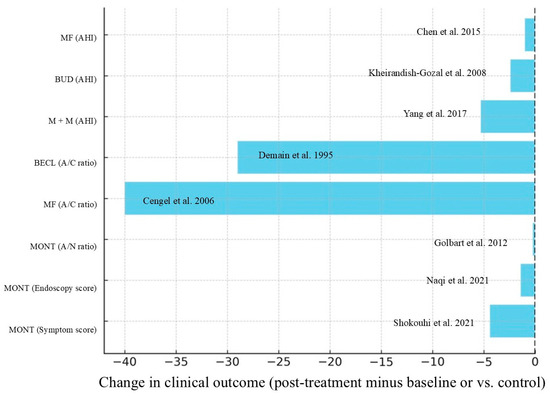
Figure A1.
Descriptive forest plot grouped by outcome type [14,32,36,39,42,51,52,54]. Legend: A/C Ratio, Adenoid-to-Choana Ratio; A/N Ratio, Adenoid-to-Nasopharynx Ratio; AHI, Apnoea–Hypopnea Index; BECL, Beclometasone; BUD, budesonide; M+M, combined montelukast and mometasone; MF, mometasone furoate; MONT, montelukast.
References
- Bitners, A.C.; Arens, R. Evaluation and Management of Children with Obstructive Sleep Apnea Syndrome. Lung 2020, 198, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Chae, K.Y. Obstructive sleep apnea syndrome in children: Epidemiology, pathophysiology, diagnosis and sequelae. Korean J. Pediatr. 2010, 53, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Kaditis, A.G.; Alonso Alvarez, M.L.; Boudewyns, A.; Alexopoulos, E.I.; Ersu, R.; Joosten, K.; Larramona, H.; Miano, S.; Narang, I.; Trang, H.; et al. Obstructive sleep disordered breathing in 2- to 18-year-old children: Diagnosis and management. Eur. Respir. J. 2016, 47, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, F.; Zucchini, L.; Pellitteri, F.; Palone, M.; Lombardo, L. Obstructive Sleep Apnea in Developmental Age: 22-Item Pediatric Sleep Questionnaire for an Observational Descriptive Investigation. Children 2023, 10, 1265. [Google Scholar] [CrossRef]
- Nosetti, L.; Zaffanello, M.; Simoncini, D.; Dellea, G.; Vitali, M.; Amoudi, H.; Agosti, M. Prioritising Polysomnography in Children with Suspected Obstructive Sleep Apnoea: Key Roles of Symptom Onset and Sleep Questionnaire Scores. Children 2024, 11, 1228. [Google Scholar] [CrossRef]
- Lein, A.; Altumbabic, H.; Đešević, M.; Baumgartner, W.D.; Salkic, A.; Umihanic, S.; Ramaš, A.; Harčinović, A.; Kosec, A.; Brkic, F.F. Association of adenoid hypertrophy and clinical parameters with preoperative polygraphy in pediatric patients undergoing adenoidectomy. Eur. Arch. Otorhinolaryngol. 2025, 282, 1075–1084. [Google Scholar] [CrossRef]
- Sant’Ana, J.P.; Mastrandonakis, I.C.F.; Silva, R.S.B.; Duprat, A.C.; Floriano, C.G.; Miyake, M.M. Reliability of nasofibroscopy for the evaluation of adenoid hypertrophy and its correlation with clinical symptoms. Braz. J. Otorhinolaryngol. 2023, 89, 101307. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, R. Pediatric Obstructive Sleep Apnea: Diagnostic Challenges and Management Strategies. Cureus 2024, 16, e75347. [Google Scholar] [CrossRef]
- Kuhle, S.; Hoffmann, D.U.; Mitra, S.; Urschitz, M.S. Anti-inflammatory medications for obstructive sleep apnoea in children. Cochrane Database Syst. Rev. 2020, 1, Cd007074. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Bhattacharjee, R.; Bandla, H.P.R.; Gozal, D. Antiinflammatory therapy outcomes for mild OSA in children. Chest 2014, 146, 88–95. [Google Scholar] [CrossRef]
- Liming, B.J.; Ryan, M.; Mack, D.; Ahmad, I.; Camacho, M. Montelukast and Nasal Corticosteroids to Treat Pediatric Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. Otolaryngol. Head. Neck Surg. 2019, 160, 594–602. [Google Scholar] [CrossRef] [PubMed]
