Combination Therapy for Overcoming Multidrug Resistance in Breast Cancer Through Hedgehog Signaling Pathway Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PTX-Loaded PEG-PLA Nanoparticles and CYP-Loaded PEG-PLGA Nanoparticles
2.3. Quantitation of CYP and PTX
2.4. P-gp Expression in MCF-7 and MCF-7/Adr Cells
2.5. Nanoparticle Accumulation in MCF-7 and MCF-7/Adr Cells
2.6. Cytotoxicity Study
2.7. Cell Apoptosis Analysis
2.8. Penetration of Three-Dimensional Multicellular Tumor Spheroids
2.9. In Vivo Antitumor Efficacy
2.10. TUNEL Assay
2.11. Ki-67 Assay
2.12. Perfusion and Microvessel Density Observation
2.13. Blood Test
2.14. Statistical Analysis
3. Results and Discussion
3.1. Characterization of CYP NPs and PTX NPs
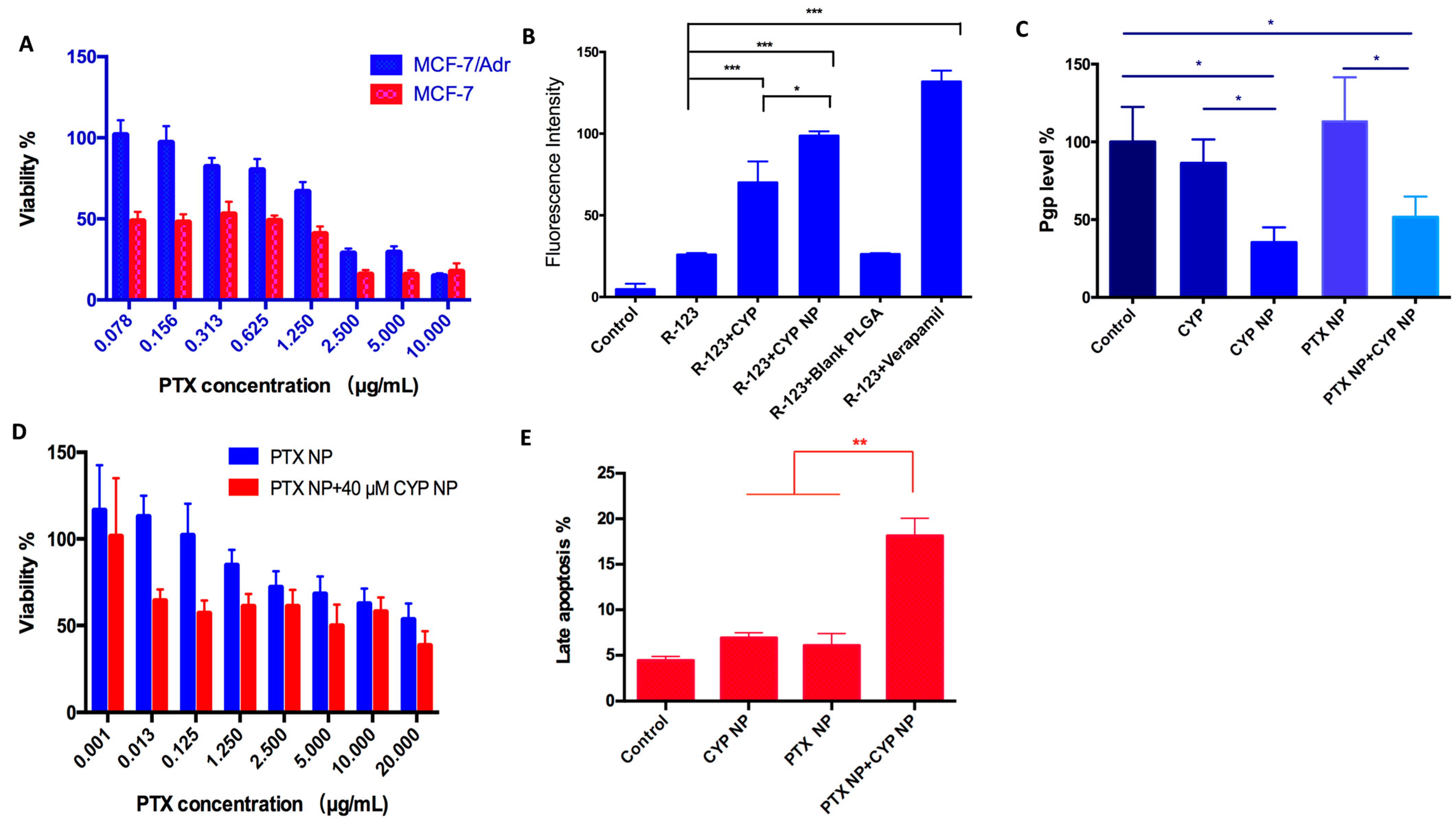
3.2. Repressive P-gp Expression and Enhanced P-gp Substrate Internalization in Resistant Cells by CYP Formulations
3.3. Enhanced P-gp Substrate Penetration in Resistant Tumor Spheroids by CYP Formulations
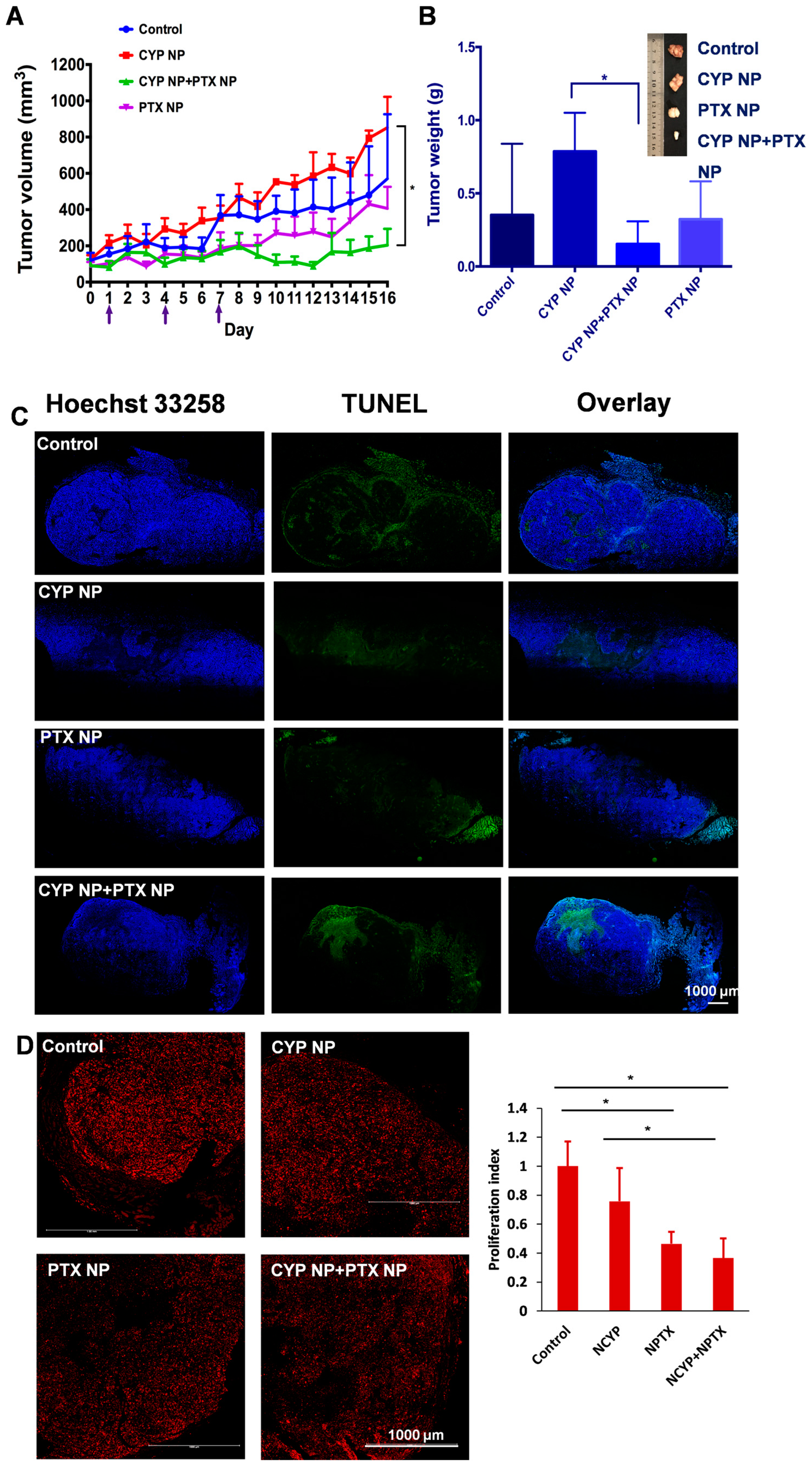
3.4. Therapeutic Efficacy in Resistant Breast Cancer Xenograft Mice
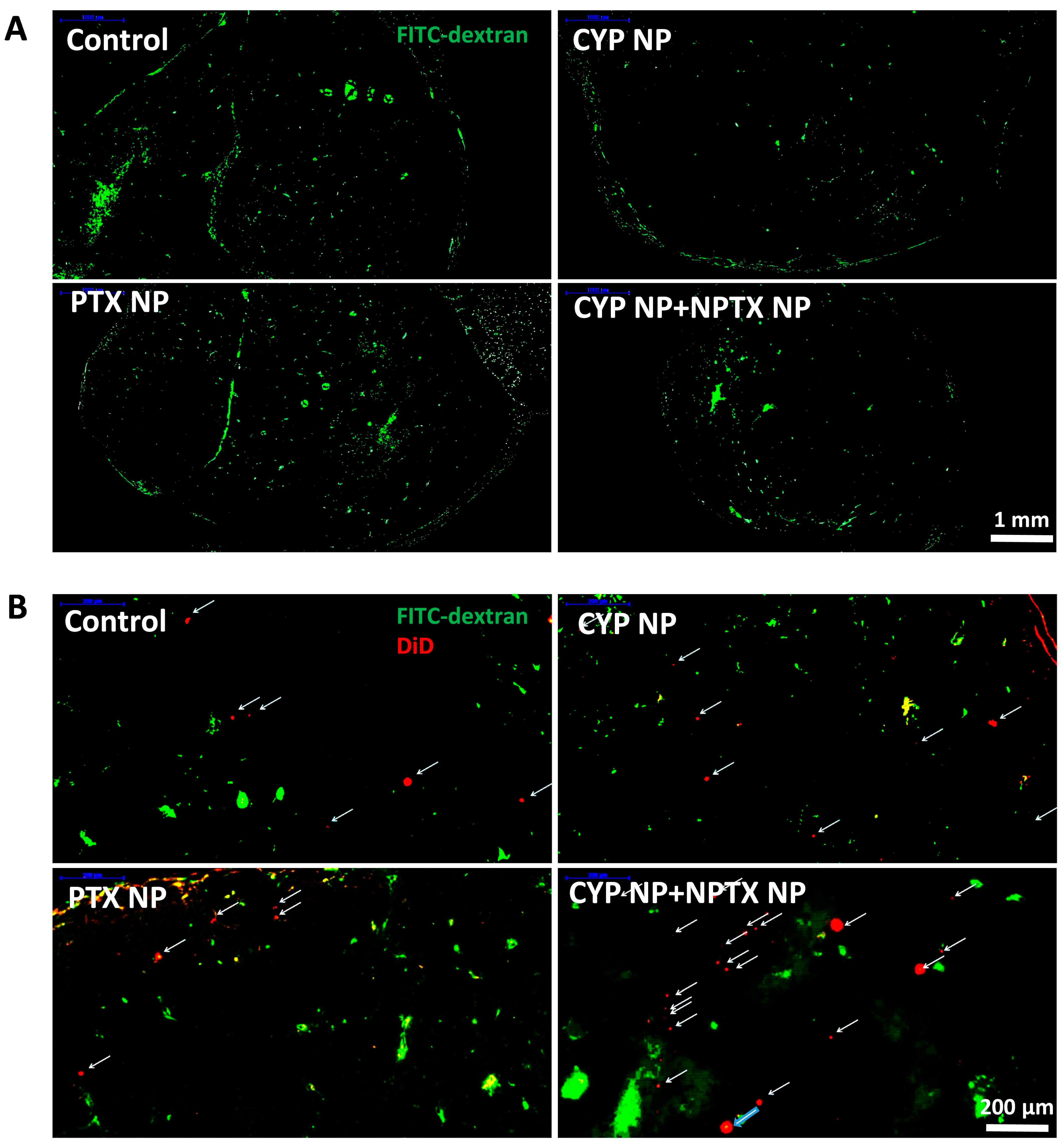
3.5. Enhanced Biodistribution and Blood Perfusion of Nanoparticles in Tumor by CYP Formulation
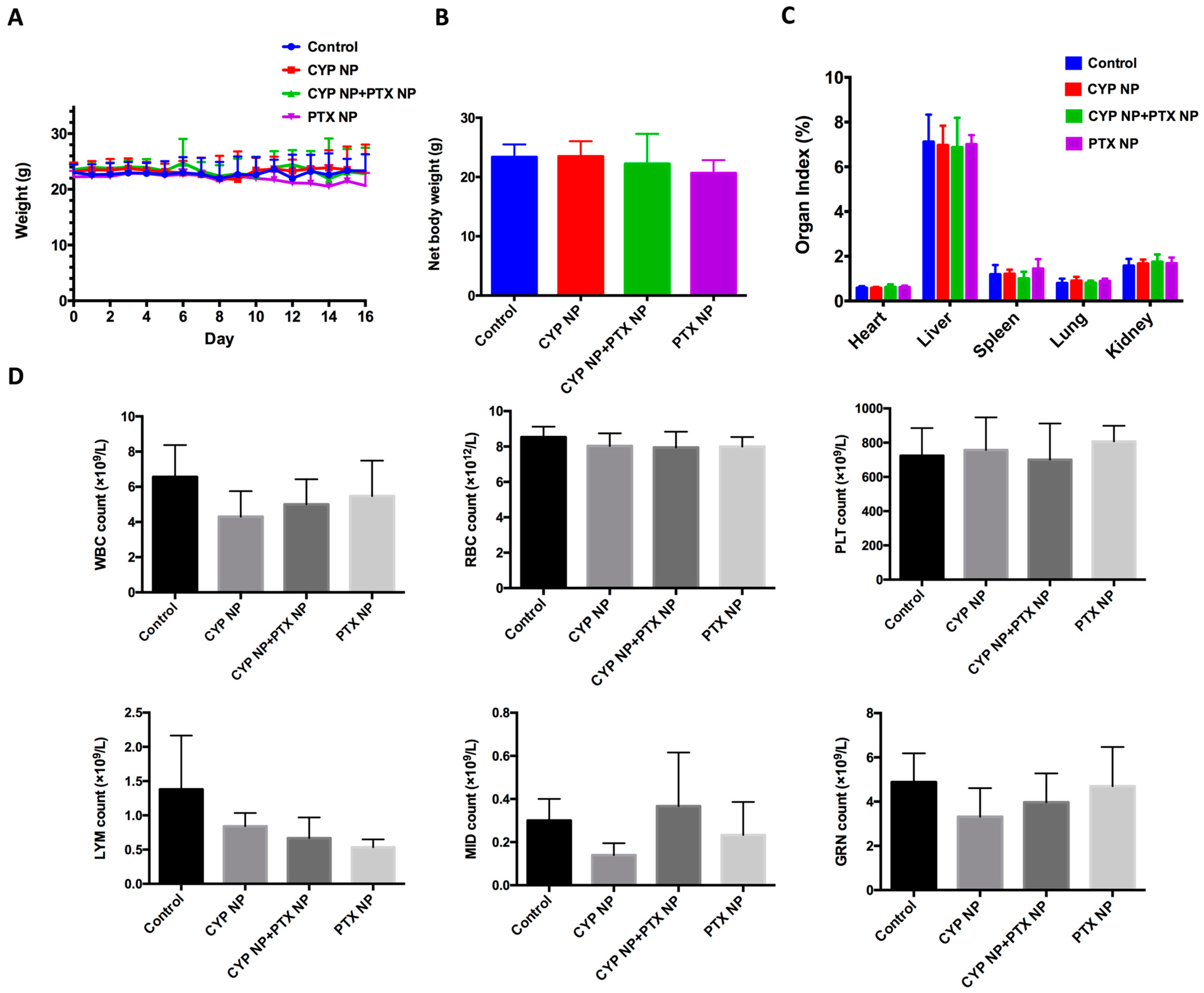
3.6. Safety Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, F.U.; Sufiyan Chhipa, A.; Mishra, V.; Gupta, V.K.; Rawat, S.G.; Kumar, A.; Pathak, C. Molecular and cellular paradigms of multidrug resistance in cancer. Cancer Rep. 2022, 5, e1291. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; To, K.K.W.; Chen, Z.S.; Fu, L. ABC transporters affects tumor immune microenvironment to regulate cancer immunotherapy and multidrug resistance. Drug Resist. Updat. 2023, 66, 100905. [Google Scholar] [CrossRef]
- Dong, J.; Yuan, L.; Hu, C.; Cheng, X.; Qin, J.J. Strategies to overcome cancer multidrug resistance (MDR) through targeting P-glycoprotein (ABCB1): An updated review. Pharmacol. Ther. 2023, 249, 108488. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Jing, J.; Wu, Z.; Wang, J.; Luo, G.; Lin, H.; Fan, Y.; Zhou, C. Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies. Signal Transduct. Target. Ther. 2023, 8, 315. [Google Scholar] [CrossRef]
