Preparation and Therapeutic Evaluation of Engineered Semaglutide and Statin–Lipid Conjugate-Based Nanoparticle
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Rosuvastatin–Lipid Conjugate (RLC)
2.3. Preparation and Stability Optimization of SRLC NPs
2.4. Nanoparticle Analysis Using Electron Microscopy
2.5. In Silico Computer Simulation of Nanoformulation
2.6. Cytotoxicity Assay of SRLC NPs in Fibroblast Cell
2.7. Preparation of RITC-Labeled Semaglutide
2.8. In Vivo Pharmacokinetic (PK) Analysis
2.9. High-Fat Diet (HFD) Animal Experiment
2.10. Statistical Analysis
3. Results and Discussion
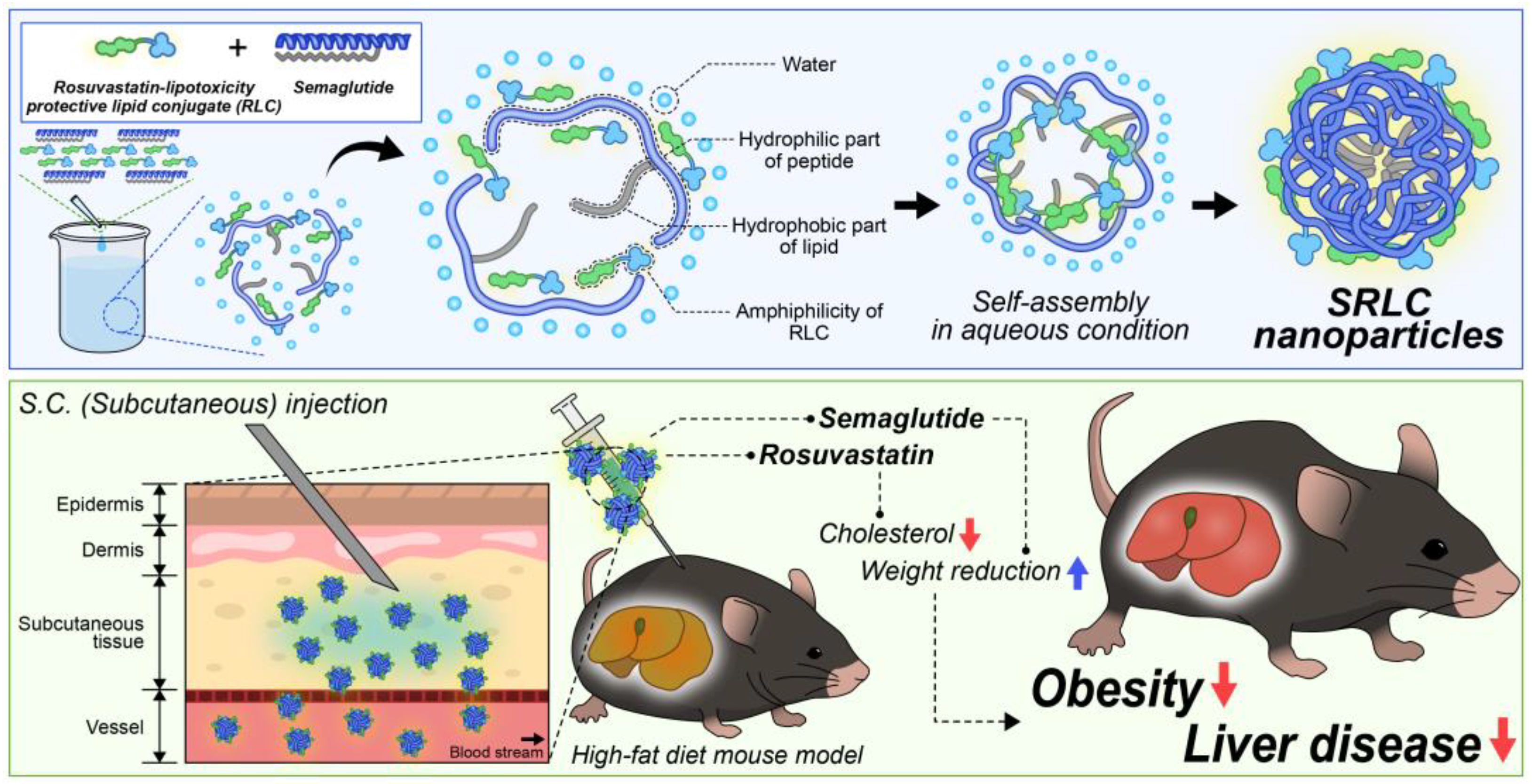
3.1. Synthesis of RLC
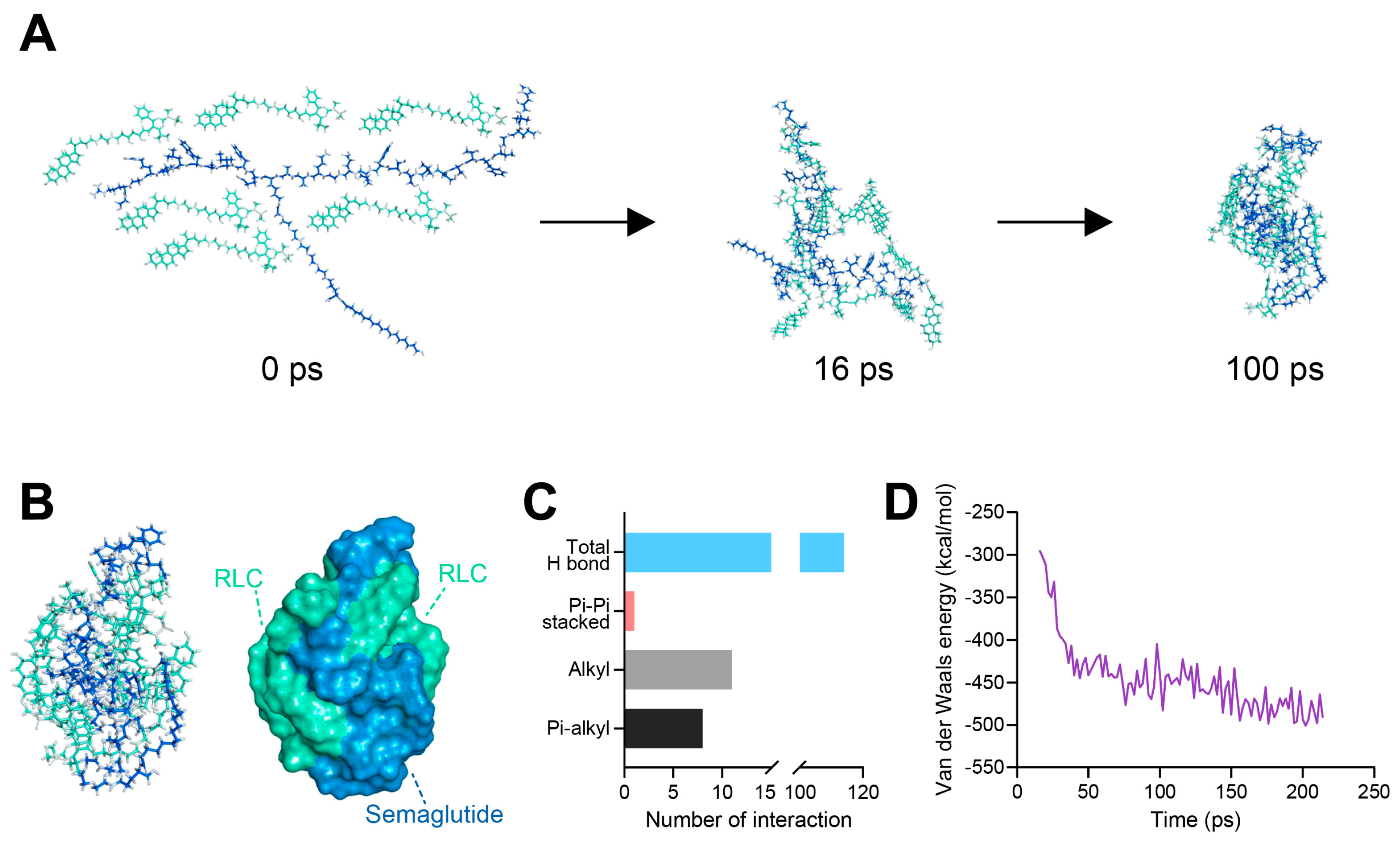
3.2. In Silico Molecular Dynamics (MD) Simulation of SRLC NPs
3.3. Characterization of SRLC NPs
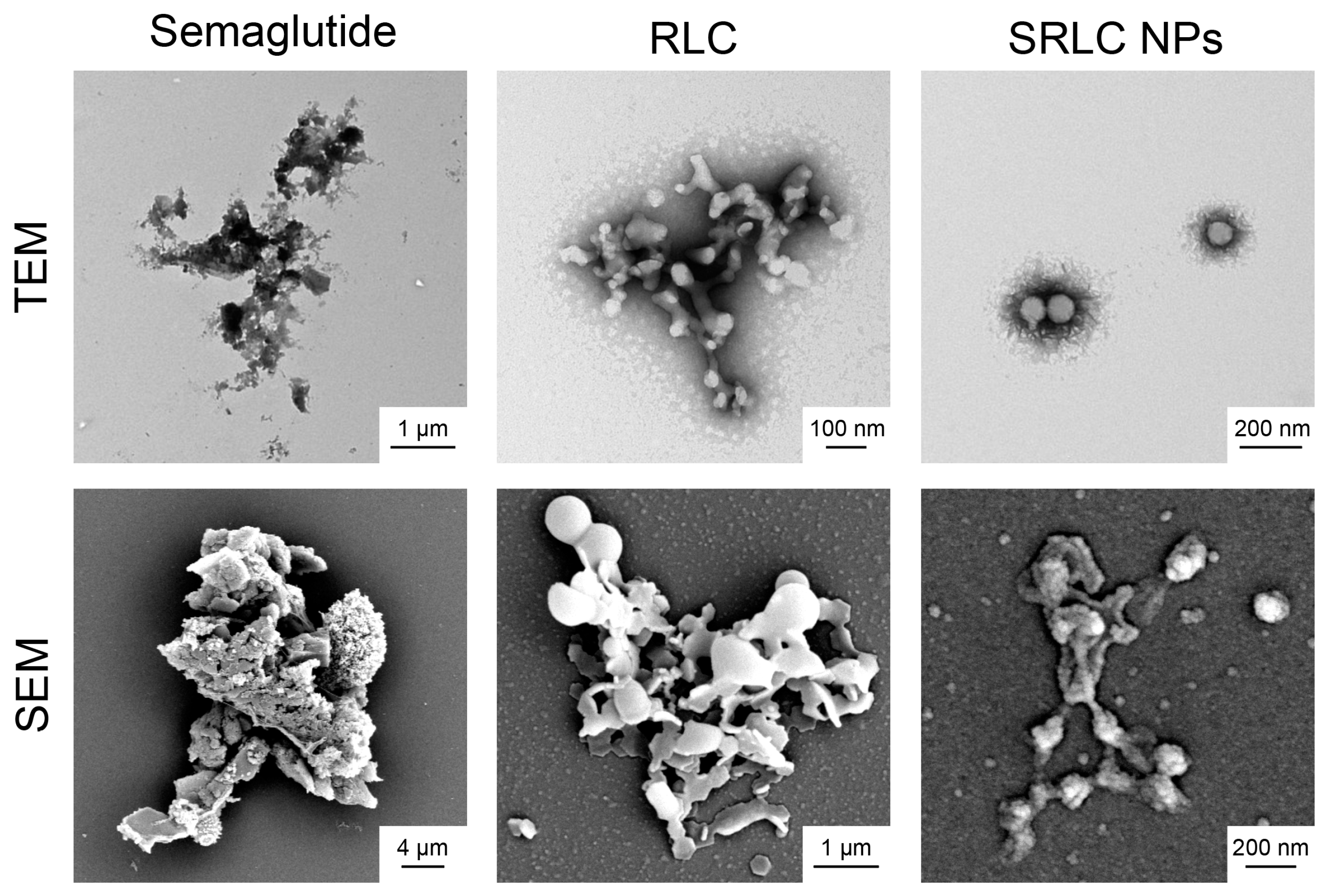
3.4. Morphological Analysis of SRLC NPs
3.5. Cytotoxicity Assay of SRLC NPs on Fibroblast Cell
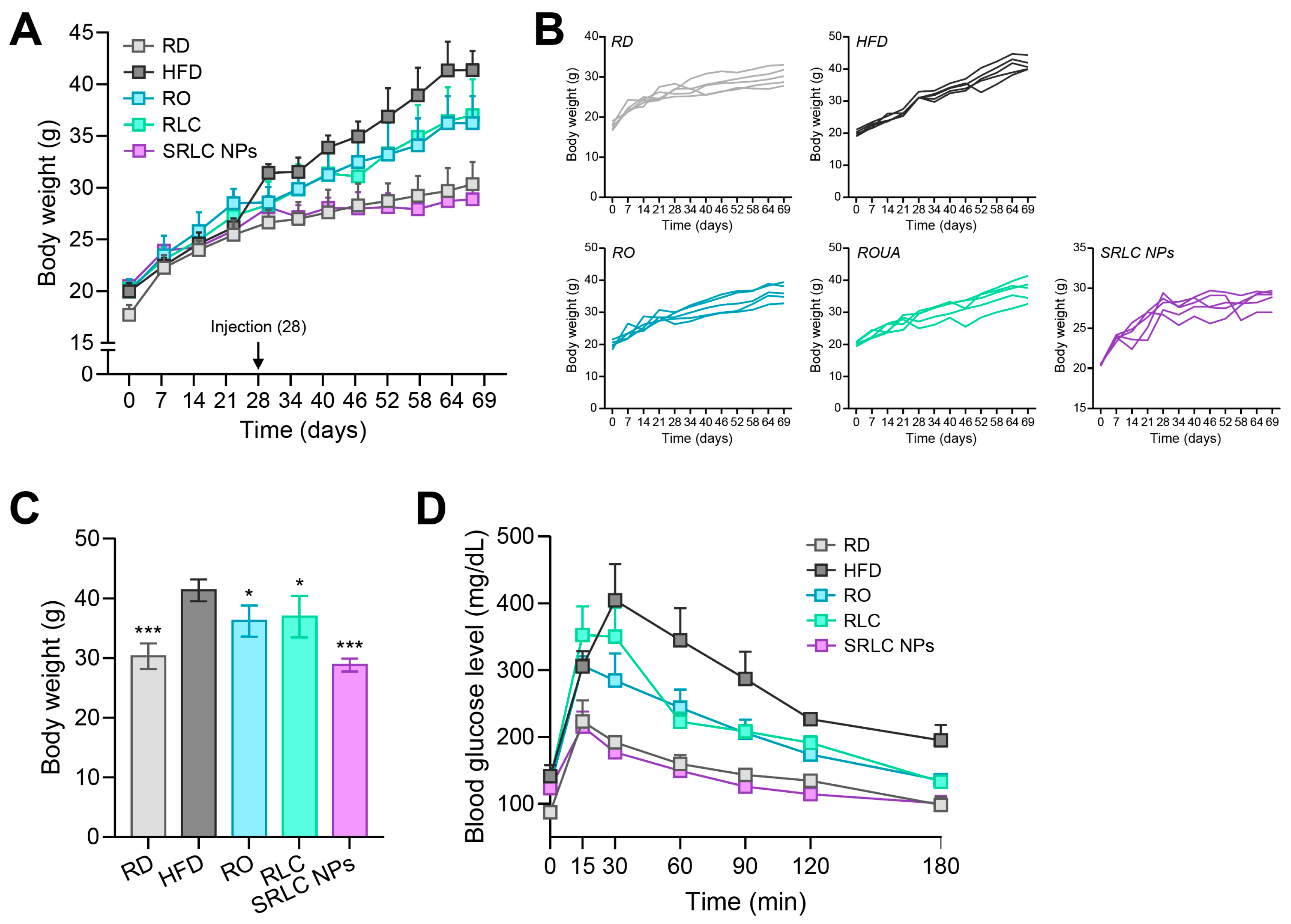
3.6. Anti-Obesity Effect of SRLC NPs in High-Fat Diet (HFD)-Fed Mouse Model
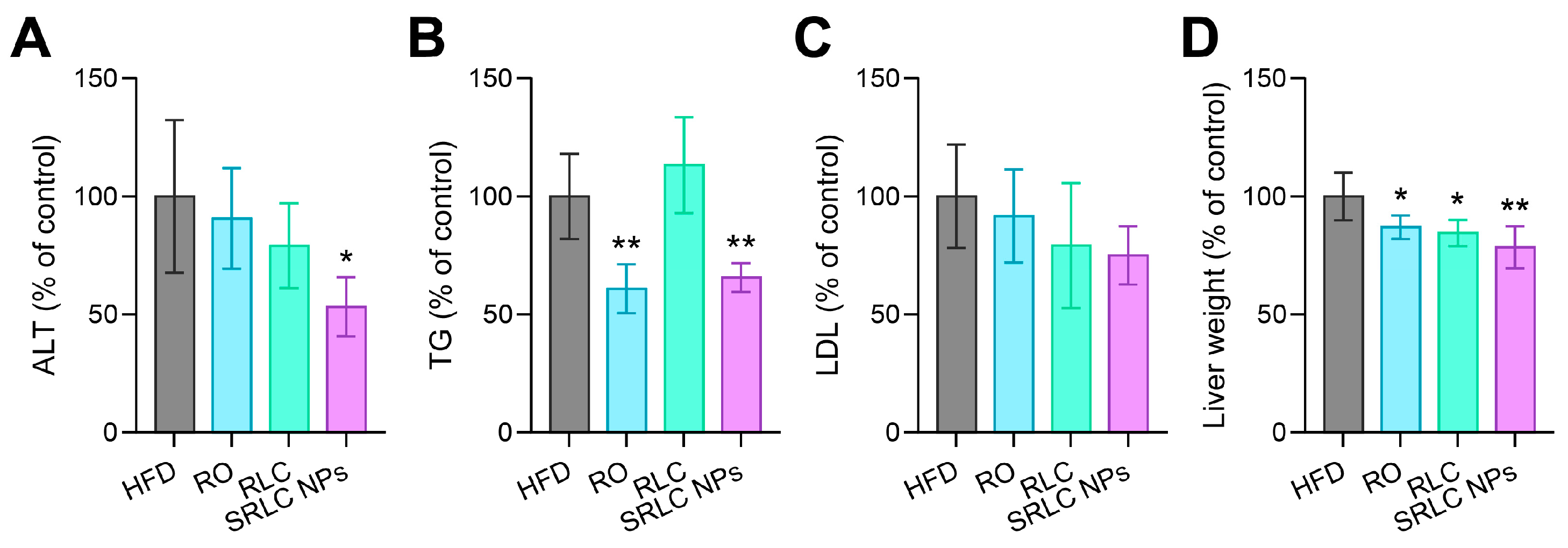
3.7. Clinical Pathological Evaluation of the Therapeutic Effects of SRLC NPs in High-Fat Diet (HFD)-Fed Mouse Model
3.8. Histopathological Evaluation of the Therapeutic Effects of SRLC NPs in High-Fat Diet (HFD)-Fed Mouse Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| DLS | Dynamic light scattering |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | Dimethyl sulfoxide |
