The Modulation of Cell Plasticity by Budesonide: Beyond the Metabolic and Anti-Inflammatory Actions of Glucocorticoids
Abstract
1. Introduction
2. Physicochemical Properties of Budesonide
2.1. Chemical Structure and Properties
2.2. Bioavailability and Carriers for Delivery
2.3. Pharmacokinetic Parameters
2.4. Molecular Targets and Mechanisms of Action
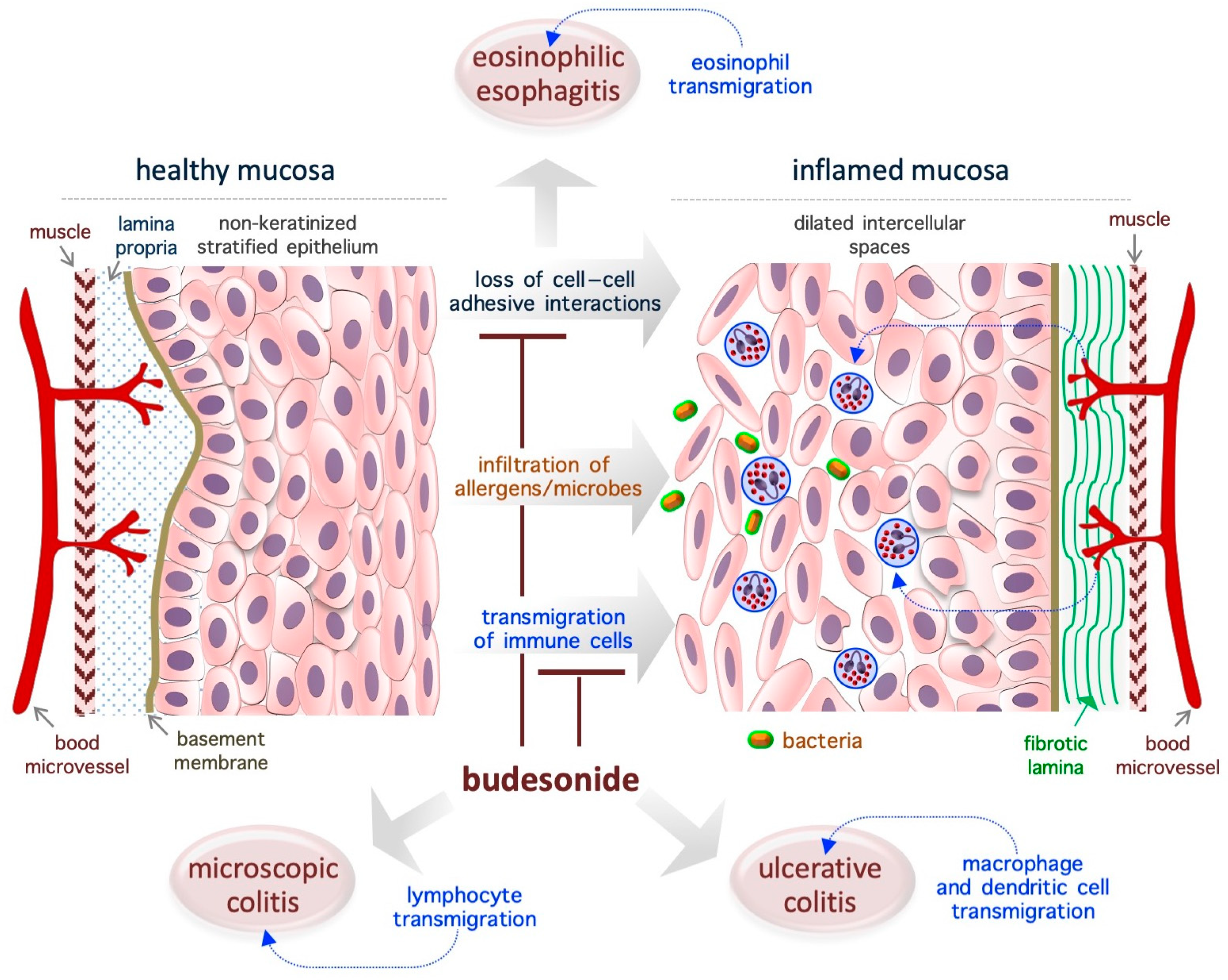
3. Budesonide-Mediated Mucosal Re-Epithelialization
3.1. Eosinophilic Esophagitis
3.2. Ulcerative Colitis
3.3. Microscopic Colitis
3.4. Asthma
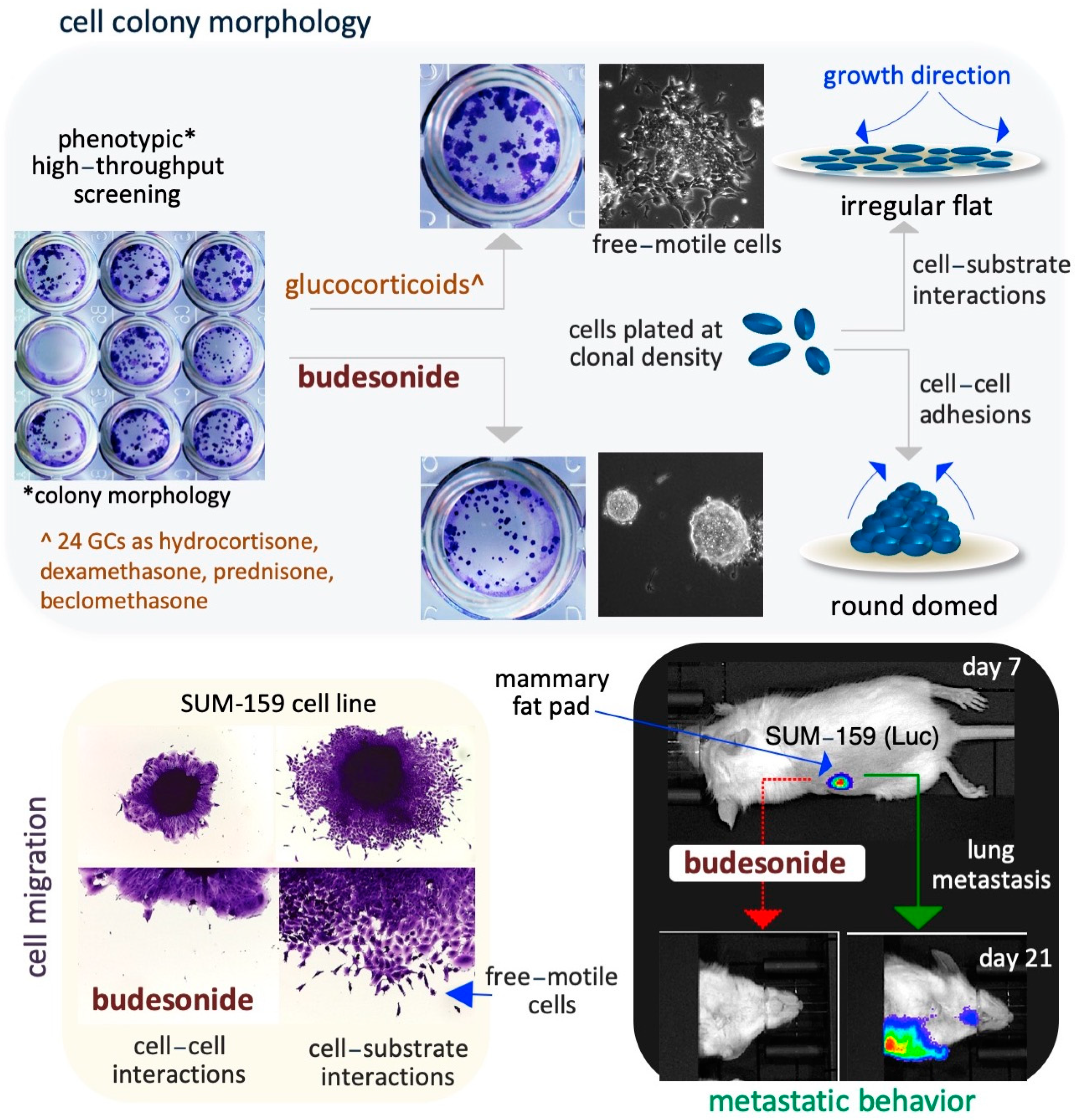
4. Budesonide-Mediated Inhibition of Plasticity in Stem and Cancer Cells
4.1. Lung Cancer Cells
4.2. Breast Cancer Cells
4.3. Pancreatic Cancer Cells
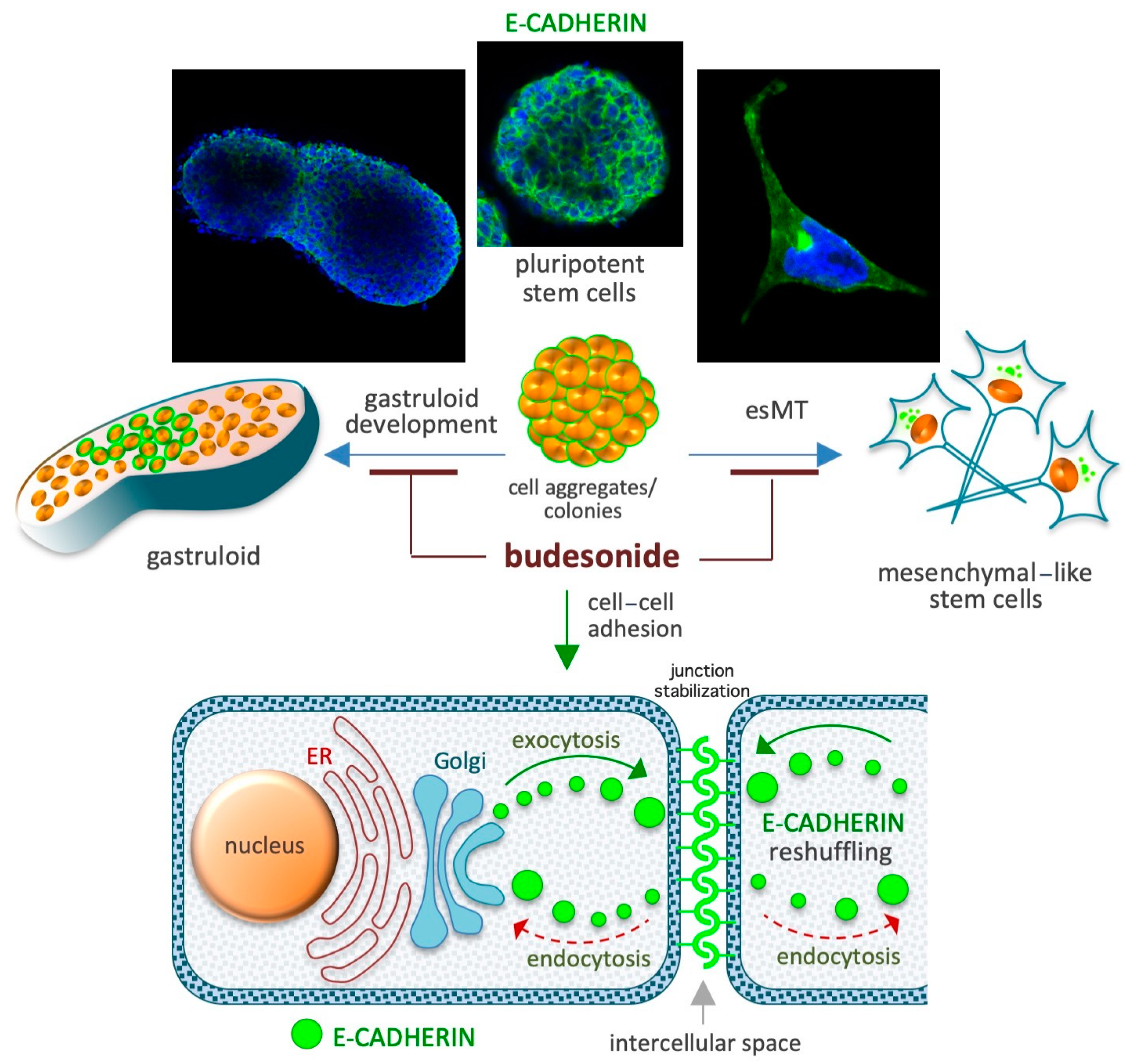
5. Budesonide-Mediated Stabilization of Cell–Cell Interactions
5.1. Preservation of the Naïve Ground State in Stem Cells
5.2. Naïve-to-Primed Transition in Pluripotent Stem Cells
5.3. Gastruloid Development
5.4. Gastroenteric Mucosa
5.5. Airway Mucosa
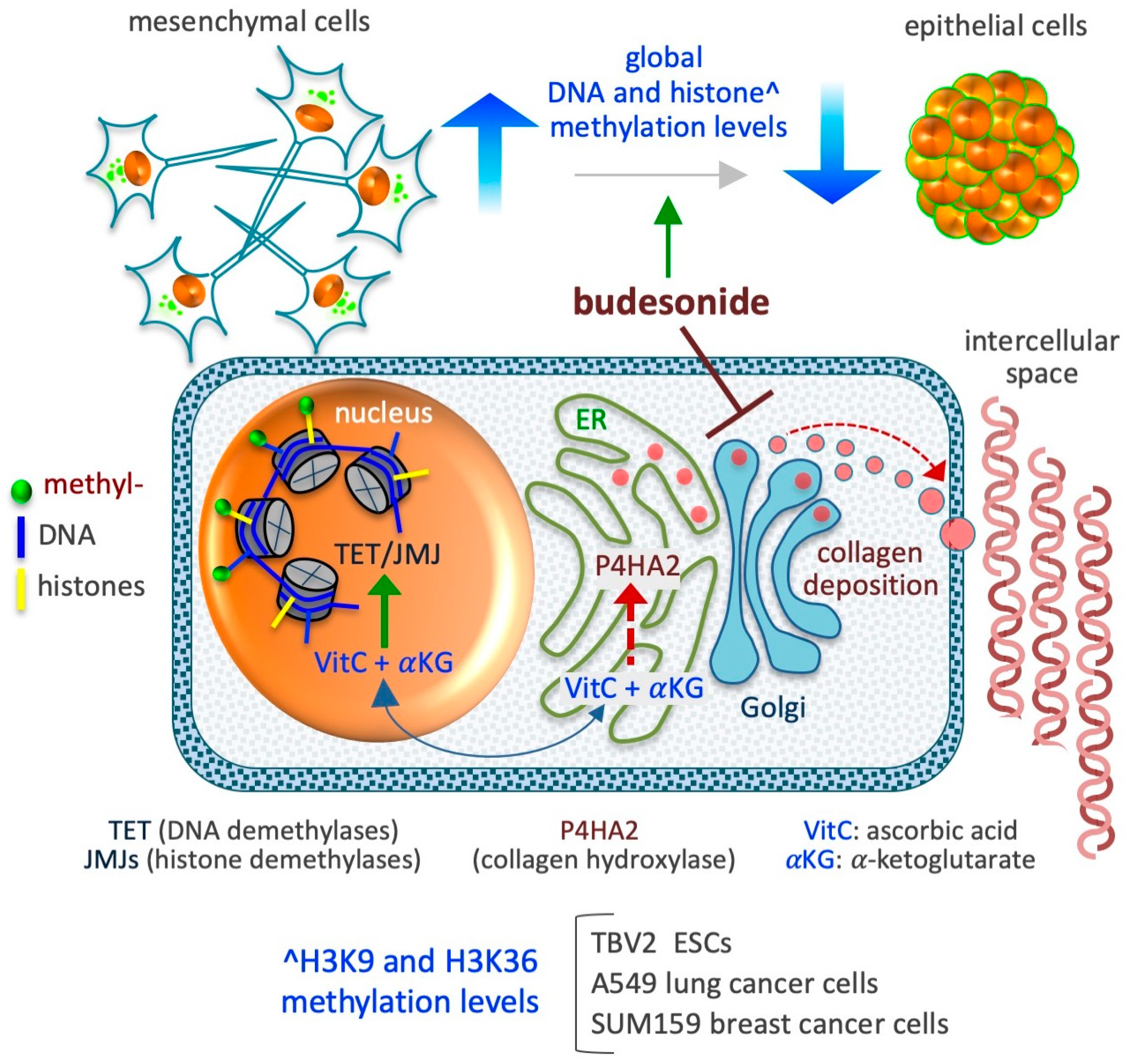
6. Budesonide-Mediated Inhibition of Collagen Deposition and Modulation of the Epigenetic Landscape
6.1. DNA Methylation
6.2. Histone Methylation
6.3. Histone Deacetylation
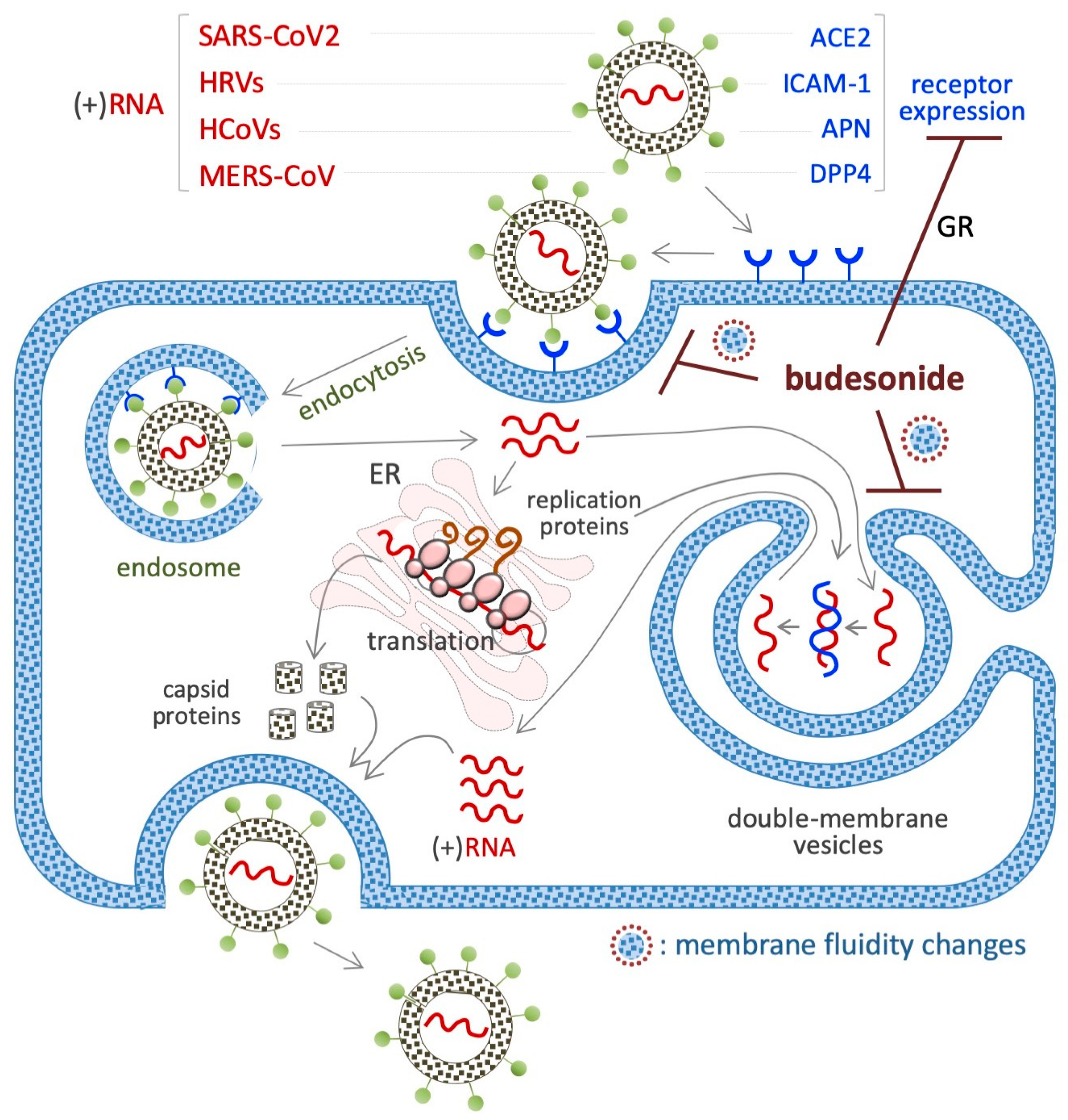
7. Budesonide-Mediated Inhibition of Replication of SARS-CoV-2 and Single-Stranded (+)RNA Virus
7.1. SARS-CoV2 (+)RNA Virus
7.2. HRVs (+)RNA Virus
7.3. HCoV (+)RNA Virus
7.4. MERS-CoV (+)RNA Virus
7.5. Viral Mimetic dsRNA
8. Concluding Remarks and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ACE2 | angiotensin-converting enzyme-2 |
| αKG | alpha-ketoglutarate |
| ASM | airway smooth muscle |
| ATF4 | activating transcription factor 4 |
| BUD | budesonide |
| cAMP | cyclic adenosine monophosphate |
| CDH1 | Cadherin 1 |
| CDKN1C | Cyclin-Dependent Kinase Inhibitor 1C |
| COX-2 | cyclooxygenase-2 |
| c-MYC | Cellular Myelocytomatosis |
| ECM1 | extracellular matrix protein 1 |
| EGF | extracellular signal-regulated kinase |
| EoE | eosinophilic esophagitis |
| EMT | epithelial-to-mesenchymal transition |
| ER | endoplasmic reticulum |
| ESCs | embryonic stem cells |
| esMT | embryonic-stem-to-mesenchymal transition |
| FGF | fibroblast growth factor |
| GCs | Glucocorticoids |
| GPCR | G protein-coupled receptor Gα |
| GR | glucocorticoid receptor |
| HCoV | human coronavirus |
| HDAC | histone deacetylase |
| 5hMC | 5-hydroxymethylcytosine |
| HRVs | human rhinoviruses |
| HTS | high-throughput screening |
| ICAM-1 | intercellular adhesion molecule-1 |
| IDs | inflammatory diseases |
| IGF-2 | Insulin-like Growth Factor 2 |
| IFN-β | type I interferon-beta |
| IL-1β | Interleukin-1 β |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| JMJ | Jumonji |
| LIF | leukemia inhibitory factor |
| LMNB1 | extracellular matrix glycoprotein laminin B1 |
| MC | microscopic colitis |
| MERS | Middle East respiratory syndrome |
| MPO | myeloperoxidase |
| N3CR1 | nuclear receptor subfamily 3 group C member 1 |
| NF-kB | nuclear factor kappa-B |
| Nrf2 | nuclear factor erythroid 2-related factor |
| PDAC | pancreatic adenocarcinoma |
| P4HA | prolyl-collagen hydroxylation |
| P450/CYP3A | cytochrome P4503A |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| TET | ten-eleven translocation |
| TGFβ1 | transforming growth factor-β1 |
| TGM1_3 | transglutaminase enzymes 1 and 3 |
| TNF | tumor necrosis factor |
| UC | ulcerative colitis |
| VitC | vitamin C |
| WNT | Wingless and Int-1 |
References
- Chrousos, G.P. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 1995, 332, 1351–1362. [Google Scholar] [CrossRef]
- Benedek, T.G. History of the development of corticosteroid therapy. Clin. Exp. Rheumatol 2011, 29 (Suppl. S68), 5–12. [Google Scholar] [PubMed]
- Ellul-Micallef, R.; Hansson, E.; Johansson, S.A. Budesonide: A new corticosteroid in bronchial asthma. Eur. J. Respir. Dis. 1980, 61, 167–173. [Google Scholar]
- Ellul-Micallef, R.; Johansson, S.A. Acute dose-response studies in bronchial asthma with a new corticosteroid, budesonide. Br. J. Clin. Pharmacol. 1983, 15, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.A.; Andersson, K.E.; Brattsand, R.; Gruvstad, E.; Hedner, P. Topical and systemic glucocorticoid potencies of budesonide and beclomethasone dipropionate in man. Eur. J. Clin. Pharmacol. 1982, 22, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, E.; Thalen, A.; Brattsand, R.; Gustafsson, J.A.; Johansson, U.; Roempke, K.; Saartok, T. Correlation between chemical structure, receptor binding, and biological activity of some novel, highly active, 16 alpha, 17 alpha-acetal-substituted glucocorticoids. Mol. Pharmacol. 1984, 25, 70–78. [Google Scholar] [CrossRef]
- Adcock, I.M.; Mumby, S. Glucocorticoids. Handb. Exp. Pharmacol. 2017, 237, 171–196. [Google Scholar] [CrossRef]
- Nilsson, K.; Andersson, M.; Beck, O. Phospholipid removal combined with a semi-automated 96-well SPE application for determination of budesonide in human plasma with LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 970, 31–35. [Google Scholar] [CrossRef]
- Streel, B.; Cahay, B.; Klinkenberg, R. Using total error concept for the validation of a liquid chromatography-tandem mass spectrometry method for the determination of budesonide epimers in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2290–2300. [Google Scholar] [CrossRef]
- Berg, S.; Melamies, M.; Rajamaki, M.; Vainio, O.; Peltonen, K. Liquid chromatography tandem mass spectrometry determination of total budesonide levels in dog plasma after inhalation exposure. Anal. Bioanal. Chem. 2012, 402, 1209–1215. [Google Scholar] [CrossRef]