- Carballo, G.B.; Honorato, J.R.; de Lopes, G.P.F.; Spohr, T.C.L.d.S.e. A highlight on Sonic hedgehog pathway. Cell Commun. Signal. 2018, 16, 11. [Google Scholar] [CrossRef]
- Chai, J.Y.; Sugumar, V.; Alshanon, A.F.; Wong, W.F.; Fung, S.Y.; Looi, C.Y. Defining the Role of GLI/Hedgehog Signaling in Chemoresistance: Implications in Therapeutic Approaches. Cancers 2021, 13, 4746. [Google Scholar] [CrossRef]
- Lospinoso Severini, L.; Ghirga, F.; Bufalieri, F.; Quaglio, D.; Infante, P.; Di Marcotullio, L. The SHH/GLI signaling pathway: A therapeutic target for medulloblastoma. Expert Opin. Ther. Targets 2020, 24, 1159–1181. [Google Scholar] [CrossRef]
- Bertrand, N.; Guerreschi, P.; Basset-Seguin, N.; Saiag, P.; Dupuy, A.; Dalac-Rat, S.; Dziwniel, V.; Depoortère, C.; Duhamel, A.; Mortier, L. Vismodegib in neoadjuvant treatment of locally advanced basal cell carcinoma: First results of a multicenter, open-label, phase 2 trial (VISMONEO study): Neoadjuvant Vismodegib in Locally Advanced Basal Cell Carcinoma. eClinicalMedicine 2021, 35, 100844. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Du, S.; Wang, C.; Zhang, Y.; Wang, H.; Fan, Y.; Gao, Y.; Gu, L.; Huang, Q.; Wang, B.; et al. Inhibition of primary cilia-hedgehog signaling axis triggers autophagic cell death and suppresses malignant progression of VHL wild-type ccRCC. Cell Death Dis. 2024, 15, 739. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Liu, L.; Liu, F.; Li, A.H.; Zhu, Y.; Wen, H.; Xue, X.; Tian, Z.; Sun, H.; et al. Targeted inhibition of the HNF1A/SHH axis by triptolide overcomes paclitaxel resistance in non-small cell lung cancer. Acta Pharmacol. Sin. 2024, 45, 1060–1076. [Google Scholar] [CrossRef] [PubMed]
- Murone, M.; Rosenthal, A.; de Sauvage, F.J. Sonic hedgehog signaling by the Patched–Smoothened receptor complex. Curr. Biol. 1999, 9, 76–84. [Google Scholar] [CrossRef]
- Hu, A.; Song, B.-L. The interplay of Patched, Smoothened and cholesterol in Hedgehog signaling. Curr. Opin. Cell Biol. 2019, 61, 31–38. [Google Scholar] [CrossRef]
