Intranasal Corticosteroids and Oral Montelukast for Paediatric Obstructive Sleep Apnoea: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
- (1)
- The paediatric population.
- (2)
- Assessed the effects of intranasal corticosteroids and/or montelukast in treating Obstructive Sleep Apnoea (OSA), sleep disordered breathing (SDB), and adenoid hypertrophy (AH).
- (3)
- Employed a randomised controlled design.
- (4)
- Reported the clinical and/or instrumental outcomes.
2.2. Statistics
3. Results
4. Discussion
4.1. Intranasal Steroids and Dosages
4.2. Montelukast
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AH | Adenoid Hypertrophy |
| AHI | Apnoea–Hypopnea Index |
| ATH | Adenotonsillar Hypertrophy |
| ADI | Oxygen Desaturation Index |
| A/N ratio | Adenoid-to-Nasopharynx ratio |
| A/C ratio | Adenoid-to-Choana ratio |
| oAHI | Obstructive Apnoea–Hypopnea Index |
| OME | Otitis Media with Effusion |
| OSA | Obstructive Sleep Apnoea |
| PSQ | Paediatric Sleep Questionnaire |
| PSG | Polysomnography |
| RCS | Randomised Controlled Study |
| RCT | Randomised Controlled Trial |
| SDB | Sleep Disordered Breathing |
Appendix A
Appendix B
| A | B |
|---|---|
| p-values | LN(p-value) |
| A2: p-value1 | =LN(A2) |
| A3: p-value2 | =LN(A3) |
| ... | ... |
| A11: p-value10 | =LN(A11) |
| SUM: | =SUM(B2:B11) |
| X2 = −2 × SUM: | =−2 × B12 |
| combined p-value: | =CHISQ.DIST.RT(B13, 2 × COUNT(A2:A11)) |
| Reference | Design | Pop (Age) | N | In/Ex | Tx vs. Ctrl | Dur | O1 | O2 | Out (Tx) | Out (Ctrl) | Stat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liu (2017) [48] | Two-stage, parallel, Rnd, DB, DD | Child. 6–12 yrs AH + AR | 240 tot (MF vs. Placebo; Stage 2: 4 groups) | In: AH ≥ 75% rhinoph., sympt. ≥ 12 mo, positive allergy tests Ex: Seasonal rhinitis, craniofacial anomalies, recent ster./ATB | Stage 1 (6 wks): MF 50 µg/nostril/day vs placebo Stage 2 (8 wks, non-responders): MF + Oxymet vs. MF + Placebo vs. Placebo + Oxymet vs. Placebo + Placebo | 6 wks + 8 wks | A/C reduction, increased nasal volume | Symptom reduction (congestion, snoring, etc.) | A/C: 87.2→27.3; Nasal vol. 11.2→16.8; TSS: 16.5→4.8 | A/C: 85.1→83.4; Nasal vol. 11.1→11.2; TSS: 15.3→14.9 | p < 0.05 (A/C, nasal vol., TSS, congestion, snoring) |
| Hashemi Jazi (2011) [49] | Prosp., Rnd, longitudinal | Child. 2–10 yrs AH | 39 tot (20 Flutic. vs. 19 Azithro) | In: AH + OSA sympt. (apnoea, snoring, hypernasality) Ex: Craniofacial, neuromusc. anomalies, recurrent infections, ster./ATB <4 wks | Nasal Fluticasone (1 puff/nostril × 2/day ×1 wk, then 1/day × 5 wks) vs Azithromycin (12 mg/kg × 5 days in cycles ×6 wks) | 6 wks | AH severity reduction, OSA sympt. reduction | Reduced nasal obstr., snoring, mouth breathing | Obstr. 51–100%: 70%→40%; Snoring: 85%→10%; Mouth Breathing: 60%→10%; Apnoea: 10%→5% | Obstr. 51–100%: 79%→47%; Snoring: 95%→5%; Mouth Breathing: 68%→5%; Apnoea: 15%→0% | Obstr. p = 0.004; Snoring/Mouth Breathing p < 0.001; Apnoea p = 0.02 |
| Evangelisti (2022) [50] | Unblinded, open-label | Child. 3–10 yrs Severe OSAS (AHI > 10) | 28 tot (15 vs. 13) | In: Severe OSAS (PSG), awaiting A-T Ex: Chronic cardiopulm., craniofacial, genetic disorders, epilepsy, severe obesity | Oral Betamethasone (0.1 mg/kg/day × 7 days) + Nasal Beclometasone vs. Nasal Beclometasone alone | 7 days | Improved SpO2 (mean, min.), SCR reduction | ODI reduction, time < 90% SpO2 | SCR: 12.6→8.3; Mean SpO2: 95.3→97.0; Min SpO2: 78.8→89.2; ODI: 11.7→3.0; <90% time: 1.75→0.0 | SCR: 12.2→12.3; Mean SpO2: 95.6→95.5; Min SpO2: 82.5→77.8; ODI: 12.3→11.3; <90% time: 1.35→2.0 | p = 0.0001 (SCR, Mean SpO2), p = 0.001 (Min SpO2), p < 0.0001 (ODI, desaturation <90% time) |
| Reference | Design | Pop (Age) | N | In/Ex | Tx vs. Ctrl | Dur | O1 | O2 | Out (Tx) | Out (Ctrl) | Stat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gelardi (2013) [58] | Rnd, blinded, PC study | Child. 4–12 yrs AH/sub-obstr. tonsils | 45 tot (27 vs. 18) | In: AH/sub-obstr. tonsils, SDB/OME > 6 mo, no immunosuppr. < 3 mo Ex: Cardiac, bronch. disorders, iodine allergy, TB | Aerosal® (micronized NaCl + iodine) 10 sessions vs. Placebo (no aerosol) | ~2 wks (10 sessions) + 3-mo follow-up | ≥25% hypertrophy reduction (clinical-endoscopic) | Hearing (≥10 dB), tympanograms, SpO2, nasal cytology | ≥25% reduction: 44.4%; Hearing +5 dB; Tymp. improvement 29.6% | ≥25% reduction: 22.2%; Hearing 0 dB; Tymp. 5.6% | p = 0.204 (hypertrophy), p = 0.018 (hearing), p = 0.064 (tymp.) |
| Hood (2021) [60] | MC, DB, Rnd, CT | Child. 3–7.99 yrs with SCA + SDB | Expected 200 tot | In: SCA (HbSS/HbSβ0), SDB, no current mont. Ex: Adverse mont. reactions, developmental disorders, participation in other trials | Montelukast 4 mg/day vs. Placebo | 12 wks | Primary: ↑ cognitive processing speed (secondary: AH reduction) | Executive function, cerebral perfusion, SDB parameters | Mont. (n = 100): Processing speed +8 pts; A/N ratio 0.81→0.57 | Ctrl (n = 100): No change; A/N ratio unchanged | Power 90% for Δ = 8 pts cognitive; α = 0.025; ITT analysis |
