Synergistic Antioxidant and Anti-Ferroptosis Therapy via BPNS-Encapsulated Thermoresponsive Chitosan Hydrogel for Spinal Cord Injury Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Acquisition
2.2. Preparation of Hydrogels
2.3. Characterization of BPNS and Hydrogels
2.4. Cytotoxicity Assay
2.5. Cell Therapy
2.6. ROS Determination
2.7. In Vivo Animal Models
2.8. BBB Score, Bladder, and Hindlimb Posture
2.9. Detection of Iron, Glutathione, and Malondialdehyde Content
2.10. Histological Analysis
2.11. Immunohistochemical Analysis
2.12. Western Blotting
2.13. Transcriptomic Analysis
2.14. Statistical Analysis
3. Results
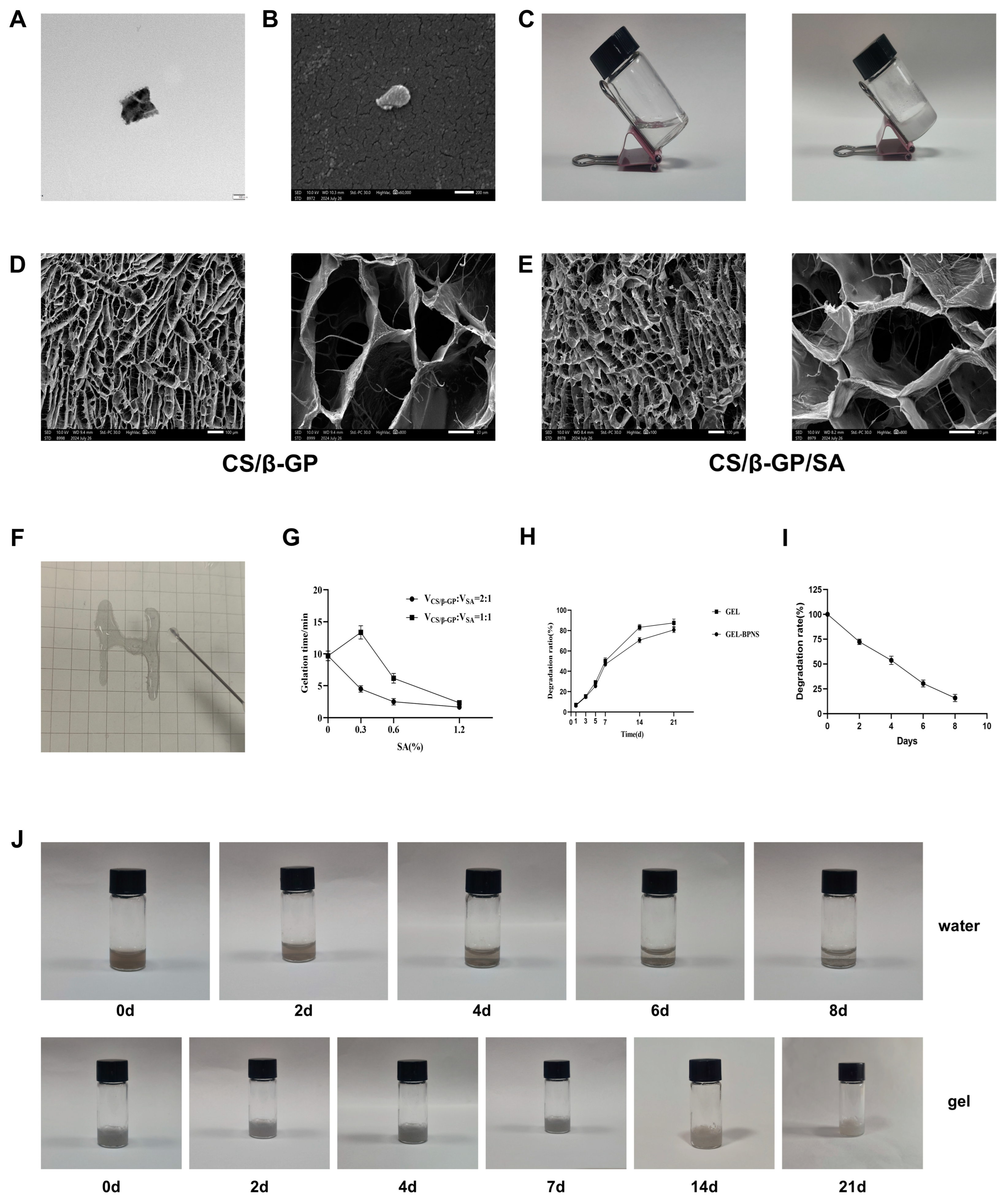
3.1. Synthesis and Detection of the Composite
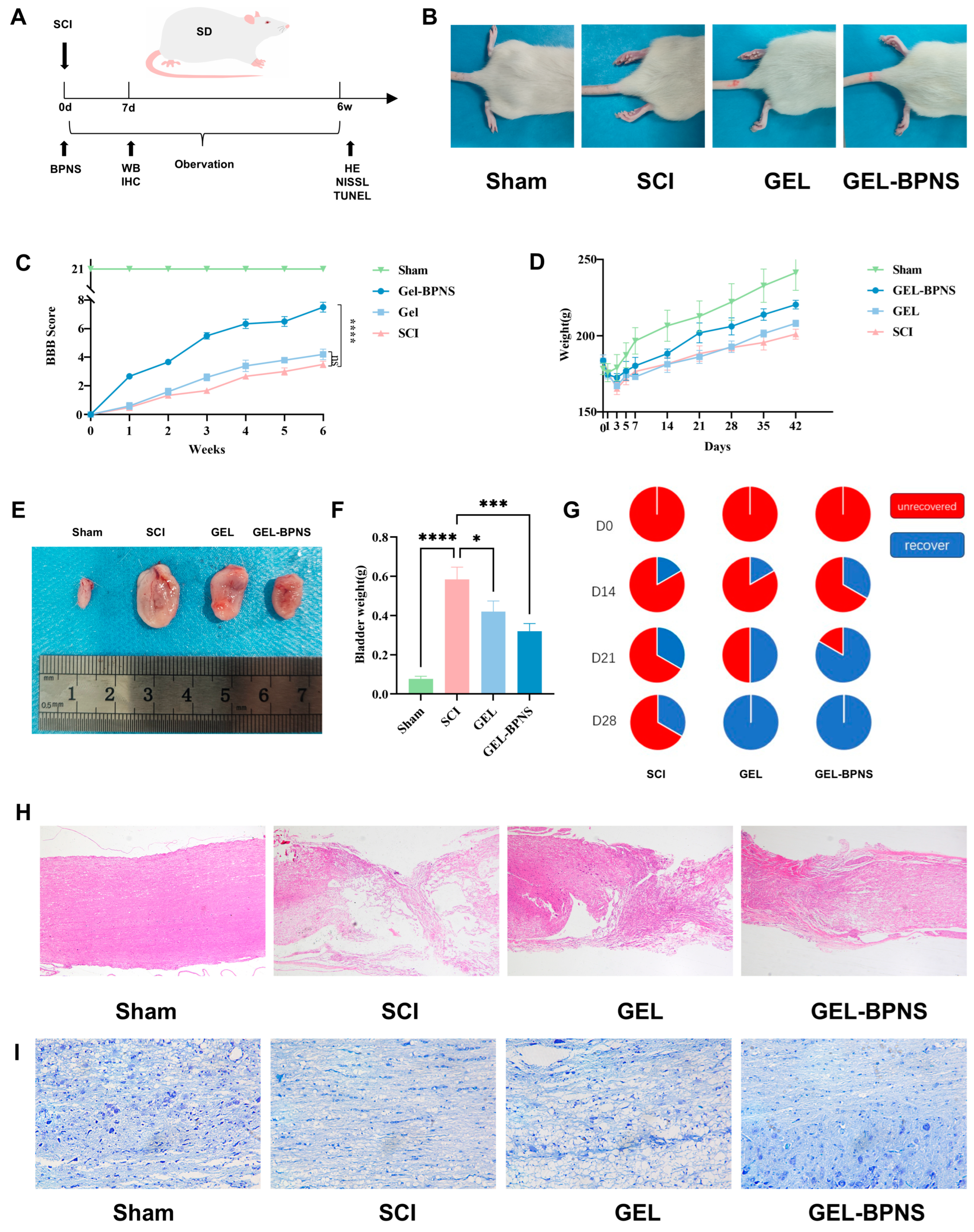
3.2. Composite Promotes Spinal Cord Injury Rat Functional Recovery
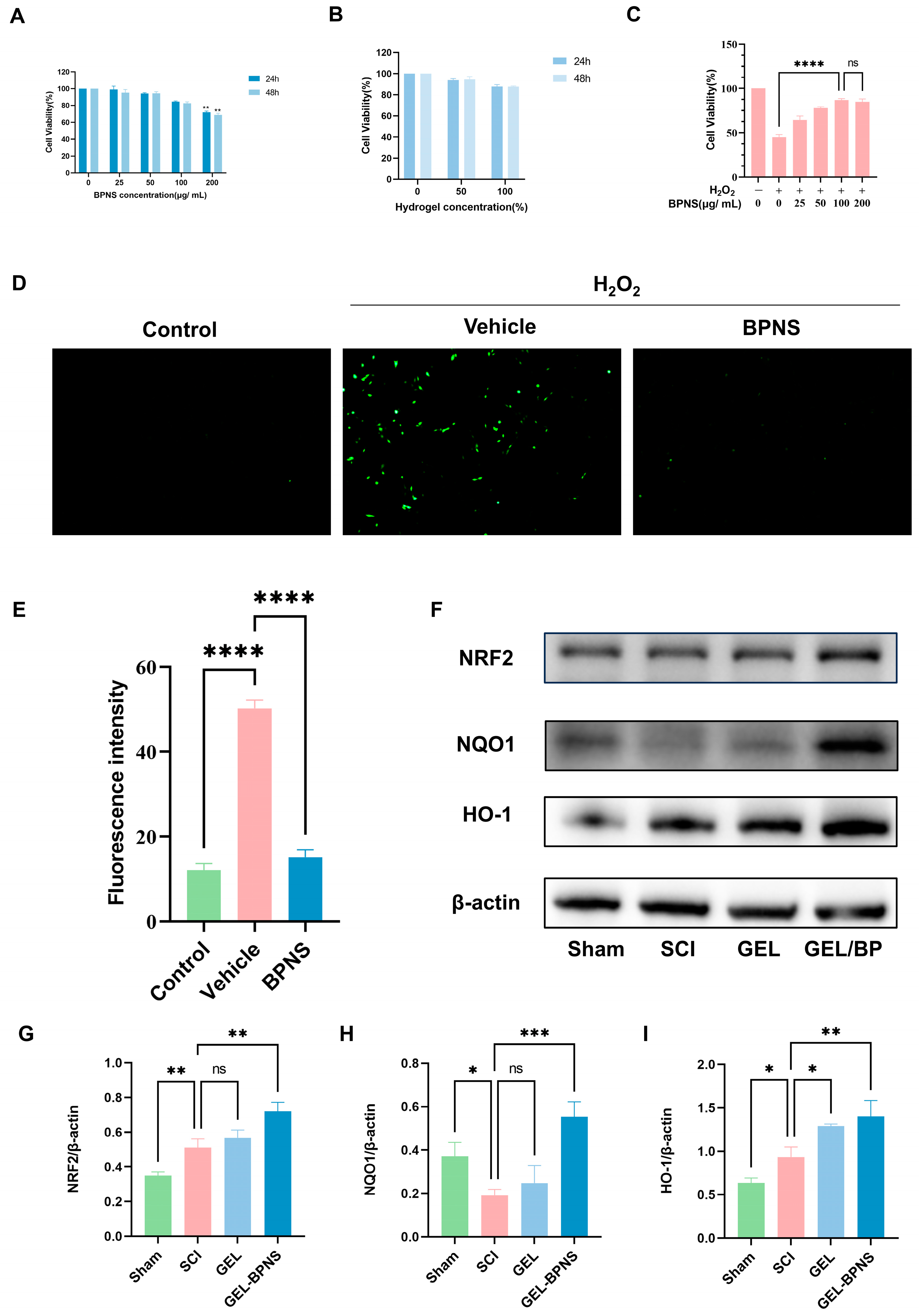
3.3. Compound Cytotoxicity and Antioxidant Properties
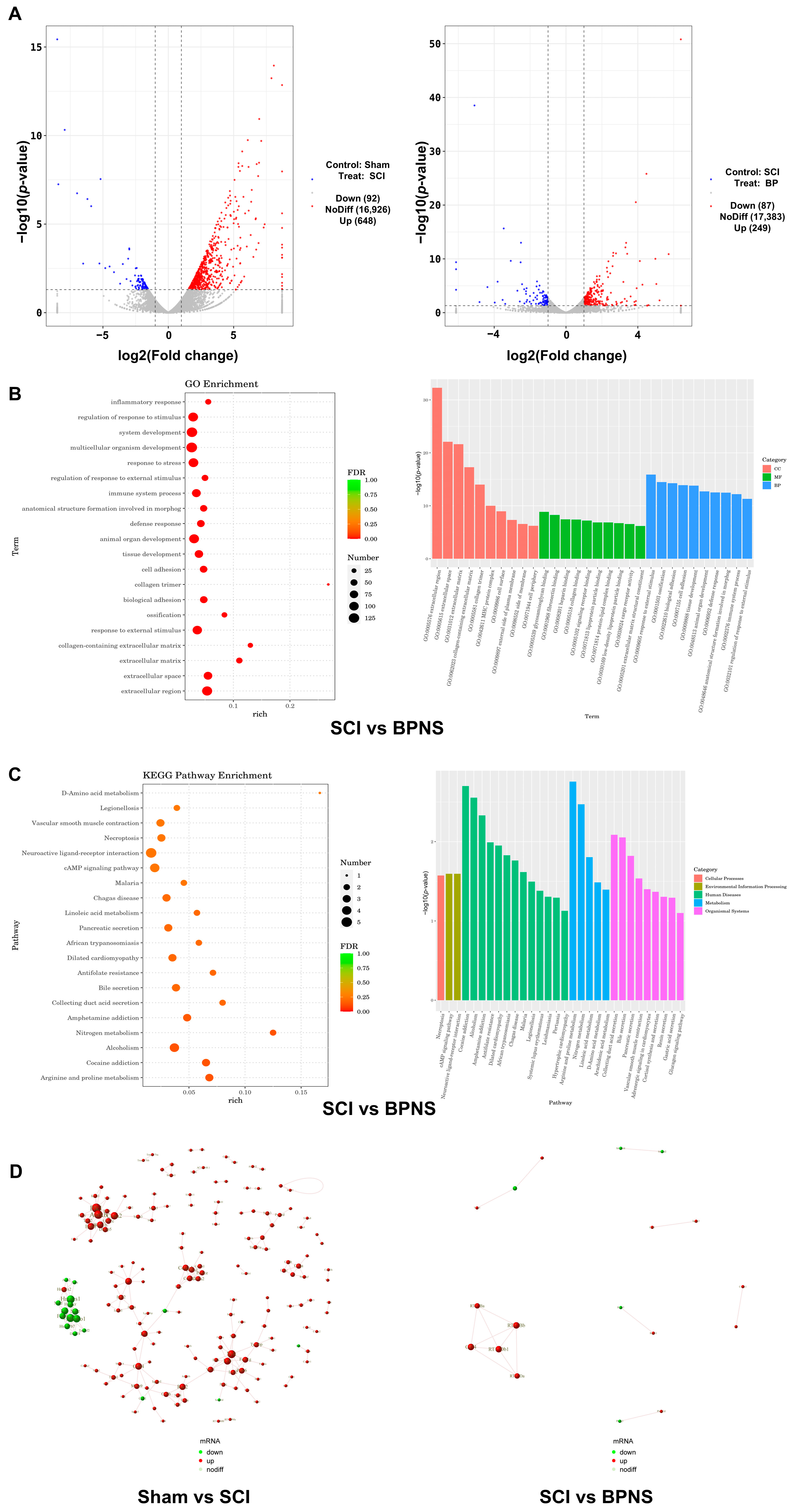
3.4. Transcriptomic Analysis
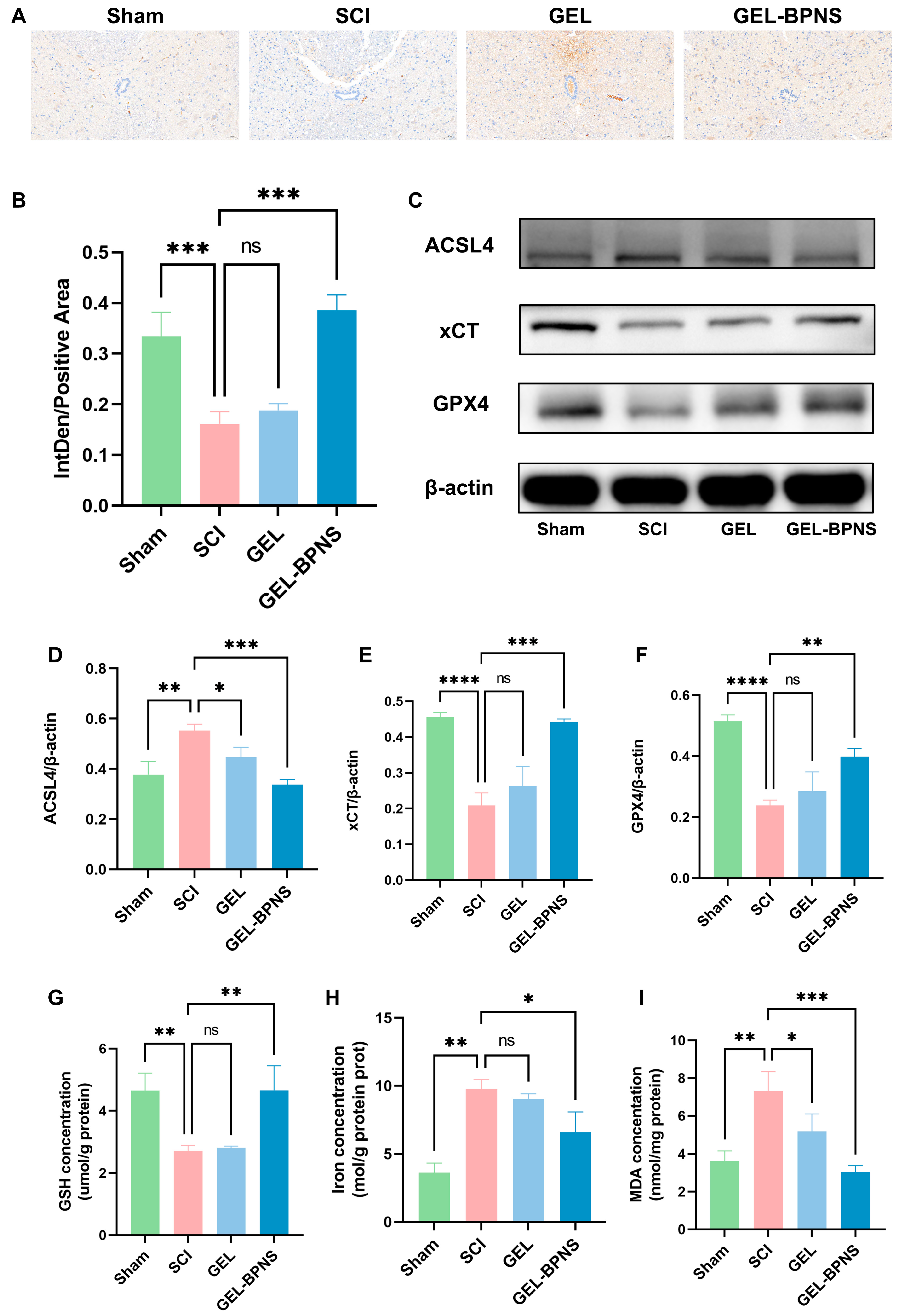
3.5. The Composite Inhibits Ferroptosis After SCI by Activating the xCT/GSH/GPX4 Signaling Pathway
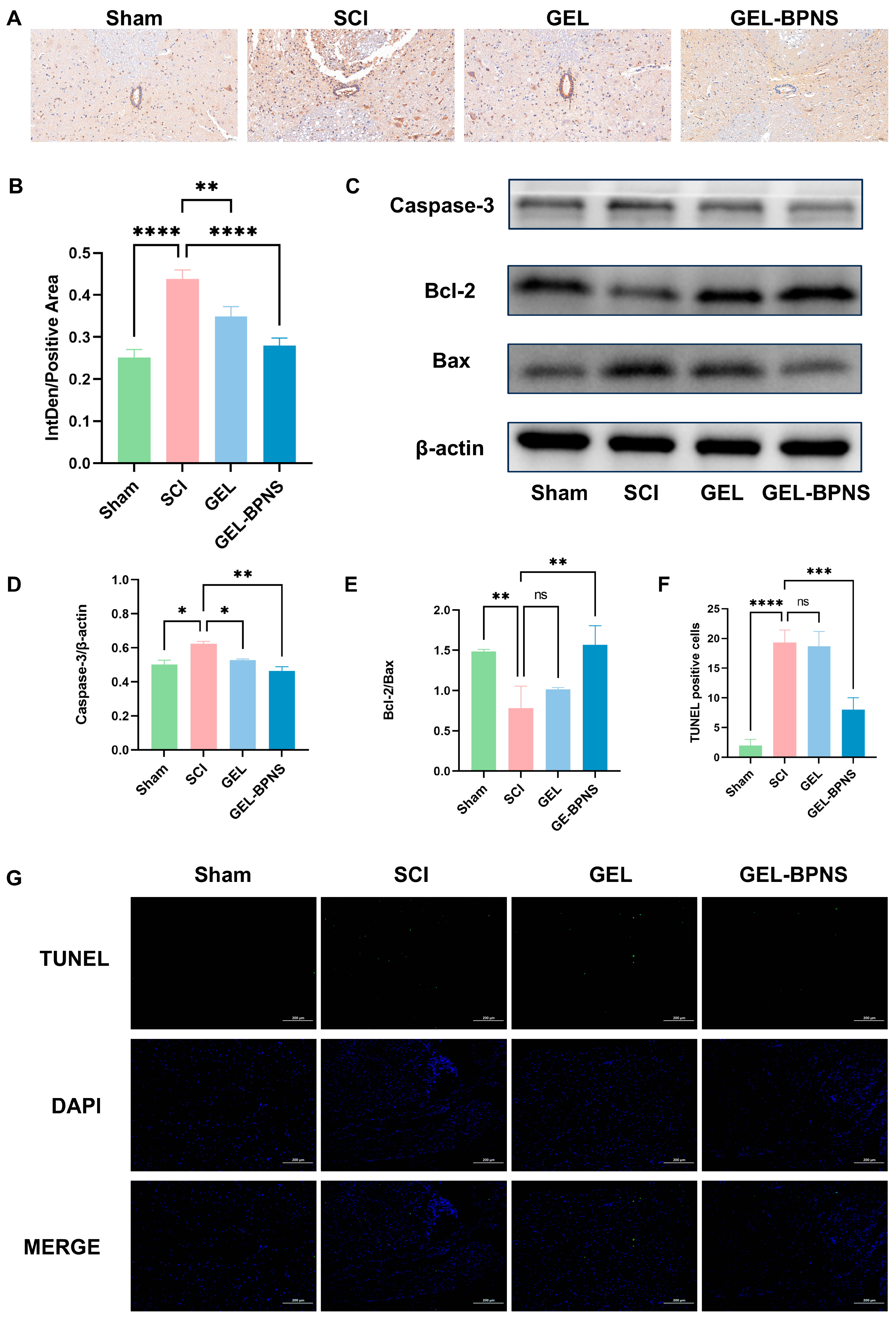
3.6. Composite Inhibits Apoptosis After SCI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonald, J.W.; Sadowsky, C. Spinal-cord injury. Lancet 2002, 359, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Crispo, J.A.G.; Kuramoto, L.K.; Cragg, J.J. Global burden of spinal cord injury: Future directions. Lancet Neurol. 