- Casas, B.S.; Adolphe, C.; Lois, P.; Navarrete, N.; Solís, N.; Bustamante, E.; Gac, P.; Cabané, P.; Gallegos, I.; Wainwright, B.J.; et al. Downregulation of the Sonic Hedgehog/Gli pathway transcriptional target Neogenin-1 is associated with basal cell carcinoma aggressiveness. Oncotarget 2017, 8, 84006–84018. [Google Scholar] [CrossRef]
- Das, S.; Samant, R.S.; Shevde, L.A. Nonclassical activation of Hedgehog signaling enhances multidrug resistance and makes cancer cells refractory to smoothened-targeting Hedgehog inhibition. J. Biol. Chem. 2013, 288, 11824–11833. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Mondal, G.; Wen, D.; Mahato, R.I. Combination therapy of paclitaxel and cyclopamine polymer-drug conjugates to treat advanced prostate cancer. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 391–401. [Google Scholar] [CrossRef]
- Shao, H.; Liu, W.; Liu, M.; He, H.; Zhou, Q.-L.; Zhu, S.-F.; Gao, S. Asymmetric Synthesis of Cyclopamine, a Hedgehog (Hh) Signaling Pathway Inhibitor. J. Am. Chem. Soc. 2023, 145, 25086–25092. [Google Scholar] [CrossRef]
- Taipale, J.; Chen, J.K.; Cooper, M.K.; Wang, B.; Mann, R.K.; Milenkovic, L.; Scott, M.P.; Beachy, P.A. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 2000, 406, 1005–1009. [Google Scholar] [CrossRef]
- Chen, J.K.; Taipale, J.; Young, K.E.; Maiti, T.; Beachy, P.A. Small molecule modulation of Smoothened activity. Proc. Natl. Acad. Sci. USA 2002, 99, 14071–14076. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Johnson, R.P.; Senft, S.C.; Pan, E.Y.; Krakowski, A.C. Advanced basal cell carcinoma: What dermatologists need to know about treatment. J. Am. Acad. Dermatol. 2022, 86, S14–S24. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.; Lázaro, L.R.; Gunnlaugsson, T.; Scanlan, E.M. Glycosidase activated prodrugs for targeted cancer therapy. Chem. Soc. Rev. 2022, 51, 9694–9716. [Google Scholar] [CrossRef]
- Jin, H.; Wang, L.; Bernards, R. Rational combinations of targeted cancer therapies: Background, advances and challenges. Nat. Rev. Drug Discov. 2023, 22, 213–234. [Google Scholar] [CrossRef]