| Tracy (1998) [59] | DB-PC-Rnd | Child. 3–11 yrs Chronic OME | 61 tot (20 vs. 19 vs. 20) (3 groups) | In: OME > 3 mo, >3 AOM episodes in 6 mo Ex: Systemic ster. <6 mo, tubes, beclom. allergy, nasal spray < 2 wks | 1) ATB (amoxic./sulf.) 2) ATB + nasal beclom. 336 µg/day 3) ATB + nasal placebo | 12 wks | Middle ear effusion resolution (tympanometry/otoscopy) | Ear pain, hearing, tympanic mobility | G2 (ATB+bec.): Faster effusion resolution, improved tympanic/otoscopy scores (p ≤ 0.05 at 4–8 wks) | G1/G3: Slower improvement, “catch-up” at 12 wks | p ≤ 0.01 (right tymp. pressure), p ≤ 0.004 (symptoms), p ≤ 0.05 (effusion) |
| Wang (2017) [62] | Cross-sect. | Child. <18 yrs with OME | Data on 1.94 × 109 visits | In: OME diagnosis Ex: Acute otitis media, ATB use, recent surgery | Intranasal steroids (various) vs. No use | Retrosp. 2005–2012 | Intranasal steroid prescription frequency in OME | QoC implications, appropriateness | Steroids 10.0% (95% CI 6.3–15.5) OR = 3.58 | Steroids 3.5% (95% CI 3.1–3.9) | p = 0.002 (OR), p < 0.001 (risk diff.) |
| Sobhy (2013) [33] | Rnd prosp. parallel | Child. 3–13 yrs Post-adenoidectomy | 200 tot (100 vs. 100) | In: Adenoidectomy, residual symptoms, no ster. < 1 yr Ex: Epistaxis, immunodef., genetic/neuromusc. disorders | Mom. furoate 40 µg/nostril/day × 12 wks vs. Saline | 12 mo | Prevention of nasal obstr., rhinorrhea, post-surg. snoring recurrence | QoL, re-surgery reduction | Nasal obstr.: 2.31→0.73; Discharge: 2.16→0.67; Snoring: 2.27→0.79 | Nasal obstr.: 2.33→1.49; Discharge: 2.12→1.53; Snoring: 2.25→1.44 | OR = 2.89 p = 0.001 (obstr.), OR = 3.21 p = 0.0001 (discharge), OR = 2.95 p = 0.0001 (snore) |
| Zhao (2023) [65] | Prosp. Rnd CT | Child. 2–9 yrs AH | 93 tot (31 AAT, 32 Xiaoxian + AAT, 30 Montel.) | In: AH + nasal congestion, mouth br., snoring, high OSA-18 Ex: Prev. adenoidectomy, polyps, septal dev., recent ster. | AAT vs. Xiaoxian + AAT vs Mont. 4–5 mg/day | 1 mo + 6-mo follow-up | Improvement in congestion, mouth br., snoring | QoL, recurrence rate | OSA-18: 69.56→53.47; Congestion: 1.63→0.50; Mouth br.: 1.56→0.47; Snoring: 1.88→0.38; Sec. sympt.: 3.0→0.72 | OSA-18: 67.43→58.50; Congestion: 1.67→0.57; Snoring: 1.87→1.0; Sec. sympt.: 2.87→1.32 | OSA-18: p = 0.004, Open-mouth p = 0.006, Snore p = 0.008, Sec. sympt. p = 0.0014 |
| Bilgili (2023b) [61] | Prosp. Rnd longit. | Child. 4–14 yrs ET dysfunction + AH | 100 tot | In: AH, ETD, snoring, mouth br., no prev. surgery Ex: Acute nas./otol. infections, craniofacial disorders, recent ster./ATB | Azelastine + Flutic. dipr. 2×/day (548 µg + 200 µg total/day) | 3 mo, No Ctrl | A/C reduction (endoscopic), ETS-7, tubomanometry | OME resolution, nasal symptoms | A/C 82→37%, ETS-7: 6.36→9.72, OME resolved 66% | N/A (no ctrl) | p < 0.01 (A/C), p = 0.001 (ETS-7, adenoid volume) |
| Bilgili (2023a) [66] | Prosp. Rnd longit. | Child. 4–13 yrs AH | 65 tot No Ctrl | In: AH > 6 mo, no ster. < 4 wks, no allergies/atopy Ex: Prev. surgery, craniofacial disorders | Azelastine+Flutic. (MP-AzeFlu) 2×/day (548 µg azel. + 200 µg flutic.) | 24 wks, No Ctrl | A/C reduction, symptom improvement (obstr., snoring, apnoea, etc.) | Need for surgery, QoL | A/C: 3.57→1.74, Total sympt.: 15.63→2.31, Snoring: 2.82→0.62, Apnoea: 2.52→0.22 | N/A (no ctrl) | p < 0.01– < 0.001 (A/C, all symptoms) |
Appendix C
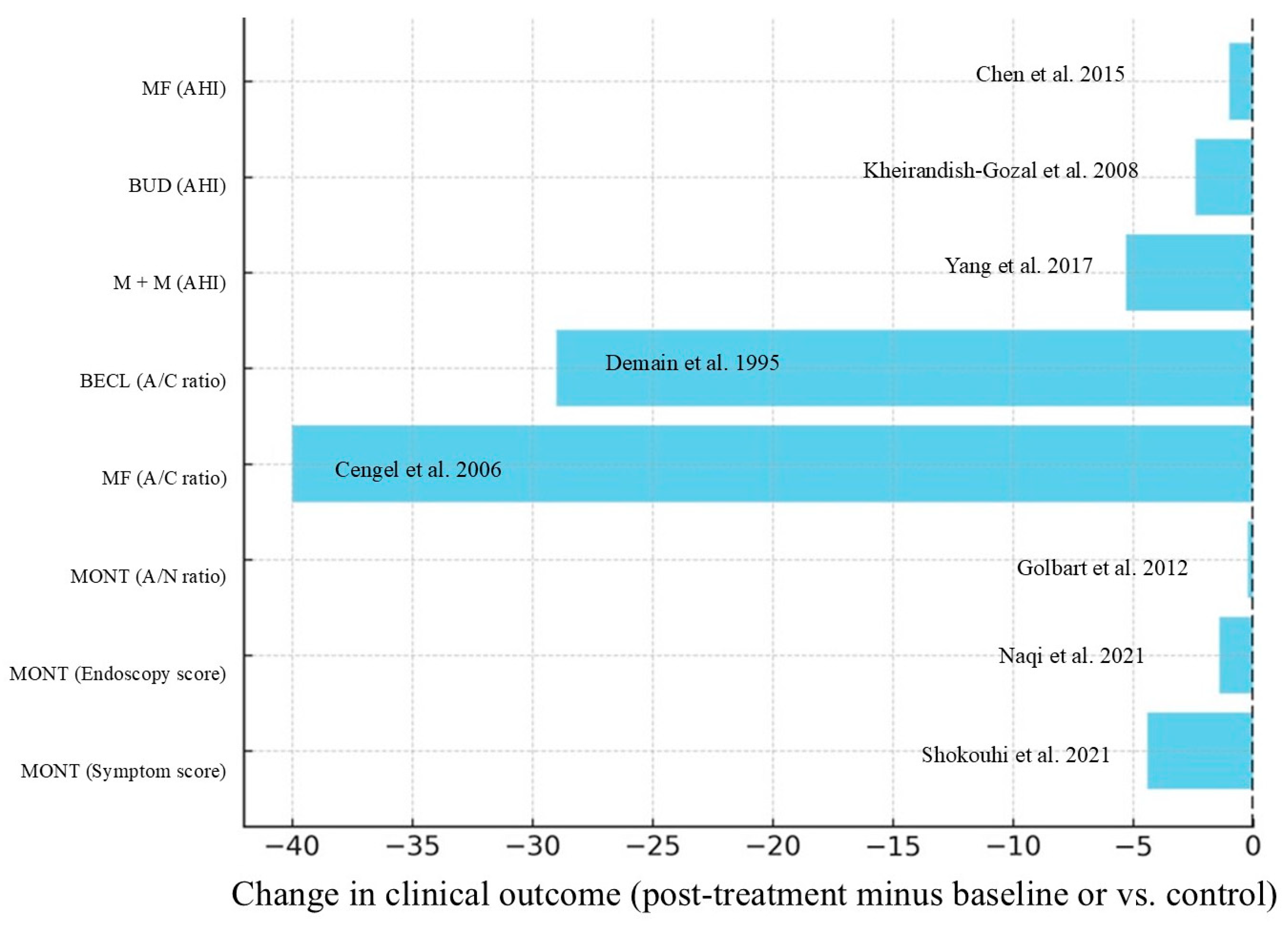
References
- Bitners, A.C.; Arens, R. Evaluation and Management of Children with Obstructive Sleep Apnea Syndrome. Lung 2020, 198, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Chae, K.Y. Obstructive sleep apnea syndrome in children: Epidemiology, pathophysiology, diagnosis and sequelae. Korean J. Pediatr. 2010, 53, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Kaditis, A.G.; Alonso Alvarez, M.L.; Boudewyns, A.; Alexopoulos, E.I.; Ersu, R.; Joosten, K.; Larramona, H.; Miano, S.; Narang, I.; Trang, H.; et al. Obstructive sleep disordered breathing in 2- to 18-year-old children: Diagnosis and management. Eur. Respir. J. 2016, 47, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, F.; Zucchini, L.; Pellitteri, F.; Palone, M.; Lombardo, L. Obstructive Sleep Apnea in Developmental Age: 22-Item Pediatric Sleep Questionnaire for an Observational Descriptive Investigation. Children 2023, 10, 1265. [Google Scholar] [CrossRef]
- Nosetti, L.; Zaffanello, M.; Simoncini, D.; Dellea, G.; Vitali, M.; Amoudi, H.; Agosti, M. Prioritising Polysomnography in Children with Suspected Obstructive Sleep Apnoea: Key Roles of Symptom Onset and Sleep Questionnaire Scores. Children 2024, 11, 1228. [Google Scholar] [CrossRef]
- Lein, A.; Altumbabic, H.; Đešević, M.; Baumgartner, W.D.; Salkic, A.; Umihanic, S.; Ramaš, A.; Harčinović, A.; Kosec, A.; Brkic, F.F. Association of adenoid hypertrophy and clinical parameters with preoperative polygraphy in pediatric patients undergoing adenoidectomy. Eur. Arch. Otorhinolaryngol. 2025, 282, 1075–1084. [Google Scholar] [CrossRef]
- Sant’Ana, J.P.; Mastrandonakis, I.C.F.; Silva, R.S.B.; Duprat, A.C.; Floriano, C.G.; Miyake, M.M. Reliability of nasofibroscopy for the evaluation of adenoid hypertrophy and its correlation with clinical symptoms. Braz. J. Otorhinolaryngol. 2023, 89, 101307. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, R. Pediatric Obstructive Sleep Apnea: Diagnostic Challenges and Management Strategies. Cureus 2024, 16, e75347. [Google Scholar] [CrossRef]
