Evaluation of Olive Oil-Based Formulations Loaded with Baricitinib for Topical Treatment of Alopecia Areata
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Biological Tissue for Ex Vivo Permeation Study
2.3. Olive Oils
2.4. Preparation of Olive Oil-Based Formulations
2.5. Physicochemical Characterization of Oils Formulations
2.5.1. Chromatographic Operating Conditions for the Quantification of BCT
2.5.2. pH Measurements
2.5.3. Extensibility Test
2.5.4. Rheological Studies
2.6. Antioxidant Properties
2.7. In Vitro Release Studies of Oils Fomulations
2.8. Ex Vivo Permeation of Oils Formulation Through Skin
2.8.1. Calculation of the Permeation Parameters
2.8.2. Amount of BCT Retained in the Skin
2.9. Tolerance and Efficacy Studies
2.9.1. In Vivo Tolerance Study by Evaluating the Biomechanical Properties of Human Skin
2.9.2. Efficacy Study of Oils Formulations on Hair Growth in Mice
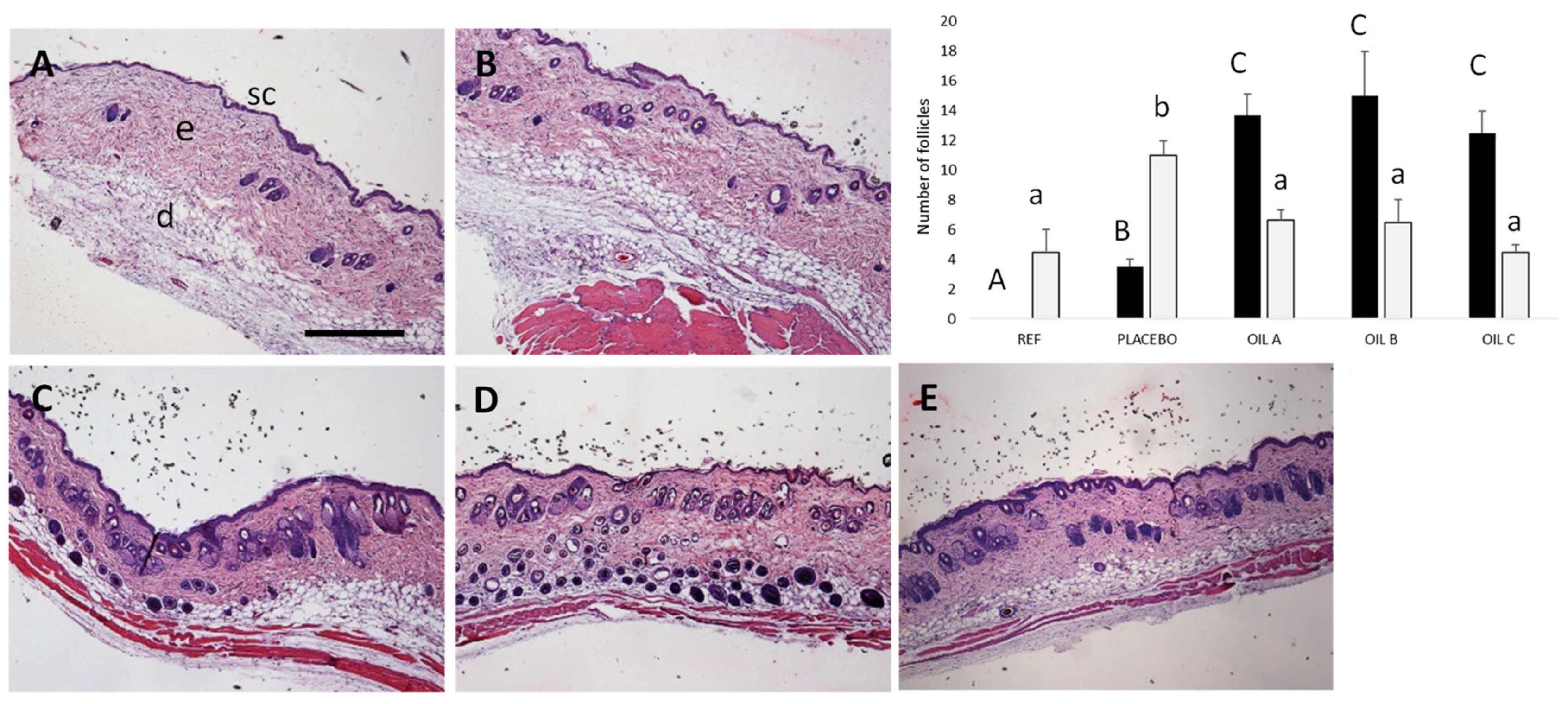
2.9.3. Histological Analysis
2.10. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of Oils
Rheological Behavior
3.2. Antioxidant Test
3.3. In Vitro Release of Oils Formulations
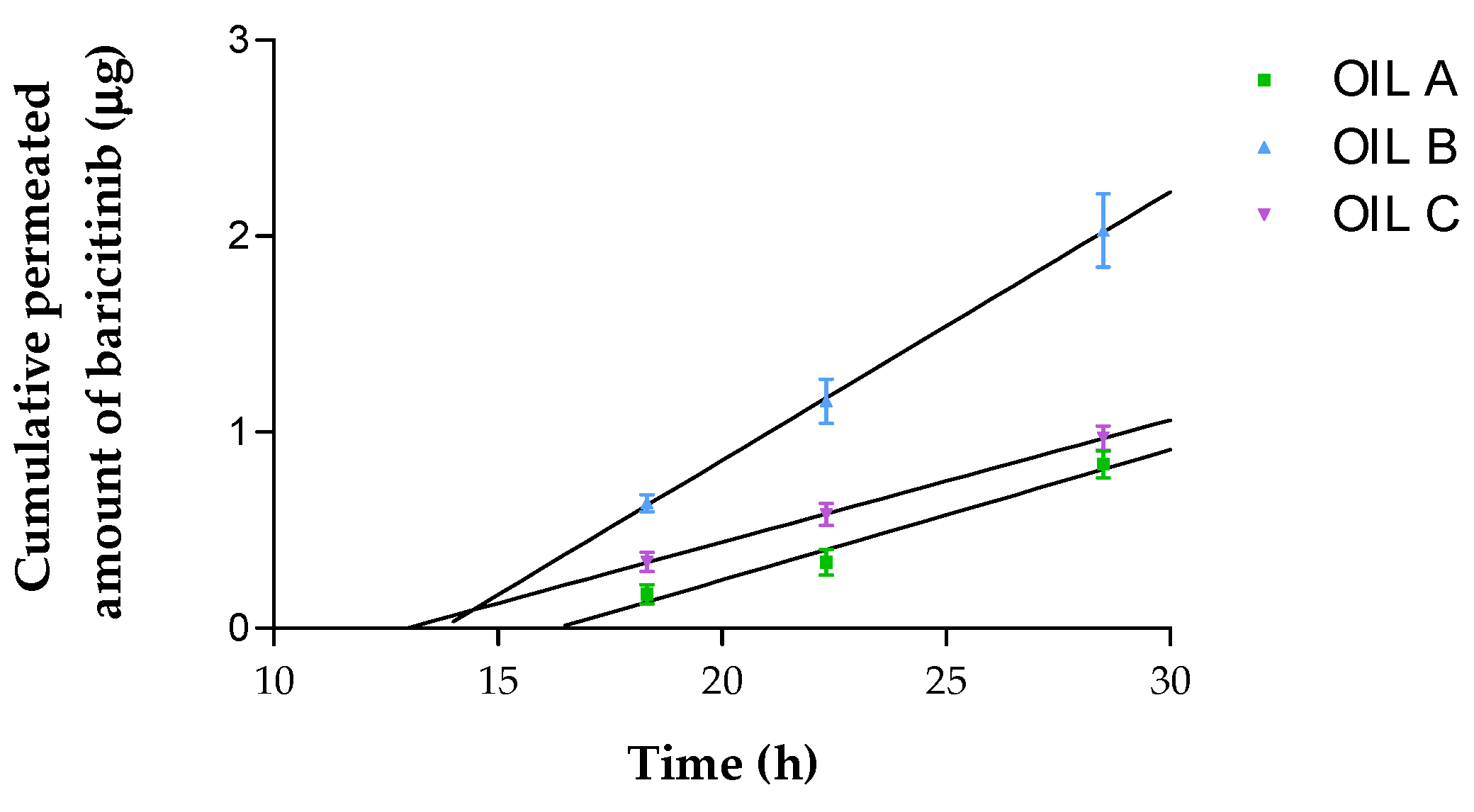
3.4. Ex Vivo Permeation of Oils Through Human Skin
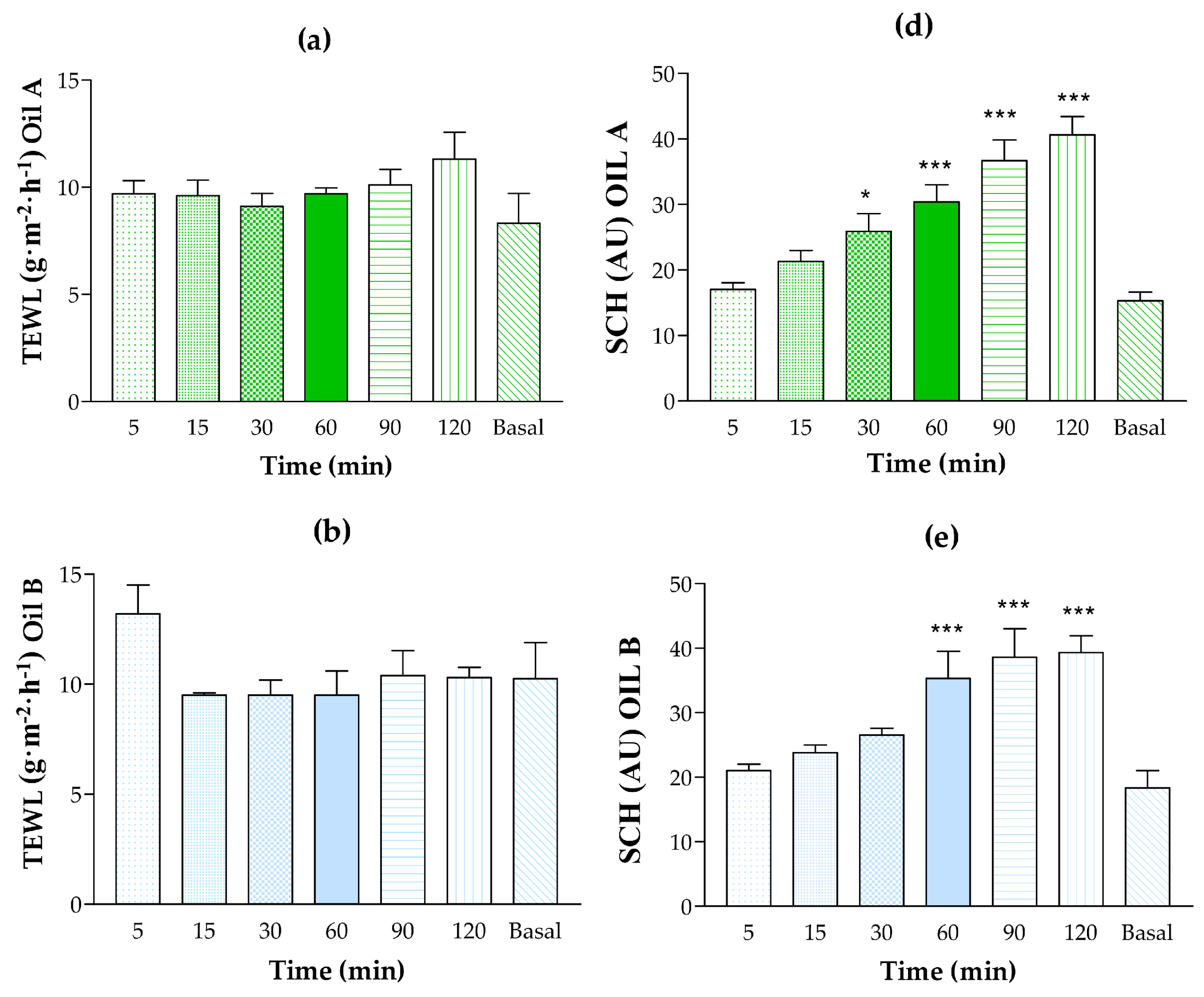
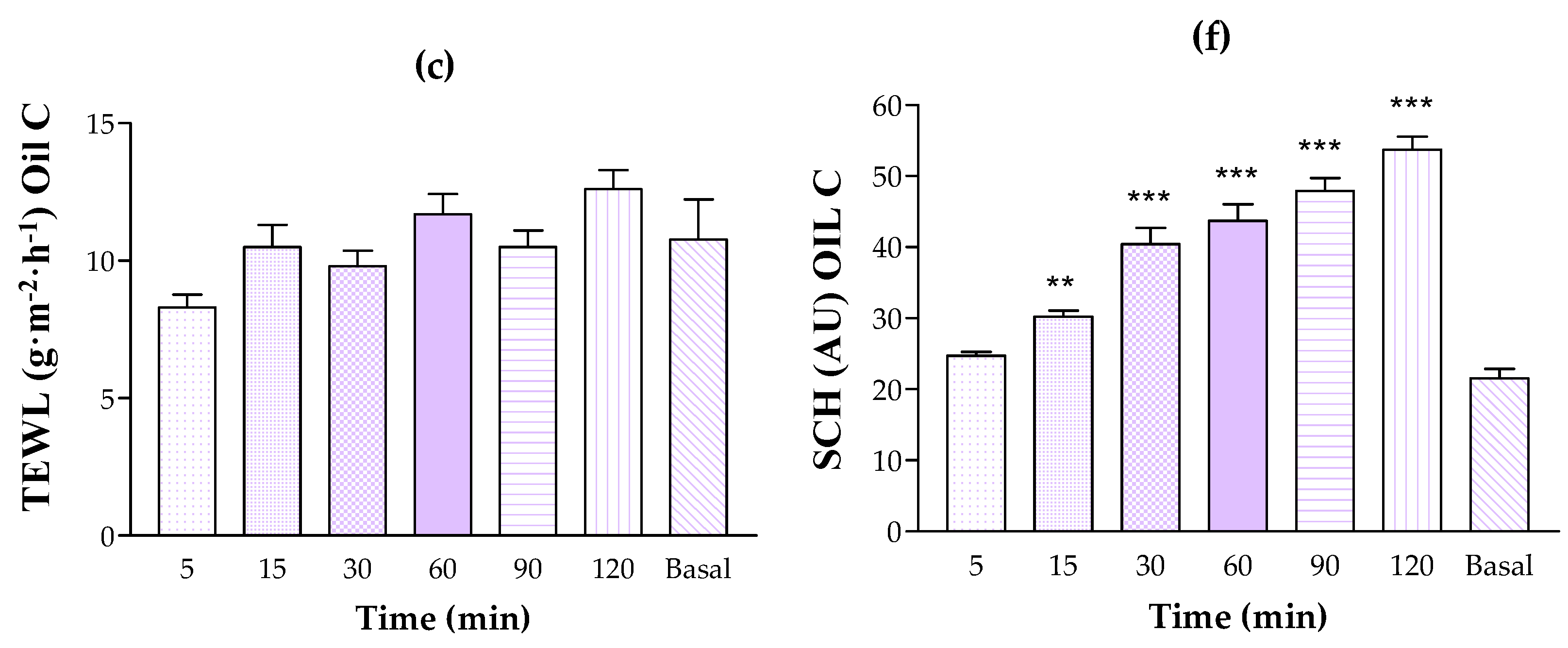
3.5. Skin Tolerance in Humans and Hair Growth Efficacy in Mice of Oil-Based Formulations
3.5.1. Human Skin Tolerance Test
3.5.2. Efficacy Study of Oils Formulations on Hair Growth in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John, K.K.G.; Brockschmidt, F.F.; Redler, S.; Herold, C.; Hanneken, S.; Eigelshoven, S.; Giehl, K.A.; De Weert, J.; Lutz, G.; Kruse, R.; et al. Genetic Variants in CTLA4 Are Strongly Associated with Alopecia Areata. J. Investig. Dermatol. 2011, 131, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Leung, P.S.C.; Huntley, A.C.; Gershwin, M.E. The Autoimmune Basis of Alopecia Areata: A Comprehensive Review. Autoimmun. Rev. 2015, 14, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; De Jong, A.; Harel, S.; Destefano, G.M.; Rothman, L.; et al. Alopecia Areata Is Driven by Cytotoxic T Lymphocytes and Is Reversed by JAK Inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wan, S.; Xie, B.; Song, X. Novel Potential Therapeutic Targets of Alopecia Areata. Front. Immunol. 2023, 14, 1148359. [Google Scholar] [CrossRef]