- Wei, X.; Song, M.; Li, W.; Huang, J.; Yang, G.; Wang, Y. Multifunctional nanoplatforms co-delivering combinatorial dual-drug for eliminating cancer multidrug resistance. Theranostics 2021, 11, 6334–6354. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, J.; Yuan, Y.; Modh, H.; Yang, Z. Combined Therapy in Cancer Treatment BT—Drug Delivery to Tumors: Recent Strategies and Techniques. In Drug Delivery to Tumors; Teng, L., Yang, Z., Li, C., Eds.; Springer Nature Singapore: Singapore, 2025; pp. 89–108. [Google Scholar] [CrossRef]
- Gong, J.; Shi, T.; Liu, J.; Pei, Z.; Liu, J.; Ren, X.; Li, F.; Qiu, F. Dual-drug codelivery nanosystems: An emerging approach for overcoming cancer multidrug resistance. Biomed. Pharmacother. 2023, 161, 114505. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y. Combination therapy based on nano codelivery for overcoming cancer drug resistance. Med. Drug Discov. 2020, 6, 100024. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, S.; Jiang, J.; Gao, Y.; Wang, Y.; Zhao, Y.; Zhang, J.; Zhang, M.; Huang, Y. Targeting lactate metabolism and immune interaction in breast tumor via protease-triggered delivery. J. Control. Release 2023, 358, 706–717. [Google Scholar] [CrossRef]
- Yusuf, R.Z.; Duan, Z.; Lamendola, D.E.; Penson, R.T.; Seiden, M. V Paclitaxel Resistance: Molecular Mechanisms and Pharmacologic Manipulation. Curr. Cancer Drug Targets 2003, 3, 1–19. [Google Scholar] [CrossRef]
- Tong, S.-W.; Xiang, B.; Dong, D.-W.; Qi, X.-R. Enhanced antitumor efficacy and decreased toxicity by self-associated docetaxel in phospholipid-based micelles. Int. J. Pharm. 2012, 434, 413–419. [Google Scholar] [CrossRef]
- Bordallo, E.; Rieumont, J.; Tiera, M.J.; Gómez, M.; Lazzari, M. Self-assembly in aqueous solution of amphiphilic graft copolymers from oxidized carboxymethylcellulose. Carbohydr. Polym. 2015, 124, 43–49. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Ma, Y.-C.; Zhang, W.-J.; Yang, Z.-Z.; Liang, D.-S.; Wu, Z.-F.; Qi, X.-R. Combination therapy with micellarized cyclopamine and temozolomide attenuate glioblastoma growth through Gli1 down-regulation. Oncotarget 2017, 8, 42495–42509. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Wang, A.; Yang, Z.; Liu, Y.; Qi, X. Enhance Cancer Cell Recognition and Overcome Drug Resistance Using Hyaluronic Acid and α-Tocopheryl Succinate Based Multifunctional Nanoparticles. Mol. Pharm. 2015, 12, 2189–2202. [Google Scholar] [CrossRef]