- Kuhle, S.; Hoffmann, D.U.; Mitra, S.; Urschitz, M.S. Anti-inflammatory medications for obstructive sleep apnoea in children. Cochrane Database Syst. Rev. 2020, 1, Cd007074. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Bhattacharjee, R.; Bandla, H.P.R.; Gozal, D. Antiinflammatory therapy outcomes for mild OSA in children. Chest 2014, 146, 88–95. [Google Scholar] [CrossRef]
- Liming, B.J.; Ryan, M.; Mack, D.; Ahmad, I.; Camacho, M. Montelukast and Nasal Corticosteroids to Treat Pediatric Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. Otolaryngol. Head. Neck Surg. 2019, 160, 594–602. [Google Scholar] [CrossRef] [PubMed]
- de Benedictis, F.M.; Bush, A. Corticosteroids in respiratory diseases in children. Am. J. Respir. Crit. Care Med. 2012, 185, 12–23. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Bandla, H.P.; Gozal, D. Montelukast for Children with Obstructive Sleep Apnea: Results of a Double-Blind, Randomized, Placebo-Controlled Trial. Ann. Am. Thorac. Soc. 2016, 13, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Goldbart, A.D.; Greenberg-Dotan, S.; Tal, A. Montelukast for children with obstructive sleep apnea: A double-blind, placebo-controlled study. Pediatrics 2012, 130, e575–e580. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Lu, T.; Qiu, Y.; Li, X.; Liu, Y.; Tai, J.; Guo, Y.; Zhang, J.; Wang, S.; Zhao, J.; et al. The efficacy and safety of montelukast in children with obstructive sleep apnea: A systematic review and meta-analysis. Sleep. Med. 2021, 78, 193–201. [Google Scholar] [CrossRef]
- Chohan, A.; Lal, A.; Chohan, K.; Chakravarti, A.; Gomber, S. Systematic review and meta-analysis of randomized controlled trials on the role of mometasone in adenoid hypertrophy in children. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1599–1608. [Google Scholar] [CrossRef]
- Liu, H.T.; Lin, Y.C.; Kuan, Y.C.; Huang, Y.H.; Hou, W.H.; Liou, T.H.; Chen, H.C. Intranasal corticosteroid therapy in the treatment of obstructive sleep apnea: A meta-analysis of randomized controlled trials. Am. J. Rhinol. Allergy 2016, 30, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, A.G.; Chervoneva, I.; Cielo, C.M.; Bradford, R.; Cornaglia, M.A.; Tapia, I. Secondary Analysis of a Randomized Controlled Trial of Intranasal Corticosteroids in the Treatment of Mild to Moderate Pediatric Obstructive Sleep Apnea. In TP73. TP073 PEDIATRIC SLEEP; American Thoracic Society International Conference Abstracts; American Thoracic Society: New York, NY, USA, 2021; p. A3345. [Google Scholar]
- Alanazi, F.; Alruwaili, M.; Alanazy, S.; Alenezi, M. Efficacy of montelukast for adenoid hypertrophy in paediatrics: A systematic review and meta-analysis. Clin. Otolaryngol. 2024, 49, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leng, S.; Hu, Q.; Li, Y.; Wei, Y.; Lu, Y.; Qie, D.; Yang, F. Pharmacological interventions for pediatric obstructive sleep apnea (OSA): Network meta-analysis. Sleep. Med. 2024, 116, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.; Krishna, M.M.; Franco, A.J.; Jekov, L.; Sudo, R.Y.U.; Cabral, T.D.D. Efficacy of combination therapy with mometasone and montelukast versus mometasone alone in treatment of adenoid hypertrophy in children: A systematic review and meta-analysis. Am. J. Otolaryngol. 2025, 46, 104566. [Google Scholar] [CrossRef]
- Yoon, S.; Baik, B.; Park, T.; Nam, D. Powerful p-value combination methods to detect incomplete association. Sci. Rep. 2021, 11, 6980. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.J. The harmonic mean p-value for combining dependent tests. Proc. Natl. Acad. Sci. USA 2019, 116, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- van Dijk, S.H.B.; Brusse-Keizer, M.G.J.; Bucsán, C.C.; van der Palen, J.; Doggen, C.J.M.; Lenferink, A. Artificial intelligence in systematic reviews: Promising when appropriately used. BMJ Open 2023, 13, e072254. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Kumar, M.V.; Su, E.; Flores Miranda, A.; Saha, A.; Sussman, J. Evaluating the efficacy of artificial intelligence tools for the automation of systematic reviews in cancer research: A systematic review. Cancer Epidemiol. 2024, 88, 102511. [Google Scholar] [CrossRef]
- Islam, N.; van der Schaar, M. Use of generative artificial intelligence in medical research. BMJ 2024, 384, q119. [Google Scholar] [CrossRef]
- Ganjavi, C.; Eppler, M.B.; Pekcan, A.; Biedermann, B.; Abreu, A.; Collins, G.S.; Gill, I.S.; Cacciamani, G.E. Publishers’ and journals’ instructions to authors on use of generative artificial intelligence in academic and scientific publishing: Bibliometric analysis. BMJ 2024, 384, e077192. [Google Scholar] [CrossRef]
- Won, S.; Morris, N.; Lu, Q.; Elston, R.C. Choosing an optimal method to combine P-values. Stat. Med. 2009, 28, 1537–1553. [Google Scholar] [CrossRef]
- Berlucchi, M.; Salsi, D.; Valetti, L.; Parrinello, G.; Nicolai, P. The role of mometasone furoate aqueous nasal spray in the treatment of adenoidal hypertrophy in the pediatric age group: Preliminary results of a prospective, randomized study. Pediatrics 2007, 119, e1392–e1397. [Google Scholar] [CrossRef]