- Passeron, T.; King, B.; Seneschal, J.; Steinhoff, M.; Jabbari, A.; Ohyama, M.; Tobin, D.J.; Randhawa, S.; Winkler, A.; Telliez, J.B.; et al. Inhibition of T-Cell Activity in Alopecia Areata: Recent Developments and New Directions. Front. Immunol. 2023, 14, 1243556. [Google Scholar] [CrossRef]
- Strazzulla, L.C.; Wang, E.H.C.; Avila, L.; Lo Sicco, K.; Brinster, N.; Christiano, A.M.; Shapiro, J. Alopecia Areata: Disease Characteristics, Clinical Evaluation, and New Perspectives on Pathogenesis. J. Am. Acad. Dermatol. 2018, 78, 1–12. [Google Scholar] [CrossRef]
- Craiglow, B.G.; King, B.A. Killing Two Birds with One Stone: Oral Tofacitinib Reverses Alopecia Universalis in a Patient with Plaque Psoriasis. J. Investig. Dermatol. 2014, 134, 2988–2990. [Google Scholar] [CrossRef]
- Mohammadi-Meyabadi, R.; Beirampour, N.; Garrós, N.; Alvarado, H.L.; Limón, D.; Silva-Abreu, M.; Calpena, A.C.; Mallandrich, M. Assessing the Solubility of Baricitinib and Drug Uptake in Different Tissues Using Absorption and Fluorescence Spectroscopies. Pharmaceutics 2022, 14, 2714. [Google Scholar] [CrossRef]
- Taylor, P.C.; Laedermann, C.; Alten, R.; Feist, E.; Choy, E.; Haladyj, E.; De La Torre, I.; Richette, P.; Finckh, A.; Tanaka, Y. A JAK Inhibitor for Treatment of Rheumatoid Arthritis: The Baricitinib Experience. J. Clin. Med. 2023, 12, 4527. [Google Scholar] [CrossRef]
- Radi, G.; Simonetti, O.; Rizzetto, G.; Diotallevi, F.; Molinelli, E.; Offidani, A. Baricitinib: The First Jak Inhibitor Approved in Europe for the Treatment of Moderate to Severe Atopic Dermatitis in Adult Patients. Healthcare 2021, 9, 1575. [Google Scholar] [CrossRef]
- Nezamololama, N.; Fieldhouse, K.; Metzger, K.; Gooderham, M. Emerging Systemic JAK Inhibitors in the Treatment of Atopic Dermatitis: A Review of Abrocitinib, Baricitinib, and Upadacitinib. Drugs Context 2020, 9, 2020-8-5. [Google Scholar] [CrossRef] [PubMed]