- Borges, N.C.; Astigarraga, R.B.; Sverdloff, C.E.; Borges, B.C.; Paiva, T.R.; Galvinas, P.R.; Moreno, R.A. Budesonide quantification by HPLC coupled to atmospheric pressure photoionization (APPI) tandem mass spectrometry. Application to a comparative systemic bioavailability of two budesonide formulations in healthy volunteers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 236–242. [Google Scholar] [CrossRef]
- Li, X.; Tong, H.; Xu, B.; Deng, Y.; Li, Y.; Huang, J.; Mao, Y.; Liu, M.; Zhang, P.; Guo, S. A sensitive and high-throughput LC-ESI-MS/MS method to detect budesonide in human plasma: Application to an evaluation of pharmacokinetics of budesonide intranasal formulations with and without charcoal-block in healthy volunteers. Drug Dev. Ind. Pharm. 2021, 47, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ponzetto, F.; Parasiliti-Caprino, M.; Settanni, F.; Nonnato, A.; Mengozzi, G.; Ghigo, E.; Giordano, R. Simultaneous Measurement of Cortisol, Cortisone, Dexamethasone and Additional Exogenous Corticosteroids by Rapid and Sensitive LC-MS/MS Analysis. Molecules 2022, 28, 248. [Google Scholar] [CrossRef]
- Rower, J.E.; Anderson, D.J.; Sherwin, C.M.; Reilly, C.A.; Ballard, P.L.; Mcevoy, C.T.; Wilkins, D.G. Development and validation of an assay for quantifying budesonide in dried blood spots collected from extremely low gestational age neonates. J. Pharm. Biomed. Anal. 2019, 167, 7–14. [Google Scholar] [CrossRef]
- Jonsson, G.; Astrom, A.; Andersson, P. Budesonide is metabolized by cytochrome P450 3A (CYP3A) enzymes in human liver. Drug Metab. Dispos. 1995, 23, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.; Otley, A. Budesonide in the treatment of inflammatory bowel disease. Expert. Rev. Clin. Immunol. 2011, 7, 419–428. [Google Scholar] [CrossRef]
- Dilger, K.; Schwab, M.; Fromm, M.F. Identification of budesonide and prednisone as substrates of the intestinal drug efflux pump P-glycoprotein. Inflamm. Bowel Dis. 2004, 10, 578–583. [Google Scholar] [CrossRef]
- Szefler, S.J. Pharmacodynamics and pharmacokinetics of budesonide: A new nebulized corticosteroid. J. Allergy Clin. Immunol. 1999, 104, S175–S183. [Google Scholar] [CrossRef]
- Claytor, J.; Kumar, P.; Ananthakrishnan, A.N.; Colombel, J.F.; Agrawal, M.; Ungaro, R.C. Mild Crohn’s Disease: Definition and Management. Curr. Gastroenterol. Rep. 2023, 25, 45–51. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef]
- Leigh, R.; Mostafa, M.M.; King, E.M.; Rider, C.F.; Shah, S.; Dumonceaux, C.; Traves, S.L.; Mcwhae, A.; Kolisnik, T.; Kooi, C.; et al. An inhaled dose of budesonide induces genes involved in transcription and signaling in the human airways: Enhancement of anti- and proinflammatory effector genes. Pharmacol. Res. Perspect. 2016, 4, e00243. [Google Scholar] [CrossRef]
- Toropainen, T.; Velaga, S.; Heikkila, T.; Matilainen, L.; Jarho, P.; Carlfors, J.; Lehto, V.P.; Jarvinen, T.; Jarvinen, K. Preparation of budesonide/gamma-cyclodextrin complexes in supercritical fluids with a novel SEDS method. J. Pharm. Sci. 2006, 95, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.G.; Bayiha, J.C.; Dufour, G.; Cataldo, D.; Evrard, B.; Silva, L.C.; Deleu, M.; Mingeot-Leclercq, M.P. Changes in membrane biophysical properties induced by the Budesonide/Hydroxypropyl-beta-cyclodextrin complex. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Bayiha, J.C.; Evrard, B.; Cataldo, D.; De Tullio, P.; Mingeot-Leclercq, M.P. The Budesonide-Hydroxypropyl-beta-Cyclodextrin Complex Attenuates ROS Generation, IL-8 Release and Cell Death Induced by Oxidant and Inflammatory Stress. Study on A549 and A-THP-1 Cells. Molecules 2020, 25, 4882. [Google Scholar] [CrossRef]
- Dufour, G.; Bigazzi, W.; Wong, N.; Boschini, F.; De Tullio, P.; Piel, G.; Cataldo, D.; Evrard, B. Interest of cyclodextrins in spray-dried microparticles formulation for sustained pulmonary delivery of budesonide. Int. J. Pharm. 2015, 495, 869–878. [Google Scholar] [CrossRef]
- Xian, S.; Zhu, J.; Wang, Y.; Song, H.; Wang, H. Oral liposomal delivery of an activatable budesonide prodrug reduces colitis in experimental mice. Drug Deliv. 2023, 30, 2183821. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Ahmad, A.; Kanika; Kumar, A.; Vyawahare, A.; Sakla, R.; Nadeem, A.; Siddiqui, N.; Raza, S.; Khan, R. Caffeic Acid-Conjugated Budesonide-Loaded Nanomicelle Attenuates Inflammation in Experimental Colitis. Mol. Pharm. 2023, 20, 172–182. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; D/O Segar Singh, S.K.; Chetty Annan, N.; Bhattamisra, S.K.; Gorain, B.; Mohd Amin, M.C.I. Budesonide-Loaded Pectin/Polyacrylamide Hydrogel for Sustained Delivery: Fabrication, Characterization and In Vitro Release Kinetics. Molecules 2021, 26, 2704. [Google Scholar] [CrossRef]
- Vafaei, S.Y.; Abdolghaffari, A.H.; Mahjub, R.; Eslami, S.M.; Esmaeili, M.; Abdollahi, M.; Atyabi, F.; Dinarvand, R. Budesonide-Loaded Hyaluronic Acid Nanoparticles for Targeted Delivery to the Inflamed Intestinal Mucosa in a Rodent Model of Colitis. Biomed. Res. Int. 2022, 2022, 7776092. [Google Scholar] [CrossRef]
- Ali, H.; Weigmann, B.; Neurath, M.F.; Collnot, E.M.; Windbergs, M.; Lehr, C.M. Budesonide loaded nanoparticles with pH-sensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases. J. Control Release 2014, 183, 167–177. [Google Scholar] [CrossRef]
- Kompella, U.B.; Bandi, N.; Ayalasomayajula, S.P. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Yathavan, B.; Ellis, A.; Jedrzkiewicz, J.; Subrahmanyam, N.; Khurana, N.; Pulsipher, A.; Alt, J.A.; Ghandehari, H. Systemic administration of budesonide in pegylated liposomes for improved efficacy in chronic rhinosinusitis. J. Control Release 2023, 360, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Ferri, D.; Costero, A.M.; Gavina, P.; Parra, M.; Merino, V.; Teruel, A.H.; Sancenon, F.; Martinez-Manez, R. Efficacy of budesonide-loaded mesoporous silica microparticles capped with a bulky azo derivative in rats with TNBS-induced colitis. Int. J. Pharm. 2019, 561, 93–101. [Google Scholar] [CrossRef]