2023, 22, 976–978. [Google Scholar] [CrossRef]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal cord injury: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Wang, X.; Niu, X.; Wang, Y.; Liu, Y.; Yang, C.; Chen, X.; Qi, Z. C–C motif chemokine ligand 2/C–C motif chemokine receptor 2 pathway as a therapeutic target and regulatory mechanism for spinal cord injury. Neural Regen. Res. 2025, 20, 2231–2244. [Google Scholar] [CrossRef]
- Jendelova, P. Therapeutic Strategies for Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 3200. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wan, B.; Gong, G.; Yin, J. ROS: Executioner of regulating cell death in spinal cord injury. Front. Immunol. 2024, 15, 1330678. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, C.; Liang, J.X.; Zheng, F.F.; Qi, X.Y.; Gao, F. Targeting Mitochondrial Oxidative Stress: Potential Neuroprotective Therapy for Spinal Cord Injury. J. Integr. Neurosci. 2023, 22, 153. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Z.; Jing, S.; Wang, B.; Ye, Z.; Xiong, W.; Liu, Y.; Liu, Y.; Xu, C.; Kumeria, T.; et al. Repair spinal cord injury with a versatile anti-oxidant and neural regenerative nanoplatform. J. Nanobiotechnol. 2024, 22, 351. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Li, J.; Li, Z.; Quan, J.; Liu, X.; Tang, Y.; Liu, B. The Latest View on the Mechanism of Ferroptosis and Its Research Progress in Spinal Cord Injury. Oxidative Med. Cell. Longev. 2020, 2020, 6375938. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yuan, S.; Shi, L.; Li, J.; Ning, G.; Kong, X.; Feng, S. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 2021, 54, e12992. [Google Scholar] [CrossRef]