| DPBS | Dulbecco’s phosphate-buffered saline |
| DW | Distilled water |
| EDA | Ethylenediamine |
| EDC | 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide |
| eWAT | Epididymal white adipose tissue |
| FBS | Fetal bovine serum |
| H&E | Hematoxylin and eosin staining |
| HFD | High-fat diet |
| iBAT | Interscapular brown adipose tissue |
| MD | Molecular dynamics |
| NHS | N-hydroxysuccinimide |
| NMR | Nuclear magnetic resonance |
| OGTT | Oral glucose tolerance test |
| ORO | Oil Red O staining |
| PK | Pharmacokinetic |
| RD | Regular diet |
| RITC | Rhodamine B isothiocyanate |
| RLC | Rosuvastatin–lipid conjugate |
| RO | Rosuvastatin calcium |
| RP-HPLC | Reversed-phase high-performance liquid chromatography |
| sWAT | Subcutaneous white adipose tissue |
| SEM | Scanning electron microscopy |
| SRLC NPs | Semaglutide–RLC nanoparticles |
| TBE | 2,2,2-tribromoethanol |
| TEM | Transmission electron microscopy |
| TFA | Trifluoroacetic acid |
| UDCA | Ursodeoxycholic acid |
References
- Chan, W.K. Comparison between obese and non-obese nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S58–S67. [Google Scholar]
- Milic, S.; Lulic, D.; Stimac, D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar]