- Sguizzato, M.; Ferrara, F.; Baraldo, N.; Bondi, A.; Guarino, A.; Drechsler, M.; Valacchi, G.; Cortesi, R. Bilosomes and Biloparticles for the Delivery of Lipophilic Drugs: A Preliminary Study. Antioxidants 2023, 12, 2025. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Cai, J.; Wang, Z.; Zhang, W.; Xu, J.; Zhang, D.; Gong, D. Facile fabrication of diatomite biosilica-based nasal drug delivery vehicle for enhanced treatment of allergic rhinitis. Colloids Surf. B Biointerfaces 2024, 234, 113715. [Google Scholar] [CrossRef]
- Pinheiro Do Nascimento, L.; Tsapis, N.; Reynaud, F.; Desmaele, D.; Moine, L.; Vergnaud, J.; Abreu, S.; Chaminade, P.; Fattal, E. Mannosylation of budesonide palmitate nanoprodrugs for improved macrophage targeting. Eur. J. Pharm. Biopharm. 2022, 170, 112–120. [Google Scholar] [CrossRef]
- Slavkova, M.; Lazov, C.; Spassova, I.; Kovacheva, D.; Tibi, I.P.; Stefanova, D.; Tzankova, V.; Petrov, P.D.; Yoncheva, K. Formulation of Budesonide-Loaded Polymeric Nanoparticles into Hydrogels for Local Therapy of Atopic Dermatitis. Gels 2024, 10, 79. [Google Scholar] [CrossRef]
- Padula, C.; Machado, I.P.; Vigato, A.A.; De Araujo, D.R. New Strategies for Improving Budesonide Skin Retention. Pharmaceutics 2021, 14, 30. [Google Scholar] [CrossRef]
- Barratt, J.; Lafayette, R.A.; Rovin, B.H.; Fellstrom, B. Budesonide delayed-release capsules to reduce proteinuria in adults with primary immunoglobulin A nephropathy. Expert. Rev. Clin. Immunol. 2023, 19, 699–710. [Google Scholar] [CrossRef]
- Del Vecchio, L.; Allinovi, M.; Comolli, S.; Peiti, S.; Rimoldi, C.; Locatelli, F. Drugs in Development to Treat IgA Nephropathy. Drugs 2024, 84, 503–525. [Google Scholar] [CrossRef]
- Awad, A.; Hollis, E.; Goyanes, A.; Orlu, M.; Gaisford, S.; Basit, A.W. 3D printed multi-drug-loaded suppositories for acute severe ulcerative colitis. Int. J. Pharm. X 2023, 5, 100165. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Viano, I.; Ong, J.J.; Luzardo-Alvarez, A.; Gonzalez-Barcia, M.; Basit, A.W.; Otero-Espinar, F.J.; Goyanes, A. 3D printed tacrolimus suppositories for the treatment of ulcerative colitis. Asian J. Pharm. Sci. 2021, 16, 110–119. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, C.; Yu, H.; Shen, K.; Assam, P.N.; Gillen, M.; Liu, Y.; Dorinsky, P. Pharmacokinetics and Tolerability of Budesonide/Glycopyrronium/Formoterol Fumarate Dihydrate and Glycopyrronium/Formoterol Fumarate Dihydrate Metered Dose Inhalers in Healthy Chinese Adults: A Randomized, Double-blind, Parallel-group Study. Clin. Ther. 2019, 41, 897–909. [Google Scholar] [CrossRef]
- Gupta, S.K.; Hill, M.; Vitanza, J.M.; Farber, R.H.; Desai, N.K.; Williams, J.; Song, I.H. Pharmacokinetics of budesonide oral suspension in children and adolescents with eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Dilger, K.; Alberer, M.; Busch, A.; Enninger, A.; Behrens, R.; Koletzko, S.; Stern, M.; Beckmann, C.; Gleiter, C.H. Pharmacokinetics and pharmacodynamic action of budesonide in children with Crohn’s disease. Aliment. Pharmacol. Ther. 2006, 23, 387–396. [Google Scholar] [CrossRef]
- O’donnell, S.; O’morain, C.A. Therapeutic benefits of budesonide in gastroenterology. Ther. Adv. Chronic Dis. 2010, 1, 177–186. [Google Scholar] [CrossRef]
- Moore, C.D.; Roberts, J.K.; Orton, C.R.; Murai, T.; Fidler, T.P.; Reilly, C.A.; Ward, R.M.; Yost, G.S. Metabolic pathways of inhaled glucocorticoids by the CYP3A enzymes. Drug Metab. Dispos. 2013, 41, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.; Seale, J.P. Clinical pharmacokinetics of inhaled budesonide. Clin. Pharmacokinet. 2001, 40, 427–440. [Google Scholar] [CrossRef]
- Van Den Bosch, J.M.; Westermann, C.J.; Aumann, J.; Edsbacker, S.; Tonnesson, M.; Selroos, O. Relationship between lung tissue and blood plasma concentrations of inhaled budesonide. Biopharm. Drug Dispos. 1993, 14, 455–459. [Google Scholar] [CrossRef]
- Corvino, A.; Granato, E.; Scognamiglio, A.; Fiorino, F.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Cirino, G.; Cerqua, I.; et al. A New Process for the Synthesis of Budesonide 21-Phosphate and Evaluation in a Murine Model of Inflammation. Molecules 2024, 29, 4514. [Google Scholar] [CrossRef]
- Barrette, A.M.; Roberts, J.K.; Chapin, C.; Egan, E.A.; Segal, M.R.; Oses-Prieto, J.A.; Chand, S.; Burlingame, A.L.; Ballard, P.L. Antiinflammatory Effects of Budesonide in Human Fetal Lung. Am. J. Respir. Cell Mol. Biol. 2016, 55, 623–632. [Google Scholar] [CrossRef]
- Nunez, F.J.; Johnstone, T.B.; Corpuz, M.L.; Kazarian, A.G.; Mohajer, N.N.; Tliba, O.; Panettieri, R.A., Jr.; Koziol-White, C.; Roosan, M.R.; Ostrom, R.S. Glucocorticoids rapidly activate cAMP production via G(alphas) to initiate non-genomic signaling that contributes to one-third of their canonical genomic effects. FASEB J. 2020, 34, 2882–2895. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.W.; Miao, C.Y.; Liu, L.; Zhou, J.; Su, D.F.; Wang, Y.X.; Jiang, C.L. Rapid inhibitory effect of glucocorticoids on airway smooth muscle contractions in guinea pigs. Steroids 2006, 71, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Triggiani, M.; Granata, F.; Petraroli, A.; Loffredo, S.; Frattini, A.; Staiano, R.I.; Monaco, G.; Marone, G. Inhibition of secretory phospholipase A2-induced cytokine production in human lung macrophages by budesonide. Int. Arch. Allergy Immunol. 2009, 150, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Davidow, L.; Arvanites, A.C.; Blanchard, J.; Lam, K.; Xu, K.; Oza, V.; Yoo, J.W.; Ng, J.M.; Curran, T.; et al. Glucocorticoid compounds modify smoothened localization and hedgehog pathway activity. Chem. Biol. 2012, 19, 972–982. [Google Scholar] [CrossRef]