- Olamiju, B.; Friedmann, A.; King, B. Treatment of Severe Alopecia Areata with Baricitinib. JAAD Case Rep. 2019, 5, 892–894. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Kontzias, A.; Yamaoka, K.; Tanaka, Y.; Laurence, A. Janus Kinase Inhibitors in Autoimmune Diseases. Ann. Rheum. Dis. 2013, 72, ii111–ii115. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, A.; Dai, Z.; Xing, L.; Cerise, J.E.; Ramot, Y.; Berkun, Y.; Sanchez, G.A.M.; Goldbach-Mansky, R.; Christiano, A.M.; Clynes, R.; et al. Reversal of Alopecia Areata Following Treatment with the JAK1/2 Inhibitor Baricitinib. EBioMedicine 2015, 2, 351–355. [Google Scholar] [CrossRef]
- Taheri, M.; Amiri-Farahani, L. Anti-Inflammatory and Restorative Effects of Olives in Topical Application. Dermatol. Res. Pract. 2021, 2021, 9927976. [Google Scholar] [CrossRef]
- Visioli, F.; Davalos, A.; López de las Hazas, M.C.; Crespo, M.C.; Tomé-Carneiro, J. An Overview of the Pharmacology of Olive Oil and Its Active Ingredients. Br. J. Pharmacol. 2020, 177, 1316–1330. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Barros, L.; Soares, M.E.; Bastos, M.L.; Pereira, J.A. Antioxidant Activity and Phenolic Contents of Olea europaea L. Leaves Sprayed with Different Copper Formulations. Food Chem. 2007, 103, 188–195. [Google Scholar] [CrossRef]
- Garrós, N.; Bustos-Salgados, P.; Domènech, Ò.; Rodríguez-Lagunas, M.J.; Beirampour, N.; Mohammadi-Meyabadi, R.; Mallandrich, M.; Calpena, A.C.; Colom, H. Baricitinib Lipid-Based Nanosystems as a Topical Alternative for Atopic Dermatitis Treatment. Pharmaceuticals 2023, 16, 894. [Google Scholar] [CrossRef]
- Mohammadi-Meyabadi, R.; Mallandrich, M.; Beirampour, N.; Garrós, N.; Espinoza, L.C.; Sosa, L.; Suñer-Carbó, J.; Rodríguez-Lagunas, M.J.; Garduño-Ramírez, M.L.; Calpena-Campmany, A.C. Lipid-Based Formulation of Baricitinib for the Topical Treatment of Psoriasis. Pharmaceutics 2024, 16, 1287. [Google Scholar] [CrossRef]