- Baker, A.; Grobler, A.; Davies, K.; Griffiths, A.; Hiscock, H.; Kubba, H.; Peters, R.L.; Ranganathan, S.; Rimmer, J.; Rose, E.; et al. Effectiveness of Intranasal Mometasone Furoate vs Saline for Sleep-Disordered Breathing in Children: A Randomized Clinical Trial. JAMA Pediatr. 2023, 177, 240–247. [Google Scholar] [CrossRef]
- Chan, C.C.; Au, C.T.; Lam, H.S.; Lee, D.L.; Wing, Y.K.; Li, A.M. Intranasal corticosteroids for mild childhood obstructive sleep apnea--a randomized, placebo-controlled study. Sleep. Med. 2015, 16, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, T.S. Role of intranasal steroid in the prevention of recurrent nasal symptoms after adenoidectomy. Int. J. Otolaryngol. 2013, 2013, 603493. [Google Scholar] [CrossRef]
- Yilmaz, H.B.; Celebi, S.; Sahin-Yilmaz, A.; Oysu, C. The role of mometasone furoate nasal spray in the treatment of adenoidal hypertrophy in the adolescents: A prospective, randomized, cross-over study. Eur. Arch. Otorhinolaryngol. 2013, 270, 2657–2661. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Chakravarti, A. A double-blind randomized placebo-controlled trial of topical intranasal mometasone furoate nasal spray in children of adenoidal hypertrophy with otitis media with effusion. Am. J. Otolaryngol. 2014, 35, 766–770. [Google Scholar] [CrossRef]
- Cengel, S.; Akyol, M.U. The role of topical nasal steroids in the treatment of children with otitis media with effusion and/or adenoid hypertrophy. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Lepcha, A.; Kurien, M.; Job, A.; Jeyaseelan, L.; Thomas, K. Chronic adenoid hypertrophy in children-is steroid nasal spray beneficial? Indian. J. Otolaryngol. Head. Neck Surg. 2002, 54, 280–284. [Google Scholar] [CrossRef]
- Criscuoli, G.; D’Amora, S.; Ripa, G.; Cinquegrana, G.; Mansi, N.; Impagliazzo, N.; Pisacane, A. Frequency of surgery among children who have adenotonsillar hypertrophy and improve after treatment with nasal beclomethasone. Pediatrics 2003, 111, e236–e238. [Google Scholar] [CrossRef]
- Demain, J.G.; Goetz, D.W. Pediatric adenoidal hypertrophy and nasal airway obstruction: Reduction with aqueous nasal beclomethasone. Pediatrics 1995, 95, 355–364. [Google Scholar] [CrossRef]
- Gudnadottir, G.; Ellegård, E.; Hellgren, J. Intranasal Budesonide and Quality of Life in Pediatric Sleep-Disordered Breathing: A Randomized Controlled Trial. Otolaryngol. Head. Neck Surg. 2018, 158, 752–759. [Google Scholar] [CrossRef]
- Hong, H.; Chen, F.; Zheng, X.; Liao, W.; Liao, Z.; Cao, Y.; He, H.; Zhu, Z.; Fan, Y. Decreased frequency of adenoidectomy by a 12-week nasal budesonide treatment. Ther. Clin. Risk Manag. 2017, 13, 1309–1316. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics 2008, 122, e149–e155. [Google Scholar] [CrossRef]
- Esteitie, R.; Emani, J.; Sharma, S.; Suskind, D.L.; Baroody, F.M. Effect of fluticasone furoate on interleukin 6 secretion from adenoid tissues in children with obstructive sleep apnea. Arch. Otolaryngol. Head. Neck Surg. 2011, 137, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Brouillette, R.T.; Manoukian, J.J.; Ducharme, F.M.; Oudjhane, K.; Earle, L.G.; Ladan, S.; Morielli, A. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J. Pediatr. 2001, 138, 838–844. [Google Scholar] [CrossRef]
- Demirhan, H.; Aksoy, F.; Ozturan, O.; Yildirim, Y.S.; Veyseller, B. Medical treatment of adenoid hypertrophy with “fluticasone propionate nasal drops”. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 773–776. [Google Scholar] [CrossRef]
- Ciprandi, G.; Varricchio, A.; Capasso, M.; Varricchio, A.M.; De Lucia, A.; Ascione, E.; Avvisati, F.; Capristo, C.; Marseglia, G.L.; Barillari, U. Intranasal flunisolide treatment in children with adenoidal hypertrophy. Int. J. Immunopathol. Pharmacol. 2007, 20, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Varricchio, A.; Tortoriello, G.; Capasso, M.; De Lucia, A.; Marchisio, P.; Varricchio, A.M.; Mansi, N.; Giordano, L.; Liberatore, G.; Di Gioacchino, M.; et al. Prevention of surgery in children with adenoidal hypertrophy treated with intranasal flunisolide: A 12-month follow-up. J. Biol. Regul. Homeost. Agents 2009, 23, 95–101. [Google Scholar] [PubMed]
- Liu, W.; Zhou, L.; Zeng, Q.; Luo, R. Combination of mometasone furoate and oxymetazoline for the treatment of adenoid hypertrophy concomitant with allergic rhinitis: A randomized controlled trial. Sci. Rep. 2017, 7, 40425. [Google Scholar] [CrossRef]
- Jazi, S.M.; Barati, B.; Kheradmand, A. Treatment of adenotonsillar hypertrophy: A prospective randomized trial comparing azithromycin vs. fluticasone. J. Res. Med. Sci. 2011, 16, 1590–1597. [Google Scholar]
- Evangelisti, M.; Barreto, M.; Di Nardo, G.; Del Pozzo, M.; Parisi, P.; Villa, M.P. Systemic corticosteroids could be used as bridge treatment in children with obstructive sleep apnea syndrome waiting for surgery. Sleep. Breath. 2022, 26, 879–885. [Google Scholar] [CrossRef]
- Naqi, S.A.; Ashfaq, A.H.; Umar, M.A.; Karmani, J.K.; Arshad, N. Clinical outcome of Montelukast Sodium in Children with Adenoid Hypertrophy. Pak. J. Med. Sci. 2021, 37, 362–366. [Google Scholar] [CrossRef]
- Shokouhi, F.; Meymaneh Jahromi, A.; Majidi, M.R.; Salehi, M. Montelukast in Adenoid Hypertrophy: Its Effect on Size and Symptoms. Iran. J. Otorhinolaryngol. 2015, 27, 443–448. [Google Scholar] [PubMed]