- de Benedictis, F.M.; Bush, A. Corticosteroids in respiratory diseases in children. Am. J. Respir. Crit. Care Med. 2012, 185, 12–23. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Bandla, H.P.; Gozal, D. Montelukast for Children with Obstructive Sleep Apnea: Results of a Double-Blind, Randomized, Placebo-Controlled Trial. Ann. Am. Thorac. Soc. 2016, 13, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Goldbart, A.D.; Greenberg-Dotan, S.; Tal, A. Montelukast for children with obstructive sleep apnea: A double-blind, placebo-controlled study. Pediatrics 2012, 130, e575–e580. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Lu, T.; Qiu, Y.; Li, X.; Liu, Y.; Tai, J.; Guo, Y.; Zhang, J.; Wang, S.; Zhao, J.; et al. The efficacy and safety of montelukast in children with obstructive sleep apnea: A systematic review and meta-analysis. Sleep. Med. 2021, 78, 193–201. [Google Scholar] [CrossRef]
- Chohan, A.; Lal, A.; Chohan, K.; Chakravarti, A.; Gomber, S. Systematic review and meta-analysis of randomized controlled trials on the role of mometasone in adenoid hypertrophy in children. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1599–1608. [Google Scholar] [CrossRef]
- Liu, H.T.; Lin, Y.C.; Kuan, Y.C.; Huang, Y.H.; Hou, W.H.; Liou, T.H.; Chen, H.C. Intranasal corticosteroid therapy in the treatment of obstructive sleep apnea: A meta-analysis of randomized controlled trials. Am. J. Rhinol. Allergy 2016, 30, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, A.G.; Chervoneva, I.; Cielo, C.M.; Bradford, R.; Cornaglia, M.A.; Tapia, I. Secondary Analysis of a Randomized Controlled Trial of Intranasal Corticosteroids in the Treatment of Mild to Moderate Pediatric Obstructive Sleep Apnea. In TP73. TP073 PEDIATRIC SLEEP; American Thoracic Society International Conference Abstracts; American Thoracic Society: New York, NY, USA, 2021; p. A3345. [Google Scholar]
- Alanazi, F.; Alruwaili, M.; Alanazy, S.; Alenezi, M. Efficacy of montelukast for adenoid hypertrophy in paediatrics: A systematic review and meta-analysis. Clin. Otolaryngol. 2024, 49, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leng, S.; Hu, Q.; Li, Y.; Wei, Y.; Lu, Y.; Qie, D.; Yang, F. Pharmacological interventions for pediatric obstructive sleep apnea (OSA): Network meta-analysis. Sleep. Med. 2024, 116, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.; Krishna, M.M.; Franco, A.J.; Jekov, L.; Sudo, R.Y.U.; Cabral, T.D.D. Efficacy of combination therapy with mometasone and montelukast versus mometasone alone in treatment of adenoid hypertrophy in children: A systematic review and meta-analysis. Am. J. Otolaryngol. 2025, 46, 104566. [Google Scholar] [CrossRef]
- Yoon, S.; Baik, B.; Park, T.; Nam, D. Powerful p-value combination methods to detect incomplete association. Sci. Rep. 2021, 11, 6980. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.J. The harmonic mean p-value for combining dependent tests. Proc. Natl. Acad. Sci. USA 2019, 116, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- van Dijk, S.H.B.; Brusse-Keizer, M.G.J.; Bucsán, C.C.; van der Palen, J.; Doggen, C.J.M.; Lenferink, A. Artificial intelligence in systematic reviews: Promising when appropriately used. BMJ Open 2023, 13, e072254. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Kumar, M.V.; Su, E.; Flores Miranda, A.; Saha, A.; Sussman, J. Evaluating the efficacy of artificial intelligence tools for the automation of systematic reviews in cancer research: A systematic review. Cancer Epidemiol. 2024, 88, 102511. [Google Scholar] [CrossRef]