- Gao, W.; Xiang, B.; Meng, T.-T.; Liu, F.; Qi, X.-R. Chemotherapeutic drug delivery to cancer cells using a combination of folate targeting and tumor microenvironment-sensitive polypeptides. Biomaterials 2013, 34, 4137–4149. [Google Scholar] [CrossRef] [PubMed]
- Faupel-Badger, J.M.; Ginsburg, E.; Fleming, J.M.; Susser, L.; Doucet, T.; Vonderhaar, B.K. 16-kDa Prolactin Reduces Angiogenesis, but Not Growth of Human Breast Cancer Tumors In Vivo. Horm. Cancer 2010, 1, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Nicoulin, V.; Peloso, C.; Curia, S.; Richard, J.; Lopez-Noriega, A. In Vitro and In Vivo Hydrolytic Degradation Behaviors of a Drug-Delivery System Based on the Blend of PEG and PLA Copolymers. ACS Appl. Mater. Interfaces 2023, 15, 55495–55509. [Google Scholar] [CrossRef]
- de Oliveira, M.A.; Araújo, R.S.; Mosqueira, V.C.F. PEGylated and functionalized polylactide-based nanocapsules: An overview. Int. J. Pharm. 2023, 636, 122760. [Google Scholar] [CrossRef]
- Wei, P.-S.; Chen, Y.-J.; Lin, S.-Y.; Chuang, K.-H.; Sheu, M.-T.; Ho, H.-O. In situ subcutaneously injectable thermosensitive PEG-PLGA diblock and PLGA-PEG-PLGA triblock copolymer composite as sustained delivery of bispecific anti-CD3 scFv T-cell/anti-EGFR Fab Engager (BiTEE). Biomaterials 2021, 278, 121166. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG–PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef]
- Sinha, S.; Tripathi, A.K.; Pandey, A.; Naik, P.; Pandey, A.; Verma, V.S. Self-assembled PEGylated micelles for precise and targeted drug delivery: Current challenges and future directions. Biocatal. Agric. Biotechnol. 2024, 60, 103296. [Google Scholar] [CrossRef]
- Som Chaudhury, S.; Nandi, M.; Kumar, K.; Ruidas, B.; Sur, T.K.; Prasad, P.; Chakrabarti, S.; De, P.; Sil, J.; Das Mukhopadhyay, C. Rodent Model Preclinical Assessment of PEGylated Block Copolymer Targeting Cognition and Oxidative Stress Insults of Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 2036–2050. [Google Scholar] [CrossRef] [PubMed]