- Angulo, P. Obesity and nonalcoholic fatty liver disease. Nutr. Rev. 2007, 65, S57–S63. [Google Scholar]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar]
- Wilding, J.P.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar]
- Davies, M.; Pieber, T.R.; Hartoft-Nielsen, M.-L.; Hansen, O.K.; Jabbour, S.; Rosenstock, J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: A randomized clinical trial. JAMA 2017, 318, 1460–1470. [Google Scholar]
- Blundell, J.; Finlayson, G.; Axelsen, M.; Flint, A.; Gibbons, C.; Kvist, T.; Hjerpsted, J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 2017, 19, 1242–1251. [Google Scholar]
- Kweon, S.; Lee, J.H.; Yang, S.B.; Park, S.J.; Subedi, L.; Shim, J.H.; Cho, S.S.; Choi, J.U.; Byun, Y.; Park, J.; et al. Design of chimeric GLP-1A using oligomeric bile acids to utilize transporter-mediated endocytosis for oral delivery. Biomater. Res. 2023, 27, 83. [Google Scholar]
- Buckley, S.T.; Bækdal, T.A.; Vegge, A.; Maarbjerg, S.J.; Pyke, C.; Ahnfelt-Ronne, J.; Madsen, K.G.; Schéele, S.G.; Alanentalo, T.; Kirk, R.K.; et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci. Transl. Med. 2018, 10, 7047. [Google Scholar]
- Granhall, C.; Donsmark, M.; Blicher, T.M.; Golor, G.; Sondergaard, F.L.; Thomsen, M.; Bækdal, T.A. Safety and Pharmacokinetics of Single and Multiple Ascending Doses of the Novel Oral Human GLP-1 Analogue, Oral Semaglutide, in Healthy Subjects and Subjects with Type 2 Diabetes. Clin. Pharmacokinet. 2019, 58, 781–791. [Google Scholar]