- Rana, R.; Carroll, C.E.; Lee, H.J.; Bao, J.; Marada, S.; Grace, C.R.; Guibao, C.D.; Ogden, S.K.; Zheng, J.J. Structural insights into the role of the Smoothened cysteine-rich domain in Hedgehog signalling. Nat. Commun. 2013, 4, 2965. [Google Scholar] [CrossRef]
- Recchia, A.D.; Dominicis, A.; D’amore, V.M.; Fabiano, T.; Al Jaf, A.I.A.; Peria, S.; Basoli, F.; Rainer, A.; Marinelli, L.; Di Leva, F.S.; et al. Pharmacological targeting of smoothened receptor cysteine-rich domain by Budesonide promotes in vitro myelination. Front. Mol. Neurosci. 2024, 17, 1473960. [Google Scholar] [CrossRef]
- Orta, M.L.; Dominguez, I.; Pastor, N.; Cortes, F.; Mateos, S. The role of the DNA hypermethylating agent Budesonide in the decatenating activity of DNA topoisomerase II. Mutat. Res. 2010, 694, 45–52. [Google Scholar] [CrossRef]
- Laserna-Mendieta, E.J.; Navarro, P.; Casabona-Frances, S.; Savarino, E.V.; Amorena, E.; Perez-Martinez, I.; Guagnozzi, D.; Blas-Jhon, L.; Betore, E.; Guardiola-Arevalo, A.; et al. Swallowed topical corticosteroids for eosinophilic esophagitis: Utilization and real-world efficacy from the EoE CONNECT registry. United Eur. Gastroenterol. J. 2024, 12, 585–595. [Google Scholar] [CrossRef]
- Hirano, I.; Dellon, E.S.; Gupta, S.K.; Katzka, D.A.; Collins, M.H.; Wojtowicz, A.M.; Terreri, B.; Zhang, W.; Boules, M.; Bhatia, S.; et al. Safety of an investigational formulation of budesonide (budesonide oral suspension) for eosinophilic oesophagitis: An integrated safety analysis of six phase 1–3 clinical trials. Aliment. Pharmacol. Ther. 2023, 57, 1117–1130. [Google Scholar] [CrossRef]
- Gupta, S.K.; Vitanza, J.M.; Collins, M.H. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2015, 13, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Straumann, A.; Lucendo, A.J.; Miehlke, S.; Vieth, M.; Schlag, C.; Biedermann, L.; Vaquero, C.S.; Ciriza De Los Rios, C.; Schmoecker, C.; Madisch, A.; et al. Budesonide orodispersible tablets maintain remission in a randomized, placebo-controlled trial of patients with eosinophilic esophagitis. Gastroenterology 2020, 159, 1672–1685.e5. [Google Scholar] [CrossRef] [PubMed]
- Lucendo, A.J.; Molina-Infante, J.; Arias, A.; Von Arnim, U.; Bredenoord, A.J.; Bussmann, C.; Amil Dias, J.; Bove, M.; Gonzalez-Cervera, J.; Larsson, H.; et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur. Gastroenterol. J. 2017, 5, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Lucendo, A.J.; Miehlke, S.; Schlag, C.; Vieth, M.; Von Arnim, U.; Molina-Infante, J.; Hartmann, D.; Bredenoord, A.J.; Ciriza De Los Rios, C.; Schubert, S.; et al. Efficacy of Budesonide Orodispersible Tablets as Induction Therapy for Eosinophilic Esophagitis in a Randomized Placebo-Controlled Trial. Gastroenterology 2019, 157, 74–86. [Google Scholar] [CrossRef]
- O’shea, K.M.; Aceves, S.S.; Dellon, E.S.; Gupta, S.K.; Spergel, J.M.; Furuta, G.T.; Rothenberg, M.E. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology 2018, 154, 333–345. [Google Scholar] [CrossRef]
- Dellon, E.S.; Hirano, I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology 2018, 154, 319–332. [Google Scholar] [CrossRef]
- Racca, F.; Pellegatta, G.; Cataldo, G.; Vespa, E.; Carlani, E.; Pelaia, C.; Paoletti, G.; Messina, M.R.; Nappi, E.; Canonica, G.W.; et al. Type 2 Inflammation in Eosinophilic Esophagitis: From Pathophysiology to Therapeutic Targets. Front. Physiol. 2021, 12, 815842. [Google Scholar] [CrossRef]
- Adel-Patient, K.; Campeotto, F.; Grauso, M.; Guillon, B.; Moroldo, M.; Venot, E.; Dietrich, C.; Machavoine, F.; Castelli, F.A.; Fenaille, F.; et al. Assessment of local and systemic signature of eosinophilic esophagitis (EoE) in children through multi-omics approaches. Front. Immunol. 2023, 14, 1108895. [Google Scholar] [CrossRef]
- Shoda, T.; Wen, T.; Aceves, S.S.; Abonia, J.P.; Atkins, D.; Bonis, P.A.; Caldwell, J.M.; Capocelli, K.E.; Carpenter, C.L.; Collins, M.H.; et al. Consortium of Eosinophilic Gastrointestinal Disease, Researchers Eosinophilic oesophagitis endotype classification by molecular, clinical, and histopathological analyses: A cross-sectional study. Lancet Gastroenterol. Hepatol. 2018, 3, 477–488. [Google Scholar] [CrossRef]
- Sherrill, J.D.; Kc, K.; Wu, D.; Djukic, Z.; Caldwell, J.M.; Stucke, E.M.; Kemme, K.A.; Costello, M.S.; Mingler, M.K.; Blanchard, C.; et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol. 2014, 7, 718–729. [Google Scholar] [CrossRef]
- Rochman, M.; Travers, J.; Miracle, C.E.; Bedard, M.C.; Wen, T.; Azouz, N.P.; Caldwell, J.M.; Kc, K.; Sherrill, J.D.; Davis, B.P.; et al. Profound loss of esophageal tissue differentiation in patients with eosinophilic esophagitis. J. Allergy Clin. Immunol. 2017, 140, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.; Wang, N.; Stringer, K.F.; Mishra, A.; Fulkerson, P.C.; Abonia, J.P.; Jameson, S.C.; Kirby, C.; Konikoff, M.R.; Collins, M.H.; et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J. Clin. Investig. 2006, 116, 536–547. [Google Scholar] [CrossRef]
- Sherrill, J.D.; Kiran, K.C.; Blanchard, C.; Stucke, E.M.; Kemme, K.A.; Collins, M.H.; Abonia, J.P.; Putnam, P.E.; Mukkada, V.A.; Kaul, A.; et al. Analysis and expansion of the eosinophilic esophagitis transcriptome by RNA sequencing. Genes. Immun. 2014, 15, 361–369. [Google Scholar] [CrossRef]
- Blanchard, C.; Mingler, M.K.; Vicario, M.; Abonia, J.P.; Wu, Y.Y.; Lu, T.X.; Collins, M.H.; Putnam, P.E.; Wells, S.I.; Rothenberg, M.E. IL-13 involvement in eosinophilic esophagitis: Transcriptome analysis and reversibility with glucocorticoids. J. Allergy Clin. Immunol. 2007, 120, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xian, M.; Wang, Y.; Wang, C.; Zhang, L. Budesonide repairs decreased barrier integrity of eosinophilic nasal polyp epithelial cells caused by PM2.5. Clin. Transl. Allergy 2021, 11, e12019. [Google Scholar] [CrossRef] [PubMed]