- Nene, S.; Devabattula, G.; Vambhurkar, G.; Tryphena, K.P.; Khatri, D.K.; Godugu, C.; Singh, P.K.; Srivastava, S. Topical Delivery of Baricitinib-Impregnated Nanoemulgel: A Promising Platform for Inhibition of JAK—STAT Pathway for the Effective Management of Atopic Dermatitis. Drug Deliv. Transl. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- El Moussaoui, S.; Fernández-Campos, F.; Alonso, C.; Limón, D.; Halbaut, L.; Garduño-Ramirez, M.L.; Calpena, A.C.; Mallandrich, M. Topical Mucoadhesive Alginate-Based Hydrogel Loading Ketorolac for Pain Management after Pharmacotherapy, Ablation, or Surgical Removal in Condyloma Acuminata. Gels 2021, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, D.; Alcover, M.M.; Sessa, M.; Halbaut, L.; Guill, C.; Boix-montañ, A.; Fisa, R. Topical Amphotericin B Semisolid Dosage Form for Cutaneous Leishmaniasis: Physicochemical Characterization, Ex Vivo Skin Permeation and Biological Activity. Pharmaceutics 2020, 12, 149. [Google Scholar] [CrossRef]
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. Paper-Based DPPH Assay for Antioxidant Activity Analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of Antiradical Properties of Antioxidants Using DPPH-Assay: A Critical Review and Results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Garrós, N.; Mallandrich, M.; Beirampour, N.; Mohammadi, R.; Domènech, Ò.; Rodríguez-Lagunas, M.J.; Clares, B.; Colom, H. Baricitinib Liposomes as a New Approach for the Treatment of Sjögren’s Syndrome. Pharmaceutics 2022, 14, 1895. [Google Scholar] [CrossRef]
- Pérez-González, N.; Rodríguez-Lagunas, M.J.; Calpena-Campmany, A.C.; Bozal-de Febrer, N.; Halbaut-Bellowa, L.; Mallandrich, M.; Clares-Naveros, B. Caspofungin-Loaded Formulations for Treating Ocular Infections Caused by Candida spp. Gels 2023, 9, 348. [Google Scholar] [CrossRef]
- Cañadas-Enrich, C.; Abrego, G.; Alvarado, H.L.; Calpena-Campmany, A.C. Pranoprofen Quantification in Ex Vivo Corneal and Scleral Permeation Samples: Analytical Validation. J. Pharm. Biomed. Anal. 2018, 160, 109–118. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Clin. Rev. Educ. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Suzuki, K.; Ponnappan, A.; VanDeusen, J.B.; Cooper, M.A.; Florea, S.M.; Freud, A.G.; Robinson, M.L.; Durbin, J.; Caligiuri, M.A. Fatal Leukemia in Interleukin 15 Transgenic Mice Follows Early Expansions in Natural Killer and Memory Phenotype CD8+ T Cells. J. Exp. Med. 2001, 193, 219–231. [Google Scholar] [CrossRef]