- Rosenstock, J.; Allison, D.; Birkenfeld, A.L.; Blicher, T.M.; Deenadayalan, S.; Jacobsen, J.B.; Serusclat, P.; Violante, R.; Watada, H.; Davies, M. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: The PIONEER 3 randomized clinical trial. JAMA 2019, 321, 1466–1480. [Google Scholar] [CrossRef] [PubMed]
- Trandafir, L.M.; Dodi, G.; Frasinariu, O.; Luca, A.C.; Butnariu, L.; Tarca, E.; Moisa, S.M. Tackling Dyslipidemia in Obesity from a Nanotechnology Perspective. Nutrients 2022, 14, 3774. [Google Scholar] [CrossRef]
- Alshareeda, A.T.; Khatijah, M.Z.N.; Al-Sowayan, B.S. Nanotechnology: A revolutionary approach to prevent breast cancer recurrence. Asian J. Surg. 2023, 46, 13–17. [Google Scholar] [CrossRef]
- Jakhar, D.K.; Vishwakarma, V.K.; Singh, R.; Jadhav, K.; Shah, S.D.; Arora, T.; Verma, R.K.; Yadav, H.N. Fat fighting liraglutide based nano-formulation to reverse obesity: Design, development and animal trials. Int. J. Pharm. 2023, 634, 122585. [Google Scholar]
- Engin, A.B.; Engin, E.D.; Engin, A. Targeted Nano-Based Systems for the Anti-Obesity Agent’s Delivery. In Obesity and Lipotoxicity; Springer: Berlin/Heidelberg, Germany, 2024; pp. 657–676. [Google Scholar]