- Islam, N.; van der Schaar, M. Use of generative artificial intelligence in medical research. BMJ 2024, 384, q119. [Google Scholar] [CrossRef]
- Ganjavi, C.; Eppler, M.B.; Pekcan, A.; Biedermann, B.; Abreu, A.; Collins, G.S.; Gill, I.S.; Cacciamani, G.E. Publishers’ and journals’ instructions to authors on use of generative artificial intelligence in academic and scientific publishing: Bibliometric analysis. BMJ 2024, 384, e077192. [Google Scholar] [CrossRef]
- Won, S.; Morris, N.; Lu, Q.; Elston, R.C. Choosing an optimal method to combine P-values. Stat. Med. 2009, 28, 1537–1553. [Google Scholar] [CrossRef]
- Berlucchi, M.; Salsi, D.; Valetti, L.; Parrinello, G.; Nicolai, P. The role of mometasone furoate aqueous nasal spray in the treatment of adenoidal hypertrophy in the pediatric age group: Preliminary results of a prospective, randomized study. Pediatrics 2007, 119, e1392–e1397. [Google Scholar] [CrossRef]
- Baker, A.; Grobler, A.; Davies, K.; Griffiths, A.; Hiscock, H.; Kubba, H.; Peters, R.L.; Ranganathan, S.; Rimmer, J.; Rose, E.; et al. Effectiveness of Intranasal Mometasone Furoate vs Saline for Sleep-Disordered Breathing in Children: A Randomized Clinical Trial. JAMA Pediatr. 2023, 177, 240–247. [Google Scholar] [CrossRef]
- Chan, C.C.; Au, C.T.; Lam, H.S.; Lee, D.L.; Wing, Y.K.; Li, A.M. Intranasal corticosteroids for mild childhood obstructive sleep apnea--a randomized, placebo-controlled study. Sleep. Med. 2015, 16, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, T.S. Role of intranasal steroid in the prevention of recurrent nasal symptoms after adenoidectomy. Int. J. Otolaryngol. 2013, 2013, 603493. [Google Scholar] [CrossRef]
- Yilmaz, H.B.; Celebi, S.; Sahin-Yilmaz, A.; Oysu, C. The role of mometasone furoate nasal spray in the treatment of adenoidal hypertrophy in the adolescents: A prospective, randomized, cross-over study. Eur. Arch. Otorhinolaryngol. 2013, 270, 2657–2661. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Chakravarti, A. A double-blind randomized placebo-controlled trial of topical intranasal mometasone furoate nasal spray in children of adenoidal hypertrophy with otitis media with effusion. Am. J. Otolaryngol. 2014, 35, 766–770. [Google Scholar] [CrossRef]
- Cengel, S.; Akyol, M.U. The role of topical nasal steroids in the treatment of children with otitis media with effusion and/or adenoid hypertrophy. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Lepcha, A.; Kurien, M.; Job, A.; Jeyaseelan, L.; Thomas, K. Chronic adenoid hypertrophy in children-is steroid nasal spray beneficial? Indian. J. Otolaryngol. Head. Neck Surg. 2002, 54, 280–284. [Google Scholar] [CrossRef]
- Criscuoli, G.; D’Amora, S.; Ripa, G.; Cinquegrana, G.; Mansi, N.; Impagliazzo, N.; Pisacane, A. Frequency of surgery among children who have adenotonsillar hypertrophy and improve after treatment with nasal beclomethasone. Pediatrics 2003, 111, e236–e238. [Google Scholar] [CrossRef]
- Demain, J.G.; Goetz, D.W. Pediatric adenoidal hypertrophy and nasal airway obstruction: Reduction with aqueous nasal beclomethasone. Pediatrics 1995, 95, 355–364. [Google Scholar] [CrossRef]
- Gudnadottir, G.; Ellegård, E.; Hellgren, J. Intranasal Budesonide and Quality of Life in Pediatric Sleep-Disordered Breathing: A Randomized Controlled Trial. Otolaryngol. Head. Neck Surg. 2018, 158, 752–759. [Google Scholar] [CrossRef]