- Whiting, D.A. The Histopathology of Alopecia Areata in Vertical and Horizontal Sections. Dermatol. Ther. 2001, 14, 297–305. [Google Scholar] [CrossRef]
- Schramm, G.A. Practical Approach to Rheology and Rheometry, 2nd ed.; Gebrueder HAAKE GmbH: Karlsruhe, Germany, 2004. [Google Scholar]
- Sosa, L.; Clares, B.; Alvarado, H.L.; Bozal, N.; Domenech, O.; Calpena, A.C. Amphotericin B Releasing Topical Nanoemulsion for the Treatment of Candidiasis and Aspergillosis. Nanomedicine 2017, 13, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, J.; Stefaniak, A.; Eloff, F.; John, S.; Agner, T.; Chou, T.C.; Nixon, R.; Steiner, M.; Franken, A.; Kudla, I.; et al. International Guidelines for the In Vivo Assessment of Skin Properties in Non-Clinical Settings: Part 2. Transepidermal Water Loss and Skin Hydration. Ski. Res. Technol. 2013, 19, 265–278. [Google Scholar] [CrossRef]
- Matsui, N.; Ohnishi, A.; Uyama, A.; Sugino, T.; Terada, T.; Tsukamoto, M. Wearable System for Continuous Estimation of Transepidermal Water Loss. Electronics 2024, 13, 4779. [Google Scholar] [CrossRef]
- Godwin, D.A.; Kim, N.H.; Felton, L.A. Influence of Transcutol® CG on the Skin Accumulation and Transdermal Permeation of Ultraviolet Absorbers. Eur. J. Pharm. Biopharm. 2002, 53, 23–27. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. In Vivo Skin Permeation of Sodium Naproxen Formulated in Pluronic F-127 Gels: Effect of Azone and Transcutol. Drug Dev. Ind. Pharm. 2005, 31, 447–454. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.H.; Korting, H.C. The PH of the Skin Surface and Its Impact on the Barrier Function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef]
- Masaki, H. Role of Antioxidants in the Skin: Anti-Aging Effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Shalaby, K. Effect of Olive Oil Acidity on Skin Delivery of Diclofenac: In Vitro Evaluation and Ex Vivo Skin Permeability Studies. J. Biomed. Nanotechnol. 2022, 18, 234–242. [Google Scholar] [CrossRef]