- Ying, Y.; Huang, Z.; Tu, Y.; Wu, Q.; Li, Z.; Zhang, Y.; Yu, H.; Zeng, A.; Huang, H.; Ye, J.; et al. A shear-thinning, ROS-scavenging hydrogel combined with dental pulp stem cells promotes spinal cord repair by inhibiting ferroptosis. Bioact. Mater. 2023, 22, 274–290. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Fang, Y.; Liu, C.; Zhang, H. Ferroptosis in Osteoarthritis: Current Understanding. J. Inflamm. Res. 2024, 17, 8471–8486. [Google Scholar] [CrossRef]
- Zhang, C.; Zhai, T.; Zhu, J.; Wei, D.; Ren, S.; Yang, Y.; Gao, F.; Zhao, L. Research Progress of Antioxidants in Oxidative Stress Therapy after Spinal Cord Injury. Neurochem. Res. 2023, 48, 3473–3484. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, M.; Feng, L.; Zhong, W.; Zhang, L.; Du, R.; Sun, J.; Wang, C.; Du, J. Nanozyme Hydrogels Promote Nerve Regeneration in Spinal Cord Injury by Reducing Oxidative Stress. ACS Appl. Mater. Interfaces 2024, 16, 59949–59961. [Google Scholar] [CrossRef]
- Springer, J.E.; Azbill, R.D.; Knapp, P.E. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat. Med. 1999, 5, 943–946. [Google Scholar] [CrossRef]
- He, W.; Li, Z.-q.; Gu, H.-y.; Pan, Q.-l.; Lin, F.-x. Targeted Therapy of Spinal Cord Injury: Inhibition of Apoptosis Is a Promising Therapeutic Strategy. Mol. Neurobiol. 2024, 61, 4222–4239. [Google Scholar] [CrossRef]
- Oltval, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ashwell, K.W.S.; Waite, P. Advances in Secondary Spinal Cord Injury: Role of Apoptosis. Spine 2000, 25, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Eswaraiah, V.; Zeng, Q.; Long, Y.; Liu, Z. Black Phosphorus Nanosheets: Synthesis, Characterization and Applications. Small 2016, 12, 3480–3502. [Google Scholar] [CrossRef]