- Wang, Z.; Wu, X.; Liu, J.; Wang, Y.; Zhang, Y.; Wu, Y.; Kang, Y.; Zhang, R.; Li, J.; Liu, D. Effects of oral cysteine leukotriene receptor antagonist-montelukast on adenoid lymphoid tissue: A histopathological study under light microscope. Front. Pharmacol. 2023, 14, 1285647. [Google Scholar] [CrossRef]
- Yang, D.Z.; Liang, J.; Zhang, F.; Yao, H.B.; Shu, Y. Clinical effect of montelukast sodium combined with inhaled corticosteroids in the treatment of OSAS children. Medicine 2017, 96, e6628. [Google Scholar] [CrossRef]
- Ras, A.E.; Hamed, M.H.; Abdelalim, A.A. Montelukast combined with intranasal mometasone furoate versus intranasal mometasone furoate; a comparative study in treatment of adenoid hypertrophy. Am. J. Otolaryngol. 2020, 41, 102723. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Pourroshani, B.; Eftekhari, K.; Malekiantaghi, A.; Ashournia, P.; Shafiei, A. Effect of Combination Montelukast and Nasal Mometasone on Childhood Adenoid Hypertrophy. Iran. J. Otorhinolaryngol. 2024, 36, 391–397. [Google Scholar] [CrossRef]
- Tuhanıoğlu, B.; Erkan, S.O. Evaluation of the effects of montelukast, mometasone furoate, and combined therapyon adenoid size: A randomized, prospective, clinical trial with objective data. Turk. J. Med. Sci. 2017, 47, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Gelardi, M.; Iannuzzi, L.; Greco Miani, A.; Cazzaniga, S.; Naldi, L.; De Luca, C.; Quaranta, N. Double-blind placebo-controlled randomized clinical trial on the efficacy of Aerosal in the treatment of sub-obstructive adenotonsillar hypertrophy and related diseases. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1818–1824. [Google Scholar] [CrossRef]
- Tracy, J.M.; Demain, J.G.; Hoffman, K.M.; Goetz, D.W. Intranasal beclomethasone as an adjunct to treatment of chronic middle ear effusion. Ann. Allergy Asthma Immunol. 1998, 80, 198–206. [Google Scholar] [CrossRef]
- Hood, A.M.; Stotesbury, H.; Kölbel, M.; DeHaan, M.; Downes, M.; Kawadler, J.M.; Sahota, S.; Dimitriou, D.; Inusa, B.; Wilkey, O.; et al. Study of montelukast in children with sickle cell disease (SMILES): A study protocol for a randomised controlled trial. Trials 2021, 22, 690. [Google Scholar] [CrossRef]
- Bilgili, A.M.; Durmaz, H.; Dilber, M. Eustachian Tube Dysfunction in Children with Adenoid Hypertrophy: The Effect of Intranasal Azelastine-Fluticasone Spray Treatment on Middle Ear Ventilation and Adenoid Tissue. Ear Nose Throat J. 2023, 102, 198–203. [Google Scholar] [CrossRef]
- Wang, D.E.; Lam, D.J.; Bellmunt, A.M.; Rosenfeld, R.M.; Ikeda, A.K.; Shin, J.J. Intranasal Steroid Use for Otitis Media with Effusion: Ongoing Opportunities for Quality Improvement. Otolaryngol. Head. Neck Surg. 2017, 157, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Chakravarti, A. Role of mometasone furoate aqueous nasal spray for management of adenoidal hypertrophy in children. J. Laryngol. Otol. 2014, 128, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W.H.; Pathadka, S.; Qin, S.X.; Fung, L.W.Y.; Yan, V.K.C.; Yiu, H.H.E.; Bloom, C.I.; Wong, I.C.K.; Chan, E.W.Y. Neuropsychiatric events associated with montelukast in patients with asthma: A systematic review. Eur. Respir. Rev. 2023, 32, 230079. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, J.; Wang, M.Y.; Hou, Z.W.; Shi, H.S.; Zhang, X.X. Effect of oral Xiao-xian decoction combined with acupoint application therapy on pediatric adenoid hypertrophy: A randomized trial. Medicine 2023, 102, e32804. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, A.M.; Durmaz, H.; Dilber, M. Efficacy of Topical Azelastine and Fluticasone Dipropionate Combination in Children with Adenoid Hypertrophy. Ear Nose Throat J. 2023, 102, 28–34. [Google Scholar] [CrossRef]


| Reference | Design | Pop (Age) | N | In/Ex | Tx vs. Ctrl | Dur | O1 | O2 | Out (Tx) | Out (Ctrl) | Stat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bhargava (2014) [35] | DB-RCT | Child. 2–12 yrs, AH ± OME | 100 (30 vs. 32) | In: AH G3–4 ≥ 3 mo, no resp. prev. treatments Ex: prev. adenoidect., ster. < 1 yr, craniofacial disord., Down synd., recurr. acute inf. | MF 200 µg/day (2 puffs/nostril/day) vs. Saline | 24 wks | OME resol., adenoid size reduct. | PTA, sympt., QoL | OME resol. 93% | OME resol. 50% | p = 0.04 sympt.; p = 0.0001 (adenoid reduct. Tx, NS in Ctrl); p = 0.0004 (OME); p < 0.0001 (PTA); p = 0.0001 (QoL) |
| Berlucchi (2007) [30] | RCT | Child. 3–7 yrs, AH ≥75% | 60 tot (27 vs. 30; 57 compl.) | In: choan. obstruct. ≥75%, 3–7 yrs Ex: tonsil hypert., allergies, ster. < 4 wks, acute inf. < 2 wks, craniofacial disord. | MF 50 µg/day (1 puff/nostril/day) vs. Placebo | 40 days + 3 mo maint. | Adenoid pad reduct. (avoid adenoidect.) | Obstr. sympt. (obstruct., rhinorrhea, cough, snoring, apnoea), AE | Choan. obstr. 88.5%→64% Tot sympt. 11→3 | 76.5%→76% Tot sympt. 10→9 | p < 0.001 (choan. obstr., total sympt., nas. obstr.), p = 0.00005 (snor.), p = 0.00034 (apnoea) |
| Cengel (2006) [36] | CRS | Child. 3–15 yrs, AH ± OME ≥3 mo | 122 tot (67 vs. 55) | In: AH/OME ≥ 3 mo, ≥2 ATB courses Ex: ster. < 4 wks, immunodef., MF allergy, craniofacial disord. | MF 100 µg/day (1 puff/nostril/day) vs. No Tx | 6 wks | AH reduct. (choan. %), OME resol. | Sympt. improv. (snoring, obstr., mouth breath., apnoea) | OME resol. 42.2%, A/C ratio 80→40 | OME resol. 14.5%, A/C ratio 70→80 | p < 0.001 (OME, A/C ratio, sympt.) |