- Pinto, S.; Viegas, J.; Cristelo, C.; Pacheco, C.; Barros, S.; Buckley, S.T.; Garousi, J.; Gräslund, T.; Santos, H.A.; Sarmento, B. Bioengineered Nanomedicines Targeting the Intestinal Fc Receptor Achieve the Improved Glucoregulatory Effect of Semaglutide in a Type 2 Diabetic Mice Model. ACS Nano 2024, 18, 28406–28424. [Google Scholar] [CrossRef]
- Eissa, N.G.; Elsabahy, M.; Allam, A. Engineering of smart nanoconstructs for delivery of glucagon-like peptide-1 analogs. Int. J. Pharm. 2021, 597, 120317. [Google Scholar]
- Kim, G.L.; Song, J.G.; Han, H.-K. Enhanced oral efficacy of semaglutide via an ionic nanocomplex with organometallic phyllosilicate in type 2 diabetic rats. Pharmaceutics 2024, 16, 886. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Che, J.; Zhang, Y.; Yin, T.; Gou, J.; Tang, X.; Wang, Y.; He, H. Sodium glycocholate liposome encapsulated semaglutide increases oral bioavailability by promoting intestinal absorption. Int. J. Pharm. 2024, 665, 124669. [Google Scholar] [CrossRef]
- Sandhu, J.S.; Das, R.; Mehta, D.K.; Dhanawat, M. Virtue of Nanotechnology in Confronting Obesity: Recent Advances. Nanosci. Nanotechnol.-Asia 2021, 11, 1–12. [Google Scholar]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of nanotechnology in medical field: A brief review. Glob. Health J. 2023, 7, 70–77. [Google Scholar]