- Hou, J.; Wang, H.; Ge, Z.; Zuo, T.; Chen, Q.; Liu, X.; Mou, S.; Fan, C.; Xie, Y.; Wang, L. Treating Acute Kidney Injury with Antioxidative Black Phosphorus Nanosheets. Nano Lett. 2020, 20, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Wang, H.; Huang, S.; Xia, F.; Dresselhaus, M.S. The renaissance of black phosphorus. Proc. Natl. Acad. Sci. USA 2015, 112, 4523–4530. [Google Scholar] [CrossRef]
- He, Z.; Chen, W.; Hu, K.; Luo, Y.; Zeng, W.; He, X.; Li, T.; Ouyang, J.; Li, Y.; Xie, L.; et al. Resolvin D1 delivery to lesional macrophages using antioxidative black phosphorus nanosheets for atherosclerosis treatment. Nat. Nanotechnol. 2024, 19, 1386–1398. [Google Scholar] [CrossRef]
- Lu, H.; Wei, J.; Liu, K.; Li, Z.; Xu, T.; Yang, D.; Gao, Q.; Xiang, H.; Li, G.; Chen, Y. Radical-Scavenging and Subchondral Bone-Regenerating Nanomedicine for Osteoarthritis Treatment. ACS Nano 2023, 17, 6131–6146. [Google Scholar] [CrossRef] [PubMed]
- Favron, A.; Gaufrès, E.; Fossard, F.; Phaneuf-L’Heureux, A.-L.; Tang, N.Y.W.; Lévesque, P.L.; Loiseau, A.; Leonelli, R.; Francoeur, S.; Martel, R. Photooxidation and quantum confinement effects in exfoliated black phosphorus. Nat. Mater. 2015, 14, 826–832. [Google Scholar] [CrossRef]
- Biswas, R.; Mondal, S.; Ansari, M.A.; Sarkar, T.; Condiuc, I.P.; Trifas, G.; Atanase, L.I. Chitosan and Its Derivatives as Nanocarriers for Drug Delivery. Molecules 2025, 30, 1297. [Google Scholar] [CrossRef]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Nawrotek, K.; Marqueste, T.; Caron, G.; Modrzejewska, Z.; Zarzycki, R.; Decherchi, P. Reconstruction of the Injured Spinal Cord by Implantation of a Hydrogel based on Chitosan and β-Glycerol Phosphate-motor Behavior and Ventilatory Assessments. Procedia Eng. 2013, 59, 226–232. [Google Scholar] [CrossRef]