| Yilmaz (2013) [34] | DB-RXO | Adolesc. 12–18 yrs, AH | 28 (30 init., 2 lost) | In: nasal obstruct. ≥ 6 mo Ex: ster. < 1 yr, immunodef., prev. adenoidect. | MF 200 µg/day (6 wks) vs. Saline, 3-wk wash-out, Tx crossover | 6 wks + 6 wks | Adenoid vol. (NS) | Total sympt., QoL | TSS: 6.56→4.31 | TSS: 6.50→5.33 | p = 0.000 (total sympt.), p = 0.428 (adenoid vol.) |
| Baker (2023) [31] | MC-DB-RCT | Child. 3–12 yrs, SDB ≥ 2 wks | 276 tot (138 vs. 138) 9.4% lost | In: SDB score ≥ −1, No prev. adenotonsillect., BMI <97th perc., no ster. < 6 wks | MF 100 µg/day (1 puff/nostril/day) vs. Saline | 6 wks | SDB sympt. resol. | ENT eval., QoL (PedsQL), PSQ-SDB, OSA-5, parental satisf. | SDB resol. 44%, PSQ-SDB 0.51→0.38, OSA-5 6.2→3.6 | SDB resol. 41%, PSQ-SDB 0.53→0.40, OSA-5 5.5→3.8 | p = 0.51 (SDB), p = 0.00 (PSQ-SDB, OSA-5, QoL, satisf.) |
| Chan (2015) [32] | RCT | Child. 6–18 yrs, mild OSA | 62 tot (31 vs. 31; 50 compl.) | In: OAHI 1–5, snoring ≥ 3 nights/wk Ex: craniofacial disord., elev. BMI, nasal/phar. surg. | MF 200 µg/day (2 puff/nostril/day) vs Placebo | 4 mo | OAHI change (PSG pre/post) | ODI, snoring freq., adenoid/tonsil size (endosc.), sleep param. | OAHI 2.7→1.7, ODI −0.6, snoring 75%→54.5% | OAHI 2.5→2.9, ODI +0.7, snoring unchanged | p = 0.039 (OAHI), p = 0.037 (ODI), p = 0.031 (snoring) |
| Sobhy (2013) [33] | RCT | Child. 3–13 yrs post-adenoidect. | 200 tot (100 vs. 100) | In: post-adenoidect. with persistent sympt. Ex: ster. < 1 yr, epistaxis, immunodef., genetic/neuromusc. disord. | MF 40 µg/day vs. Saline | 12 mo | Sympt. recurrence prev. (nasal obstruct., rhinorrhea, snoring) | QoL, reduct. reintervention | Obstr. 2.31→0.73, Nasal secr. 2.16→0.67, Snor. 2.27→0.79 | Obstr. 2.33→1.49, Nasal secr. 2.12→1.53, Snor. 2.25→1.44 | p = 0.001 (obstr.), p = 0.0001 (secr./snor.), p = 0.003 (X-ray) |
| Reference | Design | Pop (Age) | N | In/Ex | Tx vs. Ctrl | Dur | O1 | O2 | Out (Tx) | Out (Ctrl) | Stat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lepcha (2002) [37] | DB-RCT | Child. 3–12 yrs | 31 tot (5 lost) | In: AH (clinical + X-ray), no ster. < 1 yr, no nasal spray < 2 wks, no immunodef./epistaxis. Ex: see inclusion criteria | Beclom. 200 µg/day vs. Placebo | 8 wks | Improv. obstruct. sympt. + adenoid size reduct. (X-ray/endosc.) | Eval. nasal secretion, OME, AE | Nasal Block: −1.54 ± 0.97; Snoring: −1.31 ± 1.18; Endosc.: −0.38 ± 0.51 | Nasal Block: −1.69 ± 1.11; Snoring: −1.77 ± 1.01; Endosc.: −0.08 ± 0.28 | p = NS symptom scores (p ≥ 0.07) |
| Criscuoli (2003) [38] | Rnd single-blind, crossover | Child. ~3.8 yrs | 60 tot (53 compl.) | In: obstruct. ≥ 6 mo, AH/NF > 0.5, planned A-T. Ex: ster. < 1 yr, epistaxis, immunodef., URTI < 2 wks | Beclom. 400 µg/day vs Saline (2-wk cross.), then 200 µg/day “open” 24 wks | 4 wks (cross.) + 24 wks (open) (total ~28 wks); follow-up 100 wks | ≥50% reduct. nasal obstruction index (NOI) score | Freq. adenotonsillect., AE, obstruct. meas. at 24, 52, and 100 wks | Nasal Block: −1.54 ± 0.97; Snoring: −1.31 ± 1.18; Mild improv. X-ray/endosc. (NS) | N/A (placebo 2 wks), then all open-label beclom. | p > 0.05 for radiogr. and endosc. diff.; no severe AE reported |
| Demain (1995) [39] | DB-PC-RXO | Child. 5–11 yrs | 20 tot (17 compl. 8 wks, 14 compl. 24 wks) | In: Chron. nasal obstruct. sympt., A/C ratio ~90%, no ster. < 12 mo, no immunodef./epistaxis | Beclom. 336 µg/day (2 puff x2/day) vs. Placebo (8 wks), then “open” 168 µg/day for 16 wks | 8-wk cross + 16 wks open (total 24 wks) | Adenoid size reduct. (rhinoscopy: −29% at 24 wks) Obstruct. sympt. reduct. (−82% score) | Reduct. snoring, rhinolalia, enuresis in 8/9, fewer ATB, improv. OME pressures | A/C ratio 91%→−14/−15% at 4 wks, −29% at 24 wks; sympt. score: −82% | Ctrl: minimal ratio changes (0%→−2%); sympt. scores essentially unchanged | p = 0.0002 and 0.0006 (A/C reduct.), p≈0.05 (sympt.); carryover eff. in RXO |
| Reference | Design | Pop (Age) | N | In/Ex | Tx vs. Ctrl | Dur | O1 | O2 | Out (Tx) | Out (Ctrl) | Stat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gudnadottir (2018) [40] | Pros, Rnd, DB, PC | Child. 4–10 yrs with SDB | 60 tot (30 vs. 30) | In: SDB ≥ 3 mo, no steroids < 4 wks, no prev. AT surgery, no craniofacial disorders. Ex: acute infections, severe SDB | Budesonide 128 µg/day vs. Placebo | 6 wks | OSA-18 reduction (QoL in SDB) | Snoring, apnoeas, nasal obstruction, QoL (VAS), adenoid size | OSA-18: 65.2→45.7 (Δ −19.5) | OSA-18: 54.8→47.3 (Δ −7.5) | OSA-18: p = 0.0014 Snoring: p = 0.0020 Caregiver concern: p = 0.0057 QoL (VAS): p < 0.001 |
| Hong (2017) [41] | Rnd, DB | Prepubertal child. 6–8 yrs | 100 tot (92 compl.) | In: nasal obstruction + snoring ≥ 6 mo, A/N ratio > 0.5, AHI ≥ 2, scheduled adenoidectomy. Ex: steroids < 6 mo, epistaxis, immunodef., URTI < 2 wks, required tonsillectomy | Nebulised budesonide (1 mg/2 mL/day, 2 wks) + subsequent nasal spray 64 µg/nostril/day vs. Saline 2 mL/day for 2 wks | Tot 26 wks (2 wks DB + 12 wks open-label + follow-up) | NOI reduction ≥ 2 + reduced adenoidectomy rate | Snoring, nasal secretion, OME, AE, growth | NOI: 3.41→1.92 Snoring: 3.39→2.01 Adenoidectomy: 30.77% | NOI: 3.37→3.32 Snoring: 3.42→3.38 Adenoidectomy: 73.33% | NOI, snoring, secretion: p < 0.001 Adenoidectomy: p = 0.002 |