- Hong, H.; Chen, F.; Zheng, X.; Liao, W.; Liao, Z.; Cao, Y.; He, H.; Zhu, Z.; Fan, Y. Decreased frequency of adenoidectomy by a 12-week nasal budesonide treatment. Ther. Clin. Risk Manag. 2017, 13, 1309–1316. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics 2008, 122, e149–e155. [Google Scholar] [CrossRef]
- Esteitie, R.; Emani, J.; Sharma, S.; Suskind, D.L.; Baroody, F.M. Effect of fluticasone furoate on interleukin 6 secretion from adenoid tissues in children with obstructive sleep apnea. Arch. Otolaryngol. Head. Neck Surg. 2011, 137, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Brouillette, R.T.; Manoukian, J.J.; Ducharme, F.M.; Oudjhane, K.; Earle, L.G.; Ladan, S.; Morielli, A. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J. Pediatr. 2001, 138, 838–844. [Google Scholar] [CrossRef]
- Demirhan, H.; Aksoy, F.; Ozturan, O.; Yildirim, Y.S.; Veyseller, B. Medical treatment of adenoid hypertrophy with “fluticasone propionate nasal drops”. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 773–776. [Google Scholar] [CrossRef]
- Ciprandi, G.; Varricchio, A.; Capasso, M.; Varricchio, A.M.; De Lucia, A.; Ascione, E.; Avvisati, F.; Capristo, C.; Marseglia, G.L.; Barillari, U. Intranasal flunisolide treatment in children with adenoidal hypertrophy. Int. J. Immunopathol. Pharmacol. 2007, 20, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Varricchio, A.; Tortoriello, G.; Capasso, M.; De Lucia, A.; Marchisio, P.; Varricchio, A.M.; Mansi, N.; Giordano, L.; Liberatore, G.; Di Gioacchino, M.; et al. Prevention of surgery in children with adenoidal hypertrophy treated with intranasal flunisolide: A 12-month follow-up. J. Biol. Regul. Homeost. Agents 2009, 23, 95–101. [Google Scholar] [PubMed]
- Liu, W.; Zhou, L.; Zeng, Q.; Luo, R. Combination of mometasone furoate and oxymetazoline for the treatment of adenoid hypertrophy concomitant with allergic rhinitis: A randomized controlled trial. Sci. Rep. 2017, 7, 40425. [Google Scholar] [CrossRef]
- Jazi, S.M.; Barati, B.; Kheradmand, A. Treatment of adenotonsillar hypertrophy: A prospective randomized trial comparing azithromycin vs. fluticasone. J. Res. Med. Sci. 2011, 16, 1590–1597. [Google Scholar]
- Evangelisti, M.; Barreto, M.; Di Nardo, G.; Del Pozzo, M.; Parisi, P.; Villa, M.P. Systemic corticosteroids could be used as bridge treatment in children with obstructive sleep apnea syndrome waiting for surgery. Sleep. Breath. 2022, 26, 879–885. [Google Scholar] [CrossRef]
- Naqi, S.A.; Ashfaq, A.H.; Umar, M.A.; Karmani, J.K.; Arshad, N. Clinical outcome of Montelukast Sodium in Children with Adenoid Hypertrophy. Pak. J. Med. Sci. 2021, 37, 362–366. [Google Scholar] [CrossRef]
- Shokouhi, F.; Meymaneh Jahromi, A.; Majidi, M.R.; Salehi, M. Montelukast in Adenoid Hypertrophy: Its Effect on Size and Symptoms. Iran. J. Otorhinolaryngol. 2015, 27, 443–448. [Google Scholar] [PubMed]
- Wang, Z.; Wu, X.; Liu, J.; Wang, Y.; Zhang, Y.; Wu, Y.; Kang, Y.; Zhang, R.; Li, J.; Liu, D. Effects of oral cysteine leukotriene receptor antagonist-montelukast on adenoid lymphoid tissue: A histopathological study under light microscope. Front. Pharmacol. 2023, 14, 1285647. [Google Scholar] [CrossRef]
- Yang, D.Z.; Liang, J.; Zhang, F.; Yao, H.B.; Shu, Y. Clinical effect of montelukast sodium combined with inhaled corticosteroids in the treatment of OSAS children. Medicine 2017, 96, e6628. [Google Scholar] [CrossRef]