- Harish Prashanth, K.V.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Wang, C.; Liu, C.; Liang, C.; Qu, X.; Zou, X.; Du, S.; Zhang, Q.; Wang, L. Role of Berberine Thermosensitive Hydrogel in Periodontitis via PI3K/AKT Pathway In Vitro. Int. J. Mol. Sci. 2023, 24, 6364. [Google Scholar] [CrossRef]
- Yuan, N.; Shao, K.; Huang, S.; Chen, C. Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: A review. Int. J. Biol. Macromol. 2023, 240, 124321. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qing, Y.; Lou, Y.; Li, R.; Wang, H.; Wang, X.; Ying, B.; Tang, X.; Qin, Y. Injectable thermosensitive black phosphorus nanosheet- and doxorubicin-loaded hydrogel for synergistic bone tumor photothermal-chemotherapy and osteogenesis enhancement. Int. J. Biol. Macromol. 2023, 239, 124209. [Google Scholar] [CrossRef]
- Pan, W.; Dai, C.; Li, Y.; Yin, Y.; Gong, L.; Machuki, J.O.a.; Yang, Y.; Qiu, S.; Guo, K.; Gao, F. PRP-chitosan thermoresponsive hydrogel combined with black phosphorus nanosheets as injectable biomaterial for biotherapy and phototherapy treatment of rheumatoid arthritis. Biomaterials 2020, 239, 119851. [Google Scholar] [CrossRef]
- Zhang, N.; Hu, J.; Liu, W.; Cai, W.; Xu, Y.; Wang, X.; Li, S.; Ru, B. Advances in Novel Biomaterial-Based Strategies for Spinal Cord Injury Treatment. Mol. Pharm. 2024, 21, 4764–4785. [Google Scholar] [CrossRef]
- Dong, R.; Zheng, S.; Cheng, X. Designing hydrogel for application in spinal surgery. Mater. Today Bio 2025, 31, 101536. [Google Scholar] [CrossRef]