- Ordas, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Barnes, E.L.; Loftus, E.V., Jr.; Kappelman, M.D. Effects of Race and Ethnicity on Diagnosis and Management of Inflammatory Bowel Diseases. Gastroenterology 2021, 160, 677–689. [Google Scholar] [CrossRef]
- Sherlock, M.E.; Macdonald, J.K.; Griffiths, A.M.; Steinhart, A.H.; Seow, C.H. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2015, 2015, CD007698. [Google Scholar] [CrossRef]
- Xue, N.N.; He, M.; Li, Y.; Wu, J.Z.; Du, W.W.; Wu, X.M.; Yang, Z.Z.; Zhang, C.G.; Li, Q.Y.; Xiao, H. Periplaneta americana extract promotes intestinal mucosa repair of ulcerative colitis in rat. Acta Cir. Bras. 2020, 35, e202001002. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis. Mon. 2019, 65, 100851. [Google Scholar] [CrossRef]
- Naganuma, M.; Aoyama, N.; Tada, T.; Kobayashi, K.; Hirai, F.; Watanabe, K.; Watanabe, M.; Hibi, T. Complete mucosal healing of distal lesions induced by twice-daily budesonide 2-mg foam promoted clinical remission of mild-to-moderate ulcerative colitis with distal active inflammation: Double-blind, randomized study. J. Gastroenterol. 2018, 53, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.E.; D’amato, M.; Ng, S.C.; Pardi, D.S.; Ludvigsson, J.F.; Khalili, H. Microscopic colitis. Nat. Rev. Dis. Primers 2021, 7, 39. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Fernandez-Banares, F.; Sato, T.; Pardi, D.S. Microscopic colitis: Etiopathology, diagnosis, and rational management. Elife 2022, 11, e79397. [Google Scholar] [CrossRef]
- Pervez, A.; Siddique, K.; Khan, M.A.S. A Literature Review of Microscopic Colitis. Cureus 2024, 16, e52862. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Pardi, D.S. Diagnosis and Pharmacological Management of Microscopic Colitis in Geriatric Care. Drugs Aging 2024, 41, 113–123. [Google Scholar] [CrossRef]
- Langner, C.; Aust, D.; Ensari, A.; Villanacci, V.; Becheanu, G.; Miehlke, S.; Geboes, K.; Munch, A. Working Group of Digestive Diseases of the European Society Of, PathologyThe European Microscopic Colitis, Group Histology of microscopic colitis-review with a practical approach for pathologists. Histopathology 2015, 66, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Pardi, D.S. Diagnosis and Management of Microscopic Colitis. Am. J. Gastroenterol. 2017, 112, 78–85. [Google Scholar] [CrossRef]
- Sehgal, K.; Tome, J.; Kamboj, A.K.; Dierkhising, R.A.; Pardi, D.S.; Khanna, S. The natural history of histological changes in microscopic colitis. Ther. Adv. Gastroenterol. 2023, 16, 17562848231168237. [Google Scholar] [CrossRef] [PubMed]
- Miehlke, S.; Guagnozzi, D.; Zabana, Y.; Tontini, G.E.; Kanstrup Fiehn, A.M.; Wildt, S.; Bohr, J.; Bonderup, O.; Bouma, G.; D’amato, M.; et al. European guidelines on microscopic colitis: United European Gastroenterology and European Microscopic Colitis Group statements and recommendations. United Eur. Gastroenterol. J. 2021, 9, 13–37. [Google Scholar] [CrossRef]
- Tome, J.; Sehgal, K.; Kamboj, A.K.; Comstock, B.; Harmsen, W.S.; Khanna, S.; Pardi, D.S. Budesonide maintenance in microscopic colitis: Clinical outcomes and safety profile from a population-based study. Am. J. Gastroenterol. 2022, 117, 1311–1315. [Google Scholar] [CrossRef]
- Escudero-Hernandez, C.; Van Beelen Granlund, A.; Bruland, T.; Sandvik, A.K.; Koch, S.; Ostvik, A.E.; Munch, A. Transcriptomic Profiling of Collagenous Colitis Identifies Hallmarks of Nondestructive Inflammatory Bowel Disease. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 665–687. [Google Scholar] [CrossRef] [PubMed]
- Royce, S.G.; Cheng, V.; Samuel, C.S.; Tang, M.L. The regulation of fibrosis in airway remodeling in asthma. Mol. Cell Endocrinol. 2012, 351, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Mottais, A.; Riberi, L.; Falco, A.; Soccal, S.; Gohy, S.; De Rose, V. Epithelial-Mesenchymal Transition Mechanisms in Chronic Airway Diseases: A Common Process to Target? Int. J. Mol. Sci. 2023, 24, 12412. [Google Scholar] [CrossRef]
- Tsartsali, L.; Hislop, A.A.; Mckay, K.; James, A.L.; Elliot, J.; Zhu, J.; Rosenthal, M.; Payne, D.N.; Jeffery, P.K.; Bush, A.; et al. Development of the bronchial epithelial reticular basement membrane: Relationship to epithelial height and age. Thorax 2011, 66, 280–2855. [Google Scholar] [CrossRef] [PubMed]
- Roche, W.R.; Beasley, R.; Williams, J.H.; Holgate, S.T. Subepithelial fibrosis in the bronchi of asthmatics. Lancet 1989, 1, 520–524. [Google Scholar] [CrossRef]
- D’aniello, C.; Cermola, F.; Palamidessi, A.; Wanderlingh, L.G.; Gagliardi, M.; Migliaccio, A.; Varrone, F.; Casalino, L.; Matarazzo, M.R.; De Cesare, D.; et al. Collagen Prolyl Hydroxylation-Dependent Metabolic Perturbation Governs Epigenetic Remodeling and Mesenchymal Transition in Pluripotent and Cancer Cells. Cancer Res. 2019, 79, 3235–3250. [Google Scholar] [CrossRef]
- Comes, S.; Gagliardi, M.; Laprano, N.; Fico, A.; Cimmino, A.; Palamidessi, A.; De Cesare, D.; De Falco, S.; Angelini, C.; Scita, G.; et al. L-Proline induces a mesenchymal-like invasive program in embryonic stem cells by remodeling H3K9 and H3K36 methylation. Stem Cell Rep. 2013, 1, 307–321. [Google Scholar] [CrossRef]
- Minchiotti, G.; D’aniello, C.; Fico, A.; De Cesare, D.; Patriarca, E.J. Capturing Transitional Pluripotency through Proline Metabolism. Cells 2022, 11, 2125. [Google Scholar] [CrossRef]
- Casalino, L.; Comes, S.; Lambazzi, G.; De Stefano, B.; Filosa, S.; De Falco, S.; De Cesare, D.; Minchiotti, G.; Patriarca, E.J. Control of embryonic stem cell metastability by L-proline catabolism. J. Mol. Cell Biol. 2011, 3, 108–122. [Google Scholar] [CrossRef]
- Ibello, E.; Saracino, F.; Delle Cave, D.; Buonaiuto, S.; Amoroso, F.; Andolfi, G.; Corona, M.; Guardiola, O.; Colonna, V.; Sainz, B., Jr.; et al. Three-dimensional environment sensitizes pancreatic cancer cells to the anti-proliferative effect of budesonide by reprogramming energy metabolism. J. Exp. Clin. Cancer Res. 2024, 43, 165. [Google Scholar] [CrossRef]