- Lee, D.N.; Yang, S.B.; Kweon, S.; Lee, J.H.; Lee, K.J.; Ryu, Y.; Shin, D.W.; Kim, Y.J.; Lee, Y.K.; Park, J. Design and development of novel self-assembled catechol-modified bile acid conjugates as pH-responsive apical sodium-dependent bile acid transporter targeting nanoparticles. Biomaterials 2024, 308, 122539. [Google Scholar]
- Buniyamin, I.; Akhir, R.M.; Asli, N.A.; Khusaimi, Z.; Malek, M.F.; Mahmood, M.R. Nanotechnology applications in biomedical systems. Curr. Nanomater. 2022, 7, 167–180. [Google Scholar]
- Astrup, A.; Carraro, R.; Finer, N.; Harper, A.; Kunesova, M.; Lean, M.; Niskanen, L.; Rasmussen, M.; Rissanen, A.; Rössner, S. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. 2012, 36, 843–854. [Google Scholar]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; Le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar]
- Morofuji, Y.; Nakagawa, S.; Ujifuku, K.; Fujimoto, T.; Otsuka, K.; Niwa, M.; Tsutsumi, K. Beyond Lipid-Lowering: Effects of Statins on Cardiovascular and Cerebrovascular Diseases and Cancer. Pharmaceuticals 2022, 15, 151. [Google Scholar] [CrossRef]
- de Boer, L.M.; Oorthuys, A.O.J.; Wiegman, A.; Langendam, M.W.; Kroon, J.; Spijker, R.; Zwinderman, A.H.; Hutten, B.A. Statin therapy and lipoprotein(a) levels: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2022, 29, 779–792. [Google Scholar]