- Chandra, N.S.; Gorantla, S.; Priya, S.; Singhvi, G. Insight on updates in polysaccharides for ocular drug delivery. Carbohydr. Polym. 2022, 297, 120014. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr. Polym. 2019, 207, 143–159. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Zhao, Y.; Gao, G. Mitochondria regulation in ferroptosis. Eur. J. Cell Biol. 2020, 99, 151058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wan, Y.; Xie, H.; Mu, Y.; Du, P.; Wang, D.; Wu, X.; Ji, H.; Wan, L. Degradation Chemistry and Stabilization of Exfoliated Few-Layer Black Phosphorus in Water. J. Am. Chem. Soc. 2018, 140, 7561–7567. [Google Scholar] [CrossRef]
- Ziletti, A.; Carvalho, A.; Campbell, D.K.; Coker, D.F.; Castro Neto, A.H. Oxygen Defects in Phosphorene. Phys. Rev. Lett. 2015, 114, 046801. [Google Scholar] [CrossRef]
- Alizadeh, A.; Moradi, L.; Katebi, M.; Ai, J.; Azami, M.; Moradveisi, B.; Ostad, S.N. Delivery of injectable thermo-sensitive hydrogel releasing nerve growth factor for spinal cord regeneration in rat animal model. J. Tissue Viability 2020, 29, 359–366. [Google Scholar] [CrossRef]
- Carrêlo, H.; Jiménez-Rosado, M.; Vieira, T.; Da Rosa, R.R.; Perez-Puyana, V.M.; Silva, J.C.; Romero, A.; Borges, J.P.; Soares, P.I.P. A Thermoresponsive injectable drug delivery system of chitosan/β-glycerophosphate with gellan gum/alginate microparticles. Int. J. Biol. Macromol. 2024, 271, 131981. [Google Scholar] [CrossRef]
- Zöller, K.; To, D.; Bernkop-Schnürch, A. Biomedical applications of functional hydrogels: Innovative developments, relevant clinical trials and advanced products. Biomaterials 2025, 312, 122718. [Google Scholar] [CrossRef]