- Mahanty, J.; Rasheed, S.H.; Kumar, S.; Singh, H.; Sharma, A. Potential of Essential Oils as Alternative Permeation Enhancers for Transdermal Delivery. World J. Tradit. Chin. Med. 2023, 9, 258–269. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Vera-García, R.; Silva-Abreu, M.; Domènech, Ò.; Badia, J.; Rodríguez-Lagunas, M.J.; Clares, B.; Calpena, A.C. Topical Pioglitazone Nanoformulation for the Treatment of Atopic Dermatitis: Design, Characterization and Efficacy in Hairless Mouse Model. Pharmaceutics 2020, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, W.; Fang, L.; Liu, C. Investigation of Controlled Release Molecular Mechanism of Oil Phase in Spilanthol Emulsion: Development and In Vitro, In Vivo Characterization. AAPS PharmSciTech 2019, 20, 227. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Cruz, G.; León-López, A.; Cruz-Gómez, V.; Jiménez-Alvarado, R.; Aguirre-Álvarez, G. Collagen Hydrolysates for Skin Protection: Oral Administration and Topical Formulation. Antioxidants 2020, 9, 181. [Google Scholar] [CrossRef]
- Husein-ElAhmed, H.; Husein-ElAhmed, S. Comparative Efficacy of Oral Janus Kinase Inhibitors and Biologics in Adult Alopecia Areata: A Systematic Review and Bayesian Network Meta-Analysis. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.; Freitas, E.; Torres, T. Efficacy and Safety of Baricitinib in Patients with Alopecia Areata: Evidence to Date. Drugs Context 2023, 12, 2023-6-2. [Google Scholar] [CrossRef]
- Anzai, A.; Wang, E.H.C.; Lee, E.Y.; Aoki, V.; Christiano, A.M. Pathomechanisms of Immune-Mediated Alopecia. Int. Immunol. 2019, 31, 439–447. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Segura-Fernández-nogueras, M.V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández-González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin Barrier Function in Psoriasis and Atopic Dermatitis: Transepidermal Water Loss and Temperature as Useful Tools to Assess Disease Severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef]




| Oil A | Oil B | Oil C | |
|---|---|---|---|
| Squalene (mg/mL) | 1500 | 1000 | 150 |