- Gomez-Rubio, P.; Zock, J.P.; Rava, M.; Marquez, M.; Sharp, L.; Hidalgo, M.; Carrato, A.; Ilzarbe, L.; Michalski, C.; Molero, X.; et al. Reduced risk of pancreatic cancer associated with asthma and nasal allergies. Gut 2017, 66, 314–322. [Google Scholar] [CrossRef]
- Willey, R.F.; Godden, D.J.; Carmichael, J.; Preston, P.; Frame, M.H.; Crompton, G.K. Twice daily inhalation of a new corticosteroid, budesonide, in the treatment of chronic asthma. Eur. J. Respir. Dis. Suppl. 1982, 122, 138–142. [Google Scholar] [PubMed]
- Del Valle, I.; Rudloff, S.; Carles, A.; Li, Y.; Liszewska, E.; Vogt, R.; Kemler, R. E-cadherin is required for the proper activation of the Lifr/Gp130 signaling pathway in mouse embryonic stem cells. Development 2013, 140, 1684–1692. [Google Scholar] [CrossRef]
- Soncin, F.; Mohamet, L.; Eckardt, D.; Ritson, S.; Eastham, A.M.; Bobola, N.; Russell, A.; Davies, S.; Kemler, R.; Merry, C.L.; et al. Abrogation of E-cadherin-mediated cell-cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal. Stem Cells 2009, 27, 2069–2080. [Google Scholar] [CrossRef]
- Wong, S.H.M.; Fang, C.M.; Chuah, L.H.; Leong, C.O.; Ngai, S.C. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit. Rev. Oncol. Hematol. 2018, 121, 11–22. [Google Scholar] [CrossRef]
- Sharaireh, A.M.; Fitzpatrick, L.M.; Ward, C.M.; Mckay, T.R.; Unwin, R.D. Epithelial cadherin regulates transition between the naive and primed pluripotent states in mouse embryonic stem cells. Stem Cells 2020, 38, 1292–1306. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, X.; Wang, Y.; Chen, X.; Chen, W.; Dan, S.; She, S.; Hu, W.; Dai, J.; Hu, J.; et al. Translational and post-translational control of human naive versus primed pluripotency. iScience 2022, 25, 103645. [Google Scholar] [CrossRef] [PubMed]
- Soncin, F.; Mohamet, L.; Ritson, S.; Hawkins, K.; Bobola, N.; Zeef, L.; Merry, C.L.; Ward, C.M. E-cadherin acts as a regulator of transcripts associated with a wide range of cellular processes in mouse embryonic stem cells. PLoS ONE 2011, 6, e21463. [Google Scholar] [CrossRef]
- Ying, Q.L.; Wray, J.; Nichols, J.; Batlle-Morera, L.; Doble, B.; Woodgett, J.; Cohen, P.; Smith, A. The ground state of embryonic stem cell self-renewal. Nature 2008, 453, 519–523. [Google Scholar] [CrossRef]
- Pieters, T.; Van Roy, F. Role of cell-cell adhesion complexes in embryonic stem cell biology. J. Cell Sci. 2014, 127, 2603–2613. [Google Scholar] [CrossRef]
- Cermola, F.; Amoroso, F.; Saracino, F.; Ibello, E.; De Cesare, D.; Fico, A.; Cobellis, G.; Scalera, E.; Casiraghi, C.; D’aniello, C.; et al. Stabilization of cell-cell adhesions prevents symmetry breaking and locks in pluripotency in 3D gastruloids. Stem Cell Rep. 2022, 17, 2548–2564. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, F.; Ibello, E.; Saracino, F.; Cermola, F.; Ponticelli, G.; Scalera, E.; Ricci, F.; Villetti, G.; Cobellis, G.; Minchiotti, G.; et al. Budesonide Analogues Preserve Stem Cell Pluripotency and Delay 3D Gastruloid Development. Pharmaceutics 2023, 15, 1897. [Google Scholar] [CrossRef]
- D’aniello, C.; Habibi, E.; Cermola, F.; Paris, D.; Russo, F.; Fiorenzano, A.; Di Napoli, G.; Melck, D.J.; Cobellis, G.; Angelini, C.; et al. Vitamin C and l-Proline Antagonistic Effects Capture Alternative States in the Pluripotency Continuum. Stem Cell Rep. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- D’aniello, C.; Fico, A.; Casalino, L.; Guardiola, O.; Di Napoli, G.; Cermola, F.; De Cesare, D.; Tate, R.; Cobellis, G.; Patriarca, E.J.; et al. A novel autoregulatory loop between the Gcn2-Atf4 pathway and (L)-Proline [corrected] metabolism controls stem cell identity. Cell Death Differ. 2015, 22, 1094–1105. [Google Scholar] [CrossRef]
- Glover, H.J.; Holliday, H.; Shparberg, R.A.; Winkler, D.; Day, M.; Morris, M.B. Signalling pathway crosstalk stimulated by L-proline drives mouse embryonic stem cells to primitive-ectoderm-like cells. Development 2023, 150, dev201704. [Google Scholar] [CrossRef]
- Van Den Brink, S.C.; Van Oudenaarden, A. 3D gastruloids: A novel frontier in stem cell-based in vitro modeling of mammalian gastrulation. Trends Cell Biol. 2021, 31, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.E.; Santos, S.D. The ever-growing world of gastruloids: Autogenous models of mammalian embryogenesis. Curr. Opin. Genet. Dev. 2023, 82, 102102. [Google Scholar] [CrossRef]
- Cermola, F.; D’aniello, C.; Tate, R.; De Cesare, D.; Martinez-Arias, A.; Minchiotti, G.; Patriarca, E.J. Gastruloid Development Competence Discriminates Different States of Pluripotency. Stem Cell Rep. 2021, 16, 354–369. [Google Scholar] [CrossRef]
- Hashmi, A.; Tlili, S.; Perrin, P.; Lowndes, M.; Peradziryi, H.; Brickman, J.M.; Martinez Arias, A.; Lenne, P.F. Cell-state transitions and collective cell movement generate an endoderm-like region in gastruloids. Elife 2022, 11, e59371. [Google Scholar] [CrossRef]
- Riethmacher, D.; Brinkmann, V.; Birchmeier, C. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc. Natl. Acad. Sci. USA 1995, 92, 855–859. [Google Scholar] [CrossRef]
- Bandyopadhyay, C.; Schecterson, L.; Gumbiner, B.M. E-cadherin activating antibodies limit barrier dysfunction and inflammation in mouse inflammatory bowel disease. Tissue Barriers 2021, 9, 1940741. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.I.; Lees, C.W. Genetics of ulcerative colitis. Inflamm. Bowel Dis. 2011, 17, 831–848. [Google Scholar] [CrossRef]