| Kheirandish-Gozal (2008) [42] | DB, Rnd, crossover | Child. 6–12 yrs with mild OSAS | 62 tot (48 vs. 32) | In: mild OSAS (AHI 2–7), habitual snoring, hypertrophic AH Ex: asthma with prev. therapy, recent steroids, immunodef., nasal surg., craniofacial abnormalities | Budesonide 64 µg/day vs. Placebo (saline) | 6 wks | AHI and N/P ratio reduction | Sleep architecture, SpO2 | OAHI: 3.7→1.3 N/P: 0.71→0.57 Nadir SpO2: 88.9%→91.4% | OAHI: 2.9→4.0 N/P: 0.77→0.77 Nadir SpO2: 90.1%→88.5% | p < 0.0001 (AHI, N/P) p = 0.004 (SpO2) |
| Reference | Design | Pop (Age) | n | In/Ex | Tx vs. Ctrl | Dur | O1 | O2 | Out (Tx) | Out (Ctrl) | Stat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Esteitie (2011) [43] | Rnd, prosp., open-label, parallel-group | Child. 2–12 yrs OSA (AHI ≥5/h) | 24 tot (11 vs. 13) | In: OSAS (PSG), scheduled for A-T, BMI < 95th perc., no recent steroids, no uncontrolled asthma Ex: craniofacial anomalies, systemic disorders | Fluticasone furoate 55 µg/nostril/day vs. No Tx | 2 wks | Adenoid tissue IL-6 reduction | Other inflamm. markers (IL-10, TGF-β, etc.) | IL-6: 44 (13–311) pg/mL | IL-6: 133 (10–674) pg/mL | p = 0.05 (IL-6); NS other cytokines |
| Brouillette (2001) [44] | Rnd, triple-blind, PC, parallel | Child. 1–10 yrs mild-mod. OSA | 25 tot (13 vs. 12) | In: OSA (AHI > 1/h), hypertrophic AH/tonsils, no acute inf., no steroids < 3 wks Ex: severe OSA, craniofacial anomalies, steroid allergies | Nasal Fluticasone: 200 µg/day (1st wk), then 100 µg/day (5 wks) vs. Placebo | 6 wks | AHI reduction | ODI, arousal, SpO2 desat., adenoid/tonsil size, parental sympt. score | AHI: 10.7→5.8 ODI: 7.0→2.9 Arousal: 6.1→2.7 | AHI: 10.9→13.1 ODI: 5.6→5.4 Arousal: 4.1→3.9 | AHI: p = 0.04 ODI: p = 0.03 Arousal: p = 0.05 |
| Demirhan (2010) [45] | Prosp., Rnd, PC | Child. 4–16 yrs AH | 45 tot (25 vs. 20) | In: AH with indication for A-T, sympt. > 6 mo, no recent steroids, no allergies Ex: allergic rhinitis, turbinate hypertrophy, chronic disorders | Fluticasone Prop. nasal drops 400 µg/day vs Saline | 8 wks | Reduction A/C ratio, obstr. sympt., apnoeas, snoring | Total sympt. score, tonsil size, otologic parameters | Total sympt.: 13.72→2.96 A/C: 86.9%→56.2% Surgery avoided ~76% | Total sympt.: 14.85→14.65 A/C: 87.2%→85.2% Surgery 80% | p < 0.05 (sympt., A/C) No OR/RR data |
| Reference | Design | Pop (Age) | N | In/Ex | Tx vs. Ctrl | Dur | O1 | O2 | Out (Tx) | Out (Ctrl) | Stat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ciprandi (2007) [46] | Prosp., parallel-group | Child. 3–6 yrs (mean 4.5) | 178 tot (139 vs. 39) | In: AH GIII/IV, surgical indication. No steroids < 1 yr, no acute conditions < 2 wks | Nasal Flunisolide (dose in drops per weight, 2×/day, Rinowash) vs. Saline solution | 8 wks | AH grade reduction (I–IV), avoid adenoidectomy | Nasal obstruction sympt., potential surgery prevention, NS AE | AH reduction in 72.6% (AH IV: 41.7%→8.6%; Surgery avoided in 46/58) | AH reduction in 30.7% (AH IV: 15.4%→12.8%) | p < 0.02 (overall AH reduction); p < 0.04 (GIV reduction) |
| Varricchio (2009) [47] | [abstract] (Possibly Prosp. study) | Child. with AH GIII/IV (age n.r.) | 178 tot (group details n.r.) | In: AH GIII/IV (endoscopic); Exclusions not specified | Nasal Flunisolide (dose n.r.) 8 wks vs. Saline, 12-mo follow-up | 8 wks + 6–12 mo follow-up | AH grade reduction (endoscopic) | Maintenance of AH reduction (especially in allergic subjects), need for surgery, NS AE | Significant AH reduction at 8 wks (p < 0.01), maintained in allergic subjects (p < 0.05) | n.d. | p < 0.01 (initial AH reduction), p < 0.05 (maintenance in allergic subjects) |
| Reference | Design | Pop (Age) | N | In/Ex | Tx vs. Ctrl | Dur | O1 | O2 | Out (Tx) | Out (Ctrl) | Stat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Naqi (2021) [51] | RCT | Child. 4–12 yrs AH | 60 tot (30 vs. 30) | In: Symptomatic AH (snoring, apnoea, mouth breathing) + endoscopic/X-ray confirmation Ex: Obesity, acute infections, prev. A-T, recent ster./ATB | Montelukast 5 mg/day vs. Placebo | 3 mo | Adenoid reduction (endoscopy, X-ray) | Improvement in snoring, apnoea, mouth breathing | Endoscopy 3.77→2.37, X-ray 87.23%→51.33% | Endoscopy 3.57→3.43, X-ray 81.16%→77.83% | p ≤ 0.0001 (endo, X-ray), p = 0.007 (snoring), p ≤ 0.0001 (mouth breathing, sleep) |
| Goldbart (2012) [14] | DB-PC | Child. 2–10 yrs Mild-mod. OSAS | 46 tot (23 vs. 23) | In: AHI < 10, habitual snoring, AH Ex: Obesity, craniofacial anomalies, recent ster./mont. | Montelukast 4 or 5 mg/day (12 wks) vs. Placebo | 12 wks | AHI reduction, adenoid size reduction | OSAS symptom improvement, saturation | A/N ratio 0.81→0.57, AHI 6.0→3.6, OAI 3.9→1.7 | N/A | p < 0.001 (A/N ratio), p = 0.07 (AHI), p < 0.01 (OAI) |
| Kheirandish-Gozal (2016) [13] | DB-RCT-PC | Child. 2–10 yrs Mild-mod. OSA | 64 tot (28 vs. 29; 57 compl.) | In: AHI > 2, no prev. A-T, no recent ster./mont. Ex: Severe OSA, chronic conditions, craniofacial anomalies | Montelukast 4 mg/day <6 yrs, 5 mg/day ≥6 yrs (16 wks) vs. Placebo | 16 wks | AHI and OSA severity reduction (PSG) | Improvement in ODI3%, min. SpO2, arousal index | AHI 9.2→4.2, ODI3 7.2→2.8, Ad. size 2.4→2.0, SpO2 nadir 85.2→91.0 | AHI 8.2→8.7, ODI3 7.0→6.8, Ad. size 2.5→2.4, SpO2 nadir 84.8→86.1 | p < 0.0001 (AHI, SpO2), p = 0.001 (ODI3), p < 0.001 (Ad. size), p = 0.01 (arousal) |