- Takakura, Y.; Takahashi, Y. Strategies for persistent retention of macromolecules and nanoparticles in the blood circulation. J. Control. Release 2022, 350, 486–493. [Google Scholar] [CrossRef]
- Haroon, H.B.; Hunter, A.C.; Farhangrazi, Z.S.; Moghimi, S.M. A brief history of long circulating nanoparticles. Adv. Drug Deliv. Rev. 2022, 188, 114396. [Google Scholar] [CrossRef]
- Shu, M.; Tang, J.; Chen, L.; Zeng, Q.; Li, C.; Xiao, S.; Jiang, Z.; Liu, J. Tumor microenvironment triple-responsive nanoparticles enable enhanced tumor penetration and synergetic chemo-photodynamic therapy. Biomaterials 2021, 268, 120574. [Google Scholar] [CrossRef]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of Polymeric Nanoparticles for Blood–Brain Barrier Transfer—Strategies and Challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qi, Y.; Liu, G.; Song, Y.; Jiang, X.; Du, B. Size-Dependent In Vivo Transport of Nanoparticles: Implications for Delivery, Targeting, and Clearance. ACS Nano 2023, 17, 20825–20849. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Xiang, J.; Wei, Q.; Tang, Y.; Piao, Y.; Shao, S.; Zhou, Z.; Tang, J.; Li, Z.-C.; Shen, Y. Role of Micelle Size in Cell Transcytosis-Based Tumor Extravasation, Infiltration, and Treatment Efficacy. Nano Lett. 2023, 23, 3904–3912. [Google Scholar] [CrossRef]
- Nguyen, L.N.M.; Ngo, W.; Lin, Z.P.; Sindhwani, S.; MacMillan, P.; Mladjenovic, S.M.; Chan, W.C.W. The mechanisms of nanoparticle delivery to solid tumours. Nat. Rev. Bioeng. 2024, 2, 201–213. [Google Scholar] [CrossRef]
- Silva, R.; Vilas-Boas, V.; Carmo, H.; Dinis-Oliveira, R.J.; Carvalho, F.; de Lourdes Bastos, M.; Remião, F. Modulation of P-glycoprotein efflux pump: Induction and activation as a therapeutic strategy. Pharmacol. Ther. 2015, 149, 1–123. [Google Scholar] [CrossRef]
- Wang, A.; Liang, D.; Liu, Y.; Qi, X. Roles of ligand and TPGS of micelles in regulating internalization, penetration and accumulation against sensitive or resistant tumor and therapy for multidrug resistant tumors. Biomaterials 2015, 53, 160–172. [Google Scholar] [CrossRef]