| Tocopherol (mg/mL) | 30 | 40 | 15 |
| Hydroxytyrosol (mg/mL) | 1 | 10 | 0.1 |
| Tyrosol (mg/mL) | 50 | 150 | 0.5 |
| Formulation | Baricitinib Content (μg/mL) |
|---|---|
| Oil A | 219.96 ± 40.28 |
| Oil B | 158.33 ± 35.79 |
| Oil C | 149.08 ± 34.57 |
| Formulation | Rheological Behavior Model and Stretch Ramp-Down | Viscosity at 100 s−1 (mPa·s) |
|---|---|---|
| Oil A | Newtonian (Newton, r = 0.9999) | 68.67 ± 0.10 |
| Oil B | Newtonian (Newton, r = 0.9999) | 78.15 ± 0.01 |
| Oil C | Newtonian (Newton, r = 1) | 59.64 ± 0.05 |
| Sample | DPPH % of Reduction (Mean ± SD) | ||
|---|---|---|---|
| 10 ppm | 100 ppm | 1000 ppm | |
| Oil A− | −4.212 ± 1.758 | −3.796 ± 0.450 | 10.088 ± 1.531 |
| Oil B− | −5.720 ± 3.602 | −0.7280 ± 0.392 | 17.680 ± 0.392 |
| Oil C− | −1.352 ± 0.238 | −4.368 ± 2.298 | 0.708 ± 1.261 |
| Oil A | −8.736 ± 6.398 | 1.300 ± 1.250 | 15.184 ± 1.729 |
| Oil B | −5.668 ± 3.235 | 2.808 ± 0.6619 | 17.836 ± 1.288 |
| Oil C | −2.808 ± 0.2548 | −0.1560 ± 1.544 | 12.012 ± 0.3821 |
| BCT solution | −1.300 ± 2.944 | −2.860 ± 0.9215 | −13.931 ± 1.808 |
| Parameter | Oil A | Oil B | Oil C | Tukey’s Multiple Comparison Test | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Oil A vs. Oil B | Oil A vs. Oil C | Oil B vs. Oil C | |
| AUC 0–52 (μg·h) | 2158.00 ± 215.4 | 1399.00 ± 127.30 | 1675.00 ± 159.30 | p < 0.001 | p < 0.01 | p > 0.05 |
| Efficiency (%) | 62.90 ± 6.20 | 40.80 ± 4.20 | 48.80 ± 5.10 | p < 0.001 | p < 0.01 | p > 0.05 |
| MRT (h) | 19.30 ± 1.30 | 30.80 ± 3.00 | 26.60 ± 2.70 | p < 0.001 | p < 0.01 | p > 0.05 |
| A52 (μg) | 65.90 ± 6.80 | 46.30 ± 4.90 | 42.60 ± 4.10 | p < 0.001 | p < 0.001 | p > 0.05 |
| Parameter | Oil A | Oil B | Oil C | Tukey’s Multiple Comparison Test | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Oil A vs. Oil B | Oil A vs. Oil C | Oil B vs. Oil C | |
| J (μg/h) | 0.066 ± 0.011 | 0.137 ± 0.003 | 0.062 ± 0.001 | p < 0.001 | p > 0.05 | p < 0.001 |
| J/sup (μg/h/cm2) | 0.104 ± 0.017 | 0.214 ± 0.005 | 0.097 ± 0.001 | p < 0.001 | p > 0.05 | p < 0.001 |
| Kp (cm/h) | 0.003 ± 0.001 | 0.015 ± 0.000 | 0.005 ± 0.000 | p < 0.001 | p < 0.001 | p < 0.001 |