- Lee, K.J.; Lee, Y.M.; Yang, S.B.; Lee, J.H.; Kim, H.; Lim, J.H.; Park, J. A novel chemically engineered multifunctional statin conjugate as self-assembled nanoparticles inhibiting bile acid transporters. J. Control. Release 2024, 372, 885–900. [Google Scholar]
- Byrne, P.; Demasi, M.; Jones, M.; Smith, S.M.; O’Brien, K.K.; DuBroff, R. Evaluating the Association Between Low-Density Lipoprotein Cholesterol Reduction and Relative and Absolute Effects of Statin Treatment A Systematic Review and Meta-analysis. JAMA Intern. Med. 2022, 182, 474–481. [Google Scholar]
- Yu, D.B.; Liao, J.K. Emerging views of statin pleiotropy and cholesterol lowering. Cardiovasc. Res. 2022, 118, 413–423. [Google Scholar]
- Adhyaru, B.B.; Jacobson, T.A. Safety and efficacy of statin therapy. Nat. Rev. Cardiol. 2018, 15, 757–769. [Google Scholar]
- Fu, J.C.; Zhou, Z.B.; Fan, M.D. A Synergistic Semaglutide-targeted Fluorescent Nanodrug Delivery System and its Therapeutic Efficacy in Diabetic Nephropathy Treatment. J. Inorg. Organomet. Polym. Mater. 2024, 1–10. [Google Scholar]
- Lee, J.H.; Lim, H.; Ma, G.; Kweon, S.; Park, S.J.; Seo, M.; Lee, J.H.; Yang, S.B.; Jeong, H.G.; Park, J. Nano-anticoagulant based on carrier-free low molecular weight heparin and octadecylamine with an albumin shuttling effect. Nat. Commun. 2024, 15, 6769. [Google Scholar] [CrossRef]