- Veider, F.; Haddadzadegan, S.; Sanchez Armengol, E.; Laffleur, F.; Kali, G.; Bernkop-Schnürch, A. Inhibition of P-glycoprotein-mediated efflux by thiolated cyclodextrins. Carbohydr. Polym. 2024, 327, 121648. [Google Scholar] [CrossRef]
- Xu, M.; Li, L.; Liu, Z.; Jiao, Z.; Xu, P.; Kong, X.; Huang, H.; Zhang, Y. ABCB2 (TAP1) as the downstream target of SHH signaling enhances pancreatic ductal adenocarcinoma drug resistance. Cancer Lett. 2013, 333, 152–158. [Google Scholar] [CrossRef]
- Honorato, J.R.; Hauser-Davis, R.A.; Saggioro, E.M.; Correia, F.V.; Sales-Junior, S.F.; Soares, L.O.S.; Lima, L.d.R.; Moura-Neto, V.; Lopes, G.P.d.F.; Spohr, T.C.L.d.S. Role of Sonic hedgehog signaling in cell cycle, oxidative stress, and autophagy of temozolomide resistant glioblastoma. J. Cell. Physiol. 2020, 235, 3798–3814. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, S.; Zhao, Y.; Shi, C.; Liu, P.; Zhang, C.; Lei, Y.; Zhang, B.; Bai, B.; Huang, Y.; et al. Anti-proliferation of breast cancer cells with itraconazole: Hedgehog pathway inhibition induces apoptosis and autophagic cell death. Cancer Lett. 2017, 385, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Zi, Y.; Yang, K.; He, J.; Wu, Z.; Liu, J.; Zhang, W. Strategies to enhance drug delivery to solid tumors by harnessing the EPR effects and alternative targeting mechanisms. Adv. Drug Deliv. Rev. 2022, 188, 114449. [Google Scholar] [CrossRef]
- Niu, B.; Wu, Y.; Zhou, M.; Lin, R.; Ge, P.; Chen, X.; Zhou, H.; Zhang, X.; Xie, J. Precise delivery of celastrol by PEGylated aptamer dendrimer nanoconjugates for enormous therapeutic effect via superior intratumor penetration over antibody counterparts. Cancer Lett. 2023, 579, 216461. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, W.; Lock, L.L.; Tang, J.; Sui, M.; Sun, W.; Cui, H.; Xu, D.; Shen, Y. The Role of Micelle Size in Tumor Accumulation, Penetration, and Treatment. ACS Nano 2015, 9, 7195–7206. [Google Scholar] [CrossRef]
- Agarwal, R.; Jurney, P.; Raythatha, M.; Singh, V.; Sreenivasan, S.V.; Shi, L.; Roy, K. Effect of Shape, Size, and Aspect Ratio on Nanoparticle Penetration and Distribution inside Solid Tissues Using 3D Spheroid Models. Adv. Healthc. Mater. 2015, 4, 2269–2280. [Google Scholar] [CrossRef]