- Muise, A.M.; Walters, T.D.; Glowacka, W.K.; Griffiths, A.M.; Ngan, B.Y.; Lan, H.; Xu, W.; Silverberg, M.S.; Rotin, D. Polymorphisms in E-cadherin (CDH1) result in a mis-localised cytoplasmic protein that is associated with Crohn’s disease. Gut 2009, 58, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Petrova, Y.I.; Spano, M.M.; Gumbiner, B.M. Conformational epitopes at cadherin calcium-binding sites and p120-catenin phosphorylation regulate cell adhesion. Mol. Biol. Cell 2012, 23, 2092–2108. [Google Scholar] [CrossRef] [PubMed]
- Seoudi, S.S.; Allam, E.A.; El-Kamel, A.H.; Elkafrawy, H.; El-Moslemany, R.M. Targeted delivery of budesonide in acetic acid induced colitis: Impact on miR-21 and E-cadherin expression. Drug Deliv. Transl. Res. 2023, 13, 2930–2947. [Google Scholar] [CrossRef]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- De Boer, W.I.; Sharma, H.S.; Baelemans, S.M.; Hoogsteden, H.C.; Lambrecht, B.N.; Braunstahl, G.J. Altered expression of epithelial junctional proteins in atopic asthma: Possible role in inflammation. Can. J. Physiol. Pharmacol. 2008, 86, 105–112. [Google Scholar] [CrossRef]
- Heijink, I.H.; Brandenburg, S.M.; Noordhoek, J.A.; Postma, D.S.; Slebos, D.J.; Van Oosterhout, A.J. Characterisation of cell adhesion in airway epithelial cell types using electric cell-substrate impedance sensing. Eur. Respir. J. 2010, 35, 894–903. [Google Scholar] [CrossRef]
- Xiao, C.; Puddicombe, S.M.; Field, S.; Haywood, J.; Broughton-Head, V.; Puxeddu, I.; Haitchi, H.M.; Vernon-Wilson, E.; Sammut, D.; Bedke, N.; et al. Defective epithelial barrier function in asthma. J. Allergy Clin. Immunol. 2011, 128, 549–556. [Google Scholar] [CrossRef]
- Veronesi, G.; Guerrieri-Gonzaga, A.; Infante, M.; Bonanni, B. Chemoprevention studies within lung cancer screening programmes. Ecancermedicalscience 2015, 9, 597. [Google Scholar] [CrossRef]
- Veronesi, G.; Szabo, E.; Decensi, A.; Guerrieri-Gonzaga, A.; Bellomi, M.; Radice, D.; Ferretti, S.; Pelosi, G.; Lazzeroni, M.; Serrano, D.; et al. Randomized phase II trial of inhaled budesonide versus placebo in high-risk individuals with CT screen-detected lung nodules. Cancer Prev. Res. 2011, 4, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, G.; Lazzeroni, M.; Szabo, E.; Brown, P.H.; Decensi, A.; Guerrieri-Gonzaga, A.; Bellomi, M.; Radice, D.; Grimaldi, M.C.; Spaggiari, L.; et al. Long-term effects of inhaled budesonide on screening-detected lung nodules. Ann. Oncol. 2015, 26, 1025–1030. [Google Scholar] [CrossRef]
- Wattenberg, L.W.; Wiedmann, T.S.; Estensen, R.D.; Zimmerman, C.L.; Steele, V.E.; Kelloff, G.J. Chemoprevention of pulmonary carcinogenesis by aerosolized budesonide in female A/J mice. Cancer Res. 1997, 57, 5489–5492. [Google Scholar] [PubMed]
- Wattenberg, L.W.; Wiedmann, T.S.; Estensen, R.D.; Zimmerman, C.L.; Galbraith, A.R.; Steele, V.E.; Kelloff, G.J. Chemoprevention of pulmonary carcinogenesis by brief exposures to aerosolized budesonide or beclomethasone dipropionate and by the combination of aerosolized budesonide and dietary myo-inositol. Carcinogenesis 2000, 21, 179–182. [Google Scholar] [CrossRef][Green Version]
- Pereira, M.A.; Li, Y.; Gunning, W.T.; Kramer, P.M.; Al-Yaqoub, F.; Lubet, R.A.; Steele, V.E.; Szabo, E.; Tao, L. Prevention of mouse lung tumors by budesonide and its modulation of biomarkers. Carcinogenesis 2002, 23, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Tao, L.H.; Wang, W.; Gunning, W.T.; Lubet, R. Chemoprevention: Mouse colon and lung tumor bioassay and modulation of DNA methylation as a biomarker. Exp. Lung Res. 2005, 31, 145–163. [Google Scholar] [CrossRef]
- Alyaqoub, F.S.; Tao, L.; Kramer, P.M.; Steele, V.E.; Lubet, R.A.; Gunning, W.T.; Pereira, M.A. Prevention of mouse lung tumors and modulation of DNA methylation by combined treatment with budesonide and R115777 (ZarnestraMT). Carcinogenesis 2006, 27, 2442–2447. [Google Scholar] [CrossRef][Green Version]
- Galbraith, A.R.; Seabloom, D.E.; Wuertz, B.R.; Antonides, J.D.; Steele, V.E.; Wattenberg, L.W.; Ondrey, F.G. Chemoprevention of Lung Carcinogenesis by Dietary Nicotinamide and Inhaled Budesonide. Cancer Prev. Res. 2019, 12, 69–78. [Google Scholar] [CrossRef]
- Pereira, M.A.; Tao, L.; Liu, Y.; Li, L.; Steele, V.E.; Lubet, R.A. Modulation by budesonide of DNA methylation and mRNA expression in mouse lung tumors. Int. J. Cancer 2007, 120, 1150–1153. [Google Scholar] [CrossRef]
- Tao, L.; Li, Y.; Wang, W.; Kramer, P.M.; Gunning, W.T.; Lubet, R.A.; Steele, V.E.; Pereira, M.A. Effect of budesonide on the methylation and mRNA expression of the insulin-like growth factor 2 and c-myc genes in mouse lung tumors. Mol. Carcinog. 2002, 35, 93–102. [Google Scholar] [CrossRef]
- Ciechomska, M.; Roszkowski, L.; Maslinski, W. DNA Methylation as a Future Therapeutic and Diagnostic Target in Rheumatoid Arthritis. Cells 2019, 8, 953. [Google Scholar] [CrossRef]
- D’aniello, C.; Cermola, F.; Patriarca, E.J.; Minchiotti, G. Vitamin C in Stem Cell Biology: Impact on Extracellular Matrix Homeostasis and Epigenetics. Stem Cells Int. 2017, 2017, 8936156. [Google Scholar] [CrossRef] [PubMed]
- D’aniello, C.; Cermola, F.; Patriarca, E.J.; Minchiotti, G. Metabolic-Epigenetic Axis in Pluripotent State Transitions. Epigenomes 2019, 3, 13. [Google Scholar] [CrossRef]
- Barnes, P.J. Inhaled Corticosteroids. Pharmaceuticals 2010, 3, 514–540. [Google Scholar] [CrossRef]
- Adenuga, D.; Caito, S.; Yao, H.; Sundar, I.K.; Hwang, J.W.; Chung, S.; Rahman, I. Nrf2 deficiency influences susceptibility to steroid resistance via HDAC2 reduction. Biochem. Biophys. Res. Commun. 2010, 403, 452–456. [Google Scholar] [CrossRef]