| Shokouhi (2015) [52] | DB-RCT-PC | Child. 4–12 yrs AH ≥75% | 60 tot (30 vs. 30) | In: AH ≥ 75% (endo), nasal obstr. (snoring, apnoea, mouth breathing) Ex: Prev. A-T, ster./mont. < 4 wks, genetic/systemic disorders | Montelukast 5 mg/day vs. Placebo (12 wks) | 12 wks | Adenoid reduction (endo, A/N ratio) | Symptom improvement (snoring, mouth breathing, sleep) | 76% adenoid reduction, Sympt. 7.7→3.3 | 3% adenoid reduction, Sympt. 7.4→6.7 | p < 0.0001 (total sympt., mouth breathing), p < 0.007 (snoring) |
| Wang (2023) [53] | DB-RCT prosp. | Child. 3–8 yrs Mod-sev. AH | 20 tot (10 vs. 10) | In: AH ≥ 50%, no mont. allergy, no acute infections Ex: Tonsillar hypertrophy, OME, sinusitis, recent ster./ATB | Montelukast 5 mg/day vs. Placebo (4 wks) | 4 wks | Histopathological evaluation of adenoids (germinal centres, inflammatory infiltration) | Blood lymphocyte count, epithelial cysts, inflammation grade | Germ centres: 8.7, Cysts: 0.0, Inflamm. infiltration: 1.1 | Germ centres: 16.5, Cysts: 0.6, Inflamm. infiltration: 2.0 | p = 0.029 (germ centres), p = 0.024 (cysts), p = 0.040 (infiltration) |
| Reference | Design | Pop (Age) | n | In/Ex | Tx vs. Ctrl | Dur | O1 | O2 | Out (Tx) | Out (Ctrl) | Stat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yang (2017) [54] | RCT | Child. 2–8 yrs, Mild-mod. OSAS | 195 tot (65 Mont., 61 Mom., 57 Comb.) | In: AHI 5–10/h, snoring, obstruct. apnoeas Ex: Prev. A-T, drug allergies, craniofacial anomalies, severe obesity | Montelukast 5 mg/day vs. Nasal Mometasone 50 µg/day vs. Comb. (Mont. + Mom.) | 12 wks | AHI reduction, ↑ min SaO2 | Reduction in symptom scores (snoring, hyperventilation, restless sleep) | AHI: baseline 6.9→1.61, Min SaO2: 90.16→94.8, A/N ratio: 0.75→0.50, Snoring: 3.59→1.51 (Data across 3 groups: Mont. baseline 7.25→1.3, Mom. baseline 6.1→1.15) | N/A | p < 0.01 (AHI, snoring), p < 0.05 (min SaO2, mouth br.), p < 0.05 (A/N ratio) |
| Ras (2020) [55] | Prosp. Rnd | Child. 3–10 yrs, AH G3/4 | 100 tot (50 vs 50) | In: A/N ratio > 50%, AH G3/4, no severe OSAS, no recent ster./mont. Ex: Chronic conditions, craniofacial anomalies, allergies | Nasal Mom. (100 µg/day) + Mont. (4/5 mg/day) vs. Mom. alone | 3 mo + 3 mo follow-up | Adenoid volume reduction (A/N ratio), endoscopic improvement | VAS symptom score, recurrence rate | A/N ratio: 52.8 ± 11.3, Endoscopic improvement 68%, Recurrence 23.5% | A/N ratio: 62.88 ± 12.1, Endoscopic improvement 36%, Recurrence 55.5% | p = 0.001–0.02 (rhino, mouth br., snore, A/N ratio, endosc., recurrence) |
| Jafari (2024) [56] | Rnd, DB, PC | Child. 2–14 yrs, AH | 96 tot (51 vs. 45) | In: AH diagnosis (clinical + X-ray), obstruct. symptoms Ex: Drug allergies, genetic/neuromusc. conditions, recurrent infections, ster./ATB < 2 wks | Mont. 5 mg/day + Nasal Mom. (50 µg/puff/na/day) vs. Mom. (50 µg/puff/na/day) + placebo | 2 mo | Clinical score reduction (snoring, mouth br., nasal voice) | Reduced need for adenoidectomy, QoL | Clinical score 9.1→6.4, A/N ratio 0.80→0.74 | Clinical score 8.9→6.6, A/N ratio 0.80→0.75 | p = 0.117 (clinical score), p = 0.161 (A/N ratio); no significant difference between groups |
| Tuhanioglu and Erkan (2017) [57] | Rnd, prosp. | Child. 4–10 yrs, AH 3–4 | 120 tot (4 groups of 30) | In: AH ≥ 50%, obstruct. sympt., no immediate adenoidectomy Ex: Acute infections, craniofacial anomalies, ster. <3 wks, allergies | G1 Nasal Mom. (50 µg/day) G2 Mont. (4/5 mg/day) G3 Mom. + Mont. G4 No Tx (Ctrl) | 3 mo | Adenoid size reduction (X-ray, endoscopy) | Symptom improvement (scale 0–10) | Airway clearance: Mont. −22.51%, Mom. −21.76%, Comb. −21.79%; Obstr. sympt. −14.77/−17.03/−17.54% | Ctrl: −12.46% (adenoid), −8.8% (obstr. sympt.) | p < 0.05 (vs. control); no significant difference among the 3 Tx |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaffanello, M.; Pietrobelli, A.; Nosetti, L.; Antoniazzi, F.; Frassoldati, R.; Piacentini, G. Intranasal Corticosteroids and Oral Montelukast for Paediatric Obstructive Sleep Apnoea: A Systematic Review. Pharmaceutics 2025, 17, 588. https://doi.org/10.3390/pharmaceutics17050588
Zaffanello M, Pietrobelli A, Nosetti L, Antoniazzi F, Frassoldati R, Piacentini G. Intranasal Corticosteroids and Oral Montelukast for Paediatric Obstructive Sleep Apnoea: A Systematic Review. Pharmaceutics. 2025; 17(5):588. https://doi.org/10.3390/pharmaceutics17050588
Chicago/Turabian StyleZaffanello, Marco, Angelo Pietrobelli, Luana Nosetti, Franco Antoniazzi, Rossella Frassoldati, and Giorgio Piacentini. 2025. "Intranasal Corticosteroids and Oral Montelukast for Paediatric Obstructive Sleep Apnoea: A Systematic Review" Pharmaceutics 17, no. 5: 588. https://doi.org/10.3390/pharmaceutics17050588
APA StyleZaffanello, M., Pietrobelli, A., Nosetti, L., Antoniazzi, F., Frassoldati, R., & Piacentini, G. (2025). Intranasal Corticosteroids and Oral Montelukast for Paediatric Obstructive Sleep Apnoea: A Systematic Review. Pharmaceutics, 17(5), 588. https://doi.org/10.3390/pharmaceutics17050588