| Tl (h) | 16.31 ± 1.65 | 13.76 ± 1.48 | 12.96 ± 1.35 | p < 0.05 | p < 0.01 | p > 0.05 |
| P1 (cm) | 0.299 ± 0.005 | 1.232 ± 0.003 | 0.399 ± 0.000 | p < 0.001 | p < 0.001 | p < 0.001 |
| P2 (1/h) | 0.010 ± 0.001 | 0.012 ± 0.001 | 0.013 ± 0.001 | p > 0.05 | p < 0.05 | p > 0.05 |
| Css (ng/mL) | 0.198 ± 0.033 | 0.409 ± 0.009 | 0.186 ± 0.002 | p < 0.001 | p > 0.05 | p < 0.001 |
| Qret (ng/cm2) | 1875.00 ± 124.32 | 468.75 ± 54.38 | 125.00 ± 12.37 | p < 0.001 | p < 0.001 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beirampour, N.; Mallandrich, M.; Bustos-Salgado, P.; Domínguez-Villegas, V.; Garrós, N.; Mohammadi-Meyabadi, R.; Clares-Naveros, B.; Romero-Olid, M.N.; Pérez-Cano, F.J.; Girbal, M.; et al. Evaluation of Olive Oil-Based Formulations Loaded with Baricitinib for Topical Treatment of Alopecia Areata. Pharmaceutics 2025, 17, 475. https://doi.org/10.3390/pharmaceutics17040475
Beirampour N, Mallandrich M, Bustos-Salgado P, Domínguez-Villegas V, Garrós N, Mohammadi-Meyabadi R, Clares-Naveros B, Romero-Olid MN, Pérez-Cano FJ, Girbal M, et al. Evaluation of Olive Oil-Based Formulations Loaded with Baricitinib for Topical Treatment of Alopecia Areata. Pharmaceutics. 2025; 17(4):475. https://doi.org/10.3390/pharmaceutics17040475
Chicago/Turabian StyleBeirampour, Negar, Mireia Mallandrich, Paola Bustos-Salgado, Valeri Domínguez-Villegas, Núria Garrós, Roya Mohammadi-Meyabadi, Beatriz Clares-Naveros, Maria Nuria Romero-Olid, Francisco J. Pérez-Cano, Marina Girbal, and et al. 2025. "Evaluation of Olive Oil-Based Formulations Loaded with Baricitinib for Topical Treatment of Alopecia Areata" Pharmaceutics 17, no. 4: 475. https://doi.org/10.3390/pharmaceutics17040475
APA StyleBeirampour, N., Mallandrich, M., Bustos-Salgado, P., Domínguez-Villegas, V., Garrós, N., Mohammadi-Meyabadi, R., Clares-Naveros, B., Romero-Olid, M. N., Pérez-Cano, F. J., Girbal, M., Rodríguez-Lagunas, M. J., Suñer-Carbó, J., & Calpena, A. C. (2025). Evaluation of Olive Oil-Based Formulations Loaded with Baricitinib for Topical Treatment of Alopecia Areata. Pharmaceutics, 17(4), 475. https://doi.org/10.3390/pharmaceutics17040475