- Natarajan, R.; Northrop, N.; Yamamoto, B. Fluorescein Isothiocyanate (FITC)-Dextran Extravasation as a Measure of Blood-Brain Barrier Permeability. Curr. Protoc. Neurosci. 2017, 79, 9.58.1–9.58.15. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, T.; Shen, S.; She, X.; Tuo, Y.; Hu, Y.; Pang, Z.; Jiang, X. Cyclopamine disrupts tumor extracellular matrix and improves the distribution and efficacy of nanotherapeutics in pancreatic cancer. Biomaterials 2016, 103, 12–21. [Google Scholar] [CrossRef]
- Habib, T.N.; Altonsy, M.O.; Ghanem, S.A.; Salama, M.S.; Hosny, M.A. Optimizing combination therapy in prostate cancer: Mechanistic insights into the synergistic effects of Paclitaxel and Sulforaphane-induced apoptosis. BMC Mol. Cell Biol. 2024, 25, 5. [Google Scholar] [CrossRef] [PubMed]
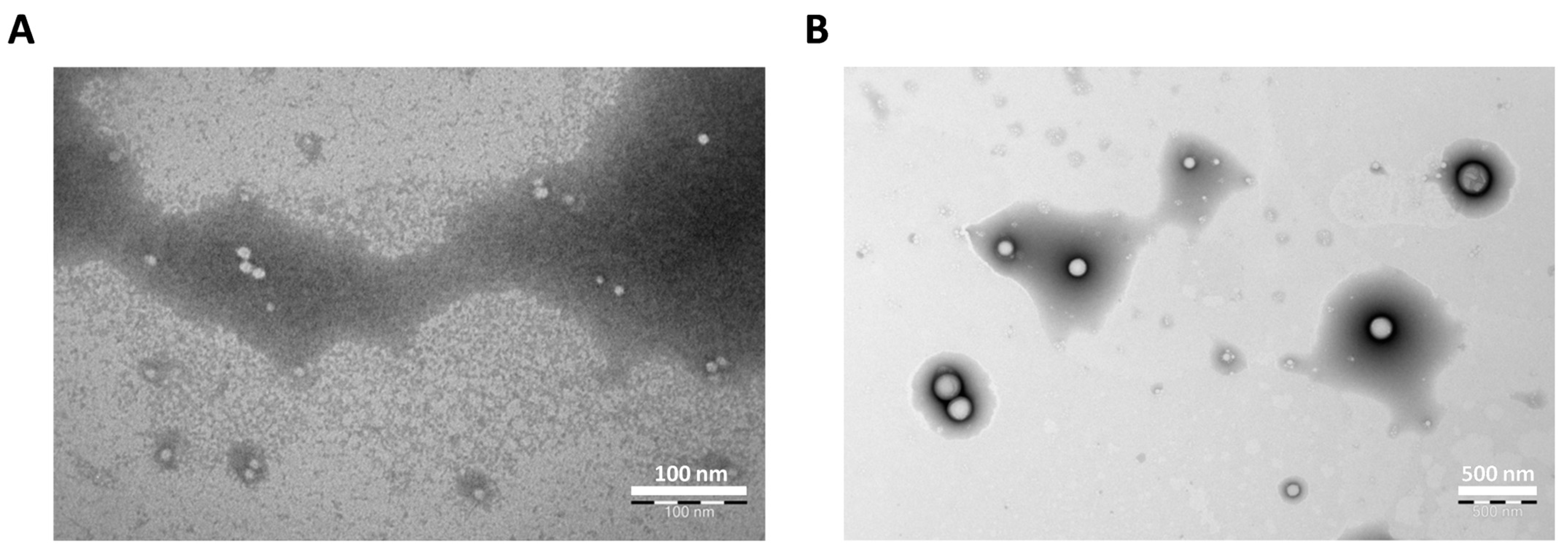

| Size (nm) | Polydispersity Index | Zeta Potential (mV) | Encapsulation Efficiency (%) | |
|---|---|---|---|---|
| PTX NP | 28.36 ± 6.68 | 0.245 ± 0.06 | −9.82 ± 5.63 | 90.56 ± 2.30 |
| CYP NP | 201.52 ± 31.95 | 0.228 ± 0.05 | −18.63 ± 4.8 | 98.24 ± 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yang, Y.; Qi, X. Combination Therapy for Overcoming Multidrug Resistance in Breast Cancer Through Hedgehog Signaling Pathway Regulation. Pharmaceutics 2025, 17, 572. https://doi.org/10.3390/pharmaceutics17050572
Liu Y, Yang Y, Qi X. Combination Therapy for Overcoming Multidrug Resistance in Breast Cancer Through Hedgehog Signaling Pathway Regulation. Pharmaceutics. 2025; 17(5):572. https://doi.org/10.3390/pharmaceutics17050572
Chicago/Turabian StyleLiu, Yujie, Yiliang Yang, and Xianrong Qi. 2025. "Combination Therapy for Overcoming Multidrug Resistance in Breast Cancer Through Hedgehog Signaling Pathway Regulation" Pharmaceutics 17, no. 5: 572. https://doi.org/10.3390/pharmaceutics17050572
APA StyleLiu, Y., Yang, Y., & Qi, X. (2025). Combination Therapy for Overcoming Multidrug Resistance in Breast Cancer Through Hedgehog Signaling Pathway Regulation. Pharmaceutics, 17(5), 572. https://doi.org/10.3390/pharmaceutics17050572