- Ras, A.E.; Hamed, M.H.; Abdelalim, A.A. Montelukast combined with intranasal mometasone furoate versus intranasal mometasone furoate; a comparative study in treatment of adenoid hypertrophy. Am. J. Otolaryngol. 2020, 41, 102723. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Pourroshani, B.; Eftekhari, K.; Malekiantaghi, A.; Ashournia, P.; Shafiei, A. Effect of Combination Montelukast and Nasal Mometasone on Childhood Adenoid Hypertrophy. Iran. J. Otorhinolaryngol. 2024, 36, 391–397. [Google Scholar] [CrossRef]
- Tuhanıoğlu, B.; Erkan, S.O. Evaluation of the effects of montelukast, mometasone furoate, and combined therapyon adenoid size: A randomized, prospective, clinical trial with objective data. Turk. J. Med. Sci. 2017, 47, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Gelardi, M.; Iannuzzi, L.; Greco Miani, A.; Cazzaniga, S.; Naldi, L.; De Luca, C.; Quaranta, N. Double-blind placebo-controlled randomized clinical trial on the efficacy of Aerosal in the treatment of sub-obstructive adenotonsillar hypertrophy and related diseases. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1818–1824. [Google Scholar] [CrossRef]
- Tracy, J.M.; Demain, J.G.; Hoffman, K.M.; Goetz, D.W. Intranasal beclomethasone as an adjunct to treatment of chronic middle ear effusion. Ann. Allergy Asthma Immunol. 1998, 80, 198–206. [Google Scholar] [CrossRef]
- Hood, A.M.; Stotesbury, H.; Kölbel, M.; DeHaan, M.; Downes, M.; Kawadler, J.M.; Sahota, S.; Dimitriou, D.; Inusa, B.; Wilkey, O.; et al. Study of montelukast in children with sickle cell disease (SMILES): A study protocol for a randomised controlled trial. Trials 2021, 22, 690. [Google Scholar] [CrossRef]
- Bilgili, A.M.; Durmaz, H.; Dilber, M. Eustachian Tube Dysfunction in Children with Adenoid Hypertrophy: The Effect of Intranasal Azelastine-Fluticasone Spray Treatment on Middle Ear Ventilation and Adenoid Tissue. Ear Nose Throat J. 2023, 102, 198–203. [Google Scholar] [CrossRef]
- Wang, D.E.; Lam, D.J.; Bellmunt, A.M.; Rosenfeld, R.M.; Ikeda, A.K.; Shin, J.J. Intranasal Steroid Use for Otitis Media with Effusion: Ongoing Opportunities for Quality Improvement. Otolaryngol. Head. Neck Surg. 2017, 157, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Chakravarti, A. Role of mometasone furoate aqueous nasal spray for management of adenoidal hypertrophy in children. J. Laryngol. Otol. 2014, 128, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W.H.; Pathadka, S.; Qin, S.X.; Fung, L.W.Y.; Yan, V.K.C.; Yiu, H.H.E.; Bloom, C.I.; Wong, I.C.K.; Chan, E.W.Y. Neuropsychiatric events associated with montelukast in patients with asthma: A systematic review. Eur. Respir. Rev. 2023, 32, 230079. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, J.; Wang, M.Y.; Hou, Z.W.; Shi, H.S.; Zhang, X.X. Effect of oral Xiao-xian decoction combined with acupoint application therapy on pediatric adenoid hypertrophy: A randomized trial. Medicine 2023, 102, e32804. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, A.M.; Durmaz, H.; Dilber, M. Efficacy of Topical Azelastine and Fluticasone Dipropionate Combination in Children with Adenoid Hypertrophy. Ear Nose Throat J. 2023, 102, 28–34. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).