- Yang, S.-B.; Lee, D.-N.; Lee, J.-H.; Seo, M.; Shin, D.W.; Lee, S.; Lee, Y.-H.; Park, J. Design and Evaluation of a Carrier-Free Prodrug-Based Palmitic–DEVD–Doxorubicin Conjugate for Targeted Cancer Therapy. Bioconjugate Chem. 2023, 34, 333–344. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-J.; Yang, S.-B.; Lee, J.-H.; Seo, B.; Won, H.-S.; Park, J. Preparation and Therapeutic Evaluation of Engineered Semaglutide and Statin–Lipid Conjugate-Based Nanoparticle. Pharmaceutics 2025, 17, 480. https://doi.org/10.3390/pharmaceutics17040480
Lee K-J, Yang S-B, Lee J-H, Seo B, Won H-S, Park J. Preparation and Therapeutic Evaluation of Engineered Semaglutide and Statin–Lipid Conjugate-Based Nanoparticle. Pharmaceutics. 2025; 17(4):480. https://doi.org/10.3390/pharmaceutics17040480
Chicago/Turabian StyleLee, Kyeong-Ju, Seong-Bin Yang, Jae-Hyeon Lee, Bison Seo, Hyung-Sik Won, and Jooho Park. 2025. "Preparation and Therapeutic Evaluation of Engineered Semaglutide and Statin–Lipid Conjugate-Based Nanoparticle" Pharmaceutics 17, no. 4: 480. https://doi.org/10.3390/pharmaceutics17040480
APA StyleLee, K.-J., Yang, S.-B., Lee, J.-H., Seo, B., Won, H.-S., & Park, J. (2025). Preparation and Therapeutic Evaluation of Engineered Semaglutide and Statin–Lipid Conjugate-Based Nanoparticle. Pharmaceutics, 17(4), 480. https://doi.org/10.3390/pharmaceutics17040480