- Panicker, J.N.; Fowler, C.J.; Kessler, T.M. Lower urinary tract dysfunction in the neurological patient: Clinical assessment and management. Lancet Neurol. 2015, 14, 720–732. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Zhao, Y.-p.; Ye, M.-Z.; Wang, L.; Lan, T.-S.; Wang, Y.; Qi, Z.-Q. Chimeric CNS-targeting-peptide engineered exosomes for experimental autoimmune encephalomyelitis therapy. Int. Immunopharmacol. 2023, 124, 110835. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, H.; Li, J.; Wang, X.; Misra, H.; Li, Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 2012, 50, 264–274. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Tie, H.; Tian, W.; Zhao, Y.; Qin, L.; Guo, S.; Li, Q.; Bao, C. Eriodictyol regulated ferroptosis, mitochondrial dysfunction, and cell viability via Nrf2/HO-1/NQO1 signaling pathway in ovarian cancer cells. J. Biochem. Mol. Toxicol. 2023, 37, e23368. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Y.; Zhao, C.; Zhang, H.; Pu, Y.; Yin, L. Copper induces oxidative stress and apoptosis of hippocampal neuron via pCREB/BDNF/ and Nrf2/HO-1/NQO1 pathway. J. Appl. Toxicol. 2022, 42, 694–705. [Google Scholar] [CrossRef]
- Li, F.J.; Long, H.Z.; Zhou, Z.W.; Luo, H.Y.; Xu, S.G.; Gao, L.C. System Xc -/GSH/GPX4 axis: An important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy. Front. Pharmacol. 2022, 13, 910292. [Google Scholar] [CrossRef]
- Boland, K.; Flanagan, L.; Prehn, J.H. Paracrine control of tissue regeneration and cell proliferation by Caspase-3. Cell Death Dis. 2013, 4, e725. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Chen, C.; Xie, B.; Fang, Z.; Hu, W.; Chen, J.; Fu, H.; He, H. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury. J. Neuroinflamm. 2017, 14, 143. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Ndong, J.C.; Hettinghouse, A.; Sun, G.; Chen, C.; Zhang, C.; Liu, R.; Liu, C.J. Progranulin deficiency exacerbates spinal cord injury by promoting neuroinflammation and cell apoptosis in mice. J. Neuroinflamm. 2019, 16, 238. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, S.; Yang, M. Recent progress and challenges in the treatment of spinal cord injury. Protein Cell 2023, 14, 635–652. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, Y.; Wang, X.; Zeng, W.; Zhang, Z.; Zhang, Z.; Qi, Z. Synergistic Antioxidant and Anti-Ferroptosis Therapy via BPNS-Encapsulated Thermoresponsive Chitosan Hydrogel for Spinal Cord Injury Regeneration. Pharmaceutics 2025, 17, 573. https://doi.org/10.3390/pharmaceutics17050573
Liu Y, Wang Y, Wang X, Zeng W, Zhang Z, Zhang Z, Qi Z. Synergistic Antioxidant and Anti-Ferroptosis Therapy via BPNS-Encapsulated Thermoresponsive Chitosan Hydrogel for Spinal Cord Injury Regeneration. Pharmaceutics. 2025; 17(5):573. https://doi.org/10.3390/pharmaceutics17050573
Chicago/Turabian StyleLiu, Yang, Yingkai Wang, Xiangzi Wang, Wanchen Zeng, Zehong Zhang, Zhengmian Zhang, and Zhongquan Qi. 2025. "Synergistic Antioxidant and Anti-Ferroptosis Therapy via BPNS-Encapsulated Thermoresponsive Chitosan Hydrogel for Spinal Cord Injury Regeneration" Pharmaceutics 17, no. 5: 573. https://doi.org/10.3390/pharmaceutics17050573
APA StyleLiu, Y., Wang, Y., Wang, X., Zeng, W., Zhang, Z., Zhang, Z., & Qi, Z. (2025). Synergistic Antioxidant and Anti-Ferroptosis Therapy via BPNS-Encapsulated Thermoresponsive Chitosan Hydrogel for Spinal Cord Injury Regeneration. Pharmaceutics, 17(5), 573. https://doi.org/10.3390/pharmaceutics17050573