- Heinen, N.; Meister, T.L.; Klohn, M.; Steinmann, E.; Todt, D.; Pfaender, S. Antiviral Effect of Budesonide against SARS-CoV-2. Viruses 2021, 13, 1411. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Song, J.H.; Ahn, J.H.; Lee, G.S.; Ahn, H.; Yoon, S.I.; Kang, S.G.; Kim, P.H.; Jeon, S.M.; Choi, E.J.; et al. Antiviral and anti-inflammatory activity of budesonide against human rhinovirus infection mediated via autophagy activation. Antivir. Res. 2018, 151, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Yamaya, M.; Nishimura, H.; Deng, X.; Sugawara, M.; Watanabe, O.; Nomura, K.; Shimotai, Y.; Momma, H.; Ichinose, M.; Kawase, T. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir. Investig. 2020, 58, 155–168. [Google Scholar] [CrossRef]
- Matsuyama, S.; Kawase, M.; Nao, N.; Shirato, K.; Ujike, M.; Kamitani, W.; Shimojima, M.; Fukushi, S. The Inhaled Steroid Ciclesonide Blocks SARS-CoV-2 RNA Replication by Targeting the Viral Replication-Transcription Complex in Cultured Cells. J. Virol. 2020, 95, e01648-20. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Nicolau, D.V., Jr.; Langford, B.; Mahdi, M.; Jeffers, H.; Mwasuku, C.; Krassowska, K.; Fox, R.; Binnian, I.; Glover, V.; et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): A phase 2, open-label, randomised controlled trial. Lancet Respir. Med. 2021, 9, 763–772. [Google Scholar] [CrossRef]
- Finney, L.J.; Glanville, N.; Farne, H.; Aniscenko, J.; Fenwick, P.; Kemp, S.V.; Trujillo-Torralbo, M.B.; Loo, S.L.; Calderazzo, M.A.; Wedzicha, J.A.; et al. Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon. J. Allergy Clin. Immunol. 2021, 147, 510–519. [Google Scholar] [CrossRef]
- Patriarca, E.J.; Cermola, F.; D’aniello, C.; Fico, A.; Guardiola, O.; De Cesare, D.; Minchiotti, G. The Multifaceted Roles of Proline in Cell Behavior. Front. Cell Dev. Biol. 2021, 9, 728576. [Google Scholar] [CrossRef]
- Yamaya, M.; Nishimura, H.; Nadine, L.; Kubo, H.; Nagatomi, R. Formoterol and budesonide inhibit rhinovirus infection and cytokine production in primary cultures of human tracheal epithelial cells. Respir. Investig. 2014, 52, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef]
- Heijink, I.H.; Jonker, M.R.; De Vries, M.; Van Oosterhout, A.J.; Telenga, E.; Ten Hacken, N.H.; Postma, D.S.; Van Den Berge, M. Budesonide and fluticasone propionate differentially affect the airway epithelial barrier. Respir. Res. 2016, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, C.; Hetelekides, S.; Eliseeva, S.I.; Georas, S.N.; Veazey, J.M. Budesonide promotes airway epithelial barrier integrity following double-stranded RNA challenge. PLoS ONE 2021, 16, e0260706. [Google Scholar] [CrossRef]
- Tunek, A.; Sjodin, K.; Hallstrom, G. Reversible formation of fatty acid esters of budesonide, an antiasthma glucocorticoid, in human lung and liver microsomes. Drug Metab. Dispos. 1997, 25, 1311–1317. [Google Scholar] [PubMed]
- Miller-Larsson, A.; Mattsson, H.; Hjertberg, E.; Dahlback, M.; Tunek, A.; Brattsand, R. Reversible fatty acid conjugation of budesonide. Novel mechanism for prolonged retention of topically applied steroid in airway tissue. Drug Metab. Dispos. 1998, 26, 623–630. [Google Scholar]
- Van Den Brink, K.I.; Boorsma, M.; Staal-Van Den Brekel, A.J.; Edsbacker, S.; Wouters, E.F.; Thorsson, L. Evidence of the in vivo esterification of budesonide in human airways. Br. J. Clin. Pharmacol. 2008, 66, 27–35. [Google Scholar] [CrossRef]
- Brattsand, R.; Miller-Larsson, A. The role of intracellular esterification in budesonide once-daily dosing and airway selectivity. Clin. Ther. 2003, 25, C28–C41. [Google Scholar] [CrossRef]
- Nave, R.; Fisher, R.; Mccracken, N. In vitro metabolism of beclomethasone dipropionate, budesonide, ciclesonide, and fluticasone propionate in human lung precision-cut tissue slices. Respir. Res. 2007, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Redmer, T.; Diecke, S.; Grigoryan, T.; Quiroga-Negreira, A.; Birchmeier, W.; Besser, D. E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep. 2011, 12, 720–726. [Google Scholar] [CrossRef]
- Cheung, K.J.; Gabrielson, E.; Werb, Z.; Ewald, A.J. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 2013, 155, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Chen, S.C.; Prasad, M.; He, L.; Wang, X.; Choesmel-Cadamuro, V.; Sawyer, J.K.; Danuser, G.; Montell, D.J. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 2014, 157, 1146–1159. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.J.H.; Gumbiner, B.M.; Kwon, Y.V. Remodeling of E-cadherin subcellular localization during cell dissemination. Mol. Biol. Cell 2023, 34, ar46. [Google Scholar] [CrossRef]
- Na, T.Y.; Schecterson, L.; Mendonsa, A.M.; Gumbiner, B.M. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc. Natl. Acad. Sci. USA 2020, 117, 5931–5937. [Google Scholar] [CrossRef]
- Troyanovsky, S.M. Adherens junction: The ensemble of specialized cadherin clusters. Trends Cell Biol. 2023, 33, 374–387. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patriarca, E.J.; D’Aniello, C.; De Cesare, D.; Cobellis, G.; Minchiotti, G. The Modulation of Cell Plasticity by Budesonide: Beyond the Metabolic and Anti-Inflammatory Actions of Glucocorticoids. Pharmaceutics 2025, 17, 504. https://doi.org/10.3390/pharmaceutics17040504
Patriarca EJ, D’Aniello C, De Cesare D, Cobellis G, Minchiotti G. The Modulation of Cell Plasticity by Budesonide: Beyond the Metabolic and Anti-Inflammatory Actions of Glucocorticoids. Pharmaceutics. 2025; 17(4):504. https://doi.org/10.3390/pharmaceutics17040504
Chicago/Turabian StylePatriarca, Eduardo Jorge, Cristina D’Aniello, Dario De Cesare, Gilda Cobellis, and Gabriella Minchiotti. 2025. "The Modulation of Cell Plasticity by Budesonide: Beyond the Metabolic and Anti-Inflammatory Actions of Glucocorticoids" Pharmaceutics 17, no. 4: 504. https://doi.org/10.3390/pharmaceutics17040504
APA StylePatriarca, E. J., D’Aniello, C., De Cesare, D., Cobellis, G., & Minchiotti, G. (2025). The Modulation of Cell Plasticity by Budesonide: Beyond the Metabolic and Anti-Inflammatory Actions of Glucocorticoids. Pharmaceutics, 17(4), 504. https://doi.org/10.3390/pharmaceutics17040504






